Abstract
Ornamental orchid breeding programs have been conducted to develop commercially valuable cultivars with improved characteristics of commercial interest, such as size, flower color, pattern, shape, and resistance to pathogens. Conventional breeding, including sexual hybridization followed by selection of desirable characteristics in plants, has so far been the main method for ornamental breeding, but other techniques, including mutation induction by polyploidization and gamma irradiation, and biotechnological techniques, such as genetic transformation, have also been studied and used in ornamental breeding programs. Orchids are one of the most commercially important families in floriculture industry, having very particular reproductive biology characteristics and being a well-studied group of ornamentals in terms of genetic improvement. The present review focuses on the conventional and biotechnological techniques and approaches specially employed in breeding Phalaenopsis orchids, the genus with highest worldwide importance as an ornamental orchid, highlighting the main limitations and strengths of the approaches. Furthermore, new opportunities and future prospects for ornamental breeding in the CRISPR/Cas9 genome editing era are also discussed. We conclude that conventional hybridization remains the most used method to obtain new cultivars in orchids. However, the emergence of the first biotechnology-derived cultivars, as well as the new biotechnological tools available, such as CRISPR-Cas9, rekindled the full potential of biotechnology approaches and their importance for improve ornamental orchid breeding programs.
Subject terms: Plant breeding, Agricultural genetics
Introduction
In crop production, knowledge about the heritability of important traits, such as adaptation, resistance, yield, color of flowers, among others, can feed into better strategies and tools for genetic improvement techniques.
Plant breeding is the art, science, and technology of improving plants for the benefit of humankind (Semiarti et al. 2020a). Plant breeding has been developed and evolved together with the history of the agriculture, beginning with the selection of higher yield plants as the source of seeds for the next planting season, the evolution of different techniques of conventional breeding, and more recently, the use of biotechnological methods such as genetic transformation and genome editing (Priyadarshan 2019). Plant breeding has increased the diversity of crops and genotypes and assisted breeders and geneticists to better understand how to select and fix new heritable traits in crops for the development of agriculture. Breeding programs also enable the selection of suitable parents for hybridization (Yuan et al. 2015), and conservation of genotypes from various species in germplasm banks is essential for preserving the genetic variability of a group of species assisting breeders (Vendrame et al. 2014).
Floriculture currently represents one of the sectors of agriculture and horticulture that invests the most into newly available technologies (Cardoso and Vendrame 2022).
In an economical context, orchids are one of the most important products in the floriculture industry, with the genus Phalaenopsis being the one with the greatest global demand in terms of cut and potted floriculture (Hsing et al. 2016; Liang et al. 2020). The longer shelf life (greater than 30 days), efficient control of flowering induction and development, the presence of flowers with multiple color combinations and diverse architecture, and size and number of inflorescences (Liang et al. 2020; Tong et al. 2020) explain their success among all ornamental plants.
The Netherlands has been for a long time the global leader of orchid trade with a spotlight to the Phalaenopsis, also commercially called the Dutch Phalaenopsis. In 2020, the value of Dutch Phalaenopsis orchids was €422 million with 117 million units sold, holding first position among the most popular house plants sold (Royal Flora Holland 2020). Global production of Phalaenopsis orchids has reached 300 million plants per year and exportation value of these orchids in Taiwan (the main competitor of the Netherlands), as documented until 2012, has also increased proportionally (Yuan et al. 2015).
The success of these two countries in the Phalaenopsis trade is directly associated with the development, evolution, and maintenance of breeding programs, and the success in the development of new cultivars and groups of cultivars with new characteristics of marketable interest (Lee et al. 2020). Another factor contributing to the successful cultivation and commercialization of orchids is the development of efficient clonal propagation systems, using techniques such as micropropagation and protocorm-like bodies (PLBs). These techniques have enabled the production of millions of clonal plantlets annually, creating a world market for trading cultivars, plantlets, and cultivation/propagation technologies (Cardoso and Vendrame 2022).
The Orchidaceae have, probably, the highest hybridization capacity among all plant families in the world. This is due to the weak barriers for hybridization and unusual type of plant embryo development, which result in the hybrid seeds with very limited reserves (no endosperm) that develop into protocorms (Yeung 2022). These limited nutritional reserves of orchid embryos require specific associations between mycorrhiza fungi and orchids for orchid seed germination and seedling development (Chen et al. 2022). However, a specific in vitro method developed for orchid seed germination, called asymbiotic germination, uses a specific culture medium to mimics the association with fungus, aiming to promote a rapid and efficient system for germination and development of seedlings from hybrid-derived seeds rescued (Balilashaki et al. 2015).
All these characteristics render orchids one of the most hybridized, interesting, and intriguing ornamentals in the world. Phalaenopsis is also one of the most studied genera in terms of breeding. The Phalaenopsis genus comprises around 63–70 species (Cribb and Schuiteman 2012), commonly referred to as moth orchids, native to the tropics and subtropics areas of Asia, Australia and the Pacific Ocean islands (Huang et al. 2015). The Phalaenopsis species and hybrids live as epiphytes, are monopodial with undeveloped pseudobulbs and succulent leaves, have high water and nutrient efficiency, and exhibit crassulacean acid metabolism photosynthesis with CO2 uptake at night (Chuang et al. 2014). The number of artificial hybrids surpass 39 thousand hybrids only in the International Orchid Register Database (RHS 2023). The above factors demonstrate the importance of Phalaenopsis orchids as a model for the genetic development of novel approaches for improvement of ornamental orchids and other flower crops.
Previous reviews on orchids, including Phalaenopsis have focused on biotechnological methods/tools (Vendrame and Khoddamzadeh 2016) and breeding applications (Enoki and Takahara 2022), such as orchid molecular biology (Hsiao et al. 2011a), the use of tissue culture (Khatun et al. 2020) and other biotechnological methods (Hossain et al. 2013), and genomics (Tsai et al. 2017) and omics approaches (Balilashaki et al. 2019). However, none of these reviews explored extensively the literature for each technique or compared the advantages and limitations of conventional hybridization versus modern biotechnological methods in orchids. In this review we include some practical examples of how mixed techniques using both biotechnology and conventional breeding tools are useful for the development of new generations of orchid cultivars.
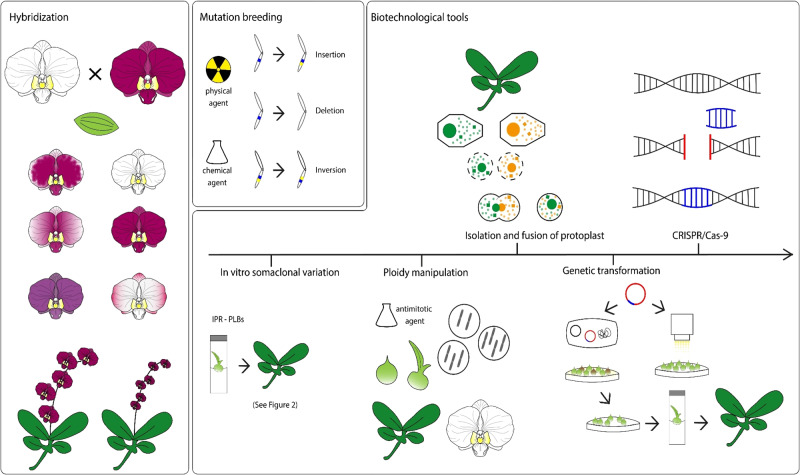
Thus, the aim of this review is to describe and discuss the main biotechnological and conventional approaches employed for orchid breeding programs, with a focus on Phalaenopsis orchids (Fig. 1), including an outline of future prospects.
Fig. 1. Biotechnological and conventional approaches for orchid breeding.
Conventional breeding by hand-cross hybridization and mutation breeding using chemical and physical agents; Biotechnological tools, in vitro somaclonal variation including the Induction, Proliferation and Regeneration of PLBs (IPR-PLBs), ploidy manipulation, isolation and fusion of protoplasts, Agrobacterium tumefaciens-mediated transformation and particle bombardment and, Clustered Regularly Interspaced Short Palindromic Repeats (CRISPR/Cas-9) genome editing.
Orchid breeding by hybridization or conventional breeding
Artificially created orchid hybrids exhibit improved characteristics, such as size, number of flowers, shape, longevity, color and fragrance pattern of flowers, stalk length, blooming periods, and early flowering. Furthermore, leaf appearance and plant architecture, number of viable seeds, the convenience of cultivation, and resistance to pathogens are targeted (Semiarti et al. 2020b).
Current large-scale Phalaenopsis breeding programs based on hybridization have focused on aspects related to ornamental features, such as uniformity of the induction and development of flowering using low temperatures, plant architecture, size and number of inflorescences, the color and size of the flowers and, in some groups of cultivars, the production of compact plants for commercialization in small pots (see https://www.anthura.nl/products/orchid-pot/?lang=en and https://www.floricultura.com/en/orchidaceae/floriclone-phalaenopsis/).
Phalaenopsis orchid genotypes used as progenitors in breeding programs, usually exhibit large flowers with white, pink, and striped color patterns, whereas the novelty cultivars exhibit innovative coloration such as yellow, red, white/rose with red spots or stripes (Fig. 2A), and also fragrance in some cases (Lee et al. 2020). The introduction of other genetic sources for new types, such as small and compact orchids, also called dwarf or mini, are also considered for the market (Vo et al. 2019). Most commercial varieties are generally tetraploids while wild-species are diploids (Lee et al. 2020).
Fig. 2. Conventional hybridization by hand-cross pollination, seed obtaining and in vitro development of seedlings of Phalaenopsis orchids.
A typical tetraploid cultivar of Phalaenopsis used in breeding programs; B details of the flower labellum and the column where the pollinia and the stigma of the flower used for hand-cross pollination are located; C fruit of Phalaenopsis with seeds (fws) and a seedless fruit (sef); D plastic container used to store orchid seeds at low temperatures; E protocorm development derived from seeds of immature fruits; F protocorm development from seeds of mature fruits; G regeneration of seedlings of the hybrid progeny F1.
The procedure for hand-cross pollination, the most used conventional breeding approach, consists of different phases. It starts with the selection of parents containing hereditary characteristics of interest, followed by hand-cross pollination between the progenitors, their fertilization and subsequent fruit and seed development towards fruit and seed maturity within 4–14 months depending on the orchid genera. The immature or mature seeds are then rescued, established in vitro, and germinated, using the technique called “asymbiotic in vitro seed germination” (Fig. 2A–G).
Rescue of zygotic embryos from intra- and intergeneric crosses, performed by in vitro asymbiotic germination, has been applied widely for Phalaenopsis breeding (Tsai 2014) and produced the most of actual groups of cultivars with thousands of hybrids. The asymbiotic seed germination of orchids accelerates obtaining the progeny, improves the germination and development of seedlings and it is used for breeding, due to the high heterozygosity of parentals and the high number of seeds (more than hundred thousand seeds per fruit) from each crossing (Zanello and Cardoso 2019). Seedlings are later acclimatized and cultivated in plastic pots in a greenhouse, aiming at the selection of progenies with specific and aimed features.
However, conventional breeding techniques in orchids present some limitations, such as the high rates of cross incompatibility in some orchid cultivars, particularly those with large genomes (Azadi et al. 2016), which leads to no fruit production, the production of fruits without seeds, or seeds with no embryos; the latter two resulting in time and effort waste by the breeder. In our experience with breeding different commercial group of orchids, the limitation varies according to the genus. Cattleya and Dendrobium hybrids may produce fruits containing seeds without embryos; Oncidium genera crossings could result in low fruit set and formation and also the development of fruits without seeds, and in Phalaenopsis the main limitation is the long time required for the formation of large fruits associated with the production of fruits without seeds (Fig. 2C).
When hybridization is successful with seed and embryo formation, seeds can be stored at low temperatures for 6–12 months (Fig. 2C, D), or are directly germinated in vitro with the formation of protocorms (Fig. 2E, F), followed by seedling development (Fig. 2G).
Also, the lifecycle for growing Phalaenopsis (2–3 years) leads to 4–7 years for obtaining a novel cultivar, whereas additional 2–3 years are required for the production of large quantities of clonal plantlets that in fact reach the growers and flower markets. The large-scale production of plantlets from a novel cultivar is achieved by cloning the selected individuals using micropropagation, e.g., through in vitro shoot proliferation, or by PLBs formation from different types of somatic tissues (Cardoso et al. 2020).
