Abstract
Between 30% and 70% of patients with breast cancer have pre-existing chronic conditions, and more than half are on long-term non-cancer medication at the time of diagnosis. Preliminary epidemiological evidence suggests that some non-cancer medications may affect breast cancer risk, recurrence, and survival. In this nationwide cohort study, we assessed the association between medication use at breast cancer diagnosis and survival. We included 235,368 French women with newly diagnosed non-metastatic breast cancer. In analyzes of 288 medications, we identified eight medications positively associated with either overall survival or disease-free survival: rabeprazole, alverine, atenolol, simvastatin, rosuvastatin, estriol (vaginal or transmucosal), nomegestrol, and hypromellose; and eight medications negatively associated with overall survival or disease-free survival: ferrous fumarate, prednisolone, carbimazole, pristinamycin, oxazepam, alprazolam, hydroxyzine, and mianserin. Full results are available online from an interactive platform (https://adrenaline.curie.fr). This resource provides hypotheses for drugs that may naturally influence breast cancer evolution.
Subject terms: Breast cancer, Epidemiology
Preliminary epidemiological evidence suggests that some non-cancer medications may affect breast cancer risk, recurrence, and survival. In this study, the authors utilized a nationwide database of breast cancer patients to estimate the association between frequently used drugs taken prior to diagnosis and breast cancer prognosis. And they identified 16 drugs associated with breast cancer outcomes.
Introduction
Breast cancer (BC) is the most frequent cancer in women and the leading cause of cancer deaths in women worldwide. Its incidence increases with age, as does the incidence of many other chronic diseases, such as hypertension, diabetes, and dyslipidemia. Between 30% and 70% of patients with BC suffer from pre-existing comorbid conditions at BC diagnosis1–3 and more than half are already taking medication (chronic treatment with non-cancer drugs)4,5.
There is a strong complex interplay between comorbid conditions and concomitant medication, but most previous studies on this topic have focused on either comorbid conditions1,3,6,7 or concomitant medication4,8. Several studies have reported epidemiological evidence for associations between non-cancer treatments, such as aspirin or non-steroidal anti-inflammatory drugs, and lower BC risk9. Other medications, such as statins10,11, beta-blockers12,13, and metformin11, have been shown to be associated with lower rates of BC recurrence or better survival after BC. Conversely, several frequently prescribed medications have been shown to increase the risk of BC14,15 or to interact with BC treatments16–19.
In France, all the medical and administrative information relating to the reimbursement of healthcare expenses is collected and aggregated within the National Health Data System20 (Système National des Données de Santé, SNDS). The SNDS is among the largest and most exhaustive health data resources worldwide, covering approximately 70 million people in considerable detail. It provides a wealth of information that can be used to generate real-world evidence to develop precision medicine or support public health decision-making21,22.
We hypothesize that several concomitant medications taken prior to diagnosis may modify the natural course of BC. The main objective of this study designated ADRENALINE (Atlas of Drugs, Comorbidities, and Cancer Treatment Survival Interaction), is to analyze the impact of medication use in the six months preceding the diagnosis of BC on overall survival (OS, main outcome), and disease-free survival (DFS, secondary outcome) in a very large cohort of French women diagnosed with BC. Using SNDS data for a published cohort of patients with BC23, we identified all the non-cancer medications commonly prescribed in the six months preceding BC diagnosis. We adjusted for approximately a hundred confounding factors to identify medications with a significant positive or negative association with DFS or OS. We then used mediation analyzes to estimate the extent to which these associations could be explained by a change in BC subtype or nodal status at diagnosis due to the use of the medications concerned. Finally, we built an interactive tool to explore the distribution of comorbid conditions, medications used at BC diagnosis, their co-occurrence, and the association between these medications and survival (available as a public resource from https://adrenaline.curie.fr).
Results
Characteristics of the patients and tumors
The analyzes included 235,368 patients with BC in total, of whom 12.1% relapsed or died and 6.6% died during follow-up. The median follow-up time was 54.6 months for OS and 53.9 months for DFS. Median age at diagnosis was 60 years (Table 1). The distribution of BC subtypes was as follows: luminal (65.1%), TNBC (7.7%), HER2-positive (8.4%) (18.9% undefined tumors). Most patients had node-negative disease (81.2%), received radiotherapy (85.3%) and endocrine therapy (70.4%), and approximately one-third received chemotherapy (38.3%).
Table 1.
Characteristics of the patients in the total population, patients without medication at the time of BC diagnosis, and patients on at least one medication at the time of BC diagnosis
| Category | Variable | Class | Total | No concomitant medication | At least one drug |
|---|---|---|---|---|---|
| n (%) | 235 368 (100%) | 56 510 (24%) | 178 858 (76%) | ||
| Pre-exposure covariates | |||||
|
Socio- demographic |
Age at diagnosis (years) | 60.0 [50.0, 69.0] | 52.0 [45.0, 61.0] | 63.0 [53.0, 71.0] | |
| Age at diagnosis (years, classes) | <30 | 1 124 (0.5) | 477 (0.8) | 647 (0.4) | |
| 30-39 | 10 539 (4.5) | 4 631 (8.2) | 5 908 (3.3) | ||
| 40-49 | 43 206 (18.4) | 17 582 (31.1) | 25 624 (14.3) | ||
| 50-59 | 58 003 (24.6) | 18 297 (32.4) | 39 706 (22.2) | ||
| 60-69 | 64 042 (27.2) | 10 994 (19.5) | 53 048 (29.7) | ||
| 70-79 | 39 163 (16.6) | 3 458 (6.1) | 35 705 (20.0) | ||
| 80+ | 19 291 (8.2) | 1 071 (1.9) | 18 220 (10.2) | ||
| Deprivation index (quintiles) | 1st quintile (least deprived) | 46 323 (19.7) | 12 369 (21.9) | 33 954 (19.0) | |
| 2nd quintile | 46 688 (19.8) | 11 657 (20.6) | 35 031 (19.6) | ||
| 3rd quintile | 45 984 (19.5) | 11 021 (19.5) | 34 963 (19.5) | ||
| 4th quintile | 46 183 (19.6) | 10 504 (18.6) | 35 679 (19.9) | ||
| 5th quintile (most deprived) | 45 992 (19.5) | 9 538 (16.9) | 36 454 (20.4) | ||
| Overseas départements | 4 198 (1.8) | 1 421 (2.5) | 2 777 (1.6) | ||
| GP consultations* | 5.0 [3.0, 9.0] | 2.0 [1.0, 4.0] | 6.0 [4.0, 10.0] | ||
| GP consultations* (classes) | 0 | 12 964 (5.5) | 8 421 (14.9) | 4 543 (2.5) | |
| 1–5 | 109 539 (46.5) | 38 489 (68.1) | 71 050 (39.7) | ||
| 6–11 | 32 701 (13.9) | 1 308 (2.3) | 31 393 (17.6) | ||
| 12+ | 80 164 (34.1) | 8 292 (14.7) | 71 872 (40.2) | ||
| Gynecologist visits** | 0.0 [0.0, 1.0] | 1.0 [0.0, 1.0] | 0.0 [0.0, 1.0] | ||
| Gynecologist visits** (classes) | 0 | 127 785 (54.3) | 28 225 (49.9) | 99 560 (55.7) | |
| 1 | 65 868 (28.0) | 17 694 (31.3) | 48 174 (26.9) | ||
| 2–3 | 35 290 (15.0) | 8 941 (15.8) | 26 349 (14.7) | ||
| 4+ | 6 425 (2.7) | 1 650 (2.9) | 4 775 (2.7) | ||
| Mammographic screening before diagnosis | No | 146 945 (62.4) | 38 843 (68.7) | 108 102 (60.4) | |
| Yes | 88 423 (37.6) | 17 667 (31.3) | 70 756 (39.6) | ||
| Comorbid conditions | Comorbid conditions (binary) | No | 124 652 (53.0) | 44 872 (79.4) | 79 780 (44.6) |
| Yes | 110 716 (47.0) | 11 638 (20.6) | 99 078 (55.4) | ||
| Comorbid condition category | Cardiovascular | 60 146 (25.6) | 2 931 (5.2) | 57 215 (32.0) | |
| Endocrine and metabolism | 51 588 (21.9) | 3 522 (6.2) | 48 066 (26.9) | ||
| Psychiatric disorders | 30 372 (12.9) | 4 713 (8.3) | 25 659 (14.3) | ||
| Frailty (proxy) | 11 888 (5.1) | 1 181 (2.1) | 10 707 (6.0) | ||
| Pulmonary | 10 883 (4.6) | 750 (1.3) | 10 133 (5.7) | ||
| Rheumatologic disease and connective tissue diseases | 7 918 (3.4) | 413 (0.7) | 7 505 (4.2) | ||
| Gastrointestinal | 7 519 (3.2) | 752 (1.3) | 6 767 (3.8) | ||
| Neurologic | 6 983 (3.0) | 746 (1.3) | 6 237 (3.5) | ||
| Liver | 2 668 (1.1) | 324 (0.6) | 2 344 (1.3) | ||
| Kidney | 2 524 (1.1) | 93 (0.2) | 2 431 (1.4) | ||
| Other | 1 015 (0.4) | 103 (0.2) | 912 (0.5) | ||
| Immune | 635 (0.3) | 84 (0.1) | 551 (0.3) | ||
| Post-exposure covariates | |||||
| BC biology | Inferred BC subtype | luminal | 153 109 (65.1) | 34 117 (60.4) | 118 992 (66.5) |
| TNBC | 18 149 (7.7) | 5 532 (9.8) | 12 617 (7.1) | ||
| HER2 + | 19 722 (8.4) | 5 974 (10.6) | 13 748 (7.7) | ||
| Undefined | 44 388 (18.9) | 10 887 (19.3) | 33 501 (18.7) | ||
| Nodal status | Node-negative | 191 164 (81.2) | 45 282 (80.1) | 145 882 (81.6) | |
| Node-positive | 44 204 (18.8) | 11 228 (19.9) | 32 976 (18.4) | ||
| BC treatment | Breast surgery | Partial mastectomy | 173 173 (73.6) | 40 238 (71.2) | 132 935 (74.3) |
| Mastectomy | 62 195 (26.4) | 16 272 (28.8) | 45 923 (25.7) | ||
| Radiotherapy | No | 34 683 (14.7) | 8 294 (14.7) | 26 389 (14.8) | |
| Yes | 200 685 (85.3) | 48 216 (85.3) | 152 469 (85.2) | ||
| Chemotherapy | No | 145 116 (61.7) | 29 421 (52.1) | 115 695 (64.7) | |
| Yes | 90 252 (38.3) | 27 089 (47.9) | 63 163 (35.3) | ||
| Endocrine therapy | No | 69 713 (29.6) | 18 628 (33.0) | 51 085 (28.6) | |
| Yes | 165 655 (70.4) | 37 882 (67.0) | 127 773 (71.4) | ||
The number of patients, and the percentage of patients (in parentheses), are reported for categorical variables. The median value, and the interquartile range (in parentheses), are reported for continuous variables. *Number of general practitioner (GP) visits in the year preceding BC diagnosis.
