Abstract
We describe use of developing chicken embryos as a model to study neuronal spread and virulence of pseudorabies virus (PRV). At embryonic day 12, β-galactosidase-expressing PRV strains were injected into the vitreous humor of one eye, and virus replication and spread from the eye to the brain were measured by β-galactosidase activity and the recovery of infectious virus from tissues. The wild-type PRV strain, Becker, replicated in the eye and then spread to the brain, causing extensive pathology characterized by edema, hemorrhage, and necrosis that localized to virally infected tissue. The attenuated vaccine strain, Bartha, replicated in the eye and spread throughout specific regions of the brain, producing little to no overt pathology. Becker mutants lacking membrane proteins gE or gI replicated in the eye and were able to spread to the brain efficiently. The pathology associated with replication of these mutants in the brain was intermediate to that induced by Becker or Bartha. Mixed infection of a gE deletion mutant and a gI deletion mutant restored the pathogenic phenotype to wild-type levels. These data indicate that the replication of virus in embryonic brain tissue is not sufficient to induce the characteristic pathological response and that the gE and gI gene products actively affect pathological responses in the developing chicken brain.
Historically, the chicken embryo has been used for propagation of many viruses, including pseudorabies virus (PRV; a swine alphaherpesvirus) (1, 5, 16, 19, 23). Some of the earliest references to alphaherpesvirus infection of chicken embryos noted the pathogenesis and neurotropism of both PRV and herpes simplex virus (HSV) (1, 5, 34, 41, 48). Nahmias and coworkers determined that following inoculation of the chorioallantoic membrane (CAM), HSV type 2 (HSV-2) but not HSV-1 strains could form pocks (41). This work was extended by Stevens and colleagues, who identified HSV-1 strains which not only could form pocks on the CAM but also would then invade and kill the embryo (17, 18). In this report, we describe the use of developing chicken embryos as a model to study neuronal spread and virulence of PRV.
It is noteworthy that while chicken embryos are highly susceptible to infection by PRV, older animals are much less sensitive. The extent of productive infection and subsequent mortality of hatched chickens was age dependent, dose dependent, and route dependent (45). Within a few hours after hatching, chicks became resistant to previously lethal intranasal and intramuscular challenges, while intracerebral challenges continued to cause pathogenesis, signs of disease, and mortality. This is reminiscent of the natural host infection where PRV causes a lethal infection of fetal and neonatal animals but a more benign infection of adults (23, 51). Thus, one long-term objective in establishing this model is to understand the molecular basis for age-dependent sensitivity to infection.
Another long-term objective was to identify PRV genes required to cause disease and death in animals (virulence genes). In the 1980s, Lomniczi et al. used intracranial injection of PRV into day-old chicks to identify PRV virulence genes (32). The strategy was based on the striking result that despite injection directly into the brain, animals survived infection by the attenuated PRV vaccine strain Bartha but were killed by injection of the Ka strain of PRV (6). Not only did Bartha not replicate in the brains of the day old chick, but it was rapidly cleared (32). The mutations in Bartha that led to its attenuation were identified by marker rescue experiments with cloned DNA segments from the virulent Ka strain (25, 33, 38, 39). When defects in the gC, gE, and Ul21 genes in Bartha were repaired, full virulence was restored. These gene products were also required for full virulence in swine, the natural host of PRV (25, 33, 39). None of these genes are required for efficient virus replication in cultured cells, although gE is required for efficient cell-cell spread in some but not all cell lines (22, 37). How these gene products affect virulence remains an area of active research.
We describe a model where primary infection occurs inside the embryonic chicken eye and then spreads by neuronal routes to the brain. This route of infection was chosen for several reasons. First, the eye is one of the largest and most accessible structures in the developing embryo. This enabled the precise delivery of the inoculum to an enclosed site, physically confining the inoculum and preventing unwanted infection of other tissues. Second, the eye is spatially removed from the brain, yet major structures such as the retina and muscles are directly connected to the brain via well-defined nerves. This physically separates replication in the periphery from spread to and replication in the brain. Third, the development and structure of the chick visual system are well characterized both in cell biology and neurobiology, and so we can make specific predictions about the routes by which virus is transported to the brain. Fourth, retinal ganglion cells on the surface of the retina are directly available for infection essentially as a monolayer, and these neurons are in synaptic contact with other cells in the retina as well as the brain. Finally, the unique tight junctions of the pigmented epithelial cells separate the neural retina from the circulation, reducing immune surveillance at the time of infection.
We demonstrate that this model is a useful and facile experimental paradigm for the characterization of PRV neuronal spread and virulence. It provides a sensitive, inexpensive, and high-throughput means of characterizing viral mutants defective in virulence and neuronal spread. Importantly, the model reports quantitatively and qualitatively on these phenotypes consistent with other animal model systems used to date. A noteworthy exception is the infection by the attenuated Bartha strain. Lomniczi et al. had noted that in the brain of a day-old chick, Bartha was rapidly cleared from the central nervous system (CNS) and the animals survived the infection (32). In contrast, we find that Bartha replicated in the eye and spread to and throughout the embryonic brain. Moreover, despite significant replication and the presence of infectious virus, the pathology produced by Bartha infection was markedly reduced and animals survived several days longer than animals infected with wild-type virus. Mutant viruses carrying defined mutations in gE and gI were studied to determine if these two known virulence genes defective in Bartha were responsible for this dramatic reduction in pathology.
(A portion of this work was submitted as a senior undergraduate thesis by G.S.Y.)
MATERIALS AND METHODS
Viruses and cells.
The PRV strains used in this study are given in Table 1. Strains Becker-Blu (BeBlu), PRV-99Blu (26), PRV-98Blu, and PRV-91Blu are isogenic with the wild-type Becker strain. They all express a hybrid protein comprised of the first seven amino acids of gG fused to β-galactosidase. These viruses were constructed by cotransfecting a plasmid bearing the gG-lacZ fusion gene with viral DNA according to the method of Mettenleiter and Rauh (36). Southern blot analysis confirmed the insertion of the gG-lacZ fusion gene at the gG locus. The construction of Bartha-Blu (BaBlu), PRV-91, PRV-98, and PRV-99 have been described elsewhere (49, 50). All virus strains were propagated and titered on PK15 cells. PK15 cells were grown at 37°C in Dulbecco modified Eagle medium (DMEM) supplemented with 10% fetal calf serum (FCS) (Gibco/BRL).
TABLE 1.
