Abstract
Mitochondrial structure often determines the function of these highly dynamic, multifunctional, eukaryotic organelles, which are essential for maintaining cellular health. The dynamic nature of mitochondria is apparent in descriptions of different mitochondrial shapes [e.g., donuts, megamitochondria (MGs), and nanotunnels] and crista dynamics. This review explores the significance of dynamic alterations in mitochondrial morphology and regulators of mitochondrial and cristae shape. We focus on studies across tissue types and also describe new microscopy techniques for detecting mitochondrial morphologies both in vivo and in vitro that can improve understanding of mitochondrial structure. We highlight the potential therapeutic benefits of regulating mitochondrial morphology and discuss prospective avenues to restore mitochondrial bioenergetics to manage diseases related to mitochondrial dysfunction.
Mitochondria are not kidney beans
Mitochondria (see Glossary) were discovered in the 1800s [1], and the advent of high-resolution electron microscopy (EM) techniques [2] allowed Palade to identify their unique double membrane in the 1950s [3,4]. Additional studies throughout the mid-1900s [1] revealed the link between mitochondrial structure and function [5], and continual EM advancements have enabled 3D renderings of cellular ultrastructure [6]. Across the 1990s, developments in plastic section electron tomography (ET), which remains clinically relevant today, allowed researchers to explicate the complexity of mitochondrial internal micro-compartments, known as cristae [7]. Today, cryo-ET, using principles of rapid freezing, allows for 3D reconstruction of mitochondria and localizing associated large protein complexes (e.g., ATP synthase [8]) in their native state, further elucidating the mitochondrial structure–function interplay [9].
Recent advances in research have revealed mitochondria as dynamic organelles with heterogeneous shapes and networks both within and among cell types and tissues (Figure 1). For example, mitochondrial morphology can range from a single compact (e.g., in cardiac tissue [10]) or swollen (e.g., in brown adipose tissue [11]) structure to highly branched networks (as in skeletal muscle [12-14]) [15]. Phenotypes can exist ubiquitously across tissue types, although certain tissue types or disease states are more likely to display certain morphologies: for example, liver mitochondria shift between spherical and donut- or c-shaped forms [16,17], cardiac muscle cells have elongated mitochondria arranged in parallel [18], and skeletal muscles have highly connected mitochondrial networks [19]. These phenotypes are best analyzed via high-resolution 3D technology. For instance, low-resolution techniques may mistake nanotunnels for fragmentation [20] and utilizing EM thin sections alone may falsely show cup- or stomatocyte-shaped mitochondria as mitochondrial donuts [21]. Mitochondria can also display distinct morphologies and biochemical properties within a given cell [12,22]: populations near the plasma membrane play essential roles in the functional coupling of ATP-guided ion channels [23], whereas perinuclear networks regulate gene transcription under hypoxic conditions by increasing reactive oxygen species (ROS) in the nucleus [24]. The variation in structure between these subpopulations may reflect differences in function [13,25]. Different intra- and intertissue mitochondrial networks and populations also display different responses to substrates, inhibitors, and oxidative stress, highlighting their complexity, diversity, and plasticity [18,22,26,27].
Figure 1. 3D structures of mitochondria.
3D structures of mitochondria beyond the kidney bean shape, which may have altered cristae dynamics. The eight so-far observed mitochondrial shapes are compact, branching, nanotunnels, mitochondrial donut, elongated, large volume, small volume, and megamitochondria, and these structures, as obtained from 3D reconstructions from serial block face scanning electron microscopy, may have functional implications.
In addition to producing energy in the form of ATP, mitochondria are involved in calcium signaling, apoptosis, and the oxidative stress response [15,28]. Mitochondrial dynamics and heterogeneity are crucial for regulating these processes and maintaining cellular homeostasis both within individual cells and across an organism [12,29]. Several mechanisms, including mitochondrial biogenesis, transport, mitophagy, fusion, and fission, regulate mitochondrial structure and function [29,30]. Other cellular components, such as lipid droplets and the endoplasmic reticulum (ER), can also influence mitochondrial structure through interorganellar contact sites that modulate mitochondrial biochemical properties, functional capacity, and dynamics [19,31-33].
Studies of 3D mitochondrial structures indicate that structural changes may occur during aging and that these changes may be associated with functional deficiencies and the development of mitochondria-dependent diseases [10,14]. Additionally, a recent review summarized how mitochondrial phenotypic frequencies may change in response to stimuli [15]. As we explore here, the differential responses to aging among disparate tissue types, which can include the presence of unique mitochondrial structures [34], may be a key pathological factor. However, the relative frequencies of these unique shapes, including MGs, nanotunnels, donuts, and elongated, branched, and compact morphologies (Table 1), must be further elucidated in disease states.
Table 1.
Eight 3D phenotypes of mitochondria
| Phenotype | Proposed functional relevance | Potential disease relevance | Refs |
|---|---|---|---|
| Small volume | Potentially occur posterior to fission. Often found in regions of high energy need so they may deliver energy rapidly. Also commonly observed throughout the aging process. | Reduced volume may be representative of Parkinson’s and Alzheimer’s disease. | [10,14,15] |
| Large volume | Potentially occur posterior to fusion. The larger volume allows for increased energy generation by a few mitochondria. Potentially linked to a loss of membrane potential. | Excessive enlargement may be linked to heart failure. | [11,123] |
| Compact | Exhibit enhanced oxidative phosphorylation and potential rearrangement of cristae organization. | May be associated with oxidative stress-related conditions, such as ischemia–reperfusion injury and aged heart. | [10,15] |
| Elongated | Exhibit a higher surface area:volume ratio, potentially for increased interactions. | Excessive elongation may occur in neurodegenerative diseases. | [15] |
| Megamitochondria | Potentially a sign of mitochondrial swelling or a compensatory mechanism for cellular stress. | May arise in liver diseases, such as alcoholic liver disease and nonalcoholic fatty liver disease. | [40,41,46] |
| Nanotunnel | Possibly a sign of mitochondrial immobility. Enables ion communication between mitochondria and potential mitochondrial dynamic protein transfers. | Arise in skeletal muscle of mtDNA disease, in cardiomyopathy, and cardiac calcium impairment. | [48,49] |
| Mitochondria donut |
Potentially a sign of incomplete fission or a compensatory mechanism for increasing surface area interactions at the cost of cristae. | Arise in the brain of individuals with cognitive decline. | [17,35-37,72] |
| Branching | An interconnected network of mitochondria that can enable distribution of energetics in the mitochondria. | Altered branching may be observed in cancers. | [10] |
Here, we first describe mitochondrial donuts, MGs, and tunnels in different cell types and in response to different stimuli before discussing how these shapes are related to the organization and orientation of their cristae. We then build on the overview of these internal and overall structures by describing how fusion and fission events, contacts with other organelles, and other structural regulators impact mitochondrial shape. Finally, we explore the potential clinical implications of improved knowledge of mitochondrial structures and dynamics.
Mitochondrial shapes
Mitochondria can be donuts
Ahmad et al. reported that donut-shaped mitochondria may be an early cellular stress marker [35]. They showed that ROS production induced by complex I and III inhibitors resulted in the transient and reversible transition from tubular to donut-shaped mitochondrial structures, which are associated with increased mitochondrial ROS levels [35]. The formation of these structures may also exhibit tissue dependency. Bleck et al. observed in healthy adult mice that, compared with oxidative muscle fibers, glycolytic muscle fibers showed a higher frequency of donut-shaped mitochondria [19]. In contrast, donut-shaped mitochondria may arise more frequently in the liver due to metabolic stress [17]. Donut-shaped mitochondria can also appear in disease states: for example, the memory impairment characteristic of Alzheimer’s disease occurs concomitantly with an increased prevalence of neuronal donut-shaped mitochondria [36].
