Abstract
Background
Dengue fever caused by dengue virus is a tropical disease and is among the deadliest vector-borne diseases. The humid and hot summers of Pakistan support the probation of the vectors responsible for the transmission of viral and other parasitic diseases.
Methodology
A retrospective study, from 2012- 2019, of dengue infected individuals from the Punjab province of Pakistan was carried out to analyze epidemiology, clinical and laboratory findings of subjects with dengue virus infection. Data was derived from National Institute of Health (NIH) followed by Dengue control program of Pakistan, covering the incidence rate in 36 districts of Punjab and Islamabad Capital Territory (ICT) respectively. Patients data including the presence of dengue specific antigen or/and antibodies such as NS1 and IgG/IgM were observed. The study also included the analysis of demographic data, geographic data, and the month-wise distribution of dengue cases to examine seasonal trends.
Results
We analyzed 25,682 dengue infected individuals. The statistical analysis revealed a significant association between genders in which male population was more affected by dengue than females. It was also noted that the middle age group was the most affected age group while the highest number of cases were reported in October. Rawalpindi and Lahore were the most affected cities in Punjab province while Islamabad represented the highest number of cases during the recent outbreak in 2019. The IgM and IgG antibodies were highly prevalent among the infected patients.
Conclusion
Dengue is endemic in Pakistan, circulating throughout the year. Highest number of cases were observed in the month of October, September and November respectively. Association between climate change and vector-borne diseases need to be investigated in Pakistan as they significantly influence the timing and intensity of dengue and other disease outbreaks. Further exploration of hematological parameters is required to better diagnose and treat the disease. For the effective control of dengue outbreaks, awareness campaigns on sewage management and vector control along with social factors are strongly recommended for better control and eradication of the disease.
Keywords: Dengue, Epidemiology, Antibodies, NS1, Punjab
Introduction
Dengue is a deadly emerging and re-emerging viral infection, which is prevalent in the tropical and subtropical regions throughout the globe [1, 2]. The mortality and morbidity of Dengue fever has made it a major public health concern. Dengue fever is caused by the dengue virus (DENV), a single-stranded RNA virus that belongs to the genus Flavivirus of the family Flaviviridae in Amarillovirales order. DENV has been classified into four distinct serotypes (DEN-1, DEN-2, DEN-3, DEN-4) and ten different genotypes based on the variation of sequence in the virus's envelope gene [3, 4]. Two species of Aedes mosquito, Aedes aegypti (A. aegypti) and Aedes albopictus (A. albopictus are mainly responsible for the transmission of the dengue virus) [5, 6]. The dengue virus infection is categorized into three different classes ranging from asymptomatic and moderate dengue fever to severe dengue shock syndrome (DSS) and dengue hemorrhagic fever (DHF), which may lead to death [7, 8]. The normal mortality rate of DENV infection is less than 1%; however, it usually stands 1–5% when there is no proper management, care and clinical administration [9, 10]. According to the World Health Organization (WHO), half of the world population is now at risk because of the dramatic growth of DENV infection incidence in recent decades. DENV infects approximately 100–400 million individuals each year throughout the world [11].
In Pakistan, the DENV is endemic since 1995 due to temperate climate of the country [12, 13]. Pakistan is located in South Asia between longitudes 61° and 75.5° east, latitudes 23.45° and 36.75° north, and the sixth most populated country of the world [14]. Several vector-borne diseases, including leishmaniases, dengue hemorrhagic fever, West Nile virus infection, malaria, scrub typhus, typhus, Crimean-Congo hemorrhagic fever, and Japanese encephalitis, have been reported in the region. These occurrences can be attributed to the subtropical location and favorable climatic conditions for vectors. [1, 14, 15]. In 1994, the first outbreak of dengue fever due to the serotypes DEN-1 and DEN-2 was reported, which led to thousands of morbidities [13]. A second outbreak involving DEN-3 serotype occurred in Karachi in 2005 with the dramatic increase of severe DHF patients [16]. Since 2006, every year, the dengue outbreaks and co-circulation of various dengue serotypes have been reported. In Lahore, Punjab, a major epidemic occurred in 2011 which reported more than 50,000 cases, this was followed by a huge dengue outbreak in Khyber Pakhtunkhwa province of Pakistan in 2013 [2, 17, 18]. Due to the rich fauna, artificial water reservoirs for various purposes, floods from heavy rainfall, open irrigation channels, and vast agricultural land of Pakistan provide a plenty of breeding sites for mosquito vector (s) [2]. The activities of dengue virus vectors vary according to seasons, various geographical areas of the country. It has been observed that incidence of cases typically increases after the rainy seasons [2, 8]. Primary exposure to the DENV infection leads to the development of lifelong immunity against the particular serotype, while secondary infection may lead to the development of life-threatening conditions of the disease such as DSS and DHF [4, 19]. A specific type of antibody, known as immune globulin, can be produced in response to infection by the same serotype of DENV.. However, the DSS and DHF mediated by an antibody-dependent enhancement (ADE) mechanism might occur if the causative agent is a different serotype [20].
