Abstract
Open-surfaced water sources have been used to irrigate vegetable farms in cities. Open-surface water often contains unmonitored concentrations of health-threatening contaminants that pose health risks, especially when used to produce vegetables for human consumption. However, information on levels of heavy metals and faecal coliform bacteria in such vegetables in selected sites, especially in Greater Accra Metropolitan Area (GAMA) of Ghana is rare. This study examines the levels of heavy metals and faecal coliform in two vegetables-lettuce and bell pepper - that were cultivated using open-surface wastewater from drains and constructed reservoirs at different locations of the GAMA. Using concurrent mixed methods, questionnaires were administered to 67 vegetable farmers, followed by the collection of vegetable samples from three urban farm sites, Haatso and Dzorwulu and Weija irrigation scheme site (WISS) for laboratory analysis. The concentrations of Lead (Pb), Mercury (Hg) and Cadmium (Cd) were determined using atomic absorption spectroscopy after microwave digestion of the vegetables while total faecal coliform was quantified using MacConkey-Endo broth method. The results from all three sites showed that the concentrations of Cd (=0.001 μg/mg) and Pb (=0.005 μg/mg) in lettuce were within the World Health Organization's (WHO) permissible levels. However, the Hg (≥0.309 μg/mg) and faecal coliform (>5 count/100 ml) in the vegetables from all three sites exceeded the WHO permissible limits. Therefore, consumers of vegetables from such urban farms are exposed to health risks associated with Hg and faecal coliforms. There is the need to intensify education on the health risks of consuming vegetables produced from open-surface water sources from the observed sites. The enforcement of existing phytosanitary standards to enhance food safety and the quality of urban vegetables is also recommended.
Keywords: Irrigated farmlands, Lettuce and Bell pepper, Polluted water, GAMA, Urban farming, Faecal coliform
1. Introduction
The world is experiencing rapid urbanization, with a growing dependence on urban agriculture to meet increasing human demand for healthy vegetables to augment dietary needs [[1], [2], [3]]. Urban and peri-urban agriculture encompasses production, processing, distribution and sale of food particularly vegetables within cities and city peripheries [4,5]. In this context, "urban vegetables" are plants like lettuce (Lactuca sativa) and bell pepper (Capsicum annuum) grown for their edible parts in different parts of an urban area.
In Ghana, Mordor Intelligence report [6] suggests that the vegetable market share was around USD 0.95 billion in 2024 and is projected to reach USD 1.2 billion in 2029. Apart from export of vegetables from Ghana to other countries, estimates point out that, total vegetable produced in the country was respectively 786,457, 787,160, 788,010 and 788,396 metric tons in 2017, 2018, 2019 and 2020 [7].
Unfortunately, rapid rate of urbanization (56.7%) in Ghana and unpredictable rainfall patterns resulting in scarcity of freshwater, have forced many small-scale farmers to use polluted or contaminated wastewater sources for urban vegetable farming [8,9]. This practice does not only raise concerns about quality of the agricultural produce but also gives rise to potential food safety and health risks for urban families [10,11] who rely on these vegetables in the Greater Accra Metropolitan Area (GAMA) of Ghana.
Wastewater, originating from urban households, industrial, and commercial activities [12,13], often contains unwholesome physical, chemical, and microbial substances, making it unsuitable for irrigating crops intended for human consumption [14,15]. The contamination of wastewater sources by toxic faecal coliform bacteria (E. coli which indicates faecal contamination) and heavy metals (HMs) like lead (Pb), nickel (Ni), cadmium (Cd), chromium (Cr), and mercury (Hg) render many urban water systems unwholesome. Escherichia coli (E. coli) bacteria and heavy metals compromise water systems including dams, reservoirs, lakes rivers and streams making them unsuitable for drinking, irrigation and sustaining aquatic life [[16], [17], [18], [19]]. However, in many urban areas like GAMA where vegetable cultivation is a notable farming practice, these polluted water systems continue to serve as the primary source of irrigation water for vegetable farming [19,20].
Although, recycling or treating wastewater for urban agriculture may offer a viable solution [17], persistent lack of institutional oversight in monitoring urban agriculture phytosanitary conditions exacerbates concerns about the use of wastewater and contaminated streams. As a result, several studies have already established strong links between irrigation water quality and the resulting agricultural produce [10,11]. Some of these studies indicate that urban families are exposed to contaminated farm produce due to the use of polluted surface water sources for irrigation [10]. Notably, the recent study by Onalenna and Rahube [21] identified presence of blaTEM gene in soil and vegetables irrigated with wastewater, raising legitimate concerns for urban vegetable production for consumption both locally and for export.
Ghana's efforts to diversify its export base by promoting non-traditional crops like tomatoes, chili peppers, eggplants and bottle gourds, among others like sweet peppers, onions/shallots, okra, carrots, cauliflower, cabbage, lettuce and cucumber, faced challenges [22,6]. According to GIRSAL [22], the European Union (EU) imposed a ban on vegetable imports from Ghana in 2015 due to the discovery of banned substances in some of the vegetables. As a result, the export of fresh chili to the UK, which was valued at about US$ 5.473 million in 2014 (Fig. 1), reduced to US$ 1.312 in 2015 and continued to decline.
Fig. 1.
Chili export from Ghana to the UK from 2012 to 2021: Source [22].
The ban had a significant impact on the livelihoods of farmers across both rural and urban areas, many of whom relied on income from vegetable production for their daily sustenance. In response to a sustainable urban vegetable production drive to boost the international competitiveness of Ghana, the GhanaVeg program was established. The program aims to enhance vegetable production throughout the country. Hence, it is worth investigating the current quality of urban vegetable produce to ascertain the programme's impact on GAMA.
Although, several studies have investigated the presence of heavy metals [[23], [24], [25], [26], [27]] and bacteriological substances [28,29] in urban vegetables, little knowledge exists in terms of spatial differences in the locations of urban vegetable production sites and water sources in GAMA. In addition, most studies focused on well-known repositories of heavy metal-contaminated wastewater sources like Korle Lagoon. For instance, Osae et al. [23] evaluated the concentration of heavy metals in vegetables in the catchment area of the Korle Lagoon and found that the heavy metal concentration in lettuce was far above the recommended World Health Organization's (WHO) guideline level. However, studies comparing the level of heavy metals and faecal coliform contamination of vegetables irrigated using presumably wastewater and water from official irrigation schemes are limited.
The current study takes a different approach by employing a unique combination of experimental and social research incorporating interviews to gain an in-depth understanding from the farmers' perspectives. To the best of our knowledge, no previous study has employed this hybrid exploratory concurrent mixed methods research design of combining laboratory experiments on ‘potentially contaminated vegetables’ and farmer surveys to understand farmers' choices of water sources used in vegetable farming in GAMA. Our study, therefore seeks to fill the above research gaps by analysing the levels of heavy metals and faecal contamination in two urban vegetables from three different locations in GAMA. Specifically, our study objective aims to examine the spatial differences in heavy metal and faecal contaminants in the lettuce and bell pepper cultivated by farmers in Dzorwulu, Haatso and Weija irrigation scheme site (WISS), with the first two sites using wastewater from drains, while the latter uses water reservoir under official irrigation scheme. The study also examines the socioeconomic attributes of the farmers and their motivation for the choice of irrigation water source used in producing the vegetables. This research thus contributes to the broader field of sustainable urban agriculture by addressing differences in locations of farms under diverse irrigation schemes, offering insights into the complex spatial ecology of urban vegetable farming, water quality and food safety.