Our understanding of donut-shaped mitochondria has evolved alongside the techniques that allow us to model them, including EM [36] and live-cell imaging [37]. Donut-shaped mitochondria form when the opposite ends of a mitochondrion fuse [37], although the mechanism underlying this process remains largely unknown. While it has been suggested that donut-shaped mitochondria can form independent from fission, these fission-independent models likely show collapsed mitochondria that phenotypically appear as cup shaped, which represent a fission-independent response to mitochondrial membrane potential loss [21]. Donut mitochondria have been observed under hypoxic conditions [37], and are associated with reduced mitochondrial fusion events and changes in osmotic pressure that lead to diminished neuronal synapses [36,38]. When returned to nonstressed conditions, donut-shaped mitochondria readily revert to other morphologies [37], suggesting that the donut shape may represent an adaptive response for increasing mitochondrial surface area for organelle contacts [15] or resisting mitophagy [39]. Donut-shaped mitochondria maintain the overall mitochondrial membrane potential (generated by an electrochemical gradient across the inner mitochondrial membrane). This suggests that donut formation may protect against mitochondrial function loss by reducing the Gibbs free energy of the system, which inversely increases membrane potential [38,39]. However, it is unclear if other mitochondrial structures depend on Gibbs free energy, as well as associated thermodynamic temperature. While past results have found that even under uncouplers, mitochondria donut diameters tend to be approximately 1.3 μm [37], it is unclear if donut size may determine the bending energy, a major barrier to donut formation that may be counterbalanced by stress-inducible mitochondrial osmotic pressure [38]. Furthermore, as the study of mitochondrial donuts continues, advanced methods must be utilized, as fluorescence and thin EM techniques may mistakenly identify discoid-, curved-, and vase-shaped mitochondria as donuts [21].
Mitochondria can be mega
Mitochondria that swell to two to three times their typical size (from an average length of ~5 μm up to 10 μm) while maintaining outer membrane integrity are characterized as MGs [40]. Although these phenotypes can reach the size of the nucleus [41], ultrastructural or 3D reconstruction techniques are useful to identify them [40,41]. MGs form in response to diseases, such as nonalcoholic fatty liver disease [41], and environmental or cellular stressors [40]. However, the effect of MG formation on cell function is unknown.
The mechanism underlying MG formation is not fully understood, but mitochondrial fission and fusion defects are likely contributors. During stress, fusion increases mitochondrial complementation and maximizes oxidative capacity as a compensatory mechanism [42]. MG formation may also result from stress-induced hyperfusion [43], the accumulation of damaged or dysfunctional mitochondria due to fission or fusion defects [44], and mitochondrial membrane potential–dependent changes in mitochondrial fission and fusion [45].
The ER-associated degradation (ERAD) pathway also regulates mitochondrial size, and ERAD deficiency leads to MG formation, suggesting that MG formation may be regulated through inter-organelle mechanisms [46]. Given there is a known association between ER stress and mitochondrial elongation [47], Zhou et al. investigated how ERAD deficiency affects mitochondria and found that deficiency in the ERAD component Sel1L promotes MG formation in brown adipocytes, possibly due to inhibited fission or accelerated fusion [46]. This finding suggests that MG formation may be affected by changes in expression of specific proteins at the mitochondrial–ER contact sites (MERCs).
Mitochondria can form tunnels
Investigations of several diseases have uncovered various specialized mitochondrial shapes. In Alzheimer’s disease, 3D EM revealed a novel elongated mitochondrial phenotype known as ‘mitochondria-on-a-string’ [20] – later termed nanotunnels. As reviewed [48], mitochondrial nanotunnels are thin (<200-nm diameter) double membranes that connect adjacent mitochondria (up to 30 μm in distance) and can transport proteins and molecules across the mitochondrial membrane into connected organelles. Nanotunnels have been detected in diseased tissue and are hypothesized to result from incomplete fission [48], although it is unclear whether their formation requires specific types of fission (e.g., midpoint or endpoint). The effect of nanotunnel formation on the distribution of dynamic mitochondrial proteins and the structure dependence of protein localization also requires investigation. The spatial orientation of mitochondrial proteins may reveal nanotunnel formation mechanisms, although the specific pathways involved in healthy and disease conditions remain underexplored.
In human skeletal tissue samples from a patient with mitochondrial disease and large-scale mitochondrial DNA (mtDNA) deletion, Vincent and colleagues identified an 18.1-fold increase in nanotunnel formation compared with healthy samples [13], suggesting a correlation between nanotunnel formation and mtDNA mutations, although other mechanisms may be involved. Mitochondrial fragmentation due to excessive fission indicates cell damage [42], and nanotunnels may form in response to cell damage caused by mtDNA mutations. There are other examples of proteins involved in nanotunnel formation: ryanodine receptor 2 (RYR2)-dependent calcium homeostasis is known to regulate nanotunnel formation [49], and activating transcription factor 4 (ATF4) and other ER stress proteins could play a role in this regulation [50]; little else is known about these regulatory mechanisms and would benefit from further exploration.
One potential complication of studying nanotunnels is that they are among the mitochondrial shapes that arise under hypoxic conditions [48] and therefore may result from improper, hypoxia-inducing fixation techniques [51]. Thus, the standardization of fixation and quantification techniques must be improved to ensure that mitochondrial structures represent altered metabolic conditions rather than improper fixation [52].
Connecting mitochondrial shape to cristae dynamics and membrane potential
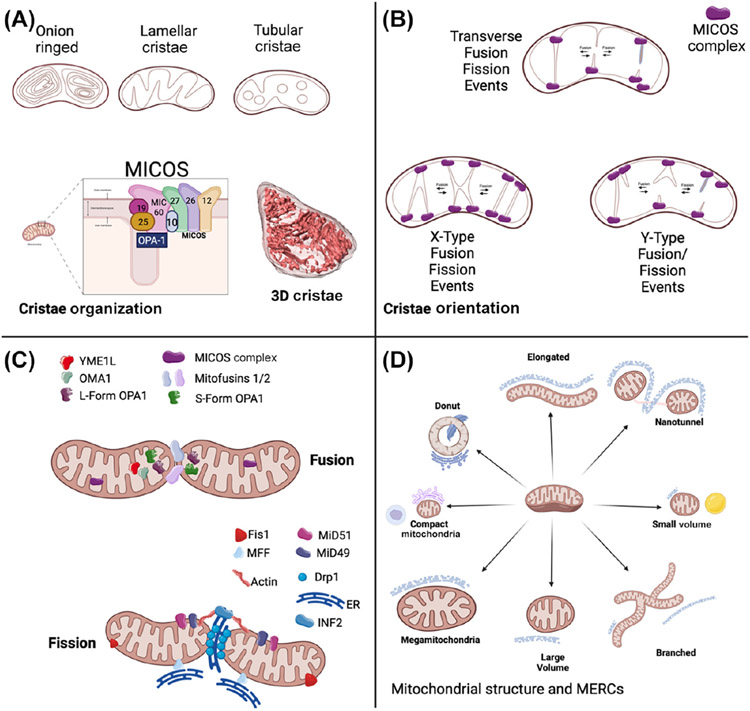
The diversity and heterogeneity of mitochondrial shapes, including those described in the preceding text, affect the spatial orientation of cristae and are modulated by the mitochondrial contact site and cristae organizing system (MICOS) complex [53], as well as mitochondrial signaling pathways (Figure 2A). Although cristae have unique dynamics independent of mitochondrial structures, common fusion proteins, including optic atrophy 1 (OPA1), which is epistatic to the MICOS complex [54], and YME1L, regulate their dynamics through crista junction closing and opening [55]. Cristae are tubular or lamellar under normal conditions [56]; abnormal shapes, such as spherical [55] and onion-ring cristae [57] (Figure 2A), arise in abnormal conditions, such as mitochondrial dysfunction and mitochondrial disorder, respectively. These abnormal shapes may optimize intracristal spaces, but their functional implications remain understudied [57]. In some mitochondrial phenotypes, such as MGs, a paucity or abnormality of cristae is common [41,46]. However, while the cristae structural differences may confer functional changes [15], the interdependence between cristae shape and mitochondrial phenotype remains poorly explicated.
Figure 2. Current state of mitochondrial dynamics beyond their phenotype.
(A) Mitochondrial cristae can take on several morphologies, potentially due to the mitochondrial contact site and cristae organizing system (MICOS) complex, and may be imaged in 3D. (B) Mitochondrial cristae can form x- and y-type fusion and fission events, indicating cristae-specific dynamics. (C) Known factors involved in mitochondrial dynamics and potential new regulators. Fission can be mitochondrial fission factor (MFF)- or mitochondrial fission 1 protein (FIS1)-mediated, but the roles of these regulators and others, such as YME1L, in regulating 3D mitochondrial shape remain unclear. (D) Contact sites have several key phenotypes, which may depend on mitochondrial shape. Abbreviations: MERCs, mitochondria–endoplasmic reticulum contact sites; OPA1, optic atrophy 1.
