Abstract
Background
As comprehensive surgical management for gastric cancer becomes increasingly specialized and standardized, the precise differentiation between ≤T1 and ≥T2 gastric cancer before endoscopic intervention holds paramount clinical significance.
Objective
To evaluate the diagnostic efficacy of contrast-enhanced gastric ultrasonography in differentiating ≤T1 and ≥T2 gastric cancer.
Methods
PubMed, Web of Science, and Medline were searched to collect studies published from January 1, 2000 to March 16, 2023 on the efficacy of either double contrast-enhanced gastric ultrasonography (D-CEGUS) or oral contrast-enhanced gastric ultrasonography (O-CEGUS) in determining T-stage in gastric cancer. The articles were selected according to specified inclusion and exclusion criteria, and the quality of the included literature was assessed using the Quality Assessment of Diagnostic Accuracy Studies-2 scale. Meta-analysis was performed using Stata 12 software with data from the 2 × 2 crosslinked tables in the included literature.
Results
In total, 11 papers with 1124 patients were included in the O-CEGUS analysis, which revealed a combined sensitivity of 0.822 (95% confidence interval [CI] = 0.753–0.875), combined specificity of 0.964 (95% CI = 0.925–0.983), and area under the summary receiver operating characteristic (sROC) curve (AUC) of 0.92 (95% CI = 0.89–0.94). In addition, five studies involving 536 patients were included in the D-CEGUS analysis, which gave a combined sensitivity of 0.733 (95% CI = 0.550–0.860), combined specificity of 0.982 (95% CI = 0.936–0.995), and AUC of 0.93 (95% CI = 0.91–0.95). According to the I2 and P values of the forest plot, there was obvious heterogeneity in the combined specificities of the included papers. Therefore, the two studies with the lowest specificities were excluded from the O-CEGUS and D-CEGUS analyses, which eliminated the heterogeneity among the remaining literature. Consequently, the combined sensitivity and specificity of the remaining studies were 0.794 (95% CI = 0.710–0.859) and 0.976 (95% CI = 0.962–0.985), respectively, for the O-CEDUS studies and 0.765 (95% CI = 0.543–0.899) and 0.986 (95% CI = 0.967–0.994), respectively, for the D-CEGUS studies. The AUCs were 0.98 and 0.99 for O-CEGUS and D-CEGUS studies, respectively.
Conclusion
Both O-CEGUS and D-CEGUS can differentiate ≤T1 gastric cancer from ≥T2 gastric cancer, thus assisting the formulation of clinical treatment strategies for patients with very early gastric cancer. Given its simplicity and cost-effectiveness, O-CEGUS is often favored as a staging method for gastric cancer prior to endoscopic intervention.
Keywords: Gastric ultrasonography, Contrast agent, Gastric cancer, T-stage, Meta-analysis
Introduction
Gastric cancer has been listed as the malignancy with the fifth-highest incidence rate and the fourth-highest mortality rate worldwide, according to the Global Cancer Statistics 2020 report [1]. Because of the complexity of gastric cancer management, adequate preoperative staging is prerequisite for rationalizing patient treatment options with the specialization and standardization of comprehensive surgical management for gastric cancer.
Surgical resection and partial combination chemotherapy have been clinically preferred when patients have progressive gastric cancer (i.e., stage ≥T2 gastric cancer) [2]. However, studies previously demonstrated that endoscopic submucosal dissection (ESD) is a feasible option for treating early gastric cancer (i.e., stage ≤T1 gastric cancer) without lymph node metastasis, provided the indications are met. Because ESD is a less invasive procedure with comparable near- and long-term efficacy as traditional surgical procedures [3], it can potentially change the future paradigm of gastric cancer management. Therefore, accurately differentiating stage ≤T1 gastric cancer from stage ≥T2 gastric cancer before endoscopic intervention carries clinical significance.
Currently, the primary methods for diagnosing gastric lesions are gastroscopy, endoscopic ultrasonography (EUS), computed tomography (CT), double contrast-enhanced gastric ultrasonography (D-CEGUS), and oral contrast-enhanced gastric ultrasonography (O-CEGUS) [4–7]. Gastroscopy allows direct visualization and biopsy of gastric cancer. However, it does not determine the depth of tumor infiltration (i.e., T-staging). EUS is considered mandatory preoperatively for patients recommended for ESD [2]. However, it is invasive, it is susceptible to inflammation and the probe angle, and it is demanding on the operator. CT utilizes ionizing radiation, which is harmful to patients, and it has limitations for observing the submucosal hypodense zone (stage ≤T1).
CEGUS is a widely accessible and radiation-free imaging technique that can display the hierarchical structure of the gastric wall and lesions, and it is gradually gaining clinical acceptance as an essential tool for the mass screening of gastric cancer [5–8]. Despite many clinical studies on the feasibility of contrast-enhanced gastric ultrasonography in the preoperative evaluation of gastric cancer, its applicability in clinically staging gastric cancer remains unclear. Therefore, this meta-analysis examined the diagnostic utility of O-CEGUS/D-CEGUS in differentiating ≤T1 and ≥T2 gastric cancer for selecting suitable treatments.
Methods
Search strategy and selection criteria
We searched PubMed (National Center for Biotechnology Information, Bethesda, MD, USA), Web of Science (Thomson Reuters, Toronto, Canada), and Medline (using OvidSP, US National Library of Medicine, Bethesda, MD, USA) to identify studies published from January 1, 2000 to March 16, 2023 on the use of either O-CEGUS or D-CEGUS in differentiating stage ≤T1 and stage ≥T2 gastric cancer (based on the American Joint Committee on Cancer [AJCC]/International Union against Cancer [UICC] TNM staging system). The medical subject headings used for the search were “((gastr* OR stomach) AND (carcinoma OR cancer OR tumor OR neoplas* OR disease) AND ultraso*).” Additionally, “(NOT endoscop*)” was used to reduce the number of results. In addition, all published meta-analyses on similar topics were reviewed.
This study included both English or Chinese reports of clinical trials and cohort studies evaluating the diagnostic efficacy of either O-CEGUS or D-CEGUS in staging gastric cancer or determining the depth of infiltration. Eligible studies encompassed patients with well-defined postoperative pathological findings (gold standard), particularly pertaining to the T-stage according to the AJCC/UICC TNM staging system category (T1, T2, T3, and T4). Moreover, patients were required to undergo a comprehensive preoperative examination using O-CEGUS or D-CEGUS. In addition, the included studies provided sufficient information to construct a 2 × 2 column table to categorize the diagnostic accuracy of CEGUS for stage ≤T1 or ≥T2 gastric cancer (true positive, false positive, false negative, and true negative; Table 1).
Table 1.
