Abstract
Understanding how climate change and demographic factors may shape future population exposure to viruses such as Zika, dengue, or chikungunya, transmitted by Aedes mosquitoes is essential to improving public health preparedness. In this study, we combine projections of cumulative monthly Aedes-borne virus transmission risk with spatially explicit population projections for vulnerable demographic groups to explore future county-level population exposure across the conterminous United States. We employ a scenario matrix—combinations of climate scenarios (Representative Concentration Pathways) and socioeconomic scenarios (Shared Socioeconomic Pathways)—to assess the full range of uncertainty in emissions, socioeconomic development, and demographic change. Human exposure is projected to increase under most scenarios, up to + 177% at the national scale in 2080 under SSP5*RCP8.5 relative to a historical baseline. Projected exposure changes are predominantly driven by population changes in vulnerable demographic groups, although climate change is also important, particularly in the western region where future exposure would be about 30% lower under RCP2.6 compared to RCP8.5. The results emphasize the crucial role that socioeconomic and demographic change play in shaping future population vulnerability and exposure to Aedes-borne virus transmission risk in the United States, and underline the importance of including socioeconomic scenarios in projections of climate-related vector-borne disease impacts.
Keywords: Climate impacts, Shared Socioeconomic Pathways, Vector-borne diseases, Aedes mosquitoes, Representative Concentration Pathways, Scenarios, Vulnerability
1. Introduction
Aedes mosquitoes can transmit dengue, chikungunya and Zika viruses (Chouin-Carneiro et al 2016, Epelboin et al 2017). The geographic range of Aedes mosquitoes has expanded in the conterminous United States over the past 2–3 decades (Hahn et al 2016, Kraemer et al 2019). Sporadic, autochthonous transmission of all three viruses has occurred recently in south Florida and Texas (Brunkard et al 2007, Ramos et al 2008, Trout et al 2010, Kendrick et al 2014, Hotez 2018, Rosenberg et al 2018, CDC 2020a, 2020b). Given these recent trends, it is essential to understand how climatic and demographic changes may influence the transmission of these viruses during the 21st century.
Estimating future population exposure (i.e. the number of persons exposed to a risk of vector-borne disease transmission) to Aedes-borne virus transmission risk under changing climatic conditions requires an understanding of (i) the expansion and redistribution of Aedes vectors due to climate change, (ii) the differential vulnerability of local population groups, and (iii) the growth and future spatial distribution of vulnerable populations. While the influence of climate change on the expansion and redistribution of Aedes mosquitoes and Aedes-borne virus transmission risk has been explored in a wide range of studies (e.g. Caminade et al 2012, Fischer et al 2013, Proestos et al 2015,Campbellet al 2015,Tjadenet al 2017,Liu-Helmersson et al 2019a,b, Ryan et al 2019), the use of projected population growth rates and patterns to estimate future population vulnerability and exposure to Aedes mosquitoes and Aedes-borne virus transmission risk is less common (Monaghan et al 2016, Kraemer et al 2019,Messinaet al 2019).The omission of these population projections, and lack of consideration of population subgroups, is potentially problematic. It may lead to an overestimation of the role that climate change plays in shaping future population exposure to vector-borne diseases (VBDs) and introduces systematic bias into climate-related health adaptation planning (Ebi et al 2016, Suk 2016), and may lead to skewed estimates of impact across sociodemographic subgroups of the population.
In the past few years, the climate change research community has been engaged in the operationalization of a new scenario framework that facilitates the integration of future demographic and socioeconomic characteristics—through scenarios—into climate impacts, adaptation, and vulnerability (IAV) studies (Moss et al 2010). This scenario framework is made up of climate scenarios—Representative Concentration Pathways, RCPs (van Vuuren et al 2011)—and socioeconomic scenarios—Shared Socioeconomic Pathways, SSPs (O’Neill et al 2017)—combined together into a scenario matrix (Ebi et al 2014). This framework (hereafter referred as SSP*RCP framework) is being increasingly used in IAV studies to explore future population exposure—under socioeconomic and climatic uncertainty—to a wide range of climate-related risks such as extreme heat (e.g. Jones et al 2018, Rohat et al 2019), inland and coastal flooding (e.g. Alfieri et al 2015, Brown et al 2018), fire risk (Knorr et al 2016), air pollution (Chowdhury et al 2018), and food security (e.g. Hasegawa et al 2014). The SSP*RCP framework has been applied to some VBD-related studies (e.g. Monaghan et al 2016, Li et al 2019a, Messina et al 2019). However, uncertainty in future population vulnerability and exposure to VBDs could be much more readily assessed if the SSP*RCP framework approach was applied more broadly and thoroughly across many different VBDs, particularly given the wide range of future socioeconomic pathways that exist.
In this paper, we apply the SSP*RCP framework to assess future population exposure to Aedes-borne virus transmission risk (VTR) in the conterminous United-States (hereafter referred as CONUS), at the county-level, up to 2080, under four consistent combinations of climate and socioeconomic scenarios. We combine projections of cumulative monthly risk of Aedes-borne virus transmission (under two climate scenarios) with population projections for a number of vulnerable demographic groups (under three socioeconomic/demographic scenarios). Using a scenario matrix, we explore separately the relative contribution of climate change and demographic growth to future exposure, and assess the avoided exposure due to strong mitigation options or to different socioeconomic pathways.
2. Data and methods
2.1. Scenario setting
We explored future population exposure to Aedes-borne VTR under several climate and socioeconomic scenarios, spanning the wide range of uncertainties in future emission levels, socioeconomic development, and demographic growth. We employed the lowest and highest fossil fuel emission scenarios, RCP2.6 and RCP8.5. The former assumes strong mitigation options and a rapid decline in emissions by the middle of the century, while the latter assumes continued growth of emissions throughout the century (van Vuuren et al 2011). The projected increase in global average temperature for 2081–2100 ranges from 0.3°–1.7 °C under RCP2.6 to 2.6°–4.8 °C under RCP8.5, relative to 1986–2005 (Stocker et al 2013). It has been suggested that the RCP8.5 scenario is increasingly unlikely given that coal use is projected to taper off and clean energy costs are falling (Hausfather and Peters 2020).
We combined these two emission scenarios with three socioeconomic/demographic scenarios—SSP1, SSP3, and SSP5—covering the full range of uncertainty in demographic growth in the United States (figure S1 (available online at stacks.iop.org/ERL/15/084046/mmedia)). Along with assumptions of population growth among different demographic groups, these scenarios also depict varying levels of socioeconomic development in terms of economic growth, environmental awareness, education, spatial patterns of urban development, technological development, health equity, and economic inequalities (O’Neill et al 2017). SSP1, named Sustainability, depicts medium population growth in the United States, along with economic development that places large emphasis on human well-being and achieving development goals, reducing inequality, concentrating urbanization, and increasing sustainable consumption. By contrast, SSP3, named Regional Rivalry, depicts overall population decline in the United States, along with increased inequality, reduced health and education investments, slowing global economic growth, and strong governmental focus on regional security with a subsequent reduction in immigration. Finally, SSP5, named Fossil-fueled Development, depicts high population growth in the United States driven primarily by immigration, along with a high technological development, strong investments to enhance human and social capital, and a rapid growth of the global economy through heavy use of fossil fuel resources.
Although a given RCP can be consistent with several SSPs, not all SSP*RCP combinations are consistent, and some require more mitigation efforts than others (Kriegler et al 2012, 2014). SSP1 and SSP5 can theoretically lead to emission levels depicted under RCP2.6 (requiring massive mitigation efforts under SSP5), but this is not the case for SSP3 (Rogelj et al 2018). Similarly, the socioeconomic development depicted under SSP1 is not consistent with the high emission levels associated with RCP8.5. Bearing in mind these implausible combinations, we employed the SSP*RCP combinations depicted in table 1. To enable isolating the individual contribution of climate change and population growth on future human exposure, we also explored future population exposure under combinations of (i) baseline climate and future population and (ii) baseline population and future climate (see section 2.2).
Table 1.
Combinations of climate and socioeconomic scenarios to explore future population exposure and to isolate the climate and population (pop.) effects on Aedes-borne VTR (see section 2.2). Combinations that are assessed are indicated with ‘Yes’ and those that are not are indicated with ‘No (implausible)’. The historical baseline for population is 2010 and the historical baseline for climate is 1960–1990.
| Historical | SSP1 | SSP3 | SSP5 | |
|---|---|---|---|---|
|
| ||||
| Historical | Baseline | Pop. effect | Pop. effect | Pop. effect |
| RCP2.6 | Climate effect | Yes | No (implausible) | Yes |
| RCP8.5 | Climate effect | No (implausible) | Yes | Yes |
2.2. Exposure projections, individual effects, and avoided exposure
We defined the population exposure in a given county and for a given population group as being the combination of the cumulative monthly transmission risk of Aedes-borne virus with the population count. Population exposure is therefore expressed in terms of person-months of exposure per year, in line with metrics used in other climate impact studies, e.g. (Martens et al 1999, Caminade et al 2014, Jones et al 2015, Rohat et al 2019). The main advantage of this exposure metric lies in that it accounts for the duration (in months) of the exposure event. We assessed population exposure to Aedes-borne VTR for baseline and future (years ‘2050’ and ‘2080’) for different population groups separately (see section 2.4), under the four SSP*RCP combinations detailed in section 2.1. Using the scenario matrix and combinations with baseline climate or baseline population (table 1), we isolated the population and climate effects. The population effect represents the changes in population exposure due to changes in population growth (as a function of demographic/socioeconomic conditions) only, while the climate effect represents the changes in population exposure due to climate change only (Jones et al 2015). We also computed the interaction effect between the two, that is, the difference between the total projected change in exposure and the sum of the climate and population effects. The interaction effect is interesting in that it represents the process by which concurrent changes in population and climatic conditions affect the population exposure (Rohat et al 2019). We explored the population, climate, and interaction effects at the county scale for the four SSP*RCP combinations separately for both increased and decreased exposure.
