Abstract
Here we report that administration of retinoids can alter the outcome of an acute murine cytomegalovirus (MCMV) infection. We show that a crucial viral control element, the major immediate-early enhancer, can be activated by retinoic acid (RA) via multiple RA-responsive elements (DR2) that bind retinoid X receptor-retinoic acid receptor (RAR) heterodimers with apparent dissociation constants ranging from 15 to 33 nM. Viral growth is dramatically increased upon RA treatment of infected tissue culture cells. Using synthetic retinoid receptor-specific agonists and antagonists, we provide evidence that RAR activation in cells is required for mediating the response of MCMV to RA. Oral administration of RA to infected immunocompetent mice selectively exacerbates an infection by MCMV, while cotreatment with an RAR antagonist protects against the adverse effects of RA on MCMV infection. In conclusion, these chemical genetic experiments provide evidence that an RAR-mediated pathway can modulate in vitro and in vivo infections by MCMV.
Retinoids, a group of vitamin A derivatives, have been found to play important roles in development, growth, reproduction, vision, and general homeostasis of numerous tissues. The cellular responses to extracellular retinoids are mediated principally by members of the steroid-thyroid superfamily of intracellular hormone receptors, which include the retinoic acid (RA) receptors (RARs) and the retinoid X receptors (RXRs) (reviewed in reference 12). The RAR subfamily binds two naturally occurring ligands, all-trans-RA (ATRA) and 9-cis-RA, whereas RXR subfamily members bind 9-cis-RA exclusively (12, 34). These particular receptors are nuclear proteins that, upon ligand activation, function as heterodimeric transcription factors to control expression of target genes by binding to specific DNA sequences, termed RA response elements (RAREs) (reviewed in reference 34). In addition, these two families of nuclear receptors may indirectly influence biological processes by remodeling chromatin, interacting with transcriptional coregulators, or cross-talking with other signalling pathways (12, 13, 24, 29, 50).
In response to environmental stimuli, intracellular signalling events can play an important role in modulating the outcome of an infection. In this regard, recent findings from in vitro studies point toward a functional link between a diverse group of pathogenic viruses and a direct or indirect component of the vitamin A signalling pathway (reviewed in reference 20). Examples of these viruses include human papillomavirus, Epstein-Barr virus, human cytomegalovirus (HCMV), hepatitis B virus, and human immunodeficiency virus. However, the elaboration and extent to which extracellular agents (such as retinoids) drive or restrict the infectious program of a virus are not well understood. Elucidating host cell-virus interactions that determine the outcome of virus infection is central to understanding the processes of viral pathogenesis. To this end, we have investigated the involvement of retinoids in modulating the CMV infectious program. Retinoids are known to influence HCMV at a variety of different levels in vitro. For instance, exposure of cells to RA can enhance viral gene expression and the susceptibility to infection via differentiation- and nondifferentiation-driven events (3–5, 21, 22, 30, 40) and can reactivate viral expression in latently infected tissue culture cells (51). At the level of viral gene expression, the HCMV genome depends on a hierarchy of interactions among the host-encoded and viral immediate-early (IE) genes in the infected cell. In this regard, the HCMV major IE promoter (MIEP) has been shown to respond to physiological concentrations of RA via three high-affinity binding sites for RXR-RAR heterodimers (6, 19). The biological significance of the HCMV MIEP in vivo is underscored by transgenic animal model systems which show that the pattern of expression controlled by the HCMV MIEP corresponds to sites of natural infection in humans (9, 26). This pattern of expression and cellular sites of natural infection closely overlaps with the cell-specific distribution pattern of RARs (8). Taken together, these studies indicate that RA may indirectly or directly affect HCMV, and hence they predict the potential influence of RA in altering a CMV infection in vivo. The question of whether retinoids have any impact on the infectious disease process remains open because of the species-specific restriction in the ability of HCMV to replicate. Infection of mice with murine CMV (MCMV) has provided a good model to study the pathogenesis of CMV. We have therefore studied the effect of retinoids on MCMV infection in vitro and in vivo.
Here, we present evidence that MCMV is susceptible to regulation by natural and synthetic retinoids at a number of different levels. We show that in tissue culture cells RA can activate the MCMV enhancer and can also selectively promote viral growth. The stimulatory effects of RA on enhancer activity and viral growth can be prevented by treatment with an RAR-specific antagonist. In vivo, we demonstrate that oral administration of RA to infected mice worsens an acute infection by MCMV but not other pathogenic viruses. By contrast, we provide evidence that oral dosage of an RAR antagonist to infected mice can protect against the adverse effects of RA in MCMV infection. These observations thus define a novel pathogenetic pathway in the infectious program of CMV and have important implications for understanding control mechanisms of viruses outside immunoregulatory pathways.
MATERIALS AND METHODS
Cells and viruses.
NIH 3T3 murine fibroblasts, NT-2/D1 human teratocarcinoma cells, TK−143B human osteosarcoma cells, and Vero cells were propagated in Dulbecco’s modified essential medium (DMEM) supplemented with 2 mM glutamine, 100 U of penicillin per ml, 100 μg of gentamicin per ml, and 10% fetal bovine serum, except for the NIH 3T3 cells, for which 10% calf serum was used. The Smith strain of MCMV used in this study was kindly provided by Ann Campbell (Eastern Virginia Medical School). RM408, RM427, and RM461 MCMV recombinants were originally obtained from Edward Mocarski (Stanford University). RM408 carries the lacZ gene under control of the MCMV MIEP-enhancer (nucleotides −146 to +50) inserted in place of a 79-bp HpaI fragment in the ie2 promoter (35). RM461 and RM427 each carry the lacZ gene under control of the HCMV MIEP-enhancer (nucleotides −219 to −19). In RM461, the insertion is in a HindIII site between sgg1 and ie2 (48). In RM427, the insertion replaces a 79-bp fragment between two HpaI sites in the ie2 promoter (48). In addition, RM427 carries a spontaneous 323-nucleotide deletion in the sgg1 gene. Stocks of tissue-propagated MCMVs were prepared in and titers were determined by standard plaque assay on NIH 3T3 cells. For in vivo assays, salivary gland-passaged MCMV was used. Salivary glands from weanling BALB/c.ByJ mice inoculated intraperitoneally with 103 PFU of MCMV were harvested on day 15 of infection, homogenized in a 10% suspension, and cleared by centrifugation. The vaccinia virus strain WR and herpes simplex virus type 1 used in these studies were initially obtained from Persephone Burrow and Pietro Sanna (The Scripps Research Institute), respectively. Viral stocks for vaccinia virus WR and herpes simplex virus type 1 were prepared and viral titers were determined by standard plaque assay on human TK−143B and Vero cells, respectively.
Retinoids.
ATRA was purchased from Sigma Chemical Co. (St. Louis, Mo.). 9-cis-RA and LG100069 {4-[1-(3,5,5,8,8-pentamethyl-5,6,7,8-tetrahydro-2-naphthyl)ethenyl]benzoic acid} were provided by Rich Heyman (Ligand Pharmaceuticals Inc., San Diego, Calif.). TTNPB {(E)-4-[2-(5,5,8,8-tetramethyl-5,6,7,8-tetrahydro-2-naphthalenyl)-1-propenyl]benzoic acid} was prepared as described by Loeliger et al. (33). The RAR antagonist AGN 193109 {4-[(5,6-dihydro-5,5-dimethyl-8-(4-methylphenyl)-2-naphthalenyl)-ethynyl]benzoic acid} was synthesized as described by Johnson et al. (23). For cell culture assays, retinoid stock solutions (10 mM) were made in dimethyl sulfoxide and/or ethanol and stored under argon at −70°C. Further dilutions were made in DMEM supplemented with charcoal resin-treated serum before use, except in the experiments shown in Fig. 4A and B, where the serum was not charcoal treated. For animal treatments, retinoids were prepared as a suspension in corn oil (50 mg of ATRA per kg of body weight and 25 mg of AGN 193109 per kg) immediately before use. Control animals received the corn oil alone. All retinoids were handled under subdued lighting.
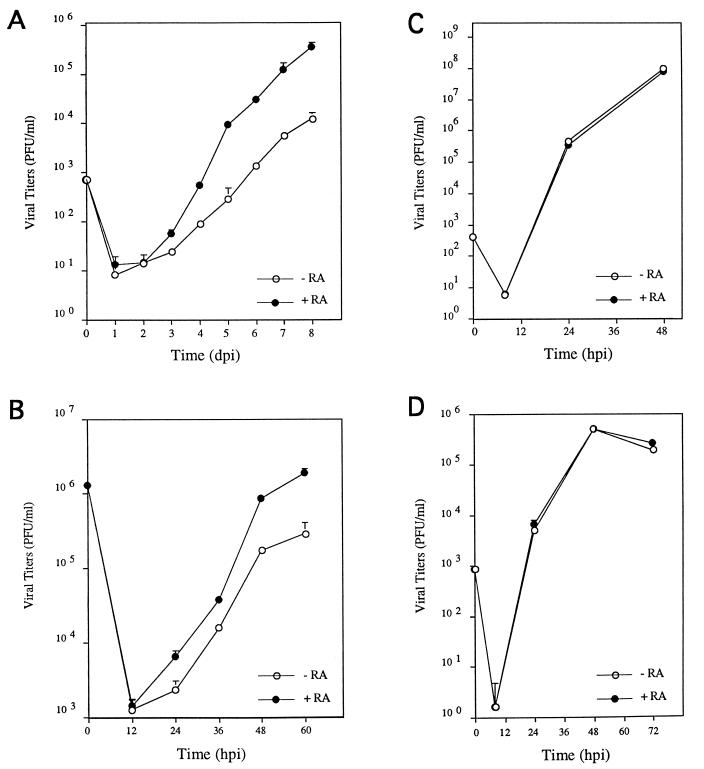
FIG. 4.
