Abstract
The human JC polyomavirus (JCV) is the etiologic agent of the fatal central nervous system (CNS) demyelinating disease progressive multifocal leukoencephalopathy (PML). PML typically occurs in immunosuppressed patients and is the direct result of JCV infection of oligodendrocytes. The initial event in infection of cells by JCV is attachment of the virus to receptors present on the surface of a susceptible cell. Our laboratory has been studying this critical event in the life cycle of JCV, and we have found that JCV binds to a limited number of cell surface receptors on human glial cells that are not shared by the related polyomavirus simian virus 40 (C. K. Liu, A. P. Hope, and W. J. Atwood, J. Neurovirol. 4:49–58, 1998). To further characterize specific JCV receptors on human glial cells, we tested specific neuraminidases, proteases, and phospholipases for the ability to inhibit JCV binding to and infection of glial cells. Several of the enzymes tested were capable of inhibiting virus binding to cells, but only neuraminidase was capable of inhibiting infection. The ability of neuraminidase to inhibit infection correlated with its ability to remove both α(2-3)- and α(2-6)-linked sialic acids from glial cells. A recombinant neuraminidase that specifically removes the α(2-3) linkage of sialic acid had no effect on virus binding or infection. A competition assay between virus and sialic acid-specific lectins that recognize either the α(2-3) or the α(2-6) linkage revealed that JCV preferentially interacts with α(2-6)-linked sialic acids on glial cells. Treatment of glial cells with tunicamycin, but not with benzyl N-acetyl-α-d-galactosaminide, inhibited infection by JCV, indicating that the sialylated JCV receptor is an N-linked glycoprotein. As sialic acid containing glycoproteins play a fundamental role in mediating many virus-cell and cell-cell recognition processes, it will be of interest to determine what role these receptors play in the pathogenesis of PML.
Approximately 70% of the human population worldwide is seropositive for JC virus (JCV). Like other polyomaviruses, JCV establishes a lifelong latent or persistent infection in its natural host (40, 49, 50, 68, 72). Reactivation of JCV in the setting of an underlying immunosuppressive illness, such as AIDS, is thought to lead to virus dissemination to the central nervous system (CNS) and subsequent infection of oligodendrocytes (37, 40, 66, 68). Reactivation of latent JCV genomes already present in the CNS has also been postulated to contribute to the development of progressive multifocal leukoencephalopathy (PML) following immunosuppression (19, 48, 55, 70, 75). Approximately 4 to 6% of AIDS patients will develop PML during the course of their illness (10). In the CNS, JCV specifically infects oligodendrocytes and astrocytes. Outside the CNS, JCV genomes have been identified in the urogenital system, in the lymphoid system, and in B lymphocytes (2, 17, 18, 30, 47, 59). In vitro, JCV infects human glial cells and, to a limited extent, human B lymphocytes (3, 4, 39, 41, 42). Recently, JCV infection of tonsillar stromal cells and CD34+ B-cell precursors has been described (47). These observations have led to the suggestion that JCV may persist in a lymphoid compartment and that B cells may play a role in trafficking of JCV to the CNS (4, 30, 47).
Virus-receptor interactions play a major role in determining virus tropism and tissue-specific pathology associated with virus infection. Viruses that have a very narrow host range and tissue tropism, such as JCV, are often shown to interact with high affinity to a limited number of specific receptors present on susceptible cells (26, 44). In some instances, virus tropism is strictly determined by the presence of specific receptors that mediate binding and entry (7, 16, 27, 35, 46, 53, 56, 67, 73, 74, 76). In other instances, however, successful entry into a cell is necessary but not sufficient for virus growth (5, 8, 45, 57). In these cases, additional permissive factors that interact with viral regulatory elements are required.
The receptor binding characteristics of several polyomaviruses have been described. The mouse polyomavirus (PyV) receptor is an N-linked glycoprotein containing terminal α(2-3)-linked sialic acid (12–14, 22, 28). Both the large and small plaque strains of PyV recognize α(2-3)-linked sialic acid. The small-plaque strain also recognizes a branched disialyl structure containing α(2-3)- and α(2-6)-linked sialic acids. Neither strain recognizes straight-chain α(2-6)-linked sialic acid. The ability of the large- and small-plaque strains of PyV to differentially recognize these sialic acid structures has been precisely mapped to a single amino acid in the major virus capsid protein VP1 (21). The large-plaque strains all contain a glycine at amino acid position 92 in VP1, and the small-plaque strains all contain a negatively charged glutamic acid at this position (21). In addition to forming small or large plaques, these strains also differ in the ability to induce tumors in mice (20). This finding suggests that receptor recognition plays an important role in the pathogenesis of PyV.
The cell surface receptor for lymphotropic papovavirus (LPV) is an O-linked glycoprotein containing terminal α(2-6)-linked sialic acid (26, 33, 34). Infection with LPV is restricted to a subset of human B-cell lines, and recognition of specific receptors is a major determinant of the tropism of LPV for these cells (26).
Unlike the other members of the polyomavirus family, infection of cells by simian virus 40 (SV40) is independent of cell surface sialic acids. Instead, SV40 infection is mediated by major histocompatibility complex (MHC)-encoded class I proteins (5, 11). MHC class I proteins also play a role in mediating the association of SV40 with caveolae, a prerequisite for successful targeting of the SV40 genome to the nucleus of a cell (1, 63). Not surprisingly, SV40 has been shown not to compete with the sialic acid-dependent polyomaviruses for binding to host cells (15, 26, 38, 58).
Very little is known about the early steps of JCV binding to and infection of glial cells. Like other members of the polyomavirus family, JCV is known to interact with cell surface sialic acids (51, 52). A role for sialic acids in mediating infection of glial cells has not been described. It is also not known whether the sialic acid is linked to a glycoprotein or a glycolipid. In a previous report, we demonstrated that JCV bound to a limited number of cell surface receptors on SVG cells that were not shared by the related polyomavirus SV40 (38). In this report, we demonstrate that virus binding to and infection of SVG cells is dependent on an N-linked glycoprotein containing terminal α(2-3)- and α(2-6)-linked sialic acids. Competitive binding assays with sialic acid-specific lectins suggest that the virus preferentially interacts with α(2-6)-linked sialic acids. We are currently evaluating the role of this receptor in determining the tropism of JCV for glial cells and B cells.
MATERIALS AND METHODS
Cells, virus, and antibody.
The human glial cell line SVG was established by transformation of human fetal glial cells by an origin-defective SV40 mutant and has been previously described (41). SVG cells were maintained in a humidified 37°C CO2 incubator in Eagle’s minimum essential medium (E-MEM; Mediatech Inc., Herndon, Va.) supplemented with 10% heat-inactivated fetal bovine serum (Mediatech). The hybridoma PAB597, which produces an antibody to SV40 V antigen, was obtained from E. Harlow and maintained in RPMI 1640 Hybrimax medium (Sigma, St. Louis, Mo.) supplemented with 10% heat-inactivated fetal bovine serum (Mediatech). The monoclonal antibody PAB597 has previously been shown to cross-react with JCV VP1 (6). The hybrid Mad-1/SVEΔ virus was constructed by insertion of the regulatory region of SV40 into the regulatory region of the Mad-1 strain of JCV (Mad-1/SVE) (69). Propagation of Mad-1/SVE in human glial cells led to deletions and alterations exclusively in the regulatory region. The rearranged regulatory region contains the origin of replication, the TATA box, and 78 bp of the first 98-bp repeat from JCV and one complete 72-bp repeat from SV40. Most of one of the 72-bp pair repeats and the 21-bp repeats from SV40 were deleted. The virus is termed Mad-1/SVEΔ to indicate this fact. A comparison of the restriction patterns of Mad-1/SVEΔ DNA with the prototype Mad-1 DNA were identical except for the regulatory region changes just discussed (69). No additional alterations were apparent following subsequent passage of Mad-1/SVEΔ in human fetal glial cells (69). We sequenced the VP1 gene of the chimeric virus and found that it is identical to published sequence of VP1 from the prototype Mad-1 strain.