Mutation breeding
Mutation is a natural consequence of the modifications occurring during DNA replication or ineffectiveness of DNA repair and is one of the bases of genetic variation in plants. Artificial application of particular chemicals, such as colchicine, oryzalin, trifluralin, pronamide and amiprofos-methyl (Vilcherrez-Atoche et al. 2022), or physical agents, such as gamma-rays, X-rays and ion-beams to increase induction and frequency of mutations in plant cells has been used for producing novel cultivars of ornamental plants (Melsen et al. 2021). This is referred to as mutation breeding. This technique can reduce the time for the development of new cultivars of orchids, depending on the goals, methods, and mutagens chosen, resulting in high rates of plants with genomic mutations, such as polyploidization, or by specific punctual genic mutations (Li et al. 2021; Vilcherrez-Atoche et al. 2022). However, undesirable effects of mutation breeding in the tissues can also occur and include: unpredictable mutants, plant death due to lethal doses or time of exposure to the mutagenic agent, or by lethal mutations resulted from the treatment and the generation of chimeric plants, that makes difficult the selection of solid and stable mutants (Kim 2020).
Irradiation is employed as an indirect tool for mutagenesis and is one of the most commonly used approaches for floricultural species, used both under in vitro or in vivo conditions. When radiation penetrates living cells, energy in the form of both waves and particles interacts with the atoms and molecules in the cells, thereby producing free radicals, which may modify or destroy the vital components of the cells (Oladosu et al. 2016).
Gamma rays are the main type of radiation used in plant mutation breeding because they penetrate deep into the biological matter and cause point mutations and small deletions. The main advantages of this technique were observed when the irradiation modifies one or few traits in the organism, maintaining most of the genetic background of the cultivar (Kim 2020; Anne and Lim 2020). Its application for superior genotypes aiming at the expected point mutations gathers small changes in traits, such as flower color, disease-resistance, associated with good genome of superior cultivars used in floriculture (Lestari et al. 2018).
In Phalaenopsis, studies with gamma irradiation have been reported (Table 1) and aimed at changes in flower color, size, morphology, shelf-life and flowering (Anne and Lim 2020; Magdalita et al. 2022). Induction of early flowering (less than 2 years) in P. aphrodite and P. amabilis was reported using gamma ray irradiation in lower doses (15–25 Gy) (Magdalita et al. 2020; Widiarsih and Dwimahyani 2013). Furthermore, gamma irradiation was used in protocorms of P. amabilis with successful development of mutant seedlings showing resistance to Dickeya dadantii, which causes soft-rot disease (Putri et al. 2021).
Table 1.
Gamma-irradiation mutation breeding of Phalaenopsis.
| Phalaenopsis species/cultivar | Explant | Radiation Doses (Gy) | Primary findings | Reference |
|---|---|---|---|---|
| - | Pollen | - | - 60–80 Grays (Gy) were optimum | (Zhang et al. 2009a) |
| - | PLB | 15, 20, 25, 30, 60, 90, 120 of 60 Co-γ ray |
- Low doses of radiation did not affect Phalaenopsis PLBs growth, but high doses decreased survival rate, multiplication coefficient, and differentiation ratio - 50–68 Gy was reported as the LD50 for Phalaenopsis PLBs |
(Zhang et al. 2009b) |
| P. aphrodite cv. F141780 | Axillary bud of floral stalk from mutant seedlings | 0, 15, 20, 25. and 30 of 60Co-γ ray | - The low dosage materials have small genetic distance and strong genetic stability, while the high-dosage ones have long and weak genetic distance and stability, respectively | (Ma and Zhang 2011) |
| P. amabilis Bl. | Ready-to-plant in vitro plantlet | 0, 5, 10, 15, 20. and 25 |
- One plant from the 25 Gy treatment developed one flower spike - Early flowering mutant was achieved since most orchids take 2 years to flower after acclimatization |
(Widiarsih and Dwimahyani 2013) |
| P. aphrodite | Germinating embryos | 10, 15, 20 and 25 Gy |
- 10 and 15 Gy resulted in the highest mean number of regenerants, after the untreated ones - 25 Gy was inhibitory to growth. - 15 Gy induced early flowering (1 year and 8 months), shortening juvenility - 15 Gy induced dwarf plants and inflorescences; changes in color of flowers from white to light purple |
(Magdalita et al. 2020; 2022) |
| P. amabilis | Protocorms | 0, 5 10, 15 and 20 Gy | - Successful identification of soft-rot pathogen (Dickeya dadantii) resistance plantlets from irradiated and non-irradiated protocorms | (Putri et al. 2021) |
PLB protocorm-like bodies, LD50 half lethal dose.
Gamma irradiation has also been applied to breeding of other important commercial orchid genera, such as Cymbidium (Kim et al. 2020a), Dendrobium (Billore et al. 2019; Sherpa et al. 2022) and Oncidium (Ahmad et al. 2006). The main results of these studies include the induction of early flowering plants (Sherpa et al. 2022), yellow-leaf phenotype (Kim et al. 2020a) or leaf chimeras (Kim et al. 2020b).
Biotechnological tools applied for Phalaenopsis breeding
Biotechnological tools have shown promising results in producing new orchid cultivars with greater efficiency and precision achieved using conventional breeding (hybridization or conventional mutation breeding) (Hsiao et al. 2011a; Semiarti et al. 2020b).
Some breeding goals can only be achieved through genetic transformation (Hsing et al. 2016), and its benefits and efficiency to meet challenges of the world’s modern agriculture have been demonstrated for a large number of important food and industrial crops and ornamental species (Noman et al. 2017). Furthermore, the use of CRISPR/Cas9 represents a simple and efficient genome editing technology for inducing mutation in target genes for breeding purposes, thereby enabling the transformation of plants for color, fragrance, size and shelf-life, and conferring resistance to abiotic and biotic stresses (Corte et al. 2019).
In vitro somaclonal variation
PLBs are specific organs of orchids, which originate from somatic tissues, such as protocorms, leaves, shoot tips, among others that are regenerated under in vitro conditions and are conventionally used for large-scale propagation of different orchid genera, including Phalaenopsis. The technique known as Induction, Proliferation, and Regeneration of Protocorm-Like Bodies (IPR-PLBs) can also be used as a spontaneous source of mutations originated from somaclonal variations (SVs). Conventionally, SVs result in mutant genotypes, also called somaclones, and include undesirable genetic or epigenetic variations in Phalaenopsis orchids, such as the absence of flower parts (Cardoso et al. 2020).
The origin of the SV phenomenon under in vitro conditions is derived from activation of different chromosomal changes (including polyploidization), transposons, retrotransposons, methylation and demethylation of DNA (Manchanda et al. 2018). The origin of SV observed in PLBs is not completely understood, but PLBs are more susceptible to somaclonal variation compared to conventional micropropagation, which uses shoot tips or inflorescence nodes as explants that directly regenerate shoots. The frequency of SVs in the IPR-PLB technique can range according to different endogenous factors, such as the genotype, the age and the origin of tissues used as explant, and exogenous factors, such as culture medium, with emphasis on plant growth regulators and other additives. Also, the frequency, number and period of subcultures, cultivation conditions and especially the long-term subculture are important factors affecting the SV frequency (Lee et al. 2016). The increase in the frequency of SVs in Phalaenopsis using the IPR-PLBs technique can also be achieved by the use of UV radiation or by the use of the mutagenic agent Ethyl Methane Sulfonate (Kurniadi et al. 2023).
Most of the SV that occur in vitro using IPR-PLBs or those that remain in the ex vitro stage show undesired characteristics, such as morphological abnormalities in the development of orchid leaves (“creased leaves”), inflorescences and flowers, or even in the absence of specific structures within the flowers, such as altered development of petals and sepals or the absence of labellum and pollinia (Cardoso et al. 2020).
Although the SVs are undesirable in clonal in vitro propagation systems, this type of variation from original tissues has been used for breeding purposes in ornamental plants, mainly when derived from punctual stable mutations in the plant tissues. The use of SVs-derived plants in breeding programs are strategic and the obtaining of interesting somaclones, from the agricultural point of view, and these benefits were demonstrated in different crop species, including SVs-derived genotypes showing dwarfism, abiotic and biotic stresses tolerance, and changes in color of flowers and fruits (Rajan and Singh 2021).
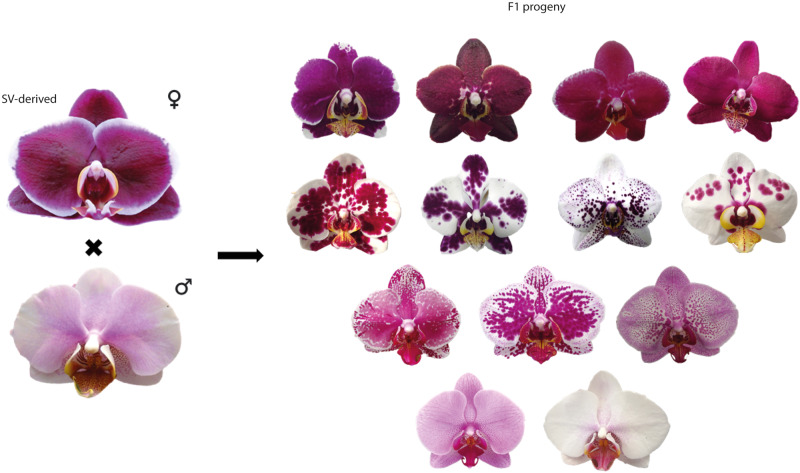
Therefore, somaclonal variation also emerged as a potential technique for breeding in ornamentals (Eeckhaut et al. 2020). At the end of the 20th century, a new group of commercial Phalaenopsis varieties emerged with non-conventional flower colors, characterized by blotches of spots resembling a clown face with painted red or magenta-black spots in their flowers; commercially called “Harlequin” Phalaenopsis. Current studies with the first “Harlequin” Phalaenopsis cultivars that appeared in the market around 1996, demonstrated that the flower colors were triggered by a single point mutation in in vitro plantlets derived from PLBs, used to clone P. Golden Peoker “Brother” (Hsu et al. 2019; Lee et al. 2020). This mutation was named “ES”, since the first SV-derived plants flowered at the Ever-Spring Nursery. Eventually, clones derived from micropropagated meristems also presented around 30% of plants mutated, enlarging the “Harlequin” genotypes, maintaining this irregular distribution of red spots or blotches of spots in their flowers, but with segregation of flower color, establishing the fundamental genetic basis for all subsequent Harlequin-type cultivars currently available in the market (Lin 2021).
P. “Everspring Black” (Fig. 3) is one of these SV-derived cultivars from P. Golden Peoker “Brother”, with intense and solid red-marron color in petals and sepals. The recent conventional hybridization performed between the “Harlequin”-type P. Everspring Black with P. “PH501” (Fig. 3), a cultivar showing conventional flower color, resulted in a progeny showing a good diversity of colors in flowers among individuals, most of them showing characteristics of “Harlequin” (Fig. 3—F1 progenies), proving that the SV characteristic is hereditary and can be used to generate new cultivars by conventional hybridization.
Fig. 3. Heritability of the ‘Harlequin’ trait in Phalaenopsis orchids.
F1 progeny obtained from conventional hybridization of Phalaenopsis “Everspring Black” (female parent), a cultivar derived from single point mutation of somaclonal variation (SV-derived) of Protocorm-like bodies of P. Golden Peoker “Brother”, and the commercial cultivar called P. “PH501” (male parent). Original figures from Jean C. Cardoso.
These observations about the occurrence of SVs in PLBs are also reported in the micropropagation of the Oncidium “Milliongolds”, one of the worldwide distributed Oncidium orchids with yellow flowers (Wang et al. 2019).