Effect of RA on MCMV growth. (A) NIH 3T3 cells were exposed to 9-cis-RA at 10−5 M (+RA) or vehicle (−RA) for 4 h. Subsequently, the cells were infected with MCMV (Smith strain) at an MOI of 0.01 PFU/cell and reexposed to 9-cis-RA at 10−5 M (+RA) or vehicle (−RA). At the different times after infection indicated, the presence of extracellular virus in the cultures was determined. Each data point represents the average and standard deviation for three separate cultures. Similar results were obtained with 10−5 M ATRA (data not shown). dpi, days postinfection. (B) Same as panel A except that MCMV infections were carried out at an MOI of 10. hpi, hours postinfection. (C) Same as panel A except that NIH 3T3 cells were infected with vaccinia virus at an MOI of 0.01 PFU/cell. In this case, the presence of intracellular virus in the cultures was determined. (D) Same as panel A except that NIH 3T3 cells were infected with herpes simplex virus type 1 at an MOI of 0.01 PFU/cell. Growth of herpes simplex virus type 2 in NIH 3T3 cells was also unaffected by treatment with 9-cis-RA at 10−5 M (data not shown).
Plasmid constructions and transfections.
The reporter constructs pON405, containing MCMV MIEP-enhancer sequences from position −2000 to +50 relative to the start site, and pON407, containing 196 bp of the MCMV MIEP-enhancer (from position −146 to +50 relative to the start site) linked to the Escherichia coli lacZ indicator gene were kindly provided by Edward Mocarski (11, 35). The reporter plasmids MDR2a and MDR2b, encoding the two different RAREs, were constructed by inserting the double-stranded oligonucleotides 5′-TATTGACCTTTTGTACTGGG-3′ (MDR2a) and 5′-TATTGACCTTATGTACGTGC-3′ (MDR2b) at the HindIII-BamHI sites upstream of the herpes simplex virus thymidine kinase gene promoter, tkCAT (pRSCAT4 [49]). The β-galactosidase expression vector RSVβgal and the luciferase expression vector tkLuc were used as internal controls in transfection assays (6).
Transfections were performed by the calcium phosphate coprecipitation method as previously described (19). Cellular extracts were prepared as described previously and assayed for β-galactosidase, luciferase, or chloramphenicol acetyltransferase (CAT) activity (6). β-Galactosidase activity was expressed as the normalized response, which is the β-galactosidase activity divided by the luciferase activity. For the CAT assays, cell extracts containing the same amount of β-galactosidase activity were used. The CAT activity was quantitated by using a Molecular Dynamics Phosphorimager system with ImageQuant software.
DNA binding assays and determination of the apparent equilibrium dissociation constant.
The human RARβ and RXRβ proteins were expressed in the baculovirus system as previously described (10). Binding reactions were performed as described by Angulo et al (6) with recombinant RARβ and RXRβ and 0.2 to 0.8 ng of 32P-labeled annealed MDR2a or MDR2b oligonucleotide probe. Gels were imaged and quantitated by using a Molecular Dynamics PhosphorImager system with ImageQuant software. The apparent equilibrium dissociation constants (KDs) for the RXR-RAR-DNA complex for sites MDR2a and MDR2b were determined by equilibrium binding analyses. A series of standard binding reaction mixtures containing a constant amount of RXR and/or RAR were incubated with increasing concentrations of double-stranded MDR2a or MDR2b oligonucleotides. Bound and free DNAs were separated by electrophoretic mobility shift assay (EMSA), and the radioactivity in each fraction was quantitated. Data were plotted as 1/[bound DNA] versus 1/[free DNA]. The y intercept of such a plot is 1/[active protein]. The slope of this plot is the apparent KD divided by the concentration of active protein. In this analysis, the value of the apparent KD is only an estimate of the true KD, since the nonspecific binding of protein with the poly(dI-dC) present in each reaction mixture and the potential dissociation of the receptor-DNA complex in the gel are not taken into account.
In vitro infections.
NIH 3T3 cells (approximately 3 × 105 per well of six-well plates) were exposed to the different retinoids or the vehicle in DMEM supplemented with 3% charcoal resin-treated calf serum for 4 h and were infected with MCMV at the different multiplicities of infection (MOIs) indicated in the figure legends (ranging from 0.01 PFU per cell in the multistep growth curves to 10 PFU per cell for single-step growth curves). After a 1-h adsorption period, the virus inoculum was removed, the cultures were washed three times with phosphate-buffered saline, and fresh DMEM supplemented with 3% charcoal resin-treated serum containing the retinoids or the solvent alone was added. Every 24 h after infection, the cultures were fed with fresh medium containing the retinoids or the solvent. At different times after infection, the supernatants of three independent cultures were harvested, frozen, and thawed, and the infectious virus was quantitated by standard plaque assay on NIH 3T3 cells. Vaccinia virus and herpes simplex virus type 1 infections of NIH 3T3 cells were carried out in the same manner as described above for MCMV infections, using an MOI of 0.01 PFU per cell and DMEM supplemented with 3% charcoal resin-treated calf serum containing the retinoids. Infectious vaccinia virus from cellular extracts and cell-free herpes simplex virus type 1 in the cultures were quantitated by standard plaque assay on TK−143B and Vero cells, respectively.
Mouse treatments and in vivo infections.
Six-week-old female BALB/c.ByJ mice from the Scripps Research Institute breeding colony were used under specified pathogen-free conditions. Mice were treated by intragastric intubation with 200 μl of corn oil or the different retinoids (50 mg of ATRA per kg and/or 25 mg of AGN 193109 per kg) in corn oil 2 h prior to infection and on days 2, 4, 7, 9, 11, and 14 after infection. Animals were injected intraperitoneally with various amounts of salivary gland passage MCMV (Smith strain). When the viral load in spleens was analyzed, 4 days after inoculation, mice (five per group) were euthanized and their spleens were removed, weighed, and harvested as a 10% (wt/vol) tissue homogenate, and virus titers were determined on NIH 3T3 cells by standard plaque assay. To compare levels of lethality of MCMV in mice treated with the different retinoids and those treated with the solvent alone, infected animals were observed daily and monitored for death up to and including day 15 postinoculation. In a first set of experiments, the 50% lethal dose (LD50) was calculated by the method of Reed and Muench (42), using six mice per group and serial fivefold dilutions of MCMV (ranging from 5 × 103 to 1 × 106 PFU/mouse). In a second set of experiments (shown in Fig. 7), a logit regression curve, relating survival to log dose of virus, was fit to each group (three to six mice per group), and the LD50 was estimated for each group from the logit regression model (14). In this case, the virus dose ranged from 3 × 104 to 3 × 105 PFU/mouse in steps of 1 × 104 in the group of animals treated with ATRA, whereas in the group of animals treated with solvent alone, the virus dose ranged from 9 × 104 to 8 × 105 PFU/mouse, again in steps of 1 × 104. Vaccinia virus infections were carried out in a manner similar to that described above for the MCMV infections.
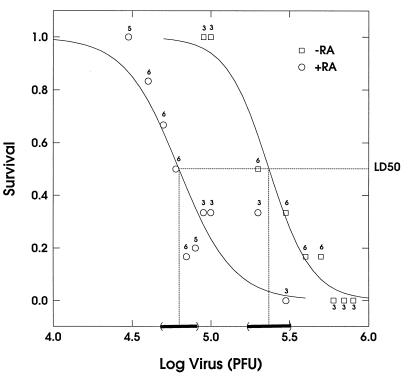
FIG. 7.
Levels of lethality of MCMV in mice treated with ATRA and those treated with solvent alone. BALB/c.ByJ mice were pretreated by intragastric intubation with vehicle (corn oil) (−RA) or 50 mg of ATRA per kg in vehicle (+RA) 2 h before intraperitoneal infection with different doses of MCMV (Smith strain). Treatment with vehicle or ATRA in vehicle was repeated on days 2, 4, 7, 9, 11, and 14 after infection. Mice were monitored daily for survival up to and including day 15. Logit regression curves relating survival to the log (to the base 10) dose of virus were fit to each group (deviance = 2.10 and P = 0.95 for the control treated mice; deviance = 6.80 and P = 0.56 for the mice treated with RA). The points indicate the observed survival at each dose, with the integers above the points denoting the numbers of animals tested at that dose. The LD50 for each group was estimated from the fitted logit regression line as 6.27 × 104 for the group of mice treated with RA and 2.34 × 105 for the control group. The bars along the x axis represent approximate 95% confidence intervals for the LD50s. A formal test of the equivalence of the LD50s for the two groups would be rejected at an alpha level of less than 0.001.
Histopathology.
Organs for histological sections were fixed in Bouin’s solution before being dehydrated and embedded in paraffin by standard procedures. Sections (7 μm thick) were cut with a rotary microtome and stained with hematoxylin and eosin for examination.
RESULTS
Positive regulation of the MCMV enhancer by RA.