Cristae harbor the machinery for oxidative phosphorylation and ATP production [56], and their organization is intricately linked to energy production efficiency and overall mitochondrial health [54,58]. Advanced imaging techniques, such as 3D MINFLUX nanoscopy, have revealed the complex organization of cristae and the MICOS complex [53], which is involved in structural cristae remodeling [59]. Cristae organization disruptions and alterations are implicated in neuro-degenerative, metabolic, and cardiovascular diseases [60,61].
Notably, the interplay between crista shapes and the mitochondrial permeability transition (mPT) must be further explored. Though the molecular mechanism is unknown, elevated calcium may open an mPT pore to an unspecified channel, resulting in ruptured cristae and eventual cell death [62]. As mPT pores are regulated by matrix cyclophilin D, they may consist of ATP synthase dimers [62]; however, this suggestion remains controversial [63]. Beyond the role of mPT in disease [64], the mPT has clear roles in modulating membrane potential [62]. This offers implications in mPT regulating membrane potential-dependent mitochondrial structure, such as the formation of cup-shaped cristae upon loss of membrane potential [21]. Because individual cristae can have distinct membrane potentials [59], and because ATP synthase dimerization regulates crista morphology [65], the interplay between mPT, ATP synthase dimerization, cristae structure, and mitochondrial structure must be explicated, possibly through cryo-ET, which enables visualization of ATP synthases [8].
Cristae undergo ongoing and dynamic membrane remodeling events, which may be implicated in mPT pore formation or shape tendency. In these continuous cycles, similar to OPA1-mediated cristae junction flux [55], cristae are continuously remodeled by transverse, x-type, y-type, and other types of cristae fusion and fission (Figure 2B) [60,66]. However, this process remains poorly elucidated. For example, crista dynamics may be determined by the spatial orientation of the mitochondrial MICOS complex, which may depend on the mitochondrial shape. Yet, it is unclear whether the relative rates of types of crista remodeling events affect their membrane potential, tendency toward crista organizational types, or mitochondrial shape, especially in the case of pathological conditions.
New players in the regulation of mitochondrial shape
Fusion and fission
Discussions of mitochondrial dynamics often include a common set of core proteins, including OPA1, dynamin-related protein 1, mitofusin 1, and mitofusin 2. However, the current understanding has evolved beyond these factors (Figure 2C). In addition to its role in cristae remodeling [67], OPA1, when in its long, active form, plays an important role in fusion. Upstream, OPA1 can be inhibited through cleavage by YME1L and OMA1 [68], resident proteases that modulate mitochondrial dynamics. Similarly, actin constricts to enact fission, and other mitochondrial dynamics proteins, including MiD49 and MiD51, also play a role in fission [69]. Notably, actin constriction prefission is orchestrated by ER-associated IFN2 [70], highlighting the interconnectedness of MERCs and mitochondrial fusion–fission dynamics.
Although past studies have suggested that mitochondrial networks are determined entirely by fusion and fission dynamics [30], the involvement of various fission factors, such as mitochondrial fission factor (MFF) and mitochondrial fission 1 protein (FIS1) [71], in determining mitochondrial phenotype remains unclear. Kleele et al. demonstrated that distinct forms of fission occur based on spatial orientation of FIS1 and MFF [71], suggesting that protein distribution may contribute to mitochondrial morphology. A recent study found that FIS1 overexpression results in increased donut formation [72], but whether MFF overexpression has similar effects has not yet been explored. Although previous studies have shown that mitochondrial fission events are integral to mitochondrial morphology outcomes [29], the specific contributions of unique 3D phenotypes, such as MGs, and contact sites require further exploration.
Sex-dependent modulators
Estrogen, which declines with aging, is associated with changes in mitochondrial morphology. In ovariectomized monkeys, donut-shaped mitochondria are a pathological indicator of age-related Alzheimer’s disease, and normal mitochondrial structure and function can be rescued by estrogen treatment [36]. Notably, an in vivo mouse study showed that 17β-estradiol (E2) in mitochondrial membranes decreases membrane viscosity, which can have adverse effects given that optimal membrane fluidity is necessary for proper mitochondrial function [73]. Mitochondrial membrane potential changes may occur concomitantly with alterations in membrane fluidity [74], and mitochondrial cristae have functionally independent membrane potentials [59]. Therefore, it could be informative to examine whether E2 inner-membrane localization occurs, and, if so, whether such localization is cristae-dependent or E2 binds to specific areas of the inner mitochondrial membrane. Furthermore, sex-dependent differences in mitochondrial structure across aging remain unclear, which has implications for uncovering if estrogen may modulate mitochondrial structure and confer a protective effect.
Nucleoids
Nucleoids contain mtDNA, which encodes several proteins and RNAs (tRNA and rRNA) involved in protein synthesis, mechanistically enabling submitochondrial organization and maintenance of mtDNA [75]. As reviewed [76], nucleoids, dictated by tissue type and age, often have integral roles in modulating metabolism. Lewis et al. convincingly demonstrated that mtDNA signaling involves MERCs [77]. Nucleoid transportation at MERCs is further determined by the MICOS complex [78], suggesting the potential involvement of cristae. Although fusion and fission are understood to contribute to mitochondrial genome integrity through the maintenance of nucleoids distributed to daughter mitochondria [79], it is unclear how different mitochondrial shapes affect nucleoid replication and distribution. For example, the large size of MGs may affect MERC formation [46] and consequently impact nucleoid distribution. The mechanisms underlying the formation of different mitochondrial shapes may also impact the number and distribution of nucleoids; for example, if MGs are formed from multiple fusion events [41], they may contain more than one nucleoid, which may impact their function [76]. Thus, further study is needed to understand how changes in mitochondrial structure affect mtDNA and vice versa. Utilizing new techniques, such as Mitomate tracker [80], for the automated quantification of nucleoid distribution in tissues where mitochondrial phenotypes are naturally found (e.g., liver cells for MG [41]), may offer greater insight into this topic.
MICOS complex
The MICOS complex is involved in aging [10,14,81] and, as described in the preceding text, cristae organization (Figure 2A) [66], and may also affect overall mitochondrial shape [10,14]. As previously reviewed [60], the MICOS complex contains multiple proteins, although their independent roles and necessity for MICOS complex functionality remain unknown. One such protein, mitofilin, is important in nucleoid organization and metabolism [82], suggesting that nucleoid distribution may involve the MICOS complex or that its various components play essential roles beyond metabolism. The MICOS complex also interacts with the sorting and assembly machinery complex [83], but the necessity of these two complexes for the formation of specific mitochondrial shapes remains unclear.
Tissue-dependent deletion of the MICOS complex and its specific proteins may advance our understanding of how the MICOS complex affects the gross mitochondrial structure and cristae spacing. Tools have recently been developed to enact overexpression of MICOS complex components [84]. However, floxed models for the deletion of MICOS complex components are lacking, and it remains unclear whether the MICOS complex subunits can be deleted in a tissue-specific manner to better identify the specific functions of individual components.
Other potential modulators
As this section highlighted, our understanding of mitochondrial modulators must continue to evolve. One promising avenue is the study of mitochondrial transporters, specialized proteins that mediate the movement of essential small molecules that are critical in maintaining cellular energy production, metabolism, and redox balance (Box 1). Mitochondrial processes involving the internal diffusion of metabolites and ions are influenced both by the shape of the inner membrane, in turn, affected by crista dynamics, and transport across the membrane, making transporters novel potential upstream regulators of mitochondrial structure. Understanding their integrated functions in mitochondrial biology may lead to the identification of therapeutic targets and novel modulators of mitochondrial structure in various diseases.
Box 1. Mitochondrial transporters – facilitators of cellular metabolism that may affect structure.