Finally, we estimated the relative avoided exposure due (i) to shifts in climatic conditions, that is, a shift from a high (RCP8.5) to a low (RCP2.6) emission scenario (using baseline population conditions), and (ii) to shifts in population growth patterns due to socioeconomic/demographic conditions, that is, a shift from a high (SSP5) to a medium (SSP1) population growth scenario or a shift from a high (SSP5) to a low (SSP3) population growth scenario (using baseline climatic conditions).
2.3. Aedes-borne virus transmission risk (VTR)
We retrieved projections of Aedes-borne virus transmission risk (VTR) from (Ryan et al 2019), for baseline as well as for future time-periods—’2050’ (2040–2069) and ‘2080’ (2070–2099)—under both RCP2.6 and RCP8.5. Briefly, (Ryan et al 2019) employed a temperature-driven empirically parametrized model of viral transmission (by the vectors Ae. aegypti and Ae. albopictus) coupled to baseline and future downscaled temperature projections from four general circulation models (GCMs, see Table S1 and Hijmans et al 2005) to estimate future cumulative monthly transmission risk on a 1/12° spatial grid. The temperature bounds suitable for virus transmission (posterior probability of temperature suitability > 97.5%) are 21.3–34.0 °C for Ae. aegypti and 19.9–29.4 °C for Ae. albopictus (see Ryan et al 2019) for full details of the modelling approach). Here we used the projections of cumulative monthly transmission risk performed with the baseline and four GCMs, and aggregated them to the county scale (using area-weighted mean) for each time-period and RCP. We employed the multi-model ensemble mean to explore future transmission risk and the interquartile range (IQR) to display inter-model uncertainties arising from differing representations of climate processes in GCMs.
2.4. Selection and projection of vulnerable population groups
Because some population groups are more vulnerable to Aedes-borne diseases than others (Beard et al 2016), we assessed future exposure for a number of potentially vulnerable population groups, in addition to the exposure of the whole population. Population groups with higher vulnerability to Aedes-borne diseases include those who are more likely to be bitten by Aedes mosquitoes and those who are more likely to suffer adverse health conditions if infected by Aedes-borne viruses. Aedes mosquitoes are primarily daytime biters and prefer to take blood meals after sunrise and in late afternoon, although at least one study has shown that they will bite in the evening under artificial lights (Chadee and Martinez 2000). Groups more likely to be exposed to Aedes bites include children (more likely to play outside) and outdoor workers (Bennett and Mcmichael 2010, Schulte et al 2016). Those who tend to have homes that are more permeable (e.g. open windows instead of air conditioning and broken window screens) are also more likely to receive mosquito bites (Reiter et al 2003, Radke et al 2012). In this regard, low-income communities appear especially vulnerable, as they are less likely to possess and/or to use air conditioning (Hernández and Bird 2010). Many of these at-risk communities are located in the United States-Mexico (US-MX) border region. For example, Brownsville, TX, a community located at the US-MX border, has seen sporadic transmission of Aedes-borne viruses. A dengue outbreak investigation in 2005 determined that 85% of the population had air-conditioning while 61% reported screens on windows and doors (Ramos et al 2008). Aedes mosquitoes thrive in urban environments (e.g. Eisen and Moore 2013) and typically oviposit in artificial, water-filled containers (Hiscox et al 2013); this is particularly true for Ae. aegypti, but also to a lesser extent for Ae. albopictus (Roche et al 2015). Additionally, Ae. albopictus, like Ae. aegypti exhibits highly anthropophilic biting behavior (Delatte et al 2010). Aedes aegypti preferentially feeds on humans; Ae. albopictus is a more catholic feeder but also has a high mammalian affinity. For example, studies of Ae.Albopictus in the northeastern United States indicated that 90% of bloodmeals were mammalian, and 58% of those were human (Faraji et al 2014). Furthermore, Ae. albopictus has been implicated repeatedly in chikungunya outbreaks worldwide, indicating its role in virus transmission (Benedict et al 2007, Rezza et al 2007, Bonizzoni et al 2013, Weaver and Lecuit 2015). Urban populations are, therefore, considered more vulnerable than rural ones, as urbanites are more likely to be in contact with Aedes mosquitoes, increasing the potential for virus transmission (Salje et al 2019). Finally, the elderly are more likely to suffer adverse health effects if infected by Aedes-borne viruses (Brien et al 2009, Dye 2014, Badawi et al 2018), hence making this group highly vulnerable.
2.4.1. Total population, elderly, and children.
Population projections at the county level in the United States were retrieved from (Hauer 2019), who used the Hamilton-Perry method (Swanson et al 2010) to project age-sex-race/ethnicity (ASRE) cohorts up to 2100 under the five SSPs. We retrieved projections for all ASRE cohorts (i.e. the total population), for elderly (ASRE cohorts older than 65 years), and for children (ASRE cohorts comprised between 5–14 years).
2.4.2. Urban population.
We retrieved spatial population projections under the SSPs from (Gao 2017), who downscaled to a 1/100° grid the 1/8° spatial projections of (Jones and O’Neill 2016). This set of projections differentiates the urban and rural populations and accounts for SSP-specific assumptions of urban development. Using these projections, we computed the share of urban population (over the total population) at the county-level under each SSP and each 10 year period from 2010 to 2080. We then combined the SSP-, time-, and county-specific shares of urban population with the county-level population projections retrieved from (Hauer 2019), yielding county-level projections of urban population under each SSP.
2.4.3. Outdoor workers.
We considered outdoor workers as those people who have occupations in which > 70% of the work performed is outside, according to the Bureau of Labor Statistics (see table S2; BLS, 2017). We retrieved county-level data on occupation of the employed population from the American Community Survey (ACS) estimates, spanning yearly from 2010 to 2017. We then computed the ratio of outdoor workers over the working age population (20–64 years) for each county, averaged across the period 2010–2017. This ratio ranges from 8.7% to 48.0%, with most counties being close to the national average ratio of ~ 23% (figure S2). Assuming constant county-specific ratios, we applied the population projections of 20–64 years ASRE cohorts—retrieved from (Hauer 2019)—to project the future number of outdoor workers at the county-level under each SSP.
2.4.4. Low-income population.
We retrieved national-scale projections of population in poverty under each SSP from Rao et al (2019), which were generated by combining Gini projections with GDP and population projections, assuming lognormal income distributions. We combined these projections with SSP-based national population projections (KC and Lutz, 2017) to estimate SSP-specific compound annual growth rates (AGRs) of poverty reduction for each 10 year step spanning 2010–2080. Assuming changes in poverty rates to be homogeneously spread across the country, we applied the SSP- and time-specific national AGRs of poverty reduction to the historical county-level shares of low-income population (that is, below the national poverty threshold; data retrieved for different age-sex cohorts from the ACS estimates for year 2012) and employed the ASRE population projections retrieved from (Hauer 2019) to estimate future low-income populations at the county-level under each SSP.
3. Results and discussion
3.1. Population projections
The total population of the conterminous United-States (CONUS) is projected to shift from approximately 301 million (M) in 2010 to 472 M in 2050 and to 627 M in 2080 under SSP5, plateau at 465 M in 2080 under SSP1, and decrease to 298 M in 2080 under SSP3 (figure 1). The urban CONUS population shows similar trends, with a slightly higher growth rate compared to total population, due to the increased urbanization depicted under all the SSPs (Jiang and O’Neill, 2017). The primary drivers of urbanization include income growth and the desire for sustainable and compact living (SSP1), varying levels of economic growth (SSP3 and SSP5), as well as technological advances and increases in agricultural productivity (SSP5) (Jiang and O’Neill, 2017). In contrast, the increase in outdoor population is slower than that of the total population, because of the relatively lower increase in working age population. Nevertheless, the number of outdoor workers still largely increases under SSP1 and SSP5, shifting respectively from 35 M in 2010 to 41 M and 58 M in 2080 (figure 1).
Figure 1.
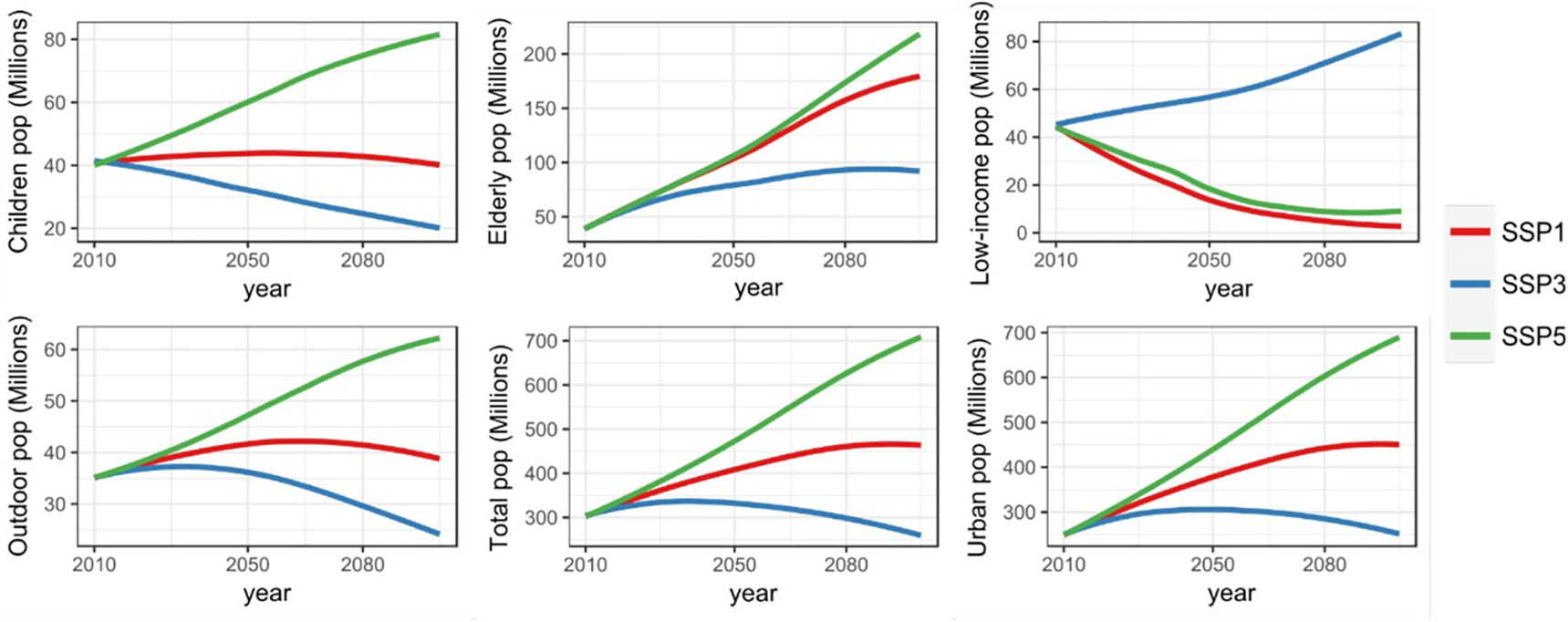
Population projections of the total population and the five vulnerable population groups, under SSP1, SSP3, and SSP5, for the conterminous United-States.