As a first step in exploring the role of retinoids in modulating MCMV, we examined the ability of its MIEP-enhancer to be regulated by RA. To test this possibility, we used a recombinant plasmid (pON405) containing the murine MIEP-enhancer that regulates expression of the bacterial lacZ reporter enzyme, β-galactosidase. The reporter plasmid was initially tested for RA responsiveness after transient transfection into human embryonal teratocarcinoma NT-2/D1 cells. NT-2/D1 cells endogenously express unstimulated levels of retinoid receptors (RARs and RXRs) that can efficiently transactivate reporter plasmids containing high-affinity binding sites for RAR-RXR heterodimers (6, 44). In these experiments, ATRA selectively induced (6- to 10-fold) the expression from the MIEP-containing reporter construct in a concentration-dependent manner (Fig. 1A and B and data not shown). The murine MIEP responded to ATRA with a half-maximal response at ∼5 nM ATRA, suggesting the physiological significance of the observed RA induction (data not shown).
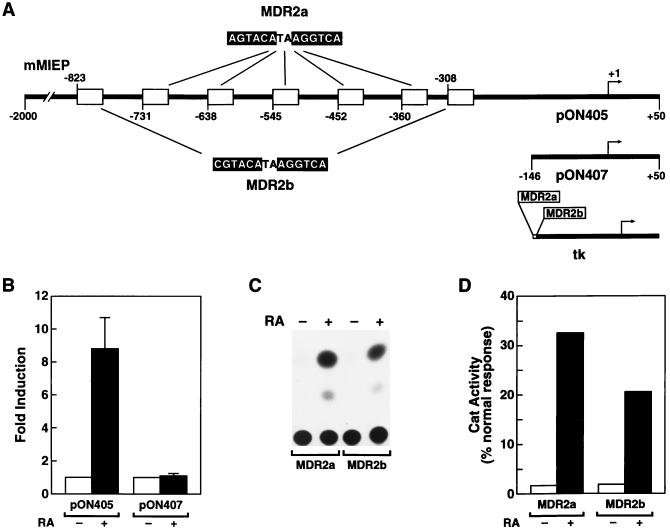
FIG. 1.
RAREs in the MCMV enhancer. (A) Schematic representation of the MCMV MIEP sequence from position −2000 to +50 present in the reporter construct pON405. The locations of the seven potential RAREs are marked by open boxes. The AGGTCA-related motifs of the two types of elements, named MDR2a and MDR2b, are indicated. The numbers refer to the nucleotide position relative to the transcription start site. A schematic of the MCMV MIEP deletion mutant present in the reporter construct pON407 in which sequences from position −146 to −2000 are abolished is shown below. One copy each of MDR2a and MDR2b was independently transferred to the heterologous promoter of the CAT gene expression vector tkCAT to generate the reporter plasmids MDR2a and MDR2b shown in the lower portion. (B) NT-2/D1 cells were transfected with 5 μg of either the pON405 or pON407 reporter plasmid and incubated for 36 h with 10−5 M ATRA (+) or the vehicle (−). Transfection efficiency was standardized by cotransfection of 5 μg of tkLuc. The fold induction of β-galactosidase activity was calculated for each construct by taking the activity in the absence of ATRA as 1. Data shown represent the means and standard deviations of triplicate determinations. (C) Five micrograms of the reporter plasmids MDR2a and MDR2b was cotransfected with 5 μg of the β-galactosidase pRSVβgal internal control expression vector into NT-2/D1 cells and then treated for 36 h with 10−5 M ATRA (lanes +) or with vehicle (lanes −). The figure shows the result of a representative assay for CAT enzyme activity from cell lysates. Similar results were obtained in three independent experiments. (D) The response of MDR2a and MDR2b to RA (b+) is plotted as the normalized activity observed in these experiments, calculated for each construct by taking the activity in the absence of ATRA (−) as 1.
To determine whether the selectivity of the ATRA induction is dependent on the enhancer sequences, we analyzed a deletion mutant of the MIEP reporter construct that has previously been shown to eliminate enhancer activity in the transient-expression assay (15). Truncation of the MIEP reporter construct at nucleotide position −146 abolished the response to ATRA (Fig. 1A and B), suggesting that enhancer sequences located upstream of position −146 mediate a stimulatory effect of ATRA on the murine MIEP. Inspection of this sequence region revealed 10 candidate direct repeat sequences that closely resemble RAREs, with their tandem repeats separated by 2 nucleotides. Previous studies have shown that a responsive element for ATRA is composed of tandem repeats of the canonical half-site AGGTCA, in which optimal receptor binding is determined by a spacer of 2 (DR2 element) or 5 (DR5 element) nucleotides between each half-site (12). Seven of these putative RAREs are located within the boundaries of the defined enhancer domain (15) and on the basis of sequence homology can be grouped into two types of elements, named MDR2a and MDR2b (a schematic representation of these sites is shown in Fig. 1A). In comparison with the consensus core motif (5′-A/GGT/GTCA-3′), MDR2a repeats show the best match, with 92% identity, while MDR2b elements have an 83% match to the consensus sequence. To investigate whether these elements could confer ATRA inducibility on an heterologous promoter, a single copy of MDR2a and MDR2b sequences was individually transferred to the herpes simplex virus thymidine kinase promoter, which is nonresponsive to ATRA. The transcriptional activities of these reporter constructs, in the absence and presence of ATRA, were investigated in the transient-transfection assay. Single copies of both MDR2a and MDR2b elements were able to confer ATRA inducibility to the herpes simplex virus thymidine kinase promoter (Fig. 1C and D). In comparison with the MDR2b element, the MDR2a element confers a stronger response to ATRA (Fig. 1C and D). These data provide evidence that the MCMV enhancer contains at least seven RAREs of the DR2 configuration and suggest that these elements might directly interact with RXR-RAR heterodimers.
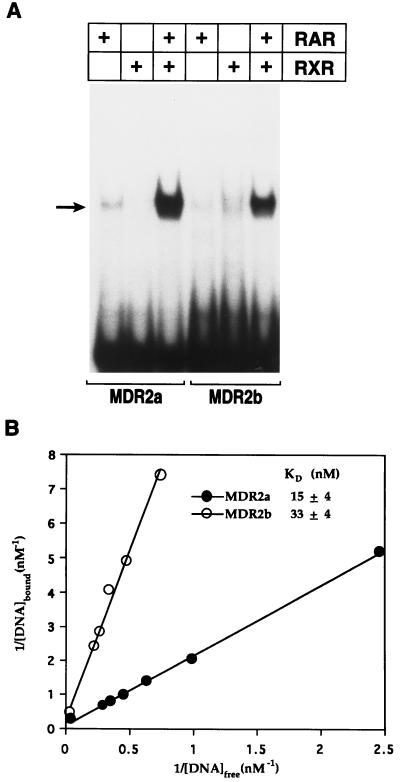
Accordingly, EMSAs with 32P-labeled DNA probes were used to demonstrate a direct interaction between the MDR2a and MDR2b elements and purified recombinant RAR and RXR proteins. Under the conditions used in these experiments, RAR and RXR alone weakly bind the DNA probes but a major DNA complex could be formed when the probes were incubated with both RAR and RXR (Fig. 2A), indicating that MDR2a and MDR2b bind RXR-RAR heterodimers more efficiently than either RXR or RAR homodimers. The heterodimeric complex could be specifically competed by unlabeled probe but not by a nonspecific oligonucleotide (data not shown). We next sought to investigate the binding affinity of the RAR-RXR heterodimers to the MDR2a and MDR2b sites by determining the apparent equilibrium dissociation constant (KD) of heterodimers to each site by using the EMSA system. The apparent KD measurements derived from double-reciprocal plots of RXR-RAR binding reactions were 15 and 33 nM for MDR2a and MDR2b, respectively (Fig. 2B). The binding constants for receptor heterodimers bound to these elements are within the physiological concentration range of these nuclear receptor proteins. In good agreement, the strengths of MDR2a- and MDR2b-mediated RA activation in cells (Fig. 1D) correlate with the affinity to which they bind RXR-RAR heterodimers (Fig. 2B). Taken together, these experiments provide evidence to support the conclusion that the viral enhancer binds RXR-RAR in vivo.
FIG. 2.
Binding of RAR-RXR heterodimers to the MCMV enhancer RAREs. (A) Baculovirus-derived preparations of RARβ or RXRβ were incubated either independently or in combination with 32P-labeled probes representing the MDR2a and MDR2b elements and analyzed in a gel mobility retardation assay. The arrow indicates the major specific nucleoprotein complex detected. (B) The apparent dissociation constants (KD) were determined by using double-reciprocal plots (see Materials and Methods for details). The figure shows the results of a representative experiment, and the apparent KD data represent average values from three separate determinations.