**Number of gynecologist visits in the year preceding BC diagnosis.
GP general practitioner, BC breast cancer, TNBC triple-negative breast cancer.
Comorbid conditions
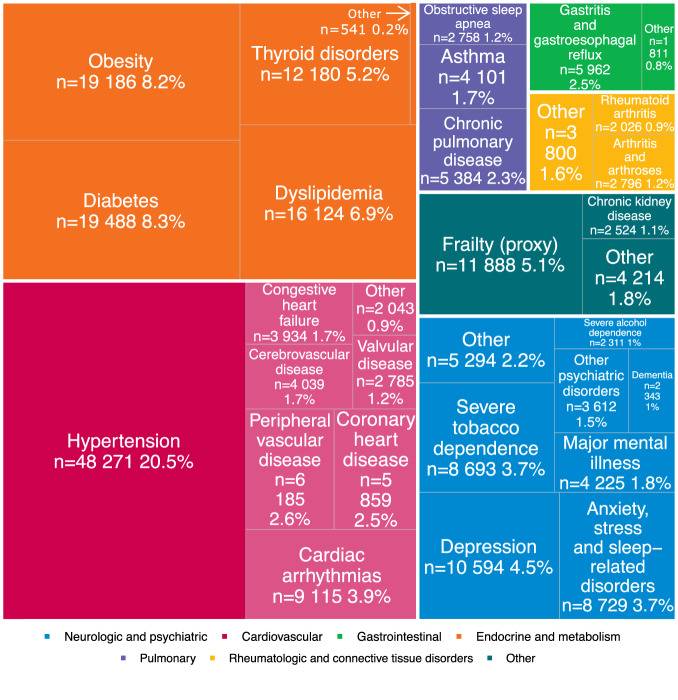
At least one comorbid condition was present at diagnosis in 47.0% of patients. The frequency and number of comorbid conditions increased with age and deprivation index (Supplementary Fig. 1). Cardiovascular diseases were the most frequent (25.6% of patients), followed by endocrine/metabolic diseases (21.9%) and psychiatric (12.9%) disorders (Table 1). The top three comorbid diagnoses were hypertension (20.5%), diabetes (8.3%) and obesity (8.2%) (Fig. 1). Strong associations were found between certain comorbid conditions, such as hypertension and diabetes (n = 10,760), or hypertension and dyslipidemia (n = 10,643, Supplementary Fig. 2). An interactive viewer of associations between comorbid conditions is available online (Supplementary Fig. 3, https://adrenaline.curie.fr/static/network_comor/index.html). No comorbid condition was found in 53.0% of patients, some of whom were taking non-specific medications, such as vitamins (21.1%), analgesics (14.1%), or sex hormones and modulators of the genital system (15.4%) (Supplementary Fig. 4).
Fig. 1. Distribution of comorbid conditions (by disease) in the total population.
Diseases are color-coded by category. Percentages of the total population are reported. In each category, comorbid conditions with fewer than 2000 cases were regrouped into the “Other” category to improve readability. In the neurologic and psychiatric diseases category, “Other” includes anorexia or bulimia (n = 114, 0%), cognitive disabilities (n = 791, 0.3%), epilepsy (n = 1278, 0.5%), hemiplegia, paraplegia or palsy (n = 1600, 0.7%), multiple sclerosis (n = 719, 0.3%), other substance use disorder (n = 244, 0.1%), and Parkinson’s disease (n = 989, 0.4%). In the cardiovascular diseases category, “Other” includes coagulopathy (n = 743, 0.3%“), hemoglobinopathy (n = 104, 0%), and pulmonary embolism (n = 1217, 0.5%). In the gastrointestinal diseases category, “Other” includes inflammatory bowel disease (n = 1022, 0.4%), pancreatic disease (n = 232, 0.1%), and peptic ulcer disease (n = 576, 0.2%). In the endocrine and metabolic diseases category, “Other” includes other endocrine disorders (n = 541, 0.2%). In the rheumatologic and connective tissue disorders category, “Other” includes connective tissue diseases (n = 1102, 0.5%), fibromyalgia (n = 324 0.1%), osteoporosis (n = 1817, 0.8%), and rheumatic diseases (n = 664, 0.3%). In the other diseases category, “Other” includes hereditary metabolic disorders (n = 459, 0.2%), myopathies, or disorders of muscles (n = 562, 0.2%), HIV/AIDS (n = 316, 0.1%), organ or tissue transplant (n = 186 0.1%), other immune deficiency (n = 141, 0.1%), chronic hepatitis (n = 1001, 0.4%), cirrhosis (n = 879, 0.4%), and steatosis and hereditary diseases (n = 1046, 0.4%). The data can be further explored on the interactive ADRENALINE web application (https://adrenaline.curie.fr/comorbidity_description). Source data are provided as a Source Data file. Abbreviations: HIV: human immunodeficiency virus; AIDS: acquired immunodeficiency syndrome.
Concomitant medication
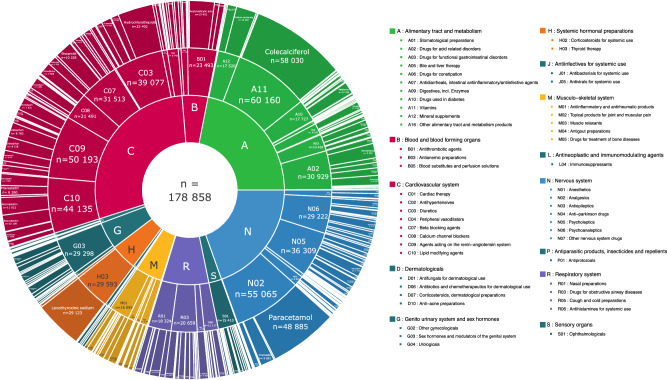
Approximately three quarters of patients were on at least one concomitant medication in the six months preceding diagnosis (76.0%), and the frequency and number of medications increased with age and deprivation index (Supplementary Fig. 5). The three main anatomical classes were drugs targeting the alimentary tract and metabolism (ATC A), the cardiovascular system (ATC C) and the nervous system (ATC N), with colecalciferol, paracetamol, and levothyroxine the three medications most frequently reported (Fig. 2, Supplementary Data 1, adrenaline.curie.fr/comed_description). Some medications were often prescribed together (Supplementary Fig. 6): vitamins (A11) and mineral supplements (A12, n = 13,918); diuretics (C03) and agents acting on the renin-angiotensin system (C09, n = 26,456); or psycholeptics (N05) and psychoanaleptics (N06, n = 16,706). Co-prescriptions can be explored further with an online interactive tool (Supplementary Fig. 7, https://adrenaline.curie.fr/static/network_comor/index.html). The number of concomitant medications taken at diagnosis was correlated with the number of comorbid conditions (Supplementary Fig. 8), and there was a strong association between comorbid conditions and concomitant medications from the corresponding therapeutic class (Supplementary Fig. 4), such as cardiovascular conditions and agents acting on the renin-angiotensin system (55.0%).
Fig. 2. Distribution of concomitant medications by ATC code, for ATC level 1 (inner ring), ATC level 2 (middle ring), and ATC level 5 (outer ring).
Concomitant medications are color-coded by ATC level. Raw data for ATC classes for which the data cannot be read on the graph can be accessed in Supplementary Data 1 or via the interactive display available online at https://adrenaline.curie.fr/comed_description. Source data are provided as a Source Data file. ATC Anatomical Therapeutic Chemical.
