Abstract
It is well known that DNA damage can cause apoptosis. However, whether apoptosis and its metabolites contribute to DNA repair is largely unknown. In this study, we found that apoptosis‐deficient Fasmut and Bim− /− mice show significantly elevated DNA damage and premature cellular senescence, along with a significantly reduced number of 16,000 g apoptotic vesicles (apoVs). Intravenous infusion of mesenchymal stromal cell (MSC)‐derived 16,000 g apoVs rescued the DNA damage and premature senescence in Fasmut and Bim −/− mice. Moreover, a sublethal dose of radiation exposure caused more severe DNA damage, reduced survival rate, and loss of body weight in Fasmut mice than in wild‐type mice, which can be recovered by the infusion of MSC‐apoVs. Mechanistically, we showed that apoptosis can assemble multiple nuclear DNA repair enzymes, such as the full‐length PARP1, into 16,000 g apoVs. These DNA repair components are directly transferred by 16,000 g apoVs to recipient cells, leading to the rescue of DNA damage and elimination of senescent cells. Finally, we showed that embryonic stem cell‐derived 16,000 g apoVs have superior DNA repair capacity due to containing a high level of nuclear DNA repair enzymes to rescue lethal dose‐irradiated mice. This study uncovers a previously unknown role of 16,000 g apoVs in safeguarding tissues from DNA damage and demonstrates a strategy for using stem cell‐derived apoVs to ameliorate irradiation‐induced DNA damage.
Keywords: apoptosis, apoptotic vesicles, DNA damage repair, DDR‐apoV complexes, embryonic stem cells, mesenchymal stromal cells, PARP1, premature senescence, radiation damage
1. INTRODUCTION
Apoptosis is a highly conserved process that occurs in both physiological and pathological conditions in our daily life (Fuchs & Steller, 2011). Billions of cells experience apoptosis every day (Arandjelovic & Ravichandran, 2015), generating a substantial number of apoptotic vesicles (apoVs), which are critical for maintaining organ and body homeostasis (F. Lei et al., 2023; Liu et al., 2018; Ma et al., 2023; Rausch et al., 2023). ApoVs, a specific type of extracellular vesicles (EVs) released during apoptosis, include apoptotic bodies ranging from 1 to 5 µm, apoptotic microvesicles ranging from 0.1 to 1 µm, and apoptotic exosomes less than 150 nm in diameter, carrying proteins, nucleic acids, lipids, and other molecules inherited from the parent cells (Akers et al., 2013; Liu et al., 2018; Lotvall et al., 2014; J. Wang et al., 2021; Yáñez‐Mó et al., 2015; Zhang et al., 2022). During apoptosis, plentiful nuclear‐related components, including proteins and nucleic acids, are packaged into apoVs, setting them apart from physiologically released EVs (Akers et al., 2013; Elzanowska et al., 2021; He et al., 2009; Malkin & Bratman, 2020; Qu et al., 2022; Yáñez‐Mó et al., 2015; Zhang et al., 2022). The role of these inherited nuclear materials in apoVs has not yet been fully elucidated.
DNA damage occurs routinely in the human body, resulting from various factors like UV radiation, X‐rays, γ‐rays, chemical agents, and reactive oxygen species (ROS) (Chatterjee & Walker, 2017; Jeggo et al., 2016; Liao et al., 2022; Norbury & Zhivotovsky, 2004; Schumacher et al., 2021). Accumulation of DNA damage can lead to senescence and cancer (Jeggo et al., 2016; Schumacher et al., 2021). It is widely known that cells possess a complicated DNA repair system to address DNA damage, but whether the apoptotic metabolism is involved in DNA repair remains unknown (Chatterjee & Walker, 2017).
Radiation damage is considered a significant clinical disorder that must be prevented in warfare and even in daily life, a challenge that is projected to take on increasing importance in the future (Mettler & Voelz, 2002; Sage & Shikazono, 2017; Santivasi & Xia, 2014). Nevertheless, the Critical Medicines List for Radiological and Nuclear Emergencies provided by the World Health Organization (WHO) in 2023 does not include any medicines specifically designed to promote DNA damage repair. Thus, there is a pressing necessity for developing practical therapeutic approaches for repairing DNA damage.
In this study, we showed that apoptosis deficiency‐induced reduction of apoVs in mouse models leads to multi‐organ premature cellular senescence due to accelerated DNA damage. Systemic infusion of apoVs can rescue apoptosis deficiency‐induced premature senescence and DNA damage, revealing that apoptotic metabolism contributes to DNA repair.
2. MATERIALS AND METHODS
The detailed methods for cell culture, characterization of apoVs and EVs (including NTA and nano FCM), western blot, immunofluorescence staining, flow cytometry analysis, proteomic analysis, cell cycle analysis and RT‐qPCR are provided in the supplementary materials.
2.1. Mice
B6.129S1‐Bcl2l11tm1.1Ast/J (Bim −/−) and B6.MRL‐Faslpr/J (Fasmut ) mice were purchased from the Jackson Laboratory. C57BL/6J mice were purchased from Sun Yat‐sen University, Guangzhou, China. Approval for the animal study protocol was obtained from the Institutional Animal Care and Use Committee at Sun Yat‐sen University (SYSU‐IACUC‐2022‐001472).
2.2. Antibodies and reagents
All antibodies, chemical reagents, kits, RNA, and other resources are listed in Supplementary Table 1.
2.3. Apoptosis‐deficient mouse models
Apoptosis‐deficient mouse models include Fasmut , Bim −/−, and Z‐VAD‐FMK‐induced mice in this study. The 16,000 g mBMMSC‐apoV treatment for Fasmut and Bim −/− mice started at the age of four months, at which time they began to receive a weekly intravenous injection of 20 µg (4 × 109) mBMMSC‐apoVs per mouse for two consecutive months. The control group received PBS injection instead. To obtain Z‐VAD‐FMK‐induced mice, we injected C57BL/6 mice once a week with a dosage of 12.5 µg Z‐VAD‐FMK per gram of body weight for two consecutive months. The treatment group received an additional injection of 20 µg (4 × 109) apoVs per mouse on the third day after Z‐VAD‐FMK injection each week, also for a duration of two months.
2.4. Irradiated mouse models
To obtain an irradiated mouse model, we used an X‐ray irradiator (RS2000, Rad Source, the USA) to irradiate mice at a dose rate of 1.025 grey per minute, with a sublethal dose of 5.5 Gy or lethal dose of 6 Gy. Following irradiation, the treatment group of mice received intravenous injection of 16,000 g apoVs at a dosage of 20 µg (4 × 109) per mouse, while the control group was given PBS instead. To establish a cellular model of radiation injury, we irradiated mBMMSCs with a dose of 10 Gy at a dose rate of 1.338 grey per minute. The irradiated cells were immediately co‐cultured with 16,000 g apoVs.
2.5. Isolation of 16,000 g tissue‐derived EVs
To prepare the 16,000 g tissue‐derived EV samples, equal amounts (50 mg) of tissue samples were measured and placed in 1 mL 10% Liberase TM solution. To isolate EVs located within the interstitial tissues, they were then minced and ground in the solution and transferred to a 37°C shaker set at a speed of 180 rpm for 30 min for digestion as previously reported (Crescitelli et al., 2021). Following digestion, sequential centrifugations were conducted at 800 g for 10 min and 2,000 g for 10 min to obtain supernatants. After centrifuging at 16,000 g for 30 min, tissue‐derived EVs at 16,000 g were collected from the supernatants. These vesicles were subsequently used for further analysis.
2.6. Isolation of 16,000 g apoVs and 120,000 g EVs from cultured cells
Apoptosis induction in MSCs or ESCs was conducted following previously documented procedures with modifications (Qu et al., 2022). Cells were treated with 500 nM staurosporine (STS) in α‐MEM medium for 6–14 h (6 h for human BMMSCs and UCMSCs, 9 h for ESCs, 12‐14 h for mouse BMMSCs). ApoVs were obtained from apoptotic cells through sequential centrifugation. After centrifugation at 800 g for 10 min and subsequently at 2,000 g for 10 min at 4°C, the supernatant was centrifuged at 16,000 g for 30 min to isolate apoVs. Isolation of 120,000 g EVs from cultured MSCs was performed as described in previous study (Zhang et al., 2022).
2.7. Senescence‐associated β‐galactosidase (SA‐β‐Gal) staining
The cellular senescence assays of frozen sections and BMMSCs were performed following the instructions provided by the Cellular Senescence Assay Kit (Merck Millipore, Germany).