Virus purification and labeling.
The preparation and labeling of JCV virions has been described elsewhere (38). In brief, 108 SVG cells were infected with 3,200 hemagglutination units of virus for 1 h at 37°C. At 3 weeks postinfection, when the cells showed extensive cytopathic effect, they were removed from the dishes by scraping and pelleted by centrifugation at 2,000 rpm for 30 min. The resulting cell pellet was suspended in 30 ml of the supernatant and subjected to three freeze-thaw cycles. Deoxycholic acid was then added to 0.25%, and the suspension was incubated at 37°C for 1 h. Cell debris was removed by centrifugation at 5,000 rpm, and the supernatants were layered on a cushion of cesium chloride (1.34 g/ml). Virus was banded by centrifugation for 24 h at 35,000 rpm in an SW55Ti rotor. This virus band was removed and dialyzed extensively against phosphate-buffered saline (PBS; 137 mM NaCl, 2.682 mM KCl, 8.1 mM Na2HPO4, 1.47 mM KH2PO4 [pH 7.2]). Purified virus was stored in 100-μl aliquots at −80°C. Virus titers were determined by hemagglutination assay. For virus labeling, 2.0 mg of gradient-purified JCV was dialyzed overnight in labeling buffer (0.05 M boric acid, 0.2 M NaCl [pH 9.2]). The virus was then incubated for 8 h at room temperature with 50 μl of a 5.0-mg/ml solution of fluorescein isothiocyanate (FITC) (Sigma) dissolved in dimethyl sulfoxide (Sigma) (25). The FITC-labeled virus was purified by centrifugation over a cushion of cesium chloride. The FITC-labeled virus band was visualized with a hand-held UV light, removed, and dialyzed extensively against PBS (pH 7.2). The ratio of FITC to protein was determined by spectrophotometry.
Enzymes, lectins, and glycosylation inhibitors.
Neuraminidase from Vibrio cholerae hydrolyzes α(2-3)- and α(2-6)-linked terminal sialic acids present in various gangliosides, glycoproteins, mucopolysaccharides, oligosaccharides, and polysaccharides and was obtained from Calbiochem-Novabiochem Corp., La Jolla, Calif. Recombinant α(2-3)-specific neuraminidase was obtained from New England Biolabs, Beverly, Mass. Peptide N-glycosidase (PNGase F) is an amidase which cleaves between the innermost GlcNAc and asparagine residues of high-mannose, hybrid, and complex oligosaccharides from N-linked glycoproteins and was obtained from New England Biolabs. Phospholipases A2 and C were from Boehringer Mannheim Corp., Indianapolis, Ind. Trypsin was obtained from Sigma. The digoxigenin-labeled lectins Maackia amurensis (MAA) and Sambucus nigra bark (SNA) were obtained from Boehringer Mannheim. MAA specifically binds to α(2-3)-linked sialic acid, and SNA specifically binds to α(2-6)-linked sialic acid. An antidigoxigenin FITC-labeled antibody was also obtained from Boehringer Mannheim. The inhibitor of N-linked glycosylation tunicamycin was obtained from Boehringer Mannheim. The inhibitor of O-linked glycosylation benzyl N-acetyl-α-d-galactosaminide (benzylGalNAc) was obtained from Oxford Glycosciences, Bedford, Mass.
Hemagglutination assays.
Human type O erythrocytes (RBCs) were obtained from a healthy volunteer by venipuncture. The cells were washed several times in Alsever’s buffer (0.1 M d-glucose, 0.027 M sodium citrate, 0.07 M NaCl [pH 6.5]) and stored for up to 2 weeks at 4°C. Before use, the cells were washed three times in PBS and adjusted to a concentration of 0.5% in PBS. For the hemagglutination inhibition assays, RBCs were incubated for 1 h at 37°C with neuraminidase (0.2 U/ml), α(2-3)-specific neuraminidase (5,000 U/ml), PNGase F (10,000 U/ml), or trypsin (10.0 μg/ml). The cells were then washed three times in PBS and added (50 μl) to serially diluted JCV in 96-well round-bottom culture dishes. Hemagglutination titers were read as the reciprocal of the highest dilution of virus that hemagglutinated.
Virus binding assays.
For virus binding assays, SVG cells were removed from culture dishes by incubation in Versene (0.15 M NaCl, 0.002 M KCl, 0.006 M Na2HPO4, 0.001 M KH2PO4, 0.001 M EDTA) and suspended at a concentration of 105 cells/ml in PBS. The cells were incubated on ice with increasing concentrations of FITC-labeled JCV virions or with an equivalent amount of FITC-labeled bovine serum albumin as a negative control. After a 60-min incubation, the cells were washed once in PBS containing 0.05 mg of propidium iodide per ml, washed twice in PBS, and fixed in 1.0 ml of PBS containing 1% paraformaldehyde. The cells were analyzed on a Becton Dickinson FACScalibur flow cytometer using CellQuest software.
Lectin binding and competition assays.
Lectins that specifically recognize either the α(2-3) (MAA) or α(2-6) (SNA) linkage of sialic acid were used to determine whether JCV interacts with α(2-3)- or α(2-6)-linked sialic acids on SVG cells. The lectins are tagged with digoxigenin, and their binding to cells is detected with antidigoxigenin FITC-labeled antibodies. To assess the effectiveness of neuraminidase and α(2-3)-specific neuraminidase on sialic acid removal, SVG cells were treated for 1 h with neuraminidase or α(2-3)-specific neuraminidase. The cells were then washed three times in PBS and incubated with a subsaturating amount of either MAA (10 μg/ml) or SNA (10 μg/ml) for 30 min at 4°C. The cells were washed in PBS and incubated with a sheep antidigoxigenin FITC-labeled antibody at 4°C for an additional 30 min. The cells were washed and fixed in PBS containing 1% paraformaldehyde, and lectin binding was assayed by flow cytometry. For the competition assay, SVG cells were incubated with a 500-fold excess (5.0 mg/ml) of unlabeled cesium chloride-purified JCV for 30 min at 4°C. SNA (10 μg/ml) or MAA (10 μg/ml) was then added and incubated for 30 min at 4°C. The cells were washed with PBS and incubated with sheep antidigoxigenin antibody for an additional 30 min at 4°C. After a final wash in PBS, the cells were fixed and lectin binding was assayed by flow cytometry.
Infectivity assays.
