Highlights
-
•
Intrahepatic cholestasis of pregnancy (ICP) is a pregnancy-related condition with a global incidence of 0.5%–1.0%. The study aimed to reveal new diagnostic opportunities in laboratory assessment of the condition.
-
•
Aspartate aminotransferase to platelet ratio index (APRI) and Fibrosis-4 index (FIB-4) levels were higher in women with ICP than in controls.
-
•
APRI and FIB-4 are promising markers in predicting complications related to ICP.
Key words: APRI, FIB-4, intrahepatic cholestasis of pregnancy, obstetric cholestasis, pregnancy
Abstract
BACKGROUND
Intrahepatic cholestasis of pregnancy is a pregnancy-related liver condition that is characterized by elevated liver function tests and/or bile acids in the presence of pruritis.
OBJECTIVE
The study aimed to assess the aspartate aminotransferase to Platelet Ratio Index and Fibrosis-4 Index scores in intrahepatic cholestasis of pregnancy.
STUDY DESIGN
The prospective study was carried out by assessing 142 women: 71 whose pregnancies were complicated by intrahepatic cholestasis of pregnancy and 71 without intrahepatic cholestasis of pregnancy. The Fibrosis-4 Index score and aspartate aminotransferase to Platelet Ratio Index were assessed.
RESULTS
Our findings indicate that both aspartate aminotransferase to Platelet Ratio Index and Fibrosis-4 Index scores were reliable indicators of intrahepatic cholestasis of pregnancy, correlating with important complications of the condition.
CONCLUSION
This study provides valuable information to help clinicians better diagnose and perform the management of intrahepatic cholestasis of pregnancy.
AJOG Global Reports at a Glance.
Why was this study conducted?
Intrahepatic cholestasis of pregnancy (ICP) is a pregnancy-related condition with a global incidence of 0.5%–1.0%. The study aimed to reveal new diagnostic opportunities in laboratory assessment of the condition.
Key findings
Aspartate aminotransferase to platelet ratio index (APRI) and Fibrosis-4 index (FIB-4) levels were higher in women with ICP than in controls. Besides that we found the negative association between APRI and FIB-4 levels and gestation age at delivery.
What does this add to what is known?
APRI and FIB-4 are promising markers in predicting complications related to ICP.
Introduction
Intrahepatic cholestasis of pregnancy (ICP) is a liver disease that is characterized by the appearance of pruritis that cannot be explained by other causes, with a global incidence of 0.5% to 1.0%.1,2
The laboratory diagnosis of ICP is based on the assessment of serum bile acid (BA) levels and liver function tests, such as aspartate aminotransferase (AST), alanine aminotransferase (ALT), and others.3 Assessment of serum BA levels is considered the definitive biochemical marker in the diagnosis of ICP and monitoring of ICP patients’ condition.2 Based on BA values, cholestasis gravidarum can be classified as mild (BA, 10–39 μmol/L) and severe (BA, ≥40 μmol/L).4 Alanine aminotransferase and aspartate aminotransferase refer to liver enzymes and are markers of hepatocyte damage. At the same time, ALT and AST levels are frequently elevated in ICP, and their increase may precede the rise in BA values by 1–2 weeks.5 According to the presented data, ALT and AST values increase significantly in 85% of cases of intrahepatic cholestasis of pregnancy, in some cases reaching levels up to 25 times higher than their baseline values.6
Although biopsy is the most specific test to assess the nature and severity of liver condition, it has disadvantages, such as the risk of serious complications.7 Therefore, some scores have been designed to replace liver biopsy in terms of predicting liver injury.7 In the calculation of some of these scores, complete blood count and routine biochemical parameters are used.7, 8, 9 A promising marker in the diagnosis of ICP is the aspartate aminotransferase to platelet ratio index (APRI).10 The APRI score has been shown to be a useful tool in diagnosing and predicting the progression of liver cirrhosis and fibrosis, as it is a noninvasive test that correlates with liver biopsy results. At the same time, few studies would focus on the assessment of APRI level in women with ICP.11,12
The Fibrosis-4 (FIB-4) score, which is a noninvasive marker, is effective in predicting fibrosis in liver conditions.3,13,14 The FIB-4 score efficacy in various liver diseases has been appreciated, and it has been argued that it could be a promising marker for highlighting the progression of liver disease.7, 8, 9,14 However, there is little data regarding the role of FIB-4 score in ICP.7 Thus, further studies are needed to assess the role of APRI and FIB-4 in the diagnosis of ICP.