2. Materials and methods
2.1. Study area
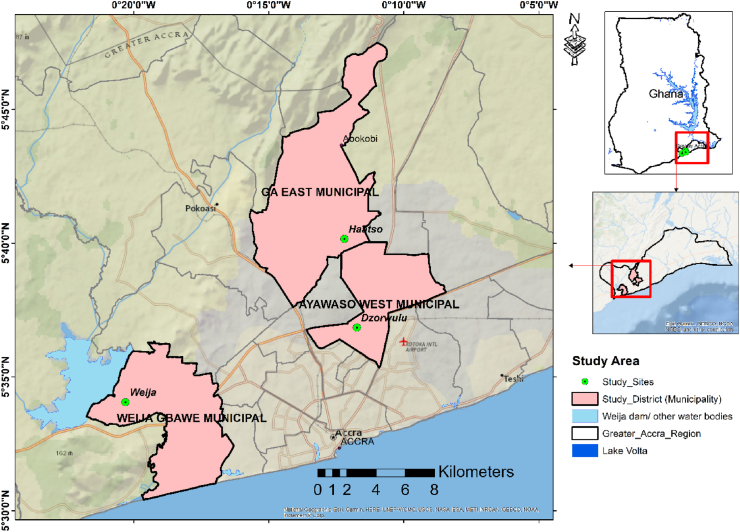
The study was conducted on farm sites in three different Municipalities in the Greater Accra Metropolitan Area (GAMA) as shown in Fig. 2. The locations of the farm sites were Haatso in Ga East Municipality (GEM), Dzorwulu in the Ayawaso West Municipality (AWM), and Weija (WISS) in Weija-Gbawe Municipality (WGM). Dzorwulu and Haatso are 17 km and 19 km respectively away from Weija which is in the eastern part of GAMA (see Fig. 2). The Weija irrigation scheme site (WISS) is about 21 km from Accra central. The farmers take their irrigation water from the Weija reservoir which is one of the two reservoirs supplying drinking water to many homes in the GAMA. The WISS is a formal irrigation scheme recently renovated by the Ghana Irrigation Development Authority (GIDA) with support from the Food and Agricultural Organization (FAO) and the Ministry of Food and Agriculture (MoFA) of Ghana. The Weija reservoir takes its source from Densu which is a major river that flows towards the south-eastern portion of the country (please refer to Fig. 2 for the locations of the vegetable production sites).
Fig. 2.
Map of the study area and sites.
The Densu River traverses three regions in Ghana. It takes its source from the Atiwa Mountains in the Eastern Region and passes through the Central Region to GAMA. In the GAMA, the river passes through the Ga South Municipality and enters the sea at Bortianor. Increasing anthropogenic activities along the path of Densu River affect the water quality in the reservoir which has the potential to also affect the quality of water for domestic and agricultural purposes [30,31]. These activities include mining (both legal and illegal), industrialisation, urbanization and commercial activities. Due to these activities, the river was labelled among the highly polluted rivers in Ghana [32]. Most farms in the Haatso and Dzorwulu areas get their water supply sources from drains or streams such as the Onyasia Stream [27], which is one of the tributaries of the Odaw River in GAMA [28]. Other sources include other natural and human-induced drains, raw wastewater, storms, and grey water. These water sources often contain high faecal contamination with dilution changes between locations and seasons. These polluted streams are sometimes supplemented by farmers with pipe-borne water [33,34]. A few farmers also use partially treated effluents from wastewater treatment system ponds that can be found around the Burma Camp military facility by puncturing the sewage pipes [27]. The three farm sites host the cultivation of major vegetables such as okra, pepper, carrot, cabbage, and lettuce farms for local consumption in the GAMA and Kasoa in the Central Region. Vegetable farming is the major economic activity for about 55% of these municipalities' economically active farming population [28].
2.2. Survey of vegetable farmers
The study generally adopted the concurrent mixed methods approach, where experimental laboratory research and a semi-structured survey were designed and implemented together. The survey was a stratified convenient farm habitat assessment [35,36] involving sampling of vegetable (lettuce and bell pepper) farmers across the three sites. The initial intention of the study was to use quota sampling to sample 90 vegetable farmers, 30 from each site. However, a total of 67 vegetable farmers were conveniently sampled, 22, 21, and 24 from Haatso, Dzorwulu, and WISS respectively. The shortfall was because some of the identified vegetable farmers were unwilling to participate in the survey.
A semi-structured survey questionnaire was used. The questionnaire aimed at soliciting farmers’ socio-economic attributes (age, education, crops, and inputs) and their opinions on sources of water for farming. The study was interested in the perceived impact of the water and chemicals being used on the vegetables and experiences of vegetable farming, the sustainability and health risks of their activities. The questionnaire was pre-tested to ensure its reliability. The pre-testing was conducted using 15 non-selected farmers, 5 each from the three study sites. After a successful pre-test of the questionnaire, a one-on-one individual interview of the selected farmers was conducted, following ethical standards.
Ethical compliance was observed by obtaining informed consent from all respondents (orally or written). This assures anonymity of their identity and confidentiality of their information. Confidentiality was assured by using codes for each respondent instead of using their names. Also, farmers who were not comfortable with the survey were exempted. The identity of those who willingly participated were protected throughout the study.
2.3. Vegetable sample collection, preparation and analyses
The experimental laboratory analysis was conducted to measure the levels of bacteria pathogens and heavy metals in samples of ready-to-sell fresh lettuce (lactuca sativa) and bell pepper (capsicum annuum) collected from farms in all the three sites. The vegetable samples from Haatso and Dzorwulu served as the primary experimental samples because the study's major objective was to assess contaminants in lettuce and pepper caused by wastewater usage. The vegetable samples from the WISS were used as a control sample because the farms were irrigated using fresh water from the Weija reservoir, which is not wastewater. The WISS was usually monitored by the Ghana Irrigation Development Authority (GIDA) to ensure that the water used for irrigating the farms within the irrigation scheme is good. The samples were collected into transparent plastic bags with zip-lock closures that were labelled with the site's information. The samples were transferred to the University's laboratory at a temperature of 4 °C in an ice chest for analysis. To prevent microbial contamination, the laboratory apparatus used for the microbiological analysis were all sterilized using accepted practices. The Petri dishes and test tubes were washed with soap, followed by a water rinse, and then allowed to air dry. To prevent fungus and other cross-contaminations, the inoculation room was sprayed with 3% thymol. Before and following use, the workbenches were cleaned with ethanol at a concentration of about 80%. To guarantee there were no residues of thymol before the room was used, the room was made to dry for about an hour. All inoculum transfers were carried out in the inoculation chamber to prevent medium contamination. Before and after the transfer of samples, inoculation loops or bent wires were sterilized until they were red hot under a naked flame. Additionally, the media- and sample-containing test tubes' opening ends were flamed before and after transfers. Before measuring 100 ml of De-Ionized (DI) water into a beaker, the pH meter was calibrated. The pH of the mixed puree was measured using the pH meter after 100 g of the vegetables and 100 ml of the DI water were added to a clean blender and blended for 3 min.
2.4. Media preparation and faecal coliform tests
We used specific media for coliform bacteria detection in the lettuce and bell pepper purees. The medium [37,38] for total coliform detection and enumeration was MacConkey(M)-Endo broth [39] and that for Faecal Coliform (FC) was Modified(M)-FC broth base [40]. For Escherichia coli (E. coli), tryptone water was used.