Association between concomitant medication and survival
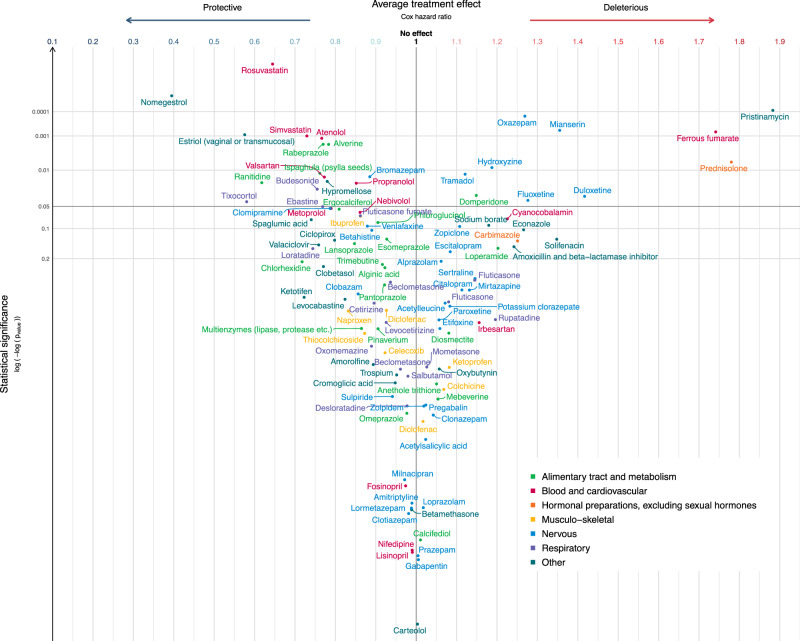
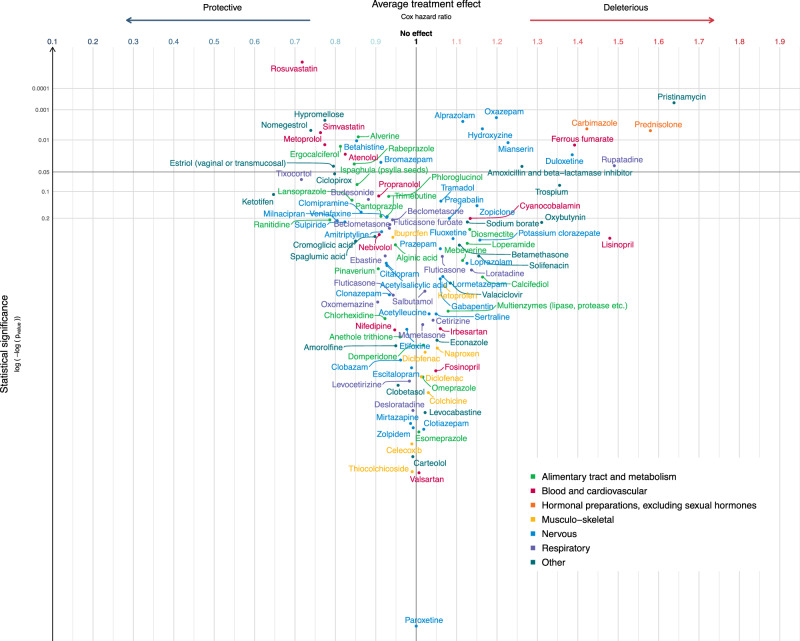
The 288 medications selected for the analyzes included 113 (39%) that passed the adjustment quality test (Figs. 3 and 4, Supplementary Data 2, 3). Before adjustment for multiple tests, 32 medications were significantly associated with OS (n = 25, Fig. 5A), or DFS (n = 23, Fig. 6A), including 16 medications associated with both OS and DFS: rabeprazole, alverine, ferrous fumarate, atenolol, simvastatin, rosuvastatin, prednisolone, oxazepam, bromazepam, hydroxyzine, mianserin, duloxetine, estriol (vaginal or transmucosal), nomegestrol, pristinamycin, and hypromellose. Among these 16 drugs, all medications targeting the alimentary tract and metabolism (rabeprazole, alverine) were protective, as were medications targeting the cardiovascular system (atenolol, simvastatin, and rosuvastatin) and the genitourinary system (estriol vaginal or transmucosal, nomegestrol). All medications targeting the blood and blood-forming organs (ferrous fumarate) and hormonal system (prednisolone) were deleterious, as were antibiotics (pristinamycin) (Figs. 5A, 6A). For medications targeting the nervous system, we observed mixed-class effects in the association between survival and the intake of benzodiazepines, with bromazepam being protective (HR OS: 0.89, 95% CI: 0.80 to 0.98; HR DFS: 0.91 95% CI: 0.84 to 0.99) whereas oxazepam (HR OS: 1.27, 95% CI: 1.12 to 1.44; HR DFS: 1.20, 95% CI: 1.07 to 1.34) was deleterious.
Fig. 3. Estimated average treatment effect (ATE) for overall survival (OS) for the 113 medications passing the adjustment quality test.
Medications are represented by circles color-coded by ATC level and linked to the full name of the medication. The ATE (i.e. the Cox hazard ratio, HR) is plotted on the x-axis. Lower HRs (protective effect of the medication, increasing overall survival in breast cancer) are displayed on the left. Higher HRs (deleterious effect of the medication, decreasing overall survival in breast cancer) are displayed on the right. An HR of 1 (no effect of the medication on overall survival in breast cancer) is indicated by a vertical line. We used two-sided Wald tests with robust covariances for statistical inference. No adjustment for multiple comparisons was made at this stage of the pipeline. Statistical significance is plotted on the y-axis. Lower p-values (high statistical significance) are displayed at the top. Higher p-values (low statistical significance) are displayed at the bottom. An interactive display is available via the ADRENALINE web application (https://adrenaline.curie.fr/survival_analysis). Source data are provided as a Source Data file. *Estriol (vaginal or transmucosal). ATE average treatment effect, ATC Anatomical Therapeutic Chemical.
Fig. 4. Estimated average treatment effect (ATE) for disease-free survival (DFS) for the 113 medications passing the adjustment quality test.
Medications are represented by circles color-coded by ATC level and linked to the full name of the medication. The ATE (i.e. the Cox hazard ratio, HR) is plotted on the x-axis. Lower HRs (protective effect of the medication, increasing disease-free survival in breast cancer) are displayed on the left. Higher HRs (deleterious effect of the medication, decreasing disease-free survival in breast cancer) are displayed on the right. An HR of 1 (no effect of the medication on disease-free survival in breast cancer) is indicated by a vertical line. We used two-sided Wald tests with robust covariances for statistical inference. No adjustment for multiple comparisons was made at this stage of the pipeline. Statistical significance is plotted on the y-axis. Lower p-values (high statistical significance) are displayed at the top. Higher p-values (low statistical significance) are displayed at the bottom. An interactive display is available via the ADRENALINE web application (https://adrenaline.curie.fr/survival_analysis). Source data are provided as a Source Data file. Abbreviations: ATE: average treatment effect; ATC: Anatomical Therapeutic Chemical.
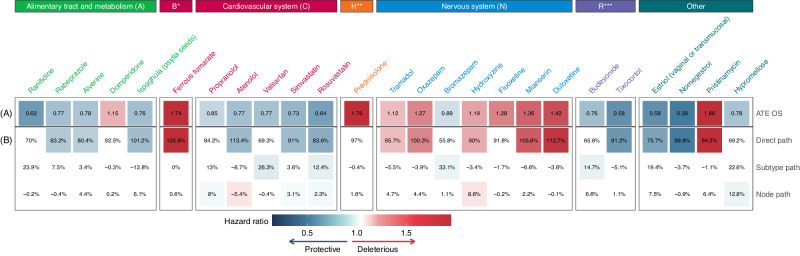
Fig. 5. Mediation analyzes for the 25 medications with a significant protective or deleterious effect for overall survival (OS).
Medications are grouped and color-coded by ATC level. Significant protective associations are shown in blue. Significant deleterious associations are shown in red. Non-significant associations are shown in white. A Average treatment effect (ATE) of the medication (Cox hazard ratio, HR). B Breakdown of the ATE into three path-specific effects (expressed as percentages): direct path, subtype path and node path. Source data are provided as a Source Data file. ATE average treatment effect, ATC Anatomical Therapeutic Chemical, HR hazard ratio, B* blood and blood-forming organs, H** systemic hormonal preparations, excluding sex hormones and insulins, R*** respiratory system.
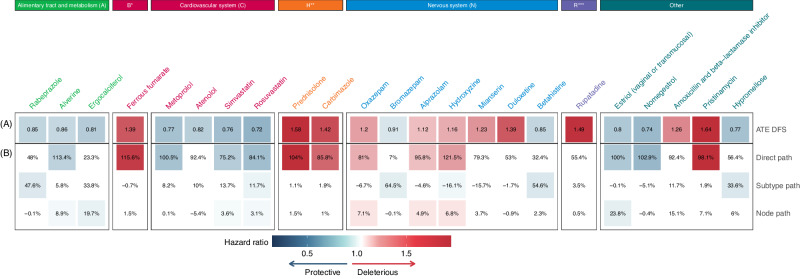
Fig. 6. Mediation analyzes for the 23 medications with a significant protective or deleterious effect for disease-free survival (DFS).
Medications are grouped and color-coded by ATC level. Significant protective associations are shown in blue. Significant deleterious associations are shown in red. Non-significant associations are shown in white. (A) Average treatment effect (ATE) of the medication (Cox hazard ratio, HR). (B) Breakdown of the ATE into three path-specific effects (expressed as percentages): direct path, subtype path, and node path. Source data are provided as a Source Data file. ATE average treatment effect, ATC Anatomical Therapeutic Chemical, HR hazard ratio, A* alimentary tract and metabolism, B** blood and blood-forming organs, H*** systemic hormonal preparations, excluding sex hormones and insulins, R**** respiratory system.
Mediation analyzes
The 32 medications significantly associated with a decrease or increase in DFS or OS were selected for mediation analyzes, in which the observed associations were broken down into: (1) direct effect, including the inherent effect of the medication and any effect through other pathways not involving a difference in BC subtype or nodal status, (2) indirect effect through pathways involving a difference in BC subtype; (3) indirect effect through pathways involving a difference in nodal status. The associations between concomitant medication and survival were generally almost entirely attributable to direct effects (Figs. 5B and 6B). Among the 16 medications significantly associated with both OS and DFS, a significant percentage of the estimated ATE could be attributed to differences in tumor subtype for rabeprazole (7.5% non-significant for OS; 47.6% for DFS), rosuvastatin (12.4% for OS; 11.7% for DFS), bromazepam (33.1% for OS; 64.5% for DFS), and hypromellose (22.6% non-significant for OS; 33.6% for DFS); and a significant percentage of the estimated ATE could be attributed to differences in nodal status at diagnosis for alverine (4.4% non-significant for OS; 8.9% for DFS), simvastatin (3.1% for OS; 3.6% for DFS), rosuvastatin (2.3% for OS; 3.1% for DFS), oxazepam (4.4% non-significant for OS; 7.1% for DFS), hydroxyzine (8.6% for OS; 6.8% for DFS), estriol vaginal or transmucosal (7.5% non-significant for OS; 23.8% for DFS), and hypromellose (12.8% for OS; 6% non-significant for DFS). For atenolol, we observed a protective association with survival overall (HR OS 0.77 95% CI 0.65 to 0.90; HR DFS 0.82 95% CI 0.70 to 0.97), but atenolol use was associated with more frequent lymph node involvement at BC diagnosis (−5.4% of the effect).