Material and Methods
The prospective study was carried out by assessing 142 women, divided into 2 groups: 71 whose pregnancies were complicated by ICP (L1) and 71 women without ICP (L0). Pregnant women were enrolled in the study in the ICP group (L1) on the onset of the symptoms and the next case without ICP for the L0. The diagnosis of ICP was confirmed by assessing clinical symptoms and BA levels. Women with known coagulopathy; preeclampsia; hemolysis, elevated liver enzymes, low platelet count syndrome; acute hepatitis; and drug-induced liver injury were excluded from the study. The women's ALT, AST, and platelet numbers were assessed to calculate the FIB-4 score and APRI. The aspartate aminotransferase/platelet ratio index is calculated using the formula: ([AST/upper limit of the normal values] × 100)/number of platelets (109/L).12,15 FIB-4 score was calculated using Sterling's formula [age (years) × AST (U/L)/number of platelets (109/L) × √ALT(U/L)].7,14,16
SPSS software (version 21, IBM, Aemonk NY) was used to conduct the statistical analysis. The arithmetic mean±standard deviation values were calculated to describe the numeric indicators, and a t test to compare the 2 means was applied. In addition, the median (interquartile interval) was calculated. To compare categorical variables in groups, the χ² test was used with Yates’ correction. The Pearson correlation coefficient (r) was calculated to measure a linear correlation between 2 variables. The receiver operating characteristics (ROC) curve was used to assess sensitivity and specificity in examining model efficiency. At the same time, the Youden index and the cutoff values for each analyzed indicator were calculated. P<.05 was considered statistically significant.
Results
The age of pregnant women included in the study was 18–43 years (29.5±6.3) years in the L1 and 27.3±5.4 years in the L0 (Table 1). Thus, the BA values assessed in the L1 BA ranged between 10.0–211.3 μmol/L, the mean values being 34.7±37.7 μmol/L. In the L0, the mean BA values were 3.3±1.6) μmol/L, ranging from 1.0–7.8 μmol/L. Considering the classification of ICP, we mention that mild condition was found in 50 women (70.4% [95% confidence interval (CI), 58.9–80.5%]) and severe condition was found in 21 women (29.6% [95% CI, 19.5– 41.1]), which correlates with the data in the literature.
Table 1.
Baseline characteristics of patients
| Number | Criteria | L1 (n=71) | L0 (n=71) | 95% CI | P values |
|---|---|---|---|---|---|
| 1 | Age (y) | 29.5±6.3; 30.0 (25.0–34.0) | 27.3±5.4; 27.0 (23.0–31.0) | 0.25–4.14 | .0270 |
| 2 | BA (μmol/L) | 34.7±37.7; 18.9 (11.1–44.0) | 3.3±1.6; 3.1 (2.1–4.4) | 22.54–40.25 | ˂.0001 |
| 3 | ALT (U/L) | 141.9 ±178.4; 76.0 (22.0–181.0) | 19.2±22.0; 13.0 (9.0–17.0) | 80.52–164.87 | ˂.0001 |
| 4 | AST (U/L) | 87.1±93.2; 57.8 (28.0–128.0) | 20.6±10.5; 17.0 (14.0–22.0) | 44.49–88.50 | ˂.0001 |
| 5 | Platelet (109/L) | 247.3±57.3; 243.0 (209.0–275.0) | 240.7±71.7; 230.0 (190.0–278.0) | −14.93 to 28.13 | .5456 |
| 6 | APRI | 1.20±1.20; 0.73 (0.38–1.74) | 0.30±0.10; 0.25 (0.18–0.34) | 0.61–1.18 | ˂.0001 |
| 7 | FIB-4 | 0.97±0.59; 0.81 (0.53–1.20) | 0.61±0.25; 0.55 (0.42–0.70) | 0.20–0.51 | ˂.0001 |
ALT, alanine aminotransferase; APRI, aspartate aminotransferase to platelet ratio index; AST, aspartate aminotransferase; BA, bile acids; CI, confidence intervals; FIB-4, Fibrosis-4 index; ICP, intrahepatic cholestasis of pregnancy; L0, with ICP; L1, without ICP.
