Abstract
Background
Little is known about adjuvant therapy (AT) omission and use outside of randomized trials. We aimed to assess the patterns of AT omission and use in a cohort of upfront resected pancreatic cancer patients in a real-life scenario.
Methods
From January 2019 to July 2022, 317 patients with resected pancreatic cancer and operated upfront were prospectively enrolled in this prospective observational trial according to the previously calculated sample size. The association between perioperative variables and the risk of AT omission and AT delay was analyzed using multivariable logistic regression.
Results
Eighty patients (25.2%) did not receive AT. The main reasons for AT omission were postoperative complications (38.8%), oncologist’s choice (21.2%), baseline comorbidities (20%), patient’s choice (10%), and early recurrence (10%). At the multivariable analysis, the odds of not receiving AT increased significantly for older patients (odds ratio [OR] 1.1, p < 0.001), those having an American Society of Anesthesiologists score ≥II (OR 2.03, p = 0.015), or developing postoperative pancreatic fistula (OR 2.5, p = 0.019). The likelihood of not receiving FOLFIRINOX as AT increased for older patients (OR 1.1, p < 0.001), in the presence of early-stage disease (stage I–IIa vs. IIb–III, OR 2.82, p =0.031; N0 vs. N+, OR 3, p = 0.03), and for patients who experienced postoperative major complications (OR 4.7, p = 0.009). A twofold increased likelihood of delay in AT was found in patients experiencing postoperative complications (OR 3.86, p = 0.011).
Conclusions
AT is not delivered in about one-quarter of upfront resected pancreatic cancer patients. Age, comorbidities, and postoperative complications are the main drivers of AT omission and mFOLFIRINOX non-use.
ClinicalTrials registration: NCT03788382.
Supplementary Information
The online version contains supplementary material available at 10.1245/s10434-024-14951-4.
Keywords: Pancreatic cancer, Adjuvant therapy, Pancreatic surgery, Failure to rescue, Postoperative complications
Pancreatic cancer (PC) is a world-leading cause of cancer-related death, with an overall 5-year relative survival rate of 12% and an increased annual incidence of 1%.1 In the last two decades, robust evidence has demonstrated that adjuvant therapy (AT) leads to improved survival.2,3 The 5-year update of the PRODIGE-24 randomized trial recently corroborated the outstanding long-term results of adjuvant mFOLFIRINOX,4,5 which has become the standard of care following upfront pancreatectomy since 2019; however, little is known about the applicability of such a therapeutic combination outside controlled trials. Given the high rate of complications associated with pancreatic resections6 and the toxicity profile of mFOLFIRINOX, it can be postulated that only patients with outstanding performance status constituted the study population of the PRODIGE-24 trial, thus introducing a significant selection bias in the study results.
Several reports have thus far shown that the AT omission rate ranges from 30 to 50%,6–8 and in these cases, the chance of long-term survival is reduced. For example, almost a decade ago, Merkow et al. reported the magnitude and detailed risk factors for AT omission, demonstrating that even non-life-threatening complications (e.g., pneumonia, urinary tract infection, or surgical site infection) may contribute to the AT omission that occurred in 56.4% of patients experiencing a complicated postoperative course.9 Notably, that study was published when the most common therapeutic regimen was gemcitabine, which historically showed a significantly better toxicity profile compared with mFOLFIRINOX.5 Finally, AT access is considered a quality metric in pancreas surgery to evaluate systems performance at institutional levels.10
Identifying preoperative factors associated with AT omission is paramount to maximizing the multimodal treatment of PC and reducing the fraction of patients receiving incomplete therapy (namely, surgery only). In fact, in the current era, some clinicians are more likely to recommend neoadjuvant therapy even for resectable PC (rPC), for patients deemed to be at high risk of failing to be initiated on AT, and to increase patient likelihood of receiving all intended therapy.11–13
To the authors’ knowledge, this is the first study to rigorously depict real-life use of AT after PC resection, defining primarily (1) the pattern of AT omission and use; (2) factors associated with AT omission; and (3) factors associated with AT delay. Second, the adherence to national guidelines was assessed.
Methods
Study Design
This was a single-center, prospective, observational study conducted from 1 January 2019 through 27 July 2022 at the Unit of Pancreatic Surgery, Pancreas Institute, University of Verona, Verona, Italy. The local Ethics Committee prospectively approved the collection of patient data (PAD-R, #1101CESC). The trial protocol is available at ClinicalTrials.gov (NCT03788382). The authors were responsible for the study design, data analysis, and contents of this article. The principles of the Declaration of Helsinki14 and the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE)15 guidelines were followed to conduct the trial and report the study, respectively.
Patient Selection and Data Collection
Preoperative, intraoperative, and postoperative data of consecutive patients who underwent upfront resection (pancreatoduodenectomy, distal pancreatectomy, total pancreatectomy), and a final pathology reporting PC, were collected. Patients who received surgery after neoadjuvant therapy for PC were not included since there is no consensus on administering further chemotherapy after pancreatectomy in this circumstance. Patients who experienced in-hospital death after surgery were also excluded.