Infectivity was scored by two different methods. For the infection inhibition assays with neuraminidases, proteases, and phospholipases, we developed an assay to score infected cells by flow cytometry. The assay conditions were optimized by mixing SVG cells with HeLa cells at various ratios and staining for T antigen, which is constitutively expressed only in the SVG cells. The percentage of T-antigen-positive cells scored by flow cytometry accurately reflected the percentage of SVG cells in the mixed cell population. For the experiment, uninfected control cells, untreated infected cells, and enzyme-treated and infected cells were washed three times in PBS and fixed in 70% ethanol in PBS for 30 min at room temperature. The cells were washed three times in PBS and then incubated with anti-V-antigen monoclonal antibody (undiluted hybridoma supernatant) for 45 min at room temperature. The cells were then washed three times in PBS and incubated with for an additional 45 min with goat anti-mouse FITC-labeled antibody (Jackson Immunoresearch, West Grove, Pa.). The cells were washed three times in PBS and fixed in 1% paraformaldehyde in PBS. The cells were then analyzed by flow cytometry using Cellquest software. Uninfected SVG cells that had been incubated with both antibodies were used as a negative control.
For the infection inhibition assays with tunicamycin and benzylGalNAc, SVG cells were grown on coverslips, and infected cells were scored by indirect immunofluorescence assay. SVG cells were untreated or treated with tunicamycin (0.002 mg/ml) for 4, 8, and 24 h or with benzylGalNAc (10 μM) for 24 h. The cells were then incubated with JCV for 1 h at 37°C in the presence of inhibitor. The cells were then washed in PBS, and at 72 h postinfection, the coverslips were fixed with 70% ethanol. The percentage of infected cells was determined by indirect immunofluorescence assay of V-antigen-expressing cells. This was done by incubating the fixed cells with a 1:10 dilution of the anti-V-antigen monoclonal antibody PAB597 for 45 min at room temperature. The cells were then washed three times in PBS and incubated with a 1:50 dilution of goat anti-mouse FITC-labeled antibody for 45 min at room temperature. The cells were washed once with PBS, once with PBS containing 0.02% Evans blue (counterstain), and twice with PBS. V-antigen-positive cells were visualized on a Zeiss epifluorescence microscope and scored by counting. On average, 300 fields containing between 70 and 100 cells per field were counted.
RESULTS
Neuraminidase and PNGase F inhibit hemagglutination.
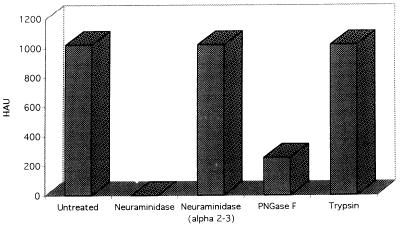
All of the polyomaviruses except SV40 hemagglutinate RBCs from various species. Human type O RBCs are the preferred cell type used to titrate JCV, although the virus will hemagglutinate other RBC types. As a first approach to characterizing JCV receptors, we tested a panel of enzymes for the ability to inhibit JCV-mediated hemagglutination of human type O RBCs. The RBCs were collected by venipuncture and washed three times in Alsever’s buffer before use in the assay. The washed RBCs were then suspended at a concentration of 0.5% (vol/vol) in PBS and incubated for 1 h at 37°C with or without either neuraminidase, α(2-3)-specific neuraminidase, PNGase F, or trypsin. After enzyme treatment of the RBCs, the cells were washed extensively in PBS and added to a dilution series of virus in 96-well round-bottom microtiter plates. After incubation for 2 h at 4°C, the hemagglutination titers of virus were scored as the reciprocal of the highest dilution of virus that caused hemagglutination. Neuraminidase and PNGase F both inhibited hemagglutination (Fig. 1). In contrast, α(2-3)-specific neuraminidase and trypsin had no effect on hemagglutination (Fig. 1). The ability of the neuraminidases to inhibit hemagglutination correlated with their known ability to remove both α(2-3)- and α(2-6)-linked sialic acids. The ability of PNGase F to inhibit suggests that N-linked glycoproteins are an important determinant of hemagglutination.
FIG. 1.
Neuraminidase and PNGase F inhibit JCV-mediated hemagglutination. Human type O RBCs were either untreated or treated with neuraminidase (0.2 U/ml), α(2-3)-specific neuraminidase (5,000 U/ml), PNGase F (10,000 U/ml), or trypsin (10.0 μg/ml) as indicated. The cells were then added (0.5% suspension) to twofold serial dilutions of JCV. Hemagglutination units (HAU) are expressed as the reciprocal of the highest dilution of JCV that still hemagglutinated. α(2-3)-specific neuraminidase and trypsin had no effect on hemagglutination. Neuraminidase totally abrogated hemagglutination, and PNGase F reduced hemagglutination fourfold.
Effects of enzymes on virus binding to glial cells.
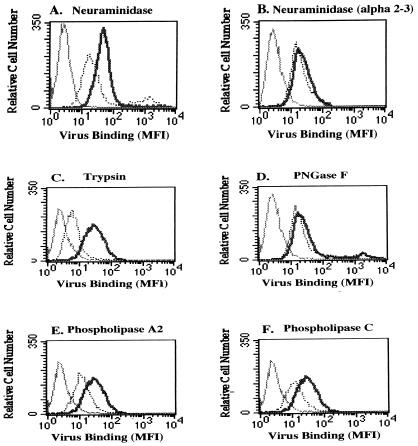
To characterize the molecular determinants of virus binding to glial cells, we measured the binding of FITC-labeled virus to enzyme treated SVG cells by flow cytometry. SVG cells were either untreated or treated for 1 h with increasing concentrations of neuraminidase, α(2-3)-specific neuraminidase, PNGase F, trypsin, phospholipase A2, or phospholipase C. Cell viability was determined by staining the cells with propidium iodide. Only concentrations of enzyme that maintained greater than 85% viability were used to measure their effects on virus binding. Similar to our results with the hemagglutination assay, neuraminidase inhibited virus binding to glial cells but α(2-3)-specific neuraminidase had no effect on virus binding (Fig. 2A and B). These results suggest that α(2-6)-linked sialic acids are also important for virus binding to SVG cells. In contrast to the effects of PNGase F on hemagglutination, this enzyme had very little effect on virus binding to SVG cells (Fig. 2D). Also, in contrast to the hemagglutination results, treatment of SVG cells with trypsin significantly reduced virus binding (Fig. 2C). Two other enzymes, phospholipase A2 and phospholipase C, also inhibited virus binding to SVG cells (Fig. 2E and F). These results suggest that virus binds to a wide variety of cell surface ligands on SVG cells.
FIG. 2.
Effects of enzymes on the binding of FITC-labeled JCV to SVG cells. SVG cells were either untreated (thick solid lines) or treated (dotted lines) with the indicated enzymes. FITC-labeled JCV was then added to the cells, and binding was measured by flow cytometry. MFI, mean fluorescence intensity. Thin solid lines here and in Fig. 3 and 5 are cells without added virus. Neuraminidase, trypsin, phospholipase A2, and phospholipase C all inhibited virus binding (compare thick solid lines to the dotted lines in panels A, C, E, and F). α(2-3)-specific neuraminidase and PNGase F did not inhibit virus binding significantly (compare thick solid lines with the dotted lines in panels B and D).