Cemortan. Assessment of aspartate aminotransferase to platelet ratio index and Fibrosis-4 index score on women with intrahepatic cholestasis of pregnancy. Am J Obstet Gynecol Glob Rep 2024.
In line with the study's aim, ALT, AST, and platelet were assessed among participants. Thus, ALT values ranged from 6.0 to 1121.0 U/L in L1 and 5.3 to 138.8 U/L in L0. Increased ALT levels above the reference values were detected in 49 (69.0% [95% CI, 56.6–78.9%]) cases in the research group compared with 7 (9.9% [95% CI, 4.0–18.3]) cases in the control group (χ² 49.564, P=.0001). Mean ALT values in L1 were found to be 141.9±178.4 U/L vs 19.2±22.0 U/L in L0.
Although AST values were elevated less than ALT values, the elevation of AST levels above the reference values was detected in 53 (74.6% [95% CI, 62.3–83.5]) cases in the L1 compared with 9 (12.7% [95% CI, 4.2–18.8]) cases in the L0 (χ²=52.935, P=.0001). AST values ranged from 11.0–657.0 U/L in L1 and 11.0–71.2 U/L in L0. Mean AST values in L1 were found to be 87.1±93.2 U/L vs 20.6±10.5 U/L in L0.
It should be noted that in 10 cases (14.1% [95% CI, 6.8–25.8]) of L1, on the background of increased BA levels, liver function test values were within the normal range, indicating the heterogeneity of the condition.
In addition, we were interested in assessing platelet values in recruited women. Therefore, the mean value of the platelets in women with ICP was 247.3±57.3 109/L, compared with 240.7±71.7 109/L in L0.
As previously mentioned, a promising indicator in the diagnosis of ICP is the APRI score. The mean values of APRI in L1 were 1.2±1.2 compared with 0.3±0.1 in L0 (Figure 1). Analyzing the correlation between the APRI score and different indicators assessed in the study, it was detected that a negative correlation with the term when delivery occurred (r=−0.421, P=.01). In addition, the positive correlation was found with the meconium-stained amniotic fluid (r= 0.260, P=.05), and the BA level (r= 0.385, P=.01).
Figure 1.
APRI and FIB-4 levels in women included in the study (U/L)
APRI, aspartate aminotransferase to platelet ratio index; FIB-4, Fibrosis-4 index; ICP, intrahepatic cholestasis of pregnancy.
Cemortan. Assessment of aspartate aminotransferase to platelet ratio index and Fibrosis-4 index score on women with intrahepatic cholestasis of pregnancy. Am J Obstet Gynecol Glob Rep 2024.
The mean values of FIB-4 in L1 were 0.97±0.59 compared with 0.61±0.25 in L0, the difference being statistically significant. Analyzing the data obtained through the correlation of the FIB-4 score, it was found a positive correlation with the BA level (r=0.288, P=.05), cesarean delivery rate (r=0308, P=.01), and the amount of postpartum blood loss in women recruited in the study (r=0.237, P=.05). At the same time, a negative correlation was found with the gestational age at birth (r=−0.333, P=.01).
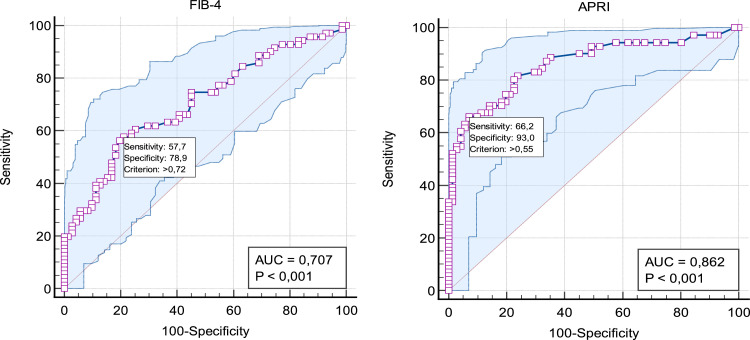
When evaluating the informative value of ALT, AST, and APRI in ICP diagnosis based on ROC, it is considered very good, with the area under the curve (AUC) ranging from 0.81–0.90 (Table 2).
