Abstract
Supportive evidence that apoptosis contributes to loss of CD4+ lymphocytes in human immunodeficiency virus type 1 (HIV-1)-infected humans comes from an apparent lack of abnormal apoptosis in apathogenic lentivirus infections of nonhuman primates, including HIV-1 infection of chimpanzees. Two female chimpanzees were inoculated, one cervically and the other intravenously, with HIV-1 derived from the LAI/LAV-1b strain, which was isolated from a chimpanzee infected with the virus for 8 years. Within 6 weeks of infection, both recipient chimpanzees developed a progressive loss of CD4+ T cells which correlated with persistently high viral burdens and increased levels of CD4+ T-cell apoptosis both in vitro and in vivo. Lymph nodes from both animals also revealed evidence of immune hyperactivation. Intermediate levels of T-cell apoptosis in both peripheral blood and lymph nodes were seen in a third chimpanzee that had been infected with the LAI/LAV-1b strain for 9 years; this animal has maintained depressed CD4/CD8 T-cell ratios for the last 3 years. Similar analyses of cells from 4 uninfected animals and 10 other HIV-1-infected chimpanzees without loss of CD4+ cells revealed no difference in levels of apoptosis in these two control groups. These results demonstrate a correlation between immune hyperactivation, T-cell apoptosis, and chronic loss of CD4+ T cells in HIV-1-infected chimpanzees, providing additional evidence that apoptosis is an important factor in T-cell loss in AIDS. Furthermore, the results show that some HIV-1 strains are pathogenic for chimpanzees and that this species is not inherently resistant to HIV-1-induced disease.
Human immunodeficiency virus type 1 (HIV-1) infection in humans is associated with rapid turnover of CD4+ T cells, high viral burdens, and a chronic state of immune hyperactivation which, paradoxically, results in defective T-cell function and a gradual decline in CD4+-T-cell numbers (4, 6, 22, 28, 32, 52). The cause of T-cell loss is likely to be multifactorial, and several mechanisms have been proposed, including virus induction of lymphocyte apoptosis (2, 32). Abnormally high levels of lymphocyte apoptosis in peripheral blood and lymph nodes (LN) from HIV-1- and HIV-2-infected persons have been demonstrated (31, 35, 37, 45), but the underlying mechanism(s) of this phenomenon remains undefined (41). Apoptosis may be either a primary mechanism of T-cell loss in AIDS or an epiphenomenon of infection (1, 43).
Compelling evidence that apoptosis plays an important role in the pathogenesis of primate lentivirus disease comes from studies of natural and experimental infections of nonhuman primates with simian immunodeficiency viruses (SIVs) and HIV-1. In general, the SIVs are not pathogenic in their natural African host species but induce an AIDS-like disease in experimentally infected Asian macaques (27). CD4+-T-cell apoptosis appears to be present only in those nonhuman primate species in which SIV infection is pathogenic (13). Consistent with this observation, chimpanzees (Pan troglodytes) can be persistently infected with HIV-1 but generally do not develop HIV-related disease or exhibit elevated CD4+-T-cell apoptosis (13, 15, 23, 24, 26, 29, 48).
Recently, CD4+-T-cell dysfunction and depletion in two HIV-1-infected chimpanzees (C-499 and C-455) at the Yerkes Regional Primate Research Center were reported (40, 49). C-499 had been infected with at least two strains of HIV-1 (SF2 and LAI/LAV-1b) for about 10 years, while C-455 rapidly developed loss of CD4+ lymphocytes following transfusion of blood from C-499. Approximately 2 years before euthanasia of C-499 due to AIDS, we obtained blood from this animal, and virus, which we designated HIV-1JC499, was isolated. Subsequently, we inoculated two chimpanzees with this strain, one (C-384) intravenously and the other (C-166) via the cervical os. Both animals developed gradual but continuous declines in the percentages and absolute numbers of CD4+ T cells in peripheral blood and LN and have maintained high levels of cell-free HIV-1 in plasma (see below). If apoptosis is indeed an important mechanism for loss of CD4+ lymphocytes in HIV-1-induced disease, then one would expect to find increased levels of apoptotic cells in these two animals compared to uninfected and asymptomatic HIV-1-infected chimpanzees. To test this hypothesis, we examined peripheral blood mononuclear cells (PBMC) and LN tissues for evidence of abnormal T-cell apoptosis. Our results demonstrate (i) that the virus isolated from C-499 establishes high viral burdens and can induce hematologic abnormalities in chimpanzees and (ii) that there is a correlation between induction of CD4+-T-cell apoptosis and chronic loss of CD4+ T cells in HIV-1-infected chimpanzees.
MATERIALS AND METHODS
Animals.
Adult male and female chimpanzees were housed in biosafety level 2 facilities at the Laboratory for Experimental Medicine and Surgery in Primates (LEMSIP), New York University, or at the Coulston Foundation (Alamogordo, N.Mex.). All animals were maintained in accordance with the Animal Welfare Act, institutional guidelines, and standard practices for the humane care and use of chimpanzees in biomedical research. C-384 was inoculated intravenously with 1 ml of approximately 500 tissue culture infectious doses of HIV-1JC499; C-166 was exposed without trauma via the cervical os to about 600 tissue culture infectious doses in a volume of 0.25 ml, as described previously (21). Chimpanzees were observed daily for signs of illness or changes in behavior and were routinely monitored to evaluate their hematologic, virologic, and immunologic status. Before all procedures, chimpanzees were anesthetized by intramuscular injection of ketamine hydrochloride (10 mg/kg of body weight). Peripheral LN biopsies were performed by standard sterile surgical techniques. Blood and tissue samples were shipped overnight to the University of Alabama at Birmingham for analysis.
Mononuclear cell isolation and culture.
Chimpanzee PBMC were isolated from heparinized blood by density gradient centrifugation over lymphocyte separation medium (Organon Teknika, Durham, N.C.), washed, and resuspended at 106 cells/ml in complete medium (RPMI 1640 supplemented with 10% heat-inactivated fetal bovine serum [FBS], 2 mM l-glutamine, 0.075% [wt/vol] sodium bicarbonate, penicillin [100 IU/ml], streptomycin [100 μg/ml], 0.8% amphotericin B [Fungizone], and gentamicin sulfate [55 μg/ml]). PBMC either were analyzed immediately in ex vivo studies or were cultured for 48 h (37°C, 5% CO2) in complete medium alone (resting-cell culture) or in the presence of staphylococcal enterotoxin B (SEB) (1 μg/ml) or phytohemagglutinin (PHA) (10 μg/ml). Some archived PBMC samples stored in liquid nitrogen vapor in RPMI 1640 containing 35% FBS, 10% dimethyl sulfoxide, and gentamicin sulfate (50 μg/ml) were also evaluated. Immediately before use, cells were thawed, washed, counted, and resuspended in complete medium.
Measurement of plasma viral RNA.
HIV-1 virion RNA in EDTA-treated plasma was measured with the Amplicor HIV-1 Monitor test kit (Roche Diagnostic Systems) according to the manufacturer’s instructions.
Flow cytometric analysis.
Phenotypic analysis of mononuclear cells was performed in a FACSCalibur flow cytometer (Becton Dickinson, Mountain View, Calif.) with monoclonal antibodies (MAbs) specific for human cell surface antigen CD2, CD4, CD8, CD14, or CD20 conjugated to the fluorochrome phycoerythrin or fluorescein isothiocyanate (all from Becton Dickinson). Viable lymphocytes were gated according to forward and side scatter characteristics, and 10,000 events were analyzed per sample.