Virus strains used in this study
| Strain | Genotypea | Comments | Reference |
|---|---|---|---|
| BaBlu | gG, ΔgI, ΔgE, ΔUS9, ΔUS2, gC,b Ul21,b gM,b others? | Expresses lacZ gene at gG locus | 49 |
| BeBlu | gG | Expresses lacZ gene at gG locus | This study |
| PRV-91Blu | gG, ΔgE | Expresses lacZ gene at gG locus; isogenic with BeBlu | This study |
| PRV-98Blu | gG, ΔgI | Expresses lacZ gene at gG locus; isogenic with BeBlu | This study |
| PRV-99Blu | gG, ΔgE, ΔgI | Expresses lacZ gene at gG locus; isogenic with BeBlu | 26 |
gG was inactivated by insertion of the lacZ gene as described in Materials and Methods. The prefix “Δ” indicates that the gene is deleted in the strain.
Point mutations have been identified in the gene (see text).
Eggs.
Fertile White Leghorn chicken eggs (Gallus domesticus) were obtained weekly from a local supplier (Avian Services, Frenchtown, N.J.). Eggs were stored at 10°C for up to 1 week prior to incubation, during which time further development was curtailed. Embryonic day 1 (E1) began on the day when the eggs were placed in a 37.5°C humidified incubator (Carolina Biological Supply Company, Burlington, N.C.). Eggs were positioned pointed end down in the incubator and were rotated mechanically on a 4-h cycle. On E9, the eggs were turned such that the blunt end of the egg was down and were kept in this position in an humidified incubator for 2 h. A candling light was used to ascertain the position of the embryo in the egg, and a pencil mark was made on the shell directly over the embryo. A 26-gauge needle was then used to puncture a small hole in the air sac of the egg which is found on the blunt end of the egg. This allows for a separation of the vascularized CAM from the dry white membrane and the shell. After this time, eggs remained in a horizontal position with the pencil mark facing up. A hacksaw blade was then used to cut a rectangular hole approximately 2 by 3 cm in the shell centered over the pencil mark. The rectangular piece of eggshell was pried away from the dry, white membrane below, using sterile forceps. The dry, white membrane was then separated from the CAM beneath it, allowing the vascularized CAM to sink down into the egg and fill the airspace. The window was sealed with cellophane tape, and the eggs were returned to the incubator until injections were performed. Experimental protocols were approved by the Animal Welfare Committee and were consistent with the regulations of the American Association for Accreditation of Laboratory Animal Care and those in the Animal Welfare Act (Public Law 99-198). All animals were confined to a biosafety level 2 laboratory.
Intraocular injections.
Intraocular injections were performed on E12 embryos (E12 = stage 38 [21]). At this time, most areas of the brain have formed connections with eye structures (44). Immediately prior to injection, virus stocks were thawed and sonicated briefly, and cells and cellular debris were removed by centrifugation at 3,000 × g in a microcentrifuge for 5 min. This cleared virus suspension was diluted to 108 PFU/ml (unless otherwise noted) in DMEM–10% FCS, and 1 μl (105 PFU) was loaded into a 10-μl Hamilton syringe. Tungsten needles were used to puncture the sclera of the right eye of the embryo. While the eye of the embryo was held in place with a tungsten needle, the Hamilton syringe was inserted at the midtemporal side of the eye into the center of the vitreous humor, and 1 μl of inoculum was injected slowly with the bevel of the needle facing toward the posterior of the eye. The needle was held in place momentarily and then removed gradually to minimize leakage of virus from the injection site. The window was resealed with cellophane tape, and the egg was returned to the incubator.
To control for death of the embryo due to the injection procedure itself or to constituents of the medium in which the virus was delivered, medium alone or inoculum which had been cleared of virus by centrifugation was injected into the eye. In the worse case set of such experiments, 18% of the animals died and 82% survived beyond 168 h (n = 33), at which point the experiment was terminated. Animals that died within 24 h of injection were considered to have died as a result of the injection procedure and were discarded. Typically, less than 10% of the animals died within 24 h.
Determination of LD50 and mean time to death.
Animals were injected with various amounts of virus ranging from 102 to 106 PFU per animal in a volume of 1 μl. Control injections of DMEM–10% FCS alone were also performed. Death was assessed by absence of movement, shrinkage of blood vessels, and failure of blood vessels to bleed when punctured. LD50, defined as the number of PFU required to kill 50% of the animals within 168 h (time just before hatching), was determined by the graphic interpolation method of Reed and Muench (46). For determination of mean time to death, a standard inoculum of 105 PFU was used. Animals were examined at least every 6 h to determine the time of death. Mean time to death, defined as the average time required to kill an animal injected with 105 PFU of virus, were determined by graphic interpolation of the data presented in Fig. 1. Animals were considered to have survived the infection if they lived beyond 168 h after injection. Surviving animals were euthanized at this time, and none were allowed to hatch (hatching would occur at E21).
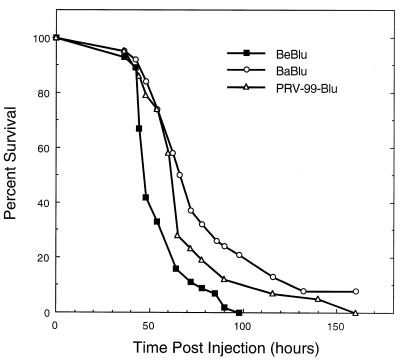
FIG. 1.
Survival curves of PRV strains in the chicken embryo eye model. Embryos were injected on E12 with 105 PFU of virus in a volume of 1 μl. Virus was delivered into the vitreous body of the right eye, using a 10-μl Hamilton syringe. Animal survival was monitored at least every 6 h over a 168-h period. The numbers of embryos used were 45, 38, and 43 for BeBlu, BaBlu, and PRV-99Blu, respectively.
Tissue processing.
At various times after infection, animals were sacrificed by decapitation and the brain was removed from the skull. Brains were placed in X-Gal (5-bromo-4-chloro-3-indolyl-β-d-galactopyranoside) buffer (1 mg of X-Gal per ml, 10 mM potassium ferrocyanide, 10 mM potassium ferricyanide, 2 mM MgCl2, 0.01% sodium deoxycholate, and 0.02% Nonidet P-40 in 0.1 M phosphate buffer, pH 7.4) for 1 h at 37°C. Tissue was stored in 4% paraformaldehyde in phosphate-buffered saline at 4°C until photographed.
Recovery of infectious virus from tissue.
The tissue from three infected animals was used per time point. Tissue was harvested, frozen in liquid nitrogen immediately after dissection, and kept at −70°C until titer determination. The tissue from the three animals was weighed, pooled, and ground with a mortar and pestle under liquid nitrogen to produce a fine powder. Approximately 100 mg was suspended in 1 ml of DMEM–10% FCS. Samples were treated with three cycles of freezing at −70°C and thawing at 37°C to release virus from infected cells, sonicated briefly, and centrifuged at 3,000 × g in a microcentrifuge for 5 min to pellet cell and tissue debris. Virus in the supernatant was quantitated by duplicate plaque assays on PK15 cells. Titers were expressed as the number of PFU per organ.