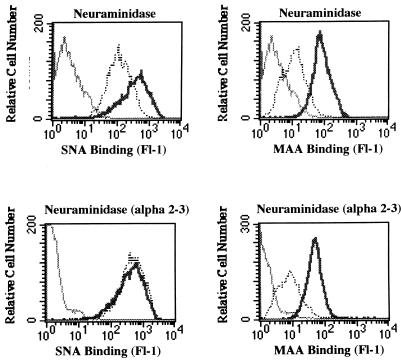
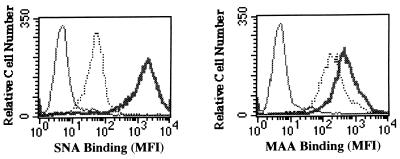
As a control for the effectiveness and specificity of the two neuraminidases, we measured the binding of an α(2-6)-linked sialic acid-specific lectin (SNA) and an α(2-3)-linked sialic acid-specific lectin (MAA) to untreated and enzyme treated SVG cells. As expected, neuraminidase inhibited the binding of both SNA and MAA to SVG cells, and α(2-3)-specific neuraminidase inhibited only the binding of MAA to SVG cells (Fig. 3).
FIG. 3.
Effects of neuraminidase and α(2-3)-specific neuraminidase on the binding of SNA and MAA to SVG cells. SVG cells were either untreated (thick solid lines) or treated (dotted lines) with neuraminidase or with α(2-3)-specific neuraminidase as indicated. The cells were then incubated with the digoxigenin-labeled lectins SNA or MAA. Lectin binding was detected by using an antidigoxigenin FITC-labeled antibody and flow cytometry. MFI, mean fluorescence intensity. Neuraminidase inhibited the binding of both SNA and MAA to the SVG cells. Treatment of the SVG cells with the α(2-3)-specific neuraminidase inhibited only binding of MAA to the cells.
Effects of enzymes on infection of glial cells by JCV.
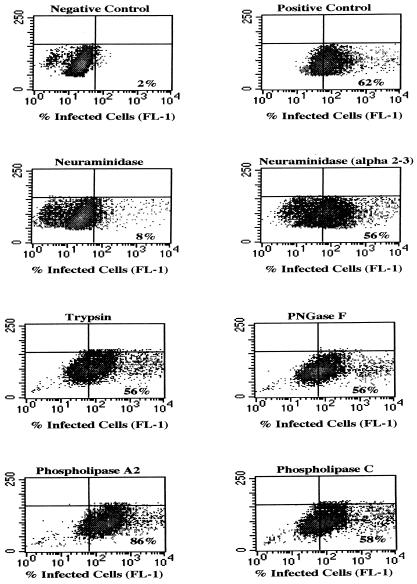
To determine the nature of specific receptors that mediate infection, we treated SVG cells with neuraminidase, α(2-3)-specific neuraminidase, PNGase F, trypsin, phospholipase A2, and phospholipase C under the same conditions used in the binding assays. The cells were then infected with virus and assayed for the presence of the late viral protein, V antigen, by flow cytometry. In contrast to their effects on virus binding, treatment of glial cells with trypsin, phospholipase A2, and phospholipase C did not inhibit infection (Fig. 4). PNGase F and α(2-3)-specific neuraminidase also had no effect on infection. The only enzyme that inhibited infection was neuraminidase (Fig. 4). These results are consistent with those of the hemagglutination assay and indicate that functional interactions between JCV and the cell surface are critically dependent on the presence of α(2-6)-linked sialic acids.
FIG. 4.
Effects of enzymes on virus infection of SVG cells. SVG cells were either untreated or treated with neuraminidase, α(2-3)-specific neuraminidase, trypsin, PNGase F, phospholipase A2, or phospholipase C as indicated. The cells were then infected with JCV, and the percentage of infected cells was scored by flow cytometric analysis of V-antigen expression. The negative control represents uninfected cells that were incubated with anti-V-antigen antibody and secondary FITC-labeled antibody. The positive control represent untreated, infected cells, stained with anti-V-antigen antibody and secondary FITC-labeled antibody. The mean fluorescence intensity of V-antigen-positive cells is shown on the x axis, and the percentage of V-antigen-positive cells relative to the negative control is indicated in the bottom right quadrant of each density plot. Treatment of SVG cells with α(2-3)-specific neuraminidase, trypsin, PNGase F, phospholipase A2, or phospholipase C did not inhibit infection significantly. In fact, treatment with phospholipase A2 slightly enhanced infection. Treatment of the SVG cells with neuraminidase, however, inhibited infection by 87%.
Effect of virus on lectin binding to SVG cells.
To determine whether JCV preferentially interacts with either α(2-3)- or α(2-6)-linked sialic acids, a binding competition assay was performed. In the first experiment, we examined whether α(2-3)-linked (MAA) or α(2-6)-linked (SNA) sialic acid-specific lectins could inhibit the binding of FITC-labeled virus to cells. Preincubation of the cells with MAA and SNA alone or in combination had no effect on virus binding (not shown). This result may be due to the fact that virus binds to ligands other than those containing sialic acid or that virus binding to sialic acids is of higher affinity than lectin binding. We therefore reversed the experiment and examined whether binding of unlabeled virus to SVG cells could block the subsequent binding of either SNA or MAA to the cells. We found that an excess of unlabeled virus reduced the binding of SNA to SVG cells 30-fold, whereas the binding of MAA was reduced only 2-fold (Fig. 5). This result suggests that virus binds preferentially to α(2-6)-linked sialic acids on SVG cells (Fig. 5).
FIG. 5.
JCV binding to SVG cells preferentially inhibits the subsequent binding of SNA to the cells. SVG cells were incubated in the absence (thick solid lines) or presence (dotted lines) of a 500-fold excess of unlabeled JCV. Equivalent amounts of the sialic acid specific lectins SNA or MAA were then added as indicated. The binding of SNA and MAA to the SVG cells was detected with an antidigoxigenin FITC-labeled antibody. MFI, mean fluorescence intensity. JCV inhibited the binding of SNA 30-fold and inhibited the binding of MAA twofold.
An inhibitor of N-linked glycosylation but not an inhibitor of O-linked glycosylation inhibits infection of glial cells by JCV.
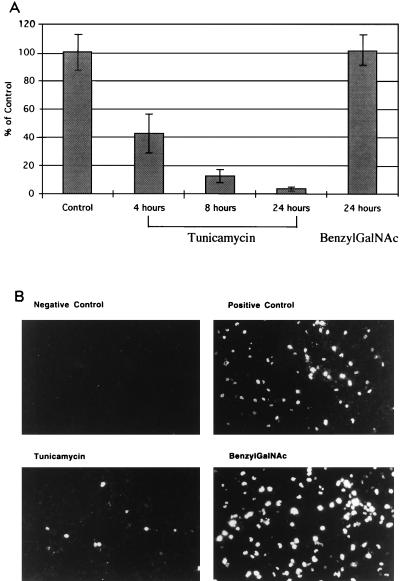
The effect of PNGase F on hemagglutination indicates that N-linked glycoproteins are important in mediating hemagglutination. PNGase F, however, had little to no effect in inhibiting virus binding to or infection of SVG cells. This discrepancy may be due to differences in the accessibility of N-linked glycoproteins to cleavage by PNGase F on RBCs versus SVG cells. We therefore used specific inhibitors of N-linked and O-linked glycosylation to determine whether N- or O-linked glycoproteins or glycolipids play a significant role in mediating infection of glial cells by JCV. SVG cells growing on coverslips were incubated for 4, 8, or 24 h in the presence of the N-linked glycosylation inhibitor tunicamycin or for 24 h in the presence of the O-linked glycosylation inhibitor benzyl GalNAc. The cells were then infected with virus for 1 h at 37°C in the presence of inhibitor. The cells were then washed three times with PBS to remove the inhibitor and any unbound virus. At 72 h postinfection, the cells were fixed in 70% ethanol and stained with the anti-V-antigen monoclonal antibody. V-antigen-positive cells were scored by indirect immunofluorescence assay. Treatment of the cells with tunicamycin inhibited infection at all time points tested (Fig. 6). In contrast, treatment of the cells for 24 h with benzylGalNAc had no effect on infection by JCV (Fig. 6). These results confirm a role for N-linked glycoproteins in mediating infection and rule out a significant role for O-linked glycoproteins or glycolipids.
