Abstract
HIV population viral load (PVL) can reflect antiretroviral therapy (ART) program effectiveness and transmission potential in a community. Using nationally representative data from household surveys conducted in Zimbabwe, Malawi, and Zambia in 2015–16, we examined the association between various VL measures and the probability of at least one recent HIV-1 infection in the community. We used Limiting-antigen (LAg) Avidity enzyme immunoassay (EIA), VLS (HIV RNA <1000 copies/mL), and ARVs in the blood to identify recent HIV-1 cases.
Among 1,510 EAs across the three surveys, 52,036 adults aged 15–59 years resided in 1,363 (90.3%) EAs with at least one HIV-positive adult consenting to interview and blood draw and whose VL was tested. Mean HIV prevalence across these EAs was 13.1% (95% confidence intervals [CI] 12.7–13.5). Mean VLS prevalence across these EAs was 58.7% (95% CI 57.3–60.0).
In multivariable analysis, PVL was associated with a recent HIV-1 case in that EA (adjusted odds ratio [AOR]: 1.4, 95% CI 1.2–1.6, p=0.001). VLS prevalence was inversely correlated with recent infections (AOR: 0.3, 95% CI 0.1–0.6, p=0.004). The 90-90-90 indicators, namely, the prevalence of HIV diagnosis, ART coverage, and VLS at the EA level, were inversely correlated with HIV recency at the EA level.
We found a strong association between PVL and VLS prevalence and recent HIV-1 infection at the EA level across three southern African countries with generalized HIV epidemics. These results suggest that population-based measures of VLS in communities may serve as a proxy for epidemic control.
Keywords: HIV, southern Africa, population viral load, HIV incidence, 90-90-90 indicators
Background:
In 2014, UNAIDS set the ambitious goal of ending the AIDS epidemic by 2030.1 To achieve this goal, UNAIDS set a three-part target, namely that 90% of all people living with HIV (PLHIV) will know their status, 90% of those diagnosed with HIV will receive sustained antiretroviral therapy (ART), and 90% of people receiving ART will have viral load suppression (VLS) by 2020 (also known as 90-90-90). The underlying rationale for these targets is that high viral load (VL) in blood and genital fluid is the primary determinant of HIV transmission, and therefore, reduction of the VL in HIV-positive individuals reduces the risk of HIV transmission.2
Recent surveys in high-HIV-burden, resource-constrained settings in sub-Saharan Africa suggest that the UNAIDS targets are achievable.3,4 Population-based HIV Impact Assessment (PHIA)i surveys, launched in 2014, are HIV-focused, household-based, nationally representative, cross-sectional surveys of adults and children in 14 high-burden countries. Initial findings from the PHIA surveys in 11 countries reveal encouraging results regarding the 90-90-90 targets. For example, VLS among those on ART ranges from 76% in Cote d’Ivoire5 to 92% in Eswatini (formerly Swaziland)6. However, the overall question remains whether achieving these targets leads to a reduction in HIV transmission and, in turn, to a lower incidence rate and eventual containment of the epidemic.
Community viral load (CVL), defined as an aggregate measure of individual VLs of PLHIV in care in a specific community, was initially used to evaluate HIV treatment programs and proxy for HIV incidence.7–9 The US Centers for Disease Control and Prevention (CDC) subsequently defined population viral load (PVL) as an aggregate VL measure that includes data from individuals unaware of their HIV-positive status. They recommended using PVL as a measure of the effectiveness of ART programs10. However, the limitations, such as the possibility of ecological fallacy, sampling bias, missing data, and HIV prevalence in the community, raised questions about the value of CVL as a standard measure of an ART program and modeling potential for ongoing transmission.7–9,11,12
Two recent studies addressed those concerns and showed that the PVL or prevalence of viremia correlated with HIV incidence. One study used a sample of people who inject drugs and men who have sex with men drawn from participants of the baseline assessment of a cluster-randomized trial across 22 cities in India. The other study analyzed the data from prospective population cohorts in rural KwaZulu-Natal, South Africa.13 To date, though, no national studies have directly examined the strength of association between VL aggregate measures and the occurrence of recent HIV infection in small communities.
Neighborhoods are a basic unit of analysis for a variety of community-based HIV-related interventions. Through community-level studies, it is possible to identify the best strategies for achieving the desired outcomes.14 We report an analysis of the association between PVL and VLS and the probability of at least one recent HIV-1 infection at the community level using data from PHIA surveys conducted in three contiguous southern African countries, Zimbabwe, Malawi, and Zambia.
Methods
We used nationally representative data from the PHIA surveys implemented in Zimbabwe, Malawi, and Zambia, analyzed at the surveys’ smallest geographic area, enumeration areas (EA), representing a community. Each survey had a cross-sectional, two-stage stratified cluster sample design. In the first stage, a systematic random sample of each country’s EAs was drawn based on probability proportional to size. In the second stage, a random sample of households was drawn from each EA using an equal probability method. Adult women and men living in residential households, and visitors who slept in the household the night before the survey, were eligible to participate if they were willing and cognitively able to provide consent. Age was self-reported, and eligible ages were defined as 15 years or older in Zimbabwe, 15–64 years in Malawi, and 15–59 years in Zambia.
Most (84%−89%) of the approximately 13,000–15,000 selected households in each country agreed to participate, and 77–82% of eligible adults in consenting households completed both an interview and HIV testing.
