Abstract
Phytochemicals are compounds found in plants that possess a variety of bioactive properties, including antioxidant and immunomodulatory properties. Recent studies have highlighted the potential of phytochemicals in targeting specific signalling pathways involved in cytokine storm, a life-threatening clinical condition resulting from excessive immune cell activation and oversupply of proinflammatory cytokines. Several studies have documented the immunomodulatory effects of phytochemicals on immune function, including their ability to regulate essential cellular and molecular interactions of immune system cells. This makes them a promising alternative for cytokine storm management, especially when combined with existing chemotherapies. Furthermore, phytochemicals have been found to target multiple signalling pathways, including the TNF-α/NF-κB, IL-1/NF-κB, IFN-γ/JAK/STAT, and IL-6/JAK-STAT. These pathways play critical roles in the development and progression of cytokine storm, and targeting them with phytochemicals represents a promising strategy for controlling cytokine release and the subsequent inflammation. Studies have also investigated certain families of plant-related constituents and their potential immunomodulatory actions. In vivo and in vitro studies have reported the immunomodulatory effects of phytochemicals, which provide viable alternatives in the management of cytokine storm syndrome. The collective data from previous studies suggest that phytochemicals represent a potentially functional source of cytokine storm treatment and promote further exploration of these compounds as immunomodulatory agents for suppressing specific signalling cascade responses. Overall, the previous research findings support the use of phytochemicals as a complementary approach in managing cytokine storm and improving patient outcomes.
Keywords: Cytokines, Cytokine release syndrome, Cytokine storm, Immunomodulation, Inflammatory pathways, Phytochemicals
Introduction
Medicinal plants have been successfully utilized topically and internally since ancient civilisations to treat various health concerns across cultures.1 Specifically, herbal compounds have been crucial in drug development, particularly in the treatment of cancer and infectious disorders.2 Phytochemicals refer to substances or chemicals derived from plants with unique structures and activities.3 These substances are vital for plant development, physiological activities, and defence.4 Phytochemicals are abundant in vegetables, fruits, nuts, and seeds.5 Researching into the phytochemical family is an enormous undertaking due to its diversity.
Reports have documented the antioxidant properties of phytochemicals.6 Some phytochemicals were reported to precisely modified signal transduction processes, including regulating antioxidant enzyme synthesis and promoting antioxidant effects in cells.7 In addition, the antioxidative properties of phytochemicals are essential in preventing neurological disorders, such as Alzheimer’s disease, by minimizing oxygen radicals, neutralizing carcinogenic metabolism, treating and impeding oxidative stress-induced chronic illnesses.8,9 Studies have also found that some phytochemicals prevented carcinogenesis, combat microbial infections, inhibited ATP synthase, and promoted skin regeneration.10,11 Advancements in plant extraction technology have turned phytochemicals into a more effective, safer and potentially vital components in the development of plant-based medicines.12 It is worth noting that approximately one-third of the drugs currently approved by the Food and Drug Administration (FDA) were derived from plants, underscoring the extensive utilization and advantages of medicinal plants.13
Phytochemicals offer several benefits in modulating immune functions, including the maintenance of health through immune system support and the modulation of essential cellular and molecular interactions within the immune system. Cytokines are proteins produced by immune cells and play diverse biological roles. They play crucial roles in coordinating innate immune response by promoting local protective inflammation in acute phase responses.14 Cytokines are instrumental in initiating and regulating adaptive immune responses.15 Consequently, this review explores the valuable proof and evidence of phytochemicals in regulating immune responses, particularly in the context of cytokine storms.
Lymphocytes and macrophages are the primary sources of pro-inflammatory cytokines.16 In certain disease conditions, an excessive and uncontrolled systemic hyperinflammatory response can occur, which is characterized by elevated levels of pro-inflammatory cytokines such as interleukin-1 (IL-1), IL-6, interferons (INFs), and tumor necrosis factor-alpha (TNF-α). This phenomenon is known as a cytokine storm or cytokine release syndrome, which can lead to multiple organ dysfunction and development of acute respiratory distress syndrome (ARDS).17-20 Cytokine activation is nevertheless beneficial in combating infections and malignancies but could be detrimental to the host if released excessively.
Cytokine storm is a rapidly developing and life-threatening clinical condition. Historically, the term cytokine storm was invented by Ferrara et al21 in 1993 to describe the clinical manifestation of graft-versus-host disease. The phrase was later employed in numerous inflammatory diseases, including autoimmune conditions, organ transplantation, as well as in the context of cancer chimeric antigen receptor (CAR-T) cell therapy, which was related to the symptoms that followed the treatment of certain blood cancers,22 and. Currently, the term “cytokine storm” has gained increased attention, particularly in infectious diseases such as influenza, severe acute respiratory syndrome coronavirus (SARS-CoV), and coronavirus disease 2019 (COVID-19).22-28 This is due the implication of cytokine storm on the severity of these diseases.
The oversupply of inflammatory cytokines and unrestrained immune cell activation during cytokine storm could result in various pathological conditions, such as continuous fever, arthralgia, myalgia, capillary leak disorder, hypotension, hemophagocytic lymphohistiocytosis (HLH), ARDS, and multi-organ failure.29
Clinical data from several studies on COVID-19 infection have proven that cytokine storm is life-threatening if untreated. The phenomenon is also one of the leading fatal causes of COVID-19.30-32 Numerous types of cytokines have been reported to contribute towards the pathogenesis of cytokine storms, thus a single drug treatment might be ineffective. Pathophysiological characteristics of cytokine storm could arise from the effects of pro-inflammatory cytokines, including TNF-α, IL-1, IL-4, IL-6, IL-7, IL-18, and IFN-γ.33 Accordingly, effective cytokine storm-reducing strategies may require the suppression of hyperinflammatory responses and modulation of the immune responses.34
Relevant keywords were employed in searching multiple data sources which included PubMed, SpringerLink, ScienceDirect, Google Scholar, and Scopus. In this review, the entire content of pertinent articles was acquired to enable the best literature-based resources accessibility. Furthermore, the data were selected based on the significance and strong understanding of the immunomodulating agent extracted from a plant source and their relationship with cytokine release immuno-pathogenicity to provide an unbiased viewpoint.
The chemical nature of compounds with immunomodulatory effects
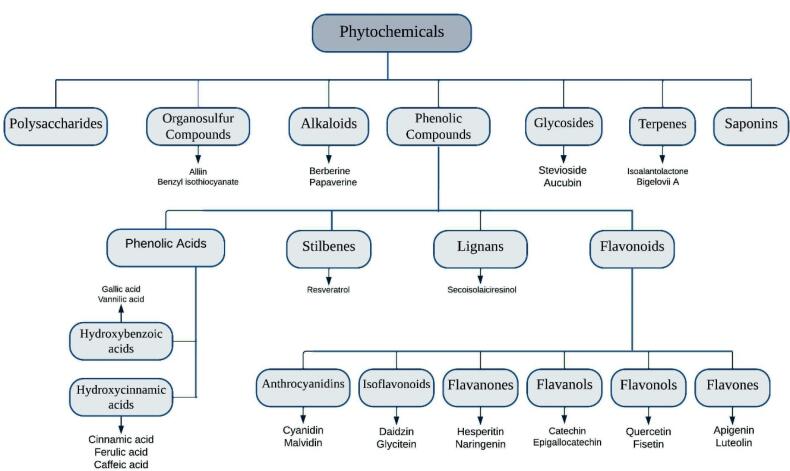
Over the years, the interest in natural-derived medicines, particularly phytochemicals, has grown tremendously, resulting in the identification of several active compounds currently categorized as alkaloids, polyphenols, glycosides, organosulfurs, saponin, carotenoids, and terpenes (see Figure 1).35,36 Immunomodulators are compounds that regulate or normalise pathophysiological processes.37 Researchers have been interested in the immunomodulatory properties of plant- derived phytochemical compounds. Consequently, research investigations on immunomodulatory phytochemicals and their active molecular constituents have led to the development of novel immunomodulatory agents to supplement existing chemotherapies. Nevertheless, most researches have focused on the discovery and investigation of specific families of plant-related chemicals and their potential immunomodulatory effects.38,39
Figure 1.
Phytochemicals classification
Polyphenols
Polyphenols contain at least one aromatic ring with one or more hydroxyl groups, making them one of the largest groups of phytochemicals.40 Recent studies reported that phenolic compounds are associated with the positive effects of medicinal herbs.41 Phenolic acids, flavonoids, lignans, and stilbenes are the primary subclasses of polyphenols.42 In more details, based on the variations in their generic structure, particularly the degree of oxidation of the oxygenated heterocyclic C ring, flavonoids can be classified into different categories, including flavones, flavonols, flavanols, anthocyanidins, flavanones, and isoflavonoids (Figure 1).43
Studies on plant extracts and phytochemicals have demonstrated the anti-inflammatory effects of polyphenols in preventing the progression of chronic illnesses.44-47 Polyphenolics also possess superior antioxidant activities, while others such as flavonoids and flavonols demonstrated immunomodulatory actions.47-49 Flavanones are another group of polyphenols with an immunomodulatory activity that reduces the intensity of inflammatory responses. Moreover, flavanones extracted from Citrus by-products have been found to possess in vitro antioxidant and anti-inflammatory properties.50
Some polyphenols such as luteolin and quercetin affects the equilibrium between pro- and anti-inflammatory upregulations by suppressing IL-1β and TNF-α synthesis while promoting IL-10 release.51 Fouad et al52 investigated the immunomodulatory effects of naringenin in acute lung injury (ALI) model in rats. The findings of their study demonstrated that naringenin reduced the expression of TNF-α, nuclear factor kappa B (NF-κB), and inducible nitric oxide synthase (iNOS) and significantly diminished the secretion and action of the pro-inflammatory cytokine (IL-6).
Zhang and colleagues explored the efficacy of apigenin as a pre-treatment for LPS-induced inflammation in human macrophages. As reported, apigenin markedly inhibited TNF-α, IL-1β-induced NF-κB activation, and IL-6 production.53 Furthermore, apigenin has been demonstrated to suppress adhesion molecules [vascular cell adhesion protein 1 (VCAM1) and IL-6-induced intercellular adhesion molecule (ICAM-1)] and chemokines (CCL5).53 Consequently, several natural immunomodulating drugs could produce inhibitory efficacy against inflammatory cytokines, thus enabling the possibility of targeting cytokine storm cascades when employed as an immunomodulatory agent during cytokine storm treatments.
Alkaloids
Alkaloids are nitrogen-containing compounds, and are one of the most common phytochemicals in plants. They are found in families such as Apocynaceae, Amaryllidaceae, Asteraceae, and Papaveraceae families, and have significant biological activities and pharmacological effects due to their nitrogen-containing frameworks with a negative oxidation potential.54 Moreover, phytochemicals in this class exhibit anti-inflammatory and antioxidant properties, as well as enzymatic inhibitory activities, which contribute significantly to their role in the treatment of neurological disorders.55,56
Moreover, alkaloids have demonstrated anticancer, antibacterial, and analgesic attributes.57,58 Alkaloids, such as colchicine when administered at pharmacological doses exhibited immunomodulatory effects including diminishing cytokines production, such as IFN-γ, IL-1β, IL-6, and IL-18.59 Studies also indicated that colchicine suppresses inflammatory cytokines via multiple mechanisms, including the interruption of inflammasome activation [one of the major pathways to limit pro-inflammatory cytokine (IL-1, IL-6, TNF-α) release] and recruitment of more macrophages and neutrophils.60-62
Compared to polyphenols, alkaloids have a narrow therapeutic margin and exhibit activity at extremely low doses. However, their potential cytotoxic effects should not be overlooked, with the exception of chelerythrine and chelidonine.63 Additionally, certain alkaloids have been known to cause gastrointestinal side effects, such as diarrhea, nausea, and cramps.64
Glycosides
Glycosides are composed of two chemically and functionally independent parts, wherein the glycone (saccharide) portion is linked to another functional group via a glycosidic bond.65 Many plants store glycosides as inactive compounds that can be activated by enzymatic reactions.66 The pharmacological effects of glycoside are generally attributed to its aglycone moiety, whereas its glycone moiety determines its water solubility.