Patients received AT at the authors’ institution or in other centers according to the patient’s region of residence and preference. The chemotherapy regimen was assigned at the discretion of the treating oncologist. For patients receiving AT in other institutions, data were obtained by direct contact with the patient or the treating oncologist. In detail, the following information was collected: AT administration (yes/no), reasons for AT omission, time to AT start (days), and type of chemotherapy prescribed.
Outcomes
The primary endpoints were (1) the proportion of patients not accessing AT at all; (2) the investigation of perioperative factors associated with AT omission; and (3) AT delay. As a secondary endpoint, adherence to the Italian Association of Medical Oncology (AIOM) guidelines was evaluated, considering the introduction in the recommendations of FOLFIRINOX that occurred in October 2019 (9 months after the PRODIGE-24 study). Therefore, for the purposes of this study, from January 2019 to October 2019, gemcitabine was considered the standard of care, while onwards, FOLFIRINOX was considered standard of care.
Statistical Analysis
A precision-based approach was used to calculate the sample size. Assuming up to 85% of the subjects would have received AT (based on previous historical institutional data), the study would require a sample size of 317 patients to estimate the expected proportion with 5% absolute precision and 95% confidence. Continuous variables were expressed as medians with interquartile range (IQR) and were compared using the non-parametric Mann–Whitney U test. Categorical variables were presented as frequencies with percentages and were compared using the Chi-square test or Fisher’s exact test.
The association between clinicopathological and perioperative data and AT administration was tested using multivariable logistic regression models (backward regression, Wald test, p <0.05 for variable entry, p > 0.1 for removal). Variables were selected for model entry according to their clinical relevance and statistically significant association with the outcome of interest at univariable analysis (p < 0.01). Analysis of the area under the receiving operating characteristic (ROC) curve was performed to identify the best age threshold associated with the likelihood of adjuvant treatment omission, using the Youden Index (J). For AT delay, the cut-off chosen was 12 weeks. Modeling was performed with no missing data.
Statistical analyses were performed using the MedCalc software (MedCalc, Oostende, Belgium).
Results
A total of 1122 patients with periampullary disease received pancreatectomy over the study period. After applying the inclusion and exclusion criteria, 317 patients were enrolled as planned. Fig. 1 of Electronic supplementary material (ESM) shows the study flowchart. The median age was 70 years (IQR 9), and the sexes were almost equally balanced. The proportion of ASA class > 2 and Charlson Age Comorbidity Index (CACI) ≥ 4 patients was 31.8% and 30%, respectively. Table 1 reports the general characteristics of the study population.
Table 1.
General characteristics of the study population [n = 317]
| Variable | Total |
|---|---|
| Demographics | |
| Age, years [median (IQR)] | 70 (9) |
| Sex, Female | 162 (51.1) |
| BMI, kg/m2 [median, IQR)] | 23.7 (4.8) |
| Underweight—BMI < 18.5 | 14 (4.5) |
| Normal—BMI 18.5–25.0 | 185 (58.3) |
| Overweight—BMI 25.0–30.0 | 96 (30.3) |
| Obese—BMI >30.0 | 22 (6.9) |
| Smoking, current or past | 179 (56.5) |
| Alcohol abuse, current or past | 45 (14.2) |
| FH of pancreatic cancer | 33 (10.4) |
| PH of other malignancies | 20 (6.3) |
| ASA score III–IV | 101 (31.8) |
| CACI ≥ 4 | 95 (30.0) |
| Rectal colonization by MDR bacteria | 27 (8.5) |
| Biliary stenting | 141 (44.5) |
| Weight loss | 192 (60.6) |
| Anemia | 108 (34.1) |
| Cholangitis within 6 weeks from surgery | 20 (6.3) |
| CA19-9 serum levels, U/mL [median (IQR)] | 98 (62) |
| Surgical data | |
| Pancreaticoduodenectomy | 199 (62.8) |
| Distal splenopancreatectomy | 97 (30.6) |
| Total pancreatectomy | 21 (6.6) |
| Minimally invasive approach | 31 (9.8) |
| Vascular resection | 22 (6.9) |
| Estimated blood loss [median (IQR)] | 420 (500) |
| Pathology data | |
| AJCC staging (8th edition) | |
| Stage I | 42 (13.2) |
| Stage II | 109 (34.4) |
| Stage III | 166 (52.4) |
| R status, R0 | 244 (76.8) |
| Lymph nodes examined [median (IQR)] | 39 (21) |
| N status | |
| N0 | 48 (15.1) |
| N1 | 104 (32.8) |
| N2 | 165 (52.1) |
Data are expressed as n (%) unless otherwise specified
CACI Charlson Age Comorbidity Index30, FH family history, PH personal history, IQR interquartile range, BMI body mass index, ASA American Society of Anesthesiologists, MDR multidrug resistance, AJCC American Joint Committee on Cancer
Pattern of Adjuvant Therapy Omission
After surgery, 80/317 patients (25.2%) did not receive AT, whereas 217 patients (74.8%) eventually did receive AT. AT was omitted due to postoperative complications lasting >12 weeks or clinical deterioration leading to an Eastern Cooperative Oncology Group performance status (ECOG PS) score of 2 in 38.8% of cases. Omission due to oncologist’s choice occurred in 21.2% of patients, mainly for early-stage cases (stage I–IIA). Other reasons were baseline comorbidities (CACI >3) in 20% of cases, patient choice (10%), and very early recurrence on postoperative restaging (10%). Table 2 summarizes the oncological data, including reasons for AT omission, time to AT initiation, and the regimens prescribed.
