Abstract
Intestinal ultrasound (IUS) is emerging as a key tool to achieving the therapeutic target of transmural healing in inflammatory bowel disease (IBD). IUS is a non-invasive, radiation-free, imaging modality comparable to MRI, CT and ileocolonoscopy (IC). With the appropriate training and equipment, IUS can be an easily repeatable bedside test for IBD diagnosis and disease monitoring, including treatment response. Core to successful high quality IUS employment are appropriate training and expert techniques; however, the training pathway will not be explored in this review. Given the increasing shift towards objective assessment for tight disease control, gastroenterologist-led IUS should be incorporated into the armamentarium of imaging modalities alongside radiologists, to enhance our diagnostic and monitoring toolbox. This comprehensive review aims to outline the current literature around IUS and propose the placement of IUS in a treat-to-target algorithm in IBD. Ultimately, IUS facilitates timely management decisions to optimise patient care with potential to revolutionise patient outcomes, moving towards transmural healing as the holy grail of therapy in IBD.
Introduction
Inflammatory bowel diseases (IBD), comprised of Crohn’s disease (CD) and ulcerative colitis (UC), are chronic immune-mediated gastrointestinal disorders characterised by periods of activity and remission. Diagnosis relies on clinical, biochemical, endoscopic and histological parameters. 1 Intestinal ultrasound (IUS) exhibits increasing global uptake due to technological advancement, training opportunities and increasing expertise. 2–4
The updated Selecting Therapeutic Targets in Inflammatory Bowel Disease (STRIDE II) guidelines raise treatment expectations. Therapeutic targets focus on clinical and endoscopic healing (EH), however, aspirational targets include histologic and transmural healing (TH) which reduce relapse rates, hospitalisation and need for surgery. 1 Although ileocolonoscopy (IC) is the gold-standard for disease assessment, 5 myriad limitations include need for anaesthesia/sedation, invasiveness, high costs with challenging access. Cross-sectional imaging has limitations: CT imparts radiation and requires iodine-based intravenous (i.v.) contrast, thus is not recommended for surveillance 5,6 ; magnetic resonance enterography (MRE) involves long image acquisition times and often requires contrast. 6,7 IUS presents a safe, accurate, cost-effective alternative for transmural assessment. Cost comparison of these modalities is however needed. IUS can be performed during gastroenterology assessment. 8–10 This review aims to summarise how IUS is gaining recognition in IBD practice and illustrate IUS as an indispensable objective tool to guide therapy and limit disease progression and complications. 4
Imaging modalities in IBD: how does IUS compare?
Cross-sectional imaging is essential in IBD, as small bowel involvement is common, a ‘blind spot’ given limits in the reach of conventional endoscopy. 11 Symptoms inconsistently predict endoscopic activity: up to 18% of symptomatic patients with CD lack ulceration at IC while some without symptoms display severe lesions. 12–14 CT, MRI and IUS can be used to examine small bowel extent, skip lesions, strictures, fistulae or other penetrating complications. 5,15 Emerging literature suggests all three have comparable diagnostic accuracy in diagnosis, therapeutic response and complication identification 16–21 (Table 1). Notably, gastroenterologist-led IUS focuses on the intestine while other intra-abdominal/pelvic organs are not formally examined, a limitation that requires clear disclosure to patients. Where visualisation of the latter structures may be required, radiologic assessment should be sought formally.
Table 1.
Advantages of IUS as point-of-care tool
Convenience
|
|---|
Disease
diagnosis
|
Disease
monitoring
|
Disease
severity
|
Disease
therapy
|
IUS, intestinal ultrasound.
CTE
CTE has high accuracy for active small bowel inflammation, requiring oral and i.v. contrast. 22 CTE detects mural healing and therapeutic response, with modest correlation with clinical, biochemical or endoscopic activity indices. 23 Whilst CT detects small bowel pathology with equivalent sensitivity and specificity to MRI, radiation limits repeatability. 16,17,22 CT/CTE is most relevant in acute or emergency settings, where urgent assessment of potential complications is warranted. 6 Clinical and/or serological findings may guide appropriate CT/CTE utility to minimise recurrent radiation exposure. 5
MRE
MRE is preferred over CT without significant difference in disease localisation (p = 1.0), bowel wall thickening (BWT) (p = 1.0), wall enhancement (p = 1.0) or detection of fistulae (p = 0.08), lymphadenopathy (p = 1.0) and perivisceral fat enhancement (p = 0.31). Additionally, MRI is superior in per segment analysis in detecting ileal wall enhancement (p = 0.02) and strictures (p = 0.04). 22 Ample literature supports high correlation between MRE and endoscopy in assessment of inflammatory IBD activity. 20,24,25 MRE is however time-consuming and costly, somewhat limited by breath-holding ability and claustrophobia. 26 Risks of contrast agents when employed, such as nephrogenic systemic fibrosis and substance accumulation within the basal ganglia have been documented, however, the former is usually minimised through routine renal function testing and appropriate patient selection, and the clinical significance of the latter remains unclear. 27
IUS
A landmark prospective multicentre trial comparing the diagnostic accuracy of MRE and IUS for the extent and activity of newly-diagnosed and relapsed CD (METRIC) confirmed both are accurate with high sensitivity for detecting small intestinal disease. 21 MRE sensitivity was 97% (95% CI 91–99) for detecting terminal ileal disease with IUS sensitivity 91% (95% CI 79–97). 21 Colonic disease is universally more challenging: MRE sensitivity was 41% [26-58] while IUS was 49% [33-65], not statistically significant. 21 Unlike IUS, the accuracy of MR for distal colonic disease is enhanced with rectal contrast. 28 Literature demonstrates comparable agreement between IUS and MRE in detecting IBD location and activity, 19,29 although MRE is more sensitive in assessing disease extent. 19
Standard monitoring in IBD
Clinical, biochemical and endoscopic parameters are used to assess IBD activity and therapeutic response with a shift towards objective improvement to guide therapy. 1,5,30 Notably, imaging cannot replace endoscopy and biopsies for dysplasia surveillance and tissue diagnosis. 5
Biochemical markers
C-reactive protein (CRP) >5 mg l−1 has high specificity for detecting endoscopic IBD activity but low sensitivity in excluding a flare. 31 Faecal calprotectin (fcal), a neutrophil-derived protein with high sensitivity and low specificity for intestinal inflammation, correlates with endoscopic disease in IBD diagnosis, relapse and treatment response, particularly in the colon 5,32–34 with values <150 μg/g suggesting endoscopic healing (EH). 1 It is more specific and sensitive than CRP, unaffected by extraintestinal pathology. 35 Abnormal CRP and fcal should prompt exclusion of infection and confirmation with endoscopy or imaging. 5 Whilst blood and stool biomarkers are compositely used to predict disease activity and therapeutic response, they cannot predict disease location nor extent, and can be falsely negative. 1,36
Endoscopy
EH in CD is associated with improved long-term outcomes, reduction in bowel damage, relapse, surgery risk and complications even in clinical remission. 37,38 IC or flexible sigmoidoscopy ± biopsies remain the reference standard for disease activity assessment. 5 Mucosal healing (MH) is associated with long-term corticosteroid-free clinical remission, reduced risk of colectomy and inflammation at 5 years. 39,40 Histologic healing is associated with reduced complications including hospitalisation, corticosteroid use 41 and cancer prevention. 42 However, histological targets are tempered by lack of established incremental gain over MH alone. 43
Video capsule endoscopy (VCE) is an adjunctive modality for small bowel evaluation. VCE is well-tolerated, minimally invasive, without sedation requirement; 44,45 however, access is not ubiquitous due to cost and local expertise. 46 Patency capsule clearance may be necessary 5,45 as VCE retention occurs in 2–13% due to stenoses/strictures. 47 It is considered safe to proceed to VCE if the patency capsule is passed after 30 h or confirmed radiologically. 48
Advantages of IUS compared to other monitoring techniques
Transmural healing (TH) is an aspirational target for CD beyond endoscopic MH, imparting further beneficial outcomes 49 with IUS well-placed to assess TH (Figure 1). Patient experience and preference are increasingly recognised in monitoring strategies, given the potential impact on adherence and outcome, yet have not directed algorithms nor guidelines to date. 5 IUS is well-tolerated, easily repeatable with opportunity to engage patients in real-time. 50 Otherwise, patients need to return to their provider for information. 35,51 Bedside IUS facilitates timely medical decision-making 35,51 a distinct advantages over MR/CT. 50 Patients report enhanced disease understanding and confidence in making informed treatment decisions. 51 Nearly all (99%) patients in the METRIC trial rated IUS as acceptable compared to 88% for MRE. 21 IUS also exhibits high interobserver agreement; 51,52 in the METRIC cohort, κ coefficient (k) was 0.64 for small bowel disease diagnosis and 0.63 for relapsing disease. 53 In UC, interobserver agreement for BWT is almost perfect with intraclass correlation coefficient (ICC) of 0.96, with substantial agreement for colour Doppler imaging (CDI) k = 0.63, disease activity k = 0.77 and ICC of 0.93 for disease severity emphasising IUS reliability. 54 Real-time interobserver agreement is also demonstrated in CD: six blinded operators exhibited moderate agreement on BWT and stratification, vascularisation, and lymphadenopathy and substantial agreement on lesion location, and presence of fistula, phlegmon and abscess. Not so for mesenteric adiposity changes, lesion extent, narrowing and prestenotic dilatation with poor agreement demonstrated. 55 In certain populations, IUS may be less prone to technical difficulties compared with MRE, without motion artifact causing image degradation 29 or bowel preparation. 50 There are recognised challenges in performance of IUS, such as increased abdominal adiposity limiting resolution of bowel loops in addition to limitations in anatomic resolution.