FIG. 6.
(A) Tunicamycin but not benzylGalNAc inhibits infection of SVG cells by JCV. SVG cells were either untreated or treated with tunicamycin (0.002 mg/ml) for 4, 8, or 24 h or with benzylGalNAc (10 μM) for 24 h. The cells were then infected with JCV and at 72 h postinfection were stained with an anti-V-antigen monoclonal antibody. The percentage of infected cells was scored by counting V-antigen-positive cells. On average, 300 microscopic fields (40×) containing between 70 and 100 cells were counted for each sample. The data are plotted as a percentage of the positive control, which was set at 100. (B) Representative photomicrographs of SVG cells either untreated (positive control) or treated with tunicamycin (8 h) or benzylGalNAc (24 h).
DISCUSSION
The presence of terminal sialic acid on many cell surface ligands plays an important role in cell-cell and virus-cell recognition processes (23, 24, 54, 61). For example, the immunoglobulin superfamily-related lectins sialoadhesin and CD22 recognize their ligands in the context of α(2-3)- and α(2-6)-linked sialic acids, respectively (54, 71). Sialic acids also serve as specific receptors for several human viruses, including orthomyxoviruses, rotaviruses, and many polyomaviruses (9, 14, 28, 29, 32–34, 43, 60–62, 77). In each case, virus infectivity is mediated by recognition of specific sialic acid linkages to the underlying glycan. All of the polyomaviruses except SV40 use sialic acid as a major component of their cellular receptors (14, 28, 33, 34, 43, 60, 62). As the crystal structures of both SV40 and the PyV have been solved, detailed structural comparisons have been made between these two viruses (36, 64, 65). The critical difference between PyV and SV40 with respect to the ability to interact with sialic acid is an eight-amino-acid truncation in the SV40 virion attachment protein, VP1 (36, 65). These eight amino acids form part of an external loop on the surface of the PyV capsid that makes direct contact with sialic acid residues. Detailed structural analysis has also been performed on two strains of PyV that differ in the ability to recognize α(2-3)- or α(2-6)-linked sialic acids (64, 65). A small-plaque variant of PyV appears to recognize both α(2-3)-linked sialic acid and α(2-6)-linked sialic acid, whereas a large-plaque variant recognizes only the α(2-3) linkage. The critical difference between these two strains is that the small-plaque variant contains a glycine at amino acid 92 of VP1 and the large-plaque strain contains a glutamic acid at this position (21). It has been suggested that the presence of a negatively charged glutamate at this position interferes with the binding of branched α(2-6)-linked sialic acid chains. This possibility is supported by recent structural analyses of recombinant PyV VP1 complexed with a branched oligosaccharide containing the α(2-6)-linked sialic acid (64).
The interactions between the human polyomavirus JCV and its cellular receptor have not been studied in detail. In a previous report, we demonstrated that labeled JCV binds to a limited number of cell surface receptors on the human glial cell line SVG (38). Also in that report, we demonstrated that JCV and SV40 do not share MHC class I proteins as receptors (38). Here we demonstrate that hemagglutination, virus binding to SVG cells, and virus infection of SVG cells are inhibited by enzymatic removal of α(2-3)- and α(2-6)-linked sialic acids from RBCs and SVG cells. Removal of only α(2-3)-linked sialic acids by a recombinant α(2-3)-specific neuraminidase did not inhibit hemagglutination, virus binding, or virus infection. This finding indicates that the presence of the α(2-6) linkage is critical for mediating functional interactions between JCV and its receptor. In an attempt to distinguish whether JCV preferentially binds to either the α(2-3)- or the α(2-6)-linked sialic acid or to both simultaneously, we examined the abilities of specific lectins to block this interaction. We found that lectins were not able to block hemagglutination, virus binding to glial cells, or virus infection of glial cells. This result was not totally unexpected, as lectins are known to interact with weak affinity to their ligands. Presumably the avidity of virus binding to cells allows the virus to displace the bound lectins. We next examined whether virus binding to glial cells could block the binding of either the α(2-3)- or the α(2-6)-linked sialic acid-specific lectins to glial cells. We found that virus binding had a profound inhibitory effect on the binding of the α(2-6)-specific lectin but had only a minimal inhibitory effect on the binding of the α(2-3)-specific lectin. As the α(2-6)-linked sialic acid-specific lectin recognizes only straight-chain sialic acids on the cell surface, our data strongly suggest that JCV binds to straight-chain α(2-6)-linked sialic acids. In support of this view, the Mad-1 strain of JCV, from which our chimeric virus is derived, has an asparagine residue at position 92 and a glutamate at position 93 of VP1. Studies with PyV indicate that the presence of glutamate at this position is inhibitory to the recognition of α(2-6)-linked sialic acid in a branched disialyl configuration. We feel that it is therefore unlikely that JCV VP1 recognizes a branched α(2-6)-linked sialic acid.
Our initial results from the hemagglutination assay suggested that the JCV receptor was an N-linked glycoprotein, as PNGase F inhibited hemagglutination. We were unable to confirm this in the virus binding or infection studies, as PNGase F did not inhibit either binding or infection. In fact, in the virus binding assays we found that several enzymes in addition to neuraminidase inhibited. These included trypsin, phospholipase A2, and phospholipase C. The fact that phospholipases inhibited binding raised the possibility that the JCV receptor might be a glycolipid. Glycolipids have been implicated as playing a role in mediating interactions between BK virus and cell surfaces (62). As virus binding studies alone cannot distinguish between specific and nonspecific interactions with cells, we sought to determine which cell surface components were important for mediating infection. In contrast to the wide variety of enzymes that inhibited virus binding, we found that only neuraminidase was capable of inhibiting infection. The recombinant α(2-3)-linked sialic acid-specific neuraminidase had no effect on infection despite its ability to reduce α(2-3)-specific lectin binding. As neither phospholipases nor PNGase F inhibited infection, we tested known inhibitors of N- and O-linked glycosylation to ascertain whether the JCV receptor was an N-linked glycoprotein, an O-linked glycoprotein, or a glycolipid. The N-linked glycosylation inhibitor tunicamycin was a potent inhibitor of virus infection. The O-linked glycosylation inhibitor benzylGalNAc had no effect on infection. We observed some toxicity with the tunicamycin when cells were treated either with higher concentrations of inhibitor or for longer time periods. However, the ability of tunicamycin to inhibit infection of SVG cells by JCV was readily demonstrable at doses and time points that were not toxic to the cells. Tunicamycin has been reported to inhibit SV40 DNA replication by interfering with N-linked glycosylation of the SV40 T antigen (31). This effect was not seen in SV40-transformed COS cells presumably because T antigen is constitutively expressed to high levels in such cells (31). We also examined the effect of tunicamycin on the expression of SV40 V antigen in SVG cells and found no effect. This indicates that tunicamycin inhibits infection not at the level of DNA replication but rather at an earlier step in the life cycle of the virus. The fact that trypsin did not inhibit infection indicates that the JCV receptor is a trypsin-insensitive N-linked glycoprotein. This is not unusual, as many cell surface glycoproteins including MHC class I protein are not cleaved by trypsin.