Mitochondrial ADP/ATP carriers play crucial roles in cellular energy production by transporting ADP into the mitochondrial matrix for ATP synthesis and exporting ATP to fuel the cell. To meet the inorganic phosphate requirements for cellular oxidative phosphorylation and ATP synthesis, the mitochondrial proton/phosphate symporter transports inorganic phosphate from the cytosol into the mitochondrial matrix [106]. Most mitochondrial transporters belong to the solute carrier family 25 (SLC25), which are typically embedded in the mitochondrial inner membrane and play critical roles in supplying this organelle with essential metabolic intermediates and coenzymes. Recently, three independent research groups discovered that SLC25A51 serves as a mammalian NAD+ transporter [107-109], revealing how mitochondria are supplied with this key cofactor that fuels most cellular redox reactions. NAD+ functions as both an electron acceptor (in its oxidized form) and donor (in its reduced form) [107-109] and is an important signaling molecule in numerous cellular processes. The identification of SLC25A51/MCART1 as the primary mitochondrial NAD+ transporter represents a significant advancement in understanding cellular metabolism.
Mitochondrial transporters play crucial roles in cellular metabolism and are linked to various diseases, including muscular dystrophy [110]. They may also support drug delivery applications; for example, reduced mitochondrial NAD+ levels and protein hyperacetylation result in an anti-tumor effect when combined with aspirin [111]. The impacts of mitochondrial transporters are evident in aging populations: mitochondrial dysfunction, oxidative stress, inflammation, and genomic instability have been proposed as hallmarks of aging [10,112,113]. SLC25 transporters facilitate vital processes, and growing evidence indicates that some SLC25 family members are involved in pathological states, including cancer [114]. Similarly, SLC1A5 was recently found to mediate mitochondrial glutamine metabolism in cancer, indicating that SLC transporters may also modulate levels of ROS [115]. As previously reviewed [116], ROS can affect calcium signaling in mitochondria, suggesting further involvement of MERCs. Cup-shaped and donut mitochondria are suggested to be adaptive mechanisms to respond to osmotic stress and oxidative damage that occurs following the loss of mitochondrial membrane potential [21]. Thus, SLC25 members, including potentially SLC25A51, may modulate mitochondrial structure by promoting osmotic stress. However, it remains unclear whether SLC transporters can also mechanistically shift mitochondrial structures to those emblematic of disease states. More work is needed pointedly focused on testing how transporter function can result in changes in mitochondrial structure, which may uncover potential avenues for intervention.
Mitochondrial shapes are influenced by their organellar connectome
Mitochondrial structures should be considered in the context of the entire organellar connectome, including MERCs and contacts with lipid droplets and autophagic materials [31]. As noted previously, recent findings have shown that MERCs have regulatory roles in both fission [32] and fusion [33], thereby modulating mitochondrial morphology. However, it is unclear whether different types of contact sites with unique biochemical roles exist. For example, centers of donut-shaped mitochondria are often filled with sarcoplasmic reticulum (SR), ER, or lipid droplet contact sites (Figure 2D) [19]. Tangentially, mitochondria form contacts with lipid droplets to perform fatty acid oxidation [85]. Studies of these lipid droplets show that their mitochondrial contact sites can either be anchored or formed in ‘kiss-and-run’ interactions, each of which has distinct functional implications and formation pathways [85,86]. As organelle recruitment may differ across distinct structures, future studies should examine how different mitochondrial shapes affect MERCs.
Beyond MERCs, deeper investigation is needed into how mitochondrial interactions with other organelles, such as recycling machinery [87], may dictate morphology. Notably, artificial transgene tethers have arisen as a potential therapy to regulate MERC formation, which may thereby alter mitochondrial morphology [32,33,88]. This application represents a potentially valuable opportunity to reduce cardiac modeling [88]. However, no transgenes have been identified as potential tethers for other mitochondrial contact sites.
MERC formation may facilitate the interorganellar transfer of mtDNA, Ca2+, or other ions [89]. Lavorato and colleagues reported that decreased RYR2 activity, which is associated with SR Ca2+ imbalances, increased nanotunneling without changing fission rates [49]. This phenomenon may be connected to ATF4-mediated pathways, which are associated with mitochondrial Ca2+ homeostasis [90]. Thus, nanotunnel formation represents a compensatory cellular response to Ca2+ dysregulation, which is further supported by the increased rate of nanotunnel formation in disease states [13,48], suggesting an interplay between impaired MERC formation and the emergence of structures representative of disease states.
The contributions of different crista types and the involvement of mitochondrial proteins, such as the MICOS complex or fission factors, in the formation of contact sites, also remain unclear. However, the current understanding of how MERCs dictate MICOS complex orientation [91] may help explain how MERCs can dictate cristae structure.
Future clinical considerations of mitochondrial structure
mtDNA changes were first considered to be functionally linked to myopathies in the 1980s [92], and the role of mitochondria in medicine has continued to evolve ever since [93]. However, despite the known association of mitochondrial structure with pathologies [36,41], it still has limited clinical applications, and the functional impacts of different mitochondrial shapes, including their relative frequencies in different disease states and tissues, remain unclear. Certain mitochondrial phenotypes, such as donut mitochondria, may serve as markers of disease [36], and recent studies have noted changes in the prevalence of certain mitochondrial phenotypes in response to specific pathological conditions [13,15,41]. Frequency changes for specific mitochondrial shapes could further indicate cellular stress, inflammation, or metabolic alterations. Notably, mitochondrial mass, stress conditions, and motility dynamically affect fusion and fission rates, resulting in heterogenous micropopulations [94]. Further elucidation of how these factors affect the drivers of fission, such as MFF or FIS1 [71], may support clinical diagnosis.
Different mitochondrial distributions have been established for subsarcolemmal, perinuclear, and intermyofibrillar regions of mammalian cardiac muscle [26,95], but whether these distribution differences affect mitochondrial function has not been determined. Further, various mitochondrial populations have displayed differences in relative energetics [96], and variations in the aging rates of tissues [34] may indicate tissue- [14] or population-dependent mitochondrial regulation mechanisms contributing to pathology. However, many studies have focused on murine models; a comprehensive understanding of mitochondrial structures and functions will require the characterization of mitochondrial phenotypic frequencies across various tissue and cell types from various organisms, including humans, to account for potential tissue-specific effects.
Research must examine the processes and pathways affected by mitochondrial genomes to elucidate how ancestry affects population health studies. The presence of several different alleles within one patient, known as mtDNA heteroplasmy, may also contribute to differences in mitochondrial shape as mtDNA may affect both mitochondrial shape and organelle–organelle interactions [77]. However, it is unclear whether mtDNA alterations affect mitochondrial shape across ancestry, race, ethnicity, and geographical order. mtDNA has a high mutation rate, and some mutations are deleterious, predisposing carriers toward certain mitochondrial diseases. Moreover, depletion of mtDNA induces mitochondria fission factors, impacts 3D formation, and changes the actin cytoskeleton [97]. Therefore, identifying female germline mtDNA mutations, learning how these mutations become predominant, and determining the possible pathways impacted are important avenues for understanding mtDNA-related inheritable diseases and interactions with mitochondrial structures.
A program using deep learning processes and large-input, open-source software could determine whether mitochondrial shapes contribute to disease prognosis and associated diagnosis. Future clinical applications will likely require machine learning techniques to render 3D mitochondrial structures. For example, the recently developed MitoEM program has successfully segmented mitochondrial images in both human and mouse tissues, representing a promising avenue in accelerating the speed of mitochondrial reconstruction and subsequent quantification [98]. However, this pipeline has not been tested in clinical applications, and EM technique standardization is still lacking [99]. Moreover, current machine learning techniques may struggle to accurately survey mitochondrial phenotypic frequencies in human biopsy tissue without a dedicated database or atlas. While drastic improvements must be made in EM techniques, not limited to validation, technique standardization, and dedicated databases for accurate phenotypic surveys in human biopsy tissues, as these technologies continue to rapidly improve, clinical applications may be a promising future avenue.