Table 2.
Oncology data
| Variable | Total |
|---|---|
| Adjuvant therapy omission | 80 (25.2) |
| Pattern of failure | |
| Postoperative complications | 31 (38.8) |
| Deemed not necessarya | 17 (21.2) |
| Baseline comorbidities (CACI ≥4) | 16 (20) |
| Patient’s choice | 8 (10) |
| Early recurrence | 8 (10) |
| Chemotherapy regimen | |
| Gemcitabine, monotherapy | 99 (41.8) |
| mFOLFIRINOX | 91 (38.4) |
| Gemcitabine-based polychemotherapy | 37 (15.6) |
| Other regimens | 10 (4.2) |
| Time discharge-chemotherapy start, weeks [median (IQR)] | 8 (4.0) |
Data are expressed as n (%) unless otherwise specified
CACI Charlson Age Comorbidity Index, IQR interquartile range
aR0, or Stage I or Stage IIA
Perioperative Factors Associated with Adjuvant Therapy Omission
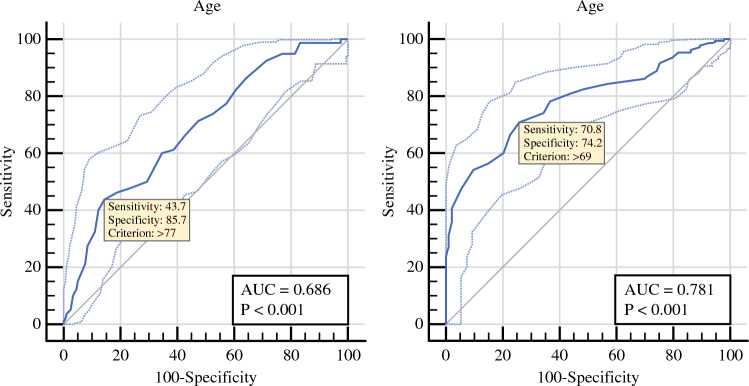
Patients not accessing AT were more frequently older, had a higher ASA score (III or IV) and a CACI index, and received a microscopically non-radical (R1) surgery (all p < 0.001) [Table 3]. The ROC curve analysis found a cut-off value for not receiving AT of 78 years (AUC 0.686; p < 0.001) (Fig. 1).
Table 3.
Association between perioperative factors and adjuvant therapy access
| Variable | Total [n = 317] |
No adjuvant therapy [n = 80] | Adjuvant therapy [n = 237] | p-value |
|---|---|---|---|---|
| Baseline data | ||||
| Age, years [median (IQR)] | 71 (14) | 75 (12) | 70 (12) | <0.001 |
| <60 years | 44 (13.9) | 3 (3.8) | 41 (17.3) | <0.001 |
| −69 years | 98 (13.9) | 18 (22.5) | 80 (33.8) | |
| 70–79 years | 127 (40.1) | 35 (43.8) | 92 (38.8) | |
| ≥80 years | 48 (15.1) | 24 (30) | 24 (10.1) | |
| Sex (male:female) | 155:162 | 44 (55) | 111 (46.8) | 0.245 |
| BMI, kg/m2 [median (IQR)] | 23.7 (3.8) | 23.6 (4.6) | 23.8 (4.8) | 0.906 |
| Underweight – BMI <18.5 | 14 (4.4) | 5 (6.3) | 9 (3.8) | 0.473 |
| Normal – BMI 18.5–25.0 | 184 (58) | 41 (51.9) | 143 (60.3) | |
| Overweight – BMI 25.0–30.0 | 100 (31.5) | 28 (35) | 72 (30.4) | |
| Obese – BMI >30.0 | 19 (6) | 6 (7.5) | 13 (5.5) | |
| Smoking, current or past | 179 (56.5) | 47 (58.7) | 132 (55.3) | 0.742 |
| Alcohol abuse, current or past | 45 (14.2) | 14 (17.5) | 31 (13.1) | 0.328 |
| FH of pancreatic cancer | 33 (10.4) | 9 (11.3) | 24 (10.1) | 0.776 |
| ASA score III–IV | 101 (31.9) | 38 (47.5) | 63 (26.6) | <0.001 |
| CACI [median (IQR)] | 2 (0) | 6 (1) | 5 (2) | <0.001 |
| Weight loss | 192 (60.6) | 50 (62.5) | 142 (59.9) | 0.233 |
| Biliary stenting | 141 (44.5) | 31 (38.7) | 110 (46.4) | 0.683 |
| Cholangitis within 6 weeks from surgery | 20 (6.3) | 4 (5) | 16 (6.8 | 0.233 |
| CA19-9, U/mL [median (IQR)]a | 98 (162) | 115 (230) | 97 (152) | 0.576 |
| Surgical data | 0.417 | |||
| Pancreatoduodenectomy | 199 (62.8) | 49 (61.2) | 150 (63.3) | 0.456 |
| Distal splenopancreatectomy | 96 (30.3) | 23 (28.7) | 73 (30.8) | – |
| Total pancreatectomy | 22 (6.9) | 8 (10) | 14 (5.9) | – |
| Minimally invasive approach | 31 (9.8) | 12 (15) | 19 (8) | 0.069 |
| Vascular resection | 22 (6.9) | 6 (7.5) | 16 (6.8) | 0.820 |
| Estimated blood loss [median (IQR)] | 420 (500) | 460 (473) | 420 (470) | 0.683 |
| Pathological data | ||||
| Stage I | 42 (13.2) | 11 (13.8) | 31 (13.1) | 0.454 |
| Stage II | 110 (34.7) | 32 (40) | 78 (32.9) | – |
| Stage III | 165 (52.1) | 37 (46.2) | 128 (54) | – |
| R status, R1 | 73 (23) | 25 (31.2) | 48 (20.3) | 0.043 |
| N status | ||||
| N0 | 48 (15.1) | 14 (17.5) | 34 (14.3) | 0.760 |
| N1 | 114 (36) | 29 (36.2) | 85 (35.9) | – |
| N2 | 155 (48.9) | 37 (46.2) | 118 (49.8) | – |
Bold values indicate statistically significant
Data are expressed as n (%) unless otherwise specified
CACI Charlson Age Comorbidity Index30, IQR interquartile range, BMI body mass index, FH family history, PH personal history, ASA American Society of Anesthesiologists, CACI Charlson Age Comorbidity Index
aN = 282 (89.9%); in 35 cases, the CA19-9 was not expressed
Fig. 1.