Biomarker collection
HIV home-based testing and counseling (HBTC) was conducted in each household following national guidelines in each country. Individuals with a nonreactive result on the screening test (Determine™ HIV-1/2 [Abbott Molecular Inc., Des Plaines, Illinois, United States]) were reported as HIV-negative. Individuals with a reactive screening test result underwent confirmatory testing. In Zambia and Malawi, Uni-Gold™ (Trinity Biotech, plc. Wicklow, Ireland), and in Zimbabwe, First Response® HIV 1–2.0 Card Test (Premier Medical Corporation Ltd., Nani Daman, India) were used as confirmatory tests. Those with a reactive result on both screening and confirmatory tests were classified as HIV positive. Additional, confirmatory testing using the Geenius HIV 1/2 Supplemental Assay (Bio-Rad, Hercules, California, United States) was conducted on all samples that tested HIV positive during HBTC.ii
To measure the HIV-1 VL (HIV RNA copies per mL) from the plasma of confirmed HIV-positive participants, the Abbott m2000 System (Abbott Molecular Inc., Chicago, Illinois, United States) was used in Zimbabwe and Malawi, and the COBAS TaqMan 48 Analyzer and the COBAS AmpliPrep/COBAS TaqMan HIV-1 Test, v2.0 (Roche Molecular Diagnostics, Branchburg, New Jersey, United States) was used in Zambia.
VL from dried blood spot samples from adults with insufficient volume of plasma was measured using the NucliSENS easyQ platform (bioMerieux, Marcy l’Etoile, France) in Zimbabwe, the open-mode protocol for the Abbott RealTime HIV-1 assay (Abbott Molecular Inc., Chicago, Illinois, United States) in Malawi, and the COBAS AmpliPrep/COBAS Taqman HIV-1 Qualitative Test version 2.0 (Abbott Molecular, Wiesbaden, Germany) in Zambia.
A laboratory-based recent infection testing algorithm, which employed a combination of the Limiting-antigen (LAg) Avidity enzyme immunoassay (EIA) (Sedia Biosciences Corporation, Portland, Oregon, United States), VL, and antiretroviral (ARV) detection was used to distinguish recent from long-term infection. Specimens with normalized optical density (ODn) values > 1.5 were classified as long-term infections, as were specimens with VLS, classified as HIV RNA < 1,000 copies/mL (due to elite controllers who naturally suppress their viremia or persons under suppressive ART), and any specimens with detectable ARV. Specimens with ODn values ≤ 1.5 and VL > 1000 copies/mL and had non-detectable ARV were classified as recent infections.
ARV detection assays
To qualitatively detect ARVs, a single DBS was eluted, and chromatographic separation was carried out on a Luna 5μm PFP column (110 Å, 50 × 2 mm) (Phenomenex, Torrance, California, USA). Each ARV was detected using an API 4000 LC/MS/MS instrument (Applied Biosystems, Foster City, California, USA). This qualitative method used a limit of detection of 0.02 μg/ml for each ARV, with a signal-to-noise ratio of at least 5:1 for all ARVs. Samples with concentrations above 0.02 μg/ml were considered positive for each ARV. Testing was conducted at the University of Cape Town, South Africa.
Three ARVs were selected as markers for the most commonly prescribed first- and second-line regimens in each country. In Malawi and Zambia, samples were tested for efavirenz, atazanavir, and lopinavir. Samples from participants who were virally suppressed or self-reported on ART, but had no evidence of the first three compounds, were tested for nevirapine. In Zimbabwe, samples were tested for efavirenz, nevirapine, and lopinavir.
Statistical analysis
In this study, we aggregated the data at the level of EA. In census parlance, an EA is the smallest geographic unit that groups many households for enumeration purposes15.
We calculated different population-level VL measures, as defined by CDC and other studies in the literature,10,12,13,16, as well as population-level VLS to evaluate the association between these measures and recent HIV diagnoses. PVL was defined as the arithmetic mean of log10 HIV RNA of all HIV-positive individuals either diagnosed (aware of their HIV status) or undiagnosed (unaware of their status), regardless of their treatment status (whether or not linked and retained in an HIV care and treatment program) in the EA; ‘unaware VL’ as the arithmetic mean of log10 HIV RNA in HIV-positive individuals reporting to be unaware of their HIV status; and ‘on-ART VL’ as the arithmetic mean of log10 HIV RNA in people reporting current use of ART or having detectable ARV in the blood.iii We defined the prevalence of VLS as the proportion of individuals with HIV in the EA who were virologically suppressed. We estimated population ART coverage as the proportion of PLHIV reporting current ART use or having a detectable ARV in the blood. Awareness was defined as reporting being HIV positive or having detectable ARV in the blood.
In our sensitivity analysis, we used alternative HIV RNA cut-offs for viremic individuals of more than 400 copies per mL and more than 40 copies per mL.
HIV incidence depends not only on the VLs of PLHIV but also on the relative sizes of the infected and uninfected population as well as rates and patterns of their contacts.17 We used HIV prevalence and the total number of survey participants at the EA level to account for infected and uninfected populations and the mean age of the female population at the EA level to account for the chance of a sexual encounter. We used logistic regression to estimate the probability of one recent HIV-1 infection, adjusted for EA-level variables, e.g., HIV prevalence (three categories: HIV prevalence less than 10%, 10%−20%, and more than 20%), the total number of survey participants (two categories: less than 50 and equal to or more than 50 participants) and mean age of the female population as a continuous variable. Survey weights accounting for non-response using Chi-squared automatic interaction detector (CHAID) analysis and non-coverage, and the probability of selection was applied, and variances were estimated using jackknife replicate weights.