M-Endo broth [39]: 4.8 g of dehydrated endo medium was suspended in 100 ml of distilled water containing 2 ml of 95% ethanol. The solution was covered with aluminium foil and heated on a hot plate while stirring with a magnetic stirrer. The solution was removed from the heat source immediately after boiling began. It was allowed to cool at room temperature. It was dispensed into Petri dishes.
M − FC broth [40]: 3.7 g of dehydrated FC medium was suspended in 100 ml of distilled water. About 1 ml of a 1% solution of rosolic acid in 0.2 N NaOH was added, and the solution was covered with aluminium foil. It was heated whilst stirring. The solution was taken off the hotplate immediately after boiling began. Cooling was done at room temperature and the broth was dispensed into Petri dishes. Rosolic Acid: 0.08 g of NaOH was dissolved in 10 ml distilled water. 0.1 g of rosolic acid was dissolved in the NaOH solution.
Tryptone water [39]: 20 g of tryptone and 5 g of calcium chloride were weighed and dissolved in distilled water and made up to 1000 ml using distilled water. A pH of 7.5 was recorded. About 6 mls each of the solutions were dispensed into MacConkey bottles and autoclaved at 115 °C for 10 min.
Preparation of Petri dishes: About 47 mm microbiological grade Petri dishes were labelled on the underside. Absorbent pads were deposited in each dish using a pair of sterile forceps. A sterile pipette was used to dispense 2 ml of the appropriate medium onto the absorbent paper.
Incubation: Incubation of total coliforms was done at 37 °C for 24 h in a Millipore incubator. Incubation for faecal coliform was done at 44.5 °C for 24 h.
Faecal coliform bacteria identification and counting: After leaving the solution for over 24 h, developed total coliform in the vegetables appeared pinkish and grey in colour with a metallic sheen. Faecal coliforms were yellow or blue in colour.
2.5. Heavy metal concentration analysis
This section explains the procedures used to prepare the samples to determine the concentration (mg/kg) of the heavy metals (Pb, Cd, Fe, Hg, As, Se) in the vegetables.
2.5.1. Determination of heavy metals in vegetables (Pb, Cd, Fe)
Sample preparation: To remove all surface and tissue water, vegetables were dried in an oven at 105 °C. The dried vegetables were ground into a powder, and 1 g of that powder was placed in a clean porcelain crucible together with 1 ml of strong nitric acid. To allow the contents to char, the crucible was put on a hot plate set at 200 °C for 2 h. The crucible was removed from the hot plate and allowed to cool for 30 min 1 ml of concentrated nitric was added and the crucible was placed on the hot plate again for 30 min. The crucible was allowed to cool. The crucible and its content were placed in a furnace at 400 °C for 3 h after which it was allowed to cool for 1 h.
The ash was dissolved with 10% 7 M nitric acid and the sample was filtered into a 100 ml volumetric flask. DI water was added to the 100 ml mark, and the solution was sent for metal concentration measurement using atomic absorption spectrophotometry (PINAAcle 900T PerkinElmer Inc., Massachusetts, United States).
2.5.2. Determination of heavy metals in vegetables (Hg, As, Se)
Sample preparation: Five grams (5 g) of dried sample was placed in an Erlenmeyer flask and 10 ml of concentration. HNO3 was added to it. The sample was then warmed on a hot plate until solubilization. The temperature was increased to near boiling until the solution began to turn brown. After allowing the sample to cool down, 10 ml of conc. HNO3 was again added and thereafter returned to the hotplate to reduce the volume to 5–10 ml. About 30% of H202 was added to the sample when it was allowed to cool. The sample was again heated, and the process was repeated until the solution was clear. About 2 ml of concentrated HCl was added after the sample was cooled and then it was heated again. After cooling, the sample was transferred into a 100 ml volumetric flask. Dilution was done with the American Society for Testing and Materials (ASTM) type 1 water [41]. According to ASTM [41], this water can be described as ultrapure based on their standard. The insoluble material was allowed to separate after which the sample was analysed using the hydride generation/atomic absorption spectrometric method for As and Se. The cold vapour atomic absorption spectrometric method was used for Hg analysis.
2.6. Statistical analysis
The farmers' socio-economic attributes were described using the frequency (n) and percentages (%) while the levels of heavy metals (mg/kg) and coliform (count/100 ml) were estimated using the mean. The results and discussion section are discussed next.
3. Results and discussions
3.1. Background profile of vegetable farmers
The basic profile of the vegetable farmers at the three study sites is represented in Table 1. The findings revealed that 60 (89.6%) of the respondents were males and 7 (10.4%) were females. This finding is not new to this study site but has been observed in other similar studies conducted in Ghana (see Refs. [10,27]). Women do not dominate in farming but rather are involved in the vending of the produce [42]. In Table 1, majority of the farmers are in the age range of 41–50 years (53.7%) followed by the 51–60 years age group (19.4%), and 7.5% are those who are 61 years and above. The lowest age group is 18–30 years, which is the most youthful. In terms of education, majority had Senior High School/’O’ level education (41.7%), followed by Middle/Junior High School (29.9%). Those educated up to primary school level were 17.9% and the least educational attainment was 4.5%, and this is for respondents that had non-formal education. As far as crop varieties that were planted are concerned, 32.8% grew more than four crops, 29.9% grew three varieties of crops, 19.4% grew two varieties and 17.9% grew only one variety. The most common fertilizer used was organic fertilizer according to 53.7% of the farmers. Those who used NPK and Potassium fertilizer were 22.4% for each fertilizer type. Only a few (1.5%) did not apply fertilizer. These basic profiles of the respondents can be seen in Table 1.
Table 1.
Basic socioeconomic profile of the respondents.