Polyploidization
Polyploidy plays a significant role in breeding of different orchid genera, such as Phalaenopsis (Lee et al. 2020), Cymbidium, Dendrobium and Oncidium (Li et al. 2021) and Brassolaeliocattleya (Vilcherrez-Atoche et al. 2023). A recent review reported information about the natural and artificial occurrence of polyploidy in orchids, including Phalaenopsis (Vilcherrez-Atoche et al. 2022). Antimitotic agents, such as colchicine or oryzalin, are conventionally used, aiming at self-polyploidization of different orchid genera. In Phalaenopsis 80% of the studies used colchicine as an antimitotic agent for polyploidy induction (Vilcherrez-Atoche et al. 2022), but interestingly, natural polyploids of different orchid genera are also reported, originated from high number of endo-reduplicated cells reported in different types of orchid tissues (Vilcherrez-Atoche et al. 2022) due to the higher rates of endopolyploid tissues, such as observed in protocorms and PLBs of Phalaenopsis (Chen et al. 2011). Similar observations were reported for Brassolaeliocattleya orchids (Vilcherrez-Atoche et al. 2023), with the occurrence of 11.5% polyploids seedlings derived from protocorms without the treatment with antimitotic agents. Another source of polyploidy in Phalaenopsis is the use of unreduced pollen grains, of natural origin (0.68–1.78%) and which can also be induced or increased in frequency by the use of colchicine (up to 10%) (Wu et al. 2022).
Polyploids have been used in Phalaenopsis breeding to generate large flowers and inflorescences with multiple flowers, plant size and leaf thickness (Chen et al. 2010; Lee et al. 2020). In fact, Lee et al. (2020) reported the predominance of tetraploid cultivars (80% of 60 total cultivars analyzed) in the floriculture industry, with only one diploid reported. Tetraploid hybrids of Phalaenopsis have superior horticultural and ornamental characteristics compared to diploids, such as high vigorous leaves (larger, longer and thicker), rapid development, good adaptation to pot cultivation and greenhouse management, longer inflorescences containing higher number of flowers, higher diameter of flowers, petals and sepals, which in general is considered one of the most important floriculture products in the world.
Haploid and double-haploid technology
Although uncommon in orchid breeding, gametic embryogenesis technology is a powerful breeding tool used to provide haploid and double-haploid plants for the agricultural industry. This technology, together with genetic transformation, is currently used for the most important crops propagated by seeds in the world, such as maize, wheat, barley, and Triticum and Brassica genera aiming at the production of inbred and homozygous lines (Zargar et al. 2022).
The development of this technology could provide important advances in ornamental breeding programs by producing homozygous lineages, followed by hybridization and production of F1 hybrid seeds. In floriculture, haploids and double haploids have been developed for some species, such as Chrysanthemum (Wang et al. 2014) and Gerbera (Li et al. 2020) by using ovule culture, and in Anthurium using anther culture (Winarto et al. 2010).
In orchids, the natural high number of seeds in fruits, commonly hundreds of thousands to millions of seeds per fruit (Anghelescu et al. 2023), is a desirable trait that benefits the use of double-haploid technology aiming at the production of F1 hybrid seeds from inbred lines. The in vitro asymbiotic germination of seeds is simpler, faster and more efficient method for orchid propagation, compared with current micropropagation systems. Haploid and double-haploid plants can also support genetic studies, such as genetic map construction, molecular breeding and transgeny (Thaneshwari et al. 2018).
Genetic transformation
Genetic transformation is a useful technique to transfer specific genes of interest into plants, otherwise impossible through conventional hybridization. Genetic transformation can be accomplished by either direct or indirect methods, the former through particle bombardment (e.g., gene gun), the latter by Agrobacterium tumefasciens specific strains with high virulence. The virulence mechanism of A. tumefaciens induces the transfer of the target DNA fragments with characteristics of interest into the plant species (Semiarti et al. 2020a). For instance, Agrobacterium-mediated transformation and particle bombardment approaches have been reported as efficient and reliable alternatives for introducing specific foreign genes into orchid genomes (Wang et al. 2019) and enables the better comprehension of gene functions in Phalaenopsis (Semiarti et al. 2007). The particle bombardment system (gene gun) is an efficient and a rapid physical process without any biological host limitations, which has been applied for the transformation of orchid tissues (Chew et al. 2019).
Some disadvantages of genetic transformation of orchids using Agrobacterium-mediated modification include the limited host range and the lack of a rapid transient gene (Chew et al. 2019). However, the use of super-binary vector and super-virulent bacterial strains, such as EHA101 and EHA105 (Subramaniam and Rathinam 2010) promotes in some monocots, including orchids, successfully transformation outcomes (Nopitasari et al. 2020; Tong et al. 2020; Semiarti et al. 2020a). Nonetheless, the Agrobacterium-mediated transformation system is often preferred because it is a simpler method compared to particle bombardment. Additionally, the copy number of the transgenes produced is reduced, resulting in fewer problems associated with transgene co-suppression and instability (Chai et al. 2002; Mishiba et al. 2005). Genetic transformation systems through Agrobacterium have also been studied in different orchid genera, such as Cattleya (Zhang et al. 2010), Cymbidium (Chin et al. 2007b), Dendrobium (Teixeira da Silva et al. 2016; Utami et al. 2018), Vanda (Shrestha et al. 2007a), Oncidium and Odontoglossum (Raffeiner et al. 2009).
In Phalaenopsis, disease-resistance related genes have been transferred including two disease-resistance genes; Gastrodia antifungal protein (GAFP) and Neutrophils Peptide-I (NPI) conferring resistance to Colletotrichum gloeosporioides Sacc. (Li et al. 2013); wasabi defensin gene isolated from Wasabia japonica to produce Phalaenopsis with resistance to Erwinia carotovora (Sjahril et al. 2006); gene encoding Cymbidium Mosaic Virus (CymMV), coat protein to give protection against CymMV infection (Liao et al. 2004) and anti-microbial peptide from sweet pepper ferredoxin-like protein cDNA (Pflp) to obtain resistance to Erwinia carotovora (Chan et al. 2005). These reports concluded that CymMV resistance was RNA-mediated via post-transcriptional gene silencing mechanism (Liao et al. 2004; Chan et al. 2005).
Besides these disease-resistance genes, the rice plant-derived glutathione S-transferases (gst) gene, which is associated with protecting cells against different stresses (Chin et al. 2007a) and lipid transfer protein (ltp) encoding gene, responsible for cold tolerance, were transferred to Phalaenopsis (Qin et al. 2011).
Genetic transformation aiming at improvements in size of plants and color of flowers was also reported. Miniaturized plants of P. “Sogo Yukidian SPM313”, were obtained by ectopic overexpression of the OsGA2ox6 gene (Hsieh et al. 2020), expanding the possibilities for miniaturized Phalalaenopsis plants for the flower market. Su and Hsu (2003; 2010) performed the transformation of Phalaenopsis using particle bombardment to deliver the cytochrome P450 gene for enhancing its anthocyanin content and reported a change in the petal color from pink to magenta in the resulting Phalaenopsis hybrid (Chen et al. 2011). Similar results were obtained by Lou et al. (2023) with the use of plasmids carrying genes correlated to flowers (PhCHS-5 and PhF3′5′H) and which resulted in changes in flower color in Phalaenopsis and petunia.
Few reports have discussed the performance or efficiency of genetic transformation in Phalaenopsis, but some reports still point to low transformation efficiency as one of the difficulties in using this technique (Lou et al. 2023). In addition, most of the studies are related to disease-resistance, showing that genetic transformation systems in Phalaenopsis are not well established and their transformation efficiency is still low (Li et al. 2021). The choice of the Agrobacterium strain is an important parameter that influences transformation efficiency (Subramaniam et al. 2008). Among the 21 studies on Agrobacterium-mediated transformation of Phalaenopsis orchids, nine studies used the strain EHA101, eight studies used the strain EHA105 and seven studies used the strain LBA4404 (Table 2). Some of these A. tumefaciens strains were also used in other genera of orchids. The strain EHA101 is the most used in Cattleya, Cymbidium, Dendrobium and Vanda, while for Oncidium and Erycina pusilla the strain EHA105 was the most employed for genetic transformation’ (Mii and Chin 2018). Interestingly, strain EHA105 was the only one used in Phalaenopsis since 2016, showing better transformation efficiency.
Table 2.
Genetic transformation of Phalaenopsis by Agrobacterium tumefaciens and particle bombardment.
| Method | Phalaenopsis species/ cultivar | Explant | Agrobacterium strain | Genes | Primary findings | Reference |
|---|---|---|---|---|---|---|
| Agrobacterium-mediated | Phalaenopsis [Doritaenopsis Coral Fantasy × Phalaenopsis (Baby Hat × Ann Jessica)] | Cell clumps derived from friable calli | EHA101 | hpt, nptII, and gus |
- 10 (strain EHA101) and 24 (strain LBA4404) hygromycin-resistant regenerated plants were obtained per gram of cells after 10 h co-cultivation with Agrobacterium - 500 µM of acetosyringone enhanced the infection rate, increasing the number of GUS spots (42.4) compared to the control (10.4) |
(Belarmino and Mii 2000) |
| Agrobacterium-mediated | Phalaenopsis lines (T0, T5, T10, and Hikaru) | PLBs | LBA4404 | hpt, nptII and gus |
- L1.5-3 mgL−1 hygromycin were suitable for transformed PLBs selection - Percentage of transformed (rooted plantlets) PLBs was 1.5–14.6% - Transversely bisected PLBs were more efficiently transformed (16.2%) than the intact PLBs (8.4%) |
(Chai et al. 2002) |
| Particle bombardment | P. TS444 [(New Eagle × Pinlong Cinderella) × Dtps. Taisuco Red] | Petals | - | F3′5′H P450, gfp |
- Phalaenopsis petals bombarded with the F3′5′H gene showed change in color from pink to magenta - The highest frequency of transformation was obtained with bombarding helium pressure of 10.3 MPa. - P450 gene was successfully transformed into Phalaenopsis petals |
(Su and Hsu 2003) |
| Particle bombardment | P. TS340 [P. Taisuco Kochdiam × P. Taisuco Kaaladian] | PLBs | - | CymMV cp, htp, gus, gfp |
- 20 transgenic lines were obtained after hygromycin selection - Transgenic Phalaenopsis transformed with cp gene resulted in protection against CymMV infection. - CymMV resistance was RNA-mediated through PTGS |
(Liao et al. 2004) |
|
Agrobacterium-mediated Particle bombardment |
P. TS97K [P. amabilis W1–10 X P. 0061mabilis W1–22] | PLBs | EHA105 | CymMV cp, pflp, gfp, gus, hpt |
- Putative transformants with CymMV cp by particle bombardment transformation did not regenerate after hygromycin selection, so PLB were re-transformed with pflp - cp expression increased after CymMV infection - Transgenic lines showed strong resistance to CymMV and Erwinia carotovora - CymMV resistance was transgene-mediated through PTGS |
(Chan et al. 2005) |
| Agrobacterium-mediated |
P. S122-2 × S153 and S153 × S119-4 |
Immature protocorms | EHA101 | hpt, nptII, and gus |
- The highest transformation efficiency (1.93%) based on hygromycin-resistant plant was obtained when protocorms were subcultured on the medium with acetosyringone 2 days before inoculation - Acetosyringone enhanced the efficiency of Agrobacterium infection at inoculation and during pre-culture - Meropenem (5 mg L−1), was effective to eliminate Agrobacterium. - 88 lines of transgenic plants were obtained from independent protocorms, in which two presented an abnormal leaf shape |
(Mishiba et al. 2005) |
| Agrobacterium- | P. Wataboushi “#6.13” | Embryonic cell suspension culture | EHA101 | htp, nptII and wasabi defensin gene |