2.8. Comet assay
After the experimental treatment, mBMMSCs were harvested by briefly centrifuging and resuspended into cold PBS at a concentration of approximately 106 cells/ml. The comet assay was performed as described previously (Jiang et al., 2023). The detailed experimental procedures and the reagents employed are provided in supplementary materials. Analysis was conducted using fluorescence microscopy, selecting at least 50 cells per sample, and calculating the tail moment score using CaspLab 1.2.3_b2.
2.9. siRNA transfection
To obtain PARP1‐knockdown apoVs, siRNA‐PARP1 (Ribo, China) was initially introduced into mouse BMMSCs via Lipofectamine RNAiMAX transfection reagent (Thermo Fisher). Then the 16,000 g siPARP1‐apoVs were isolated from PARP1‐knockdown cells after inducing apoptosis with STS.
2.10. Statistical analysis
Statistical analysis was performed using GraphPad Prism 8.0 software. Independent unpaired two‐tailed Student's t‐tests were used to compare data between two groups, whereas one‐way ANOVA were employed for comparisons involving more than two groups.
3. RESULTS
3.1. Apoptosis deficiency leads to DNA damage and premature cellular senescence
Apoptosis can be induced by extrinsic or intrinsic pathway, both of which can lead to the same execution procedure (Fulda & Debatin, 2006). Fas and Bim are vital molecules in the extrinsic and intrinsic apoptotic pathways, respectively (Brunner et al., 1995; D. C. Huang & Strasser, 2000; Hughes et al., 2008). Previous studies have demonstrated reduced apoptotic rates in both Fas mutant (Fasmut ) and Bim knockout (Bim −/−) mice compared to WT mice (Bouillet et al., 1999; Herold et al., 1446; Liu et al., 2018). In this study, we showed that six‐month‐old Fasmut and Bim −/− mice exhibited hair impairment and premature senescence compared to WT mice (Figures 1a‐c and S1a‐c). To investigate whether these disorders are associated with the deficiency of apoptotic metabolism, we utilized another apoptosis‐deficient mouse model induced by Z‐VAD‐FMK, a well known pan‐caspase inhibitor (Van Noorden, 2001). Z‐VAD‐FMK was administered to WT mice via tail vein injection at a dosage of 12.5 µg per gram body weight weekly for a two‐month period. Following this treatment, the mice displayed similar hair impairment and premature cellular senescence to those observed in the Fasmut and Bim −/− mice (Figure S1a‐c). SA‐β‐Gal staining showed that elevated β‐galactosidase activity in the heart, liver, lung, kidney, and spleen of Fasmut, Bim −/−, and Z‐VAD‐FMK‐induced mice compared to age‐matched WT mice (Figures 1b,c and S1b,c). γH2AX is a widely utilized marker for DNA double‐strand breaks (DSBs), indicating DNA damage and participating in the cellular response to DNA repair (Mah et al., 2010). Here, we observed a significant elevation in the number of γH2AX foci in the heart, liver, lung, and spleen of these three apoptosis‐deficient mouse models compared to WT mice (Figures 1d,e and S1d,e).
FIGURE 1.

Apoptosis‐ deficient Fasmut mice show premature senescence and DNA damage phenotypes. (a) Representative images of 6‐month‐old Fasmut and C57BL/6 mice. Fasmut mice showed a premature ageing phenotype with hair loss and ageing morphology. (b, c) Representative images (b) and quantification (c) of SA‐β‐Gal positive areas in the heart, liver, lung, kidney, and spleen from 6‐month‐old Fasmut and C57BL/6 mice (n = 3). Scale bar = 50 µm. (d, e) Immunofluorescent (IF) staining (d) and quantification (e) of γH2AX foci in the heart, liver, lung, kidney and spleen from 6‐month‐old Fasmut and C57BL/6 mice. 30 cells were analyzed from each mouse (n = 3). Scale bar = 10 µm. (f) Immunofluorescent images (left) and quantification (right) of γH2AX foci (30 cells were analyzed from each mouse) and the comet assay (50 cells were analyzed from each mouse) of bone marrow mesenchymal stromal cells (BMMSCs) isolated from 6‐month‐old Fasmut and C57BL/6 mice (n = 3). Scale bar = 10 µm. (g, h) Representative images and quantification of SA‐β‐Gal, P16 and P21 positive BMMSCs of Fasmut and C57BL/6 mice (n = 3). Scale bar = 50 µm. (i) Western blot analysis depicted the expression of P53, P21, P16 and γH2AX in BMMSCs isolated from Fasmut and C57BL/6 mice. The graph bars show the mean ± SD. SD, standard deviation. *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001. ns, not significant.
Next, we isolated and cultured bone marrow mesenchymal stromal cells (BMMSCs) from Fasmut and Bim −/‐ mice and found an increased levels of DNA damage and cellular senescence in these BMMSCs compared to WT mouse‐derived BMMSCs, as evidenced by elevated expression of γH2AX, SA‐β‐Gal, P16 and P21 as well as increased comet tail length (Olive & Banath, 2006) (Figures 1f‐i and S1f‐k).
To investigate whether the premature cellular senescence and DNA damage phenotypes in these mouse models are associated with their defects in apoptotic metabolites, we examined the levels of apoVs in various organs including the heart, liver, spleen, lung and kidney. Previous studies have demonstrated that apoVs with an average size of 100–200 nm can be isolated through gradient centrifugation at 16,000 g4,10. Therefore, we used enzymatic digestion and gradient centrifugation to isolate EVs from 50 mg of tissue obtained from multiple organs of Fasmut , Bim −/− and Z‐VAD‐FMK‐induced mice (Figure 2a). The quantity, average size, and charge of these vesicles were measured by Zeta View. Our results showed that the quantities of 16,000 g EVs in the heart, liver, spleen, lung and kidney of these three mouse models were significantly lower than those in WT mice, while no significant differences were observed in terms of size or surface charge (Figure 2b‐d).
FIGURE 2.

The quantity of apoVs were markedly reduced in multiple organs of apoptosis‐deficient mice. (a) Schematic diagram indicating the procedures for isolating tissue‐derived 16,000 g EVs. (b, c) Representative size and membrane potential of tissue‐derived 16,000 g EVs (from the liver). Nanoparticle tracking analysis (NTA) was calibrated using standard beads of 100 nm by Zeta View. (d) NTA exhibited the total number of 16,000 g vesicles isolated from 50 mg of different tissues in C57BL/6, Fasmut , Bim−/− and Z‐VAD‐FMK induced mice. n = 3 for each group. (e, f) Nano Flow cytometry (nFCM) analysis revealed the Annexin V‐positive vesicles isolated from 50 mg of different tissues from C57BL/6, Fasmut , Bim−/− and Z‐VAD‐FMK induced mice (n = 3). (g) Liver‐derived 16,000 g EV expression of apoV specific markers caspase‐3, cleaved caspase‐3, PARP1, cleaved PARP1, caspase‐8, cleaved caspase‐8, and calreticulin. (h) Linear regression plots showing the correlation between the total number of Annexin V‐positive vesicles and the experimental parameters including the percentages of SA‐β‐Gal positive area and γH2AX foci number in different tissues (including heart, liver, lung, kidney and spleen) of C57BL/6, Fasmut , Bim−/− and Z‐VAD‐FMK induced mice (n = 3). The graph bars show the mean ± SD. *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001. ns, not significant.
Phosphatidylserine is recognized as one of the surface markers of apoVs which can be indicated by Annexin V (Rausch et al., 2023; Zhang et al., 2022). We further analyzed the expression of Annexin V in these 16,000 g EVs using nano flow cytometry (nano FCM). Consistently, the numbers of Annexin V‐positive EVs in the heart, liver, spleen, lung and kidney of Fasmut , Bim−/− and Z‐VAD‐FMK induced mice were significantly lower than that in WT mice (Figure 2e,f). Furthermore, western blot analysis showed reduced expression of apoV markers (Zhang et al., 2022), including caspase 3, cleaved caspase 3, PARP1, cleaved PARP1, caspase 8, cleaved caspase 8, and calreticulin in 16,000 g EVs isolated from apoptosis‐deficient mice when compared to those from WT mice (Figure 2g). Meanwhile, we examined the 16,000 g EVs isolated from the circulation of these three mouse models and found that the quantity of 16,000 g EVs in an equivalent serum volume (200 µL) was consistently lower compared to the WT group, accompanied with a decreased number of Annexin V‐positive EVs (Figure S2a‐c). Overall, these findings indicate that the levels of apoVs in the circulation, heart, liver, spleen, lung and kidney of Fasmut , Bim −/− and Z‐VAD‐FMK‐induced mice are lower than those in WT mice.