Impact on survival after adjustment for multiple testing
After adjustment for multiple testing, sixteen medications remained significantly associated with an increase (n = 8) or decrease (n = 8) in OS (Fig. 7) or DFS (Fig. 8), six of which were associated with both OS and DFS (simvastatin, rosuvastatin, nomegestrol, prednisolone, pristinamycin, and oxazepam). Rabeprazole (HR OS: 0.77, 95% CI: 0.65 to 0.91), alverine (HR OS: 0.78, 95% CI: 0.67 to 0.91), atenolol (HR OS: 0.77, 95% CI: 0.65 to 0.90), simvastatin (HR OS: 0.73, 95% CI: 0.61 to 0.88; HR DFS: 0.76, 95% CI: 0.63 to 0.93), rosuvastatin (HR OS: 0.64, 95% CI: 0.55 to 0.76; HR DFS: 0.72, 95% CI: 0.63 to 0.82), estriol (vaginal or transmucosal, HR OS: 0.58, 95% CI: 0.42 to 0.80), nomegestrol (HR OS: 0.39, 95% CI: 0.26 to 0.60; HR DFS: 0.74, 95% CI: 0.60 to 0.91), and hypromellose (HR DFS: 0.77, 95% CI: 0.66 to 0.91) were associated with longer survival, whereas ferrous fumarate (HR OS 1.74, 95% CI: 1.26 to 2.40), prednisolone (HR OS: 1.78, 95% CI: 1.18 to 2.69; HR DFS: 1.58, 95% CI: 1.15 to 2.18), carbimazole (HR DFS: 1.42, 95% CI: 1.11 to 1.82), pristinamycin (HR OS: 1.88, 95% CI: 1.37 to 2.58; HR DFS: 1.64, 95% CI: 1.24 to 2.16), oxazepam (HR OS: 1.27, 95% CI: 1.12 to 1.44; HR DFS: 1.20, 95% CI: 1.07 to 1.34), alprazolam (HR DFS: 1.12, 95% CI: 1.04 to 1.20), hydroxyzine (HR DFS: 1.16, 95% CI: 1.05 to 1.29), and mianserin (HR OS: 1.36, 95% CI: 1.14 to 1.61) were associated with shorter survival. We obtained similar results in sensitivity analyzes performed with two different timeframes for the identification of comorbid conditions (Supplementary Table 1).
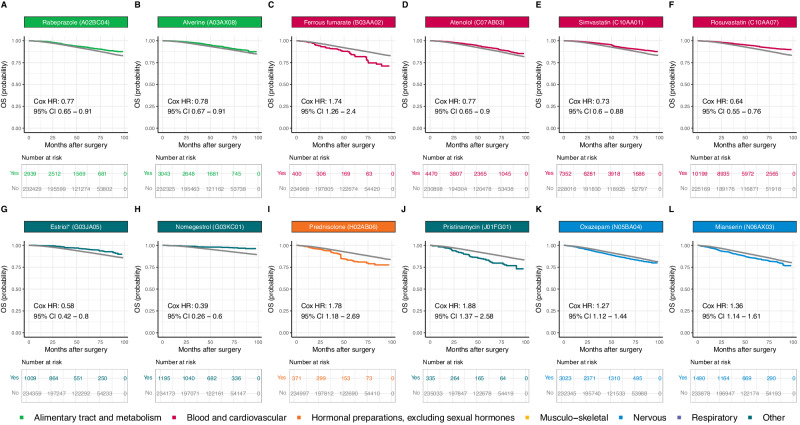
Fig. 7. Adjusted Kaplan-Meier survival curves for the 12 medications with a significant HR after adjustment for multiple testing for overall survival (OS).
A Rabeprazole (A02BC04); B Alverine (A03AX08); C Ferrous fumarate (B03AA02); D Atenolol (C07AB03); E Simvastatin (C10AA01); F Rosuvastatin (C10AA07); G Estriol* (vaginal or transmucosal) (G03JA05); H Nomegestrol (G03KC01); I Prednisolone (H02AB06); J Pristinamycin (J01FG01); K Oxazepam (N05BA04); L Mianserin (N06AX03). Survival curves for patients not on medication at the time of diagnosis are displayed in gray. Survival curves for patients with concomitant medication are color-coded by medication ATC level. The survival curves for all the other medications are available via the ADRENALINE web application (https://adrenaline.curie.fr/survival_analysis). Source data are provided as a Source Data file. * Vaginal or transmucosal. ATC Anatomical Therapeutic Chemical.
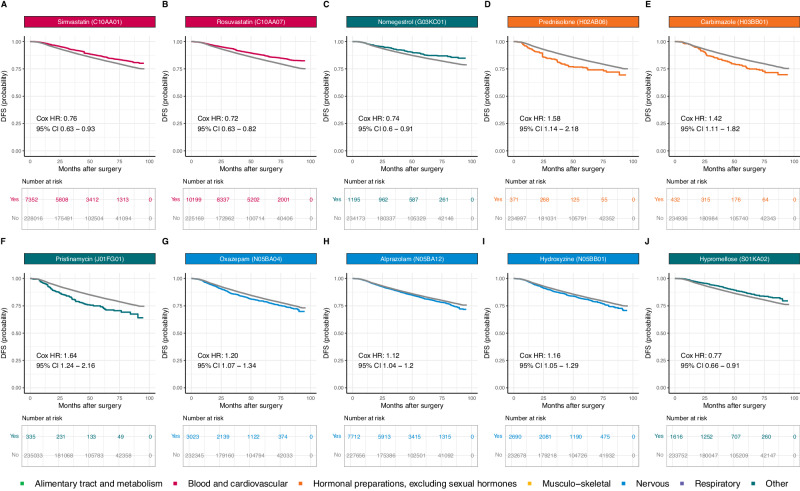
Fig. 8. Adjusted Kaplan-Meier survival curves for the 10 medications with a significant HR after adjustment for multiple testing for disease-free survival (DFS).
A Simvastatin (C10AA01); B Rosuvastatin (C10AA07); C Nomegestrol (G03KC01); D Prednisolone (H02AB06); E Carbimazole (H03BB01); F Pristinamycin (J01FG01); G Oxazepam (N05BA04); H Alprazolam (N05BA12); I Hydroxyzine (N05BB01); J Hypromellose (S01KA02). Survival curves for patients not on medication at the time of diagnosis are displayed in gray. Survival curves for patients with concomitant medication are color-coded by medication ATC level. The survival curves for all the other medications are available via the ADRENALINE web application (https://adrenaline.curie.fr/survival_analysis). Source data are provided as a Source Data file. ATC Anatomical Therapeutic Chemical.
Discussion
We performed a large, comprehensive overview of medication taken at the time of BC diagnosis and comorbid conditions, and performed extensive causal inference analyzes to investigate the association between concomitant medication and survival, adjusting for underlying disease and confounding factors. This study provides several new insights.
Little is known about the patterns of comorbid conditions at BC diagnosis, and no gold standard approach has been validated for assessing comorbidity in the cancer context24. We analyzed comorbid conditions individually, using a large list of diseases selected from an extensive literature review25–34. Consistent with previous studies35, we found that hypertension, obesity, diabetes, and dyslipidemia were the most frequent conditions, and were strongly associated. Comorbid conditions were strongly linked to concomitant medication intake, but the overlap was incomplete, probably due to the use of medications unrelated to comorbid conditions (contraceptive pills), or for undefined conditions (analgesics). We developed a causal inference pipeline to isolate the role of concomitant medication from those of the underlying condition and other confounding factors, and to decipher its direct effect on mortality through indirect effects involving modifications in BC subtype or nodal status at diagnosis. We discovered eight protective and eight deleterious associations.
Rabeprazole, a commonly used proton pump inhibitor (PPI), was associated with a 23% decrease in instantaneous risk of death. PPIs are thought to enhance the antitumor effects of chemotherapy36 and are associated with higher levels of immune infiltration37. There is a strong rationale for PPI use as adjuvant anticancer agents, possibly for deacidification of the tumor microenvironment38–40.
Atenolol, a beta-blocker, was associated with longer overall survival, consistent with the large body of preclinical41 and clinical42 data and the findings of meta-analyzes43. Multiple relevant mechanisms have been proposed for this effect44.
Two statins, simvastatin and rosuvastatin, were found to be highly protective, decreasing instantaneous risk of death by 27% and 36% and relapse or death by 24% and 28%, respectively. Statins inhibit the rate-limiting step of cholesterol biosynthesis and lower serum cholesterol concentration, but they also have pleiotropic effects on cell growth, signal transduction, differentiation, and apoptosis, thereby modulating physiological processes essential to cancer initiation and promotion45. Statins were found to be associated with a lower risk of BC recurrence46 and BC-related deaths47 in observational studies, and are currently under evaluation in two randomized clinical trials (NCT03971019, NCT04601116).
