Abstract
Objective:
To evaluate the efficacy, toxicity and survival of salvage peptide receptor radionuclide therapy (PRRT) with indigenous, direct-route 177Lu-labelled-DOTATATE in metastatic Nueroendocrine tumor (NET) patients who showed an objective response or disease stabilization following initial course of 177Lu-DOTATATE PRRT cycles and eventually developed progressive disease after a time-interval of more than 1 year; the variables influencing survival and response of salvage PRRT were also examined.
Methods:
A total of 26 progressive metastatic NET patients who received salvage PRRT with indigenous 177Lu-DOTATATE, were evaluated. Response was assessed under three broad categories as clinical symptomatic, biochemical and imaging (both molecular and morphological imaging). The Kaplan–Meier product-limit method was used to calculate progression-free survival (PFS) and overall survival (OS). Toxicity of salvage PRRT was evaluated by NCI-CTCAE v. 5.0 criteria (included complete blood counts, renal and liver function tests). Association between various variables and response and survival were analyzed using the χ2 test.
Results:
Out of the 26 patients, the complete follow-up data were not available for four patients, where only survival information was available. Thus, a total of 22 patients (median age: 55 years, range: 38–68 years, 12 men and 10 women) were included and analyzed retrospectively in study. The cumulative dose of initial course of PRRT (I-PRRT) with indigenous 177Lu-DOTATATE ranged from 800 mCi (29.6 GBq) to 1231 mCi (45.54 GBq) per patient {mean administered cumulative dose of 964 mCi (35.66 GBq) per patient}, and the salvage PRRT with indigenous 177Lu-DOTATATE comprised of a mean dose of 170 mCi (6.29GBq) per patient. The disease control rate of 68.1%, 77.3%, 63.6% and 63.6% were observed after salvage PRRT on clinical symptomatic, biochemical, molecular and morphological imaging response respectively. The median PFS after salvage PRRT was 17 months. The median OS was not attained after I-PRRT (OS-i) and salvage PRRT (OS-s). Estimated OS-i rate was 68% at 108 months and OS-s rate was 82% at 18 months. None of the patients developed Grade 3/4 hematotoxicity, nephrotoxicity and hepatotoxicity or AML/MDS after I-PRRT and salvage PRRT at median follow-up of 72 months and 12 months respectively. The highest level of toxicity was Grade 2 [seen as reversible anemia, thrombocytopenia and nephrotoxicity in 3 (13.5%), 1 (4.5%) and 2 patients (9%) respectively]. The significant p-value was not observed for any variable association.
Conclusion:
With limited therapeutic options available for progressive NET after I-PRRT and in the absence of high-grade toxicity after 177Lu-DOTATATE salvage PRRT, retreatment with PRRT may be considered as a relatively safe therapeutic option for these patients.
Advances in knowledge:
This study examined salvage retreatment PRRT with indigenous “direct-route” 177Lu-DOTATATE and registered its safety and survival benefits, indicating this could be an effective therapeutic option in this clinical setting.
Introduction
Neuroendocrine neoplasms (NENs) originate from diffuse neuroendocrine cell system and may present at many different sites in body, most frequently in gastrointestinal system. Most of NEN included a well-differentiated neuroendocrine tumor (NET), whereas around 10–20% of all NENs present with a poorly differentiated neuroendocrine carcinoma. Surgery is curative and the treatment of choice for local or locoregional disease. 1 Several systemic treatment options are available for locally advanced or metastatic NET, which includes long-acting somatostatin analogs, molecular-targeted agents, chemotherapy agents, and peptide receptor radionuclide therapy (PRRT). 2 Of these therapies, PRRT has maximum benefit in terms of response rate, progression-free survival (PFS) and overall survival (OS) and minimum toxicity profile. Recently, the prospective Phase III trial NETTER-1 has demonstrated better results in midgut NET patients, 3 which led approval of Lutathera (177Lu-DOTATATE) by the U.S. Food and Drug Administration and European Medicines Agency. Available literature of PRRT outcome in NET patients demonstrated radiological response rate in the range of 29–39% and disease stabilization in around 27–43%. 4–6 A fraction of these NET patients after certain time interval eventually shows progressive disease. In this situation, patients have limited treatment options such as molecular-targeted agents (everolimus or sunitinib), chemotherapy, and local ablative therapies with deteriorated quality of life, high-grade toxicities profile and unfavorable survival after these therapies. 7 177Lu-DOTATATE is considered as safest and effective therapy for a well-differentiated NET patient as shown by NETTER-1 trial. Therefore, retreatment after initial course of PRRT cycles by using additional 177Lu-DOTATATE cycles as salvage therapy can be considered an acceptable treatment option for these patients with progressive disease. However, available literature on salvage PRRT is scarce and reports usually small number heterogeneous group of patients with use of different radioligands, doses, cycles, follow-ups periods, response and toxicities assessment after salvage PRRT. 8
To our best knowledge, study on salvage PRRT with indigenously produced 177Lu via direct route by irradiating enriched lutetium target (82% 176Lu) at a thermal neutron flux of ~1.5×1014 n/cm2s is not available in literature. Hence, the aim of our study was to evaluate the efficacy, survival and toxicity of salvage PRRT with indigenous, direct route 177Lu-DOTATATE in metastatic NET patients who showed an objective response or disease stabilization following initial course of indigenous 177Lu-DOTATATE PRRT cycles and eventually developed progressive disease after a time interval of more than 1 year. In addition, we evaluated various parameters influencing survival and response following salvage PRRT.