The current study was aimed to examine the epidemiological and serological characteristics of dengue virus infection in Punjab province of Pakistan. Punjab is the most populous province of the Pakistan with diverse range of climate conditions. We examined the dengue positive patients from 2012 to 2019 from Punjab, outside Punjab and Islamabad.
Materials and methods
Selection of study area
Based on population, Punjab is the largest Province of Pakistan that is situated in northwestern region of Pakistan and divided in 36 districts. So this cross-sectional retrospective study was conducted in Punjab, and federally administered, a capital territory of Pakistan i.e. Islamabad from 2012–2019 and includes 34 districts of Punjab i.e. Attock, Bahawalnagar, Bahawalpur, Chakwal, Chiniot, Dera Ghazi Khan, Faisalabad, Gujranwala, Gujrat, Hafizabad, Kasur, Khanewal, Khushab, Lahore, Layyah, Lodhran, Mandi Bahuddin, Multan, Muzaffargarh, Nankana Sahib, Narowal, Okara, Pakpattan, Rahim Yar Khan, Rawalpindi, Sahiwal, Sargodha, Sheikhupura, Sialkot, Toba Tek Singh, Vehari, Jhang, Jehlum and Mianwali to examine epidemiological and clinical features of Dengue virus infection (Fig. 1).
Fig. 1.
Map of Pakistan with Punjab province in dark gray (A) and map shows Islamabad capital territory (ICT) in black color and Punjab province with its different districts in dark grey where dengue infected patients reported (B). The black bold lines show the boundaries of regions/Provinces of Pakistan
Data source
Since 2010, Pakistan has been experiencing an epidemic of dengue fever. In this study, the dengue epidemiological data from 2012 to 2019 was derived from National Institute of Health followed by Dengue control program of Pakistan, covering the incidence rate in 36 districts of Punjab and ICT respectively. The suspected patients were examined for the presence of IgG, IgM or/and NS1 antigen. The patients examined positive for the presence of either antigen (Ag), antibodies (Abs) and/or both were included in this study. The demographic information of individuals including age, gender, occupation etc. were recorded. The data regarding sign and symptoms of dengue infection (abdominal pain, enlarged liver, fever, haematemesis, skin rashes, nose and gum bleeding), previous exposure, hospital stay etc. was also recorded.
Inclusive and exclusive criteria
This study includes positive dengue patients data particularly and covering all districts of Punjab and Islamabad capital territory (ICT) respectively. In the study, 26, 582 positive patients data from 34 districts of Punjab, outside of Punjab (not resident of Punjab, who only come from other regions of Pakistan to visit during selected time period) and Islamabad territory has been selected as a sample size (n) for further epidemiological and serological analysis.
Ethical approval
The study was approved by National Institute of Health Islamabad. There was neither any sort of study-related patient interaction or interventions was involved. Furthermore, all data was analyzed anonymously and henceforth, informed consent was not prerequisite.
Case definition and laboratory investigation
Positive cases are diagnosed based on epidemiological exposure and clinical manifestation by experienced doctors and further confirmed by relevant laboratory results, such as enzyme linked immunosorbent assay (ELISA), NS1 antigen or polymerase chain reaction.
Based on dengue antibodies or NS1 antigen presence, the dengue infection has been classified as primary or secondary infection. Primary infection is defined as an IgM-negative/IgG-negative; or IgM-positive/IgG-negative on the blood sample drawn within 3 days of symptom onset. Secondary infection was defined as an IgM-negative/IgG-positive or an IgM-positive/IgG-positive result on the blood sample drawn within 3 days of symptom onset.The titers, of IgM and IgG antibodies vary based on whether the infection is primary or secondary. In the case of a primary (first) dengue infection, IgM levels are notably elevated, whereas in a secondary infection, IgM levels tend to be lower. Conversely, the levels of IgG increase during a secondary infection A patient was considered positive for dengue infection upon the detection of anti-dengue IgM. Similarly, NS1 is also considered an important biomarker for detection of dengue infection and hence was used for the rapid detection. The infection was also considered primary infection when the patients was negative for IgG and IgM antibodies but positive for NS1 antigen.