Glycosides could be classified based on their aglycone structure, for instance, sterol or flavonoid, whereas the number of saccharides in the carbohydrate unit determines whether the glycosides are mono-, di-, or trisaccharides. Glycosides could also be divided according to the glycosidic linkage between their aglycone and the carbohydrate groups (sugar moiety). Alternatively, therapeutic applications could be employed as another classification basis, such as cardiac glycosides, which are well-known for their positive effects on cardiac arrhythmia.65,67,68
The unique structure of glycosides has resulted in their wide therapeutic applications, including antioxidant, immunomodulatory effects, anticancer, and anticoagulant.69-71 Studies have shown that glycosides, such as sativoside, derived from Stevia rebaudiana leaves down-regulate pro-inflammatory cytokines production. In addition, it reduces NF-κB and pro-inflammatory cytokines levels IL-1, IL-6, IL-17a, IL-10, and TNF-α.72-75 Similarly, naringin, a glycoside derived from the flavanone naringenin and found as the primary bioactive constituent in citrus fruits. This glycoside demonstrated a neuroprotective effect in cerebral infarction by inhibiting neuronal cell apoptosis and diminishing inflammatory cytokines, such as IL-6 and TNF-α.76
Terpenes (isoprenoids)
Based on their structure and functions, terpenes are divided into several classes. The isoprene unit is the backbone of terpenes, and most terpenes consist of two or more isoprene units organised in a specific sequence.77 Terpenes are basic hydrocarbon structures, while terpenoids are modified terpenes with extra functional groups, commonly relocated or eliminated oxygen-containing groups.78
Terpenes are natural chemical compounds found in plants and animals and are well known for their diverse medicinal qualities. These compounds play a protective role against diseases and parasites in plants, animals, and microbes.79 Additionally, terpenes are also essential to plants as they are required in carbon fixation via metabolic reactions.80
Some studies have suggested the potential of terpenes in modulating cytokines due to their lipophilic properties that facilitated their rapid actions and uptakes.81 In another study, some terpenes, including carvacrol have shown enhanced production of anti-inflammatory cytokines, such as IL-10.82
Polysaccharides
Polysaccharides are macromolecular molecules with broad biological functions. They are composed of more than ten monosaccharides connected by glycosidic linkages. Various polysaccharide molecules have been extracted from organic sources and classified according to their sources as animal, microorganism, or plant polysaccharides.83 Recent studies have focused on the immunobiological effects of polysaccharides extracted primarily from Chinese herbal medicine.84,85 Several polysaccharide classes have demonstrated antioxidant, antitumor, and immunomodulatory properties. In fact, the most established mechanism is the capacity of polysaccharides to modulate macrophage function. These phytochemicals could inhibit cytokines, including IL-6 and TNF-α, while inducing cytokines, such as IL-2, IL-10, and IL-4. Prospectively, polysaccharides might be the foundation for evaluating new medicinal compounds with immunomodulatory attributes.86-88
Organosulfur compounds
Organosulfurs are widely recognized for their exceptional therapeutic characteristics and health benefits. Typically, these class of phytochemicals are found in several dietary sources, including vegetables, fruits, grains, and legumes.89 Several plant-based diets rich in organosulfur compounds have been studied for their anti-inflammatory and antioxidant properties.90 For instance, Allium cepa, Allium sativum, andPentadiplandra brazzeana contain high concentrations of organosulfur compounds such as alliin, allicin, diallyl disulfide, and diallyl trisulfide, which are responsible for the plants’ anti-inflammatory, antioxidant, anticancer, hepato- and cardioprotective properties.91,92 Garlic extracts have also been reported to modulate the release of inflammatory cytokines, including IL‐1β, IL-6, and TNF-α, demonstrating immunomodulatory-inducing abilities.93
Studies have revealed that the water fraction of garlic increased IFN-γ and IL-12 levels while suppressing the expression of inflammatory cytokines IL-1, IL-6, and TNF-α in bronchoalveolar lavage fluid.94 In another report, allicin inhibited the spontaneous and TNF-α -induced production of IL-1β and IL-8 from two different cell lines in a dose-dependent manner. The diminished cytokine production was attributed to the inhibitory effect of allicin on the breakdown of IκB in the NF-κB pathway.95 Therefore, the use of organosulfur compounds alone or combined with other phytoconstituents might be effective against disproportionate immune responses seemingly related to cytokine storm due to their various pleiotropic effects.
Table 1 summarizes the immunomodulatory properties of various natural products with respect to specific regulatory signalling pathways.
Table 1. Immunomodulatory properties of various phytochemicals and the associated regulatory signalling pathways .
| Phytochemical class | Phytochemical name | Plant source | Experimental model | Targeted inflammatory pathway | Main effect | Reference |
|---|---|---|---|---|---|---|
| Alkaloid | Berberine | Coptis chinensis | LPS-induced ARDS model in mice | NF-κB pathway | Inhibit the production of IL-1β, IL-6 and TNF-α. | 96 |
| Alkaloid | Berberine | Coptis chinensis | Female BALB/c Mice |
MAPK and NF-κB signalling pathways | Reduce the expression levels of the relative cytokines IL-2 and IL-4. | 97 |
| Alkaloid | Protostemonine | Stemona sessilifolia | C57BL/6 mice model | MAPK and NF-κB signalling pathways | Decrease generation of IL-1β, IL-6 and TNF-α in murine ALI model. Decrease the expression of iNOS, and the generation of NO. |
98 |
| Alkaloid | Tetrahydroberberrubine | Corydalis yanhusuo | LPS-induced acute lung injury in mice | MAPK, AKT and NF-kB pathways | Inhibit the activation of NF-kB p65 and JNK/p38 MAPKs. | 99 |
| Alkaloid | Tabersonine | Catharanthus roseus | LPS-induced acute lung injury in mice | NF-κB pathway, MAPK/MK2 signalling | Inhibit the production of IL-1β, IL-6 and TNF-α. Inhibit production of iNOS, NO. |
100 |
| Flavonoid | Cyanidin | Black elderberries, rubus (blackberry, raspberry) | Male C57BL/6 J mice | SirT1/NF-κB pathway | Supress block of NF-B signalling. Reduce IL-1, IL-18 expression. |
101 |
| Flavonoid | Apigenin | Cynodon dactylon, Mentha longifolia. | LPS-stimulated human monocytes, LPS-stimulated mouse macrophages. |
NF-κB pathways | Suppresses TNF release in primary human monocytes. Reduce the expression of IL-1α, IL-8 TNF-α. |
102 |
| Flavonoid | Epigallocatechin-3-gallate | Camellia sinensis L. | - HPAEpiCs (type II alveolar epithelial cells)/ A549 cells (human alveolar epithelial cell carcinoma), Male ICR mice. |
MAPK/STAT3 pathway. | Reduce TNF-α-induced oxidative stress. Suppress MAPKs phosphorylation and expression signal activators of STAT-3. |
103 |
| Flavonoid | Puerarin | Radix puerariae | Male Sprague-Dawley rats | NF-κB/ JAK2/STAT3 Signal | Reduced the levels of IL-1β, IL-6 and tumour TNF-α in cerebral tissue. | 104 |
| Flavonoid | Luteolin | Reseda luteola, other plants | Male Wistar rats | NF-κB pathway | Suppress IL-1β-stimulated inflammatory action in rat chondrocytes. Suppress the IL-1β-stimulated phosphorylation of NF-κB p65 in vitro. Decrease the IL-1β-stimulated production of NO, TNF-α, and PGE2. Decrease the expression of iNOS and COX-2. |
105 |
| Flavonoid | Luteolin | Reseda luteola, other plants | Murine model of LPS-induced Acute lung injury. | MAPK/NF-kB pathways | Decrease superoxide dismutase and catalase activity, as well as oxidative damage in lung tissue. | 106 |
| Flavonoid | Fisetin | Apples, strawberries, cucumbers and many other plants | Male BALB/c mice | NF-kB and NFAT pathways | Inhibit the Th1 and Th2 production, and reduce the ratio of CD8 + / CD4 + T cells. | 107 |
| Flavonoid | Astilbin | Smilacis Glabrae Rhizoma | LPS-induced ARDS in mice | MAPK signal pathway | Decrease pro-inflammatory cytokines release. Suppressed the activities of myeloperoxidase and malondialdehyde. Supress the expression of TNF-α and IL-6 in vivo and in vitro. |
108 |
| Flavonoid | Puerarin | Radix puerariae | LPS-induced acute lung injury in mice / RAW264.7 cell line | NF-κB pathway | Inhibit the production of IL-1β, IL-6 and TNF-α. | 109 |
| Flavonoid | Acacetin | Robinia pseudoacacia (black locust), Turnera diffusa (damiana), Betula pendula (silver birch) | sepsis-induced acute lung injury model in mice | NF-κB pathway | Regulate COX-2, iNOS. Decrease pro-inflammatory cytokine concentration. |
110 |
| Flavonoid | Hesperidin | Citrus fruits | Male BALB/c mice, MCF7 BRCA cell line. |
NF-κB pathway | Reduce IL-1 and TNF- levels in the spleen cells. Exhibit good antioxidant& anti-inflammatory properties. |
111,112 |
| Flavonoid | Hesperetin | Citrus fruits | male C57BL/6J mice | NF-κB pathway | Suppress colitis-stimulated tissue oxidative stress. Suppress TNF-α, IL-6, IL-1β, and IL-33. |
113 |
| Flavonoid | Hesperetin | Citrus fruits | LPS-induced acute lung injury in mice | MAPK signal pathway | Reduce the number of neutrophils. Reduce the level TNF-α and IL-6, in the model in vivo and in vitro. Regulate IκB degradation. |
114 |
| Flavonoid | Silymarin |
Silybum
marianum (Milk thistle) |
human hepatoma cell lines | NF-κB pathway | Inhibit the expression of TNF-α. Exhibits antiviral and anti-inflammatory effects. |
115 |
| Triterpene | Bigelovii A | Salicornia bigelovii T | LPS-induced acute lung injury in mice | NF-κB pathway MAPK pathway |
Decrease inflammatory mediators. Neutrophil infiltration. |
116 |
| Triterpene | Cucurbitacine | Hemsleya amabilis | Female BALB/c mice | JAK/ STAT3 pathway NF-κB pathway |
Suppress the expression of TNF-α, IFN-γ and IL-6. | 117 |
| Sesquiterpene | Isoalantolactone | Inula helenium L | male C57/BL6 mice | NF-κB pathway | Decrease IL-6, IL-1β, TNF-α, and NO Expression. Suppress neutrophil infiltration. |
118 |
| Polysaccharides | Kochia scoparia polysaccharide fraction | Kochia scoparia | LPS-induced ALI in mice | Not mentioned | Decrease neutrophil infiltration. Decrease IL-6 and TNF-α levels. Reduce neutrophil infiltration. |
119 |
| Polysaccharides | Dendrobium officinale -extracted polysaccharides | Dendrobium officinale | Dextran sodium sulfate -induced acute UC in mice | NLRP3 pathway β-arrestin-1 signal pathway. |
Inhibit NLRP3 inflammasome and β-arrestin-1 activation. Reduce the mRNA levels of NLRP3, IL-1β and IL-18. |
120 |