Apoptotic cell populations were identified by using a technique based on alterations in membrane permeability to the fluorescent chemical 7-amino-actinomycin D (7-AAD), as described previously (47). Briefly, 106 cultured or uncultured mononuclear cells were washed in phosphate-buffered saline (PBS) (pH 7.2) supplemented with 2% FBS and 0.1% sodium azide and were immunostained with fluorochrome-conjugated MAbs to cell surface markers. Cells were then incubated with 7-AAD (20 μg/ml in PBS supplemented with 2% FBS and 0.1% sodium azide) for 20 min at 4°C in the dark and fixed in a nonfluorescent actinomycin D solution (20 μg/ml in 1% paraformaldehyde in PBS) to block nonspecific 7-AAD labeling of living cells (9). Relevant unstained and single-stained control samples for three-color flow cytometric analysis were used to set gates prior to data capture and were analyzed with all sample sets. Apoptotic cells were identified in the FL-3 red channel as a peak of increased fluorescence (7-AADLO; fluorescence intensity, 101 to 102), while labeled cell surface antigens were detected simultaneously in channels FL-1 (fluorescein isothiocyanate) and FL-2 (phycoerythrin). For the CD4+- or CD8+-T-cell subset, the level of apoptosis was calculated as the percentage of 7-AADLO cells among all cells (7-AAD negative [7-AADNEG] plus 7-AADLO) positive for a given subset marker. 7-AAD staining and three-color fluorescence-activated cell sorter (FACS) analysis were performed on four occasions at monthly intervals on PBMC samples from progressor chimpanzees C-166, C-384, and C-487 (see Results). PBMC from at least one nonprogressor chimpanzee were included in each analysis. A total of six control chimpanzees, two of which were uninfected, were included in all statistical comparisons. PBMC from three of these six control animals were analyzed on two independent occasions, and cells from one nonprogressor, C-460, were analyzed on all four occasions. In addition, we analyzed cryopreserved preinfection PBMC from C-166 and C-384.
Quantification of apoptotic lymphocytes in LN.
LN tissues were fixed in 10% neutral buffered formalin, paraffin embedded, cut into serial thin sections (5 μm), and mounted on Colorfrost Plus slides. Apoptosis was detected with an ApopTag in situ detection kit (Oncor, Inc.), which employs TUNEL (terminal deoxynucleotidyl transferase [TdT]-mediated dUTP nick end-labeling) methodology (19). Sections were deparaffinized, rehydrated, and predigested with Oncor protein-digesting enzyme (30 min at 37°C in a humidified chamber). After quenching of endogenous peroxidase activity, samples were incubated with a TdT enzyme mix (60 min at 37°C in a humidified chamber). Control slides were incubated with a reaction buffer containing distilled water instead of TdT. Incorporated digoxigenin-labeled nucleotides were detected by using an anti-digoxigenin peroxidase-conjugated MAb fragment. Bound peroxidase activity was detected with filtered SigmaFast diaminobenzidine, producing an insoluble brown-black precipitate. Sections were counterstained in 0.5% (wt/vol) methyl green in 0.1 M sodium acetate, destained in distilled water, and dehydrated prior to mounting of coverslips with Permount.
Apoptotic cells in tissue sections were quantified by using an adaptation of the method of Muro-Cacho et al. (39). Sections were viewed at a magnification of ×400 on a Nikon Fluophot binocular microscope with a 10- by 10-grid objective, which corresponded to an area of 0.25 mm2. Four major histoarchitectural regions of the lymph node were analyzed separately. For each region, the number of TUNEL-positive cells was counted in four randomly selected grids (a total tissue area of 1 mm2) per section. For sections that contained no evidence of germinal center formation, a score of 0 was assigned. Tissue sections from three LN biopsies (taken at 3-month intervals) were analyzed for each progressor chimpanzee. Concurrently, apoptosis was quantified in sections prepared from 13 randomly selected LN biopsies from eight other nonprogressor and uninfected chimpanzees, together with preinfection LN biopsy samples from progressors C-166 and C-384. Three consecutive sections were evaluated for each LN, and mean numbers of TUNEL-positive cells were determined.
Gel electrophoresis of fragmented DNA.
DNA fragmentation analysis was performed with an Apoptotic DNA-Ladder kit (Boehringer-Mannheim, Indianapolis, Ind.) according to the manufacturer’s instructions.
Transmission electron microscopy.
LN biopsy tissues were fixed in 2% glutaraldehyde in PBS, rinsed, and postfixed in 1% buffered osmium tetroxide. Samples were dehydrated and then embedded in Spurr plastic. Ultrathin sections (100 nm) were cut and stained with uranyl acetate and lead citrate before imaging with a Philips 301 electron microscope.
Statistical analyses.
Data were analyzed by using Instat 2.00 (GraphPad software). Statistical comparisons were made with a two-tailed Student t test. Correlations were calculated by Pearson’s linear correlation analysis on log-transformed data; a P value of <0.05 was considered statistically significant.
RESULTS
Clinical status of progressor chimpanzees.
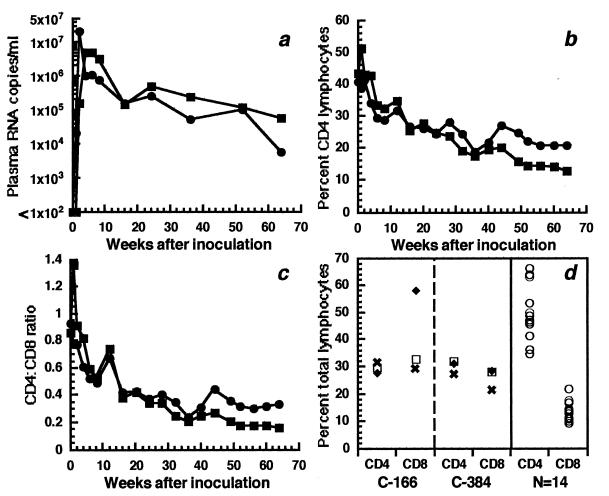
After inoculation of C-166 and C-384 with HIV-1JC499 by the cervical and intravenous routes, respectively, both animals exhibited high levels of plasma HIV-1 RNA, which peaked at 5 × 106 and 2.1 × 107 copies/ml, respectively, and then were maintained at greater than 5.3 × 104 copies/ml (Fig. 1a). That these animals had high cell-associated viral burdens was indicated by isolation of virus from PBMC and peripheral LN cells of both animals in 100% of attempts during the 15 months of follow-up. In association with high viral burdens, both animals developed progressive declines in percentages and absolute numbers of CD4+ lymphocytes (Fig. 1b), with concomitant increases in percentages and absolute numbers of CD8+ lymphocytes. These changes resulted in decreased CD4/CD8 ratios, which fell from 0.86 to 0.16 for C-166 and from 0.93 to 0.33 for C-384 during the 15 months after inoculation (Fig. 1c). This loss of CD4+ T cells in blood was also mirrored in peripheral LN, where percentages of CD4+ T cells were less than those of a large group of chimpanzees infected with other HIV-1 strains (Fig. 1d). This decrease in CD4+ T cells was accompanied by elevated levels of CD8+ T cells and resulted in lower CD4/CD8 ratios, especially in C-166, where the CD4/CD8 ratio among LN cells decreased from 1.08 at 12 weeks to 0.47 at 49 weeks after infection. These ratios are significantly lower than a mean CD4/CD8 ratio of 3.78 in LN cells from 20 biopsies of 16 chimpanzees (data for 14 animals are shown in Fig. 1d) infected with various HIV-1 strains from 4 to 191 weeks. Thus, HIV-1JC499 induced a progressive loss of CD4+-T-cell populations in these two chimpanzees; for the purposes of this study, they are defined as progressors.
