Abstract
Attenuated Edmonston measles virus (MV-Edm) is not pathogenic in standard mice. We show here that MV-Edm inoculated via the natural respiratory route has a limited propagation in the lungs of mice with a targeted mutation inactivating the alpha/beta interferon receptor. A high dose of MV-Edm administered intracerebrally is lethal for about half of these mice. To study the consequences of the availability of a high-affinity receptor for MV propagation, we generated alpha/beta interferon-defective mice expressing human CD46 with human-like tissue specificity. Intranasal infection of these mice with MV-Edm resulted in enhanced spread to the lungs and more prominent inflammatory response. Virus replication was also detected in peripheral blood mononuclear cells, the spleen, and the liver. Moreover, intracerebral inoculation of adult animals with low MV-Edm doses caused encephalitis with almost inevitably lethal outcome. We conclude that in mice alpha/beta interferon controls MV infection and that a high-affinity receptor facilitates, but is not strictly required for, MV spread and pathogenesis.
Measles remains one of the leading causes of infant death in developing countries (40) and, in rare cases, persistent measles virus (MV) infection induces the lethal neurodegenerative disease subacute sclerosing panencephalitis (SSPE) (10, 55). Direct studies of early MV replication in humans are lacking, but experimental studies in monkeys (32, 58) and histopathological observations in humans (33) suggest local replication in the respiratory mucosa a few days after infection. MV may then spread, possibly carried in pulmonary macrophages, to draining lymph nodes and from there enter the bloodstream carried in leukocytes, disseminating first to lymphoid tissues and then to tissues throughout the body (40).
MV infection of adult rodents is restricted to brain-adapted strains inoculated intracerebrally (29). These strains have substantial changes in the sequence of the receptor binding protein hemagglutinin (H) (30), alterations which may permit more efficient MV entry into rodent cells. MV entry into mouse cells is also more efficient with expression of CD46, the receptor for the MV vaccine strain Edmonston (MV-Edm) (13, 36) and probably for several wild-type strains (51, 52, 57). However, transgenic rodents expressing CD46 are not susceptible to MV infection when inoculated by the natural respiratory route (17, 39, 56). Nevertheless, MV-Edm intracerebral inoculation of neonatal transgenic mice expressing one form of CD46 in neurons resulted in disease and death (46).
To obtain mice in which MV spread can be studied, we operated at two levels. First, knowing that alpha/beta interferon controls MV infection in cultured cells (6, 28) and may do so in patients with SSPE (15, 60), we tested whether mice with a targeted mutation inactivating the alpha/beta interferon receptor (Ifnartm strain) (35) are susceptible to infection with the attenuated strain MV-Edm. Indeed we observed limited MV spread after intranasal inoculation and 50% lethality after high-dose intracerebral inoculation. Second, we produced the new transgenic line CD46Ge (Ge for genomic), which, unlike previous lines (17, 46, 56), expresses CD46 with human-like tissue specificity (34, 42), and crossed it with Ifnartm mice to obtain an Ifnartm-CD46Ge line. Respiratory inoculation of these mice with MV-Edm resulted in enhanced virus spread and more prominent lung tissue inflammation, and intracerebral infection was lethal at low virus doses.
MATERIALS AND METHODS
CD46 transgenic mice.
A yeast strain carrying the 400-kb yeast artificial chromosome (YAC) RCA1, with the CD46 gene in its center (18), was grown in selective medium, and YAC DNA was prepared (48). Briefly, yeast cells were embedded in agarose and lysed, the intact chromosomal DNA was separated by pulsed-field gel electrophoresis, a gel slice with the YAC was identified and excised, and the DNA was concentrated on a second agarose gel cast around the slice. To compact the DNA and minimize shearing forces, buffers containing NaCl and polyamines were used. After agarase digestion, YAC DNA was dialyzed against microinjection buffer (100 mM NaCl, 10 mM Tris-HCl [pH 7.5], 0.1 mM EDTA, 30 μM spermine, 70 μM spermidine). About 2 pl of purified YAC DNA (2 ng/μl) in microinjection buffer was injected into fertilized oocytes of B6C3F1 hybrids, and about 30 oocytes were transferred to each pseudopregnant foster mother. Seventeen pups were born, of which two were transgenic. One animal (Hugo) was a rare mosaic nontransmitter, whereas TgN(hCD46Ge)373Zbz (Teresa) transmitted its insert to about 50% of its progeny. The animals were held, and experiments were performed, under optimal hygienic conditions.
For genotype analysis, tails from 5- to 6-week-old mice were cut (0.5 to 1 cm in length) and DNA was prepared (26). CD46-specific PCR was based on two primer pairs: 5′-AAAGGGCAAATTACCTTAAGGGGTG and 5′-AGCACTTCGACCTAAAAATAGAGAT, amplifying 263 bp of the CD46 promoter region, and 5′-GCCAGTTCATCTTTTGACTCTATTAA and 5′-CAGCATATCCCTGCTTTAATACAAC, amplifying 249 bp of exon 14. Additionally, a primer pair (5′-CCTCGTCTTCAATAAAACATTT and 5′-AGCCCTGACAGGGGGTTAT) amplifying 369 bp of the promoter region of the MCP-like genetic element, situated about 90 kb upstream of the CD46 gene (12), was occasionally used. The results obtained with the three primer pairs were equivalent. Southern blots probed with the 263-bp PCR product of the CD46 promoter and with a Prn-p gene fragment (5), both nick translated with the Prime-it II labelling kit (Stratagene), allowed identification of CD46 homozygous mice (59).
To establish the Ifnartm-CD46Ge mutant line, a CD46Ge 2n male was crossed with a homozygous Ifnartm female. The F2 progeny were screened for an inactivating insertion in both alpha/beta interferon alleles (35) and for homozygosity for CD46 (see above). The haplotype of Ifnartm mice is H-2b, and that of Ifnartm-CD46Ge mice is H-2bk. Both the H-2b and H-2k haplotypes are highly sensitive to MV-induced encephalitis (38).
Viruses and infections.
The MV-Edm substrain used was that adapted to grow in HeLa cell spinner cultures by S. Udem; RNA from this substrain was also used to reconstitute a cDNA copy of the MV genome suitable for reverse genetics (45). The MV-P-CAT virus is a derivative of this substrain to which a transcription unit expressing the reporter protein chloramphenicol acetyltransferase (CAT) was added downstream of the phosphoprotein (P) gene (54). The rat brain-adapted CAM/RBH strain and the wild-type wtF strain were kindly supplied by S. Niewiesk, Würzburg, Germany. The wild-type Chicago-1 strain was kindly supplied by D. Griffin, Baltimore, Md.
MV-Edm, MV-P-CAT, and Chicago-1 were propagated in Vero cells as described previously (45) and used for mouse inoculation in the form of postnuclear supernatants (5 min at 800 × g). The wtF strain was grown in the Epstein-Barr virus-transformed B lymphoblastoid cell line B-LCL JP (supplied by R. S. van Binnendijk, Utrecht, The Netherlands), and the CAM/RBH strain was grown in rat brains; both strains were used for mouse inoculation as cell homogenates.
For intranasal infection, 5- to 8-week-old animals were anesthetized and then infected in both nares with 50 μl of virus in phosphate-buffered saline (PBS). For mock infections, postnuclear supernatants of uninfected cells were used. For intracerebral MV inoculation, 5- to 8-week-old animals were anesthetized and injected along the skull midline with 30 μl of virus by means of a syringe with a 27-gauge needle. Infected animals, homozygous for both mutated loci, were observed daily for clinical symptoms or death.