| Phenolic acid | Gallic acid | Bearberry, pomegranate root bark, and many other plants | C57BL/6J mice | NLRP3 pathway | Decrease IL-1β expression. Inhibit NLRP3 inflammasome activation. |
121 |
| Phenolic acid | Gallic acid | Bearberry, pomegranate root bark, and many other plants | Male BALB/c mice | NF-κB pathway | Downregulation of TNF-α /IL-1β/ /MIP-2/GCSF genes. Reduce production of IL-1β, IL-6, and TNF-α. |
122 |
| Phenolic acid | Chlorogenic acid | Chaenomeles lagenaria | LPS-induced murine RAW 264.7 macrophages / BALB/c mice | NF-κB/NLRP3 pathway | Reduce production of IL-1b& IL-18. | 123 |
| Phenolic compound | Imperatorin | Urena lobata | Male C57BL/6 mice | JAK/STAT and NF-κB | Decrease the expression of iNOS and COX-2. Inhibit IL-6 and TNFα production. |
124 |
| Phenolic compound | Isofraxidin | Sarcandra glabra and Acanthopanax senticosus | Mice in vitro / in vivo | MAPK pathway. | Reduce the production of TNF-α. Regulate proinflammatory cytokines. |
125 |
| Phenolic compound | Curcumin | Curcuma longa | WKY and SHR rats | NF-κB-mediated NLRP3 regulation. | Reduce IL-1β production. Good target for NLRP3 inflammasome-driven disorders. |
126 |
| Phenolic compound | Apocynin | Picrorhiza kurroa, and many other plants. | Adult male SPF Wistar rats |
NLRP3 inflammasome Activation. NF-κB signalling NADPH oxidase (NOX) signalling. |
Decrease levels ofNLRP3 inflammasome proteins. Reduce the serum level of TNF-α, IL-1β and IL-6. |
127 |
| Phenolic compound | Paeonol | Moutan Cortex | Trinitrobenzene sulfonic acid TNBS-induced colitis in Female BALB/c mice, colorectal cancer-derived cell line (CW-2) |
NF-κB and STAT1 | Reduce the production of iNOS protein and mRNA generated by TNF-α and IFNγ signalling. Suppress TNFα-enhanced NF-κB regulation activity and IFNγ stimulation of STAT1. |
128 |
| Phenolic compound | Gingerol | Zingiber officinale (Ginger) | Female Balb/c miceallergy model, HaCaT cell line | NF-κB/MAPK pathways | Suppress inhibited the phosphorylation of MAP kinases. Inhibit the synthesis of cytokines necessary for T cell activation and proliferation. |
129 |
| Organosulfur compounds |
Allicin | Garlic and others | Kupffer cells and male Sprague Dawley rats (treated with acrylamide). |
MAPK /NF-κB / NLRP3 inflammasomes pathways |
Reduce reactive oxygen species release. Reduced the phosphorylation of JNK, ERK, p65, p38, and IκBα. Suppressing the stimulation of the NLRP3 inflammasome. Reducing the release of IL-1β, IL-6, IL-18, and TNF-α. |
130 |
| Organosulfur compounds |
Benzyl isothiocyanate | Alliaria petiolata, and papaya seeds | Male C57BL/6 J mice | NF-κB/NLRP3 pathway | Decrease in IL-1β expression. Reduce macrophage infiltration. |
131 |
| Organosulfur compound | Alliin | Allium species (garlic, onion) | - LPS-induced RAW264.7 cell line, dextran sulfate sodium-induced colitis in ICR mice. | MAPKs-PPARγ /AP-1/ NF-κB /STAT-1 signalling pathways. | Suppress the phosphorylation of p38, JNK. Suppress the transcription of iNOS via interference with STAT-1. Reduce the activity of pro-inflammatory cytokines. |
132 |
| Glycoside | Bergenin | Bergenia ligulata and Bergenia ciliata | LPS-induced ALI in male BALB/c mice, Raw264.7 cell line. | NF-κB pathway | Inhibit production of IL-1β, IL-6, and TNF-α. Supress the activation of NF-κB by suppress the phosphorylation of NF-κB p65 unit. |
133 |
| Glycoside | Stevioside | Stevia rebaudiana | Male Wistar rats | -TLR4-MD2 and TNFR1, NF-kB |
Reduce the expression of NF-κB and proinflammatory mediators. Free radical scavenger, exert good antioxidant properties. |
74 |
| Glycoside | Stevioside | Stevia rebaudiana | Caco-2 (human colon carcinoma) cell line | NF-κB signalling | Exhibit potent immunomodulatory effects on IκBα activation and NF-κB inhibition and reduce cytokine production. Suppressed LPS-stimulated IL-1β, IL-6, and TNF-α release. |
134 |
| Glycoside | Catalpol | Rehmannia glutinosa | male C57BL/6J mice | JNK and NF-kB signalling pathways | Inhibit JNK and IKKb phosphorylation. Suppress the activation of p50/ NF-kB. Decrease mRNA levels of pro-inflammatory cytokines. |
135 |
Immunomodulating agents and the regulatory signalling pathways
Possible pharmacological targets
The TNF-α /NF-κBsignalling pathways
TNF-α is a well-recognized pro-inflammatory cytokine predominantly released by macrophages, monocytes, and T cells. It is implicated in numerous infectious and autoimmune disorders.136,137 The TNF-dependent activation of NF-κB also increases anti-apoptotic and pro-inflammatory gene transcriptions.138 Furthermore, TNF-α imbalance is the hallmark of numerous autoimmune disorders.139 Higher TNF-α concentration has been associated with poor outcomes in SARS-CoV and MERS patients.140 Nevertheless, TNF-α has also been reported to inhibit NF-B and ameliorate pulmonary symptoms in mice infected with the SARS-CoV virus.141
The TNF/NF-κB interactions could play pathogenic roles in developing cytokine storm cascades and immune system hyperactivation during cytokine storms. Consequently, suppressing NF-κB signalling pathway could assist in reducing inflammatory diseases.142 Selective TNF-α inhibition is also therapeutically helpful in treating different pathological conditions, considering the involvement of numerous other cytokines and intermediates in cytokine storms. Accordingly, TNF-α blockers, such as infliximab and adalimumab, have been used effectively in treating various immune-mediated illnesses. The administration of anti-TNF-α therapy on COVID-19 patients also limits the release of other inflammatory-enhancing mediators. Furthermore, treating patients with active rheumatoid arthritis using anti-TNF-α has resulted in a rapid vascular permeability decrement and reduced broad-spectrum cytokines release, such as IL-6 and IL-1.143-146
Several phytochemicals possess modulatory activation and inflammation ameliorative abilities. For example, quercetin, a polyphenolic component suppresses pro-inflammatory gene expressions by blocking the nuclear translocation of p50 and p65 subunits of the NF-κB receptors.147 Min et al148 also demonstrated that quercetin diminished the gene expression and production of IL-1β, IL-6, IL-8, and TNF-α in human mast cells by inhibiting IκBα degradation and p65 nuclear translocation. In another report, Chen et al149 reported the inhibition of IKK and NF-κB activations, as well as a decrease in NF-κB’s ability to bind DNA in BV-2 microglia mice treated with LPS and IFN-γ.
Other phytochemicals, such as silymarin (flavonoid),150 ursolic acid(triterpenoid),151 gingerol (phenolic compounds),152 flavopiridol (flavonoid),153 zerumbone (sesquiterpene),154 curcumin (polyphenol pigment),155 and green tea catechins- epigallocatechin-3-gallate (phenolic compounds)156 are natural immunomodulatory agents with the ability to block one or more stages in NF-κB signalling. Consequently, pharmacologically profiling of phytochemicals would enable the identification of potent inhibitors for the NF-κB signalling pathway, thus providing a solid rationale for their application in cytokine storm management.
The IL-1/NF-κBsignalling pathways
IL-1β is one of the most investigated IL-1 family members due to its prominent role in autoinflammatory disorders. It is primarily released by macrophages, monocytes, and dendritic cells.157 IL-1β derived is from inactive IL-1β precursors via NLRP3 inflammasome cleavage.158 Several studies suggested that IL-1β might contribute to the severity of COVID-19 symptoms and autoinflammatory diseases.159-161
In severe COVID-19 cases, reactive oxygen species (ROS) arising from inflammation, and infiltration activates of NLRP3, which is one of the most significant innate immune components. Hence, this process accelerates inflammation by releasing IL-1 and enhancing IL-1 precursor cleavage, which subsequently exacerbates cytokine inflammation throughout the COVID-19 infection.162,163 Therefore, a selective antagonist targeting NLRP3 might be a therapeutic target for early-stage disease cases aiming to minimise cytokine storms, alleviate complications, and reduce mortality rates.164,165 Moreover, targeting the IL-1RI receptor has been recorded as effective approach during cytokine storm treatments in certain autoimmune disorders, such as CAR-T-cell therapy-induced cytokine storm,157 and secondary HLH.166
Numerous phytochemicals have been documented to suppress NLRP3 activation by acting on various stages of inflammasome cascades and positively affecting experimental models. For example, Fan et al167 reported that tenuigenin, a triterpene isolated from the root of P. tenuifolia, inhibited the activation of NLRP3 inflammasome by repressing ROS before impeding caspase-1 cleavage and IL-1β productions in BV2 microglial cell. Several phytochemicals from different categories have also been found to target NLRP3. Such immunomodulatory agents include triterpenoid Asiatic acid,168 sesquiterpene lactone Arglabin,169 cucurbitacin,170 and iridoid glycoside scropolioside B.171
The IL-6/JAK-STAT signalling
IL‐6 is a prototypical cytokine involved in numerous biological processes, including acute-phase reactions, immune responses, and hematopoiesis.172 It is characterized by a unique receptor system that consists of two functional proteins: the standard signal transducer for cytokines related to IL-6 (gp130) and the specific receptor for IL-6R.173 IL-6 is a good target molecule for cytokine storm given that it is expressed for longer periods than TNF-α and IL-1. It is also considered a superior indicator of disease severity and a prognostic marker for various diseases associated with cytokine storms, including CAR-T-induced and COVID-19.157,174,175
Blockade of IL-6 signalling has produced rapid and significant improvements in clinical symptoms and reduction in serum cytokine levels (including IL-6, IL-8, IL-10, and IFN-γ) during cytokine storms. Therefore, targeting IL-6 antagonism holds promise as a therapeutic approach for various cytokine storms, regardless of the specific situations and cytokine profiles involved.175,176
Gallic acid, a phenolic acid naturally found in vegetables and fruits, has the ability to modulate the activation of the STAT pathway. Pandurangan et al177 reported that gallic acid attenuated STAT3 phosphorylation and decreased p65-NF-κB expressions in the colon of mice induced with dextran sodium sulfate. Similarly, Wung et al178 revealed the inhibition of IL-6-induced intercellular adhesion molecule (ICAM-1) gene expressions by resveratrol, partly via Rac-mediated pathway interferences through suppression of STAT3 phosphorylation. The safety and efficacy of phytochemicals make them a promising agent to consider for IL-6 JAK/STAT inhibition in cytokine storm therapy.
