Abstract
Starting from drug discovery, through research and development, to clinical trials and FDA approval, artificial intelligence (AI) plays a vital role in planning, developing, assessing modelling, and optimization of product attributes. In recent decades, machine-learning algorithms integrated into artificial neural networks, neuro-fuzzy logic and decision trees have been applied to tremendous domains related to drug formulation development. Optimized formulations were transformed from lab to market based on optimized properties derived from AI Technologies. Research and development in pharmaceutical industry rely upon computer-driven equipment and machine learning technology to extract data, perform simulations, modelling, and optimization to get optimum solutions. Merging AI technologies in various steps of pharmaceutical manufacture is a major challenge due to lack of in-house technologies. In silico studies based on artificial intelligence are widely applied as effective tools to screen the market needs of medications and pharmaceutical services through inspecting scientific literature and prioritizing medicines for specific illnesses or a particular patient. Specialized personnel who excel in scientific and data science with analytical knowledge are essential for transformation to smart manufacturing and offering services. However, privacy, cybersecurity, AI-dependent unemployment, and ownership rights of AI technologies require proper regulations to gain the benefits and minimize the drawbacks.
Keywords: Artificial intelligence, Challenges, Drug product, Pharmaceutical service, Predictions, Smart manufacture
1. Introduction
Discovering and identifying new drug candidates is a continuously growing process which takes relatively long time with average of ±10 years and a capital of approximately 2.6 Billion dollars worldwide (DiMasi et al., 2016). Recently, AI has been applied to all disciplines and in all daily life activities. In a matter of a second, it can extract scientific knowledge from literature, then the extracted data is used by numerous end users in various fields including publishers, researchers, large companies, and the media. AI processes including data mining, modeling and optimization based on machine-learning algorithms in the form of artificial neural networks, neuro-fuzzy logic and decision trees have been applied to numerous domains in the medical literature (Zia et al., 2022). Connections of equipment such as computers, machines and smart appliances operated with software and mounted with sensors are generally known as the internet of things (IoTs) (Lee, 2019). These complicated systems when employed in industry are capable of not only deep machine learning, real time process monitoring, but also correction of errors and optimization of working conditions to achieve high-quality products. Complicated domains of scientific data in pharmaceutical industry and pharmaceutical research inevitably necessitate application of AI to help decision-makers in data analysis, filtration, expedite the design and implementation of experiments, select optimum models and predict process outcomes (Henstock, 2019). Investments in the computational technologies such as simulation and modelling applied in the field of drug discovery and development are expected to strongly support drug research and innovation leading to emergence of new drug molecules (Kiriiri et al., 2020). Similarly, pharmaceutical services either in the clinical settings or community practice are recently showing great advancement by applying AI-technologies such as automated dispensing cabinets or robotic drug dispensing systems (e.g. Pyxis, Omnicell) that offers reduced medication errors and provides sufficient time for pharmacists to counsel patients appropriately (Jumeau et al., 2021). Applications of AI technologies in pre and post marketing of pharmaceutical products including regular medicines and vaccines have been successfully employed in pharmacovigilance programs in many countries. For example in China, special laboratories for pharmacovigilance research and evaluation were established and connected to pharmacovigilance information technology and data science innovation Center at Tsinghua University for proper control and management of pharmacovigilance data (Song et al., 2023).
In the field of clinical trials, AI technologies are finding their way into the design and implementation of adequately controlled and successful clinical trials, especially for oncology and cardiovascular medicines. However, challenges facing this sector includes lack of regulatory authorities guidance and other ethical issues such as bias and errors still need to be addressed (Askin et al., 2023). For pharmaceutical products marketing and supply chain management, AI technology became a basic tool for studying products shortage and inventories management. However, advanced data analytics need to be applied in complicated drug marketing management and stock control situations such as pandemics and other drug shortage crises to reach appropriate forecasting of market need (Nguyen et al., 2022).
This review discusses various sectors of pharmaceutical manufacture, pharmacy practice and pharmaceutical care in which AI-technologies are applied in to explore strengths and weaknesses of the new techniques with suggestions for solutions to tackle the emerging challenges and maximize the net benefits.
2. Application of AI
2.1. Applications in medical and pharmaceutical education
The presence of medical experts that have sufficient knowledge and expertise in data science and analysis, modelling, optimization and various in silico techniques will help in better exploitation of information and finding solutions for encountered problems. Starting from researchers’ education and training on new emerging technologies based on AI will undoubtedly fill the gap between pharma companies and the high technology enterprises. Searching literature through Publishers’ databases for diagnosis and treatment of infectious diseases such as COVID 19 as an example has increased in the last 5 years by researchers in academia and pharmaceutical industry (Hulsen, 2021). Hundreds of millions of articles, abstracts and patent files can be searched, integrated and valuable data can be extracted using AI software applications. Numerous hypotheses of cause-effect relationships could be made, and outcomes could be easily predicted.
2.2. Prioritizing medicines for specific illnesses
The partnership between the large multinational pharmaceutical company Pfizer Incorp. and the giant of AI technology IBM Whatson Health is a live example for expanding investment in AI technologies to foster drug development (Russo-Spena et al., 2019).