Methods and materials
Patient population
The study included patients of histopathologically proven NET who had been treated with initial course of PRRT (I-PRRT) with 4–7 cycles of indigenous 177Lu-DOTATATE and showed an objective response or stable disease (SD) on the Response Evaluation Criteria in Solid Tumors 1.1 (RECIST 1.1) criteria after I-PRRT, but eventually developed progressive disease (PD) after a time gap of more than 1 year and were retreated with salvage PRRT with indigenous 177Lu-DOTATATE. The study was approved by the local Scientific Committee and the Ethics Committee. The need to obtain informed consent was waived as this study was a retrospective analysis of patient`s data.
Patient selection for salvage PRRT
The inclusion criteria for salvage PRRT was metastatic NET patients who showed PD more than 1 year after last cycle of I-PRRT and had shown adequate tracer uptake on 68Ga-DOTATATE PET/CT (Krenning score ≥3, compared on MIP, coronal and transaxial images), preserved hematological (hemoglobin of ≥8 g dl−1, a white blood cell count of ≥3×109/l, and platelets of ≥75×109/l), renal (a glomerular filtration rate of >30 ml/min/1.73 m2), and liver (serum bilirubin ≤1.5 x upper limit of normal and ALT<2.5 x upper limit of normal) function tests, preserved functional status (Karnofsky index ≥40 and ECOG performance status ≤3), and life expectancy >3 months. Exclusion criteria for salvage PRRT were metastatic NET patients who developed PD within 1 year of last cycle of I-PRRT, insufficient tracer uptake (Krenning score ≤2, compared on MIP, coronal and transaxial images) on 68Ga-DOTATATE PET/CT, inadequate hematological, renal and liver function tests, poor performance status and life expectancy ≤3 months.
Salvage PRRT protocol
Eligible NET patients underwent pre-salvage PRRT work-up protocol which included clinical symptoms evaluation, imaging (68Ga-DOTATATE PET/ with or without contrast CT and 18F-FDG PET/CT scan), measurements of tumor markers {serum chromogranin A (CgA) level}, hematological (serum hemoglobin, white blood cell, and platelets counts), renal (serum creatinine), and liver (serum bilirubin and ALT) function tests, and determination of performance status (Karnofsky index and ECOG score) before administering salvage PRRT.
The Bhabha Atomic Research Centre (BARC), Mumbai, India supplied sterile solution of 177LuCl3 (177Lu produced in Indian nuclear reactor via direct route production) in 0.01M HCl with a specific activity of greater than 999 MBq/μg. In the institute, in-house 177Lu labeling of DOTATATE was carried with a radionuclide purity of greater than 99%. 9,10 For routine clinical use in NET patients, indigenous 177Lu-DOTATATE final product was approved by the Radiopharmaceutical Committee (RPC), the regulatory body of Department of Atomic Energy (DAE), India.
All eligible NET patients were admitted in the radionuclide therapy ward for giving salvage PRRT. A mixed amino-acid infusion (containing positively charged lysine and arginine) was given 1 h before and continued over 7.5 h after administration of indigenous 177Lu-DOTATATE. Therapeutic dose of 150–200 mCi (5.55–7.4 GBq) per patient of indigenous 177Lu-DOTATATE was given in 100 ml of normal saline over 30 min. All treated patients were monitored for 24 h for any acute adverse effects.
Follow-up after salvage PRRT
All treated patients underwent routine biweekly blood analysis for hematology (serum hemoglobin, white blood cell, and platelets counts), renal (serum creatinine), and liver (serum bilirubin and ALT) function tests to determine the toxicity profile. Any observed toxicity was recorded continuously. The Common Terminology Criteria for Adverse Events version 5.0 (CTCAE v. 5.0) of the National Cancer Institute Adverse events were used for grading toxicity profiles in these patients.
During follow-up, clinical symptoms evaluation, imaging (68Ga-DOTATATE PET/CT scan), and measurement of serum CgA (tumor marker) was done at every 3 months interval for initial 6 months after salvage PRRT cycle then after at every 6 months interval.
Outcome of salvage PRRT
Outcome of salvage PRRT was divided into three broad categories I) therapeutic outcome, II) survival outcome and III) toxicity profile
I) Therapeutic outcome: divided into clinical symptomatic, biochemical and imaging response.
A) Clinical symptomatic response: the clinical symptomatic response was evaluated based on the patient’s subjective report for tumor-related symptoms after salvage PRRT. At follow-up, the patients were directly asked about tumor-related symptoms and these symptoms reported by patient were compared with prior symptoms before salvage PRRT on an analogue scale of 0–100% as to whether clinical symptoms had “disappeared” or 90–100% improvement (complete response [CR]) or had “improved” 30%–90% improvement (partial response [PR]) or were “SD” (<30% improvement/worsen) or “worse” (≥30% increase in symptoms or new symptoms; PD).