Data analysis
Different statistical tools were used for the analysis of data. The t-test and Mann–Whitney U test were used. Different categorical variables were expressed as percentage and frequency rate. Fisher’s exact test and χ2 test were used for the analysis of categorical variables. Various comparisons among different groups were also performed using the Bonferroni adjustment method. P-value of ≤ 0.05 was considered statistically significant. The maps were created using Arc View Geographical Information Software3 (Arc GIS). Statistical Package for the Social Sciences (SPPS) (version 23.0) was used for all the statistical analyses.
Results
The current study included 26,582 individuals who were tested positive for anti-dengue antibodies or NS1 antigen from 2012 to 2019 in Punjab province of Pakistan. Among the total infected individuals, 7,301 (27.46%) were female while 18,381 (69.14%) were male. However, the 900 (3.38%) individuals had an unknown sex.The dengue positive gender-wise cases along with years is represented in Table 1. Statistical analysis revealed a significant association (p value < 0.001) dengue infection and gender. The ratio of male-infected individuals was higher (18,281, n = 25,682) than female individuals (7,301, n = 25,682).
Table 1.
The gender-wise dengue infected individuals in different years are represented
| Year | Females | Males | P value |
|---|---|---|---|
| 2012 | 12 | 28 | < 0.001* |
| 2013 | 881 | 1766 | |
| 2014 | 470 | 973 | |
| 2015 | 1246 | 2966 | |
| 2016 | 1463 | 3590 | |
| 2017 | 309 | 726 | |
| 2018 | 219 | 742 | |
| 2019 | 2701 | 7590 | |
| Total | 7301 | 18,381 |
The chi-square test was applied and p < 0.05 was considered significant at a 95% confidence interval
*p-value is significant
Dengue infection in different age groups
The age-wise cases are represented in Table 2. We observed that the middle age group (21 to 30 years) was the most affected group (8,261, n = 25,682) followed by 11 to 20 years age group (5,551, n = 25,682) and 31 to 40 years age group (5,339, 25,682) respectively. The highest number of cases (n = 10,288) were reported in the year 2019. The older individuals (> 70 years) were found to be the least affected age group (n = 238) followed by smallest age group (1 to 10 years) with 701 patients and age group 60 to 70 years with 721 cases. The statistical analysis revealed a significant association (p value < 0.001) between age group and dengue infection.
Table 2.
Age-wise cases of dengue virus infected individuals are represented
| Age group | 2012 | 2013 | 2014 | 2015 | 2016 | 2017 | 2018 | 2019 | Total | p value |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 to 10 | 3 | 140 | 43 | 124 | 113 | 23 | 30 | 225 | 701 | < 0.001* |
| 11 to 20 | 9 | 631 | 401 | 950 | 991 | 205 | 195 | 2169 | 5551 | |
| 21 to 30 | 19 | 818 | 448 | 1323 | 1653 | 337 | 300 | 3363 | 8261 | |
| 31 to 40 | 5 | 519 | 269 | 848 | 1024 | 231 | 227 | 2216 | 5339 | |
| 41 to 50 | 2 | 312 | 167 | 525 | 653 | 118 | 126 | 1273 | 3176 | |
| 51 to 60 | 1 | 129 | 73 | 295 | 385 | 76 | 59 | 677 | 1695 | |
| 60 to 70 | 1 | 72 | 24 | 116 | 176 | 33 | 20 | 279 | 721 | |
| > 70 | 27 | 15 | 34 | 59 | 12 | 5 | 86 | 238 | ||
| Total | 40 | 2648 | 1440 | 4215 | 5054 | 1035 | 962 | 10,288 | 25,682 |
The chi-square test was applied and p < 0.05 was considered significant at a 95% confidence interval
*p-value is significant
Clinical features and serological pattern of dengue infection
The clinical features revealed that fever was the most common symptom observed in individuals of all age groups and both genders. The other common symptoms included headache, fatigue, nose and gum bleeding, abdominal pain, hematemesis, skin rashes and vomiting. The patients were categorized positive for dengue virus infection based on serological analysis. The presence of anti-dengue antibodies such as IgG and/or IgM along with NS1 antigen were examined. We found that IgM antibody was the most prevalent anti-dengue antibody detected in 11,148 individuals including 7,679 males while female 3,469 individuals. Anti-dengue specific IgG antibodies were found to be the second most (n = 5,396, males = 4,155, females = 1,241) prevalent serological marker after anti-dengue IgM. The serological pattern observed in our study is represented in Table 3. A total of 1,313 individuals were positive for both IgG and IgM including 955 males while 358 females. We observed that 2,649 individuals were positive for both types of anti-dengue antibodies while also for NS1 antigen as represented in Table 3.