ROC curve analysis for prediction of adjuvant therapy omission (left) and mFOLFIRINOX omission (right). ROC receiver operating characteristic, AUC area under the curve
Regarding the association with postoperative course, approximately one of two patients experienced at least a postoperative complication (n = 149, 50.2%), whereas major complications occurred in 42 patients (13.2%) and cardiopulmonary complications occurred in 55 (17.3%) patients. Patients experiencing a complicated postoperative course were significantly less likely to receive AT (all complications, major complications, clinically relevant pancreatic fistula, abdominal collections requiring treatment, surgical site infections, sepsis and cardiopulmonary events; all p < 0.05 (Table 4). In fact, those who did not have access to AT had a longer hospital stay.
Table 4.
Association between postoperative complications and adjuvant therapy access
| Variable | Total [n = 317] |
No adjuvant therapy [n = 80] | Adjuvant therapy [n = 237] | p-Value |
|---|---|---|---|---|
| Length of stay [median (IQR)] | 10 (11) | 13 (28.0) | 9 (4.0) | < 0.001 |
| Any complication | 159 (50.2) | 55 (68.7) | 104 (43.9) | < 0.001 |
| Major complications (CD ≥3) | 42 (13.2) | 17 (40.5) | 25 (10.5) | 0.014 |
| Clinically-relevant POPFa | 49 (15.5) | 22 (27.5) | 27 (1.4) | < 0.001 |
| Postpancreatectomy hemorrhageb | 25 (7.9) | 7 (8.8) | 18 (7.6) | 0.740 |
| Chyle leaka | 9 (2.8) | 3 (3.7) | 6 (2.5) | 0.571 |
| Delayed gastric emptyingb | 29 (9.1) | 8 (10.0) | 14 (5.9) | 0.213 |
| Re-intervention | 24 (7.6) | 9 (11.3) | 15 (6.3) | 0.150 |
| Abdominal collections | 40 (12.6) | 17 (21.2) | 23 (9.7) | 0.007 |
| Surgical site infection | 18 (5.7) | 9 (11.3) | 9 (3.8) | 0.012 |
| Sepsis | 45 (14.2) | 22 (27.5) | 23 (9.7) | < 0.001 |
| Cardiopulmonary complicationsc | 55 (17.4) | 23 (28.7) | 32 (13.5) | 0.001 |
Bold values indicate statistically significant
Data are expressed as n (%) unless otherwise specified
IQR interquartile range, CD Clavien–Dindo34, POPF postoperative pancreatic fistula, ISGPF International Study Group of Pancreatic Fistula, ISGPS International Study Group for Pancreatic Surgery
aGraded according to the ISGPF31
bGraded according to the ISGPS32,33
cIncluding any cardiopulmonary event requiring treatment
At the multivariable analysis, the likelihood odds of not receiving AT increased significantly for older patients (odds ratio [OR] 1.09, 95% confidence interval [CI] 1.05–1.13, p < 0.001), those with an ASA score of III–IV (OR 2.03, 95% CI 1.14–3.6, p = 0.015), or developing clinically relevant postoperative pancreatic fistula (OR 2.5, 95% CI 1.15–6.1, p = 0.019). Notably, no pathological parameter was associated with AT initiation (Table 5).
Table 5.