These findings further suggest a potential association between the reduced quantity of apoVs and the extent of multi‐organs premature senescence and DNA damage. We conducted correlation analyses between the quantity of Annexin V‐positive EVs and both SA‐β‐Gal positivity rates and the numbers of γH2AX foci in the heart, liver, spleen, lung and kidney (Figure 2h). Interestingly, we noticed a negative correlation between the quantity of Annexin V‐positive EVs and both the SA‐β‐Gal positivity rates and the numbers of γH2AX foci (Figure 2h). These data suggest that apoV deficiency may contribute to systemic multi‐organs premature senescence and DNA damage.
3.2. 16,000 g MSC‐apoVs ameliorate premature senescence and DNA damage in apoptosis‐deficient mouse models
To further explore the potential relationship between the reduction of apoVs and the premature senescence and DNA damage phenotypes in Fasmut , Bim− /− and Z‐VAD‐FMK‐induced mice, we isolated and expanded BMMSCs from WT mice as previously reported (Miura et al., 2004). BMMSC apoptosis was induced by staurosporine (STS) and MSC‐apoVs were collected by gradient centrifugation as previously described (J. Wang et al., 2021). We intravenously infused 20 µg (4 × 109) of 16,000 g mBMMSC‐apoVs per mouse into these three mouse models once a week for two consecutive months. Significant improvements were observed in cellular senescence and DNA damage, including hair impairment, SA‐β‐Gal positivity rate and the number of γH2AX foci in the heart, liver, spleen, lung and kidney (Figures 3a‐e and S3a‐e). Furthermore, we co‐cultured Fasmut and Bim −/− BMMSCs with MSC‐apoVs for 24 h, resulting in decreased levels of cellular senescence markers, including SA‐β‐Gal, P16 and P21, as well as reduced DNA damage, as evidenced by the expression of γH2AX and comet tail length (Figures 3f‐i and S3f‐k). These findings suggest that MSC‐apoVs can improve premature cellular senescence and DNA damage.
FIGURE 3.

MSC‐apoVs ameliorate premature senescence and DNA damage phenotypes in Fasmut mice. (a) Representative images of Fasmut and MSC‐apoV‐treated Fasmut mice. Systemic injection of MSC‐apoVs for 2 months ameliorated a premature ageing phenotype with hair loss and ageing morphology of Fasmut mice. (b, c) Representative images (b) and quantification (c) of SA‐β‐Gal positive areas in the heart, liver, lung, kidney, and spleen from Fasmut and MSC‐apoV‐treated Fasmut mice (n = 5). Scale bar = 50 µm. (d, e) IF staining (d) and quantification (e) of γH2AX foci in the heart, liver, lung, kidney, and spleen from Fasmut and MSC‐apoV‐treated Fasmut mice. 30 cells were analyzed from each mouse (n = 3). Scale bar = 10 µm. (f) Immunofluorescent images (left) and quantification (right) of γH2AX foci (35 individual cells were analyzed in each group) and the comet assay (50 individual cells were analyzed in each group) of Fasmut BMMSCs with or without MSC‐apoV treatment. Cells were co‐culture with PBS or apoVs for 24 h. Scale bar = 10 µm. (g, h) Representative images (g) and quantification (h) of SA‐β‐Gal, P16 and P21 positive BMMSCs of Fasmut BMMSCs with or without MSC‐apoV treatment (n = 3). Scale bar = 50 µm. (i) Western blot analysis showed the expression of P53, P21, P16 and γH2AX in Fasmut BMMSCs with or without MSC‐apoV treatment. (j) Graphical summary illustrates MSC‐apoVs ameliorate premature senescence and DNA damage phenotypes in apoptosis‐ deficient mice. The graph bars show the mean ± SD. *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001. ns, not significant.
3.3. Apoptosis‐deficient mice are more sensitive to irradiation‐induced DNA damage
The above experimental results preliminarily demonstrated the correlation between the deficiency of apoVs and the reduced DNA repair capacity. To further confirm this correlation, we generated a classic animal model of DNA damage induced by X‐ray irradiation (Chang et al., 2016; Fooladi et al., 2022; Murphy & Kamen, 2019). First, we exposed normal WT mice and apoV‐deficient Fasmut mice to 5.5 grey sublethal dose of total‐body irradiation (TBI) by X‐ray irradiator (RS2000, Rad Source, the USA). Fasmut mice were more susceptible to radiation‐induced damage compared to WT mice (Figure 4a). All Fasmut mice either died or reached humane endpoints within 12 days, whereas WT mice exhibited significantly higher survival rates (14‐day survival rate: 4/7, 30‐day survival rate: 2/7) (Figure 4a). To confirm the radioprotective effects of apoVs, we administered 16,000 g mBMMSC‐apoVs at a dose of 20 µg (4 × 109) per Fasmut and WT mouse (Figure 4a). Additionally, we established a pre‐apoV treatment group in which Fasmut mice were given an equal dose of 16,000 g mBMMSC‐apoVs at 12 h prior to X‐ray irradiation. In Fasmut mice, both apoV and pre‐apoV treatment group exhibited improved survival rates (30‐day survival rates of 3/7 and 2/7, respectively), prolonged survival time (Figure 4a), reduced weight loss (Figure 4b), decreased numbers of γH2AX foci in multiple organs (Figure 4c‐g) and improved 24‐h survival of bone marrow cells when compared to the PBS control group (Figure 4h and Supplementary Video 1). Notably, the pre‐apoV group of Fasmut mice, which received MSC‐apoVs prior to irradiation, exhibited a survival rate similar to that of the WT group (Figure 4a).
FIGURE 4.

Apoptosis‐deficient mice are more sensitive to irradiation‐induced DNA damage. (a) Schematic diagram illustrating the sublethal total‐body irradiation (TBI) mouse model (5.5 Gy of X‐ray exposure). The mice were divided into five groups: Fasmut mice pretreated with MSC‐apoVs, and Fasmut and C57BL/6 mice treated with MSC‐apoVs or PBS immediately after radiation. Kaplan−Meier survival curves showed the survival rates of each group. n = 7 for each group. (b) Weight loss of Fasmut and WT mice was evaluated after X‐ray radiation. n = 7 for each group. (c) Representative IF images of γH2AX foci in the liver, lung, kidney and spleen from each group. Scale bar = 20 µm. (d‐g) The quantification of γH2AX foci in the liver, spleen, lung and kidney from irradiated mice. 30 cells were analyzed from each mouse (n = 3). (h) Survival rates of bone marrow cells of each group 24 h after radiation. n = 3 for each group. The graph bars show the mean ± SD. *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001. ns, not significant.
As for WT mice, the apoV group showed higher survival rates (30‐day survival rate: 5/7) than the PBS group (30‐day survival rate: 2/7), along with prolonged survival time (Figure 4a), reduced weight loss (Figure 4b), decreased numbers of γH2AX foci in multiple organs (Figure 4c‐g) and improved 24‐h survival of bone marrow cells compared to the PBS group (Figure 4h). Among the tissues we examined, the bone marrow was the most severely damaged after irradiation (Jiang et al., 2023; Mettler & Voelz, 2002). We harvested bone marrow cells from a femur and tibia, lysed the red blood cells, and counted the total number of cells as previously described (Zhao et al., 2014). We found that MSC‐apoVs significantly prevented the bone marrow cells depletion in both Fasmut and WT mice measured at 24 h post irradiation (Figure 4h). Flow cytometry analysis revealed higher survival rates of haematopoietic progenitors (lin‐, c‐kit+, Scal‐1+), B lymphocytes (B220+) monocytes (CD11b+, Ly6C+), and neutrophils (CD11b+, Ly6G+) in both MSC‐apoV and pre‐MSC‐apoV treatment groups compared to the PBS group (Figure S4b, c). Meanwhile, morphological characteristics of all surviving mice were recorded at 7, 14, and 30 days after irradiation (Figure S4a). Notably, at the day 7, the most of Fasmut mice in PBS group exhibited body curling, stiffness, and weakness, with one mortality. One WT mouse in PBS group also exhibited slightly body curling (Figure S4a). However, mice treated with MSC‐apoVs and pre‐MSC‐apoVs showed more active compared to the PBS group (Supplementary Video 1). By the day 30, the surviving mice in the MSC‐apoV and pre‐MSC‐apoV treatment groups fully recovered (Figure S4a and Supplementary Video 2).