Consistent with recent trends, all SSPs (including SSP3) depict an increase in the number of elderly (older than 65 years). Noteworthy, the increase in elderlyunderSSP1andSSP5followsasimilartrend,both shifting from approximately 39 M in 2010 to 105 M in 2050, and reaching 158 M (174 M) under SSP1 (SSP5) by 2080, that is, a ~ 5-fold increase compared to 2010. Conversely, the ageing of the society leads to a progressive decrease in the number of children under SSP1—and to a rapid decrease under SSP3, linked to the total population decline. The number of children increases only under SSP5, due to the high immigration-driven demographic growth.
Finally, the low-income population decreases under SSP1 and SSP5—due to economic growth, enhancement of social capital, and strong decrease in economic inequalities—, shifting from 45 M (baseline) to 5 M (9 M) in 2080 under SSP1 (SSP5). In contrast, SSP3 depicts an increase in the net number of low-income population—despite the decline of the total population—reaching 71 M in 2080, mainly due to the progressive decline in social welfare programs, long-term economic downturn, and increased economic inequalities.
Spatial patterns of population projections indicate great variations across regions (figure S3) and counties (figure 2). Despite the high demographic growth depicted under SSP5, a number of counties—predominantly located in the Midwest and South—have a declining population. SSP1 also leads to very contrasting spatial patterns, with some regions (such as Florida, California, and southern Texas) showing great population growth (> + 50% in 2080 relative to 2010), while a number of counties in the Midwest and South show a large population decline of −25% to more than −50% in 2080 (relative to 2010). Noteworthy, some counties that have been rapidly growing in the past decades still show a high population growth under SSP3, despite the overall decline of the population. Altogether, the contrasting trends and spatial patterns of population projections of the vulnerable groups are likely to influence future levels and spatial patterns of population exposure.
Figure 2.
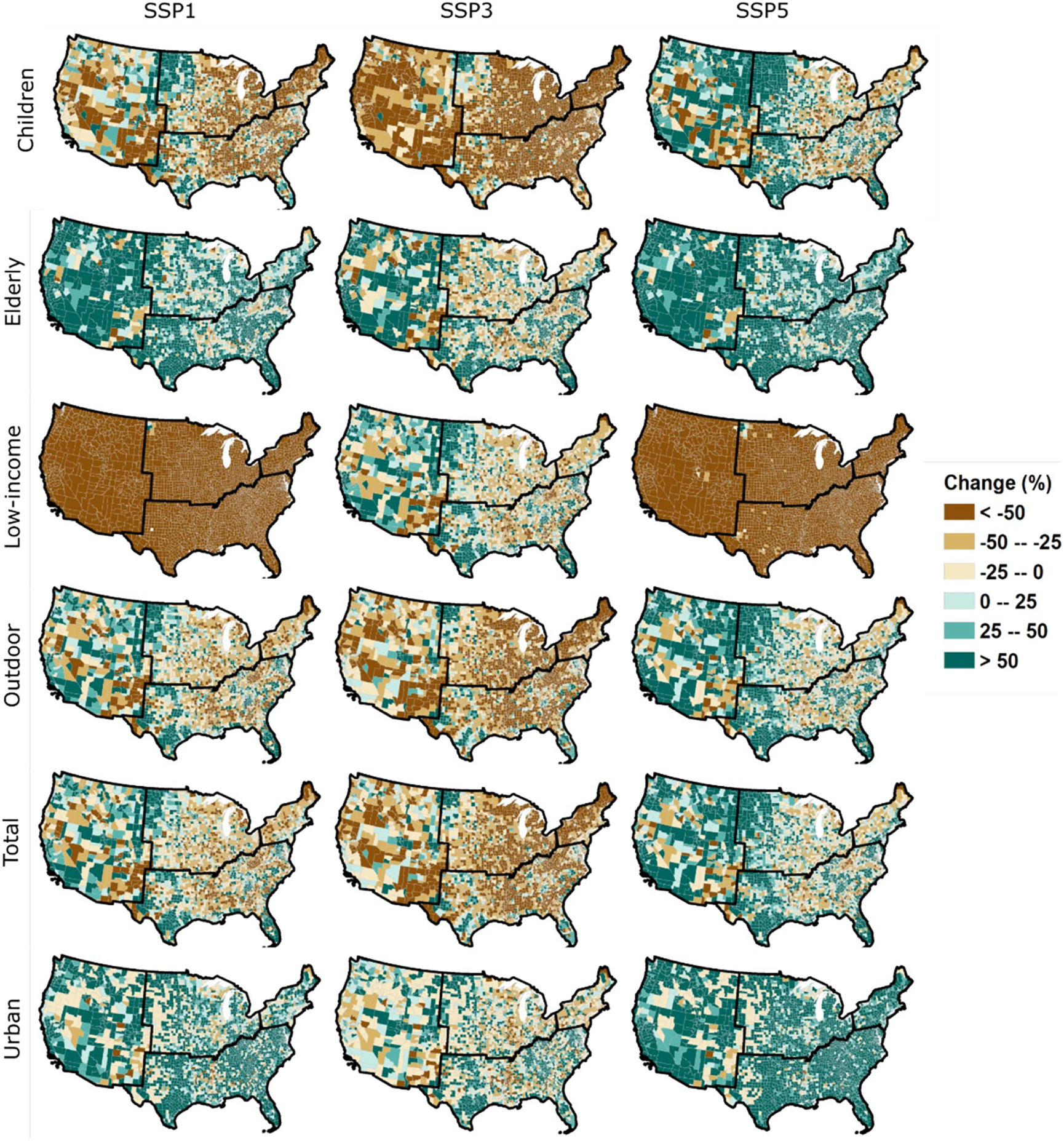
County-level spatial patterns of change in population (for year 2080 relative to year 2010), for the different population groups, under SSP1, SSP3, and SSP5.
3.2. Projections of cumulative monthly transmission risk of Aedes-borne virus
At the national level (CONUS) a significant increase in temperature suitability for VTR by the vector Ae. aegypti is projected under RCP8.5, with the multi-model spatial average cumulative monthly transmission risk shifting from approximately 2.8 months at baseline to 3.5 (IQR = 0.3) months in 2050 and 4.0 (0.1) months in 2080 (figure 3(a) and table S3). Under this stronger climate change scenario, some southern counties attain year-round transmission risk in 2080, while the maximum baseline cumulative monthly transmission risk is less than 10 months. Noteworthy, RCP8.5 leads to a much smaller increase in temperature suitability for VTR by the vector Ae. albopictus, with the CONUS-averaged cumulative monthly transmission risk shifting from 3.1 months to 3.4 (0.2) months in 2050 and 2080. This is due to the comparatively lower maximum temperature threshold of this species (29.4 °C for Ae. albopictus compared to 34.0 °C for Ae. aegypti) that is increasingly exceeded under the RCP8.5 scenario, particularly in the South. In contrast, climate change as depicted under the RCP2.6 scenario has little influence on the CONUS-averaged cumulative monthly VTR by Aedes mosquitoes, leading only to a slight increase (0.1 month) for both vectors (table S3).
Figure 3.