| Age of Respondents (Frequencies and Percentages) | ||||||
|---|---|---|---|---|---|---|
| Study Sites | 18–30 | 31–40 | 41–50 | 51–60 | 61+ | Total N (%) |
| Haatso | 2 (3.0%) | 4 (6.0%) | 11 (16.4%) | 3 (4.4%) | 2 (3.0%) | 22 (32.8%) |
| Dzorwulu | 2 (3.0%) | 2 (3.0%) | 14 (20.9%) | 1 (1.4%) | 2 (3.0%) | 21 (31.3%) |
| WISS | 1 (1.5%) | 2 (3.0%) | 11 (16.4%) | 9 (13.4) | 1 (1.5%) | 24 (35.8%) |
| Total | 5 (7.5%) | 8 (11.9%) | 36 (53.7) | 13 (19.4%) | 5 (7.5%) | 67 (100%) |
| Education level of Respondents (Frequencies and Percentages) | ||||||
|
Study Sites |
No Education |
Non-Formal Education |
Primary |
Middle/Junior High School |
SHS/O'Level/ Technical |
Total N (%) |
| Haatso | 1 (1.49%) | 1 (1.5%) | 2 (3.0%) | 4 (6.0%) | 14 (20.8%) | 22 (32.8%) |
| Dzorwulu | 2 (3.0%) | 2 (3.0%) | 1 (1.5%) | 5 (7.5%) | 11 (16.4%) | 21 (31.3%) |
| WISS | 1 (1.49%) | – | 9 (13.4) | 11 (16.4%) | 3 (4.5%) | 24 (35.8%) |
| Total | 4 (6.0%) | 3 (4.5%) | 12(17.9%) | 20 (29.9%) | 28 (41.7%) | 67 (100%) |
| Main Varieties of crop(s) cultivated (Frequencies and Percentages) | ||||||
|
Study Sites |
1 Variety |
2 Varieties |
3 Varieties |
4+ Varieties |
Total N (%) |
|
| Haatso | 3 (4.5%) | 5 (7.5%) | 6 (9.0) | 8 (11.9%) | 22 (32.8%) | |
| Dzorwulu | 4 (6.0%) | 2 (3.0%) | 7 (10.4%) | 8 (11.9%) | 21 (31.3%) | |
| WISS | 5 (7.5%) | 6 (8.9%) | 7 (10.4%) | 6 (9.0%) | 24 (35.8%) | |
| Total | 12 (17.9%) | 13 (19.4) | 20 (29.9%) | 22 (32.8%) | 67 (100%) | |
| Types of Fertilizers Used on Farms (Frequencies and Percentages) | ||||||
|
Study Sites |
Organic manure |
NPK |
Potassium nitrate |
I do not use fertilizer |
Total N (%) |
|
| Haatso | 11 (16.4%) | 6 (9.0) | 5 (7.5%) | – | 22(32.8%) | |
| Dzorwulu | 8 (11.9%) | 5 (7.5%) | 7 (10.4%) | 1 (1.49%) | 21 (31.3%) | |
| WISS | 17 (25.37%) | 4 (6.0%) | 3 (4.5%) | – | 24 (35.8%) | |
| Total | 36 (53.7%) | 15 (22.4%) | 15 (22.4%) | 1 (1.5%) | 67 (100%) | |
Note: All figures in parentheses are the percentages of the total sample size (N = 67).
3.2. Main sources of water for vegetable farms and reasons for using them
The Onyasia stream was found to be the primary source of water for the vegetable farmers at the Haatso and Dzorwulu farm sites (see Table 2). Approximately 26.8% of the farmers in Haatso and about 25.4% of the farmers in Dzorwulu used this water as their primary irrigation water source. A total of 52.2% of the farmers stated that they used the stream as their main irrigation water source, which receives effluents from numerous drains and dwellings in the city.
Table 2.
Percent distribution of the main sources of water used by vegetable farmers.
| Study Sites | Main sources of water |
Total N (%) | ||
|---|---|---|---|---|
| Stream/Dam | Dug-outs/Well | Pipe | ||
| Haatso | 18 (26.8%) | 3 (4.5%) | 1 (1.5%) | 22 (32.8) |
| Dzorwulu | 17 (25.4%) | 2 (3%) | 2 (3%) | 21 (31.4) |
| WISS | 24 (35.8%) | – | – | 24 (35.8) |
| Total | 59 (88.0%) | 5 (7.5%) | 3(4.5%) | 67 (100.0) |
Note: All figures not in parentheses are the frequency responses (N = 67).
The finding of the main sources of water for vegetable farming in Accra confirms the claim by [42] that vegetable farmers in urban and peri-urban areas mostly rely on wastewater flowing in streams. While environmentalists and water conservation experts have argued for the reuse of wastewater in aquacultural activities to ensure sustainable water resources management which is in line with the United Nations Sustainable Development Goal 6, wastewater has to be treated to meet some quality standards as specified by [43] for it to be safe to be used to water crops for human consumption. This is because using untreated wastewater to water crops can pose serious health risks to humans. An example of such health risk is salmonella bacteria outbreaks which are common in fruits and vegetables.
Table 3 shows the responses of respondents (farmers) from the three study sites, where closeness of the water source to the farm was the main reason why most farmers used their current water source for irrigating their vegetable farms. For instance, 41%, 38%, and 54% of the respondents at Haatso, Dzorwulu and WISS attributed their use of the current water sources to closeness to their farms. At Haatso, the other two main reasons were that they could access it for free (16.2%) and that it is reliable to use (18.2%). At Dzorwulu the two other main reasons were that they are available for free (23.8%). According to the farmers, that water is what is available to them (14.2%) to use on their farms. Similar findings were reported for Weija. On the whole, the closeness of the water source to the farm, availability, and reliability are very important when it comes to consideration in the use of water for irrigation [44].
Table 3.
Reasons for using a particular water source.
| Study Sites | Reasons for using a particular water source to water vegetables |
N (%) | ||||
|---|---|---|---|---|---|---|
| It is good | That is what is available | It is free | It is reliable | It is closer to my farm | ||
| Haatso | 2 (9.1%) | 3 (13.6%) | 4 (18.2%) | 4 (18.2%) | 9 (40.9%) | 22 (100.0) |
| Dzorwulu | 3 (14.2%) | 3 (14.2%) | 5 (23.8%) | 2 (9.5%) | 8 (38.1%) | 21 (100.0) |
| WISS | 1(4.2%) | 4 (16.7%) | 3 (12.5%) | 3 (12.5%) | 13 (54.2%) | 24 (100.0) |
| Total | 6 (8.9%) | 10 (14.8%) | 12 (18%) | 9 (13.4%) | 30 (44.8%) | 67 (100.0) |
Note: All figures not in parenthesis are the frequency responses (N = 67).
3.3. Concentration of selected heavy metals in lettuce from the 3 farm sites
Heavy metal concentrations in the lettuce samples from Haatso, Dzorwulu, and Weija are presented in Table 4. The results show a mean Hg concentration of 0.036 × 104 mg/kg in lettuce from Haatso, 0.116 × 104 mg/kg for Dzorwulu, and 0.4860 × 104 mg/kg for Weija irrigation scheme site (WISS). In other words, the Hg concentration in lettuce from Haatso (360 mg/kg) was lower than those from Dzorwulu (1160 mg/kg) and Weija (4860 mg/kg). The results also show that lettuce from Dzorwulu and Weija had a Hg concentration above the maximum WHO permissible level of 1000 mg/kg for human consumption. The concentrations of Pb and Cd from all the irrigation sites were within WHO [43] recommended limits for human consumption. However, the sample collected from the WISS, which does not use wastewater but river/dam, recorded high levels of mercury. This may be due to the high levels of mercury in the irrigation water since some studies have already reported high levels of Hg in the Densu River which is used for irrigation by the farmers at the Weija irrigation scheme site [45]. It should be noted that the high concentration of Hg in the vegetables can also come from the soil or from the fertilizer the farmers use on their farms.
Table 4.
The concentration of heavy metals in lettuce.
| Heavy Metal | Mean chemical concentration mg/kg in lettuce |
|||
|---|---|---|---|---|
| Haatso | Dzorwulu | WISS | WHO Standard | |
| Hg (μg mg x104) | 360 | 1160 | 4860 | ≤1000 |
| Pb (μg mg x104) | 50 | 50 | 50 | ≤1000 |
| Cd (μg mg x104) | 30 | 30 | 30 | ≤200 |
It should be indicated that although mercury can be released into the environment from natural sources, from the soil, or the fertilizer the farmers used, anthropogenic factors along the Densu River could be the main source of heavy metals in the vegetables [45]. For instance, mercury is used in mining along the Densu River, especially those engaged in illegal mining [46] and indiscriminate dumping of solid and electronic waste [47].
