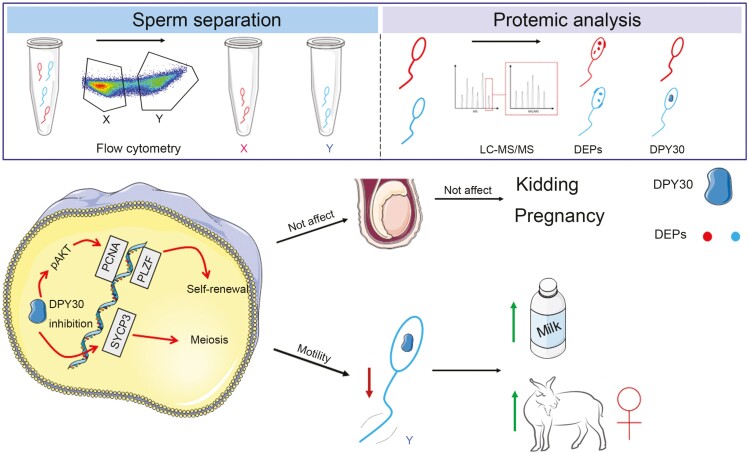
Abstract
The demand for goat milk products has increased exponentially with the growth of the global population. The shortage of dairy products will be addressed extraordinarily by manipulating the female rate of goat offspring to expand the goat population and goat milk yield. No studies have reported bioinformatic analyses of X- and Y-bearing sperm of dairy goats, although this will contribute to exploring novel and applied sex-skewing technologies. Regulatory subunit of the histone methyltransferase complex (DPY30) was determined to be the key differentially expressed protein (DEP) among 15 DEPs identified in the present study. The spatiotemporal expression of DPY30 strongly suggested a functional involvement of the protein in spermatogenesis. DPY30 promoted meiosis via upregulating SYCP3, which played a crucial role in mediating sex ratio skewing in goats. Although DPY30 suppressed the self-renewal of spermatogonia stem cells through AKT/PLZF, DPY30 inhibition in the testis did not induce testicular dysgenesis. Based on the biosafety assessment in mice testes, lentivirus-mediated DPY30 knockdown in bucks’ testes increased X-bearing sperm proportion and female kids’ rate (22.8 percentage points) without affecting sperm quality, pregnancy rate, and kidding rate. This study provides the first evidence of the DEGs in the sexed sperm of dairy goats. DPY30 inhibition in the testes of bucks increased the female kids’ rate without influencing reproductive performance. The present study provides evidence for expanding the female dairy goat population to address the concern of dairy product shortage.
Keywords: proteomics, sex control, DPY30, self-renewal, AKT
DPY30 silence in buck’s testis could increase the female offspring rate without affecting the reproductive performance of goats. We explore a rapid, cheap, safe, and applicable sex-skewing method in dairy goat.
Graphical Abstract
Graphical Abstract.
1. Introduction
By 2050, the global food supply will need to increase by 70% to accommodate the growing global population (Stratonovitch and Semenov, 2015). However, the developing breeding and reproduction technologies has hindered the further development of dairy products (Kahi and Wasike, 2019). Therefore, to meet the projected global demand for dairy products, it is essential to utilize modern biotechnologies to support sustainable livestock production. Indeed, goat milk products have been considered to be an important economic commodity in many countries, capable of meeting a wide range of individual nutritional needs. Sexing technology could control livestock offspring sex via human intervention to address several practical concerns, including food shortage, long breeding cycles (Thomas et al., 2014), welfare (castration) (Maes et al., 2020), and CO2 emissions (Xie et al., 2020). Meanwhile, it is a strategic way to maximize the meat and milk production. Female animals are crucial for population expansion and the development of the dairy industry (Shi et al., 2017), while male animals have a higher capacity for meat production due to better feed efficiency and lean/fat ratio.
Although sexed sperm separated by flow cytometry had been commercially applied in dairy cattle, the technology was challenging to popularize and apply in dairy goat farms due to the higher cost. The price of sexed sperm was > 3 times higher than normal semen. The long and tortuous cervix with 4 to 7 cervical rings of goats influenced the efficiency of artificial insemination (AI), such that a higher insemination dose was required in goats compared to cattle to guarantee a higher pregnancy rate (Furstoss et al., 2015; Sathe, 2018). Based on this, the high cost has hindered its large-scale commercial application. Meanwhile, sperm separated by flow cytometry resulted in a low pregnancy rate due to the damage caused by DNA dye (Hoechst33342) and the freezing/thawing process (Thomas et al., 2019). Although TLR7/8 activation-mediated X- and Y-bearing sperm separation produced 80% accurate XX embryos, the sperm sexing method could not be applied to AI due to the irreversible activation of the glycolytic pathway in sperm cells (Umehara et al., 2020). The SRY (sex determining region Y) antibody specifically recognized goat Y-bearing sperm which caused additional damage induced by increased cell membrane permeability (Soleymani et al., 2019). Currently, there is a lack of suitable and applied routes to manipulate the offspring sex of dairy goat. Further research on sexing technology must focus on developing novel, rapid, safe, and applicable approaches in goats. At the same time, bioinformatic analysis between X- and Y-bearing sperm was more likely to provide evidence to explore new sexing technology.
Proteomics is suitable for identifying the different biomarkers between X- and Y-bearing sperm cells, as protamine in spermatozoa causes the transcriptionally inactive state (Buttress et al., 2022). At present, the studies on proteomic analyses of X- and Y-bearing sperm have been performed in dairy cattle (Chen et al., 2012; De Canio et al., 2014; Scott et al., 2018), so there was no direct evidence to investigate the DEPs of X- and Y-bearing sperm in dairy goats. Performing such studies would provide more information for developing new sexing technologies. The DEPs located in the cell membrane could be used for immunological separation of X- and Y-bearing sperm (Soleymani et al., 2021). The DEPs involved in sperm motility and sex determination should be regulated by the gene editing and RNA interference (lentivirus-mediated) to influence X- and Y-bearing sperm fertilization and XX and XY embryo development (Xie et al., 2020). In summary, proteomic analyses of X- and Y-bearing sperm are essential for developing novel, effective sexing technology.
The DEPs in the sexed sperm of dairy goats and the involvement of DEPs in regulating sex determination have never been well studied before, and the relevant research will provide evidence for expanding the dairy goat population and increasing milk production. In the present study, we aimed to identify the crucial DEPs of X- and Y-bearing sperm in dairy goats. The involvement of DEPs in testis expression patterns and molecular regulatory mechanisms in spermatogonial stem cells would also be investigated morphologically and mechanistically. Prior to sex-skewing analyses in goats, the biosafety of gene editing critical DEP in mouse testis should be evaluated to avoid economic losses in farms. Based on these results, the effects of crucial DEP on offspring sex were investigated in dairy goats. Our findings aimed to explore a simple sex-skewing method mediated by regulatory crucial DEP expression during the spermatogenesis process, which provides insights into genetic targets for controlling the offspring sex rate in dairy goats.
Materials and Methods
Animals
In this study, all animal experimental procedures were approved by the Animal Care and Use Committee of Northwest A&F University (DK2022070).
X- and Y-bearing sperm separation
The semen was collected as per conventional artificial vaginal method from male Xinong Saanen dairy goats (2 to 3 years old) at the Xinong Experimental Station of Northwest A&F University in Yangling (34°14ʹN; 107°59ʹE), Shaanxi, China.
The fresh goat semen was diluted with semen diluent (1:3) at a temperature of 37 °C (He et al., 2021). After dilution, the sample was collected for fresh semen analysis and simultaneously used for subjective quality control analyses. The diluted semen was transferred to the Ding Yuan Cattle Breeding Company (Zhengzhou, Henan Province, China) after being warmed in a water bath and wrapped in insulation. Hoechst33342 (28 μM/m) was used to stain the DNA of sperm for 45 min, allowing the separation of X- and Y-bearing sperm by flow cytometry (SX MoFlo). The isolated samples were adequately stored in liquid nitrogen for an appropriate period of time. The separation efficiency was then evaluated by flow cytometry after staining with Hoechst33342 (100 μM/m). The X- and Y-bearing sperm from at least three bucks were analyzed.
Mass spectrometry and protein identification
Mass spectrometry was performed at Novogene Bio Technology Company (Beijing, China). The LC-MS/MS analysis was performed on an Orbitrap Fusion (Thermo Q exactive HF-X) mass spectrometer. Sperm protein was extracted with protein lysis buffer (100 mM ammonium bicarbonate, 6 M urea, 0.2 % SDS). Samples were then treated with trypsin (1:50), triethylammonium bicarbonate buffer (TEAB, 50 mM), and incubated overnight at 37 °C. Samples were collected and dried by lyophilization. Peptides were dissolved and eluted with 80% acetonitrile and 0.1% formic acid. To quantify the differentially expressed proteins in X- and Y-bearing sperm, LC‐MS/MS was performed to obtain MS1 (primary level of mass spectrometry) and MS2 (secondary level of mass spectrometry) spectra data. Three biological replicate samples (sexed sperm from three bucks) for LC-MS/MS were analyzed. MS/MS spectra were automatically searched against the NCBI protein database (Capra hircus). Only proteins with an adjusted P-value <0.05 and fold change values of greater than ±1.2 were considered as differentially expressed.
