Abstract
Recombinant adenovirus vectors (AdV) have been considered a potential vehicle for performing gene therapy in patients suffering from Duchenne muscular dystrophy but are limited by a cellular and humoral immune response that prevents long-term transgene expression as well as effective transduction after AdV readministration. Conventional immunosuppressive agents such as cyclosporine and FK506, which act by interfering with CD3-T-cell receptor-mediated signaling via calcineurin, are only partially effective in reversing these phenomena and may also produce substantial organ toxicity. We hypothesized that activation of redundant T-cell activation pathways could limit the effectiveness of these drugs at clinically tolerable doses. Therefore, we have tested the ability of immunomodulatory immunoglobulins (Ig) with different modes of action to facilitate AdV-mediated gene transfer to adult dystrophic (mdx) mice. When used in isolation, immunomodulatory Ig (anti-intercellular adhesion molecule-1, anti-leukocyte function-associated antigen-1, anti-CD2, and CTLA4Ig) were only mildly effective in mitigating cellular and/or humoral immunity against adenovirus capsid proteins and the therapeutic transgene product, dystrophin. However, the combination of FK506 plus CTLA4Ig abrogated the immune response against adenovirus proteins and dystrophin to a degree not achievable with the use of either agent alone. At 30 days after AdV injection, >90% of myofibers could be found to express dystrophin with little or no evidence of a cellular immune response against transduced fibers. In addition, the humoral immune response was markedly suppressed, and this was associated with increased transduction efficiency following vector readministration. These data suggest that by facilitating both primary and secondary transduction after AdV administration, combined targeting of CD3-T-cell receptor-mediated signaling via calcineurin and the B7:CD28 costimulatory pathway could greatly increase the potential utility of AdV-mediated gene transfer as a therapeutic modality for genetic diseases such as Duchenne muscular dystrophy that will require long-term transgene expression and repeated vector delivery.
Duchenne muscular dystrophy (DMD) is an X-linked genetic and ultimately fatal disorder that afflicts approximately 1 in 3,500 male newborns. The primary defect is an absence of dystrophin (17), a subsarcolemmal protein believed to play an important role in providing structural reinforcement to the muscle cell surface membrane (30, 35). First-generation adenovirus vectors (AdV), which have been made replication defective by deleting early region 1 (E1) from the vector genome, have been used to achieve AdV-mediated transfer of a 6.3-kb dystrophin minigene in vivo (2, 8, 28, 36). AdV infect nonreplicating cells such as skeletal muscle fibers with a relatively high degree of efficiency (1, 2), and it has recently been reported that AdV-mediated dystrophin minigene transfer is capable of ameliorating muscle function in an animal model of DMD, the mdx mouse (8, 41). However, for this beneficial effect to be realized, animals must be either immunologically immature (8) or actively immunosuppressed with potent drug therapy (41). In the presence of an intact immune system, CD8+ cytotoxic T lymphocytes (CTLs) destroy the AdV-infected myofiber population (2, 34, 42) and also produce an accompanying worsening of muscle contractile function (33, 34).
Although substantial progress has been made in developing less immunogenic vectors through the inactivation (45) or deletion (5, 12, 16) of viral genome elements, this approach has at least two inherent limitations with respect to the treatment of monogeneic recessive disorders such as DMD. First, since AdV particle neutralization by antibodies directed against inoculum capsid proteins is believed to be the principal mechanism preventing effective readministration of AdV (22, 44), it is doubtful that this problem can be overcome by further modification of the vector genome. Second, the therapeutic transgene protein product would itself represent a neoantigen that could, depending upon its own intrinsic immunogenicity, stimulate host cellular immunity with attendant elimination of AdV-infected cells. Indeed, the magnitude and nature of host immune responses to foreign gene transfer appear to vary considerably depending upon the specific transgene product being expressed (7, 31). For this reason, it is exceedingly important that proposed immunosuppressive regimens be tested not only with nontherapeutic marker genes as has been the case in many prior studies (14, 20, 33, 34, 42, 46) but also with the specific therapeutic transgene of clinical interest.
Based on the above considerations, the development of safe and effective methods for downregulating the host immune response against both adenoviral capsid proteins and dystrophin is a likely prerequisite to the eventual application of any type of AdV-mediated gene transfer in DMD patients. Distinct stages of cell-cell interaction between antigen-presenting cells (APCs) and T cells are normally involved in the induction of an antigen-specific immune response (for a review, see reference 15). These include (i) adhesion between the APC and the T cell, (ii) recognition of foreign antigen presented to T-cell receptors located in the CD3 complex on the T-cell surface, and (iii) costimulation of the T cell by accessory molecules present on the APC, which triggers subsequent T-cell proliferation and effector function. Commonly employed immunosuppressive drugs such as cyclosporine and FK506 exert their effects by blocking T-cell signaling events associated with the CD3-T-cell receptor pathway, thereby inhibiting interleukin-2 production (11, 21, 27). We have previously reported that FK506, which blocks T-cell signaling by calcineurin, a Ca2+- and calmodulin-dependent phosphatase (27), significantly increased the level of dystrophin gene expression after a single delivery of AdV to muscles of mdx mice (28). However, FK506 was only partially effective in blocking the generation of antibodies against adenoviral capsid proteins and permitting further dystrophin gene expression after a second AdV injection (28). Although this problem might theoretically be overcome through the use of higher drug doses, in clinical practice this approach is often limited by substantial organ toxicity as well as an increased risk of host infection. Furthermore, even in the presence of maximally tolerated doses of FK506 or related compounds, T-cell activation could potentially occur via redundant signaling pathways that are unaffected by blockade of CD3-T-cell receptor-mediated lymphocyte activation (11, 21). In this regard, it is particularly noteworthy that T-lymphocyte activation induced by the interaction between B7-1 (CD80) or B7-2 (CD86) accessory molecules on APCs and CD28 molecules present on T cells, which constitutes perhaps the most important costimulation pathway (9, 15), is distinct from the CD3-T-cell receptor signaling pathway and therefore not inhibited by either cyclosporine or FK506 (11, 21).
Adhesion molecule pairings between intercellular adhesion molecule (ICAM)-1 and leukocyte function-associated antigen (LFA)-1, as well as between LFA-3 and CD2, have been shown to be important in facilitating foreign antigen recognition by T lymphocytes in vivo (4, 13, 19). Whereas the former interaction appears to be largely dependent upon the presence of T-cell activation, the latter is reported to be essentially independent of this parameter, thus suggesting the possibility of differential roles for these adhesion pairs (32). In addition, the fusion protein CTLA4Ig (26), which has a higher avidity for B7 molecules than CD28 does and an inhibitory effect on CD28-mediated T-cell activation (9, 15, 26, 39), has been shown to produce organ allograft acceptance in animal models (15, 24, 25) as well as persistent transgene expression after liver-directed AdV-mediated gene transfer (22). Therefore, in the present study, we have employed immunomodulatory immunoglobulins (Ig) to impede these specific adhesion and costimulatory molecule interactions to determine whether short-term interference with receptor-ligand pairings normally involved in T-cell activation enhances the efficacy of AdV-mediated dystrophin gene transfer in adult dystrophic (mdx) mice. Furthermore, we have attempted to ascertain the existence of any additive or synergistic effects when a combinatorial strategy is used to inhibit both CD3-T-cell receptor-mediated signaling via calcineurin and the CD28-mediated costimulatory pathway. Here we report that the latter approach in particular markedly abrogates the immune response against adenoviral proteins and the dystrophin transgene product for primary as well as secondary AdV-mediated dystrophin gene transfer, thereby expanding the potential utility of this modality as a therapeutic option for DMD.