Receptor recognition by both the mouse virus PyV and the monkey virus LPV have been shown to play significant roles in defining virus tropism and pathogenesis. Both viruses utilize glycoproteins terminating in either α(2-3)- or α(2-6)-linked sialic acid. Our data demonstrate that JCV also utilizes glycoproteins containing terminal sialic acids as specific receptors. It is likely that receptor recognition by JCV plays an important role in defining virus tropism and pathogenesis. We are currently examining the role of specific cellular receptors in defining the tropism of JCV for glial cells and B cells.
ACKNOWLEDGMENTS
This work was supported by American Cancer Society Internal Research Grant IRG-45-37, Rhode Island Foundation Medical Research Grants 5-29899 and 5-29810, and by Public Health Service grant CA71878 from the National Cancer Institute.
We thank Eugene O. Major, Leonard Norkin, and Ed Harlow for viral strains, cell lines, and hybridomas. We also thank Peter Shank for critical reading of the manuscript.
REFERENCES
- 1.Anderson H A, Chen Y, Norkin L C. Bound simian virus 40 translocates to caveolin enriched membrane domains, and its entry is inhibited by drugs that selectively disrupt caveolae. Mol Biol Cell. 1996;7:1825–1834. doi: 10.1091/mbc.7.11.1825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Arthur R R, Dagostin S, Shah K V. Detection of BK virus and JC virus in urine and brain tissue by the polymerase chain reaction. J Clin Microbiol. 1989;27:1174–1179. doi: 10.1128/jcm.27.6.1174-1179.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Assouline J G, Major E O. Human fetal Schwann cells support JC virus multiplication. J Virol. 1991;65:1002–1006. doi: 10.1128/jvi.65.2.1002-1006.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Atwood W J, Amemiya K, Traub R, Harms J, Major E O. Interaction of the human polyomavirus, JCV, with human B-lymphocytes. Virology. 1992;190:716–723. doi: 10.1016/0042-6822(92)90909-9. [DOI] [PubMed] [Google Scholar]
- 5.Atwood W J, Norkin L C. Class I major histocompatibility proteins as cell surface receptors for simian virus 40. J Virol. 1989;63:4474–4477. doi: 10.1128/jvi.63.10.4474-4477.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Atwood W J, Wang L, Durham L C, Amemiya K, Traub R G, Major E O. Evaluation of the role of cytokine activation in the multiplication of JC virus (JCV) in human fetal glial cells. J Neurovirol. 1995;1:40–49. doi: 10.3109/13550289509111009. [DOI] [PubMed] [Google Scholar]
- 7.Bai M, Harfe B, Freimuth P. Mutations that alter an Arg-Gly-Asp (RGD) sequence in the adenovirus type 2 penton base protein abolish its cell rounding activity and delay virus reproduction in flat cells. J Virol. 1993;67:5198–5205. doi: 10.1128/jvi.67.9.5198-5205.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bass D M, Greenberg H B. Strategies for the identification of icosahedral virus receptors. J Clin Invest. 1992;89:3–9. doi: 10.1172/JCI115575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bass D M, Mackow E, Greenberg H B. Identification and partial characterization of rhesus rotavirus binding glycoprotein on murine enterocytes. Virology. 1991;183:602–610. doi: 10.1016/0042-6822(91)90989-o. [DOI] [PubMed] [Google Scholar]
- 10.Berger J R, Concha M. Progressive multifocal leukoencephalopathy: the evolution of a disease once considered rare. J Neurovirol. 1995;1:5–18. doi: 10.3109/13550289509111006. [DOI] [PubMed] [Google Scholar]
- 11.Breau W C, Atwood W J, Norkin L C. Class I major histocompatibility proteins are an essential component of the simian virus 40 receptor. J Virol. 1992;66:2037–2045. doi: 10.1128/jvi.66.4.2037-2045.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cahan L D, Paulson J C. Polyoma virus adsorbs to specific sialyloligosaccharide receptors on erythrocytes. Virology. 1980;103:505–509. doi: 10.1016/0042-6822(80)90208-1. [DOI] [PubMed] [Google Scholar]
- 13.Cahan L D, Singh R, Paulson J C. Sialyloligosaccharide receptors of binding variants of polyomavirus. Virology. 1983;130:281–289. doi: 10.1016/0042-6822(83)90083-1. [DOI] [PubMed] [Google Scholar]
- 14.Chen M H, Benjamin T. Roles of N-glycans with alpha 2,6 as well as alpha 2,3 linked sialic acid in infection by polyoma virus. Virology. 1997;233:440–442. doi: 10.1006/viro.1997.8596. [DOI] [PubMed] [Google Scholar]
- 15.Clayson E T, Compans R W. Characterization of simian virus 40 receptor moieties on the surfaces of Vero C1008 cells. J Virol. 1989;63:1095–1100. doi: 10.1128/jvi.63.3.1095-1100.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dalgleish A, Beverly P C, Clapham P, Crawford D, Greaves M, Weiss R. The CD4 (T4) antigen is an essential component of the receptor for the AIDS retrovirus. Nature. 1984;312:763–767. doi: 10.1038/312763a0. [DOI] [PubMed] [Google Scholar]
- 17.Dorries K, Vogel E, Gunther S, Czub S. Infection of human polyomaviruses JC and BK in peripheral blood leukocytes from immunocompetent individuals. Virology. 1994;198:59–70. doi: 10.1006/viro.1994.1008. [DOI] [PubMed] [Google Scholar]
- 18.Dubois V, Lafon M E, Ragnaud J M, Pellegrin J L, Damasio F, Baudouin C, Michaud V, Fleury H J A. Detection of JC virus DNA in the peripheral blood leukocytes of HIV-infected patients. AIDS. 1996;10:353–358. doi: 10.1097/00002030-199604000-00001. [DOI] [PubMed] [Google Scholar]
- 19.Elsner C, Dorries K. Evidence of human polyomavirus BK and JC infection in normal brain tissue. Virology. 1992;191:72–80. doi: 10.1016/0042-6822(92)90167-n. [DOI] [PubMed] [Google Scholar]
- 20.Freund R, Calderone A, Dawe C J, Benjamin T L. Polyomavirus tumor induction in mice, effects of polymorphisms of VP1 and large T antigen. J Virol. 1991;65:335–341. doi: 10.1128/jvi.65.1.335-341.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Freund R, Garcea R L, Sahli R, Benjamin T L. A single amino acid substitution in polyomavirus VP1 correlates with plaque size and hemagglutination behavior. J Virol. 1991;65:350–355. doi: 10.1128/jvi.65.1.350-355.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fried H, Cahan L, Paulson J. Polyoma virus recognizes specific sialyloligosaccharide receptors on host cells. Virology. 1981;109:188–192. doi: 10.1016/0042-6822(81)90485-2. [DOI] [PubMed] [Google Scholar]
- 23.Hanasaki K, Varki A, Powell L D. CD22-mediated cell adhesion to cytokine-activated human endothelial cells. J Biol Chem. 1995;270:7533–7542. doi: 10.1074/jbc.270.13.7533. [DOI] [PubMed] [Google Scholar]