B) Biochemical response: before and after salvage PRRT, serum CgA levels (tumor markers) were measured. Percentage change of serum CgA levels were evaluated whether there was normalization of tumor markers as CR, 30-75% decrease in values of tumor markers as PR, <30% decrease or increase in values of tumor markers as SD and ≥30% increase in values of tumor markers as PD.
C) Imaging response: the PET Response Criteria in Solid Tumors (PERCIST) criteria was used for molecular based imaging response evaluation, where complete resolution of abnormal uptake in previously avid lesions was considered as CR, ≥30% reduction in tracer uptake as PR, >30% increase in tracer uptake or appearance newly avid lesions as PD and neither PR nor PD as SD on 68Ga-DOTATATE imaging according to the Hicks criteria as detailed by Wahl et al. 11,12 For morphological based imaging response evaluation, the Response Evaluation Criteria in Solid Tumors (RECIST) 1.1 criteria was used. 13
Disease control rate (DCR) was defined as CR+PR+SD for clinical symptomatic, biochemical and imaging response evaluation criteria
II) Survival outcome
Progression-free survival (PFS) was measured from the time of salvage PRRT until progression of disease on morphological imaging
OS was measured from the date of first cycle of I-PRRT (OS-i) and from date of salvage PRRT (OS-s) to the date of patient death from any cause or the date at last clinical follow-up of patient.
III) Toxicity assessment
Hematotoxicity, nephrotoxicity, hepatotoxicity and also nausea, vomiting, and hormonal crisis as acute toxicity after salvage PRRT were documented and graded as per the NCI-CTCAE v. 5.0.
The outcome of salvage PRRT was evaluated during the follow-up. This follow-up period ranged from 3 to 24 months with median duration of 12 months after salvage PRRT. For analysis of salvage PRRT outcome, the last follow-up data was used in all NET patients.
Statistical analysis
Discrete variables were summarized by counts (percentages) and continuous variables by their median (range), unless stated otherwise. The CR, PR, SD, and PD were calculated in each of response evaluation categories (clinical symptomatic, biochemical, PERCIST on 68Ga-DOTATATE, and RECIST 1.1) as aforementioned. The Kaplan-Meier method was used to determine median point estimate with 95% confidence interval (CI) for PFS, OS-i and OS-s. The χ2 was used to test the association of following categorical variables with survival and response of salvage PRRT and a p-value of less than 0.05 considered statistically significant: patient age at start of salvage PRRT, site of primary tumor, Ki-67 index, serum CgA levels, disease burden before salvage PRRT, 68Ga-DOTATATE and 18F-FDG avidity in primary or metastatic lesions. All statistical analyses were performed using IBM SPSS software v. 21.
Results
A total of 26 NET patients were received salvage PRRT in our institute during 2019 to 2020. Out of these patients, four patients were lost to follow-up. On telephonic communication, these four patients developed progressive disease and eventually died after time gap of 12–18 months following salvage PRRT.
Therefore, a total 22 patients (median age of 55 years, range 38–68 years, 12 men and 10 women) were included and analyzed retrospectively in study. The sites of primary tumors were as follows: pancreas in nine patients, small intestine in seven patients, large intestine in two patients, lung in one patient and unknown primary site in three patients. The study included WHO 2019 Grade I NET in 4 patients, Grade II in 15 patients and remaining 3 patients without grading because of unavailability of Ki-67 index. The liver (n = 13 patients) and skeleton (n = 6 patients) were common sites for metastatic disease. High FDG avidity (SUVmax>5), high disease burden (number of metastatic lesions > 6), and elevated serum CgA levels (>400 ng ml−1) was found in 4 patients, 7 patients and 10 patients respectively. The performance status with ECOG score of 3 and 2 was observed in 4 patients and 18 patients respectively and normal GFR was noted in 20 patients before salvage PRRT.
Before salvage PRRT, cumulative dose of I-PRRT ranged from 800 mCi (29.6 GBq) to 1231 mCi (45.54 GBq) per patient with an average administered cumulative dose of 964 mCi (35.66 GBq) per patient and number of I-PRRT cycles ranged from 4 to 7 cycles per patient and an average of 5 cycles administered per patient. The duration of follow-up after first cycle of I-PRRT ranged from 48 to 108 months with a median duration of 72 months.
The time interval between the last cycle of I-PRRT and salvage PRRT ranged from 13 to 36 months with an average time interval of 28.5 months. The dose of salvage PRRT ranged from 150 mCi (5.55 GBq) to 200 mCi (7.4 GBq) per patient with an average of 170 mCi (6.29 GBq) per patient and all NET patients received single cycle of salvage PRRT. The details of patients’ characteristics in the study population have been demonstrated in Table 1.
Table 1.
Patient characteristics
| Patients characteristics | Number of Patients |
|---|---|
| Total no. of progressive NET patients received salvage PRRT with indigenous 177Lu-DOTATATE | 26 |
| Total no. of progressive NET patients included and analyzed in the study | 22 |
| Sex (Male:Female) | 12:10 |
| Progressive/metastatic disease before salvage PRRT | 22 |
| Liver metastatic site prior salvage PRRT | 13 |
| Skeleton metastatic site prior salvage PRRT | 6 |
| High FDG avidity (SUVmax >5) prior salvage PRRT | 4 |
| Disease burden (number of metastatic lesions > 6) prior salvage PRRT | 7 |
| Site of primary disease | |
| Small intestine | 7 |
| Large intestine | 2 |
| Pancreas | 9 |
| Mediastinal | 1 |
| Unknown primary site | 3 |
| WHO Grade | |
| Grade 1 | 4 |
| Grade 2 | 15 |
| Unavailable | 3 |
NET, neuroendocrine tumor; PRRT, peptide receptor radionuclide therapy.