Concluding remarks
Future studies of 3D mitochondrial structures will be facilitated by developing microscopy techniques [99], such as serial block-face scanning electron microscopy (SEM) [100], correlative light EM [101], transmission EM [99,102], focused ion beam-SEM [103], cryo-ET [9], and mass spectrometry imaging [104] (Box 2). Using these techniques in tandem to define protein distribution and the 3D structures of mitochondria and cristae may clarify how different protein concentrations regulate mitochondrial phenotypes, such as through specific concentrations of proteins in distinct geographies. These methods may be further combined with techniques, such as mitoRACE, that provide information on energy metabolism [22]. Although these techniques are not yet widely used in clinical settings, they may reveal useful information about mitochondrial ultrastructure for clinical contexts.
Box 2. Emerging technologies to consider for surveying mitochondrial dynamics.
2D and 3D imaging may be able to detect unique mitochondrial phenotypes, although this identification requires new standards for mitochondrial characterization. Mitochondrial shape could serve as a cost-effective indicator of disease progression, and 3D imaging may be able to distinguish between healthy and diseased clinical samples.
Current 3D imaging techniques reveal diverse mitochondrial structures, including nanotunnels, megamitochondria, hyperbranching, and fragmentation [52]. For example, focused ion beam (FIB)-scanning electron microscopy (SEM) combines FIB milling with SEM to generate high-resolution 3D reconstructions of subcellular structures [52,103]. FIB-SEM has been used to study mitochondrial morphology, cristae organization, and MERCs in various cell types under different conditions [51,117] and can reveal alterations in mitochondrial structure that clarify the contributions of mitochondrial morphology to disease progression and pathogenesis.
Alternatively, cryo-ET can produce 3D images of the mitochondrial native state [9,118]. Beyond the mitochondrial ultrastructure, the high resolution of cryo-ET can render protein complexes [9], including changes in respiratory chain supercomplexes [119]; ATP synthase [8]; tethers of mitochondrial contact sites, such as those tethering mitoribosomes to the inner mitochondria membrane [120]; and cytoskeletal filament interactions [121]. These cryo-ET-derived nanoscale details provide greater mechanistic insight into the formation of mitochondria shapes and associated contact sites.
Recent advances have led to clinical applications of correlative light electron microscopy (CLEM), a powerful imaging approach that combines the molecular specificity of fluorescence microscopy with the ultrastructural resolution of electron microscopy [122]. CLEM has been used to study real-time changes in mitochondrial protein localization and oligomerization [101]. CLEM allows researchers to visualize specific proteins or organelles within the context of the overall cellular architecture at high resolution, including changes in mitochondrial morphology, enabling a more comprehensive understanding of mitochondrial structure and function [122].
High-resolution mass spectrometry imaging (MSI), another powerful imaging technique, simultaneously visualizes multiple isotopes within a single sample [104], enabling researchers to study the distribution of specific elements within mitochondria as well as potential mitochondrial alterations in disease states. MSI can also be used to investigate various aspects of mitochondrial function, including the distribution of proteins and lipids and the turnover of mitochondrial components. The developing technology Multicolor 3D MINFLUX [53] can assess the spatial orientation of proteins within the mitochondria, such as the MICOS complex, which may have implications for overall activation. Thus, future investigations may uncover how protein distribution affects mitochondrial morphology.
We believe that accurate quantification of mitochondrial shapes is essential for characterizing their roles in cellular functions and disease, and that we must define parameters for classifying distinct phenotypes, including the distances of nanotunnels and the sizes of donuts and MGs. Importantly, our understanding of mitochondrial structure must consider the potential pluralistic hallmarks of disease states (Figure 3, Key figure). Future research may explore how the presence and frequency of these structures differ between tissues and investigate the mechanisms of abnormal mitochondrial morphology formation in various diseases (see Outstanding questions for additional topics of study). For example, recent studies have shown that the MICOS complex is mechanistically implicated in cardiac dysfunction [81], but the clinical impacts of MICOS complex expression changes in different disease states must be further explored. In conclusion, future study of mitochondrial shape, arrangement, and heterogeneity, in addition to identifying novel regulators, will be crucial to understanding their roles in cellular homeostasis and how they are altered to respond to the physiological state and energy demands of the cell.
Outstanding questions.
Are altered mitochondrial shapes related to human pathology? Can the regulation of mitochondrial morphology mitigate pathological development?
Are differences in machinery or genome activation across mitochondrial phenotypes related to mtDNA inheritance and mitochondrial function?
Do homotypic (same type) and heterotypic (different types) fusion or fission events govern the formation of distinct mitochondrial shapes?
Does the spatial orientation of the MICOS complex determine mitochondrial shapes?
What are the effects of x-type, y-type, and other types of cristae cycle dynamics?
Does the relative orientation of cristae differ among different mitochondrial shapes?
Do alterations in cristae types and orientations precede changes in mitochondrial shapes?
What roles do cristae play in modulating mitochondrial proteins or related carriers (e.g., SLC25)? Do interactions of SLC25A46 with OPA1 and the MICOS complex alter cristae orientation?
Do tissue-specific differences in the presence and frequency of mitochondrial shapes exist? What mechanisms are involved in establishing abnormal mitochondrial morphologies in various disease states?
Do differences in protein distribution determine 3D phenotypes?
Are the relative frequencies of various mitochondrial shapes associated with positive or negative disease states?
Why are some forms of MERCs associated with specific mitochondrial shapes?
Can artificial intelligence and other techniques be used to detect patterns in mitochondrial shape frequency?
What molecular mechanisms underlie tissue-specific expression and regulation of mitochondrial transporters in various metabolic contexts? Do these mechanisms crosstalk with mitochondrial shapes?
How do variations in mitochondrial shapes impact the localization and activity of mitochondrial transporters?
How do the shape and curvature of the inner mitochondrial membrane influence the distribution and shape of cristae or the organization of mitochondrial transporters?
Key figure
Mitochondrial structural hallmarks of disease
Figure 3.
Based on our review, we propose the following essential aspects to consider when researching mitochondrial structures and how their structure may influence their function: cristae types [124]; cristae orientation and fusion/fission events [66,124]; mitochondrial fusion and fission [30,71]; organelle interactions [31]; nucleoid distribution changes [76,77]; tissue-dependent changes [10,11,14,34]; and relative mitochondrial 3D structural phenotypes [15].
Highlights.
Mitochondrial shape heterogeneity in disease may inform novel therapeutic targets.
3D electron microscopy is developing as a potential clinical diagnostics tool for exploring mitochondrial pathology.
Characterizing mitochondrial structure may elucidate tissue-dependent differences and aid in understanding different cell types and conditions associated with different health outcomes.
Acknowledgments
S.M.C. is funded by National Institutes of Health (NIH) grants R01HL165729 and R01GM151746. A.K. is funded by NIH grants R01HL147818, R03HL155041, R01HL157584, R01DK135764, R21TW012635 and R01HL144941. A.H. is funded by The UNCF/Bristol-Myers Squibb E.E. Just Faculty Fund; Burroughs Wellcome Fund (BWF) Career Award at the Scientific Interface (ID #1021868.01); BWF Ad-hoc Award; NIH Small Research Pilot Subaward to 5R25HL106365-12 from the National Institutes of Health PRIDE Program (DK020593); Vanderbilt Diabetes and Research Training Center Alzheimer’s Disease Pilot & Feasibility Program; and Science Diversity Leadership Grant #2022-253529 from the Chan Zuckerberg Initiative’s Donor-Advised Fund of the Silicon Valley Community Foundation. M.R.M. is funded by Howard Hughes Medical Institute Hanna H. Gray Fellows Program Faculty Phase Grant #GT15655 and BWF Postdoctoral Diversity Enrichment Program Transition to Faculty Grant #1022604.
Glossary
- Activating transcription factor 4 (ATF4)
transcription factor that plays a key role in the cellular response to stress, including endoplasmic reticulum stress, and is known to induce the integrated stress response.
- ATP
nucleotide produced by mitochondria during cellular respiration that serves as the primary energy currency of cells through its interconversion with ADP.
- Cristae
partitions dictated by invaginations and separated from the rest of the inner mitochondrial membrane through cristae junctions. These infoldings increase the inner membrane surface area, allowing for increased ATP synthesis. Cristae are known to take on a multitude of shapes, including onion, tubular, and lamellar. Previously reviewed by [56].
- Endoplasmic reticulum (ER)
cellular organelle involved in the synthesis of lipids, proteins, and the regulation of calcium levels. Close physical contact sites between ER and mitochondria, known as MERCs, with biochemical isolated fractions called mitochondria-associated membranes (MAMs).