Table 3.
Serological outcomes of patients are represented. The IgG presence shows the secondary dengue infection and IgM presence shows primary dengue infection. Whereas both presences show primary and secondary infection at a time
| Anti-dengue antibodies/ antigen | Male | Female | Total |
|---|---|---|---|
| IgG | 4,155 | 1,241 | 5,396 |
| IgM | 7,679 | 3,469 | 11,148 |
| NS1 | 1,939 | 587 | 2,526 |
| IgG + IgM | 955 | 358 | 1,313 |
| IgG + NS1 | 1,141 | 191 | 1,332 |
| NS1 + IgM | 1001 | 317 | 1,318 |
| NS1 + IgG + IgM | 2261 | 388 | 2649 |
| Total | 25,682 |
Region-wide distribution of dengue infection
Dengue positive individuals in various region/district of the Punjab province along with cases of outside of Punjab province are reported in Table 4. We have also summarized the cases of Capital territory Islamabad in Table 4. The data reveals that Rawalpindi (n = 7,215 cases) was the more affected region followed by Lahore (n = 2,958 cases) and Multan (n = 386 cases). It is important to mention that the capital territory of Islamabad alone represented the highest number of cases. We observed more than 10, 000 dengue positive cases in 2019 alone in Islamabad. We also reported 709 cases of those patients that were diagnosed in Punjab; however, they were resident of the Punjab province, represented in Table 4. Some of the regions represented the lowest number of cases such as Layyah, Khushab, and Jhelum which might be due their favorable climate condition.
Table 4.
District wise dengue positive cases in Punjab province
| District | 2012 | 2013 | 2014 | 2015 | 2016 | 2017 | 2018 | 2019 | Total |
|---|---|---|---|---|---|---|---|---|---|
| Attock | - | 3 | 12 | 19 | 23 | 15 | 13 | - | 85 |
| Bahawalnagar | 1 | 4 | - | 1 | 6 | 2 | 2 | - | 16 |
| Bahawalpur | - | 16 | 2 | 6 | 12 | 1 | 2 | - | 39 |
| Chakwal | - | 4 | 4 | 7 | 11 | 12 | 42 | - | 80 |
| Chiniot | - | 2 | 1 | 2 | - | 2 | - | - | 7 |
| Dera Ghazi Khan | - | 3 | - | 1 | 4 | 4 | 1 | - | 13 |
| Faisalabad | 12 | 21 | 1 | 12 | 100 | 9 | 12 | - | 167 |
| Gujranwala | 1 | 6 | 4 | 3 | 11 | 14 | 9 | - | 48 |
| Gujrat | - | 2 | 3 | 1 | 2 | 2 | - | - | 10 |
| Hafizabad | 1 | 2 | - | - | 1 | 2 | 2 | - | 8 |
| Kasur | - | 13 | - | - | 15 | 3 | - | - | 31 |
| Khanewal | - | 3 | - | 2 | 5 | 2 | 1 | - | 13 |
| Khushab | - | 1 | - | - | 1 | 2 | - | - | 4 |
| Lahore | 10 | 1510 | 97 | 142 | 1108 | 74 | 17 | - | 2958 |
| Layyah | 1 | 1 | - | - | - | - | 1 | - | 3 |
| Lodhran | - | 2 | - | 1 | 3 | - | 2 | - | 8 |
| Mandi Bahuddin | - | 4 | 4 | 2 | 3 | - | - | - | 13 |
| Multan | - | 9 | 2 | 362 | 6 | 3 | 4 | - | 386 |
| Muzaffargarh | - | 2 | - | 9 | 6 | 3 | - | - | 20 |
| Nankana Sahib | 1 | 1 | 1 | 1 | 7 | 4 | - | - | 15 |
| Narowal | 1 | 6 | 4 | 8 | 2 | 2 | - | 23 | |
| Okara | 1 | 3 | 2 | - | 2 | 1 | - | - | 9 |
| Outside Punjab | - | 21 | 22 | 322 | 150 | 132 | 62 | - | 709 |
| Pakpattan | 1 | 4 | 1 | 1 | 4 | 1 | - | - | |
| Rahim Yar Khan | - | 3 | 1 | 1 | 10 | 2 | - | - | 17 |
| Rawalpindi | - | 893 | 1212 | 3194 | 1159 | 334 | 423 | - | 7215 |
| Sahiwal | - | 6 | 1 | 4 | 7 | - | 1 | - | 19 |
| Sargodha | 1 | 5 | 3 | 2 | 18 | 5 | 2 | - | 36 |
| Sheikhupura | 4 | 80 | 60 | 10 | 25 | 8 | 1 | - | 188 |
| Sialkot | - | 5 | 1 | - | 4 | 1 | 1 | - | 12 |
| Toba Tek Singh | - | 4 | 1 | 4 | 8 | 1 | 4 | - | 22 |
| Vehari | - | 5 | 4 | 6 | - | 2 | - | 17 | |
| Jhang | 4 | - | - | 1 | 11 | 1 | - | - | 17 |
| Jehlum | 2 | - | - | 2 | - | - | - | - | 4 |
| Mianwali | 1 | - | - | 1 | 2 | 2 | 5 | - | 11 |
| Islamabad | - | - | 95 | - | 2314 | 393 | 353 | 10,292 | 13,447 |
Month-wise distribution of dengue cases
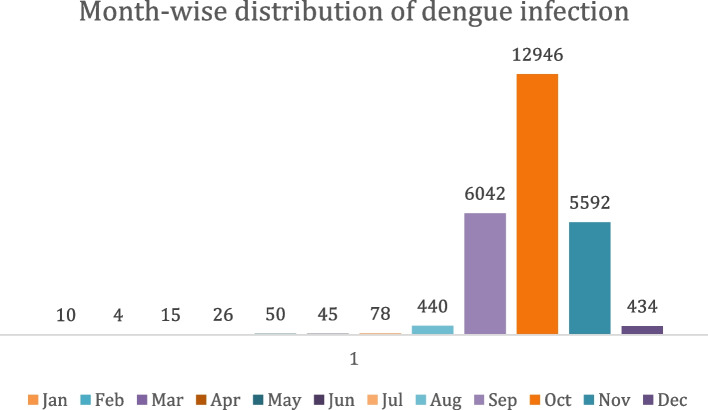