Outcome of salvage PRRT
I) Therapeutic outcome of salvage PRRT
A) Clinical symptomatic response: on clinical symptomatic response evaluation in 22 patients, CR was found in 1 patient (4.5%), PR in 1 patient (4.5%), SD in 13 patients (59.1%), whereas PD was found in 7 patients (31.9%).
B) Biochemical response: Out of 22 patients, CR was found in 1 patient (4.5%), PR in 1 patient (4.5%), SD in 15 patients (68.3%), whereas PD was found in 5 patients (22.7%) on biochemical response evaluation.
C) Imaging response: CR was not found in any one of patients on imaging response evaluation after salvage PRRT. Out of 22 patients, PR in 2 patients (9%), SD in 12 patients (54.6%) and PD in 8 patients (36.4%) on 68Ga-DOTATATE imaging by using PERCIST criteria.
The RECIST 1.1 criteria was applied in 22 patients after salvage PRRT, we found PR in 1 patient (4.5%), SD in 13 patients (59.1%) and 8 in 46 patients (36.4%) on as shown in Table 2.
Table 2.
Therapeutic response of salvage PRRT
| Response | Clinical symptomatic response evaluation (No. of patients) | Biochemical response evaluation (No. of patients) | PERCIST (No. of patients) 68Ga-DOTATATE | RECIST 1.1 (No. of patients) |
|---|---|---|---|---|
| Complete response | 1 (4.5%) | 1 (4.5%) | 0 | 0 |
| Partial response | 1 (4.5%) | 1 (4.5%) | 2 (9%) | 1 (4.5%) |
| Stable disease | 13 (59.1%) | 15 (68.3%) | 12 (54.6%) | 13 (59.1%) |
| Progressive disease | 7 (31.9%) | 5 (22.7%) | 8 (36.4%) | 8 (36.4%) |
PRRT, peptide receptor radionuclide therapy.
II) Survival outcome
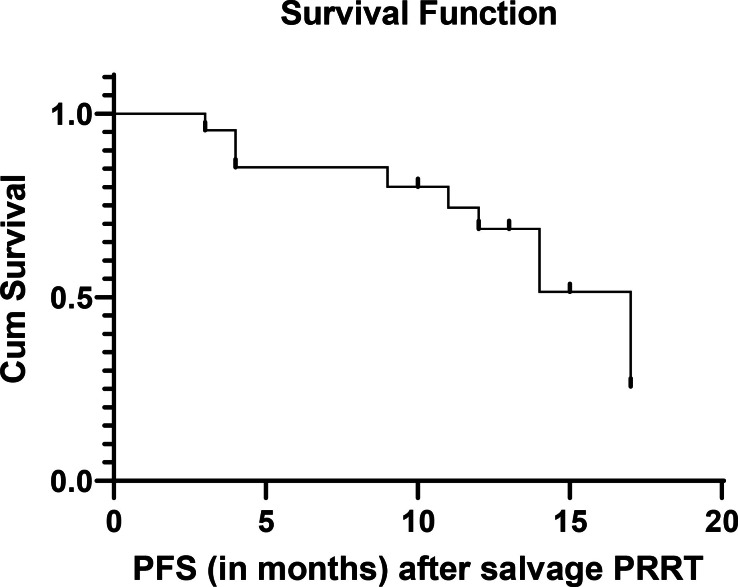
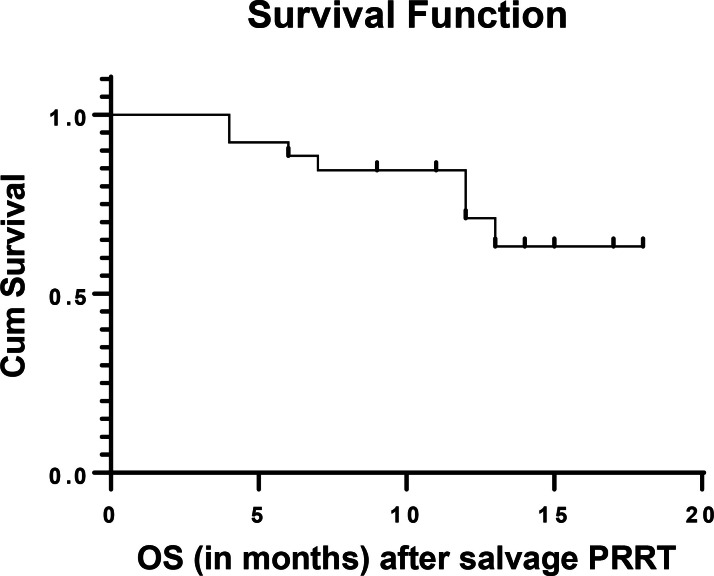
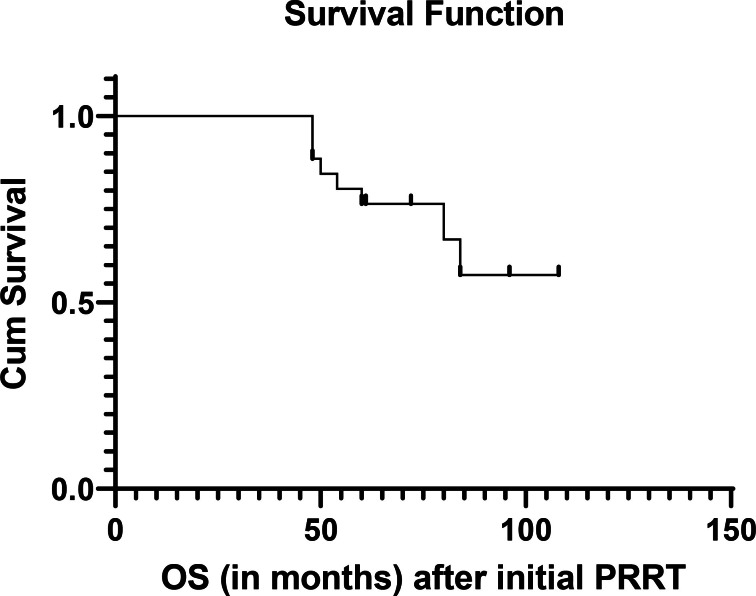
The observed median PFS of the population was 17 months after salvage PRRT as shown in Figure 1. The median OS-i and OS-s were not attained at a median follow-up period of 72 months and12 months respectively. Estimated OS-i was 68% at 108 months and OS-s was 82% at 18 months as depicted in Figure 2 (OS-s) and Figure 3 (OS-i).
Figure 1.
PFS after salvage PRRT. PFS, progression-free survival; PRRT, peptide receptor radionuclide therapy.
Figure 2.
OS after salvage PRRT (OS-s). OS, overall survival; PRRT, peptide receptor radionuclide therapy.
Figure 3.
OS after initial PRRT (OS-i). OS, overall survival; PRRT, peptide receptor radionuclide therapy.
III) Toxicity assessment
As per NCI-CTCAE 5.0, acute toxicities of nausea (Grade 1) and vomiting (Grade 1) were seen in four patients (18%) and two patients (9%) respectively within 24 h after the administration of salvage PRRT. Transient anemia was seen in 18 patients {Grade 1 in 15 patients (68.3%) and Grade 2 in 3 patients (13.5 %)}. Out of these 18 patients, 13 patients had low hemoglobin levels before salvage PRRT. Transient thrombocytopenia and leucopenia was seen in 6 patients {Grade 1 in 5 patients (22.7%) and Grade 2 in 1 patient (4.5 %)} and 2 patients {Grade 1 in 2 patients (9%)} respectively. Four patients were demonstrated nephrotoxicity of Grade 1 and Grade 2 in two patients each respectively. No Grade 3/4 hematological and nephrotoxicity was found in any of 22 patients during the follow-up period as shown in Table 3. Association of survival with various variables