Cross-contingency table of D-CEGUS/O-CEGUS in differentiating ≤T1 and ≥T2 gastric cancer
| D-CEGUS/O-CEGUS | Gold standard | |
|---|---|---|
| ≤T1 | T2-T4 | |
| ≤T1 | TP | FP |
| T2-T4 | FN | TN |
D-CEGUS Double contrast-enhanced gastric ultrasonography, O-CEGUS Oral contrast-enhanced gastric ultrasonography, TP true positives, FP false positives, FN false negatives, TN true negatives
Furthermore, studies involving animal experiments, reviews, correspondence, case reports, expert opinions, and editorials were excluded. Additionally, to ensure the robustness of the findings, only the largest studies were included in cases of overlap among study populations. Adhering to the guidelines outline in the Preferred Reporting Items for Systematic Reviews, the present investigation aimed to maintain methodological rigor and transparency throughout the review process [9].
Procedures
After excluding duplicates using EndNote X9, two researchers (ZY, XYY) independently screened the literature and extracted information based on aforementioned selection criteria. Generally, the researchers first read the titles and abstracts to exclude unsuitable studies. Then, the researchers thoroughly read the full texts of the included studies to further eliminate literature with incomplete or inadequate information.
The data that were extracted from the selected literature study included the name of first author of the study, the year of publication, and country (Table 2). Two researchers (ZY and XYY) independently assessed the quality of the included literature using RevMan 5.3 software (Copenhagen: The Nordic Cochrane Center, The Cochrane Collaboration, 2014) and Quality Assessment of Diagnostic Accuracy Studies-2 (University, Bristol, UK) [10]. Two investigators discussed and resolved any disagreement during the selection process, and a third investigator (YJY) was involved if a consensus was not reached between the two investigators.
Table 2.
The basic information of the included studies
| Author | Year | Country | PL (C/E) | Design(R/P) | Blind | Number | Gender(F/M) | Average age ±SD (years) | TNM edition | Agents | Machine | Scheme(D/O) | ≤T1 VS ≥T2 | |||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| TP | FP | FN | TN | |||||||||||||
| Cheng-long Wang | 2009 | China | C | R | DB | 59 | 24/38 | 56.0 ± 11.4 | UICC 6th | Contrast medium | Acuson Sequoia 512 | O | 2 | 1 | 1 | 55 |
| D | 2 | 0 | 1 | 56 | ||||||||||||
| Jian Cui | 2010 | China | C | R | DB | 59 | N | N | UICC 6th | water | Acuson Sequoia 512 | O | 2 | 15 | 1 | 41 |
| D | 2 | 6 | 1 | 50 | ||||||||||||
| Kengo Satoa | 2017 | Japan | E | R | DB | N | N | N | The Japanese versionc | Water | APLIO XG/SSA-790A, APLIO 500 TUS一A500 | O | 89 | 13 | 11 | 79 |
| Shuxiang Zhang | 2018 | China | E | R | DB | 72 | 27/45 | 46.9±11.3 | UICC 8th | Contrast medium | GE Voluson730 | O | 14 | 2 | 6 | 50 |
| Ping He | 2019 | China | E | R | DB | 54 | 18/36 | 61±9.70 | AJCC 8th | water | Philips iU22 | D | 7 | 0 | 1 | 46 |
| Q.Z. Gai | 2021 | China | E | R | DB | 109 | 41/68 | 51.37±11.45 | AJCC 8th | Contrast medium | Philips iU22 | O | 20 | 2 | 5 | 82 |
| Zhixiang Gao | 2022 | China | E | R | DB | 46 | N | N | N | Water | Philips iU22 | O | 3 | 3 | 0 | 40 |
| LING-LING Wu | 2023 | China | E | P | DB | 108 | 29/79 | 61.7±11.2 | AJCC 8th | Contrast medium | GE LOGIQ E9 | O | 16 | 3 | 3 | 86 |
| Liang Wang | 2019 | China | E | R | DB | 158 | 52/106 | 59.5±10.6 | N | Contrast medium | Acuson Sequoia 512 | D | 20 | 3 | 12 | 123 |
| Junling Wang | 2021 | China | E | R | DB | 206 | 95/111 | 59.7±11.3 | AJCC 8th | Contrast medium | Acuson Sequoia 512 | D | 10 | 3 | 1 | 192 |
| Tao Yu | 2015 | China | E | R | DB | 40 | 13/27 | 49(25~73)b | AJCC 7th | Contrast medium | GE Logiq E9 | O | 5 | 1 | 0 | 34 |
| Zhijun Liu | 2015 | China | E | P | DB | 264 | N | N | N | Contrast medium | Toshiba Aplio 400 | O | 32 | 4 | 4 | 224 |
| Sheng-Ri Liao | 2004 | China | E | R | DB | 125 | 47/78 | N | AJCC 6th | Water | Toshiba 6000, Aloka 2000, DU-6 | O | 5 | 2 | 4 | 114 |
| Sainan Wang | 2022 | China | E | R | DB | 50 | 28/22 | 25.5±25 | N | Contrast medium | MINDRAY Resona 7T | O | 8 | 0 | 4 | 38 |
aThis study included 190 patients with a total of 200 lesions, of which 8 lesions were unclear
bMedian age (range)
cThe TNM criteria of the Japanese Classification of Gastric Carcinoma
PL publication language, SD Standard deviation, C Chinese, E English, P Prospective study, R Retrospective study, DB Double blind design, N Not unclear, TP True positive, FP False positive, FN False negative, TN True negative, UICC Union for International Cancer Control, AJCC American Joint Committee on Cancer, D Double contrast enhanced gastric ultrasonograp, O Oral contrast-enhanced gastric ultrasonography
Statistical analysis
We presented true-positive, false-negative, false-positive, and true-negative data for the differential diagnosis of gastric cancer at stage ≤T1 or ≥T2 by O-CEGUS and/or D-CEGUS in each study separately to construct 2 × 2 cross-tabulation tables, which were used to calculate the sensitivity and specificity of gastric filling ultrasonography for differentiating stage ≤T1 gastric cancer from stage ≥T2 gastric cancer. If both ultrasonographic protocols were analyzed in a single study (O-CEGUS and D-CEGUS), we separately collected data for both procedures in our analyses. In addition, when documenting the accuracy of a study by different readers, we agreeably used the numbers provided by the first reader.
Statistical analyses were performed using Stata 14.0 (Stata, College Station, TX, USA) and MetaDisc 1.4 (Ramón y Cajal Hospital, Madrid, Spain) [11]. Data from each independent study were used to calculate the combined sensitivity, specificity, positive likelihood ratio (PLR), negative likelihood ratio (NLR), and diagnostic advantage ratio with their corresponding 95% confidence intervals (CIs). PLR was defined as the occurrence of a positive O-CEGUS/D-CEGUS diagnosis in patients with stage ≤T1 gastric cancer in discriminating stage ≤T1 gastric cancer from stage ≥T2 gastric cancer. NLR was defined as the likelihood of a negative O-CEGUS/D-CEGUS diagnosis in patients with stage ≥T2 gastric cancer in distinguishing stage ≤T1 gastric cancer from stage ≥T2 gastric cancer. The diagnostic odds ratio was defined as the ratio of the likelihood of a positive O-CEGUS/D-CEGUS result in patients with stage ≤T1 gastric cancer to the likelihood of a positive O-CEGUS/D-CEGUS result in patients with stage ≥T2 gastric cancer.