FIG. 1.
Evaluation of chimpanzees C-166 (▪) and C-384 (•) after cervical and intravenous inoculation, respectively, of HIV-1JC499. (a) Plasma HIV-1 RNA levels. (b) Percentages of CD4+ T cells in peripheral blood. (c) CD4/CD8 ratios in peripheral blood. (d) Percentages of CD4+ and CD8+ T cells in peripheral lymph nodes. For C-166 and C-384, the LN biopsies were done 12 (✖), 24 (□), and 49 (⧫) weeks after inoculation. All of the control LN biopsies were performed on chimpanzees infected with different HIV-1 strains for various times.
Included in the analysis were 4 uninfected chimpanzees (not included in Table 1) and 11 chimpanzees infected with various HIV-1 strains by different routes and for different times (Table 1). With one exception, none of these infected chimpanzees exhibited persistent declines in CD4+-T-cell numbers or CD4/CD8 ratios, and all had lower viral burdens than C-166 and C-384; therefore, animals in this group were defined as nonprogressors. The one exception was C-487, which had been infected with HIV-1LAI/LAV-1b for about 9 years. Between 6 and 22 months after the initial infection, this animal had been subjected to a series of specific and nonspecific inoculations to stimulate its immune system, which resulted in multiple transient episodes of increased viral replication (16). Viral burdens have remained relatively high, as indicated by isolation of virus from C-487’s PBMC, by standard coculture techniques, on 100% of attempts during 9 years of infection. In addition, during the last 3 years, levels of HIV-1 virion RNA in C487’s plasma varied between 2.4 × 103 and 1.3 × 104 RNA copies/ml. For the first 2 years after infection, C-487 had CD4/CD8 ratios of between 1.0 and 2.25; however, at about 2 years, the numbers of CD8+ T lymphocytes began to increase such that the CD4/CD8 ratio decreased, and for the past 3 years, this ratio has fluctuated between 0.2 and 0.3, with absolute numbers of CD4+ T cells between 443 and 900/μl of blood (data not shown). Based on these data (and those obtained in the present study), C-487 is designated a progressor, but with the caveats that it has been infected for 9 years, whereas C-166 and C-384 have been infected for 15 months, and that C-487’s hematologic profile has been relatively stable for several years.
TABLE 1.
HIV-1-infected chimpanzees evaluated
| Animal | HIV-1 strain(s) | Route(s)a | Mo infectedb | % Δ CD4+ cellsc | FACS | TUNEL |
|---|---|---|---|---|---|---|
| Progressors | ||||||
| C-166 | JC499 | CvOs | 10–13 | −61.9 | + | + |
| C-384 | JC499 | IV | 10–13 | −40.3 | + | + |
| C-487 | LAV-1b | IV | 106–110 | −58.3 | + | + |
| Nonprogressors | ||||||
| C-090 | IIIB | CvOs | 47, 48 | −2.5 | + | NDd |
| C-092 | IIIB | CvOs | 20 | −15.8 | + | ND |
| C-304 | IIIB | CvOs | 17, 26 | 5.7 | ND | + |
| C-382 | IIIB/E402 | CvOs/CvOs | 50/8 | 32.4 | ND | + |
| C-424 | IIIB | CvOs | 2 | 24.3 | ND | + |
| C-441 | SF2 | IV | 12 | 22.6 | + | ND |
| C-460 | IIIB/E402 | CvOs/IV | 46–49/29–32 | −17.5 | + | + |
| C-498 | IIIB | CvOs | 2 | 0.5 | ND | + |
| C-534 | IIIB/E402 | IV | 2/2 | −12.1 | + | + |
| C-1196 | LAV-1b | Rectal | 12 | 11.1 | + | ND |
CvOs, virus deposited without trauma at the cervical os, as described previously (21); IV, virus inoculated via the femoral vein; rectal, virus deposited approximately 10 cm into the rectum. If two strains and routes are shown, the animal was inoculated with the second HIV-1 strain at a later time and became dually infected.
Numbers are months that the animals had been infected with HIV-1 when the analyses were done. If an animal was inoculated sequentially with two HIV-1 strains, that is, superinfected, the time after inoculation relative to both strains is given.
Values are percentages of change in percentages of CD4+ lymphocytes in peripheral blood from the time of inoculation until the time at which the analyses were done. For animals for which multiple PBMC samples were analyzed, the values shown are averages. A negative value reflects loss of CD4+ T cells.
ND, not done.
Ex vivo apoptosis in chimpanzee CD4+ and CD8+ T cells.
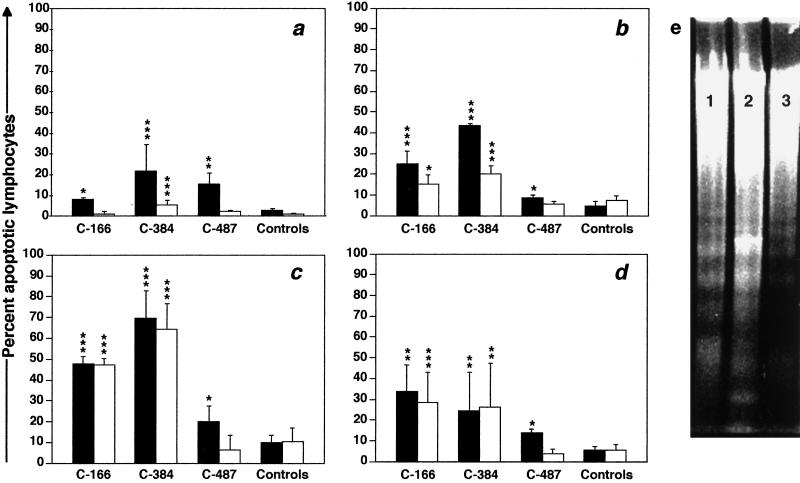
Immediately after separation from whole blood, PBMC were evaluated for percentages of apoptotic T cells by 7-AAD staining and three-color FACS analysis (Fig. 2). Low levels (means of 2 to 5% for CD4+ T cells and <1% for CD8+ T cells) of lymphocyte apoptosis were detected ex vivo in PBMC from nonprogressor chimpanzees and in preinfection samples from C-166 and C-384. Compared to values for the control animals, ex vivo CD4+-T-cell apoptosis was significantly increased for all three progressor chimpanzees (Fig. 3a), with maximal levels in samples from C-384 (21%); only C-384 had significant increases in ex vivo apoptosis in CD8+ T cells. Agarose gel electrophoresis of DNA isolated from PBMC from C-166 and C-384 revealed a classic oligonucleosomal laddering pattern, confirming that cells undergoing apoptosis were present ex vivo (data not shown). When the data for those HIV-1-infected chimpanzees analyzed by 7-AAD FACS were combined, a clear correlation (P < 0.0035) between the extent of ex vivo CD4+-T-cell apoptosis and in vivo CD4+-T-cell depletion (expressed as the change in percentage of CD4+ T cells from the time of infection to the time of sampling) was observed (Fig. 4).
FIG. 2.