3.4. 16,000 g MSC‐apoVs promote DNA repair in vitro
To further confirm the DNA repair capabilities of 16,000 g mBMMSC‐apoVs, we established an in vitro BMMSC DNA damage model using X‐ray irradiation (Zuo et al., 2019). BMMSCs were irradiated with a dose of 10 grey, and MSC‐apoV treatment was administered after the irradiation (Figure 5a). BMMSC samples were collected at different time points for further analysis. First, MSC‐apoVs were stained with PKH26 and co‐cultured with the irradiated BMMSCs (Figure 5a). We observed a significant uptake of MSC‐apoVs in the irradiated BMMSCs at 1 h post irradiation, where MSC‐apoVs colocalized with DNA double‐strand break marker γH2AX (Figure 5a). These finding indicate that MSC‐apoVs can be effectively internalized and utilized by irradiated BMMSCs. Then, irradiated BMMSCs co‐cultured with MSC‐apoVs for 1 h were collected for qPCR analysis (Figure 5b). The results revealed that treatment with MSC‐apoVs led to an increase in mRNA expression for various proteins associated with cell cycle, DNA single‐strand repair, homologous recombination (HR), and non‐homologous end joining (NHEJ) pathways, including chk1, chk2, Msh5, PARP1, XRCC1, BRCA1, BARD1, XRCC4, XRCC6, Prkdc, NHEJ1 and Lig4. DNA DSBs are the most serious type of DNA damage caused by irradiation (Santivasi & Xia, 2014). PARP1 and 53BP1 are considered critical sensors for DSBs during irradiation‐induced damage (D. Huang & Kraus, 2022; Panier & Boulton, 2014). It is suggested that the repair of DNA DSBs mainly involves in HR and NHEJ pathways (Chatterjee & Walker, 2017; Shrivastav et al., 2008). BRCA1 and KU70 are proteins that initially bind to the sites of DNA breaks in these two pathways, respectively (Caestecker & Van de Walle, 2013; Pastwa & Blasiak, 2003). We found an increased fluorescence intensity of 53BP1, PARP1, BRCA1 and KU70 in the nuclei of the MSC‐apoV treatment group, suggesting that MSC‐apoVs may accelerate the recruitment of these proteins contributing to DNA DSB repair (Figure 5c,d). When cells experienced severe DNA damage, they activate self‐protective mechanisms and arrest the cell cycle to prevent cells with significant DNA errors from entering the mitotic phase (Hustedt & Durocher, 2016). Here, cell cycle analysis revealed an increase in G0/G1 phase cells in the MSC‐apoV treatment group, indicating that MSC‐apoVs may prolong G0/G1 phase arrest to allow more time for DNA repair (Figure 5e). Western blot analysis revealed that the expression of γH2AX started to decrease in the MSC‐apoV group at 1 h after irradiation and was significantly lower than that in PBS group at 8 h post irradiation, which was confirmed by immunofluorescence staining (Figure 5f‐i). Additionally, the comet assay demonstrated a significant reduction in DNA DSBs in the MSC‐apoV group compared to the PBS group (Figure 5f,h). Furthermore, western blot analysis showed that the expression levels of DNA repair proteins, including PARP1, BRCA1 and KU70, were significantly higher in the MSC‐apoV group compared to the PBS group at 0.5 and 1 h post irradiation. This suggests that MSC‐apoVs can accelerate the activation of DNA repair pathways, including both NHEJ and HR pathways, in irradiated BMMSCs (Figure 5i). Overall, our findings show that MSC‐apoVs can accelerate the repair of irradiation‐induced DNA DSBs in cultured BMMSCs.
FIGURE 5.

MSC‐apoVs promote DNA repair in vitro. (a) Fluorescence images of BMMSCs co‐culture with PKH26‐labelled MSC‐apoVs after radiation. (b) Transcription expression analysis of DNA repair‐related‐genes in irradiated BMMSCs (10 Gy) with MSC‐apoVs or PBS treatment (n = 3). (c, d) Immunofluorescence analysis revealed the recruitment of DNA repair proteins PARP1, 53BP1, BRCA1 and KU70 in irradiated BMMSCs following co‐culture with MSC‐apoVs. (e) Cell cycle analysis of irradiated BMMSCs with MSC‐apoVs or PBS treatment at 1 h post irradiation (n = 3). (f‐h) Immunofluorescent images and quantification of γH2AX foci (n = 35) and the comet assay (n = 50) of irradiated BMMSCs with MSC‐apoVs or PBS treatment at 4 h post irradiation. (i) Western blot analysis of irradiated BMMSCs with MSC‐apoVs or PBS treatment at 0.5, 1, 2, 4 and 8 h post irradiation. Scale bar = 10 µm. The graph bars show the mean ± SD. *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001. ns, not significant.
3.5. PARP1 plays a crucial role in MSC‐apoV‐mediated DNA repair
To investigate the components responsible for DNA repair, we focused on poly (ADP‐ribose) polymerase 1, also known as PARP1, which plays a crucial role in both DNA repair and apoptosis (Chaudhuri & Nussenzweig, 2017; D. Huang & Kraus, 2022; Kumar et al., 2022). Previous studies have demonstrated PARP1's role in recognizing and binding to DNA DSBs, serving as an early sensor of DNA damage (Chaudhuri & Nussenzweig, 2017; Ghosh et al., 2016; D. Huang & Kraus, 2022). It facilitates the recruitment of repair factors to the damaged sites, including Ku70/Ku80 heterodimer and BRCA1, which are essential for initiating the NHEJ and HR process (Chaudhuri & Nussenzweig, 2017; Li & Yu, 2013; Singh et al., 2021; Zada et al., 2021). PARP1 is known to be cleaved during apoptosis (Kumar et al., 2022; Mashimo et al., 2021; Soldani et al., 2001). However, to our surprise, we detected full‐length PARP1 in apoVs using western blot analysis (Figure 6b). To explore the functional role of PARP1 within MSC‐apoVs, we knocked down PARP1 in BMMSCs using siRNA and induced apoptosis to collect PARP1 knockdown MSC‐apoVs (si‐PARP1‐apoVs). We used western blotting to confirm the knockdown efficiency of PARP1 (Figure 6a,b). Co‐culturing the si‐PARP1‐apoVs with irradiated BMMSCs revealed a significant reduction in the efficiency of DNA repair compared to MSC‐apoV group (Figure 6c). Immunofluorescence staining of γH2AX and comet assay showed that MSC‐apoVs have reduced capacity to repair DNA damage after PARP1 knockdown (Figure 6d‐g). Western blot analysis showed a slower recruitment of DNA repair proteins PARP1, BRCA1, Rad52 and KU70 in the siPARP1‐apoVs group compared to the apoV group at 0.5 and 1 h post irradiation (Figure 6c). In conclusion, our findings suggest that PARP1 plays a crucial role in MSC‐apoV‐mediated DNA repair.
FIGURE 6.
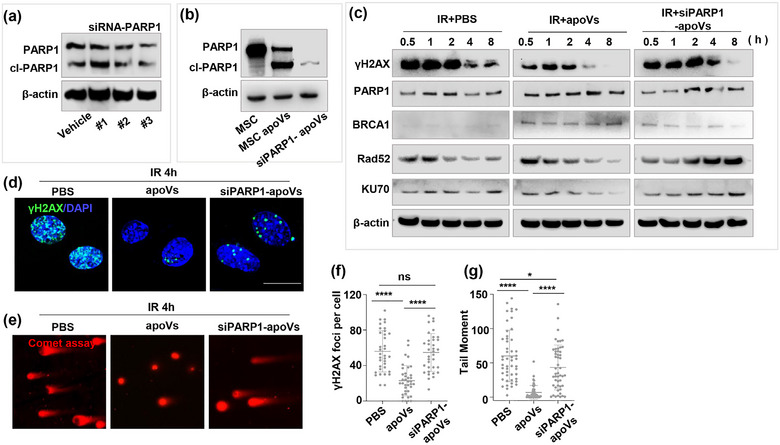
PARP1 plays a crucial role in MSC‐apoV‐mediated DNA repair. (a, b) Western blot analysis depicted the siRNA knockdown efficiency of PARP1 in BMMSCs and BMMSC‐apoVs. (c) Western blot analysis revealed the DNA repair efficiency in irradiated BMMSCs treated with PBS, MSC‐apoVs, or siPARP1‐MSC‐apoVs. (d‐g) γH2AX immunofluorescence staining (n = 35 for each group) and comet assay analysis (n = 50 for each group) of irradiated BMMSCs with PBS, MSC‐apoV, and siPARP1‐MSC‐apoV treatment at 4 h post irradiation. Scale bar = 20 µm. The graph bars show the mean ± SD. *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001. ns, not significant.