Because O-CEGUS and D-CEGUS are two different examination protocols, forest plots were used to present the combined sensitivity and specificity of O-CEGUS and D-CEGUS for differentiating stage ≤T1 gastric cancer from stage ≥T2 gastric cancer along with summary receiver operating characteristic (sROC) curves. Moreover, κ statistics were used to quantitatively assess the concordance between the two investigators in evaluating the quality of the literature. Heterogeneity in test precision was examined for each forest plot using the index of inconsistency (I2) and the Cochran Q statistic [12]. In the analysis, P ≥ 0.05 denoted a lack of no significant difference, indicating no heterogeneity among the studies, and the effect sizes could be combined using a fixed-effects model. However, P < 0.05 suggested a significant difference, denoting heterogeneity among the studies exists, and the effect sizes could be combined using a random-effects model. Low, moderate, and high heterogeneity were indicated by I2 ≤25%, 25% < I2 ≤ 50%, and I2 > 50%, respectively [13]. Heterogeneity among the studies was assumed when I2 > 50% and P < 0.05, and a random-effects model was used for statistical analysis.
Diagnostic analysis was performed using sROC curves. After plotting the corresponding sROC curves, the area under the curve (AUC) was calculated to determine its diagnostic value. Publication bias analysis was performed using Deek’s test for studies that could provide information on the diagnostic tetragonal table for discriminating stage ≤T1 gastric cancer from stage ≥T2 gastric cancer. Publication bias was considered to exist among the included literature if P < 0.05. Finally, sensitivity analysis was used to verify the stability of the model.
O-CEGUS/D-CEGUS scanning
Before the O-CEGUS examination, all patients fasted for more than 6–8 h. The ultrasonographic scan began with an essential two-dimensional ultrasound examination using an abdominal probe to identify various stomach lesions. Next, the patients were instructed to consume oral contrast or water (500–800 mL) to fill the gastric cavity, followed by scans in the supine and lateral positions. The presence, location, size, echogenic features, morphology, and stomach wall hierarchy of gastric lesions were imaged and recorded [2, 5, 6]. D-CEGUS was performed by pushing 2.4 mL of intravenous contrast (SonoVue®) through the median cubital vein along with O-CEGUS. Afterward, the whole stomach was scanned in the contrast pulse sequencing mode, and all related information was recorded [14].
The T-staging criteria for both D-CEGUS and O-CEGUS relied on the five-layer structure of the gastric wall. T1 denoted tumor invasion confined to the first three layers from the stomach cavity outward, specifically within the mucosa or submucosa. T2 indicated tumor invasion to the fourth layer, extending outward to the muscular propria. T3 represented tumor penetration of the fifth layer, signifying invasion of the serous layer. Lastly, T4 indicated tumor penetration through the serous layer, with possible invasion into adjacent tissues or organs. These defined criteria provide a structured framework for accurately staging gastric cancer based on the depth of tumor invasion, facilitating effective clinical decision-making and treatment planning.
Results
Literature retrieval results
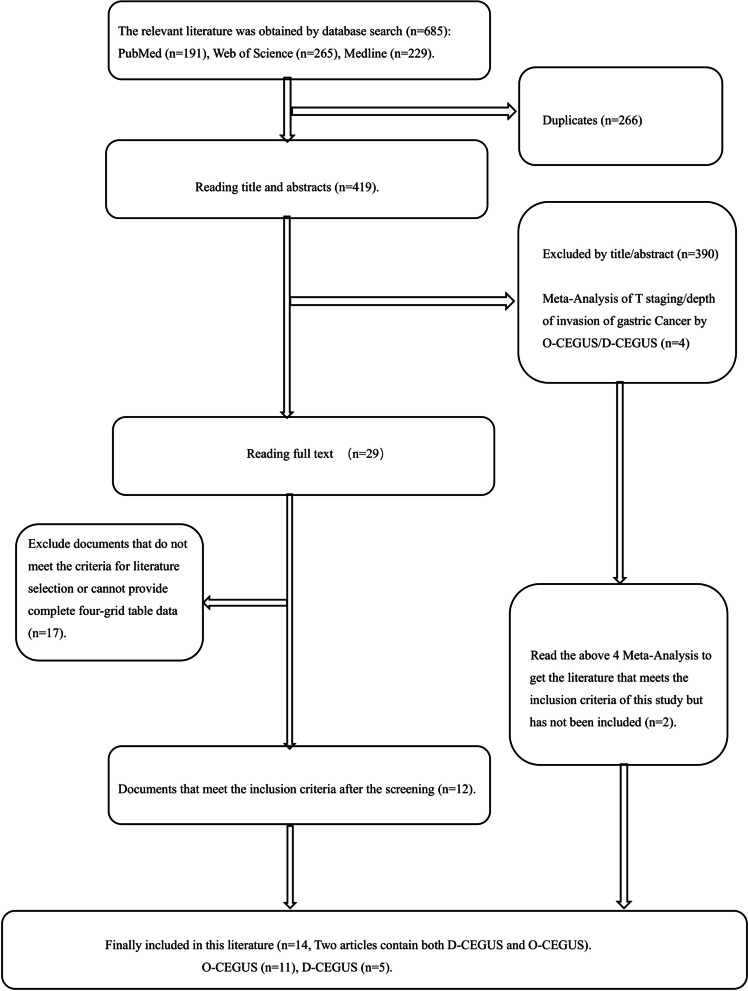
Using the search strategy, 685 papers were obtained, of which 419 papers remained after de-duplication using EndNote. After reading the titles and abstracts, 29 papers were chosen for full reading, and 17 did not meet the inclusion criteria or did not provide sufficient data to the complete four-cell table for differentiating stage ≤T1 and stage ≥T2 gastric cancer. Thus, 12 studies [15–26] were finally included. Among the 390 papers excluded from the initial screening, four meta-analyses related to the T-stage or depth of infiltration of gastric cancer diagnosed by either O-CEGUS or D-CEGUS were identified. Of these, two studies were found to provide sufficient data to complete the four-cell table (true positive, false positive, true negative, false negative) for differentiating stage ≤T1 and stage ≥T2 gastric cancer and were included [14, 27]. Altogether, 14 publications were included in this meta-analysis. The basic information of all included articles is presented in Table 2.