MATERIALS AND METHODS
Preparation of recombinant adenoviruses.
Adenovirus recombinants containing the 6.3-kb human dystrophin minigene (AdV-Dys) were constructed by using E1/E3-deleted replication-defective serotype 5 human adenovirus as previously outlined in detail (2, 34), where the transgene cDNA was driven by cytomegalovirus promoter/enhancer elements inserted into the E1 region. The absence of contamination by E1-containing replication-competent AdV was confirmed by using a sensitive PCR screening assay as previously described (29). AdV titers were determined by spectrophotometry at 260 nm and are expressed as particles per milliliter (2, 34).
Immunomodulatory reagents.
Rat IgG directed against murine adhesion molecules ICAM-1 (anti-CD54, hybridoma YN1/1.7; American Type Culture Collection, Rockville, Md.) and LFA-1 (anti-CD11a, hybridoma M17/4; American Type Culture Collection) were purified over protein G from hybridoma supernatant. Based upon a regimen previously described for cardiac allograft preservation in mice (19), AdV-injected mdx mice were treated with 100 μg of each monoclonal antibody by intraperitoneal (i.p.) injection daily, beginning the day of AdV-Dys administration and continuing for a total of 6 days. Rat IgG directed against murine CD2 (4, 13) (hybridoma 12–15; gift of P. Altevogt, Heidelberg, Germany) and an irrelevant (control) rat IgG were similarly purified and injected i.p. by using the same dosing regimen. Human CTLA4Ig (gift of P. Linsley, Bristol-Meyers Squibb, Seattle, Wash.) is a soluble fusion protein containing the extracellular domain of the CTLA4 receptor together with the Fc domain of IgG, which inhibits T-cell signaling via the B7:CD28 costimulation pathway (9, 15, 26). By using a dosing regimen previously reported to prolong transgene expression in mice for several months after AdV-mediated gene transfer to liver (22), CTLA4Ig was administered i.p. at a dose of 200 μg on days 0, 2, and 10 after AdV-Dys injection of mdx muscles. For mice treated with FK506 (5 mg/kg of body weight/day subcutaneously), this immunosuppressive regimen was selected based upon our prior demonstration of sustained dystrophin expression 2 months after AdV-Dys delivery to mdx mice (28); the drug was begun on the day prior to AdV-Dys injection and continued until the animals were euthanized.
Animal procedures and experimental protocols.
Dystrophin-deficient mdx mice were purchased from The Jackson Laboratory (Bar Harbor, Maine) and entered into the study at 30 to 50 days of age. Prior to AdV injection, the mice were anesthetized with ketamine (130 mg/kg) and xylazine (20 mg/kg) by injection into muscles other than those used for AdV-Dys injection. Target muscles were then surgically exposed to permit AdV-Dys injection under direct visualization. At the end of the designated experimental period, the mice were euthanized by anesthetic overdose. All animal procedures were approved by the institutional animal ethics committee.
(i) Use of different immunomodulatory Ig in isolation.
mdx mice received 20 μl of purified AdV-Dys (7 × 1011 particles/ml) in the anterior tibialis muscle. The animals were treated with one of the following: (i) anti-ICAM-1/LFA-1, (ii) anti-CD2, (iii) CTLA4Ig, or (iv) control rat IgG as described above. At 30 days after AdV-Dys administration, injected muscles were excised and frozen for immunohistochemistry analysis; sera were also collected for detection of antibodies against adenoviral proteins and dystrophin (see below).
(ii) Use of combinatorial approach to block calcineurin and CD28 costimulation pathways.
Animals were divided into three dosing groups: (i) CTLA4Ig alone, (ii) FK506 alone, and (iii) FK506 plus CTLA4Ig. The dosing regimens for both CTLA4Ig and FK506 were as described above. Each group again received AdV-Dys in the right anterior tibialis muscle on day 0 (first administration), followed by the same dose of AdV-Dys delivered to the left anterior tibialis on day 20 (second administration); this sequence was selected to allow direct comparison with our prior study of FK506 therapy in the setting of AdV-mediated dystrophin gene transfer (28). All animals were subsequently euthanized on day 30, and the AdV-Dys-injected muscles as well as sera were collected.
Dystrophin immunostaining and quantitation of inflammatory response.
Excised muscles were embedded in mounting medium and snap-frozen in isopentane precooled with liquid N2. Transverse cryostat sections (6-μm thick) were obtained from the midportion of the muscle and then fixed on slides in 1% acetone. Immunohistochemical procedures were carried out to detect dystrophin expression by using a polyclonal antidystrophin (C terminus) primary antibody and biotinylated secondary antibody with subsequent visualization by peroxidase staining, as previously outlined in detail (2, 28). Muscle sections were also counterstained with hematoxylin and eosin to allow detection of inflammatory cell infiltration within AdV-injected muscles. Microscopically visualized sections were photographed by video camera (magnification, ×100) and the image was captured on a Macintosh computer with a frame-grabber. Analysis of the number of dystrophin-positive myofibers on the entire muscle cross-section was performed by using the public domain program NIH Image (version 1.49). To quantify the magnitude of inflammation in AdV-Dys-injected muscles, a standard point-counting technique was employed and the area fraction of inflammation was then determined as previously described (6). Briefly, three to four randomly selected microscopic fields per muscle were selected, and a 100-point grid was superimposed onto each captured image by using a stereology software package (Stereology Toolbox; Morphometrix, Davis, Calif.). An abnormal point was defined as either falling upon inflammatory cells or a myofiber invaded by such cells. The area fraction of inflammation was calculated by dividing the number of abnormal points by the total number of points falling on the tissue section and is expressed as a percentage.
Measurement of humoral immune responses.
The host antibody response to adenovirus capsid proteins was measured by an enzyme-linked immunosorbent assay (ELISA) as previously described (34). Briefly, Nunc Maxisorb microtiter plates (GIBCO, Gaithersburg, Md.) were coated with heat-inactivated AdV particles (108/well) overnight in 100 μl of sterile phosphate-buffered saline (PBS) (pH 7.2). Serum obtained from each individual mouse was then diluted in ELISA buffer (0.5% bovine serum albumin, 0.05% Tween 20 in PBS) and applied to the microtiter plate wells, and the wells were incubated overnight at 4°C. Reactivity to AdV was determined by incubation with horseradish peroxidase conjugates with goat anti-mouse IgG (1:1,000; Serotec, Toronto, Ontario, Canada) for 1 h, followed by a washing step and the addition of enzyme substrate [100-μl/well concentration of 0.1 mg of 2,2′-azino-bis(3-ethylbenzthiazoline 6-sulfonic acid)diammonium per ml in 0.1 M citrate buffer, (pH 4.5)] and 0.01% H2O2. Absorbance was read at 405 nm on an Easy Reader-400AT (SLT Lab Instruments, Salzburg, Austria). Sera from naive non-AdV-injected mdx mice served to establish background absorbance values, and data from all experimental groups are expressed as a percent of the naive serum value.
