Summary
Background
Current labelling advises discontinuation of metformin when estimated glomerular filtration rate (eGFR) < 30 ml/min/1.73 m2 due to increased risk of lactic acidosis. However, in real-world practice, the risk-benefit ratios remain uncertain. We examined the risk associations of discontinued-metformin use with cardiorenal and clinical outcomes in patients with type 2 diabetes (T2D) and advanced chronic kidney disease.
Methods
In this territory-wide, retrospective cohort and target trial emulation study, we included Chinese patients attending the Hong Kong Hospital Authority (HA) and enrolled in the Risk-Assessment-and-Management-Programme-for-Diabetes-Mellitus (RAMP-DM) from 2002 to 2019. Patients were stratified by discontinuation of metformin within six months after reaching eGFR < 30 ml/min/1.73 m2 from January 1, 2002 to December 31, 2018, and followed up until December 31 2019. We excluded patients who had observational time <6 months from eGFR < 30 ml/min/1.73 m2, and had their eGFR measured during a hospitalisation episode due to acute kidney injury, or missing diagnosis date of diabetes. We compared the risk associations of metformin discontinuation with clinical outcomes. The primary outcomes were major adverse cardiovascular events (MACE), end-stage kidney disease (ESKD), cancer, and all-cause mortality. A Cox-model with time-dependent exposure and covariates was used to estimate the hazard ratio (HR) of outcomes in a propensity-score overlap-weighted cohort. The risk of occurrence of lactic acidosis (serum lactate > 5.0 mmol/L with a concomitant blood pH < 7.35 or ICD-9 codes of 276.2) in discontinued-metformin versus continued-metformin users was assessed in a separate register-based cohort.
Findings
A total of 33,586 metformin users with new-onset eGFR < 30 ml/min/1.73 m2 were included in the study, 7500 (22.3%) of whom discontinued metformin within 6 months whereas 26,086 (77.7%) continued use of metformin. During a median follow-up of 3.8 (IQR: 2.2–6.1) years, 16.4% (5505/33,586), 30.1% (10,113/33,586), and 7.1% (2171/30,682) had incident MACE, ESKD, and cancer respectively, and 44.4% (14,917/33,586) died. Compared to continued-metformin use, discontinuation was associated with higher risk of MACE (weighted and adjusted HR = 1.40, 95% CI: 1.29–1.52), ESKD (HR = 1.52, 1.42–1.62), and death (HR = 1.22, 1.18–1.27). No association was observed for cancer (HR = 0.93, 0.85–1.01). Discontinued-metformin users had higher change in HbA1c change at 6-month of follow-up versus continued-metformin users (weighted mean HbA1c level change: 0.5% [0.4–0.6%] versus 0.2% [0.1–0.2]). In the separate register-based cohort (n = 3235), null association was observed between metformin use and risk of lactic acidosis (weighted HR = 0.94 [0.53–1.64]).
Interpretation
Our results suggest that discontinuation of metformin in patients with T2D and chronic kidney disease may be associated with increased risk of cardiovascular-renal events. Use of metformin below eGFR of 30 ml/min/1.73 m2 may be associated with cardiovascular, renal, and mortality benefits that need to be weighed against the risk of lactic acidosis, but further research is needed to validate these findings.
Funding
CUHK Impact Research Fellowship Scheme.
Keywords: Metformin, Cardiovascular disease, Mortality, Therapeutics, Diabetes, Chronic kidney disease, Lactic acidosis
Research in context.
Evidence before this study
We searched PubMed with the terms “diabetes”, “chronic kidney disease (CKD)”, “metformin”, “hyperkalemia”, “death”, “mortality”, “cardiovascular disease”, “major adverse cardiovascular events (MACE)”, “cancer”, and “end-stage kidney disease (ESKD) or “end stage renal disease (ESRD)” for original articles and reviews published up to June 30, 2023. Most studies on the associations between metformin use and risk of clinical outcomes including all-cause mortality, cardiovascular (CVD), MACE, and ESKD were focused on people with T2D and CKD with eGFR ≥ 30 ml/min/1.73 m2. These studies have reported reduced risk for CVD, all-cause mortality, and CV-events with metformin use. A retrospective cohort of 10,426 patients with T2D reported metformin users had 35% reduced risk for all-cause mortality and ESKD, especially in those with eGFR of 30–45 ml/min/1.73 m2. However, the associations between discontinued use of metformin in patients with T2D and advanced-CKD (eGFR < 30 ml/min/1.73 m2) remains uncertain.
Added value of this study
In this cohort study that included 33,586 patients with T2D and advanced CKD, discontinued metformin was associated with higher risk of MACE, ESKD, as well as cardiovascular-cause, pneumonia-cause, and all-cause mortalities, compared with continued metformin use.
Implications of all the available evidence
In this real-world study, we adopted an emulated target trial approach and observed that discontinuation of metformin was associated with increased risk of cardiovascular events. Use of metformin below eGFR of 30 ml/min/1.73 m2 may be associated with cardio-renal and mortality benefits that need to be weighed against the risks of lactic acidosis. Pending confirmatory evidence from randomised controlled trials, our data support the possibility of continued metformin use in people with diabetes and advanced CKD for cardiovascular protection.
Introduction
Chronic kidney disease (CKD) is a growing global health challenge. In 2021, 10% of the world’s population is affected by diabetes, which is one of the primary causes of CKD.1 The burden of cardiovascular disease (CVD) also increases continuously as kidney function declines, and patients with both diabetes and CKD have an exceptionally high risk of CVD.2 Metformin is the first-line oral glucose-lowering drug (GLD) for individuals with type 2 diabetes (T2D).3 Traditionally, metformin was contraindicated in patients with reduced kidney function due to the paucity of randomised controlled trial (RCT) data and early reports of lactic acidosis, primarily associated with phenformin.4 However, increasing real-world evidence supports its safety in patients with a broad range of kidney function. In 2016, the labelling of metformin was changed to include its use in patients with estimated glomerular filtration rate (eGFR) of 30–60 ml/min/1.73 m2 (CKD stage G3).5 In 2020, a consensus statement recommended continuing metformin at half maximum dose and increased frequency of eGFR monitoring in patients with eGFR of 30–44 ml/min/1.73 m2 (CKD stage G3b) with its discontinuation when eGFR falls below 30 ml/min/1.73 m2.6
Use of metformin in CKD stage G4 (eGFR 15–29 ml/min/1.73 m2) has been controversial. Metformin is renally cleared and the risk of metformin-associated lactic acidosis (MALA) might be increased when eGFR fell below 30 ml/min/1.73 m2 as compared with preserved kidney function.7, 8, 9, 10 In observational studies, the incidence rates of MALA varied from 3.0 to 138 per 100, 000 person-years amongst metformin users,11 though not necessarily higher than non-metformin treated controls.7,12 Discontinuation of metformin in patients with T2D was associated with increased glycated haemoglobin (HbA1c) of 0.92–1.09%.13 The loss of insulin-sensitizing effects of metformin can be pronounced in patients with advanced CKD who are generally insulin-resistant due to uremia, obesity and chronic inflammation.14 Most patients who discontinued metformin required addition or intensification of alternative GLDs such as insulin, which is associated with weight gain, high-risk of hypoglycaemia and often multiple daily injections.13,15 Loss of putative anti-inflammatory and antioxidative effects of metformin may worsen cardiovascular-renal outcomes.16
No RCTs to date have examined the strategy of continuation versus discontinuation of metformin in patients with upon reaching CKD stage G4 on end-stage kidney disease (ESKD) and CVD.8 In the absence of RCT data, we used real-world data to emulate a target trial and investigated the associations of discontinuation versus continuation of metformin therapy with the risk of all-cause mortality, major adverse cardiovascular events (MACE), all-site cancer, and ESKD among Chinese patients with T2D established on metformin therapy when eGFR reached 30 ml/min/1.73 m2. We hypothesized that metformin discontinuation was associated with higher risk of clinical outcomes in patients with T2D and advanced CKD. We compared glycemic trajectories between discontinued-metformin versus continued-metformin users, and the risk of lactic acidosis between these two groups in a separate complementary, register-based cohort.
Methods
Data sources
This is a real-world population-based cohort study of Chinese patients with T2D attending Hong Kong Hospital Authority (HA) enrolled in the Risk-Assessment-and-Management-Programme-for-Diabetes-Mellitus (RAMP-DM) from January 1, 2002 to December 31, 2019.17, 18, 19 Hong Kong has 7.5 million population mainly of Chinese descent with universal health coverage provided by the government-funded HA. The HA manages all hospitals and clinics with on-site drug dispensing, and over 90% of people with diabetes were captured in the territory-wide electronic health record (EHR) system. The HA database is a patient-level database curated from the HA-EHR system including a cohort of 581,811 patients with diabetes who underwent structured assessment through the RAMP-DM module.18 Patients in this module were referred by their doctors to undergo periodic structured assessments, such as feet, eye, and laboratory tests, in hospital-based diabetes centres or community-based clinics guided by a uniform template. Additional details on the RAMP-DM module can be found elsewhere.18
Study design
In this real-world study, we adopted an emulated target trial approach20 to compare the effect of discontinuation versus continuation of metformin on clinical outcomes in patients with T2D who reached eGFR < 30 ml/min/1.73 m2. Further details on protocol of the emulated target trial, biases consideration and statistical analysis strategies are described in Supplementary Methods. This study was reported according to the Reporting of studies Conducted using Observational Routinely-collected Data statement for pharmacoepidemiology (RECORD-PE) guidelines,21 and analysis of the anonymized HA database has been approved by the Joint-NTEC-CUHK Clinical Research Ethics Committee.