Several sex hormones used either locally (vaginal or transmucosal estriol treatment) or systemically (nomegestrol) were associated with longer survival in our cohort of BC patients when used prior to diagnosis, with HRs of 0.58 and 0.39 for OS, and 0.80 and 0.74 for DFS, respectively. The relationship between sex hormones and BC progression remains incompletely understood. Sex hormones used systematically have previously been associated with an increased risk of BC48–50, or with an increased risk of relapse when use post-diagnosis51. Conversely, post-diagnosis vaginal estrogen therapy has been tentatively associated with a lower risk of BC recurrence52, specific mortality53, or all-cause mortality54, although safety concerns have been raised in patients currently treated with aromatase inhibitors54. While our findings do not address the risk of BC incidence or the impact of post-diagnosis hormone use, they suggest that pre-diagnosis sex hormones may be associated with decreased mortality and relapse in BC patients, possibly through changes in tumor biology at diagnosis, as 23.8% of the protective association we observed between vaginal or transmucosal estriol and DFS was mediated by a decreased likelihood of lymph node involvement.
The two remaining protective associations suggested by our results were novel. The use of hypromellose eye drops in the six months prior to BC was associated with a decrease in instantaneous risk of 22% for OS and 23% for DFS. Notably, a significant part of the protective association was mediated by a change in BC subtype and nodal status at BC diagnosis. Alverine use prior to BC diagnosis was also associated with an improved prognosis in our results (HR OS: 0.78; HR DFS: 0.86). Further investigation of these novel observations is warranted.
Eight medications were negatively associated with survival. Preclinical study suggested a role for ferroportin and iron regulation in BC progression and prognosis55,56. Here, we found that ferrous fumarate was associated with poorer survival (HR OS: 1.74; HR DFS: 1.39). However, we cannot exclude the possibility of a residual confounding bias due to an underreporting of anemia and low ferritinemia in reimbursement claims. Similarly, we found that two antidepressants, hydroxyzine and mianserin, were associated with decreased survival, but we cannot exclude that these associations could be explained by residual confounding bias due to depression, which has been shown to increase both all-cause and specific mortality in patients with BC57. The 42% decrease in instantaneous risk for DFS suggested for carbimazole could also be explained by insufficient adjustment for hyperthyroidism, a condition that may be associated with poorer prognosis, possibly through higher baseline mammographic density58,59.
Pristinamycin, an antibiotic used primarily against staphylococcal and streptococcal infections, was also associated with higher mortality. The negative impact of antibiotics on oncological outcomes – particularly in patients treated with immunotherapy – is attracting growing interest60, but there is currently no observational data for BC. Our study revealed that prednisolone was associated with a 78% decrease in instantaneous risk for OS and a 58% for DFS, in line with several preclinical studies suggesting that glucocorticoids may promote BC progression and metastasis61–63, and with an epidemiologic study reporting that the use of glucocorticoids was associated with a decreased risk of stage I-II BCs but an increased risk of stage III-IV BCs64. Given the intensive use of glucocorticoids as adjunctive therapy during chemotherapy, we believe that further research on this topic is urgently needed.
Finally, oxazepam and alprazolam were found to be deleterious, whereas other benzodiazepines had no effect (prazepam HR OS: 1.00, 95% CI: 0.86 to 1.17) or a protective effect (bromazepam, HR OS: 0.89, 95% CI: 0.80 to 0.98), suggesting that medications from the same therapeutic class may differentially modify the course of BC. Consistent with our findings, differential effects of benzodiazepines on cancer outcomes have been observed in observational studies of BC risk65 or pancreatic cancer survival66, possibly reflecting differences in pharmacological properties and potential interactions with cancer-related pathways66–68.
We provide here a unique resource, uniting on the same interactive platform an extensive overview of the causal impact of non-oncological medications on a very large, exhaustive cohort of patients with BC. We applied a stringent methodology to minimize confounding bias, and identified sixteen medications affecting relapse or mortality. Our study has several limitations. First, given the very conservative strategy used to correct for multiple testing, we cannot exclude the possibility that several other medications tested had a genuine effect on survival. Second, while most chronic medications are prescription-only drugs, the observed associations for certain medications (e.g. paracetamol or vitamins) may be subject to potential mismeasurement bias due to the lack of over-the-counter purchase data in the SNDS. Third, while the inclusion of comorbid conditions recorded up to 6 months after BC diagnosis allowed us to include conditions noted solely during BC hospitalizations, we could not rule out mismeasurement bias due to the inclusion of comorbid conditions triggered by BC diagnosis and treatment, such as depressive symptoms or postoperative acute phlebitis. However, in the two sensitivity analyzes we performed, our results were not affected by the timeframe chosen for the identification of comorbid conditions in hospital discharge reports, suggesting robustness of our study with respect to such measurement bias. Fourth, we could not overcome indication bias for some molecules (e.g. insulin), which were removed from the analyzes due to insufficient adjustment quality. Fifth, despite our high-dimensional adjustment and conservative quality check strategy, we cannot exclude the presence of unmeasured confounding bias for some molecules, which may weaken the causal interpretation of the results. These include lifestyle and behavioral factors such as diet, body mass index, smoking, alcohol consumption, or physical activity. While the presence of proxy indicators in our adjustment set (e.g., severe obesity, diabetes, hypertension, severe tobacco and alcohol dependence, deprivation index) may mitigate this limitation, we acknowledge the importance of direct measures of these variables in future research. Sixth, due to our limited follow-up period, with a median of four and a half years, our results relate predominantly to early deaths and recurrences. Finally, while our study provides foundational insights into the association between pre-diagnosis medication use and BC outcomes, we recognize that the absence of dose-response analysis limits the granularity of our findings. Future investigations are needed to determine the potential dose-dependent effects of pre-diagnosis medications on disease progression and patient survival.
Because our primary objective was to examine the effect of medication use prior to BC diagnosis in a population of patients with BC, our results do not reflect the effect of medication on BC risk, nor do they reflect the effect of medication use after cancer diagnosis or in a non-cancer population. Similarly, while we estimated the magnitude of the observed effect attributable to a change in BC subtype or lymph node involvement at diagnosis, we did not perform such mediation analysis for other BC biological characteristics, such as tumor stage, nor for post-diagnosis drug use, whose mediation portion remained included in the direct path-specific effect.
This work opens up new perspectives. From a research and development standpoint, academic or industrial researchers investigating molecules or pathways could confirm their hypotheses on human epidemiologic data and evaluate the magnitude of the effect on real-world evidence with this platform. This resource can also provide new hypotheses for drugs that may naturally influence BC evolution, from its presentation at diagnosis (subtype, lymph node involvement) to its long-term prognosis (overall survival, disease-free survival). The prospect of improving BC prognosis with affordable medications is particularly appealing, in a context in which the costs of innovative oncologic therapies could jeopardize healthcare systems. Hence, research to evaluate the effect of the drugs identified in this study after cancer diagnosis, including dose-response analyzes, is urgently needed.
Methods
Ethics and data protection
This study was conducted in the framework of a partnership between Institut Curie and INCa and was performed in accordance with institutional and ethical rules concerning research based on data from patients. The study was authorized by the French data protection agency (Commission nationale de l’informatique et des libertés—CNIL, under registration number 920017). In accordance with French regulations applicable to the SNDS, no informed consent was required.
Data source and study population
We conducted a nationwide retrospective study with the published FRESH (French Early Breast Cancer Cohort) cohort23. The data released from the SNDS database available at the French National Cancer Institute (INCa)20,69 which included (i) demographic data, (ii) hospital discharge reports, (iii) outpatient care, and (iv) long-term illness (LTI) records. The FRESH cohort includes all women with non-metastatic BC newly diagnosed between January 1, 2011, and December 31, 2017, identified by a tag with a diagnosis code for BC in long-term illness records; or in at least one hospital discharge report within the period considered. The cohort excludes: (1) patients under the age of 18 years at inclusion (2) patients not affiliated to the principal national health insurance coverage plan (“Régime Général”), (3) patients not undergoing breast surgery in the year preceding or following inclusion, (4) patients with a concomitant cancer at another site, (5) patients with evidence of prior BC at diagnosis, (6) patients with distant metastases at BC diagnosis, and (7) patients with missing or inconsistent data (Supplementary Fig. 9). The date of BC diagnosis was taken as the date of either the earliest breast core biopsy in the year before the first breast surgery, or the earliest fine-needle aspiration cytology, or the earliest breast imaging procedure or the date of the first BC treatment. Details about the available data are provided in the Supplementary Methods.
Concomitant medication
Concomitant medications were identified from outpatient drug delivery data the six months preceding BC diagnosis. Medications were classified according to the World Health Organization ATC (Anatomical Therapeutic Chemical) classification. We excluded diagnostic agents, medications used for cancer treatment and medications with no systemically active molecule (Supplementary Table 2). For medications based on combinations of molecules (e.g. beta-blocker and diuretics), we considered the individual components separately, with specific splitting rules applied for sex hormones (Supplementary Fig. 10). Chronic exposure to concomitant medication was coded as: (i) “yes” if the patient had received at least three months of the full dose in the six months preceding BC diagnosis; (ii) “no” otherwise. The decision rules for defining three months of full-dose treatment depended on the presentation and dose schedule of the medication, as described in the Supplementary Methods and Supplementary Table 3. In our analyzes, we focused specifically on chronic exposures to concomitant medication. We restricted the analysis to medications taken by at least 300 patients, to ensure sufficient statistical power. The reference group for comparison included both patients who were never exposed to the medication and patients who had non-chronic exposure to this medication.
Comorbid conditions
In total, we identified 52 comorbid conditions, belonging to 12 categories (Supplementary Data 4), as proposed in previous studies25–34. The presence of a disease at BC diagnosis was detected on the basis of procedure codes in the year before BC diagnosis up to BC diagnosis, and of diagnosis codes in the year before BC diagnosis up to 180 days after BC diagnosis. We used diagnosis codes up to 180 days after diagnosis to include the comorbid conditions noted by the surgeon at the time of first surgery for BC. As sensitivity analyzes, we tested two additional timeframes for diagnosis codes: (i) the year prior to BC diagnosis up to BC surgery, and (ii) the one-year period prior to BC surgery. Further details are provided in the Supplementary Methods.