The humoral immune response to human dystrophin in AdV-Dys-injected animals was assessed by using a previously described immunocytochemical detection system (28). Human muscle biopsy specimens were obtained from individuals without histological evidence of neuromuscular disease. Mouse sera from each AdV-Dys-injected experimental group were pooled, while sera from naive non-AdV-injected mdx mice served as a negative control. Sections of normal human skeletal muscle were then blocked with 10% goat serum in PBS for 1 h and then incubated with serial dilutions of pooled mouse sera (up to a maximum dilution of 1:70,000) in blocking buffer overnight. Secondary antibody (1:200) consisted of a biotinylated anti-mouse IgG raised in horse (Vector, Burlingame, Calif.) which was applied for 1 h. Sections were then reacted with Cy3-conjugated streptavidin (1:1,000) for 20 min (Jackson ImmunoResearch, West Grove, Pa.), mounted, and viewed by epifluorescence microscopy. Mouse sera which generated visually detectable sarcolemmal staining on human muscle sections were considered to contain antibodies against human dystrophin. The antibody titer was expressed as the highest dilution of mouse serum giving a positive response.
RESULTS
Effects of immunomodulation on dystrophin expression and muscle inflammation after primary AdV-Dys administration.
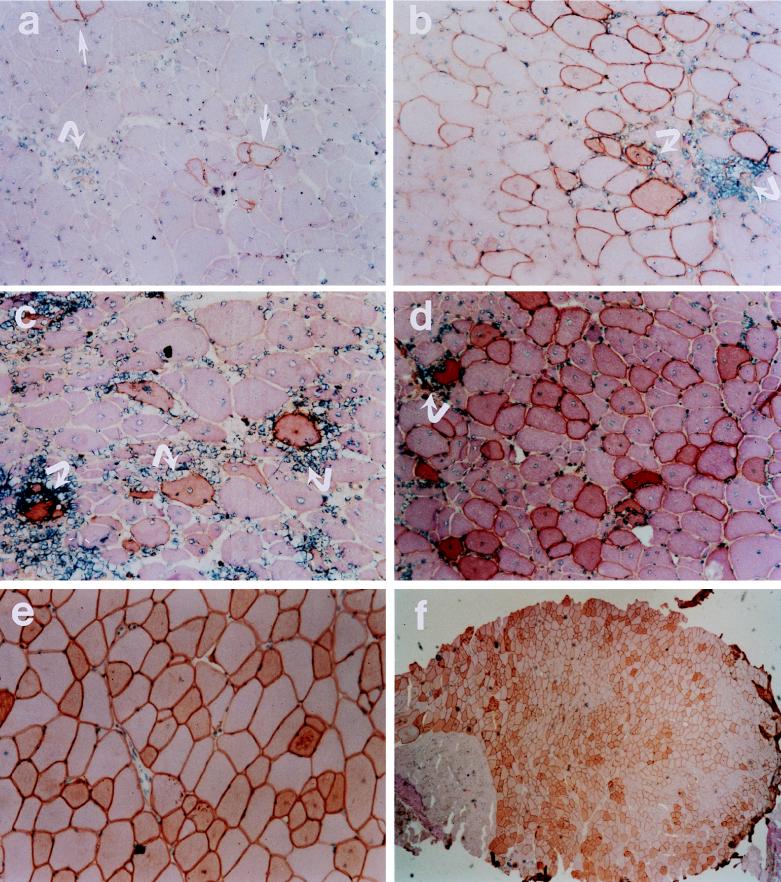
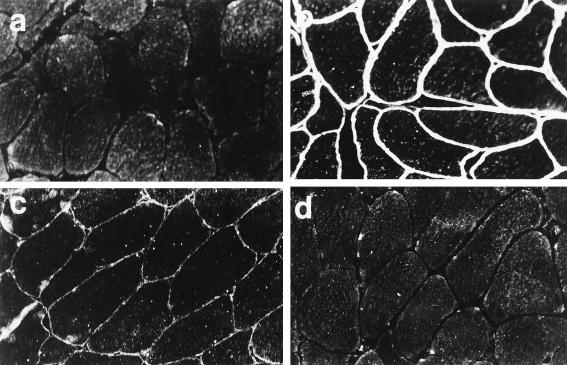
Representative micrographs from different experimental groups are shown in Fig. 1. In the immunocompetent control mdx mice (Fig. 1a), there were scattered foci of inflammatory cell infiltration within those regions containing occasional dystrophin-positive fibers. Although there were greater numbers of dystrophin-positive fibers present in the anti-ICAM-1/LFA-1 (Fig. 1b), anti-CD2 (Fig. 1c), and CTLA4Ig (Fig. 1d) groups as compared to the control, a considerable degree of inflammatory cell infiltration was also noted. However, the addition of FK506 to CTLA4Ig led to markedly reduced inflammatory cell invasion of dystrophin-positive regions within AdV-Dys-injected mdx muscles (Fig. 1e and f). In keeping with this finding, mdx mice treated with FK506 plus CTLA4Ig also demonstrated substantially higher numbers of dystrophin-positive fibers, and in some instances, ∼90% of myofibers expressed dystrophin 30 days after primary AdV-Dys administration, as shown in Fig. 1f.
FIG. 1.
Representative micrographs of adult mdx muscles 30 days after primary AdV-mediated dystrophin gene transfer. Dystrophin immunohistochemistry was monitored by hematoxylin and eosin counterstaining in the following groups: control (a), anti-ICAM-1/LFA-1 (b), anti-CD2 (c), CTLA4Ig (d), and FK506 plus CTLA4Ig (e). Magnification, ca. ×200. Dystrophin-expressing myofibers are identified by dark staining of the sarcolemma, as illustrated by the straight arrows in panel a. Examples of mononuclear inflammatory cell infiltration of dystrophin-expressing fibers are shown by the curved arrows. It is important to note that substantial muscle inflammation was observed in all groups receiving immunomodulatory Ig alone, whereas inflammatory cell infiltration with the combination of FK506 plus CTLA4Ig was minimal or absent. In addition, the vast majority of muscle fibers expressed recombinant dystrophin in the FK506 plus CTLA4Ig group, as shown by the low-magnification (×40) micrograph in panel f.