- The use of fine cell aggregates and 25 mg L−1of hygromycin resulted in high yield of hygromycin-resistant calli (19 calli per gram of cell co-cultivated with Agrobacterium) - About 30 transgenic plantlets per gram of embryonic suspension calli were obtained - Transformed Phalaenopsis showed strong resistance to Erwinia carotovora infection - One of 15 (6.7%) transgenic plants tested showed susceptibility and died. |
(Sjahril et al. 2006) |
| Agrobacterium- | P. Wataboushi “#6.13” | Embryonic cell suspension culture | EHA101 | htp, nptII and gus |
- Cefotaxime at 300 mg L−1 and carbenicillin at 500 mg L−1 suppressed Agrobacterium re-growth while meropenem at 3-5 mg L−1 presented the same effect - The highest transformation efficiency (10.3, 19.7, and 34.8 hygromycin-resistant calli, PLB and plantlet per gram of cell, respectively) was obtained when cells were infected for 2 h with Agrobacterium. |
(Sjahril and Mii 2006) |
| Agrobacterium- | Phalaenopsis derived from the cross between 2 elite clones | Protocorms | EHA101 | htp, nptII, gus and gst |
- 68 transgenic plants derived independent protocorms were obtained from 6325 mature seeds - Expression of GST was only detected in the plantlets that showed resistance to hygromycin - Regenerated plants grown in greenhouse bloomed within 2 years |
(Chin et al. 2007a) |
| Agrobacterium- | P. amabilis (L.) Blume | Protocorms | LBA4404 | nptII and BP/KNAT1 gene |
- Transformation frequency of 0.1 and 0.3% was obtained when protocorms were co-cultivated with Agrobacterium (harboring BP/KNAT1 gene) for 4 days on medium containing kanamycin and carbenicillin. - 139 transgenic plantlets were regenerated from 12 starting protocorms producing kanamycin-resistant shoots |
(Semiarti et al. 2007) |
| Agrobacterium- | P. bellina | PLBs | LBA4404 | hptII, gfp and gus |
- 53% PLB formation frequency was obtained in MS½ medium containing 0.8 µM 2,4-D - Phal. bellina PLBs were sensitive to hygromycin. Percentage of surviving PLBs were 100, 43, 32 and 23% at hygromycin 0, 1, 2 and 3 mg L−1. - The highest percentage of gfp transient expression was obtained for 45–90 min inoculation time |
(Maziah and Fern 2008) |
| Agrobacterium- | P. violacea | PLBs | EHA101, EHA105 |
gusA hpt mgfp5 |
- Transformation efficiency of EHA105 was higher than EHA101 - The highest percentage of gus expression was obtained using PLBs (5 mm), EHA105 with OD600nm of 0.6 |
(Subramaniam et al. 2008) |
| Agrobacterium- | P. violacea | PLBs | EHA101, EHA105 | gusA, gfp, hpt | - Agrobacterium is attracted to Phal. violacea exudates but chemotaxis is not a blocking step in Agrobacterium-mediated transformation | (Subramaniam et al. 2008) |
| Agrobacterium- | P. violacea | PLBs | EHA101, EHA105 | gus, gfp |
- Based on the gfp expression, strain EHA105 was better than EHA101 - Strain EHA105 in MS½ supplemented with 5% banana Mas extract, 200 mg L−1 L-cysteine, 60 µM silver nitrate, pH 5.5 increased T-DNA delivery frequency |
(Julkifle et al. 2010) |
| Agrobacterium- | P. amabilis (L.) Blume | Protocorms | LBA4404 | gfp and nptII |
- Coconut water and tomato extract increased transformation frequency (6.8–16.6%) of regenerated shoots - Tomato extract (100-150 mg L−1) during pre-culture in NP medium improved the transformation efficiency - Of the 210 plantlets examined, 191 were positive for gfp gene fragment |
(Semiarti et al. 2010) |
| Agrobacterium- | P. violacea | PLBs |
EHA 101 EHA 105 |
hptII, gusA and gfp |
- Agrobacterium is attracted to exudates from Phal. violacea explants - The highest frequency of transient gus expression was obtained with strain EHA105 co-cultivated in MS½ medium supplemented with 200 mg L−1 L-cysteine and 60 µM silver nitrate at 24 °C |
(Subramaniam and Rathinam 2010) |
| Particle bombardment |
P. TS444, Phal. TS440 (P. Taipei Gold × Dtps. Sun-Chen Beauty), P. TS340, P. Hwafeng Red jewel, P. New Cinderella |
petals | - | gfp, gusA, P450 | - Helium pressure of 220 psi provided the best transformation result | (Su and Hsu 2010) |
| Particle bombardment | P. aphrodite subsp. formosana, P. “Wedding Promenade”, P. equestris, P. “Luchia Lady” | PLB | - | gusA, gfp, sense-PeUFGT3 |
- CHS, CHI and ANS might not be the key genes for red color formation since their expression was not significantly different between red and white flowers - Downregulation of PeUFGT3 manipulates flower color development |
(Chen et al. 2011) |
| Agrobacterium- | P. amabilis | Leaf-derived embryogenic calli | LBA4404 | nptII and ltp |
- P. amabilis transformed with ltp exhibited strong cold stress tolerance at 10/7°C with green, healthy and vivid leaves - The highest transformation efficiency was 12.16%, when infected calli were co-cultivated 0.4 (OD600) A. tumefaciens for 20 min. - Obtention of 470 transgenic plants derived from independent PLBs. |
(Qin et al. 2011) |
| Agrobacterium- | P. Dtps.Tailin Angel | PLBs | LBA4404 | gafp and npi, |
- The highest frequency of PLB induction (85%) was obtained in MS½ medium with 10.0 mg L−16-BA and 1.0 mg L−1NAA - Cefotaxime sodium concentrations had no significant differences in protocorm induction - Protocorms and roots were not produced at 10 mg L−1 kanamycin - gafp and npi were transferred to Phalaenopsis, indicating its resistance to Colletotrichum gloeosporioides Sacc |
(Li et al. 2013) |
| Agrobacterium- | P. aphrodite cv. M1663 and P. aphrodite subsp. formosana | Protocorms | EHA105 |
hptII, gfp gus |
- 74 transgenic seedlings overexpressing Ubi:eGFP were obtained after three successive hygromycin selections and grown in greenhouse conditions, presenting normal morphology - Transformation frequency was 1.2–5.2% - All 22 transgenic lines showed HptII banding but five plants did not show GFP bands |
(Hsing et al. 2016) |
| Particle bombardment | P. bellina | PLBs | - | Sgfp, hptII, gfp and gusA | - Three hygromycin-resistant lines out of 160 bombarded individual PLBs, achieved an efficiency of 1.88% | (Chew et al. 2019) |
| Agrobacterium- | P. Sogo Yukidian “SPM313” | PLBs | EHA105 | OsGA2ox6, gus |
- Out of approximately 400 PLBs on the selection medium, only three PLB groups survived - Transgenic Phalaenopsis displayed green, shorter and wider leaves, thicker roots and much shorter flower spikes (10 cm vs 33 cm) than the nontrans- genic line with a normal flower size and blooming ability |
(Hsieh et al. 2020) |
| Agrobacterium- | P. amabilis (L.) Blume | Protocorms | EHA105 | CRISPR/Cas9, hpt |
- Transformation efficiency using V2T1 gene is 1.6%, higher than transformation efficiency using V2T2 gene (1.3%) - Agrobacterium-mediated transformation to deliver CRISPR/Cas9 was successfully achieved- A color change from green to pale yellowish was detected in the transformants |
(Nopitasari et al. 2020) |
| Agrobacterium- | P. amabilis | Protocorm | EHA105 |
CRISPR/Cas9 PDS3T1 sgRNA PDS3T2 sgRNAis |
- 0.96% PDS transformants obtained from PDS3T2 lines and 0.9% from PDS3T1 lines. - Transformant showed color changes in the leaf tissue (albino phenotype) |
(Semiarti et al. 2020a) |
| Agrobacterium- | P. equestris | Protocorms | - | hptII, MAD |
- The gene-editing efficiencies of the sgRNAs were 100% (MADS8: 7/7; MADS36: 12/12 and MADS44: 2/2). - 46 transformants derived from 20 explants contained triple mutants |
(Tong et al. 2020) |
Hpt hygromycin phosphotransferase, nptII neomycin phosphotransferase II, gus β-glucuronidase, PLB protocorm-like bodies, CymMV Cymbidium mosaic virus, CymMV cp Cymbidium mosaic virus coat protein, pflp sweet pepper ferredoxin-like protein, gfp green fluorescent protein, PTGS post-transcriptional gene silencing, gst glutathione S-transferases, ltp lipid transfer protein, gafp gastrodia antifungal protein, npi neutrophils peptide-I, OsGA2ox6 gibberellin 2-oxidase 6, PDS3 phyotoene desaturase-3, VAR2 variegate 2, RT-PCR reverse transcriptase polymerase chain reaction, pflp sweet pepper ferredoxin-like protein, F3′5′H flavonoid-3′,5′-hydroxylase, NP New Phalaenopsis.
The target explants used for the genetic transformation of Phalaenopsis in the reported studies were PLBs, protocorms, callus (Sjahril et al. 2006; Sjahril and Mii 2006) and flower petals (Table 2). PLBs were also the most frequently used explant in Dendrobium (Teixeira da Silva et al. 2016), and successfully used in Cymbidium (Chin et al. 2007b), Oncidium (Liau et al. 2003) and Vanda (Shrestha et al. 2007a).
In Phalaenopsis breeding programs, PLBs have been used as target tissues for genetic transformation specifically due to their high regeneration efficiency and their clonal nature, thereby increasing the transformation efficiency (Chew et al. 2018; Subramaniam et al. 2008). The transformation efficiency of intact and transversely bisected PLBs was also studied for Agrobacterium-mediated transformation of Phalaenopsis for the gus gene, showing that transversely bisected PLBs exhibited a higher transformation efficiency (16.2%) than intact PLBs (8.4%) (Chai et al. 2002).
Selectable marker genes are also used to verify the transgenic event, select the transformants from the non-transformants and evaluate the transformation efficiency in the culture medium supplemented with a selective agent (Hsiao et al. 2011b;). In most cases, genetic transformation has been performed using antibiotic-resistance genes, such as the hygromycin phosphotransferase (hpt) gene and the neomycin phosphotransferase II (nptII) gene (Table 2). The use of the BP/KNAT1 gene of Arabidopsis as a visible marker of transformation was studied in P. amabilis protocorms (Semiarti et al. 2007) as well as in Vanda tricolor and Coelogyne pandurata, resulting in multiple transformant shoots (Semiarti et al. 2011).
We here conducted a literature review using 17 reports on the use of the β-glucuronidase (gus) gene and nine reports involving the green fluorescent protein (gfp) gene (Table 2). The difference between the analysis of these two genes is that the gus assay is destructive, while gfp does not cause any damage to the cells. In Dendrobium, gus assays were the most frequently used approach for selection of transgenic lineages (Teixeira da Silva et al. 2016).
Genetic transformation completely changed agriculture in this century, with large predominance of transgenic than conventional cultivars in worldwide agricultural crops, such as soybean, maize and cotton. However, the benefits of transgenics cultivars still face challenges: the difficulties of transfer of target genes to solve key problems of floriculture and the difficulties in releasing these cultivars for commercialization due to strong regulatory laws adopted in some countries, leading to certain discouragement in producing and testing the cultivation of transgenic cultivars.
Isolation and fusion of protoplasts
Protoplasts are plant cells that have had their cell walls removed via mechanical destruction or enzymatic treatment (Eeckhaut et al. 2013). Protoplasts still have the capacity for totipotency, i.e., the ability for regeneration of a complete plant from a single cell (Davey et al. 2010; Naing et al. 2021). The protoplast culture technique comprises three main steps—protoplast isolation and cultivation, and regeneration of complete plants. Isolation of protoplasts is generally achieved using enzymatic digestion of cell wall components and its success depends on the degree of cell wall breakdown and the release of intact protoplasts. Variables such as cell wall thickness, temperature, pH and concentration of osmotic stabilizers and the time of the digestion duration are important for the successful release and isolation of protoplasts (Li et al. 2018; Naing et al. 2021).
The formulation of the culture medium and of the experimental system for the culture, the density of protoplasts and the growth of protoplasts in the culture medium must be considered during the culture of isolated protoplasts. The process of regeneration of protoplasts to buds is represented by five phases, which range from the formation of a new cell wall and a constant mitotic division to a series of morphogenic processes such as the formation of colonies and then callus that finally allow the generation of growth and development of tissues (Naing et al. 2021). According to Eeckhaut et al. (2013), the success of plant regeneration from protoplasts mainly depends on the type of donor material used during the isolation process (Davey et al. 2010).
Studies on the cultivation of protoplasts in the genus Phalaenopsis with successful protocols for the stages of isolation, cultivation and regeneration of complete plants have also been reported (Ichihashi and Shigemura 2002; Machmudi et al. 2019; Shrestha et al. 2007b) (Table 3).
Table 3.
