Abstract
Background:
Tamoxifen has been successfully administered as adjunctive therapy for breast cancer. However, the effect of tamoxifen as an estrogen agonist and antagonist can cause pathological changes in the uterus. The agonist effect may stimulate endometrial proliferation leading to endometrial polyps, hyperplasia, and, rarely, endometrial cancer.
Objective:
We present the case of tamoxifen-treated breast cancer case to better understand one of the most serious consequences, endometrial cancer.
Case presentation:
A 37-year-old woman came to our centre with complaints of abnormal vaginal bleeding. She has diagnosed with grade I infiltrative ductal carcinoma in 2018, with primary complaints of right breast mass and axillary lymphadenopathy. During this period, adjuvant chemotherapy was given tamoxifen 20 mg once daily. There were no complaints or relapses at a six-month follow-up over three years. In the fourth year, the patient complained of vaginal bleeding. A vaginal biopsy was performed, and the results showed low-grade endometrioid-type endometrial carcinoma. Total hysterectomy and bilateral salpingo-oophorectomy were performed with the resultant mass of up to half of the myometrial lining with metastatic negative parallax lymph nodes.
Conclusion:
Following tamoxifen therapy, endometrial cancer is more likely to occur in patients. Patients who experience irregular vaginal hemorrhage should have hysteroscopy or uterine ultrasound performed, and if the cause is unknown, a biopsy should be performed.
Keywords: endometrial cancer, tamoxifen, premenopausal, cancer treatment
1. BACKGROUND
In the decreasing the risk of a patient developing estrogen-positive breast cancer, selective estrogen receptor modulators (SERMs) have become the current option. However, the effect of tamoxifen as an estrogen agonist and antagonist can cause pathological changes in the uterus. The agonist effect may stimulate endometrial proliferation leading to endometrial polyps, hyperplasia, and, rarely, endometrial cancer.[1,2] There are no recommendations that suggest gynecological testing for endometrial cancer in premenopausal women taking tamoxifen.[3,4] In this article, we will look at a tamoxifen-treated breast cancer case to better understand one of the most serious consequences, endometrial cancer.
2. CASE PRESENTATION
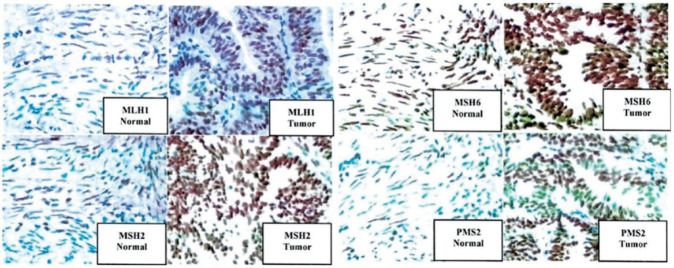
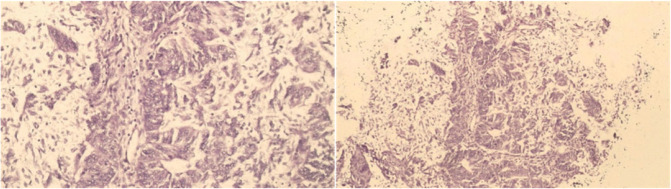
A 37-year-old woman came to our centre with complaints of abnormal vaginal bleeding. She has diagnosed with grade I infiltrative ductal carcinoma in 2018, with primary complaints of right breast mass and axillary lymphadenopathy. Immunohistochemical analysis showed ER-positive 80%, PR positive 80%, HER2+1, and low KI67 <20% (Figure 1). Right mastectomy was performed with sentinel lymph node biopsy (SLNB). Histopathological examination showed superior, inferior medial, and lateral resection, clear base margins, and five negative sentinel lymph node metastases with mixed endometrioid histopathological diagnoses and papillary serous carcinoma (Figure 2). During this period, adjuvant chemotherapy was given tamoxifen 20 mg once daily.
Figure 1. Immunohistochemical analysis revealed that ER-positive 80%, PR positive 80%, HER2 +1, and KI67 low <20%.
Figure 2. The histopathological examination showed mixed endometrioid and papillary serous carcinoma.