Western blotting
To determine the specific protein expression by Western blotting (WB), the total proteins of goat cells (separated sperm, treated mGSCs-I-SB) were extracted with RIPA lysis buffer containing protease and phosphatase inhibitors (Roche). The detailed experimental procedures were mentioned in the previous article (He et al., 2022a). Briefly, denatured proteins (high‐temperature water bath) were subjected to electrophoresis at 80 V for 30 min, followed by 120 V for approximately 80 min. The proteins were then transferred to PVDF membranes at a constant voltage of 16 V at different times. The proteins were then blocked with 5% skim milk powder for 2 h before primary antibodies were added, and the incubation continued for 10 h. For specific visualization of the protein bands, the membranes were incubated with the corresponding secondary antibodies (HRP linked) for 2 h. This experiment was carried out in three independent biological replicates and three technical replicates of each biological replicate.
The following primary antibodies were used: rabbit anti-DPY30 (1:1000, Abmart), rabbit anti-DYNLL2 (1:1000, Abclonal), rabbit anti-pSTAT3 (1:2000, Abmart), rabbit anti-pAKT (1:2000, Cell Signaling Technology), rabbit anti-tAKT (1:3000, Cell Signaling Technology), rabbit anti-pAMPK (1:2000, Cell Signaling Technology), rabbit anti-pERK1/2 (1:2000, Cell Signaling Technology), rabbit anti-PLZF (1:1000, Proteintech), rabbit anti-PCNA (1:5000, Abcam), and rabbit anti-pSMAD3 (1:1000, Zen Bio).
Adenovirus package
Adenovirus packaging was performed using the AdEasy adenoviral system. The DPY30 gene sequence (Capra hircus) was ligated to the Ad-Track-CMV vector to construct the Ad-Track-CMV-DPY30 expression vectors, followed by recombination in BJ5183 E. coli cells. The recombinant adenoviral plasmid Ad-DPY30 was linearized (endoenzyme Pac I) and then transfected into HEK-293A adenovirus packaging cells to obtain packaged recombinant adenovirus expressing DPY30. Third-generation adenovirus was used to overexpress DPY30.
Quantitative RT-PCR
Quantitative real-time PCR was used to evaluate the transcription levels of specific genes in various goat tissues, testes of different ages, and treated cells (mGSCs-I-SB, GC-1). For RNA isolation, frozen tissues were crushed in liquid nitrogen. TRIzol was used to extract total RNA for cDNA preparation using Evo M-MLV RT Kit (Accurate Biology, China). RT-PCR (two-step Real-Time PCR System) was established using the SYBR Green kit (Accurate Biology, China). Based on the ΔΔCT method, the housekeeping genes (GAPDH and β-ACTIN) were utilized to normalize the relative expression of the target genes. Experiments were repeated in three independent biological replications (three goats or three treatments), and three replicates were included in each experiment. The RT-PCR primer sequences are listed in Supplementary Tables S3 and S4.
Immunohistochemical staining
To prepare paraffin-embedded tissues, goat and mouse testes were fixed in a fixative solution. For morphological analysis, tissues were dewaxed, rehydrated, and stained with hematoxylin and eosin (H&E). Tissue sections were visualized with microscope inspection after dehydration.
Immunohistochemistry (IHC) was performed to analyze the expression and localization of DPY30 in the testes of goats of different ages. Prior to staining, the dewaxed sections were heated in sodium citrate buffer and washed with diH2O. The tissues were then blocked with 3% bovine serum albumin (BSA) for 30 min and incubated with primary antibody (10 h; anti-DPY30, 1:100, ABclonal) and secondary antibodies (50 min). Then, the chromogenic reaction was developed with DAB and counterstained with hematoxylin. After dehydration and mounting of coverslips, the slides were histologically examined. The DPY30-positive cells were captured in three randomly selected microscopic fields and repeated in three goats or mice.
Immunofluorescence
Immunofluorescence (IF) was used to detect the signals of specific proteins in goat and mouse testes. After heating in EDTA antigen retrieval buffer (pH 8.0), the dewaxed sections were blocked with 3% BSA. Following that, slides were incubated with specific primary antibodies overnight at 4 °C and with the corresponding secondary antibody at room temperature for 1 h. The nuclei were stained with DAPI, and the sections were immediately incubated in spontaneous fluorescence quenching reagent for 5 min. Afterward, protein signals were captured by fluorescence microscopy.
To investigate protein expression in treated goat cells (mGSCs-I-SB), IF was subjected to label the signals of the indicated protein. Adherent cells were fixed with 4 % paraformaldehyde, permeabilized with 0.5% Triton X-100, and blocked with 5 % BSA. Subsequently, the cells were incubated with the primary antibody (10 h) and the corresponding secondary antibody (30 min). DAPI counterstaining was used to visualize the nuclei. Finally, an anti-fluorescence quenching agent was utilized and photographed using a fluorescence microscope.
Quantification of fluorescence intensity was performed in three randomly selected microscopic fields and repeated in three independent animals and treated cells. The following primary antibodies were used: rabbit anti-DPY30 (1:100, Abmart), rabbit anti-pAKT (1:50, Abmart), rabbit anti-PLZF (1:50, Proteintech), rabbit anti-C-KIT (1:100, Proteintech), and rabbit anti-STRA8 (1:100, Abmart).
Cell culture and treatment
The HEK-293A cells, HEK-293T cells, and GC-1cells (spg mouse spermatogonial germ cell line) were cultured in DMEM supplemented with 10 % FBS (Every Green, China), and antibiotics (1% penicillin, 1% streptomycin). The immortalized dairy goat male germline stem cell line (mGSCs-I-SB) was cultured in DMEM supplemented with 10% FBS (Gibco), and antibiotics (1% penicillin, 1% streptomycin). Retinoic acid (1 µM) was used to induce the differentiation and meiosis of mGSCs-I-SB cells.
Based on the cloned goat sequences of DPY30 (Supplementary Table S2), the reconstruction plasmid pAd-Track-CMV-DPY30 (pAd-track-DPY30) was constructed by joining the DPY30 CDS fragment and linearized pAd-Track-CMV. In order to overexpress DPY30 in mGSCs-I-SB cells, adenovirus-DPY30 (Ad-DPY30, 1 × 1010 PFU/mL, 20 µL) was generated by transfecting the adenovirus plasmids (pAd-track-DPY30) into HEK-293A cells.
Lentivirus package
DPY30 expression in the goats and mice testes was silenced by lentiviral-mediated shRNA knockdown. HEK-293T cells were transfected with PLL3.7-shRNA, pMD2.G, and psPAX2 to produce lentiviruses. Afterward, the lentiviral particles were concentrated using the Lenti-X concentrator kit (Takara, Japan), and the lentiviral titer was measured using the QuickTiter Lentivirus Titer kit (Biodragon, China). Lentivirus of goat and mouse were transfected into mGSCs-I-SB and GC-1 cells to detect interference efficiency by RT-PCR, respectively. The primer sequences used for shRNA are listed in Supplementary Table S1.
Lentivirus injection
Prior to the study in bucks, male mice received DPY30 interference lentivirus. Ten male BALB/c mice (10-wk old, 25 ± 2 g) were randomly divided into two groups (n = 5) after purchase from the Experimental Animal Corporation of Dossy (Chengdu, China). A total of 109 TU/mL lentivirus (50 µL) was injected into both testes of the mice using 30G needles. The animals were individually housed in a natural light–dark cycle (12 h light and 12 h dark) with free access to food and water. After a spermatogenic cycle (35 d; Oakberg, 1956), mouse testes were harvested to detect the integrity of the seminiferous tubules to evaluate the biosafety in model animals prior to application in bucks.
Nine bucks were randomly divided into two groups: control (n = 6) and lentivirus (n = 3), with identical housing, feeding, and management. A total of 3 mL (109 TU/mL) of lentivirus (Qin et al., 2015) was injected into the testes of bucks by testicular multiple point injection (10 injection sites, from deep to shallow) using a 30G needle (60 mm in length). A second injection was performed at 21 d after the first injection to enhance the knockdown effect. The semen was used for quality analysis and artificial insemination of does after one spermatogenic cycle (47 d, since the first injection; França et al., 1999).
Estrous synchronization and artificial insemination
Three hundred and two multiparous does of similar age and reproductive performance were estrus synchronized and mated. The does underwent estrous synchronization followed by artificial insemination. Estrous synchronization was conducted by an intravaginal controlled internal drug release (CIDR) insert (Pharmacia & Upjohn, USA). FSH (30 IU,12 d and 13 d) and PG (0.25 mg, 16 d) were administered after the CIDR insert, respectively. The does were mated twice, at the onset of estrus and 12 h later, with diluted fresh semen (108 sperm cells) from control and treated bucks via artificial insemination. All the synchronized does received semen randomly within 1 month. The pregnant does received the same ration and management, and the pregnancy rate (number of kidded does/number of bred does), kidding rate (number of born kids/number of kidded does), and sex rate (numbed of female kids/number of kids) were recorded in both groups. All animal data were used for analyses.
Computer-aided semen analysis (CASA) system
To assess whether the lentivirus affected the reproductive performance of the bucks, semen from control and treated goats was collected as per conventional artificial vaginal method, and the diluted sperm quality (1:3, 10 μL) was analyzed by the CASA system (Song Jing Tian Lun, China). Parameters related to sperm quality, including sperm motility, viability, density, and straight-line velocity (VSL), were statistically analyzed. Three visual fields of sperm cells (6 control goats and 3 treated goats) were randomly selected to obtain the report.