If applied correctly in pharmaceutical manufacture, AI would eventually reduce costs and demolish the time of drug discovery. It will also expedite the experimental part, predict side effects and potential toxicity as well as facilitating clinical trials leading to shorter product life-cycle (Agrawal, 2018). Personalized drug delivery systems (PDDs) have recently been developed for specific patients suffering from unique illnesses related to their genetic characteristics such as certain types of cancer, metabolic disorders, and other inherited diseases. Using digital tools can be the ideal solution for such personal disease conditions (Raijada et al., 2021).
2.3. Technologies used in prediction of drug-drug and drug-disease interactions
Many AI models have been proposed to solve problems in different domains including the medical and pharmaceutical fields (Fig. 1), most of which are based on machine learning principles (MLP). The most recent proposed models are capable of both problem solving and decision making using seven layers and customized to various machine learning algorithms (Wu and Bouvry, 2023).
Fig. 1.
Machine Learning activities in the medical and pharmaceutical field.
AI technologies are recently applied in what is called personalized medicine. In this technique patients’ diagnosed with the same illness or disease such as cancer, are treated differently based on their disease history, cause, lifestyle, age and genetics (Johnson et al., 2021). The extent of drug efficacy in some people being higher than others is also linked to personal pharmacodynamics and drug pharmacokinetics. Therefore, linking these together can create invaluable cause-effect information and make use of data records in hospitals and clinics which can be mined when necessary to figure out the underlying causes and propose solutions for similar medical problems. Machine learning is classified into three main categories, supervised, unsupervised and reinforced learning (Pouyanfar et al., 2018) as described in Fig. 2. In supervised learning, the data set is divided into two categories, one for training and one for testing and network is trained on full examples of inputs and outputs data records. Unsupervised learning on the contrary, has no pretraining on records containing outputs. In reinforcement learning, the network learns from its own actions and rewards and is used to solve problems needing sequential decisions. There are three main types of algorithms that are widely applied in building models from complex domain data and making predictions. First, include the Naïve Bayesian (NB) which detects uncertainty in data based on probabilities of outcomes and characterized by being suitable for high noise and small number of data records. Second, artificial neural networks (ANN) which are widely used in the medical and pharmaceutical field and characterized by being flexible and dynamic where it can be updated continuously with new data (Chen et al., 2020a). Third, the support vector machines (SVM) which is characterized by high capability and accuracy in dealing with classification and predictions of executed nonlinear data. SVM is widely implemented in drug design and discovery applications for simulated screening and prediction of pharmacological activities (Maltarollo et al., 2019). Numerous searchable databases are available for extraction and prediction of drug-drug interactions and adverse effects using AI technology. These include - DrugBank, Drug-drug interactions (DDI) Extraction 2013 corpus, Side effect resource (SEDR), FDA Adverse Event Reporting system (FAERS) and Medline are all used for predicting interactions and adverse drug reactions from huge biomedical and pharmacological data (Qiu et al., 2021).
Fig. 2.
Machine Learning Classification.
2.4. Applications of AI during formulation design and optimization
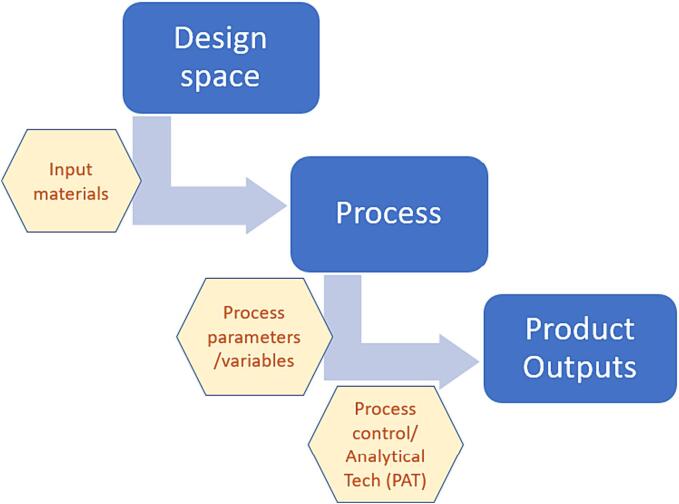
Different software programs based on AI are being used in the design and optimization of pharmaceutical formulations. These were developed following the theory of quality by design first established by Dr. Joseph M Juran (Juran and by Design, 1992) that focuses on planning quality in products and services through creation of features necessary to achieve customer satisfaction. Goals are achieved through three major axes, the preset objectives, understanding product and process parameters, and control of quality using scientific approach and risk management. There are four basic goals of QbD in the pharmaceutical industry that aim at maximizing benefits and achievements. These include product quality specifications, process capability (product and process design, understanding and control), product development, risk analysis and change management (Yu et al., 2014). The American Food and Drug Administration (FDA) adopted the QbD and process analytical technology (PAT) as tools for optimization of method and product critical characteristics (Fig. 3) during development stages to minimize failure and support high product quality attributes (Murphy et al., 2016). To achieve the second goal (product and process design), several widely used applications based on quality by design are applied and collectively known as design of experiment (DoE). These include; factorial design, central composite design, Box Behnken design which are based on mathematical modeling and statistical evaluations of the created model prediction co-efficient (Rakić et al., 2014). Other optimization techniques for modeling and predictions are based on artificial neural networks and characterized by better predictions of complex domains showing non-linear relationships between variables than the multiple linear regression techniques (Koletti et al., 2020). Integrating QbD, PAT and other predictive tools is quite challenging in pharmaceutical process design and optimization and requires high-level technology and experienced personnel to apply them effectively.