RESULTS
Our objective was to determine how late-stage chicken embryos would respond to PRV infection and if we would observe spread of the infection from the eye to relevant areas of the brain. To facilitate the rapid identification of infected tissue, we used viruses expressing the enzyme β-galactosidase, whose activity can be screened visually by formation of blue pigment in the presence of X-Gal substrate. The disruption of the gG gene by the lacZ reporter gene has been shown to have little or no effect on the growth or virulence of PRV in any system studied to date (8, 24, 49).
Measurement of virulence.
Virulence was assessed by determination of LD50 and mean time to death. The LD50 for BeBlu was 58 PFU, and the mean time to death was 46 h. The LD50 for the attenuated BaBlu strain was 5,600 PFU, and the mean time to death was 68 h (Table 2). These numbers compare favorably to those obtained for mice: the LD50 for Becker was reported to be 200 PFU (192-h period), with a mean time to death of about 65 h (15, 52). We have not determined the LD50 for Bartha in mice, but the mean time to death is approximately 120 h after eye injection (15).
TABLE 2.
Virulence of PRV strains in the chicken embryo eye model
| Strain | LD50a (PFU) | Mean time to deathb (h) |
|---|---|---|
| BeBlu | 5.8 × 101 (31c) | 46 (45) |
| BaBlu | 5.6 × 103 (56) | 68 (38) |
| PRV-99Blu | 2.0 × 102 (31) | 61 (43) |
Determined by the graphic interpolation method of Reed and Muench as described in Materials and Methods. Animals surviving beyond 168 h postinjection were considered to have survived the virus challenge.
Determined by using an inoculum of 105 PFU. Animals were monitored at least every 6 h for survival. Animals which survived the challenge were not included in the determination.
Number of animals tested.
The data for mean time to death in the chicken embryo model are shown graphically in Fig. 1. We speculate that the genetic variation in the outbred embryos contributes in large part to the variation. In this experiment, 3 of 38 embryos injected with BaBlu survived to 168 h and probably would have hatched successfully.
The Bartha strain is attenuated in part because of the deletion in the US region that removes coding sequences for gI, gE, US9, and US2 (31, 35, 42). Of these genes, glycoproteins gE and gI have been demonstrated to be required for virulence and spread in rodent and swine model systems (2, 9, 22, 24, 26–29, 32, 35). We assessed the contributions of these gene products after infection of the chicken embryo with PRV mutants that have defined deletions of gE and gI. The lacZ gene was crossed into the gG locus as described in Materials and Methods. PRV-99Blu is isogenic with BeBlu and deleted for both gE and gI. The LD50 of PRV-99Blu was 200 PFU, and the mean time to death was 61 h (Table 2; Fig. 1). The relative virulence (LD50 and mean time to death) of the three PRV strains tested in the chicken embryo (BeBlu > PRV-99Blu > BaBlu) are similar to those reported in rodent and swine systems (9, 39). We next examined gross tissue pathology and virus replication at the site of primary infection and in the brain.
Infection of the eye.
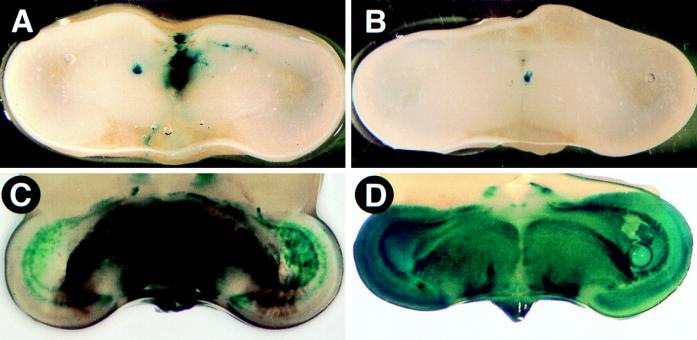
Eyes infected with 105 PFU of any PRV strain displayed a characteristic loss of pigment from the retinal pigmented epithelium. This striking phenotype could be detected as early as 48 h after infection and was marked by 72 h after infection (Fig. 2B). No such depigmentation was observed in the uninjected eye (Fig. 2A). When lower titers of virus were injected, more focal areas of depigmentation were observed, and these areas did not consolidate over time. The retinal pigmented epithelium was not affected when medium alone was injected (data not shown). No other overt signs of viral infection were obvious on the body of the infected embryo, suggesting that viral infection was limited to the injected eye.
FIG. 2.
Closeup images of the eyes from a PRV-infected embryo. The uninjected (A) and injected (B) eyes from a BaBlu-infected embryo were harvested at 72 h after infection.
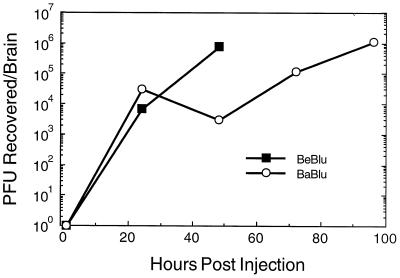
β-Galactosidase activity was detected in dissected retinal tissue and could occasionally be visualized on the sclera near the injection site (data not shown). Virus replication in the eye was determined more precisely by direct titer of infectious virus (Fig. 3). At the indicated times after injection, the infected eyes from three embryos were harvested and pooled, and virus was recovered quantitated from the tissue and as described in Materials and Methods. At 1 h after injection (time zero), approximately 1% of the inoculum could be recovered from the injected eyes of both BeBlu- and BaBlu-infected embryos. Infectious virus titers increased with time such that by 48 h after infection, the titer of BeBlu increased nearly 3 orders of magnitude. BaBlu replication in the eye was slower but reached approximately the same final titer as for BeBlu. Taken together, these data suggest that both virus strains replicated in the eye and neither produced gross changes that would indicate the difference in virulence between the strains. Nevertheless, the reduced rate of BaBlu replication may have slowed spread to the brain, thereby prolonging the life of the infected animal. To address this possibility, we next determined if both viruses were capable of spread from the eye to the brain.
FIG. 3.
Replication of PRV strains in the eye. At the indicated times, the inoculated eyes from three embryos were harvested and pooled, and infectious virus was released from tissue as described in Materials and Methods. Virus titer was determined by plaque assay on PK15 cells in duplicate.
Both BeBlu and BaBlu spread to the brain.