We used the simulation extrapolation method for fitting models with additive measurement error to ensure that wide confidence intervals (CI) in small EAs that resulted from measuring HIV prevalence and VLS at the EA level did not bias our results.18
Survey protocols for the Malawi, Zambia, and Zimbabwe PHIAs were approved by the CDC Institutional Review Board (IRB), the Columbia University Medical Center IRB, and the local IRB in each country (National Health Science Research Committee of Malawi, the Tropical Disease Research Center in Zambia, and the Medical Research Council of Zimbabwe).
Results:
We collected data from 1,510 EAs in three countries: 499 EAs (155 urban and 344 rural) in Zimbabwe between October 2015 and August 2016, 500 EAs (160 urban and 340 rural) in Malawi between November 2015 and August 2016, and 511 EAs (194 urban 317 rural) in Zambia between March and August 2016. We included 1,363 (90.3%) EAs with at least one HIV-infected adult consenting to an interview and blood draw, whose VL was successfully measured. We used the blood samples from a total of 52,036 adults aged 15–59 years who resided in these EAsiv. Across the EAs, 58.6% (95% CI 58.2%–59.1%) of the participants were female. Among the 1,363 EAs, 92.6%, 7.0% and 0.4% had 0, 1 and 2 recent HIV-1 cases, respectively.
The mean age of women and men aged 15–59 at the EA level was 30.2 (95% CI 30.2–30.3) years and 30.0 (95% CI 30.0–30.1) years, respectively. Mean HIV prevalence at the EA level was 13.1% (95% CI 12.7%–13.5%). HIV prevalence was less than 10% in 499 (36.8%) EAs, 10–20% in 587 (42.9%) EAs and more than 20% in 277 (20.2%) EAs. Overall, amongst PLHIV across all EAs, 74.9% (95% CI 73.7%–76.1%) knew their HIV serostatus, 66.5% (95% CI 65.2%–67.9%) were on ART and 58.7% (95% CI 57.3%–60.0%) had VLS. Across these EAs, 38.2% (95% CI 36.9%–39.6%) of all PLHIV had HIV-1 RNA more than 1000 copies/mL (prevalence of viremia). (Table 1)
Table 1.
HIV-related characteristics of 1,363 enumeration areas with at least one HIV-positive adult, participating in Population-based HIV Impact Assessment surveys in Zambia, Zimbabwe and Malawi (2015–2016)
| Mean | Jackknife Std. Err. | (95% CI) | |
|---|---|---|---|
| Age of the female population in the EA (years) | 30.2 | 0.02 | (30.2–30.3) |
| Age of the male population in the EA (years) | 30.0 | 0.03 | (30.0–30.1) |
| Number of survey adult participants in the EA | 43 | 0.55 | (42–44) |
| Proportion of adults ever tested for HIV in the EA | 74.5% | 0.3% | (74.0–75.0) |
| HIV prevalence in the EA | 13.1% | 0.2% | (12.7–13.5) |
| Proportion of PLHIV aware of their HIV status | 74.9% | 0.6% | (73.7–76.1) |
| Proportion of PLHIV currently on ART | 66.5% | 0.7% | (65.2–67.9) |
| Prevalence of virologic suppression (VL< 1000 copies/mL) | 58.7% | 0.7% | (57.3–60.0) |
| Prevalence of viremia with VL≥ 1000 copies/mL | 38.2% | 0.7% | (36.9–39.6) |
| Prevalence of viremia with VL≥ 400 copies/mL | 40.2% | 0.7% | (38.8–41.6) |
| Prevalence of viremia with VL≥ 40 copies/mL | 46.3% | 0.7% | (44.8–47.7) |
Note:
ART: antiretroviral therapy
EA: Enumeration area
PLHIV: People living with HIV
VL: Viral load
Across EAs, median PVL was 2.5 log10 copies per mL (IQR 2.0–3.3) (339 copies per mL, 95% CI 108–2,065), median unaware VL was 4.3 log10 copies per mL (IQR 3.5–4.8) (19,335 copies per mL, CI 3,138–63,374) and median on-ART VL was 2.2 log10 copies per mL (IQR 1.9–2.9) (163 copies per mL, 95% CI 71–757). (Table 1 and Table 2)
Table 2.
Viral Load Measures of HIV-Positive Adults
| Median (log10) | Interquartile Range (IQR) | Median copies/mL | IQR | |
|---|---|---|---|---|
| Median population viral load | 2.5 | (2.0–3.3) | 339 | (108–2,065) |
| Median viral load of those unaware of HIV status | 4.3 | (3.5–4.8) | 19,335 | (3,138–63,374) |
| Median viral load of currently on ART | 2.2 | (1.9–2.9) | 163 | (71–757) |
In multivariable analysis, PVL and unaware VL were significantly associated with a recent HIV-1 case in an EA. Controlling for other variables in the model, for one unit increase in log10 PVL, the odds of an EA with at least one recent HIV infection increased by 1.37 (95% CI 1.2–1.6, p <0.001) and for unaware VL by 1.38 (95% CI 1.1–1.8, p <0.05). The correlation between on-ART VL with a recent HIV-1 case in an EA was statistically insignificant. (Table 3)
Table 3.