3.4. Concentration of selected heavy metals in pepper from the 3 farm sites
The concentrations of Hg, Pb, and Cd in pepper samples from Haatso, Dzorwulu, and WISS are shown in Table 5. Except for Hg, all the concentrations were within the maximum permissible limit set by WHO [43]. The results show low Hg concentration in vegetable samples from Haatso (0.0150 * 104 mg/kg) and Dzorwulu (0.0280 * 104 mg/kg) compared to that of the Weija irrigation scheme site (0.309 * 104 mg/kg) which is on the high side. The amount of Hg obtained in this study is significantly elevated compared with the maximum acceptable limit set by WHO. The Onyasia Stream serves as the main water source for the farms in Haatso and Dzorwulu, and this may be the source of pollution of the vegetables, or it may be due to uptake from the soil which eventually accumulates in the sampled vegetables.
Table 5.
Mean concentrations of heavy metals in pepper.
| Heavy Metal | Mean Chemical Concentration mg/kg in Pepper |
|||
|---|---|---|---|---|
| Haatso | Dzorwulu | WISS | WHO Standard | |
| Hg (μg mg x10000) | 150 | 280 | 3090 | <1000 |
| Pb (μg mg x10000) | 50 | 50 | 50 | <1000 |
| Cd (μg mg x10000) | 30 | 30 | 30 | <200 |
Wastewater can considerably contribute to the accumulation of heavy metals in soils and crops [48,49]. Apart from airborne contamination from traffic, the application of manures, fertilizers, and pesticides are additional sources of heavy metals in irrigated agriculture farms [50,51]. Despite Ghana's lower level of industrialisation, untreated but diluted wastewater (wastewater mixed with stream/stormwater) is often used for vegetable cultivation in urban areas [33]. For instance, Accra is home to roughly 1000 active vegetable producers and these vegetables are consumed by about 200,000 urban residents daily [29]. Heavy metals, often known as trace metals, are one of the most enduring contaminants in wastewater. The environment and human health are negatively impacted by high concentrations of heavy metals released into water bodies in several ways [52]. Wastewater that is released into the Onyasia River may contain heavy metal pollutants, which may also end up being absorbed via the soil and bioaccumulating by the vegetables that are irrigated with the stream water. This study shows that three common heavy metals—lead, mercury, and cadmium—pose severe risks to both humans and the environment [53].
The finding in this paper is similar to that of [23] who also assessed heavy metal concentrations in some vegetables and their corresponding soil from a farm in the Korle Lagoon's catchment. They found that heavy metals in lettuce were more than the recommended guideline level. Specifically, Fe and Zn in the vegetables were above the recommended guideline level. Due to these findings, they posit that vegetables grown around the Korle Lagoon are not good for human consumption because they are carcinogenic while few are noncarcinogenic to both adults and children. Accumulation of heavy metals in tissues, organs and cells of the human body can produce health effects such as reduced growth, short and long-term morbidities, organ and nervous system damage, and in extreme cases, death [54].
3.5. Comparison of faecal coliform counts in lettuce and pepper in the three sites
Table 6 lists the faecal coliform and total coliform counts in the lettuce and pepper samples taken from the three farm sites. The findings show that coliforms and faecal coliforms were present in the lettuce and pepper samples taken from the irrigation sites, and their levels were higher than the geometric mean count recommended by the WHO [43]. This suggests that eating the lettuce and pepper from the three irrigated sites is unsafe for the general population's health. The level of faecal coliforms suggests that water used for irrigation of the vegetables is the most likely source of the faecal contamination even though the water has not been tested to confirm this assertion. The good thing about this finding is that, faecal coliform and total coliform counts in the samples from the WISS are lower than those of Haatso and Dzorwulu.
Table 6.
Faecal and total coliform contamination levels of lettuce and pepper from the vegetable farms.
| Study site | Sample | Faecal coliform, Col. count/100 ml |
Total coliform Col. count/100 ml |
|---|---|---|---|
| WISS | Lettuce | >5 | >5 |
| Pepper | >5 | >5 | |
| Haatso | Lettuce | >6 = (TNTC) | >6 |
| Pepper | >6 | >6 | |
| Dzorwulu | Lettuce | >6 | >6 |
| Pepper | >6 | >6 |
This study supports the assertions made by [27,33] that the use of contaminated water for the cultivation of vegetables poses risks to people's health. Since fresh produce and vegetable farmers in urban and peri-urban areas of Ghana and other West African countries depend on water from various wastewater sources to irrigate their crops, the consumers of these crops are likely to be exposed to diseases like typhoid fever. This is because, vegetables bioaccumulate the bacterial, biological, and chemical pollutants found in most streams and wastewater sources [29].
Changing risky behaviour seems to be motivated in most cases by the knowledge of a health threat associated with the behaviour [55]. Hence, a lack of awareness of the outcome of risky behaviour may not elicit behavioural change in people or society. For most farmers, the lack of understanding of the potential health risks or hazardous nature and effect of wastewater use on their produce and consumers makes it difficult to change their behaviour. In situations where they want to change the source of water for irrigation but there are no alternative sources, they have no option but to continue to use the contaminated source. Risk perception relating to hazards is usually based on group or individual's experiences and the prevailing environmental conditions [56]. This may mean that vegetable farmers may see no problem with the use of their sources of water for farming if the community accepts, does not complain about it and have not experienced any negative health outcomes attributed to the use of their farming products. Again, vegetable farmers may continue with risky farming practices like using wastewater sources probably because they are not aware of the level of pollution and health consequences of their actions. It will also continue when authorities are not enforcing regulations on best farming practices, or simply because consumers are not perturbed with the harm that they are ingesting. They will therefore continue to use these water sources for their urban farming. This study together with others like [57] points out that, these water sources may be the cause of heavy metal contaminants.
Among agricultural sources of heavy metals in plants, it can be said that the most common sources are pesticides, fertilizers, and sewage sludge [58] to mention only a few. Fertilisers, especially the organic and inorganic types are accountable for producing a lot of heavy metals in the soil [59]. Pesticides are very important for plant growth and for a growing population as those found in urban areas of West Africa including Ghana. Some crops cannot grow in the urban environment without the application of pesticides. This is because pesticides protect most crops in tropical countries like Ghana in the wake of climate change. It should be noted that the farmers interviewed said they mainly use organic fertilizer on their farms. The point is that unless the soil is contaminated with heavy metals from a particular source, natural sources of heavy metals in soils may not reach the levels found in the vegetables in the sites under investigation. The obvious source of contamination may be the water from the river used to irrigate the crops.
Research conducted by [34] through the examination of both conventional and unconventional vegetables utilizing wastewater sourced from specific regions in Accra shows that the irrigation water derived from wastewater exhibited the highest levels of manganese (Mn), followed by iron (Fe), zinc (Zn), lead (Pb), copper (Cu), and nickel (Ni). However, chromium (Cr), cadmium (Cd), and cobalt (Co) concentrations remained within the thresholds established by the World Health Organization (WHO) and the Food and Agriculture Organization (FAO). They also found that iron (Fe), manganese (Mn), copper (Cu), lead (Pb) and nickel (Ni) levels were higher in wastewater-irrigated soils than in groundwater-irrigated soils respectively. These are interesting studies that show that using untreated wastewater on crops has a high concentration of heavy metals in them. However, evidence from studies like [60,61] suggests that mining and municipal solid wastes are the key sources of heavy metal contamination in the Weija reservoir.