Three-color FACS analysis of CD4+ and CD8+ cells in PBMC samples from nonprogressor chimpanzee C-460 (a to c) and progressor C-166 (d to f) after culture for 48 h in the absence of mitogens. (a and d) Gates for 7-AADNEG live and 7-AADLO apoptotic cells in the FL-3 channel. (b and e) Percentages of CD4+ and CD8+ T cells in the 7-AADNEG population. (c and f) Percentages of apoptotic CD4+ and CD8+ T cells in the 7-AADLO population. In this example, the percentage of apoptotic CD4+ T cells in C-460’s PBMC is 5% (2 ÷ 39), and that in C-166’s PBMC is 22% (5 ÷ 23).
FIG. 3.
Percentages of apoptotic CD4+ (closed bars) and CD8+ (open bars) T cells in PBMC samples from progressor and control (nonprogressor and uninfected) chimpanzees (n = 8). (a) Ex vivo apoptosis. (b) In vitro apoptosis after culture for 48 h in medium alone. (c and d) In vitro apoptosis after culture for 48 h in the presence of PHA (10 μg/ml) or SEB (1 μg/ml), respectively. Values for the three progressor chimpanzees are means of four different PBMC samples collected at 1-month intervals. Error bars represent two standard errors of the mean. ∗, P < 0.05; ∗∗, P < 0.005; ∗∗∗, P < 0.0005 (all relative to control values). (e) Agarose gel electrophoresis of low-molecular-weight DNA in PBMC from C-166 (lane 1), C-384 (lane 2), and C-487 (lane 3) after culture for 48 h in medium alone.
FIG. 4.
Correlation between ex vivo apoptosis of CD4+ T cells and in vivo CD4+ T-cell loss in HIV-1-infected chimpanzees: ▪, C-166; •, C-384; ▴, C-487; ✖, nonprogressors (those analyzed by FACS; see Table 1). The slope of the line, the correlation coefficient (r2), and the P value were determined by linear regression; placement of the line is an approximation.
In vitro apoptosis in chimpanzee CD4+ and CD8+ T cells.
After 48 h in culture, in vitro apoptosis of resting CD4+ and CD8+ T cells from control chimpanzees increased marginally over ex vivo levels (up to 5 and 7% of CD4+ and CD8+ cells, respectively). However, significantly higher levels of T-cell apoptosis were detected in resting cultured PBMC from C-166 and C-384 (Fig. 3b), with mean values of 25 and 44%, respectively, for CD4+ cells and lower levels for CD8+ cells. There was a hierarchy for percentages of apoptotic lymphocytes in cultured resting CD4+ T cells: apoptosis in PBMC from C-384 and C-166 was greater than that in C-487 (P < 0.05), which in turn was greater than that in nonprogressors (P < 0.05).
Similar response patterns were seen for both progressors and nonprogressors after culture with mitogens for 48 h (Fig. 3c and d). While activation-induced apoptosis of both T-cell subsets increased marginally in nonprogressors following stimulation with either PHA or SEB, levels of apoptosis were significantly higher for progressor chimpanzees. In general, PHA stimulation induced apoptosis in a higher percentage of cells than did SEB treatment. As with the cultured resting PBMC, the same hierarchy in the level of apoptosis of CD4+ T cells was observed after PHA stimulation: that of cells from C-166 and C-384 was greater than that of cells from C-487, which was greater than that in PBMC from the nonprogressors. SEB treatment also induced significantly more CD4+-T-cell apoptosis in PBMC from progressors than in those from nonprogressors, but there were no significant differences in responses to SEB among the three progressor chimpanzees. With respect to CD8+ T cells, both mitogens induced significant increases in this lymphocyte subset only in PBMC from C-166 and C-384. Results similar to those discussed above were found for PBMC of all three progressor chimpanzees after 24 or 72 h in culture with these two mitogens (data not shown). Detection by agarose gel electrophoresis of fragmented DNA in cultures of PBMC from C-166, C-384, and C-487 confirmed that apoptotic cells were present (Fig. 3e).
Lymphocyte apoptosis in peripheral LN.
LN tissues obtained by biopsy from all three progressor chimpanzees had histopathologic changes consistent with HIV-induced follicular hyperplasia (7). In the progressor animals, germinal centers were prominent with well-defined mantle zones and contained scattered macrophages and mitotic figures (Fig. 5a). Paracortical areas generally appeared normal, but occasionally areas of lymphocyte dropout, macrophage infiltration, and vascular hyperplasia were seen. Follicular involution and widespread paracortical lymphocyte depletion were not observed. Most of the LN biopsy samples from nine control chimpanzees showed no evidence of histopathologic change. Secondary follicles were detected infrequently, and no paracortical T-cell depletion was noted (Fig. 5b).
FIG. 5.
Immune hyperactivation and lymphocyte apoptosis in LN from progressor chimpanzees. (a) Follicular hyperplasia in a hematoxylin-eosin-stained LN section from C-384. Magnification, ×40. (b) Histology of normal LN tissue from nonprogressor C-534. Magnification, ×40. (c) TUNEL-positive lymphocytes in germinal center of LN from C-166. Magnification, ×100. (d) TUNEL-positive lymphocytes in paracortex of LN from C-384. Magnification, ×1,000. (e) Tingible body macrophage containing multiple phagocytosed apoptotic nuclei in germinal center of LN from C-166. Magnification, ×1,000. (f) TUNEL-stained LN tissue from nonprogressor C-304. Magnification, ×400. (g and h) Transmission electron micrograph of LN biopsy tissue from C-384, showing apoptotic lymphocytes with highly condensed chromatin, cytoplasmic condensation, and plasmalemmal zeiosis. Magnification, ×27,000.
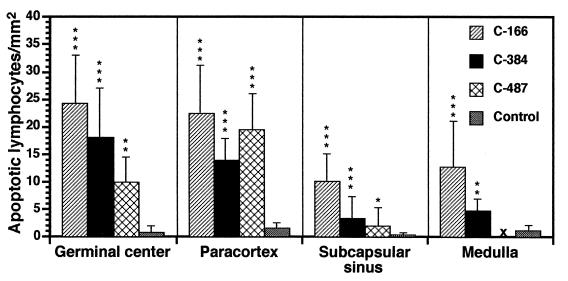
Tissue sections prepared from LN biopsies from progressor and control (nonprogressor and uninfected) chimpanzees were analyzed by the TUNEL technique for evidence of lymphocyte apoptosis. In postinfection samples from the three progressor chimpanzees, increases in apoptotic cells were detected in all LN areas, particularly in germinal centers and paracortices (Fig. 5c and d and 6). A few large macrophages containing multiple phagocytosed apoptotic nuclei were seen in some germinal centers (Fig. 5e), as was reported previously for HIV-1-infected humans (39). There was no apparent increase over time in lymphocyte apoptosis in LN sections from progressor chimpanzees. In sections from the nonprogressor chimpanzees, TUNEL-positive cells were rare and often were completely absent (Fig. 5f). There were no apoptotic cells in reagent control slides.
Transmission electron microscopy also revealed many lymphocytes with apoptotic morphology (condensed chromatin, cytoplasmic swelling, nuclear fragmentation, and plasmalemmal zeiosis) in LN biopsy specimens from C-166 and C-384 (Fig. 5g and h). Although there appeared to be an association between in vivo loss of CD4+ T cells and the extent of lymphocyte apoptosis in LN biopsies, all correlation analyses failed to show statistical significance.