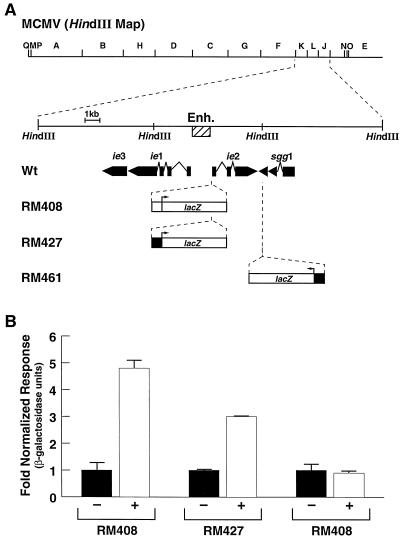
While the data presented above clearly show that RAR and RXR can regulate the viral enhancer, they do not address whether RA is able to exert control of the enhancer during an infection. To demonstrate that the enhancer can be regulated by RA in the context of an infection, we carried out infection experiments with MCMV recombinants containing an insertion of a lacZ reporter gene under the control of either the HCMV or MCMV MIEP. The segment of the HCMV MIEP (nucleotides −219 to −19) present in RM461 and RM427 recombinant viruses and the segment of the MCMV MIEP (nucleotides −146 to +50) present in RM408 recombinant virus have been shown to be nonresponsive to ATRA and 9-cis-RA (6) (Fig. 1A and B). In the recombinants RM408 and RM427, the lacZ reporter gene is positioned in the immediate vicinity of the endogenous MCMV enhancer, and its expression has been shown to be controlled by the enhancer (35) (Fig. 3A). By contrast, in the recombinant RM461 virus, the lacZ reporter is positioned in a locus outside the influence of the MCMV enhancer (48) (Fig. 3A). Thus, these recombinants contain lacZ inserts in which their different positions in the genome determine their differential responsiveness to regulation by the enhancer. If the endogenous MCMV enhancer is responsive to RA, reporter gene activity would be enhanced by RA in RM408- and RM427-infected cells but not in RM461-infected cells. In the experiment whose results are shown in Fig. 3B, murine NIH 3T3 fibroblasts were infected with either RM408, RM427, or RM461 and were exposed to 9-cis-RA or the vehicle alone, and the amount of β-galactosidase activity present in each infection was quantitated. As predicted, infection of cells with RM408 and RM427 showed increased (three- to fivefold) levels of reporter gene activity upon exposure to 9-cis-RA, while the reporter gene activity in RM461-infected cells remained unchanged (Fig. 3B). These results indicate that RA can stimulate the enhancer activity of MCMV in the context of an infection.
FIG. 3.
Activation of the MCMV enhancer by RA in the context of the infection. (A) A schematic representation of the HindIII map of the MCMV genome is shown on the top line. The HindIII-J, -K, and -L fragments have been expanded to show the region containing the MIE genes (ie1, ie2, and ie3) and the sgg1 gene. The hatched box depicts the murine MIEP-enhancer (Enh.). The recombinant viruses RM408, RM427, and RM461, which carry an insertion of the lacZ reporter gene, are shown in the lower portion. The position and orientation of the insertion for each virus are shown. The black box depicts the HCMV promoter-enhancer sequences from position −219 to −19. The empty box depicts position −146 to +50 from the MCMV promoter-enhancer. Wt, wild type. (B) NIH 3T3 cells were exposed to 9-cis-RA (10−5 M) (+) or the vehicle (−), infected with MCMV recombinant RM408, RM427, or RM461 at an MOI of 0.1 PFU/cell, and assayed 48 h later for β-galactosidase activity. Shown is the normalized β-galactosidase activity observed in these experiments, calculated in each case by taking the activity in the absence of RA as 1. Error bars indicate standard deviations.
RA enhances the growth of MCMV in tissue culture.
We next evaluated the potential role of RA in influencing viral growth. For this purpose, NIH 3T3 cells were infected with MCMV at an MOI of 0.01 in the presence and absence of 9-cis-RA, and the amount of infectious virus produced was determined by plaque assay at different times postinfection. As shown in Fig. 4A, the rate of growth exhibited by MCMV increased markedly in the presence of 9-cis-RA or ATRA (see also Fig. 5B, and data not shown). The magnitude of this response varied from a 20- to 100-fold enhancement in growth yield. The growth response of MCMV to 9-cis-RA is selective, as growth of vaccinia virus and herpes simplex virus (two viruses for which functional RAREs in their genomes have not been described) in NIH 3T3 cells is unaffected by treatment with exogenous 9-cis-RA (Fig. 4C and D).
FIG. 5.
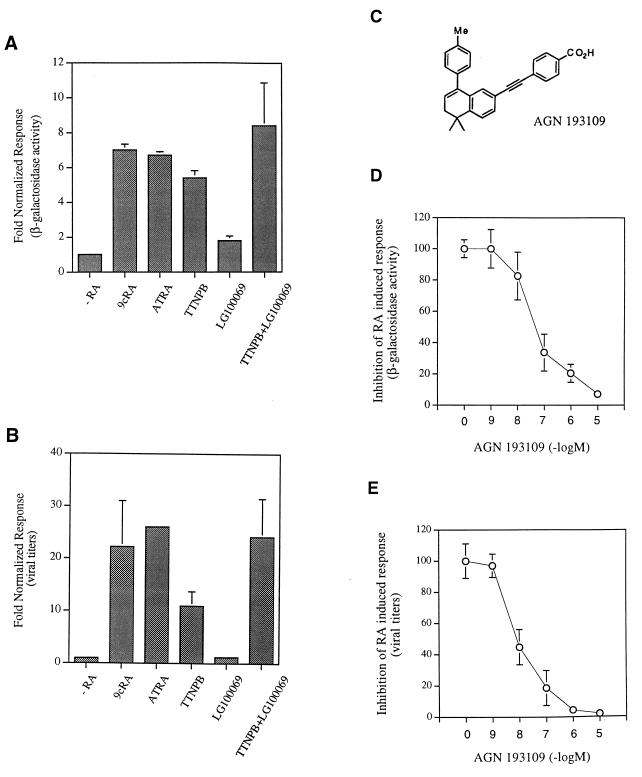
RAR activation in cells is required for mediating the induction of MCMV by RA. (A) NT-2/D1 cells were transfected with 5 μg of pON405 and incubated for 36 h with the vehicle (−RA), 9-cis-RA (9cRA) at 10−5 M, ATRA at 10−5 M, TTNPB at 10−7 M, LG100069 at 10−7 M, or a combination of TTNPB and LG100069 at 10−7 M each. Transfection efficiency was standardized by cotransfection of 5 μg of tkLuc. The response of pON405 to the different retinoids is plotted as the fold induction of β-galactosidase activity observed in these experiments, calculated for each construct by taking the activity in the absence of RA as 1. Each bar represents the average and standard deviation of triplicate determinations. (B) NIH 3T3 cells were exposed to 9-cis-RA at 10−5 M, ATRA at 10−5 M, TTNPB at 10−7 M, LG100069 at 10−7 M, a combination of TTNPB and LG100069 at 10−7 M each, or the vehicle (−RA) for 4 h. Subsequently, the cells were infected with MCMV (RM461) at an MOI of 0.01 PFU/cell and reexposed to the different retinoids or the vehicle (−RA). On day 5 after infection, the presence of extracellular virus in the cultures was determined. The fold enhancement of MCMV replication was calculated in each case by taking the amount of virus in the absence of RA as 1. Each bar represents the average and standard deviation for three separate cultures. (C) Structure of the RAR antagonist. The 50% inhibitory concentrations of AGN 193109 are 9 (±1) nM for RARα, 7 (±3) nM for RARβ, and 5 (±1) nM for RARγ. The 50% inhibitory concentrations were determined by performing transfection assays on CV-1 cells with a reporter construct, MTV-4(R5G)-LUC, containing four copies of the DR-5 RARE R5G and expression plasmids for either RARα, -β, or -γ and using a 10−8 M dose of ATRA (25). (D) NT-2/D1 cells were cotransfected with 5 μg of pON405 and incubated for 36 h with the vehicle or 10−6 M 9-cis-RA in the presence or absence of the indicated increasing concentrations of the retinoid antagonist AGN 193109 or the vehicle. Transfection efficiency was standardized by cotransfection of 5 μg of tkLuc. β-Galactosidase activity is expressed as the normalized response, which is the β-galactosidase activity divided by the luciferase activity. Activation obtained with 9-cis-RA treatment alone was considered 100%. The data presented are representative of results of triplicate experiments. (E) NIH 3T3 cells were exposed to the vehicle or 9-cis-RA at 5 × 10−5 M in the presence or absence of the indicated concentrations of the retinoid antagonist AGN 193109 or the vehicle. Subsequently, cells were infected with MCMV (RM461) at an MOI of 0.01 PFU/cell and reexposed to the different retinoids. On day 5 after infection, the presence of extracellular virus was determined. The amount of virus obtained with 9-cis-RA treatment alone was considered 100%. Each data point represents the average and standard deviation for three separate cultures.
In the type of growth curve examined as described above, the yield of virus at the various harvesting points reflects a cumulative rate of growth from multiple rounds of replication and infection in the culture. To determine the level of growth rate enhancement in a single round of infection, a one-step growth analysis of MCMV in the presence and absence of exogenously added 9-cis-RA was performed. In this experiment, the cultures were infected with an MOI of 10 to ensure that 100% of the cells were infected. Figure 4B shows that in a single-step growth analysis, 9-cis-RA treatment results in an approximately eightfold enhancement in growth of MCMV. The greater RA-induced enhancement of MCMV growth at low MOI than at high MOI is most likely attributable to a cumulative effect from multiple rounds of replication in the multistep growth curve. However, it is possible that an IE protein could be required for viral growth at low MOI but may play less of a role at high MOI, as has been suggested for HCMV (38). A similar fold enhancement in viral multiplication at high MOI is also observed upon administration of ATRA (data not shown). In this regard, it is noteworthy that while ATRA binds only RARs, in living cells it can be converted to 9-cis-RA, which binds and activates both RARs and RXRs. Taken together, these results are consistent with the suggestion that retinoid-induced MCMV multiplication is mediated by RARs and/or RXRs.
Response of MCMV to RAR-specific and RXR-specific ligands.