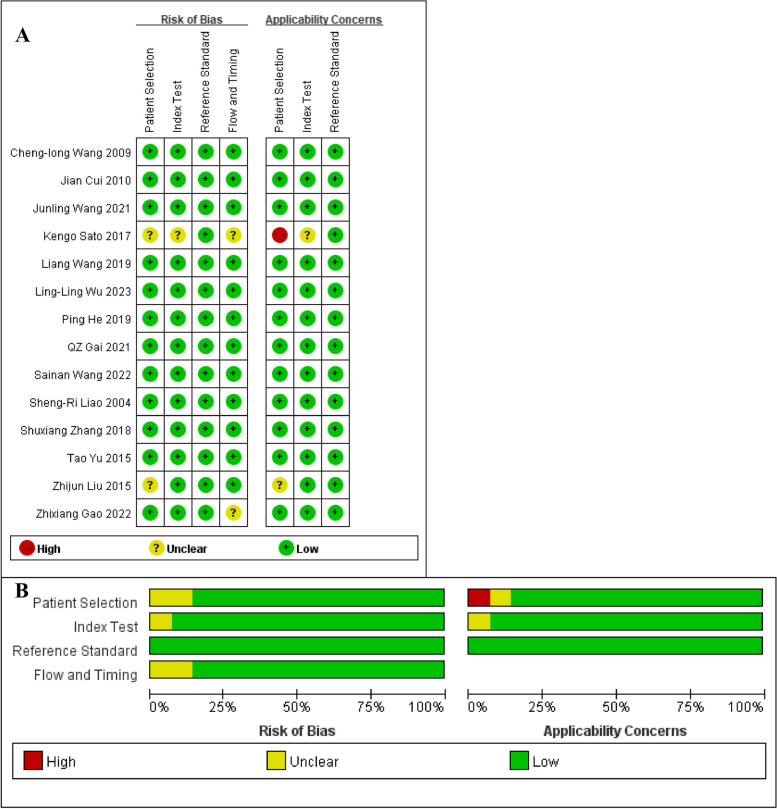
Because O-CEGUS and D-CEGUS are performed using two different operation protocols, we analyzed the two protocols separately. Among the 14 included studies, two investigated the use of both O-CEGUS and D-CEGUS in staging early gastric cancer, and thus, these studies were included in both the O-CEGUS and D-CEGUS analyses. Collectively, a pool of 11 papers, as well as another pool of five papers, were included in the meta-analysis on either O-CEGUS or D-CEGUS in staging early gastric cancer (≤T1 stage gastric cancer). The flow chart of the literature screening is presented in Fig. 1. There was high agreement between the two observers in the assessment of the quality of the literature (κ = 0.900). In summary, 1124 patients in 11 papers on O-CEGUS in staging gastric cancer were included in this meta-analysis, with the study populations ranging from 40 to 264, and 536 patients in five papers on D-CEGUS in gastric cancer staging were included in the meta-analysis, with the study populations ranging 54 to 206. Furthermore, the CEGUS-based diagnosis was confirmed by surgical pathology results. All of the information about the included studies and evaluation of the quality of the literature is presented in Table 2 and Fig. 2.
Fig. 1.
Flow chart of literature screening
Fig. 2.
Risk of bias and applicability concerns. A Quality evaluation of the included studies (n = 14). Red indicates high risk, green indicates low risk, and yellow indicates incomplete information that cannot be assessed. B Authors’ judgments about each domain presented as percentages across the included studies
Meta-analysis
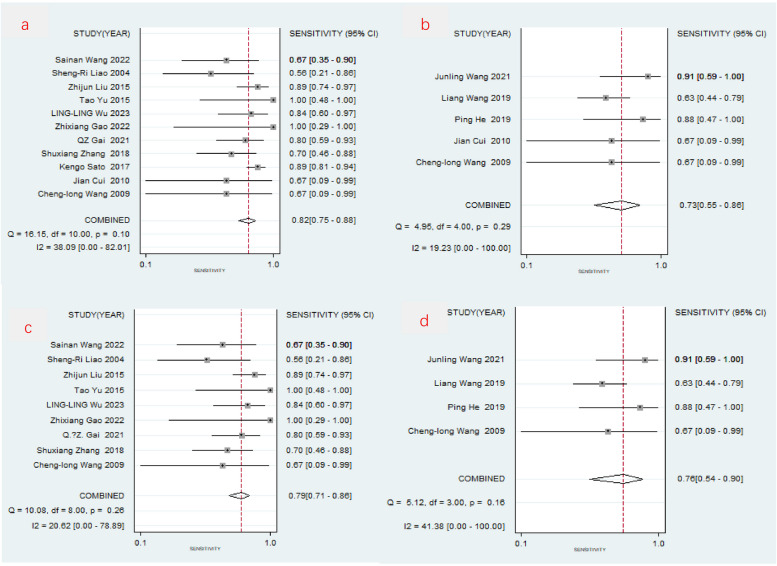
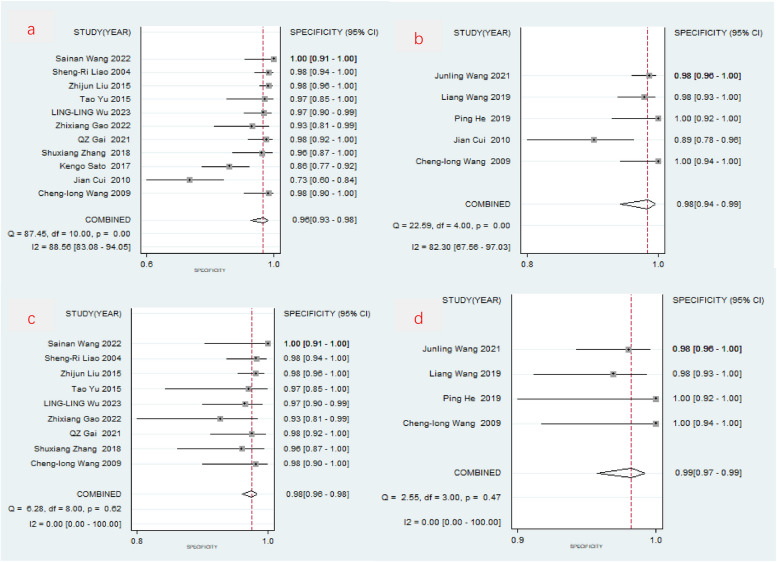
O-CEGUS: The combined sensitivity of the 11 O-CEGUS–related papers was 0.822 (95% CI = 0.753–0.875; Fig. 3a), the specificity was 0.964 (95% CI = 0.925–0.983), the PLR was 23.016 (95% CI = 11.199–47.302, Fig. 4a), the NLR was 0.184 (95% CI = 0.133–0.256), and the diagnostic odds ratio was 124.850 (95% CI = 62.039–251.250). The AUC was 0.92 (95% CI = 0.89–0.94), suggesting O-CEGUS had high accuracy in diagnosing gastric cancer (Fig. 5a). The forest plot revealed a sensitivity I2 of 38.09% (P = 0.10) and a specificity I2 of 88.56% (P < 0.01), suggesting significant heterogeneity regarding the specificity of the included literature, and further analysis of the sources of heterogeneity was needed.