The IFN-γ/JAK/STAT signalling
IFN-γ signalling plays a crucial role in inflammatory and other immunological responses, contributing to the prevention of viral and bacterial infections.179 IFN-γ is predominantly released by NK and activated T cells and is a potent macrophage activator.180 Moreover, STAT1 phosphorylation is regulated by JAK1/TYK2 or JAK1/JAK1, which is vital for signalling via the IFN-γ and related receptor class.181 Studies have revealed that IFN-γ plays a significant role in several cytokine storm-related diseases.182 These findings are supported by evidence indicating that elevated IFN-γ levels are ineffective against infections and lead to immunopathology due to impaired NK function, as evidenced by primary HLH cases.183
Although specific investigations have raised doubts about the role of IFN-γ blockers due to worsened prognosis of severe COVID-19 patients by generating secondary infections in COVID-19 cases, INF-γ blockades could be the core of the treatment.184 Numerous studies have reported that several phytochemicals possess potent abilities to reduce or block IFN-γ activation pathways. In a study conducted by Yang and colleagues, it was found that berberine, a natural isoquinoline alkaloid, inhibited the IFN-γ signalling pathway in DSS-induced ulcerative colitis. Regarding mechanisms, berberine regulates the IFN-γ signalling pathway via interaction with the genes responsible for encoding IFN-γ. Furthermore, IRF8 decreased significantly in ulcerative colitis mice treated with berberine.185 Ishiguro et al128 found that paeonol (polyphenolic product) reduced IFNγ-induced STAT1 activations, TNF-α-induced NF-κB transcriptional activities, and IFN-γ and TNFα-induced iNOS mRNA expressions.
Conclusion
Cytokine storm is a life-threatening condition that has been the subject of several studies, aimed at developing immunomodulatory drugs that target specific cytokines. Plant-derived immunosuppressants are a potential alternative for treating cytokine storm syndrome. A combination therapy comprising plant-derived immunosuppressants and some medications may be successful. Therefore, further studies are needed to understand the processes of phytochemically derived immunomodulating agents in different physiological situations and to gain greater insights into their therapeutic applications.
Acknowledgments
We gratefully acknowledge the generous support and valuable contribution of NatureCeuticals SDN BHD in funding this research.
Competing Interests
The authors declare no conflict of interest.
Ethical Approval
Not applicable.
Funding
This review article was part of a research project that was supported by NatureCeuticals SDN BHD, which provided financial support for the research.
References
- 1.Halberstein RA. Medicinal plants: historical and cross-cultural usage patterns. Ann Epidemiol. 2005;15(9):686–99. doi: 10.1016/j.annepidem.2005.02.004. [DOI] [PubMed] [Google Scholar]
- 2.Atanasov AG, Waltenberger B, Pferschy-Wenzig EM, Linder T, Wawrosch C, Uhrin P, et al. Discovery and resupply of pharmacologically active plant-derived natural products: a review. Biotechnol Adv. 2015;33(8):1582–614. doi: 10.1016/j.biotechadv.2015.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tyagi S, Singh G, Sharma A, Aggarwal G. Phytochemicals as candidate therapeutics: an overview. Int J Pharm Sci Rev Res. 2010;3(1):53–5. [Google Scholar]
- 4.Santino A, Taurino M, De Domenico S, Bonsegna S, Poltronieri P, Pastor V, et al. Jasmonate signaling in plant development and defense response to multiple (a)biotic stresses. Plant Cell Rep. 2013;32(7):1085–98. doi: 10.1007/s00299-013-1441-2. [DOI] [PubMed] [Google Scholar]
- 5.Liu RH. Health-promoting components of fruits and vegetables in the diet. Adv Nutr. 2013;4(3):384S–92S. doi: 10.3945/an.112.003517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.do Nascimento LD, de Moraes AAB, da Costa KS, Pereira Galúcio JM, Taube PS, Costa CML, et al. Bioactive natural compounds and antioxidant activity of essential oils from spice plants: new findings and potential applications. Biomolecules. 2020;10(7):988. doi: 10.3390/biom10070988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hayes JD, McMahon M, Chowdhry S, Dinkova-Kostova AT. Cancer chemoprevention mechanisms mediated through the Keap1-Nrf2 pathway. Antioxid Redox Signal. 2010;13(11):1713–48. doi: 10.1089/ars.2010.3221. [DOI] [PubMed] [Google Scholar]
- 8.He L, He T, Farrar S, Ji L, Liu T, Ma X. Antioxidants maintain cellular redox homeostasis by elimination of reactive oxygen species. Cell PhysiolBiochem. 2017;44(2):532–53. doi: 10.1159/000485089. [DOI] [PubMed] [Google Scholar]
- 9.Zhang YJ, Gan RY, Li S, Zhou Y, Li AN, Xu DP, et al. Antioxidant phytochemicals for the prevention and treatment of chronic diseases. Molecules. 2015;20(12):21138–56. doi: 10.3390/molecules201219753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ahmad Z, Hassan SS, Azim S. A therapeutic connection between dietary phytochemicals and ATP synthase. Curr Med Chem. 2017;24(35):3894–906. doi: 10.2174/0929867324666170823125330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Babashah S, Bakhshinejad B, Tahmasebi Birgani M, Pakravan K, Cho WC. Regulation of microRNAs by phytochemicals: a promising strategy for cancer chemoprevention. Curr Cancer Drug Targets. 2018;18(7):640–51. doi: 10.2174/1568009617666170623124710. [DOI] [PubMed] [Google Scholar]
- 12.Yuan H, Ma Q, Ye L, Piao G. The traditional medicine and modern medicine from natural products. Molecules. 2016;21(5):559. doi: 10.3390/molecules21050559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cragg GM, Newman DJ, Snader KM. Natural products in drug discovery and development. J Nat Prod. 1997;60(1):52–60. doi: 10.1021/np9604893. [DOI] [PubMed] [Google Scholar]
- 14.Dinarello CA. Historical insights into cytokines. Eur J Immunol. 2007;37(Suppl 1):S34–45. doi: 10.1002/eji.200737772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Holdsworth SR, Gan PY. Cytokines: names and numbers you should care about. Clin J Am Soc Nephrol. 2015;10(12):2243–54. doi: 10.2215/cjn.07590714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Arango Duque G, Descoteaux A. Macrophage cytokines: involvement in immunity and infectious diseases. Front Immunol. 2014;5:491. doi: 10.3389/fimmu.2014.00491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kim JS, Lee JY, Yang JW, Lee KH, Effenberger M, Szpirt W, et al. Immunopathogenesis and treatment of cytokine storm in COVID-19. Theranostics. 2021;11(1):316–29. doi: 10.7150/thno.49713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fajgenbaum DC, June CH. Cytokine storm. N Engl J Med. 2020;383(23):2255–73. doi: 10.1056/NEJMra2026131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Behrens EM, Koretzky GA. Cytokine storm syndrome: looking toward the precision medicine era. Arthritis Rheumatol. 2017;69(6):1135–43. doi: 10.1002/art.40071. [DOI] [PubMed] [Google Scholar]
- 20.Matthay MA, Ware LB, Zimmerman GA. The acute respiratory distress syndrome. J Clin Invest. 2012;122(8):2731–40. doi: 10.1172/jci60331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ferrara JL, Abhyankar S, Gilliland DG. Cytokine storm of graft-versus-host disease: a critical effector role for interleukin-1. Transplant Proc. 1993;25(1 Pt 2):1216–7. [PubMed] [Google Scholar]
- 22.Grupp SA, Kalos M, Barrett D, Aplenc R, Porter DL, Rheingold SR, et al. Chimeric antigen receptor-modified T cells for acute lymphoid leukemia. N Engl J Med. 2013;368(16):1509–18. doi: 10.1056/NEJMoa1215134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.London NR, Zhu W, Bozza FA, Smith MC, Greif DM, Sorensen LK, et al. Targeting Robo4-dependent Slit signaling to survive the cytokine storm in sepsis and influenza. Sci Transl Med. 2010;2(23):23ra19. doi: 10.1126/scitranslmed.3000678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Imus PH, Blackford AL, Bettinotti M, Luznik L, Fuchs EJ, Huff CA, et al. Severe cytokine release syndrome after haploidentical peripheral blood stem cell transplantation. Biol Blood Marrow Transplant. 2019;25(12):2431–7. doi: 10.1016/j.bbmt.2019.07.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wadia PP, Tambur AR. Yin and yang of cytokine regulation in solid organ graft rejection and tolerance. Clin Lab Med. 2008;28(3):469–79. doi: 10.1016/j.cll.2008.08.001. [DOI] [PubMed] [Google Scholar]
- 26.Davila ML, Riviere I, Wang X, Bartido S, Park J, Curran K, et al. Efficacy and toxicity management of 19-28z CAR T cell therapy in B cell acute lymphoblastic leukemia. Sci Transl Med. 2014;6(224):224ra25. doi: 10.1126/scitranslmed.3008226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Teijaro JR, Walsh KB, Cahalan S, Fremgen DM, Roberts E, Scott F, et al. Endothelial cells are central orchestrators of cytokine amplification during influenza virus infection. Cell. 2011;146(6):980–91. doi: 10.1016/j.cell.2011.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pedersen SF, Ho YC. SARS-CoV-2: a storm is raging. J Clin Invest. 2020;130(5):2202–5. doi: 10.1172/jci137647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mangalmurti N, Hunter CA. Cytokine storms: understanding COVID-19. Immunity. 2020;53(1):19–25. doi: 10.1016/j.immuni.2020.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lau SKP, Lau CCY, Chan KH, Li CPY, Chen H, Jin DY, et al. Delayed induction of proinflammatory cytokines and suppression of innate antiviral response by the novel Middle East respiratory syndrome coronavirus: implications for pathogenesis and treatment. J Gen Virol. 2013;94(Pt 12):2679–90. doi: 10.1099/vir.0.055533-0. [DOI] [PubMed] [Google Scholar]