Fig. 1 shows the study design and time frame definitions.22 The index date was defined as the first measurement of eGFR reaching <30 ml/min/1.73 m2 after excluding values measured during a hospitalisation episode due to acute kidney injury (AKI), which was defined by International Classification of Diseases, 9th-Revision (ICD-9) codes of 584.6 We firstly identified patients who reached an eGFR < 30 ml/min/1.73 m2 and treated with metformin during the 6-month period before the index date. We adopted the landmark design with a landmark time at 6 months after the index date to classify discontinued- and continued-metformin users to minimizing immortal time bias during the period from the index date to the date of metformin discontinuation.23,24 We opted for a 6-month landmark time, aligning with a clinically meaningful period for individuals with diabetes in the Hong Kong EHR system because the doctors seldom wrote a prescription for a period of over 6 months. We compared the strategies of continued-metformin use within first 6 months after eGFR reaching <30 ml/min/1.73 m2 (index date) versus discontinued-metformin use, which was defined as the stopping or absence of dispensation of metformin within first 6 months after the index date. Reinitiating metformin users were defined as those who subsequently restarted metformin after initial discontinuation within 6 months of index date. A metformin user was defined as a patient who has been exposed to metformin for longer than 90 days during the observation period. The follow-up period started at 6 months after the index date and ended at the earliest date of outcomes of interest or censor date (December 31, 2019). The baseline period was defined as one-year period to index date for definition of baseline covariates. We used the most recent laboratory values closest to the index date as baseline data (Fig. 1).
Fig. 1.
Graphical depiction of study design and time frame definitions.Abbreviations: eGFR, estimated glomerular filtration rate; Rx, prescription; ESKD, end-stage kidney disease; AKI, acute kidney injury.
Outcomes
The primary outcomes were incidence of MACE, ESKD, all-site cancer, and all-cause and cause-specific mortality using the validated principal discharge diagnosis in ICD-9 and death codes (ICD-10).25 MACE was defined as the first occurrence of nonfatal myocardial infarction (ICD-9 code: 410), non-fatal stroke (ICD-9 code: 430, 431, 434 and 436), and CV-death (Supplementary Table S1). We defined ESKD as dialysis or kidney-replacement therapy (ICD-9 code) or eGFR < 15 ml/min/1.73 m2 on at least two occasions separated by ≥90 days, excluding eGFR values measured during AKI hospitalisations (ICD-9 code: 584). We calculated eGFR from plasma creatinine values using the CKD-epidemiology (CKD-EPI) Equation. We used principal discharge diagnoses to define first event of all-site cancer (ICD-9: 140:208). Causes of death was defined by ICD-10 codes in the HA database and classified as CV-cause, cancer-cause, and non-CV and non-cancer cause, as well as pneumonia-cause mortality (Supplementary Table S1).
The secondary outcomes were changes in HbA1c between discontinued-metformin and continued-metformin users at 6 months of follow-up after index date. We assessed the risk of hospitalisation due to severe hypoglycaemia (SH) defined by ICD-9 codes19 (250.8, 250.81, 250.82, 250.83, and 251.2), and use of GLD following metformin discontinuation.
Assessment of metformin and other medications
We obtained individual-level longitudinal dispensing data of medications from the HA database, including name, dose, frequency, duration (days), start and end dates. We coded metformin and other medications according to its active ingredients by the Anatomical Therapeutic Chemical (ATC) Classification System,19 which included insulin, sulfonylureas, thiazolidinediones, dipeptidyl-peptidase-4-inhibitors (DPP-4is), alpha-glucosidase-inhibitors (AGIs), glucagon-like peptide-1 receptor analogue (GLP-1 RAs), and sodium-glucose co-transporter-2 inhibitors (SGLT2is), statins, renin-angiotensin-system inhibitors (RASi), and other medications (Supplementary Table S2). Fixed-dose combination formulations were counted as two different medications based on the active ingredient. Time-varying exposure to metformin and other medications were based on start and end dates of dispensing records within each follow-up year for each patient, while prior treatment time was defined based on start and end dates of dispensing records to the index date in the EHR system. We calculated the time-weighted mean daily metformin dose by multiplying the mean daily dose by the duration of each prescription for each patient, summing these products for all patients, and then dividing by the total duration of prescriptions during the follow-up period, expressed as the formula: Time-weighted mean dose = (Σ[mean daily dose ∗ duration of each prescription])/Total duration of each prescription.7,26
Time-fixed and time-varying covariates
Baseline covariates included clinical and laboratory data collected during structured diabetes risk assessments, including sex, age, sociodemographic, history of complications, physical examination (blood pressure, body mass index [BMI], waist circumference [WC]) and laboratory values. These included eGFR, HbA1c, lipids (triglyceride [TG], low-density lipoprotein cholesterol [LDL-C], high-density lipoprotein cholesterol [HDL-C], total cholesterol [TC]), and urine albumin-creatinine-ratio (uACR). We retrieved laboratory values for HbA1c, lipids, eGFR, comorbidities, and dispensing records as time-varying covariates during the baseline and observation periods from the HA database. The comorbidities were identified through hospitalisations due to renal, CVD, cancer, and chronic obstructive pulmonary disease (COPD) as defined by ICD-9 codes and/or self-reported medical history (such as diabetic retinopathy) at the time of the assessment. We used the RAMP-DM module data to document smoking, BMI and waist circumference at baseline. We updated age, diabetes duration, laboratory values and comorbidities for the analysis.
Statistical analysis
The descriptive statistics in this study were reported as counts (percentages), mean (standard deviation, SD), or median (interquartile range, IQR). Standardized mean difference (SMD) was used to assess the balance of each covariate in the between-group comparisons.
We performed risk association analyses on clinical outcomes in discontinued-metformin versus continued-metformin users after propensity-score overlap-weighting (PS-OW) in patients who reached an eGFR < 30 ml/min/1.73 m2. The PS represented the probability of each patient receiving metformin based on their measured covariates. Overlap-weighting is a PS-based method that attempts to mimic important attributes of RCTs. Compared with the classic PS-based methods, such as matching and inverse-probability of treatment-weighting, the OW-method had better performance in terms of a clinically relevant target population, covariate balance, and precision.27 A multivariate logistic model was used to calculate the PS, and the effect size of selected covariates was used to balance all confounders at baseline for each patient using the OW-approach (Table 1 and Supplementary Table S3). We calculated eGFR slope from the structured risk assessment to eGFR reaching 30 ml/min/1.73 m2 (index date) as part of the baseline PS score.28 We calculated incidence rates of outcomes of interest expressed as 1000 person-years. The Cox-model with time-varying metformin exposure was applied to the PS-OW weighted cohort to adjust for metformin switching and/or discontinuation or reinitiation after index date and other time-varying covariates, including HbA1c, lipids, insulin, other GLDs, RASis, and statins.25 Cox-model with time-fixed exposure and covariates was applied for ESKD, all-cause and cause-specific mortality to avoid overestimating the association due to discontinued metformin at occurrence of events. Scaled-Schoenfeld-residual plots were used to check for violations of the assumption of proportional hazards. Results were expressed as hazard ratio (HR) with 95% confidence interval (CI).
Table 1.