Fig. 3.
Integration of quality by design (QbD) and PAT into process and product cycle.
Applications of experimental design methodology are diverse, including chemometric approaches for method development based on QbD such as those used in separation of complex mixtures using liquid chromatography (Cela et al., 2013). The technique enables researchers to model system behavior using mathematical quadratic equations followed by optimization of the process through searching the model for the best solution of the problem under test.
In many modeling experiments, the central composite design proved to be the best mathematical and statistical model-generating design by which critical independent factors and their interactions can be accurately determined.
Artificial neural networks are more capable of doing modeling, optimization and when linked to fuzzy logic can help in optimization, prediction and decision making in what is called Design expert systems (Mukherjee and Deshpande, 1995). Decision making performed by fuzzy logic rule is based on previous knowledge of relations between variables and converting to linguistic variables and formulating the If-Then function. ANNs are capable of deep learning through associated algorithms and are classified into three main categories that can be supervised or unsupervised: the multilayer perceptron networks (MLP), recurrent neural networks (RNNs) and the convolutional neural networks (CNNs). The most efficient and recent type is the CNNs which are characterized by being a dynamic system with the ability to deal with complex domains in pattern recognition, systems modeling and signal propagation (Paul et al., 2021).
2.5. AI real-time applications in pharmaceutical process monitoring and control
For new developmental processes in the life-cycle of pharmaceutical products, the regulatory authorities such as food and drug administration (FDA) and the European Medicines Agency (EMA) require quality by design and process analytical technology (PAT) approved designs as per the ICH 2004 guidelines (Zobel-Roos et al., 2019). Recent advances in biotechnology-based manufacturing such as biomolecules, proteins, vaccines, and peptides necessitate optimization of the development process and place large controls on the inherently multivariate product and process characteristics. Modeling of developmental processes involving large biological molecules seems to be a complex and multifunctional approach that needs to be looked at as a sole use technology for each specific molecule. However, long term sustainable development of biologic manufacture needs to be manipulated by dedicated facilities and special modeling techniques and for gaining the benefit of both sustainable development and reduction of costs and hazards (Zobel-Roos et al., 2017). A clear, well-established and fully understood workflow of manufacturing steps including; process development, piloting, engineering, process controls and manufacturing operations are strongly needed before setting a multistep modeling technique for large scale biopharmaceutical production to achieve the required benefits (Kroll et al., 2017). During life cycle of the product, multiscale modeling includes molecular modeling for drug discovery, drug-target interactions, pharmacokinetic simulations, design of experiments, cost modeling, advanced process control and process analytical technologies, and fluid dynamics for engineering and production (Zobel-Roos et al., 2019). Being complicated systems, experience-based, high cost makes application of such technologies a big challenge to small or intermediate pharmaceutical corporations. Currently, many PAT tools are used for monitoring and control of various pharmaceutical unit operations such as solid mixing, granulation, tableting and coating. These tools include nondestructive analytical techniques such as Near Infra-red spectroscopy (NIRS), Raman Spectroscopy, hyperspectral imaging and mass spectrometry that are capable of providing continuous verification of intermediate product quality attributes (Kim et al., 2021).
3. Virtual models for formulation design and production
Examples of efficient models that mimics the original product life-cycle in a virtual theme is often called a digital twin and is looked at as the model that can be used to recreate the process design, engineering and production in the same way as that of original real-time production (Chen et al., 2020b). The whole pharmaceutical manufacturing industry is now shifting to fully digitalization by virtue of recent advancements in artificial intelligence to increase efficiency and productivity of this vital economic sector (Tao et al., 2018).
The term Industry 4.0 is defined in the literature as the integration between real-time pharmaceutical production systems and the information technology systems leading to fully digital or smart manufacturing. This can be achieved through application of different IT technologies including cyber-physical systems which enabled digital process monitoring and control, risk management leading to saving time and costs plus maximizing productivity (Dalenogare et al., 2018). There is general agreement on the Digital twin and Industry 4.0 technologies by all leading pharmaceutical companies in the world and there is great coincidence of their aims with FDA and ICH guidelines set for high quality products. Technologies such as Pharma 4 enable production of a well-controlled manufacturing strategy and operational models strengthened by PAT and QbD (Hariry et al., 2022). It integrates the use of the internet of things, advanced process monitoring and control using sensors into a completely digital cyber physical system (Wasalathanthri et al., 2020). A detailed structure of the digital twin includes three components, the physical product, the virtual product, and the Communication system in-between. Data received from the physical product includes critical process parameters data obtained directly from the linked equipment, and data on product critical quality attributes obtained through attached sensors (Fig. 4). The collected information from virtual products is stored on a cloud platform and used as a source for the virtual product where modeling, optimization and predictions can be conducted. Simultaneous comparisons between real-time simulations and physical processes can be monitored and investigated through the communication platform interface. Examples of modeling software used for the virtual product includes MATLAB, Aspen-ONE and STAR-CCM (Khawaja and Scott, 2011, Arrieta, 2019). These powerful computing systems can do multitasks including discovery of data patterns, track performance, identify potential problems and suggest corrective actions in a user interactive mode. The communication interface between the virtual and physical sections of the digital twin framework is composed of large capacity clouds that can be used for storage, visualization, analysis, integration, and management of data in a fully digitalized system (Tao et al., 2020).