Swim-up test
Diluted semen was centrifuged (500 × g, 5 min) to determine whether the motility of X- and Y-bearing sperm was significantly different. Then, the semen was incubated at 37 °C for 2 h to screen for the sperm with higher motility (Katska-Ksiazkiewicz et al., 2004). After staining with Hoechst33342 (100 μM/m), the sperm in the supernatant were collected for flow cytometric analysis of X and Y proportions. The portion of the unstained cell population served as the negative control. Three biological replicates and three technical replicates were performed for each time point.
Statistics analysis
Given the homogeneity of the bucks (age, weight, and breeds), bucks were not included as a covariate in the statistical analyses. The number of replicate samples for each experiment is indicated in the respective figure legends. SPSS version 26 was used for statistical analyses to assess the statistical significance of the differences between groups and to calculate P values. Statistical graphs were generated utilizing GraphPad Prism 6, with data presented as the mean ± standard deviation (x ± S). Statistical comparisons between groups were analyzed for significance by two-tailed Student’s t-test between two groups and one-factor ANOVA for multiple groups.
Results
The identification and testicular expression profiles of crucial differentially expressed protein in the X- and Y-bearing sperm of bucks
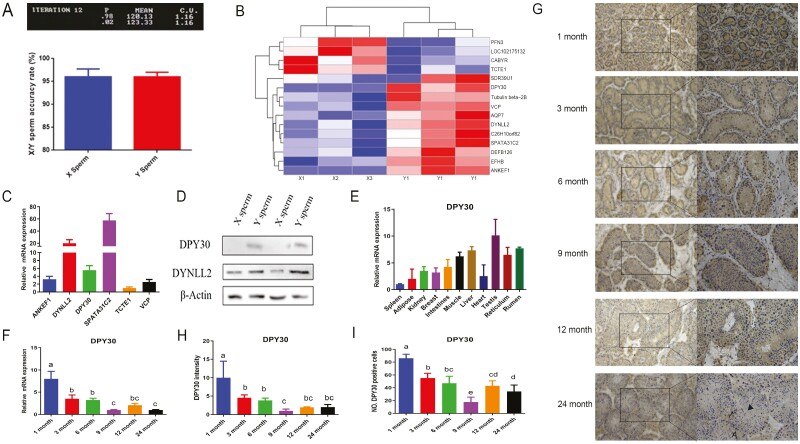
High-purity (>95%) X- and Y-bearing sperm (Fig. 1 A) were obtained via flow cytometer, and 15 differentially expressed proteins (DEPs) were identified with LC-MS/MS (Fig. 1 B). Of these, there were 11 upregulated proteins in Y-bearing sperm, 2 of whom (DPY30, ANKEF) were specifically expressed proteins (Table 1). The remaining 4 DEPs were upregulated in X-bearing sperm (Table 2).
Figure 1.
The identification and testicular expression profiles of crucial differentially expressed protein in the X- and Y-bearing of bucks. A. Representative image and statistical analysis of purity analysis of X- and Y-bearing sperm from three bucks. “P” represented the percentage of X/Y purity. B. Differentially expressed proteins were presented with the heatmap. C. The transcriptional level of differentially expressed proteins of X- and Y-bearing sperm in dairy goat testis. D. Western blotting was performed to determine the expression of DPY30 and DYNLL2 in X- and Y-bearing sperm. E. DPY30 mRNA level was evaluated in various tissues of goat. F. Expression levels of DPY30 gene in goat testes of different ages. G. The immunohistochemical of DPY30 was performed in goat testes of different ages (scale bar = 100 μm). A: Spermatogonia; black arrow: spermatocyte; triangle: spermatid. H. The grey scale values (H) were performed. I. The number of DPY30 positive cells (H) was analyzed. At least three biological replicate samples (bucks) for immunohistochemistry were analyzed. (G, H). Three biological replicates were evaluated in (RT-PCR, WB), with three technical replicates per biological replicate (C–F). Data were compared by one-way ANOVA. (a–d) Values with different letters are specific to each group and show significant differences (P < 0.05) (D-I).
Table 1.
Upregulated proteins of dairy goat in Y-bearing sperm
| Accession number | Protein | Gene |
|---|---|---|
| XP_005685257.3 | Epimerase family protein SDR39U1 isoform X1 | SDR39U1 |
| XP_005686451.1 | Protein dpy-30 homolog | DPY30 |
| XP_017894737.1 | Tubulin beta-2B chain | Tubulin beta-2B |
| XP_017907601.1 | Transitional endoplasmic reticulum ATPase | VCP |
| XP_005684118.1 | Aquaporin-7 | AQP7 |
| XP_017919600.1 | Dynein light chain 2, cytoplasmic | DYNLL2 |
| XP_017896621.1 | Uncharacterized protein C10orf82 homolog | C26H10orf82 |
| XP_013821539.2 | Spermatogenesis-associated protein 31C2 | SPATA31C2 |
| NP_001279991.1 | β-Defensin 126 precursor | DEFB126 |
| XP_017908716.1 | EF-hand domain-containing family member B | EFHB |
| XP_005687868.1 | Ankyrin repeat and EF-hand domain-containing protein 1 | ANKEF1 |
Table 2.
Upregulated proteins of dairy goat in X-bearing sperm
| Accession number | Protein | Gene |
|---|---|---|
| XP_017906102.1 | Profilin-3 | PFN3 |
| XP_017910325.1 | Fumarylacetoacetate hydrolase domain-containing protein 2 | LOC102175132 |
| XP_005697095.1 | Calcium-binding tyrosine phosphorylation-regulated protein isoform | CABYR |
| XP_017894763.1 | T-complex-associated testis-expressed protein | TCTE1 |
Two DEPs (DPY30, DYNLL2) identified by LC-MS/MS were further investigated with immunoblotting, which showed significant congruence effects (Fig. 1C). Protein bands of DPY30 were not detected in X-bearing sperm (Fig. 1 C), as indicated by LC-MS/MS. DPY30 expression is the third of the six DEPs examined to be reported functionally (Fig. 1 D) and in different tissues of goats (Fig. 1 E). These findings taken together show that DPY30 was significantly expressed in the testis and was specifically expressed in goat Y-bearing sperm compared to X-bearing sperm. DPY30 was identified as the crucial differentially expressed protein between X- and Y-bearing sperm based on its expression pattern in dairy goats.
Spatial and temporal developmental protein expression profiles provide a powerful strategy for understanding how morphological diversity can be generated. In the testes of 1-mo-old bucks, DPY30 was substantially expressed, whereas in those of 3-, 6-, and 9-mo-old goats, the expression was gradually reduced (Fig. 1G-I). In 12- and 24-mo-old goats, DPY30 expression has tended to be stable eventually with an increasing trend compared to 9-mo-old goats. Spermatogonia located on the basement membrane are labelled with A, nucleoid division was observed in spermatocytes (black arrow) and spermatid’s size is small with morphological changes (triangle). Round and elongated spermatids appeared in the seminiferous tubules indicating the sexual maturity of the bucks, which could influence DPY30 expression. Similar results were obtained via RT-PCR (Fig. 1 F). Overall, DPY30 was highly expressed in the testes of kids and low in the testes of young and adult bucks.
DPY30 ameliorates self-renewal attenuated by retinoic acid via AKT
DPY30 colocalized with PLZF (self-renewal marker protein) and C-KIT (differentiation marker), but not with STRA8 (meiosis marker protein; Fig. S1A-C). Based on these results, it was suggested that DPY30 might interact with PLZF and C-KIT to participate in the process of self-renewal and differentiation, but the detailed mechanisms and the regulatory factors involved remained unclear. To address this issue, mGSCs-I-SB cells were treated with adenoviral overexpression of DPY30 (Ad-DPY30) and retinoic acid (RA, the differentiation inducer in stem cells).
The goat DPY30 gene coding region (300 bp) was cloned (Supplementary Fig. S2A, B). Based on the sequences, a goat Ad-DPY30 adenovirus was generated using a recombinant adenovirus vector (Ad-Track-CMV-DPY30) to greatly enhance the expression of DPY30 in mGSCs-I-SB cells (Supplementary Fig. S2C-D).