- 24.Hanasaki K, Varki A, Stamenkovic I, Bevilacqua M P. Cytokine-induced beta-galactosidase alpha-2,6-sialyltransferase in human endothelial cells mediates alpha-2,6-sialylation of adhesion molecules and CD22 ligands. J Biol Chem. 1994;269:10637–10643. [PubMed] [Google Scholar]
- 25.Harlow E, Lane D. Antibodies: a laboratory manual. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1988. [Google Scholar]
- 26.Haun G, Keppler O T, Bock C T, Herrmann M, Zentgraf H, Pawlita M. The cell surface receptor is a major determinant restricting the host range of the B-lymphotropic papovavirus. J Virol. 1993;67:7482–7492. doi: 10.1128/jvi.67.12.7482-7492.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Haywood A M. Virus receptors: binding, adhesion strengthening, and changes in viral structure. J Virol. 1994;68:1–5. doi: 10.1128/jvi.68.1.1-5.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Herrmann M, Wilhelm von der Leith C, Stehling P, Reutter W, Pawlita M. Consequences of subtle sialic acid modification on the murine polyomavirus receptor. J Virol. 1997;71:5922–5931. doi: 10.1128/jvi.71.8.5922-5931.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hofling K, Brossmer R, Klenk H, Herrler G. Transfer of an esterase-resistant receptor analog to the surface of influenza C virions results in reduced infectivity due to aggregate formation. Virology. 1996;218:127–133. doi: 10.1006/viro.1996.0172. [DOI] [PubMed] [Google Scholar]
- 30.Houff S A, Major E O, Katz D A, Kufta C V, Sever J L, Pittaluga S, Roberts J R, Gitt J, Saini N, Lux W. Involvement of JC virus-infected mononuclear cells from the bone marrow and spleen in the pathogenesis of progressive multifocal leukoencephalopathy. N Engl J Med. 1988;318:301–305. doi: 10.1056/NEJM198802043180507. [DOI] [PubMed] [Google Scholar]
- 31.Huang T, Hsu M. Inhibition of DNA replication of adenovirus type 5 and Simian virus 40 by tunicamycin. Virology. 1991;182:889–893. doi: 10.1016/0042-6822(91)90636-p. [DOI] [PubMed] [Google Scholar]
- 32.Ito T, Suzuki Y, Mitnaul L, Vines A, Kida H, Kawaoka Y. Receptor specificity of influenza A viruses correlates with the agglutination of erythrocytes from different animal species. Virology. 1997;227:493–499. doi: 10.1006/viro.1996.8323. [DOI] [PubMed] [Google Scholar]
- 33.Keppler O T, Herrmann M, Oppenlander M, Meschede W, Pawlita M. Regulation of susceptibility and cell surface receptor for the B-lymphotropic papovavirus by N glycosylation. J Virol. 1994;68:6933–6939. doi: 10.1128/jvi.68.11.6933-6939.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Keppler O T, Stehling P, Herrmann M, Kayser H, Grunow D, Reutter W, Pawlita M. Biosynthetic modulation of sialic acid-dependent virus-receptor interactions of two primate polyoma viruses. J Biol Chem. 1995;270:1308–1314. doi: 10.1074/jbc.270.3.1308. [DOI] [PubMed] [Google Scholar]
- 35.Klatzman D, Champagne E, Chamaret S, Gruest J, Guetard D, Hercend T, Gluckman J, Montagnier L. T lymphocyte T4 molecule behaves as the receptor for human retrovirus LAV. Nature. 1984;312:767–768. doi: 10.1038/312767a0. [DOI] [PubMed] [Google Scholar]
- 36.Liddington R, Yan Y, Moulai J, Sahli R, Benjamin T, Harrison S. Structure of simian virus 40 at 3.8 Å resolution. Nature. 1991;354:278–284. doi: 10.1038/354278a0. [DOI] [PubMed] [Google Scholar]
- 37.Lipton H L. Is JC Virus latent in the brain. Ann Neurol. 1991;29:433–434. doi: 10.1002/ana.410290415. [DOI] [PubMed] [Google Scholar]
- 38.Liu C K, Hope A P, Atwood W J. The human polyomavirus, JCV, does not share receptor specificity with SV40 on human glial cells. J Neurovirol. 1998;4:49–58. doi: 10.3109/13550289809113481. [DOI] [PubMed] [Google Scholar]
- 39.Major E O, Amemiya K, Elder G, Houff S A. Glial cells of the human developing brain and B cells of the immune system share a common DNA binding factor for recognition of the regulatory sequences of the human polyomavirus, JCV. J Neurosci Res. 1990;27:461–471. doi: 10.1002/jnr.490270405. [DOI] [PubMed] [Google Scholar]
- 40.Major E O, Amemiya K, Tornatore C S, Houff S A, Berger J R. Pathogenesis and molecular biology of progressive multifocal leukoencephalopathy, the JC virus-induced demyelinating disease of the human brain. Clin Microbiol Rev. 1992;5:49–73. doi: 10.1128/cmr.5.1.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Major E O, Miller A E, Mourrain P, Traub R G, de Widt E, Sever J. Establishment of a line of human fetal glial cells that supports JC virus multiplication. Proc Natl Acad Sci USA. 1985;82:1257–1261. doi: 10.1073/pnas.82.4.1257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Major E O, Vacante D A. Human fetal astrocytes in culture support the growth of the neurotropic human polyomavirus, JCV. J Neuropathol Exp Neurol. 1989;48:425–436. doi: 10.1097/00005072-198907000-00004. [DOI] [PubMed] [Google Scholar]
- 43.Mantyjarvi R, Arstila P, Meurman O. Hemagglutination by BK virus, a tentative new member of the papovavirus family. Infect Immun. 1972;6:824–828. doi: 10.1128/iai.6.5.824-828.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Marsh M, Helenius A. Virus entry into animal cells. Adv Virus Res. 1989;36:107–151. doi: 10.1016/S0065-3527(08)60583-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mei Y F, Wadell G. Molecular determinants of adenovirus tropism. Curr Top Microbiol Immunol. 1995;199:213–228. doi: 10.1007/978-3-642-79586-2_11. [DOI] [PubMed] [Google Scholar]
- 46.Mendelsohn C L, Wimmer E, Racaniello V R. Cellular receptor for poliovirus: molecular cloning, nucleotide sequence, and expression of a new member of the immunoglobulin superfamily. Cell. 1989;56:855–865. doi: 10.1016/0092-8674(89)90690-9. [DOI] [PubMed] [Google Scholar]
- 47.Monaco M G C, Atwood W J, Gravell M, Tornatore C S, Major E O. JCV infection of hematopoetic progenitor cells, primary B lymphocytes, and tonsillar stromal cells: implication for viral latency. J Virol. 1996;70:7004–7112. doi: 10.1128/jvi.70.10.7004-7012.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mori M, Kurata H, Tajima M, Shimada H. JC virus detection by in situ hybridization in brain tissue from elderly patients. Ann Neurol. 1991;29:428–432. doi: 10.1002/ana.410290414. [DOI] [PubMed] [Google Scholar]