Fig. 4.
Connections of real-time process monitoring and virtual components of digital twins.
3.1. Applications of digital twin technology in the medical field and pharmacy practice
Digital twin (DT) framework technologies were applied in many fields including finance, product engineering, automotive transport, and aerospace. In the healthcare sector DT are applied successfully in training programs for healthcare practitioners as in simulated patients and simulated surgical operations. It has been also applied in testing of drugs’ pharmacokinetic/pharmacodynamic behavior using modeling and simulation applications (Tao et al., 2018) and applications addressing the public health of populations (Bruynseels et al., 2018). Another example is the treatment of rare diseases using new AI-suggested remedies which are based on individual responses to drugs and specific patient characteristics such as genetics and analytics obtained from historical data (Vermesan and Ovidiu, 2021). Automated Pharmacy practice in medication dispensing, inventory and supply chain management either in hospital or community pharmacy has been applied widely to gain more benefits such as reducing service time, minimization of dispensing errors and maximization of business return (Mathy et al., 2020). The role of pharmacists in healthcare services has been expanded in the last 5 years, new tasks such as vaccine administration, testing patients’ samples for COVID 19, counselling on types of vaccines and their efficacy. AI-based applications helped pharmacists in counselling and provided accurate, Error-free data on medications, side effects, interactions, and specific patients, through automated platforms for medication use. Automated pharmacy can provide huge amounts of data that can serve public health planning and management as well as financial control on investments. (Al Meslamani, 2023). However, extensive training of new pharmacists on using these techniques is needed either during their undergraduate field training, apprenticeship or early carrier periods.
3.2. Applications in drug development and approval of new drug entities
Innovative technologies of modelling and simulation of biological processes such as physiologically based pharmacokinetic (PBPK) have been used extensively in the last decade to predict drug pharmacokinetics from knowledge of specific patient physiology and drug characteristics. This tool enabled regulating authorities making the right decisions related to accepting or modifying new drug registrations and the stages of clinical trials, and even clinical study designs (Zhao et al., 2011). Physiologically based pharmacokinetic (PBPK) modeling was applied widely in predicting drug toxicity and determination of drug concentrations in various tissues using animal data integrated with in-vitro and computational models. This technique has led to valuable saving in processing time, resources and reduced the dependency on animals in the preliminary studies. It enabled making of multiple solutions or comparisons for the same treatment using various routes of administration, different forms of the active pharmaceutical ingredient, distribution in different tissues, metabolism and elimination by several compartments (Fisher et al., 2018). These in silico techniques are invaluable for expediting preclinical research and provide comprehensive data for personalized medicines based on individualized pharmacokinetic and pharmacodynamic studies. However, software availability and experienced researchers are two major challenges for expanding this kind of technology to reach most of the pharma groups. Deep learning algorithms were recently applied in diagnosis and individualized psychiatric medical treatments using pharmacogenomic, neuroimaging, and gene-environment interaction complex data (Lin et al., 2020). More interestingly and fast growing is the development of monoclonal antibodies for treating cancer and immunological diseases. Molecular dynamics and Computational techniques based on simulation and modeling of antibodies’ structure, sequencing and target interactions have been applied to achieve high antibody target specificity, binding affinity and reduce immunogenicity. Huge databases such as protein data bank (PDB) and antibody structural database (SAbDab) are used to search antibody structure and properties for specific targeted therapy (Kim et al., 2023).
3.3. Artificial intelligence during post-manufacture and marketing stage
Besides drug discovery and development, drug marketing and distribution are two areas where AI is widely used, and its benefits far exceed the cost of the applied technology. Most medium and large pharmaceutical companies are relying on AI technology in maximizing profits and minimizing costs and time for product manufactures. In marketing and distribution stages, data on product characteristics, sales, customers, report analysis and human resources, ongoing quality and stability studies are all managed by AI software programs. Small pharmaceutical companies are achieving great interest in business transformation through adopting AI in all its processes starting from research and development, through production and marketing (Kulkov, 2021). Applications of AI are extended to address market forecasting and link sales to the success of marketing channels, market needs, drug shortages in pandemics and disease crises, and sales prediction. Additionally, evaluation of performance of medical representatives and impacts of their activities on the sales are among the benefits of newly developed smart AI solutions for human resources. Raw material as well as pharmaceutical products storage and distribution are also transformed to smart processes through digitalized warehousing operated by application of programmed robotics in large companies. Finally, to achieve safe and proficient drug supply chains, companies are shifting to the use of recent AI-based blockchain technology which enables smart and secure independent data management (Xu et al., 2023).