The month-wise pattern of dengue cases is represented in Fig. 2. Highest number of cases were observed in the month October (n = 12,946) followed by September (n = 6,042), and November (n = 5,592). Similar cases were recorded in August and December which were 440 and 434, respectively. The lowest number of cases were recorded in winter which steadily increased from February (n = 4) to March (n = 15), April (n = 26), May (n = 50) and then July (n = 78). A slight decrease in cases was observed in June (n = 45) which could be linked to the increased temperature experienced in June.
Fig. 2.
Month-wise dengue cases are represented. October represented the highest number of cases
Discussion
Dengue, a vector-borne viral disease that infects about 100 to 400 million individuals annually around the globe [21]. The burden of dengue virus infection has increased alarmingly around the globe in recent years. Although the risk of dengue infection exists in more than 128 countries, about 70% cases are reported from Asia [22, 23]. The number of cases reported to the World Health Organization in the last two decades has increased by more than eight-fold. The humid and hot summers of Pakistan increase the propagation of Aedes species that have been known vectors for dengue virus transmission. The dengue virus infection was identified in 1994 in Pakistan, although cases were experienced in 1982, however no information was available at that time [24]. Thousands of cases were reported in Karachi in 2005 and later in the entire country in 2010 [25].
In our study, we examined the epidemiological and serological features of dengue virus infection in the Punjab, the most populous province of Pakistan. We observed 25,682 dengue infected individuals in which 7,301 were female while 18,381 were male individuals and the statistical analysis revealed significant association between the gender and dengue infection. Our results are in accordance with the previous studies which reported higher risk of dengue infection in male individuals in other regions of the country [4, 26, 27]. Higher prevalence in male individuals could be possibly linked to the lifestyle of male individuals and their increased exposure to the environment in our society. The age-wise distribution of the cases is represented in Table 2. The middle age group (21 to 30 years) was observed as the most affected group. The high frequency of cases in the age group of 21 to 30 years in this study was due to larger population size in that age category. The statistical analysis revealed a significant association (p value < 0.001) between age group and dengue infection. Previous studies have also reported the young population as the most vulnerable group to viral infections which could also be linked to their exposure to the environment. The increased incidence in the populous districts such as Rawalpindi and Lahore as represented in Table 4 could be linked to the standing of these regions in trade and industrial activities along with climate conditions and more testing facilities. The highest number of cases were reported from Rawalpindi which is situated within the federal capital territory of Islamabad and is considered as hub for trades and business. Another reason could be travel of individuals from rural areas to urban areas. Some of the region represented the lowest number of cases such as Jhelum, Khushab, and Rahim Yar Khan which are known for extreme temperatures. The extreme temperature could also be linked with the lower number of cases in such regions which could affect the propagation of vectors responsible for dengue transmission. Previous studies have reported that climate variation can potentially increase the dengue transmission via the alteration in vector’s prevalence as well as changes in human behavior [28, 29]. Dengue outbreaks have been observed to be associated with climate change globally [30]. The main determinant of climate variability in Asia are the monsoons. It has been reported that dengue outbreaks have significantly coincided with the post-monsoon season of variable rainfall and vectors of dengue virus are more abundant in the post-monsoon season [31].