Isolation and fusion of Phalaenopsis protoplasts.
| Phalaenopsis species/cultivar | Original material | Culture of protoplast | Fusion of protoplast | Transient gene expression system | Primary Findings | Reference |
|---|---|---|---|---|---|---|
| P. “Wataboushi” (PP2776) | Callus induced of shoot tip | Isolation: 2% Cellulase, 0.5% Macerozyme, 0.1%, Driserase 0.1%, Pectlyase 100 mM CaCl2. 2H2O, 5 mM MES, 0.6 M sorbitol | No | No | Yield of 3.8 × 105 protoplast per g fresh weight | (Shrestha et al. 2007b) |
| P. aphrodite subsp. Formosana | Petals | Isolation: 1% Cellulase, 0.7 M Mannitol, 20 mM KCl, 20 mM MES. | No | Yes | - Maximum yield was 1.9 × 105 and viability of transfected protoplasts was 77.5% | (Lin et al. 2018) |
| Phalaenopsis hybrid cultivar “Ruili Beauty” | Leaves | Isolation: 1% Cellulase, 0.7% Macerozyme, 0.4 M mannitol | No | Yes |
- Maximum yield was 5.9 × 106 and percentage of viable protoplasts was 57.9% - Protoplast transformation efficiency was 41.7% |
(Li et al. 2018) |
| P. amabilis | Foliar mesophyll | Isolation: 2% Cellulase, 1% Macerozyme, 5 mM MES, 0.5 M sorbitol | No | No | - Number of isolate protoplasts was 1.1 × 106 and the percentage of viable protoplasts was 83.88%. | (Machmudi et al. 2019) |
| Phalaenopsis spp. | Leaves | Isolation: 1.2% Cellulase, 0.6% Macerozyme. | No | No | - Maximum yield was 1.8 × 107 and percentage of viable protoplasts was 92.8% | (Ren et al. 2021) |
Despite the challenges with uses of orchid protoplasts, research on protoplast culture technique in orchids showed that it is an efficient tool with a high rate of regeneration of stable plants and could be used to improve biotechnological tools for the development of new cultivars as well as for biological studies in orchids (Hossain et al. 2013). A few examples of the biotechnological techniques in which protoplasts can be used as study material are somatic hybridization, protoplast transformation and somaclonal variation induction (Davey et al. 2010).
Somatic hybridization via protoplast fusion is an interesting technique for genetic improvement in plants. This technique uses a total or partial combination of somatic cells obtained from different plants (cultivars, genera, or species) for producing hybrid cells with different genetic or cytoplasmic combinations, thereby generating different somatic hybrids or somatic cybrids, respectively (Grosser et al. 2010). Somatic cell fusion may be performed independently or combined with other treatments, such as electrofusion or polyethylene glycol (PEG) addition (Davey et al. 2010). Somatic hybridization through protoplast fusion for the genetic improvement of plants offers some advantages, such as fusion of somatic cells from incompatible species by sexual hybridization, due to multiple intrinsic barriers (Davey et al. 2010).
In several species of ornamental plants, including orchids, there have been reports on the development of somatic hybrids using protoplast fusion by electrofusion, PEG and increased pH/Calcium which result in new cultivar development (Naing et al. 2021). However, no reports about protoplast fusion and somatic hybrids have been obtained in Phalaenopsis. Advances in protoplast culture technology in orchids breeding could increase the number of novel cultivars and the genetic variability (Shrestha et al. 2007b). In general, it is expected that the new tools, such as genome editing by CRIPR/Cas, with the use of the protoplasts, restore the importance of studies with this biotechnological tool to generate a new group of ornamental plants with desired and innovative characteristics (Naing et al. 2021). Recently, Xia et al. (2022) demonstrated that protoplasts enable the development and identification of two multiplex genome editing tools applied for Phalaenopsis orchids.
Phalaenopsis in the CRISPR/Cas9 genome-editing era
Currently, orchids belonging to the genus Phalaenopsis exhibit the highest rate of transcriptomic information available in the databases for the Orchidaceae (Chao et al. 2017). Consequently, studies concerning the identification and functional analysis of genes are important to understand the various biological processes involved, (such as their reproductive biology and plant growth) and the extraordinary characteristics that these orchids have, for the development of breeding programs (Cai et al. 2015; Bai et al. 2023; Suputri et al. 2024).
Some tools, such as mutants or transgenic plants, assist in conducting genetic and functional studies in plants. However, such studies are challenging in the case of orchids due to the limited availability of mutants and the long time consumed in the production of transgenic orchids owing to their long-life cycle (Lin et al. 2018). The Agrobacterium-mediated genetic transformation has been used for delivering Clustered Regularly Interspaced Short Palindromic Repeats (CRISPR/Cas9). A methodology using the CRISPR/Cas9 KO system was developed using PDS3 as the marker gene, with promising results and a flower color change in the transformed leaf tissue (Semiarti et al. 2020a). The flower color change was also observed by Nopitasari et al. (2020).
The CRISPR/Cas genome editing technology has been used in several plant species, successfully achieving the required modifications and manipulations in the plant genome (Wang et al. 2019). The CRISPR/Cas technology has been used in Phalaenopsis (Tong et al. 2020; Xia et al. 2022), presenting great potential application for studies in orchid breeding. Tong et al. (2020) developed multiple mutants of P. equestris in the MADS genes (important for flowering initiation and flower development) using CRISPR/Cas9 and A. tumefaciens for mediating the transformation. Xia et al. (2022) reported the successful application of different types of genome editing tools—PTG-Cas9-HPG and RMC-Cpf1-HPG multiplex genome editing systems—in Phalaenopsis using genome editing in protoplasts as explants and PEG transformation method. In addition, mutant genes for inducing early flowering in Phalaenopsis, such as Gibberellic acid insensitive (GAI) gene, have been identified for use in CRISPR/Cas9 tools (Suputri et al. 2024).
Another biotechnological tool namely the transient expression system of protoplasts allows conducting genetic, functional, regulatory and signaling studies (Fraiture et al. 2014). The technique involves subjecting the protoplasts to PEG treatments or mechanical electroporation to deliver the plasmid containing the foreign DNA into the protoplast (Davey et al. 2010). The transient expression system of protoplasts allows the study of the transcriptome and how it responds to the expression of the genes of interest. In addition, this system could be a useful tool for the structural, functional, and regulatory analysis of the expressed genes (Lin et al. 2018). The use of transient gene expression system in orchids using protoplasts was reported in recent studies with Cymbidium (Ren et al. 2020,2021; Yang et al. 2021), Dendrobium (Li et al. 2021) and Phalaenopsis (Li et al. 2018; Lin et al. 2018) and have been emerged in the literature, due to the large information in the data banks and various advances in the areas of phylogenetics, genetics and molecular mechanisms in these genera.
An efficient protocol for the isolation, transfection, and expression of transient genes was established for protoplasts obtained from the foliar mesophyll of P. “Ruili Beauty” (Li et al. 2018), successfully detecting the T1R1 protein in the nucleus and the cell membrane of the transformed protoplasts. Lin et al. (2018) also established an efficient protocol for protoplasts obtained from the petals of the tetraploid P. aphrodite subsp. formosana, with the observation and analysis of the protein localization and the interaction among the protein PaCDKA and PaCYCD3. In addition, the study also provided insights into genes associated with the cell cycle (dependent on PaE2F/PaDP) and hormonal response through the DR5v2 auxin reporter.
The transient protoplast gene expression system also serves as a platform for multiple investigations regarding the biological processes in plants (Xia et al. 2022). Therefore, it is expected that this system will be used independently and in combination with other techniques, such as CRISPR/Cas9 high-performance screening and protoplast regeneration (Gaillochet et al. 2021). Thus, it will contribute to exponentially increasing the orchid mutant libraries, which would serve as important tools for understanding the functions of genes in orchids (Xia et al. 2022).
Conclusions and future prospects
Conventional breeding techniques in orchids, such as controlled crosses followed by selection of genotypes containing specific features, continue to be the most widely used methods for orchid breeding, generating the largest number of commercial cultivars, including the Phalaenopsis multi-flora types, currently one of the high-commercialized in the world’s floriculture trade (Lee et al. 2020). Intergeneric hybridization also provides important opportunities for generations of new orchid cultivars, such as the new indigo or dark blue cultivar called Rhynchonopsis Tariflor Blue Kid “1030-4”, an intergeneric hybrid between P. Fire Cracker and Rhynchostylis coelestis used as the source of blue flowers (Wu et al. 2022). This example of new intergeneric hybrids in orchids (Phalaenopsis x Rhynchostylis), highlights the high and still largely unexplored potential of this technique to obtain new desired plant characteristics, likely to gain importance in the future of floriculture and orchid cultivation.
The recently developed “Harlequin” cultivars group in Phalaenopsis, firstly obtained through in vitro somaclonal variation (SV), emphasize the importance of biotechnological techniques in floriculture industry. In fact, most breeding programs in floriculture are carried out in developed countries by private companies, holding most of the world’s genetic diversity of ornamental species, including orchids. However, this high concentration of technology and genetics in commercial companies to some extent limits the public access to knowledge of the research in this area.
Some innovative features expected in the new generations of biotech cultivars and that could contribute to floriculture are: exotic flowers with pure colors such as true blue (Liang et al. 2020), similar to those developed for rose (Nakamura 2010) and chrysanthemum (Noda et al. 2017), black (Ahmad et al. 2022) and red; the differential color distribution among and along flower organs to provide specific color combination, flowers with scent (Huang et al. 2021), and finally fluorescent flowers and plants, such as those obtained by transgeny in petunias (Chin et al. 2018). Beyond such ornamental characteristics, it is also expected that these technologies could be useful to increase water efficiency, nutrient uptake, resistance to abiotic and biotic stresses, such as gray mold (Zhao et al. 2018) and to increase the sustainability and the resilience of ornamental plant cultivation to climatic changes.
The use of biotechnology for the genetic improvement of crops continues to be one of the great milestones of the current agricultural revolution. The use of transgenic cultivars in the world has increased more than 100-fold in the last 30 years, with more than 190 million hectares with cultivated varieties derived from biotechnology, with special applicability of Genetic Modification (GMs) to large crops such as soybean, corn, cotton and canola (Turnbull et al. 2021). However, ornamental plant cultivars derived from biotechnology are still scarce in the flower market, and are limited by regulatory and profitability issues (Boutigny et al. 2020), but also by the limited studies related to genomic and transcriptome sequencing associated with most ornamental plants.
The uses of novel “omics” tools and genome editing are emerging in orchids (Li et al. 2021; Xia et al. 2022) as a result of recent advances in whole-genome sequencing (Zheng et al. 2021). Recent advances in technologies with reduced costs and that accelerate the processes of genomic sequencing in ornamental plants, such as in Phalaenopsis orchids (Hsu et al. 2020), as well as making them more available around the world, have emerged in the expansion of information about target genes that could be introduced by biotechnological tools and that could generate new groups of cultivars of ornamental plants. Furthermore, the emergence of more efficient technologies for genomic editing, such as the CRISPR/Cas9 technology, allows the development for cultivars that are more efficient in the use of natural resources, more productive, and possess characteristics that are difficult to obtain by conventional breeding programs (Ahn et al. 2020), without the complex regulatory process found with transgenic cultivars. Therefore, these advances and also the confirmed heredity of target-traits introduced by biotechnology, will enable, in the short-medium term, the employment and expansion of cultivars derived from modern biotechnological methods.
Acknowledgements
CMI and JAVA thanks to Coordenação de Aperfeiçoamento de Pessoal de Nível Superior - Brasil (CAPES) - Finance Code 001. CMI and JCC thank to São Paulo Research Foundation (FAPESP) grant 2020/09426-4 and n° 2018/20673-3. JCC thanks to Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq, Brazil) for funding the Project 311083/2018-8. This study was financed in part by the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior - Brasil (CAPES) - Finance Code 001. This work is supported by the USDA National Institute of Food and Agriculture, Hatch project 7001563. The authors would like to especially thank the Editor of Heredity, Prof. Dr. Frank Hailer, for his great efforts and intellectual and editorial contributions to improving the quality of this article.
Author contributions
CMI: conceptualization, data curation, investigation, methodology, project administration, visualization, writing—original draft. JAV-A: data curation, investigation, validation, writing—review & editing. JCC: conceptualization, validation, funding acquisition, supervision, validation, writing, review, and editing. MAG: writing, reviewing, and editing. WAV: writing, reviewing, and editing.
Competing interests
The authors declare no competing interests.