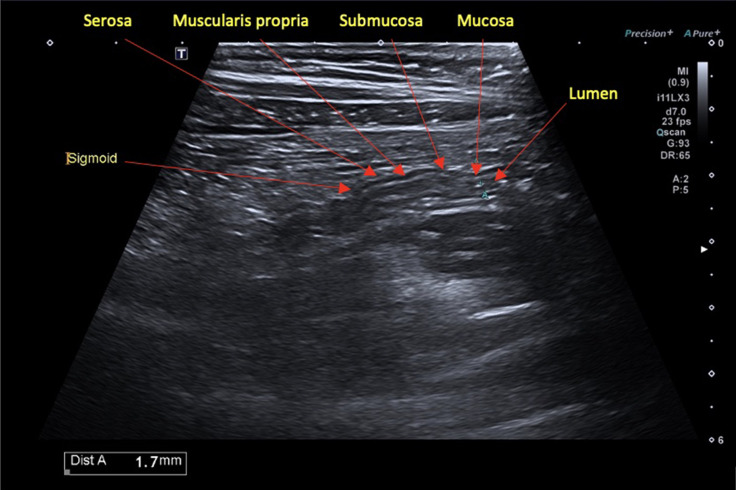
Figure 1.
Normal BWT seen in longitudinal view of the sigmoid colon. BWT, bowel wall thickening
IUS findings
Bowel wall thickness (BWT)
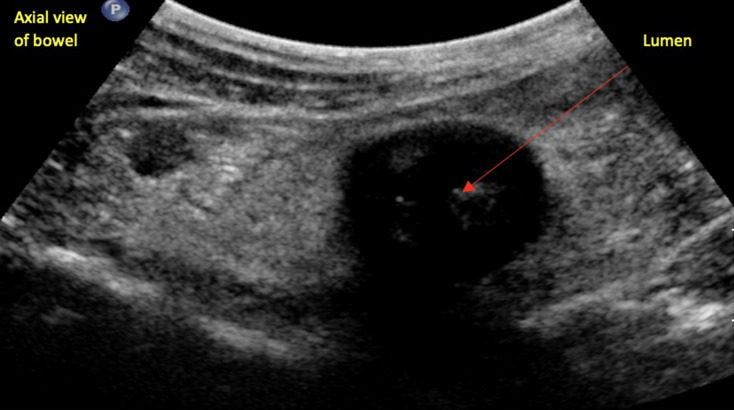
BWT is the most reliable and important measure of IBD activity with good interobserver agreement (k 0.72–1). 3,18,56 BWT is measured perpendicular to the wall in axial and longitudinal axes, from the mucosal interface to the serosa, avoiding mucosal folds and haustra 3,4,57 (Figure 1). A threshold value of >3 mm is agreed to be pathological, with a sensitivity of 88% and specificity of 93%, more accurate compared to a cut-off of 4 mm (75 and 97% respectively). 57,58 While some advocate for a continuous scoring of BWT, others categorise, e.g. Ultrasound Global Assessment of Disease Activity Score, BWT of 4.0–6.0 is mild inflammation; 6.1–8.0 moderate; and >8.0 severe. 57 BWT is considered a surrogate marker of transmural inflammation with thickening predictive of disease recurrence and surgery risk. 59,60
Bowel wall stratification (BWS)
IUS is the only modality that clearly depicts wall layering 4,57 (Figure 2). Interreader variability for detecting BWS is fairly reliable with k −0.22–0.85. 56 Focal and/or extensive disruption in BWS correlates with active disease. 3 Chronic inflammatory changes often lead to architectural alterations involving the intestinal submucosa, characterised by increased BWT and echogenicity. 57
Figure 2.
Complete loss of bowel wall stratification.
Hyperaemia
Increased vascularity of the bowel wall due to inflammation is assessed by colour Doppler imaging (CDI), set to detect low flow, small calibre vessels. 57 Normal bowel has minimal vascularity on CDI; however, increased intramural and extramural flow strongly correlates with histologic inflammation 61 (Figure 3a and b) with grading as suggested (Table 2) . There is variable interobserver agreement with k 0.53–0.89, 56 likely due to its semi-quantitative and subjective assessment, contingent upon machine settings and physiologic variability. 4 Reliable depiction of CDI requires careful, consistent machine calibration, breath-holding, and standard machine settings. 63 It is predominantly qualitative thus should be interpreted with some caution. Deep bowel loops may reveal falsely low signal, and inflamed bowel may not have detectable flow. However, CDI remains one of the most important parameters contributing to inflammatory activity.
Figure 3.
(a) Increased signal on colour Doppler imaging of the terminal ileum. (b) Grade 4 hyperaemia on Doppler with extension into mesentery.
Table 2.
Limberg grade description 61,62
| Grade 0 No bowel wall thickening, no vascularisation |
|---|
| Grade 1 Bowel wall thickening, no vascularisation |
| Grade 2 Bowel wall thickening with short stretches of vascularity |
| Grade 3 Bowel wall thickening with long stretches of vascularity |
| Grade 4 Bowel wall thickening with long stretches of vascularity reaching into the mesentery |
Lymphadenopathy
Mesenteric lymph nodes (LN) are common yet non-specific, often present in healthy individuals, especially children. 4,64,65 LNs > 10 mm (short axis) are more likely to be pathologic. 3,65 Interobserver LN assessment is reproducible (k 0.56–0.90). 56 However, the lack of association with clinical or biochemical activity and poor specificity excludes LNs from most activity indices. 63
Mesenteric fat hypertrophy (MFH)
Creeping fat or MFH due to fibro-fatty proliferation is important pathophysiologically, 66 appearing as hyperechoic, homogeneous tissue on the mesenteric aspect of diseased bowel. 3,64,67 Although it has poor interobserver agreement (k 0.14–0.69), 56 MFH is associated most strongly with histological grade of inflammation compared with focal hyperechogenicity without fat wrapping or stratified pattern. 68
Extramural complications: strictures, fistulae, abscesses
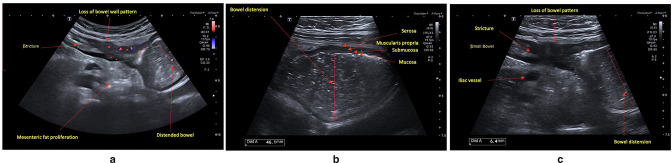
Strictures are common in CD, leading to clinical and subclinical obstruction (Figure 4a, b and c). Defined as luminal narrowing, strictures exhibit increased BWT, pre-stenotic dilatation, with luminal diameter greater than 25–30 mm often associated with hyperperistalsis of the prestenotic segment. 3,69 Early strictures may not exhibit proximal dilatation. Discerning the relative contribution of inflammatory vs fibrotic components (potentially reversible vs irreversible) remains challenging, impacting optimal medical vs surgical/endoscopic dilation intervention. 70 IUS techniques are rapidly evolving to address this conundrum: CDI, elastography, echostratification and contrast-enhanced ultrasound (CEUS) may provide valuable insight. 3,71 Strictures associated with inflammation may exhibit BWS loss and hyperaemia. Stratification loss may also signify smooth muscle hypertrophy, refractory to medical therapy (CDI may help to discern the two); fibrotic strictures can exhibit echogenicity and hypovascularity portending need for endoscopic dilatation or surgery. 3,64,72,73 IUS detection is highly accurate compared to CT/MRI and gross pathology: pooled sensitivity of 79% (95% CI 71–84%) and specificity 92% (95% CI 87–96%). 9,74 IUS also affords real-time assessment of intestinal motility, distinguishing collapsed bowel or functional contractions in strictures and dysmotility associated with obstruction. 64
Figure 4.
(a) Stricture with pre-stenotic features of hyperaemia, loss of wall stratification and marked mesenteric inflammatory fat (b). Dilated small bowel segment with stagnant fluid contents suggestive of bowel obstruction (c). Luminal narrowing with per-stenotic dilation and loss of wall stratification within a stricture.