DISCUSSION
While nonhuman primate models of AIDS have provided much information regarding the pathogenesis of HIV-1 (15), attempts to induce AIDS-like disease in any animal species with HIV-1 per se generally have been unsuccessful. However, the recent report by Novembre et al. (40) of development of AIDS in a chimpanzee (C-499) infected with HIV-1 for about 10 years and rapid loss of CD4+ lymphocytes in a recipient of blood from C-499 demonstrated that the virus harbored by C-499 (designated HIV-1JC) had evolved into a strain of HIV-1 pathogenic for chimpanzees. More than 1 year before euthanasia of C-499, we obtained blood from this animal and isolated virus, which we designated HIV-1JC499. The fact that the two chimpanzees we inoculated with this isolate initially had high levels of cell-free HIV-1 in plasma and developed sequelae consistent with HIV-1-related disease within a few weeks suggests that infection with HIV-1JC499 ultimately might induce immunodeficiency and AIDS in chimpanzees. Together, the results of these two studies by ourselves and Novembre et al. (40) clearly show that chimpanzees are not inherently resistant to the pathogenic effects of HIV-1. To the contrary, the data indicate that disease induction in this species more than likely is a property of the HIV-1 strain with which the animals are infected.
Disease in C-499, as well as that in the two chimpanzees inoculated with HIV-1JC499, was induced by viral quasispecies derived primarily from HIV-1LAI/LAV-1b (40, 51). Although C-499 was inoculated with three HIV-1 strains (LAI/LAV-1b, SF2, and NDK), only infection with LAI/LAV-1b and SF2, and not that with NDK, was confirmed (17, 18). Furthermore, phylogenetic analysis of env, p17gag, and nef gene sequences of HIV-1JC499 suggests that this pathogenic quasispecies is probably composed of chimeric variants that resulted from recombinational events between HIV-1LAI/LAV-1b and HIV-1SF2 (51). Although the HIV-1JC isolate described by Novembre et al. (40) and HIV-1JC499 are closely related, these two viral isolates are not identical; HIV-1JC499 was isolated from C-499’s PBMC approximately 18 months before HIV-1JC. Both isolates, however, appear to be pathogenic for chimpanzees.
Novembre et al. (40) associated the enhanced pathogenicity of their HIV-1JC isolate with a putative acquired ability to induce syncytium formation in chimpanzee PBMC in vitro. However, the original stock of HIV-1LAI/LAV-1b used to inoculate C-499 replicates efficiently in chimpanzee macrophages, forms syncytia with both human and chimpanzee PBMC, and is cytopathic for chimpanzee CD4+ T cells in vitro (20, 50). It is unlikely, therefore, that syncytium formation is responsible for disease induction or is a major mode of CD4+-T-cell loss in HIV-1JC- or HIV-1JC499-infected chimpanzees. The ability to induce lymphocyte apoptosis, as demonstrated here, appears to be a specific early effect of infection with HIV-1JC499 (and perhaps HIV-1JC). Long-term infection of C-487 with HIV-1LAI/LAV-1b appears to have elicited increased levels of lymphocyte apoptosis, but neither apoptosis nor depletion of CD4+ T cells was observed during the first few years of HIV-1LAI/LAV-1b infection of C-487 or in another chimpanzee (C-1196) (Table 1) infected with the same virus stock. It is of interest, however, that at 35 months after inoculation of HIV-1LAI/LAV-1b, C-1196 was maintaining a relatively high viral burden, with 2.2 × 104 virion RNA copies per ml of plasma.
Other investigators have shown a correlation between apathogenic HIV and SIV infections in nonhuman primates and a lack of chronic immune hyperactivation and apoptosis (13, 23, 24, 26, 48). In the present study, we ascertained whether lymphocytes from chimpanzees C-166 and C-384 exhibited increased levels of apoptosis by evaluating three populations of cells: (i) uncultured T cells obtained directly from peripheral blood (ex vivo), (ii) PBMC cultured for 48 h with or without mitogens (in vitro), and (iii) peripheral LN cells (in vivo). Compared to levels found in a cohort of uninfected and asymptomatic HIV-1-infected chimpanzees, significant increases in T-cell apoptosis were detected in all three cell populations in both HIV-1JC499-infected animals. Moreover, susceptibility to apoptosis does not appear to be a peculiar property of lymphocytes from C-166 and C-384, since PBMC and LN cells obtained from these two chimpanzees before infection did not exhibit abnormal levels of lymphocyte apoptosis. During evaluation of the group of asymptomatic HIV-1-infected animals, we identified one chimpanzee, C-487, with intermediate levels of apoptosis. This animal had been infected for approximately 9 years with HIV-1LAI/LAV-1b, one of the strains with which C-499, which succumbed to AIDS, was infected. For simplicity, C-487 was designated a progressor, although its historical hematologic and virologic profiles were different from those of the two chimpanzees infected for only 15 months with HIV-1JC499.
Levels of apoptosis detected by the 7-AAD FACS assay in PBMC of the nonprogressor chimpanzees in our study are consistent with those for HIV-1-infected asymptomatic chimpanzees published by others (24). Likewise, levels of apoptosis in PBMC of the three progressors are comparable to those described for HIV-infected humans (25). Furthermore, the distribution of apoptotic cells within different LN regions was similar to that reported by Finkel et al. (14) and Muro-Cacho et al. (39), who showed that the majority were in the light zone of secondary follicles, with fewer such cells in paracortical regions and sinuses. Although exact quantification of stained cells in tissue sections is difficult, the differences in the number of TUNEL-positive LN cells between progressor and nonprogressor chimpanzees were striking and were consistent with results of the PBMC analyses. The lower sensitivity of this assay may explain why no significant correlation between lymphocyte apoptosis in LN biopsies and in vivo T-cell depletion was found, even though there appeared to be an obvious trend.
During the span of 4 months when lymphocytes in C-166 and C-384 were evaluated, there appeared to be little, if any, increase in the extent of apoptosis. Whether specific factors correlate with the degree of apoptosis in HIV-infected humans is not clear. Some investigators have found that apoptosis is independent of disease progression and viral burden (38, 39, 46), whereas others have reported correlations with disease progression and apoptosis of cultured PBMC or only of cultured CD8+ cells (10, 25, 30, 42). While differences in the cohorts studied or culture conditions used in the various studies could explain these discrepancies, it is possible that these correlations depend on the HIV-1 strain and the course of infection in each individual and therefore cannot be established with a cross-sectional analysis. Although it is clear that apoptosis is a function of overall immune activation, the quantifiable apoptosis in HIV-infected individuals is likely to be the result of two or more competing processes. This idea is supported by recent reports showing that chemical inhibition of apoptosis in PBMC from infected humans results in increased HIV replication, and it was suggested that apoptosis may be a protective mechanism to limit virus production (3, 11). Thus, during the clinically asymptomatic stage, there may be a steady-state level of HIV-1 established by virus production, cell killing, and regeneration of susceptible CD4+ cells. Such a scenario is consistent with the data of Ho et al. (28), Wei et al. (52), and Mellors et al. (36) showing that levels of viremia stabilize and become relatively constant 6 to 12 months after primary infection.