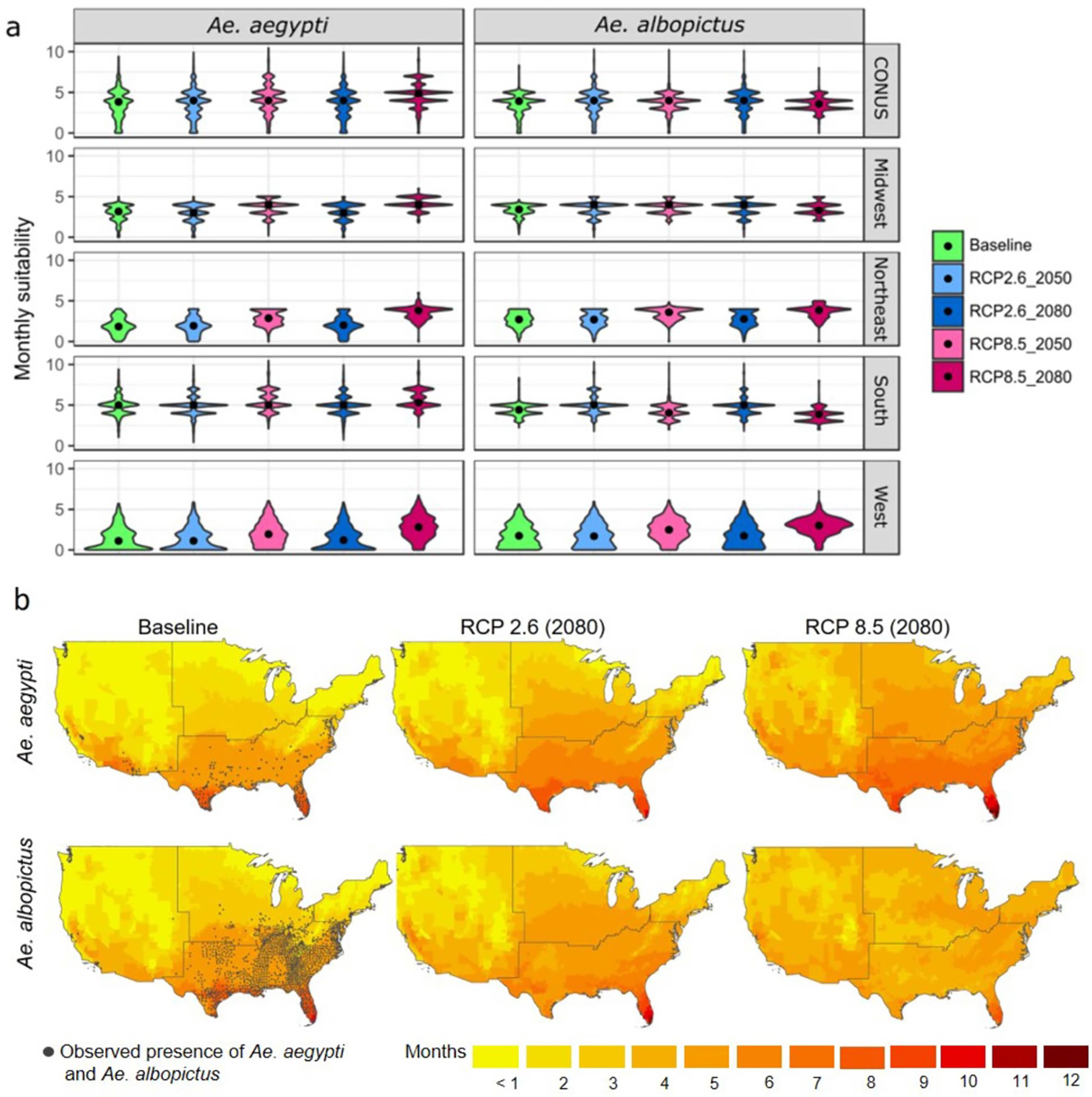
Multi-model averaged cumulative monthly VTR by Ae. aegypti and Ae. albopictus, projected under RCP2.6 and RCP8.5, represented as (a) the national and regional distribution of county-level results for baseline, 2050, and 2080; and as (b) county-level maps for baseline and 2080. Observed Ae. aegypti and Ae. albopictus presence locations are from. The VTR data are reproduced from Ryan et al (2019); CC BY 4.0. Observed Ae. aegypti and Ae. albopictus presence locations are from Kraemer et al (2015); CC0 BY 1.0.
The CONUS-averaged results exhibit large regional disparities (figure 3(b); difference plots are shown in figure S4). The increase in cumulative monthly VTR by Ae. aegypti due to climate change under RCP8.5 is particularly reinforced in the West and Northeast, where it doubles in 2080, relative to baseline. In the Midwest, all counties are projected to be suitable for virus transmission in 2080, as the minimum cumulative monthly transmission risk is 2.0 (0.1) months under this scenario, compared to 0 months at baseline. The number of counties in the West showing no temperature suitability year-round also largely decreases under this scenario (figure 3(b)). For Ae. albopictus, the RCP2.6 scenario leads to a significant increase cumulative monthly VTR in certain areas of the South, with values in the most at-risk counties shifting from 8.3 months to 11.0 (0.6) months in 2080. Under RCP8.5, VTR decreases significantly in the South (from 4.4 to 3.8 (0.4) months in average in 2080), but increases in the West (from 1.8 to 2.9 (0.1) months in average).
3.3. Future population exposure
Aggregated at the CONUS scale, results show an increase in total population exposure to Ae. aegypti VTR under all scenario combinations (figure 4), shifting from approximately 1.14 billion (B) person-months per year in 2010 to 1.50 (IQR = 0.01) B under SSP3*RCP8.5, 1.90 (0.16) B under SSP1*RCP2.6, 2.58 (0.22) B under SSP5*RCP2.6, and up to 3.16 (0.03) B under SSP5*RCP8.5 by 2080, i.e. an increase in exposure ranging from 32% to 177% relative to 2010. In comparison, the increase in total population exposure to Ae. albopictus VTR is lesser, shifting from approximately 1.15 B person-months in 2010 to 1.92 (0.007) B under SSP1*RCP2.6 and 2.61 (0.009) B under SSP5*RCP2.6, i.e. an increase of 127% the baseline level. Noteworthy, total population exposure to Ae. albopictus VTR (i) remains stable at 1.15 (0.09) B person-months under SSP3*RCP8.5 and (ii) is greater under SSP5*RCP2.6 (2.61 (0.009) B) than under SSP5*RCP8.5 (2.42 (0.19) B), because of the restricting effect of a comparatively stronger climate change on temperature suitability for VTR by Ae. albopictus under RCP8.5.
Figure 4.
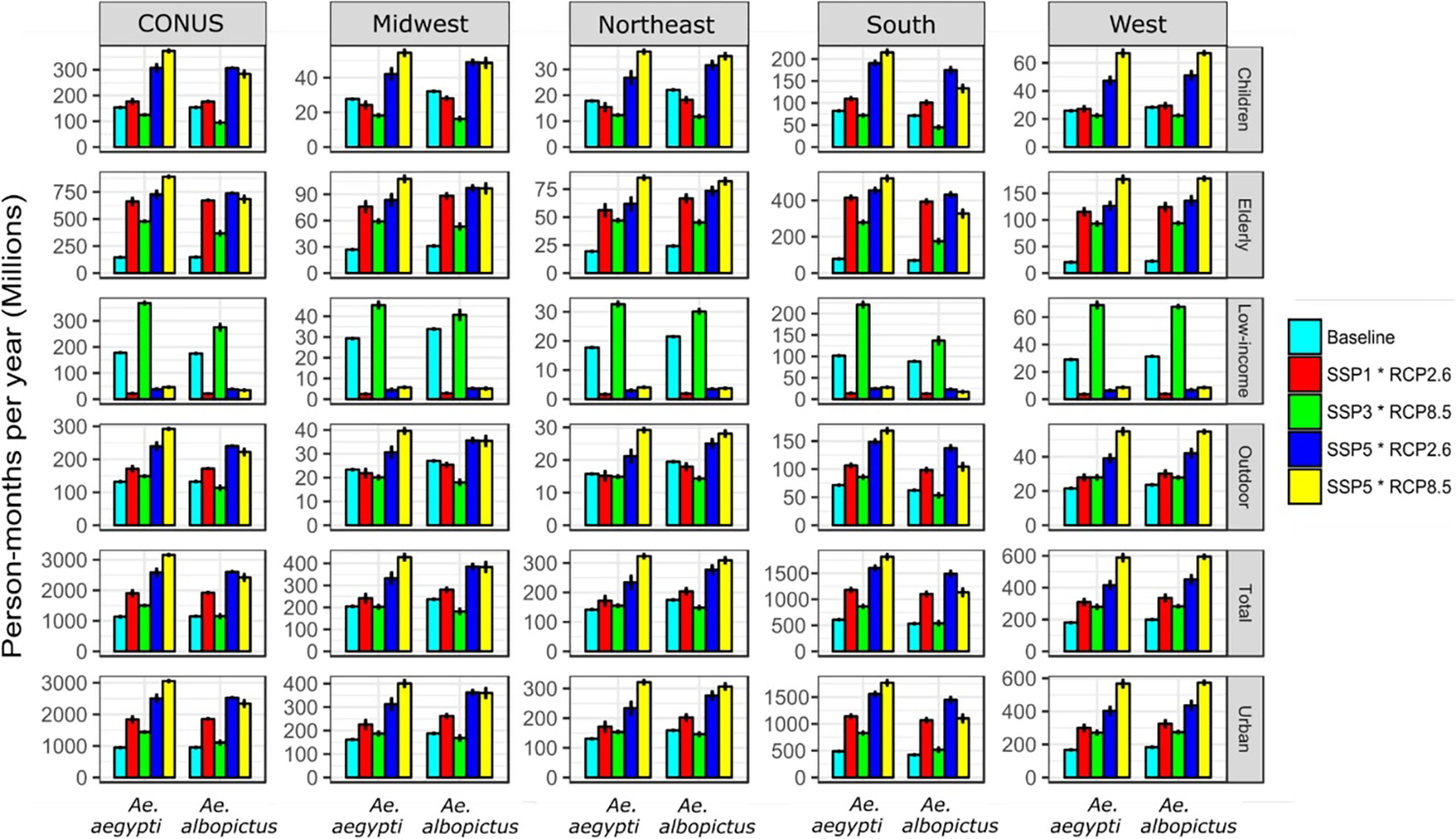
Multi-model projections of population exposure (in millions of person-months per year) to Aedes-borne VTR, aggregated at the continental (CONUS) and regional scale, for the historical period (year 2010, Baseline) and for 2080 under four SSP*RCP combinations. Results are presented separately for the different population groups and the two Aedes mosquitoes. Errors bars represent the multi-model interquartile ranges (IQRs).