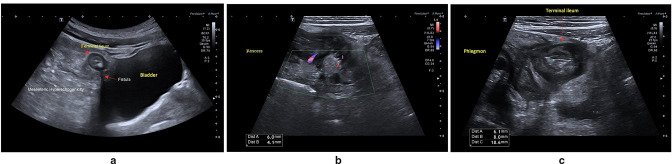
IUS can detect fistulae, sinus tracts and fissures 3,57,64 with similar accuracy to CT and MRI 22,75 (Figure 5a). Fistulae result from extramural fissures arising from deep intestinal ulcerations communicating with other tissues, whereas sinus tracts are linear extensions/blind-ends. 3,64 Both sinus tracts and fistulae are hypoechoic irregularities arising from thickened bowel with or without gas, with a diameter <2 cm. 3,19,76 In one systematic review, IUS had a pooled sensitivity of 74% (95% CI 67–79%) in detection of enteric fistulae compared with surgery. 16
Figure 5.
(a) Enterovesical fistula with echogenic inflammatory fat. (b) Penetrating terminal ileal disease with hypoechoic area (6*4.1 mm) suspicious for abscess with marked inflammatory for echo. (c) Severs increase in BWT in the terminal ileum hypoechoic area deep to inflamed loop suggestive of phlegmon. BWT, bowel wall thickening.
Compared with surgery, IUS has a pooled sensitivity of 84% (95% CI 79–88%) and specificity 93% (95% CI 89–95%) for abscess detection. 16 Microperforations may appear as inflammatory perienteric masses without extraluminal air (phlegmon). Spiculation or hypoechoic stranding from the outer serosa 57,77 are harbingers of penetrating complications: abscesses (Figure 5b) or poorly defined inflammatory masses (Figure 5c): hypoechoic masses with or without echogenic gas. 57 An inflammatory mass can be differentiated from abscess with CDI or CEUS. 3,57,64 Interobserver agreement is excellent for detection of stenosis in IBD using IUS (k 0.81–1), whereas for fistulae it varies. 56
IUS can characterise small bowel motility in real-time although standardisation is needed. Peristalsis is non-specific, however, can be reduced or absent in diseased bowel. Free intra-abdominal fluid juxtaposed to diseased bowel is non-specific but can reflect disease severity. 3,64
Utility of IUS in CD
Diagnosis, disease extent and activity
Although important for quality of life, clinical symptoms correlate poorly with objective inflammation. In the CALM trial, use of symptoms to guide treatment in CD resulted in lower rates of EH compared to patients with composite clinical and biomarker assessments. 36 IUS is comparable in diagnosing CD compared to MRE and IC 19,21 with diagnostic sensitivity of 94%, specificity of 97%, a positive-predictive value of 97% and negative-predictive value of 94%. 19 The highest diagnostic performance is in the ileum, sigmoid and descending colon, with lower accuracy in the duodenum, proximal jejunum and rectum. 10,78 Allocca et al 52 showed IUS had 92% sensitivity and 100% specificity for presence of ulcers at colonoscopy, with high diagnostic accuracy compared to MRE and IC: 91% for disease localisation, 96% for ulceration. 52 Repeatability during follow up and timely therapeutic response measurement is key: Kucharzik et al. 67 conducted the multicentre TRUST study, serially following CD patients over 12 months demonstrating IUS changes in response to therapy. 79
Post-operative recurrence
IUS confers high accuracy in detecting recurrence compared to IC, 64,80 whereas clinical and biomarkers correlate poorly with IC. 81 Although fcal is accurate, a moderate false-positive rate in discerning post-operative recurrence exits 82 which may improve when combined with IUS. 64 BWT correlates with endoscopic recurrence and lack of therapeutic response signifying surgical risk. 59,83,84 BWT > 3 mm had 79% sensitivity and 95% specificity for post-surgical endoscopic recurrence (Rutgeerts’ score ≥i2): sensitivity 93% for detecting severe post-surgical recurrence (Rutgeerts’ score ≥i3). 60
Utility of IUS in UC
Diagnosis, disease extent and activity
Evidence for IUS in UC is evolving with IC and biopsies the mainstay of UC diagnosis. 3,5,20,85 In UC, symptom correlation is still imperfect. 1,86 Persistent, low-grade inflammation portends worse outcomes, including hospitalisation and corticosteroid need. 41 IUS can be used to detect BWT in active UC 3 although absence of BWT does not exclude active inflammation, and CDI is an important tool to detect mild to moderate inflammation limited to the mucosa. It is accurate in detecting inflammation and defining extent proximal to the rectum. 3,87 TRUST&UC, a multicentre prospective observational study demonstrated IUS as a sensitive tool detecting disease activity with increased BWT in 88.5% of those flaring with left-sided or pancolitis. 35 Whilst rectal assessment can be challenging, a transperineal approach is promising 88 and IUS remains valuable conferring more than 70% sensitivity in detecting disease proximal to the rectum, with up to 97% sensitivity in the sigmoid and descending colon. 10 Loss of BWS, increased hyperaemia and MFH are also features of greater disease activity. 4,10,35
The TRUST group demonstrated BWT and CDI mirrored improvements in clinical scores and fcal at 3 months, with changes seen as early as 2 weeks. 35 This rapid IUS response to intensified therapy with subsequent response on clinical indices cements the role of IUS in UC monitoring. 35 Early evidence also predicts corticosteroid response in guiding salvage therapy timing in acute severe UC. 89
Adjunctive techniques in IUS
Small intestine contrast ultrasonography (SICUS)
Ingestion of a neutral oral contrast agent, (500–800 ml polyethylene-glcyol/PEG) 30 min pre-examination increases sensitivity for detection of proximal small bowel disease, strictures and penetrating complications. 3,64,76 Pallota et al showed SICUS had 97.5% sensitivity and 100% specificity in identifying at least one stricture (k = 0.93) and sensitivity of 75 and 100% specificity for at least two or more strictures (k = 0.78). 76 Additionally, SICUS had 96% sensitivity and 90.5% specificity (k = 0.88) in identifying fistulae in 27/28 patients as well as 100% sensitivity and 95% specificity (k = 0.89) in detecting intraabdominal abscesses in 10 patients undergoing bowel surgery. 76 Despite this, oral contrast is not commonly employed clinically, as it prolongs exam time and is not preferred by patients.
Transperineal ultrasound (TPUS)
A small high frequency curvilinear or linear transducer on the perineum can visualise the anal canal and surrounding soft tissues to characterise penetrating, perianal disease. 57,64 Presence of internal and external fistulae, location within the anal canal and clock-face representation can be accurately documented. 57 TPUS has high diagnostic accuracy compared with both endoanal ultrasound: sensitivity of 84.9% for fistulae detection 90 ; and MRI, with a sensitivity of 90.6%, with excellent agreement between TPUS and MRI (k = 0.783). 91 The accuracy in detecting abscesses has pooled sensitivity of 86% (95% CI 67–99%) and positive-predictive value of 90% (95% CI 76–99) in a meta-analysis. 92 Whilst TPUS is currently useful in screening for perianal disease, its role in assessing disease severity and monitoring response remains to be clarified, and operator expertise must be acknowledged. 92
Contrast-enhanced ultrasound (CEUS)
Although CEUS utility with standard greyscale B mode IUS for luminal assessment of disease activity is limited globally, it can aid in differentiation between an inflammatory mass vs abscess. IUS with microbubble contrast, comprised of 2–6 μm lipid-coated gas particles 57 is used to augment perfusion assessment. A retrospective review demonstrated 100% specificity for abscess detection among 71 inflammatory masses in 50 patients, resulting in k = 0.972 for differentiating abscess vs phlegmon. 93 The role of CEUS in activity assessment is contentious, with lack of agreement on peak enhancement or area under the curve thresholds, adding significant complexity to standard examination, limiting uptake.
Shear wave elastography (SWE)
SWE is an exciting IUS application measuring shear wave speed generated by an acoustic pressure wave through bowel as a marker of tissue density and stiffness in real-time, 57,64,94 reflecting fibrosis vs inflammation in strictures. SWE is higher in fibrosis with AUC of 0.822 when using cut-off 22.55 kPa as a discriminator between mild-moderate and severe fibrosis. 95 Combined with CEUS, SWE can accurately identify inflammation and smooth-muscle hypertrophy. 95 However, its application is limited as standardisation of measures across machines is lacking.