Multivariable analysis (logistic model) of PVL measures and the adjusted odds of at least one recent HIV-1 infection in an enumeration area.
| (Model 1) | (Model 2) | (Model 3) | ||||
|---|---|---|---|---|---|---|
| Variable | Population viral load (AOR) | 95% CI | Unaware viral load (AOR) | 95% CI | On-ARV viral load (AOR) | 95% CI |
| HIV prevalence | ||||||
| <10% (Reference) | 1.00 | - | 1.00 | - | 1.00 | - |
| 10%−20% | 2.45** | [1.39–4.33] | 1.42 | [0.77–2.58] | 2.34** | [1.28–4.25] |
| >20% | 4.71*** | [2.58–8.61] | 2.34** | [1.25–4.41] | 4.40*** | [2.34–8.26] |
| Number of HIV-positive adults residing in EA who participated in survey | ||||||
| <50 (reference) | 1.00 | - | 1.00 | - | 1.00 | - |
| ≥50 | 1.98** | [1.25–3.14] | 1.72* | [1.07–2.74] | 1.77* | [1.11–2.82] |
| Mean age of female population | 0.91* | [0.84–0.98] | 0.91* | [0.84–1.00] | 0.89** | [0.82–0.97] |
| Population VL in EA | 1.37*** | [1.15–1.63] | ||||
| Unaware VL in EA | 1.38* | [1.06–1.81] | ||||
| On-ARV VL in EA | 1.05 | [0.84–1.32] | ||||
| Observations | 1363 | 907 | 1262 |
All the variables are aggregated and weighted at EA-level
p < 0.05,
p < 0.01,
p < 0.001
PVL defined as the arithmetic mean of log10 HIV RNA of all HIV-positive individuals either diagnosed (aware of their HIV status) or undiagnosed (unaware of their status), regardless of their treatment status (whether or not linked and retained in an HIV care and treatment program) in the EA; ‘Unaware VL’ is defined as the arithmetic mean of log10 HIV RNA in HIV-positive individuals unaware of their HIV status; and ‘on-ART VL’ is defined as the arithmetic mean of log10 HIV RNA in people reporting current use of ART or having a detectable ARV in the blood.
AOR: adjusted odds ratio
ART: antiretroviral therapy
EA: Enumeration area
PLHIV: People living with HIV
VL: Viral load
Prevalence of HIV awareness, ART coverage, and VLS among all HIV-positive adults were all inversely correlated with recent infections. Controlling for other variables, for 1% increase in the proportion of awareness of HIV status, ART coverage or VLS, the odds of an EA with at least one recent HIV infection decreased by 0.16 (95% CI 0.07–0.36, p <0.001), 0.25 (95% CI 0.11–0.57, p <0.001) or 0.28 (95% CI 0.12–0.63], p <0.01), respectively. (Table 4)
Table 4.
Results of the multivariable analysis (logistic model) to examine the relationship between three UNAIDS targets (90-90-90) and the adjusted odds of at least one recent HIV-1 infection in a survey enumeration area.
| (Model 1) | (Model 2) | (Model 3) | ||||
|---|---|---|---|---|---|---|
| Prevalence of HIV diagnosis among PLHIV | 95% CI | ART coverage among PLHIV | 95% CI | Prevalence of VLS among PLHIV | 95% CI | |
| HIV prevalence <10% (Reference) | 1.00 | - | 1.00 | - | 1.00 | - |
| 10%−20% | 2.68*** | [1.50,4.80] | 2.56** | [1.44,4.55] | 2.48** | [1.40,4.37] |
| >20% | 5.35*** | [2.87,9.94] | 4.94*** | [2.68,9.08] | 4.80*** | [2.62,8.78] |
| Number of HIV-positive adults residing in EA who participated in survey <50 (reference) | 1.00 | - | 1.00 | - | 1.00 | - |
| ≥50 | 2.04** | [1.28,3.25] | 1.96** | [1.24,3.10] | 1.98** | [1.25,3.14] |
| Mean age of female population | 0.91* | [0.84,0.99] | 0.91* | [0.84,0.99] | 0.91* | [0.84,0.99] |
| Prevalence of HIV diagnosis (awareness among PLHIV) | 0.16*** | [0.07,0.36] | ||||
| ART coverage among PLHIV | 0.25*** | [0.11,0.57] | ||||
| Prevalence of VLS among PLHIV | 0.28** | [0.12,0.63] | ||||
| Observations | 1363 | 1363 | 1363 |
All the variables are aggregated and weighted at EA-level
p < 0.05,
p < 0.01,
p < 0.001
ART: antiretroviral therapy
EA: Enumeration area
PLHIV: People living with HIV
VL: Viral load
Figures 1a, 1b, and 1c illustrate the adjusted predicted probability of a recent HIV infection at the EA level. In this sample, as PVL and unaware VL increase, the likelihood of having a recent HIV infection at the EA level also increases. On-ART VL was not correlated with a recent HIV infection. Figures 2a, 2b, and 2c show that the three 90-90-90 indicators, namely prevalence of HIV awareness, ART coverage, and VLS at the EA level, are inversely correlated with a recent case of HIV infection at the EA level. All three indicators appear equally impactful on the probability of having a recent infection at the EA level, but the effect diminishes as each indicator’s prevalence increases. For instance, when the prevalence of VLS changes from 40% to 80%, the predicted probability of a recent HIV infection at the EA level drops by 37%, from 0.08 (95% CI 0.06–0.10) to 0.05 (95% CI 0.04–0.07), holding all other covariates at mean value. On average, every 1% increase in VLS in an EA decreased the predicted probability of one recent infection by 8.9%.
Fig.1.