D-CEGUS: The combined sensitivity (Fig. 3b) of the five D-CEGUS–related papers included in the study was 0.733 (95% CI = 0.550–0.860), the specificity (Fig. 4b) was 0.982 (95% CI = 0.936–0.995), the PLR was 40.349 (95% CI = 10.060–161.839), the NLR was 0.272 (95% CI = 0.149–0.497), and the diagnostic odds ratio was 148.286 (95% CI = 24.182–909.317). The AUC was 0.93 (95% CI = 0.91–0.95), suggesting that D-CEGUS had high accuracy in diagnosing gastric cancer (Fig. 5b). The forest plot revealed a sensitivity I2 of 19.23% (P = 0.29) and a specificity I2 of 82.30% (P < 0.01), suggesting significant heterogeneity in the specificity of the included literature, and further analysis of the sources of heterogeneity was needed.
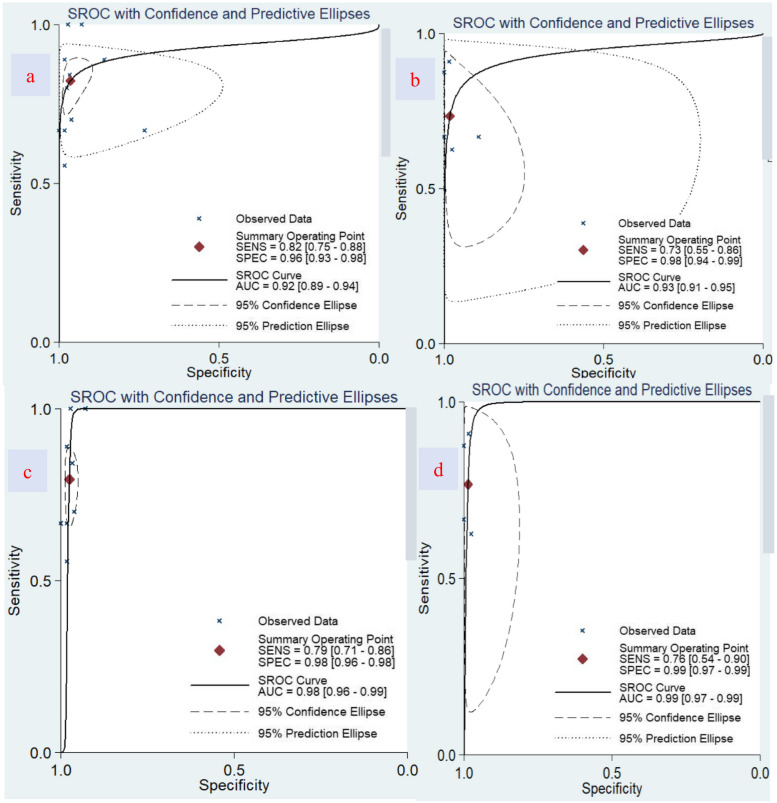
Heterogeneous analysis: After excluding the studies by Cui et al. (17) and Kengo Sato et al. (20), which had the lowest specificities, heterogeneity was not noticed among the remaining nine studies on O-CEGUS (sensitivity: I2 = 20.62%, P = 0.26; specificity: I2 = 0.00%, P = 0.62) or the remaining four studies on D-CEGUS (sensitivity: I2 = 41.38%, P = 0.16; specificity: I2 = 0.00%, P = 0.47; Figs. 3c,d, 4c, d). The combined sensitivity and specificity of the nine O-CEGUS papers were 0.794 (95% CI = 0.710–0.859) and 0.976 (95% CI = 0.962–0.985), respectively, and the AUC was 0.98 (Fig. 5c). The combined sensitivity and specificity of the four D-CEGUS papers were 0.765 (95% CI = 0.543–0.899) and 0.986 (95% CI = 0.967–0.994), respectively, and the AUC was 0.99 (Fig. 5d). The indicators of the sensitivity forest plot are presented in Fig. 3, and the indicators of the specificity forest plot are presented in Fig. 4.
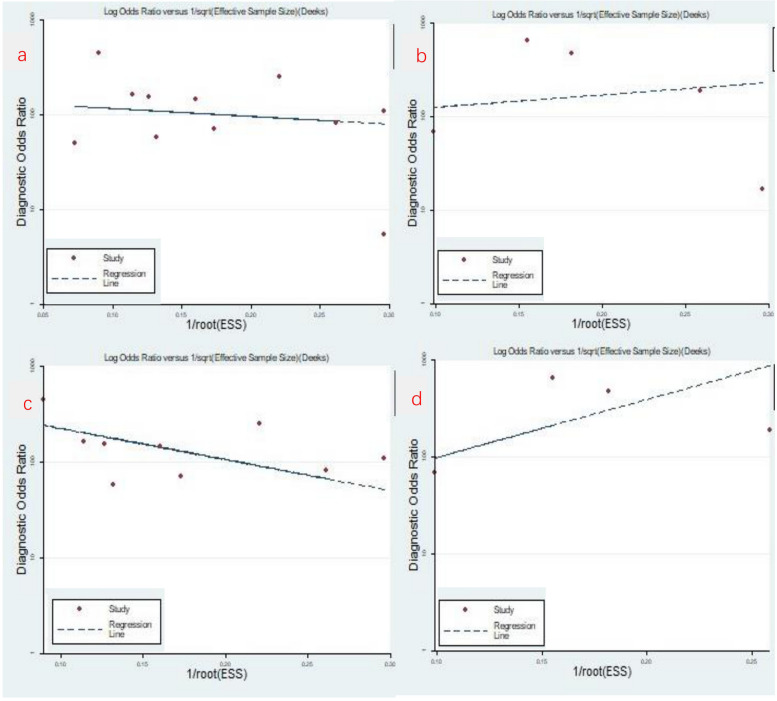
Publication bias: The literature included in this study was further examined for publication bias. Funnel plots, as depicted in Fig. 6a–d, were used to scrutinize the presence of publication bias among studies on O-CEGUS and D-CEGUS both before and after excluding sources of heterogeneity. The P-value derived from Deek’s test was 0.739 for the studies on O-CEGUS and 0.793 for the studies on D-CEGUS. The results suggest the absence of publication bias in this meta-analysis.
Sensitivity analysis: The stability and reliability of this meta-analysis were also evaluated by sensitivity analysis, in which one study was excluded at a time and the remaining data were combined to observe whether the heterogeneity and pooled effects changed. The results did not reveal any significant change in the heterogeneity and pooled effects of the meta-analysis in all cases, thereby indicating that this meta-analysis had high stability.
Fig 3.
The combined sensitivity of O-CEGUS/D-CEGUS for discriminating ≤T1 and ≥T2 gastric cancer. a The sensitivity forest plot of the 11 included O-CEGUS–related papers. b The sensitivity forest plot of the five included D-CEGUS–related papers. c Sensitivity forest plots of nine O-CEGUS–related papers after excluding the sources of heterogeneity. d Sensitivity forest plots of four D-CEGUS–related papers after exclusion the sources of heterogeneity
Fig. 4.