Clinical profiles of patients categorized by metformin discontinuation when eGFR declined to <30 ml/min/1.73 m2 before and after propensity score (PS) overlap-weighting.
| Variables | Overall | Before PS overlap-weighting |
After PS overlap-weighting |
||||
|---|---|---|---|---|---|---|---|
| Continuation | Discontinuation | SMD | Continuation | Discontinuation | SMD | ||
| Number of patients, % | 33,586 | 26,086 (77.7) | 7500 (22.3) | 26,086 | 7500 | ||
| Sex, % | 0.407 | <0.001 | |||||
| Men | 13,970 (41.6) | 9691 (37.2) | 4279 (57.1) | 51.7 | 51.7 | ||
| Women | 19,616 (58.4) | 16,395 (62.8) | 3221 (42.9) | 48.3 | 48.3 | ||
| Age at index date, years | 74.3 (10.3) | 75.0 (9.9) | 71.7 (11.4) | 0.312 | 72.9 (10.6) | 72.9 (10.9) | <0.001 |
| Duration of diabetes, years | 14.7 (8.6) | 14.8 (8.6) | 14.3 (8.4) | 0.059 | 14.5 (8.7) | 14.5 (8.4) | <0.001 |
| Family history of diabetes, % | 9641 (28.7) | 7262 (27.8) | 2379 (31.7) | 0.085 | 30.2 | 30.2 | <0.001 |
| Use of tobacco, % | 0.247 | ||||||
| Never | 25,000 (70.3) | 20,048 (76.8) | 4952 (66.0) | 69.1 | 69.1 | ||
| Ever | 5579 (16.6) | 4009 (15.4) | 1570 (20.9) | 19.6 | 19.6 | ||
| Current | 3007 (9.0) | 2029 (7.8) | 978 (13.0) | 11.3 | 11.3 | ||
| Body mass index, kg/m2 | 25.8 (4.2) | 25.9 (4.3) | 25.6 (4.0) | 0.064 | 25.7 (4.2) | 25.7 (4.0) | <0.001 |
| Waist circumference, cm | 90.4 (10.4) | 90.4 (10.4) | 90.1 (10.3) | 0.031 | 90.2 (10.4) | 90.2 (10.2) | <0.001 |
| Systolic BP, mmHg | 141.9 (15.5) | 141.6 (15.3) | 142.8 (16.0) | 0.075 | 142.4 (15.7) | 142.4 (15.8) | <0.001 |
| Diastolic BP, mmHg | 72.5 (9.3) | 72.1 (9.1) | 73.7 (9.7) | 0.161 | 73.1 (9.4) | 73.1 (9.5) | <0.001 |
| HDL-C, mmol/L | 1.2 (0.4) | 1.2 (0.4) | 1.2 (0.4) | 0.062 | 1.2 (0.4) | 1.2 (0.4) | <0.001 |
| LDL-C, mmol/L | 2.4 (0.9) | 2.4 (0.9) | 2.5 (1.0) | 0.134 | 2.5 (1.0) | 2.5 (1.0) | <0.001 |
| Total cholesterol, mmol/L | 4.4 (1.1) | 4.4 (1.1) | 4.5 (1.3) | 0.110 | 4.4 (1.2) | 4.4 (1.2) | <0.001 |
| Triglyceride, mmol/L | 1.8 (1.3) | 1.8 (1.2) | 1.8 (1.4) | 0.045 | 1.8 (1.3) | 1.8 (1.3) | <0.001 |
| Blood haemoglobin, g/dL | 11.3 (1.8) | 11.3 (1.8) | 11.3 (1.8) | 0.051 | 11.3 (1.9) | 11.3 (1.8) | <0.001 |
| HbA1c, % | 7.4 (1.5) | 7.4 (1.5) | 7.6 (1.7) | 0.102 | 7.5 (1.5) | 7.5 (1.7) | <0.001 |
| eGFR, ml/min/1.73 m2 | 26.9 (3.0) | 27.0 (3.0) | 26.6 (3.1) | 0.102 | 27.0 (3.1) | 27.0 (3.0) | <0.001 |
| eGFR slope | −3.9 (2.7) | −3.7 (2.5) | −4.6 (3.2) | 0.332 | −4.3 (2.9) | −4.3 (2.8) | <0.001 |
| Medications | |||||||
| Glucose-lowering drugs (GLDs) | |||||||
| Insulin | 8045 (24.0) | 5632 (21.6) | 2413 (32.2) | 0.240 | 28.7 | 28.7 | <0.001 |
| Sulfonylureas | 22,703 (67.6) | 17,501 (67.1) | 5202 (69.4) | 0.049 | 68.3 | 68.3 | <0.001 |
| AGIs | 616 (1.8) | 427 (1.6) | 189 (2.5) | 0.062 | 2.2 | 2.2 | <0.001 |
| TZD | 774 (2.3) | 587 (2.3) | 187 (2.5) | 0.016 | 2.5 | 2.5 | <0.001 |
| DPP-4i | 3605 (10.7) | 2547 (9.8) | 1058 (14.1) | 0.134 | 12.7 | 12.7 | <0.001 |
| GLP-1RA | 26 (0.1) | 15 (0.1) | 11 (0.1) | 0.028 | 0.1 | 0.1 | <0.001 |
| SGLT2i | 103 (0.3) | 74 (0.3) | 29 (0.4) | 0.018 | 0.3 | 0.3 | <0.001 |
| Number of GLDs | 0.257 | <0.001 | |||||
| 0 | 6520 (19.4) | 5364 (20.6) | 1156 (15.4) | 17.1 | 17.1 | ||
| 1 | 19,630 (58.4) | 15,553 (59.6) | 4077 (54.4) | 56.1 | 56.1 | ||
| 2 | 6211 (18.5) | 4371 (16.8) | 1840 (24.5) | 22.2 | 22.2 | ||
| 3 | 1225 (3.6) | 798 (3.0) | 427 (5.7) | 0.5 | 0.5 | ||
| RASi | 26,508 (78.9) | 20,427 (78.3) | 6081 (81.1) | 0.069 | 80.2 | 80.2 | <0.001 |
| Statins | 19,326 (57.5) | 14,829 (56.8) | 4497 (60.0) | 0.063 | 58.9 | 58.9 | <0.001 |
| Non-statins lipid-modifying | 2051 (6.1) | 1533 (5.9) | 518 (6.9) | 0.042 | 6.6 | 6.6 | <0.001 |
| Alpha-blockers | 2092 (6.2) | 1490 (5.7) | 602 (8.0) | 0.092 | 7.5 | 7.5 | <0.001 |
| Medication treatment time, years | |||||||
| Insulin | 1.2 (3.0) | 1.2 (3.0) | 1.4 (3.2) | 0.074 | 1.4 (3.1) | 1.4 (3.2) | <0.001 |
| Metformin | 7.6 (4.3) | 7.8 (4.3) | 7.2 (4.4) | 0.140 | 7.8 (4.1) | 7.8 (3.8) | <0.001 |
| Sulfonylureas | 6.0 (4.7) | 6.1 (4.7) | 5.6 (4.6) | 0.113 | 5.7 (4.6) | 5.7 (4.7) | <0.001 |
| AGIs | 0.2 (0.9) | 0.2 (0.8) | 0.2 (0.9) | 0.037 | 0.2 (0.9) | 0.2 (0.9) | <0.001 |
| TZD | 0.1 (0.5) | 0.1 (0.5) | 0.1 (0.5) | 0.011 | 0.1 (0.5) | 0.1 (0.5) | <0.001 |
| DPP-4i | 0.2 (0.9) | 0.2 (0.9) | 0.3 (0.9) | 0.070 | 0.3 (0.9) | 0.3 (0.9) | <0.001 |
| GLP-1RA | 0.0 (0.1) | 0.0 (0.0) | 0.0 (0.1) | 0.029 | 0.0 (0.1) | 0.0 (0.1) | <0.001 |
| SGLT2i | 0.0 (0.1) | 0.0 (0.1) | 0.0 (0.1) | 0.024 | 0.0 (0.1) | 0.0 (0.1) | <0.001 |
| RASi | 5.2 (4.4) | 5.3 (4.4) | 5.2 (4.3) | 0.024 | 5.2 (4.3) | 5.2 (4.4) | <0.001 |
| Other antihypertensives | 1.5 (2.9) | 1.5 (3.0) | 1.4 (2.8) | 0.020 | 1.4 (2.9) | 1.4 (2.9) | <0.001 |
| Statins | 3.0 (3.7) | 3.0 (3.7) | 2.9 (3.7) | 0.016 | 3.0 (3.7) | 3.0 (3.7) | <0.001 |
| Alpha-blockers | 0.2 (1.1) | 0.2 (1.1) | 0.3 (1.2) | 0.051 | 0.3 (1.2) | 0.3 (1.2) | <0.001 |
| Complications history | |||||||
| CVD | 12,204 (36.3) | 9385 (36.0) | 2819 (37.6) | 0.033 | 37.4 | 37.4 | <0.001 |
| Stroke | 5624 (16.7) | 4336 (16.6) | 1288 (17.2) | 0.015 | 17.2 | 17.2 | <0.001 |
| CHD | 7372 (21.9) | 5695 (21.8) | 1677 (22.4) | 0.013 | 22.4 | 22.4 | <0.001 |
| AMI | 2335 (7.0) | 1755 (6.7) | 580 (7.7) | 0.039 | 7.5 | 7.5 | <0.001 |
| Heart failure | 4722 (14.1) | 3592 (13.8) | 1130 (15.1) | 0.037 | 14.8 | 14.8 | <0.001 |
| COPD | 1515 (4.5) | 1219 (4.7) | 296 (3.9) | 0.036 | 4.2 | 4.2 | <0.001 |
| Diabetics retinopathy | 4444 (13.2) | 3355 (12.9) | 1089 (14.5) | 0.048 | 13.9 | 13.9 | <0.001 |
| Period of index year | 0.076 | <0.001 | |||||
| 2002–2006 | 2558 (7.6) | 1931 (7.4) | 627 (8.4) | 8.1 | 8.1 | ||
| 2007–2010 | 5398 (16.1) | 4103 (15.7) | 1295 (17.3) | 16.8 | 16.8 | ||
| 2011–2014 | 11,097 (33.0) | 8801 (33.7) | 2296 (30.6) | 31.2 | 31.2 | ||
| 2015–2018 | 14,533 (43.3) | 11,251 (43.1) | 3282 (43.8) | 43.9 | 43.9 | ||
SMD, standardized mean difference; RASi, renin angiotensin system inhibitors; BP, blood pressure; HDL-C, high-density lipoprotein cholesterol; LDL-C, low-density lipoprotein cholesterol; eGFR, estimated glomerular filtration rate; AGIs: alpha-glucosidase inhibitors; TZDs: thiazolidinediones; DPP-4i: dipeptidyl peptidase 4 inhibitors; GLP1-RA: glucagon-like peptide-1 receptor analogues; SGLT2i: sodium-glucose co-transporter 2 inhibitors; GLDs: glucose-lowering drugs; CVD, cardiovascular disease; IHD, Ischemic heart disease; AMI, acute myocardial infarction.