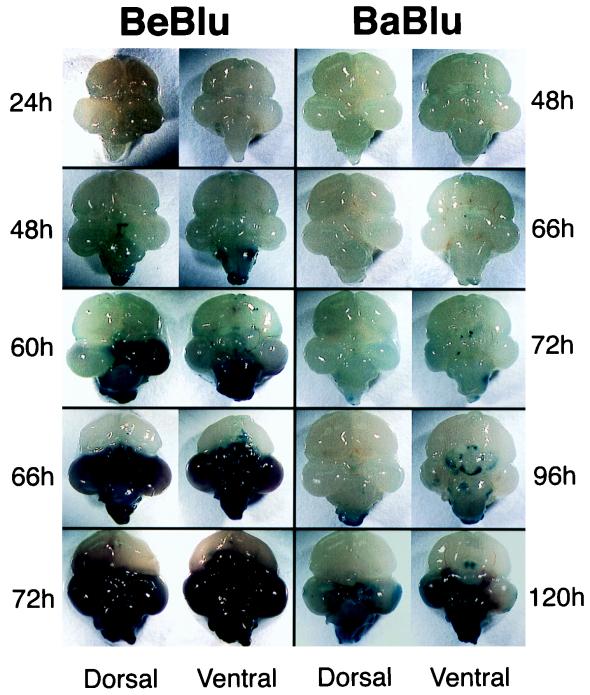
E12 embryos were injected with 105 PFU of BeBlu or BaBlu into the vitreous humor of the right eye. At the indicated times postinjection, embryos were sacrificed, and the brains carefully removed and placed in a solution containing X-Gal to identify lacZ expressing tissue at or near the surface of the tissue. The contrast of blue infected tissue with colorless uninfected tissue enabled a more direct assessment of hemorrhage, necrosis, and edema. A detailed histopathological analysis of PRV infection of the chicken embryo CNS is the subject of a study in progress.
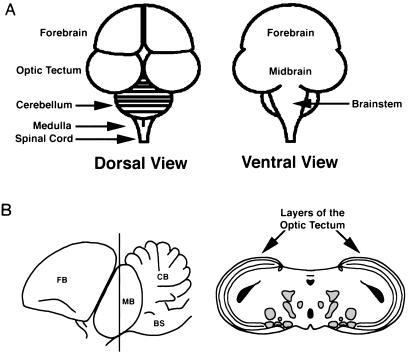
A schematic representation of the chicken embryo brain is shown in Fig. 4A for orientation of the data in Fig. 5. Figure 5 illustrates the progression of infection of BeBlu and BaBlu in the chicken embryo brain. The most obvious difference between the BeBlu- and BaBlu-infected tissues was the extent of edema, hemorrhage, and necrosis elicited by BeBlu infection that was either absent or considerably reduced during BaBlu infection. These markers of pathological infection occurred considerably faster for BeBlu infection than for the minor pathology observed for BaBlu infection. These data correlate with the lower virulence of BaBlu than of to BeBlu.
FIG. 4.
Schematic drawing of the chicken embryo brain. (A) Dorsal and ventral views of the embryonic brain. (B) Left, lateral view of the chick brain. FB, forebrain; MB, midbrain; BS, brainstem; CB, cerebellum. Right, coronal section through the midbrain, the position of which is indicated on the left side by the dark line through the midbrain. Areas shaded in gray represent known retinorecipient regions in addition to the optic tectum. Areas shaded in black represent ventricles.
FIG. 5.
Progression of PRV infection in the chicken embryo brain. At the indicated times after injection into the right eye, the brains from infected embryos were removed and placed in X-Gal. Only infected tissue close to the surface of the brain or exposed by necrosis was stained blue. Dorsal and ventral views of each brain are shown. Note that the times that the brains were collected are different for Be-Blu- and Ba-Blu-infected embryos. It is important to note that many of the BeBlu-infected animals sacrificed at 48 h had more extensive infection than the examples illustrated and that in some cases their brains resembled the 66-h brain. This inherent variability is likely due to the use of genetically outbred animals but nevertheless emphasizes the speed with which BeBlu infection exerts its pathology.
The pattern of virus spread in the brain, as marked by the appearance of β-galactosidase activity in unfixed tissue, also differed markedly between the two viruses (Fig. 5). β-Galactosidase staining of the brainstem and the dorsal midbrain was evident in BeBlu-infected embryos at 48 h. By 60 h, the infection had spread through one lobe of the midbrain and throughout the brainstem, and these areas were marked by edema, hemorrhage, and a softening of tissue. At 66 h, both lobes of the midbrain and the brainstem were necrotic. At 72 h, the entire midbrain lost integrity and was easily lost during dissection. Hemorrhaging and edema were obvious in the remaining tissue. Despite this tissue destruction, the adjacent forebrain showed no signs of infection.
The first signs of BaBlu infection were apparent in the brainstem, medulla, and central, ventral midbrain between 66 and 72 h. By 96 h, infection had spread through the brainstem, medulla, spinal cord, cerebellum, and ventral midbrain. By 120 h, infection was detected in the optic tectum of the midbrain. Despite this extensive spread, we saw little overt pathology of the type observed for BeBlu.
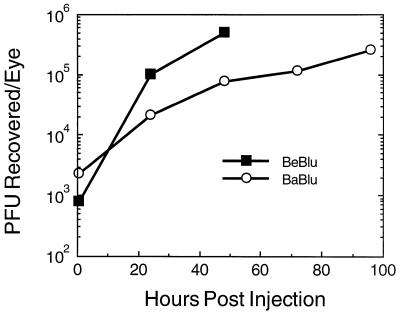
The X-Gal substrate only penetrates the superficial layers of the brain and provides a limited and qualitative estimate of virus replication. Therefore, we measured the concentration of infectious virus in brain extracts (Fig. 6). At the indicated times after injection, three embryo brains were harvested and pooled, and virus was recovered from the tissue and quantitated as described in Materials and Methods. As shown in Fig. 6, similar amounts of infectious virus could be recovered from the brains of both BeBlu- and BaBlu-infected embryos as early as 24 h after infection. These data indicate that both strain are transported from the eye and replicate in the brain. We noted in this experiment that less infectious BaBlu than BeBlu was recovered at late times, which may reflect the observation that day-old chicks can clear Bartha after intracranial inoculation (31).
FIG. 6.
Replication of PRV strains in the brain. At the indicated times after injection, brains from three infected embryos were removed and pooled, and infectious virus was released from tissue as described in Materials and Methods. Virus titer was determined by plaque assay on PK15 cells in duplicate.