Table 2.
Sensitivity and specificity of biochemical tests assessed in women with ICP
| Marker | AUC ROC | 95% CI | P values | Youden index | Cutoff values | Se (%) | Sp (%) |
|---|---|---|---|---|---|---|---|
| BA, µmol/L | 1.00 | 1.00–1.0 | <.0001 | 1.0000 | >7.80 | 100.0 | 100.0 |
| ALT, U/L | 0.85 | 0.79–0.92 | <.0001 | 0.6338 | >18.80 | 81.7 | 81.7 |
| AST, U/L | 0.87 | 0.81–0.93 | <.0001 | 0.6197 | >26.80 | 80.3 | 81.7 |
| APRI | 0.86 | 0.79–0.91 | <.0001 | 0.5915 | >0.55 | 66.2 | 92.9 |
| FIB-4 | 0.70 | 0.62–0.78 | <.0001 | 0.3662 | >0.72 | 57.7 | 78.8 |
ALT, alanine aminotransferase; APRI, aspartate aminotransferase to platelet ratio index; AST, aspartate aminotransferase; AUC, area under the curve; BA, bile acids; CI, confidence intervals; FIB-4, Fibrosis-4 index; ICP, intrahepatic cholestasis of pregnancy; ROC, receiver operating characteristics; Se, sensitivity; Sp, specificity.
Cemortan. Assessment of aspartate aminotransferase to platelet ratio index and Fibrosis-4 index score on women with intrahepatic cholestasis of pregnancy. Am J Obstet Gynecol Glob Rep 2024.
However, the informative value of FIB-4 is considered to be satisfactory (AUC ROC, 0.70), Figure 2. Hence, the APRI score was found to be more specific than the FIB-4 score, with a specificity of 92.9% vs 78.8%, respectively. In addition, the APRI score had a higher sensibility of 66.2% than the FIB-4 score, which had a sensibility of 57.7%.
Figure 2.
ROC curves for FIB-4 and APRI
APRI, aspartate aminotransferase to platelet ratio index; FIB-4, Fibrosis-4 index; ICP, intrahepatic cholestasis of pregnancy; ROC, receiver operating characteristics.
Cemortan. Assessment of aspartate aminotransferase to platelet ratio index and Fibrosis-4 index score on women with intrahepatic cholestasis of pregnancy. Am J Obstet Gynecol Glob Rep 2024.
Comment
Principal findings
According to our data, both APRI and FIB-4 scores were reliable indicators of ICP. Hence, the abovementioned indicators correlated with important complications of the condition, such as meconium-stained amniotic fluid, the amount of postpartum blood loss in women, and the gestational age at birth.
Results
The APRI score has been shown to be a useful tool in diagnosing and predicting liver cirrhosis and fibrosis, as it is a noninvasive test that correlates with liver biopsy results.11,12 Thus, in a meta-analysis of 40 studies, researchers concluded that an APRI score >1.0 has a sensitivity of 76% and a specificity of 72% for predicting cirrhosis. In addition, the same meta-analysis revealed that an APRI score >0.7 has a sensitivity of 77% and a specificity of 72% for predicting significant liver fibrosis.11 A recently published study has highlighted the association of APRI values with serum BA levels in ICP, and with the meconium-stained amniotic fluid in women with ICP.10 Another study revealed that the APRI score assessed in the first trimester may be associated with the development of ICP in the subsequent gestational period.17
The reason why markers such as APRI and FIB-4 can be used to determine fibrosis in liver pathologies is thrombocytopenia because of portal hypertension and increased AST and ALT levels because of liver injury.7 Even if the main application of the FIB-4 score is to determine fibrosis in liver disease, the authors highlighted that FIB-4 is an important marker of prediction in ICP, where fibrosis is minimal or absent.3,7 These results suggested that liver lesions, which do not progress with fibrosis, cannot be detected only by a single marker but need the use of several parameters. Even if liver fibrosis cannot be demonstrated morphologically in women with ICP, molecular changes can be observed.3,7 In women with ICP, a liver biopsy reveals bile plugs in the hepatocytes and canaliculus without dilatation or damage and centrilobular cholestasis without inflammation.17 These pathology results imply that ICP is a reversible condition.17
Clinical implications
Prediction and prevention of perinatal complications have a major impact on modern obstetrics.17 Hence, we found a negative association between APRI and FIB-4 levels and gestation age at delivery; in addition, APRI was shown to be a promising marker in predicting the presence of meconium-stained amniotic fluid. FIB-4 was shown to be a promising marker in predicting c-section rate and the total amount of postpartum blood loss in women with ICP.