Among the clinical features, fever was the most common symptom along with headache, fatigue, nose and gum bleeding, abdominal pain, hematemesis, skin rashes and vomiting. The patients were categorized positive for dengue virus infection based on serological analysis. The presence of anti-dengue antibodies such as IgG and/or IgM along with NS1 antigen are represented in Table 3.
The highest number of cases were observed in the year 2019 in Islamabad as represented in Table 4. Previously, the highest number of dengue cases have been reported in the same year worldwide [32]. Our study suggests the immediate proper monitoring of epidemiological, serological and clinical features of dengue infection in the regions. The co-epidemics of dengue infection with other viral infections such as Covid-19 and polio could aggravate the less developed and overburdened health system of Pakistan countries [33–35]. For the effective control of dengue outbreaks, social factors along with sewage management and vector control are strongly recommended. We also suggest an awareness campaign on the management, control, and eradication of such diseases.
Conclusion
From the current study, it could be concluded that dengue infection was increased in Pakistan. Male individuals and the young population were the most vulnerable group to dengue infection. The federal capital represented the highest number of cases in 2019. Rawalpindi and Lahore were the most affected regions of the Punjab province while highest number of cases were reported in October. Further investigations are needed to explore the elevation in hematological and other parameters associated with dengue virus infection. The study limitations include the usage of antibodies based detections i.e. IgM detection for diagnosis of dengue which might have cross-reactivity related issues between other circulating flaviviruses. Further exploration of hematological parameters is required to better diagnose and treat the disease. We also suggest that the link between climate change and vector-borne diseases need to be investigated in Pakistan as they significantly influence the timing and intensity of dengue and other disease outbreaks. Public awareness and preventive strategies are highly recommended to overcome the burden of viral diseases including dengue, polio and Covid-19.
Authors’ contributions
All authors contributed equally. All authors are agree with and approved the current version of manuscript for submission.
Funding
The authors declare that no funds, grants, or other support were received during the preparation of this manuscript.
Availability of data and materials
Data related to this manuscript is available to researchers from Tanzeel Zohra upon request.
Declarations
Ethics approval and consent to participate
All authors consented to participate.
Consent for publication
All authors consented for the publication of their work.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Tanzeel Zohra, Email: tanzeelzohra@gmail.com, Email: t.zohra@nih.org.pk.
Muhammad Ayaz, Email: ayazuop@gmail.com, Email: mayaz@uom.edu.pk.