IUS activity scores: utility and limitations
There is no consensus on an optimal IUS scoring index. 96 The recently derived International Bowel Ultrasound Segmental Activity Score (IBUS-SAS) uses reliable sonographic components including BWT, BWS, CDS and inflammatory fat. 63 The Simple Sonographic Score comprises BWT and Doppler, 97 developed using statistically significant parameters in a retrospective then prospective evaluation of IUS with endoscopy with an AUROC of 0.836 when applied to a prospective cohort for external validation. 97 Due to lack of prospective validation, widespread use of scores is limited. Currently, a large international multicentre trial is in progress to establish this. 98
Targeting transmural remission (TR): IUS in clinical practice
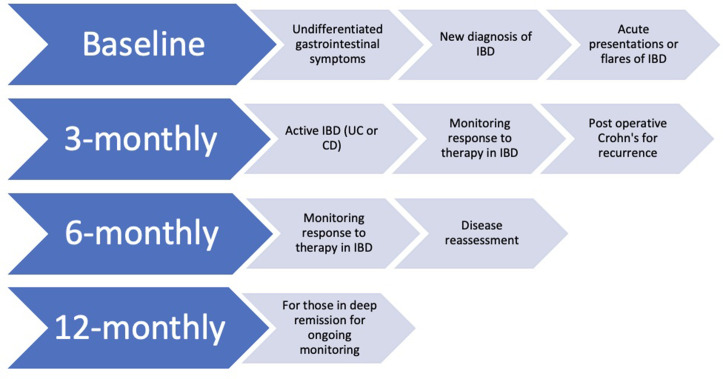
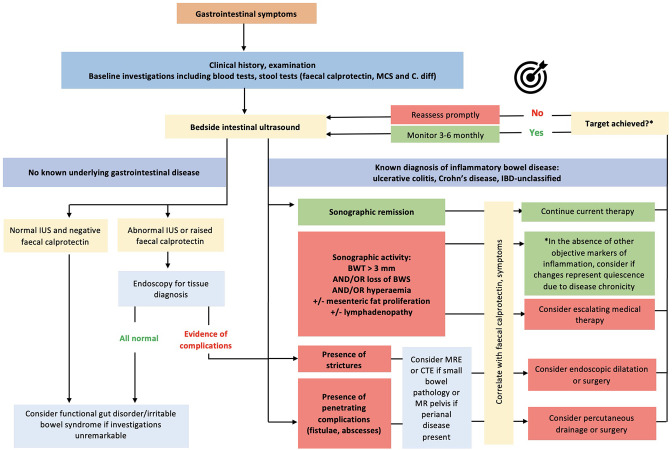
Transmural remission (TR), defined as the resolution of mucosal ulcerations, transmural disease and extramural disease 99 is associated with sustained clinical remission, reduced need for medical escalation, surgery and prevention of bowel disease progression with better outcomes than endoscopic remission 49,100,101 (Figure 1). We propose an algorithm for timing IUS at index, followed by routine surveillance 36 (Figure 6). IUS performed at baseline for those with suspected IBD or symptoms, as recommended by ECCO-ESGAR, to ensure exclusion of complications and proximal disease in IBD. 5 In established IBD, serial exam intervals are contingent upon treatment trajectory: to evaluate therapeutic response, after drug initiation, evaluation at 12 weeks is ideal. Earlier evaluation may be important as the TRUST UC showed changes at 2 weeks. 35 Post-operative IUS should occur within 3–6 months with fcal, which may obviate endoscopic confirmation and optimise resource use. Use of MRE/CTE is important where IUS is limited or for complex perianal disease, complex post-operative anatomy or need for exclusion of extraintestinal manifestations, such as sacroiliitis. IUS should be correlated with biomarkers to objectively guide timely clinical decisions. Presence of complications may necessitate additional imaging such as MRE prior to endoscopic or surgical intervention.
Figure 6.
Timing of IUS. CD, Crohn’s disease; IBD, inflammatory bowel disease; IUS, intestinal ultrasound; UC, ulcerative colitis.
What is the treatment target in IUS and is it achievable?
A recent systematic review established much needed expert consensus on IUS treatment response and transmural remission. 102 The presence of complications including stenoses portends poorer response and clinical outcomes compared to uncomplicated luminal inflammatory disease, likely related to the reversibility of disease and respective damage. 102 High quality, large population studies are needed regarding monitoring and resolution of CD-related intestinal complications. Colonic disease tends to respond faster than ileal disease. 102 Another important reportable aspect of response is reduction in length of disease, in addition to improvement in BWT (>25%), BWS, hyperaemia, mesenteric inflammatory fat proliferation, and lymphadenopathy. 35 Incremental levels of healing offer superior outcomes in IBD, although a minority of patients will achieve deeper TH (~25%) and histological healing (~10%) with conventional therapy (Figure 7).
Figure 7.
A proposed algorithm on how to incorporate IUS in treat to transmural healing. BWT, bowel wall thickening; CTE, CT enterography; IBD, inflammatory bowel disease; IUS, intestinal ultrasound; MRE, magnetic resonance enterography
Future directions
Despite its paradigm-changing potential, widespread uptake of IUS remains challenging. 64,103 Incorporation of IUS into routine IBD practice requires specialised training, service delivery adaptation, with investment into ultrasound technology and funding model evolution. 103 Current funding models favour MRE or CTE. Coordinated efforts between national and international groups beyond Europe are needed to encourage IUS in guidelines for IBD monitoring and must include patient experience and preference in their consensus. 64,103 Broadening IUS applicability to other intestinal disorders has been suggested given its timely nature including functional motility gastrointestinal disorders and visualisation of faecal loading, 64 however further prospective studies are warranted to validate such use.
Conclusion
IUS is integral to future patient-centred, innovative models of IBD care. Monitoring strategies should align with patient preferences and foster patient engagement. Multidisciplinary care is the foundation of excellence in care, combining expertise in imaging from both specialist gastroenterologists coordinating medical care with experts in diagnostic imaging, to leverage the benefits of all dedicated small bowel imaging modalities. Integration of IUS into clinical practice will facilitate early and accurate IBD disease assessment to enable objective monitoring and expedited therapeutic decision-making, improving patient experience, outcomes and quality of life.
Footnotes
Acknowledgements: AA is supported by scholarships and grants from Crohn’s and Colitis Australia, the Australian Commonwealth Government Research Training Program (University of Melbourne), the Gastroenterogical Society of Australia and Celltrion Healthcare. KN is supported by the Helmsley Trust
Conflicts of Interest: AA has no conflicts; RV has no conflicts; BC received consulting fees/speaker’s honoraria from Janssen, AbbVie, Takeda, Gilead and Novartis and research grants from Pfizer, Ferring and Janssen; RVB has received Grant/Research support/Speaker fees (all paid to employer for research support): AbbVie, Ferring, Janssen, Shire, Takeda, Emerge Health; shareholder in Biomebank; KN reports advisory board fees from AbbVie, Janssen, Pfizer, Ferring and Takeda; speaker’s fees from AbbVie, Janssen and Pfizer; and research support from AbbVie, Janssen
Funding: Open access publishing facilitated by The University of Melbourne, as part of the Wiley - The University of Melbourne agreement via the Council of Australian University Librarians
Contributors: AA, BC, RB and KN developed the manuscript outline. AA, KN drafted the manuscript. All authors edited and approved the final review prior to its publication.
Contributor Information
Aysha H. Al-Ani, Email: aysha.alani@mh.org.au, Department of Medicine, University of Melbourne, Melbourne, Australia ; Department of Gastroenterology, The Royal Melbourne Hospital, Melbourne, Australia .
Rose Vaughan, Email: vaughanrose@gmail.com, Department of Medicine, University of Melbourne, Melbourne, Australia ; Department of Gastroenterology, The Royal Melbourne Hospital, Melbourne, Australia .
Britt Christensen, Email: britt.christensen@unimelb.edu.au, Department of Medicine, University of Melbourne, Melbourne, Australia ; Department of Gastroenterology, The Royal Melbourne Hospital, Melbourne, Australia .
Robert V. Bryant, Email: robert.bryant@sa.gov.au, Department of Gastroenterology, The Queen Elizabeth Hospital, Woodville, Australia ; School of Medicine, University of Adelaide, Adelaide, Australia .
Kerri L. Novak, Email: knovak@ucalgary.ca, Department of Gastroenterology, The University of Calgary, Alberta, Australia .