Table 3.
Treatment-adverse events
| Adverse events ↓/CTCAE Grade*→ | Grade 1 | Grade 2 | Grade 3 | Grade 4 |
|---|---|---|---|---|
| During/after salvage PRRT in number of NET patients | ||||
| Nausea | 4 (18%) | 0 | 0 | 0 |
| Vomiting | 2 (9%) | 0 | 0 | 0 |
| Anemia | 15 (68.3) | 3 (13.5%) | 0 | 0 |
| Leucopenia | 2 (9%) | 0 | 0 | 0 |
| Thrombocytopenia | 5 (22.7%) | 1 (4.5%) | 0 | 0 |
| Nephrotoxicity | 2 (9%) | 2 (9%) | 0 | 0 |
| Hepatotoxicity | 0 | 0 | 0 | 0 |
CTCAE, Common Terminology Criteria for Adverse Events; NET, neuroendrocine tumor; PRRT, peptide receptor radionuclide therapy.
In this study, significant p-value for response and survival association with various variables such as site of primary tumor, Ki-67 index, serum CgA levels, disease burden before salvage PRRT, 68Ga-DOTATATE and 18F-FDG avidity in primary or metastatic lesions was not found. This is likely because of a smaller number of patients in each subgroup of variables that might have resulted into non-significant p-value or non-comparable test.
Discussion
The clinical treatment guidelines are not available for relapsed/progressive cases of NET following PRRT. Hence, the management of these cases is extremely difficult in the presence of limited therapeutic options. One of latest therapeutic option for these patients is a targeted α therapy by using 225Ac-DOTATATE. Other alternate therapeutic options for progressive NET patient are molecular-targeted therapies such as Everolimus and Sunitinib, conventional oral chemotherapy such as Temozolomide and Capecitabine, all of which constitute valid alternatives. Everolimus and sunitinib are approved treatment options for advanced progressive gastro-pancreatic-intestinal /lung NET and advanced progressive pancreatic NET respectively. The RADIANT-4 study demonstrated a median PFS of 11 months in advanced progressive gastro-pancreatic-intestinal /lung NET patients treated with everolimus. 14 However, the use of everolimus in NET patients were associated with common side-effects, e.g. stomatitis (>60%), diarrhea (30%), fatigue (30%), infections (20%–29%), pneumonitis (12%–16%) and hyperglycemia (10%–13%). Because of these side-effects, dose reduction or treatment interruption of everolimus is required in around 60% of NET patients. Sometimes use of everolimus may be led to life-threatening side-effects such as serious infections, sepsis, thromboembolic events and hence require regular monitoring of patients during this therapy. Similar to PFS shown by the RADIANT-4 study, a median PFS of 11.4 months was found in a randomized trial 15 on sunitinib in pancreatic NET patients with common side-effects of diarrhea (59%), nausea (45%), asthenia (34%), vomiting (34%), fatigue (32%), hypertension (26%), lymphopenia (26%) and hair color changes (29%).