- ER-associated degradation (ERAD) pathway
helps to ensure the quality control of newly synthesized proteins through the degradation of misfolded or unassembled proteins in the ER.
- Intracristal space
region enclosed by cristae within the mitochondrial inner membrane, in which oxidative phosphorylation predominantly occurs.
- Lipid droplets
organelles that hydrolyze and store neutral lipids.
- Membrane fluidity
measure of microviscosity referring to the dynamic flexibility of lipid bilayers which is influenced by the collection of phospholipids contained, the nature of their attached fatty acids (length and saturation), and the incorporation of cholesterol and/or its derivatives.
- MINFLUX
super-resolution microscopy technique that allows imaging with extremely high spatial resolution of proteins, including mitochondrial and cristae proteins [53].
- Mitochondria
dynamic double-membraned cellular organelles with pluralistic roles extending to ATP synthesis, apoptosis, and calcium signaling.
- Mitochondria–ER contact sites (MERCs)
shorter than 50 nm between the ER and the outer mitochondrial membrane. Previously reviewed by [105].
- Mitochondrial contact site and cristae organizing system (MICOS) complex
Consists of seven known protein subunits spanning the cristae junctions of the inner mitochondria membrane, with roles including cristae architecture remodeling. Previously reviewed by [60,61].
- Mitochondrial fission 1 protein (FIS1)
A mitochondrial outer membrane protein involved in mitochondrial fission through the recruitment of Drp1.
- Mitochondrial fission factor (MFF)
mitochondrial outer membrane protein involved in mitochondrial fission through the recruitment of Drp1. Aids in determining a fission type that is distinct from that of FIS1.
- Mitochondrial permeability transitions (mPTs)
events in which the inner mitochondrial membrane undergoes a sudden increase in permeability to ions and solutes, such as calcium, which can result in a loss of membrane potential and decreased ATP synthesis.
- Mitophagy
selective degradation or removal of mitochondria through autophagy, often targeting damaged mitochondria.
- mtDNA heteroplasmy
different sequences of mtDNA within the same individual, indicating mutations.
- Nucleoids
dynamic structures composed of active mtDNA and nucleoid-associated proteins. Previously reviewed by [76].
- OMA1
mitochondrial inner membrane protease involved in the regulation of mitochondrial dynamics through the proteolytic processing of OPA1.
- Optic atrophy 1 (OPA1)
mitochondrial inner membrane protein that is a principal regulator of mitochondrial fusion.
- rRNA
structural and functional component of the ribosome, the cellular machinery responsible for protein synthesis. As with tRNAs and mRNAs, mitochondria have their own set of rRNA which is derived from mtDNA.
- Ryanodine receptor 2 (RYR2)
calcium-release channel primarily found in the sarcoplasmic reticulum of cardiac muscle cells, with mutations of RYR2 altering nanotunnel presence.
- Transverse fusion–fission events
cristae fusion and fission events that happen across their inner boundary membrane.
- X-type fusion–fission events
cristae merging and fission events that happen across their length, wherein adjoining cristae appear in an 'X' shape.
- YME1L
mitochondrial inner membrane member of the AAA (ATPases associated with diverse cellular activities) family that proteolytically process OPA1 to modulate mitochondrial fusion.
- Y-type fusion–fission events
cristae merging and fission events that happen across their length, wherein adjoining cristae appear in a 'Y' shape.
Footnotes
Declaration of interests
No interests are declared.
References
- 1.Ernster L and Schatz G (1981) Mitochondria: a historical review. J. Cell Biol 91, 227s–255s [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ruska E. (1987) The development of the electron microscope and of electron microscopy. Biosci. Rep 7, 607–629 [DOI] [PubMed] [Google Scholar]
- 3.Palade GE (1952) The fine structure of mitochondria. Anat. Rec 114, 427–451 [DOI] [PubMed] [Google Scholar]
- 4.Palade GE (1953) An electron microscope study of the mitochondrial structure. J. Histochem. Cytochem 1, 188–211 [DOI] [PubMed] [Google Scholar]
- 5.Hackenbrock CR (1966) Ultrastructural bases for metabolically linked mechanical activity in mitochondria. I. Reversible ultrastructural changes with change in metabolic steady state in isolated liver mitochondria. J. Cell Biol 30, 269–297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bron C. et al. (1990) Three-dimensional electron microscopy of entire cells. J. Microsc 157, 115–126 [DOI] [PubMed] [Google Scholar]
- 7.Mannella CA et al. (1994) The internal compartmentation of rat-liver mitochondria: tomographic study using the high-voltage transmission electron microscope. Microsc. Res. Tech 27, 278–283 [DOI] [PubMed] [Google Scholar]
- 8.Davies KM et al. (2014) Visualization of ATP synthase dimers in mitochondria by electron cryo-tomography. J. Vis. Exp 91, e51228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Danev R. et al. (2019) Cryo-electron microscopy methodology: current aspects and future directions. Trends Biochem. Sci 44, 837–848 [DOI] [PubMed] [Google Scholar]
- 10.Vue Z. et al. (2023) Three-dimensional mitochondria reconstructions of murine cardiac muscle changes in size across aging. Am. J. Physiol. Heart Circ. Physiol 325, H965–H982 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Crabtree A. et al. (2023) Defining mitochondrial cristae morphology changes induced by aging in brown adipose tissue. Adv. Biol. (Weinh) 8, e2300186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Glancy B. et al. (2015) Mitochondrial reticulum for cellular energy distribution in muscle. Nature 523, 617–620 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vincent AE et al. (2019) Quantitative 3D mapping of the human skeletal muscle mitochondrial network. Cell Rep. 26, 996–1009.e4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vue Z. et al. (2023) 3D reconstruction of murine mitochondria reveals changes in structure during aging linked to the MICOS complex. Aging Cell 22, e14009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Glancy B. et al. (2020) The functional impact of mitochondrial structure across subcellular scales. Front. Physiol 11, 541040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ding W-X et al. (2012) Electron microscopic analysis of a spherical mitochondrial structure. J. Biol. Chem. 287, 42373–42378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lionetti L. et al. (2014) High-lard and high-fish-oil diets differ in their effects on function and dynamic behaviour of rat hepatic mitochondria. PLOS ONE 9, e92753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Seppet EK et al. (2001) Functional complexes of mitochondria with Ca, MgATPases of myofibrils and sarcoplasmic reticulum in muscle cells. Biochim. Biophys. Acta 1504, 379–395 [DOI] [PubMed] [Google Scholar]
- 19.Bleck CK et al. (2018) Subcellular connectomic analyses of energy networks in striated muscle. Nat. Commun 9, 1–11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Trushina E. (2016) A shape shifting organelle: unusual mitochondrial phenotype determined with three-dimensional electron microscopy reconstruction. Neural Regen. Res 11, 900–901 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Miyazono Y. et al. (2018) Uncoupled mitochondria quickly shorten along their long axis to form indented spheroids, instead of rings, in a fission-independent manner. Sci. Rep 8, 350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Willingham TB et al. (2019) MitoRACE: evaluating mitochondrial function in vivo and in single cells with subcellular resolution using multiphoton NADH autofluorescence. J. Physiol 597, 5411–5428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lawrie AM et al. (1996) A role for calcium influx in the regulation of mitochondrial calcium in endothelial cells. J. Biol. Chem 271, 10753–10759 [DOI] [PubMed] [Google Scholar]
- 24.Al-Mehdi A-B et al. (2012) Perinuclear mitochondrial clustering creates an oxidant-rich nuclear domain required for hypoxia-induced transcription. Sci. Signal 5, ra47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kirkwood SP et al. (1986) Mitochondrial reticulum in limb skeletal muscle. Am. J. Physiol. Cell Physiol 251, C395–C402 [DOI] [PubMed] [Google Scholar]
- 26.Koves TR et al. (2005) Subsarcolemmal and intermyofibrillar mitochondria play distinct roles in regulating skeletal muscle fatty acid metabolism. Am. J. Physiol. Cell Physiol 288, C1074–C1082 [DOI] [PubMed] [Google Scholar]
- 27.Chance B and Williams GR (1955) Respiratory enzymes in oxidative phosphorylation: I. Kinetics of oxygen utilization. J. Biol. Chem 217, 383–393 [PubMed] [Google Scholar]