To investigate the extent of BaBlu and BeBlu spread from the eye to deep layers of the brain, we stained coronal sections of unfixed and unperfused brains for β-galactosidase activity. In Fig. 7, we illustrate sections of the BeBlu- or BaBlu-infected midbrain at 48 h postinfection and at a late time point when death was imminent (a schematic representation of these sections is shown in Fig. 4B). We note that many retinorecipient regions of the brain lie in this region and that it shows the most pronounced tissue pathology. At 48 h postinjection, X-Gal-stained tissue was evident in the midbrain of both BaBlu- and BeBlu-infected embryos. At this time, BeBlu-infected embryos (Fig. 7A) typically showed more staining than BaBlu-infected embryos (Fig. 7B), and the sites stained through the midbrain differed somewhat between the two viruses (see also Fig. 5). A section through the midbrain of a BeBlu-infected embryo at 60 h postinfection is shown in Fig. 7C. X-Gal staining was evident in deeper layers of the midbrain, as was a substantial amount of hemorrhaging and necrosis. In striking contrast, a section though the midbrain of a BaBlu-infected embryo at 96 h after infection revealed that virtually the entire section was infected with the exception of the outer layers of the midbrain (Fig. 7D). Only trace amounts of hemorrhaging and no necrosis were seen in the midbrain. It was evident during sectioning that unlike BeBlu-infected tissue, the integrity of BaBlu-infected tissue was relatively intact. Taken together, these data indicate that the replication and spread of virus throughout the brain alone are not sufficient to induce the extensive tissue pathology observed in the case of BeBlu infection.
FIG. 7.
PRV infection of the midbrain after intraocular inoculation. The brains of infected embryos were sliced in the coronal plane through the midbrain by using a razor blade and placed in X-Gal substrate buffer for 1 h. (A) Section through the midbrain of a BeBlu-infected embryo at 48 h postinfection; (B) section through the midbrain of a BaBlu-infected embryo at 48 h postinfection; (C) section through the midbrain of a BeBlu-infected embryo at 60 h postinfection; (D) section through the midbrain of a BaBlu-infected embryo at 96 h postinfection.
Role of gE and gI in infection of the chicken embryo.
In rodent model systems and in the natural host, deletion of gE and/or gI from the PRV genome results in virus strains that are less virulent than the parental wild-type strain (2, 9, 22, 24, 26–29, 32, 35). In these studies, the mean time to death was extended, symptoms were reduced, but the LD50 was approximately the same as for wild-type virus. gE and gI form a heterodimer found in infected cells and in the virus particle. These molecules appear to function together to promote both virulence and spread of virus. We next addressed the roles of gE and gI during infection of the chicken embryo.
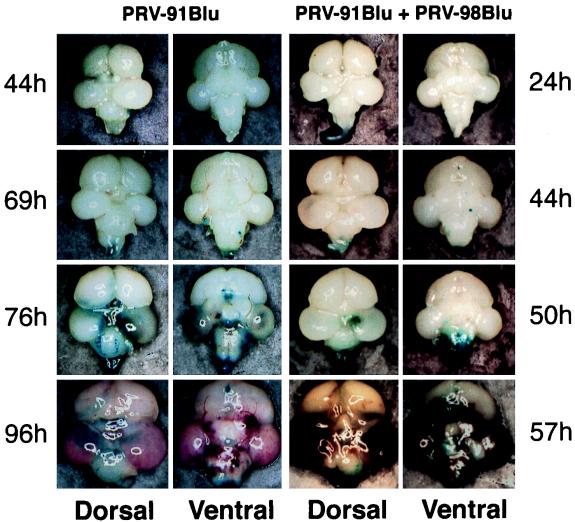
The progression of infection of PRV-91Blu (gE−) measured by X-Gal staining is shown in Fig. 8; similar results were obtained after infection by gI− virus (PRV-98Blu) or gE− gI− double-deletion virus (PRV-99Blu) (not shown). At 44 h after injection, X-Gal staining was first evident on the brainstem. By 69 h, infection had spread throughout brainstem, medulla, and spinal cord. Infection of the optic tectum was not observed until 76 h after injection. At this time, there was extensive infection of the brainstem, medulla, spinal cord, cerebellum, and ventral sites in the midbrain. Gross pathological injury was not obvious until 76 h, when hemorrhaging was observed in the most infected areas. By 96 h, the entire brain was inflamed and hemorrhage in infected areas was extensive. However, the severe necrosis and loss of tissue integrity observed in BeBlu infections was rarely found. PRV-91Blu infection was more destructive than BaBlu infection (compare Fig. 5 and 8), but the appearance of tissue damage in PRV-91Blu infection was noticeably slower than observed for BeBlu infection. These data suggest that gE and gI play an important role in the production of hemorrhaging, swelling, and necrosis in the chicken embryo brain. However, the data also demonstrate that BaBlu harbors mutations in addition to the deletions of gE and gI that affect the virulence of PRV in the chicken embryo. Experiments studying other genes defective in Bartha are in progress. For example, we have infected chicken embryos with PRV509 (a deletion of gC in Becker) and find that it is as virulent as BeBlu (data not shown).
FIG. 8.
Progression of infection of gE and gI mutant PRV strains in the chicken embryo brain. At the indicated times after injection into the right eye, the brains from infected embryos were removed and placed in X-Gal. Only infected tissue close to the surface of the brain or exposed by necrosis was stained blue. Dorsal and ventral views of each brain are shown. PRV-91Blu + PRV-98Blu, coinfection experiment where equal amounts of the gE mutant PRV-91Blu and the gI mutant PRV-98Blu were mixed and injected. Note that the times that the brains were collected are different for the two infections.
gE and gI form a heterodimer which is thought to be the functional complex affecting virulence and spread of PRV in rodent and swine infections system (13). We tested this idea in the chicken model by coinfecting a chicken eye with a mixture of PRV-91Blu (gE− gI+) and PRV-98Blu (gE+ gI−) and determined if the mutants complemented each other as has been demonstrated in the rodent eye model system (14). Equal numbers of PFU of PRV-91Blu and PRV-98Blu were mixed, 105 total PFU were injected into the right eye of E12 chicken embryos, and the progression of infection of the brain was assessed (Fig. 8). After coinfection, the spread of virus to the brain mimicked that of a virulent BeBlu infection (compare Fig. 8 with Fig. 5) and was distinct from that of either PRV-91Blu or PRV-98Blu alone. These data suggest that gE and gI function together and play critical roles in the virulence of PRV in the chicken embryo eye model, as evidenced by the rapid destruction of brain tissue. The mechanism by which these viral gene products induce this pathology is currently under investigation.