The combined specificity of O-CEGUS/D-CEGUS for discriminating ≤T1 and ≥T2 gastric cancer. a The specificity forest plot of the 11 included O-CEGUS-related papers. b The specificity forest plot of the five included D-CEGUS–related papers. c Specificity forest plots of nine O-CEGUS–related papers after excluding the sources of heterogeneity; Figure 4d: Specificity forest plots of four D-CEGUS–related papers after excluding the sources of heterogeneity
Fig. 5.
The sROC curves of O-CEGUS/D-CEGUS for discriminating ≤T1 and ≥T2 gastric cancer. a The sROC curves of the 11 included O-CEGUS–related papers. b The sROC curves of the five included D-CEGUS–related papers. c The sROC curves of nine O-CEGUS–related papers after excluding the sources of heterogeneity. d The sROC curves of four D-CEGUS–related papers after excluding the sources of heterogeneity. An empty circle represents each study’s sensitivity/specificity. A filled black circle represents the summary point for sensitivity/specificity. Dotted closed line, 95% confidence interval of the summary point; dashed closed line, 95% prediction region
Fig. 6.
Funnel plot for publication bias among the included studies. a Funnel plot of 11 papers related to O-CEGUS. b Funnel plot of five papers related to D-CEGUS. c Funnel plot of nine papers related to O-CEGUS after excluding the sources of heterogeneity. d Funnel plot of four papers related to D-CEGUS after excluding the sources of heterogeneity. Each red circle represents a study
Discussion
O-CEGUS and D-CEGUS have been feasibly used to diagnose gastric lesions, and their diagnostic value has gradually gained clinical recognition and attention [2]. However, their clinical efficacy in staging gastric cancer remains undefined. Our present study used meta-analysis to investigate the diagnostic efficacy of O-CEGUS and D-CEGUS in distinguishing stage ≤T1 and stage ≥T2 gastric cancer, and detailed analyses revealed that the AUCs for O-CEGUS and D-CEGUS were higher than 0.9 in the differential diagnosis of stage ≤T1 versus stage ≥T2 gastric cancer, thereby suggesting that CEGUS has diagnostic utility in staging early gastric cancer. We noticed the existence of high heterogeneity in the specificity of the included literature, and thus, the two papers with lowest specificities were excluded from the O-CEGUS and D-CEGUS forest plots, which subsequently eliminated the heterogeneity among the remaining papers. Consequently, nine studies related to O-CEGUS and four studies related to D-CEGUS were included in the next round of meta-analysis, which still gave AUCs > 0.9 for the efficacy of CEGUS in differentiating stage ≤T1 and stage ≥T2 gastric cancer. Thus, our meta-analysis proved both either O-CEGUS and D-CEGUS possessed high diagnostic efficacy in differentiating stage ≤T1 and stage ≥T2 gastric cancer.
Our present findings align closely with those of several previous studies conducted on similar grounds. Zhang et al. performed a meta-analysis involving seven studies, of which four were used for accuracy comparisons and two were used for sensitivity comparisons. The results revealed that O-CEGUS effectively differentiated early and advanced gastric cancer with AUC as high as 0.937 [28]. Similarly, Xu et al. conducted meta-analysis of six studies, finding that DCEUS exhibited an overall sensitivity and specificity of 0.94 and 0.91, respectively, in identifying T1–T2 and T3–T4 gastric cancer [29]. Furthermore, the meta-analysis by Zhang et al. incorporated eight studies on D-CEGUS, and its sensitivity and specificity for determining the T-stage of gastric cancer were 0.78 and 0.98, respectively for T1, 0.81 and 0.96, respectively, for T2, 0.88 and 0.85, respectively, for T3, and 0.81 and 0.96, respectively, for T4. The authors concluded that D-CEGUS had better accuracy than CT and EUS in differentiating T1–T2 and T3–T4 gastric cancer [30]. Lastly, the most recent meta-analysis by Nan et al. encompassed 21 studies on O-CEGUS extracted from various databases, and the study revealed AUCs of 0.93, 0.82, 0.87, and 0.97 for T1, T2, T3, and T4 gastric cancer, respectively [31].
However, our study differs significantly from the aforementioned research. Notably, the studies by Xu et al. [29], Zhang et al. [30], and Nan et al. [31] did not directly evaluate the diagnostic performance of CEGUS in distinguishing early gastric cancer from late-stage gastric cancer, which is crucial for guiding endoscopic intervention in patients with gastric cancer. The study by Zhang et al. [28] only analyzed the diagnostic performance of O-CEGUS in distinguishing early gastric cancer from late-stage gastric cancer without evaluating the clinical value of D-CEGUS in gastric cancer, and it included fewer O-CEGUS–related articles than the present study.
Taken together, there is sufficient evidence, along with outcomes, to recommend both O-CEGUS and D-CEGUS for everyday clinical practice in determining and differentiating the T-stage of gastric cancer, particularly in accurately discerning stage ≤T1 and stage ≥T2 gastric cancer before commencing endoscopic intervention.
Furthermore, our study both focused on the diagnostic efficacy of O-CEGUS/D-CEGUS for discriminating stage ≤T1 versus stage ≥T2 gastric cancer and analyzed O-CEGUS and D-CEGUS separately. Despite the smaller number of studies for D-CEGUS than for C-CEGUS, both methods exhibited the capability to differentiate between ≤T1 and ≥T2 gastric cancer. Consequently, these two different CEGUS methodologies represent viable alternatives aiding surgeons in selecting suitable treatment strategies. In addition, our findings suggest that although the diagnostic efficacy of D-CEGUS in differentiating ≤T1 and ≥T2 gastric cancer mirrors that of O-CEGUS, the former requires the administration of intravenous contrast agents such as Sonazoid® and SonoVue®. Given the potential risks of adverse reactions associated with Sonazoid® [32, 33] and SonoVue® [34, 35] alongside the higher examination costs of D-CEGUS, preliminary recommendations favor O-CEGUS for patients with gastric cancer undergoing ESD. This approach aims to streamline clinical decision-making, optimize patient outcomes, and maximize cost-effectiveness and convenience.
This study had several limitations. First, the limited number of studies on D-CEGUS in differentiating ≤T1 and ≥T2 gastric cancer might have hindered the comprehensive establishment of its efficacy and feasibility. Second, the predominantly Chinese origin of the included studies, with one article conducted in Japan, raises questions about the generalization of the conclusions regarding the application of CEGUS in gastric cancer staging. Third, the predominance of retrospective cohort studies among the included studied introduced the potential for recall and selection biases, necessitating further scrutiny of the accuracy of the data. Fourth, variation in ultrasound equipment across studies could contribute to diagnostic inaccuracy. Fifth, the use of different versions of TNM staging in the included studies might have limited the generalization and application of the findings. Lastly, although the diagnostic efficacy of EUS versus O-CEGUS/D-CEGUS for differentiating ≤T1 and ≥T2 gastric cancer is an area of interest, further validation through comparative trials is needed to draw definitive conclusions.