- 31.Li Y, Chen M, Cao H, Zhu Y, Zheng J, Zhou H. Extraordinary GU-rich single-strand RNA identified from SARS coronavirus contributes an excessive innate immune response. Microbes Infect. 2013;15(2):88–95. doi: 10.1016/j.micinf.2012.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ragab D, Salah Eldin H, Taeimah M, Khattab R, Salem R. The COVID-19 cytokine storm; what we know so far. Front Immunol. 2020;11:1446. doi: 10.3389/fimmu.2020.01446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Shimizu M. Clinical features of cytokine storm syndrome. In: Cron RQ, Behrens EM, eds. Cytokine Storm Syndrome. Cham: Springer International Publishing; 2019. p. 31-41. 10.1007/978-3-030-22094-5_3. [DOI]
- 34.Mahmudpour M, Roozbeh J, Keshavarz M, Farrokhi S, Nabipour I. COVID-19 cytokine storm: the anger of inflammation. Cytokine. 2020;133:155151. doi: 10.1016/j.cyto.2020.155151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Islam MA, Alam F, Solayman M, Khalil MI, Kamal MA, Gan SH. Dietary phytochemicals: natural swords combating inflammation and oxidation-mediated degenerative diseases. Oxid Med Cell Longev. 2016;2016:5137431. doi: 10.1155/2016/5137431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Liu RH. Potential synergy of phytochemicals in cancer prevention: mechanism of action. J Nutr. 2004;134(12 Suppl):3479S–85S. doi: 10.1093/jn/134.12.3479S. [DOI] [PubMed] [Google Scholar]
- 37.Puri A, Saxena R, Saxena RP, Saxena KC, Srivastava V, Tandon JS. Immunostimulant activity of Nyctanthes arbor-tristis L. J Ethnopharmacol. 1994;42(1):31–7. doi: 10.1016/0378-8741(94)90020-5. [DOI] [PubMed] [Google Scholar]
- 38.Sultan MT, Butt MS, Qayyum MM, Suleria HA. Immunity: plants as effective mediators. Crit Rev Food Sci Nutr. 2014;54(10):1298–308. doi: 10.1080/10408398.2011.633249. [DOI] [PubMed] [Google Scholar]
- 39. Nair A, Chattopadhyay D, Saha B. Plant-derived immunomodulators. In: Ahmad Khan MS, Ahmad I, Chattopadhyay D, eds. New Look to Phytomedicine. Academic Press; 2019. p. 435-99. 10.1016/b978-0-12-814619-4.00018-5. [DOI]
- 40.Croft KD. Dietary polyphenols: antioxidants or not? Arch BiochemBiophys. 2016;595:120–4. doi: 10.1016/j.abb.2015.11.014. [DOI] [PubMed] [Google Scholar]
- 41.Rodriguez-Mateos A, Vauzour D, Krueger CG, Shanmuganayagam D, Reed J, Calani L, et al. Bioavailability, bioactivity and impact on health of dietary flavonoids and related compounds: an update. Arch Toxicol. 2014;88(10):1803–53. doi: 10.1007/s00204-014-1330-7. [DOI] [PubMed] [Google Scholar]
- 42.Spencer JP, Abd El Mohsen MM, Minihane AM, Mathers JC. Biomarkers of the intake of dietary polyphenols: strengths, limitations and application in nutrition research. Br J Nutr. 2008;99(1):12–22. doi: 10.1017/s0007114507798938. [DOI] [PubMed] [Google Scholar]
- 43.Mir IA, Tiku AB. Chemopreventive and therapeutic potential of “naringenin,” a flavanone present in citrus fruits. Nutr Cancer. 2015;67(1):27–42. doi: 10.1080/01635581.2015.976320. [DOI] [PubMed] [Google Scholar]
- 44.Giovinazzo G, Gerardi C, Uberti-Foppa C, Lopalco L. Can natural polyphenols help in reducing cytokine storm in COVID-19 patients? Molecules. 2020;25(24):5888. doi: 10.3390/molecules25245888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Andriantsitohaina R, Auger C, Chataigneau T, Étienne-Selloum N, Li H, Martínez MC, et al. Molecular mechanisms of the cardiovascular protective effects of polyphenols. Br J Nutr. 2012;108(9):1532–49. doi: 10.1017/s0007114512003406. [DOI] [PubMed] [Google Scholar]
- 46.Azab A, Nassar A, Azab AN. Anti-inflammatory activity of natural products. Molecules. 2016;21(10):1321. doi: 10.3390/molecules21101321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yahfoufi N, Alsadi N, Jambi M, Matar C. The immunomodulatory and anti-inflammatory role of polyphenols. Nutrients. 2018;10(11):1618. doi: 10.3390/nu10111618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Liskova A, Samec M, Koklesova L, Samuel SM, Zhai K, Al-Ishaq RK, et al. Flavonoids against the SARS-CoV-2 induced inflammatory storm. Biomed Pharmacother. 2021;138:111430. doi: 10.1016/j.biopha.2021.111430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kopustinskiene DM, Jakstas V, Savickas A, Bernatoniene J. Flavonoids as anticancer agents. Nutrients. 2020;12(2):457. doi: 10.3390/nu12020457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Khan MK, Zill EH, Dangles O. A comprehensive review on flavanones, the major citrus polyphenols. J Food Compost Anal. 2014;33(1):85–104. doi: 10.1016/j.jfca.2013.11.004. [DOI] [Google Scholar]
- 51.Comalada M, Ballester I, Bailón E, Sierra S, Xaus J, Gálvez J, et al. Inhibition of pro-inflammatory markers in primary bone marrow-derived mouse macrophages by naturally occurring flavonoids: analysis of the structure-activity relationship. BiochemPharmacol. 2006;72(8):1010–21. doi: 10.1016/j.bcp.2006.07.016. [DOI] [PubMed] [Google Scholar]
- 52.Fouad AA, Albuali WH, Jresat I. Protective effect of naringenin against lipopolysaccharide-induced acute lung injury in rats. Pharmacology. 2016;97(5-6):224–32. doi: 10.1159/000444262. [DOI] [PubMed] [Google Scholar]
- 53.Zhang X, Wang G, Gurley EC, Zhou H. Flavonoid apigenin inhibits lipopolysaccharide-induced inflammatory response through multiple mechanisms in macrophages. PLoS One. 2014;9(9):e107072. doi: 10.1371/journal.pone.0107072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Cordell GA, Quinn-Beattie ML, Farnsworth NR. The potential of alkaloids in drug discovery. Phytother Res. 2001;15(3):183–205. doi: 10.1002/ptr.890. [DOI] [PubMed] [Google Scholar]
- 55.Dall’Acqua S. Plant-derived acetylcholinesterase inhibitory alkaloids for the treatment of Alzheimer’s disease. Botanics. 2013;3:19–28. doi: 10.2147/btat.s17297. [DOI] [Google Scholar]
- 56.Hussain G, Rasul A, Anwar H, Aziz N, Razzaq A, Wei W, et al. Role of plant derived alkaloids and their mechanism in neurodegenerative disorders. Int J Biol Sci. 2018;14(3):341–57. doi: 10.7150/ijbs.23247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Khan H, Alam W, Alsharif KF, Aschner M, Pervez S, Saso L. Alkaloids and colon cancer: molecular mechanisms and therapeutic implications for cell cycle arrest. Molecules. 2022;27(3):920. doi: 10.3390/molecules27030920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Martino E, Casamassima G, Castiglione S, Cellupica E, Pantalone S, Papagni F, et al. Vinca alkaloids and analogues as anti-cancer agents: looking back, peering ahead. Bioorg Med Chem Lett. 2018;28(17):2816–26. doi: 10.1016/j.bmcl.2018.06.044. [DOI] [PubMed] [Google Scholar]
- 59.Cronstein BN, Sunkureddi P. Mechanistic aspects of inflammation and clinical management of inflammation in acute gouty arthritis. J Clin Rheumatol. 2013;19(1):19–29. doi: 10.1097/RHU.0b013e31827d8790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Slobodnick A, Shah B, Krasnokutsky S, Pillinger MH. Update on colchicine, 2017. Rheumatology (Oxford) 2018;57(suppl_1):i4–i11. doi: 10.1093/rheumatology/kex453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Fordham JN, Kirwan J, Cason J, Currey HL. Prolonged reduction in polymorphonuclear adhesion following oral colchicine. Ann Rheum Dis. 1981;40(6):605–8. doi: 10.1136/ard.40.6.605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Reyes AZ, Hu KA, Teperman J, Wampler Muskardin TL, Tardif JC, Shah B, et al. Anti-inflammatory therapy for COVID-19 infection: the case for colchicine. Ann Rheum Dis. 2021;80(5):550–7. doi: 10.1136/annrheumdis-2020-219174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Zielińska S, Czerwińska ME, Dziągwa-Becker M, Dryś A, Kucharski M, Jezierska-Domaradzka A, et al. Modulatory effect of Chelidonium majus extract and its alkaloids on LPS-stimulated cytokine secretion in human neutrophils. Molecules. 2020;25(4):842. doi: 10.3390/molecules25040842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Salvo F, Fourrier-Réglat A, Bazin F, Robinson P, Riera-Guardia N, Haag M, et al. Cardiovascular and gastrointestinal safety of NSAIDs: a systematic review of meta-analyses of randomized clinical trials. Clin PharmacolTher. 2011;89(6):855–66. doi: 10.1038/clpt.2011.45. [DOI] [PubMed] [Google Scholar]
- 65. Bartnik M, Facey PC. Glycosides. In: Badal S, Delgoda R, eds. Pharmacognosy. Boston: Academic Press; 2017. p. 101-61. 10.1016/b978-0-12-802104-0.00008-1. [DOI]
- 66. Brito-Arias M. N-glycosides. Springer; 2007.
- 67.Prassas I, Diamandis EP. Novel therapeutic applications of cardiac glycosides. Nat Rev Drug Discov. 2008;7(11):926–35. doi: 10.1038/nrd2682. [DOI] [PubMed] [Google Scholar]
- 68. Soto-Blanco B. Herbal glycosides in healthcare. In: Mandal SC, Nayak AK, Dhara AK, eds. Herbal Biomolecules in Healthcare Applications. Academic Press; 2022. p. 239-82. 10.1016/b978-0-323-85852-6.00021-4. [DOI]
- 69.Nenaah G. Antimicrobial activity of Calotropis procera Ait (Asclepiadaceae) and isolation of four flavonoid glycosides as the active constituents. World J Microbiol Biotechnol. 2013;29(7):1255–62. doi: 10.1007/s11274-013-1288-2. [DOI] [PubMed] [Google Scholar]
- 70.Lee W, Yang S, Lee C, Park EK, Kim KM, Ku SK, et al. Aloin reduces inflammatory gene iNOS via inhibition activity and p-STAT-1 and NF-κB. Food Chem Toxicol. 2019;126:67–71. doi: 10.1016/j.fct.2019.02.025. [DOI] [PubMed] [Google Scholar]
- 71.Dong X, Fu J, Yin X, Cao S, Li X, Lin L, et al. Emodin: a review of its pharmacology, toxicity and pharmacokinetics. Phytother Res. 2016;30(8):1207–18. doi: 10.1002/ptr.5631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Boonkaewwan C, Toskulkao C, Vongsakul M. Anti-inflammatory and immunomodulatory activities of stevioside and its metabolite steviol on THP-1 cells. J Agric Food Chem. 2006;54(3):785–9. doi: 10.1021/jf0523465. [DOI] [PubMed] [Google Scholar]
- 73.Chatsudthipong V, Muanprasat C. Stevioside and related compounds: therapeutic benefits beyond sweetness. Pharmacol Ther. 2009;121(1):41–54. doi: 10.1016/j.pharmthera.2008.09.007. [DOI] [PubMed] [Google Scholar]