- 49.Norkin L C. Papovaviral persistent infections. Microbiol Rev. 1982;46:384–425. doi: 10.1128/mr.46.4.384-425.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Norkin L C, Steinberg V I, Kosz-Vnenchak M. Human glioblastomas persistently infected with SV40 carry non-defective viral genomes. J Virol. 1985;53:658–666. doi: 10.1128/jvi.53.2.658-666.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Padgett B, Rogers C, Walker D. JC virus, a human polyomavirus associated with progressive multifocal leukoencephalopathy. Infect Immun. 1977;15:656–662. doi: 10.1128/iai.15.2.656-662.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Padgett B, Walker D. Virologic and serologic studies of progressive multifocal leukoencephalopathy. Prog Clin Biol Res. 1983;105:107–117. [PubMed] [Google Scholar]
- 53.Philipson L, Lonberg-Holm K, Petterson U. Virus receptor interaction in an adenovirus system. J Virol. 1968;2:1064–1075. doi: 10.1128/jvi.2.10.1064-1075.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Powell L D, Varki A. I-type lectins. J Biol Chem. 1995;270:14243–14246. doi: 10.1074/jbc.270.24.14243. [DOI] [PubMed] [Google Scholar]
- 55.Quinlivan E B, Norris M, Bouldin T W, Suzuki K, Meeker R, Smith M S, Hall C, Kenney S. Subclinical central nervous system infection with JC virus in patients with AIDS. J Infect Dis. 1992;166:80–85. doi: 10.1093/infdis/166.1.80. [DOI] [PubMed] [Google Scholar]
- 56.Racaniello V R. Cell receptors for picornaviruses. Curr Top Microbiol Immunol. 1990;161:1–22. doi: 10.1007/978-3-642-75602-3_1. [DOI] [PubMed] [Google Scholar]
- 57.Rossman M G. Viral cell recognition and entry. Protein Sci. 1994;3:1712–1725. doi: 10.1002/pro.5560031010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sanjuan N, Zijlstra M, Carroll J, Jaenisch R, Benjamin T. Infection by polyomavirus of murine cells deficient in class I major histocompatibility complex proteins. J Virol. 1992;66:4587–4590. doi: 10.1128/jvi.66.7.4587-4590.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Schneider E M, Dorries K. High frequency of polyomavirus infection in lymphoid cell preparations after allogeneic bone marrow transplantation. Transplant Proc. 1993;25:1271–1273. [PubMed] [Google Scholar]
- 60.Seganti L, Mastromarino P, Superti F, Sinibaldi L, Orsi N. Receptors for BK virus on human erythrocytes. Acta Virol. 1981;25:177–181. [PubMed] [Google Scholar]
- 61.Shi W, Chammas R, Varki N M, Powell L, Varki A. Sialic acid 9-O-acetylation on murine erythroleukemia cells affects complement activation, binding to I-type lectins, and tissue homing. J Biol Chem. 1996;271:31526–31532. doi: 10.1074/jbc.271.49.31526. [DOI] [PubMed] [Google Scholar]
- 62.Sinibaldi L, Goldoni P, Pietropaolo V, Cattani L, Peluso C, Di Taranto C. Role of phospholipids in BK virus infection and haemagglutination. Microbiologica. 1992;15:337–344. [PubMed] [Google Scholar]
- 63.Stang E, Kartenbeck J, Parton R G. Major histocompatibility complex class I molecules mediate association of SV40 with caveolae. Mol Biol Cell. 1997;8:47–57. doi: 10.1091/mbc.8.1.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Stehle T, Harrison S C. High-resolution structure of a polyomavirus VP1-oligosaccharide complex: implications for assembly and receptor binding. EMBO J. 1997;16:5139–5148. doi: 10.1093/emboj/16.16.5139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Stehle T, Yan Y, Benjamin T L, Harrison S C. Structure of murine polyomavirus complexed with an oligosaccharide receptor fragment. Nature. 1994;369:160–163. doi: 10.1038/369160a0. [DOI] [PubMed] [Google Scholar]
- 66.Telenti A, Marshall W, Aksamit A, Smilack J, Smith T. Detection of JC virus by polymerase chain reaction in cerebrospinal fluid. Eur J Clin Microbiol Infect Dis. 1992;11:253–254. doi: 10.1007/BF02098091. [DOI] [PubMed] [Google Scholar]
- 67.Tomassini J E, Graham D, DeWitt C M, Lineberger D W, Rodkey J A, Colonno R J. cDNA cloning reveals that the major group rhinovirus receptor on HeLa cells in intercellular adhesion molecule 1. Proc Natl Acad Sci USA. 1989;86:4907–4911. doi: 10.1073/pnas.86.13.4907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Tornatore C, Amemiya K, Atwood W, Conant K, Major E O, Berger J. JC virus—current concepts and controversies in the molecular virology and pathogenesis of progressive multifocal leukoencephalopathy. Rev Med Virol. 1994;4:197–219. [Google Scholar]
- 69.Vacante D A, Traub R, Major E O. Extension of JC virus host range to monkey cells by insertion of a simian virus 40 enhancer into the JC virus regulatory region. Virology. 1989;170:353–361. doi: 10.1016/0042-6822(89)90425-x. [DOI] [PubMed] [Google Scholar]
- 70.Vago L, Cinque P, Sala E, Nebuloni M, Caldarelli R, Racca R, Ferrante P, Trabattoni G, Costanzi G. JCV-DNA and BKV-DNA in the CNS tissue and CSF of AIDS patients and normal subjects. Study of 41 cases and review of the literature. J Acquired Immune Defic Syndr Hum Retrovirol. 1996;12:139–146. doi: 10.1097/00042560-199606010-00006. [DOI] [PubMed] [Google Scholar]
- 71.Vinson M, Anton van der Merwe P, Kelm S, May A, Jones E, Crocker P R. Characterization of the sialic acid binding site in sialoadhesin by site directed mutagenesis. J Biol Chem. 1996;271:9267–9272. doi: 10.1074/jbc.271.16.9267. [DOI] [PubMed] [Google Scholar]
- 72.Walker D L, Frisque R J. The biology and molecular biology of JC virus. In: Salzman N P, editor. The Papovaviridae. New York, N.Y: Plenum Press; 1986. pp. 327–377. [Google Scholar]
- 73.Weiss R A. Cellular receptors and viral glycoproteins involved in retroviral entry. In: Levy J, editor. The Retroviridae. New York, N.Y: Plenum Press; 1993. pp. 1–108. [Google Scholar]
- 74.Weiss R A, Tailor C S. Retrovirus receptors. Cell. 1995;82:531–533. doi: 10.1016/0092-8674(95)90024-1. [DOI] [PubMed] [Google Scholar]
- 75.White F A, III, Ishaq M, Stoner G L, Frisque R J. JC virus DNA is present in many human brain samples from patients without progressive multifocal leukoencephalopathy. J Virol. 1992;66:5726–5734. doi: 10.1128/jvi.66.10.5726-5734.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Wickham T J, Mathias P, Cheresh D A, Nemerow G R. Integrins αVβ3 and αVβ5 promote adenovirus internalization but not virus attachment. Cell. 1993;73:309–319. doi: 10.1016/0092-8674(93)90231-e. [DOI] [PubMed] [Google Scholar]
- 77.Willoughby R E, Yolken R H, Schnaar R L. Rotaviruses specifically bind to the neutral glycosphingolipid asialo-GM1. J Virol. 1990;64:4830–4835. doi: 10.1128/jvi.64.10.4830-4835.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]