We next sought to explore whether an RAR or an RXR agonist is sufficient to mediate the increased growth and enhancer activation. For these experiments we used the metabolically stable RA analogs TTNPB and LG100069, which are known to be highly selective and potent ligands for the RAR and RXR families, respectively (10, 28). These synthetic retinoids eliminate complications resulting from interconversion and cross-receptor binding encountered with the natural retinoids. Accordingly, we analyzed the ability of these synthetic retinoids to activate reporter constructs containing the viral enhancer in transfection assays. Figure 5A shows that enhancer activity could be stimulated by 9-cis-RA (sevenfold), ATRA (sevenfold), and TTNPB (sixfold) but not LG100069 (<twofold), indicating the requirement for RAR ligand activation functions to mediate transcriptional enhancement. This result is consistent with our previous studies showing that the RXR partner of an RXR-RAR heterodimer bound to the HCMV enhancer RAREs is unable to activate transcription on its own (3, 6). To investigate whether RXR can contribute to enhancer activation, the RAR- and RXR-specific ligands were simultaneously added, and the resulting transcriptional activation was compared with that obtained with TTNPB alone. Figure 5A shows that a small enhancement in the efficacy of TTNPB activation was elicited in the presence of LG100069. These results suggest that RXR activation can play a role in modulating RA activation of the enhancer, although the response appears to be highly dependent on RAR transactivation functions.
To determine whether activation of RAR or RXR also plays a differential role in regulating MCMV growth, we evaluated viral growth in the presence of the various synthetic ligands. In these experiments, NIH 3T3 cells were infected with MCMV at an MOI of 0.01 in the presence of the different synthetic retinoids. Figure 5B shows that MCMV growth could be stimulated by TTNPB (11-fold) but not LG100069 (less than 2-fold), demonstrating the inability of RXR to exclusively contribute to ligand activation and indicating that an RAR-induced pathway is sufficient for mediating induction of MCMV growth. When TTNPB and LG100069 were added together, a higher level of MCMV growth (22-fold) was induced than with TTNPB alone (Fig. 5B). The combination of TTNPB and LG100069 induced viral growth to levels comparable to those found for 9-cis-RA (21-fold) or ATRA (25-fold), thus suggesting that RXR activation plays a role in the presence of a transcriptionally active RAR. These results lead us to propose that the RAR pathway plays the predominant role in promoting viral growth by RA.
An RAR-selective antagonist can block the response of MCMV to RA.
To establish that activation of RAR by RA leads to an increase in enhancer activity and viral growth, we next tested whether a synthetic antagonist selective for RAR is able to suppress the stimulatory effects of RA. A number of retinoid antagonists have been described and shown to specifically block RAR activation by exogenous agonists in vitro (2, 7, 16, 17, 23, 31, 45–47, 52). One of these compounds, AGN 193109 (Fig. 5C), which binds all three RAR subtypes with affinities in the low-nanomolar range, has been shown to be a potent inhibitor of RA activation (23). Recent studies with this compound have shown that it can not only antagonize RA activation as a competitive inhibitor (neutral antagonist) but also inhibit the basal gene transcriptional activity of unliganded RAR and hence function as an inverse agonist (25). When increasing amounts of the RAR antagonist were added to a fixed concentration of 9-cis-RA, the degree of transcriptional activation from the MCMV enhancer was inhibited at a molar ratio of antagonist to agonist of 1:1 (Fig. 5D). Thus, AGN 193109 is an effective antagonist of a ligand (9-cis-RA) that interacts with high affinity with both RARs and RXRs which bind the MCMV enhancer. AGN 193109 does not bind RXRs and thus is unlikely to prevent 9-cis-RA from directly interacting with RXRs. Note that as shown above, RXR ligands alone are relatively inactive in stimulating the multiple RXR-RAR heterodimers bound to the enhancer (Fig. 5A). Since the antagonist (AGN 193109) binds with high affinity to RARs without any agonist activity, AGN 193109 likely inhibits the 9-cis-RA effect of the RAR partner of the heterodimer. We therefore conclude that ligand activation of RAR is required for mediating the transcriptional activation of the enhancer by retinoids.
Since the growth rate of MCMV is affected by retinoids, we also investigated whether activation of RAR is also necessary for mediating the enhanced growth response. In these experiments, NIH 3T3 cells were infected with MCMV at an MOI of 0.01 and treated with a fixed concentration of 9-cis-RA in the presence of increasing concentrations of AGN193109. Figure 5E clearly shows that AGN 193109 can effectively antagonize the stimulatory effect of 9-cis-RA in a dose-responsive manner where maximal antagonism is achieved at equimolar concentrations with 9-cis-RA. As expected, AGN 193109 also effectively antagonized the ATRA-induced growth of MCMV (data not shown). In the absence of exogenously added ATRA or 9-cis-RA, AGN 193109 did not result in any marked inhibition of MCMV growth (data not shown), suggesting that AGN 193109 functions as a neutral antagonist under these conditions of cell culture and infection. Taking the data together, we conclude that RAR activation in cells is required for mediating the effects of exogenous RA on MCMV.
Treatment of MCMV-infected animals with exogenous RA selectively leads to more disease and increased susceptibility to lethal infection.
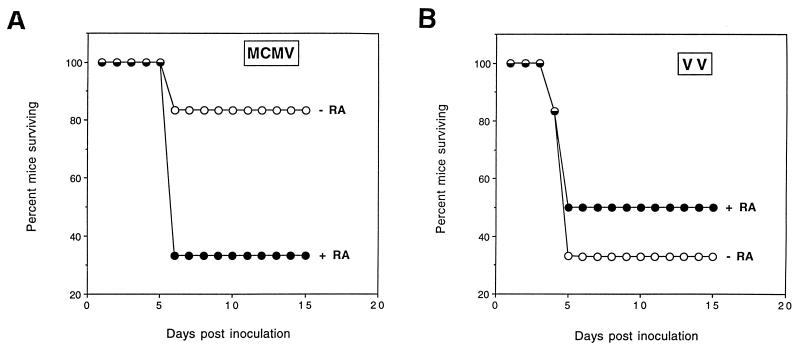
The pharmacokinetic properties of natural retinoids have been the subject of several studies. For the BALB/c mouse it has been shown that following a single initial oral administration of ATRA, the plasma peak time of this retinoid occurs between 1 and 3 h (1). When frequent doses of ATRA are administered after the first dose, ATRA plasma levels are reduced, indicating that intermittent dosing of this retinoid, instead of a daily dosing, may better maintain long-term plasma ATRA levels. On the basis of these observations, we chose an administration regimen in which animals are exposed to an initial dose of ATRA at 2 h prior to infection and to subsequent doses every other day after infection. In these experiments, then, infected animals were exposed to ATRA for short pulses of time. In control experiments, uninfected animals administered this level of ATRA on alternate days all survived the treatment period and showed no overt signs of illness (data not shown). Accordingly, as a first step to assess whether exogenous ATRA treatment alters the susceptibility to infection, 6-week-old BALB/c mice were inoculated with 105 PFU of MCMV and every other day were orally administered 50 mg of ATRA per kg in corn oil or given corn oil alone. Under these conditions of infection, 83% of the control mice survived the infection, whereas only 33% of the ATRA-treated animals survived (Fig. 6A). To evaluate whether this effect was specific to a pathogenic MCMV infection, we examined the survival of ATRA-treated and untreated mice inoculated with 2 × 107 PFU of vaccinia virus. (Vaccinia virus is not influenced by RA in tissue culture [Fig. 4C]). Figure 6B shows that 33 and 50% of the untreated and ATRA-treated animals, respectively, survived vaccinia virus infection. These results suggest that oral administration of ATRA can selectively increase the susceptibility to a lethal MCMV infection.
FIG. 6.
Oral administration of ATRA selectively increases the susceptibility of mice to MCMV infection. BALB/c.ByJ mice (six per group) were pretreated by intragastric intubation with vehicle (corn oil) (−RA) or 50 mg of ATRA per kg in vehicle (+RA) 2 h before intraperitoneal infection with 1 × 105 PFU of MCMV (Smith strain) (A) or 2 × 107 PFU of vaccinia virus (VV) (B). Treatment with vehicle or ATRA in vehicle was repeated on days 2, 4, 7, 9, 11, and 14 after infection. Mice were monitored daily for survival up to and including day 15. Survival curves were statistically analyzed by the log rank test and showed that RA treatment significantly influenced the rate of mortality from MCMV (P value of 0.01) but not from vaccinia virus (P value of 0.64).
To quantify what influence ATRA has on the outcome of an MCMV infection, the LD50 was experimentally calculated. In these experiments the LD50 was determined by the method of Reed and Muench (42) (see Materials and Methods for details), using six mice per group with serial fivefold dilutions. When titrated in control treated mice, 2 × 105 PFU of MCMV was equivalent to one LD50, but when mice were treated with ATRA during infection, only 6 × 104 PFU was equivalent to one LD50. Furthermore, in an additional experiment using a different stock of virus and serial onefold viral dilutions, identical results were obtained (Fig. 7). In this experiment, the calculated LD50 was 2.34 × 105 for control treated mice and 6.27 × 104 for mice exposed to ATRA (Fig. 7). Thus, ATRA treatment of MCMV-infected mice appears to mildly exacerbate an acute infection. To test whether the effects of ATRA on the acute MCMV infection are mediated by activation of RAR, AGN 193109 was coadministered with ATRA. In these experiments, mice (six mice per group) were inoculated with serial fivefold dilutions of virus, and each group was cotreated with 50 and 25 mg of ATRA and RAR antagonist per kg, respectively. The determined LD50 indicates that whereas the dose of MCMV required to cause 50% mortality in mice treated with ATRA was 6 × 104 PFU, the LD50 in mice cotreated with ATRA and RAR antagonist was 5 × 105. These results show that an RAR antagonist can block the ATRA-induced effects in an acute infection, providing direct evidence that these adverse effects are mediated by RARs. When AGN 193109 was administered to MCMV-infected mice, in the absence of exogenous ATRA, the determined LD50 did not significantly increase in comparison with that for control infected mice (data not shown). Together, these results lead us to conclude that ligand activation of RARs can play a role in modulating an MCMV infection in vivo.