DISCUSSION
In this report, we describe a simple system using late-stage chicken embryos to study brain infection and resulting pathogenesis by PRV after intravitreal infection. These studies are facilitated by the detailed characterization of structure and development of the chicken embryo eye and its neural circuitry. In the developing retina, retinal ganglion cell (RGC) genesis occurs primarily between E3 and E6 (10), and the RGC layer of the retina has formed by E12 (44). The retina, however, does not achieve its final adult architecture until 22 days after hatching (44). Axons of the earliest-generated RGC arrive at the optic tectum late on E6 (11) and have been shown to contact the dendritic surfaces of tectal cells in the midbrain by E9. Synaptic transmission from RGC to tectal cells can first be demonstrated on E10, and by E11 retinal connections with the tectum are well established (44). From E8 to E9 onward, selective RGC apoptosis results in a decrease in the number of RGC by approximately 40% until the final number of RGC is reached at E18. RGC do not degenerate until after their axons have reached the optic tectum. Photoreceptors in the retina first respond to light stimuli on E18. Based on these facts, we initiated our experiments in E12 embryos, a time in development when the RGC layer has formed and substantial connections exist between the retina and the brain. In addition, the choice of E12 embryos also allowed ample time to follow the course of viral infection before hatching occurred at E21.
All viral strains tested were capable of replication the E12 eye. The site of replication was most likely the neural retina, based on β-galactosidase staining. After primary replication, virus then appears in the brain in well-defined areas. We have initiated studies to identify the route of spread from eye to the brain. Preliminary characterization of the sites first infected by BaBlu and BeBlu suggest that infection occurs through the III (oculomotor), and V (trigeminal) cranial nerves and via the retina through infection of axon terminals which project to the isthmo-optic nucleus (4). Infection of the brainstem, medulla, and cerebellum (Fig. 5) is consistent with infection via the III and V nerves (43), and infection of the thalamus (not shown) at later times in infection is consistent with infection occurring via the V nerve (43). Infection of the deep layers of the optic tectum is consistent with the infection of the isthmo-optic nucleus (40). Currently, we have little evidence of spread via retinal ganglion cell infection; the outermost layers of the optic tectum are not consistently or heavily labeled early after infection as would be expected if spread were by the optic nerve.
As a control for nonneuronal spread to the brain, we examined blood, yolk, heart, gut, and liver and found no signs of infection by either recovery of infectious virus or tissue pathology (not shown). Thus, dissemination of infection throughout the embryo did not occur at the level of detection that we used. All of our data are consistent with viral infection of the brain occurring through neurons which innervate the eye.
We have demonstrated by using LD50 and mean time to death measurements that the chicken embryo responds to infection in a hierarchy similar to that observed in other animal models studied to date (8, 9, 32, 33, 39). The Becker strain is more virulent than gE or gI mutants, and the Bartha strain is the least virulent, although it still kills the animals. The lethality of the Bartha strain is presumably due to its extensive spread throughout the brains of infected animals such that a critical region of the brain required for the survival of the embryo is destroyed. The response to virulent PRV infection of the embryonic brain is striking, and the severity of CNS pathology correlates well with the LD50 and mean time to death measurements of the PRV strains tested. This stands in contrast to infection by attenuated strains where replication occurs, but pathology is reduced or absent. Thus, we believe the chicken embryo model provides an assay for identification of PRV virulence factors that influence the pathogenesis of any animal infection.
The known mutations in Bartha include deletions of gE, gI, US9, and US2 (31, 35, 42) and point mutations in the genes encoding gC (47), gM (12), and UL21 (25, 33). Our preliminary data suggest that gE and gI function together to enhance virulence; however, the tissue pathology induced by the gE or gI mutant strains was intermediate to that of Becker and Bartha. This finding indicates that other factors contribute to the severity of tissue pathology induced by the Becker strain. The functions of the US9, US2, UL21, and gM proteins are unclear at present, as is their putative role in PRV pathogenesis. It is interesting to note that the US9 gene is also deleted in another attenuated PRV strain, Norden (31, 35, 42). The US9 gene encodes a tail-anchored, type II membrane protein found in cellular membranes and in the virus envelope (7). The role of this molecule in PRV pathogenesis is currently being examined more closely.
The capacity of BaBlu, but not BeBlu, to replicate in late-stage embryonic brains may decrease as development progresses. This speculation follows from our observation that even though considerable numbers of cells exhibit β-galactosidase staining (Fig. 7D), the amount of infectious virus recovered from the tissue was about the same as that recovered from BeBlu-infected animals in which fewer cells were infected (compare Fig. 6 and 7A). Lomniczi and colleagues found that the day-old chicken brain was not permissive for Bartha (32). Thus, the apparent reduced replication of BaBlu observed in late-stage embryos may reflect the gradual acquisition of this property during development. Preliminary evidence suggests that when E14 embryos receive an intracranial inoculation of Bartha, approximately 50% of the animals survive beyond 128 h and probably would have survived hatching. In contrast, after intracranial injection of Becker, all animals die between 48 and 72 h after infection. It will be of interest to determine the nature of this antivirus defense system.
One useful feature of the chicken embryo is the ease with which cells can be cultured. Preliminary results of assays using primary cultured retinal cells isolated from E9 chicken embryos indicate that there are striking differences in infections by Becker and Bartha (3). The two strains infect these cells with equal efficiency, and single-step growth experiments revealed that the kinetics and amount of infectious virus production were also similar. However, the cytopathic effect elicited by the Becker strain was considerably greater than that elicited by the Bartha strain; after 24 h of infection at high multiplicity, Becker-infected cells had lysed and released from the substrate whereas the Bartha-infected cells appeared intact and remained attached to the dish despite having produced similar amounts of infectious virus. If this were also occurring in vivo, it might explain the frank hemorrhage and necrotic response observed in the brains of BeBlu- but not BaBlu-infected embryos. The genetic differences between Bartha and Becker which contribute to this phenotype in vitro are currently being addressed.
The chicken embryo brain is truly dynamic in that both neuronal cells and glia continue to differentiate until late times in development (near hatching) (20, 30). This observation must certainly influence the ability of PRV strains to replicate and spread. Nevertheless, viral genes that affect virulence in swine and rodents also have the same effect in the chick embryo. We assume this means that these host-virus interactions are highly conserved in the many hosts of PRV. Thus, we are hopeful that the chicken embryo model will enable identification of host factors or cells involved in antiviral defense in the brain.
ACKNOWLEDGMENTS
We thank E. L. Senecal for her hard work and invaluable advice and insight in establishing the utility of the chicken eye model. Thanks go to all the members of the Enquist laboratory for critical reviews of the manuscript and insight during the course of this work. Nick Brecha provided helpful suggestions and insight on the route of infection. We also thank E. Mocarski for helpful criticism and reviewer 1 for suggesting an alternative interpretation of the data.
B.W.B. was supported by a postdoctoral fellowship from the Medical Research Council of Canada. This work was supported by NINDS grant 1R0133506 to L.W.E.