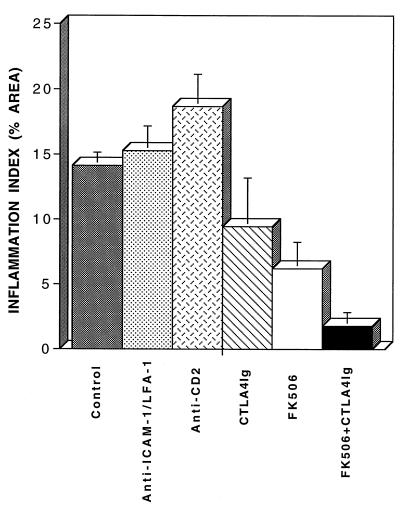
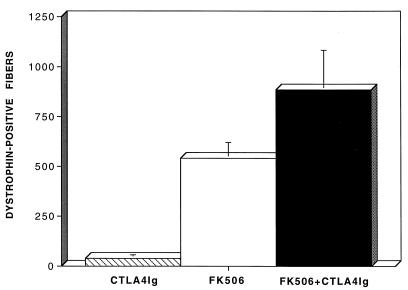
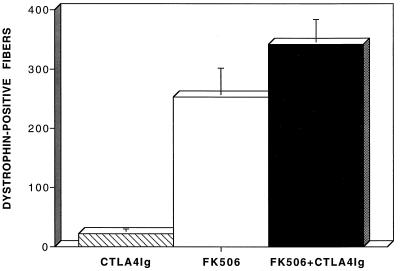
The observations described above were expanded upon in each group of animals through quantitative assessment of the magnitude of inflammation at 30 days post-AdV-Dys injection. These data are illustrated in Fig. 2. Surprisingly, the level of cellular inflammation in the anti-ICAM-1/LFA-1 and anti-CD2 groups was actually equal to or greater than that observed in immunocompetent controls, possibly due to more intense antigenic stimulation by the greater numbers of dystrophin-positive myofibers found at this time point. In contrast, the use of either CTLA4Ig or FK506 alone reduced the level of inflammation below that of control mdx mice. Importantly, the greatest decrement in inflammatory cell invasion of myofibers after AdV-Dys injection of mdx mouse muscles occurred in the FK506 plus CTLA4Ig group, where an eightfold reduction compared to that of immunocompetent controls was observed. Figure 3 shows that FK506 plus CTLA4Ig also produced a substantially higher number of dystrophin-positive fibers at 30 days than either agent alone. By contrast, the mean number of dystrophin-positive fibers at 30 days after primary AdV-Dys administration to immunocompetent control mdx animals was relatively low (35 ± 5 [mean standard error] myofibers/muscle) as previously reported (2, 28), and this was only mildly improved upon in the anti-ICAM-1/LFA-1 and anti-CD2 groups (data not shown).
FIG. 2.
Effects of different immunomodulatory regimens on muscle inflammation after primary AdV-mediated dystrophin gene transfer. In comparison to that of immunocompetent control mdx animals, the levels of inflammatory cell infiltration in anti-ICAM-1/LFA-1 and anti-CD2 groups were equivalent or even increased. In contrast, the use of either CTLA4Ig or FK506 alone reduced the level of inflammation. However, the greatest impact on the cellular immune response to AdV-Dys administration was observed in the FK506 plus CTLA4Ig group, where the degree of inflammatory cell infiltration of AdV-Dys-injected muscles was markedly abrogated in comparison to that of immunocompetent control mice.
FIG. 3.
Effects of immunomodulation on the level of dystrophin expression after primary AdV-Dys administration to adult mdx mouse muscles. The total number of dystrophin-expressing myofibers on an entire cross-section of the anterior tibialis muscle was determined 30 days after AdV-Dys administration. Values are expressed as means ± standard errors (n = 3 to 4 animals/group). As can be seen, the combination of FK506 plus CTLA4Ig led to a major increase in transduction efficiency over that attained when either CTLA4Ig or FK506 was used in isolation.
Effects of immunomodulation on humoral immunity to adenovirus capsid proteins.
Adoptive transfer of antisera obtained from both AdV-Dys-immunized and AdV-LacZ-immunized animals leads to equivalent large reductions in the efficiency of subsequent AdV-mediated dystrophin gene transfer to mdx mice (unpublished data). This suggests that antibodies generated against adenovirus capsid proteins, as well as perhaps other undefined serum factors induced by AdV administration, play a major role in reducing transduction efficiency after vector readministration to mdx muscle tissue. Accordingly, we assessed the effects of immunomodulation on production of antiadenovirus antibodies after AdV-Dys delivery to adult mdx mouse muscles.
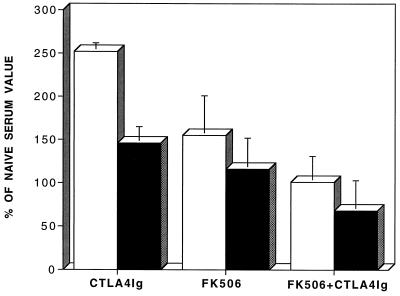
In sera of immunocompetent mdx mice (1:1,000 dilution) examined 30 days after AdV-Dys administration, the signal for antiadenovirus antibodies detected by ELISA amounted to ∼300% of background (i.e., naive serum) values. Although the different immunomodulatory Ig tested were able to produce only a minimal blunting of this response when used in isolation, CTLA4Ig appeared to be the most effective in this regard. However, Fig. 4 shows that in contrast to the mild decrease in antiadenovirus antibodies observed with CTLA4Ig alone, the humoral immune response against adenovirus capsid proteins was substantially reduced in the two groups of FK506-treated mice. Additionally, as was the case for cellular immunity, the reduction in humoral immune responses by FK506 was further enhanced by the addition of CTLA4Ig. In fact, the signal for antiadenovirus antibodies obtained by ELISA in the sera of animals treated with FK506 plus CTLA4Ig did not exceed background levels found in naive mdx animals not exposed to AdV-Dys.
FIG. 4.
Effects of immunomodulation on antiadenovirus antibody production after AdV-mediated dystrophin gene transfer to adult mdx mouse muscles. Antiadenoviral antibodies were quantitated by ELISA and are expressed as a percentage of background values obtained from a negative control (i.e., naive non-AdV-injected) mdx mouse. All data are mean values ± standard errors (n = 3 to 4 animals/group). Primary AdV-Dys administration (day 0) was followed by secondary administration on day 20, and sera from mdx mice were then obtained on day 30. The combination of FK506 plus CTLA4Ig achieved the greatest reduction of the humoral immune response against adenoviral capsid proteins. Open bars, 1:1,000 dilution; closed bars, 1:10,000 dilution.
Effects of immunomodulation on humoral immunity to the therapeutic transgene product.
Antibodies against the transgene-encoded protein (human minidystrophin) were detected by employing an immunohistochemical assay in which serially diluted sera from AdV-Dys-injected mdx mice were reacted with sections of normal human skeletal muscle, as shown in Fig. 5. All experimental groups demonstrated the presence of antidystrophin antibodies at 30 days after AdV-Dys administration. In this regard, sera obtained from the immunocompetent control, anti-ICAM-1/LFA-1, and anti-CD2 groups showed detectable antidystrophin antibodies at dilutions exceeding 1:70,000. The humoral immune response against dystrophin was less pronounced in mdx mice treated with either CTLA4Ig or FK506 alone, in which antidystrophin antibodies were detectable only up to dilutions of 1:35,000 or 1:2,500, respectively. However, in keeping with the marked reduction of antiadenovirus antibodies described earlier, the combination of FK506 plus CTLA4Ig also resulted in the greatest decrease in antidystrophin antibodies, which were detectable only with sera diluted up to 1:200.
FIG. 5.