REFERENCES
- 1. Turner D, Ricciuto A, Lewis A, D’Amico F, Dhaliwal J, Griffiths AM, et al. . STRIDE-II: an update on the selecting therapeutic targets in inflammatory bowel disease (STRIDE) . Initiative of the International Organization for the Study of IBD (IOIBD): Determining Therapeutic Goals for Treat-to-Target Strategies in IBD Gastroenterology 2021. ; 160: 1570 – 83 . [DOI] [PubMed] [Google Scholar]
- 2. Fraquelli M, Castiglione F, Calabrese E, Maconi G . Impact of intestinal ultrasound on the management of patients with inflammatory bowel disease: how to apply scientific evidence to clinical practice . Dig Liver Dis 2020. ; 52: 9 – 18 . doi: 10.1016/j.dld.2019.10.004 [DOI] [PubMed] [Google Scholar]
- 3. Maconi G, Nylund K, Ripolles T, Calabrese E, Dirks K, Dietrich CF, et al. . EFSUMB recommendations and clinical guidelines for intestinal ultrasound (GIUS) in inflammatory bowel diseases . Ultraschall Med 2018. ; 39: 304 – 17 . doi: 10.1055/s-0043-125329 [DOI] [PubMed] [Google Scholar]
- 4. Nylund K, Maconi G, Hollerweger A, Ripolles T, Pallotta N, Higginson A, et al. . EFSUMB recommendations and guidelines for gastrointestinal ultrasound . Ultraschall Med 2017. ; 38: e1 - 15 . doi: 10.1055/s-0042-115853 [DOI] [PubMed] [Google Scholar]
- 5. Maaser C, Sturm A, Vavricka SR, Kucharzik T, Fiorino G, Annese V, et al. . ECCO-ESGAR guideline for diagnostic assessment in IBD part 1: initial diagnosis, monitoring of known IBD, detection of complications . J Crohns Colitis 2019. ; 13: 144 – 64 . doi: 10.1093/ecco-jcc/jjy113 [DOI] [PubMed] [Google Scholar]
- 6. Masselli G, Gualdi G . CT and MR enterography in evaluating small bowel diseases: when to use which modality? Abdom Imaging 2013. ; 38: 249 – 59 . doi: 10.1007/s00261-012-9961-8 [DOI] [PubMed] [Google Scholar]
- 7. Chatterji M, Fidler JL, Taylor SA, Anupindi SA, Yeh BM, Guglielmo FF . State of the art MR enterography technique . Top Magn Reson Imaging 2021. ; 30: 3 – 11 . doi: 10.1097/RMR.0000000000000263 [DOI] [PubMed] [Google Scholar]
- 8. Novak K, Tanyingoh D, Petersen F, Kucharzik T, Panaccione R, Ghosh S, et al. . Clinic-based point of care transabdominal ultrasound for monitoring crohn’s disease: impact on clinical decision making . J Crohns Colitis 2015. ; 9: 795 – 801 . doi: 10.1093/ecco-jcc/jjv105 [DOI] [PubMed] [Google Scholar]
- 9. Novak KL, Jacob D, Kaplan GG, Boyce E, Ghosh S, Ma I, et al. . Point of care ultrasound accurately distinguishes inflammatory from noninflammatory disease in patients presenting with abdominal pain and diarrhea . Can J Gastroenterol Hepatol 2016. ; 2016: 4023065 . doi: 10.1155/2016/4023065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Parente F, Greco S, Molteni M, Cucino C, Maconi G, Sampietro GM, et al. . Role of early ultrasound in detecting inflammatory intestinal disorders and identifying their anatomical location within the bowel . Aliment Pharmacol Ther 2003. ; 18: 1009 – 16 . doi: 10.1046/j.1365-2036.2003.01796.x [DOI] [PubMed] [Google Scholar]
- 11. Wilkens R, Novak KL, Lebeuf-Taylor E, Wilson SR . Impact of intestinal ultrasound on classification and management of crohn’s disease patients with inconclusive colonoscopy . Can J Gastroenterol Hepatol 2016. ; 2016. doi: 10.1155/2016/8745972 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Colombel JF, Sandborn WJ, Reinisch W, Mantzaris GJ, Kornbluth A, Rachmilewitz D, et al. . Infliximab, azathioprine, or combination therapy for crohn’s disease . N Engl J Med 2010. ; 362: 1383 – 95 . doi: 10.1056/NEJMoa0904492 [DOI] [PubMed] [Google Scholar]
- 13. Hanauer SB, Feagan BG, Lichtenstein GR, Mayer LF, Schreiber S, Colombel JF, et al. . Maintenance infliximab for crohn’s disease: the ACCENT I randomised trial . Lancet 2002. ; 359: 1541 – 49 . doi: 10.1016/S0140-6736(02)08512-4 [DOI] [PubMed] [Google Scholar]
- 14. Lémann M, Mary J-Y, Colombel J-F, Duclos B, Soule J-C, Lerebours E, et al. . A randomized, double-blind, controlled withdrawal trial in crohn’s disease patients in long-term remission on azathioprine . Gastroenterology 2005. ; 128: 1812 – 18 . doi: 10.1053/j.gastro.2005.03.031 [DOI] [PubMed] [Google Scholar]
- 15. Samuel S, Bruining DH, Loftus EV, Becker B, Fletcher JG, Mandrekar JN, et al. . Endoscopic skipping of the distal terminal ileum in crohn’s disease can lead to negative results from ileocolonoscopy . Clin Gastroenterol Hepatol 2012. ; 10: 1253 – 59 . doi: 10.1016/j.cgh.2012.03.026 [DOI] [PubMed] [Google Scholar]
- 16. Panés J, Bouzas R, Chaparro M, García-Sánchez V, Gisbert JP, Martínez de Guereñu B, et al. . Systematic review: the use of ultrasonography, computed tomography and magnetic resonance imaging for the diagnosis, assessment of activity and abdominal complications of crohn’s disease . Aliment Pharmacol Ther 2011. ; 34: 125 – 45 . doi: 10.1111/j.1365-2036.2011.04710.x [DOI] [PubMed] [Google Scholar]
- 17. Puylaert CAJ, Tielbeek JAW, Bipat S, Stoker J . Grading of crohn’s disease activity using CT, MRI, US and scintigraphy: a meta-analysis . Eur Radiol 2015. ; 25: 3295 – 3313 . doi: 10.1007/s00330-015-3737-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Horsthuis K, Bipat S, Bennink RJ, Stoker J . Inflammatory bowel disease diagnosed with US, MR, scintigraphy, and CT: meta-analysis of prospective studies . Radiology 2008. ; 247: 64 – 79 . doi: 10.1148/radiol.2471070611 [DOI] [PubMed] [Google Scholar]
- 19. Castiglione F, Mainenti PP, De Palma GD, Testa A, Bucci L, Pesce G, et al. . Noninvasive diagnosis of small bowel crohn’s disease: direct comparison of bowel sonography and magnetic resonance enterography . Inflamm Bowel Dis 2013. ; 19: 991 – 98 . doi: 10.1097/MIB.0b013e3182802b87 [DOI] [PubMed] [Google Scholar]
- 20. Ordás I, Rimola J, Rodríguez S, Paredes JM, Martínez-Pérez MJ, Blanc E, et al. . Accuracy of magnetic resonance enterography in assessing response to therapy and mucosal healing in patients with crohn’s disease . Gastroenterology 2014. ; 146: 374 – 82 . doi: 10.1053/j.gastro.2013.10.055 [DOI] [PubMed] [Google Scholar]
- 21. Taylor SA, Mallett S, Bhatnagar G, Baldwin-Cleland R, Bloom S, Gupta A, et al. . Diagnostic accuracy of magnetic resonance enterography and small bowel ultrasound for the extent and activity of newly diagnosed and relapsed crohn’s disease (METRIC): a multicentre trial . Lancet Gastroenterol Hepatol 2018. ; 3: 548 – 58 . doi: 10.1016/S2468-1253(18)30161-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Fiorino G, Bonifacio C, Peyrin-Biroulet L, Minuti F, Repici A, Spinelli A, et al. . Prospective comparison of computed tomography enterography and magnetic resonance enterography for assessment of disease activity and complications in ileocolonic crohn’s disease . Inflamm Bowel Dis 2011. ; 17: 1073 – 80 . doi: 10.1002/ibd.21533 [DOI] [PubMed] [Google Scholar]
- 23. Bruining DH, Loftus EV, Ehman EC, Siddiki HA, Nguyen DL, Fidler JL, et al. . Computed tomography enterography detects intestinal wall changes and effects of treatment in patients with crohn’s disease . Clin Gastroenterol Hepatol 2011. ; 9: 679 – 83 . doi: 10.1016/j.cgh.2011.04.025 [DOI] [PubMed] [Google Scholar]
- 24. Rimola J, Rodriguez S, García-Bosch O, Ordás I, Ayala E, Aceituno M, et al. . Magnetic resonance for assessment of disease activity and severity in ileocolonic crohn’s disease . Gut 2009. ; 58: 1113 – 20 . doi: 10.1136/gut.2008.167957 [DOI] [PubMed] [Google Scholar]
- 25. Oussalah A, Laurent V, Bruot O, Bressenot A, Bigard M-A, Régent D, et al. . Diffusion-weighted magnetic resonance without bowel preparation for detecting colonic inflammation in inflammatory bowel disease . Gut 2010. ; 59: 1056 – 65 . doi: 10.1136/gut.2009.197665 [DOI] [PubMed] [Google Scholar]