Missing data for time-varying covariates in each Cox-model was imputed using the chained-equations with multiple imputations by R ‘smcfcs’ package with inclusion of all covariates, outcome of interest, and exposure. The data were tested for non-missing completely at random (MCAR) using ‘MissMech’ package. The default number of imputed datasets (m = 5) was used to generate imputations. This imputation method performs well for different types of missing patterns including non-MCAR.29
Subgroup and sensitivity analyses
We performed subgroup analyses on separate PS-OW cohorts based on sex and pre-existing CVD, comparing the risk associations of metformin discontinuation with outcomes of interest. We limited our analysis to those with uACR data at baseline (n = 25,694) and adjusted for uACR in evaluating the risk associations, as well as conducted subgroups analysis by uACR categories (<3, 3–30, and >30 mmol/L). We also repeated the analyses by including those who restarted metformin after 6 months of index date (n = 1602), as well as limited to those with a second eGFR < 30 ml/min/1.73 m2 within 6 months of index date. Additionally, we applied multiple PS-based approaches including 1:1 PS matching (PSM), inverse probability of treatment weights (IPTW), and Cox marginal-structural-model (Cox-MSM) model to address confounding by indication, time-varying confounders related to treatment initiation or discontinuation, and competing risk of death for clinical events of interest in this study.30
Metformin discontinuation and HbA1c response
The HbA1c and use of GLDs (percentage use of insulin, sulfonylurea, DPP-4is and other agents) upon reaching eGFR < 30 ml/min/1.73 m2 were examined. We compared the absolute changes and percentage changes in HbA1c from the index date to 6-month follow-up (nearest value within 3rd-9th month window) between discontinued-metformin and continued-metformin users. We balanced the potential confounders in the PS-OW cohort and compared the weighted mean HbA1c change at 6-month of follow-up between discontinued- and continued-metformin users.
Incidence of lactic acidosis in the register-based cohort
We conducted a complementary analysis to determine the occurrence of lactic acidosis in a separate register-based cohort - the Hong Kong Diabetes Register (HKDR) since data on serum lactate and blood pH and detailed medical notes were not available in the RAMP-DM module. The HKDR17 is an ongoing research-driven quality improvement program with a structured-protocol for periodic comprehensive assessment of risk factors and complications with capture of outcome data retrieved from the EHR system. Personal data and clinical measurements were documented during structured assessments upon enrolment to the HKDR, with patients’ informed consent. Laboratory assessments such as serum lactate, pH, lipids, HbA1c and eGFR, and use of medications were curated from the EHR system. We retrieved data from 30,503 patients with diabetes enrolled in 2001–2020 observed until 30 June 2021. Using the same inclusion and exclusion criteria as the main analysis, we identified 3235 metformin-users in whom eGFR decreased to <30 ml/min/1.73 m2 with 2413 (74.6%) metformin users and 822 (25.4%) non-metformin users during the follow-up period (Supplementary Fig. S1).
We defined lactic acidosis events by using laboratory values (serum lactate > 5.0 mmol/L with a concomitant blood pH < 7.35) and ICD-9 codes of 276.2 during the follow-up period. We reviewed all medical records of patients who experienced lactic acidosis that met the definition. Lactic acidosis events that occurred more than one month apart were considered separate events.
Negative control design for detecting confounding and bias
To address potential biases and uncontrolled confounding, we applied a negative control design and balanced the groups of discontinued-metformin and continued-metformin.31 We used principal discharge diagnosis ICD-9 codes to define the occurrence of upper gastrointestinal bleeding (UGIB) (Supplementary Table S1) as a negative control outcome, expecting null association between discontinuing metformin and the risk of UGIB. Amongst 33,586 patients in the main analysis, we excluded 1934 patients with history of UGIB and repeated analysis in the OW-PS weighted cohort of 31,652 metformin users (7136 discontinued and 24,516 continued) who reached a baseline eGFR < 30 ml/min/1.73 m2.
All analyses were implemented using R software (Version 4.0.0). We used PSweight and survey packages to fit the PS-OW weighted models and Cox-models. A two-sided P value of <0.05 was considered statistically significant.
Role of the funding source
The funder of the study had no role in study design, data collection, data analysis, data interpretation, or writing of this report. AY and EL have accessed and verified the data. EC is the guarantor of this work with final responsibility for the decision to submit for publication.
Results
Study patients
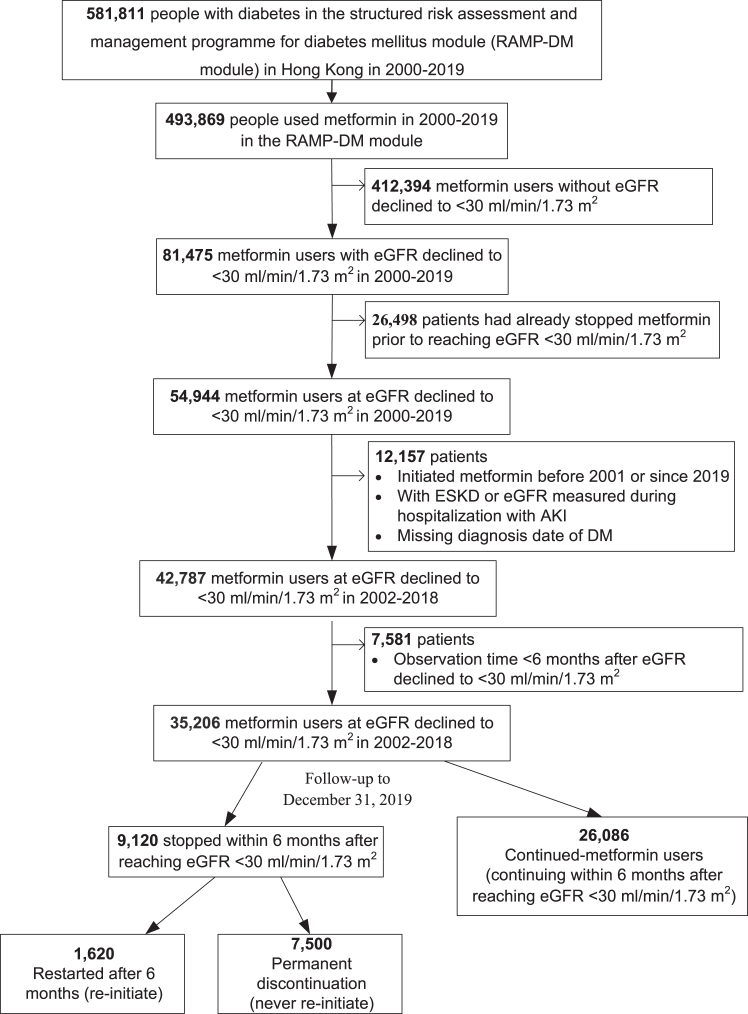
In our main analysis, we identified 493,869 patients who were initiated on metformin from January 1, 2000 to December 31, 2019 in our population-based cohort (Fig. 2). We excluded patients with eGFR ≥ 30 ml/min/1.73 m2 (n = 412,394) throughout the observation period and those who had already stopped metformin prior index date (n = 26,498). We also excluded patients (n = 12,157) who met the following criteria: enrolled in 2000–2001 to avoid bias from incomplete case records in the first two years of establishment of the EHR system,19,32 had observational time <6 months from the index date, and had their eGFR measured during a hospitalisation episode due to AKI, or missing diagnosis date of diabetes. This yielded 35,206 metformin users with baseline data when eGFR declined <30 ml/min/1.73 m2 from January 1, 2002 to December 31, 2018, including 9120 discontinued users and 26,086 continued users. In the main analysis (n = 33,586, 41.6% men and 58.4% women), we compared the effects of permanently discontinued-metformin during the follow-up period (n = 7500) versus continued-metformin (n = 26,086) (Fig. 2).