Representative micrographs illustrating immunohistochemical detection of antibodies generated against human dystrophin following AdV-Dys delivery to adult mdx mice. Pooled sera from the different experimental groups were serially diluted and reacted with sections of normal human skeletal muscle (magnification, ×400). (a) Naive mdx (i.e., non-AdV-injected) mouse sera (1:400 dilution) generated no sarcolemmal staining, consistent with an absence of antidystrophin antibodies. (b) Control mdx (AdV-Dys-injected without immunosuppression) mouse sera, on the other hand, produced strong sarcolemmal staining at a dilution of 1:35,000, consistent with a high level of antidystrophin antibodies. (c) CTLA4Ig-treated mdx mouse sera allowed only very faint sarcolemmal staining at the same 1:35,000 dilution. (d) FK506-plus-CTLA4Ig-treated mdx mouse sera generated no detectable sarcolemmal staining at a dilution of 1:400, similar to the results of the naive group shown in panel a.
Effects of immunomodulation on the efficiency of dystrophin gene transfer after secondary AdV-Dys administration.
Given the above-described effects of FK506 plus CTLA4Ig on humoral immunity after AdV-Dys administration, we further assessed the ability of the different immunomodulatory regimens to facilitate secondary AdV-mediated dystrophin gene transfer. The mean number of dystrophin-positive fibers in the two FK506-treated groups at 10 days after AdV-Dys readministration was substantially higher than that observed in mdx mice treated with CTLA4Ig (Fig. 6) or the other immunomodulatory Ig in isolation. Importantly, as was the case for primary AdV-Dys delivery, the addition of CTLA4Ig to FK506 led to a further major increase in the level of dystrophin expression (as compared to either CTLA4Ig or FK506 alone) after secondary AdV-Dys administration. Thus, the findings are consistent with the fact that FK506 plus CTLA4Ig was most effective in achieving a global reduction in antibody generation against both adenovirus capsid proteins and dystrophin.
FIG. 6.
Effects of immunomodulation on dystrophin expression after secondary AdV-mediated gene transfer to adult mdx mice. The total number of dystrophin-expressing myofibers on an entire cross-section of the anterior tibialis muscle was determined 10 days after AdV-Dys readministration. Values are expressed as means ± standard errors (n = 3 to 4 animals/group). As was the case after primary AdV-Dys administration, the combination of FK506 plus CTLA4Ig achieved the highest transduction efficiency following secondary AdV-mediated dystrophin gene transfer to adult mdx mouse muscles.
DISCUSSION
This is the first study to compare the abilities of different immunomodulatory Ig to facilitate effective primary as well as secondary AdV-mediated dystrophin gene transfer to dystrophic mouse muscle tissue. The short-term administration of neutralizing Ig directed against cell adhesion molecules during the period corresponding to initial AdV capsid particle exposure and early dystrophin transgene expression could theoretically prevent molecular interactions normally required for CD3-T-cell receptor-mediated recognition of these foreign antigens. Administration of CTLA4Ig, on the other hand, should not interfere with antigen recognition but rather with the subsequent step of costimulation that is generally needed to achieve optimal T-cell activation and clonal expansion. Both of these strategies by themselves have previously been demonstrated to successfully induce specific tolerance to allografted organs in experimental animals (4, 19, 24, 25), thus raising the possibility that a state of tolerance to both vector proteins and dystrophin might also be achievable.
In the present study, short-term interference with cell adhesion and costimulatory molecules was able to only partially abrogate undesirable immune responses to AdV-mediated dystrophin gene transfer. Indeed, the equal or even greater degree of muscle inflammation observed in the anti-ICAM-1/LFA-1 and anti-CD2 groups in comparison to that of control mdx animals at 1 month after AdV delivery suggests that these treatments simply delayed the onset of cellular immunity against AdV-infected myofibers. However, among the immunomodulatory Ig regimens examined, CTLA4Ig was found to be the most effective in blunting cellular and humoral immune responses resulting from AdV-mediated gene transfer. In addition to the present study, two other groups have reported on the effects of CTLA4Ig administration in the context of AdV-mediated gene transfer (14, 22, 23). Guerette et al. (14) reported a low efficacy of CTLA4Ig in preventing cellular and humoral immune responses after AdV-mediated transfer of the LacZ reporter gene to nondystrophic murine skeletal muscles. Kay et al. (22, 23), on the other hand, found that CTLA4Ig prevented cellular infiltration and allowed prolonged transgene expression for several months after liver-directed AdV-mediated transfer of the human α-1 antitrypsin gene. Reported differences among studies in the immunosuppressive effects of CTLA4Ig after AdV-mediated gene transfer may be related to a number of factors. First, different intrinsic immunogenicities of the transgene products examined likely played an important role in determining the intensity of ensuing immune responses. Second, the use of murine CTLA4Ig (22, 23) may offer greater therapeutic advantage in mouse models. In particular, at the latest time point (day 10) in which human CTLA4Ig was administered in the present study, generation of anti-human neutralizing antibodies could theoretically have been sufficient to limit its effectiveness. Therefore, it is possible that murine rather than human CTLA4Ig would have been more efficacious in preventing AdV-triggered immune responses. Along these same lines, a lack of neutralizing antibodies against the murine analog could also permit more prolonged treatment with CTLA4Ig, although it should be noted that this strategy did not appear to offer any additional benefit over shorter-term CTLA4Ig administration in the context of liver-directed AdV-mediated gene transfer (23).
The nature of the host immune response to AdV delivery can also vary as a function of the AdV-injected target tissue being studied (20, 46). This may be related to different modes of antigen presentation and priming of T-cell subsets in the different organ systems and could even differ between healthy and dystrophic skeletal muscles since the latter contain numerous macrophages that could act as professional APCs. Under these conditions, it is conceivable that AdV infection of resident macrophages within dystrophic muscle could amplify cellular as well as humoral immune responses. Whereas cellular immunity directed against adenoviral proteins alone appears able to destroy AdV-infected cells in lung (47) and liver (43), in skeletal muscle it has been suggested that adenoviral antigens are of little importance in this regard (37, 42). This conclusion was based upon the observation that animals showing natural immunological tolerance to transgene-encoded proteins did not demonstrate destructive cellular immune responses against AdV-infected myofibers (37, 42).
Given this apparent predominance of transgene-encoded proteins as targets of the CTL attack after AdV infection of skeletal muscle (37, 42), a noteworthy finding in this study was the highly immunogenic nature of the human dystrophin protein when expressed in adult mdx mice. Since dystrophin normally maintains an intracellular location, in the present study it is likely that necrosis of AdV-infected cells (due to either CTL attack or incomplete protection from the underlying disease process as a result of subtherapeutic recombinant dystrophin levels) allowed for dystrophin exposure to the extracellular milieu with subsequent antibody formation. This occurred despite the presence in mdx muscles of a small number (<1%) of revertant fibers able to express murine dystrophin due to somatic cell backmutations (presumably during embryonic development) of the gene (18), which could theoretically confer some degree of immunological tolerance to exogenously supplied dystrophin. Although the lack of tolerance to human dystrophin observed in our study could be related to species differences in dystrophin protein structure, it should be noted that antidystrophin antibodies have also been documented after murine dystrophin gene transfer to mdx mice via myoblast transplantation (38). The present study cannot resolve the question of whether antidystrophin antibodies played a direct role in the eventual elimination of AdV-infected fibers by way of antibody-dependent cellular cytotoxicity (3). However, it has been reported that the presence of antidystrophin antibodies per se does not appear to produce an accelerated loss of dystrophin-positive fibers after transplantation of normal murine myoblasts to mdx mice (38).