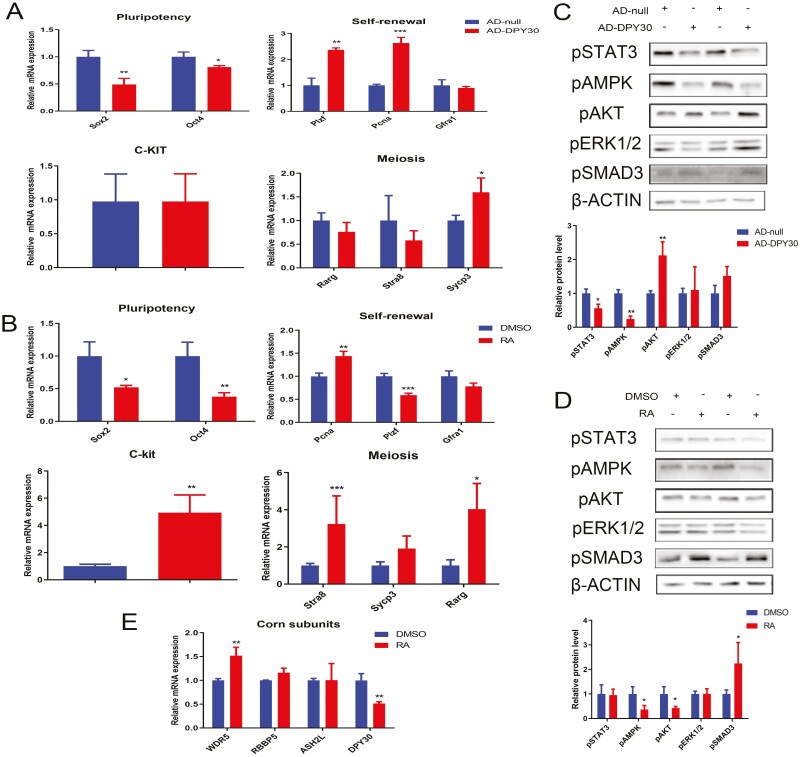
DPY30 upregulation stimulated self-renewal (PLZF, PCNA) and meiosis (SYCP3), whereas the pluripotency of mGSCs-I-SB was inhibited (SOX2, OCT4) (Fig. 2A). Spermatogenesis depends on the continuous self-renewal of spermatogonial stem cells (SSCs) to maintain a stable number of SSCs in the testis. Although pluripotency and self-renewal were always synchronously regulated, these two processes were independent in stem cells (Xu et al., 2022). DPY30 regulated pluripotency and self-renewal via modulating relative genes respectively. We found that DPY30 was involved in the developmental regulation of spermatogonial stem cells, indicating that DPY30 may be modulated by RA. At the same time, RA activated differentiation (C-KIT) and meiosis (STRA8, RARG) and suppressed pluripotency (Sox2, OCT4) and self-renewal (PLZF) in mGSCs-I-SB cells (Fig. 2B). DPY30, specifically expressed in Y-bearing sperm, could promote meiosis via SYCP3.
Figure 2.
DPY30 ameliorates self-renewal attenuated by retinoic acid via AKT. A. The cells were transfected with Ad-DPY30 for 48 h; the effects of Ad-DPY30 on the pluripotency, self-renewal, differentiation, and meiosis were measured in mGSCs-I-SB. B. The cells were treated with RA for 48 h; the effects of RA on pluripotency, self-renewal, differentiation, and meiosis were measured in mGSCs-I-SB. C. The cells were transfected with Ad-DPY30 for 72 h; the effects of Ad-DPY30 on the signaling proteins were measured in mGSCs-I-SB. D. The cells were treated with RA for 72 h; the effect of RA on the signaling proteins was measured in mGSCs-I-SB. E. The cells were treated with RA for 48 h; the effect of RA on the WDR5, RBBP5, ASH2L, and DPY30 was measured by RT-PCR in mGSCs-I-SB. Three biological replicates were evaluated in (RT-PCR, WB), with three technical replicates per biological replicate (A–E). Data were presented as the mean ± standard deviation (A–E). Data were compared by two-tailed Student’s t-test, *P < 0.05, **P < 0.01, ***P < 0.001(A–E).
The common pathways involved in stem cell development were measured with DPY30 overexpression and RA treatment, both of which altered the activity of AMPK and AKT (Fig. 2C, D). Of these, DPY30 promoted phosphor-AKT, of whom was inhibited with RA (Fig. 2C, D). WDR5, RBBP5, ASH2L, and DPY30 are components of the WRAD complex, which is a core subunit of the H3K4 methyltransferase (SET1/MLL) and participates in H3K4 methylation and transcriptional regulation (Su et al., 2023). Meanwhile, DPY30 was suppressed by RA (Fig. 2E), so that pAKT could be attenuated with RA mediated DPY30 inhibition.
DPY30 promoted self-renewal of goat male germline stem cells via AKT/PLZF
Although we had confirmed that RA mediated DPY30 inhibition affected AKT, the underlying molecular processes may go unrecognized. Meanwhile, RA inversely regulated PLZF expression compared to Ad-DPY30 transfection (Fig. 2A, B), suggesting that RA might attenuate DPY30 expression, which contributed to PLZF inhibition. Therefore, we further assessed the mechanism of DPY30 mediated self-renewal regulation to evaluate the feasibility of DPY30 inhibition in bucks testis.
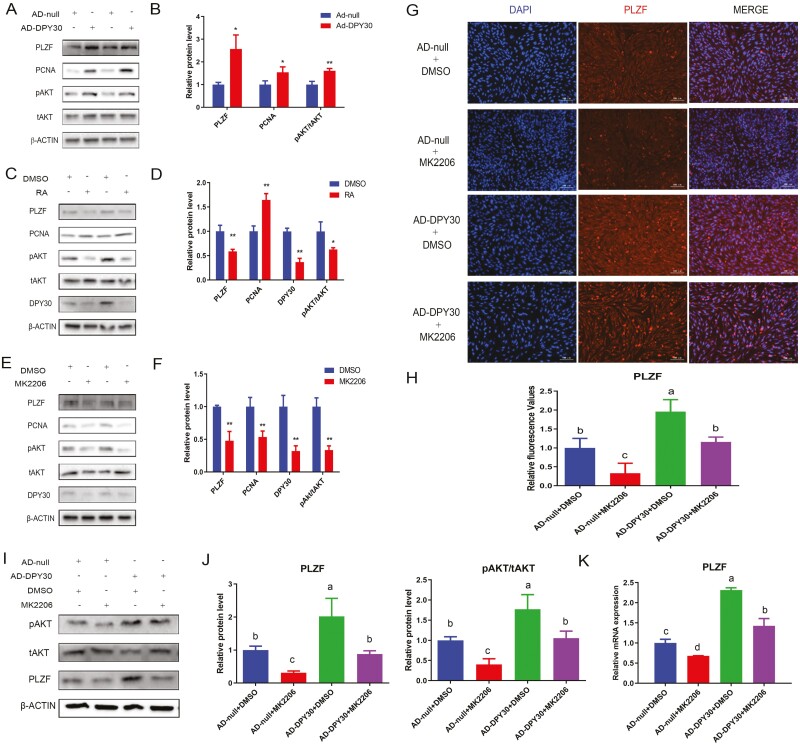
The above results (Fig. 2A-E) were further confirmed by immunoblotting (Fig. 3A-D). DPY30 overexpression increased the PLZF and PCNA protein level via activating AKT (Fig. 3A, B), whereas RA inhibited PLZF by decreasing AKT, which was mediated by DPY30 inhibition (Fig. 3C-D). Consistently, the AKT inhibitor (MK2206) suppressed PLZF and PCNA (Fig. 3E, F). Notably, MK2206 and Ad-DPY30 cooperated in an opposing manner to regulate the expression of the PLZF, and MK2206 attenuated the stimulation of PLZF induced by Ad-DPY30 (Fig. 3G, H). Similar results for PLZF were obtained via western blotting (Fig. 3I, J) and RT-PCR (Fig. 5K). Overall, the self-renewal regulation of mGSCs-I-SB was mainly modulated by DPY30 (inhibited by RA) through AKT-mediated PLZF and PCNA.
Figure 3.
DPY30 promoted self-renewal of goat male germline stem cells via AKT/PLZF. A. The mGSCs-I-SB cells were transfected with Ad-DPY30 for 72 h. Cell lysates were immunoblotted with the indicated antibodies. B. The quantification analyses (A) were shown. C. The mGSCs-I-SB cells were treated with RA for 72 h. Cell lysates were immunoblotted with the indicated antibodies. D. The quantification analyses (C) were shown. E. The mGSCs-I-SB cells were treated with MK2206 for 72 h. Cell lysates were immunoblotted with the indicated antibodies. F. The quantification analyses (E) were shown. G. The mGSCs-I-SB cells were transfected with Ad-DPY30 (24 h), followed by MK2206 (1 μM) treatment for 48 h; PLZF expression was measured by immunofluorescence. H. The quantification analyses (G) were shown. I. The mGSCs-I-SB cells were transfected with Ad-DPY30 (24 h), followed by MK2206 (1 μM) treatment for 48 h; cell lysates were immunoblotted with the indicated antibodies. J. The quantification analyses (G) were shown. K. The mGSCs-I-SB cells were transfected with Ad-DPY30 (24 h), followed by MK2206 (1 μM) treatment for 24 h; RT–qPCR with primers for PLZF was used to analyze the levels of the gene expression. Three biological replicates were evaluated in (RT-PCR, WB, IF), with three technical replicates per biological replicate (A-K). Data were presented as the mean ± standard deviation (A-K). Data were compared by two-tailed Student’s t test, *P < 0.05, **P < 0.01, ***P < 0.001 (A-F). Data were compared by one-way ANOVA, a–d Values with different letters are specific to each group and show significant differences (P < 0.05) (G-K).
Figure 5.