Univariable and multivariable logistic regression for the risk of not receiving adjuvant chemotherapy according to selected variables
| Variable | Univariable [OR (95% CI)] |
p-Value | Multivariable [OR (95% CI)] |
p-Value |
|---|---|---|---|---|
| Baseline data | ||||
| Age, years | 1.09 (1.05–1.12) | < 0.001 | 1.10 (1.01–1.14) | < 0.001 |
| ASA score | ||||
| I–II, | Ref | |||
| III–IV | 2.5 (1.48–4.2) | < 0.001 | 2.03 (1.14–3.6) | 0.015 |
| CACI | 1.51 (1.25–1.82) | < 0.001 | ||
| CA19-9, U/mL | 4 (1.38–11.53) | 0.010 | ||
| Surgical data | 1.00 (0.99–1) | 0.437 | ||
| Minimally invasive approach | ||||
| No | Ref | |||
| Yes | 2.02 (0.93–4.38) | 0.073 | ||
| Pathological data | ||||
| Staging | ||||
| Stage IA–IIA | Ref | |||
| Stage IIB–III | 0.89 (0.44–1.78) | 0.749 | ||
| R status | ||||
| R0 | Ref | |||
| R1 | 1.79 (1.01–3.16) | 0.045 | ||
| N status | ||||
| N0 | Ref | |||
| N1–2 | 0.76 (0.38–1.51) | 0.437 | ||
| Postoperative data | ||||
| Any complication, no/yes | 2.81 (1.64–4.81) | < 0.001 | ||
| Major complication (CD ≥3), no/yes | 2.28 (1.16–4.5) | 0.016 | ||
| Abdominal collection, no/yes | 2.51 (1.26–4.99) | 0.008 | ||
| Surgical site infection, no/yes | 3.21 (1.22–8.4) | 0.017 | ||
| Sepsis, no/yes | 3.52 (1.93–6.77) | < 0.001 | ||
| Cardiopulmonary complication, no/yes | 2.58 (1.4–4.76) | 0.002 | ||
| CR-POPF | 2.95 (1.56–5.55) | < 0.001 | 2.5 (1.15–6.18) | 0.019 |
Guidelines Adherence
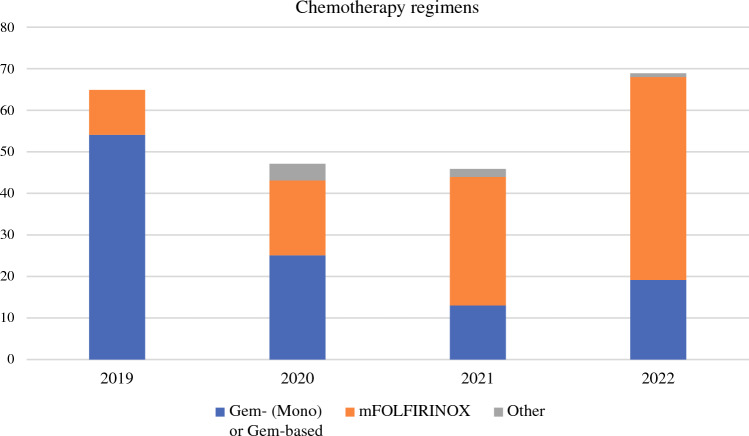
Adjuvant FOLFIRINOX utilization increased approximately fivefold over the study period (from 16.1% to 71%) (Fig. 2). Patients not receiving FOLFIRINOX were significantly older (median age 65 vs. 74 years, p < 0.001), had a history of alcohol abuse, higher ASA and CACI score, and displayed more advanced N stage (all p < 0.05) [electronic supplementary material (ESM) Table S1]. The ROC curve analysis found the cut-off value for not receiving AT with mFOLFIRINOX was > 69 years (AUC 0.781, p < 0.001) (Fig. 1).
Fig. 2.
Chemotherapy regimens prescribed during the study period (for guideline adherence evaluation; study period November 2019–July 2022). Gem gemcitabine, mono monotherapy
At the multivariable analysis, the likelihood of not receiving mFOLFIRINOX increased for older patients (OR 1.10, 95% CI 1.06–1.14, p < 0.001), patients who experienced major complications during the postoperative course (OR 4.7, 95% CI 1.46–15.3, p = 0.009), and in less advanced cases, either in terms of stage (stage I–IIa vs. stage IIb–III: OR 2.82 95% CI 1.09–7.25, p =0.031) or nodal involvement (N0 vs. N+, OR 3, 95% CI 1.11–8.53, p = 0.030) [ESM Table S2].
Adjuvant Therapy Delay
Overall, 237 patients initiated AT after a median of 8 weeks from surgery (IQR 6–10), and 22 (9.3%) eventually did initiate AT after 12 weeks. On multivariable analysis, major complications were associated with a more than twofold increased likelihood of delay in AT (OR 3.86, 95% CI 1.35–11.05, p = 0.011) [ESM Table S3].
Discussion
The main aim of this study was to depict a real-life scenario of the use of AT after upfront PC resection, focusing on its omission and the associated factors. A prospective data collection was performed to investigate this aspect thoroughly. In line with some single-center findings,10,16–19 we found that one-quarter of patients (25.2%) do not receive any chemotherapy after surgery—a significantly lower rate when compared with previous registry- or population-based reports where this proportion settles at around 40–50%.9,20–22
We report that postoperative complications predicted AT therapy and, specifically, FOLFIRINOX omission. Clinical deterioration following a complicated postoperative course has been found to have a pivotal role in negatively influencing access to AT.9,22–24 Of note, in a recent multicenter study, Henry et al. demonstrated that the detrimental effect of major postoperative complications on survival is largely mediated by AT omission.25 The strict association between a complicated postoperative course and the likelihood of AT omission also impacts treatment selection. Indeed, the patient allocation to either a treatment or another (in the case of resected PC, upfront surgery vs. neoadjuvant therapy) also needs to be weighed on the likelihood of recovering from possible major complications. In this sense, large-scale studies focusing on the identification of patients at higher risk of failure to rescue after pancreatic surgery,26 effective prehabilitation strategies, or a bundle of perioperative interventions to improve the recovery,27 are eagerly awaited.