Fig. 2.
Flowchart of selection patients.Abbreviations: eGFR, estimated glomerular filtration rate; ESKD, end-stage kidney disease; AKI, acute kidney injury.
The overall median follow-up time was 3.8 (IQR: 2.2–6.1) years (149,149 person-years). The median of follow-up to death was 3.4 (IQR: 1.9–5.6) years for discontinued-metformin users and 3.9 (IQR: 2.3–6.3) years for continued-metformin users. The median metformin mean daily dose at baseline was 1000 [IQR: 1000, 1000] mg in metformin users. The mean age at baseline was 74.3 ± 10.3 years, with a mean diabetes duration of 14.7 ± 8.6 years. At baseline, discontinued-metformin users were more likely to have diabetic retinopathy, and treated with insulin, DPP-4is, RASi and statins than continued-metformin users. In the PS-OW weighted cohort, all characteristics, including HbA1c, eGFR, and history of complications, were well-balanced between the two groups at index date (Table 1).
Among the 7500 discontinued metformin users in our main analysis, 36.2% of patients had hospitalisation records within 6 months of the index date. For the primary cause of hospitalisations, 5.3% were due to CVD, 4.9% were due to heart failure, 3.4% for coronary heart disease (CHD), and 2.2% for pneumonia.
Metformin use, incidence of clinical outcomes and mortality
During the follow-up period, 16.4% (5505/33,586), 30.1% (10,113/33,586), and 7.1% (2171/30,682) had incident MACE, ESKD, and cancer respectively, and 44.4% (14,917/33,586) died. The respective crude incidence (per-1000-person-years) in discontinued-metformin and continued-metformin users were 45.7-and-38.4 events for MACE, 149.8-and-67.7 events for ESKD, 15.5-and-16.0 events for cancer, and 120.5-and-94.7 events for all-cause mortality. Compared to continued-metformin, discontinued-metformin was associated with higher risk of MACE (weighted and adjusted HR = 1.40, 95% CI: 1.29–1.52), ESKD (HR = 1.52, 95% CI: 1.52–1.62), and all-cause mortality (HR = 1.22, 95% CI: 1.18–1.27) (Fig. 3, Fig. 4). No association was observed between metformin discontinuation and risk of cancer (HR = 0.93, 95% CI: 0.85–1.01). A higher risk of CV-specific mortality was observed in discontinued-metformin users (HR = 1.23, 95% CI: 1.13–1.34). A higher risk of non-CV and non-cancer (HR = 1.16, 95% CI: 1.07–1.26), and pneumonia (HR = 1.06, 95% CI: 1.00–1.13) cause-specific mortalities were observed in discontinued-metformin users. Cancer cause-specific mortality was similar between the two groups (HR = 1.09, 95% CI: 0.97–1.23, Fig. 3).
Fig. 3.
Weighted and adjusted hazard ratio (HRs) for the association between discontinuation of metformin and risk of clinical events among patients with type 2 diabetes and advanced chronic kidney disease (eGFR reached 30 ml/min/1.73 m2). Weighted and adjusted HRs and 95% CIs of MACE, ESKD, and cancer were yielded using Cox model with time-varying metformin use, adjusting for time-varying HbA1c, lipids, age, medications, and comorbidities in the overlap-weighted cohort. To evaluate the association between metformin discontinuation and risk of cancer, we excluded prevalent cancer cases and separately adopted the overlap-weighted propensity score matching in the incident cancer cohort. Results of all-cause and cause-specific mortality were yielded using time-fixed Cox model in the overlap-weighed cohort. Abbreviations: MACE, major adverse cardiovascular events; ESKD, end-stage kidney disease; eGFR, estimated glomerular filtration rate.
Fig. 4.
Weighted cumulative incidence Kaplan–Meier curves for clinical events among patients with type 2 diabetes and advanced chronic kidney disease (eGFR reached 30 ml/min/1.73 m2). Cumulative incidence curves were estimated in the overlap-weighted cohort. Abbreviations: MACE, major adverse cardiovascular events; ESKD, end-stage kidney disease.
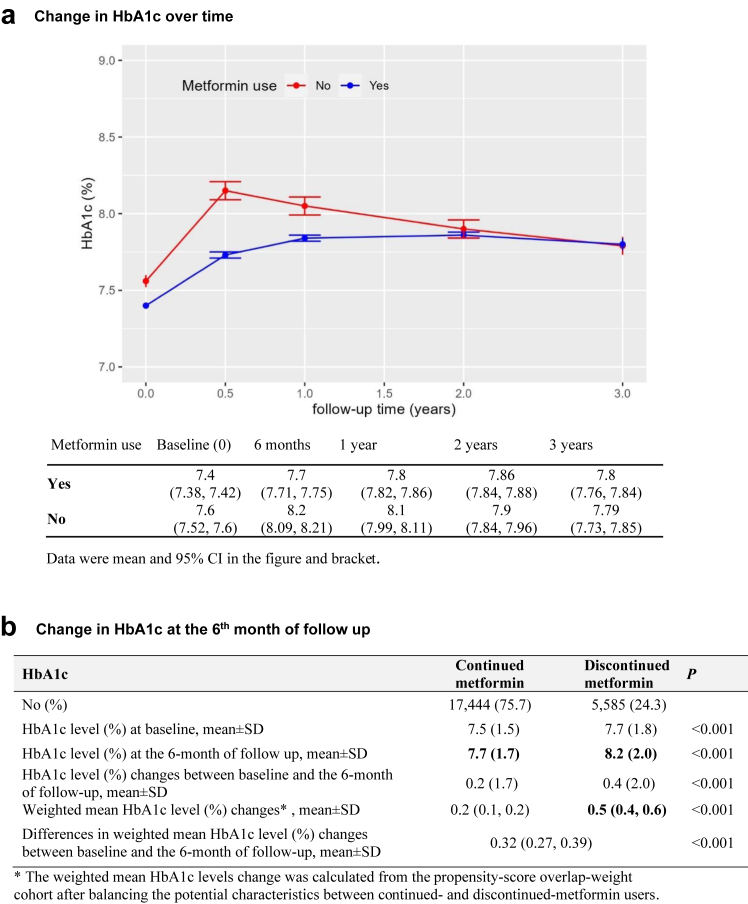
Glycemic control, SH, and GLD use following metformin discontinuation
We included 23,029 patients with HbA1c data at the 6-month of index date (Supplementary Table S4). HbA1c rose in the first 6 months following eGFR < 30 ml/min/1.73 m2 with larger increases in the discontinued-metformin group (Fig. 5A). The respective mean ± SD HbA1c levels at baseline and at the 6th-month of follow-up was 7.7 ± 1.7% versus 8.2 ± 2.0% in 5585 discontinued-metformin users and 7.4 ± 1.5% versus 7.8 ± 1.7% in 17,444 continued-metformin users. After balancing baseline characteristics between the two groups (Supplementary Table S4), discontinued-metformin users had larger increase in HbA1c at the 6th-month of follow-up in the PS-OW cohort (weighted mean HbA1c value change: 0.5% [0.4–0.6%] versus 0.2% [0.1–0.2%]) while long-term trends in HbA1c did not differ (Fig. 5B). eGFR increased in continued-metformin users within first two years after eGFR reaching < 30 ml/min/1.73 m2, while it decreased in the discontinued-metformin users (Supplementary Table S5).
Fig. 5.
Changes in HbA1c and its 95% confidence interval (CI) over time (a) and the 6-month of follow-up by metformin continuations after reaching eGFR reached 30 ml/min/1.73 m2.
The proportion of use of insulin and DPP-4is increased for discontinued-metformin versus continued-metformin users (32.2%-to-46.3% versus 21.6%-to-29.7% for insulin; 14.1%-to-20.6% versus 9.8%-to-15.0% for DPP-4is). The proportion of sulfonylureas use at the 6-month was 52.9% for discontinued-metformin users and 57.8% for continued-metformin users. During the follow-up period, the respective crude incidence of first hospitalisation due to SH was 22.7 and 20.8 events per-1000-patient-years in discontinued- and continued-metformin users. After balancing all characteristics of the two groups in the PS-OW weighted cohort (Supplementary Table S4), a high risk of SH was observed in discontinued-metformin users (HR = 1.12, 95% CI: 1.09–1.16).
Subgroup and sensitivity analyses
The higher risk of main outcomes in discontinued-metformin users were observed in both men and women (Supplementary Fig. S2). Risk associations of metformin discontinuation with MACE, ESKD, and all-cause mortality were consistent in patients with and without pre-existing CVD (Supplementary Fig. S3).