- 26. Allocca M, Danese S, Laurent V, Peyrin-Biroulet L . Use of cross-sectional imaging for tight monitoring of inflammatory bowel diseases . Clin Gastroenterol Hepatol 2020. ; 18: 1309 – 23 . doi: 10.1016/j.cgh.2019.11.052 [DOI] [PubMed] [Google Scholar]
- 27. Pasquini L, Napolitano A, Visconti E, Longo D, Romano A, Tomà P, et al. . Gadolinium-based contrast agent-related toxicities . CNS Drugs 2018. ; 32: 229 – 40 . doi: 10.1007/s40263-018-0500-1 [DOI] [PubMed] [Google Scholar]
- 28. Santillan CS . MR imaging techniques of the bowel . Magn Reson Imaging Clin N Am 2014. ; 22: 1 – 11 . doi: 10.1016/j.mric.2013.07.004 [DOI] [PubMed] [Google Scholar]
- 29. Barber JL, Maclachlan J, Planche K, Furman M, Crespi D, Bab N, et al. . There is good agreement between MR enterography and bowel ultrasound with regards to disease location and activity in paediatric inflammatory bowel disease . Clin Radiol 2017. ; 72: 590 – 97 . doi: 10.1016/j.crad.2017.02.008 [DOI] [PubMed] [Google Scholar]
- 30. Peyrin-Biroulet L, Sandborn W, Sands BE, Reinisch W, Bemelman W, Bryant RV, et al. . Selecting therapeutic targets in inflammatory bowel disease (STRIDE): determining therapeutic goals for treat-to-target . Am J Gastroenterol 2015. ; 110: 1324 – 38 . doi: 10.1038/ajg.2015.233 [DOI] [PubMed] [Google Scholar]
- 31. Mosli MH, Zou G, Garg SK, Feagan SG, MacDonald JK, Chande N, et al. . C-reactive protein, fecal calprotectin, and stool lactoferrin for detection of endoscopic activity in symptomatic inflammatory bowel disease patients: A systematic review and meta-analysis . Am J Gastroenterol 2015. ; 110: 802 – 19 . doi: 10.1038/ajg.2015.120 [DOI] [PubMed] [Google Scholar]
- 32. van Rheenen PF, Van de Vijver E, Fidler V . Faecal calprotectin for screening of patients with suspected inflammatory bowel disease: diagnostic meta-analysis . BMJ 2010. ; 341: c3369 . doi: 10.1136/bmj.c3369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. DʼHaens G, Ferrante M, Vermeire S, Baert F, Noman M, Moortgat L, et al. . Fecal calprotectin is a surrogate marker for endoscopic lesions in inflammatory bowel disease . Inflamm Bowel Dis 2012. ; 18: 2218 – 24 . doi: 10.1002/ibd.22917 [DOI] [PubMed] [Google Scholar]
- 34. Vinding KK, Elsberg H, Thorkilgaard T, Belard E, Pedersen N, Elkjaer M, et al. . Fecal calprotectin measured by patients at home using smartphones—A new clinical tool in monitoring patients with inflammatory bowel disease . Inflamm Bowel Dis 2016. ; 22: 336 – 44 . doi: 10.1097/MIB.0000000000000619 [DOI] [PubMed] [Google Scholar]
- 35. Maaser C, Petersen F, Helwig U, Fischer I, Roessler A, Rath S, et al. . Intestinal ultrasound for monitoring therapeutic response in patients with ulcerative colitis: results from the TRUST&UC study . Gut 2020. ; 69: 1629 – 36 . doi: 10.1136/gutjnl-2019-319451 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Colombel J-F, Panaccione R, Bossuyt P, Lukas M, Baert F, Vaňásek T, et al. . Effect of tight control management on crohn’s disease (CALM): a multicentre, randomised, controlled phase 3 trial . Lancet 2017. ; 390: 2779 – 89 . doi: 10.1016/S0140-6736(17)32641-7 [DOI] [PubMed] [Google Scholar]
- 37. Neurath MF, Travis SPL . Mucosal healing in inflammatory bowel diseases: a systematic review . Gut 2012. ; 61: 1619 – 35 . doi: 10.1136/gutjnl-2012-302830 [DOI] [PubMed] [Google Scholar]
- 38. Ungaro RC, Yzet C, Bossuyt P, Baert FJ, Vanasek T, D’Haens GR, et al. . Deep remission at 1 year prevents progression of early crohn’s disease . Gastroenterology 2020. ; 159: 139 – 47 . doi: 10.1053/j.gastro.2020.03.039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Shah SC, Colombel JF, Sands BE, Narula N . Mucosal healing is associated with improved long-term outcomes of patients with ulcerative colitis: A systematic review and meta-analysis . Clin Gastroenterol Hepatol 2016. ; 14: 1245 – 55 . doi: 10.1016/j.cgh.2016.01.015 [DOI] [PubMed] [Google Scholar]
- 40. Frøslie KF, Jahnsen J, Moum BA, Vatn MH, IBSEN Group . Mucosal healing in inflammatory bowel disease: results from a norwegian population-based cohort . Gastroenterology 2007. ; 133: 412 – 22 . doi: 10.1053/j.gastro.2007.05.051 [DOI] [PubMed] [Google Scholar]
- 41. Bryant RV, Burger DC, Delo J, Walsh AJ, Thomas S, von Herbay A, et al. . Beyond endoscopic mucosal healing in UC: histological remission better predicts corticosteroid use and hospitalisation over 6 years of follow-up . Gut 2016. ; 65: 408 – 14 . doi: 10.1136/gutjnl-2015-309598 [DOI] [PubMed] [Google Scholar]
- 42. Ullman TA, Itzkowitz SH . Intestinal inflammation and cancer . Gastroenterology 2011. ; 140: 1807 – 16 . doi: 10.1053/j.gastro.2011.01.057 [DOI] [PubMed] [Google Scholar]
- 43. Narula N, Aruljothy A, Alshahrani A-A, Fadida M, Al-Saedi M, Marshall JK, et al. . Histologic remission does not offer additional benefit for ulcerative colitis patients in endoscopic remission . Aliment Pharmacol Ther 2020. ; 52: 1676 – 82 . doi: 10.1111/apt.16147 [DOI] [PubMed] [Google Scholar]
- 44. Fletcher JG, Fidler JL, Bruining DH, Huprich JE . New concepts in intestinal imaging for inflammatory bowel diseases . Gastroenterology 2011. ; 140: 1795 – 1806 . doi: 10.1053/j.gastro.2011.02.013 [DOI] [PubMed] [Google Scholar]
- 45. Swaminath A, Legnani P, Kornbluth A . Video capsule endoscopy in inflammatory bowel disease: past, present, and future redux . Inflamm Bowel Dis 2010. ; 16: 1254 – 62 . doi: 10.1002/ibd.21220 [DOI] [PubMed] [Google Scholar]
- 46. Mitselos IV, Christodoulou DK, Katsanos KH, Tatsioni A, Rapti A, Eliakim R, et al. . The role of small bowel capsule endoscopy and ileocolonoscopy in patients with nonspecific but suggestive symptoms of crohn’s disease . Eur J Gastroenterol Hepatol 2016. ; 28: 882 – 89 . doi: 10.1097/MEG.0000000000000644 [DOI] [PubMed] [Google Scholar]
- 47. Nemeth A, Kopylov U, Koulaouzidis A, Wurm Johansson G, Thorlacius H, Amre D, et al. . Use of patency capsule in patients with established crohn’s disease . Endoscopy 2016. ; 48: 373 – 79 . doi: 10.1055/s-0034-1393560 [DOI] [PubMed] [Google Scholar]
- 48. Lewis BS . Expanding role of capsule endoscopy in inflammatory bowel disease . World J Gastroenterol 2008. ; 14: 4137 – 41 . doi: 10.3748/wjg.14.4137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Lafeuille P, Hordonneau C, Vignette J, Blayac L, Dapoigny M, Reymond M, et al. . Transmural healing and MRI healing are associated with lower risk of bowel damage progression than endoscopic mucosal healing in crohn’s disease . Aliment Pharmacol Ther 2021. ; 53: 577 – 86 . doi: 10.1111/apt.16232 [DOI] [PubMed] [Google Scholar]
- 50. Buisson A, Gonzalez F, Poullenot F, Nancey S, Sollellis E, Fumery M, et al. . Comparative acceptability and perceived clinical utility of monitoring tools: A nationwide survey of patients with inflammatory bowel disease . Inflamm Bowel Dis 2017. ; 23: 1425 – 33 . doi: 10.1097/MIB.0000000000001140 [DOI] [PubMed] [Google Scholar]
- 51. Friedman AB, Asthana A, Knowles SR, Robbins A, Gibson PR . Effect of point-of-care gastrointestinal ultrasound on decision-making and management in inflammatory bowel disease . Aliment Pharmacol Ther 2021. ; 54: 652 – 66 . doi: 10.1111/apt.16452 [DOI] [PubMed] [Google Scholar]
- 52. Allocca M, Fiorino G, Bonifacio C, Furfaro F, Gilardi D, Argollo M, et al. . Comparative accuracy of bowel ultrasound versus magnetic resonance enterography in combination with colonoscopy in assessing crohn’s disease and guiding clinical decision-making . J Crohns Colitis 2018. ; 12: 1280 – 87 . doi: 10.1093/ecco-jcc/jjy093 [DOI] [PubMed] [Google Scholar]