In addition to increased apoptosis of CD4+ T cells, significant ex vivo and in vitro CD8+-T-cell apoptosis was detected in PBMC from C-384 and C-166, as has been described for HIV-1-infected humans (8, 10, 23, 33, 37). Increased CD8+-T-cell apoptosis has also been detected in both uninfected and SIV-infected African and Asian monkeys and in one HIV-infected chimpanzee (13); however, the number of animals evaluated in each instance, especially in the apathogenic models, was too small to be meaningful. The presence of significant ex vivo CD8+-T-cell apoptosis in C-166 and C-384 may be relevant to the enhanced pathogenicity of HIV-1JC499, particularly in light of a recent report demonstrating Fas ligand-mediated killing by SIV-infected cells of CD8+ cytotoxic T lymphocytes (CTL) expressing Fas (54). This mechanism, in concert with clonal exhaustion of HIV-specific CTL clones, probably by activation-induced cell death, could reduce the overall frequency of virus-specific CTL and contribute to virus escape from immune surveillance (8, 10, 44). Conversely, the lack of CD8+-T-cell apoptosis in PBMC from C-487 may account for the continued maintenance of elevated levels of this T-cell subset and the relative stability over the past few years of this animal’s clinical condition and hematologic parameters.
Whether the mechanisms that effect apoptosis of CD4+ and CD8+ cells are the same or different also is unclear. There is a general consensus that Fas-Fas ligand interactions mediate CD4+-T-cell apoptosis, but not all investigators invoke this same cell-killing method as being responsible for the majority of CD8+-T-cell apoptosis (13). Involvement in HIV infection of the apoptosis pathway initiated by tumor necrosis factor and its receptor has been reported (5). Other proposed mechanisms for inducing apoptosis include direct interactions between gp120 Env and the CD4 molecule, which could not affect CD8+ T cells directly (12, 53). However, soluble Tat protein, which is secreted by HIV-infected cells, can interact with cellular components, resulting in enhanced expression of Fas (12, 34); this mechanism could affect CD8+ T cells. It will be of interest to determine specific mechanisms of apoptosis in HIV-1-infected chimpanzees and whether they differ from those in HIV-1-infected humans.
One factor that does appear to have a direct relationship to lymphocyte apoptosis in HIV-1-infected humans is the degree of immune hyperactivation in PBMC and LN (14, 39). All LN sections from the three progressor chimpanzees, but none of those from nonprogressors, demonstrated follicular hyperplasia. Preliminary analyses also indicate that follicular hyperplasia in the progressors is associated with trapping of viral p24gag antigen in germinal centers in a pattern consistent with its presence on follicular dendritic cells. Such trapping of antigens in HIV-1-infected chimpanzees has not been reported previously and was not evident in germinal centers from nonprogressor animals. Thus, in the three HIV-1-infected chimpanzees described here, loss of CD4+ T cells was associated with immune hyperactivation and an increase in lymphocyte apoptosis. These observations, which are in broad agreement with published studies on HIV-1-infected humans and SIV-infected macaques, provide additional evidence that increased apoptosis is a correlate of in vivo CD4+-T-cell depletion and is likely to be an important mode of T-cell loss in HIV infection. Future use of HIV-1JC499 in the HIV-1-chimpanzee model will provide an ideal system to identify specific correlations, time of onset, and mechanisms of induction of activation-induced apoptosis in HIV disease.
FIG. 6.
TUNEL-positive (apoptotic) lymphocytes in major histoarchitectural regions of LN tissue from progressor and control (nonprogressor and uninfected) chimpanzees. Three consecutive tissue sections were analyzed for each LN biopsy. ×, not done due to lack of available tissue. ∗, P < 0.05; ∗∗, P < 0.005; ∗∗∗, P < 0.0005. Error bars represent two standard errors of the mean.
ACKNOWLEDGMENTS
We thank James Mahoney and the veterinary staff at LEMSIP and Patrice Frost at the Coulston Foundation for inoculation and collection of blood and tissue samples, Elizabeth Muchmore for coordination of the studies at LEMSIP and Ali Javadian for coordination of those at the Coulston Foundation, Pamela May and Jackie Stallworth for assistance with lymphocyte preparation and immunostaining of PBMC for FACS analysis, Marion Spell for FACS data collection, Yafen Niu for determining HIV-1 RNA levels in plasma, Marilyn Shackleford for preparing histologic specimens, and Nancy Brissie for electron microscopy.
This work was supported in part by Pasteur Merieux Serum et Vaccins, the French National AIDS Research Agency (ANRS), NIH grant U01 AI28147, and an NIH grant to the University of Alabama at Birmingham Center for AIDS Research for shared facilities. I.C.D. was supported by NIH grant T32 RR07003.
REFERENCES
- 1.Ameisen J C. From cell activation to cell depletion. The programmed cell death hypothesis of AIDS pathogenesis. In: Andrieu J-M, Lu W, editors. Cell activation and apoptosis in HIV infection. New York, N.Y: Plenum Press; 1995. pp. 139–163. [PubMed] [Google Scholar]
- 2.Ameisen J C, Capron A. Cell dysfunction and depletion in AIDS: the programmed cell death hypothesis. Immunol Today. 1991;12:102–105. doi: 10.1016/0167-5699(91)90092-8. [DOI] [PubMed] [Google Scholar]
- 3.Antoni B A, Sabbatini P, Rabson A B, White E. Inhibition of apoptosis in human immunodeficiency virus-infected cells enhances virus production and facilitates persistent infection. J Virol. 1995;69:2384–2392. doi: 10.1128/jvi.69.4.2384-2392.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ascher M S, Sheppard H W. AIDS as immune system activation: a model for pathogenesis. Clin Exp Immunol. 1988;73:165–167. [PMC free article] [PubMed] [Google Scholar]
- 5.Badley A D, Dockrell D, Simpson M, Schut R, Lynch D H, Leibson P, Paya C V. Macrophage-dependent apoptosis of CD4+ T lymphocytes from HIV-infected individuals is mediated by FasL and tumor necrosis factor. J Exp Med. 1997;185:55–64. doi: 10.1084/jem.185.1.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bentin J, Tsoukas C, McCutchan J A, Spector S E, Richman D D, Vaughan J H. Impairment in T-lymphocyte responses during early infection with the human immunodeficiency virus. J Clin Immunol. 1989;9:159–168. doi: 10.1007/BF00916944. [DOI] [PubMed] [Google Scholar]