Failure to recover from complications and baseline comorbidities accounted for about 60% of AT omission causes. Age might be the trade union between these two factors as it has traditionally been found to be a strong driver of AT omission.16,18,20,22,28 In the present study, increasing age remained independently associated with AT omission or not receiving FOLFIRINOX (p < 0.001). The likelihood of not receiving AT steadily increased with age, reaching up to 50% in octogenarians. A previous study focusing on AT use in octogenarians reported similar findings (47.4% of AT use), yet it demonstrated the beneficial oncological effects on survival.29 Of note, while the ROC curve analysis found a cut-off of 78 years for not receiving AT (AUC 0.686, p < 0.001), that cut-off decreased to 70 years for not receiving FOLFIRINOX (AUC 0.781, p < 0.001). This relatively low cut-off, combined with the finding that having an ASA score of III–IV led to a more than twofold increased likelihood of not receiving FOLFIRINOX, made us speculate that in the presence of a septuagenarian patient with relevant comorbidities, at the time of preoperative consultation, a multidisciplinary evaluation with a geriatric and oncologic assessment is desirable. More in general, a comprehensive baseline evaluation integrating surgical, anesthesiologic, oncologic, psychologic and nutritional assessment could help in identifying patients at high risk of futile operations and selecting the most appropriate treatment algorithm, including the option of neoadjuvant therapy for those who are expected not to receive AT.31,32
Another surprising finding of this study is that in 21.2% of cases, the oncologist decided not to prescribe any AT due to early-stage PC. This result, already reported by Xia et al.,18 may seem inexplicable given both the tumor biology of PC, which is thought to be aggressive and micrometastatic since the very early stages, and current clinical guideline recommendations. However, given the rarity of such a condition, data on the efficacy of AT after resection of early-stage PC are scant,33 and no definitive conclusion could be derived about its actual benefit.
Regarding the compliance to guidelines rate, our findings report that FOLFIRINOX prescription increased fivefold during the study period (from 16 to 71%), reflecting a steadily increased acceptance of the guideline recommendation. This is concordant with the recently published GARIBALDI survey, promoted by the AIOM, which presented an overall guidelines adherence of 69% in resected PC patients. However, both the survey and this study were developed at the point of introducing FOLFIRINOX as first-option AT into practice, which occurred in October 2019, compared with the results of PRODIGE-24 published in January 2019.34 Incorporating guidelines require time to complete as clinical experience and caseload increase. Thus, any further consideration may become inappropriate.
This study has some limitations. First, given the overall number of resected PCs undergoing neoadjuvant therapy, a selection bias applies to the cohort of patients who were selected for surgery; however, the authors could not critically find major drivers of patient selection. Second, the oncological consultation was not centralized at the authors’ institution. Third, we could not collect perioperative nutritional, performance status, and laboratory data, which may have driven the oncological decision not to prescribe AT. Finally, we do not present data on AT completion, tolerance and dose modification, as well as survival data to support the importance of AT completion. Such endpoints will be evaluated in a separate study after concluding appropriate patient follow-up.
Conclusion
One-quarter of patients who underwent upfront pancreatectomy for resected PC still do not receive AT due to a mix of age- and comorbidity-dependent factors, but primarily due to an adverse postoperative course. There was a steady uptake of adjuvant FOLFIRINOX over the study period. The implications of dose density/intensity and chemotherapy completion on oncologic outcomes warrant future investigation.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgments
The authors thank Dr. Nicola Quintarelli for her assistance in data collection.
Author contributions
Conceptualization: SP, GM, CB, RS. Data curation: SP, AC, FC, MDP, GL, ES. Formal analysis: SP, FC, MDP, GL, ES. Funding acquisition: CB, FC. Investigation: SP, GM. Methodology: SP, GM. Project administration: SP, RS, CB. Resources: SP, GM, RS. Supervision: RS, CB. Validation: SP, GM, CB, RS. Visualization: SP, GM, ES. Writing—original draft: All authors. Writing—review and editing: All authors.
Funding
Open access funding provided by Università degli Studi di Verona within the CRUI-CARE Agreement. Fondazione Italiana Malattie Pancreas (FIMP) – Italian Ministry of Health (CUP_J38D19000690001). FC was supported by Fondazione Umberto Veronesi.
Data availability
The data supporting this study’s findings are available from the corresponding author (SP) upon reasonable request.
Disclosure
Salvatore Paiella and Erica Secchettin receive consultancy honoraria from AlphaTau. Giuseppe Malleo, Gabriella Lionetto, Alice Cattelani, Fabio Casciani, Matteo De Pastena, Claudio Bassi, and Roberto Salvia have no conflicts of interest to declare in relation to this work.
Footnotes
Claudio Bassi: Deceased.