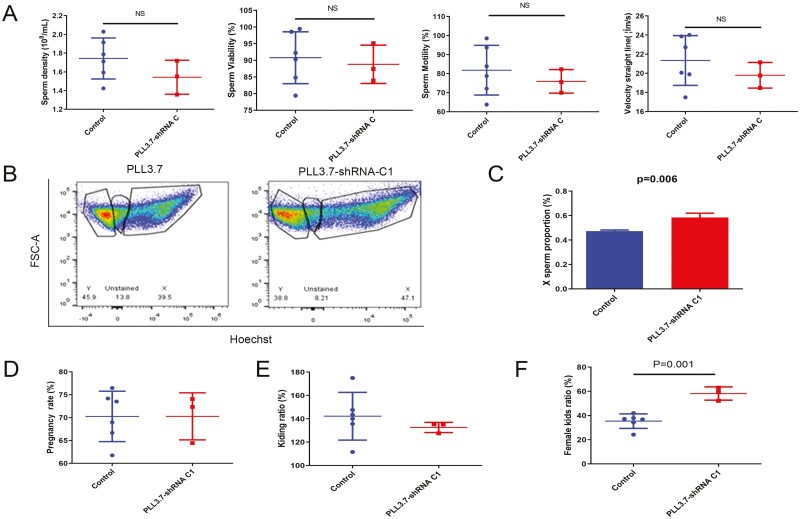
DPY30 inhibition in bucks’ testis increased female kids’ ratio. A. Testes were treated with lentivirus for 43 d; the effect of DPY30 interference lentivirus on goat sperm quality. B and C. The swim-up sperm was stained with Hoechst33342 and measured by flow cytometry to detect the X-bearing sperm proportion. Representative image (B) and statistical analyses (C) of X-bearing sperm proportion in swim-up sperm. D. Testes were treated with lentivirus for 43 d, and the treated semen was used for artificial insemination; the effect of DPY30 interference lentivirus on pregnancy rate of does. E. Testes were treated with lentivirus for 43 d, and the treated semen was used for artificial insemination; the effect of DPY30 interference lentivirus on the kidding rate of does. F. Testes were treated with lentivirus for 43 d, and the treated semen was used for artificial insemination; the effect of DPY30 interference lentivirus on female kids’ rate of offspring. All images are representative of n = 6 (Control) and n = 3 (PLL3.7-shRNA C1) independent experiments (A-F). Data were presented as the means ± standard deviation (A-F). Data were compared by two-tailed Student’s t test, *P < 0.05, **P < 0.01, ***P < 0.001(A-F).
DPY30 inhibition in testis could not induce testicular dysgenesis in mouse
It has been established that DPY30 plays a role in the self-renewal regulation, as reported above, and that DPY30 inhibition may result in testicular dysgenesis in bucks. The practicality of DPY30 suppression was evaluated in mice, and the underlying processes were examined to prevent further economic losses in dairy goat farms.
PLL3.7-shRNA M1 (lentivirus M1) was used to inhibit DPY30 expression in mice with a higher interference efficacy (Supplementary Figs. S3A and 4A). After DPY30 knockdown, pAKT (Fig. 4 B) and PLZF (Fig. 4C, E) were subsequently inhibited (Fig. 4C, D) in the testis, although C-KIT and STRA8 expression were unaltered (Fig. S4A-D). Surprisingly, the colocalization of DPY30 and PLZF was attenuated with DPY30 silence (Fig. 4C, F).
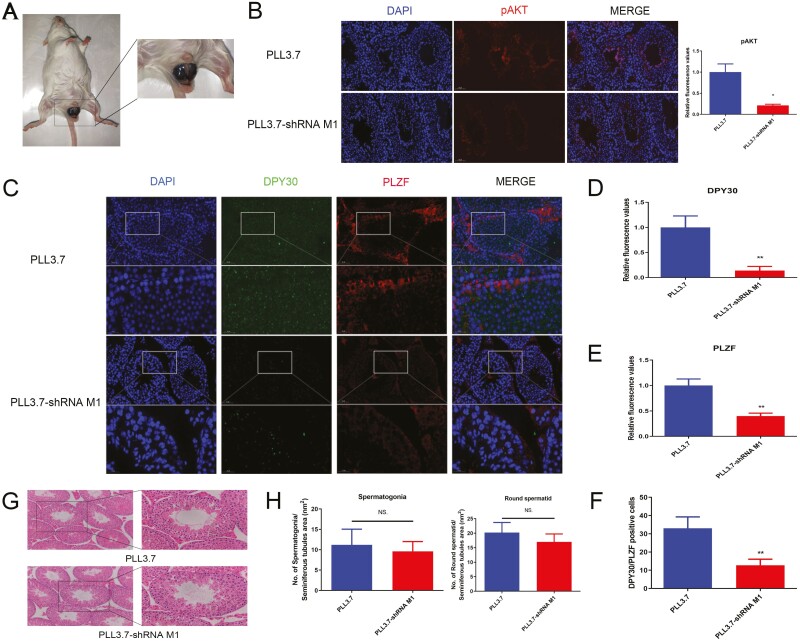
Figure 4.
DPY30 inhibition in testis could not induce testicular dysgenesis in mouse. A. The injection of DPY30 interference lentivirus in mice testis. B. Testes were treated with lentivirus for 35 d; the expression of pAKT in testes was analyzed. C. Testes were treated with lentivirus for 35 d; the expression of DPY30 and PLZF was analyzed in the testes. D. The quantitative analysis of DPY30 expression in C. E. The quantitative analysis of PLZF expression in C. F. The DPY30/PLZF positive cell number in C. G. Testes were treated with lentivirus for 35 d; the effect of DPY30 interference lentivirus on mice testicular morphology; H. Testes were treated with lentivirus for 35 d; the effect of DPY30 interference lentivirus on cell counts (spermatogonia, round spermatid cell) in mice testis were calculated. At least three biological replicate samples (mice) for immunohistochemistry and immunofluorescence were analyzed. (B-H) Data were presented as the means ± standard deviation (B-H). Data were compared by two-tailed Student’s t test, *P < 0.05, **P < 0.01, ***P < 0.001 (B-H).
In the above results, we found that DPY30 could regulate PLZF and SYCP3 expression, which may influence the development of spermatogonia and round spermatid cells. In lentivirus M1 treated mice, no obvious morphological changes were detected in the seminiferous tubules (Fig. 4G). There were no appreciable variations in the number of mouse germ cells (spermatogonia, round spermatid) between groups (Fig. 4H). It has been proposed that changes take place at the molecular level before pathological modifications and alterations that may be detected under a microscope (Mehrotra et al., 2006). Collectively, although DPY30 inhibition in mouse testes suppressed self-renewal via AKT/ PLZF, lentivirus M1 failed didn’t testicular dysgenesis in mice.
DPY30 inhibition in bucks’ testis increased female kids’ ratio
It has been proved that lentivirus-mediated DPY30 knockdown in mouse testis has no effect on seminiferous tubule integrity. As such, it is, however, very efficient and practical to induce DPY30 knockdown in buck testes.
Goat DPY30 interference lentiviruses were packaged and screened to obtain the lentivirus C1 with higher interference efficiency (Fig. S3B). Sperm quality was unaffected by lentivirus C1, indicating that sperm movement properties may not be directly impacted by DPY30 knockdown for 47d (seminiferous epithelium cycle) (Fig. 5A). Meanwhile, the proportion of X-bearing sperm was increased in lentivirus C1 treated bucks’ sperm which were screened by the swim-up test (Fig. 5B, C). It was indicated that the proportion of X-bearing sperm increased with higher motility. Subsequently, the treated semen was used for artificial fertilization of 302 oestrous does (Table 3). Of these, 212 does became pregnant and gave birth to 292 kids (Table 4). Although female kids’ rate increased significantly (35.40 % ± 6.00 % (control group) vs 58.20 % ± 5.50 % (lentivirus C1-treated group), P = 0.001 (Fig. 5F) in the offspring of DPY30 interference lentiviruses treated bucks compared to control goats, there was no difference in pregnancy rate and kidding rate between groups (Fig. 5D, F). In summary, Lentivirus-mediated DPY30 inhibition in the testes of bucks increased the female rate of goat kids without influencing reproductive performance.
Table 3.
The effect of DPY30 interference lentivirus on does’ reproductive performance
| No. of breeding | No. of pregnancy | No. of kids | Breeding ratio, % | Pregnancy ratio, % | |
|---|---|---|---|---|---|
| Control | |||||
| Control 1 | 34 | 21 | 31 | 61.76 | 147.62 |
| Control 2 | 34 | 25 | 35 | 73.53 | 140.00 |
| Control 3 | 29 | 20 | 35 | 68.97 | 175.00 |
| Control 4 | 34 | 26 | 29 | 76.47 | 111.54 |
| Control 5 | 31 | 23 | 33 | 74.19 | 143.48 |
| Control 6 | 21 | 14 | 19 | 66.67 | 135.71 |
| Lentivirus groups | |||||
| Lentivirus 1 | 45 | 29 | 37 | 64.44 | 127.59 |
| Lentivirus 2 | 47 | 34 | 46 | 72.34 | 135.29 |
| Lentivirus 3 | 27 | 20 | 27 | 74.07 | 135.00 |
Table 4.
The effect of DPY30 interference lentivirus on female kids’ rate of offspring
| No. of female kids | No. of male kids | Female kid ratio, % | |
|---|---|---|---|
| Control | |||
| Control 1 | 13 | 18 | 41.94 |
| Control 2 | 13 | 22 | 37.14 |
| Control 3 | 12 | 23 | 34.29 |
| Control 4 | 11 | 18 | 37.93 |
| Control 5 | 8 | 25 | 24.24 |
| Control 6 | 7 | 12 | 36.84 |
| Lentivirus groups | |||
| Lentivirus 1 | 22 | 15 | 59.46 |
| Lentivirus 2 | 24 | 22 | 52.17 |
| Lentivirus 3 | 17 | 10 | 62.96 |
Discussion
Owing to an ever-increasing world population, and the growing demand for animal-based food proteins (milk and meat), future global consumption of animal food products is projected to increase (Nyalala et al., 2021). Sex-skewing of animals could expand the livestock population and promote the production of milk (female) and meat (male) within a short period (Xie et al., 2020). This study provides insights into genetic targets for expanding the livestock populations via human intervention to address concerns about animal food shortages.