- 28.Glancy B. (2020) Visualizing mitochondrial form and function within the cell. Trends Mol. Med 26, 58–70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Westrate LM et al. (2014) Mitochondrial morphological features are associated with fission and fusion events. PLoS One 9, e95265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zamponi N. et al. (2018) Mitochondrial network complexity emerges from fission/fusion dynamics. Sci. Rep 8, 363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kim Y. et al. (2021) Reorganization of the mitochondria-organelle interactome during postnatal development in skeletal muscle. bioRxiv, Published online June 17, 2021. 10.1101/2021.06.16.448433 [DOI] [Google Scholar]
- 32.Friedman JR et al. (2011) ER tubules mark sites of mitochondrial division. Science 334, 358–362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Abrisch RG et al. (2020) Fission and fusion machineries converge at ER contact sites to regulate mitochondrial morphology. J. Cell Biol 219, e201911122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Schaum N. et al. (2020) Ageing hallmarks exhibit organspecific temporal signatures. Nature 583, 596–602 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ahmad T. et al. (2013) Computational classification of mitochondrial shapes reflects stress and redox state. Cell Death Dis. 4, e461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hara Y. et al. (2014) Presynaptic mitochondrial morphology in monkey prefrontal cortex correlates with working memory and is improved with estrogen treatment. Proc. Natl. Acad. Sci 111, 486–491 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Liu X and Hajnóczky G (2011) Altered fusion dynamics underlie unique morphological changes in mitochondria during hypoxia–reoxygenation stress. Cell Death Differ. 18, 1561–1572 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Long Q. et al. (2015) Modeling of mitochondrial donut formation. Biophys. J 109, 892–899 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhou Y. et al. (2020) Topology-dependent, bifurcated mitochondrial quality control under starvation. Autophagy 16, 562–574 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wakabayashi T. (2002) Megamitochondria formation - physiology and pathology. J. Cell. Mol. Med 6, 497–538 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Shami GJ et al. (2021) Three-dimensional ultrastructure of giant mitochondria in human non-alcoholic fatty liver disease. Sci. Rep 11, 3319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Horn A. et al. (2020) Mitochondrial fragmentation enables localized signaling required for cell repair. J. Cell Biol 219, e201909154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tondera D. et al. (2009) SLP-2 is required for stress-induced mitochondrial hyperfusion. EMBO J. 28, 1589–1600 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Galloway CA et al. (2012) Mitochondrial morphology – emerging role in bioenergetics. Free Radic. Biol. Med 53, 2218–2228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Twig G. et al. (2008) Fission and selective fusion govern mitochondrial segregation and elimination by autophagy. EMBO J. 27, 433–446 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zhou Z. et al. (2020) Endoplasmic reticulum-associated degradation regulates mitochondrial dynamics in brown adipocytes. Science 368, 54–60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lebeau J. et al. (2018) The PERK arm of the unfolded protein response regulates mitochondrial morphology during acute endoplasmic reticulum stress. Cell Rep. 22, 2827–2836 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Vincent AE et al. (2017) Mitochondrial nanotunnels. Trends Cell Biol. 27, 787–799 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lavorato M. et al. (2017) Increased mitochondrial nanotunneling activity, induced by calcium imbalance, affects intermitochondrial matrix exchanges. PNAS 114, E849–E858 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sasaki K. et al. (2020) Mitochondrial translation inhibition triggers ATF4 activation, leading to integrated stress response but not to mitochondrial unfolded protein response. Biosci. Rep 40, BSR20201289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hinton A. et al. (2023) A comprehensive approach to sample preparation for electron microscopy and the assessment of mitochondrial morphology in tissue and cultured cells. Adv. Biol. (Weinh) 7, e2200202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Garza-Lopez E. et al. (2022) Protocols for generating surfaces and measuring 3D organelle morphology using Amira. Cells 11, 65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Pape JK et al. (2020) Multicolor 3D MINFLUX nanoscopy of mitochondrial MICOS proteins. Proc. Natl. Acad. Sci 117, 20607–20614 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Glytsou C. et al. (2016) Optic atrophy 1 is epistatic to the core MICOS component MIC60 in mitochondrial cristae shape control. Cell Rep. 17, 3024–3034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hu C. et al. (2020) OPA1 and MICOS regulate mitochondrial crista dynamics and formation. Cell Death Dis. 11, 1–17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Cogliati S. et al. (2016) Mitochondrial cristae: where beauty meets functionality. Trends Biochem. Sci 41, 261–273 [DOI] [PubMed] [Google Scholar]
- 57.Vincent AE et al. (2016) The spectrum of mitochondrial ultrastructural defects in mitochondrial myopathy. Sci. Rep 6, 30610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Cogliati S et al. (2013) Mitochondrial cristae shape determines respiratory chain supercomplexes assembly and respiratory efficiency. Cell 155, 160–171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wolf DM et al. (2019) Individual cristae within the same mitochondrion display different membrane potentials and are functionally independent. EMBO J. 38, e101056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Anand R. et al. (2021) Emerging roles of the MICOS complex in cristae dynamics and biogenesis. Biology (Basel) 10, 600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Eramo MJ et al. (2020) The ‘mitochondrial contact site and cristae organising system’ (MICOS) in health and human disease. J. Biochem 167, 243–255 [DOI] [PubMed] [Google Scholar]
- 62.Giorgio V. et al. (2013) Dimers of mitochondrial ATP synthase form the permeability transition pore. Proc. Natl. Acad. Sci. U. S. A 110, 5887–5892 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Carroll J. et al. (2019) Persistence of the permeability transition pore in human mitochondria devoid of an assembled ATP synthase. Proc. Natl. Acad. Sci 116, 12816–12821 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Bonora M. et al. (2020) Physiopathology of the permeability transition pore: molecular mechanisms in human pathology. Biomolecules 10, 998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Paumard P. et al. (2002) The ATP synthase is involved in generating mitochondrial cristae morphology. EMBO J. 21, 221–230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kondadi AK et al. (2020) Cristae undergo continuous cycles of membrane remodelling in a MICOS-dependent manner. EMBO Rep. 21, e49776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Frezza C. et al. (2006) OPA1 controls apoptotic cristae remodeling independently from mitochondrial fusion. Cell 126, 177–189 [DOI] [PubMed] [Google Scholar]
- 68.Anand R. et al. (2014) The i-AAA protease YME1L and OMA1 cleave OPA1 to balance mitochondrial fusion and fission. J. Cell Biol 204, 919–929 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Losón OC et al. (2013) Fis1, Mff, MiD49, and MiD51 mediate Drp1 recruitment in mitochondrial fission. Mol. Biol. Cell 24, 659–667 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Korobova F. et al. (2013) An actin-dependent step in mitochondrial fission mediated by the ER-associated formin INF2. Science 339, 464–467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kleele T. et al. (2021) Distinct fission signatures predict mitochondrial degradation or biogenesis. Nature 593, 435–439 [DOI] [PubMed] [Google Scholar]
- 72.Suh J. et al. (2023) Mitochondrial fragmentation and donut formation enhance mitochondrial secretion to promote osteogenesis. Cell Metab. 35, 345–360.e7 [DOI] [PubMed] [Google Scholar]
- 73.Reiter RJ et al. (2014) Melatonin reduces lipid peroxidation and membrane viscosity. Front. Physiol 5, 377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Ricchelli F. et al. (2001) Disaccharide modulation of the mitochondrial membrane fluidity changes induced by the membrane potential. IUBMB Life 51, 111–116 [DOI] [PubMed] [Google Scholar]