3.6. Apoptosis generates apoV complexes containing abundant DNA repair‐related proteins
ApoVs can inherit nuclear proteins from the parent cells (Zhang et al., 2022). To determine details of 16,000 g apoVs contain DNA repair‐related proteins, we conducted proteomic analysis on 16,000 g mBMMMSC‐apoVs extracted from six independent mice. The results of GO enrichment analysis revealed the presence of 1948 nuclear proteins in MSC‐apoVs (Figure 7a). Further analysis using GO pathway enrichment showed that these proteins were predominantly associated with DNA repair, cell cycle regulation, chromatin organization, and RNA transcription (Figure 7b). We next examined the 112 identified DNA repair‐related proteins and found that MSC‐apoVs contain various proteins related to DNA repair pathways including DNA replication, DSB repair pathways (HR and NHEJ), mismatch repair and nucleotide excision repair (Figure 7c). Similarly, in the KEGG pathway enrichment analysis of proteins related to Genetic Information Processing, we identified 43 proteins associated with replication and repair (Figure S5a,b). Through further KEGG pathway enrichment analysis, we found that these proteins are also involved in multiple DNA repair pathways (Figure S5c,d). We performed western blot analysis to confirm that apoVs contained numerous DNA repair‐related proteins inherited from the parent cells, including PARP1, Rad51, Rad52, XLF, PCNA, DDB1, XRCC1 and PRIM1 (Figure 7d). Additionally, we conducted 3D‐reconstruction of apoVs using a 3D imaging system of Elyra 7 Lattice SIM (Zeiss, Germany) (Figure 7e). We observed numerous different DNA repair enzymes within the apoVs. To further elucidate the crucial role of these DNA repair‐related proteins, we conducted a protein‐protein interaction network analysis and identified that PARP1 plays a critical role (Figure S5e).
FIGURE 7.

Apoptosis generates apoV complexes containing abundant DNA repair‐related proteins. (a) GO cellular component enrichment analysis revealed that BMMSC‐apoVs contain 1948 protein species derived from nucleus. (b) GO biological process enrichment analysis showed that among these nuclear proteins, 112 were related to DNA repair pathways. (c) Further GO biological process analysis was performed on these 112 DNA repair‐related proteins identified above. (d) Western blot demonstrated that MSC‐apoVs inherited a significant amount of DNA repair enzymes from the parent cells, including Rad51, Rad52, XLF, PARP1, PCNA, PRIM1, DDB1 and XRCC1. (e) 3D‐reconstruction of apoVs using a 3D imaging system of Elyra 7 Lattice SIM (Zeiss, Germany). Representative images showed numerous different DNA repair enzymes within the apoVs. Scale bar = 200 nm.
3.7. Embryonic stem cells (ESC)‐apoVs can effectively treat lethal dose‐irradiated mice
Through our above investigations, we established the relationship between MSC‐apoVs and DNA repair, demonstrating the therapeutic potential of MSC‐apoVs for irradiation damage. We next compared DNA repair capacity between 16,000 g apoVs derived from apoptotic mBMMSCs and 120,000 g EVs from cultured mBMMSCs. And we found that apoVs possess higher levels of DNA repair enzymes, as well as a greater diversity of these enzymes, than 120,000 g EVs (Figure S6a). The production of apoVs from individual cell is significantly higher than that of 120,000 g EVs (Figure S6b‐d). When using an equivalent quantity of MSC‐apoVs or 120,000 g EVs for irradiated cells, we found that MSC‐apoVs exhibited superior therapeutic effects compared to 120,000 g EVs (Figure S6e‐g).
Additionally, we compared 16,000 g apoVs derived from three different sources: human BMMSCs, umbilical cord mesenchymal stromal cells (UCs), and ESCs. Western blot analysis revealed that ESC‐apoVs had the highest level of DNA repair enzymes, including PARP1, PCNA, PRIM1, DDB1, Rad51, Rad52, KU70, KU80, XLF and XRCC1 (Figure 8a). ESC‐apoVs showed enhanced efficiency to repair DNA DSBs in cultured BMMSCs (Figure 8b,c). To further assess the efficacy of ESC‐apoVs in treating irradiation‐induced impairment, we administered them to mice subjected to a six grey lethal dose of TBI by X‐ray and found that ESC‐apoVs showed superior effects for improving mouse survival rate and bone marrow cell survival compared to MSC‐apoVs group (Figure 8d‐f). These findings highlight the significant clinical application potential of ESC‐apoVs as a promising approach to control severe irradiation damage.
FIGURE 8.

ESC‐apoVs provide effective treatment for lethal dose‐irradiated mice. (a) Western blot analysis was performed to compare the levels of DNA repair proteins in apoVs derived from human embryonic stem cells (ESCs), umbilical cord mesenchymal stromal cells (UCs), and bone marrow mesenchymal stromal cells (BMMSCs). (b) Evaluation of the DNA repair efficiency of ESC‐apoVs in an in vitro cellular model. Western blot analysis revealed the DNA repair efficiency in irradiated BMMSCs (12 Gy) when treated with PBS, UC‐apoVs, and ESC‐apoVs. (c) Immunofluorescent images and quantification of γH2AX foci (n = 35) and the comet assay (n = 50) of irradiated BMMSCs. Scale bar = 20 µm. (d, e) The C57BL/6 mice were treated with ESC‐apoVs, UC‐apoVs or PBS immediately after lethal TBI (6 Gy). Kaplan−Meier survival curves and weight loss were calculated to assess the treatment efficacy. n = 7 for each group. (f) Survival rates of total bone marrow cells, haematopoietic progenitors (lin‐, c‐kit+, Scal‐1+), monocytes (CD11b+, Ly6C+), neutrophils (CD11b+, Ly6G+), and B lymphocytes (B220+) in irradiated mice from each group at 24 h post irradiation. n = 3 for each group. The graph bars show the mean ± SD. *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001. ns, not significant.
4. DISCUSSION
Apoptosis is a highly controlled process responsible for maintaining tissue homeostasis and eliminating potentially harmful cells, such as those with DNA damage or mutations (Arandjelovic & Ravichandran, 2015; Fuchs & Steller, 2011). In our daily life, DNA damage is a frequent occurrence within the body, induced by various endogenous and exogenous factors (Chatterjee & Walker, 2017; Jeggo et al., 2016). To maintain genomic stability, cells have evolved complicated DNA repair mechanisms that can correct different types of DNA lesions (Chatterjee & Walker, 2017). Apoptosis and DNA damage repair are two fundamental physiological processes that occur daily life. Traditionally, severe DNA damage has been thought to trigger apoptosis, which serves as a mechanism to eliminate cells with severe DNA damage and reduce the risk of tumour formation (Norbury & Zhivotovsky, 2004; J. Y. Wang, 2001). However, it is unknown whether apoptotic metabolism contributes to the DNA repair. Previous studies have demonstrated that apoptotic cells not only supply a substantial quantity of apoVs (Liu et al., 2018), but also release various beneficial metabolites, such as spermidine (Medina et al., 2020). In this study, we provide the first evidence that apoptosis can generate a significant amount of apoVs containing DNA repair enzymes (DDR‐apoV complexes), which play a crucial role in DNA damage repair. These DDR‐apoV complexes contain key proteins involved in various DNA repair pathways and constitute a valuable legacy left by cells upon their apoptosis to maintain genomic stability. Meanwhile, our findings highlight the importance of studying the interplay between apoptosis and DNA repair, and there are still many fascinating questions that await further investigation in this field. For instance, our study raises the questions about the mechanism that regulates the selective packaging of beneficial substances into apoVs and the potential of regulating apoV packaging processes during programmed cell death.
ApoVs were previously thought to be metabolic waste cleared by macrophages in vivo (Arandjelovic & Ravichandran, 2015). However, starting from our initial discovery in 2018 concerning the role of circulating apoVs in maintaining bone homeostasis, we have gradually uncovered their therapeutic effects, biocompatibility, and in vivo metabolic pathways in various diseases (F. Lei et al., 2023; Liu et al., 2018; Ma et al., 2023; Ou et al., 2022; J. Wang et al., 2021; Zheng et al., 2021). Recently, there has been growing interest in studying the roles of EVs carrying nuclear materials, such as PCNA (Q. Lei et al., 2021), splicing factors (Pavlyukov et al., 2018), telomeres (Lanna et al., 2022) and gene transfer (Cai et al., 2014). However, physiological exosomes carrying nuclear materials are relatively rare compared to apoVs, and our understanding of the functions associated with nuclear materials in vesicles remains limited. ApoVs inherit a significant amount of nuclear material from the parent cells, including proteins and nucleic acids, serving as an excellent model for studying the roles of nuclear materials in vesicles. Here, we provided the first evidence that apoptosis can generate apoV complexes containing a large number of DNA repair enzymes and demonstrated the important role of PARP1 within these complexes for DNA damage repair. These findings suggest that the apoV system potentially contributes to the daily maintenance of DNA stability.