Research implications
Previous studies demonstrate that the abovementioned markers can be useful tools in predicting cases of ICP; however, not much is known about the long-term implications of APRI and FIB-4 in the diagnosis and management of the condition. Therefore, it is necessary to perform a study involving a larger number of participants, and other biomarkers are needed to confirm those findings. This information provided will be important in the clinical counseling of women diagnosed with ICP.
Strengths and limitations
However, the results of this study need to be interpreted in light of its limitations. The study was based on a relatively small sample size; in addition, it presents a heterogeneity between groups (age); thus, the results may not be generalizable to larger populations. Despite some limitations and the small sample used, the study provided important information regarding the assessment of APRI and FIB-4 scores in the diagnosis of ICP in pregnant women.
Conclusions
The results of our study suggested that the assessment of APRI and FIB-4 scores in women with ICP is an important step in the diagnosis and management of the condition. In addition, our data showed the correlation between those markers and the gestational age at birth, as well as the amount of postpartum blood loss.
CRediT authorship contribution statement
Maria Cemortan: Writing – original draft, Data curation, Conceptualization. Corina Iliadi-Tulbure: Writing – review & editing, Validation, Resources. Irina Sagaidac: Writing – review & editing, Methodology, Conceptualization. Olga Cernetchi: Writing – review & editing, Validation, Supervision, Project administration.
Footnotes
The authors report no conflict of interest.
The study obtained ethical approval (identification number 46, from February 28, 2020) from the Ethics Committee of the Nicolae Testemitanu State University of Medicine and Pharmacy, Chisinau, Republic of Moldova. Written informed consent was obtained from all participants; all methods were carried out in accordance with relevant guidelines and regulations (study registration number ISRCTN21187408, https://www.isrctn.com/ISRCTN21187408).
Cite this article as: Cemortan M, Iliadi-Tulbure C, Sagaidac I, et al. Assessment of aspartate aminotransferase to Platelet Ratio Index and Fibrosis-4 Index score on women with intrahepatic cholestasis of pregnancy. Am J Obstet Gynecol Glob Rep 2024;XX:x.ex–x.ex.