RNA analysis: reverse transcription-PCR, Northern blots, and in situ hybridization.
For RNA extraction from organs (11), animals were sacrificed and tissues were removed and snap frozen in liquid nitrogen. The minus-strand primer 5′-TTATAACAATGATGGAGGGTAGGC, hybridizing to the last 24 nucleotides of the nucleocapsid (N) mRNA (43), was used for reverse transcription. PCR was based on the primer pair 5′-GATGGAGGGTAGGCGGATGTTGTTCTGGC–5′-ACTCGGTATCACTGCCGAGGATGCAAGGC, amplifying 474 bases of the N mRNA.
For Northern analysis 5 μg of RNA was separated by electrophoresis on a 1% formaldehyde agarose gel and analyzed with a digoxigenin (DIG)-labelled probe (DIG RNA labelling kit; Boehringer, Mannheim, Germany) corresponding to 851 bp of the MV N mRNA (9) or with a control DIG-labelled β-actin RNA probe (Boehringer).
MV N-specific mRNA was detected in tissue sections with DIG-labelled N RNA of negative polarity. After deparaffinization, 2-μm-thick sections were processed as instructed by the manufacturer (Boehringer) with the following modifications. Prehybridization was at 37°C for 2 h without proteinase K pretreatment. One hundred microliters of DIG-labelled N RNA probe (30 pg/μl in hybridization buffer with Denhardt’s solution) was added to each section, and the sections were incubated at 68°C overnight in a humid chamber. Immunological detection was done with the DIG-nucleic acid detection kit (Boehringer). The sections were developed for 6 h at room temperature in the dark.
Protein analysis: Western blot, CAT assay, and histopathology.
Mouse organs were snap frozen in liquid nitrogen. Human tissues were collected 9 to 15 h postmortem. The tissues were homogenized with an Ultra-Turrax T25 (IKA, Staufen, Germany) tissue grinder in PBS buffer containing 0.5% Nonidet P-40 and 0.5% deoxycholate. The homogenates were centrifuged in an Eppendorf centrifuge for 10 min at 2,000 rpm. The obtained supernatant was recovered, and the protein concentration was determined by the bicinchoninic acid assay (Pierce). The homogenates were subjected to nonreducing sodium dodecyl sulfate–11% polyacrylamide gel electrophoresis, and the separated proteins were transferred to an Immobilon-P nylon membrane (Millipore). CD46 was detected with a rabbit antiserum (4).
For CAT assays 800 μg of protein from each tissue was processed according to the supplier’s protocol (Promega). Briefly, the same volume of the reaction mixture containing [14C]chloramphenicol (10 μCi; Du Pont), acetyl coenzyme A (0.07 mg; Sigma), and 250 mM Tris HCl (pH 7.5) was used per reaction, and the reaction mixture was incubated for 90 min at 37°C. Acetylated and nonacetylated forms of chloramphenicol were separated on thin-layer silica gels (Sigma) and visualized by autoradiography.
Mouse peripheral blood mononuclear cells (PBMC) were isolated from blood samples by a density separation medium (Lympholyte-M; Cedarlane Laboratories Ltd., Hornby, Ontario, Canada) according to the supplier’s protocol.
For histopathology animals were sacrificed and the brains were removed and fixed by immersion in 4% PBS-buffered formaldehyde for 24 h at room temperature. The tissues were embedded in paraffin and processed by standard techniques. Sagittal and coronal sections were cut at 2 μm and stained with hematoxylin-eosin (HE). Lung tissues were processed in the same way. Glial fibrillary acidic protein (GFAP) immunostaining for the detection of immunoreactive astrocytes was performed with rabbit anti-GFAP polyclonal antibodies (Calbiochem) and biotinylated swine anti-rabbit antibodies. Antibody detection was done with avidin-biotin-peroxidase (Vectastain Elite ABC and DAB-substrate kits; Vector Labs, Burlingame, Calif.).
RESULTS
Transgenic mice expressing human CD46 with human-like tissue specificity.
CD46, a human cell surface protein for which no murine homolog is available, is produced ubiquitously as four major isoforms and protects host cells from complement activation (31). These isoforms arise by alternative splicing and differ in the presence or absence of a short, heavily O-glycosylated domain (named B) and in having one of two cytoplasmic tails (named 1 and 2). To allow transfer to mice of the whole human CD46 gene, including the unmapped locus control region(s), we selected a large YAC covering part of the regulator of complement activation locus on human chromosome 1. In this YAC the 50-kb CD46 gene is preceded by the CR1-like and the MCP-like genetic elements (18). After microinjection of purified YAC DNA into mouse oocytes, two transgenic animals were obtained, of which one transmitted the human CD46 gene; its progeny are referred to as CD46Ge.
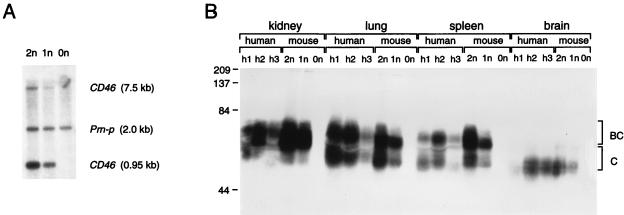
The presence of the CD46 gene in litters was monitored by PCR, and homozygous mice were subsequently identified by Southern blotting. Figure 1A presents an analysis of the genomes of two transgenic mice and one control animal. Two probes, one recognizing two EcoRI fragments (0.95 and 7.5 kb) in the CD46 promoter and one homologous to a 2-kb fragment of an endogenous mouse gene (Prn-p; internal standard), were used. The CD46 signals from the homozygous animal (2n) were approximately twice as intense as those of the hemizygous animal (1n), whereas no signal was detected in the control animal (0n).
FIG. 1.
Genome analysis (A) and protein expression (B) of CD46Ge transgenic mice. (A) Genomic analysis of transgenic and control mice. Tail DNAs were digested with EcoRI, separated on an agarose gel, blotted onto a nylon membrane, and hybridized with two probes, one recognizing two fragments (0.95 and 7.5 kb) in the human CD46 promoter region and one recognizing a 2-kb fragment of the endogenous mouse Prn-p gene. Lane 2n, CD46 homozygous mouse; lane 1n, CD46 hemizygous mouse; lane 0n, control mouse. (B) CD46 protein expression in four organs obtained at autopsies of three humans (h1, h2, and h3) compared to the same organs from a CD46 homozygous mouse (2n), a CD46 hemizygous mouse (1n), and a control mouse (0n). Thirty micrograms of protein from human tissue homogenates or 15 μg of protein from mouse tissue homogenates were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis, transferred to a nylon membrane, and reacted against a polyclonal rabbit anti-CD46 serum. The molecular masses of marker proteins are indicated on the left in kilodaltons. The approximate positions of the two major isoforms of the CD46 proteins (termed BC and C) are indicated on the right. CD46 proteins produced in different organs have different molecular masses due to differential splicing and heterogeneous N- and O-glycosylation. Note that in humans different patterns of CD46 expression have been recognized (19). Individuals h1 and h2 expressed approximately equivalent amounts of BC and C isoforms, whereas individual h3 may have had predominantly BC expression. CD46Ge mice express equivalent amounts of the BC and C isoforms. The low quantity of protein detected in certain human autopsy samples, e.g., the brain of h1, may be because of partial protein degradation.