Footnotes
Associate editor: Frank Hailer.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Carla Midori Iiyama, Email: carlaiiyama@gmail.com.
Jean Carlos Cardoso, Email: jeancardoso@ufscar.br.
References
- Ahmad Z, Hassan AA, Idris NA, Basiran MN, Tanaka A, Shikazono N, et al. Effects of ion beam irradiation on Oncidium lanceanum. J Nuclerar Relat Technol. 2006;3:1–8. [Google Scholar]
- Ahmad S, Chen J, Chen G, Huang J, Zhou Y, Zhao K, Lan S, Liu Z, Peng D. Why Black Flowers? An Extreme Environment and Molecular Perspective of Black Color Accumulation in the Ornamental and Food Crops. Front Plant Sci. 2022;13:885176. doi: 10.3389/fpls.2022.885176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahn CH, Ramya M, An HR, Park PM, Kim YJ, Lee SY, et al. Progress and challenges in the improvement of ornamental plants by genome editing. Plants. 2020;9(6):687 . doi: 10.3390/plants9060687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anghelescu NE, Vafaee Y, Ahmadzadeh K, Chen JT (2023) Asymbiotic seed germination in terrestrial orchids: problems, progress, and prospects. In: Tiwari P, Chen JT. (eds) Advances in orchid biology, biotechnology, and omics. Springer, Singapore
- Anne S, Lim JH. Mutation breeding using gamma irradiation in the development of ornamental plants: a review. Flower Res J. 2020;28:102–115. doi: 10.11623/frj.2020.28.3.01. [DOI] [Google Scholar]
- Azadi P, Bagheri H, Nalousi AM, Nazari F, Chandler SF. Current status and biotechnological advances in genetic engineering of ornamental plants. Biotechnol Adv. 2016;34:1073–1090. doi: 10.1016/j.biotechadv.2016.06.006. [DOI] [PubMed] [Google Scholar]
- Bai Y, Ma Y, Chang Y, Zhang W, Deng Y, Zhang N, et al. Identification and transcriptome data analysis of ARF family genes in five Orchidaceae species. Plant Mol Biol. 2023;112:85–98. doi: 10.1007/s11103-023-01354-4. [DOI] [PubMed] [Google Scholar]
- Balilashaki K, Gantait S, Naderi R, Vahedi M. Capsule formation and asymbiotic seed germination in some hybrids of Phalaenopsis, influenced by pollination season and capsule maturity. Physiol Mol Biol Plants. 2015;21(3):341–347. doi: 10.1007/s12298-015-0309-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balilashaki K, Zakizadeh H, Olfati JA, Vahedi M, Kumar A, Indracanti M. Recent advances in Phalaenopsis orchid improvement using omics approaches. Plant Tiss Cult Biotech. 2019;29(1):133–149. doi: 10.3329/ptcb.v29i1.41986. [DOI] [Google Scholar]
- Belarmino MM, Mii M. Agrobacterium-mediated genetic transformation of a Phalaenopsis orchid. Plant Cell Rep. 2000;19:435–442. doi: 10.1007/s002990050752. [DOI] [PubMed] [Google Scholar]
- Billore V, Mirajkar SJ, Suprasanna P, Jain M. Gamma irradiation induced effects on in vitro shoot cultures and influence of monochromatic light regimes on irradiated shoot cultures of Dendrobium sonia orchid. Biotechnol Rep. 2019;22:e00343. doi: 10.1016/j.btre.2019.e00343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boutigny A-L, Dohin N, Pornin D, Rolland M. Overview and detectability of the genetic modifications in ornamental plants. Hortic Res. 2020;7:11. doi: 10.1038/s41438-019-0232-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai J, Liu X, Vanneste K, Proost S, Tsai W-C, Liu K-W, et al. The genome sequence of the orchid Phalaenopsis equestris. Nat Genet. 2015;47:65–72. doi: 10.1038/ng.3149. [DOI] [PubMed] [Google Scholar]
- Cardoso JC, Vendrame WA. Innovation in propagation and cultivation of ornamental plants. Horticulturae. 2022;8:229. doi: 10.3390/horticulturae8030229. [DOI] [Google Scholar]
- Cardoso JC, Zanello CA, Chen JT. An overview of orchid protocorm-like bodies: Mass propagation, biotechnology, molecular aspects, and breeding. Int J Mol Sci. 2020;21(3):985. doi: 10.3390/ijms21030985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chai ML, Xu CJ, Senthil KK, Kim JY, Kim DH. Stable transformation of protocorm-like bodies in Phalaenopsis orchid mediated by Agrobacterium tumefaciens. Sci Hortic. 2002;96:213–224. doi: 10.1016/S0304-4238(02)00084-5. [DOI] [Google Scholar]
- Chan Y-L, Lin K-H, Sanjaya, Liao L-J, Chen W-H, Chan M-T. Gene stacking in Phalaenopsis orchid enhances dual tolerance to pathogen attack. Transgenic Res. 2005;14:279–288. doi: 10.1007/s11248-005-0106-5. [DOI] [PubMed] [Google Scholar]
- Chao Y-T, Yen S-H, Yeh J-H, Chen W-C, Shih M-C. Orchidstra 2.0—a transcriptomics resource for the orchid family. Plant Cell Physiol. 2017;58(1):e9. doi: 10.1093/pcp/pcw220. [DOI] [PubMed] [Google Scholar]
- Chen WH, Tang CY, Kao YL. Polyploidy and variety improvement of Phalaenopsis orchids. Acta Hortic. 2010;878:133–138. doi: 10.17660/ActaHortic.2010.878.14. [DOI] [Google Scholar]
- Chen WH, Hsu CY, Cheng HY, Chang H, Chen HH, Ger MJ. Downregulation of putative UDP-glucose: Flavonoid 3-O-glucosyltransferase gene alters flower coloring in Phalaenopsis. Plant Cell Rep. 2011;30:1007–1017. doi: 10.1007/s00299-011-1006-1. [DOI] [PubMed] [Google Scholar]
- Chen XG, Wu YH, Li NQ, Gao JY. What role does the seed coat play during symbiotic seed germination in orchids: an experimental approach with Dendrobium officinale. BMC Plant Biol. 2022;22:375. doi: 10.1186/s12870-022-03760-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chew Y-C, Halim MHA, Abdullah WMANW, Abdullah JO, Lai KS. Highly efficient proliferation and regeneration of protocorm-like bodies (PLBs) of the threatened orchid, Phalaenopsis bellina. Sains Malaysiana. 2018;47:1093–1099. doi: 10.17576/jsm-2018-4706-03. [DOI] [Google Scholar]
- Chew Y-C, Abdullah WMANW, Kok D-XA, Ong-Abdullah J, Mahmood M, Lai K-S. Development of an efficient particle bombardment transformation system for the endemic orchid, Phalaenopsis bellina. Sains Malaysiana. 2019;48:1867–1877. doi: 10.17576/jsm-2019-4809-07. [DOI] [Google Scholar]
- Chin DP, Mishiba K, Mii M. Agrobacterium-mediated transformation of protocorm-like bodies in Cymbidium. Plant Cell Rep. 2007;26:735–743. doi: 10.1007/s00299-006-0284-5. [DOI] [PubMed] [Google Scholar]
- Chin DP, Mii M, Mishiba KI. Production of transgenic Phalaenopsis plants by introducing glutathione S-transferase gene into protocorms at an early stage after germination. Acta Hortic. 2007;743:101–105. doi: 10.17660/ActaHortic.2007.743.13. [DOI] [Google Scholar]
- Chin DP, Shiratori I, Shimizu A, Kato K, Mii M, Waga I. Generation of brilliant green fluorescent petunia plants by using a new and potent fluorescent protein transgene. Sci Rep. 2018;8(1):16556. doi: 10.1038/s41598-018-34837-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chuang HT, Huang KL, Shen RS, Miyajima I, Hsu ST. Using cut-column pollination method to overcome crossing barriers in Phalaenopsis sunrise goldmour ‘KHM637. J Fac Agric Kyushu Univ. 2014;59:265–271. doi: 10.5109/1467626. [DOI] [Google Scholar]
- Corte LED, Mahmoud LM, Moraes TS, Mou Z, Grosser JW, Dutt M. Development of improved fruit, vegetable, and ornamental crops using the CRISPR/cas9 genome editing technique. Plants. 2019;8(12):601. doi: 10.3390/plants8120601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cribb P, Schuiteman A. Phalaenopsis-distribution and ecology. Renziana. 2012;2:10–13. [Google Scholar]
- Davey MR, Anthony P, Patel D, Power JB (2010) Plant protoplasts: isolation, culture and plant regeneration. In: Plant cell culture: essential methods, Wiley-Blackwell, New York, pp 153–173.