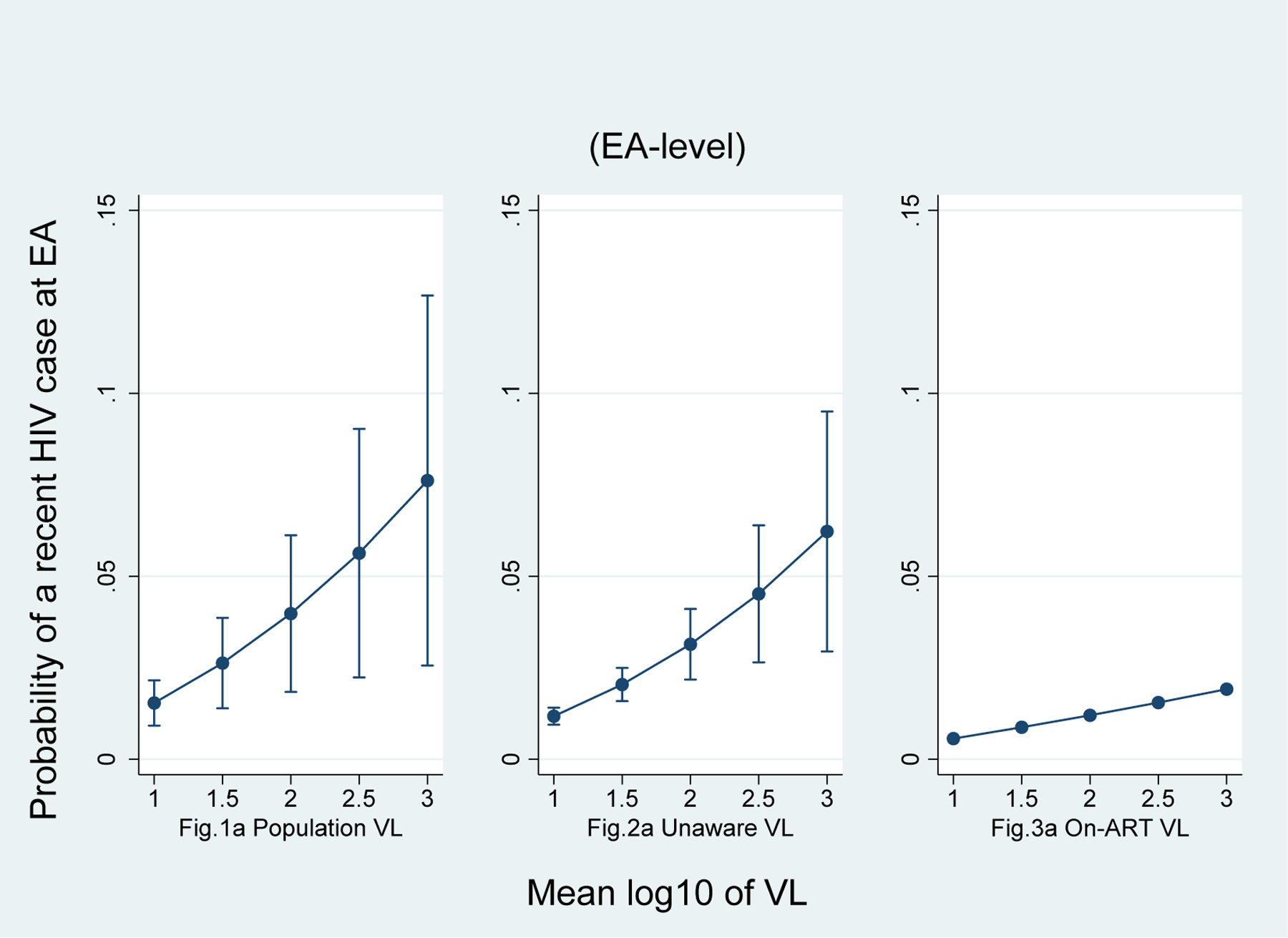
Adjusted predicted probability of at least one recent HIV case
Note:
ART: antiretroviral therapy
EA: Enumeration area
PLHIV: People living with HIV
VL: Viral load
Fig.2.
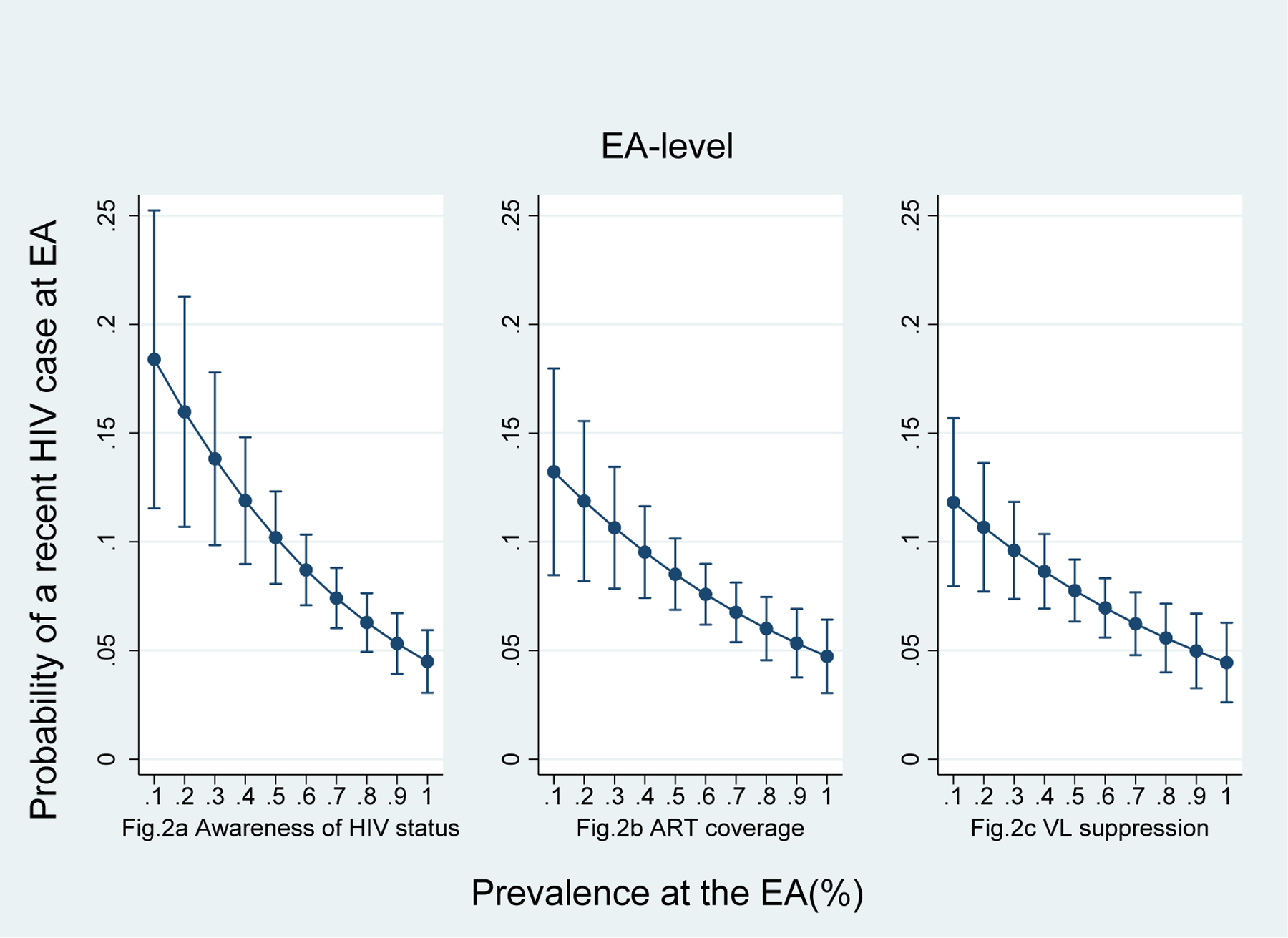
Adjusted predicted probability of at least one recent HIV case
Note:
ART: antiretroviral therapy
EA: Enumeration area
PLHIV: People living with HIV
VL: Viral load
Table 5 reports the results when alternative cut-offs for the prevalence of viremia were used. The adjusted odds ratio (AOR) associated with a one percent increase in viremia was 5.33 (95% CI 2.35–12.10, p <0.001) when the cut-off point for viremia was set at a VL> 1000 copies/mL. The AOR was lower when viremia was set at a VL> 400 copies/mL and >40 copies/mL, (AOR: 4.33; 95% CI 1.92–9.78, p <0.001) (AOR: 3.40; 95% CI 1.50–7.73, p <0.01), respectively.
Table 5.
Results of the multivariable analysis (logistic model) to examine the relationship between viremia at different cutoff points and the adjusted odds of at least one recent HIV-1 infection in a survey enumeration area.
| (1) | (2) | (3) | ||||
|---|---|---|---|---|---|---|
| Prevalence of Viremia with VL≥ 1000 copies/mL (AOR) | 95% CI | Prevalence of Viremia with VL≥ 400 copies/mL (AOR) | 95% CI | Prevalence of Viremia with VL≥ 40 copies/mL (AOR) | 95% CI | |
| HIV prevalence <10% (Reference) | 1.00 | - | 1.00 | - | 1.00 | - |
| 10%−20% | 2.53** | [1.43,4.48] | 2.49** | [1.41,4.40] | 2.43** | [1.38,4.29] |
| >20% | 4.93*** | [2.68,9.08] | 4.78*** | [2.61,8.75] | 4.61*** | [2.53,8.41] |
| Number of HIV-positive adults residing in EA who participated in survey <50 (reference) | 1.00 | - | 1.00 | - | 1.00 | - |
| ≥50 | 2.01** | [1.26,3.19] | 1.96** | [1.24,3.11] | 1.96** | [1.24,3.11] |
| Mean age of female population (years) | 0.91* | [0.84,0.99] | 0.91* | [0.84,0.98] | 0.91* | [0.84,0.98] |