Pharmacovigilance studies performed on marketed products are also under modernization using advanced AI technology to track adverse drug reactions rapidly and concisely avoiding bias due to human errors. Skills required by pharmacists working in this area include data analysis and interpretation in addition to scientific medicinal knowledge.
3.4. Challenges to transformation to smart pharmaceutical manufacture
Many technical hurdles and challenges exist that hinder wider use and integration of digital twin technology. These include, huge data storage and classification, time needed for system maintenance and update, variable properties and capabilities of modeling and data transfer applications, lagging between physical and virtual process comparisons in real-time analyses. In addition, data security and lack of trained specialists in data science and technology plus inflated cost of virtual modeling applications make switching to fully integrated DT, remain as challenges to most of small to medium pharmaceutical facilities. Big data with multidimensions is a big challenge to data analysts, therefore new advanced technologies that can reduce the dimensions and make data usable for model training through preprocessing are essential solutions for such challenges. Ensemble techniques such as “Random Forest” are methods used to search the data domain for purification and detection of the features in data leading to improved machine learning, model generation and prediction. The random forest model is a collection of several decision trees that takes the average of results from all trees (Nabipour et al., 2020). The added value of ensemble methods is their capability to combine more than one machine learning model into one powerful model with reduced bias, variance and improved predictions (Krishnaveni et al., 2021). Several additional challenges to transformation to smart manufacturing and processing includes fear of job losses and emerging system errors as well as cybersecurity. AI technologies are supposed to help specialists in managing technical and complicated processes and facilitate decision making to senior managements without causing too much losses in job opportunities (Shinde et al., 2021).
4. Prospects of artificial intelligence in pharmaceutical industry
Currently, significant efforts and massive research funding is directed to the area of genomics and proteomics in an approach to tackle problems of viral mutations and impacts on efficacy and efficiency of vaccination following the era of COVID 19. New peptide drugs for treating cancer or other means of gene therapies dealing with genetic diseases show a growing interest between researchers and pharmaceutical enterprises. With the help of AI, novel solutions will be easily found through searching the huge literature of research ideas/articles, patent applications, new applications shared in conference proceedings, and going a step further by benefiting from combining academic research outputs and medical practice findings. Companies that specialize in production and operation of AI-based technologies for development of products’ life cycle, are now achieving multi-billion Dollars profits through selling or sharing their innovations with multinational pharmaceutical companies. Large pharmaceutical companies are continuously seeking maximization of their market values and assets through developing new drug products and innovated patents through merging with or acquisition of other companies (Dierks et al., 2018). The merge of new advanced techniques such as generative AI modules, data science and business analytics will inevitably maximize the productivity and profitability of pharmaceutical companies. Hence, continuous educational and professional training programs are offered by specialized technology companies for researchers and even administrators of various corporations on industry-relevant high technology programs. Examples of technologies of interest include but are not limited to, software engineering, business management, business analytics, data Science, machine learning, artificial intelligence, cloud computing, cyber security, and digital marketing.
5. Conclusion
To maximize the benefits of artificial intelligence in developing pharmaceutical manufacturing industry and pharmaceutical services, specialized and well-trained personnel are essential, especially those having both scientific and AI skills including data analysis, simulation, modeling, and optimization. Smart emerging AI- based technologies such as digital twins, internet of things and Pharma-4 have previously induced massive advancement of various industrial sectors including aerospace, finance, and vehicle manufacture. Being expensive and confidential, tremendous efforts are required from the pharmaceutical industry to plan for and commence acquiring these technologies to join the right track of full digitalization. Regulatory authorities worldwide are open to and encouraging employment of new AI-based technologies such as 3D modeling and dynamic applications in pharmaceutical drug discovery, development, manufacture, approval, and marketing. Therefore, fulfilling the growing needs for new products especially biopharmaceuticals, mandates all pharmaceutical businesses to overcome the challenges, speed up their plans and employ advanced techniques of AI, hire experienced staff to be able to transform to smart manufacturing. On the other hand, pharmacists working in the practice need to acquire new IT skills related to data analytics, modeling and optimization techniques to help them in decision-making.
CRediT authorship contribution statement
Ahmed M. Abdelhaleem Ali: Conceptualization, Data curation, Formal analysis, Methodology, Project administration, Resources, Writing – original draft. Majed M. Alrobaian: Funding acquisition, Investigation, Methodology, Validation, Visualization, Writing – review & editing.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Contributor Information
Ahmed M. Abdelhaleem Ali, Email: a.mali@tu.edu.sa.
Majed M. Alrobaian, Email: Majed.alrobaian@tu.edu.sa.