- 74.Casas-Grajales S, Ramos-Tovar E, Chávez-Estrada E, Alvarez-Suarez D, Hernández-Aquino E, Reyes-Gordillo K, et al. Antioxidant and immunomodulatory activity induced by stevioside in liver damage: in vivo, in vitro and in silico assays. Life Sci. 2019;224:187–96. doi: 10.1016/j.lfs.2019.03.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Boonkaewwan C, Burodom A. Anti-inflammatory and immunomodulatory activities of stevioside and steviol on colonic epithelial cells. J Sci Food Agric. 2013;93(15):3820–5. doi: 10.1002/jsfa.6287. [DOI] [PubMed] [Google Scholar]
- 76.Yang J, Yuan L, Wen Y, Zhou H, Jiang W, Xu D, et al. Protective effects of naringin in cerebral infarction and its molecular mechanism. Med Sci Monit. 2020;26:e918772. doi: 10.12659/msm.918772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Bakkali F, Averbeck S, Averbeck D, Idaomar M. Biological effects of essential oils--a review. Food Chem Toxicol. 2008;46(2):446–75. doi: 10.1016/j.fct.2007.09.106. [DOI] [PubMed] [Google Scholar]
- 78. Perveen S, Al-Taweel A. Terpenes and Terpenoids. IntechOpen; 2018. 10.5772/intechopen.71175. [DOI]
- 79.Gershenzon J, Dudareva N. The function of terpene natural products in the natural world. Nat Chem Biol. 2007;3(7):408–14. doi: 10.1038/nchembio.2007.5. [DOI] [PubMed] [Google Scholar]
- 80.Dillard CJ, German JB. Phytochemicals: nutraceuticals and human health. J Sci Food Agric. 2000;80(12):1744–56. doi: 10.1002/1097-0010(20000915)80:12<1744::aidjsfa725>3.0.co;2-w. [DOI] [Google Scholar]
- 81.Spelman K, Burns J, Nichols D, Winters N, Ottersberg S, Tenborg M. Modulation of cytokine expression by traditional medicines: a review of herbal immunomodulators. Altern Med Rev. 2006;11(2):128–50. [PubMed] [Google Scholar]
- 82.da Silva Lima M, Quintans-Júnior LJ, de Santana WA, Martins Kaneto C, Pereira Soares MB, Villarreal CF. Anti-inflammatory effects of carvacrol: evidence for a key role of interleukin-10. Eur J Pharmacol. 2013;699(1-3):112–7. doi: 10.1016/j.ejphar.2012.11.040. [DOI] [PubMed] [Google Scholar]
- 83.Chen F, Huang G. Preparation and immunological activity of polysaccharides and their derivatives. Int J Biol Macromol. 2018;112:211–6. doi: 10.1016/j.ijbiomac.2018.01.169. [DOI] [PubMed] [Google Scholar]
- 84.Shao BM, Xu W, Dai H, Tu P, Li Z, Gao XM. A study on the immune receptors for polysaccharides from the roots of Astragalus membranaceus, a Chinese medicinal herb. BiochemBiophys Res Commun. 2004;320(4):1103–11. doi: 10.1016/j.bbrc.2004.06.065. [DOI] [PubMed] [Google Scholar]
- 85.Li J, Shan L, Liu Y, Fan L, Ai L. Screening of a functional polysaccharide from Zizyphus jujuba cv Jinsixiaozao and its property. Int J Biol Macromol. 2011;49(3):255–9. doi: 10.1016/j.ijbiomac.2011.04.006. [DOI] [PubMed] [Google Scholar]
- 86.Yu Y, Shen M, Song Q, Xie J. Biological activities and pharmaceutical applications of polysaccharide from natural resources: a review. CarbohydrPolym. 2018;183:91–101. doi: 10.1016/j.carbpol.2017.12.009. [DOI] [PubMed] [Google Scholar]
- 87.Schepetkin IA, Quinn MT. Botanical polysaccharides: macrophage immunomodulation and therapeutic potential. Int Immunopharmacol. 2006;6(3):317–33. doi: 10.1016/j.intimp.2005.10.005. [DOI] [PubMed] [Google Scholar]
- 88.Gan L, Hua Zhang S, Liang Yang X, Bi Xu H. Immunomodulation and antitumor activity by a polysaccharide-protein complex from Lyciumbarbarum. Int Immunopharmacol. 2004;4(4):563–9. doi: 10.1016/j.intimp.2004.01.023. [DOI] [PubMed] [Google Scholar]
- 89.Ruhee RT, Roberts LA, Ma S, Suzuki K. Organosulfur compounds: a review of their anti-inflammatory effects in human health. Front Nutr. 2020;7:64. doi: 10.3389/fnut.2020.00064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Cerella C, Dicato M, Jacob C, Diederich M. Chemical properties and mechanisms determining the anti-cancer action of garlic-derived organic sulfur compounds. Anticancer Agents Med Chem. 2011;11(3):267–71. doi: 10.2174/187152011795347522. [DOI] [PubMed] [Google Scholar]
- 91.Ryu JH, Kang D. Physicochemical properties, biological activity, health benefits, and general limitations of aged black garlic: a review. Molecules. 2017;22(6):919. doi: 10.3390/molecules22060919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Osipova V, Polovinkina M, Gracheva Y, Shpakovsky D, Osipova A, Berberova N. Antioxidant activity of some organosulfur compounds in vitro. Arab J Chem. 2021;14(4):103068. doi: 10.1016/j.arabjc.2021.103068. [DOI] [Google Scholar]
- 93.Arreola R, Quintero-Fabián S, López-Roa RI, Flores-Gutiérrez EO, Reyes-Grajeda JP, Carrera-Quintanar L, et al. Immunomodulation and anti-inflammatory effects of garlic compounds. J Immunol Res. 2015;2015:401630. doi: 10.1155/2015/401630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Hsieh CC, Liu KF, Liu PC, Ho YT, Li WS, Peng WH, et al. Comparing the protection imparted by different fraction extracts of garlic (Allium sativum L) against Der p-induced allergic airway inflammation in mice. Int J Mol Sci. 2019;20(19):4879. doi: 10.3390/ijms20194879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Lang A, Lahav M, Sakhnini E, Barshack I, Fidder HH, Avidan B, et al. Allicin inhibits spontaneous and TNF-alpha induced secretion of proinflammatory cytokines and chemokines from intestinal epithelial cells. Clin Nutr. 2004;23(5):1199–208. doi: 10.1016/j.clnu.2004.03.011. [DOI] [PubMed] [Google Scholar]
- 96.Huang L, Zhang X, Ma X, Zhang D, Li D, Feng J, et al. Berberine alleviates endothelial glycocalyx degradation and promotes glycocalyx restoration in LPS-induced ARDS. Int Immunopharmacol. 2018;65:96–107. doi: 10.1016/j.intimp.2018.10.001. [DOI] [PubMed] [Google Scholar]
- 97.Lin WC, Lin JY. Berberine down-regulates the Th1/Th2 cytokine gene expression ratio in mouse primary splenocytes in the absence or presence of lipopolysaccharide in a preventive manner. Int Immunopharmacol. 2011;11(12):1984–90. doi: 10.1016/j.intimp.2011.08.008. [DOI] [PubMed] [Google Scholar]
- 98.Wu Y, Nie Y, Huang J, Qiu Y, Wan B, Liu G, et al. Protostemonine alleviates heat-killed methicillin-resistant Staphylococcus aureus-induced acute lung injury through MAPK and NF-κB signaling pathways. Int Immunopharmacol. 2019;77:105964. doi: 10.1016/j.intimp.2019.105964. [DOI] [PubMed] [Google Scholar]
- 99.Yu X, Yu S, Chen L, Liu H, Zhang J, Ge H, et al. Tetrahydroberberrubine attenuates lipopolysaccharide-induced acute lung injury by down-regulating MAPK, AKT, and NF-κB signaling pathways. Biomed Pharmacother. 2016;82:489–97. doi: 10.1016/j.biopha.2016.05.025. [DOI] [PubMed] [Google Scholar]
- 100.Zhang D, Li X, Hu Y, Jiang H, Wu Y, Ding Y, et al. Tabersonine attenuates lipopolysaccharide-induced acute lung injury via suppressing TRAF6 ubiquitination. BiochemPharmacol. 2018;154:183–92. doi: 10.1016/j.bcp.2018.05.004. [DOI] [PubMed] [Google Scholar]
- 101.Zhou Y, Wang S, Wan T, Huang Y, Pang N, Jiang X, et al. Cyanidin-3-O-β-glucoside inactivates NLRP3 inflammasome and alleviates alcoholic steatohepatitis via SirT1/NF-κB signaling pathway. Free Radic Biol Med. 2020;160:334–41. doi: 10.1016/j.freeradbiomed.2020.08.006. [DOI] [PubMed] [Google Scholar]
- 102.Nicholas C, Batra S, Vargo MA, Voss OH, Gavrilin MA, Wewers MD, et al. Apigenin blocks lipopolysaccharide-induced lethality in vivo and proinflammatory cytokines expression by inactivating NF-kappaB through the suppression of p65 phosphorylation. J Immunol. 2007;179(10):7121–7. doi: 10.4049/jimmunol.179.10.7121. [DOI] [PubMed] [Google Scholar]
- 103.Lee IT, Lin CC, Lee CY, Hsieh PW, Yang CM. Protective effects of (-)-epigallocatechin-3-gallate against TNF-α-induced lung inflammation via ROS-dependent ICAM-1 inhibition. J NutrBiochem. 2013;24(1):124–36. doi: 10.1016/j.jnutbio.2012.03.009. [DOI] [PubMed] [Google Scholar]
- 104.Liu X, Mei Z, Qian J, Zeng Y, Wang M. Puerarin partly counteracts the inflammatory response after cerebral ischemia/reperfusion via activating the cholinergic anti-inflammatory pathway. Neural Regen Res. 2013;8(34):3203–15. doi: 10.3969/j.issn.1673-5374.2013.34.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Fei J, Liang B, Jiang C, Ni H, Wang L. Luteolin inhibits IL-1β-induced inflammation in rat chondrocytes and attenuates osteoarthritis progression in a rat model. Biomed Pharmacother. 2019;109:1586–92. doi: 10.1016/j.biopha.2018.09.161. [DOI] [PubMed] [Google Scholar]
- 106.Kuo MY, Liao MF, Chen FL, Li YC, Yang ML, Lin RH, et al. Luteolin attenuates the pulmonary inflammatory response involves abilities of antioxidation and inhibition of MAPK and NFκB pathways in mice with endotoxin-induced acute lung injury. Food Chem Toxicol. 2011;49(10):2660–6. doi: 10.1016/j.fct.2011.07.012. [DOI] [PubMed] [Google Scholar]
- 107.Song B, Guan S, Lu J, Chen Z, Huang G, Li G, et al. Suppressive effects of fisetin on mice T lymphocytes in vitro and in vivo. J Surg Res. 2013;185(1):399–409. doi: 10.1016/j.jss.2013.05.093. [DOI] [PubMed] [Google Scholar]
- 108.Kong G, Huang X, Wang L, Li Y, Sun T, Han S, et al. Astilbin alleviates LPS-induced ARDS by suppressing MAPK signaling pathway and protecting pulmonary endothelial glycocalyx. Int Immunopharmacol. 2016;36:51–8. doi: 10.1016/j.intimp.2016.03.039. [DOI] [PubMed] [Google Scholar]
- 109.Wang X, Yan J, Xu X, Duan C, Xie Z, Su Z, et al. Puerarin prevents LPS-induced acute lung injury via inhibiting inflammatory response. MicrobPathog. 2018;118:170–6. doi: 10.1016/j.micpath.2018.03.033. [DOI] [PubMed] [Google Scholar]
- 110.Sun LC, Zhang HB, Gu CD, Guo SD, Li G, Lian R, et al. Protective effect of acacetin on sepsis-induced acute lung injury via its anti-inflammatory and antioxidative activity. Arch Pharm Res. 2018;41(12):1199–210. doi: 10.1007/s12272-017-0991-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Al-Rikabi R, Al-Shmgani H, Dewir YH, El-Hendawy S. In vivo and in vitro evaluation of the protective effects of hesperidin in lipopolysaccharide-induced inflammation and cytotoxicity of cell. Molecules. 2020;25(3):478. doi: 10.3390/molecules25030478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Garg A, Garg S, Zaneveld LJ, Singla AK. Chemistry and pharmacology of the Citrus bioflavonoid hesperidin. Phytother Res. 2001;15(8):655–69. doi: 10.1002/ptr.1074. [DOI] [PubMed] [Google Scholar]