Sexed sperm separated by flow cytometry has been successfully performed in various mammals based on the different DNA content (cattle (3.8%), sheep (4.1%), and goat (4.4%)) between X- and Y-bearing sperm (González-Marín et al., 2021). The purity of X- and Y-bearing sperm (95%) was higher than that reported in some studies, including 90% (DeJarnette et al., 2008), 91% (O’Brien and Robeck, 2006), and 88% to 92% (Rath et al., 1997). High-purity sexed sperm guarantees accurate results of proteomic analyses.
In the present study, at least 556 total proteins were identified in 6 sexed sperm samples (3 groups of X-bearing sperm and 3 groups of Y-bearing sperm), which showed a higher sequencing depth when compared to a previous study (459 proteins; Scott et al., 2018). Based on these results, more DEPs (15) were screened in this work when compared with the report (9 DEPs; Scott et al., 2018). Of these, the annotated biological functions of the DEPs were associated with sperm energy metabolism, protein processing, and cytoskeleton, which was consistent with the reported works (De Canio et al., 2014; Scott et al., 2018; Sharma et al., 2022).
The extensive and top-notch protein sequence database enables accurate and reliable protein identification and quantification which is essential for meaningful and accurate proteomic investigations. Therefore, fewer DEPs were quantified in mammalian sexed sperm. The genes located on the sex chromosome, which lacked the deep and complete database of genome assembly due to large repetitive or palindromic structures, were responsible for encoding certain undetected DEPs in sexed sperm (Jobling and Tyler-Smith, 2017). Meanwhile, an incomplete livestock protein sequencing database (Lamb et al., 2020) resulted in a lower number of identified proteins in the present study (556 proteins) compared to mouse (1244) (Vicens et al., 2017) and human (3444 proteins) sperm proteomics (Chen et al., 2021). Downstream sequencing data analysis has been most negatively affected by the incomprehensive and low-quality protein sequence databases in livestock. In the future, more accurate proteins will be identified and quantified with the development of genome assembly and protein sequence databases in livestock.
Some of the DEPs found in our work, such as Tubulin beta-2B (De Canio et al., 2014), DYNLL2 (Sharma et al., 2022), and AQP7 (Li, 2021), have been verified in published studies performed in bull sexed sperm with a comparable expression pattern. Nevertheless, the amino acid sequences of the proteins were highly conserved in cattle and goats: DYNLL2 (100%), tubulin beta-2B (100%), and AQP7 (93.33%). These reported proteins demonstrated the high predictive accuracy of DEPs in our study. Determination of DEPs in the sexed sperm of dairy goats is urgently needed, as evidenced by screening for less frequent DEPs in cattle and goat sperm. This paper was the first to provide identification clues to explore novel sexing methods that could be easily applied to dairy goats.
DPY30 is an essential component of dosage compensation in C. elegans, which is a central part of a molecular mechanism that balances sex chromosome-linked gene expression between the sexes (Wang et al., 2009). Thus, DPY30, a core subunit of the H3K4 methyltransferase component (SET1/MLL), is potentially involved in mammalian sex determination (Petty et al., 2011). At the same time, DPY30 has also been shown to contribute to the development of various stem cells. DPY30 maintained the pluripotency of ectoderm cells and was essential for embryonic development (Jiang et al., 2011). DPY30 deletion induced pancytopenia and impaired differentiation of hematopoietic stem cell (Yang et al., 2019). A similar phenomenon has also been reported in neural (Shah et al., 2020) and pancreatic (Campbell et al., 2019) stem cells. According to this, although there is no direct evidence for the regulatory mechanism of DPY30 in male germline stem cell development, DPY30 mediated H3K4 methylation (Ki et al., 2022) is potentially involved in spermatogenesis and affects sperm fertilization, which may be associated with sex determination. Therefore, it is necessary to better understand the regulatory mechanisms of DPY30 that contribute to exploring sexing technology in dairy goats.
In the previous study, DPY30 was implicated in dosage compensation and sex determination (Wang et al., 2009; Petty et al., 2011). Based on this, DPY30 was identified as the major differentially expressed protein in goat sexed sperm due to its high expression in the testis and specific expression in Y-bearing sperm. The higher expression of DPY30 indicated a strong possibility of its involvement in the regulation of spermatogenesis, which would contribute to sex skewing. Subsequently, DPY30 was then investigated for its involvement in the development of male germ line stem cells. Of these, DPY30 promoted meiosis via upregulating SYCP3 which played a crucial role in mediating sex ratio skewing in goats. However, the specific mechanism of DPY30-mediated sex skewing is still unclear in the present study. A bold guess is that DPY30 might be specifically expressed in Y-bearing spermatocytes at some point prior to entry into Meiosis II, ultimately causing localization in Y-bearing sperm. The hypothesis is that since DPY30 localizes to Y-bearing cells, it serves as an essential protein required for meiosis in Y-bearing cells but not in X-bearing cells, which could be the mechanism of DPY30 silence mediated sex ratio skewing. In our previous work, DPY30 could promote glycolysis to increase sperm motility (He et al., 2022b), so DPY30 silence could influence Y-bearing sperm movement by disrupting glycolysis. A reduction (no statistical difference) in semen quality was observed in DPY30 knockdown goats, so knockdown could have more severe effects with the higher lentivirus concentration and the longer treatment period. Because of that, attention should be paid to the potential negative effect of DPY30 knockdown on semen quality. Although, circumstantial evidence currently supports this conjecture, direct investigation should be performed in the future to provide stronger evidence.
At the same time, DPY30 was inhibited with RA, attenuating self-renewal via AKT, similar results were obtained in previous publications (Jiang et al., 2011; Cheng et al., 2023). The inconsistent results in spermatogonia were due to the different experimental conditions in the in vivo and in vitro studies, including virus types, transfection efficiency, off-target effect (shRNA). In our previous work, DPY30 overexpression could increase H3K4 recruitment at the PIK3CA transcription start site to stimulate the PI3K/AKT pathway and thereby promote the cell cycle of spermatogonia in mice (He et al., 2022b). It remains unknown whether DPY30 affects cell self-renewal and meiosis through direct interactions with the retinoic acid receptor, and PI3K (P85, P110). Although there was no direct evidence for the regulation of DPY30 on AKT, the demethylases of H3K4 (LSD1, JARID1B) have been suggested to be involved in AKT modulation (Zhang et al., 2016; Pan et al., 2019). The results of our work provided a new idea and perspective for relevant studies in the future. A similar effect of AKT on stem cell self-renewal was found in previous work (Niu et al., 2016). Accordingly, the inhibition of self-renewal caused by DPY30 knockdown could be ameliorated with an AKT activator (SC79, 740 Y-P) in the testis.
The amino acid sequence of the DPY30 was highly conserved in goats and mice (99.6%), suggesting conserved functions between the animals. Therefore, DPY30 knockdown was performed in mouse testes prior to the goat experiment to assess biosafety. DPY30 loss did not cause testicular dysgenesis in mice, which was consistent with the results obtained in goats. Meanwhile, the mechanisms involved could be studied in in vivo experiments which were unfavorable for detecting in breeding bucks with high economic value and reproductive performance. The work highlighted the need for independent experiments conducted in the model animals.
In the present study, the untreated bucks were considered as the reference group instead of the WT lentivirus to assess the effect of the lentivirus C1 on the offspring sex rate for the following reasons. Firstly, breeding bucks, with higher economic value and better reproductive performance, would be at risk of injury induced by lentivirus infection, which could cause irreversible damage and reduce breeding years. At the same time, the study employed a large number of female animals (302 does). A key difficulty was the need to persuade farms to accept and use the WT lentivirus treated semen with the associated lack of biosafety evidence, which could pose a non-pregnancy risk. Finally, published reports have used untreated male ruminants to evaluate the effect of ZFY inhibition on the sex-skewing to avoid economic losses in farms (Xi et al., 2019). According to previous researchers, it is recommended that WT lentivirus should be used with caution. Meanwhile, the related results of biosafety represented by untreated semen instead of WT lentivirus would provide more confidence to popularize and apply to farms by setting the natural breeding state as the control.
Nonetheless, we still believed that lentivirus-mediated DPY30 knockdown would indiscriminately infect the cells (Wolff and Mikkelsen, 2022) in the testis. The absence of DPY30 in male germ cells would have a greater effect on Y-bearing sperm cells due to the higher expression of DPY30. At the same time, there was no evidence for the existing possibility of differentially expressed viral protein receptors in X- and Y-bearing cells, so that all cell types were effectively transfected by VSV-G (pMD2.G plasmid) mediated second-generation lentivirus (Ou et al., 2020), which was also utilized in this work. This assertion was further supported by the fact that no information had previously been reported on the effect of lentivirus on offspring sex.
In the present study, although the does in the two groups received the same ration and management, the rate of female offspring increased (22.80 % percentage points) in the group treated with lentivirus C1. The rate of female offspring was less than 50% in the control group. Pregnant ungulate mammals under ideal nutritional conditions were preferred to produce more male kids (Douhard, 2017). Similar results were obtained in previous reports, including 37.5% (Vas et al., 2019) and 42.23% (Shourabi et al., 2022). According to a study on the effect of glucose in reproduction, too much glucose aided in the in vitro growth of male blastocysts (Cameron, 2004). Meanwhile, some proteins in Y-bearing sperm contributed to the development of the male embryo, guaranteeing the superior survival potential of the male embryo (Alomar et al., 2008). Based on these findings, we believed that DPY30 inhibition in buck testes increased the female kids’ rate (22.80 percentage points), which was a novel, cheap, fast, safe, and applicable sex-skewing technology.