- Eeckhaut T, Lakshmanan PS, Deryckere D, Van Bockstaele E, Van Huylenbroeck J. Progress in plant protoplast research. Planta. 2013;238:991–1003. doi: 10.1007/s00425-013-1936-7. [DOI] [PubMed] [Google Scholar]
- Eeckhaut T, Van Houtven W, Bruznican S, Leus L, Van Huylenbroeck J. Somaclonal variation in chrysanthemum× morifolium protoplast regenerants. Front Plant Sci. 2020;11:607171. doi: 10.3389/fpls.2020.607171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enoki S, Takahara Y (2022) Applications of biotechnological approaches in the product and breeding of Phalaenopsis orchids. In: Khan M(ed) Tropical plant species. IntechOpen, London. 10.5772/intechopen.104597
- Fraiture M, Zheng X, Brunner F (2014) An Arabidopsis and tomato mesophyll protoplast system for fast identification of early MAMP-triggered immunity-suppressing effectors. In: Plant-pathogen interactions, Springer, pp 213–230 [DOI] [PubMed]
- Gaillochet C, Develtere W, Jacobs TB. CRISPR screens in plants: approaches, guidelines, and future prospects. Plant Cell. 2021;33(4):794–813. doi: 10.1093/plcell/koab099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grosser JW, Calovic M, Louzada ES (2010) Protoplast fusion technology—somatic hybridization and cybridization. In: Plant cell culture: essential methods, Wiley-Blackwell, New York, pp 175–198
- Hossain MM, Kant R, Van PT, Winarto B, Zeng S, Teixeira da Silva JA. The application of biotechnology to orchids. Crit Rev Plant Sci. 2013;32:69–139. doi: 10.1080/07352689.2012.715984. [DOI] [Google Scholar]
- Hsiao YY, Pan Z-J, Hsu C-C, Yang Y-P, Hsu Y-C, Chuang Y-C, et al. Research on orchid biology and biotechnology. Plant Cell Physiol. 2011;52:1467–1486. doi: 10.1093/pcp/pcr100. [DOI] [PubMed] [Google Scholar]
- Hsiao Y-Y, Chen Y-W, Huang S-C, Pan Z-J, Fu C-H, Chen W-H, et al. Gene discovery using next-generation pyrosequencing to develop ESTs for Phalaenopsis orchids. BMC Genom. 2011;12:360. doi: 10.1186/1471-2164-12-360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsieh K-T, Liu S-H, Wang I-W, Chen L-J. Phalaenopsis orchid miniaturization by overexpression of OsGA2ox6, a rice GA2‑oxidase gene. Bot Stud. 2020;61:10. doi: 10.1186/s40529-020-00288-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsing HX, Lin YJ, Tong C-G, Li MJ, Chen YJ, Ko SS. Efficient and heritable transformation of Phalaenopsis orchids. Bot Stud. 2016;57:30. doi: 10.1186/s40529-016-0146-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu CC, Chen SY, Lai PH, Hsiao YY, Tsai WC, Liu Z-J, et al. Identification of high-copy number long terminal repeat retrotransposons and their expansion in Phalaenopsis orchids. BMC Genom. 2020;21:1–13. doi: 10.1186/s12864-020-07221-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu C-C, Su C-J, Jeng M-F, Chen W-H, Chen H-H. A HORT1 retrotransposon insertion in the PeMYB11 promoter causes Harlequin/black flowers in Phalaenopsis Orchids. Plant Physiol. 2019;180:1535–1548. doi: 10.1104/pp.19.00205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang H, Kuo Y-W, Chuang Y-C, Yang Y-P, Huang L-M, Jeng M-F, Chen W-H, Chen H-H. Terpene synthase-b and terpene synthase-e/f genes produce monoterpenes for Phalaenopsis bellina floral scent. Front Plant Sci. 2021;12:700958. doi: 10.3389/fpls.2021.700958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang JZ, Lin CP, Cheng TC, Chang BCH, Cheng SY, Chen Y-W, et al. A de novo floral transcriptome reveals clues into Phalaenopsis orchid flower development. PLoS One. 2015;10(5):e0123474. doi: 10.1371/journal.pone.0123474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ichihashi S, Shigemura S (2002) Phalaenopsis callus and protoplast culture. In: Proceedings of the 17th World Orchid Conference, Malaysia, pp 257–261
- Julkifle AL, Rathinam X, Sinniah UR, Subramaniam S. Optimisation of transient green fluorescent protein (GFP) gene expression in Phalaenopsis violacea orchid mediated by Agrobacterium tumefaciens-mediated transformation system. Aust J Basic Appl Sci. 2010;4(8):3424–3432. [Google Scholar]
- Khatun K, Nath U, Rahman M. Tissue culture of Phalaenopsis: present status and future prospects. J Adv Biotechnol Exp Ther. 2020;3:273. doi: 10.5455/jabet.2020.d135. [DOI] [Google Scholar]
- Kim JB. Current status on applications of conventional breeding techniques and biotechnological system in ornamentals. J Plant. Biotechnol. 2020;47:107–117. [Google Scholar]
- Kim SH, Kim SW, Ahn J-W, Ryu J, Kwon S-J, Kang B-C, et al. Frequency, Spectrum, and stability of leaf mutants induced by diverse γ-ray treatments in two cymbidium hybrids. Plants. 2020;9(4):546. doi: 10.3390/plants9040546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim SH, Jo YD, Ryu J, Hong MJ, Kang BC, Kim JB. Effects of the total dose and duration of γ-irradiation on the growth responses and induced SNPs of a Cymbidium hybrid. Int J Radiat Biol. 2020;96:545–551. doi: 10.1080/09553002.2020.1704303. [DOI] [PubMed] [Google Scholar]
- Kurniadi AS, Irawati F, Putra SED, Hardjo PH. Induction of protocorm-like bodies (PLBs) Phalaenopsis spp. Hybrids mutation through ultraviolet irradiation (UV254) and Ethyl Methane Sulfonate (EMS) J Appl Agric Sci. 2023;7:1–15. [Google Scholar]
- Lee H-J, Kim Y-E, Yoon Y-J, Jeong C-S, Lian ML, Paek K-Y, et al. Highly endoreduplicated floral organs of somaclonal variants in clonally propagated Phalaenopsis ‘Spring Dancer. Plant Cell Tiss Organ Cult. 2016;126:67–77. doi: 10.1007/s11240-016-0977-6. [DOI] [Google Scholar]
- Lee Y-I, Tseng Y, Lee Y-C, Chung M-C. Chromosome constitution and nuclear DNA content of Phalaenopsis hybrids. Sci Hortic. 2020;262:109089. doi: 10.1016/j.scienta.2019.109089. [DOI] [Google Scholar]
- Lestari EP, Yunus A, Sugiyarto S. Diversity induction of Dendrobium sylvanum orchid through in vitro irradiation of gamma Ray. Biosaintifika J Biol Biol Educ. 2018;10:691–697. doi: 10.15294/biosaintifika.v10i3.16265. [DOI] [Google Scholar]
- Li C, Dong N, Zhao Y, Wu S, Liu Z, Zhai J. A review for the breeding of orchids: current achievements and prospects. Hortic Plant J. 2021;7:380–392. doi: 10.1016/j.hpj.2021.02.006. [DOI] [Google Scholar]
- Li F, Cheng Y, Zhao X, Yu R, Li H, Wang L, et al. Haploid induction via unpollinated ovule culture in gerbera hybrida. Sci Rep. 2020;10(1):1–9. doi: 10.1038/s41598-020-58552-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J, Kuang P, Liu RD, Wang D, Wang ZN, Huang MR. Transfer of the GAFP and NPI, two disease-resistant genes, into a Phalaenopsis by Agrobacterium tumefaciens. Pak J Bot. 2013;45:1761–1766. [Google Scholar]
- Li J, Liao X, Zhou S, Liu S, Jiang L, Wang G. Efficient protoplast isolation and transient gene expression system for Phalaenopsis hybrid cultivar ‘Ruili Beauty. Vitr Cell Dev Biol - Plant. 2018;54:87–93. doi: 10.1007/s11627-017-9872-z. [DOI] [Google Scholar]
- Liang C-Y, Rengasamy KP, Huang L-M, Hsu C-C, Jeng M-F, Chen W-H, et al. Assessment of violet-blue color formation in Phalaenopsis orchids. BMC Plant Biol. 2020;20:212. doi: 10.1186/s12870-020-02402-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao L-J, Pan I-C, Chan Y-L, Hsu Y-H, Chen W-H, Chan M-T. Transgene silencing in Phalaenopsis expressing the coat protein of Cymbidium Mosaic Virus is a manifestation of RNA-mediated resistance. Mol Breed. 2004;13:229–242. doi: 10.1023/B:MOLB.0000022527.68551.30. [DOI] [Google Scholar]
- Liau C-H, You S-J, Prasad V, Hsiao H-H, Lu J-C, Yang N-S, et al. Agrobacterium tumefaciens-mediated transformation of an Oncidium orchid. Plant Cell Rep. 2003;21:993–998. doi: 10.1007/s00299-003-0614-9. [DOI] [PubMed] [Google Scholar]
- Lin H-Y, Chen J-C, Fang S-C. A protoplast transient expression system to enable molecular, cellular, and functional studies in Phalaenopsis orchids. Front Plant Sci. 2018;9:843. doi: 10.3389/fpls.2018.00843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin YY (2021) Phalaenopsis Harlequin Breeding – Past, Present and Future. Brother Orchid Nursery Co.
- Lou Y, Zhang Q, Xu Q, Yu X, Wang W, Gai R, Ming F. PhCHS5 and PhF3'5’H Genes Over-Expression in Petunia (Petunia hybrida) and Phalaenopsis (Phalaenopsis aphrodite) Regulate Flower Color and Branch Number. Plants. 2023;12(11):2204. doi: 10.3390/plants12112204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma LY, Zhang T. Effects of radiation of 60Co γ-ray on the genetic stability of Phalaenopsis aphrodite. J Anhui Agric Univ. 2011;38:802–805. [Google Scholar]
- Machmudi M, Purnobasuki H, Utami ESW. The optimization mesophyll protoplast isolation for Phalaenopsis amboinensis. Bulg J Agric Sci. 2019;25:737–743. [Google Scholar]
- Magdalita PM, Pascual AOS, Villareal RL. Characterization and flowering behavior of eleven philippine native Phalaenopsis species and gamma irradiation effects on Phalaenopsis aphrodite. Philipp J Sci. 2020;149:1–10. [Google Scholar]
- Magdalita PM, Pascual AOS, Villareal RL. Evaluation of plant and flower characteristics of selected 15-Gy irradiated Phalenopsis aphrodite. Mindanao J Sci Technol. 2022;20:236–249. doi: 10.61310/mndjstmsmag.06.22. [DOI] [Google Scholar]
- Manchanda P, Kaur A, Gosal SS (2018) Somaclonal Variation for Sugarcane Improvement. In: Gosal SS Wani SH (eds) Biotechnologies of crop improvement, Vol 1, Springer International Publishing Cham, p 299–326
- Maziah M, Fern C. Agrobacterium-mediated genetic transformation of Phalaenopsis bellina using GFP and GUS reporter genes. Pertanika J Sci Technol. 2008;16:129–139. [Google Scholar]
- Melsen K, van de Wouw M, Contreras R. Mutation Breeding in Ornamentals. HortScience. 2021;56(10):1154–1165. doi: 10.21273/HORTSCI16001-21. [DOI] [Google Scholar]
- Mii M, Chin DP (2018) Genetic transformation on orchid species: an overview of approaches and methodologies. In: Orchid Propagation: From Laboratories to Greenhouses—Methods and Protocols, pp 347–365
- Mishiba KI, Chin DP, Mii M. Agrobacterium-mediated transformation of Phalaenopsis by targeting protocorms at an early stage after germination. Plant Cell Rep. 2005;24:297–303. doi: 10.1007/s00299-005-0938-8. [DOI] [PubMed] [Google Scholar]
- Naing AH, Adedeji OS, Kim CK. Protoplast technology in ornamental plants: current progress and potential applications on genetic improvement. Sci Hort. 2021;283:110043. doi: 10.1016/j.scienta.2021.110043. [DOI] [Google Scholar]
- Nakamura N. Dream comes true: development of a blue rose “Applause” and its fragrance. J Jpn Assoc Odor Environ. 2010;41:150–156. [Google Scholar]
- Noda N, Yoshioka S, Kishimoto S, Nakayama M, Douzono M, Tanaka Y, et al. Generation of blue chrysanthemums by anthocyanin B-ring hydroxylation and glucosylation and its coloration mechanism. Sci Adv. 2017;3(7):e1602785. doi: 10.1126/sciadv.1602785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noman A, Aqeel M, Deng J, Khalid N, Sanaullah T, Shuilin H. Biotechnological advancements for improving floral attributes in ornamental plants. Front Plant Sci. 2017;8:530. doi: 10.3389/fpls.2017.00530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nopitasari S, Setiawati Y, Lawrie MD, Purwantoro A, Widada J, Sasongko AB, et al. Development of an Agrobacterium-delivered CRISPR/Cas9 for Phalaenopsis amabilis (L.) Blume genome editing system. AIP Conf Proc. 2020;2260(1):060014. doi: 10.1063/5.0015868. [DOI] [Google Scholar]
- Oladosu Y, Rafii MY, Abdullah N, Hussin G, Ramli A, Rahim HA, et al. Principle and application of plant mutagenesis in crop improvement: a review. Biotechnol Equip. 2016;30:1–16. doi: 10.1080/13102818.2015.1087333. [DOI] [Google Scholar]
- Priyadarshan PM (2019) Introduction to Plant Breeding. In: PLANT BREEDING: Classical to modern. Springer, Singapore. 10.1007/978-981-13-7095-3_1
- Putri HA, Purwito A, Sudarsono, Sukma D. Morphological, molecular and resistance responses to soft-rot disease variability among plantlets of Phalaenopsis amabilis regenerated from irradiated protocorms. Biodiversitas. 2021;22:1077–1090. doi: 10.13057/biodiv/d220301. [DOI] [Google Scholar]
- Qin X, Liu Y, Mao S, Li T, Wu H, Chu C, et al. Genetic transformation of lipid transfer protein encoding gene in Phalaenopsis amabilis to enhance cold resistance. Euphytica. 2011;177:33–43. doi: 10.1007/s10681-010-0246-4. [DOI] [Google Scholar]
- Raffeiner B, Serek M, Winkelmann T. Agrobacterium tumefaciens-mediated transformation of Oncidium and Odontoglossum orchid species with the ethylene receptor mutant gene etr1-1. Plant Cell Tiss Organ Cult. 2009;98:125–134. doi: 10.1007/s11240-009-9545-7. [DOI] [Google Scholar]
- Rajan RP, Singh G. A review on application of somaclonal variation in important horticulture crops. Plant Cell Biotech. Mol Biol. 2021;22:161–175. [Google Scholar]
- Ren R, Gao J, Lu C, Wei Y, Jin J, Wong S-M, et al. Highly efficient protoplast isolation and transient expression system for functional characterization of flowering related genes in Cymbidium orchids. Int J Mol Sci. 2020;21:2264. doi: 10.3390/ijms21072264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren R, Gao J, Yin D, Li K, Lu C, Ahmad S, et al. Highly efficient leaf base protoplast isolation and transient expression systems for orchids and other important monocot crops. Front Plant Sci. 2021;12:172. doi: 10.3389/fpls.2021.626015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Royal Flora Holland (2020) The international orchid register / RHS Gardening. https://apps.rhs.org.uk/horticulturaldatabase/orchidregister/parentageresults.asp?page=2&seedgen=Phalaenopsis
- Semiarti E, Indrianto A, Purwantoro A, Isminingsih S, Suseno N, Ishikawa T, et al. Agrobacterium-mediated transformation of the wild orchid species Phalaenopsis amabilis. Plant Biotech. 2007;24:265–272. doi: 10.5511/plantbiotechnology.24.265. [DOI] [Google Scholar]
- Semiarti E, Nopitasari S, Setiawati Y, Lawrie MD, Purwantoro A, Widada J, et al. Application of CRISPR/Cas9 genome editing system for molecular breeding of orchids. Indones J Biotech. 2020;25:61. doi: 10.22146/ijbiotech.39485. [DOI] [Google Scholar]
- Semiarti E, Indrianto A, Purwantoro YH, Martiwi INA, Feroniasanti YML, Nadifah F, Mercuriana IS, Dwiyani R, Iwakawa H, Yoshioka Y, Machida Y, Machida C. High-frequency genetic transformation of Phalaenopsis amabilis orchid using tomato extract-enriched medium for the pre-culture of protocorms. J Hortic Sci Biotechnol. 2010;85(3):205–210. doi: 10.1080/14620316.2010.11512655. [DOI] [Google Scholar]
- Semiarti E, Indrianto A, Purwantoro A, Machida Y, Machi C (2011) Agrobacterium-mediated transformation of indonesian orchids for micropropagation. In: Genetic Transformation, InTech, pp 215–240.