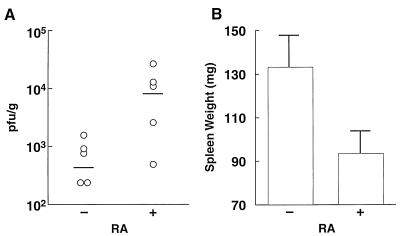
To determine whether the ATRA-treated mice undergo a more severe infection due to elevated levels of virus, the amount of infectious virus produced in the spleens of ATRA-treated or control treated animals was determined. In these experiments, control treated mice or those administered ATRA were infected with 2 × 103 PFU of MCMV and sacrificed at 4 days postinoculation. In correlation with the ATRA-enhanced mortality, a trend of higher virus titers was observed in the spleens of ATRA-treated animals in comparison with control infected mice (Fig. 8A). In additional control experiments, lymphocyte choriomeningitis virus-infected mice treated with ATRA did not show any difference in viral spleen titers in comparison with control infected mice (data not shown), further indicating the selective effect of ATRA on MCMV infection.
FIG. 8.
Oral administration of ATRA increases the severity of an MCMV infection in the spleens of infected mice. BALB/c.ByJ mice (five per group) were pretreated by intragastric intubation with vehicle (−RA) or 50 mg of ATRA per kg (+RA) 2 h before intraperitoneal infection of 5 × 103 PFU of MCMV (Smith strain). Treatment with vehicle or ATRA was repeated on day 2 after infection. All mice were sacrificed on day 4 after infection for determination of virus titers (A) and spleen weight (B). There was a trend in higher viral titers in the spleens of the ATRA-treated animals in comparison with control treated mice (P value of ∼0.05) as analyzed by the Mann-Whitney test (two tailed). Spleen weights were also significantly different (P < 0.05) as determined by Student’s t test (two-tailed). Error bars indicate standard deviations.
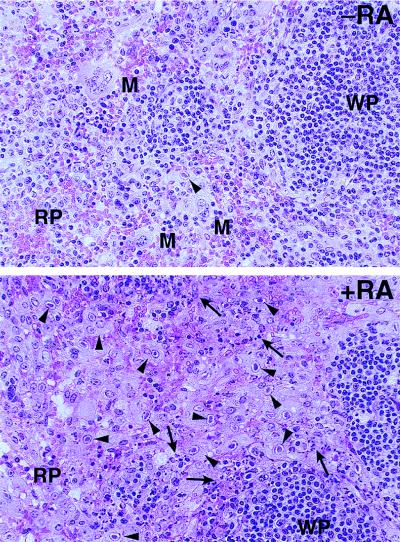
Pathological differences in the spleens of MCMV-infected mice treated with ATRA were also noted. It has been previously reported that the severity of necrosis of the spleen observed in BALB/c mice infected with MCMV correlates with high levels of viral replication (37). In this mouse strain, a hallmark of the severe necrosis inflicted by MCMV is the marked reduction in splenic weight. Spleens from control MCMV-infected mice were significantly (P < 0.05) larger than those of the ATRA-treated MCMV-infected mice (Fig. 8B). The increased sizes of spleens of the control treated animals is due to a follicular lymphoid hyperplasia characteristic of a mildly acute CMV infection (Fig. 9, top). In these spleens, there is a paucity of readily identifiable cytomegalic cells (a characteristic of a CMV-infected cell), and the red pulp has well-defined hematopoietic erythroid and myeloid tissue and shows no overt signs of necrosis (Fig. 9, top). By contrast, the smaller spleens of the ATRA-treated animals are associated with a marked absence of extramedullary hematopoietic tissue and exhibit a high density of cytomegalic cells in the red pulp, indicating an extensive infection of macrophages (Fig. 9, bottom). In these spleens, multiple sites of necrosis were readily apparent and appeared to be restricted to areas outside the lymphoid cell population in tissue closely associated with cytomegalic cells in the red pulp (Fig. 9, bottom). The pathologic features manifested by these necrotic spleens are a hallmark of a severe infection by MCMV. It is noteworthy that ATRA-treated uninfected mice have spleens larger than those of untreated control mice due to increased hematopoiesis in the spleen (32). These results show that ATRA-treated MCMV-infected animals exhibit greater virus-induced spleen damage than do untreated mice. We conclude from these experiments that treatment of infected animals with exogenous ATRA during the course of MCMV infection selectively leads to more disease and increased susceptibility to infection.
FIG. 9.
Oral administration of ATRA enhances MCMV damage in the spleens of infected mice. Splenic sections from BALB/c.ByJ mice treated by intragastric intubation with vehicle (−RA) or with 50 mg of ATRA per kg (+RA) and infected with 5 × 103 PFU of MCMV for 4 days are shown. Magnification, ×100. Some of the cytomegalic cells present are indicated by arrowheads, and the areas of necrosis are indicated by arrows. The presence of selected hematopoietic cells, such as megakaryocytes (M), is shown. The red pulp (RP) and white pulp (WP) are indicated.
DISCUSSION
The impact of molecular signals that engage and cointegrate specific intracellular signalling pathways with an infectious program of a virus is not well understood. We have addressed this issue by investigating the susceptibility of a member of the CMV family of viruses, MCMV, to retinoids in tissue culture and in an experimental animal model. Our report describes the ability of a ligand-activated RAR pathway to modulate an acute viral infection. We further demonstrate that an RAR antagonist can efficiently inhibit an RA-induced CMV infection in vitro and in vivo.
RA modulation of MCMV in vitro.
The activation of the RAR pathway by exogenous synthetic or natural RA analogs leads to a dramatic stimulation of MCMV growth in NIH 3T3 cells. This response appears to be selective for MCMV, as the growth of vaccinia virus or herpes simplex virus in NIH 3T3 cells is unaffected by RA administration. Vaccinia virus replicates in the cytoplasm and is thus not expected to be influenced by the action of nuclear hormone receptors. By contrast, replication of herpes simplex virus takes place in the nucleus; however, to date functional RAREs in its genome have not been described. Most importantly, our study shows that MCMV growth enhancement by RA can be completely inhibited by cotreatment with an RAR antagonist, demonstrating that activation of RAR is essential for mediating this response. We also found that RA induced the MCMV enhancer and that this activation could also be inhibited by an RAR antagonist. The detailed molecular mechanism by which RAR promotes the growth of MCMV remains to be determined. One possibility is that ligand-activated RARs initiate a cascade of gene expression by directly stimulating key regulatory genes of the virus. For instance, the observation that the enhancer of MCMV contains at least seven RAREs of the DR2 configuration is consistent with this possibility. Alternatively, RARs may activate host-encoded genes that subsequently play a role in promoting viral expression. Yet another possibility is that RAR indirectly promotes viral expression and replication by altering coregulatory molecules or perhaps the organization of virus-associated chromatin. While it is conceivable that any or all of these possibilities coordinate the growth enhancement of MCMV, all have in common a single transduction mediator, RAR. In principle, then, the activation or inhibition of the RAR pathway now provides a molecular basis for potentially modulating a CMV infection.
RA modulation of MCMV in vivo.
The MCMV model system enabled us to investigate contributions of exogenous RA to infected animals. This scenerio was tested in healthy adult mice challenged with an MCMV infection. In these experiments ATRA was orally administered on alternate days to infected animals during the course of the infection. In this dosage regimen, the infected animals are exposed to transient levels of exogenous ATRA lasting ∼1 to 3 h in the first dose, while in the subsequent doses ATRA persists with progressively shorter intervals due to homeostatic control in maintaining physiological levels of the natural retinoid. On the basis of several criteria, including determination of the amount of virus required to cause death, pathological changes in the spleen, and amount of infectious virus, we conclude that exogenous ATRA can exacerbate an MCMV infection. While the magnitudes of these various effects were not dramatic, they were significant. This is perhaps not an unexpected outcome, due to the poor pharmacokinetics of ATRA and the full immune competence of the animals. Indeed, the primary control of viral growth at these times of infection involves both the innate and specific immune responses (27). In this connection it is worth noting that immune molecules (such as interferon) that are known to play a major role in controlling a viral infection in vivo when administered systemically only moderately influence the outcome of an infection. We cannot exclude the possibility that the effects of exogenous ATRA on the MCMV infection in vivo are due to an unrelated mechanism such as alteration of immune effector functions. However, this possibility seems unlikely, since other pathogenic viruses such as lymphocyte choriomeningitis virus and vaccinia virus are not affected by exogenous ATRA. In addition, the effects we observe in vivo are consistent with the ability of ATRA to augment an MCMV infection in vitro. Importantly, by blocking the action of RA with an antagonist, we show that activation of RAR in vivo is necessary for mediating the response of MCMV to RA.
Conservation of RA responses between MCMV and HCMV.
The CMV family is highly species specific and is believed to have evolved by ancient cospeciation with the respective hosts. The time of divergence of MCMV and HCMV has been estimated to be around 83 million years ago (36). Thus, conservation of sequence or function is likely to signify biological significance. As highlighted in the introduction, HCMV is known to have its growth rate influenced equally by ATRA and 9-cis-RA, and it contains within its enhancer multiple RAREs. In the case of the HCMV enhancer there are fewer elements (one DR2 and two DR5s), which bind RXR-RAR heterodimers with affinities of 5, 10, and 20 nM (6). Interestingly, the MCMV and HCMV enhancers appear to respond to RA to the same level of activation. This similar response may be explained by their affinity for binding the receptors. Thus, while the HCMV enhancer has fewer RAREs than the MCMV enhancer, the elements bind more tightly to the receptors. Studies with HCMV indicate that the action of RA is relatively complex, involving multiple modes of action (20). Similarly, for MCMV the molecular and cellular basis of the response to RA is likely to be multifactorial, involving both direct and indirect effects. While further studies are required to determine the precise mechanism by which RA exerts its effects, it is clear that the analyses described here reveal a conservation in the responses of MCMV and HCMV to RA.