Other covariates
Other covariates were split into (i) pre-exposure and (ii) post-exposure covariates. Pre-exposure covariates included: (1) age at BC diagnosis, (2) the deprivation index of the area of residence70, (3) the number of general practitioner (GP) visits in the year preceding BC diagnosis, (4) the number of visits to a gynecologist in the year preceding BC diagnosis, (5) the performance of a mammographic screening in the year preceding BC diagnosis, (6) the total number of medications (molecules) to which the patient was chronically exposed to the six months preceding BC diagnosis, and (7) concomitant exposure to other medications. Post-exposure covariates included: (1) BC subtype, (2) nodal status, (3) chemotherapy status, and (4) endocrine therapy status. Further details are provided in the Supplementary Methods.
Outcomes
The primary endpoint was overall survival (OS). Disease-free survival (DFS) was evaluated as a secondary endpoint. OS was defined as the time, in months, from the first BC surgery to death or to March 1, 2019, whichever occurred first. Vital status and date of deaths were directly available in the SNDS data. DFS was defined as the time, in months, from the first BC surgery to death, loco-regional recurrence, contralateral recurrence, distant recurrence, or 30th of December 2018, whichever occurred first. Of note, we did not include the second cancer of another site (non-breast) in the definition. The occurrence of any of loco-regional recurrence, distant recurrence, or contralateral recurrence, was identified based on (i) the resumption of radiotherapy, chemotherapy, or targeted therapy at least 6 months after the end of the initial treatments, (ii) a breast surgery procedure with axillar procedure performed at least 6 months after the end of the initial treatments, (iii) the intake of an anti-cancer molecule approved only in the metastatic setting starting at least six months after initial breast surgery, or (iv) the presence of a diagnosis code of metastasis in hospitalization stays starting at least six months after initial breast surgery (Supplementary Data 5). Breast surgery was tagged with hospital procedure codes for mastectomy and partial mastectomy.
Causal inference pipeline
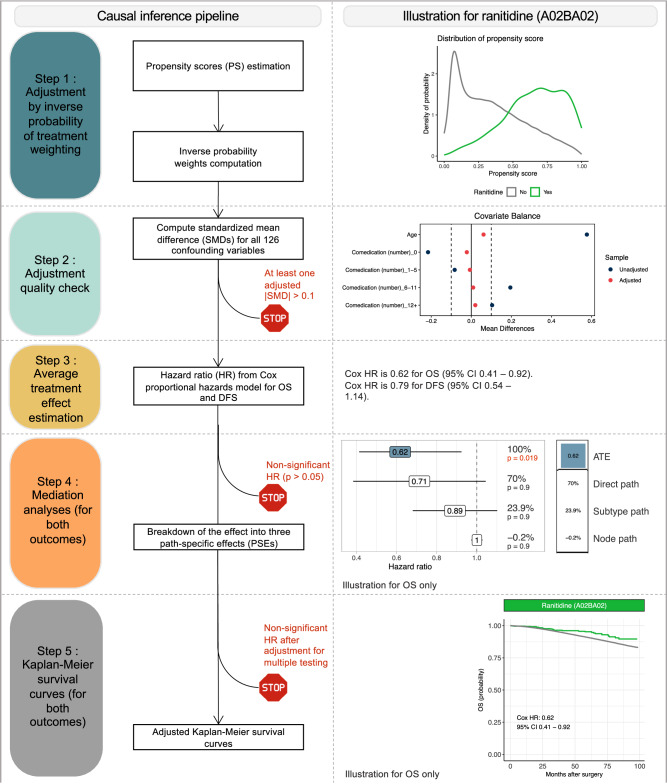
A directed acyclic graph representing the expected causal links between variables was built in accordance with expert knowledge (Supplementary Fig. 11)71, and was used to identify the pre-exposure covariates that needed to be adjusted on in the analyzes. Our goal was to estimate the average effect of each medication in the entire population (average treatment effect, ATE)71. The causal inference pipeline (Fig. 9) was run for each medication, one at a time. It could be broken down into five steps. Further details on the methods used are provided in the Supplementary Methods.
Fig. 9. Causal inference pipeline of the study (left) and illustration for one medication (ranitidine, ATC code A02BA02, right).
Details are provided in the Methods. Abbreviations: PS propensity score, IPTW inverse probability of treatment weighting, SMD standardized mean difference, ATE average treatment effect, HR hazard ratio, ATC Anatomical Therapeutic Chemical, CT chemotherapy, TNBC triple-negative breast cancer, OS overall survival, DFS disease-free survival.
Step 1: Adjustment by inverse probability of treatment weighting (IPTW)
We used inverse probability of treatment weighting (IPTW) to adjust for the confounding bias induced by pre-exposure covariates identified as confounding factors in the DAG analysis. This procedure involved: (i) estimating propensity scores (PS) i.e. the probability of receiving the drug concerned given the value of the pre-exposure covariates for each patient; (ii) weighting the dataset by assigning each patient a stabilized inverse probability weight derived from the PS. All subsequent analyzes were conducted on the weighted dataset.
Step 2: Adjustment quality check
We checked the adjustment quality a posteriori by calculating the standardized mean differences (SMDs) for each pre-exposure covariate after adjustment. In accordance with published results72, the adjustment quality was considered insufficient if any SMD had an absolute value above 0.1, in which case the molecule was discarded from subsequent analyzes.
Step 3: Average treatment effect estimation
We estimated the average treatment effect (ATE) by calculating the hazard ratio (HR) of a univariate Cox proportional hazards model fitted to the weighted population73. We used Wald tests calculated with robust covariances to draw statistical inferences about the estimated HR. The threshold for statistical significance was p = 0.05.
Step 4: Mediation analyzes
Molecules with a significant ATE were selected for mediation analyzes, which involved breaking down the ATE into several pathways passing through two potential mediators, BC subtype and nodal status (Supplementary Fig. 12). We assumed BC subtype and nodal status to be causally related. Standard direct and indirect effects were not, therefore, directly identifiable for each mediator74. It was, nevertheless, possible to break the ATE down into three path-specific effects (PSEs): (1) the effect through pathways involving neither a difference in BC subtype nor in nodal status (direct effects); (2) the effect through pathways involving a difference in nodal status only (effect through node); (3) the effect through pathways involving a difference in BC subtype (and potentially involving a difference in nodal status; effect through subtype). PSEs were estimated by a weighting approach74 and are expressed as percentages of the total effect (which may be negative).
Step 5: Kaplan–Meier survival curves
Weighted Kaplan-Meier survival curves were plotted for the molecules with a significant ATE after the Benjamini-Hochberg (BH) multiple testing procedure, and compared with an adjusted log-rank test75. The threshold for statistical significance was set at p = 0.1, due to the low power of adjusted log-rank tests76.
Web application and software
All the results are available via an interactive web application (https://adrenaline.curie.fr), also including: (i) a comprehensive descriptive overview of the database; (ii) the results of the causal inference pipeline for medication classes (ATC levels 2, 3, and 5), and for medications failing the adjustment quality test; and (iii) subgroup analyzes by BC subtype, nodal status, age, chemotherapy status, and endocrine therapy status. Analyzes were performed with R software, version 3.6.3 (see Supplementary Methods for details). All hypothesis tests were two-tailed.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Supplementary information
Description of additional supplementary files
Source data
Acknowledgements
We thank the Department of Health Data and Assessment, Health Survey Data Science and Assessment Division, French National Cancer Institute (Institut National du Cancer INCa) for providing us with access to the cancer cohort. E.D. received a PhD grant from the Ministère de l’Enseignement Supérieur et de la Recherche et de l’Innovation, allocated to École polytechnique (AMX). This study was also funded by Monoprix* (A.S.H. and F.R.) and INCa grant number 18–127 within the COMBIMMUNO (Comedications and comorbidities in breast cancer: Deciphering Interactions Between Immune Infiltration, Response to Treatment and Prognosis) project (A.S.H. and F.R.). This work was supported by an Investissement d’Avenir grant from Agence Nationale de la Recherche, reference ANR-19-P3IA-0001 (PRAIRIE 3IA Institute) (C.A.A.). The funder was not involved in study design, or in the collection, analysis, and interpretation of data, the writing of this article or the decision to submit it for publication.
Author contributions
A.S.H. and F.R. conceived the project and designed the study. E.D. carried out the statistical analysis and prepared the figures, tables, and interactive online platform. B.M., A.B., E.D.N., T.D. and S.A. participated in discussions on analysis design and refinement. C.A.A., J.A., B.A. and A.L. revised the statistical analysis and the causal framework. S.H., C.L.B.B. and P.J.B. ensured data availability and quality and participated in the design of data preparation. B.G.R., P.G., F.J., M.E., E.L., F.C., J.Y.P. and J.H. provided medical background knowledge for data identification and analysis. The manuscript was drafted by E.D., A.S.H. and C.B. and edited and approved by all authors.
Peer review
Peer review information
Nature Communications thanks Aditya Bardia, and the other, anonymous, reviewers for their contribution to the peer review of this work. A peer review file is available.
Data availability
The raw SNDS data are protected and are not available due to data privacy laws. The processed aggregated data generated in this study are provided in the Supplementary Information/Source Data file. Source data for the Figures are provided with this paper. Source data are provided with this paper.
Code availability
Code is available online (10.5281/zenodo.10777521)77.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
The online version contains supplementary material available at 10.1038/s41467-024-47002-3.