- 7.Biberfeld P, Porwit-Ksiazek A, Bottiger D, Morfeldt-Manson L, Biberfeld G. Immunohistopathology of lymph nodes in HTLV-III infected homosexuals with persistent adenopathy or AIDS. Cancer Res. 1985;45:4665s–4670s. [PubMed] [Google Scholar]
- 8.Boudet F, Lecoeur H, Gougeon M-L. Apoptosis associated with ex vivo down-regulation of Bcl-2 and up-regulation of Fas in potential cytotoxic CD8+ T lymphocytes during HIV infection. J Immunol. 1996;156:2282–2293. [PubMed] [Google Scholar]
- 9.Carbonari M, Cibati M, Cherchi M, Sbarigia D, Pesce A M, Dell’Anna L, Modica A, Fiorilli M. Detection and characterisation of apoptotic peripheral blood lymphocytes in human immunodeficiency virus infection and cancer chemotherapy by a novel flow immunocytometric method. Blood. 1994;83:1268–1277. [PubMed] [Google Scholar]
- 10.Carbonari M, Cibati M, Pesce A M, Sbarigia D, Grossi P, D’Offizi G, Luzi G, Fiorilli M. Frequency of provirus-bearing CD4+ cells in HIV type 1 infection correlates with extent of in vitro apoptosis of CD8+ but not of CD4+ cells. AIDS Res Hum Retroviruses. 1995;11:789–795. doi: 10.1089/aid.1995.11.789. [DOI] [PubMed] [Google Scholar]
- 11.Chinnaiyan A M, Woffendin C, Dixit V M, Nabel G J. The inhibition of pro-apoptotic ICE-like proteases enhances HIV replication. Nat Med. 1997;3:333–337. doi: 10.1038/nm0397-333. [DOI] [PubMed] [Google Scholar]
- 12.Cottrez F, Manca F, Dalgleish A G, Arenzana-Seisdedos F, Capon A, Groux H. Priming of human CD4+ antigen-specific T cells to undergo apoptosis by HIV-infected monocytes. A two-step mechanism involving the gp120 molecule. J Clin Invest. 1997;99:257–266. doi: 10.1172/JCI119154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Estaquier J, Idziorek T, DeBels F, Barré-Sinoussi F, Hurtrel B, Aubertin A-M, Venet A, Mehtali M, Muchmore E, Michel P, Mouton Y, Girard M, Ameisen J C. Programmed cell death and AIDS: significance of T cell apoptosis in pathogenic and nonpathogenic primate lentiviral infections. Proc Natl Acad Sci USA. 1994;91:9431–9435. doi: 10.1073/pnas.91.20.9431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Finkel T H, Tudor-Williams G, Banda N K, Cotton M F, Curiel T, Monks C, Baba T W, Ruprecht R M, Kupfer A. Apoptosis occurs predominantly in bystander cells and not in productively infected cells of HIV- and SIV-infected lymph nodes. Nat Med. 1995;1:129–134. doi: 10.1038/nm0295-129. [DOI] [PubMed] [Google Scholar]
- 15.Fultz P N. Nonhuman primate models for AIDS. Clin Infect Dis. 1993;17:S230–S235. doi: 10.1093/clinids/17.supplement_1.s230. [DOI] [PubMed] [Google Scholar]
- 16.Fultz P N, Gluckman J C, Muchmore E, Girard M. Transient increases in numbers of infectious cells in an HIV-infected chimpanzee following immune stimulation. AIDS Res Hum Retroviruses. 1992;8:313–317. doi: 10.1089/aid.1992.8.313. [DOI] [PubMed] [Google Scholar]
- 17.Fultz P N, Siegel R L, Brodie A, Mawle A C, Stricker R B, Swenson R B, Anderson D C, McClure H M. Prolonged CD4+ lymphocytopenia and thrombocytopenia in a chimpanzee persistently infected with human immunodeficiency virus type 1. J Infect Dis. 1991;163:441–447. doi: 10.1093/infdis/163.3.441. [DOI] [PubMed] [Google Scholar]
- 18.Fultz P N, Srinivasan A, Greene C R, Butler D, Swenson R B, McClure H M. Superinfection of a chimpanzee with a second strain of human immunodeficiency virus. J Virol. 1987;61:4026–4029. doi: 10.1128/jvi.61.12.4026-4029.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gavrieli Y, Sherman Y, Ben-Sasson S A. Identification of programmed cell death in situ via specific labelling of nuclear DNA fragmentation. J Cell Biol. 1992;119:493–501. doi: 10.1083/jcb.119.3.493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gendelman H E, Ehrlich G D, Baca L M, Conley S, Ribas J, Kalter D C, Meltzer M S, Poiesz B J, Nara P. The inability of human immunodeficiency virus to infect chimpanzee monocytes can be overcome by serial viral passage in vivo. J Virol. 1991;65:3853–3863. doi: 10.1128/jvi.65.7.3853-3863.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Girard M, Mahoney J, Rimsky L, Barré-Sinoussi F, Muchmore E, Weinhold K, Fultz P. HIV-1 genital infection: a chimpanzee model. In: Girard M, Valette L, editors. Retroviruses of human AIDS and related animal diseases. Lyon, France: Fondation Merieux; 1992. pp. 75–79. [Google Scholar]
- 22.Giorgi J V, Detels R. T-cell subset alterations in HIV-infected homosexual men: NIAID multicenter AIDS cohort study. Clin Immunol Immunopathol. 1989;52:10–18. doi: 10.1016/0090-1229(89)90188-8. [DOI] [PubMed] [Google Scholar]
- 23.Gougeon M-L, Garcia S, Heeney J, Tschopp R, Lecoeur H, Guetard D, Rame V, Dauguet C, Montagnier L. Programmed cell death in AIDS-related HIV and SIV infections. AIDS Res Hum Retroviruses. 1993;9:553–563. doi: 10.1089/aid.1993.9.553. [DOI] [PubMed] [Google Scholar]
- 24.Gougeon M-L, Lecoeur H, Boudet F, Ledru E, Marzabal S, Boullier S, Roue R, Nagata S, Heeney J. Lack of chronic immune activation in HIV-infected chimpanzees correlates with the resistance of T cells to Fas/Apo-1 (CD95)-induced apoptosis and preservation of a T helper 1 phenotype. J Immunol. 1997;158:2964–2976. [PubMed] [Google Scholar]
- 25.Gougeon M-L, Lecoeur H, Dulioust A, Enouf M-G, Crouvoisier M, Goujard C, Debord T, Montagnier L. Programmed cell death in peripheral lymphocytes from HIV-infected persons. Increased susceptibility to apoptosis of CD4 and CD8 T cells correlates with lymphocyte activation and with disease progression. J Immunol. 1996;156:3509–3520. [PubMed] [Google Scholar]
- 26.Heeney J, Jonker R, Koornstra W, Dubbes R, Niphuis H, DiRienzo A-M, Gougeon M-L, Montagnier L. The resistance of HIV-infected chimpanzees to progression to AIDS correlates with absence of HIV-related T-cell dysfunction. J Med Primatol. 1993;22:194–200. [PubMed] [Google Scholar]
- 27.Hirsch V M, Johnson P R. Pathogenic diversity of simian immunodeficiency viruses. Virus Res. 1994;32:183–203. doi: 10.1016/0168-1702(94)90041-8. [DOI] [PubMed] [Google Scholar]
- 28.Ho D D, Neumann A U, Perelson A S, Chen W, Leonard J M, Markowitz M. Rapid turnover of plasma virions and CD4 lymphocytes in HIV-1 infection. Nature. 1995;373:123–126. doi: 10.1038/373123a0. [DOI] [PubMed] [Google Scholar]
- 29.Johnson B K, Stone G A, Godec M S, Asher D M, Gajdusek D C, Gibbs C J. Long-term observations of human immunodeficiency virus-infected chimpanzees. AIDS Res Hum Retroviruses. 1993;9:375–378. doi: 10.1089/aid.1993.9.375. [DOI] [PubMed] [Google Scholar]