Practical implications.
The increasing use of high doses of retinoids in chemotherapy and the future availability of even more potent synthetic retinoids may make CMV infections a potential complication of retinoid therapy with RAR agonists. At present it is not known whether high doses of retinoids can lead to clinical CMV infections, although to our knowledge the high-risk immunosuppressed patients have not yet been given retinoid therapy. For MCMV, the magnitudes of the in vivo effects were significant but much lower than those observed in tissue culture. Thus, the ability of exogenous treatment with retinoids to affect humans infected with CMV may be measurable but similarly low. In the future, if retinoids prove to be a risk factor for virus infection in the clinical setting, then retinoid antagonists could have significant therapeutic value. Again, experiments with the MCMV animal model system show that cotreatment of RA-treated animals with an RAR antagonist can protect against an exacerbated infection. These results also raise the possibility that RAR antagonists might have practical potential for viruses other than CMV.
In conclusion, ligand activation of a nuclear receptor signalling pathway, the RAR pathway, can alter the outcome of in vitro and in vivo infections by MCMV. Considering that a number of viruses (e.g., hepatitis B virus, mouse mammary tumor virus, simian virus 40, and human polyomavirus BK) contain functional binding sites that enable hormonal control of expression (18, 20, 39, 41, 43, 53, 54), it seems likely that regulation of an infectious program by signalling molecules and intracellular receptors may be more widespread. If so, this adaptation may represent an important principle in viral growth control mechanisms outside the immune system.
ACKNOWLEDGMENTS
We thank Ed Mocarski for providing plasmids and recombinant viral strains and Rich Heyman for the RXR-specific agonists. We also thank Ann Campbell for the MCMV Smith strain, advice in establishing the animal model system, and many helpful discussions. We thank Kent Osborn for expert help in the pathological assessment of infected mice and Jim Koziol for help in statistical analyses of data. We thank Persephone Borrow for help with the lymphocyte choriomeningitis infections and many stimulating discussions and Michael Oldstone, Frank Chisari, Juan Carlos de La Torre, Martin Messerle, and Lindsay Whitton for comments. We are grateful to K. Zap for assistance in the preparation of the manuscript.
This work was supported by grants from the National Institutes of Health to P.G. (CA66167). P.G. is a Scholar of the Leukemia Society of America. A.A. was a fellow from the Ministerio de Educación y Ciencia (Spain).
Footnotes
Publication no. 10753-IMM of The Scripps Research Institute.
REFERENCES
- 1.Achkar C C, Bentel J M, Boylan J F, Scher H I, Gudas L J, Miller W H J. Differences in the pharmacokinetic properties of orally administered all-trans-retinoic acid and 9-cis-retinoic acid in the plasma of nude mice. Drug Metab Dispos. 1994;22:451–458. [PubMed] [Google Scholar]
- 2.Agarwal C, Chandraratna R A S, Johnson A T, Rorke E A, Eckert R L. AGN 193109 is a highly effective antagonist of retinoid action in human ectocervical epithelial cells. J Biol Chem. 1996;271:12209–12212. doi: 10.1074/jbc.271.21.12209. [DOI] [PubMed] [Google Scholar]
- 3.Angulo A, Suto C, Boehm M F, Heyman R A, Ghazal P. Retinoid activation of retinoic acid receptors but not of retinoid X receptors promotes cellular differentiation and replication of human cytomegalovirus in embryonal cells. J Virol. 1995;69:3831–3837. doi: 10.1128/jvi.69.6.3831-3837.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Angulo A, Ghazal P. Regulation of human cytomegalovirus by retinoic acid. Scand J Infect Dis. 1995;99:113–115. [PubMed] [Google Scholar]
- 5.Angulo, A., and P. Ghazal. Unpublished data.
- 6.Angulo A, Suto C, Heyman R A, Ghazal P. Characterization of the sequences of the human cytomegalovirus enhancer that mediate differential regulation by natural and synthetic retinoids. Mol Endocrinol. 1996;10:781–793. doi: 10.1210/mend.10.7.8813719. [DOI] [PubMed] [Google Scholar]
- 7.Apfel C, Bauer F, Crettaz M, Forni L, Kamber M, Kaufmann F, LeMotte P, Pirson W, Klaus M. A retinoic acid receptor alpha antagonist selectively counteracts retinoic acid effects. Proc Natl Acad Sci USA. 1992;89:7129–7133. doi: 10.1073/pnas.89.15.7129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Baskar J F, Smith P P, Ciment G S, Hoffman S, Tucker C, Tenney D J, Colberg-Poley A M, Nelson J A, Ghazal P. Developmental analysis of the cytomegalovirus enhancer in transgenic animals. J Virol. 1996;70:3215–3226. doi: 10.1128/jvi.70.5.3215-3226.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Baskar J F, Smith P P, Gajanan N, Jupp R A, Hoffmann S, Peffer N J, Tenney D J, Colberg-Poley A M, Ghazal P, Nelson J A. The enhancer domain of the human cytomegalovirus major immediate-early promoter determines cell type-specific expression in transgenic mice. J Virol. 1996;70:3207–3214. doi: 10.1128/jvi.70.5.3207-3214.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Boehm M F, McClurg M R, Pathirana C, Mangelsdorf D, White S K, Hebert J, Winn D, Goldman M E, Heyman R A. Synthesis of high specific activity [3H]-9-cis-retinoic acid and its application for identifying unusual binding properties. J Med Chem. 1994;37:408–414. doi: 10.1021/jm00029a013. [DOI] [PubMed] [Google Scholar]
- 11.Cardin R D, Abenes G B, Stoddart C A, Mocarski E S. Murine cytomegalovirus IE2, an activator of gene expression, is dispensable for growth and latency in mice. Virology. 1995;209:236–241. doi: 10.1006/viro.1995.1249. [DOI] [PubMed] [Google Scholar]
- 12.Chambon P. A decade of molecular biology of retinoic acid receptors. FASEB J. 1996;10:940–954. [PubMed] [Google Scholar]
- 13.Chen J D, Evans R M. A transcriptional co-repressor that interacts with nuclear hormone receptors. Nature. 1995;377:454–457. doi: 10.1038/377454a0. [DOI] [PubMed] [Google Scholar]
- 14.Cox D R, Snell E J. Analysis of binary data. 2nd ed. London, United Kingdom: Chapman and Hall; 1989. [Google Scholar]
- 15.Dorsch-Hasler K, Keil G M, Weber F, Jasin M, Schaffner W, Koszinowski U H. A long and complex enhancer activates transcription of the gene coding for the highly abundant immediate early mRNA in murine cytomegalovirus. Proc Natl Acad Sci USA. 1985;82:8325–8329. doi: 10.1073/pnas.82.24.8325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Eckhardt K, Schmitt G. A retinoic acid receptor α antagonist counteracts retinoid teratogenicity in vitro and reduces incidence and/or severity of malformations in vivo. Toxicol Lett. 1994;70:299–308. doi: 10.1016/0378-4274(94)90124-4. [DOI] [PubMed] [Google Scholar]
- 17.Eyrolles L, Kagechika H, Kawachi E, Fukasawa H, Lijima T, Matsushima Y, Hashimoto Y, Shudo K. Retinobenzoic acids. 6. Retinoid antagonists with a heterocyclic ring. J Med Chem. 1994;37:1508–1517. doi: 10.1021/jm00036a017. [DOI] [PubMed] [Google Scholar]
- 18.Garcia A D, Ostapchuk P, Hearing P. Functional interaction of nuclear factors EF-C, HNF-4, and RXRα with hepatitis B virus enhancer I. J Virol. 1993;67:3940–3950. doi: 10.1128/jvi.67.7.3940-3950.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ghazal P, DeMattei C, Giulietti E, Kliewer S A, Umesono K, Evans R M. Retinoic acid receptors initiate induction of the cytomegalovirus enhancer in embryonal cells. Proc Natl Acad Sci USA. 1992;89:7630–7634. doi: 10.1073/pnas.89.16.7630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ghazal P, LeBlanc J, Angulo A. Vitamin A regulation of viral growth. Rev Med Virol. 1997;7:21–34. doi: 10.1002/(sici)1099-1654(199704)7:1<21::aid-rmv179>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- 21.Gonczol E, Andrews P W, Plotkin S A. Cytomegalovirus replicates in differentiated but not undifferentiated human embryonal carcinoma cells. Science. 1984;224:159–161. doi: 10.1126/science.6322309. [DOI] [PubMed] [Google Scholar]
- 22.Heieren H H, Kim Y, Balfour H H. Human cytomegalovirus infection of kidney glomerular visceral epithelial and tubular epithelial cells in culture. Transplantation. 1988;46:426–432. doi: 10.1097/00007890-198809000-00019. [DOI] [PubMed] [Google Scholar]