Not all vulnerable population groups follow similar trends in population exposure to that of the total population. Because of continuing urbanization, the increase in exposure of urban dwellers occurs slightly faster than that of the total population. Because of the ageing population depicted under all demographic/socioeconomic scenarios, the population exposure of elderly to Aedes-borne VTR drastically increases under all scenario combinations. Exposure of this vulnerable group to Ae. aegypti increases by 230% (under SSP3*RCP8.5, 478 (5.4) million (M) person-months) up to 514% under SSP5*RCP8.5 (890 (11) M) by 2080, relative to 2010 (145 M). Conversely, the exposure of children increases only slightly under SSP1*RCP2.6 and significantly decreases under SSP3*RCP8.5—but still largely increases under SSP5*RCP2.6 and SSP5*RCP8.5 due to the high demographic growth of this population group under SSP5. Finally, the number of low-income communities exposed to transmission risk by both vectors is expected to decrease drastically under SSP1*RCP2.6, SSP5*RCP2.6, and SSP5*RCP8.5, mainly due to the decrease in the net low-income population under these two socioeconomic scenarios. In contrast, due to the increase of low-income populations depicted under SSP3, the exposure of this vulnerable group increases under SSP3*RCP8.5 and reaches 368 (6.1) M person-months in 2080 (for the vector Ae. aegypti). In comparison, this figure shrinks down to 21 (1.7) M person-months under SSP1*RCP2.6, highlighting the crucial role that socioeconomic pathways play in shaping future exposure.
In absolute numbers, the South is where the majority of exposure is located, accounting for 50%–85% of continental exposure to Ae. aegypti VTR and for 46%–64% (depending on time period, scenario combination, and population group) of continental exposure to Ae. albopictus VTR. However, the largest increase in population exposure is projected in the West, with (for instance) a total population exposure to Ae. aegypti shifting from approximately 181 M person-months (baseline) to 589 (39) M under SSP5*RCP8.5 in 2080, which represents a 225% increase relative to 2010 (as opposed to the 177% increase at the CONUS level). The West and Northeast are the only regions where SSP5*RCP8.5 leads to a greater exposure to Ae. albopictus VTR compared to SSP5*RCP2.6, due to the higher temperature suitability for Ae. albopictus under RCP8.5 in these regions. Additionally, the West and Northeast are also where the difference in exposure to Ae.aegypti VTR between SSP5*RCP8.5 and SSP5*RCP2.6 is the highest. These results suggest that climate change will be a comparatively important driver of exposure in these two regions.
3.4. Climate, population, and interaction effects
County-level spatial patterns of dominant effect (i.e. the effect responsible for the major part of the increase or decrease in exposure) show that the population effect is the dominant contributor to both increases and decreases in total population exposure to Ae. aegypti VTR under SSP1*RCP2.6 and SSP5*RCP2.6 (figure 5(a)). Under SSP5*RCP8.5, increases in total population exposure in counties in the West and Northeast are predominantly driven by the climate change effects. Noteworthy, under SSP3*RCP8.5, the climate effect dominates the increase in total population exposure in the overwhelming majority of counties, mainly due to (i) decreased total population and (ii) stronger climate change. Results for total population exposure to Ae. albopictus VTR show similar trends (figure S5), with the notable exception that the climate effect dominates the decrease in exposure in most counties of the South and Midwest under SSP3*RCP8.5 and SSP5*RCP8.5, due to the decrease in temperature suitability for Ae. albopictus forecasted in these regions under RCP8.5.
Figure 5.
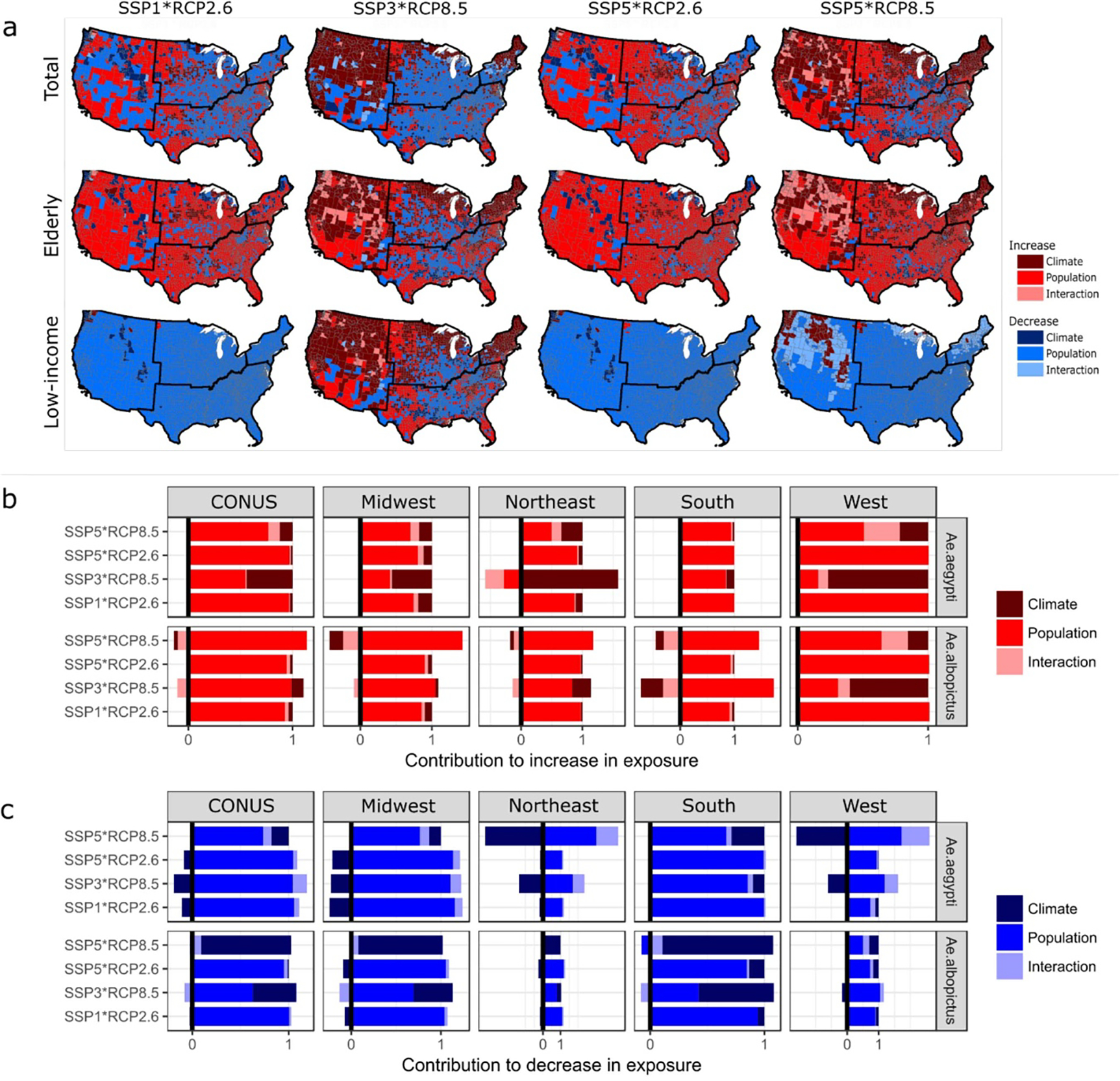
(a) Dominant effect (climate, population, or interaction) responsible for the highest increase (or decrease) in exposure at the county-level, for three population groups (see figure S6 for other population groups) and for exposure to Ae. aegypti VTR only (see figure S5 for exposure to Ae. albopictus VTR); (b) Contribution to increase in total population exposure of each individual effect, aggregated at the country (CONUS) and regional scale, and (c) same for decrease in exposure (see figures S7–S9 for results associated with other population groups). Results are presented for year 2080 only, using the multi-model mean.
While spatial patterns of dominant effects for exposure of outdoor workers, urban population, and children are rather similar to those of the total population exposure (figure S6), spatial patterns for the elderly and low-income communities show large differences. Results show that the population effect dominates the increase in elderly exposure to both Ae. aegypti (figure 5(a)) and Ae. albopictus (figure S5) in most counties, under all combinations (with the exception of SSP3*RCP8.5, where the climate effect dominates in many counties due to the slower growth of elderly population depicted under SSP3). Noteworthy, the interaction effect dominates the increase in elderly exposure to VTR by both Aedes mosquitoes in the West, highlighting the simultaneous increase in temperature suitability and growth of elderly population. Because of the strong decrease in the net number of low-income persons under SSP1 and SSP5, the population effect is the overwhelming contributor to the decrease in exposure of low-income populations (to VTR by both Aedes mosquitoes), under all scenario combination except SSP3*RCP8.5 (due to the increase in poverty described under this scenario).
Aggregated at the country and regional level (figures 5(b)/(c)), results clearly show the dominant contribution of the population effect to both increases and decreases in total population exposure to VTR by Aedes mosquitoes, with some regional exceptions under SSP3*RCP8.5 (e.g. West and Northeast regions) and Ae. albopictus-specific exceptions under SSP5*RCP8.5. This result clearly highlights the crucial role that socioeconomic pathways play in shaping future population exposure to Aedes-borne VTR in the United States.
3.5. Avoided exposure
The use of the scenario matrix also enables exploring the avoided exposure due to (i) shifts in climatic conditions (e.g. resulting from mitigation options) or to (ii) shifts in socioeconomic pathways (e.g. resulting from the implementation of different social policies). Aggregated at the national (CONUS) scale (figure 6), a shift from a high to a low emission scenario (RCP8.5—RCP2.6 shift) leads to a projected decrease in population exposure to Ae. aegypti VTR by 20% (IQR = 5.8) by 2080 (regardless of the population group accounted for), while SSP5—SSP3 and SSP5—SSP1 shifts lead to a higher projected decrease, respectively 52% and 26% (for the total population only). Although results show a dominant effect of demographic/socioeconomic scenarios on avoided exposure, climate mitigation options also play a substantial role in shaping future exposure, particularly in the Northeast and West regions, where a RCP8.5—RCP2.6 shift would lead to greater avoided exposure to Ae. aegypti VTR than a SSP5—SSP1 shift.
Figure 6.