- 75.Garrido N. et al. (2003) Composition and dynamics of human mitochondrial nucleoids. MBoC 14, 1583–1596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Lee SR and Han J (2017) Mitochondrial nucleoid: shield and switch of the mitochondrial genome. Oxidative Med. Cell. Longev 2017, e8060949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Lewis SC et al. (2016) ER-mitochondria contacts couple mtDNA synthesis with mitochondrial division in human cells. Science 353, aaf5549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Qin J. et al. (2020) ER-mitochondria contacts promote mtDNA nucleoids active transportation via mitochondrial dynamic tubulation. Nat. Commun 11, 4471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Osman C. et al. (2015) Integrity of the yeast mitochondrial genome, but not its distribution and inheritance, relies on mitochondrial fission and fusion. Proc. Natl. Acad. Sci 112, E947–E956 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Ilamathi HS et al. (2021) A new automated tool to quantify nucleoid distribution within mitochondrial networks. Sci. Rep 11, 22755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Birker K. et al. (2023) Mitochondrial MICOS complex genes, implicated in hypoplastic left heart syndrome, maintain cardiac contractility and actomyosin integrity. eLife 12, e83385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Li H. et al. (2016) Mic60/Mitofilin determines MICOS assembly essential for mitochondrial dynamics and mtDNA nucleoid organization. Cell Death Differ. 23, 380–392 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Abudu YP et al. (2021) SAMM50 acts with p62 in piecemeal basal- and OXPHOS-induced mitophagy of SAM and MICOS components. J. Cell Biol. 220, e202009092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Sohn JH et al. (2023) Liver mitochondrial cristae organizing protein MIC19 promotes energy expenditure and pedestrian locomotion by altering nucleotide metabolism. Cell Metab. 35, 1356–1372.e5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Benador IY et al. (2018) Mitochondria bound to lipid droplets have unique bioenergetics, composition, and dynamics that support lipid droplet expansion. Cell Metab. 27, 869–885.e6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Cui L and Liu P (2020) Two types of contact between lipid droplets and mitochondria. Front. Cell Dev. Biol. 8, 618322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Neikirk K. et al. (2023) Systematic transmission electron microscopy-based identification and 3D reconstruction of cellular degradation machinery. Adv. Biol 7, 2200221. [DOI] [PubMed] [Google Scholar]
- 88.Nichtová Z. et al. (2023) Enhanced mitochondria-SR tethering triggers adaptive cardiac muscle remodeling. Circ. Res 132, e171–e187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Rieusset J. (2018) The role of endoplasmic reticulum-mitochondria contact sites in the control of glucose homeostasis: an update. Cell Death Dis. 9, 1–12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Park Y. et al. (2017) mTORC1 balances cellular amino acid supply with demand for protein synthesis through post-transcriptional control of ATF4. Cell Rep. 19, 1083–1090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Tirrell PS et al. (2020) MICOS subcomplexes assemble independently on the mitochondrial inner membrane in proximity to ER contact sites. J. Cell Biol 219, e202003024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Holt IJ et al. (1988) Deletions of muscle mitochondrial DNA in patients with mitochondrial myopathies. Nature 331, 717–719 [DOI] [PubMed] [Google Scholar]
- 93.Picard M. et al. (2016) The rise of mitochondria in medicine. Mitochondrion 30, 105–116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Dalmasso G. et al. (2017) Agent-based modeling of mitochondria links sub-cellular dynamics to cellular homeostasis and heterogeneity. PLoS One 12, e0168198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Hatano A. et al. (2015) Distinct functional roles of cardiac mitochondrial subpopulations revealed by a 3D simulation model. Biophys. J 108, 2732–2739 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Porat-Shliom N. et al. (2019) Mitochondrial populations exhibit differential dynamic responses to increased energy demand during exocytosis in vivo. iScience 11, 440–449 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Srinivasan S. et al. (2017) Mitochondrial dysfunction and mitochondrial dynamics - the cancer connection. Biochim. Biophys. Acta 1858, 602–614 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Wei D. et al. (2020) MitoEM Dataset: large-scale 3D mitochondria instance segmentation from EM images. Med. Image Comput. Comput. Assist. Interv 12265, 66–76 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Neikirk K. et al. (2023) Call to action to properly utilize electron microscopy to measure organelles to monitor disease. Eur. J. Cell Biol 102, 151365. [DOI] [PubMed] [Google Scholar]
- 100.Marshall AG et al. (2023) Serial block face-scanning electron microscopy as a burgeoning technology. Adv. Biol. (Weinh) 7, e2300139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Marshall AG et al. (2023) Correlative light-electron microscopy: integrating dynamics to structure. Trends Biochem. Sci 48, 826–827 [DOI] [PubMed] [Google Scholar]
- 102.Lam J. et al. (2021) A universal approach to analyzing transmission electron microscopy with ImageJ. Cells 10, 2177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Marshall AG et al. (2023) Revisiting focused ion beam scanning electron microcopy. Trends Biochem. Sci 48, 585–586 [DOI] [PubMed] [Google Scholar]
- 104.Hogan KA et al. (2023) Using mass spectrometry imaging to visualize age-related subcellular disruption. Front. Mol. Biosci 10, 906606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Giacomello M and Pellegrini L (2016) The coming of age of the mitochondria–ER contact: a matter of thickness. Cell Death Differ. 23, 1417–1427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Ferreira GC and Pedersen PL (1993) Phosphate transport in mitochondria: past accomplishments, present problems, and future challenges. J. Bioenerg. Biomembr 25, 483–492 [DOI] [PubMed] [Google Scholar]
- 107.Luongo TS et al. (2020) SLC25A51 is a mammalian mitochondrial NAD+ transporter. Nature 588, 174–179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Girardi E. et al. (2020) Epistasis-driven identification of SLC25A51 as a regulator of human mitochondrial NAD import. Nat. Commun 11, 6145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Kory N. et al. (2020) MCART1/SLC25A51 is required for mitochondrial NAD transport. Sci. Adv 6, eabe5310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Clémençon B. et al. (2013) The mitochondrial ADP/ATP carrier (SLC25 family): pathological implications of its dysfunction. Mol. Asp. Med 34, 485–493 [DOI] [PubMed] [Google Scholar]
- 111.Li Y. et al. (2023) SLC25A51 promotes tumor growth through sustaining mitochondria acetylation homeostasis and proline biogenesis. Cell Death Differ. 30, 1916–1930 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Lejri I. et al. (2018) Mitochondria, estrogen and female brain aging. Front. Aging Neurosci 10, 124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Picca A. et al. (2019) Mitochondrial dysfunction and aging: insights from the analysis of extracellular vesicles. Int. J. Mol. Sci 20, 805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Wang Y. et al. (2021) SLC25A21 Suppresses cell growth in bladder cancer via an oxidative stress-mediated mechanism. Front. Oncol 11, 682710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Yoo HC et al. (2020) A variant of SLC1A5 is a mitochondrial glutamine transporter for metabolic reprogramming in cancer cells. Cell Metab. 31, 267–283.e12 [DOI] [PubMed] [Google Scholar]
- 116.Bertero E and Maack C (2018) Calcium signaling and reactive oxygen species in mitochondria. Circ. Res 122, 1460–1478 [DOI] [PubMed] [Google Scholar]
- 117.Wu Y. et al. (2017) Contacts between the endoplasmic reticulum and other membranes in neurons. Proc. Natl. Acad. Sci. U. S. A 114, E4859–E4867 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Liao P-C et al. (2018) Isolation of mitochondria from Saccharomyces cerevisiae using magnetic bead affinity purification. PLoS One 13, e0196632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Davies KM et al. (2018) Conserved in situ arrangement of complex I and III2 in mitochondrial respiratory chain supercomplexes of mammals, yeast, and plants. Proc. Natl. Acad. Sci. U. S. A 115, 3024–3029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Pfeffer S. et al. (2015) Organization of the mitochondrial translation machinery studied in situ by cryoelectron tomography. Nat. Commun. 6, 6019. [DOI] [PubMed] [Google Scholar]
- 121.Mageswaran SK et al. (2023) Nanoscale details of mitochondrial constriction revealed by cryoelectron tomography. Biophys. J 122, 3768–3782 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Ando T. et al. (2018) The 2018 correlative microscopy techniques roadmap. J. Phys. D. Appl. Phys 51, 443001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Vue Z. et al. (2023) Human heart failure alters mitochondria and fiber 3D structure triggering metabolic shifts. bioRxiv, Published online December 14, 2023. 10.1101/2023.11.28.569095 [DOI] [Google Scholar]
- 124.McArthur K and Ryan MT (2020) Resolving mitochondrial cristae: introducing a new model into the fold. EMBO J. 39, e105714. [DOI] [PMC free article] [PubMed] [Google Scholar]