- 23.Johnson A T, Klein E S, Gillett S J, Wang L, Song T K, Pino M E, Chandraratna R A S. Synthesis and characterization of a highly potent and effective antagonist of retinoic acid receptors. J Med Chem. 1995;38:4764–4767. doi: 10.1021/jm00024a003. [DOI] [PubMed] [Google Scholar]
- 24.Kamei Y, Xu L, Heinzel T, Torchia J, Kurokawa R, Gloss B, Lin S-C, Heyman R A, Rose D W, Glass C K, Rosenfeld M G. A CBP integrator complex mediates transcriptional activation and AP-1 inhibition by nuclear receptors. Cell. 1996;85:403–414. doi: 10.1016/s0092-8674(00)81118-6. [DOI] [PubMed] [Google Scholar]
- 25.Klein E S, Pino M E, Johnson A T, Davies P J A, Nagpal S, Thacher S M, Krasinski G, Chandraratna R A S. Identification and functional separation of retinoic acid receptor neutral antagonists and inverse agonists. J Biol Chem. 1996;271:22692–22696. doi: 10.1074/jbc.271.37.22692. [DOI] [PubMed] [Google Scholar]
- 26.Koedood M, Fichtel A, Meier P, Mitchell P J. Human cytomegalovirus (HCMV) immediate-early enhancer/promoter specificity during embryogenesis defines target tissues of congenital HCMV infection. J Virol. 1995;69:2194–2207. doi: 10.1128/jvi.69.4.2194-2207.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Koszinowski U H, Reddehase M J, Del Val M. Principles of cytomegalovirus antigen presentation in vitro and in vivo. Semin Immunol. 1992;4:71–79. [PubMed] [Google Scholar]
- 28.Kurokawa R, DiRenzo J, Boehm M, Sugarman J, Gloss B, Rosenfeld M G, Heyman R A, Glass C K. Regulation of retinoid signalling by receptor polarity and allosteric control of ligand binding. Nature. 1994;371:528–531. doi: 10.1038/371528a0. [DOI] [PubMed] [Google Scholar]
- 29.Kurokawa R, Söderström M, Hörlein A, Halachmi S, Brown M, Rosenfeld M G, Glass C K. Polarity-specific activities of retinoic acid receptors determined by a co-repressor. Nature. 1995;377:451–454. doi: 10.1038/377451a0. [DOI] [PubMed] [Google Scholar]
- 30.Lafemina R, Hayward G. Constitutive and retinoic acid-inducible expression of cytomegalovirus immediate-early genes in human teratocarcinoma cells. J Virol. 1986;58:434–440. doi: 10.1128/jvi.58.2.434-440.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lee M O, Dawson M I, Picard N, Hobbs P D, Pfahl M. A novel class of retinoid antagonists and their mechanism of action. J Biol Chem. 1996;271:11897–11903. doi: 10.1074/jbc.271.20.11897. [DOI] [PubMed] [Google Scholar]
- 32.Lindamood C, III, Cope F O, Dillehay D L, Everson M P, Giles H D, Lamon E W, McCarthy D J, Sartin J L, Hill D L. Pharmacological and toxicological properties of arotinoids SMR-2 and SMR-6 in mice. Fundam Appl Toxicol. 1990;14:15–29. doi: 10.1016/0272-0590(90)90227-b. [DOI] [PubMed] [Google Scholar]
- 33.Loeliger P, Bollag W, Mayer H. Arotinoids, a new class of highly active retinoids. Eur J Med Chem Chim Ther. 1980;15:9–15. [Google Scholar]
- 34.Mangelsdorf D J, Umesono K, Evans R M. The retinoid receptors. In: Sporn M B, Roberts A B, Goodman D S, editors. The retinoids: biology, chemistry, and medicine. New York, N.Y: Raven Press; 1994. pp. 319–349. [Google Scholar]
- 35.Manning W C, Mocarski E S. Insertional mutagenesis of the murine cytomegalovirus genome: one prominent α gene (ie2) is dispensable for growth. Virology. 1988;167:477–484. [PubMed] [Google Scholar]
- 36.McGeoch D J, Cook S, Dolan A, Jamieson F E, Telford E A R. Molecular phylogeny and evolutionary timescale for the family of mamalian herpesviruses. J Mol Biol. 1995;247:443–458. doi: 10.1006/jmbi.1995.0152. [DOI] [PubMed] [Google Scholar]
- 37.Mims C A, Gould J. Splenic necrosis in mice infected with cytomegalovirus. J Infect Dis. 1978;137:587–591. doi: 10.1093/infdis/137.5.587. [DOI] [PubMed] [Google Scholar]
- 38.Mocarski E S, Kemble G W, Lyle J M, Greaves R F. A deletion mutant in the human cytomegalovirus gene encoding IE1491aa replication defective due to a failure in autoregulation. Proc Natl Acad Sci USA. 1996;93:11321–11326. doi: 10.1073/pnas.93.21.11321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Moens U, Subramaniam N, Johansen B, Johansen T, Traavik T. A steroid hormone response unit in the late leader of the noncoding control region of the human polyomavirus BK confers enhanced host cell permissivity. J Virol. 1994;68:2398–2408. doi: 10.1128/jvi.68.4.2398-2408.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nelson J A, Groudine M. Transcriptional regulation of the human cytomegalovirus major immediate-early gene is associated with induction of DNase I-hypersensitive sites. Mol Cell Biol. 1986;6:452–461. doi: 10.1128/mcb.6.2.452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Payvar F, DeFranco D, Firestone G L, Edgar B, Wrange O, Okret S, Gustafsson J-A, Yamamoto K R. Sequence-specific binding of glucocorticoid receptor to MTV DNA at sites within and upstream of the transcribed region. Cell. 1983;35:381–392. doi: 10.1016/0092-8674(83)90171-x. [DOI] [PubMed] [Google Scholar]
- 42.Reed L J, Muench H. A simple method of estimating fifty per cent endpoints. Am J Hyg. 1938;27:493–497. [Google Scholar]
- 43.Ringold G M, Yamamoto K R, Tomkins G M, Bishop J M, Varmus H E. Dexamethasone-mediated induction of mouse mammary tumor virus RNA: a system for studying glucocorticoid action. Cell. 1975;6:299–305. doi: 10.1016/0092-8674(75)90181-6. [DOI] [PubMed] [Google Scholar]
- 44.Segars J H, Nagata T, Bours V, Medin J A, Franzoso G, Blanco J C G, Drew P D, Becker K G, An J, Tang T, Stephany D A, Neel B, Siebenlist U, Ozato K. Retinoic acid induction of major histocompatibility complex class I in NTera-2 embryonal carcinoma cells involves induction of NF-kB (p50-p65) and retinoic acid receptor β-retinoid X receptor β heterodimers. Mol Cell Biol. 1993;13:6157–6169. doi: 10.1128/mcb.13.10.6157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Standeven A M, Beard R L, Johnson A T, Boehm M F, Escobar M, Heyman R A, Chandraratna R A S. Retinoid-induced hypertriglyceridemia in rats is mediated by retinoic acid receptors. Fundam Appl Toxicol. 1996;33:264–271. doi: 10.1006/faat.1996.0164. [DOI] [PubMed] [Google Scholar]
- 46.Standeven A M, Davies P J A, Chandraratna R A S, Mader D R, Johnson A T, Thomazy V A. Retinoid-induced epiphyseal plate closure in guinea pigs. Fund Appl Toxicol. 1996;34:91–98. doi: 10.1006/faat.1996.0179. [DOI] [PubMed] [Google Scholar]
- 47.Standeven A M, Johnson A T, Escobar M, Chandraratna R A S. Specific antagonist of retinoid toxicity in mice. Toxicol Appl Pharmacol. 1996;138:169–175. doi: 10.1006/taap.1996.0110. [DOI] [PubMed] [Google Scholar]
- 48.Stoddart C A, Cardin R D, Boname J M, Manning W C, Abenes G B, Mocarski E S. Peripheral blood mononuclear phagocytes mediate dissemination of murine cytomegalovirus. J Virol. 1994;68:6243–6253. doi: 10.1128/jvi.68.10.6243-6253.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sucov H M, Murakami K K, Evans R M. Characterization of an autoregulated response element in the mouse retinoic acid receptor type β gene. Proc Natl Acad Sci USA. 1990;87:5392–5396. doi: 10.1073/pnas.87.14.5392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wade P A, Wolffe A P. Chromatin: histone acetyltransferases in control. Curr Biol. 1997;7:82–84. doi: 10.1016/s0960-9822(06)00042-x. [DOI] [PubMed] [Google Scholar]
- 51.Wolff D, Sinzger C, Drescher P, Jahn G, Plachter B. Reduced levels of IE2 gene expression and shutdown of early and late viral genes during latent infection of glioblastoma cell line U138-MG with selectable recombinants of human cytomegalovirus. Virology. 1994;204:101–113. doi: 10.1006/viro.1994.1514. [DOI] [PubMed] [Google Scholar]
- 52.Yoshimura H, Nagai M, Hibi S, Kikuchi K, Abe S, Hida T, Higashi S, Hishinuma I, Yamanaka T. A novel type of retinoic acid receptor antagonist: synthesis and structure-activity relationships of heterocyclic ring-containing benzoic acid derivatives. J Med Chem. 1995;38:3163–3173. doi: 10.1021/jm00016a020. [DOI] [PubMed] [Google Scholar]
- 53.Zuo F, Mertz J E. SV40 late gene expression is regulated by members of the steroid/thyroid hormone receptor superfamily. Proc Natl Acad Sci USA. 1995;92:8586–8590. doi: 10.1073/pnas.92.19.8586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zuo F, Kraus R J, Gulick T, Moore D D, Mertz J E. Direct modulation of simian virus 40 late gene expression by thyroid hormone and its receptor. J Virol. 1997;71:427–436. doi: 10.1128/jvi.71.1.427-436.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]