The immunosuppressive compounds cyclosporine and FK506 act by binding to members of the immunophilin class of proteins (27). The resulting drug-immunophilin complexes interfere with T-cell signaling events via calcineurin required for lymphocyte activation after stimulation of the CD3-T-cell surface receptor (11, 21, 27). There is now extensive clinical experience with these agents, which have been used primarily as a means of preventing the rejection of transplanted organs. Unfortunately, to achieve adequate levels of immunosuppression, it is frequently necessary to employ drug doses that also cause a degree of organ toxicity. To minimize such problems in the context of AdV-mediated gene transfer, it would be highly desirable to develop alternative strategies that could be used to enhance the level of immunosuppression without incurring an increase in adverse effects. The use of CTLA4Ig is particularly attractive in this regard, as it involves no apparent toxicity and has the additional advantage of allowing potential synergistic immunomodulatory effects, since its mechanism of action is distinct from the CD3-T-cell receptor-triggered pathway targeted by immunophilin-binding drugs (11, 21). In support of this concept, the addition of CTLA4Ig to anti-CD40 ligand antibody treatment has recently been reported to enhance the efficiency of primary as well as secondary AdV-mediated gene transfer to the mouse liver, whereas blockage of either the B7:CD28 or CD40:CD40 ligand pathway by itself was considerably less effective (23).
In the present study, we demonstrate that despite the use of essentially maximal FK506 therapy (approximately 10 times the usual clinical dose), superimposed inhibition of the B7:CD28 costimulatory pathway with CTLA4Ig produced a further major blunting of cellular as well as humoral immune responses directed against both adenovirus capsid proteins and recombinant dystrophin. Furthermore, the benefits of utilizing a combinatorial strategy to block both calcineurin and CD28 signaling pathways were observed after primary as well as secondary AdV-mediated dystrophin gene transfer. It should be noted that although the combination of FK506 and CTLA4Ig was able to reduce antiadenovirus antibodies to essentially undetectable levels with an accompanying improvement in secondary gene transfer, the level of myofiber transduction after secondary AdV-Dys administration was nonetheless lower than that attained following the initial AdV-Dys injection. This is consistent with previously reported findings in CD40 ligand-deficient mice (46), which also demonstrated diminished secondary transduction in the liver despite a failure to develop neutralizing antiadenovirus antibodies. Therefore, it is possible that in addition to neutralizing antibodies, other serum factors (e.g., cytokines [48]) also play a role in reducing secondary transduction efficiency and could thus serve as further targets for future therapeutic modulation of specific immune system components.
While it might be argued that vector modification is preferable to host immunosuppression as a means of preventing or mitigating undesirable immunological responses to AdV-mediated gene transfer, it is important to recognize certain inherent limitations to the former approach. As discussed earlier, there is accumulating evidence that in many instances the transgene product rather than adenoviral gene products represent the primary target of the CTL response that leads to the eventual loss of therapeutic gene expression (37, 42). Therefore, given our results indicating dystrophin itself to be highly immunogenic in the context of AdV-mediated gene transfer to dystrophin-deficient animals, one would predict that strategies involving either inactivation (45) or deletion (5, 12, 16) of adenoviral genes from the vector backbone are unlikely to be completely effective in allowing long-term persistence of dystrophin expression. Indeed, early experience with adenoviral vectors that are lacking in all viral genes appears to confirm the concern described above (5, 16). A particularly interesting development is the recent report that recombinant adeno-associated virus (AAV) vectors efficiently transduce mature skeletal muscle fibers without eliciting an immune response against transgene products that are, by contrast, immunogenic in the context of AdV-mediated gene transfer (10, 40). This suggests that AdV particles may actually act as an adjuvant and thereby boost the immune response against transgene products, including dystrophin. Application of AAV vectors to the treatment of DMD is limited, however, by a relatively small insert capacity of about 5 kb (40). Although it may be possible to further reduce the size of the current dystrophin minigene and its associated promoter elements so that these can be accommodated by the AAV vector, it is unknown whether the resulting severely truncated dystrophin protein would be functional. In addition, for both AdV (22, 44) and AAV (10, 40), the problem of humoral immunity against input viral capsid proteins and consequent inhibition of secondary transduction remains problematic in the absence of immunosuppressive therapy.
In summary, we have tested a number of strategies for providing effective immunosuppression after AdV-mediated dystrophin gene transfer in adult dystrophic (mdx) mice. Whereas interference with adhesion cell molecule function or B7:CD28 costimulation in isolation is only mildly effective in blocking undesirable immune responses, combined inhibition of CD3-T-cell receptor-mediated signaling via calcineurin and B7:CD28 costimulation markedly diminishes host immunity against both vector proteins and dystrophin. Based on the results obtained in this study, we speculate that such an approach might permit repetitive AdV-Dys administration to previously targeted muscles, thereby potentially allowing a stepwise augmentation of the level of dystrophin gene expression in dystrophic muscle tissues. Additional studies are currently in progress to test this hypothesis as well as to assess whether this strategy will lead to commensurate improvements in muscle contractile function.
ACKNOWLEDGMENTS
G.-H.G. and H.L. contributed equally to this work.
This investigation was supported by grants from the Medical Research Council of Canada, the Muscular Dystrophy Association of Canada, the Muscular Dystrophy Association, Inc. (USA), the National Research Council of Canada, and the Association Pulmonaire du Quebec. G.-H. Guibinga is a recipient of a studentship award from the Montreal Chest Institute. H. Lochmuller was supported by grants from the Deutsche Forschungsgemeinschaft and Sander-Stiftung, Germany. J. Nalbantoglu is a Research Scholar of the Fonds de la Recherche en Sante du Quebec. B. J. Petrof is the recipient of a Clinician-Scientist Award from the Medical Research Council of Canada.
We are grateful to P. Linsley for the gift of CTLA4Ig and P. Altevogt for the gift of hybridoma 12–15. We thank N. Chughtai, N. Matusiewicz, J. Bourdon, S. Prescott, and C. Allen for expert technical assistance.