References
- 1.Raza FA, Ur Rehman S, Khalid R, Ahmad J, Ashraf S, Iqbal M, Hasnain S. Demographic and clinico-epidemiological features of dengue fever in Faisalabad, Pakistan. PLoS One. 2014;9(3):e89868. doi: 10.1371/journal.pone.0089868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Suleman M, Faryal R, Aamir UB, Alam MM, Nisar N, Sharif S, Shaukat S, Khurshid A, Angez M, Umair M, Mujtaba G. Dengue outbreak in Swat and Mansehra, Pakistan 2013: an epidemiological and diagnostic perspective. Asian Pac J Trop Med. 2016;9(4):380–384. doi: 10.1016/j.apjtm.2016.03.010. [DOI] [PubMed] [Google Scholar]
- 3.Vaughn DW, Green S, Kalayanarooj S, Innis BL, Nimmannitya S, Suntayakorn S, Endy TP, Raengsakulrach B, Rothman AL, Ennis FA, Nisalak A. Dengue viremia titer, antibody response pattern, and virus serotype correlate with disease severity. J Infect Dis. 2000;181(1):2–9. doi: 10.1086/315215. [DOI] [PubMed] [Google Scholar]
- 4.Haroon M, Jan H, Faisal S, Ali N, Kamran M, Ullah F. Dengue outbreak in Peshawar: clinical features and laboratory markers of dengue virus infection. J Infect Public Health. 2019;12(2):258–262. doi: 10.1016/j.jiph.2018.10.138. [DOI] [PubMed] [Google Scholar]
- 5.Atique S, Abdul SS, Hsu CY, Chuang TW. Meteorological influences on dengue transmission in Pakistan. Asian Pac J Trop Med. 2016;9(10):954–961. doi: 10.1016/j.apjtm.2016.07.033. [DOI] [PubMed] [Google Scholar]
- 6.Li Y, Kamara F, Zhou G, Puthiyakunnon S, Li C, Liu Y, Zhou Y, Yao L, Yan G, Chen XG. Urbanization increases Aedes albopictus larval habitats and accelerates mosquito development and survivorship. PLoS Negl Trop Dis. 2014;8(11):e3301. doi: 10.1371/journal.pntd.0003301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gubler DJ. The global pandemic of dengue/dengue haemorrhagic fever: current status and prospects for the future. Ann Acad Med Singapore. 1998;27(2):227–234. [PubMed] [Google Scholar]
- 8.World Health Organization, Special Programme for Research, Training in Tropical Diseases, World Health Organization. Department of Control of Neglected Tropical Diseases, World Health Organization. Epidemic, Pandemic Alert. Dengue: guidelines for diagnosis, treatment, prevention and control. World Health Organization; 2009.
- 9.Who T. Dengue: guidelines for diagnosis, treatment, prevention and control. Geneva: WHO Library; 2009. pp. 10–12. [PubMed] [Google Scholar]
- 10.Ranjit S, Kissoon N. Dengue hemorrhagic fever and shock syndromes. Pediatr Crit Care Med. 2011;12(1):90–100. doi: 10.1097/PCC.0b013e3181e911a7. [DOI] [PubMed] [Google Scholar]
- 11.Brady OJ, Hay SI. The global expansion of dengue: how Aedes aegypti mosquitoes enabled the first pandemic arbovirus. Annu Rev Entomol. 2020;65:191–208. doi: 10.1146/annurev-ento-011019-024918. [DOI] [PubMed] [Google Scholar]
- 12.Akram DS, Igarashi A, Takasu T. Dengue virus infection among children with undifferentiated fever in Karachi. Indian J Pediatr. 1998;65(5):735–740. doi: 10.1007/BF02731055. [DOI] [PubMed] [Google Scholar]
- 13.Rasheed SB, Butlin RK, Boots M. A review of dengue as an emerging disease in Pakistan. Public Health. 2013;127(1):11–17. doi: 10.1016/j.puhe.2012.09.006. [DOI] [PubMed] [Google Scholar]
- 14.Nishtar S, Boerma T, Amjad S, Alam AY, Khalid F, ul Haq I, Mirza YA. Pakistan's health system: performance and prospects after the 18th Constitutional Amendment. The Lancet. 2013;381(9884):2193–206. doi: 10.1016/S0140-6736(13)60019-7. [DOI] [PubMed] [Google Scholar]
- 15.Nawaz M, Din M, Khan A, Khan A, Ali M, Din SU, Aslam K. Epidemiological features of cutaneous leishmaniasis endemic in hilly areas of district Karak, Khyber-Pakhtunkhwa province of Pakistan. J Parasit Dis. 2020;44(4):725–729. doi: 10.1007/s12639-020-01250-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jamil B, Hasan R, Zafar A, Bewley K, Chamberlain J, Mioulet V, Rowlands M, Hewson R. Dengue virus serotype 3, Karachi, Pakistan. Emerg Infect Dis. 2007;13(1):182. doi: 10.3201/eid1301.060376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rai MA. Control of dengue fever in Pakistan. Nature. 2011;479(7371):41. doi: 10.1038/479041d. [DOI] [PubMed] [Google Scholar]
- 18.Savioli L, Daumerie D. Sustaining the drive to overcome the global impact of neglected tropical diseases: second WHO report on neglected tropical diseases. World Health Organization; 2013.