Nuclear radiation poses a significant challenge to future society (Mettler & Voelz, 2002). However, to date, more attention has been given to drugs that inhibit DNA repair, and there has been limited research on effective DNA repair medications (Jiang et al., 2023). Although it has been suggested that some agents, such as aspirin, exhibit anti‐inflammatory and DNA repair effects in irradiation‐induced damage, clinically there is no effective therapeutic approach to treating irradiation‐induced DNA damage (Jiang et al., 2023). However, as an emerging biological therapeutic approach, the therapeutic potential of EVs, particularly apoVs, for radiation damage treatment is still unknown. This study proposes that the absence of apoVs is one of the causes of DNA damage and premature senescence. MSC‐apoV‐infusion in apoptotic‐deficient models can repair DNA damage. We surprisingly discovered that MSC‐apoVs effectively rescue irradiation‐induced DNA damaged by accelerating the repair of DNA DSBs), offering a new approach to repair DNA damage.
5. LIMITATIONS OF STUDY
ApoVs contain multiple DNA repair proteins, such as PARP1, Rad51, Rad52, non‐homologous recombination repair proteins PRKDC, KU70, KU80. This study only examines the role of PARP1 in apoV‐mediated repair for double‐strand DNA breaks. We primarily elucidated that apoVs can repair DNA damage and prevent cellular senescence by transferring of PARP1. We observed that apoVs can directly ameliorate ageing and cellular senescence, but we did not know whether apoVs have direct anti‐ageing effect since the causes of ageing are multifaceted. Our experimental results suggest that apoVs can partially ameliorate the cellular senescence and DNA damage in senescent cells. ApoVs may use multiple mechanisms to ameliorate senescent cell phenotype, such as DNA repair, upregulation of the Wnt/β‐catenin pathway (Liu et al., 2018) and regulation of autophagy (F. Lei et al., 2023).
The mechanisms of apoV generation and cargo packaging during apoptosis were not extensively investigated in this study.
6. CONCLUSION
In this study, we demonstrated that a reduction in apoV quantity leads to significant premature senescence and DNA damage, and the infusion of MSC‐apoVs can ameliorate these disorders. Meanwhile, we first revealed that apoptosis participates in DNA repair by generating DDR‐apoV complexes containing plentiful DNA repair enzymes. This discovery reveals the DNA repair role of nuclear‐related proteins in apoVs for the first time and highlights their potential therapeutic effect in the treatment of irradiation‐induced disorders. Lastly, we have identified ESC‐apoVs, which exhibit a remarkable abundance of DDR‐apoV complexes, offered effective therapy for severe irradiation damage.
AUTHOR CONTRIBUTIONS
Zhiqing Huang contributed to investigation, project administration, data curation and writing original draft. Yuzhi Zhuang and Mingchen Ma contributed to performing experimental procedures. Wenwen Li contributed to manuscript revision. Fangcao Lei and Yan Qu contributed to cell culture. Jiaqi Li and Huigen Luo contributed to illustrating all the schematic diagrams. Changzheng Li contributed to the analysis of irradiated mouse bone marrow. Lu Lu contributed to the breeding and cultivation of mice. Lan Ma, Xiao Zhang, Xiaoxing Kou and Lingjia Jiang contributed to methodology and data curation. Xueli Mao contributed to conceptualization, manuscript supervision and revision. Songtao Shi contributed to conceptualization, experimental design, data curation, formal analysis, writing and editing manuscript and supervision. All authors approved the current version of the manuscript.
CONFLICT OF INTEREST STATEMENT
The authors declare no conflicts of interest.
Supporting information
Supplementary Information
Supplementary Information
Supplementary Information
ACKNOWLEDGEMENTS
This work was supported by grants from the National Key Research and Development Program of China (2021YFA1100600 to S.S.,2022YFA104402 to M.X.), the Pearl River Talent Recruitment Program (2019ZT08Y485, 2019QN01Y138, 2019JC01Y182), the Guangdong Financial Fund for High‐Caliber Hospital Construction (174‐2018‐XMZC‐0001‐03‐0125, D‐07 to S.S., D‐11 to X.K.), and the National Natural Science Foundation of China (82170924 to X.K.).
Huang, Z. , Zhuang, Y. , Li, W. , Ma, M. , Lei, F. , Qu, Y. , Li, J. , Luo, H. , Li, C. , Lu, L. , Ma, L. , Zhang, X. , Kou, X. , Jiang, L. , Mao, X. , & Shi, S. (2024). Apoptotic Vesicles are Required to Repair DNA Damage and Suppress Premature Cellular Senescence. Journal of Extracellular Vesicles, 13, e12428. 10.1002/jev2.12428
Contributor Information
Xueli Mao, Email: maoxuel@mail.sysu.edu.cn.
Songtao Shi, Email: shisongtao@mail.sysu.edu.cn.
DATA AVAILABILITY STATEMENT
All data are available in the main text or the supplementary materials.
REFERENCES
- Akers, J. C. , Gonda, D. , Kim, R. , Carter, B. S. , & Chen, C. C. (2013). Biogenesis of extracellular vesicles (EV): Exosomes, microvesicles, retrovirus‐like vesicles, and apoptotic bodies. Journal of Neuro‐Oncology, 113, 1–11. 10.1007/s11060-013-1084-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arandjelovic, S. , & Ravichandran, K. S. (2015). Phagocytosis of apoptotic cells in homeostasis. Nature Immunology, 16, 907–917. 10.1038/ni.3253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouillet, P. , Metcalf, D. , Huang, D. C. , Tarlinton, D. M. , Kay, T. W. , Kontgen, F. , Adams, J. M. , & Strasser, A. (1999). Proapoptotic Bcl‐2 relative Bim required for certain apoptotic responses, leukocyte homeostasis, and to preclude autoimmunity. Science, 286, 1735–1738. 10.1126/science.286.5445.1735 [DOI] [PubMed] [Google Scholar]
- Brunner, T. , Mogil, R. J. , LaFace, D. , Yoo, N. J. , Mahboubi, A. , Echeverri, F. , Martin, S. J. , Force, W. R. , Lynch, D. H. , Ware, C. F. , & Green, D. R. (1995). Cell‐autonomous Fas (CD95)/Fas‐ligand interaction mediates activation‐induced apoptosis in T‐cell hybridomas. Nature, 373, 441–444. 10.1038/373441a0 [DOI] [PubMed] [Google Scholar]
- Caestecker, K. W. , & Van de Walle, G. R. (2013). The role of BRCA1 in DNA double‐strand repair: Past and present. Experimental Cell Research, 319, 575–587. 10.1016/j.yexcr.2012.11.013 [DOI] [PubMed] [Google Scholar]
- Cai, J. , Wu, G. , Tan, X. , Han, Y. , Chen, C. , Li, C. , Wang, N. , Zou, X. , Chen, X. , Zhou, F. , & He, D. (2014). Transferred BCR/ABL DNA from K562 extracellular vesicles causes chronic myeloid leukemia in immunodeficient mice. PLoS ONE, 9, e105200. 10.1371/journal.pone.0105200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang, J. , Wang, Y. , Shao, L. , Laberge, R. M. , Demaria, M. , Campisi, J. , Janakiraman, K. , Sharpless, N. E. , Ding, S. , Feng, W. , & Luo, Y. (2016). Clearance of senescent cells by ABT263 rejuvenates aged hematopoietic stem cells in mice. Nature Medicine, 22, 78–83. 10.1038/nm.4010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chatterjee, N. , & Walker, G. C. (2017). Mechanisms of DNA damage, repair, and mutagenesis. Environmental and Molecular Mutagenesis, 58, 235–263. 10.1002/em.22087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaudhuri, R. , & Nussenzweig, A. (2017). The multifaceted roles of PARP1 in DNA repair and chromatin remodelling. Nature Reviews Molecular Cell Biology, 18, 610–621. 10.1038/nrm.2017.53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crescitelli, R. , Lasser, C. , & Lotvall, J. (2021). Isolation and characterization of extracellular vesicle subpopulations from tissues. Nature Protocols, 16, 1548–1580. 10.1038/s41596-020-00466-1 [DOI] [PubMed] [Google Scholar]