- 113.Guazelli CFS, Fattori V, Ferraz CR, Borghi SM, Casagrande R, Baracat MM, et al. Antioxidant and anti-inflammatory effects of hesperidin methyl chalcone in experimental ulcerative colitis. Chem Biol Interact. 2021;333:109315. doi: 10.1016/j.cbi.2020.109315. [DOI] [PubMed] [Google Scholar]
- 114.Ye J, Guan M, Lu Y, Zhang D, Li C, Li Y, et al. Protective effects of hesperetin on lipopolysaccharide-induced acute lung injury by targeting MD2. Eur J Pharmacol. 2019;852:151–8. doi: 10.1016/j.ejphar.2019.02.042. [DOI] [PubMed] [Google Scholar]
- 115.Polyak SJ, Morishima C, Shuhart MC, Wang CC, Liu Y, Lee DY. Inhibition of T-cell inflammatory cytokines, hepatocyte NF-kappaB signaling, and HCV infection by standardized Silymarin. Gastroenterology. 2007;132(5):1925–36. doi: 10.1053/j.gastro.2007.02.038. [DOI] [PubMed] [Google Scholar]
- 116.Yan C, Guan F, Shen Y, Tang H, Yuan D, Gao H, et al. Bigelovii a Protects against lipopolysaccharide-induced acute lung injury by blocking NF-κB and CCAAT/enhancer-binding protein δ pathways. Mediators Inflamm. 2016;2016:9201604. doi: 10.1155/2016/9201604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Wang Y, Zhao GX, Xu LH, Liu KP, Pan H, He J, et al. Cucurbitacin IIb exhibits anti-inflammatory activity through modulating multiple cellular behaviors of mouse lymphocytes. PLoS One. 2014;9(2):e89751. doi: 10.1371/journal.pone.0089751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Ding YH, Song YD, Wu YX, He HQ, Yu TH, Hu YD, et al. Isoalantolactone suppresses LPS-induced inflammation by inhibiting TRAF6 ubiquitination and alleviates acute lung injury. Acta Pharmacol Sin. 2019;40(1):64–74. doi: 10.1038/s41401-018-0061-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Chen YL, Hwang TL, Fang JY, Lan YH, Chong KY, Hsieh PW. Polysaccharides from Kochia scoparia fruits protect mice from lipopolysaccharide-mediated acute lung injury by inhibiting neutrophil elastase. J Funct Foods. 2017;38:582–90. doi: 10.1016/j.jff.2017.09.060. [DOI] [Google Scholar]
- 120.Liang J, Chen S, Chen J, Lin J, Xiong Q, Yang Y, et al. Therapeutic roles of polysaccharides from Dendrobium officinale on colitis and its underlying mechanisms. CarbohydrPolym. 2018;185:159–68. doi: 10.1016/j.carbpol.2018.01.013. [DOI] [PubMed] [Google Scholar]
- 121.Lin Y, Luo T, Weng A, Huang X, Yao Y, Fu Z, et al. Gallic acid alleviates gouty arthritis by inhibiting NLRP3 inflammasome activation and pyroptosis through enhancing Nrf2 signaling. Front Immunol. 2020;11:580593. doi: 10.3389/fimmu.2020.580593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Singla E, Dharwal V, Naura AS. Gallic acid protects against the COPD-linked lung inflammation and emphysema in mice. Inflamm Res. 2020;69(4):423–34. doi: 10.1007/s00011-020-01333-1. [DOI] [PubMed] [Google Scholar]
- 123. Zeng J, Zhang D, Wan X, Bai Y, Yuan C, Wang T, et al. Chlorogenic acid suppresses miR-155 and ameliorates ulcerative colitis through the NF-κB/NLRP3 inflammasome pathway. Mol Nutr Food Res 2020:e2000452. 10.1002/mnfr.202000452. [DOI] [PubMed]
- 124.Li YZ, Chen JH, Tsai CF, Yeh WL. Anti-inflammatory property of imperatorin on alveolar macrophages and inflammatory lung injury. J Nat Prod. 2019;82(4):1002–8. doi: 10.1021/acs.jnatprod.9b00145. [DOI] [PubMed] [Google Scholar]
- 125.Niu X, Xing W, Li W, Fan T, Hu H, Li Y. Isofraxidin exhibited anti-inflammatory effects in vivo and inhibited TNF-α production in LPS-induced mouse peritoneal macrophages in vitro via the MAPK pathway. Int Immunopharmacol. 2012;14(2):164–71. doi: 10.1016/j.intimp.2012.06.022. [DOI] [PubMed] [Google Scholar]
- 126.Han Y, Sun HJ, Tong Y, Chen YZ, Ye C, Qiu Y, et al. Curcumin attenuates migration of vascular smooth muscle cells via inhibiting NFκB-mediated NLRP3 expression in spontaneously hypertensive rats. J NutrBiochem. 2019;72:108212. doi: 10.1016/j.jnutbio.2019.07.003. [DOI] [PubMed] [Google Scholar]
- 127.Jin HZ, Yang XJ, Zhao KL, Mei FC, Zhou Y, You YD, et al. Apocynin alleviates lung injury by suppressing NLRP3 inflammasome activation and NF-κB signaling in acute pancreatitis. Int Immunopharmacol. 2019;75:105821. doi: 10.1016/j.intimp.2019.105821. [DOI] [PubMed] [Google Scholar]
- 128.Ishiguro K, Ando T, Maeda O, Hasegawa M, Kadomatsu K, Ohmiya N, et al. Paeonol attenuates TNBS-induced colitis by inhibiting NF-kappaB and STAT1 transactivation. Toxicol Appl Pharmacol. 2006;217(1):35–42. doi: 10.1016/j.taap.2006.07.002. [DOI] [PubMed] [Google Scholar]
- 129.Kawamoto Y, Ueno Y, Nakahashi E, Obayashi M, Sugihara K, Qiao S, et al. Prevention of allergic rhinitis by ginger and the molecular basis of immunosuppression by 6-gingerol through T cell inactivation. J NutrBiochem. 2016;27:112–22. doi: 10.1016/j.jnutbio.2015.08.025. [DOI] [PubMed] [Google Scholar]
- 130.Nan B, Yang C, Li L, Ye H, Yan H, Wang M, et al. Allicin alleviated acrylamide-induced NLRP3 inflammasome activation via oxidative stress and endoplasmic reticulum stress in Kupffer cells and SD rats liver. Food Chem Toxicol. 2021;148:111937. doi: 10.1016/j.fct.2020.111937. [DOI] [PubMed] [Google Scholar]
- 131.Chen HW, Yen CC, Kuo LL, Lo CW, Huang CS, Chen CC, et al. Benzyl isothiocyanate ameliorates high-fat/cholesterol/cholic acid diet-induced nonalcoholic steatohepatitis through inhibiting cholesterol crystal-activated NLRP3 inflammasome in Kupffer cells. Toxicol Appl Pharmacol. 2020;393:114941. doi: 10.1016/j.taap.2020.114941. [DOI] [PubMed] [Google Scholar]
- 132.Shi L, Lin Q, Li X, Nie Y, Sun S, Deng X, et al. Alliin, a garlic organosulfur compound, ameliorates gut inflammation through MAPK-NF-κB/AP-1/STAT-1 inactivation and PPAR-γ activation. Mol Nutr Food Res. 2017;61(9):1601013. doi: 10.1002/mnfr.201601013. [DOI] [PubMed] [Google Scholar]
- 133.Yang S, Yu Z, Wang L, Yuan T, Wang X, Zhang X, et al. The natural product bergenin ameliorates lipopolysaccharide-induced acute lung injury by inhibiting NF-kappaB activition. J Ethnopharmacol. 2017;200:147–55. doi: 10.1016/j.jep.2017.02.013. [DOI] [PubMed] [Google Scholar]
- 134.Boonkaewwan C, Burodom A. Anti-inflammatory and immunomodulatory activities of stevioside and steviol on colonic epithelial cells. J Sci Food Agric. 2013;93(15):3820–5. doi: 10.1002/jsfa.6287. [DOI] [PubMed] [Google Scholar]
- 135.Zhou J, Xu G, Ma S, Li F, Yuan M, Xu H, et al. Catalpol ameliorates high-fat diet-induced insulin resistance and adipose tissue inflammation by suppressing the JNK and NF-κB pathways. BiochemBiophys Res Commun. 2015;467(4):853–8. doi: 10.1016/j.bbrc.2015.10.054. [DOI] [PubMed] [Google Scholar]
- 136.Pasquereau S, Kumar A, Herbein G. Targeting TNF and TNF receptor pathway in HIV-1 infection: from immune activation to viral reservoirs. Viruses. 2017;9(4):64. doi: 10.3390/v9040064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Lee RE, Walker SR, Savery K, Frank DA, Gaudet S. Fold change of nuclear NF-κB determines TNF-induced transcription in single cells. Mol Cell. 2014;53(6):867–79. doi: 10.1016/j.molcel.2014.01.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Karin M, Lin A. NF-kappaB at the crossroads of life and death. Nat Immunol. 2002;3(3):221–7. doi: 10.1038/ni0302-221. [DOI] [PubMed] [Google Scholar]
- 139.Chatzantoni K, Mouzaki A. Anti-TNF-alpha antibody therapies in autoimmune diseases. Curr Top Med Chem. 2006;6(16):1707–14. doi: 10.2174/156802606778194217. [DOI] [PubMed] [Google Scholar]
- 140.Mahallawi WH, Khabour OF, Zhang Q, Makhdoum HM, Suliman BA. MERS-CoV infection in humans is associated with a pro-inflammatory Th1 and Th17 cytokine profile. Cytokine. 2018;104:8–13. doi: 10.1016/j.cyto.2018.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.DeDiego ML, Nieto-Torres JL, Regla-Nava JA, Jimenez-Guardeño JM, Fernandez-Delgado R, Fett C, et al. Inhibition of NF-κB-mediated inflammation in severe acute respiratory syndrome coronavirus-infected mice increases survival. J Virol. 2014;88(2):913–24. doi: 10.1128/jvi.02576-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Karin M, Yamamoto Y, Wang QM. The IKK NF-kappa B system: a treasure trove for drug development. Nat Rev Drug Discov. 2004;3(1):17–26. doi: 10.1038/nrd1279. [DOI] [PubMed] [Google Scholar]
- 143.Silva LC, Ortigosa LC, Benard G. Anti-TNF-α agents in the treatment of immune-mediated inflammatory diseases: mechanisms of action and pitfalls. Immunotherapy. 2010;2(6):817–33. doi: 10.2217/imt.10.67. [DOI] [PubMed] [Google Scholar]
- 144.Lapadula G, Marchesoni A, Armuzzi A, Blandizzi C, Caporali R, Chimenti S, et al. Adalimumab in the treatment of immune-mediated diseases. Int J ImmunopatholPharmacol. 2014;27(1 Suppl):33–48. doi: 10.1177/03946320140270s103. [DOI] [PubMed] [Google Scholar]
- 145.Dvorak HF, Brown LF, Detmar M, Dvorak AM. Vascular permeability factor/vascular endothelial growth factor, microvascular hyperpermeability, and angiogenesis. Am J Pathol. 1995;146(5):1029–39. [PMC free article] [PubMed] [Google Scholar]
- 146.Kokkotis G, Kitsou K, Xynogalas I, Spoulou V, Magiorkinis G, Trontzas I, et al. Systematic review with meta-analysis: COVID-19 outcomes in patients receiving anti-TNF treatments. Aliment PharmacolTher. 2022;55(2):154–67. doi: 10.1111/apt.16717. [DOI] [PubMed] [Google Scholar]
- 147.Rahman I, Marwick J, Kirkham P. Redox modulation of chromatin remodeling: impact on histone acetylation and deacetylation, NF-kappaB and pro-inflammatory gene expression. BiochemPharmacol. 2004;68(6):1255–67. doi: 10.1016/j.bcp.2004.05.042. [DOI] [PubMed] [Google Scholar]