The ability of the chromosome 1 fragment to confer human-like tissue-specific expression was tested by analyzing the proteins produced in the kidney, lung, spleen, and brain. Protein extracts from human autopsy material (Fig. 1B; individuals h1, h2, and h3) were compared to extracts from CD46 homozygous, hemizygous, and negative control mice. In these and all other tissues examined and in concert with prior analyses, CD46 isoforms were visualized as broad bands, most probably due to differential splicing and to different levels of N- and O-glycosylation (31). The electrophoretic mobilities and the isoform patterns were similar in all matched tissues of mice and humans, including brain tissue, where only fast-migrating CD46 isoforms are produced (2, 19). Homozygous CD46 mice expressed more CD46 than hemizygous animals.
We also measured CD46 expression on blood cells by flow cytometry. Lymphocytes of transgenic mice (mean fluorescence, 12) reached levels comparable to those of human lymphocytes (mean fluorescence, 20), whereas mouse and human erythrocytes did not express CD46 (mean fluorescence, 0.3). Taken together, these results indicate that elements necessary for expression of the human CD46 gene were transferred to mice and suggest that differential splicing of human CD46 transcripts was faithfully reproduced in mice.
Respiratory MV infection of mice and its pathogenic consequences.
To compare the sensitivity to MV infection of CD46-expressing animals and that of alpha/beta interferon-defective animals, we inoculated these genetically modified mice intranasally with the attenuated strain MV-Edm. The mice were sacrificed 2 to 11 days postinfection (p.i.), and the lungs were removed for RNA extraction. At autopsy, macroscopic purple lesion areas were noticed in Ifnartm mouse lungs but not in CD46Ge mouse lungs. RT-PCR analysis of MV N plus-strand RNA consistently revealed considerably stronger signals in Ifnartm than in CD46Ge mice. Mock-infected mice were consistently negative (not shown).
To obtain double-mutant mice, possibly more sensitive to MV infection, the two lines were crossed. Intranasal challenge was repeated with the resulting Ifnartm-CD46Ge animals, homozygous for both mutations, and with Ifnartm mice by using 200,000 infectious units of MV-Edm or of a modified MV-Edm with an additional transcription unit expressing the reporter protein CAT (MV-P-CAT). These two viruses were transcribed at similar levels in the lungs (not shown). At autopsy, purple lesion areas in the lung tissue of infected Ifnartm-CD46Ge mice were larger than lesions in Ifnartm mice.
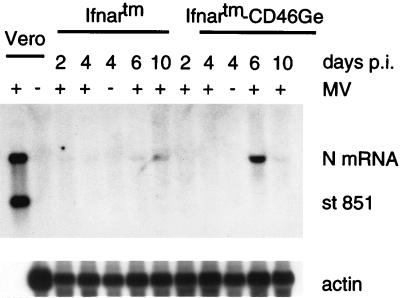
Figure 2 shows an analysis of the N mRNA produced in the lungs of Ifnartm and Ifnartm-CD46Ge mice sacrificed 2, 4, 6, or 10 days after MV-P-CAT infection and in two control mice sacrificed 4 days after mock infection. In the four mice sacrificed on days 6 and 10, a positive signal was scored. In one of these (the Ifnartm-CD46Ge mouse sacrificed at day 6), the signal was very strong. In this and other experiments, RNA from 18 Ifnartm-CD46Ge and 18 Ifnartm MV-Edm- or MV-P-CAT-infected mice sacrificed 2 to 10 days p.i. was analyzed by Northern blotting. Of the Ifnartm-CD46Ge mice, five were strongly positive, eight were positive, and five were negative. Four of the five mice with high levels of transcription were those sacrificed 6 days p.i. The five mice with no detectable transcription were sacrificed at day 2 (two of four sacrificed) or day 4 (three of five sacrificed). Of the Ifnartm mice, 8 were positive (none of four sacrificed on day 2, two of five sacrificed on day 4, three of four sacrificed on day 6, and three of five sacrificed on day 10) and 10 were negative. We conclude that MV transcription in the lungs of Ifnartm-CD46Ge mice was more efficient than that in Ifnartm mice and peaked around day 6.
FIG. 2.
Northern blot analysis of MV RNA in lungs of Ifnartm and Ifnartm-CD46Ge mice intranasally inoculated with MV-P-CAT. Mice were sacrificed at the days p.i. indicated. Five micrograms of total lung RNA was separated on a 1% formaldehyde-agarose gel, blotted to a nylon membrane, and reacted with an antisense MV N RNA probe (top panel). The blot was stripped and rehybridized with an actin RNA probe (bottom panel). As a positive control, 6 ng of total RNA from MV-infected Vero cells was used (first lane) and, as a negative control, 10 μg of total RNA from mock-infected Vero cells (second lane) was used. As additional negative controls, total RNAs from two mock-infected mice (−) were examined. A synthetic MV N plus-strand standard RNA 851 bases in length (st 851) was added to the positive control. The positions of this standard RNA, the N mRNA (about 1.7 kb), and the actin mRNA are indicated on the right. About 30,000 copies of MV N mRNA are produced in MV-infected primate cells (first lane) (9). Considering that about 800 times less RNA from Vero cells than RNA from mouse lungs was loaded, the signal in the positive control corresponds to about 40 copies of N mRNA per cell, and the signals in the mouse lung tissues correspond to a few N mRNA copies per average cell.
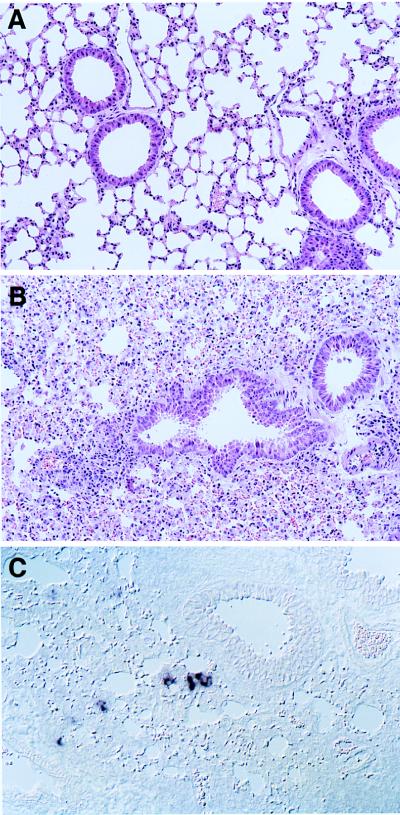
We then studied the pathogenic effects of MV-Edm replication in the lungs. Histological examination of an Ifnartm-CD46Ge animal sacrificed 6 days after infection revealed acute lung inflammation, extensive hyperemia, and diffuse hemorrhage in large areas of the lungs (Fig. 3B). In contrast, in the lung of a mock-infected animal (Fig. 3A) the alveolar lace-like structure was generally preserved and only minor hemorrhage was noticed.
FIG. 3.
Results of histological analysis (A and B) and MV RNA detection (C) in lung sections of intranasally infected Ifnartm-CD46Ge mice. After mock infection (A) or inoculation with MV-Edm (B and C), the mice were sacrificed. (C) A 2-μm-thick lung section hybridized with a MV N mRNA-specific probe.
To gain insight into the localization of MV-replicating cells in the lung, we analyzed MV RNA expression in situ. Figure 3C demonstrates a section of an MV-infected Ifnartm-CD46Ge mouse lung. Single cells and small groups of cells stained for MV N mRNA. Due to the increased cellularity and the disruption of the normal lung architecture, the specificity of cells replicating MV was difficult to determine, but groups of cells were often located near the bronchial epithelium; on the other hand, certain isolated cells were tentatively identified as macrophages. No MV transcription was detected, as expected, in mock-infected animals (not shown). This analysis indicates that MV is transcribed in epithelial cells and possibly in macrophages but overall in a small fraction of lung cells. Nevertheless, MV infection has striking pathological consequences.