Part of this study’s results have been presented at the 2023 Annual Pancreas Club Meeting, Chicago, IL, USA, May 2023; at the Digestive Disease Week Annual Meeting, Chicago, IL, USA, May 2023; and at the EAHPBA 2023 Meeting, Lyon, France, June 2023.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Salvatore Paiella, Email: Salvatore.paiella@univr.it.
Roberto Salvia, Email: Roberto.salvia@univr.it.
References
- 1.Siegel RL, Miller KD, Wagle NS, Jemal A. Cancer statistics, 2023. CA Cancer J Clin. 2023;73(1):17–48. doi: 10.3322/caac.21763. [DOI] [PubMed] [Google Scholar]
- 2.Kamarajah SK, Bundred JR, Alrawashdeh W, Manas D, White SA. A systematic review and network meta-analysis of phase III randomised controlled trials for adjuvant therapy following resection of pancreatic ductal adenocarcinoma (PDAC) HPB (Oxford). 2020;22(5):649–659. doi: 10.1016/j.hpb.2019.12.001. [DOI] [PubMed] [Google Scholar]
- 3.Flaum N, Hubner RA, Valle JW, Amir E, McNamara MG. Adjuvant chemotherapy and outcomes in patients with nodal and resection margin-negative pancreatic ductal adenocarcinoma: A systematic review and meta-analysis. J Surg Oncol. 2018;119:932–940. doi: 10.1002/jso.25440. [DOI] [PubMed] [Google Scholar]
- 4.Conroy T, Castan F, Lopez A, et al. Five-year outcomes of FOLFIRINOX vs gemcitabine as adjuvant therapy for pancreatic cancer: a randomized clinical trial. JAMA Oncol. 2022;8(11):1571–1578. doi: 10.1001/jamaoncol.2022.3829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Conroy T, Hammel P, Hebbar M, et al. FOLFIRINOX or gemcitabine as adjuvant therapy for pancreatic cancer. N Engl J Med. 2018;379(25):2395–2406. doi: 10.1056/NEJMoa1809775. [DOI] [PubMed] [Google Scholar]
- 6.Sweigert PJ, Eguia E, Baker MS, et al. Assessment of textbook oncologic outcomes following pancreaticoduodenectomy for pancreatic adenocarcinoma. J Surg Oncol. 2020;121(6):936–944. doi: 10.1002/jso.25861. [DOI] [PubMed] [Google Scholar]
- 7.Turner KM, Delman AM, Ammann AM, et al. Is there a benefit to adjuvant chemotherapy in resected, early stage pancreatic ductal adenocarcinoma? Ann Surg Oncol. 2022 doi: 10.1245/s10434-022-11580-7. [DOI] [PubMed] [Google Scholar]
- 8.Bergquist JR, Ivanics T, Shubert CR, et al. Type of resection (Whipple vs. Distal) does not affect the national failure to provide post-resection adjuvant chemotherapy in localized pancreatic cancer. Ann Surg Oncol. 2017;24(6):1731–1738. doi: 10.1245/s10434-016-5762-6. [DOI] [PubMed] [Google Scholar]
- 9.Merkow RP, Bilimoria KY, Tomlinson JS, et al. Postoperative complications reduce adjuvant chemotherapy use in resectable pancreatic cancer. Ann Surg. 2014;260(2):372–377. doi: 10.1097/SLA.0000000000000378. [DOI] [PubMed] [Google Scholar]
- 10.Abbott DE, Martin G, Kooby DA, et al. Perception is reality: quality metrics in pancreas surgery—a central pancreas consortium (CPC) analysis of 1399 patients. HPB (Oxford). 2016;18:462–469. doi: 10.1016/j.hpb.2015.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Uson Junior PLS, Dias ESD, de Castro NM, et al. Does neoadjuvant treatment in resectable pancreatic cancer improve overall survival? A systematic review and meta-analysis of randomized controlled trials. ESMO Open. 2023;8(1):100771. doi: 10.1016/j.esmoop.2022.100771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Versteijne E, Vogel JA, Besselink MG, et al. Meta-analysis comparing upfront surgery with neoadjuvant treatment in patients with resectable or borderline resectable pancreatic cancer. Br J Surg. 2018;105(8):946–958. doi: 10.1002/bjs.10870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.van Dam JL, Janssen QP, Besselink MG, et al. Neoadjuvant therapy or upfront surgery for resectable and borderline resectable pancreatic cancer: a meta-analysis of randomised controlled trials. Eur J Cancer. 2022;160:140–149. doi: 10.1016/j.ejca.2021.10.023. [DOI] [PubMed] [Google Scholar]
- 14.World Medical A World Medical Association Declaration of Helsinki: ethical principles for medical research involving human subjects. JAMA. 2013;310(20):2191–2194. doi: 10.1001/jama.2013.281053. [DOI] [PubMed] [Google Scholar]
- 15.von Elm E, Altman DG, Egger M, et al. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. Lancet. 2007;370(9596):1453–1457. doi: 10.1016/S0140-6736(07)61602-X. [DOI] [PubMed] [Google Scholar]
- 16.Akerberg D, Bjornsson B, Ansari D. Factors influencing receipt of adjuvant chemotherapy after surgery for pancreatic cancer: a two-center retrospective cohort study. Scand J Gastroenterol. 2017;52(1):56–60. doi: 10.1080/00365521.2016.1228118. [DOI] [PubMed] [Google Scholar]