In the sub-cohort of 25,694 patients with baseline uACR data, the respective proportion of patients with uACR < 3, 3–30, and >30 mg/mmol in discontinued-metformin and continued-metformin users was 18.0%-versus-27.9%, 33.0%-versus-39.6%, and 49.0%-versus-32.5% (Supplementary Table S6). We similarly observed a higher risk of MACE (HR = 1.33, 95% CI: 1.15–1.53), ESKD (HR = 1.41, 95% CI: 1.25–1.55), and all-cause mortality (HR = 1.23, 95% CI: 1.17–1.29) in discontinued-metformin (n = 5586) versus continued-metformin users (n = 20,108) in the overall PS-OW weighted cohort, and each uACR category (Supplementary Table S7). Similar associations were observed when included those who restarted metformin after discontinuation (Supplementary Table S8). In a series of sensitivity analyses, similar associations were observed when we limited our analyses to in those with 2 or more records of eGFR < 30 ml/min/1.73 m2 within the first 6 months of index date (n = 22,127) (Supplementary Table S8). Discontinued-metformin users had higher risks of death, ESKD and MACE in those with both rapid and slow eGFR decline pre-index date, stratified by the median eGFR decline of 3.4 ml/min/1.73 m2 per year (Supplementary Table S8). We observed similar associations when fitted by the 1:1 PSM (n = 7500 pairs), IPTW, and Cox-MSM models (Supplementary Table S9).
Negative outcome control analysis
In our negative control analysis, the crude incidence of UGIB in 7136 discontinued-metformin and 24,516 continued-metformin users were 7.7 and 8.2 events per-1000-person-years. After balancing all characteristics of the two groups in the PS-OW weighted cohort (Supplementary Table S10), null association was observed between discontinued-metformin versus continued-metformin with risk of UGIB (HR = 0.98, 95% CI: 0.82–1.18) (Supplementary Table S11).
Metformin discontinuation and risk of lactic acidosis in register-based cohort
In the register-based cohort, we included 3235 patients with new-onset eGFR < 30 ml/min/1.73 m2 (2413 metformin users and 822 non-metformin users). The characteristics of the two groups are shown in Supplementary Table S12. The cumulative incidence of lactic acidosis was 2.8% (91/3235) during a median follow-up of 4.3 (IQR: 2.3–7.2) years to death (16,425 person-years) (3.5% [29/822] for discontinued-metformin users, and 2.6% [62/2413] for continued-metformin users). The overall crude-incidence of first lactic acidosis event was 4.7 events (4.7 events for continued-metformin users and 4.4 events for discontinued-metformin users) per 1000 patient-years. After balancing all characteristics of the two groups in the PS-OW weighted cohort (Supplementary Table S12), null association was observed between continued-metformin use and risk of lactic acidosis (weighted HR = 0.94, 95% CI: 0.53–1.64). The crude incidence rates of deaths were 84.6 events per 1000 person-years in the overall cohort (79.0 events in continued-metformin users, and 102.9 in discontinued-metformin users). We observed the consistent higher risk of all-cause mortality (weighed HR = 1.33, 95% CI: 1.17–1.50) in discontinued-metformin versus continued-metformin users. We reviewed all clinical notes of 62 lactic acidosis events amongst 2413 continued-metformin users to evaluate causality. There were 24 events that occurred 6 months after new-onset eGFR < 30 ml/min/1.73 m2. There was evidence of cardiogenic shock in 50%, sepsis in 21%, post-cardiac resuscitation in 4%, pancreatitis in 4%, and haemorrhagic shock in 4% of patients. In the remaining cases (n = 2, 8.3%), no other causes of lactic acidosis, other than use of metformin, were identified.
Discussion
In this cohort analysis of 33,586 patients with T2D and advanced CKD, we asked an important question whether metformin should be continued in patients with T2D after reaching eGFR < 30 ml/min/1.73 m2. To our knowledge, this is the largest population-based cohort analysis showing that continued-metformin was associated with 30–60% lower risk of MACE, ESKD, and death versus discontinued-metformin in patients with T2D and advanced CKD. Similarly, the higher risk associations in discontinued-metformin versus continued-metformin were observed in both men and women, and regardless with pre-existing CVD status. After reaching eGFR < 30 ml/min/1.73 m2, HbA1c levels increased in the first 6 months, particularly among discontinued-metformin users. The use of insulin and DPP-4is increased rapidly within the first 6 months of follow-up, particularly in discontinued-metformin users. In a series of sensitivity analyses, our findings remained robust, even after adjusting for albuminuria, CVD at baseline. Continued-metformin users had a lower incidence of lactic acidosis in a separate register-based cohort.
Most observational data support the safety and potential benefits of metformin use in patients with an eGFR > 45 ml/min/1.73 m2, while little data are available in those with eGFR < 30 ml/min/1.73 m2.33 Charytan et al. conducted a secondary analysis of RCT data and observed a lower hazard of CV-mortality among metformin users versus non-metformin users (HR = 0.49, 9% CI: 0.36–0.69), with no statistically significant interaction with CKD stages.34 In a post-hoc meta-analysis, metformin was associated with lower mortality in those with normal eGFR (HR = 0.49 [95% CI: 0.32–0.75] in eGFR ≥ 90 ml/min/1.73 m2) while HRs close to the null were observed in the lower eGFR groups (HR = 0.95 [95% CI: 0.57–1.58] in eGFR < 30 ml/min/1.73 m2).33 In one study from Taiwan which included over 3000 patients with T2D and CKD stage G5, metformin was associated with higher risk of mortality (HR = 1.35, 95% CI: 1.20–1.51). Another retrospective study of 21,966 patients with T2D, however, found metformin was associated with reduced risk of death compared with sulphonylureas at eGFR < 30 ml/min/1.73 m2. However, in previous retrospective studies the non-metformin group had a higher proportion of comorbidities that could confound risk associations between metformin and outcomes.33 In our study, we used a OW-PS weighting of 49 variables to balance baseline characteristics to control for confounding by indication. We observed that discontinuation of metformin was associated with a 1.3-fold higher risk of all-cause mortality in advanced-CKD. Furthermore, null associations were observed with our negative outcome control (gastrointestinal bleeding) making our findings, supported by biological plausibility regarding the pleiotropic protective effects of metformin, unlikely to be due to chance.16
Similarly, we found discontinued-metformin use was associated with a higher risk of MACE versus continued-metformin use, irrespective of sex and pre-existing CVD status. We and others previously observed that metformin use was associated with reduced risk of MACE at eGFR ≥ 45 ml/min/1.73 m2.26,33 In a Canadian retrospective cohort of 21,996 patients with T2D and CKD, metformin use was associated with lower risk of MACE composite outcomes compared with sulfonylureas (HR = 0.67, 95% CI: 0.52–0.83).35
In this study, continuation of metformin was associated with 50% risk reduction of ESKD in patients with eGFR < 30 ml/min/1.73 m2. Our previous register-based study, including 3051 patients with T2D and eGFR of 30–44 ml/min/1.73 m2, also observed reduced risk of ESKD (HR = 0.69 [0.49–0.98]).7 Two previous studies reported 30–40% risk reduction of ESKD amongst metformin users at CKD stages G1–G2, and no increased risk at stages G4–G5. Kwon and colleagues found no significant difference in ESKD progression in a propensity-matched retrospective cohort of non-metformin (n = 306) and metformin (n = 204) users with baseline eGFR < 30 ml/min/1.73 m2 (adjusted HR = 0.8 [0.67–1.12]).10 Charlytan et al. also observed no significant differences in progression ESKD in propensity score-matched cohort of metformin and non-metformin users with CKD stage G3 (n = 4038). These differences may be explained by smaller sample size and fewer accrued events over a shorter follow-up period.
The lack of association between discontinued-metformin and risk of cancer contrasts with previous real-world studies (RWS) that have reported lower cancer incidence with metformin use. However, these studies were often affected by bias such as immortal time bias, which are not fully controlled for. Conversely, RWS that avoided these biases did not find a significant association between metformin use and risk of cancer. In our study, we addressed these biases by excluding prevalent cancer cases, performing multiple time-related approaches, and yielding robust results. While there are few data available on the association between discontinuation of metformin and risk of cancer in advanced CKD, our findings are consistent with a recent largest phase 3 RCT of metformin as adjuvant therapy for breast cancer which enrolled 3649 women with a 5-year follow-up, and found no benefit for disease-free survival or overall survival with metformin.36
In Hong Kong, non-CV and non-cancer deaths, especially pneumonia, are the leading cause of death in patients with T2D, exceeding CV-deaths.37,38 In our study, discontinued-metformin use was also associated with increased risk of non-CV and non-cancer death by 1.2-fold. Metformin may protect against pneumonia via multiple mechanisms such as reduction in pro-inflammatory cytokines and decreasing neutrophil extracellular traps (NETs).16 In a prospective study of 15,784 patients with T2D in Hong Kong, we also found metformin was independently associated with a 40% lower of pneumonia-related hospitalisation and 50% lower hazard of pneumonia-related mortality after adjusting for multiple confounders.26 Our current results suggest benefits of metformin that extend beyond cardio-renal protection to infective complications.