Limited MV systemic propagation.
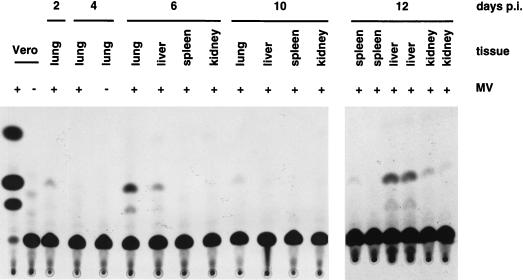
To investigate if primary MV replication in the lung was followed by systemic spread, we took advantage of the reporter gene in MV-P-CAT. A CAT assay of tissues from the same Ifnartm-CD46Ge animals utilized for RNA analysis is presented in Fig. 4. Lungs were collected from the animals sacrificed at days 2, 4, 6, and 10, and livers, spleens, and kidneys were harvested from mice sacrificed at days 6, 10, and 12. The CAT assay indicated a peak of expression in the lungs at day 6. The other organ with CAT activity at day 6 was the liver, which also tested weakly positive in the animal sacrificed at day 10 and strongly positive in the two animals sacrificed at day 12. In the spleen and kidney, CAT activity was detected only at day 12. Generally, the results of these assays confirmed the RNA analysis, but, due to the higher sensitivity, more positive samples were identified. In repeated experiments, systemic spread was always confirmed in Ifnartm-CD46Ge mice and, to a lesser extent, also in Ifnartm mice. We also isolated PBMC of infected Ifnartm-CD46Ge mice and found low-to-intermediate levels of CAT activity 4 to 12 days p.i. (not shown). This observation raises the possibility that CAT signals detected in organs may derive from circulating PBMC. The fact that in the livers the levels of CAT activity were much higher than those in PBMC is not consistent with this hypothesis. We conclude that in these mutant mice MV propagates initially in the lungs and then in other organs.
FIG. 4.
MV spread in different organs of Ifnartm-CD46Ge mice. Mice intranasally inoculated (+) or mock infected (−) with MV-P-CAT were sacrificed at the days p.i. indicated and tissues were collected, homogenized, and tested for CAT activity. As a positive control, Vero cells infected with MV-P-CAT were used (first lane), and as a negative control, mock-infected Vero cells (second lane) were used. The lung extract of a mouse sacrificed 4 days after mock infection is shown in the fifth lane.
Other parameters of mouse infection with MV were also investigated. The specific antibody response in C57BL/6, CD46Ge, Ifnartm, and Ifnartm-CD46Ge mice was monitored by Western blotting. In the last group of animals the anti-N response was strongest (a positive signal with a 1:80,000 serum dilution [not shown]) and reached its peak 21 days p.i. Neutralizing antibodies were detected in Ifnartm-CD46Ge (1:170; background of 1:30) and Ifnartm (1:60) mice but were at background level in the other two mouse lines. When the wild-type strain Chicago-1 was used to infect Ifnartm-CD46Ge mice, a 1:300 titer of neutralizing antibodies was monitored. Thus, neutralizing antibodies are produced only in alpha/beta interferon-defective animals; MV replication in CD46Ge mice may be too inefficient to elicit synthesis of neutralizing antibodies.
Intracerebral inoculation with attenuated MV is lethal.
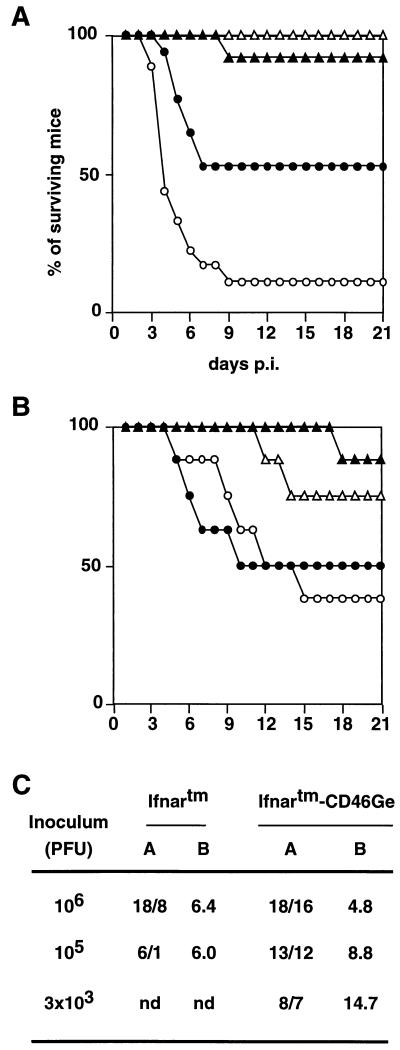
We next tested the sensitivity of genetically modified animals to intracerebral infection with a virus expected to be nonpathogenic in control mice. We inoculated 6- to 8-week-old Ifnartm-CD46Ge, Ifnartm, CD46Ge, and control mice with high doses (1 million infectious units) of MV-Edm (Fig. 5A). Sixteen of 18 infected Ifnartm-CD46Ge mice died, 2 on day 3, 8 on day 4, 2 each on days 5 and 6, and 1 each on days 7 and 9. Eight of 18 Ifnartm animals died between days 4 and 7. All of these animals showed clinical signs of neural disease, including initial hyperactivity which was followed by awkward gait, lethargy, lack of mobility, and death. In control and CD46Ge mice, 1 of a total of 24 animals died but did not develop signs of neurologic illness. We conclude that MV-Edm is pathogenic in adult mice only if their type I interferon response is defective.
FIG. 5.
Survival of mice from four strains after intracerebral inoculation with the vaccine strain MV-Edm (A and C) or with the neurotropic strain CAM/RBH (B). (A and B) Six- to 8-week-old mice were injected intracerebrally with 1 million infectious units of MV-Edm or with 104 infectious units of the rodent brain-adapted neurotropic CAM/RBH strain. Open circles, Ifnartm-CD46Ge mice; dots, Ifnartm mice; open triangles, CD46Ge mice; filled triangles, control C57BL/6 mice. The numbers of animals injected were as follows: (A) 18 Ifnartm-CD46Ge and Ifnartm and 12 CD46Ge and C57BL/6 mice; (B) 8 from each mouse strain. (C) Susceptibilities of Ifnartm and Ifnartm-CD46Ge mice to different doses of MV-Edm. Columns: A, numbers of inoculated/dead mice; B, average day of death. nd, not determined.
The differential susceptibility of the Ifnartm-CD46Ge and Ifnartm mice was more pronounced if less virus (105 infectious units) was inoculated: 12 of 13 Ifnartm-CD46Ge mice but only 1 of 6 Ifnartm mice died (Fig. 5C). If 3,000 infectious units of MV-Edm was inoculated exclusively in Ifnartm-CD46Ge mice, seven of eight animals died between 5 and 27 days after infection. Thus, though the Ifnartm-CD46Ge mortality remained nearly 90%, the incubation time was prolonged.