4. Conclusions, limitations, and future works
Conclusions: The fruit and vegetable industry has become a very important part of Ghanaian agriculture because it employs a large segment of the unemployed, provides nutrition to a large section of the population, serves as a source of income to people and provides foreign exchange to the country. Over the past years however, the export of some vegetables has been banned due to the presence of pests and other substances in the produce. Also, more vegetables are being cultivated in urban areas calling for this study to ascertain the presence of heavy metals and faecal coliform in two species of vegetables cultivated in selected locations in the Accra metropolitan area to determine their wholesomeness for human consumption. This paper is among the few to use exploratory concurrent mixed methods research design to examine the spatial differentiation in levels of heavy metals and coliform bacteria in two vegetable crops that were cultivated under different sources of irrigation water. Interestingly, the study found no spatial variation concerning total coliform and faecal coliform count in the vegetable samples at the three sites studied. The total coliform and faecal coliform count levels were higher than what is recommended by the WHO. However, heavy metal concentrations for Pb and Cd in the vegetables were below permissible levels at the sites, except for Hg, which was higher than the permissible levels set by the World Health Organization at the WISS. This paper contributes to the literature on vegetable cultivation in urban areas of Ghana using streams by pointing out that mercury (Hg) concentration in vegetable samples at the WISS, which was thought to be good was much higher compared to the other two farming sites which, on the surface does not look good. The conclusion is that the consumption of lettuce and pepper from these three sites can pose potential health risks to consumers. In particular, this study has demonstrated that the location of vegetable farms can contribute to the risk of ingestion of Hg and toxic faecal coliform bacteria from contaminated vegetables irrespective of the irrigation scheme type (formal or informal) being used by farmers. This is evident when comparing Hg concentrations in the vegetables grown at the control site (WISS) where higher values were recorded in comparison with vegetables cultivated using wastewater sources such as those in Dzorwulu and Haatso, which recorded lower values. It should however be noted that farmers in all the study sites said they access water from streams and dams that are close to their farms to irrigate their crops. Thus, it is concluded in this paper that, the vegetables produced at the study sites, especially those at the WISS have high Hg and microbial contaminants as such may not be safe for human consumption.
Limitations of the study: One of the limitations of this present study is that vegetable samples were not taken from all important hotspots of vegetable production in the Accra-Tema metropolitan area, or farming areas in the country where vegetables are cultivated for exports. Doing this would have given credence to the conclusions on the country's applicability of the findings. Another limitation of the study is that the heavy metal concentrations were not assessed for the soils and water samples of the farms where the vegetables are being grown but only on the vegetable sources. This would have helped to pinpoint the specific source of the contamination. Another limitation of this study is the small number of vegetable samples ( lettuce and bell pepper) that were used. Related to this limitation is the number of times the samples were taken. Due to financial limitations, only one sample was collected and analysed. This can call into question the extent to which the results of heavy metals and faecal contamination of vegetables in the study can be generalized. These limitations aside, we feel this is a good starting point to conduct more studies on the fruit and vegetable industry in Ghana and other countries, especially vegetables produced from urban agriculture. This is because the fruits and vegetables industry has come to stay and should be made safe and sustainable.
Recommendationsfor future works: Ghana should also come out with a good framework for quality standards. Enhanced sensitization of the populace and the farmers in urban areas as a whole on quality issues and vegetable production and sources should be embarked upon. The general public should be sensitised to the health implications of consuming crops laden with heavy metals and faecal coliform. This education drive may help reduce the risk of consumers patronising urban vegetables cultivated using polluted water. Health risk assessment for consuming faecal coliform and Hg contaminated vegetables should be evaluated in future studies as well as assessing the quality of water used to irrigate urban farms.
Research should be carried out on the vegetable farms that are being assisted in the various locations of the country. Also, research should be conducted on other vegetables like okra, onions, turia, tomatoes, herbs, cucumber and tinda to ascertain the status of heavy metals and faecal contamination in them. Further, comparative studies should be done on organic vegetables sourced from big retail stores, markets and organic home-based gardens to compare the levels of pollution in these crops in the country. In terms of the spatial location of vegetable farms, more studies should be embarked upon to compare the crops that are produced in urban areas and those cultivated in rural locations.
Funding statement
This study did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Data availability statement
Data generated for this study was used in the analysis however, data will be made available to any interested person upon request.
Additional information
.
Ethical declaration
Ethical clearance for the study was obtained from the Research and Ethics Committee of the School of Research and Graduate Studies, Wisconsin International University College, Ghana. The ethics clearance certificate number is APP/REC/0015. Also, informed consent (written or oral) was given by all the study participants before the study was conducted.
CRediT authorship contribution statement
Shine Francis Gbedemah: Writing – review & editing, Writing – original draft, Methodology, Formal analysis, Data curation, Conceptualization. Alain Attasse Gbeasor: Writing – original draft, Methodology, Investigation, Funding acquisition, Formal analysis, Data curation, Conceptualization. Gideon Selorm Hosu-Porbley: Writing – review & editing, Methodology, Formal analysis, Data curation. Louis Kusi Frimpong: Writing – review & editing, Writing – original draft, Formal analysis, Data curation. Richard Amfo-Otu: Writing – review & editing, Writing – original draft, Formal analysis, Data curation. Selase Kofi Adanu: Writing – review & editing, Writing – original draft, Formal analysis, Conceptualization. Eric Kofi Doe: Writing – review & editing, Writing – original draft, Visualization, Validation, Methodology, Formal analysis, Data curation.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this pape.