- 30.Katsikis P D, Wunderlich E S, Smith C A, Herzenberg L A, Herzenberg L A. Fas antigen stimulation induces marked apoptosis of T lymphocytes in human immunodeficiency virus-infected individuals. J Exp Med. 1995;181:2029–2036. doi: 10.1084/jem.181.6.2029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Laurent-Crawford A G, Krust B, Muller S, Riviere Y, Rey-Cuille M-A, Bechet J-M, Montagnier L, Hovanessian A G. The cytopathic effect of HIV is associated with apoptosis. Virology. 1991;185:829–839. doi: 10.1016/0042-6822(91)90554-o. [DOI] [PubMed] [Google Scholar]
- 32.Levy J A. Pathogenesis of human immunodeficiency virus infection. Microbiol Rev. 1993;57:183–289. doi: 10.1128/mr.57.1.183-289.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lewis D E, Tang D S N, Adu-Oppong A, Schober W, Rodgers J R. Anergy and apoptosis in CD8+ T cells from HIV-infected persons. J Immunol. 1994;153:412–420. [PubMed] [Google Scholar]
- 34.Li C J, Friedman D J, Wang C, Metelev V, Pardee A B. Induction of apoptosis in uninfected lymphocytes by HIV-1 Tat protein. Science. 1995;268:429–431. doi: 10.1126/science.7716549. [DOI] [PubMed] [Google Scholar]
- 35.Martin S J, Matear P M, Vyakarnam A. HIV-1 infection of human CD4+ T cells in vitro: differential induction of apoptosis in these cells. J Immunol. 1994;152:330–342. [PubMed] [Google Scholar]
- 36.Mellors J W, Kingsley L A, Rinaldo C R, Todd J A, Hoo B S, Kokka R P, Gupta P. Quantitation of HIV-1 RNA in plasma predicts outcome after seroconversion. Ann Intern Med. 1995;122:573–579. doi: 10.7326/0003-4819-122-8-199504150-00003. [DOI] [PubMed] [Google Scholar]
- 37.Meyaard L, Otto S A, Jonker R R, Mijnster M J, Keet R P M, Miedema F. Programmed death of T cells in HIV-1 infection. Science. 1992;257:217–219. doi: 10.1126/science.1352911. [DOI] [PubMed] [Google Scholar]
- 38.Meyaard L, Otto S A, Keet I P M, Roos M T L, Miedema F. Programmed death of T cells in human immunodeficiency virus infection: no correlation with progression to disease. J Clin Invest. 1994;93:982–988. doi: 10.1172/JCI117105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Muro-Cacho C A, Pantaleo G, Fauci A S. Analysis of apoptosis in lymph nodes of HIV-infected persons. Intensity of apoptosis correlates with the general state of activation of the lymphoid tissue and not with stage of disease or viral burden. J Immunol. 1995;154:5555–5566. [PubMed] [Google Scholar]
- 40.Novembre F J, Saucier M, Anderson D C, Klumpp S A, O’Neil S P, Brown C R, Hart C E, Guenthner P C, Swenson R B, McClure H M. Development of AIDS in a chimpanzee infected with human immunodeficiency virus type 1. J Virol. 1997;71:4086–4091. doi: 10.1128/jvi.71.5.4086-4091.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Oyaizu N, Pahwa S. Role of apoptosis in HIV disease pathogenesis. J Clin Immunol. 1995;15:217–231. doi: 10.1007/BF01540879. [DOI] [PubMed] [Google Scholar]
- 42.Pandolfi F, Pierdominici M, Oliva A, D’Offizi G, Mezzaroma I, Mollicone B, Giovannetti A, Rainaldi L, Quinti I, Aiuti F. Apoptosis-related mortality in vitro of mononuclear cells from patients with HIV infection correlates with disease severity and progression. J Acquired Immune Defic Syndr Hum Retrovirol. 1995;9:450–458. [PubMed] [Google Scholar]
- 43.Pantaleo G, Fauci A S. New concepts in the immunopathogenesis of HIV infection. Annu Rev Immunol. 1995;13:487–512. doi: 10.1146/annurev.iy.13.040195.002415. [DOI] [PubMed] [Google Scholar]
- 44.Pantaleo G, Soudeyns H, Demarest J F, Vaccarezza M, Graziosi C, Paolucci S, Daucher M, Cohen O J, Denis F, Biddison W E, Sekaly R P, Fauci A S. Evidence for rapid disappearance of initially expanded HIV-specific CD8+ T cell clones during primary HIV infection. Proc Natl Acad Sci USA. 1997;94:9848–9853. doi: 10.1073/pnas.94.18.9848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rey-Cuille M-A, Golobru J, Laurent-Crawford A, Krust B, Montagnier L, Hovanessian A G. HIV-2 EHO isolate has a divergent envelope gene and induces single cell killing by apoptosis. Virology. 1994;202:471–476. doi: 10.1006/viro.1994.1364. [DOI] [PubMed] [Google Scholar]
- 46.Rothen M, Gratzl S, Hirsch H H, Moroni C. Apoptosis in HIV-infected individuals is an early marker occurring independently of high viremia. AIDS Res Hum Retroviruses. 1997;13:771–779. doi: 10.1089/aid.1997.13.771. [DOI] [PubMed] [Google Scholar]
- 47.Schmid I, Uittenbogaart C H, Keld B, Giorgi J V. A rapid method for measuring apoptosis and dual-color immunofluorescence by single laser flow cytometry. J Immunol Methods. 1994;170:145–157. doi: 10.1016/0022-1759(94)90390-5. [DOI] [PubMed] [Google Scholar]
- 48.Schuitemaker H, Meyaard L, Kootstra N A, Dubbes R, Otto S A, Tersmette M, Heeney J L, Miedema F. Lack of T cell dysfunction and programmed cell death in human immunodeficiency virus type 1-infected chimpanzees correlates with absence of monocytotropic variants. J Infect Dis. 1993;168:1140–1147. doi: 10.1093/infdis/168.5.1140. [DOI] [PubMed] [Google Scholar]
- 49.Villinger F, Brar S S, Brice G T, Chikkala N F, Novembre F J, Mayne A E, Bucur S, Hillyer C D, Ansari A A. Immune and hematopoietic parameters in HIV-1-infected chimpanzees during clinical progression towards AIDS. J Med Primatol. 1997;26:11–18. doi: 10.1111/j.1600-0684.1997.tb00314.x. [DOI] [PubMed] [Google Scholar]
- 50.Watanabe M, Ringler D J, Fultz P N, MacKey J J, Boyson J E, Levine C G, Letvin N L. A chimpanzee-passaged human immunodeficiency virus isolate is cytopathic for chimpanzee cells but does not induce disease. J Virol. 1991;65:3344–3348. doi: 10.1128/jvi.65.6.3344-3348.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wei Q, Fultz P N. Extensive diversification of human immunodeficiency virus type 1 subtype B strains during dual infection of a chimpanzee that progressed to AIDS. J Virol. 1998;72:3005–3017. doi: 10.1128/jvi.72.4.3005-3017.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wei X, Ghosh S K, Taylor M E, Johnson V A, Emini E A, Deutsch P, Lifson J D, Bonhoeffer S, Nowak M A, Hahn B H, Saag M S, Shaw G M. Viral dynamics in human immunodeficiency virus type 1 infection. Nature. 1995;373:117–122. doi: 10.1038/373117a0. [DOI] [PubMed] [Google Scholar]
- 53.Westendorp M O, Frank R, Ochsenbauer C, Stricker K, Dhein J, Walczak H, Debatin K-M, Krammer P H. Sensitization of T cells to CD95-mediated apoptosis by HIV-1 Tat and gp120. Nature. 1995;375:497–500. doi: 10.1038/375497a0. [DOI] [PubMed] [Google Scholar]
- 54.Xu X-N, Screaton G R, Gotch F M, Dong T, Tan R, Almond N, Walker B, Stebbings R, Kent K, Nagata S, Stott J E, McMichael A J. Evasion of cytotoxic T lymphocyte (CTL) responses by nef-dependent induction of Fas ligand (CD95L) expression on simian immunodeficiency virus-infected cells. J Exp Med. 1997;186:7–16. doi: 10.1084/jem.186.1.7. [DOI] [PMC free article] [PubMed] [Google Scholar]