We then verified in Ifnartm-CD46Ge and Ifnartm animals the pathogenic effects of a MV strain independent of the presence of CD46 for cell entry. Since Ifnartm-CD46Ge and Ifnartm mice are not isogenic, they could have different susceptibilities to MV infection independent of the availability of the human receptor. After inoculation of 104 infectious units of the rodent brain-adapted neurotropic MV strain CAM/RBH, we observed that one of eight control animals and two of eight CD46Ge animals died 11 to 18 days p.i. (Fig. 5B). In contrast, one-half of the Ifnartm and Ifnartm-CD46Ge animals succumbed 5 to 11 days p.i. (Fig. 5B). These results indicate that the different genetic backgrounds of the Ifnartm and Ifnartm-CD46Ge mice do not measurably influence MV pathogenesis, a result which was not unexpected because their H-2 haplotypes are equivalent in terms of sensitivity to MV-induced encephalitis (38).
Pathogenic consequences of MV replication in the brain.
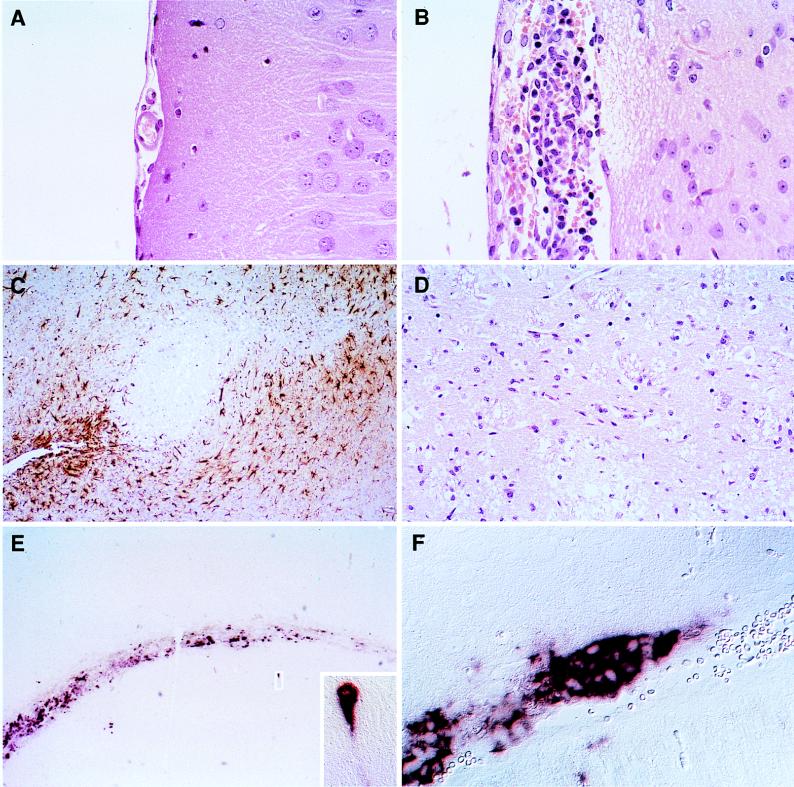
To determine the cell specificity of MV replication and to gain insight into the nature of MV-induced disease, the brains of Ifnartm-CD46Ge mice were examined 3 days after intracerebral inoculation. An HE-stained sagittal section of a mock-infected brain is shown in Fig. 6A: the meninges are thin and the parenchyma is intact. The corresponding section of a MV-Edm-infected brain (Fig. 6B) disclosed meningitis with inflammatory infiltrates of leukocytes and extensive vacuolization and necrosis of nearby brain parenchyma. Staining for GFAP revealed numerous reactive astrocytes in many brain areas. In Fig. 6C astrocytes were detected around, but not within, a region of extensive cell necrosis. An HE staining of the central area of the same region is shown at higher magnification in Fig. 6D; strong vacuolization is evident. Marked necrosis of neurons was also observed in the cerebral cortex, corpus callosum, and hypothalamus (not shown). In the brains of Ifnartm mice, less extensive pathogenic signs and no necrosis were observed, whereas in the brains of nonmutant control mice local, limited meningitis was occasionally monitored. In summary, MV-Edm-infected brains of Ifnartm-CD46Ge and, to a lesser extent, of Ifnartm mice (not shown) are characterized by severe generalized meningitis, multifocal gliosis, and marked necrosis of neurons as early as 3 days after infection. All of these pathological changes have a bilateral distribution.
FIG. 6.
Histological analysis of the consequences of MV infection and of the MV RNA distribution in the brains of Ifnartm-CD46Ge animals. A mouse inoculated with 1 million infectious units of MV-Edm (B to F) and a mock-infected mouse (A) were sacrificed 3 days p.i., their brains were prepared, and brain slices were stained as indicated below. (A, B, and D) HE-stained sections showing meningeal inflammation (B) and necrosis (B and D) of the brain tissue. (C) Immunohistochemical staining for GFAP demonstrating reactive astrocytes (brown) surrounding necrotic lesions. (E and F) In situ hybridization with a MV N-specific probe showing strong staining in the ventricular region and in scattered neurons. One infected hippocampal neuron is enlarged in the inset. In panel F, many contiguous ependymal cells are stained.
Next the replication of MV in infected brains was monitored by in situ hybridization with a MV N mRNA-specific probe (Fig. 6E and F) and by N protein immunohistochemistry (not shown). Microscopic examination of tissue sections indicated that ependymal cells lining the ventricles stained strongly, together with scattered neurons and oligodendrocytes (Fig. 6E). The inset in (Fig. 6E) shows a hippocampal neuron in which not only the cell body but also an extension, directed towards infected glial cells, is positive for MV N mRNA. At higher magnification the regions lining the ventricles revealed groups of positive cells (Fig. 6F). Occasionally, infected cells were also detected in the meninges (not shown). We interpret the distribution of infected cells in the brain to indicate that the propagation of MV infection was largely on the basis of cell-cell contact.
DISCUSSION
We show here that MV spread and pathogenesis in mice are controlled by alpha/beta interferon. Even in the absence of a high-affinity receptor for virus attachment, intranasal inoculation of Ifnartm mice results in moderate MV propagation. The availability of the high-affinity receptor CD46 facilitates MV-Edm spread and exacerbates its pathogenic consequences.
CD46 expression in humans and CD46Ge mice.
CD46 is expressed on almost all human nucleated cells in one of three patterns: predominantly the BC isoform (65% of the population), approximately equal quantities of B and C (29%), and predominantly the C isoform (6%) (31). However, in the brain the C isoform is preferentially expressed, independent of which of the three patterns is present in other tissues (19). Also, there is roughly equal expression of protein isoforms with cytoplasmic tail 1 or 2 in all tissues but the brain, where the tail-2 isoform is preferentially, if not exclusively, expressed (2, 19, 31). This pattern of isoform expression was duplicated in the transgenic mice (Fig. 1B), including that of the C isoform bearing cytoplasmic tail 2 being highly expressed in brain tissue (data not shown). Thus, the trans-acting regulatory elements necessary to reproduce human-like tissue specificity with the human CD46 gene are functional in all mouse tissues examined, including brain tissue. This transgenic mouse system can now be utilized to assess the regulation of this remarkable example of tissue-specific expression.
Additionally, since the pathogenic bacteria Streptococcus pyogenes, Neisseria gonorrhoeae, and Neisseria meningitidis use CD46 as their receptor (21, 41), CD46 mice are becoming a new tool in animal studies of bacterial cell adhesion and pathogenesis (20).
Respiratory tract MV infection of mice.
MV RNA was detected in the lungs of CD46Ge mice only by reverse transcription-PCR, but MV infection in the lungs of Ifnartm and Ifnartm-CD46Ge mice was unequivocal. Northern blots and MV replication-dependent CAT expression confirmed the in situ hybridization analysis, ruling out the possibility of detection of contaminating inoculum. Histological analysis of Ifnartm-CD46Ge mouse lungs revealed pathogenic changes comparable to those observed in intranasally infected macaques (1, 32). Neutralizing antibodies were detected.