Conclusion
Both O-CEGUS and D-CEGUS represent valuable tools for differentiating early-stage (≤T1) and advanced (≥T2) gastric cancer, thereby aiding in the selection of appropriate clinical treatment strategies for patients with very early gastric cancer. Given its simplicity and cost-effectiveness, O-CEGUS is likely to be favored as a staging method for gastric cancer prior to endoscopic interventions. The remarkable performance of CEGUS in gastric cancer staging underscores its significant clinical utility in evaluating gastric lesions, warranting further investigation and exploration into its diagnostic potential. The promising outcomes of CEGUS in this context highlights its potential to enhance patient care and treatment decision-making in the management of gastric lesions.
Acknowledgments
All authors have attested to abide by the current International Committee of Medical Journal Editors criteria for authorship and read and approved the manuscript. The manuscript has been edited and proofread by Medjaden Inc.
Abbreviations
- CEGUS
Contrast-enhanced gastric ultrasonography
- D-CEGUS
Double contrast-enhanced gastric ultrasonography
- O-CEGUS
Oral contrast-enhanced gastric ultrasonography
- sROC
Summary receiver operating characteristic
- CI
Confidence interval
- ESD
Endoscopic submucosal dissection
- EUS
Endoscopic ultrasonography
- CT
Computed tomography
- PLR
Positive likelihood ratio
- NLR
Negative likelihood ratio
- AUC
Area under the curve
Authors’ contributions
The contributions of all authors have been thoroughly reviewed and comply with the current International Committee of Medical Journal Editors criteria for authorship. Zhong Y and Xiao YY played integral roles in the literature search and data collection process, whereas Zhong Y, Xiao YY, and Ye JY were actively involved in data analysis and interpretation. The study’s conception and design were spearheaded by Huang WJ, Ye JY, and Jian GL, who also critically revised the manuscript. Zhong Y held primary responsibility for drafting the paper and revising the manuscript in collaboration with all authors. Every author has meticulously reviewed the manuscript and granted his or her approval for its submission.
Funding
This work was funded by the Medical Scientific Research Foundation of Guangdong Province of China (B2021374), the Project of Foshan “Fourteen Five” Medicine High-Level Key Specialty Construction (FSGSP145037), and the Medjaden Academy & Research Foundation for Young Scientists (MJA202306092).
Availability of data and materials
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
Declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin. 2021;71(3):209–49. doi: 10.3322/caac.21660. [DOI] [PubMed] [Google Scholar]
- 2.National Health Commission Of The People's Republic Of C Chinese guidelines for diagnosis and treatment of gastric cancer 2018 (English version) Chin J Cancer Res. 2019;31(5):707–37. doi: 10.21147/j.issn.1000-9604.2019.05.01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Park KB, Jeon CH, Seo HS, Jung YJ, Song KY, Park CH, et al. Operative safety of curative gastrectomy after endoscopic submucosal dissection (ESD) for early gastric cancer - 1:2 propensity score matching analysis: A retrospective single-center study (cohort study) Int J Surg. 2020;80:124–8. doi: 10.1016/j.ijsu.2020.06.041. [DOI] [PubMed] [Google Scholar]
- 4.Wang FH, Zhang XT, Li YF, Tang L, Qu XJ, Ying JE, et al. The Chinese Society of Clinical Oncology (CSCO): Clinical guidelines for the diagnosis and treatment of gastric cancer, 2021. Cancer Commun (Lond). 2021;41(8):747–95. doi: 10.1002/cac2.12193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Liu Z, Ren W, Guo J, Zhao Y, Sun S, Li Y, et al. Preliminary opinion on assessment categories of stomach ultrasound report and data system (Su-RADS) Gastric Cancer. 2018;21(5):879–88. doi: 10.1007/s10120-018-0798-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Liu Z, Guo J, Wang S, Zhao Y, Li J, Ren W, et al. Evaluation of transabdominal ultrasound after oral administration of an echoic cellulose-based gastric ultrasound contrast agent for gastric cancer. BMC Cancer. 2015;15:932. doi: 10.1186/s12885-015-1943-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhang X, Yao J, Zhang Y, Huang X, Wang W, Huang H. Updated Evaluation of the Diagnostic Performance of Double Contrast-Enhanced Ultrasonography in the Pre-operative T Staging of Gastric Cancer: A Meta-Analysis and Systematic Review. Front Oncol. 2022;12:844390. doi: 10.3389/fonc.2022.844390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Liu Z, Guo J, Wang S, Zhao Y, Liu Z, Li J, et al. Evaluation of Transabdominal Ultrasound with Oral Cellulose-Based Contrast Agent in the Detection and Surveillance of Gastric Ulcer. Ultrasound Med Biol. 2017;43(7):1364–71. doi: 10.1016/j.ultrasmedbio.2017.02.008. [DOI] [PubMed] [Google Scholar]
- 9.McInnes MDF, Moher D, Thombs BD, McGrath TA, Bossuyt PM, the PRISMA-DTA Group. Clifford T, Cohen JF, Deeks JJ, Gatsonis C, Hooft L, Hunt HA, Hyde CJ, Korevaar DA, Leeflang MMG, Macaskill P, Reitsma JB, Rodin R, Rutjes AWS, Salameh JP, Stevens A, Takwoingi Y, Tonelli M, Weeks L, Whiting P, Willis BH. Preferred reporting items for a systematic review and meta-analysis of diagnostic test accuracy studies: The PRISMA-DTA Statement. JAMA . 2018;319:388–396. doi: 10.1001/jama.2017.19163. [DOI] [PubMed] [Google Scholar]
- 10.Whiting PF, Rutjes AW, Westwood ME, Mallett S, Deeks JJ, Reitsma JB, et al. QUADAS-2: a revised tool for the quality assessment of diagnostic accuracy studies. Ann Intern Med. 2011;155(8):529–36. doi: 10.7326/0003-4819-155-8-201110180-00009. [DOI] [PubMed] [Google Scholar]
- 11.Zamora J, Abraira V, Muriel A, Khan K, Coomarasamy A. Meta-DiSc: a software for meta-analysis of test accuracy data. BMC Med Res Methodol. 2006;6:31. doi: 10.1186/1471-2288-6-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002;21(11):1539–58. doi: 10.1002/sim.1186. [DOI] [PubMed] [Google Scholar]
- 13.Melsen WG, Bootsma MC, Rovers MM, Bonten MJ. The effects of clinical and statistical heterogeneity on the predictive values of results from meta-analyses. Clin Microbiol Infect. 2014;20(2):123–9. doi: 10.1111/1469-0691.12494. [DOI] [PubMed] [Google Scholar]