- Semiarti E, Purwantoro A, Puspita Sari I (2020b) Biotechnology approaches on characterization, mass propagation, and breeding of indonesian orchids Dendrobium lineale (Rolfe.) and Vanda tricolor (Lindl.) with its Phytochemistry. In: Mérillon JM, Kodja H (eds) Orchids phytochemistry, biology and horticulture, Springer, pp.299-312
- Sherpa R, Devadas R, Bolbhat SN, Nikam TD, Penna S. Gamma radiation induced in-vitro mutagenesis and isolation of mutants for early flowering and phytomorphological variations in Dendrobium ‘Emma White’. Plants. 2022;11(22):3168. doi: 10.3390/plants11223168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shrestha BR, Tokuhara K, Mii M. Plant regeneration from cell suspension-derived protoplasts of Phalaenopsis. Plant Cell Rep. 2007;26:719–725. doi: 10.1007/s00299-006-0286-3. [DOI] [PubMed] [Google Scholar]
- Shrestha BR, Chin DP, Tokuhara K, Mii M. Efficient production of transgenic plants of Vanda through sonication-assisted Agrobacterium-mediated transformation of protocorm-like bodies. Plant Biotech. 2007;24:429–434. doi: 10.5511/plantbiotechnology.24.429. [DOI] [Google Scholar]
- Sjahril R, Mii M. High-efficiency Agrobacterium- mediated transformation of Phalaenopsis using meropenem, a novel antibiotic to eliminate Agrobacterium. J Hortic Sci Biotech. 2006;81:458–464. doi: 10.1080/14620316.2006.11512088. [DOI] [Google Scholar]
- Sjahril R, Dong PC, Raham SK, Yamamura S, Nakamura I, Amemiya Y, et al. Transgenic Phalaenopsis plants with resistance to Erwinia carotovora produced by introducing wasabi defensin gene using Agrobacterium method. Plant Biotech. 2006;23:191–194. doi: 10.5511/plantbiotechnology.23.191. [DOI] [Google Scholar]
- Su V, Hsu BD-D. Cloning and expression of a putative cytochrome P450 gene that influences the colour of Phalaenopsis flowers. Biotech Lett. 2003;25:1933–1939. doi: 10.1023/B:BILE.0000003989.19657.53. [DOI] [PubMed] [Google Scholar]
- Su V, Hsu BD. Transient expression of the cytochrome p450 CYP78A2 enhances anthocyanin production in flowers. Plant Mol Biol Rep. 2010;28:302–308. doi: 10.1007/s11105-009-0153-9. [DOI] [Google Scholar]
- Subramaniam S, Rathinam X. Emerging factors that influence efficiency of T-DNA gene transfer into Phalaenopsis violacea orchid via Agrobacterium tumefaciens–mediated transformation system. Int J Biol. 2010;2:64–73. doi: 10.5539/ijb.v2n2p64. [DOI] [Google Scholar]
- Subramaniam S, Vinod B, Sashi S, Rathinam X. Optimization of the transient Gusa gene transfer of Phalaenopsis violacea orchid via Agrobacterium tumefaciens: An assessment of factors influencing the efficiency of gene transfer mechanisms. Adv Nat Appl Sci. 2008;2:77–88. [Google Scholar]
- Suputri NPAEO, Prasojo IS, Prabowo LAT, Purwestri YA, Purnomo SE. Identification of early flowering mutant gene in Phalaenopsis amabilis (L.) Blume for sgRNA construction in CRISPR/Cas9 genome editing system. Braz J Biol. 2024;84:e268133. doi: 10.1590/1519-6984.268133. [DOI] [PubMed] [Google Scholar]
- Teixeira da Silva JA, Dobránszki J, Cardoso JC, Chandler SF, Zeng S. Methods for genetic transformation in Dendrobium. Plant Cell Rep. 2016;35:483–504. doi: 10.1007/s00299-015-1917-3. [DOI] [PubMed] [Google Scholar]
- Thaneshwari T, Kumari P, Aswath C. Haploid and double haploids in ornamentals a review. Int J Curr Microbiol Appl Sci. 2018;7(07):1322–1336. doi: 10.20546/ijcmas.2018.707.158. [DOI] [Google Scholar]
- Tong C, Wu F, Yuan Y, Chen Y, Lin C. High-efficiency CRISPR/Cas-based editing of Phalaenopsis orchid MADS genes. Plant Biotech J. 2020;18:889–891. doi: 10.1111/pbi.13264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai CC. A New hybrid genus Amenopsis (Orchidaceae) derived from the cross between Amesiella and Phalaenopsis. Acta Hortic. 2014;1025:57–60. doi: 10.17660/ActaHortic.2014.1025.7. [DOI] [Google Scholar]
- Tsai WC, Dievart A, Hsu CC, Hsiao YY, Chiou SY, Huang H, Chen HH. Post genomics era for orchid research. Bot Stud. 2017;58(1):1–22. doi: 10.1186/s40529-017-0213-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turnbull C, Lillemo M, Hvoslef-Eide TAK. Global regulation of genetically modified crops amid the gene edited crop boom—a review. Front Plant Sci. 2021;12:630396. doi: 10.3389/fpls.2021.630396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Utami ESW, Hariyanto S, Manuhara YSW. Agrobacterium tumefaciens-mediated transformation of Dendrobium lasianthera J.J.Sm: An important medicinal orchid. J Genet Eng Biotech. 2018;16:703–709. doi: 10.1016/j.jgeb.2018.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vendrame W, Faria RT, de, Sorace M, Sahyun SA. Orchid cryopreservation. Ciênc Agrotec. 2014;38:213–229. doi: 10.1590/S1413-70542014000300001. [DOI] [Google Scholar]
- Vendrame WA, Khoddamzadeh AA. Orchid biotechnology. Hortic Rev. 2016;44:173–228. [Google Scholar]
- Vilcherrez-Atoche JA, Iiyama CM, Cardoso JC. Polyploidization in orchids: from cellular changes to breeding applications. Plants. 2022;11:469. doi: 10.3390/plants11040469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vilcherrez-Atoche JA, Silva JC, Clarindo WR, Mondin M, Cardoso JC. In vitro Polyploidization of Brassolaeliocattleya Hybrid Orchid. Plants. 2023;12(2):281. doi: 10.3390/plants12020281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vo T-C, Seo J-W, Kim CK, Kim H-Y, Lim K-B. Breeding of the mini-type Phalaenopsis cultivar ‘yellow green’, having deep-yellow flowers with a red lip. Hort Sci Tech. 2019;37(4):540–547. [Google Scholar]
- Wang H, Dong B, Jiang J, Fang W, Guan Z, Liao Y, et al. Characterization of in vitro haploid and doubled haploid Chrysanthemum morifolium plants via unfertilized ovule culture for phenotypical traits and dna methylation pattern. Front Plant Sci. 2014;5:738. doi: 10.3389/fpls.2014.00738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang SL, Viswanath KK, Tong C-G, An HR, Jang S, Chen FC. Floral induction and flower development of orchids. Front Plant Sci. 2019;10:1–15. doi: 10.3389/fpls.2019.01258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Widiarsih S, Dwimahyani I. Gamma irradiation application for mutation breeding in early flowering moth orchid (Phalaenopsis amabilis Bl.) J Ilm Apl Isot Radiasi. 2013;9:59–66. [Google Scholar]
- Winarto B, Mattjik NA, Purwito A, Marwoto B. Improvement of selected induction culture media on callus induction in anther culture of Anthurium and a histological study on its callus formation. J Nat Indones. 2010;12(2):93–101. doi: 10.31258/jnat.12.2.93-101. [DOI] [Google Scholar]
- Wu J-Y, Hsieh T-F, Tsao C-Y, Chuang K-C. Breeding of an Indigo Phalaenopsis by Intergeneric Hybridization: Rhynchonopsis Tariflor Blue Kid ‘1030-4’. HortScience. 2022;57(3):489–490. doi: 10.21273/HORTSCI15944-21. [DOI] [Google Scholar]
- Wu T, Zhao X, Yang S, Yang J, Zhu J, Kou Y, et al. Induction of 2n pollen with colchicine during microsporogenesis in Phalaenopsis. Breed Sci. 2022;72:275–284. doi: 10.1270/jsbbs.21100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia K, Zhang D, Xu X, Liu G, Yang Y, Chen Z, et al. Protoplast technology enables the identification of efficient multiplex genome editing tools in Phalaenopsis. Plant Sci. 2022;322:111368. doi: 10.1016/j.plantsci.2022.111368. [DOI] [PubMed] [Google Scholar]
- Yang F, Gao J, Wei Y, Ren R, Zhang G, Lu C-Q, et al. The genome of Cymbidium sinense revealed the evolution of orchid traits. Plant Biotech J. 2021;19:2501–2516. doi: 10.1111/pbi.13676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeung EC. The orchid embryo — “an embryonic protocorm. Botany. 2022;100(9):691–706. doi: 10.1139/cjb-2022-0017. [DOI] [Google Scholar]
- Yuan SC, Chin SW, Chen FC. Current trends of Phalaenopsis orchid breeding and study on pollen storage. Acta Hortic. 2015;1078:19–23. doi: 10.17660/ActaHortic.2015.1078.1. [DOI] [Google Scholar]
- Zanello CA, Cardoso JC. PLBs induction and clonal plantlet regeneration from leaf segment of commercial hybrids of Phalaenopsis. J Hortic Sci Biotech. 2019;94:627–631. doi: 10.1080/14620316.2019.1600384. [DOI] [Google Scholar]
- Zargar M, Zavarykina T, Voronov S, Pronina I, Bayat M. The recent development in technologies for attaining doubled haploid plants in vivo. Agriculture. 2022;12(10):1595. doi: 10.3390/agriculture12101595. [DOI] [Google Scholar]
- Zhang L, Chin DP, Mii M. Agrobacterium-mediated transformation of protocorm-like bodies in Cattleya. Plant Cell Tiss Organ Cult. 2010;103:41–47. doi: 10.1007/s11240-010-9751-3. [DOI] [Google Scholar]
- Zhang YB, Liao FQ, Zhong HQ, Huang PP, Liu TF, Liu ZC. A preliminary study on pollen Irradiation for Phalaenopsis breeding. Fujian. J Agric Sci. 2009;24(3):237–240. [Google Scholar]
- Zhang XF, Ren YL, Shang TC, Zhang W (2009b) Effect of 60Co-γ ray irradiation on Protocorm-like Bodies growth of Phalaenopsis. Northern Horticulture
- Zhao P, Ren A, Dong P, Sheng Y, Chang X, Zhang X. The antimicrobial peptaibol trichokonin IV promotes plant growth and induces systemic resistance against Botrytis cinerea infection in moth orchid. J Phytopathol. 2018;166:346–354. doi: 10.1111/jph.12692. [DOI] [Google Scholar]
- Zheng T, Li P, Li L, Zhang Q. Research advances in and prospects of ornamental plant genomics. Hort Res. 2021;8(1):65. doi: 10.1038/s41438-021-00499-x. [DOI] [PMC free article] [PubMed] [Google Scholar]