References
- 1.Cho H, Mariotto AB, Mann BS, Klabunde CN, Feuer EJ. Assessing non-cancer-related health status of US cancer patients: other-cause survival and comorbidity prevalence. Am. J. Epidemiol. 2013;178:339–349. doi: 10.1093/aje/kws580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kimmick G, et al. Comorbidity burden and guideline-concordant care for breast cancer. J. Am. Geriatr. Soc. 2014;62:482–488. doi: 10.1111/jgs.12687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Patnaik JL, Byers T, DiGuiseppi C, Denberg TD, Dabelea D. The influence of comorbidities on overall survival among older women diagnosed with breast cancer. J. Natl Cancer Inst. 2011;103:1101–1111. doi: 10.1093/jnci/djr188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Aye PS, et al. Use of non-cancer medications in new zealand women at the diagnosis of primary invasive breast cancer: prevalence, associated factors and effects on survival. Int J. Environ. Res Public Health. 2020;17:E7962. doi: 10.3390/ijerph17217962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Calip GS, et al. Polypharmacy and adherence to adjuvant endocrine therapy for breast cancer. JOP. 2017;13:e451–e462. doi: 10.1200/JOP.2016.018317. [DOI] [PubMed] [Google Scholar]
- 6.Parés-Badell O, Banqué M, Macià F, Castells X, Sala M. Impact of comorbidity on survival by tumour location: Breast, colorectal and lung cancer (2000-2014) Cancer Epidemiol. 2017;49:66–74. doi: 10.1016/j.canep.2017.05.010. [DOI] [PubMed] [Google Scholar]
- 7.Houterman S, et al. Comorbidity has negligible impact on treatment and complications but influences survival in breast cancer patients. Br. J. Cancer. 2004;90:2332–2337. doi: 10.1038/sj.bjc.6601844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wu Y, et al. Discovery of noncancer drug effects on survival in electronic health records of patients with cancer: a new paradigm for drug repurposing. JCO Clin. Cancer Inf. 2019;3:1–9. doi: 10.1200/CCI.19.00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhao Y, et al. Association between NSAIDs use and breast cancer risk: a systematic review and meta-analysis. Breast Cancer Res. Treat. 2009;117:141–150. doi: 10.1007/s10549-008-0228-6. [DOI] [PubMed] [Google Scholar]
- 10.Ahern TP, et al. Statin prescriptions and breast cancer recurrence risk: a Danish nationwide prospective cohort study. J. Natl Cancer Inst. 2011;103:1461–1468. doi: 10.1093/jnci/djr291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Haukka, J., Niskanen, L. & Auvinen, A. Risk of cause-specific death in individuals with cancer-modifying role diabetes, statins and metformin. Int. J. Cancer10.1002/ijc.31016 (2017). [DOI] [PubMed]
- 12.Barron TI, Connolly RM, Sharp L, Bennett K, Visvanathan K. Beta blockers and breast cancer mortality: a population- based study. J. Clin. Oncol. 2011;29:2635–2644. doi: 10.1200/JCO.2010.33.5422. [DOI] [PubMed] [Google Scholar]
- 13.Powe DG, Entschladen F. Using β-blockers to inhibit breast cancer progression: Targeted therapies. Nat. Rev. Clin. Oncol. 2011;8:511–512. doi: 10.1038/nrclinonc.2011.123. [DOI] [PubMed] [Google Scholar]
- 14.Collins JA, Blake JM, Crosignani PG. Breast cancer risk with postmenopausal hormonal treatment. Hum. Reprod. Update. 2005;11:545–560. doi: 10.1093/humupd/dmi028. [DOI] [PubMed] [Google Scholar]
- 15.Støer NC, et al. Drug use and cancer risk: a drug-wide association study (DWAS) in Norway. Cancer Epidemiol. Biomark. Prev. 2021;30:682–689. doi: 10.1158/1055-9965.EPI-20-1028. [DOI] [PubMed] [Google Scholar]
- 16.Baker AF, Dorr RT. Drug interactions with the taxanes: clinical implications. Cancer Treat. Rev. 2001;27:221–233. doi: 10.1053/ctrv.2001.0228. [DOI] [PubMed] [Google Scholar]
- 17.Hamy A-S, et al. Celecoxib with neoadjuvant chemotherapy for breast cancer might worsen outcomes differentially by cox-2 expression and er status: exploratory analysis of the REMAGUS02 trial. JCO. 2019;37:624–635. doi: 10.1200/JCO.18.00636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tyler T. Drug interactions in metastatic breast cancer. J. Oncol. Pharm. Pr. 2011;17:236–245. doi: 10.1177/1078155210379167. [DOI] [PubMed] [Google Scholar]
- 19.Bibi R, et al. Prevalence of potential drug-drug interactions in breast cancer patients and determination of their risk factors. J. Oncol. Pharm. Pr. 2021;27:1616–1622. doi: 10.1177/1078155220963212. [DOI] [PubMed] [Google Scholar]
- 20.Tuppin P, et al. Value of a national administrative database to guide public decisions: From the système national d’information interrégimes de l’Assurance Maladie (SNIIRAM) to the système national des données de santé (SNDS) in France. Rev. Epidemiol. Sante Publique. 2017;65:S149–S167. doi: 10.1016/j.respe.2017.05.004. [DOI] [PubMed] [Google Scholar]
- 21.Bezin J, et al. The national healthcare system claims databases in France, SNIIRAM and EGB: Powerful tools for pharmacoepidemiology. Pharmacoepidemiol Drug Saf. 2017;26:954–962. doi: 10.1002/pds.4233. [DOI] [PubMed] [Google Scholar]
- 22.Moulis G, et al. French health insurance databases: what interest for medical research? La Revue de Mé. decine Interne. 2015;36:411–417. doi: 10.1016/j.revmed.2014.11.009. [DOI] [PubMed] [Google Scholar]
- 23.Dumas E, et al. The french early breast cancer cohort (fresh): a resource for breast cancer research and evaluations of oncology practices based on the french national healthcare system database (SNDS) Cancers (Basel) 2022;14:2671. doi: 10.3390/cancers14112671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Geraci JM, Escalante CP, Freeman JL, Goodwin JS. Comorbid disease and cancer: the need for more relevant conceptual models in health services research. JCO. 2005;23:7399–7404. doi: 10.1200/JCO.2004.00.9753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bannay A, et al. The best use of the charlson comorbidity index with electronic health care database to predict mortality. Med Care. 2016;54:188–194. doi: 10.1097/MLR.0000000000000471. [DOI] [PubMed] [Google Scholar]
- 26.Billioti de Gage S, et al. Antidepressants and hepatotoxicity: a cohort study among 5 million individuals registered in the french national health insurance database. CNS Drugs. 2018;32:673–684. doi: 10.1007/s40263-018-0537-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bouyer, B., Rudnichi, A., Dray-Spira, R., Zureik, M. & Coste, J. Thromboembolic risk after lumbar spine surgery: a cohort study on 325 000 French patients. J. Thromb. Haemost.10.1111/jth.14205 (2018). [DOI] [PubMed]
- 28.Coste J, et al. Risk of early neurodevelopmental disorders associated with in utero exposure to valproate and other antiepileptic drugs: a nationwide cohort study in France. Sci. Rep. 2020;10:17362. doi: 10.1038/s41598-020-74409-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Giral P, Neumann A, Weill A, Coste J. Cardiovascular effect of discontinuing statins for primary prevention at the age of 75 years: a nationwide population-based cohort study in France. Eur. Heart J. 2019;40:3516–3525. doi: 10.1093/eurheartj/ehz458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jabagi MJ. Risk of secondary hematologic malignancies associated with breast cancer chemotherapy and G-CSF support: A nationwide population-based cohort. Int. J. Cancer. 2020;148:375–384. doi: 10.1002/ijc.33216. [DOI] [PubMed] [Google Scholar]
- 31.Lemaitre M, et al. Association between use of thiopurines or tumor necrosis factor antagonists alone or in combination and risk of lymphoma in patients with inflammatory bowel disease. JAMA. 2017;318:1679–1686. doi: 10.1001/jama.2017.16071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Maura G, et al. Oral anticoagulants and risk of acute liver injury in patients with nonvalvular atrial fibrillation: a propensity-weighted nationwide cohort study. Sci. Rep. 2020;10:11624. doi: 10.1038/s41598-020-68304-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sbidian E, Mezzarobba M, Weill A, Coste J, Rudant J. Persistence of treatment with biologics for patients with psoriasis: a real-world analysis of 16 545 biologic-naïve patients from the French National Health Insurance database (SNIIRAM) Br. J. Dermatol. 2019;180:86–93. doi: 10.1111/bjd.16809. [DOI] [PubMed] [Google Scholar]
- 34.Semenzato, L. et al. Maladies chroniques, états de santé et risque d’hospitalisation et de décès hospitalier pour COVID-19 lors de la première vague de l’épidémie en France: Étude de cohorte de 66 millions de personnes. 41, https://www.epi-phare.fr/app/uploads/2021/02/epiphare_risques_hospitalisations_deces_covid19_20210209.pdf (2021).