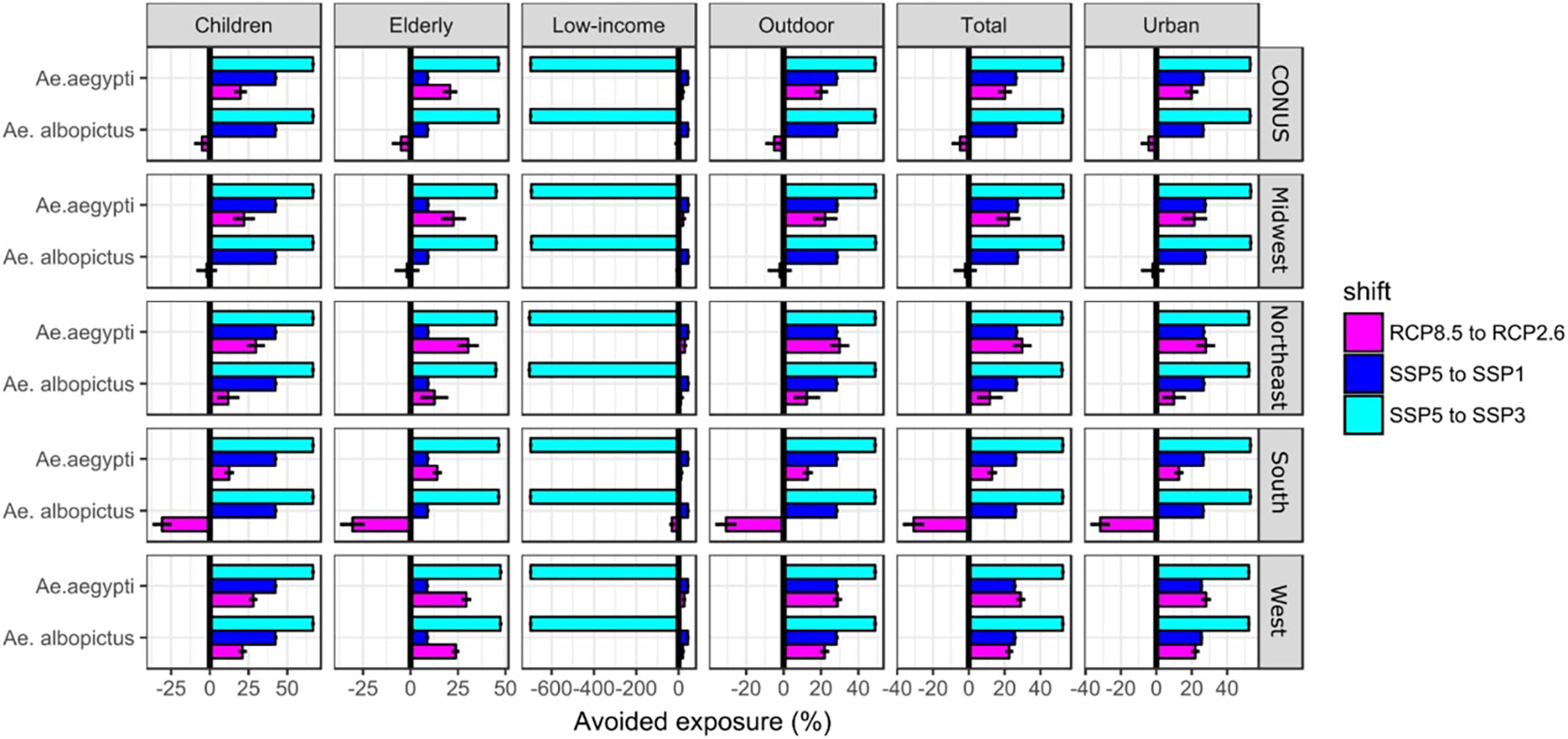
Avoided exposure to Aedes-borne VTR, in relative terms (%), due to shifts from RCP8.5 to RCP2.6 (assuming baseline socioeconomic/demographic conditions and multi-model mean), from SSP5 to SSP1, and from SSP5 to SSP3 (assuming baseline climatic conditions). Results are shown for year 2080 only and are aggregated at the country (CONUS) and regional level, for the six population groups and the two Aedes mosquitoes. Errors bars represent the multi-model interquartile ranges (IQRs).
Regarding exposure to Ae. albopictus VTR, shifts in SSPs would lead to avoided exposure of similar magnitude to that of avoided exposure to Ae. aegypti VTR, while the effect of a RCP8.5—RCP2.6 shift would be reversed. Indeed, a RCP8.5—RCP2.6 shift would not decrease, but rather increase, exposure to Ae. albopictus VTR (by 5% (7.7)), highlighting the contrasting influence of climate change scenarios on Aedes-borne VTR in the United States. Similar findings apply in the South where a RCP8.5—RCP2.6 shift would increase exposure to Ae. albopictus VTR by as much as 31% (10). The West and Northeast are the only regions where a RCP8.5—RCP2.6 would decrease population exposure to Ae. albopictus VTR (by 29% (3.3) and 12% (11) respectively).
Trends in avoided exposure for outdoor workers, children, and urban populations follow those of the total population. However, trends differ for the elderly and low-income populations. Avoided exposure of elderly due to a SSP5—SSP3 shift largely dominates the avoided exposure. Conversely, SSP5—SSP1 shift lead to very little avoided exposure, in most cases inferior to the avoided exposure due to RCP8.5—RCP2.6 shifts (for Ae. aegypti only). This is explained by the low net difference in the number of elderly between these two scenarios. Finally, due to the large difference in the number of low-income persons between SSP5 and SSP3, a SSP5—SSP3 shift would lead to increased exposure of 700% in all regions and for VTR by both Aedes mosquitoes. This highlights again the important contribution of socioeconomic development pathways to future population exposure to Aedes-borne VTR in the United-States.
4. Conclusions
We projected that population exposure to Aedes-borne VTR will increase during the 21st century across the United States, but with contrasting patterns depending on (i) the population group of concern, (ii) the species of Aedes, (iii) the emissions scenario (i.e. RCPs), and (iv) the socioeconomic pathway (i.e. SSPs). We demonstrated that the type of socioeconomic pathway plays a critical role in shaping future population vulnerability and exposure to Aedes-borne VTR, particularly when the pathway projects a decrease in certain vulnerable groups such as low-income populations. Our approach emphasizes the importance of including SSP-based population projections to ensure a more realistic portrayal of future Aedes-borne VTR under climate change scenarios. The differential exposure across the myriad SSP-RCP scenario combinations underscores the wide range of potential outcomes, and therefore the need to use scenarios to span future climatic and societal uncertainties. This framework provides insight into the substantial avoided exposure that certain social policies and mitigation efforts could trigger. One particularly unique aspect of the present study is its breakdown of population projections into potentially vulnerable subgroups. From this, we found that the trends in exposure of some vulnerable subgroups differ from that of the total population. For instance, (i) exposure of the urban population increases slightly faster than that of the total population due to the continuing urbanization, (ii) exposure of the elderly drastically increases under all SSP-RCP combinations due to the rapid ageing of the US population, and (iii) the number of low-income communities exposed to Aedes-borne VTR rapidly drops with the decrease of the net low-income population depicted under some scenarios.
While a comprehensive list of limitations is given in (Ryan et al 2019), the most important limitation of the projections of future Aedes-borne VTR is the assumption that it is only driven by changes in temperature due to climate change, when evidence suggests that land use change, urbanization, population growth, migration, and economic development play a significant role in shaping the future transmission of Aedes-borne viruses (Astrom et al 2012, Alimi et al 2015, Messina et al 2016, Kraemer et al 2019). Additionally, other climate factors such as rainfall and humidity are not accounted for in the VTR model, yet both impact Aedes survival (Halstead 2008, Schmidt et al 2018). Excluding these may affect estimates of VTR, particularly in arid regions, though human water storage practices and human-created microclimates in arid areas may mediate the influence of rainfall and ambient humidity (Beebe et al 2009, Hayden et al 2010). Another possible limitation of the VTR model is that it was parameterized for dengue virus and—although validated with human case data during the recent chikungunya and Zika epidemics (Mordecai et al 2017)—may incompletely represent transmission risk associated with chikungunya or Zika viruses, or specific dengue virus serotypes. This study is also associated with limitations related to the SSP-based projections of vulnerable population groups (see text S1), which are highly uncertain. Thus, they are most valuable as means of placing bounds of uncertainty on possible future population outcomes. Finally, the differing historical baseline population (2010) and baseline climate (1960–1990) periods may affect future exposure projections, though this effect is unlikely to be a substantial contributor to uncertainty given the uncertainty of the population projections.
We view the SSP*RCP framework as a promising tool to explore the complex interactions among socioeconomic development, climate change, and the future spread of VBDs—as recently highlighted in (Messina et al 2019). The main advantages of this framework include (i) the SSPs are being increasingly quantified (on gridded scales) for a number of relevant variables such as population growth (Jones and O’Neill 2016, Gao 2017), GDP (Murakami and Yamagata 2019), and urbanization (Gao and O’Neill 2019, Li et al 2019b), (ii) the scenarios account for the wide range of uncertainties in both socioeconomic development type and emission scenarios, (iii) the scenario matrix can be used to explore the relative contribution of climate change and socioeconomic development to the future spread of VBDs, and (iv) the growing literature on the vulnerability of populations—and of the health sector—under the SSPs (Ebi 2014, Sellers and Ebi 2018, Rao et al 2018, Zimm et al 2018, Welborn 2018, Striessnig and Loichinger 2015) can inform about the future vulnerability of exposed populations. We used the framework for VBDs, but the same framework could be consistently applied across all major sectors affected by climate change (e.g. agriculture, health, water resources).
Supplementary Material
Acknowledgments
This work was partly funded by the Swiss National Science Foundation’s Doc Mobility scholarship and by the National Institutes of Health, NIAID R01AI091843. SJR was supported in part by NSF DEB EEID 1518681. The authors declare no known conflict of interest. NCAR is supported by the National Science Foundation.