References
- 1.Ozkan S, Ceylan Y, Ozkan OV, Yildirim S. Review of a challenging clinical issue: intrahepatic cholestasis of pregnancy. World J Gastroenterol. 2015;21:7134–7141. doi: 10.3748/wjg.v21.i23.7134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Williamson C, Geenes V. Intrahepatic cholestasis of pregnancy. Obstet Gynecol. 2014;124:120–133. doi: 10.1097/AOG.0000000000000346. [DOI] [PubMed] [Google Scholar]
- 3.Gok K, Takmaz T, Kose O, et al. Can first-trimester aspartate aminotransferase/platelet ratio index score predict intrahepatic cholestasis of pregnancy? Hepatology Forum. 2023;4:30–34. doi: 10.14744/hf.2022.2022.0031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Smith DD, Rood KM. Intrahepatic cholestasis of pregnancy. Clin Obstet Gynecol. 2020;63:134–151. doi: 10.1097/GRF.0000000000000495. [DOI] [PubMed] [Google Scholar]
- 5.Wood AM, Livingston EG, Hughes BL, Kuller JA. Intrahepatic cholestasis of pregnancy: a review of diagnosis and management. Obstet Gynecol Surv. 2018;73:103–109. doi: 10.1097/OGX.0000000000000524. [DOI] [PubMed] [Google Scholar]
- 6.Bacak SJ, Thornburg LL. Liver failure in pregnancy. Crit Care Clin. 2016;32:61–72. doi: 10.1016/j.ccc.2015.08.005. [DOI] [PubMed] [Google Scholar]
- 7.Gök K, Takmaz T, Köse O, Tüten N, Bostancı MS, Özden S. Effectiveness of the fibrosis-4 score in predicting intrahepatic cholestasis of pregnancy. Eur J Arch Med Res. 2022;38:299–303. [Google Scholar]
- 8.Vilar-Gomez E, Chalasani N. Non-invasive assessment of non-alcoholic fatty liver disease: clinical prediction rules and blood-based biomarkers. J Hepatol. 2018;68:305–315. doi: 10.1016/j.jhep.2017.11.013. [DOI] [PubMed] [Google Scholar]
- 9.Xiao G, Zhu S, Xiao X, Yan L, Yang J, Wu G. Comparison of laboratory tests, ultrasound, or magnetic resonance elastography to detect fibrosis in patients with nonalcoholic fatty liver disease: a meta-analysis. Hepatology. 2017;66:1486–1501. doi: 10.1002/hep.29302. [DOI] [PubMed] [Google Scholar]
- 10.Eyisoy ÖG, Taşdemir Ü, Eriç Özdemir ME, et al. Aspartate aminotransferase to platelet ratio index (APRI) score: is it useful in patients with intrahepatic cholestasis of pregnancy? J Matern Fetal Neonatal Med. 2022;35:10137–10142. doi: 10.1080/14767058.2022.2122036. [DOI] [PubMed] [Google Scholar]
- 11.Lin ZH, Xin YN, Dong QJ, et al. Performance of the aspartate aminotransferase-to-platelet ratio index for the staging of hepatitis C-related fibrosis: an updated meta-analysis. Hepatology. 2011;53:726–736. doi: 10.1002/hep.24105. [DOI] [PubMed] [Google Scholar]
- 12.Jain P, Tripathi BK, Gupta B, Bhandari B, Jalan D. Evaluation of aspartate aminotransferase-to-platelet ratio index as a non-invasive marker for liver cirrhosis. J Clin Diagn Res. 2015;9:OC22–OC24. doi: 10.7860/JCDR/2015/13944.6814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mohammed MA, Omar NM, Mohammed SA, Amin AM, Gad DF. FICK-3 Score combining fibrosis-4, insulin resistance and cytokeratin-18 in predicting non-alcoholic steatohepatitis in NAFLD Egyptian patients. Pak J Biol Sci. 2019;22:457–466. doi: 10.3923/pjbs.2019.457.466. [DOI] [PubMed] [Google Scholar]
- 14.Sterling RK, Lissen E, Clumeck N, et al. Development of a simple noninvasive index to predict significant fibrosis in patients with HIV/HCV coinfection. Hepatology. 2006;43:1317–1325. doi: 10.1002/hep.21178. [DOI] [PubMed] [Google Scholar]
- 15.AST to platelet ratio index (APRI) calculator. Hepatitis C Online. Available at:https://www.hepatitisc.uw.edu/page/clinical-calculators/apri. Accessed September 22, 2022.
- 16.Fibrosis-4 (FIB-4) calculator. Hepatitis C Online. Available at: https://www.hepatitisc.uw.edu/page/clinical-calculators/fib-4. Accessed September 22, 2022.
- 17.Tolunay HE, Kahraman NÇ, Varlı EN, et al. First-trimester aspartate aminotransferase to platelet ratio index in predicting intrahepatic cholestasis in pregnancy and its relationship with bile acids: a pilot study. Eur J Obstet Gynecol Reprod Biol. 2021;256:114–117. doi: 10.1016/j.ejogrb.2020.11.014. [DOI] [PubMed] [Google Scholar]