References
- 1.Walther O., Nugent P., Goewey S. Transport policies and road accessibility in Ghana. SSRN Electron. J. 2023 doi: 10.2139/ssrn.4383685. [DOI] [Google Scholar]
- 2.GSS . 2021. Population & Housing Census: National Analytical Report. Accra, Ghana. [Google Scholar]
- 3.GSS . Ghana Statistical Service; Accra, Ghana: 2014. 2010 Population and Housing Census Report: Urbanisation. [Google Scholar]
- 4.Papanek A., Campbell C.G., Wooten H. Social and community benefits and limitations of urban agriculture. Environ. Data Inf. Serv. 2023;2023 doi: 10.32473/edis-fy1517-2023. [DOI] [Google Scholar]
- 5.Mead B.R., Christiansen P., Davies J.A.C., Falagán N., Kourmpetli S., Liu L., Walsh L., Hardman C.A. Is urban growing of fruit and vegetables associated with better diet quality and what mediates this relationship? Evidence from a cross-sectional survey. Appetite. 2021;163 doi: 10.1016/j.appet.2021.105218. [DOI] [PubMed] [Google Scholar]
- 6.Mordor Intelligence Vegetables in Ghana size & share analysis - growth trends & forecasts (2023-2028) 2023 https://www.mordorintelligence.com/industry-reports/ghanaian-fruit-vegetable-market [Google Scholar]
- 7.FAOSTAT . FAO; Ghana: 2023. Crops and Livestock Products.https://www.fao.org/faostat/en/#data/QCL [Google Scholar]
- 8.Dittoh S. World Bank; Washington, DC, Washington, DC: 2020. Assessment of Farmer-Led Irrigation Development in Ghana. [Google Scholar]
- 9.Malakar A., Snow D.D., Ray C. Irrigation water quality-A contemporary perspective. Water (Switzerland) 2019;11 doi: 10.3390/w11071482. [DOI] [Google Scholar]
- 10.Arimiyaw A.W., Abass K., Gyasi M.R. On-farm urban vegetable farming practices and health risk perceptions of farmers in Kumasi. Geojournal. 2020;85:943–959. doi: 10.1007/s10708-019-10003-7. 2020 v.85 no.4. [DOI] [Google Scholar]
- 11.Somda W., Tischbein B., Bogardi J.J. vol. 1. 2020. (Water Use inside Inland Valleys Agro-Systems in the Dano Basin, Burkina Faso, Water Cycle). [DOI] [Google Scholar]
- 12.Hussain I., Raschid L., Hanjra M.A., Marikar F., van der Hoek W. 2002. Wastewater Use in Agriculture: Review of Impacts and Methodological Issues in Valuing Impacts. (With an Extended List of Bibliographical References) [Google Scholar]
- 13.Saravanane R., V Ranade V., Bhandari V.M., Seshagiri Rao A. Industrial Wastewater Treatment, Recycling and Reuse. Elsevier; 2014. Urban wastewater treatment for recycling and reuse in industrial applications; pp. 283–322. [DOI] [Google Scholar]
- 14.Naidoo S., Olaniran A.O. Treated wastewater effluent as a source of microbial pollution of surface water resources. Int J Environ Res Public Health. 2013;11:249–270. doi: 10.3390/ijerph110100249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wurochekke A.A., Harun N.A., Mohamed R.M.S.R., Mohd Kassim A.H.B. Constructed wetland of lepironia articulata for household greywater treatment. APCBEE Procedia. 2014;10 doi: 10.1016/j.apcbee.2014.10.025. [DOI] [Google Scholar]
- 16.Carey J., Cook B. FAO; Rome, Italy: 2021. The Milan Urban Food Policy Pact Monitoring Framework: A Practical Handbook for Implementation. [Google Scholar]
- 17.Khan Z.I., Ahmad K., Ashraf M., Parveen R., Arshad F., Hussain A., Bibi Z., Akram N.A., Noorka I.R., Mustafa I. Risk assessment of heavy metal toxicity through contaminated vegetable from sewage water: implications for populace health. Human and Ecological Risk Assessment. 2016;22 doi: 10.1080/10807039.2015.1052959. [DOI] [Google Scholar]
- 18.Othman Y.A., Al-Assaf A., Tadros M.J., Albalawneh A. Heavy metals and microbes accumulation in soil and food crops irrigated with wastewater and the potential human health risk: a metadata analysis. Water (Basel) 2021;13:3405. doi: 10.3390/w13233405. [DOI] [Google Scholar]
- 19.Agocs M., Clarkson T., Ambre J., Becker C., Borak J., Cannella J., Kipen H., Jackson R.J., Rodnick J., Wummer B.A. Mercury toxicity. Am. Fam. Physician. 1992;46 [Google Scholar]
- 20.Edokpayi J.N., Odiyo J.O., Durowoju O.S. Impact of wastewater on surface water quality in developing countries: a case study of South Africa. Water Quality. 2017 doi: 10.5772/66561. [DOI] [Google Scholar]
- 21.Onalenna O., Rahube T.O. Assessing bacterial diversity and antibiotic resistance dynamics in wastewater effluent-irrigated soil and vegetables in a microcosm setting. Heliyon. 2022;8 doi: 10.1016/j.heliyon.2022.e09089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.GIRSAL Reviving Ghana’s fresh chili pepper export industry: Commercial chili production trial net houses by GIRSAL and partners. 2023 https://www.girsal.com/2023/04/12/reviving-ghanas-fresh-chili-pepper-export-industry-a-commercial-trial-of-production-of-chili-in-net-houses-by-girsal-and-partners/ [Google Scholar]
- 23.Osae R., Nukpezah D., Darko D.A., Koranteng S.S., Mensah A. Accumulation of heavy metals and human health risk assessment of vegetable consumption from a farm within the Korle Lagoon catchment. SSRN Electron. J. 2022 doi: 10.2139/ssrn.4241568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Abdallah C.K., Mourad K.A. Assessing the quality of water used for vegetable irrigation in Tamale Metropolis, Ghana. Sci. Rep. 2021;11 doi: 10.1038/s41598-021-84617-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Atitsogbey P., Kyereh E., Ofori H., Johnson P.-N.T., Steiner-Asiedu M. Heavy metal, microbial and pesticides residue contaminations are limiting the potential consumption of green leafy vegetables in Ghana: an overview. Heliyon. 2023;9 doi: 10.1016/j.heliyon.2023.e15466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gbogbo F., Otoo S.D., Huago R.Q., Asomaning O. High levels of mercury in wetland resources from three river basins in Ghana: a concern for public health. Environ. Sci. Pollut. Control Ser. 2017;24 doi: 10.1007/s11356-016-8309-2. [DOI] [PubMed] [Google Scholar]
- 27.Drechsel P., Keraita B. Irrigated urban vegetable production in Ghana: characteristics, benefits and risk mitigation. 2014 doi: 10.5337/2014.219. [DOI] [Google Scholar]
- 28.Adomako L.A.B., Yirenya-Tawiah D., Nukpezah D., Abrahamya A., Labi A.-K., Grigoryan R., Ahmed H., Owusu-Danquah J., Annang T.Y., Banu R.A., Osei-Atweneboana M.Y., Timire C., Tweya H., Ackon S.E.D., Nartey E., Zachariah R. Reduced bacterial counts from a sewage treatment plant but increased counts and antibiotic resistance in the recipient stream in Accra, Ghana—a cross-sectional study. Trop Med Infect Dis. 2021;6:79. doi: 10.3390/tropicalmed6020079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Amoah P., Drechsel P., Henseler M., Abaidoo R.C. Irrigated urban vegetable production in Ghana: microbiological contamination in farms and markets and associated consumer risk groups. J. Water Health. 2007;5:455–466. doi: 10.2166/wh.2007.041. [DOI] [PubMed] [Google Scholar]
- 30.Sampson E.L., Agyemang P.O. Quality of raw water from Ghana's reservoirs: a case of Weija Reservoir. J. Environ. Earth Sci. 2020 doi: 10.7176/jees/10-5-01. [DOI] [Google Scholar]
- 31.Yidana S.M., Bawoyobie P., Sakyi P., Fynn O.F. Evolutionary analysis of groundwater flow: application of multivariate statistical analysis to hydrochemical data in the Densu Basin, Ghana. J. Afr. Earth Sci. 2018;138 doi: 10.1016/j.jafrearsci.2017.10.026. [DOI] [Google Scholar]