However, infectious virus was recovered only occasionally at days 2 to 4 p.i. from lung or brain tissues and never from other organs (not shown). Virus isolation was achieved by cocultivation of mouse lung cell homogenates with indicator cells, but it was not possible to recover released virus (not shown). These data suggest that in mice a late stage of virus replication, possibly assembly or release, is inefficient. Indeed it was previously noticed that the MV titers obtained in CD46-expressing rodent cells are considerably lower than those obtained in primate cells (4, 36) and that cells of CD46 transgenic mice have different permissivities to MV infection (17).
Even in the virtual absence of detectable virus release the MV infection propagated to the three other tissues examined, spleen, liver, and kidney. Replication was highest in liver tissue, consistent with the occurrence of postmeasles hepatitis in humans (24). Since MV propagation in humans may be based on infection of lymphocytes and macrophages (40), we isolated PBMC from Ifnartm-CD46Ge and indeed found evidence for MV replication. We conclude that in interferon-defective mice MV may propagate largely in a cell-associated manner, initially in lung macrophages and then in a subset of PBMC.
Efficiency of cell entry and pathogenesis.
In mouse MV infections the efficiency of cell entry is not the principal determinant of pathogenesis. In this respect MV is different from poliovirus, because transgenic mice expressing the poliovirus receptor become highly susceptible to infection and die of poliomyelitis (25, 47).
Nevertheless, efficiency of MV cell entry and pathogenesis are linked: neurotropic MV strains selected by virus adaptation to rodent brain cells accumulate alterations in the MV attachment protein H (30). We confirmed the causality of this link by challenging Ifnartm and Ifnartm-CD46Ge mice either with a MV strain dependent on CD46 for cell entry (MV-Edm) or a rodent brain-adapted strain (CAM/RBH). The pathogenic effects of MV-Edm were more pronounced in Ifnartm-CD46Ge than in Ifnartm mice, whereas CAM/RBH was equally pathogenic in both transgenic strains. This proves that more efficient cell entry can cause enhanced pathogenesis.
In this context it is important to understand that the MV-CD46 interaction has several consequences: not only does it allow efficient virus entry, but it may also cause complement-mediated cell lysis via CD46 downregulation (53), and it may be one of the causes of suppression of cell-mediated immunity (14, 22, 37, 49). In view of these different effects, the interactions between CD46 and the H proteins of different MV strains are being characterized in detail (3, 27, 51).
MV infection of mouse brains.
MV replication is much more efficient in the rodent central nervous system than in the periphery (29). Nevertheless, brain MV spread and pathogenicity decrease with age of the mouse and are limited to certain MV strains (16). Accordingly, concentrated inocula of the attenuated strain MV-Edm failed to cause disease in adult control or CD46Ge animals. The same inocula were lethal within a few days for half of the Ifnartm mice and for almost all Ifnartm-CD46Ge animals. The extremely fast disease course in these animals (3 to 9 days), with virus propagating mostly in the easily accessible ependymal cells, implies a fundamental difference from other MV-induced brain diseases.
Less concentrated inocula (3,000 infectious units per animal) remained lethal for Ifnartm-CD46Ge mice, but the animals survived for up to 4 weeks. The recent examination of the brains of such animals at different times after infection (7) revealed progressive MV infection of neural cells, as observed in the brains of neonatally infected mice expressing a single CD46 isoform under the control of a neuron-specific promoter (NSE-CD46) (46). It is important to note another constant in the infections of neonatal NSE-CD46 mice and of adult Ifnartm-CD46Ge mice: virus RNA or antigen was often detected in contiguous cells, suggesting that in the brain MV propagation may be based largely on lateral cell-cell contacts.
Perspectives.
Mice with a defective interferon system may not be a general model for MV-induced disease, but Ifnartm-CD46Ge animals are being used for specific purposes: first, to compare the spread of standard MV and of reconstituted viruses with alterations of the envelope proteins characteristic of SSPE (7, 8, 10) in the brain; second, to address the important issue of the receptor specificities of wild-type MV strains (51) by comparing their pathogenicities in Ifnartm-CD46Ge and Ifnartm mice; and third, to test recently produced MV mutants (44, 50) in which proteins possibly required for pathogenesis (23) are inactivated. It will be instructive to compare the results of pathogenesis tests performed in Ifnartm-CD46Ge mice and rhesus macaques.
ACKNOWLEDGMENTS
We thank Lluis Montoliu for guidance in YAC transgenesis, Ulrike Müller and Michel Aguet for the Ifnartm mice, Stefan Niewiesk for MV CAM/RBH, Pius Spielhofer for MV-P-CAT, Gudrun Christiansen and Marianne König for excellent technical assistance, Bernhard Odermatt for consultation, Adriano Fontana for comments on the manuscript, Walter Bossart and Toni Cathomen for support, and Martin Billeter for continuous support and guidance.
This research was supported by grants from the Swiss National Science Foundation to R.C., J.P., and A. A. The salary of B.M. was contributed by the Swiss Serum and Vaccine Institute.
REFERENCES
- 1.Albrecht P, Lorenz D, Klutch M J, Vickers J H, Ennis F A. Fatal measles infection in marmosets: pathogenesis and prophylaxis. Infect Immun. 1980;27:969–978. doi: 10.1128/iai.27.3.969-978.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Buchholz C J, Gerlier D, Hu A, Cathomen T, Liszewski M K, Atkinson J P, Cattaneo R. Selective expression of a subset of measles virus receptor-competent CD46 isoforms in human brain. Virology. 1996;217:349–355. doi: 10.1006/viro.1996.0122. [DOI] [PubMed] [Google Scholar]
- 3.Buchholz C J, Koller D, Devaux P, Mumenthaler C, Schneider-Schaulies J, Braun W, Gerlier D, Cattaneo R. Mapping of the primary binding site of measles virus to its receptor CD46. J Biol Chem. 1997;272:22072–22079. doi: 10.1074/jbc.272.35.22072. [DOI] [PubMed] [Google Scholar]
- 4.Buchholz C J, Schneider U, Devaux P, Gerlier D, Cattaneo R. Cell entry by measles virus: long hybrid receptors uncouple binding from membrane fusion. J Virol. 1996;70:3716–3723. doi: 10.1128/jvi.70.6.3716-3723.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bueler H, Fischer M, Lang Y, Bluethmann H, Lipp H P, DeArmond S J, Prusiner S B, Aguet M, Weissmann C. Normal development and behaviour of mice lacking the neuronal cell-surface PrP protein. Nature. 1992;356:577–582. doi: 10.1038/356577a0. [DOI] [PubMed] [Google Scholar]