There are four limitations of the analyses in this paper that should be addressed in future work. The first is that lentivirus-mediated DPY30 regulation should be driven by a male germ cell-specific promoter (STRA8) to prevent it from affecting somatic cell development. The second limitation refers to the biosafety assessment of DPY30. Before alterations are visible under the microscope or become pathogenic, they occur at the molecular level. DPY30 inhibition should be analyzed with a long-term or CRISPR-mediated knockout experiment in mice and goats, which is beneficial for future practical application. This study lacked the mechanistic analyses of DPY30 in embryonic cells. Further research should reveal the effect of DPY30 on embryonic development. Finally, the results lacked the proof of concept of DPY30 lentivirus-mediated sex-skewing, and the mechanisms should be further investigated in vivo using single-cell omics and CRISPR-mediated gene editing. Indeed, these are four of the planned future lines of research.
Conclusion
In summary, this is the first study to identify differentially expressed proteins in the X- and Y-bearing sperm of dairy goat and to confirm the crucial DEP (DPY30). DPY30 promoted meiosis via SYCP3 and self-renewal of goat spermatogonial stem cells through pAKT/PLZF. DPY30 interference lentivirus mediated inhibition in the testis increased the female rate (22.8 percentage points) of goat offspring without influencing reproductive performance. The study provides evidence for the increasing female rate of goat offspring which supports the expansion of the goat population to increase milk production.
Supplementary Material
Acknowledgments
We appreciate the immortalized dairy goat male germline stem cell line (mGSCs-I-SB) donated by Prof. Jinlian Hua (College of Veterinary Medicine, Northwest A&F University, China). The research was jointly funded by the National Key Research and Development Plan of China (2021YFD1600704), the National Natural Science Foundation of China (32272828), and the Innovation Support Plan of Shaanxi Province (no. 2021TD-31).
Glossary
Abbreviations
- AI
artificial insemination
- AKT
serine/threonine kinase
- AMPK
AMP-activated protein kinase
- ASH2L
ASH2-like, histone lysine methyltransferase complex subunit
- CASA
computer-aided semen analysis
- CIDR
intravaginal controlled internal drug release
- CRISPR
Clustered Regularly Interspaced Short Palindromic Repeats
- DEPs
differentially expressed proteins
- DNA
deoxyribonucleic acid
- DPY30
dpy-30 histone methyltransferase complex regulatory subunit
- ERK1/2
extracellular-regulated kinase 1/2
- FBS
fatal bovine serum
- H3K4
histone 3 lysine 4
- HE
hematoxylin and eosin
- IF
immunofluorescence
- IHC
immunohistochemistry
- LC–MS/MS
liquid chromatography–mass spectrometry
- mGSCs
male germline stem cells
- mGSCs-I-SB
immortalized dairy goat male germline stem cell line
- MS1
primary level of mass spectrometry
- MS2
secondary level of mass spectrometry
- NCBI
National Center of Biotechnology Information
- PVDF
polyvinylidene fluoride
- RA
retinoic acid
- RBBP5
RB-binding protein 5, histone lysine methyltransferase complex subunit
- RNA
ribonucleic acid
- RT–PCR
reverse transcription polymerase chain reaction
- SDS
sodium dodecyl sulfate
- SMAD3
mothers against decapentaplegic homolog 3
- SRY
sex determining region Y
- STAT3
signal transducer and activator of transcription 3
- TEAB
triethylammonium bicarbonate
- VSL
straight-line velocity
- WB
Western blotting
- WDR5
WD repeat domain 5
- WT
wild type
Contributor Information
Huanshan He, College of Animal Science and Technology, Northwest A&F University, Yangling, Shaanxi 712100, People’s Republic of China; Gansu Key Laboratory of Biomonitoring and Bioremediation for Environmental Pollution, School of Life Sciences, Lanzhou University, Lanzhou 730000, People’s Republic of China.
Xiang Li, College of Animal Science and Technology, Northwest A&F University, Yangling, Shaanxi 712100, People’s Republic of China.
Jintao Li, College of Animal Science and Technology, Northwest A&F University, Yangling, Shaanxi 712100, People’s Republic of China.
Yong Ning, College of Animal Science and Technology, Northwest A&F University, Yangling, Shaanxi 712100, People’s Republic of China.
Jun Luo, College of Animal Science and Technology, Northwest A&F University, Yangling, Shaanxi 712100, People’s Republic of China.
Huaiping Shi, College of Animal Science and Technology, Northwest A&F University, Yangling, Shaanxi 712100, People’s Republic of China.
Conflict of interest
The authors declare that they have no competing interests.
References
- Alomar, M., Tasiaux H., Remacle S., George F., Paul D., and Donnay I... 2008. Kinetics of fertilization and development, and sex ratio of bovine embryos produced using the semen of different bulls. Anim. Reprod. Sci. 107:48–61. doi: 10.1016/j.anireprosci.2007.06.009 [DOI] [PubMed] [Google Scholar]
- Buttress, T., He S., Wang L., Zhou S., Saalbach G., Vickers M., Li G., Li P., and Feng X... 2022. Histone H2B8 compacts flowering plant sperm through chromatin phase separation. Nature 611:614–622. doi: 10.1038/s41586-022-05386-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cameron, E. Z. 2004. Facultative adjustment of mammalian sex ratios in support of the Trivers-Willard hypothesis: evidence for a mechanism. Proc. Biol. Sci. 271:1723–1728. doi: 10.1098/rspb.2004.2773 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell, S. A., McDonald C. L., Krentz N. A. J., Lynn F. C., and Hoffman B. G... 2019. TrxG complex catalytic and non-catalytic activity play distinct roles in pancreas progenitor specification and differentiation. Cell Reports 28:1830–1844.e6. doi: 10.1016/j.celrep.2019.07.035 [DOI] [PubMed] [Google Scholar]
- Chen, X., Zhu H., Wu C., Han W., Hao H., Zhao X., Du W., Qin T., Liu Y., and Wang D... 2012. Identification of differentially expressed proteins between bull X and Y spermatozoa. J. Proteomics 77:59–67. doi: 10.1016/j.jprot.2012.07.004 [DOI] [PubMed] [Google Scholar]
- Chen, H., Tang L., Hong Q., Pan T., Weng S., Sun J., Wu Q., Zeng X., Tang Y., and Luo T... 2021. Testis developmental related gene 1 (TDRG1) encodes a progressive motility-associated protein in human spermatozoa. Hum. Reprod. 36:283–292. doi: 10.1093/humrep/deaa297 [DOI] [PubMed] [Google Scholar]
- Cheng, G., An F., Cao Z., Zheng M., Zhao Z., and Wu H... 2023. DPY30 promotes the growth and survival of osteosarcoma cell by regulating the PI3K/AKT signal pathway. Eur. J. Histochem. 67:3413. doi: 10.4081/ejh.2023.3413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Canio, M., Soggiu A., Piras C., Bonizzi L., Galli A., Urbani A., and Roncada P... 2014. Differential protein profile in sexed bovine semen: shotgun proteomics investigation. Mol. Biosyst. 10:1264–1271. doi: 10.1039/c3mb70306a [DOI] [PubMed] [Google Scholar]
- DeJarnette, J. M., Nebel R. L., Marshall C. E., Moreno J. F., McCleary C. R., and Lenz R. W... 2008. Effect of sex-sorted sperm dosage on conception rates in Holstein heifers and lactating cows. J. Dairy Sci. 91:1778–1785. doi: 10.3168/jds.2007-0964 [DOI] [PubMed] [Google Scholar]
- Douhard, M. 2017. Offspring sex ratio in mammals and the Trivers-Willard hypothesis: in pursuit of unambiguous evidence. BioEssays 39:n/a–n/a. doi: 10.1002/bies.201700043 [DOI] [PubMed] [Google Scholar]
- França, L. R., Becker-Silva S. C., and Chiarini-Garcia H... 1999. The length of the cycle of seminiferous epithelium in goats (Capra hircus). Tissue Cell 31:274–280. doi: 10.1054/tice.1999.0044 [DOI] [PubMed] [Google Scholar]
- Furstoss, V., David I., Fatet A., Boissard K., Clément V., and Bodin L... 2015. Genetic and non-genetic factors related to the success of artificial insemination in dairy goats. Animal 9:1935–1942. doi: 10.1017/S1751731115001500 [DOI] [PubMed] [Google Scholar]
- González-Marín, C., Góngora C. E., Moreno J. F., and Vishwanath R... 2021. Small ruminant SexedULTRA™ sperm sex-sorting: status report and recent developments. Theriogenology. 162:67–73. doi: 10.1016/j.theriogenology.2020.12.028 [DOI] [PubMed] [Google Scholar]
- He, H., Li J., Xie Y., Li Z., Shi H., and Lu C. D... 2021. Effects of soy isoflavones on intake, body weight, sex hormones, antioxidant performance, and semen quality in Xinong Saanen goats. J. Appl. Anim. Res. 49:125–132. doi: 10.1080/09712119.2021.1901716 [DOI] [Google Scholar]
- He, H., Chen X., Li X., Yang K., Li J., and Shi H... 2022a. Lactoferrin alleviates spermatogenesis dysfunction caused by bisphenol A and cadmium via ameliorating disordered autophagy, apoptosis and oxidative stress. Int. J. Biol. Macromol. 222:1048–1062. doi: 10.1016/j.ijbiomac.2022.09.260 [DOI] [PubMed] [Google Scholar]
- He, H., Li X., Shen J., Bai S., Li C., and Shi H... 2022b. Bisphenol A exposure causes testicular toxicity by targeting DPY30-mediated post-translational modification of PI3K/AKT signaling in mice. Ecotoxicol Environ. Saf. 243:113996. doi: 10.1016/j.ecoenv.2022.113996 [DOI] [PubMed] [Google Scholar]
- Jiang, H., Shukla A., Wang X., Chen W. -yi, Bernstein B. E., and Roeder R. G... 2011. Role for Dpy-30 in ES cell-fate specification by regulation of H3K4 methylation within bivalent domains. Cell 144:513–525. doi: 10.1016/j.cell.2011.01.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jobling, M. A., and Tyler-Smith C... 2017. Human Y-chromosome variation in the genome-sequencing era. Nat. Rev. Genet. 18:485–497. doi: 10.1038/nrg.2017.36 [DOI] [PubMed] [Google Scholar]
- Kahi, A. K., and Wasike C. B... 2019. Dairy goat production in sub-Saharan Africa: current status, constraints and prospects for research and development. Asian-Australas. J. Anim. Sci. 32:1266–1274. doi: 10.5713/ajas.19.0377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katska-Ksiazkiewicz, L., Ryńska B., Gajda B., and Smorag Z... 2004. Effect of donor stimulation, frozen semen and heparin treatment on the efficiency of in vitro embryo production in goats. Theriogenology. 62:576–586. doi: 10.1016/j.theriogenology.2003.11.007 [DOI] [PubMed] [Google Scholar]
- Ki, B. S., Shim S. H., Park C., Yoo H., La H., Lee O. -H., Kwon Y., Skalnik D. G., Okada Y., Yoon H. -G.,. et al. 2022. Epigenetic regulator Cfp1 safeguards male meiotic progression by regulating meiotic gene expression. Exp. Mol. Med. 54:1098–1108. doi: 10.1038/s12276-022-00813-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamb, H. J., Hayes B. J., Nguyen L. T., and Ross E. M... 2020. The future of livestock management: a review of real-time portable sequencing applied to livestock. Genes 11:1478. doi: 10.3390/genes11121478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, S. 2021. The membrane protein differences of X/Y spermatozoa in dairy cattle and dairy goats.