REFERENCES
- 1.Acsadi G, Jani A, Massie B, Simoneau M, Holland P, Karpati G. A differential efficiency of adenovirus-mediated in vivo gene transfer into skeletal muscle cells of different maturity. Hum Mol Genet. 1994;3:579–584. doi: 10.1093/hmg/3.4.579. [DOI] [PubMed] [Google Scholar]
- 2.Acsadi G, Lochmuller H, Jani A, Huard J, Massie B, Prescott S, Simoneau M, Petrof B J, Karpati G. Dystrophin expression in muscles of mdx mice after adenovirus-mediated in vivo gene transfer. Hum Gene Ther. 1996;7:129–140. doi: 10.1089/hum.1996.7.2-129. [DOI] [PubMed] [Google Scholar]
- 3.Ahmad A, Menezes J. Antibody-dependent cellular cytotoxicity in HIV infections. FASEB J. 1996;10:258–266. doi: 10.1096/fasebj.10.2.8641559. [DOI] [PubMed] [Google Scholar]
- 4.Chavin K, Lau H T, Bromberg J S. Prolongation of allograft and xenograft survival in mice by anti-CD2 monoclonal antibodies. Transplantation. 1992;54:286–291. doi: 10.1097/00007890-199208000-00018. [DOI] [PubMed] [Google Scholar]
- 5.Clemens P R, Kochanek S, Sunada Y, Chan S, Chen H-H, Campbell K P, Caskey C T. In vivo muscle gene transfer of full-length dystrophin with an adenoviral vector that lacks all viral genes. Gene Ther. 1996;3:965–972. [PubMed] [Google Scholar]
- 6.Cruz-Orive L M, Weibel E R. Recent stereological methods for cell biology: a brief survey. Am J Physiol. 1990;258:L148–L156. doi: 10.1152/ajplung.1990.258.4.L148. [DOI] [PubMed] [Google Scholar]
- 7.Davis H L, Brazolot C L, Watkins S C. Immune-mediated destruction of transfected muscle fibers after direct gene transfer with antigen-expressing plasmid DNA. Gene Ther. 1997;4:181–188. doi: 10.1038/sj.gt.3300380. [DOI] [PubMed] [Google Scholar]
- 8.Deconinck N, Ragot T, Marechal G, Perricaudet M, Gillis J M. Functional protection of dystrophic mouse (mdx) muscles after adenovirus-mediated transfer of a dystrophin minigene. Proc Natl Acad Sci USA. 1996;93:3570–3574. doi: 10.1073/pnas.93.8.3570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Epstein F H. Costimulatory B7 molecules in the pathogenesis of infectious and autoimmune diseases. N Engl J Med. 1996;335:1369–1375. doi: 10.1056/NEJM199610313351807. [DOI] [PubMed] [Google Scholar]
- 10.Fisher K J, Jooss K, Alston J, Yang Y, Haecker S E, High K, Pathak R, Raper S E, Wilson J M. Recombinant adeno-associated virus for muscle directed gene therapy. Nat Med. 1997;3:306–312. doi: 10.1038/nm0397-306. [DOI] [PubMed] [Google Scholar]
- 11.Galvin F, Freeman G J, Razi-Wolf Z, Benacerraf B, Nadler L, Reiser H. Effects of cyclosporin A, FK506, and mycalamide A on the activation of murine CD4+ T cells by the murine B7 antigen. Eur J Immunol. 1993;23:283–286. doi: 10.1002/eji.1830230145. [DOI] [PubMed] [Google Scholar]
- 12.Gao G-P, Yang Y, Wilson J M. Biology of adenovirus vectors with E1 and E4 deletions for liver-directed gene therapy. J Virol. 1996;70:8934–8943. doi: 10.1128/jvi.70.12.8934-8943.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Guckel B, Berek C, Lutz M, Altevogt P, Schirrmacher V, Kyewski B A. Anti-CD2 antibodies induce T cell unresponsiveness in vivo. J Exp Med. 1991;174:957–967. doi: 10.1084/jem.174.5.957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Guerette B, Vilquin J T, Gingras M, Gravel C, Wood K J, Tremblay J P. Prevention of immune reactions triggered by first-generation adenoviral vectors by monoclonal antibodies and CTLA4Ig. Hum Gene Ther. 1996;7:1455–1463. doi: 10.1089/hum.1996.7.12-1455. [DOI] [PubMed] [Google Scholar]
- 15.Guinan E C, Gribben J G, Boussiotis V A, Freeman G J, Nadler L M. Pivotal role of the B7:CD28 pathway in transplantation tolerance and tumor immunity. Blood. 1994;84:3261–3282. [PubMed] [Google Scholar]
- 16.Haecker S E, Stedman H H, Balice-Gordon R J, Smith D B J, Greelish J P, Mitchell M A, Wells A, Sweeney H L, Wilson J M. In vivo expression of full-length human dystrophin from adenoviral vectors deleted of all viral genes. Hum Gene Ther. 1996;7:1907–1914. doi: 10.1089/hum.1996.7.15-1907. [DOI] [PubMed] [Google Scholar]
- 17.Hoffman E P, Brown R H J, Kunkel L M. Dystrophin: the protein product of the Duchenne muscular dystrophy locus. Cell. 1987;51:919–928. doi: 10.1016/0092-8674(87)90579-4. [DOI] [PubMed] [Google Scholar]
- 18.Hoffman E P, Morgan J E, Watkins S C, Partridge T A. Somatic reversion/suppression of the mouse mdx phenotype in vivo. J Neurol Sci. 1990;99:9–25. doi: 10.1016/0022-510x(90)90195-s. [DOI] [PubMed] [Google Scholar]
- 19.Isobe M, Yagita H, Okumura K, Ihara A. Specific acceptance of cardiac allograft after treatment with antibodies to ICAM-1 and LFA-1. Science. 1992;255:1125–1127. doi: 10.1126/science.1347662. [DOI] [PubMed] [Google Scholar]
- 20.Jooss K, Yang Y, Wilson J M. Cyclophosphamide diminishes inflammation and prolongs transgene expression following delivery of adenoviral vectors to mouse liver and lung. Hum Gene Ther. 1996;7:1555–1566. doi: 10.1089/hum.1996.7.13-1555. [DOI] [PubMed] [Google Scholar]
- 21.June C H, Ledbetter J A, Gillespie M M, Lindsten T, Thompson C B. T-cell proliferation involving the CD28 pathway is associated with cyclosporine-resistant interleukin 2 gene expression. Mol Cell Biol. 1987;7:4472–4481. doi: 10.1128/mcb.7.12.4472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kay M A, Holterman A, Meuse L, Gown A, Ochs H D, Linsley P S, Wilson C B. Long-term hepatic adenovirus-mediated gene expression in mice following CTLA4Ig administration. Nat Gene. 1995;11:191–197. doi: 10.1038/ng1095-191. [DOI] [PubMed] [Google Scholar]
- 23.Kay M A, Meuse L, Gown A M, Linsley P, Hollenbaugh D, Aruffo A, Ochs H D, Wilson C B. Transient immunomodulation with anti-CD40 ligand antibody and CTLA4Ig enhances persistence and secondary adenovirus-mediated gene transfer into mouse liver. Proc Natl Acad Sci USA. 1997;94:4686–4691. doi: 10.1073/pnas.94.9.4686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lenschow D J, Zeng Y, Thistlethwaite J R, Montag A, Brady W, Gibson M G, Linsley P S, Bluestone J A. Long-term survival of xenogeneic pancreatic islet grafts induced by CTLA4Ig. Science. 1992;257:789–792. doi: 10.1126/science.1323143. [DOI] [PubMed] [Google Scholar]
- 25.Lin H, Bolling S F, Linsley P S, Wei R-Q, Gordon D, Thompson C B, Turka L A. Long-term acceptance of major histocompatibility complex mismatched cardiac allografts induced by CTLA4Ig plus donor-specific transfusion. J Exp Med. 1993;178:1801–1806. doi: 10.1084/jem.178.5.1801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Linsley P S, Wallace P M, Johnson J, Gibson M G, Greene J L, Ledbetter J A, Singh C, Tepper M A. Immunosuppression in vivo by soluble form of the CTLA-4 T cell activation molecule. Science. 1992;257:792–795. doi: 10.1126/science.1496399. [DOI] [PubMed] [Google Scholar]