- 6.Carrigan D R, Knox K K. Identification of interferon-resistant subpopulations in several strains of measles virus: positive selection by growth of the virus in brain tissue. J Virol. 1990;64:1606–1615. doi: 10.1128/jvi.64.4.1606-1615.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cathomen T, Mrkic B, Spehner D, Drillien R, Naef R, Pavlovic J, Aguzzi A, Billeter M A, Cattaneo R. A matrix-less measles virus is infectious and elicits extensive cell fusion: consequences for pathogenesis in the brain. EMBO J. 1998;17:3899–3908. doi: 10.1093/emboj/17.14.3899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cathomen T, Naim H, Cattaneo R. Measles viruses with altered envelope protein cytoplasmic tails gain cell fusion competence. J Virol. 1998;72:1224–1234. doi: 10.1128/jvi.72.2.1224-1234.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cattaneo R, Rebmann G, Schmid A, Baczko K, ter Meulen V, Billeter M A. Altered transcription of a defective measles virus genome derived from a diseased human brain. EMBO J. 1987;6:681–688. doi: 10.1002/j.1460-2075.1987.tb04808.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cattaneo R, Schmid A, Eschle D, Baczko K, ter Meulen V, Billeter M A. Biased hypermutation and other genetic changes in defective measles viruses in human brain infections. Cell. 1988;55:255–265. doi: 10.1016/0092-8674(88)90048-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chomczynski P, Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987;162:156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- 12.Cui W, Hourcade D, Post T, Greenlund A C, Atkinson J P, Kumar V. Characterization of the promoter region of the membrane cofactor protein (CD46) gene of the human complement system and comparison to a membrane cofactor protein-like genetic element. J Immunol. 1993;151:4137–4146. [PubMed] [Google Scholar]
- 13.Dörig R E, Marcil A, Chopra A, Richardson C D. The human CD46 molecule is a receptor for measles virus (Edmonston strain) Cell. 1993;75:295–305. doi: 10.1016/0092-8674(93)80071-l. [DOI] [PubMed] [Google Scholar]
- 14.Fugier-Vivier I, Servet-Delprat C, Rivailler P, Rissoan M C, Liu Y J, Rabourdin-Combe C. Measles virus suppresses cell-mediated immunity by interfering with the survival and functions of dendritic and T cells. J Exp Med. 1997;186:813–823. doi: 10.1084/jem.186.6.813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gascon G G. Subacute sclerosing panencephalitis. Semin Pediatr Neurol. 1996;3:260–269. doi: 10.1016/s1071-9091(96)80030-1. [DOI] [PubMed] [Google Scholar]
- 16.Griffin D E, Mullinix J, Narayan O, Johnson R T. Age dependence of viral expression: comparative pathogenesis of two rodent-adapted strains of measles virus in mice. Infect Immun. 1974;9:690–695. doi: 10.1128/iai.9.4.690-695.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Horvat B, Rivailler P, Varior-Krishnan G, Cardoso A, Gerlier D, Rabourdin-Combe C. Transgenic mice expressing human measles virus (MV) receptor CD46 provide cells exhibiting different permissivities to MV infections. J Virol. 1996;70:6673–6681. doi: 10.1128/jvi.70.10.6673-6681.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hourcade D, Garcia A D, Post T W, Taillon M P, Holers V M, Wagner L M, Bora N S, Atkinson J P. Analysis of the human regulators of complement activation (RCA) gene cluster with yeast artificial chromosomes (YACs) Genomics. 1992;12:289–300. doi: 10.1016/0888-7543(92)90376-4. [DOI] [PubMed] [Google Scholar]
- 19.Johnstone R W, Loveland B E, McKenzie I F. Identification and quantification of complement regulator CD46 on normal human tissues. Immunology. 1993;79:341–347. [PMC free article] [PubMed] [Google Scholar]
- 20.Jonsson, A. B. 1998. Personal communication.
- 21.Källström H, Liszewski M K, Atkinson J P, Jonsson A B. Membrane cofactor protein (MCP or CD46) is a cellular pilus-receptor for pathogenic Neisseria. Mol Microbiol. 1997;25:639–647. doi: 10.1046/j.1365-2958.1997.4841857.x. [DOI] [PubMed] [Google Scholar]
- 22.Karp C L, Wysocka M, Wahl L M, Ahearn J M, Cuomo P J, Sherry B, Trinchieri G, Griffin D E. Mechanism of suppression of cell-mediated immunity by measles virus. Science. 1996;273:228–231. doi: 10.1126/science.273.5272.228. [DOI] [PubMed] [Google Scholar]
- 23.Kato A, Kiyotani K, Sakai Y, Yoshida T, Nagai Y. The paramyxovirus, Sendai virus, V protein encodes a luxury function required for viral pathogenesis. EMBO J. 1997;16:578–587. doi: 10.1093/emboj/16.3.578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Khatib R, Siddique M, Abbass M. Measles associated hepatobiliary disease: an overview. Infection. 1993;21:112–114. doi: 10.1007/BF01710744. [DOI] [PubMed] [Google Scholar]
- 25.Koike S, Aoki J, Nomoto A. Transgenic mouse for the study of poliovirus pathogenicity. In: Wimmer E, editor. Cellular receptors for animal viruses. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1994. pp. 463–480. [Google Scholar]
- 26.Laird P W, Zijderveld A, Linders K, Rudnicki M A, Jaenisch R, Berns A. Simplified mammalian DNA isolation procedure. Nucleic Acids Res. 1991;19:4293. doi: 10.1093/nar/19.15.4293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lecouturier V, Fayolle J, Caballero M, Carabaña J, Celma M L, Fernandez-Muñoz R, Wild T F, Buckland R. Identification of two amino acids in the hemagglutinin glycoprotein of measles virus (MV) that govern hemadsorption, HeLa cell fusion, and CD46 downregulation: phenotypic markers that differentiate vaccine and wild-type MV strains. J Virol. 1996;70:4200–4204. doi: 10.1128/jvi.70.7.4200-4204.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Leopardi R, Hyypia T, Vainionpaa R. Effect of interferon-alpha on measles virus replication in human peripheral blood mononuclear cells. APMIS. 1992;100:125–131. doi: 10.1111/j.1699-0463.1992.tb00850.x. [DOI] [PubMed] [Google Scholar]
- 29.Liebert U G, Finke D. Measles virus infections in rodents. Curr Top Microbiol Immunol. 1995;191:149–166. doi: 10.1007/978-3-642-78621-1_10. [DOI] [PubMed] [Google Scholar]
- 30.Liebert U G, Flanagan S G, Loffler S, Baczko K, ter Meulen V, Rima B K. Antigenic determinants of measles virus hemagglutinin associated with neurovirulence. J Virol. 1994;68:1486–1493. doi: 10.1128/jvi.68.3.1486-1493.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Liszewski M K, Post T W, Atkinson J P. Membrane cofactor protein (MCP or CD46): newest member of the regulators of complement activation gene cluster. Annu Rev Immunol. 1991;9:431–455. doi: 10.1146/annurev.iy.09.040191.002243. [DOI] [PubMed] [Google Scholar]
- 32.McChesney M B, Miller C J, Rota P A, Zhu Y D, Antipa L, Lerche N W, Ahmed R, Bellini W J. Experimental measles. I. Pathogenesis in the normal and the immunized host. Virology. 1997;233:74–84. doi: 10.1006/viro.1997.8576. [DOI] [PubMed] [Google Scholar]
- 33.Moench T R, Griffin D E, Obriecht C R, Vaisberg A J, Johnson R T. Acute measles in patients with and without neurological involvement: distribution of measles virus antigen and RNA. J Infect Dis. 1988;158:433–442. doi: 10.1093/infdis/158.2.433. [DOI] [PubMed] [Google Scholar]