- 53. Bhatnagar G, Quinn L, Higginson A, Plumb A, Halligan S, Tolan D, et al. . Observer agreement for small bowel ultrasound in crohn’s disease: results from the METRIC trial . Abdom Radiol (NY) 2020. ; 45: 3036 – 45 . doi: 10.1007/s00261-020-02405-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. De Voogd F, Wilkens R, Gecse K, Allocca M, Novak K, Lu C, et al. . A reliability study: strong inter-observer agreement of an expert panel for intestinal ultrasound in ulcerative colitis . J Crohns Colitis 2021. ; 15: 1284 – 90 . doi: 10.1093/ecco-jcc/jjaa267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Calabrese E, Kucharzik T, Maaser C, Maconi G, Strobel D, Wilson SR, et al. . Real-time interobserver agreement in bowel ultrasonography for diagnostic assessment in patients with crohn’s disease: an international multicenter study . Inflamm Bowel Dis 2018. ; 24: 2001 – 6 . doi: 10.1093/ibd/izy091 [DOI] [PubMed] [Google Scholar]
- 56. Fraquelli M, Sarno A, Girelli C, Laudi C, Buscarini E, Villa C, et al. . Reproducibility of bowel ultrasonography in the evaluation of crohn’s disease . Dig Liver Dis 2008. ; 40: 860 – 66 . doi: 10.1016/j.dld.2008.04.006 [DOI] [PubMed] [Google Scholar]
- 57. Lu C, Merrill C, Medellin A, Novak K, Wilson SR . Bowel ultrasound state of the art: grayscale and doppler ultrasound, contrast enhancement, and elastography in crohn disease . J Ultrasound Med 2019. ; 38: 271 – 88 . doi: 10.1002/jum.14920 [DOI] [PubMed] [Google Scholar]
- 58. Fraquelli M, Colli A, Casazza G, Paggi S, Colucci A, Massironi S, et al. . Role of US in detection of crohn disease: meta-analysis . Radiology 2005. ; 236: 95 – 101 . doi: 10.1148/radiol.2361040799 [DOI] [PubMed] [Google Scholar]
- 59. Parente F, Sampietro GM, Molteni M, Greco S, Anderloni A, Sposito C, et al. . Behaviour of the bowel wall during the first year after surgery is a strong predictor of symptomatic recurrence of crohn’s disease: a prospective study . Aliment Pharmacol Ther 2004. ; 20: 959 – 68 . doi: 10.1111/j.1365-2036.2004.02245.x [DOI] [PubMed] [Google Scholar]
- 60. Rispo A, Bucci L, Pesce G, Sabbatini F, de Palma GD, Grassia R, et al. . Bowel sonography for the diagnosis and grading of postsurgical recurrence of crohn’s disease . Inflamm Bowel Dis 2006. ; 12: 486 – 90 . doi: 10.1097/00054725-200606000-00007 [DOI] [PubMed] [Google Scholar]
- 61. Limberg B . Diagnosis of chronic inflammatory bowel disease by ultrasonography . Z Gastroenterol 1999. ; 37: 495 – 508 . [PubMed] [Google Scholar]
- 62. Sasaki T, Kunisaki R, Kinoshita H, Kimura H, Kodera T, Nozawa A, et al. . Doppler ultrasound findings correlate with tissue vascularity and inflammation in surgical pathology specimens from patients with small intestinal crohn’s disease . BMC Res Notes 2014. ; 7: 363 . doi: 10.1186/1756-0500-7-363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Novak KL, Nylund K, Maaser C, Petersen F, Kucharzik T, Lu C, et al. . Expert consensus on optimal acquisition and development of the international bowel ultrasound segmental activity score [ibus-sas]: a reliability and inter-rater variability study on intestinal ultrasonography in crohn’s disease . J Crohns Colitis 2021. ; 15: 609 – 16 . doi: 10.1093/ecco-jcc/jjaa216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Bryant RV, Friedman AB, Wright EK, Taylor KM, Begun J, Maconi G, et al. . Gastrointestinal ultrasound in inflammatory bowel disease: an underused resource with potential paradigm-changing application . Gut 2018. ; 67: 973 – 85 . doi: 10.1136/gutjnl-2017-315655 [DOI] [PubMed] [Google Scholar]
- 65. Maconi G, Di Sabatino A, Ardizzone S, Greco S, Colombo E, Russo A, et al. . Prevalence and clinical significance of sonographic detection of enlarged regional lymph nodes in crohn’s disease . Scand J Gastroenterol 2005. ; 40: 1328 – 33 . doi: 10.1080/00365510510025746 [DOI] [PubMed] [Google Scholar]
- 66. Mao R, Doyon G, Gordon IO, Li J, Lin S, Wang J, et al. . Activated intestinal muscle cells promote preadipocyte migration: a novel mechanism for creeping fat formation in crohn’s disease . Gut 2022. ; 71: 55 – 67 . doi: 10.1136/gutjnl-2020-323719 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Kucharzik T, Kannengiesser K, Petersen F . The use of ultrasound in inflammatory bowel disease . Ann Gastroenterol 2017. ; 30: 135 – 44 . doi: 10.20524/aog.2016.0105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Bhatnagar G, Rodriguez-Justo M, Higginson A, Bassett P, Windsor A, Cohen R, et al. . Inflammation and fibrosis in crohn’s disease: location-matched histological correlation of small bowel ultrasound features . Abdom Radiol (NY) 2021. ; 46: 144 – 55 . doi: 10.1007/s00261-020-02603-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Rieder F, Bettenworth D, Ma C, Parker CE, Williamson LA, Nelson SA, et al. . An expert consensus to standardise definitions, diagnosis and treatment targets for anti-fibrotic stricture therapies in crohn’s disease . Aliment Pharmacol Ther 2018. ; 48: 347 – 57 . doi: 10.1111/apt.14853 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Bettenworth D, Bokemeyer A, Baker M, Mao R, Parker CE, Nguyen T, et al. . Assessment of crohn’s disease-associated small bowel strictures and fibrosis on cross-sectional imaging: a systematic review . Gut 2019. ; 68: 1115 – 26 . doi: 10.1136/gutjnl-2018-318081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Coelho R, Ribeiro H, Maconi G . Bowel thickening in crohn’s disease: fibrosis or inflammation? diagnostic ultrasound imaging tools . Inflamm Bowel Dis 2017. ; 23: 23 – 34 . doi: 10.1097/MIB.0000000000000997 [DOI] [PubMed] [Google Scholar]
- 72. Nylund K, Jirik R, Mezl M, Leh S, Hausken T, Pfeffer F, et al. . Quantitative contrast-enhanced ultrasound comparison between inflammatory and fibrotic lesions in patients with crohn’s disease . Ultrasound Med Biol 2013. ; 39: 1197 – 1206 . doi: 10.1016/j.ultrasmedbio.2013.01.020 [DOI] [PubMed] [Google Scholar]
- 73. Maconi G, Carsana L, Fociani P, Sampietro GM, Ardizzone S, Cristaldi M, et al. . Small bowel stenosis in crohn’s disease: clinical, biochemical and ultrasonographic evaluation of histological features . Aliment Pharmacol Ther 2003. ; 18: 749 – 56 . doi: 10.1046/j.1365-2036.2003.01673.x [DOI] [PubMed] [Google Scholar]
- 74. Panes J, Bouhnik Y, Reinisch W, Stoker J, Taylor SA, Baumgart DC, et al. . Imaging techniques for assessment of inflammatory bowel disease: joint ECCO and ESGAR evidence-based consensus guidelines . J Crohns Colitis 2013. ; 7: 556 – 85 . doi: 10.1016/j.crohns.2013.02.020 [DOI] [PubMed] [Google Scholar]
- 75. Maconi G, Sampietro GM, Parente F, Pompili G, Russo A, Cristaldi M, et al. . Contrast radiology, computed tomography and ultrasonography in detecting internal fistulas and intra-abdominal abscesses in crohn’s disease: a prospective comparative study . Am J Gastroenterol 2003. ; 98: 1545 – 55 . doi: 10.1111/j.1572-0241.2003.07521.x [DOI] [PubMed] [Google Scholar]
- 76. Pallotta N, Vincoli G, Montesani C, Chirletti P, Pronio A, Caronna R, et al. . Small intestine contrast ultrasonography (SICUS) for the detection of small bowel complications in crohn’s disease: a prospective comparative study versus intraoperative findings . Inflamm Bowel Dis 2012. ; 18: 74 – 84 . doi: 10.1002/ibd.21678 [DOI] [PubMed] [Google Scholar]
- 77. Novak KL, Wilson SR . The role of ultrasound in the evaluation of inflammatory bowel disease . Semin Roentgenol 2013. ; 48: 224 – 33 . doi: 10.1053/j.ro.2013.03.003 [DOI] [PubMed] [Google Scholar]
- 78. Calabrese E, La Seta F, Buccellato A, Virdone R, Pallotta N, Corazziari E, et al. . Crohn’s disease: a comparative prospective study of transabdominal ultrasonography, small intestine contrast ultrasonography, and small bowel enema . Inflamm Bowel Dis 2005. ; 11: 139 – 45 . doi: 10.1097/00054725-200502000-00007 [DOI] [PubMed] [Google Scholar]