- Elzanowska, J. , Semira, C. , & Costa‐Silva, B. (2021). DNA in extracellular vesicles: Biological and clinical aspects. Molecular Oncology, 15, 1701–1714. 10.1002/1878-0261.12777 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fooladi, M. , Cheki, M. , Shirazi, A. , Sheikhzadeh, P. , Amirrashedi, M. , Ghahramani, F. , & Khoobi, M. (2022). Histopathological evaluation of protective effect of telmisartan against radiation‐induced bone marrow injury. Journal of Biomedical Physics and Engineering, 12, 277–284. 10.31661/jbpe.v0i0.2012-1243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuchs, Y. , & Steller, H. (2011). Programmed cell death in animal development and disease. Cell, 147, 742–758. 10.1016/j.cell.2011.10.033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fulda, S. , & Debatin, K. M. (2006). Extrinsic versus intrinsic apoptosis pathways in anticancer chemotherapy. Oncogene, 25, 4798–4811. 10.1038/sj.onc.1209608 [DOI] [PubMed] [Google Scholar]
- Ghosh, R. , Roy, S. , Kamyab, J. , Danzter, F. , & Franco, S. (2016). Common and unique genetic interactions of the poly(ADP‐ribose) polymerases PARP1 and PARP2 with DNA double‐strand break repair pathways. Dna Repair, 45, 56–62. 10.1016/j.dnarep.2016.06.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- He, B. , Lu, N. , & Zhou, Z. (2009). Cellular and nuclear degradation during apoptosis. Current Opinion in Cell Biology, 21, 900–912. 10.1016/j.ceb.2009.08.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herold, M. J. , Stuchbery, R. , Mérino, D. , Willson, T. , Strasser, A. , Hildeman, D. , & Bouillet, P. (2014). Impact of conditional deletion of the pro‐apoptotic BCL‐2 family member BIM in mice. Cell death & disease, 5, e1446 10.1038/cddis.2014.409 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang, D. , & Kraus, W. L. (2022). The expanding universe of PARP1‐mediated molecular and therapeutic mechanisms. Molecular Cell, 82, 2315–2334. 10.1016/j.molcel.2022.02.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang, D. C. , & Strasser, A. (2000). BH3‐Only proteins‐essential initiators of apoptotic cell death. Cell, 103, 839–842. 10.1016/s0092-8674(00)00187-2 [DOI] [PubMed] [Google Scholar]
- Hughes, P. D. , Hughes, P. D. , Belz, G. T. , Fortner, K. A. , Budd, R. C. , Strasser, A. , & Bouillet, P. (2008). Apoptosis regulators Fas and Bim cooperate in shutdown of chronic immune responses and prevention of autoimmunity. Immunity, 28, 197–205. 10.1016/j.immuni.2007.12.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hustedt, N. , & Durocher, D. (2016). The control of DNA repair by the cell cycle. Nature Cell Biology, 19, 1–9. 10.1038/ncb3452 [DOI] [PubMed] [Google Scholar]
- Jeggo, P. A. , Pearl, L. H. , & Carr, A. M. (2016). DNA repair, genome stability and cancer: A historical perspective. Nature Reviews Cancer, 16, 35–42. 10.1038/nrc.2015.4 [DOI] [PubMed] [Google Scholar]
- Jiang, H. , Swacha, P. , Aung, K. M. , & Gekara, N. O. (2023). Aspirin protects against genotoxicity by promoting genome repair. Cell Research, 33, 325–327. 10.1038/s41422-023-00783-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar, V. , Kumar, A. , Mir, K. U. I. , Yadav, V. , & Chauhan, S. S. (2022). Pleiotropic role of PARP1: An overview. BioTechniques, 12, 3. 10.1007/s13205-021-03038-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanna, A. , Vaz, B. , D'Ambra, C. , Valvo, S. , Vuotto, C. , Chiurchiù, V. , Devine, O. , Sanchez, M. , Borsellino, G. , Akbar, A. N. , & De Bardi, M. (2022). An intercellular transfer of telomeres rescues T cells from senescence and promotes long‐term immunological memory. Nature Cell Biology, 24, 1461–1474. 10.1038/s41556-022-00991-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lei, F. , Lei, F. , Huang, Z. , Ou, Q. , Li, J. , Liu, M. , Ma, L. , Tan, L. , Lin, Z. , & Kou, X. (2023). Apoptotic vesicles rejuvenate mesenchymal stem cells via Rab7‐mediated autolysosome formation and alleviate bone loss in aging mice. Nano Research, 16, 822–833. 10.1007/s12274-022-4709-4 [DOI] [Google Scholar]
- Lei, Q. , Lei, Q. , Gao, F. , Liu, T. , Ren, W. , Chen, L. , Cao, Y. , Chen, W. , Guo, S. , Zhang, Q. , Chen, W. , & Wang, H. (2021). Extracellular vesicles deposit PCNA to rejuvenate aged bone marrow‐derived mesenchymal stem cells and slow age‐related degeneration. Science Translational Medicine, 13, 10.1126/scitranslmed.aaz8697 [DOI] [PubMed] [Google Scholar]
- Li, M. , & Yu, X. (2013). Function of BRCA1 in the DNA damage response is mediated by ADP‐ribosylation. Cancer Cell, 23, 693–704. 10.1016/j.ccr.2013.03.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao, S. , Chen, L. , Song, Z. , & He, H. (2022). The fate of damaged mitochondrial DNA in the cell. Biochimica et Biophysica Acta (BBA) ‐ Molecular Cell Research, 1869, 119233. 10.1016/j.bbamcr.2022.119233 [DOI] [PubMed] [Google Scholar]
- Liu, D. , Kou, X. , Chen, C. , Liu, S. , Liu, Y. , Yu, W. , Yu, T. , Yang, R. , Wang, R. , Zhou, Y. , & Shi, S. (2018). Circulating apoptotic bodies maintain mesenchymal stem cell homeostasis and ameliorate osteopenia via transferring multiple cellular factors. Cell Research, 28, 918–933. 10.1038/s41422-018-0070-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lotvall, J. , Hill, A. F. , Hochberg, F. , Buzás, E. I. , Di Vizio, D. , Gardiner, C. , Gho, Y. S. , Kurochkin, I. V. , Mathivanan, S. , Quesenberry, P. , & Sahoo, S. (2014). Minimal experimental requirements for definition of extracellular vesicles and their functions: A position statement from the International Society for Extracellular Vesicles. Journal of Extracellular Vesicles, 3, 26913. 10.3402/jev.v3.26913 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma, L. , Chen, C. , Liu, D. , Huang, Z. , Li, J. , Liu, H. , Kwok, R. T. K. , Tang, B. , Sui, B. , Zhang, X. , & Tang, J. (2023). Apoptotic extracellular vesicles are metabolized regulators nurturing the skin and hair. Bioactive Materials, 19, 626–641. 10.1016/j.bioactmat.2022.04.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mah, L. J. , El‐Osta, A. , & Karagiannis, T. C. (2010). gammaH2AX: A sensitive molecular marker of DNA damage and repair. Leukemia, 24, 679–686. 10.1038/leu.2010.6 [DOI] [PubMed] [Google Scholar]
- Malkin, E. Z. , & Bratman, S. V. (2020). Bioactive DNA from extracellular vesicles and particles. Cell death & disease, 11, 584. 10.1038/s41419-020-02803-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mashimo, M. , Onishi, M. , Uno, A. , Tanimichi, A. , Nobeyama, A. , Mori, M. , Yamada, S. , Negi, S. , Bu, X. , Kato, J. , & Moss, J. (2021). The 89‐kDa PARP1 cleavage fragment serves as a cytoplasmic PAR carrier to induce AIF‐mediated apoptosis. Journal of Biological Chemistry, 296, 100046. 10.1074/jbc.RA120.014479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medina, C. B. , Mehrotra, P. , Arandjelovic, S. , Perry, J. S. , Guo, Y. , Morioka, S. , Barron, B. , Walk, S. F. , Ghesquière, B. , Krupnick, A. S. , & Lorenz, U. (2020). Metabolites released from apoptotic cells act as tissue messengers. Nature, 580, 130–135. 10.1038/s41586-020-2121-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mettler, F. A., Jr. , & Voelz, G. L. (2002). Major radiation exposure–what to expect and how to respond. New England Journal of Medicine, 346, 1554–1561. 10.1056/NEJMra000365 [DOI] [PubMed] [Google Scholar]
- Miura, M. , Chen, X. D. , Allen, M. R. , Bi, Y. , Gronthos, S. , Seo, B. M. , Lakhani, S. , Flavell, R. A. , Feng, X. H. , Robey, P. G. , & Young, M. (2004). A crucial role of caspase‐3 in osteogenic differentiation of bone marrow stromal stem cells. Journal of Clinical Investigation, 114, 1704–1713. 10.1172/jci200420427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy, M. K. , & Kamen, J. (2019). Deciding between an X‐Ray and 137Cs Irradiator—It's not just about Energy Spectra. Radiation Research, 192, 493–506. 10.1667/RR15395.1 [DOI] [PubMed] [Google Scholar]
- Norbury, C. J. , & Zhivotovsky, B. (2004). DNA damage‐induced apoptosis. Oncogene, 23, 2797–2808. 10.1038/sj.onc.1207532 [DOI] [PubMed] [Google Scholar]
- Olive, P. L. , & Banath, J. P. (2006). The comet assay: A method to measure DNA damage in individual cells. Nature Protocols, 1, 23–29. 10.1038/nprot.2006.5 [DOI] [PubMed] [Google Scholar]
- Organization, W. H. . (2023). National stockpiles for radiological and nuclear emergencies: Policy advice(2023, electronic version). ISBN 978‐92‐4‐006787‐5.