After reaching eGFR < 30 ml/min/1.73 m2, HbA1c levels rapidly increased in the first 6 months of follow-up in the overall cohort, particularly among discontinued-metformin users. Discontinued-metformin users were more likely to be prescribed with insulin at the first 6 months of follow-up and use of DPP-4is also increased. Additionally, discontinuing metformin was associated with increased risk of SH in our cohort. Our observations are in agreement with other retrospective studies which also reported discontinuation of metformin and addition of other GLDs were associated with adverse effects including weight gain and increased risk of hypoglycemic episodes. In the descriptive analysis, we observed an increase in eGFR among continued-metformin users and a decrease among discontinued-metformin users within the first two years after eGFR reaching <30 ml/min/1.73 m2. These observations do not imply causal associations and caution against hypothesizing a role for metformin in improving kidney function. While it is possible that patients with improved eGFR were more likely to continue metformin, and those with declining eGFR permanently discontinued metformin, we accounted for eGFR slope in the main analysis. Additionally, we conducted subgroup analyses based on eGFR slope in the follow-up period. These analyses showed consistently demonstrated robust associations between metformin discontinuation and risk of clinical outcomes, suggesting that this factor is unlikely to significantly impact the primary findings of our study.
Prior studies found no evidence of an increased risk of lactic acidosis with metformin use at eGFR > 30 ml/min/1.73 m2. However, at eGFR < 30 ml/min/1.73 m2, the evidence is less consistent. The reported rates of MALA at CKD stages G3–G4 (eGFR: 15–59 ml/min/1.73 m2) were 3–10 events per 10,000 patient-years.9 The wide range of estimates may be due to differing patient populations and MALA. In practice, defining a MALA event requires exclusion of other contributory causes and elevated plasma metformin levels, which are not always routinely measured. In our study, we did not observe a statistically significant difference in the risk of lactic acidosis events between continued-metformin and discontinued-metformin users. From our analysis, it can be seen that the majority of lactic acidosis cases had other contributory causes such as myocardial infarction, sepsis and only in two cases, no other cause other than metformin use was identified. These two cases also survived. A retrospective large retrospective case series, although MALA was associated with a high mortality, individuals with MALA had better outcomes than those with lactic acidosis of other origin admitted to critical care, despite higher serum lactate levels and lower pH. A pharmacokinetic study conducted on patients with diabetes and CKD stage G4, which showed that low-dose metformin treatment (<1000 mg/day) did not increase risk of lactic acidosis or other adverse safety outcomes. These results support supporting the notion of making metformin more widely available with increased monitoring and personalized dosing, as well as educating patients on sick-day rules for withholding metformin. Carefully conduced prospective research at a large scale would probably be necessary to justify metformin’s routine use in advanced CKD.7
Our study had both strengths and limitations. The strengths of this study included a large cohort of patients with T2D and advanced CKD established in a real-world setting with documentation of clinical outcome. We conducted detailed analyses and used multiple time-related statistical approaches to address indication bias with adjustment for time-varying metformin exposure and other key covariates. We applied negative control design and identified uncontrolled confounding as well as other sources of bias and potential analytic flaws. There are several limitations. Due to suboptimal adherence to the assessment protocol in the RAMP-DM module, uACR data could only be included in a sensitivity analysis. We were unable to assess metformin adherence as medication exposure was based on dispensing data. There were too few patients on SGLT2i to evaluate their potential synergistic effects with metformin on glomerular filtration. We reviewed all clinical notes of lactic acidosis events amongst metformin users for causality, however plasma metformin levels were not available. The exact reasons for discontinuation were not available, however, we can infer that the majority (64%) patients had metformin discontinued in a chronic setting upon reaching eGFR < 30 ml/min/1.73 m2, not proximate to a hospitalisation or AKI episode. Our analysis was conducted in Chinese patients with T2D and advanced CKD and further studies in other ethnicities are needed to determine generalizability of our findings. While we included key comorbidities including major cardiovascular, respiratory conditions and diabetic retinopathy, and used multiple time-related methods to minimize time-related bias and other common and significant biases, we acknowledge the challenge of entirely eliminating immortal time bias in RWS. Furthermore, we cannot exclude the possibility of residual confounding for unmeasured factors such as frailty that differed in continued and discontinued users.
In this real-world study, discontinuation of metformin below eGFR of 30 ml/min/1.73 m2 was associated with increased risk of cardiovascular-renal events regardless CVD status. Continuation of metformin may be associated with clinical and mortality benefits that need to be weighed against the risks of lactic acidosis.
Contributors
AY and EC contributed to conception of the article, statistical analysis, interpretation of results, drafting, revision and approval of the manuscript. HW, ESHL, MS, BF, and TC contributed to interpretation of results, revised the manuscript critically and approved the final version. APSK, RCWM, AOYL, and JCNC contributed to conception of the article, revised the manuscript critically and approved the final version. AY and EL have accessed and verified the data. EC is the guarantor of this work and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Data sharing statement
The dataset used in this study is unavailable due to reasons of patient confidentiality and restrictions from the Hong Kong Hospital Authority.
Declaration of interests
JCNC has received research grants and/or honoraria for consultancy or giving lectures, from AstraZeneca, Bayer, Boehringer Ingelheim, Eli-Lilly, Hua Medicine, Lee Powder, Merck Serono, Merck Sharp & Dohme, Pfizer, Servier, Sanofi and Viatris Pharmaceutical. APSK has received research grants and/or speaker honoraria from Abbott, Astra Zeneca, Bayer, Boehringer Ingelheim, Eli-Lilly, Kyowa Kirin, Merck Serono, Nestle, Novo Nordisk, Pfizer and Sanofi. AOYL has served as a member of advisory panel for Amgen, AstraZeneca, Boehringer Ingelheim and Sanofi and received research support from Amgen, Asia Diabetes Foundation, Bayer, Boehringer Ingelheim, Lee’s Pharmaceutical, MSD, Novo Nordisk, Roche, Sanofi, Sugardown Ltd, Takeda. None of these relationships had any influence on the content of the present manuscript. RCWM has received research funding from Bayer, Novo Nordisk, and Tricida Inc. for carrying out clinical trials, and has received speaker honorarium or consultancy in advisory. EC has received honoraria for giving lectures from Astra Zeneca, Boehringer Ingelheim, Abbott, P&G and received research support from Hua Medicine, Medtronic, Merck KGaA, Lee Powder. All proceeds have been donated to the Chinese University of Hong Kong to support diabetes research. Other authors have no conflicts of interest to disclose.
Acknowledgements
AY is supported by a CUHK Impact Research Fellowship Scheme. We acknowledge the Hong Kong Hospital Authority for providing anonymized clinical data for research. Part of this work has been presented as an oral abstract at the 83rd American Diabetes Association Scientific Sessions, San Diego.
Footnotes
Supplementary data related to this article can be found at https://doi.org/10.1016/j.eclinm.2024.102568.