- 34.Montoliu L, Schedl A, Kelsey G, Lichter P, Larin Z, Lehrach H, Schutz G. Generation of transgenic mice with yeast artificial chromosomes. Cold Spring Harbor Symp Quant Biol. 1993;58:55–62. doi: 10.1101/sqb.1993.058.01.009. [DOI] [PubMed] [Google Scholar]
- 35.Müller U, Steinhoff U, Reis L F, Hemmi S, Pavlovic J, Zinkernagel R M, Aguet M. Functional role of type I and type II interferons in antiviral defense. Science. 1994;264:1918–1921. doi: 10.1126/science.8009221. [DOI] [PubMed] [Google Scholar]
- 36.Naniche D, Varior-Krishnan G, Cervoni F, Wild T F, Rossi B, Rabourdin-Combe C, Gerlier D. Human membrane cofactor protein (CD46) acts as a cellular receptor for measles virus. J Virol. 1993;67:6025–6032. doi: 10.1128/jvi.67.10.6025-6032.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Niewiesk S, Eisenhuth I, Fooks A, Clegg C S, Schnorr J-J, Schneider-Schaulies S, ter Meulen V. Measles virus-induced immune suppression in the cotton rat (Sigmodon hispidus) model depends on viral glycoproteins. J Virol. 1997;71:7214–7219. doi: 10.1128/jvi.71.10.7214-7219.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Niewiesk S, Brinckmann U, Bankamp B, Sirak S, Liebert U G, ter Meulen V. Susceptibility to measles virus-induced encephalitis in mice correlates with impaired antigen presentation to cytotoxic T lymphocytes. J Virol. 1993;67:75–81. doi: 10.1128/jvi.67.1.75-81.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Niewiesk S, Schneider-Schaulies J, Ohnimus H, Jassoy C, Schneider-Schaulies S, Diamond L, Logan J S, ter Meulen V. CD46 expression does not overcome the intracellular block of measles virus replication in transgenic rats. J Virol. 1997;71:7969–7973. doi: 10.1128/jvi.71.10.7969-7973.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Norrby E, Oxman M N. Measles virus. In: Fields B N, Knipe D M, editors. Virology. New York, N.Y: Raven Press Ltd.; 1990. pp. 1013–1044. [Google Scholar]
- 41.Okada N, Liszewski M K, Atkinson J P, Caparon M. Membrane cofactor protein (CD46) is a keratinocyte receptor for the M protein of the group A streptococcus. Proc Natl Acad Sci USA. 1995;92:2489–2493. doi: 10.1073/pnas.92.7.2489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Peterson K R, Clegg C H, Li Q, Stamatoyannopoulos G. Production of transgenic mice with yeast artificial chromosomes. Trends Genet. 1997;13:61–66. doi: 10.1016/s0168-9525(97)01003-2. [DOI] [PubMed] [Google Scholar]
- 43.Radecke F, Billeter M A. Appendix: measles virus antigenome and protein consensus sequences. Curr Top Microbiol Immunol. 1995;191:181–192. doi: 10.1007/978-3-642-78621-1_12. [DOI] [PubMed] [Google Scholar]
- 44.Radecke F, Billeter M A. The nonstructural C protein is not essential for multiplication of Edmonston B strain measles virus in cultured cells. Virology. 1996;217:418–421. doi: 10.1006/viro.1996.0134. [DOI] [PubMed] [Google Scholar]
- 45.Radecke F, Spielhofer P, Schneider H, Kaelin K, Huber M, Dotsch C, Christiansen G, Billeter M A. Rescue of measles viruses from cloned DNA. EMBO J. 1995;14:5773–5784. doi: 10.1002/j.1460-2075.1995.tb00266.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rall G F, Manchester M, Daniels L R, Callahan E M, Belman A R, Oldstone M B A. A transgenic mouse model for measles virus infection of the brain. Proc Natl Acad Sci USA. 1997;94:4659–4663. doi: 10.1073/pnas.94.9.4659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ren R B, Costantini F, Gorgacz E J, Lee J J, Racaniello V R. Transgenic mice expressing a human poliovirus receptor: a new model for poliomyelitis. Cell. 1990;63:353–362. doi: 10.1016/0092-8674(90)90168-e. [DOI] [PubMed] [Google Scholar]
- 48.Schedl A, Larin Z, Montoliu L, Thies E, Kelsey G, Lehrach H, Schutz G. A method for the generation of YAC transgenic mice by pronuclear microinjection. Nucleic Acids Res. 1993;21:4783–4787. doi: 10.1093/nar/21.20.4783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Schlender J, Schnorr J J, Spielhofer P, Cathomen T, Cattaneo R, Billeter M A, ter Meulen V, Schneider-Schaulies S. Interaction of measles virus glycoproteins with the surface of uninfected peripheral blood lymphocytes induces immunosuppression in vitro. Proc Natl Acad Sci USA. 1996;93:13194–13199. doi: 10.1073/pnas.93.23.13194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Schneider H, Kaelin K, Billeter M A. Recombinant measles viruses defective for RNA editing and V protein synthesis are viable in cultured cells. Virology. 1997;227:314–322. doi: 10.1006/viro.1996.8339. [DOI] [PubMed] [Google Scholar]
- 51.Schneider-Schaulies J, Dunster L M, Kobune F, Rima B, ter Meulen V. Differential downregulation of CD46 by measles virus strains. J Virol. 1995;69:7257–7259. doi: 10.1128/jvi.69.11.7257-7259.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Schneider-Schaulies J, Schnorr J J, Brinckmann U, Dunster L M, Baczko K, Liebert U G, Schneider-Schaulies S, ter Meulen V. Receptor usage and differential downregulation of CD46 by measles virus wild-type and vaccine strains. Proc Natl Acad Sci USA. 1995;92:3943–3947. doi: 10.1073/pnas.92.9.3943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Schneider-Schaulies J, Schnorr J J, Schlender J, Dunster L M, Schneider-Schaulies S, ter Meulen V. Receptor (CD46) modulation and complement-mediated lysis of uninfected cells after contact with measles virus-infected cells. J Virol. 1996;70:255–263. doi: 10.1128/jvi.70.1.255-263.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Spielhofer P. Ph.D. thesis. Zürich, Switzerland: University of Zürich; 1995. [Google Scholar]
- 55.ter Meulen V, Stephenson J R, Kreth H W. Subacute sclerosing panencephalitis. In: Fraenkel-Conrat H, Wagner R R, editors. The viruses. 18. Virus-host interactions: receptors, persistence, and neurological diseases. New York, N.Y: Plenum Press; 1983. pp. 105–159. [Google Scholar]
- 56.Thorley B R, Milland J, Christiansen D, Lanteri M B, McInnes B, Moeller I, Rivailler P, Horvat B, Rabourdin-Combe C, Gerlier D, McKenzie I F, Loveland B E. Transgenic expression of a CD46 (membrane cofactor protein) minigene: studies of xenotransplantation and measles virus infection. Eur J Immunol. 1997;27:726–734. doi: 10.1002/eji.1830270322. [DOI] [PubMed] [Google Scholar]
- 57.Trescol-Biemont M C, Leonov S, Rabourdin-Combe C, Gerlier D. Quantification of measles virus by a virus receptor-dependent and haemagglutinin-specific T cell stimulation assay. J Immunol Methods. 1995;187:253–258. doi: 10.1016/0022-1759(95)00191-8. [DOI] [PubMed] [Google Scholar]
- 58.van Binnendijk R S, van der Heijden R W J, Osterhaus A D M E. Monkeys in measles research. Curr Top Mol Immunol. 1995;191:135–148. doi: 10.1007/978-3-642-78621-1_9. [DOI] [PubMed] [Google Scholar]
- 59.Volpe P. M.Sc. thesis. Zürich, Switzerland: University of Zürich; 1997. [Google Scholar]
- 60.Yalaz K, Anlar B, Oktem F, Aysun S, Ustacelebi S, Gurcay O, Gucuyener K, Renda Y. Intraventricular interferon and oral inosiplex in the treatment of subacute sclerosing panencephalitis. Neurology. 1992;42:488–491. doi: 10.1212/wnl.42.3.488. [DOI] [PubMed] [Google Scholar]