There were no complaints or relapses at a six-month follow-up over three years. In the fourth year, the patient complained of vaginal bleeding, an MRI was performed, and no endometrial, ovary, vaginal, or pelvic lymph node mass was seen. A positron emission tomography (PET) scan was performed to detect an endometrial group or hyperplasia, and a hypermetabolic group was found in the uterine cavity and left para iliac lymph node. There were no signs of contrast shock in other organs. A vaginal biopsy was performed, and the results showed low-grade endometrioid-type endometrial carcinoma. Total hysterectomy and bilateral salpingo-oophorectomy were performed with the resultant mass of up to half of the myometrial lining with metastatic negative parallax lymph nodes.
The informed consent was obtained from the patient before any procedure was performed. Accordingly, the patients (a 37-year-old Indonesian woman in this study) also had consented for documentation, discussion, and publication by clinical science development intention; including the patient’s early presentation to the final follow-up investigations as represented by the figure we provided in this study.
3. DISCUSSION
Tamoxifen inhibits endogenous estrogen signaling in normal and malignant breast tissue, making it a viable option for the prevention or recurrence of breast cancer in high-risk patients, such as those with atypical breast tissue hyperplasia, a history of LCIS, a five-year breast cancer risk of 1.7%, and adjunctive therapy in nonmetastatic hormone-positive breast cancer.[1,2] Tamoxifen mimics estrogen in the ovaries, bones, liver, and clotting system. Tamoxifen, based on the duration of use, has been linked to an increased chance of endometrial cancer and uterine sarcoma. Other risk variables, such as body mass index, length of treatment, and prior estrogen replacement therapy to avoid endometrial cancer while taking tamoxifen, were also considered.[3,4]
Tamoxifen use for more than five years has been linked to an increased chance of endometrial cancer in previous research. The ATLAS study found that taking tamoxifen for more than five years decreased the chance of breast cancer recurrence but raised the risk of endometrial cancer. In the absence of cancer, tamoxifen produces endometrial hyperplasia and sub-endometrial gland enlargement; as a result, endometrial thickening without atypical vaginal hemorrhage is not as indicative of further examination as a biopsy.[5,6] The American College of Obstetricians and Gynecologists states that having tamoxifen is not a justification for getting an ultrasound or endometrial sample on a regular basis. Transvaginal ultrasound (TVUS) is advised for premenopausal women taking tamoxifen who experience irregular uterine bleeding. Endometrial sampling is not required and routine follow-up is advised if the endometrial thickness is less than 4 millimeters.[7]
If an endometrial biopsy shows hyperplasia, tamoxifen should be stopped and cyclic progestin treatment started in this group of patients. In instances where there is no wish for fertility, a hysterectomy may be an option. Other experts advise that for premenopausal women taking tamoxifen, irregular vaginal bleeding be examined with hysteroscopy and uterine ultrasound, and if it persists, an ulcer biopsy be done. Any vaginal hemorrhage in postmenopausal women taking tamoxifen should be followed by a biopsy and careful monitoring. There are no evidence-based guidelines for monitoring for uterine cancer in tamoxifen users. The current proposal is for a gynecological checkup and assessment of abnormal vaginal flow once a year.[5-7]
Transvaginal ultrasound has not been demonstrated to be useful in asymptomatic patients on short-term (less than five years) tamoxifen therapy. In this research, abnormal endometrial thickness greater than 9 mm is permissible, but further invasive examinations such as dilation and curettage (D&C) are not suggested if there is no vaginal bleeding.[8] According to some research, hysteroscopic biopsy is preferable to D&C for identifying intrauterine disease in tamoxifen patients. When TVUS results are ambiguous and hysterosonography is not feasible, MRI can be used to assess the endometrium, myometrium, and related pathology. Tamoxifen alternatives, such as anastrozole, are also being researched. The study found that anastrozole was as effective and tolerable as tamoxifen in postmenopausal women with hormone-positive metastatic breast cancer. Furthermore, no endometrial swelling was noted.[9]
4. CONCLUSION
In high-risk individuals, using tamoxifen for at least five years following diagnosis is a feasible choice for avoiding breast cancer or its return. Following tamoxifen therapy, endometrial cancer is more likely to occur in patients. Tamoxifen-treated premenopausal women who experience irregular vaginal hemorrhage should have hysteroscopy or uterine ultrasound performed, and if the cause is unknown, a biopsy should be performed.