References
- Agrawal P. Artificial intelligence in drug discovery and development. J. Pharmacovigil. 2018;6(2):1000e173. doi: 10.4172/2329-6887.1000e173. [DOI] [Google Scholar]
- Arrieta A. Variability Modeling and Management of MATLAB/Simulink Models. In: Proceedings of the 23rd International Systems and Software Product Line Conference - Volume A [Internet]. 2019 Sep 9; Available from: doi: 10.1145/3336294.3342380.
- Askin S., Burkhalter D., Calado G., El Dakrouni S. Artificial intelligence applied to clinical trials: opportunities and challenges. Heal. Technol. 2023;13(2):203–213. doi: 10.1007/s12553-023-00738-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruynseels K., Santoni de Sio F., Van den Hoven J. Digital twins in health care: ethical implications of an emerging engineering paradigm. Front. Genet. 2018;9:31. doi: 10.3389/fgene.2018.00031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cela R., Ordoñez E.Y., Quintana J.B., Rodil R. Chemometric-assisted method development in reversed-phase liquid chromatography. J. Chromatogr. A. 2013;1287:2–22. doi: 10.1016/j.chroma.2012.07.081. [DOI] [PubMed] [Google Scholar]
- Chen G., Ding C., Li Y., Xiaojun Hu., Li X., Ren L.i., Ding X., Tian P., Xue W. Prediction of chronic kidney disease using adaptive hybridized deep convolutional neural network on the internet of medical things platform. IEEE Access. 2020;8:100497–100508. doi: 10.1109/access.2020.2995310. [DOI] [Google Scholar]
- Chen Y., Yang Ou., Sampat C., Bhalode P., Ramachandran R., Ierapetritou M. Digital twins in Pharmaceutical and Biopharmaceutical Manufacturing: a literature review. Processes. 2020;8(9):1088. doi: 10.3390/pr8091088. [DOI] [Google Scholar]
- Dalenogare L.S., Benitez G.B., Ayala N.F., Frank A.G. The expected contribution of industry 4.0 Technologies for Industrial Performance. Int. J. Prod. Econ. 2018;204:383–394. doi: 10.1016/j.ijpe.2018.08.019. [DOI] [Google Scholar]
- Dierks R.M., Louisa O.B., Reginster J.-Y. Critical analysis of valuation and strategical orientation of merger and acquisition deals in the pharmaceutical industry. Expert Rev. Pharmacoecon. Outcomes Res. 2018;18(2):147–160. doi: 10.1080/14737167.2018.1417040. [DOI] [PubMed] [Google Scholar]
- DiMasi J.A., Grabowski H.G., Hansen R.W. Innovation in the pharmaceutical industry: new estimates of R&D costs. J. Health Econ. 2016;47:20–33. doi: 10.1016/j.jhealeco.2016.01.012. [DOI] [PubMed] [Google Scholar]
- Fisher J.W., Yang X., Timchalk C. Handbook of Developmental Neurotoxicology. Elsevier; 2018. Physiologically based Pharmacokinetic (PBPK) models; pp. 217–228. [DOI] [Google Scholar]
- Hariry, R.E., Barenji, R.V., Paradkar, A. 2022. From Industry 4.0 to Pharma 4.0. Handbook of Smart Materials, Technologies, and Devices [Internet]. 215–36. Available from: doi: 10.1007/978-3-030-58675-1_4-1.
- Henstock P.V. Artificial intelligence for pharma: time for internal investment. Trends Pharmacol. Sci. 2019;40(8):543–556. doi: 10.1016/j.tips.2019.05.003. [DOI] [PubMed] [Google Scholar]
- Hulsen T. Literature analysis of artificial intelligence in biomedicine. Available from. 2021 doi: 10.20944/preprints202105.0056.v2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson K.B., Wei W.-Q., Weeraratne D., Frisse M.E., Misulis K., Rhee K., Zhao J., Snowdon J.L. Precision medicine, AI, and the future of personalized health Care. Clin. Transl. Sci. 2021;14(1):86–93. doi: 10.1111/cts.12884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jumeau M., Francois O., Bonnabry P. Impact of automated dispensing cabinets on dispensing errors, interruptions and pillbox preparation time. Eur. J. Hospital Pharm. [Internet]. 2021 Aug 23;30(4):237–241. doi: 10.1136/ejhpharm-2021-eahpconf.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juran, J.M. 1992. Departmental quality planning. National Productivity Review [Internet]. 11(3):287–300. Available from: hors:doi: 10.1002/npr.4040110302.
- Khawaja, H., Scott, S. 2011. CFD-DEM Simulation of propagation of sound waves in fluid particles fluidised medium. Int. J. Multiphys. [Internet]. 2011 Mar;5(1):47–60. Available from: doi: 10.1260/1750-9548.5.2.89 .