- 19.Zohra T, Saeed F, Ikram A, Khan T, Alam S, Adil M, et al. Nanomedicine as a potential novel therapeutic approach against the dengue virus. Nanomedicine. 2023;18(22):1567–84. [DOI] [PubMed]
- 20.Halstead SB. Neutralization and antibody-dependent enhancement of dengue viruses. Adv virus res. 2003;1(60):421–467. doi: 10.1016/S0065-3527(03)60011-4. [DOI] [PubMed] [Google Scholar]
- 21.Khan E, Hasan R, Mehraj V, Nasir A, Siddiqui J, Hewson R. Co-circulations of two genotypes of dengue virus in 2006 out-break of dengue hemorrhagic fever in Karachi. Pak J Clin Virol. 2008;43(2):176–179. doi: 10.1016/j.jcv.2008.06.003. [DOI] [PubMed] [Google Scholar]
- 22.Bhatt S, Gething PW, Brady OJ, Messina JP, Farlow AW, Moyes CL, Drake JM, Brownstein JS, Hoen AG, Sankoh O, Myers MF. The global distribution and burden of dengue. Nature. 2013;496(7446):504–507. doi: 10.1038/nature12060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Brady OJ, Gething PW, Bhatt S, Messina JP, Brownstein JS, Hoen AG, Moyes CL, Farlow AW, Scott TW, Hay SI. Refining the global spatial limits of dengue virus transmission by evidence-based consensus. PLoS Negl Trop Dis. 2012;6(8):e1760. doi: 10.1371/journal.pntd.0001760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chan YC, Tan HC, Seah CL, Li J, Chow VT, Salahuddin NI, Khan J. Dengue haemorrhagic fever outbreak in Karachi, Pakistan, 1994. [DOI] [PubMed]
- 25.Zohra T, Khalil AT, Saeed F, Latif B, Salman M, Ikram A, et al. Green nano-biotechnology: a new sustainable paradigm to control dengue infection. Bioinorg Chem Appl. 2022;(2022):1–25. [DOI] [PMC free article] [PubMed]
- 26.Anwar F, Tayyab M, Salman M, Abdullah, Din M, Khan J, Haq I. Dengue outbreak 2018 in district Shangla KPK; clinical features and laboratory markers of dengue virus infection. Future Virol. 2020;15(10):693–9. doi: 10.2217/fvl-2019-0130. [DOI] [Google Scholar]
- 27.Abdullah SA, Salman M, Din M, Khan K, Ahmad M, Khan FH, Arif M. Dengue outbreaks in Khyber Pakhtunkhwa (KPK), Pakistan in 2017: an integrated disease surveillance and response system (IDSRS)-based report. Pol J Microbiol. 2019;68(1):115. doi: 10.21307/pjm-2019-013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Liu-Helmersson J, Stenlund H, Wilder-Smith A, Rocklöv J. Vectorial capacity of Aedes aegypti: effects of temperature and implications for global dengue epidemic potential. PLoS ONE. 2014;9(3):e89783. doi: 10.1371/journal.pone.0089783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Liu K, Hou X, Ren Z, Lowe R, Wang Y, Li R, … Liu, Q. Climate factors and the East Asian summer monsoon may drive large outbreaks of dengue in China. Environ Res 2020;183:109190. [DOI] [PubMed]
- 30.de Oliveira-Júnior JF, Gois G, da Silva EB, Teodoro PE, Johann JA, Junior CAS. Non-parametric tests and multivariate analysis applied to reported dengue cases in Brazil. Environ Monit Assess. 2019;191(7):1–19. doi: 10.1007/s10661-019-7583-0. [DOI] [PubMed] [Google Scholar]
- 31.Baruah S, Dutta P. Seasonal prevalence of Aedes aegypti in urban and industrial areas of Dibrugarh district. Assam Trop Biomed. 2013;30(434):43. [PubMed] [Google Scholar]
- 32.World Health Organization. 2020. https://www.who.int/news-room/fact-sheets/detail/dengue-and-severe-dengue/. Accessed Dec 2020.
- 33.Din M, Asghar M, Ali M. COVID-19 and dengue coepidemics: A double trouble for overburdened health systems in developing countries. J Med Virol. 2021;93(2):601–602. doi: 10.1002/jmv.26348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Din M, Ali H, Khan M, Waris A, Ullah S, Kashif M, … Ali, M. Impact of COVID‐19 on polio vaccination in Pakistan: a concise overview. Rev Med Virol. 2020;31(4):1–8. [DOI] [PubMed]
- 35.Din M, Asghar M, Ali M. Delays in polio vaccination programs due to COVID-19 in Pakistan: a major threat to Pakistan's long war against polio virus. Public Health. 2020;189:1. doi: 10.1016/j.puhe.2020.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data related to this manuscript is available to researchers from Tanzeel Zohra upon request.