Patient Consent Form:
The informed consent was obtained from the patient before any procedure was performed. Accordingly, the patients (a 37-year-old Indonesian woman in this study) also had consented for documentation, discussion, and publication by clinical science development intention; including the patient’s early presentation to the final follow-up investigations as represented by the figure we provided in this study.
Authors contribution:
DH, MAA, MRY, ATL and RA gave a substantial contribution to the conception and design of the work. DH, MRY, ATL and RA gave a substantial contribution of data. DH, MAA and MRY gave a substantial contribution to the acquisition, analysis, or interpretation of data for the work. DH and MAA had a part in article preparing for drafting or revising it critically for important intellectual content. All authors gave final approval of the version to be published and agreed to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Conflict of interest:
There are no conflict of interest.
Financial support and sponsorship:
Nil.
REFERENCES
- 1.Gultekin IB, Imamoglu GI, Gultekin S, Yilmaz EA, Yilmaz Z, et al. Elastosonographic evaluation of endometrium in women using tamoxifen for breast cancer. Niger J Clin Pract. 2019;22(1):92–100. doi: 10.4103/njcp.njcp_387_16. [DOI] [PubMed] [Google Scholar]
- 2.Cuzick J, Sestak I, Bonanni B, Costantino JP, Cummings S, et al. Selective estrogen receptor modulators in breast cancer prevention: an updated meta-analysis of individual participant data. Lancet. 2013;381(9880):1827–34. doi: 10.1016/S0140-6736(13)60140-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Korkmazer E, Solak N, Tokgoz VY. Assessment of thickened endometrium in tamoxifen therapy. Turk J Obstet Gynecol. 2014;11(4):215–8. doi: 10.4274/tjod.82621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fleming CA, Heneghan HM, O’Brien D, McCartan DP, McDermott EW, et al. Metaanalysis of the cumulative risk of endometrial malignancy and systematic review of endometrial surveillance in extended tamoxifen therapy. Br J Surg. 2018;105(9):1098–106. doi: 10.1002/bjs.10899. [DOI] [PubMed] [Google Scholar]
- 5.Rosell J, Nordenskjold B, Bengtsson N.O, Fornander T, Hatschek T, Lindman H, Malmstrom P.O, Wallgren A, Stal O, Carstensen J. Long-term effects on the incidence of second primary cancers in a randomized trial of two and five years of adjuvant tamoxifen. Acta Oncol. 2017;56(614):617. doi: 10.1080/0284186X.2016.1273547. [DOI] [PubMed] [Google Scholar]
- 6.Davies C, Pan H, Godwin J, Gray R, Arriagada R, Raina V, Abraham M, Medeiros Alencar V.H, Badran A, Bonfill X, et al. Adjuvant Tamoxifen: Longer Against Shorter Collaborative, G. Long-term effects of continuing adjuvant tamoxifen to 10 years versus stopping at 5 years after diagnosis of oestrogen receptor-positive breast cancer: ATLAS, a randomised trial. Lancet. 2013;381:805–816. doi: 10.1016/S0140-6736(12)61963-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.van Weelden W.J, Massuger L.F, Pijnenborg J.M.A, Romano A. Anti-estrogen Treatment in Endometrial Cancer: A Systematic Review. Front. Oncol. 2019;9:359. doi: 10.3389/fonc.2019.00359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Early Breast Cancer Trialists’ Collaborative Group. Aromatase inhibitors versus tamoxifen in early breast cancer: Patient-level meta-analysis of the randomised trials. Lancet. 2015;386:1341–1352. doi: 10.1016/S0140-6736(15)61074-1. [DOI] [PubMed] [Google Scholar]
- 9.Chlebowski R.T, Schottinger J.E, Shi J, Chung J, Haque R. Aromatase inhibitors, tamoxifen, and endometrial cancer in breast cancer survivors. Cancer. 2015;121:2147–2155. doi: 10.1002/cncr.29332. [DOI] [PMC free article] [PubMed] [Google Scholar]