REFERENCES
- 1.Anderson K. Pathogenesis of herpes simplex virus infection in chick embryos. Am J Pathol. 1940;16:137–156. [PMC free article] [PubMed] [Google Scholar]
- 2.Babic N, Mettenleiter T C, Flamand A, Ugolini G. Role of essential glycoproteins gII and gp50 in transneuronal transfer of pseudorabies virus from the hypoglossal nerves of mice. J Virol. 1993;67:4421–4426. doi: 10.1128/jvi.67.7.4421-4426.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Banfield, B. W., and L. W. Enquist. Unpublished observations.
- 4.Banfield, B. W., C. Lee, S. Marks, N. Brecha, and L. W. Enquist. Unpublished observations.
- 5.Bang F. Experimental infection of the chick embryo with the virus of pseudorabies. J Exp Med. 1942;76:263–269. doi: 10.1084/jem.76.3.263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bartha A, Belák S, Benyeda J. Trypsin and heat resistance of some strains of the virus group. Acta Vet Hung. 1969;19:97–99. [PubMed] [Google Scholar]
- 7.Brideau A D, Banfield B W, Enquist L W. The Us9 gene product of pseudorabies virus, an alphaherpesvirus, is a phosphorylated, tail-anchored type II membrane protein. J Virol. 1998;72:4560–4570. doi: 10.1128/jvi.72.6.4560-4570.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Card, J. P., R. R. Miselis, and L. W. Enquist. Unpublished observations.
- 9.Card J P, Whealy M E, Robbins A K, Enquist L W. Pseudorabies virus envelope glycoprotein gI influences both neurotropism and virulence during infection of the rat visual system. J Virol. 1992;66:3032–3041. doi: 10.1128/jvi.66.5.3032-3041.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cepko C L, Austin C P, Yang X, Alexiades M, Ezzeddine D. Cell fate determination in the vertebrate retina. Proc Natl Acad Sci USA. 1996;93:589–595. doi: 10.1073/pnas.93.2.589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Crossland W J, Cowan W M, Rogers L A. Studies on the development of the chick optic tectum. IV. An autoradiographic study of the development of retino-tectal connections. Brain Res. 1975;91:1–23. doi: 10.1016/0006-8993(75)90463-1. [DOI] [PubMed] [Google Scholar]
- 12.Dijkstra J A, Mettenleiter T C, Klupp B G. Intracellular processing of pseudorabies virus glycoprotein M (gM): gM of strain Bartha lacks N-glycosylation. Virology. 1997;237:113–122. doi: 10.1006/viro.1997.8766. [DOI] [PubMed] [Google Scholar]
- 13.Enquist L W. Infection of the mammalian nervous system by pseudorabies virus (PRV) Semin Virol. 1994;5:221–231. [Google Scholar]
- 14.Enquist L W, Dubin J, Whealy M E, Card J P. Complementation analysis of pseudorabies virus gE and gI mutants in retinal ganglion cell neurotropism. J Virol. 1994;68:5275–5279. doi: 10.1128/jvi.68.8.5275-5279.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Enquist, L. W., et al. Unpublished observations.
- 16.Glover R E. Cultivation of the virus of Aujeszky’s disease on the chorioallantoic membrane of the developing egg. Br J Exp Pathol. 1939;20:150–158. [Google Scholar]
- 17.Goodman J L, Cook M L, Sederati F, Izumi K, Stevens J G. Identification, transfer, and characterization of cloned herpes simplex virus invasiveness regions. J Virol. 1989;63:1153–1161. doi: 10.1128/jvi.63.3.1153-1161.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Goodman J L, Stevens J G. Passage of herpes simplex virus type 1 on chick embryo fibroblasts confers virulence for chick embryos. Virus Res. 1986;5:191–200. doi: 10.1016/0168-1702(86)90017-1. [DOI] [PubMed] [Google Scholar]
- 19.Goodpasture E W, Woodruff A M, Buddingh G J. The cultivation of vaccine and other viruses in the chorioallantoic membrane of chick embryos. Science. 1931;74:371–372. doi: 10.1126/science.74.1919.371. [DOI] [PubMed] [Google Scholar]
- 20.Gray G E, Sanes J R. Lineage of radial glia in the chicken optic tectum. Development. 1992;114:271–283. doi: 10.1242/dev.114.1.271. [DOI] [PubMed] [Google Scholar]
- 21.Hamburger V, Hamilton H L. A series of normal stages in the development of the chick embryo. J Morphol. 1951;88:49–92. [PubMed] [Google Scholar]
- 22.Jacobs L, Rziha H-J, Kimman T G, Gielkens A L J, van Oirshot J T. Deleting valine-125 and cysteine-126 in glycoprotein gI of pseudorabies virus strain NIA-3 decreases plaque size and reduces virulence in mice. Arch Virol. 1993;131:251–264. doi: 10.1007/BF01378630. [DOI] [PubMed] [Google Scholar]
- 23.Kaplan A S. Herpes simplex and pseudorabies viruses. Virol Monogr. 1969;5:1–115. [Google Scholar]
- 24.Kimman T G, de Wind N, Oei-Lie N, Pol J M A, Berns A J M, Gielkens A L J. Contributions of single genes within the unique short region of Aujeszky’s disease virus (suid herpes virus 1) to virulence, pathogenesis and immunogenicity. J Gen Virol. 1992;73:243–251. doi: 10.1099/0022-1317-73-2-243. [DOI] [PubMed] [Google Scholar]
- 25.Klupp B G, Lomniczi B, Visser N, Fuchs W, Mettenleiter T C. Mutations affecting the UL21 gene contribute to avirulence of pseudorabies virus vaccine strain Bartha. Virology. 1995;212:466–473. doi: 10.1006/viro.1995.1504. [DOI] [PubMed] [Google Scholar]
- 26.Knapp A C, Enquist L W. Pseudorabies virus recombinants expressing functional virulence determinants gE and gI from bovine herpesvirus 1.1. J Virol. 1997;71:2731–2739. doi: 10.1128/jvi.71.4.2731-2739.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kritas S K, Nauwynck H J, Pensaert M B. Dissemination of wild-type gC-, gE-, and gI-deleted mutants of Aujeszky’s disease virus in the maxillary nerve and trigeminal ganglion of pigs after intranasal inoculation. J Gen Virol. 1995;76:2063–2066. doi: 10.1099/0022-1317-76-8-2063. [DOI] [PubMed] [Google Scholar]
- 28.Kritas S K, Pensaert M B, Mettenleiter T C. Invasion and spread of single glycoprotein deleted mutants of Aujeszky’s disease virus (ADV) in the trigeminal nervous pathway of pigs after intranasal inoculation. Vet Microbiol. 1994;40:323–334. doi: 10.1016/0378-1135(94)90120-1. [DOI] [PubMed] [Google Scholar]