Guillaume Rohat, the lead author of this paper, passed away during the review process. Guillaume was an energetic early-career scientist who inspired those around him with his passion for discovery, grasp of complexity, and ability to communicate research. He was also a wonderful friend and colleague who will be missed dearly.
Footnotes
Supplementary material for this article is available online
Data availability statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.
References
- Alfieri L, Feyen L, Dottori F and Bianchi A 2015. Ensemble flood risk assessment in Europe under high end climate scenarios Glob. Environ. Change 35 199–212 [Google Scholar]
- Alimi TO, Fuller DO, W A Q, S V H, Arevalo-Herrera M, M L Q, M V G L and J C B 2015. Predicting potential ranges of primary malaria vectors and malaria in northern South America based on projected changes in climate, land cover and human population Parasites Vectors 8 431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aström C, Rocklöv J, Hales S, Béguin A, Louis V and Sauerborn R 2012. Potential distribution of dengue fever under scenarios of climate change and economic development Ecohealth 9 448–54 [DOI] [PubMed] [Google Scholar]
- Badawi A, Velummailum R, Ryoo SG, Senthinathan A, Yaghoubi S, Vasileva D, Ostermeier E, Plishka M, Soosaipillai M and Arora P 2018. Prevalence of chronic comorbidities in dengue fever and West Nile virus: A systematic review and meta-analysis PLoS One 13 e0200200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beard CB, Eisen RJ, Barker CM, Garafolo JF, Hahn MB, Hayden MH, Monaghan AJ, Ogden NH and Schramm PJ 2016. Vector-borne diseases The Impacts of Climate Change on Human Health in the United States: A Scientific Assessment (Washington, DC: US Global Change Research Program; ) 129–56 [Google Scholar]
- Beebe NW, Cooper RD, Mottram P and Sweeney AW 2009. Australia’s dengue disk driven by human adaptation to climate change PLoS Negl. Trop. Dis. 3 e429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benedict MQ, Levine RS, Hawley WA and Lounibos LP 2007. Spread of the tiger: global risk of invasion by the mosquito Aedes albopictus Vector-Borne Zoonotic Dis. 7 76–85 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett CM and Mcmichael AJ 2010. Non-heat related impacts of climate change on working populations Glob. Health Action 3 5640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonizzoni M, Gasperi G, Chen X and James AA 2013. The invasive mosquito species Aedes albopictus: current knowledge and future perspectives Trends Parasitol. 29 460–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brien JD, Uhrlaub JL, Hirsch A, Wiley CA and Nikolich-Žugich J 2009. Key role of T cell defects in agerelated vulnerability to West Nile virus J. Exp. Med. 206 2735–45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown S, Nicholls RJ, Goodwin P, Haigh ID, Lincke D, Vafeidis AT and Hinkel J 2018. Quantifying land and people exposed to sea-level rise with no mitigation and 1.5°C and 2.0°C rise in global temperatures to year 2300 Earth’s Future 6 583–600 [Google Scholar]
- Brunkard JM, López JLR, Ramirez J, Cifuentes E, Rothenberg SJ, Hunsperger EA, Moore CG, Brussolo RM, Villarreal NA and Haddad BM 2007. Dengue fever seroprevalence and risk factors, Texas–Mexico border, 2004 Emerging Infect. Dis. 13 1477–83 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bureau of Labor Statistics, US Department of Labor 2017. Over 90 percent of protective service and construction and extraction jobs require work outdoors : the Economics Daily: U.S. Bureau of Labor Statistics The Economics Daily (available at https://www.bls.gov/opub/ted/2017/over-90-percent-of-protective-service-and-construction-and-extraction-jobs-require-work-outdoors.htm)
- Caminade C, Kovats S, Rocklov J, Tompkins AM, Morse AP, Colón-González FJ, Stenlund H, Martens P and Lloyd SJ 2014. Impact of climate change on global malaria distribution Proc. Natl Acad. Sci. U.S.A. 111 3286–91 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caminade C, Medlock JM, Ducheyne E, Mcintyre KM, Leach S, Baylis M and Morse AP 2012. Suitability of European climate for the Asian tiger mosquito Aedes albopictus: recent trends and future scenarios J. R. Soc. Interface 9 2708–17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell LP, Luther C, Moo-Llanes D, Ramsey JM, Danis-Lozano R and Peterson AT 2015. Climate change influences on global distributions of dengue and chikungunya virus vectors Phil. Trans. Soc. B 370 20140135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CDC 2020a. Areas with Zika CDC (available at: https://www.cdc.gov/zika/geo/index.html)
- CDC 2020b. Reporting and Surveillance—Zika Virus Centers for Disease Control and Prevention (available at: http://www.cdc.gov/zika/reporting/index.html)
- Chadee DD and Martinez R 2000. Landing periodicity of Aedes aegypti with implications for dengue transmission in Trinidad, West Indies J. Vector Ecol. 25 158–63 [PubMed] [Google Scholar]
- Chouin-Carneiro T, Vega-Rua A, Vazeille M, Yebakima A, Girod R, Goindin D, Dupont-Rouzeyrol M, Lourenço-de-oliveira R and Failloux A-B 2016. Differential susceptibilities of Aedes aegypti and Aedes albopictus from the Americas to Zika virus PLoS Negl. Trop. Dis. 10 e0004543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chowdhury S, Dey S and Smith KR 2018. Ambient PM 2.5 exposure and expected premature mortality to 2100 in India under climate change scenarios Nat. Commun. 9 1–10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delatte H, Desvars A, Bouétard A, Bord S, Gimonneau G, Vourc’h G and Fontenille D 2010. Blood-feeding behavior of Aedes albopictus, a vector of Chikungunya on La Réunion Vector-Borne Zoonotic Dis. 10 249–58 [DOI] [PubMed] [Google Scholar]
- Dye C 2014. After 2015: infectious diseases in a new era of health and development Phil. Trans. R. Soc. B 369 20130426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebi KL 2014. Health in the new scenarios for climate change research Int. J. Environ. Res. Public Health 11 30–46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebi KL et al. 2014. A new scenario framework for climate change research: background, process, and future directions Clim. Change 122 363–72 [Google Scholar]
- Ebi KL, Hess JJ and Isaksen TB 2016. Using uncertain climate and development Information in health adaptation planning Curr. Envir. Health Rpt. 3 99–105 [DOI] [PubMed] [Google Scholar]
- Eisen L and Moore CG 2013. Aedes (Stegomyia) aegypti in the continental United States: a vector at the cool margin of its geographic range J. Med. Entomol. 50 467–78 [DOI] [PubMed] [Google Scholar]
- Epelboin Y, Talaga S, Epelboin L and Dusfour I 2017. Zika virus: an updated review of competent or naturally infected mosquitoes PLoS Negl. Trop. Dis. 11 e0005933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faraji A, Egizi A, Fonseca DM, Unlu I, Crepeau T, Healy SP and Gaugler R 2014. Comparative host feeding patterns of the Asian tiger mosquito, Aedes albopictus, in urban and suburban Northeastern USA and implications for disease transmission PLoS Negl. Trop. Dis. 8 e3037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer D, Thomas SM, Suk JE, Sudre B, Hess A, Tjaden NB, Beierkuhnlein C and Semenza JC 2013. Climate change effects on Chikungunya transmission in Europe: geospatial analysis of vector’s climatic suitability and virus’ temperature requirements Int. J. Health Geogr. 12 51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao J 2017. Downscaling Global Spatial Population Projections from 1/8-degree to 1-km Grid Cells (Boulder, CO: National Center for Atmospheric Research; ) (https://opensky.ucar.edu/islandora/object/technotes%3A553/datastream/PDF/download/citation.pdf) [Google Scholar]
- Gao J and O’Neill BC 2019. Data-driven spatial modeling of global long-term urban land development: the SELECT model Environ. Model. Softw. 119 458–71 [Google Scholar]
- Hahn MB, Eisen RJ, Eisen L, Boegler KA, Moore CG, Mcallister J, Savage HM and Mutebi J-P 2016. Reported distribution of Aedes (Stegomyia) aegypti and Aedes (Stegomyia) albopictus in the United States, 1995–2016 (Diptera: culicidae) J. Med. Entomol. 53 1169–75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halstead SB 2008. Dengue virus-mosquito interactions Annu. Rev. Entomol. 53 273–91 [DOI] [PubMed] [Google Scholar]
- Hasegawa T, Fujimori S, Shin Y, Takahashi K, Masui T and Tanaka A 2014. Climate change impact and adaptation assessment on food consumption utilizing a new scenario framework Environ. Sci. Technol. 48 438–45 [DOI] [PubMed] [Google Scholar]
- Hauer ME 2019. Population projections for U.S. counties by age, sex, and race controlled to shared socioeconomic pathway Sci. Data 6 1–15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hausfather Z and Peters GP 2020. Emissions—the ‘business as usual’ story is misleading Nature 577 618–20 [DOI] [PubMed] [Google Scholar]
- Hayden MH, Uejio CK, Walker K, Ramberg F, Moreno R, Rosales C, Gameros M, Mearns LO, Zielinski-Gutierrez E and Janes CR 2010. Microclimate and human factors in the divergent ecology of Aedes aegypti along the Arizona, U.S./Sonora, MX border EcoHealth 7 64–77 [DOI] [PubMed] [Google Scholar]
- Hernández D and Bird S 2010. Energy burden and the need for integrated low-income housing and energy policy Poverty Public Policy 2 5–25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hijmans RJ, Cameron SE, Parra JL, Jones PG and Jarvis A 2005. Very high resolution interpolated climate surfaces for global land areas Int. J. Climatol. 25 1965–78 [Google Scholar]
- Hiscox A et al. 2013. Risk factors for the presence of Aedes aegypti and Aedes albopictus in domestic water-holding containers in areas impacted by the Nam Theun 2 Hydroelectric Project, Laos Am. J. Trop. Med. Hyg. 88 1070–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hotez PJ 2018. The rise of neglected tropical diseases in the ‘new Texas’ PLoS Negl. Trop. Dis. 12 e0005581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang L and O’Neill BC 2017. Global urbanization projections for the Shared Socioeconomic Pathways Glob. Environ. Change 42 193–9 [Google Scholar]