- Ou, Q. , Tan, L. , Shao, Y. , Lei, F. , Huang, W. , Yang, N. , Qu, Y. , Cao, Z. , Niu, L. , Liu, Y. , & Kou, X. (2022). Electrostatic charge‐mediated apoptotic vesicle biodistribution attenuates sepsis by switching neutrophil NETosis to apoptosis. Small, 18(20), e2200306. 10.1002/smll.202200306 [DOI] [PubMed] [Google Scholar]
- Panier, S. , & Boulton, S. J. (2014). Double‐strand break repair: 53BP1 comes into focus. Nature Reviews Molecular Cell Biology, 15, 7–18. 10.1038/nrm3719 [DOI] [PubMed] [Google Scholar]
- Pastwa, E. , & Blasiak, J. (2003). Non‐homologous DNA end joining. Acta Biochimica Polonica, 50, 891–908. [PubMed] [Google Scholar]
- Pavlyukov, M. S. , Yu, H. , Bastola, S. , Minata, M. , Shender, V. O. , Lee, Y. , Zhang, S. , Wang, J. , Komarova, S. , Wang, J. , & Yamaguchi, S. (2018). Apoptotic cell‐derived extracellular vesicles promote malignancy of glioblastoma via intercellular transfer of splicing factors. Cancer Cell, 34, 119–135. e110. 10.1016/j.ccell.2018.05.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qu, Y. , Qu, Y. , He, Y. , Meng, B. , Zhang, X. , Ding, J. , Kou, X. , Teng, W. , & Shi, S. (2022). Apoptotic vesicles inherit SOX2 from pluripotent stem cells to accelerate wound healing by energizing mesenchymal stem cells. Acta Biomaterialia, 149, 258–272. 10.1016/j.actbio.2022.07.009 [DOI] [PubMed] [Google Scholar]
- Rausch, L. , Flaskamp, L. , Ashokkumar, A. , Trefzer, A. , Ried, C. , Buchholz, V. R. , Obst, R. , Straub, T. , Brocker, T. , & Kranich, J. (2023). Phosphatidylserine‐positive extracellular vesicles boost effector CD8(+) T cell responses during viral infection. PNAS, 120, e2210047120. 10.1073/pnas.2210047120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sage, E. , & Shikazono, N. (2017). Radiation‐induced clustered DNA lesions: Repair and mutagenesis. Free Radical Biology and Medicine, 107, 125–135. 10.1016/j.freeradbiomed.2016.12.008 [DOI] [PubMed] [Google Scholar]
- Santivasi, W. L. , & Xia, F. (2014). Ionizing radiation‐induced DNA damage, response, and repair. Antioxid Redox Signaling, 21, 251–259. 10.1089/ars.2013.5668 [DOI] [PubMed] [Google Scholar]
- Schumacher, B. , Pothof, J. , Vijg, J. , & Hoeijmakers, J. H. J. (2021). The central role of DNA damage in the ageing process. Nature, 592, 695–703. 10.1038/s41586-021-03307-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shrivastav, M. , De Haro, L. P. , & Nickoloff, J. A. (2008). Regulation of DNA double‐strand break repair pathway choice. Cell Research, 18, 134–147. 10.1038/cr.2007.111 [DOI] [PubMed] [Google Scholar]
- Singh, J. K. , Smith, R. , Rother, M. B. , de Groot, A. J. , Wiegant, W. W. , Vreeken, K. , D'augustin, O. , Kim, R. Q. , Qian, H. , Krawczyk, P. M. , & González‐Prieto, R. (2021). Zinc finger protein ZNF384 is an adaptor of Ku to DNA during classical non‐homologous end‐joining. Nature Communications, 12, 6560. 10.1038/s41467-021-26691-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soldani, C. , Lazzè, M. C. , Bottone, M. G. , Tognon, G. , Biggiogera, M. , Pellicciari, C. E. , & Scovassi, A. I. (2001). Poly(ADP‐ribose) polymerase cleavage during apoptosis: When and where? Experimental Cell Research, 269, 193–201. 10.1006/excr.2001.5293 [DOI] [PubMed] [Google Scholar]
- Van Noorden, C. J. (2001). The history of Z‐VAD‐FMK, a tool for understanding the significance of caspase inhibition. Acta Histochemica, 103, 241–251. 10.1078/0065-1281-0060 [DOI] [PubMed] [Google Scholar]
- Wang, J. , Cao, Z. , Wang, P. , Zhang, X. , Tang, J. , He, Y. , Huang, Z. , Mao, X. , Shi, S. , & Kou, X. (2021). Apoptotic extracellular vesicles ameliorate multiple myeloma by restoring FAS‐mediated apoptosis. ACS Nano, 15, 14360–14372. 10.1021/acsnano.1c03517 [DOI] [PubMed] [Google Scholar]
- Wang, J. Y. (2001). DNA damage and apoptosis. Cell Death and Differentiation, 8, 1047–1048. 10.1038/sj.cdd.4400938 [DOI] [PubMed] [Google Scholar]
- Yáñez‐Mó, M. , Siljander, P. R. M. , Andreu, Z. , Bedina Zavec, A. , Borràs, F. E. , Buzas, E. I. , Buzas, K. , Casal, E. , Cappello, F. , Carvalho, J. , & Colás, E. (2015). Biological properties of extracellular vesicles and their physiological functions. Journal of Extracellular Vesicles, 4, 27066. 10.3402/jev.v4.27066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zada, D. , Sela, Y. , Matosevich, N. , Monsonego, A. , Lerer‐Goldshtein, T. , Nir, Y. , & Appelbaum, L. (2021). Parp1 promotes sleep, which enhances DNA repair in neurons. Molecular Cell, 81, 4979–4993.e7. 10.1016/j.molcel.2021.10.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, X. , Tang, J. , Kou, X. , Huang, W. , Zhu, Y. , Jiang, Y. , Yang, K. , Li, C. , Hao, M. , Qu, Y. , & Ma, L. (2022). Proteomic analysis of MSC‐derived apoptotic vesicles identifies Fas inheritance to ameliorate haemophilia a via activating platelet functions. Journal of Extracellular Vesicles, 11, e12240. 10.1002/jev2.12240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao, M. , Perry, J. M. , Marshall, H. , Venkatraman, A. , Qian, P. , He, X. C. , Ahamed, J. , & Li, L. (2014). Megakaryocytes maintain homeostatic quiescence and promote post‐injury regeneration of hematopoietic stem cells. Nature Medicine, 20, 1321–1326. 10.1038/nm.3706 [DOI] [PubMed] [Google Scholar]
- Zheng, C. , Zheng, C. , Sui, B. , Zhang, X. , Hu, J. , Chen, J. , Liu, J. , Wu, D. , Ye, Q. , Xiang, L. , Qiu, X. , & Liu, S. (2021). Apoptotic vesicles restore liver macrophage homeostasis to counteract type 2 diabetes. Journal of Extracellular Vesicles, 10, e12109. 10.1002/jev2.12109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuo, R. , Liu, M. , Wang, Y. , Li, J. , Wang, W. , Wu, J. , Sun, C. , Li, B. , Wang, Z. , Lan, W. , & Zhang, C. (2019). BM‐MSC‐derived exosomes alleviate radiation‐induced bone loss by restoring the function of recipient BM‐MSCs and activating Wnt/beta‐catenin signaling. Stem Cell Research & Therapy, 10, 30. 10.1186/s13287-018-1121-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Information
Supplementary Information
Supplementary Information
Data Availability Statement
All data are available in the main text or the supplementary materials.