- 29.Kritas S K, Pensaert M B, Mettenleiter T C. Role of envelope glycoproteins gI, gp63 and gIII in the invasion and spread of Aujesky’s disease virus in the olfactory nervous pathway of the pig. J Gen Virol. 1994;75:2319–2327. doi: 10.1099/0022-1317-75-9-2319. [DOI] [PubMed] [Google Scholar]
- 30.LaVail J H, Cowan W M. The development of the chick optic tectum. II. Autoradiographic studies. Brain Res. 1971;28:421–441. [PubMed] [Google Scholar]
- 31.Lomniczi B, Blankenship M L, Ben-Porat T. Deletions in the genomes of pseudorabies virus vaccine strains and existence of four isomers of the genomes. J Virol. 1984;49:970–979. doi: 10.1128/jvi.49.3.970-979.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lomniczi B, Watanabe S, Ben-Porat T, Kaplan A S. Genetic basis of the neurovirulence of pseudorabies virus. J Virol. 1984;52:198–205. doi: 10.1128/jvi.52.1.198-205.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lomniczi B, Watanabe S, Ben-Porat T, Kaplan A S. Genome location and identification of functions defective in the Bartha vaccine strain of pseudorabies virus. J Virol. 1987;61:796–801. doi: 10.1128/jvi.61.3.796-801.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lowry S P, Melnick J L, Rawls W E. Investigation of plaque formation in chick embryo cells as a biological marker for distinguishing herpes virus type 2 from type 1. J Gen Virol. 1971;10:1–9. doi: 10.1099/0022-1317-10-1-1. [DOI] [PubMed] [Google Scholar]
- 35.Mettenleiter T C, Lukacs N, Rziha H J. Pseudorabies virus avirulent strains fail to express a major glycoprotein. J Virol. 1985;56:307–311. doi: 10.1128/jvi.56.1.307-311.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mettenleiter T C, Rauh I. A glycoprotein gX-beta-galactosidase fusion gene as insertional marker for rapid identification of pseudorabies virus mutants. J Virol Methods. 1990;30:55–65. doi: 10.1016/0166-0934(90)90043-f. [DOI] [PubMed] [Google Scholar]
- 37.Mettenleiter T C, Schreurs C, Zuckermann F, Ben-Porat T. Role of pseudorabies virus glycoprotein gI in virus release from infected cells. J Virol. 1987;61:2764–2769. doi: 10.1128/jvi.61.9.2764-2769.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mettenleiter T C, Schreurs C, Zuckermann F, Ben-Porat T, Kaplan A S. Role of glycoprotein gIII of pseudorabies virus in virulence. J Virol. 1988;62:2712–2717. doi: 10.1128/jvi.62.8.2712-2717.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mettenleiter T C, Zsak L, Kaplan A S, Ben-Porat T, Lomniczi B. Role of a structural glycoprotein of pseudorabies in virus virulence. J Virol. 1987;61:4030–4032. doi: 10.1128/jvi.61.12.4030-4032.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Miceli D, Repérant J, Bavikati R, Rio J P, Volle M. Brain-stem afferents upon retinal projecting isthmo-optic and ectopic neurons of the pigeon centrifugal visual system demonstrated by retrograde transneuronal transport of rhodamine beta-isothiocyanate. Visual Neurosci. 1997;14:213–224. doi: 10.1017/s0952523800011354. [DOI] [PubMed] [Google Scholar]
- 41.Nahmias A J, Dowdle W R, Naib Z M, Highsmith A, Harwell R W, Josey W E. Relation of pock size on chorioallantoic membrane to antigenic type of herpesvirus hominis. Proc Soc Exp Biol Med. 1968;127:1022–1028. doi: 10.3181/00379727-127-32861. [DOI] [PubMed] [Google Scholar]
- 42.Petrovskis E A, Timmins J, Gierman T, Post L. Deletions in vaccine strains of pseudorabies virus and their effects on synthesis of glycoprotein gp63. J Virol. 1986;60:1166–1169. doi: 10.1128/jvi.60.3.1166-1169.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Poritsky R. Neuroanatomical pathways. Philadelphia, Pa: The W. B. Saunders Company; 1984. [Google Scholar]
- 44.Rager G H. Development of the retinotectal projection in the chicken. New York, N.Y: Springer-Verlag; 1980. [PubMed] [Google Scholar]
- 45.Ramachandran S P, Fraser G. Studies on the virus of Aujeszky’s disease. II. Pathogenicity for chicks. J Comp Pathol. 1971;81:55–62. doi: 10.1016/0021-9975(71)90055-7. [DOI] [PubMed] [Google Scholar]
- 46.Reed L J, Muench H. A simple method of estimating fifty percent endpoints. Am J Hyg. 1938;27:493–497. [Google Scholar]
- 47.Robbins A K, Ryan J P, Whealy M E, Enquist L W. The gene encoding the gIII envelope protein of pseudorabies virus vaccine strain Bartha contains a mutation affecting protein localization. J Virol. 1989;63:250–258. doi: 10.1128/jvi.63.1.250-258.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rodgers F G. Infection, haemorrhage and death of chick embryos after inoculation of herpes simplex virus on to the chorioallantoic membrane. J Gen Virol. 1973;21:187–191. doi: 10.1099/0022-1317-21-1-187. [DOI] [PubMed] [Google Scholar]
- 49.Standish A, Enquist L W, Miselis R R, Schwaber J S. Dendritic morphology of cardiac related medullary neurons defined by circuit specific infection by a recombinant pseudorabies virus expressing β-galactosidase. J Neurovirol. 1995;1:359–368. doi: 10.3109/13550289509111025. [DOI] [PubMed] [Google Scholar]
- 50.Whealy M E, Card J P, Robbins A K, Dubin J R, Rziha H J, Enquist L W. Specific pseudorabies virus infection of the rat visual system requires both gI and gp63 glycoproteins. J Virol. 1993;67:3786–3797. doi: 10.1128/jvi.67.7.3786-3797.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wittmann G, Rziha H-J. Aujeszky’s disease (pseudorabies) in pigs. In: Wittmann G, editor. Herpesvirus diseases of cattle, horses, and pigs. Boston, Mass: Kluwer Academic; 1989. pp. 230–325. [Google Scholar]
- 52.Zsak L, Zuckermann F, Sugg N, Ben-Porat T. Glycoprotein gI of pseudorabies virus promotes cell fusion and virus spread via direct cell-to-cell transmission. J Virol. 1992;66:2316–25. doi: 10.1128/jvi.66.4.2316-2325.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]