- 14.Wang L, Liu Z, Kou H, He H, Zheng B, Zhou L, et al. Double Contrast-Enhanced Ultrasonography in Pre-operative T Staging of Gastric Cancer: A Comparison With Endoscopic Ultrasonography. Front Oncol. 2019;9:66. doi: 10.3389/fonc.2019.00066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liao SR, Dai Y, Huo L, Yan K, Zhang L, Zhang H, et al. Transabdominal ultrasonography in pre-operative staging of gastric cancer. World J Gastroenterol. 2004;10(23):3399–404. doi: 10.3748/wjg.v10.i23.3399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang CL, Yang YM, Cui J, Ouyang H, Wang ZM, Ye HS, et al. Diagnostic value of double contrast-enhanced ultrasonography in pre-operative staging of gastric cancer. Zhonghua Zhong Liu Za Zhi. 2009;31(9):701–4. [PubMed] [Google Scholar]
- 17.Cui J, Yang YM, Ding LJ, Ouyang H, Ye HS, Ruan HJ, et al. Diagnostic value of contrast-enhanced ultrasonography in pre-operative T-staging of gastric cancer. Zhonghua Wei Chang Wai Ke Za Zhi. 2010;13(2):141–4. [PubMed] [Google Scholar]
- 18.Liu ZJ, Guo JT, Wang SP, Zhao Y, Li J, Ren WD, et al. Evaluation of transabdominal ultrasound after oral administration of an echoic cellulose-based gastric ultrasound contrast agent for gastric cancer. Bmc Cancer. 2015;15:932. doi: 10.1186/s12885-015-1943-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yu T, Wang XL, Zhao ZL, Liu F, Liu XT, Zhao Y, et al. Prediction of T stage in gastric carcinoma by enhanced CT and oral contrast-enhanced ultrasonography. World J Surg Oncol. 2015;13:184. doi: 10.1186/s12957-015-0577-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sato K, Saito H, Yashima K, Isomoto H, Hirooka Y. Transabdominal Ultrasonography for Assessing the Depth of Tumor Invasion in Gastric Cancer. Yonago acta medica. 2017;60(3):154–61. doi: 10.33160/yam.2017.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhang SX, Zhang Q, Chang HY, Lv B, Lu AQ. Analysis of application effects of ultrasonic aid in gastric cancer screening. Int J Clin Exp Med. 2018;11(5):5103–9. [Google Scholar]
- 22.He P, Miao LY, Ge HY, Wang TL, Ye JX, Meng LM, et al. Pre-operative Tumor Staging of Gastric Cancer: Comparison of Double Contrast-Enhanced Ultrasound and Multidetector Computed Tomography. J Ultrasound Med. 2019;38(12):3203–9. doi: 10.1002/jum.15028. [DOI] [PubMed] [Google Scholar]
- 23.Gai QZ, Li XL, Li N, Li L, Meng Z, Chen AF. Clinical significance of multi-slice spiral CT, MRI combined with gastric contrast-enhanced ultrasonography in the diagnosis of T staging of gastric cancer. Clinical & translational oncology : official publication of the Federation of Spanish Oncology Societies and of the National Cancer Institute of Mexico. 2021;23(10):2036–45. doi: 10.1007/s12094-021-02606-9. [DOI] [PubMed] [Google Scholar]
- 24.Gao ZX, Lu Q, Yan JP, Wang JF, Xu ZW, Jia JW. The value of oral contrast-enhanced ultrasonography in the diagnosis of gastric tumors in the elderly. Panminerva Medica. 2022;64(4):576–7. doi: 10.23736/S0031-0808.21.04466-9. [DOI] [PubMed] [Google Scholar]
- 25.Wang SN, Hong Y, Wang LZ. Clinical Study on the Evaluation of the Condition of Patients with Gastric Tumors and the Choice of Surgical Treatment by Gastric Ultrasonic Filling Method. Contrast Media Mol Imaging. 2022;2022:3960929. doi: 10.1155/2022/3960929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wu LL, Xin JY, Wang JJ, Feng QQ, Xu XL, Li KY. Prospective Comparison of Oral Contrast-Enhanced Transabdominal Ultrasound Imaging With Contrast-Enhanced Computed Tomography in Pre-operative Tumor Staging of Gastric Cancer. Ultrasound Med Biol. 2023;49(2):569–77. doi: 10.1016/j.ultrasmedbio.2022.10.010. [DOI] [PubMed] [Google Scholar]
- 27.Wang J, Li X, Zhang Z, Jing C, Li J. Clinical Research of Combined Application of DCEUS and Dynamic Contrast-Enhanced MSCT in Pre-operative cT Staging of Gastric Cancer. J Oncol. 2021;2021:9868585. doi: 10.1155/2021/9868585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhang YQ, Zhang JZ, Yang L, Huang SX. A meta-analysis of the utility of transabdominal ultrasound for evaluation of gastric cancer. Medicine. 2021;100(32):e26928. doi: 10.1097/MD.0000000000026928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Xu LF, Wang XD, Wu WZ, Li YX. Diagnostic Accuracy of Double Contrast-Enhanced Ultrasonography in Clarifying Tumor Depth (T Stage) of Gastric Cancer: Meta-Analysis. Ultrasound Med Biol. 2021;47(9):2483–93. doi: 10.1016/j.ultrasmedbio.2021.05.017. [DOI] [PubMed] [Google Scholar]
- 30.Zhang X, Yao J, Zhang Y, Huang X, Wang WJ, Huang HJ. Updated Evaluation of the Diagnostic Performance of Double Contrast-Enhanced Ultrasonography in the Pre-operative T Staging of Gastric Cancer: A Meta-Analysis and Systematic Review. Front Oncol. 2022;12:844390. doi: 10.3389/fonc.2022.844390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nan MM, Ye WH, Liu Y, Zhang ZB. Diagnostic accuracy of gastric filling ultrasonography in pre-operative invasion depth (T stage) of gastric cancer: Meta-analysis. Medicine. 2022;101(42):e31066. doi: 10.1097/MD.0000000000031066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Miyamoto Y, Ito T, Takada E, Omoto K, Hirai T, Moriyasu F. Efficacy of sonazoid (perflubutane) for contrast-enhanced ultrasound in the differentiation of focal breast lesions: phase 3 multicenter clinical trial. AJR Am J Roentgenol. 2014;202(4):W400–7. doi: 10.2214/AJR.12.10518. [DOI] [PubMed] [Google Scholar]
- 33.Uemura H, Sano F, Nomiya A, Yamamoto T, Nakamura M, Miyoshi Y, et al. Usefulness of perflubutane microbubble-enhanced ultrasound in imaging and detection of prostate cancer: phase II multicenter clinical trial. World J Urol. 2013;31(5):1123–8. doi: 10.1007/s00345-012-0833-1. [DOI] [PubMed] [Google Scholar]
- 34.Hu C, Feng Y, Huang P, Jin J. Adverse reactions after the use of SonoVue contrast agent: Characteristics and nursing care experience. Medicine (Baltimore). 2019;98(44):e17745. doi: 10.1097/MD.0000000000017745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Coudray S, Fabre C, Aichoun I, Perez-Martin A. Anaphylactic shock with an ultrasound contrast agent. J Med Vasc. 2017;42(6):384–7. doi: 10.1016/j.jdmv.2017.10.001. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.