Retreatment of PRRT is challenging in the progressive cases of NET, who had initially responded to PRRT. The main challenges are side-effects associated with PRRT, e.g. hematotoxicity and nephrotoxicity. These side-effects are considered as a dose limiting factors for salvage PRRT. van der Zwan et al demonstrated the safety and efficacy of salvage PRRT in one of largest series of NET patients (n = 181) who were treated with 177Lu-DOTATATE (a total cumulative dose given up to 60.5GBq and median follow-up period of 88.6 months). They observed Grade 3/4 hematotoxicity in 13 patients (7.2%) and also observed acute myeloid leukemia (AML) and myelodysplastic syndrome (MDS) in 4 patients (2.2%). But, Grade 3/4 nephrotoxicity was not seen in this study. Median PFS of 14.6 months (95% CI 12.4–16.9) and 14.2 months (95% CI 9.8–18.5) were demonstrated after retreatment PRRT and after re-retreatment PRRT respectively. They also found response rate and stabilization of disease in 26 patients (15.5%) and 100 patients (59.5%), and in 5 patients (38.5%) and 7 patients (53.8%) following retreatment PRRT and re-retreatment PRRT respectively. 16
Recently, Kim YI published a systematic review and meta-analysis article on efficacy, survival, and toxicity of salvage PRRT. A pooled proportions of hematotoxicity and nephrotoxicity in 10.8% (95%CI 5.9–16.8) and 0.7% (95%CI 0.2–1.8) respectively was observed. The overall response rate of 17.1% (95%CI 11.6–23.5) and DCR of 76.9% (95%CI 72.3–81.0) were noticed following salvage PRRT. The author also demonstrated pooled estimates of median PFS of 14.1 months (95% CI 12.2–15.9) and median OS of 26.8 months (95% CI 18.8–34.9) after salvage PRRT. It was concluded that, salvage PRRT is an effective treatment option for controlling or diminishing disease process in progressive cases of NET and to be considered as safe as I-PRRT. 8
To the best of our knowledge, our study is the first research article that evaluates the efficacy, survival and toxicity of salvage PRRT with indigenous “direct route” 177Lu-DOTATATE in progressive metastatic NET patients. In our study, we noticed salvage PRRT with indigenous 177Lu-DOTATATE a well-tolerated therapy in all NET patients. The highest level of toxicity was Grade II and seen as reversible anemia, thrombocytopenia and nephrotoxicity in three patients (13.5%), one patient (4.5%) and two patients (9%) during follow-up periods after salvage PRRT respectively. The RADIANT-4 study reported serious adverse events (Grade 3/4) in 12% of NET patients after everolimus. van der Zwan et al demonstrated Grade 3/4 hematotoxicity and AML/MDS in 7.7 and 2.2% of NET patients after salvage PRRT respectively. Other authors also reported Grade 3/4 hematotoxicity after salvage PRRT in range of 4.8–21.2%. 17–21 In our study, Grade 3/4 hematotoxicity, nephrotoxicity and hepatotoxicity and also AML/MDS was not seen in any of the patients after I-PRRT (indigenous 177Lu-DOTATATE) and salvage PRRT (indigenous 177Lu-DOTATATE) at median follow-up periods of 72 months and 12 months respectively. On survival analysis, salvage PRRT with indigenous 177Lu-DOTATATE in our study showed slightly longer PFS (a median PFS of 17 months) when indirectly compared with the RADIANT-4 study result (a median PFS of 11 months) and Yong-il Kim a meta-analysis result (a median PFS of 14.1 months) in progressive NET patients. In our study, the DCR of 68.1%, 77.3%, 63.6% and 63.6% were noticed after salvage PRRT on clinical symptomatic, biochemical, molecular and morphological imaging response evaluations respectively, these results were similar to DCR reported by other published papers on salvage PRRT. The indigenously produced 177Lu via direct (n,γ) route is used in most of the PRRT centers in India. This indigenously produced 177Lu has a number of advantages over the indirect route produced 177Lu, such as the simple chemical procedure post-irradiation in direct route, which makes production of 177Lu less technologically demanding and more cost-effective over 177Lu produced through indirect-route. With regard to the long-term outcome of direct route produced 177Lu, similar results were obtained as that of indirect-route produced 177Lu in metastatic or advanced NET patients. 22
The study had certain limitations. One limitation is that it is a retrospective single-center observational study. The study included a small sample size without control group of patients resulting into non-computable analysis of variables. Despite this, our study findings provided favorable data on toxicity, survival, and DCR of salvage PRRT with indigenous 177Lu-DOTATATE.