- Kim E.J., Kim J.H., Kim M.S., Jeong S.H., Choi D.H. Process analytical technology tools for monitoring pharmaceutical unit operations: a control strategy for continuous process verification. Pharmaceutics. 2021 Jun 21;13(6):919. doi: 10.3390/pharmaceutics13060919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J., McFee M., Fang Q., Abdin O., Kim P.M. Computational and artificial intelligence-based methods for antibody development. Trends Pharmacol. Sci. 2023;44(3):175–189. doi: 10.1016/j.tips.2022.12.005. [DOI] [PubMed] [Google Scholar]
- Kiriiri G.K., Njogu P.M., Mwangi A.N. Exploring different approaches to improve the success of drug discovery and development projects: a review. Future J. Pharm. Sci. 2020;6(1):27. doi: 10.1186/s43094-020-00047-9. [DOI] [Google Scholar]
- Koletti A.E., Tsarouchi E., Kapourani A., Kontogiannopoulos K.N., Assimopoulou A.N., Barmpalexis P. Gelatin nanoparticles for NSAID systemic administration: quality by design and artificial neural networks implementation. Int. J. Pharm. 2020;578 doi: 10.1016/j.ijpharm.2020.119118. [DOI] [PubMed] [Google Scholar]
- Krishnaveni S., Sivamohan S., Sridhar S.S., Prabakaran S. Efficient feature selection and classification through ensemble method for network intrusion detection on cloud computing. Clust. Comput. 2021;24(3):1761–1779. doi: 10.1007/s10586-020-03222-y. [DOI] [Google Scholar]
- Kroll P., Hofer A., Ulonska S., Kager J., Herwig C. Model-based methods in the Biopharmaceutical process lifecycle. Pharm. Res. 2017;34:2596–2613. doi: 10.1007/s11095-017-2308-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kulkov I. The role of artificial intelligence in business transformation: a case of Pharmaceutical companies. Technol. Soc. 2021;66 doi: 10.1016/j.techsoc.2021.101629. [DOI] [Google Scholar]
- Lee DonHee. Effects of key value co-creation elements in the Healthcare system: focusing on technology applications. Serv. Bus. 2019;13(2):389–417. doi: 10.1007/s11628-018-00388-9. [DOI] [Google Scholar]
- Lin E., Lin C.-H., Lane H.-Y. Precision psychiatry applications with pharmacogenomics: artificial intelligence and machine learning approaches. Int. J. Mol. Sci. 2020;21(3):969. doi: 10.3390/ijms21030969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maltarollo V.G., Kronenberger T., Espinoza G.Z., Oliveira P.R., Honorio K.M. Advances with support vector machines for novel drug discovery. Expert Opin. Drug Discov. 2019;14(1):23–33. doi: 10.1080/17460441.2019.1549033. [DOI] [PubMed] [Google Scholar]
- Mathy, Caryn, Christopher Pascal, Marie Fizesan, Christopher Boin, Noémie Délèze, Olivier Aujoulat. 2020. Automated Hospital Pharmacy Supply Chain and the Evaluation of Organisational Impacts and Costs. In: Supply Chain Forum: An International Journal, 21:206–18. Taylor & Francis. doi: 10.1080/16258312.2020.1784687.
- Meslamani, Ahmad Z Al. 2023. Applications of AI in Pharmacy Practice: A Look at Hospital and Community Settings. J. Med. Econ. Taylor & Francis. doi: 10.1080/13696998.2023.2249758. [DOI] [PubMed]
- Mukherjee A., Deshpande J.M. Application of artificial neural networks in structural design expert systems. Comput. Struct. 1995;54(3):367–375. doi: 10.1016/0045-7949(94)00342-z. [DOI] [Google Scholar]
- Murphy T., O’Mahony N., Panduru K., Riordan D., Walsh J. In 2016 27th Irish Signals and Systems Conference (ISSC) 2016. Pharmaceutical Manufacturing and the quality by design (QBD), process analytical technology (PAT) approach; pp. 1–7. [DOI] [Google Scholar]
- Nabipour M., Nayyeri P., Jabani H., Shahab S., Mosavi A. Predicting stock Market trends using machine learning and deep learning algorithms via continuous and Binary data; a comparative analysis. IEEE Access. 2020;8:150199–150212. doi: 10.1109/access.2020.3015966. [DOI] [Google Scholar]
- Nguyen A., Lamouri S., Pellerin R., Tamayo S., Lekens B. Data analytics in pharmaceutical supply chains: state of the art, opportunities, and challenges. Int. J. Prod. Res. 2022;60(22):6888–6907. doi: 10.1080/00207543.2021.1950937. [DOI] [Google Scholar]
- Paul D., Sanap G., Shenoy S., Kalyane D., Kalia K., Tekade R.K. Artificial intelligence in drug discovery and development. Drug Discov. Today. 2021;26(1):80–93. doi: 10.1016/j.drudis.2020.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pouyanfar S., Sadiq S.a., Yan Y., Tian H., Tao Y., Reyes M.P., Shyu M.-L., Chen S.-C., Iyengar S.S. A survey on deep learning: algorithms, techniques, and applications. ACM Computing Surveys (CSUR) 2018;51(5):1–36. doi: 10.1145/3234150. [DOI] [Google Scholar]