- 17.Weinrich M, Bochow J, Kutsch AL, et al. High compliance with guideline recommendations but low completion rates of adjuvant chemotherapy in resected pancreatic cancer: a cohort study. Ann Med Surg (Lond). 2018;32:32–37. doi: 10.1016/j.amsu.2018.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Xia BT, Habib DA, Dhar VK, et al. Early recurrence and omission of adjuvant therapy after pancreaticoduodenectomy argue against a surgery-first approach. Ann Surg Oncol. 2016;23(13):4156–4164. doi: 10.1245/s10434-016-5457-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chikhladze S, Lederer AK, Kousoulas L, et al. Adjuvant chemotherapy after surgery for pancreatic ductal adenocarcinoma: retrospective real-life data. World J Surg Oncol. 2019;17(1):185. doi: 10.1186/s12957-019-1732-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bakens MJ, van der Geest LG, van Putten M, et al. The use of adjuvant chemotherapy for pancreatic cancer varies widely between hospitals: a nationwide population-based analysis. Cancer Med. 2016;5(10):2825–2831. doi: 10.1002/cam4.921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mayo SC, Gilson MM, Herman JM, et al. Management of patients with pancreatic adenocarcinoma: national trends in patient selection, operative management, and use of adjuvant therapy. J Am Coll Surg. 2012;214(1):33–45. doi: 10.1016/j.jamcollsurg.2011.09.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wu W, He J, Cameron JL, et al. The impact of postoperative complications on the administration of adjuvant therapy following pancreaticoduodenectomy for adenocarcinoma. Ann Surg Oncol. 2014;21(9):2873–2881. doi: 10.1245/s10434-014-3722-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mackay TM, Smits FJ, Roos D, et al. The risk of not receiving adjuvant chemotherapy after resection of pancreatic ductal adenocarcinoma: a nationwide analysis. HPB (Oxford). 2020;22(2):233–240. doi: 10.1016/j.hpb.2019.06.019. [DOI] [PubMed] [Google Scholar]
- 24.Kelly KJ, Greenblatt DY, Wan Y, et al. Risk stratification for distal pancreatectomy utilizing ACS-NSQIP: preoperative factors predict morbidity and mortality. J Gastrointest Surg. 2011;15(2):250–259. doi: 10.1007/s11605-010-1390-9. [DOI] [PubMed] [Google Scholar]
- 25.Henry AC, van Dongen JC, van Goor I, et al. Impact of complications after resection of pancreatic cancer on disease recurrence and survival, and mediation effect of adjuvant chemotherapy: nationwide, observational cohort study. BJS Open. 2023;7(2):zrac174. doi: 10.1093/bjsopen/zrac174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gleeson EM, Pitt HA, Mackay TM, et al. Failure to rescue after pancreatoduodenectomy: a transatlantic analysis. Ann Surg. 2021;274(3):459–466. doi: 10.1097/SLA.0000000000005000. [DOI] [PubMed] [Google Scholar]
- 27.Powell-Brett S, Hodson J, Pande R, et al. Are physical performance and frailty assessments useful in targeting and improving access to adjuvant therapy in patients undergoing resection for pancreatic cancer? Langenbecks Arch Surg. 2023;408(1):88. doi: 10.1007/s00423-023-02828-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Eubanks A, Pepe J, Veldhuis P, de la Fuente SG. Age as a prognostic indicator for adjuvant therapy in patients who underwent pancreatic resections for cancer. J Geriatr Oncol. 2018;9(4):362–366. doi: 10.1016/j.jgo.2017.12.004. [DOI] [PubMed] [Google Scholar]
- 29.Mehtsun WT, McCleary NJ, Maduekwe UN, Wolpin BM, Schrag D, Wang J. Patterns of adjuvant chemotherapy use and association with survival in adults 80 years and older with pancreatic adenocarcinoma. JAMA Oncol. 2022;8(1):88–95. doi: 10.1001/jamaoncol.2021.5407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40(5):373–383. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 31.Bassi C, Marchegiani G, Dervenis C, et al. The 2016 update of the International Study Group (ISGPS) definition and grading of postoperative pancreatic fistula: 11 years after. Surgery. 2017;161(3):584–591. doi: 10.1016/j.surg.2016.11.014. [DOI] [PubMed] [Google Scholar]
- 32.Wente MN, Bassi C, Dervenis C, et al. Delayed gastric emptying (DGE) after pancreatic surgery: a suggested definition by the International Study Group of Pancreatic Surgery (ISGPS) Surgery. 2007;142(5):761–768. doi: 10.1016/j.surg.2007.05.005. [DOI] [PubMed] [Google Scholar]
- 33.Wente MN, Veit JA, Bassi C, et al. Postpancreatectomy hemorrhage (PPH): an International Study Group of Pancreatic Surgery (ISGPS) definition. Surgery. 2007;142(1):20–25. doi: 10.1016/j.surg.2007.02.001. [DOI] [PubMed] [Google Scholar]
- 34.Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg. 2004;240(2):205–213. doi: 10.1097/01.sla.0000133083.54934.ae. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data supporting this study’s findings are available from the corresponding author (SP) upon reasonable request.