- 32.WRC . WRC (Water Resource Commission Ghana); 2009. Ankobra River Basin - Integrated Water Resources Management Plan. [Google Scholar]
- 33.Obuobie E., Keraita B., Danso G., Amoah P., Cofie O.O., Raschid-Sally L., Drechsel P. Irrigated Urban Vegetable Production in Ghana. IWMI; Accra: 2006. Irrigated urban vegetable production in Ghana: characteristics, benefits and risks. [Google Scholar]
- 34.Lente I., Ofosu-Anim J., Brimah A.K., Atiemo S. Heavy metal pollution of vegetable crops irrigated with wastewater in Accra, Ghana. West African Journal of Applied Ecology. 2014;22 [Google Scholar]
- 35.Doe E.K., Attua E.M., Obour P.B., Quaye A.K., Fosu-Mensah B.Y. Soil health and synergy of ecological determinants of green cocoa productivity in different soil ecotypes in Ghana. Front. Sustain. Food Syst. 2023;7 doi: 10.3389/fsufs.2023.1169015. [DOI] [Google Scholar]
- 36.Osae R., Nukpezah D., Darko D.A., Koranteng S.S., Mensah A. Accumulation of heavy metals and human health risk assessment of vegetable consumption from a farm within the Korle Lagoon catchment. Heliyon. 2023;9 doi: 10.1016/j.heliyon.2023.e16005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Grabow W.O.K., Hilner C.A., Coubrough P. Evaluation of standard and modified M-FC, MacConkey, and Teepol media for membrane filtration counting of fecal coliforms in water. Appl. Environ. Microbiol. 1981;42 doi: 10.1128/aem.42.2.192-199.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Castro-Rosas J., Cerna-Cortés J.F., Méndez-Reyes E., Lopez-Hernandez D., Gómez-Aldapa C.A., Estrada-Garcia T. Presence of faecal coliforms, Escherichia coli and diarrheagenic E. coli pathotypes in ready-to-eat salads, from an area where crops are irrigated with untreated sewage water. Int. J. Food Microbiol. 2012;156 doi: 10.1016/j.ijfoodmicro.2012.03.025. [DOI] [PubMed] [Google Scholar]
- 39.MacConkey A. Lactose-fermenting bacteria in faeces. J. Hyg. 1905;5 doi: 10.1017/S002217240000259X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Presswood W.G., Strong D.K. Modification of M-FC medium by eliminating rosolic acid. Appl. Environ. Microbiol. 1978;36:90–94. doi: 10.1128/aem.36.1.90-94.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.ASTM ASTM D1193-06(2018) Standard specification for reagent water, USA. 2018 doi: 10.1520/D1193-06R18. [DOI] [Google Scholar]
- 42.Obuobie E., Danso G., Drechsel P. IWMI; 2003. Access to Land and Water for Urban Vegetable Farming in Accra.https://www.zef.de/fileadmin/user_upload/5c3a_UAM11-Land.pdf [Google Scholar]
- 43.World Health Organization (WHO) World Health Organization; Geneva, N.Y: 1989. Health Guidelines for the Use of Wastewater in Agriculture and Aquaculture: Technical Report Series No. 778. Report of a WHO Scientific Group [meeting Held in Geneva from 18 to 23 November 1987] [PubMed] [Google Scholar]
- 44.Dwumfour-Asare B., Biritwum Nyarko K., Adams A. Land tenure and water sources for urban vegetable farmers in Asante-Mampong, Ghana. Indian J. Sci. Technol. 2018;11:1–9. doi: 10.17485/ijst/2018/v11i17/107290. [DOI] [Google Scholar]
- 45.Fianko J.R., Osae S., Achel D. Impact of anthropogenic activities on the Densu River in Ghana. Water Environ. J. 2009;23 doi: 10.1111/j.1747-6593.2008.00137.x. [DOI] [Google Scholar]
- 46.Bawa S.A., Antwi-Agyei P., Domfeh M.K. Impact of the ban on illegal mining activities on raw water quality: a case-study of konongo water treatment plant, ashanti region of Ghana. Journal of Sustainable Mining. 2022;21 doi: 10.46873/2300-3960.1349. [DOI] [Google Scholar]
- 47.Eduful M., Alsharif K., Eduful A., Acheampong M., Eduful J., Mazumder L. The illegal artisanal and small-scale mining (galamsey) ‘menace’ in Ghana: is military-style approach the answer? Resour. Pol. 2020;68 doi: 10.1016/j.resourpol.2020.101732. [DOI] [Google Scholar]
- 48.Duncan A.E., Oti J., Potakey M.E. Impacts of human activities on the quality of river water: a case study of river Densu in Nsawam Adoagyiri of the Akwapim south District, eastern region of Ghana. OAlib. 2019;6 doi: 10.4236/oalib.1105785. [DOI] [Google Scholar]
- 49.Mapanda F., Mangwayana E.N., Nyamangara J., Giller K.E. The effect of long-term irrigation using wastewater on heavy metal contents of soils under vegetables in Harare, Zimbabwe. Agric. Ecosyst. Environ. 2005;107 doi: 10.1016/j.agee.2004.11.005. [DOI] [Google Scholar]
- 50.Singh A., Sharma R.K., Agrawal M., Marshall F.M. Risk assessment of heavy metal toxicity through contaminated vegetables from waste water irrigated area of Varanasi, India. Trop. Ecol. 2010;51:375–387. [Google Scholar]
- 51.McBride M.B. Toxic metals in sewage sludge-amended soils: has promotion of beneficial use discounted the risks? Adv. Environ. Res. 2003;8 doi: 10.1016/S1093-0191(02)00141-7. [DOI] [Google Scholar]
- 52.Bakare S., Denloye A.A.B., Olaniyan F.O. Cadmium, lead and mercury in fresh and boiled leafy vegetables grown in Lagos, Nigeria. Environ. Technol. 2004;25 doi: 10.1080/09593332508618465. [DOI] [PubMed] [Google Scholar]
- 53.Mahmoud N., Al-Shahwani D., Al-Thani H., Isaifan R.J. Risk assessment of the impact of heavy metals in urban traffic dust on human health. Atmosphere. 2023;14(6):1049. doi: 10.3390/atmos14061049. [DOI] [Google Scholar]
- 54.European Union (EU) Heavy metals in waste: Final report, COWI A/S, Denmark. 2002 https://t.ly/0q7uj [Google Scholar]
- 55.Renner B., Schupp H. In: The Oxford Handbook of Health Psychology. Friedman H., editor. Oxford University Press.; New York: 2011. The perception of health risks; pp. 639–666. [Google Scholar]
- 56.Garvin T. Analytical paradigms: the epistemological distances between scientists, policy makers, and the public. Risk Anal. 2001;21:443–455. doi: 10.1111/0272-4332.213124. [DOI] [PubMed] [Google Scholar]
- 57.Aftab K., Iqbal S., Khan M.R., Busquets R., Noreen R., Ahmad N., Kazimi S.G.T., Karami A.M., Al Suliman N.M.S., Ouladsmane M. Wastewater-irrigated vegetables are a significant source of heavy metal contaminants: toxicity and Health Risks. Molecules. 2023;28:1371. doi: 10.3390/molecules28031371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Alloway B.J. In: Heavy Metals in Soils. Trace Metals and Metalloids in Soils and Their Bioavailability. Alloway B.J., editor. Springer; Dordrecht, The Netherlands: 2013. Sources of heavy metals and metalloids in soils; pp. 11–50. [Google Scholar]
- 59.Khan M.N., Mobin M., Abbas Z.K., Alamri S.A. Fertilizers and their contaminants in soils, surface and groundwater. Encyclopedia of the Anthropocene. 2018;5:225–240. [Google Scholar]
- 60.Ansah E., Nukpezah D., Hogarh J.N. Levels and distribution of heavy metals in Weija reservoir, Accra, Ghana. West African Journal of Applied Ecology. 2018;26(1):74–88. [Google Scholar]
- 61.Amankwaa G., Lu Y., Liu T., Wang N., Luan Y., Cao Y., Huang W., Ni X., Gyimah E. Heavy metals concentration profile of an aquatic environment and health implications of human exposure to fish and prawn species from an urban river (Densu) Iran. J. Fish. Sci. 2020;20(2):529–546. doi: 10.22092/ijfs.2021.351023.0. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data generated for this study was used in the analysis however, data will be made available to any interested person upon request.