- 27.Liu J, Farmer J D, Lane W S, Friedman J, Weissman I, Schreiber S L. Calcineurin is a common target of cyclophilin-cyclosporin A and FKBP-FK506 complexes. Cell. 1991;66:807–815. doi: 10.1016/0092-8674(91)90124-h. [DOI] [PubMed] [Google Scholar]
- 28.Lochmuller H, Petrof B J, Pari G, Larochelle N, Dodelet V, Wang Q, Allen C, Prescott S, Massie B, Nalbantoglu J, Karpati G. Transient immunosuppression by FK506 permits a sustained high-level dystrophin expression after adenovirus-mediated dystrophin minigene transfer to skeletal muscles of adult dystrophic (mdx) mice. Gene Ther. 1996;3:706–716. [PubMed] [Google Scholar]
- 29.Lochmüller H, Huard A J J, Prescott S, Simoneau M, Massie B, Karpati G, Acsadi G. Emergence of early region 1-containing replication-competent adenovirus in stocks of replication-defective adenovirus recombinants during multiple passages in 293 cells. Hum Gene Ther. 1994;5:1485–1491. doi: 10.1089/hum.1994.5.12-1485. [DOI] [PubMed] [Google Scholar]
- 30.Menke A, Jockusch H. Decreased osmotic stability of dystrophin-less muscle cells from the mdx mouse. Nature. 1991;349:69–71. doi: 10.1038/349069a0. [DOI] [PubMed] [Google Scholar]
- 31.Michou A I, Santoro L, Christ M, Juillard V, Pavirani A, Mehtali M. Adenovirus-mediated gene transfer: influence of transgene, mouse strain and type of immune response on persistence of transgene expression. Gene Ther. 1997;4:473–482. doi: 10.1038/sj.gt.3300412. [DOI] [PubMed] [Google Scholar]
- 32.Moingeon P E, Lucich J L, Stebbins C C, Recny M A, Wallner B P, Koyasu S, Reinherz E L. Complementary roles for CD2 and LFA-1 adhesion pathways during T cell activation. Eur J Immunol. 1991;21:605–610. doi: 10.1002/eji.1830210311. [DOI] [PubMed] [Google Scholar]
- 33.Petrof B J, Acsadi G, Jani A, Massie B, Bourdon J, Matusiewicz N, Yang L, Lochmuller H, Karpati G. Efficiency and functional consequences of adenovirus-mediated in vivo gene transfer to normal and dystrophic (mdx) mouse diaphragm. Am J Respir Cell Mol Biol. 1995;13:508–517. doi: 10.1165/ajrcmb.13.5.7576685. [DOI] [PubMed] [Google Scholar]
- 34.Petrof B J, Lochmüller H, Massie B, Yang L, Macmillan C, Zhao J-E, Nalbantoglu J, Karpati G. Impairment of force generation after adenovirus-mediated gene transfer to muscle is alleviated by adenoviral gene inactivation and host CD8+ T cell deficiency. Hum Gene Ther. 1996;7:1813–1826. doi: 10.1089/hum.1996.7.15-1813. [DOI] [PubMed] [Google Scholar]
- 35.Petrof B J, Shrager J B, Stedman H H, Kelly A M, Sweeney H L. Dystrophin protects the sarcolemma from stresses developed during muscle contraction. Proc Natl Acad Sci USA. 1993;90:3710–3714. doi: 10.1073/pnas.90.8.3710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ragot T, Vincent N, Chafey P, Vigne E, Gilgenkrantz H, Couton D, Cartaud J, Briand P, Kaplan J C, Perricaudet M, Kahn A. Efficient adenovirus-mediated transfer of a human minidystrophin gene to skeletal muscle of mdx mice. Nature. 1993;361:647–650. doi: 10.1038/361647a0. [DOI] [PubMed] [Google Scholar]
- 37.Tripathy S K, Black H B, Goldwasser E, Leiden J M. Immune responses to transgene-encoded proteins limit the stability of gene expression after injection of replication-defective adenovirus vectors. Nat Med. 1996;2:545–550. doi: 10.1038/nm0596-545. [DOI] [PubMed] [Google Scholar]
- 38.Vilquin J-T, Wagner E, Kinoshita I, Roy R, Tremblay J P. Successful histocompatible transplantation in dystrophin-deficient mdx mouse despite the production of antibodies against dystrophin. J Cell Biol. 1995;131:975–988. doi: 10.1083/jcb.131.4.975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Waterhouse P, Penninger J M, Timms E, Wakeham A, Shahinian A, Lee K P, Thompson C B, Griesser H, Mak T W. Lymphoproliferative disorders with early lethality in mice deficient in ctla-4. Science. 1995;270:985–988. doi: 10.1126/science.270.5238.985. [DOI] [PubMed] [Google Scholar]
- 40.Xiao X, Li J, Samulski R J. Efficient long-term gene transfer into muscle tissue of immunocompetent mice by adeno-associated virus vector. J Virol. 1996;70:8098–8108. doi: 10.1128/jvi.70.11.8098-8108.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yang, L., H. Lochmuller, J. Luo, B. Massie, J. Nalbantoglu, G. Karpati, and B. J. Petrof. Adenovirus-mediated dystrophin minigene transfer improves muscle strength in adult dystrophic (MDX) mice. Gene Ther., in press. [DOI] [PubMed]
- 42.Yang Y, Haecker S E, Su Q, Wilson J M. Immunology of gene therapy with adenoviral vectors in mouse skeletal muscle. Hum Mol Genet. 1996;5:1703–1712. doi: 10.1093/hmg/5.11.1703. [DOI] [PubMed] [Google Scholar]
- 43.Yang Y, Ku J, Su Q, Ertl H C J, Wilson J M. Immune responses to viral antigens versus transgene product in the elimination of recombinant adenovirus-infected hepatocytes in vivo. Gene Ther. 1996;3:137–144. [PubMed] [Google Scholar]
- 44.Yang Y, Li Q, Ertl H C J, Wilson J M. Cellular and humoral immune response to viral antigens create barriers to lung-directed gene therapy with recombinant adenovirus. J Virol. 1995;69:2004–2015. doi: 10.1128/jvi.69.4.2004-2015.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yang Y, Nunes F A, Berencsi K, Gonczol E, Engelhardt J F, Wilson J M. Inactivation of E2a in recombinant adenoviruses improves the prospect for gene therapy in cystic fibrosis. Nat Gene. 1994;7:362–369. doi: 10.1038/ng0794-362. [DOI] [PubMed] [Google Scholar]
- 46.Yang Y, Su Q, Grewal I S, Schilz R, Flavell R A, Wilson J M. Transient subversion of CD40 ligand function diminishes immune responses to adenovirus vectors in mouse liver and lung tissues. J Virol. 1996;70:6370–6377. doi: 10.1128/jvi.70.9.6370-6377.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yang Y, Su Q, Wilson J M. Role of viral antigens in destructive cellular immune responses to adenovirus vector-transduced cells in mouse lungs. J Virol. 1996;70:7209–7212. doi: 10.1128/jvi.70.10.7209-7212.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yang Y, Xiang Z, Ertl H C J, Wilson J M. Upregulation of class I major histocompatibility complex antigens by interferon gamma is necessary for T-cell-mediated elimination of recombinant adenovirus-infected hepatocytes in vivo. Proc Natl Acad Sci USA. 1995;92:7257–7261. doi: 10.1073/pnas.92.16.7257. [DOI] [PMC free article] [PubMed] [Google Scholar]