- Jones B and O’Neill BC 2016. Spatially explicit global population scenarios consistent with the Shared Socioeconomic Pathways Environ. Res. Lett 11 084003 [Google Scholar]
- Jones B, O’Neill BC, Mcdaniel L, Mcginnis S, Mearns LO and Tebaldi C 2015. Future population exposure to US heat extremes Nat. Clim. Change 5 652–5 [Google Scholar]
- Jones B, Tebaldi C, O’Neill BC, Oleson K and Gao J 2018. Avoiding population exposure to heat-related extremes: demographic change vs climate change Clim. Change 146 423–37 [Google Scholar]
- Kc S and Lutz W 2017. The human core of the shared socioeconomic pathways: population scenarios by age, sex and level of education for all countries to 2100 Glob. Environ. Change 42 181–92 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kendrick K, Stanek D and Blackmore C 2014. Notes from the field: transmission of Chikungunya virus in the continental United States—Florida, 2014 MMWR Morb. Mortal Wkly. Rep. 63 1137. [PMC free article] [PubMed] [Google Scholar]
- Knorr W, Arneth A and Jiang L 2016. Demographic controls of future global fire risk Nat. Clim. Change 6 781–5 [Google Scholar]
- Kraemer MU et al. 2015. The global distribution of the arbovirus vectors Aedes aegypti and Ae. albopictus eLife 4 e08347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraemer MUG et al. 2019. Past and future spread of the arbovirus vectors Aedes aegypti and Aedes albopictus Nature Microbiology 4 854–63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kriegler E, Edmonds J, Hallegatte S, Ebi KL, Kram T, Riahi K, Winkler H and van Vuuren D P 2014. A new scenario framework for climate change research: the concept of shared climate policy assumptions Clim. Change 122 401–14 [Google Scholar]
- Kriegler E, O’Neill BC, Hallegatte S, Kram T, Lempert RJ, Moss RH and Wilbanks T 2012. The need for and use of socio-economic scenarios for climate change analysis: a new approach based on shared socio-economic pathways Glob. Environ. Change 22 807–22 [Google Scholar]
- Li S, Gilbert L, Vanwambeke SO, Yu J, Purse BV and Harrison PA 2019a. Lyme disease risks in europe under multiple uncertain drivers of change Environ. Health Perspect. 127 067010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X, Zhou Y, Eom J, Yu S and Asrar GR 2019b. Projecting global urban area growth through 2100 based on historical time series data and future Shared Socioeconomic Pathways Earth’s Future 7 351–62 [Google Scholar]
- Liu-Helmersson J, Brännström Å, Sewe MO, J C S and Rocklöv J 2019a. Estimating past, present, and future trends in the global distribution and abundance of the arbovirus vector Aedes aegypti under climate change scenarios Front Public Health 7 148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu-Helmersson J, Rocklöv J, Sewe M and Brännström Å 2019b. Climate change may enable Aedes aegypti infestation in major European cities by 2100 Environ. Res. 172 693–9 [DOI] [PubMed] [Google Scholar]
- Martens P, Kovats RS, Nijhof S, de Vries P, Livermore MTJ, Bradley DJ, Cox J and Mcmichael AJ 1999. Climate change and future populations at risk of malaria Glob. Environ. Change 9 S89–107 [Google Scholar]
- Messina JP et al. 2019. The current and future global distribution and population at risk of dengue Nat. Microbiol. 4 1508–15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Messina JP. et al. Mapping global environmental suitability for Zika virus. eLife. 2016;5:e15272. doi: 10.7554/eLife.15272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monaghan AJ, Sampson KM, Steinhoff DF, Ernst KC, Ebi KL, Jones B and Hayden MH 2016. The potential impacts of 21st century climatic and population changes on human exposure to the virus vector mosquito Aedes aegypti Clim. Change 146 487–500 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mordecai EA et al. 2017. Detecting the impact of temperature on transmission of Zika, dengue, and chikungunya using mechanistic models PLoS Negl. Trop. Dis. 11 e0005568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moss R et al. 2010. The next generation of scenarios for climate change research and assessment Nature 463 747–56 [DOI] [PubMed] [Google Scholar]
- Murakami D and Yamagata Y 2019. Estimation of gridded population and GDP scenarios with spatially explicit statistical downscaling Sustainability 11 2106 [Google Scholar]
- O’Neill BC et al. 2017. The roads ahead: narratives for shared socioeconomic pathways describing world futures in the 21st century Glob. Environ. Change 42 169–80 [Google Scholar]
- Proestos Y, Christophides GK, Ergüler K, Tanarhte M, Waldock J and Lelieveld J 2015. Present and future projections of habitat suitability of the Asian tiger mosquito, a vector of viral pathogens, from global climate simulation Phil. Trans. R. Soc. B 370 20130554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radke EG et al. 2012. Dengue outbreak in Key West, Florida, USA, 2009 Emerging Infect. Dis. 18 135–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao ND, Sauer P, Gidden M and Riahi K 2019. Income inequality projections for the Shared Socioeconomic Pathways (SSPs) Futures 105 27–39 [Google Scholar]
- Reiter P et al. 2003. Texas lifestyle limits transmission of dengue virus Emerging Infect. Dis. 9 86–89 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rezza G et al. 2007. Infection with chikungunya virus in Italy: an outbreak in a temperate region Lancet 370 1840–6 [DOI] [PubMed] [Google Scholar]
- Roche B, Léger L, L’Ambert G, Lacour G, Foussadier R, Besnard G, Barré-Cardi H, Simard F and Fontenille D 2015. The spread of Aedes albopictus in metropolitan France: contribution of environmental drivers and human activities and predictions for a near future PLoS One 10 e0125600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogelj J et al. 2018. Scenarios towards limiting global mean temperature increase below 1.5 °C Nat. Clim. Change 8 325–32 [Google Scholar]
- Rohat G, Flacke J, Dosio A, Dao H and van Maarseveen M 2019. Projections of human exposure to dangerous heat in African cities under multiple socioeconomic and climate scenarios Earth’s Future 7 528–46 [Google Scholar]
- Ramos MM et al. 2008. Epidemic dengue and dengue hemorrhagic fever at the Texas–Mexico border: results of a household-based seroepidemiologic survey, December 2005 Am. J. Trop. Med. Hyg. 78 364–9 [PubMed] [Google Scholar]
- Rosenberg R et al. 2018. Vital signs: trends in reported vectorborne disease cases—United States and territories, 2004–2016 MMWR Morb. Mortal Wkly. Rep. 67 496–501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan SJ, Carlson CJ, Mordecai EA and Johnson LR 2019. Global expansion and redistribution of Aedes-borne virus transmission risk with climate change PLoS Negl. Trop. Dis. 13 e0007213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salje H, Paul KK, Paul R, Rodriguez-Barraquer I, Rahman Z, Alam MS, Rahman M, Al-Amin HM, Heffelfinger J and Gurley E 2019. Nationally-representative serostudy of dengue in Bangladesh allows generalizable disease burden estimates eLife 8 e42869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt CA, Comeau G, Monaghan AJ, Williamson DJ and Ernst KC 2018. Effects of desiccation stress on adult female longevity in Aedes aegypti and Ae. albopictus (Diptera: culicidae): results of a systematic review and pooled survival analysis Parasites Vectors 11 267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulte PA et al. 2016. Advancing the framework for considering the effects of climate change on worker safety and health J Occup. Environ. Hyg. 13 847–65 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sellers S and Ebi KL 2018. Climate change and health under the shared socioeconomic pathway framework Int. J. Environ. Res. Public Health 15 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stocker TF, Qin D, Plattner G-K, Tignor MMB and Allen SK 2013. Climate Change 2013: The Physical Science Basis. Contribution of Working Group I to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change (Cambridge: Cambridge University Press; ) [Google Scholar]
- Striessnig E and Loichinger E 2015. Future differential vulnerability to natural disasters by level of education Vienna Yearbook Pop. Res. 13 221–40 [Google Scholar]
- Suk JE 2016. Climate change, malaria, and public health: accounting for socioeconomic contexts in past debates and future research Wiley Interdiscip. Rev. Clim. Change 7 551–68 [Google Scholar]
- Swanson DA, Schlottmann A and Schmidt B 2010. Forecasting the population of census tracts by age and sex: an example of the Hamilton–Perry method in action Popul. Res. Policy Rev. 29 47–63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tjaden NB, Suk JE, Fischer D, Thomas SM, Beierkuhnlein C and Semenza JC 2017. Modelling the effects of global climate change on Chikungunya transmission in the 21st century Sci. Rep. 7 3813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trout A et al. 2010. Locally acquired dengue–Key West, Florida, 2009–2010 MMWR Morb. Mortal. Wkly. Rep. 59 577–81 [PubMed] [Google Scholar]
- van Vuuren DP et al. 2011. The representative concentration pathways: an overview Clim. Change 109 5–31 [Google Scholar]
- Weaver SC and Lecuit M 2015. Chikungunya virus and the global spread of a mosquito-borne disease New Engl. J. Med. 372 1231–9 [DOI] [PubMed] [Google Scholar]
- Welborn L 2018. Africa and climate change—projecting vulnerability and adaptive capacity ISS Africa Rep. 2018 1–24 (https://issafrica.org/research/africa-report/africa-and-climate-change-projecting-vulnerability-and-adaptive-capacity) [Google Scholar]
- Zimm C, Sperling F and Busch S 2018. Identifying sustainability and knowledge gaps in socio-economic pathways vis-à-vis the sustainable development goals Economies 6 1–22 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