- Qiu Y., Zhang Y., Deng Y., Liu S., Zhang W. A comprehensive review of computational methods for drug-drug interaction detection. IEEE/ACM Trans. Comput. Biol. Bioinformat. 2021 May 18;19(4):1968–1985. doi: 10.1109/tcbb.2021.3081268. [DOI] [PubMed] [Google Scholar]
- Raijada D., Wac K., Greisen E., Rantanen J., Genina N. Integration of personalized drug delivery systems into digital health. Adv. Drug Deliv. Rev. 2021;176 doi: 10.1016/j.addr.2021.113857. [DOI] [PubMed] [Google Scholar]
- Rakić T., Kasagić-Vujanović I., Jovanović M., Jančić-Stojanović B., Ivanović D. Comparison of full factorial design, central composite design, and box-behnken design in chromatographic method development for the determination of fluconazole and its impurities. Anal. Lett. 2014;47(8):1334–1347. doi: 10.1080/00032719.2013.867503. [DOI] [Google Scholar]
- Russo-Spena T., Mele C., Marzullo M. Practising value innovation through artificial intelligence: the IBM Watson case. J. Creating Value. 2019;5(1):11–24. doi: 10.1177/2394964318805839. [DOI] [Google Scholar]
- Shinde A., Pawar D., Sonawane K. Automation in pharmaceutical sector by implementation of artificial intelligence platform: a way forward. Int. J. Basic Clin. Pharmacol. 2021;10(7):863. doi: 10.18203/2319-2003.ijbcp20212387. [DOI] [Google Scholar]
- Song H., Pei X., Liu Z., Shen C., Sun J., Liu Y., Zhou L., Sun F., Xiao X. Pharmacovigilance in China: evolution and future challenges. Br. J. Clin. Pharmacol. 2023;89(2):510–522. doi: 10.1111/bcp.15277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tao F., Cheng J., Qi Q., Zhang M., Zhang H.e., Sui F. Digital twin-driven product design, manufacturing and service with big data. Int. J. Adv. Manuf. Technol. 2018;94:3563–3576. doi: 10.1007/s00170-017-0233-1. [DOI] [Google Scholar]
- Tao, S., van Beek, A., Apley, D.W., Chen, W. 2020. Bayesian optimization for simulation-based design of multi-model systems. Volume 11B: 46th Design Automation Conference (DAC) [Internet]. 2020 Aug 17.doi: 10.1115/detc2020-22651.
- Vermesan, Ovidiu. Artificial Intelligence for Digitising Industry. Artificial Intelligence for Digitising Industry, 2021, pp. 1–541. Crossref, doi: 10.13052/rp-9788770226639.
- Wasalathanthri, D.P., Rehmann, M.S., Song, Y., Gu, Y., Mi, L., Shao, C., et al. 2020. Technology outlook for real‐time quality attribute and process parameter monitoring in biopharmaceutical development—A review. Biotechnol. Bioeng. [Internet]. 2020 Jul;117(10):3182–98. Available from: doi: 10.1002/bit.27461 . [DOI] [PubMed]
- Wu C., Bouvry P. In 2023 International Conference on Information Networking (ICOIN) 2023. The emerged artificial intelligence protocol for hierarchical information network; pp. 427–432. [DOI] [Google Scholar]
- Xu, Xiang, Ning Tian, Haoyu Gao, Hong Lei, Zixuan Liu, Zhiwei Liu. 2023. A survey on application of blockchain technology in drug supply chain management. In: 2023 IEEE 8th International Conference on Big Data Analytics (ICBDA), 62–71. IEEE. doi: 10.1109/icbda57405.2023.10104779.
- Yu L.X., Amidon G., Khan M.A., Hoag S.W., Polli J., Raju G.K., Woodcock J. Understanding Pharmaceutical quality by design. AAPS J. 2014;16(4):771–783. doi: 10.1208/s12248-014-9598-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao P., Zhang L., Grillo J.A., Liu Q., Bullock J.M., Moon Y.J., Song P., Brar S.S., Madabushi R., Wu T.C. Applications of physiologically based Pharmacokinetic (PBPK) modeling and simulation during regulatory review. Clin. Pharmacol. Ther. 2011;89(2):259–267. doi: 10.1038/clpt.2010.298. [DOI] [PubMed] [Google Scholar]
- Zia A., Aziz M., Popa I., Khan S.A., Hamedani A.F., Asif A.R. Artificial intelligence-based medical data mining. J. Personalized Med. 2022;12(9):1359. doi: 10.3390/jpm12091359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zobel-Roos S., Thiess H., Gronemeyer P., Ditz R., Strube J. Continuous chromatography as a fully integrated process in continuous Biomanufacturing. Continuous Biomanufacturing-Innovative Technol. Methods. 2017:369–392. doi: 10.1002/9783527699902.ch13. [DOI] [Google Scholar]
- Zobel-Roos S., Schmidt A., Mestmäcker F., Mouellef M., Huter M., Uhlenbrock L., Kornecki M., Lohmann L., Ditz R., Strube J. Accelerating biologics Manufacturing by modeling or: is approval under the QbD and PAT approaches demanded by authorities acceptable without a digital-twin? Processes. 2019;7(2):94. doi: 10.3390/pr7020094. [DOI] [Google Scholar]