| Prevalence of viremia with VL≥ 1000 copies/mL | 5.33*** | [2.35,12.10] | ||||
| Prevalence of viremia with VL≥ 400 copies/mL | 4.33*** | [1.92,9.78] | ||||
| Prevalence of viremia with VL≥ 40 copies/mL | 3.40** | [1.50,7.73] | ||||
| Observations | 1363 | 1363 | 1363 |
All the variables are aggregated and weighted at EA-level
p < 0.05,
p < 0.01,
p < 0.001
AOR: Adjusted odds ration
ART: antiretroviral therapy
EA: Enumeration area
PLHIV: People living with HIV
VL: Viral load
We did not find any statistically significant difference among countries.
Discussion
Drawing from nationally representative, population-based data from three high prevalence countries in southern Africa, we found a strong association at the community level between recent HIV-1 infection and PVL, unaware VL, and each of the three 90-90-90 targets: awareness of HIV diagnosis, being on ART and VLS. To our knowledge, this is the first report of such an association at the community level. Since neighborhood factors have been linked to HIV prevalence, uptake of HIV care and treatment, and HIV testing and counseling,14,19–21 the results of this study may inform a variety of community-based HIV-related interventions and strategies for reducing the number of new HIV infections in similar settings.
Our study shows that the prevalence of viremia in an EA was more strongly associated with a recent case of HIV than PVL. These findings are similar to those reported in a large study conducted across 22 cities in India, which reported a correlation of 0.81 (95% CI 0.62–0.91; p<0.0001) between the prevalence of viremia and HIV incidence, as compared to an association of 0.51 (0.16–0.74; p=0.007) for PVL and HIV incidence16. Our study also shows that the likelihood of recent HIV infection was significantly lower in high prevalence settings with lower PVL and lower detectable viremia levels, which is consistent with the finding of previous studies13,16.