- 148.Min YD, Choi CH, Bark H, Son HY, Park HH, Lee S, et al. Quercetin inhibits expression of inflammatory cytokines through attenuation of NF-kappaB and p38 MAPK in HMC-1 human mast cell line. Inflamm Res. 2007;56(5):210–5. doi: 10.1007/s00011-007-6172-9. [DOI] [PubMed] [Google Scholar]
- 149.Chen JC, Ho FM, Pei-Dawn Lee C, Chen CP, Jeng KC, Hsu HB, et al. Inhibition of iNOS gene expression by quercetin is mediated by the inhibition of IkappaB kinase, nuclear factor-kappa B and STAT1, and depends on heme oxygenase-1 induction in mouse BV-2 microglia. Eur J Pharmacol. 2005;521(1-3):9–20. doi: 10.1016/j.ejphar.2005.08.005. [DOI] [PubMed] [Google Scholar]
- 150.Manna SK, Mukhopadhyay A, Van NT, Aggarwal BB. Silymarin suppresses TNF-induced activation of NF-kappa B, c-Jun N-terminal kinase, and apoptosis. J Immunol. 1999;163(12):6800–9. [PubMed] [Google Scholar]
- 151.Shishodia S, Majumdar S, Banerjee S, Aggarwal BB. Ursolic acid inhibits nuclear factor-kappaB activation induced by carcinogenic agents through suppression of IkappaBalpha kinase and p65 phosphorylation: correlation with down-regulation of cyclooxygenase 2, matrix metalloproteinase 9, and cyclin D1. Cancer Res. 2003;63(15):4375–83. [PubMed] [Google Scholar]
- 152.Kim SO, Chun KS, Kundu JK, Surh YJ. Inhibitory effects of [6]-gingerol on PMA-induced COX-2 expression and activation of NF-kappaB and p38 MAPK in mouse skin. Biofactors. 2004;21(1-4):27–31. doi: 10.1002/biof.552210107. [DOI] [PubMed] [Google Scholar]
- 153.Takada Y, Aggarwal BB. Flavopiridol inhibits NF-kappaB activation induced by various carcinogens and inflammatory agents through inhibition of IkappaBalpha kinase and p65 phosphorylation: abrogation of cyclin D1, cyclooxygenase-2, and matrix metalloprotease-9. J Biol Chem. 2004;279(6):4750–9. doi: 10.1074/jbc.M304546200. [DOI] [PubMed] [Google Scholar]
- 154.Takada Y, Murakami A, Aggarwal BB. Zerumbone abolishes NF-kappaB and IkappaBalpha kinase activation leading to suppression of antiapoptotic and metastatic gene expression, upregulation of apoptosis, and downregulation of invasion. Oncogene. 2005;24(46):6957–69. doi: 10.1038/sj.onc.1208845. [DOI] [PubMed] [Google Scholar]
- 155.Singh S, Aggarwal BB. Activation of transcription factor NF-κB is suppressed by curcumin (diferuloylmethane)(∗) J Biol Chem. 1995;270(42):24995–5000. doi: 10.1074/jbc.270.42.24995. [DOI] [PubMed] [Google Scholar]
- 156.Yang F, Oz HS, Barve S, de Villiers WJ, McClain CJ, Varilek GW. The green tea polyphenol (-)-epigallocatechin-3-gallate blocks nuclear factor-kappa B activation by inhibiting I kappa B kinase activity in the intestinal epithelial cell line IEC-6. Mol Pharmacol. 2001;60(3):528–33. [PubMed] [Google Scholar]
- 157.Norelli M, Camisa B, Barbiera G, Falcone L, Purevdorj A, Genua M, et al. Monocyte-derived IL-1 and IL-6 are differentially required for cytokine-release syndrome and neurotoxicity due to CAR T cells. Nat Med. 2018;24(6):739–48. doi: 10.1038/s41591-018-0036-4. [DOI] [PubMed] [Google Scholar]
- 158.Pétrilli V, Dostert C, Muruve DA, Tschopp J. The inflammasome: a danger sensing complex triggering innate immunity. CurrOpin Immunol. 2007;19(6):615–22. doi: 10.1016/j.coi.2007.09.002. [DOI] [PubMed] [Google Scholar]
- 159.Zhang W, Zhao Y, Zhang F, Wang Q, Li T, Liu Z, et al. The use of anti-inflammatory drugs in the treatment of people with severe coronavirus disease 2019 (COVID-19): the perspectives of clinical immunologists from China. Clin Immunol. 2020;214:108393. doi: 10.1016/j.clim.2020.108393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.He L, Ding Y, Zhang Q, Che X, He Y, Shen H, et al. Expression of elevated levels of pro-inflammatory cytokines in SARS-CoV-infected ACE2 + cells in SARS patients: relation to the acute lung injury and pathogenesis of SARS. J Pathol. 2006;210(3):288–97. doi: 10.1002/path.2067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Scambler T, Holbrook J, Savic S, McDermott MF, Peckham D. Autoinflammatory disease in the lung. Immunology. 2018;154(4):563–73. doi: 10.1111/imm.12937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Dostert C, Pétrilli V, Van Bruggen R, Steele C, Mossman BT, Tschopp J. Innate immune activation through Nalp3 inflammasome sensing of asbestos and silica. Science. 2008;320(5876):674–7. doi: 10.1126/science.1156995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Cruz CM, Rinna A, Forman HJ, Ventura AL, Persechini PM, Ojcius DM. ATP activates a reactive oxygen species-dependent oxidative stress response and secretion of proinflammatory cytokines in macrophages. J Biol Chem. 2007;282(5):2871–9. doi: 10.1074/jbc.M608083200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Pan P, Shen M, Yu Z, Ge W, Chen K, Tian M, et al. SARS-CoV-2 N protein promotes NLRP3 inflammasome activation to induce hyperinflammation. Nat Commun. 2021;12(1):4664. doi: 10.1038/s41467-021-25015-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Swanson KV, Deng M, Ting JP. The NLRP3 inflammasome: molecular activation and regulation to therapeutics. Nat Rev Immunol. 2019;19(8):477–89. doi: 10.1038/s41577-019-0165-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Eloseily EM, Weiser P, Crayne CB, Haines H, Mannion ML, Stoll ML, et al. Benefit of anakinra in treating pediatric secondary hemophagocytic lymphohistiocytosis. Arthritis Rheumatol. 2020;72(2):326–34. doi: 10.1002/art.41103. [DOI] [PubMed] [Google Scholar]
- 167.Fan Z, Liang Z, Yang H, Pan Y, Zheng Y, Wang X. Tenuigenin protects dopaminergic neurons from inflammation via suppressing NLRP3 inflammasome activation in microglia. J Neuroinflammation. 2017;14(1):256. doi: 10.1186/s12974-017-1036-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Guo W, Liu W, Jin B, Geng J, Li J, Ding H, et al. Asiatic acid ameliorates dextran sulfate sodium-induced murine experimental colitis via suppressing mitochondria-mediated NLRP3 inflammasome activation. Int Immunopharmacol. 2015;24(2):232–8. doi: 10.1016/j.intimp.2014.12.009. [DOI] [PubMed] [Google Scholar]
- 169.Manayi A, Nabavi SM, Khayatkashani M, Habtemariam S, Khayat Kashani HR. Arglabin could target inflammasome-induced ARDS and cytokine storm associated with COVID-19. Mol Biol Rep. 2021;48(12):8221–5. doi: 10.1007/s11033-021-06827-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Yuan R, Zhao W, Wang QQ, He J, Han S, Gao H, et al. Cucurbitacin B inhibits non-small cell lung cancer in vivo and in vitro by triggering TLR4/NLRP3/GSDMD-dependent pyroptosis. Pharmacol Res. 2021;170:105748. doi: 10.1016/j.phrs.2021.105748. [DOI] [PubMed] [Google Scholar]
- 171.Zhu T, Zhang L, Ling S, Duan J, Qian F, Li Y, et al. Scropolioside B inhibits IL-1β and cytokines expression through NF-κB and inflammasome NLRP3 pathways. Mediators Inflamm. 2014;2014:819053. doi: 10.1155/2014/819053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Kishimoto T. Interleukin-6: from basic science to medicine--40 years in immunology. Annu Rev Immunol. 2005;23:1–21. doi: 10.1146/annurev.immunol.23.021704.115806. [DOI] [PubMed] [Google Scholar]
- 173.Hibi M, Murakami M, Saito M, Hirano T, Taga T, Kishimoto T. Molecular cloning and expression of an IL-6 signal transducer, gp130. Cell. 1990;63(6):1149–57. doi: 10.1016/0092-8674(90)90411-7. [DOI] [PubMed] [Google Scholar]
- 174. Blanco-Melo D, Nilsson-Payant BE, Liu WC, Uhl S, Hoagland D, Møller R, et al. Imbalanced host response to SARS-CoV-2 drives development of COVID-19. Cell 2020;181(5):1036-45.e9. 10.1016/j.cell.2020.04.026. [DOI] [PMC free article] [PubMed]
- 175.Tanaka T, Narazaki M, Kishimoto T. Immunotherapeutic implications of IL-6 blockade for cytokine storm. Immunotherapy. 2016;8(8):959–70. doi: 10.2217/imt-2016-0020. [DOI] [PubMed] [Google Scholar]
- 176.Kang S, Tanaka T, Narazaki M, Kishimoto T. Targeting interleukin-6 signaling in clinic. Immunity. 2019;50(4):1007–23. doi: 10.1016/j.immuni.2019.03.026. [DOI] [PubMed] [Google Scholar]
- 177.Pandurangan AK, Mohebali N, Esa NM, Looi CY, Ismail S, Saadatdoust Z. Gallic acid suppresses inflammation in dextran sodium sulfate-induced colitis in mice: possible mechanisms. Int Immunopharmacol. 2015;28(2):1034–43. doi: 10.1016/j.intimp.2015.08.019. [DOI] [PubMed] [Google Scholar]
- 178.Wung BS, Hsu MC, Wu CC, Hsieh CW. Resveratrol suppresses IL-6-induced ICAM-1 gene expression in endothelial cells: effects on the inhibition of STAT3 phosphorylation. Life Sci. 2005;78(4):389–97. doi: 10.1016/j.lfs.2005.04.052. [DOI] [PubMed] [Google Scholar]
- 179.Barber GN. The interferons and cell death: guardians of the cell or accomplices of apoptosis? Semin Cancer Biol. 2000;10(2):103–11. doi: 10.1006/scbi.2000.0313. [DOI] [PubMed] [Google Scholar]
- 180.Luo W, Li YX, Jiang LJ, Chen Q, Wang T, Ye DW. Targeting JAK-STAT signaling to control cytokine release syndrome in COVID-19. Trends Pharmacol Sci. 2020;41(8):531–43. doi: 10.1016/j.tips.2020.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Schindler C, Plumlee C. Inteferons pen the JAK-STAT pathway. Semin Cell Dev Biol. 2008;19(4):311–8. doi: 10.1016/j.semcdb.2008.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Tisoncik JR, Korth MJ, Simmons CP, Farrar J, Martin TR, Katze MG. Into the eye of the cytokine storm. Microbiol Mol Biol Rev. 2012;76(1):16–32. doi: 10.1128/mmbr.05015-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Bhattacharyya M, Ghosh MK. Hemophagoctic lymphohistiocytosis--recent concept. J Assoc Physicians India. 2008;56:453–7. [PubMed] [Google Scholar]
- 184.Ritchie AI, Singanayagam A. Immunosuppression for hyperinflammation in COVID-19: a double-edged sword? aLancet. 2020;395(10230):1111. doi: 10.1016/s0140-6736(20)30691-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Yang T, Ma X, Wang R, Liu H, Wei S, Jing M, et al. Berberine inhibits IFN-γ signaling pathway in DSS-induced ulcerative colitis. Saudi Pharm J. 2022;30(6):764–78. doi: 10.1016/j.jsps.2022.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]