Appendix ASupplementary data
References
- 1.Sun H., Saeedi P., Karuranga S., et al. IDF diabetes Atlas: global, regional and country-level diabetes prevalence estimates for 2021 and projections for 2045. Diabetes Res Clin Pract. 2021;183 doi: 10.1016/j.diabres.2021.109119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jankowski J., Floege J., Fliser D., Böhm M., Marx N. Cardiovascular disease in chronic kidney disease: pathophysiological insights and therapeutic options. Circulation. 2021;143(11):1157–1172. doi: 10.1161/CIRCULATIONAHA.120.050686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bailey C.J. Metformin: historical overview. Diabetologia. 2017;60(9):1566–1576. doi: 10.1007/s00125-017-4318-z. [DOI] [PubMed] [Google Scholar]
- 4.Holman R.R., Paul S.K., Bethel M.A., Matthews D.R., Neil H.A. 10-year follow-up of intensive glucose control in type 2 diabetes. N Engl J Med. 2008;359(15):1577–1589. doi: 10.1056/NEJMoa0806470. [DOI] [PubMed] [Google Scholar]
- 5.Inzucchi Silvio E. FDA drug safety communication: FDA revises warnings regarding use of the diabetes medicine metformin in certain patients with reduced kidney function. https://www.fda.gov/drugs/drug-safety-and-availability
- 6.Navaneethan S.D., Zoungas S., Caramori M.L., et al. Diabetes management in chronic kidney disease: synopsis of the 2020 KDIGO clinical practice guideline. Ann Intern Med. 2020;174(3):385–394. doi: 10.7326/M20-5938. [DOI] [PubMed] [Google Scholar]
- 7.Yang A., Lau E.S.H., Wu H., et al. Attenuated risk association of end-stage kidney disease with metformin in type 2 diabetes with eGFR categories 1-4. Pharmaceuticals. 2022;15(9):1140. doi: 10.3390/ph15091140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kim M.H., Oh H.J., Kwon S.H., et al. Metformin use and cardiovascular outcomes in patients with diabetes and chronic kidney disease: a nationwide cohort study. Kidney Res Clin Pract. 2021;40(4):660–672. doi: 10.23876/j.krcp.20.222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Inzucchi S.E., Lipska K.J., Mayo H., Bailey C.J., McGuire D.K. Metformin in patients with type 2 diabetes and kidney disease: a systematic review. JAMA. 2014;312(24):2668–2675. doi: 10.1001/jama.2014.15298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kwon S., Kim Y.C., Park J.Y., et al. The long-term effects of metformin on patients with type 2 diabetic kidney disease. Diabetes Care. 2020;43(5):948–955. doi: 10.2337/dc19-0936. [DOI] [PubMed] [Google Scholar]
- 11.Lalau J.D., Kajbaf F., Protti A., Christensen M.M., De Broe M.E., Wiernsperger N. Metformin-associated lactic acidosis (MALA): moving towards a new paradigm. Diabetes Obes Metab. 2017;19(11):1502–1512. doi: 10.1111/dom.12974. [DOI] [PubMed] [Google Scholar]
- 12.Lazarus B., Wu A., Shin J.I., et al. Association of metformin use with risk of lactic acidosis across the range of kidney function: a community-based cohort study. JAMA Intern Med. 2018;178(7):903–910. doi: 10.1001/jamainternmed.2018.0292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Khunti K., Godec T.R., Medina J., et al. Patterns of glycaemic control in patients with type 2 diabetes mellitus initiating second-line therapy after metformin monotherapy: retrospective data for 10 256 individuals from the United Kingdom and Germany. Diabetes Obes Metab. 2018;20(2):389–399. doi: 10.1111/dom.13083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nakashima A., Kato K., Ohkido I., Yokoo T. Role and treatment of insulin resistance in patients with chronic kidney disease: a review. Nutrients. 2021;13(12):4349. doi: 10.3390/nu13124349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Khunti K., Gomes M.B., Kosiborod M., et al. Metformin discontinuation in patients beginning second-line glucose-lowering therapy: results from the global observational DISCOVER study programme. BMJ Open. 2020;10(8) doi: 10.1136/bmjopen-2019-034613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chow E., Yang A., Chung C.H.L., Chan J.C.N. A clinical perspective of the multifaceted mechanism of metformin in diabetes, infections, cognitive dysfunction, and cancer. Pharmaceuticals. 2022;15(4):442. doi: 10.3390/ph15040442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chan J.C., Lim L.-L., Luk A.O., et al. From Hong Kong diabetes register to JADE program to RAMP-DM for data-driven actions. Diabetes Care. 2019;42(11):2022–2031. doi: 10.2337/dci19-0003. [DOI] [PubMed] [Google Scholar]
- 18.Wu H., Lau E.S.H., Yang A., et al. Data resource profile: the Hong Kong diabetes surveillance database (HKDSD) Int J Epidemiol. 2021;51(2):e9–e17. doi: 10.1093/ije/dyab252. [DOI] [PubMed] [Google Scholar]
- 19.Yang A., Wu H., Lau E.S., et al. Trends in glucose-lowering drug use, glycemic control, and severe hypoglycemia in adults with diabetes in Hong Kong, 2002–2016. Diabetes Care. 2020;43(12):2967–2974. doi: 10.2337/dc20-0260. [DOI] [PubMed] [Google Scholar]
- 20.Hernán M.A., Robins J.M. Using big data to emulate a target trial when a randomized trial is not available. Am J Epidemiol. 2016;183(8):758–764. doi: 10.1093/aje/kwv254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Langan S.M., Schmidt S.A., Wing K., et al. The reporting of studies conducted using observational routinely collected health data statement for pharmacoepidemiology (RECORD-PE) BMJ. 2018;363 doi: 10.1136/bmj.k3532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schneeweiss S., Rassen J.A., Brown J.S., et al. Graphical depiction of longitudinal study designs in health care databases. Ann Intern Med. 2019;170(6):398–406. doi: 10.7326/M18-3079. [DOI] [PubMed] [Google Scholar]
- 23.Mi X., Hammill B.G., Curtis L.H., Lai E.C., Setoguchi S. Use of the landmark method to address immortal person-time bias in comparative effectiveness research: a simulation study. Stat Med. 2016;35(26):4824–4836. doi: 10.1002/sim.7019. [DOI] [PubMed] [Google Scholar]
- 24.Morgan C.J. Landmark analysis: a primer. J Nucl Cardiol. 2019;26(2):391–393. doi: 10.1007/s12350-019-01624-z. [DOI] [PubMed] [Google Scholar]
- 25.Yang A., Shi M., Lau E.S.H., et al. Clinical outcomes following discontinuation of renin-angiotensin-system inhibitors in patients with type 2 diabetes and advanced chronic kidney disease: a prospective cohort study. EClinicalMedicine. 2023;55 doi: 10.1016/j.eclinm.2022.101751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yang A., Shi M., Wu H., et al. Long-term metformin use and risk of pneumonia and related death in type 2 diabetes: a registry-based cohort study. Diabetologia. 2021;64(8):1760–1765. doi: 10.1007/s00125-021-05452-0. [DOI] [PubMed] [Google Scholar]
- 27.Thomas L.E., Li F., Pencina M.J. Overlap weighting: a propensity score method that mimics attributes of a randomized clinical trial. JAMA. 2020;323(23):2417–2418. doi: 10.1001/jama.2020.7819. [DOI] [PubMed] [Google Scholar]
- 28.Cheung J.T.K., Yang A., Wu H., et al. Initiation of sodium-glucose cotransporter-2 inhibitors at lower HbA1c threshold attenuates eGFR decline in type 2 diabetes patients with and without cardiorenal disease: a propensity-matched cohort study. Diabetes Res Clin Pract. 2023;195 doi: 10.1016/j.diabres.2022.110203. [DOI] [PubMed] [Google Scholar]
- 29.Murad H., Dankner R., Berlin A., Olmer L., Freedman L.S. Imputing missing time-dependent covariate values for the discrete time Cox model. Stat Methods Med Res. 2020;29(8):2074–2086. doi: 10.1177/0962280219881168. [DOI] [PubMed] [Google Scholar]
- 30.Pazzagli L., Linder M., Zhang M., et al. Methods for time-varying exposure related problems in pharmacoepidemiology: an overview. Pharmacoepidemiol Drug Saf. 2018;27(2):148–160. doi: 10.1002/pds.4372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lipsitch M., Tchetgen Tchetgen E., Cohen T. Negative controls: a tool for detecting confounding and bias in observational studies. Epidemiology. 2010;21(3):383–388. doi: 10.1097/EDE.0b013e3181d61eeb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yang A., Wu H., Lau E.S.H., et al. Glucose-lowering drug use, glycemic outcomes, and severe hypoglycemia: 18-Year trends in 0·9 million adults with Diabetes in Hong Kong (2002-2019) Lancet Reg Health West Pac. 2022;26 doi: 10.1016/j.lanwpc.2022.100509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Orloff J., Min J.Y., Mushlin A., Flory J. Safety and effectiveness of metformin in patients with reduced renal function: a systematic review. Diabetes Obes Metab. 2021;23(9):2035–2047. doi: 10.1111/dom.14440. [DOI] [PubMed] [Google Scholar]
- 34.Charytan D.M., Solomon S.D., Ivanovich P., et al. Metformin use and cardiovascular events in patients with type 2 diabetes and chronic kidney disease. Diabetes Obes Metab. 2019;21(5):1199–1208. doi: 10.1111/dom.13642. [DOI] [PubMed] [Google Scholar]
- 35.Whitlock R.H., Hougen I., Komenda P., Rigatto C., Clemens K.K., Tangri N. A safety comparison of metformin vs sulfonylurea initiation in patients with type 2 diabetes and chronic kidney disease: a retrospective cohort study. Mayo Clin Proc. 2020;95(1):90–100. doi: 10.1016/j.mayocp.2019.07.017. [DOI] [PubMed] [Google Scholar]
- 36.Goodwin P.J., Chen B.E., Gelmon K.A., et al. Effect of metformin vs placebo on invasive disease-free survival in patients with breast cancer: the MA.32 randomized clinical trial. JAMA. 2022;327(20):1963–1973. doi: 10.1001/jama.2022.6147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wu H., Lau E.S., Ma R.C., et al. Secular trends in all-cause and cause-specific mortality rates in people with diabetes in Hong Kong, 2001–2016: a retrospective cohort study. Diabetologia. 2020;63(4):757–766. doi: 10.1007/s00125-019-05074-7. [DOI] [PubMed] [Google Scholar]
- 38.Magliano D.J., Chen L., Carstensen B., et al. Trends in all-cause mortality among people with diagnosed diabetes in high-income settings: a multicountry analysis of aggregate data. Lancet Diabetes Endocrinol. 2022;10(2):112–119. doi: 10.1016/S2213-8587(21)00327-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.