Our analyses showed that the likelihood of detecting a recent HIV case was higher when the prevalence of viremia was defined as more than 1000 copies per mL compared to lower thresholds (>400 copies per mL and >40 copies per mL).
Accurate HIV incidence is the best measure of an HIV treatment program’s effectiveness for HIV prevention. However, this study’s findings show that PVL and the prevalence of viremia can be an alternative way to measure program effectiveness. The ‘on-ART VL,’ the most widely used measure in the literature, was not correlated with the probability of a recent HIV infection in an EA, consistent with findings in other studies.7,13 On-ART VL is the most available data from the surveillance databases7,9,11; however, it does not include those unaware of their HIV serostatus, with higher VLs and who potentially are more likely to transmit the virus.
Consistent with previous studies,16,25, we found a negative correlation between recent infection probability in an EA and ART coverage. As expected, population ART coverage was as strong a predictor of HIV incidence as the prevalence of VLS. Additionally, our study found an inverse association between the prevalence of HIV awareness and a recent case of HIV infection. We found the three UNAIDS targets significantly correlated with each other and predictors of the occurrence of a recent HIV infection in an EA. Our findings support the use of ART coverage as an acceptable alternative in resource-limited settings where VL tests may not be available.
We found considerable variation in EA-level HIV prevalence, awareness of HIV status, ART-coverage, and VLS at the EA level across the three surveys. These findings support the revision in international agencies’ strategy to optimize resources in the provision of HIV testing and treatment at targeted sub-national areas.26,27 They emphasize identifying places where prevention and treatment services are most needed. Evaluating and monitoring data in smaller geographic areas is key to measuring program effectiveness and prioritizing resource allocation. While HIV epidemics in the countries included in this study are categorized as generalized, focusing on specific locales where sub-populations are at higher risk for HIV transmission could considerably improve the impact of the programs on HIV incidence.
Across these three high prevalence countries, new infections were rare events, occurring in only 7% of the EAs. Considering the annual incidence of HIV measured in PHIA surveys among adults ages 15 to 49 years in Malawi, Zimbabwe, and Zambia of 0.32 (95% CI 0.16–0.48), 0.44 (95% CI 0.25–0.62), 0.64 (95% CI 0.42–0.86), respectively,4 this finding is very promising for epidemic control in these countries, a critical geographic region in terms of the global epidemic.
With a cross-sectional survey design, we could not assess whether there was a causal relationship between high population viral suppression and low incidence at the community level. Neither could we adjust the results for individual characteristics such as age, sex, or sexual HIV risk behaviors since most EAs had no more than one recent HIV case. While this study is, by definition, an ecological study, where the unit of observation is the EA population, it does not suffer from the usual weaknesses of ecological studies. For example, the most troublesome issues in ecological studies are the biases that can occur if ascertainment of incidence or exposure, or both, differs from one place to another.28 In this study, however, these biases were unlikely because of similarities in the protocols, methods, and training of data collectors. Additionally, we used the LAg-Avidity EIA with an empirically derived window period for identifying recent infections, which can potentially misclassify a proportion of long-term HIV infections as recent. However, the inclusion of VL and ARV detection assay data substantially reduced the chance of misclassification.29
Our findings provide further support for the 90-90-90 targets and suggest that the prevalence of viremia and PVL are useful indicators in HIV-focused population surveys. Since the VL measurement data came from population-based surveys, our findings were less likely to be subject to the biases typically associated with health facility-based VL data. A representative sample of the general population allowed us to arrive at reliable community-level estimates. These results suggest expanding and maintaining high levels of VLS at the community level may be essential to HIV epidemic control in settings similar to these three countries. The next round of PHIA surveys may provide data to assess the longitudinal utility of PVL to monitor the effectiveness of HIV care and treatment programs.
Acknowledgments and Funding
We would like to acknowledge the participants of the ZIMPHIA, MPHIA, and ZAMPHIA surveys, and the tremendous dedication and commitment of the Ministries of Health and national statistical bureaus. This research has been supported by the President’s Emergency Plan for AIDS Relief (PEPFAR) through CDC under the terms of Cooperative Agreement #1U2GGH001226-01. The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the funding agencies.
Footnotes
The Population-Based HIV Impact Assessment (PHIA) is a multi-country project funded by the United States President’s Emergency Plan for AIDS Relief (PEPFAR) to conduct national HIV-focused surveys that describe the status of the HIV epidemic. The surveys were led by the ministries of health of each country.
Confirmatory testing using the Geenius HIV 1/2 Supplemental Assay was not part of the national algorithm but was added to the protocol after concurrence of all parties involved.
We imputed value of 1 if VL was below detection level to calculate the mean log10 viral loads.
We excluded the participants 60 to 64 years old from Malawi and Zimbabwe data to be consistent across the countries.
References:
- 1.UNAIDS. 90-90-90: an ambitious treatment target to help end the AIDS epidemic. Geneva, Switzerland: UNAIDS, 2014. [Google Scholar]
- 2.Quinn TC, Wawer MJ, Sewankambo N, et al. Viral load and heterosexual transmission of human immunodeficiency virus type 1. Rakai Project Study Group. The New England journal of medicine 2000; 342(13): 921–9. [DOI] [PubMed] [Google Scholar]
- 3.Justman JE, Mugurungi O, El-Sadr WM. HIV Population Surveys — Bringing Precision to the Global Response. New England Journal of Medicine 2018; 378: 1859–61. [DOI] [PubMed] [Google Scholar]
- 4.PHIA Project. 2018. https://phia.icap.columbia.edu/countries-overview/ (accessed 10/20/2018, 2018).