Conclusion
In summary, salvage PRRT with indigenous 177Lu-DOTATATE may be considered as a safe therapeutic option with reasonable efficacy and survival data for progressive/relapses cases of NET after I-PRRT. The main challenge for salvage PRRT in progressive NET patients is high grade hematotoxicity and nephrotoxicity, this was absent after average administered cumulative dose of 964 mCi (35.66 GBq) per patient of I-PRRT and average of 170 mCi (6.29GBq) per patient of salvage PRRT in the present study with indigenous 177Lu-DOTATATE, at median follow-up periods of 72 months and 12 months respectively. The application of salvage PRRT had produced survival benefits in progressive NET patients studied. On indirect comparison with literature data, the median PFS of salvage PRRT with indigenous 177Lu-DOTATATE was slightly longer than median PFS of everolimus and sunitinib demonstrated by the RADIANT-4 study and clinical trial on sunitinib respectively. However, prospective, randomized controlled trials in large number of progressive NET patients are required to further validate the survival benefits.
Contributor Information
Keerti Sitani, Email: keerti.sitani@gmail.com, Radiation Medicine Centre, Bhabha Atomic Research Centre, Tata Memorial Centre Annexe, JerbaiWadia Road, Parel, Mumbai, India ; Homi Bhabha National Institute, Mumbai, India .
Rahul Parghane, Email: rahul_parghane@yahoo.co.in, Radiation Medicine Centre, Bhabha Atomic Research Centre, Tata Memorial Centre Annexe, JerbaiWadia Road, Parel, Mumbai, India ; Homi Bhabha National Institute, Mumbai, India .
Sanjay Talole, Email: sdtalole@gmail.com, Homi Bhabha National Institute, Mumbai, India ; Department of Biostatistics, ACTREC, Tata Memorial Centre, Mumbai, India .
Sandip Basu, Email: drsanb@yahoo.com, Radiation Medicine Centre, Bhabha Atomic Research Centre, Tata Memorial Centre Annexe, JerbaiWadia Road, Parel, Mumbai, India ; Homi Bhabha National Institute, Mumbai, India .
REFERENCES
- 1. Pavel M, Öberg K, Falconi M, et al. . ESMO guidelines committee. electronic address: clinicalguidelines@ESMO.org. gastroenteropancreatic neuroendocrine neoplasms: ESMO clinical practice guidelines for diagnosis, treatment and follow-up . Ann Oncol 2020. ; 31: 844 – 60 . [DOI] [PubMed] [Google Scholar]
- 2. Modlin IM, Moss SF, Oberg K, Padbury R, Hicks RJ, Gustafsson BI, et al. . Gastrointestinal neuroendocrine (carcinoid) tumours: current diagnosis and management . Med J Aust 2010. ; 193: 46 – 52 . doi: 10.5694/j.1326-5377.2010.tb03742.x [DOI] [PubMed] [Google Scholar]
- 3. Strosberg J, El-Haddad G, Wolin E, et al. . Phase 3 trial of 177lu-dotatate for midgut neuroendocrine tumors . N Engl J Med 2017. ; 376: 125 – 35 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Kwekkeboom DJ, de Herder WW, Kam BL, van Eijck CH, van Essen M, Kooij PP, et al. . Treatment with the radiolabeled somatostatin analog [177 lu-dota 0,tyr3]octreotate: toxicity, efficacy, and survival . J Clin Oncol 2008. ; 26: 2124 – 30 . doi: 10.1200/JCO.2007.15.2553 [DOI] [PubMed] [Google Scholar]
- 5. Brabander T, van der Zwan WA, Teunissen JJM, Kam BLR, Feelders RA, de Herder WW, et al. . Long-term efficacy, survival, and safety of [177lu-dota0,tyr3]octreotate in patients with gastroenteropancreatic and bronchial neuroendocrine tumors . Clin Cancer Res 2017. ; 23: 4617 – 24 . doi: 10.1158/1078-0432.CCR-16-2743 [DOI] [PubMed] [Google Scholar]
- 6. Bodei L, Cremonesi M, Grana CM, Fazio N, Iodice S, Baio SM, et al. . Peptide receptor radionuclide therapy with 1 7 7 Peptide receptor radionuclide therapy with . Eur J Nucl Med Mol Imaging 2011. ; 38: 2125 – 35 . doi: 10.1007/s00259-011-1902-1 [DOI] [PubMed] [Google Scholar]
- 7. Garcia-Carbonero R, Sorbye H, Baudin E, Raymond E, Wiedenmann B, Niederle B, et al. . ENETS consensus guidelines for high-grade gastroenteropancreatic neuroendocrine tumors and neuroendocrine carcinomas . Neuroendocrinology 2016. ; 103: 186 – 94 . doi: 10.1159/000443172 [DOI] [PubMed] [Google Scholar]
- 8. Kim YI . Salvage peptide receptor radionuclide therapy in patients with progressive neuroendocrine tumors: a systematic review and meta-analysis . Nucl Med Commun 2021. ; 42: 451 – 58 . doi: 10.1097/MNM.0000000000001350 [DOI] [PubMed] [Google Scholar]