- 79. Kucharzik T, Wittig BM, Helwig U, Börner N, Rössler A, Rath S, et al. . Use of intestinal ultrasound to monitor crohn’s disease activity . Clin Gastroenterol Hepatol 2017. ; 15: 535 – 42 . doi: 10.1016/j.cgh.2016.10.040 [DOI] [PubMed] [Google Scholar]
- 80. Paredes JM, Ripollés T, Cortés X, Moreno N, Martínez MJ, Bustamante-Balén M, et al. . Contrast-enhanced ultrasonography: usefulness in the assessment of postoperative recurrence of crohn’s disease . J Crohns Colitis 2013. ; 7: 192 – 201 . doi: 10.1016/j.crohns.2012.03.017 [DOI] [PubMed] [Google Scholar]
- 81. De Cruz P, Kamm MA, Hamilton AL, Ritchie KJ, Krejany EO, Gorelik A, et al. . Crohn’s disease management after intestinal resection: a randomised trial . Lancet 2015. ; 385: 1406 – 17 . doi: 10.1016/S0140-6736(14)61908-5 [DOI] [PubMed] [Google Scholar]
- 82. Wright EK, Kamm MA, De Cruz P, Hamilton AL, Ritchie KJ, Krejany EO, et al. . Measurement of fecal calprotectin improves monitoring and detection of recurrence of crohn’s disease after surgery . Gastroenterology 2015. ; 148: 938 – 47 . doi: 10.1053/j.gastro.2015.01.026 [DOI] [PubMed] [Google Scholar]
- 83. Ripollés T, Paredes JM, Martínez-Pérez MJ, Rimola J, Jauregui-Amezaga A, Bouzas R, et al. . Ultrasonographic changes at 12 weeks of anti-TNF drugs predict 1-year sonographic response and clinical outcome in crohn’s disease: A multicenter study . Inflamm Bowel Dis 2016. ; 22: 2465 – 73 . doi: 10.1097/MIB.0000000000000882 [DOI] [PubMed] [Google Scholar]
- 84. Castiglione F, de Sio I, Cozzolino A, Rispo A, Manguso F, Del Vecchio Blanco G, et al. . Bowel wall thickness at abdominal ultrasound and the one-year-risk of surgery in patients with crohn’s disease . Am J Gastroenterol 2004. ; 99: 1977 – 83 . doi: 10.1111/j.1572-0241.2004.40267.x [DOI] [PubMed] [Google Scholar]
- 85. Feuerstein JD, Isaacs KL, Schneider Y, Siddique SM, Falck-Ytter Y, Singh S, et al. . AGA clinical practice guidelines on the management of moderate to severe ulcerative colitis . Gastroenterology 2020. ; 158: 1450 – 61 . doi: 10.1053/j.gastro.2020.01.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Arias MT, Vande Casteele N, Vermeire S, de Buck van Overstraeten A, Billiet T, Baert F, et al. . A panel to predict long-term outcome of infliximab therapy for patients with ulcerative colitis . Clin Gastroenterol Hepatol 2015. ; 13: 531 – 38 . doi: 10.1016/j.cgh.2014.07.055 [DOI] [PubMed] [Google Scholar]
- 87. Ordas I, Rimola J, Rodriguez S, Gallego M, Ricart E, Panes J . Imaging of the colon in inflammatory bowel disease: ready for prime time? Curr Drug Targets 2012. ; 13: 1252 – 60 . doi: 10.2174/138945012802429714 [DOI] [PubMed] [Google Scholar]
- 88. Sagami S, Kobayashi T, Aihara K, Umeda M, Morikubo H, Matsubayashi M, et al. . Transperineal ultrasound predicts endoscopic and histological healing in ulcerative colitis . Aliment Pharmacol Ther 2020. ; 51: 1373 – 83 . doi: 10.1111/apt.15767 [DOI] [PubMed] [Google Scholar]
- 89. Smith RL, Taylor KM, Friedman AB, Swaine AP, Gibson DJ, Gibson PR . Early assessment with gastrointestinal ultrasound in patients hospitalised for A flare of ulcerative colitis and predicting the need for salvage therapy: A pilot study . Ultrasound Med Biol 2021. ; 47: 1108 – 14 . doi: 10.1016/j.ultrasmedbio.2020.12.001 [DOI] [PubMed] [Google Scholar]
- 90. Maconi G, Ardizzone S, Greco S, Radice E, Bezzio C, Bianchi Porro G . Transperineal ultrasound in the detection of perianal and rectovaginal fistulae in crohn’s disease . Am J Gastroenterol 2007. ; 102: 2214 – 19 . doi: 10.1111/j.1572-0241.2007.01441.x [DOI] [PubMed] [Google Scholar]
- 91. Maconi G, Tonolini M, Monteleone M, Bezzio C, Furfaro F, Villa C, et al. . Transperineal perineal ultrasound versus magnetic resonance imaging in the assessment of perianal crohn’s disease . Inflamm Bowel Dis 2013. ; 19: 2737 – 43 . doi: 10.1097/01.MIB.0000436274.95722.e5 [DOI] [PubMed] [Google Scholar]
- 92. Maconi G, Greco MT, Asthana AK . Transperineal ultrasound for perianal fistulas and abscesses - A systematic review and meta-analysis . Ultraschall Med 2017. ; 38: 265 – 72 . doi: 10.1055/s-0043-103954 [DOI] [PubMed] [Google Scholar]
- 93. Ripollés T, Martínez-Pérez MJ, Paredes JM, Vizuete J, García-Martínez E, Jiménez-Restrepo DH . Contrast-enhanced ultrasound in the differentiation between phlegmon and abscess in crohn’s disease and other abdominal conditions . Eur J Radiol 2013. ; 82: e525 - 31 . doi: 10.1016/j.ejrad.2013.05.043 [DOI] [PubMed] [Google Scholar]
- 94. Chen Y-J, Mao R, Li X-H, Cao Q-H, Chen Z-H, Liu B-X, et al. . Real-time shear wave ultrasound elastography differentiates fibrotic from inflammatory strictures in patients with crohn’s disease . Inflamm Bowel Dis 2018. ; 24: 2183 – 90 . doi: 10.1093/ibd/izy115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Lu C, Gui X, Chen W, Fung T, Novak K, Wilson SR . Ultrasound shear wave elastography and contrast enhancement: effective biomarkers in crohn’s disease strictures . Inflamm Bowel Dis 2017. ; 23: 421 – 30 . doi: 10.1097/MIB.0000000000001020 [DOI] [PubMed] [Google Scholar]
- 96. Goodsall TM, Jairath V, Feagan BG, Parker CE, Nguyen TM, Guizzetti L, et al. . Standardisation of intestinal ultrasound scoring in clinical trials for luminal crohn’s disease . Aliment Pharmacol Ther 2021. ; 53: 873 – 86 . doi: 10.1111/apt.16288 [DOI] [PubMed] [Google Scholar]
- 97. Novak KL, Kaplan GG, Panaccione R, Afshar EE, Tanyingoh D, Swain M, et al. . A simple ultrasound score for the accurate detection of inflammatory activity in crohn’s disease . Inflamm Bowel Dis 2017. ; 23: 2001 – 10 . doi: 10.1097/MIB.0000000000001174 [DOI] [PubMed] [Google Scholar]
- 98. Bots S, Nylund K, Löwenberg M, Gecse K, Gilja OH, D’Haens G . Ultrasound for assessing disease activity in IBD patients: A systematic review of activity scores . J Crohns Colitis 2018. ; 12: 920 – 29 . doi: 10.1093/ecco-jcc/jjy048 [DOI] [PubMed] [Google Scholar]
- 99. Wilkens R, Novak KL, Maaser C, Panaccione R, Kucharzik T . Relevance of monitoring transmural disease activity in patients with crohn’s disease: current status and future perspectives . Therap Adv Gastroenterol 2021. ; 14. doi: 10.1177/17562848211006672 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Fernandes SR, Rodrigues RV, Bernardo S, Cortez-Pinto J, Rosa I, da Silva JP, et al. . Transmural healing is associated with improved long-term outcomes of patients with crohn’s disease . Inflamm Bowel Dis 2017. ; 23: 1403 – 9 . doi: 10.1097/MIB.0000000000001143 [DOI] [PubMed] [Google Scholar]
- 101. Messadeg L, Hordonneau C, Bouguen G, Goutorbe F, Reimund JM, Goutte M, et al. . Early transmural response assessed using magnetic resonance imaging could predict sustained clinical remission and prevent bowel damage in patients with crohn’s disease treated with anti-tumour necrosis factor therapy . J Crohns Colitis 2020. ; 14: 1524 – 34 . doi: 10.1093/ecco-jcc/jjaa098 [DOI] [PubMed] [Google Scholar]
- 102. Ilvemark J, Hansen T, Goodsall TM, Seidelin JB, Al-Farhan H, Allocca M, et al. . Defining transabdominal intestinal ultrasound treatment response and remission in inflammatory bowel disease: systematic review and expert consensus statement . J Crohns Colitis 2022. ; 16: 554 – 80 . doi: 10.1093/ecco-jcc/jjab173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Asthana AK, Friedman AB, Maconi G, Maaser C, Kucharzik T, Watanabe M, et al. . Failure of gastroenterologists to apply intestinal ultrasound in inflammatory bowel disease in the asia-pacific: a need for action . J Gastroenterol Hepatol 2015. ; 30: 446 – 52 . doi: 10.1111/jgh.12871 [DOI] [PubMed] [Google Scholar]