- 5.CIPHIA team. CIPHIA Summary Sheet. 2018. https://phia.icap.columbia.edu/wp-content/uploads/2018/08/CIPHIA_Cote-DIvoire-SS_FINAL.pdf (accessed 1/19/2019, 2019).
- 6.SHIMS2 team. SHIMS2 Summary Sheet. 2017. https://phia.icap.columbia.edu/wp-content/uploads/2017/11/Swaziland_new.v8.pdf (accessed 1/19/2019, 2019).
- 7.Montaner JSG, Lima VD, Barrios R, et al. Association of highly active antiretroviral therapy coverage, population viral load, and yearly new HIV diagnoses in British Columbia, Canada: a population-based study. The Lancet 2010; 376(9740): 532–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wood E Longitudinal community plasma HIV-1 RNA concentrations and incidence of HIV-1 among injecting drug users: a prospective cohort study. BMJ (Clinical research ed) 2009; 338: b1649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Das M, Chu PL, Santos G-M, et al. Decreases in Community Viral Load Are Accompanied by Reductions in New HIV Infections in San Francisco. PLOS ONE 2010; 5(6): e11068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Centers for Disease Control and Prevention (CDC). Guidance on community viral load: a family of measures, definitions, and methods for calculation, 2011.
- 11.Castel AD, Befus M, Willis S, et al. use of the community viral load as a population-based biomarker of HIV burden. AIDS 2012; 26(3): 345–53. [DOI] [PubMed] [Google Scholar]
- 12.Miller WC, Powers KA, Smith MK, Cohen MS. Community viral load as a measure for the assessment of HIV treatment as prevention. The Lancet Infectious Diseases 2013; 13(5): 459–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tanser F, Vandormael A, Cuadros D, et al. Effect of population viral load on prospective HIV incidence in a hyperendemic rural African community. Science translational medicine 2017; 9(420): eaam8012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Latkin CA, German D, Vlahov D, Galea S. Neighborhoods, and HIV: A social-ecological approach to prevention and care. American Psychologist 2013; 68(4): 210–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.ICF International. Demographic and Health Survey Sampling and Household Listing Manual. MEASURE DHS, Calverton, Maryland, USA: ICF International, 2012. [Google Scholar]
- 16.Solomon SS, Mehta SH, McFall AM, et al. Community viral load, antiretroviral therapy coverage, and HIV incidence in India: a cross-sectional, comparative study. The Lancet HIV 2016; 3(4): e183–e90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Boerma JT, Weir SS. Integrating demographic and epidemiological approaches to research on HIV/AIDS: the proximate-determinants framework. The Journal of infectious diseases 2005; 191(Supplement_1): S61–S7. [DOI] [PubMed] [Google Scholar]
- 18.Hardin JW, Schmiediche H, Carroll RJ. The simulation extrapolation method for fitting generalized linear models with additive measurement error. Stata Journal 2003; 3(4): 1–13. [Google Scholar]
- 19.Burns PA, Snow RC. The built environment & the impact of neighborhood characteristics on youth sexual risk behavior in Cape Town, South Africa. Health & place 2012; 18(5): 1088–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fendrich M, Avci O, Johnson TP, Mackesy-Amiti ME. Depression, substance use, and HIV risk in a probability sample of men who have sex with men. Addictive behaviors 2013; 38(3): 1715–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Carlson M, Brennan RT, Earls F. Enhancing adolescent self-efficacy and collective efficacy through public engagement around HIV/AIDS competence: a multilevel, cluster randomized controlled trial. Social science & medicine 2012; 75(6): 1078–87. [DOI] [PubMed] [Google Scholar]
- 22.WHO. Technical and operational considerations for implementing HIV viral load testing: an interim technical update. Geneva, Switzerland: WHO, 2014. [Google Scholar]
- 23.Dorward J, Drain PK, Garrett N. Point-of-care viral load testing and differentiated HIV care. The Lancet HIV 2018; 5(1): e8–e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Morrison CS, Homan R, Mack N, et al. Assays for estimating HIV incidence: updated global market assessment and estimated economic value. Journal of the International AIDS Society 2017; 20(3): n/a-N.PAG. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tanser F, Bärnighausen T, Grapsa E, Zaidi J, Newell M-L. High Coverage of ART Associated with Decline in Risk of HIV Acquisition in Rural KwaZulu-Natal, South Africa. Science 2013; 339(6122): 966–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.(UNAIDS) JUNPoHA. Location, Location: Connecting People Faster to HIV Services. Geneva: UNAIDS, 2013. [Google Scholar]
- 27.President’s Emergency Plan for AIDS Relief (PEPFAR). PEPFAR 3.0: Controlling the Epidemic: Delivering on the Promise of an AIDS-free Generation. Washington, DC: PEPFAR, 2014. [Google Scholar]
- 28.Morgenstern H Ecologic studies in epidemiology: concepts, principles, and methods. Annual review of public health 1995; 16(1): 61–81. [DOI] [PubMed] [Google Scholar]
- 29.Kassanjee R, Pilcher CD, Busch MP, et al. Viral load criteria and threshold optimization to improve HIV incidence assay characteristics-a CEPHIA analysis. AIDS (London, England) 2016; 30(15): 2361. [DOI] [PMC free article] [PubMed] [Google Scholar]