- 35.Ng HS, Vitry A, Koczwara B, Roder D, McBride ML. Patterns of comorbidities in women with breast cancer: a Canadian population-based study. Cancer Causes Control. 2019;30:931–941. doi: 10.1007/s10552-019-01203-0. [DOI] [PubMed] [Google Scholar]
- 36.Wang B-Y, et al. Intermittent high dose proton pump inhibitor enhances the antitumor effects of chemotherapy in metastatic breast cancer. J. Exp. Clin. Cancer Res. 2015;34:109. doi: 10.1186/s13046-015-0220-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hamy A-S, et al. Comedications influence immune infiltration and pathological response to neoadjuvant chemotherapy in breast cancer. OncoImmunology. 2019;9:1677427. doi: 10.1080/2162402X.2019.1677427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fais S. Evidence-based support for the use of proton pump inhibitors in cancer therapy. J. Transl. Med. 2015;13:368. doi: 10.1186/s12967-015-0735-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lu, Z.-N., Tian, B. & Guo, X.-L. Repositioning of proton pump inhibitors in cancer therapy. Cancer Chemother. Pharmacol. 1–13 10.1007/s00280-017-3426-2 (2017). [DOI] [PubMed]
- 40.Spugnini E, Fais S. Proton pump inhibition and cancer therapeutics: a specific tumor targeting or it is a phenomenon secondary to a systemic buffering? Semin. Cancer Biol. 2017;43:111–118. doi: 10.1016/j.semcancer.2017.01.003. [DOI] [PubMed] [Google Scholar]
- 41.Cole SW, Sood AK. Molecular pathways: beta-adrenergic signaling in cancer. Clin. Cancer Res. 2012;18:1201–1206. doi: 10.1158/1078-0432.CCR-11-0641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Spera G, et al. Beta blockers and improved progression-free survival in patients with advanced HER2 negative breast cancer: a retrospective analysis of the ROSE/TRIO-012 study. Ann. Oncol. 2017;28:1836–1841. doi: 10.1093/annonc/mdx264. [DOI] [PubMed] [Google Scholar]
- 43.Childers WK, Hollenbeak CS, Cheriyath P. β-Blockers reduce breast cancer recurrence and breast cancer death: a meta-analysis. Clin. Breast Cancer. 2015;15:426–431. doi: 10.1016/j.clbc.2015.07.001. [DOI] [PubMed] [Google Scholar]
- 44.Pantziarka P, Bryan BA, Crispino S, Dickerson EB. Propranolol and breast cancer—a work in progress. ecancermedicalscience. 2018;12:ed82. doi: 10.3332/ecancer.2018.ed82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Demierre M-F, Higgins PDR, Gruber SB, Hawk E, Lippman SM. Statins and cancer prevention. Nat. Rev. Cancer. 2005;5:930–942. doi: 10.1038/nrc1751. [DOI] [PubMed] [Google Scholar]
- 46.Harborg S, et al. Statin use and breast cancer recurrence in postmenopausal women treated with adjuvant aromatase inhibitors: a Danish population-based cohort study. Breast Cancer Res Treat. 2020;183:153–160. doi: 10.1007/s10549-020-05749-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Borgquist S, Broberg P, Tojjar J, Olsson H. Statin use and breast cancer survival – a Swedish nationwide study. BMC Cancer. 2019;19:54. doi: 10.1186/s12885-018-5263-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Collaborative Group on Hormonal Factors in Breast Cancer. Type and timing of menopausal hormone therapy and breast cancer risk: individual participant meta-analysis of the worldwide epidemiological evidence. Lancet394, 1159–1168 (2019). [DOI] [PMC free article] [PubMed]
- 49.Chlebowski RT, et al. Association of menopausal hormone therapy with breast cancer incidence and mortality during long-term follow-up of the women’s health initiative randomized clinical trials. JAMA. 2020;324:369–380. doi: 10.1001/jama.2020.9482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Chlebowski RT, et al. Breast cancer after use of estrogen plus progestin and estrogen alone: analyses of data from 2 women’s health initiative randomized clinical trials. JAMA Oncol. 2015;1:296–305. doi: 10.1001/jamaoncol.2015.0494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kenemans P, et al. Safety and efficacy of tibolone in breast-cancer patients with vasomotor symptoms: a double-blind, randomised, non-inferiority trial. Lancet Oncol. 2009;10:135–146. doi: 10.1016/S1470-2045(08)70341-3. [DOI] [PubMed] [Google Scholar]
- 52.Durna EM, et al. Hormone replacement therapy after a diagnosis of breast cancer: cancer recurrence and mortality. Med. J. Aust. 2002;177:347–351. doi: 10.5694/j.1326-5377.2002.tb04835.x. [DOI] [PubMed] [Google Scholar]
- 53.McVicker, L. et al. Vaginal estrogen therapy use and survival in females with breast cancer. JAMA Oncol e234508 10.1001/jamaoncol.2023.4508 (2023). [DOI] [PMC free article] [PubMed]
- 54.Cold, S. et al. Systemic or vaginal hormone therapy after early breast cancer: a danish observational cohort study. JNCI: Journal of the National Cancer Institute djac112 (2022) 10.1093/jnci/djac112. [DOI] [PMC free article] [PubMed]
- 55.Huang X. Does iron have a role in breast cancer? Lancet Oncol. 2008;9:803–807. doi: 10.1016/S1470-2045(08)70200-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Pinnix ZK, et al. Ferroportin and iron regulation in breast cancer progression and prognosis. Sci. Transl. Med. 2010;2:43ra56. doi: 10.1126/scitranslmed.3001127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Nadalin V, Cotterchio M, Kreiger N. Antihistamine use and breast cancer risk. Int. J. Cancer. 2003;106:566–568. doi: 10.1002/ijc.11240. [DOI] [PubMed] [Google Scholar]
- 58.Kanbayti IH, Rae WID, McEntee MF, Ekpo EU. Are mammographic density phenotypes associated with breast cancer treatment response and clinical outcomes? a systematic review and meta-analysis. Breast. 2019;47:62–76. doi: 10.1016/j.breast.2019.07.002. [DOI] [PubMed] [Google Scholar]
- 59.Yang H, et al. Hyperthyroidism is associated with breast cancer risk and mammographic and genetic risk predictors. BMC Med. 2020;18:225. doi: 10.1186/s12916-020-01690-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Elkrief A, Derosa L, Kroemer G, Zitvogel L, Routy B. The negative impact of antibiotics on outcomes in cancer patients treated with immunotherapy: a new independent prognostic factor? Ann. Oncol. 2019;30:1572–1579. doi: 10.1093/annonc/mdz206. [DOI] [PubMed] [Google Scholar]
- 61.Crozier M, et al. Frequently used antiemetic agent dexamethasone enhances the metastatic behaviour of select breast cancer cells. PLOS ONE. 2022;17:e0274675. doi: 10.1371/journal.pone.0274675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Zhidkova EM, et al. A brief overview of the paradoxical role of glucocorticoids in breast cancer. Breast Cancer (Auckl.) 2020;14:1178223420974667. doi: 10.1177/1178223420974667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Mitre-Aguilar IB, et al. The role of glucocorticoids in breast cancer therapy. Curr. Oncol. 2022;30:298–314. doi: 10.3390/curroncol30010024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Cairat M, et al. Use of systemic glucocorticoids and risk of breast cancer in a prospective cohort of postmenopausal women. BMC Med. 2021;19:186. doi: 10.1186/s12916-021-02004-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Iqbal U, et al. Is Long-term use of benzodiazepine a risk for cancer? Med. (Baltim.) 2015;94:e483. doi: 10.1097/MD.0000000000000483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Cornwell AC, et al. Lorazepam stimulates il6 production and is associated with poor survival outcomes in pancreatic cancer. Clin. Cancer Res. 2023;29:3793–3812. doi: 10.1158/1078-0432.CCR-23-0547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Huang X-P, et al. Allosteric ligands for the pharmacologically dark receptors GPR68 and GPR65. Nature. 2015;527:477–483. doi: 10.1038/nature15699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Tanabe K, Kozawa O, Iida H. Midazolam suppresses interleukin-1β-induced interleukin-6 release from rat glial cells. J. Neuroinflammation. 2011;8:68. doi: 10.1186/1742-2094-8-68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Bousquet PJ, et al. Cancer care and public health policy evaluations in France: Usefulness of the national cancer cohort. PLoS One. 2018;13:e0206448. doi: 10.1371/journal.pone.0206448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Rey G, Jougla E, Fouillet A, Hémon D. Ecological association between a deprivation index and mortality in France over the period 1997 − 2001: variations with spatial scale, degree of urbanicity, age, gender and cause of death. BMC Public Health. 2009;9:33. doi: 10.1186/1471-2458-9-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Hernán, M. A. & Robins, J. M. Causal Inference: What If. (Boca Raton: Chapman & Hall/CRC., 2020).
- 72.Austin PC. An Introduction to Propensity Score Methods for Reducing the Effects of Confounding in Observational Studies. Multivar. Behav. Res. 2011;46:399–424. doi: 10.1080/00273171.2011.568786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Cole SR, Hernán MA. Adjusted survival curves with inverse probability weights. Comput Methods Prog. Biomed. 2004;75:45–49. doi: 10.1016/j.cmpb.2003.10.004. [DOI] [PubMed] [Google Scholar]
- 74.VanderWeele TJ, Vansteelandt S, Robins JM. Effect decomposition in the presence of an exposure-induced mediator-outcome confounder. Epidemiology. 2014;25:300–306. doi: 10.1097/EDE.0000000000000034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Chatton A, et al. G-computation, propensity score-based methods, and targeted maximum likelihood estimator for causal inference with different covariates sets: a comparative simulation study. Sci. Rep. 2020;10:9219. doi: 10.1038/s41598-020-65917-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Le Borgne F, Giraudeau B, Querard AH, Giral M, Foucher Y. Comparisons of the performance of different statistical tests for time-to-event analysis with confounding factors: practical illustrations in kidney transplantation. Stat. Med. 2016;35:1103–1116. doi: 10.1002/sim.6777. [DOI] [PubMed] [Google Scholar]
- 77.Dumas, E. Concomitant medication, comorbidity and survival in patients with breast cancer - rt2lab/adrenaline: ADRENALINE - analysis code. Zenodo 10.5281/zenodo.10777521 (2024). [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Description of additional supplementary files
Data Availability Statement
The raw SNDS data are protected and are not available due to data privacy laws. The processed aggregated data generated in this study are provided in the Supplementary Information/Source Data file. Source data for the Figures are provided with this paper. Source data are provided with this paper.
Code is available online (10.5281/zenodo.10777521)77.