- 9. Basu S, Parghane RV, Kamaldeep N, Chakrabarty S . Peptide receptor radionuclide therapy of neuroendocrine tumors . Semin Nucl Med 2020. ; 50: 447 – 64 : S0001-2998(20)30058-1 . doi: 10.1053/j.semnuclmed.2020.05.004 [DOI] [PubMed] [Google Scholar]
- 10. Das T, Chakraborty S, Kallur KG, Venkatesh M, Banerjee S . Preparation of patient doses of (177)lu-DOTA-TATE using indigenously produced (177)lu: the indian experience . Cancer Biother Radiopharm 2011. ; 26: 395 – 400 . doi: 10.1089/cbr.2010.0881 [DOI] [PubMed] [Google Scholar]
- 11. Wahl RL, Jacene H, Kasamon Y, Lodge MA, et al. . From RECIST to PERCIST: evolving considerations for PET response criteria in solid tumors . J Nucl Med 2009. ; 50 Suppl 1: 122S - 50S . doi: 10.2967/jnumed.108.057307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Parghane RV, Talole S, Prabhash K, Basu S, et al. . Clinical response profile of metastatic/advanced pulmonary neuroendocrine tumors to peptide receptor radionuclide therapy with 177lu-DOTATATE . Clin Nucl Med 2017. ; 42: 428 – 35 . doi: 10.1097/RLU.0000000000001639 [DOI] [PubMed] [Google Scholar]
- 13. Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, et al. . New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1) . Eur J Cancer 2009. ; 45: 228 – 47 . doi: 10.1016/j.ejca.2008.10.026 [DOI] [PubMed] [Google Scholar]
- 14. Yao JC, Fazio N, Singh S, Buzzoni R, Carnaghi C, Wolin E, et al. . Everolimus for the treatment of advanced, non-functional neuroendocrine tumours of the lung or gastrointestinal tract (RADIANT-4): a randomised, placebo-controlled, phase 3 study . Lancet 2016. ; 387: 968 – 77 : S0140-6736(15)00817-X . doi: 10.1016/S0140-6736(15)00817-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Raymond E, Dahan L, Raoul J-L, Bang Y-J, Borbath I, Lombard-Bohas C, et al. . Sunitinib malate for the treatment of pancreatic neuroendocrine tumors . N Engl J Med 2011. ; 364: 501 – 13 . doi: 10.1056/NEJMoa1003825 [DOI] [PubMed] [Google Scholar]
- 16. van der Zwan WA, Brabander T, Kam BLR, Teunissen JJM, Feelders RA, Hofland J, et al. . Salvage peptide receptor radionuclide therapy with [177lu-dota,tyr3]octreotate in patients with bronchial and gastroenteropancreatic neuroendocrine tumours . Eur J Nucl Med Mol Imaging 2019. ; 46: 704 – 17 . doi: 10.1007/s00259-018-4158-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Zemczak A, Gut P, Pawlak D, Kołodziej M, Królicki L, Kos-Kudła B, et al. . The safety and efficacy of the repeated prrt with [90y]y/[177lu]lu-dotatate in patients with net . Int J Endocrinol 2021. ; 2021: 6615511 . doi: 10.1155/2021/6615511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Sabet A, Haslerud T, Pape U-F, Sabet A, Ahmadzadehfar H, Grünwald F, et al. . Outcome and toxicity of salvage therapy with 177lu-octreotate in patients with metastatic gastroenteropancreatic neuroendocrine tumours . Eur J Nucl Med Mol Imaging 2014. ; 41: 205 – 10 . doi: 10.1007/s00259-013-2547-z [DOI] [PubMed] [Google Scholar]
- 19. Yordanova A, Mayer K, Brossart P, Gonzalez-Carmona MA, Strassburg CP, Essler M, et al. . Safety of multiple repeated cycles of 177lu-octreotate in patients with recurrent neuroendocrine tumour . Eur J Nucl Med Mol Imaging 2017. ; 44: 1207 – 14 . doi: 10.1007/s00259-017-3652-1 [DOI] [PubMed] [Google Scholar]
- 20. Vaughan E, Machta J, Walker M, Toumpanakis C, Caplin M, Navalkissoor S . Retreatment with peptide receptor radionuclide therapy in patients with progressing neuroendocrine tumours: efficacy and prognostic factors for response . Br J Radiol 2018. ; 91( 1091 ): 20180041 . doi: 10.1259/bjr.20180041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Rudisile S, Gosewisch A, Wenter V, Unterrainer M, Böning G, Gildehaus FJ, et al. . Salvage PRRT with 177lu-DOTA-octreotate in extensively pretreated patients with metastatic neuroendocrine tumor (NET): dosimetry, toxicity, efficacy, and survival . BMC Cancer 2019. ; 19( 1 ): 788 . doi: 10.1186/s12885-019-6000-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Sitani K, Parghane RV, Talole S, Basu S . Long-term outcome of indigenous 177lu-DOTATATE PRRT in patients with metastatic advanced neuroendocrine tumours: a single institutional observation in a large tertiary care setting . Br J Radiol 2021. ; 94( 1117 ): 20201041 . doi: 10.1259/bjr.20201041 [DOI] [PMC free article] [PubMed] [Google Scholar]