- Maes, D. G. D., Dewulf J., Piñeiro C., Edwards S., and Kyriazakis I... 2020. A critical reflection on intensive pork production with an emphasis on animal health and welfare. J. Anim. Sci. 98:S15–S26. doi: 10.1093/jas/skz362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehrotra, R., Gupta A., Singh M., and Ibrahim R... 2006. Application of cytology and molecular biology in diagnosing premalignant or malignant oral lesions. Mol. Cancer 5:11. doi: 10.1186/1476-4598-5-11 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Niu, B., Li B., Wu C., Wu J., Yan Y., Shang R., Bai C., Li G., and Hua J... 2016. Melatonin promotes goat spermatogonia stem cells (SSCs) proliferation by stimulating glial cell line-derived neurotrophic factor (GDNF) production in Sertoli cells. Oncotarget 7:77532–77542. doi: 10.18632/oncotarget.12720 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nyalala, I., Okinda C., Kunjie C., Korohou T., Nyalala L., and Chao Q... 2021. Weight and volume estimation of poultry and products based on computer vision systems: a review. Poult. Sci. 100:101072. doi: 10.1016/j.psj.2021.101072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Brien, J. K., and Robeck T. R... 2006. Development of sperm sexing and associated assisted reproductive technology for sex preselection of captive bottlenose dolphins (Tursiops truncatus). Reprod. Fertil. Dev. 18:319–329. doi: 10.1071/rd05108 [DOI] [PubMed] [Google Scholar]
- Oakberg, E. F. 1956. Duration of spermatogenesis in the mouse and timing of stages of the cycle of the seminiferous epithelium. Am. J. Anat. 99:507–516. doi: 10.1002/aja.1000990307 [DOI] [PubMed] [Google Scholar]
- Ou, X., Liu Y., Lei X., Li P., Mi D., Ren L., Guo L., Guo R., Chen T., Hu J.,. et al. 2020. Characterization of spike glycoprotein of SARS-CoV-2 on virus entry and its immune cross-reactivity with SARS-CoV. Nat. Commun. 11:1620. doi: 10.1038/s41467-020-15562-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan, H. M., Lang W. Y., Yao L. J., Wang Y., and Li X. L... 2019. shRNA-interfering LSD1 inhibits proliferation and invasion of gastric cancer cells via VEGF-C/PI3K/AKT signaling pathway. World J. Gastrointest. Oncol. 11:622–633. doi: 10.4251/wjgo.v11.i8.622 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petty, E., Laughlin E., and Csankovszki G... 2011. Regulation of DCC localization by HTZ-1/H2AZ and DPY-30 does not correlate with H3K4 methylation levels. PLoS One. 6:e25973. doi: 10.1371/journal.pone.0025973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin, J., Xu H., Zhang P., Zhang C., Zhu Z., Qu R., Qin Y., and Zeng W... 2015. An efficient strategy for generation of transgenic mice by lentiviral transduction of male germline stem cells in vivo. J. Anim. Sci. Biotechnol. 6:59. doi: 10.1186/s40104-015-0058-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rath, D., Johnson L. A., Dobrinsky J. R., Welch G. R., and Niemann H... 1997. Production of piglets preselected for sex following in vitro fertilization with X and Y chromosome-bearing spermatozoa sorted by flow cytometry. Theriogenology. 47:795–800. doi: 10.1016/s0093-691x(97)00035-6 [DOI] [PubMed] [Google Scholar]
- Sathe, S. R. 2018. Laparoscopic artificial insemination technique in small ruminants-A procedure review. Front. Vet. Sci. 5:266. doi: 10.3389/fvets.2018.00266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott, C., de Souza F. F., Aristizabal V. H. V., Hethrington L., Krisp C., Molloy M., Baker M. A., and Dell'Aqua J. A... 2018. Proteomic profile of sex-sorted bull sperm evaluated by SWATH-MS analysis. Anim. Reprod. Sci. 198:121–128. doi: 10.1016/j.anireprosci.2018.09.010 [DOI] [PubMed] [Google Scholar]
- Shah, K., King G. D., and Jiang H... 2020. A chromatin modulator sustains self-renewal and enables differentiation of postnatal neural stem and progenitor cells. J. Mol. Cell. Biol. 12:4–16. doi: 10.1093/jmcb/mjz036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma, V., Verma A. K., Sharma P., Pandey D., and Sharma M... 2022. Differential proteomic profile of X- and Y- sorted Sahiwal bull semen. Res. Vet. Sci. 144:181–189. doi: 10.1016/j.rvsc.2021.11.013 [DOI] [PubMed] [Google Scholar]
- Shi, H., Zhang T., Li C., Wang J., Huang J., and Li Z... 2017. trans-10,cis-12-conjugated linoleic acid affects expression of lipogenic genes in mammary glands of lactating dairy goats. J. Agric. Food Chem. 65:9460–9467. doi: 10.1021/acs.jafc.7b02377 [DOI] [PubMed] [Google Scholar]
- Shourabi, E., Hakimi H., Baqeri A., Gharagozlou F., Vojgani M., Foroutannejad M., Baghbanani R. H., Mobedi E., and Akbarinejad V... 2022. Evidence that Murciano-Granadina does with longer anogenital distance are more fertile and prolific and produce heavier and male-biased litters. Anim. Reprod. Sci. 244:107047. doi: 10.1016/j.anireprosci.2022.107047 [DOI] [PubMed] [Google Scholar]
- Soleymani, B., Parvaneh S., and Mostafaie A... 2019. Goat polyclonal antibody against the sex determining region Y to separate X- and Y-chromosome bearing spermatozoa. Rep. Biochem. Mol. Biol. 8:326–334. http://rbmb.net/article-1-360-en.html. [PMC free article] [PubMed] [Google Scholar]
- Soleymani, B., Mansouri K., Rastegari-Pouyani M., Parvaneh S., Khademi F., Sharifi Tabar M., and Mostafaie A... 2021. Production of monoclonal antibody against recombinant bovine sex-determining region Y (SRY) and their preferential binding to Y chromosome-bearing sperm. Reprod. Domest. Anim. 56:270–277. doi: 10.1111/rda.13821 [DOI] [PubMed] [Google Scholar]
- Stratonovitch, P., and Semenov M. A... 2015. Heat tolerance around flowering in wheat identified as a key trait for increased yield potential in Europe under climate change. J. Exp. Bot. 66:3599–3609. doi: 10.1093/jxb/erv070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su, W. C., Mao X. -M., Li S. -Y., Luo C. -Y., Fan R., Jiang H. -F., Zhang L. -J., Wang Y. -T., Su G. -Q., and Shen D. -Y... 2023. DPY30 promotes proliferation and cell cycle progression of colorectal cancer cells via mediating H3K4 trimethylation. Int. J. Med. Sci. 20:901–917. doi: 10.7150/ijms.80073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas, J. M., Lock S. L., Poock S. E., Ellersieck M. R., Smith M. F., and Patterson D. J... 2014. Delayed insemination of nonestrous cows improves pregnancy rates when using sex-sorted semen in timed artificial insemination of suckled beef cows. J. Anim. Sci. 92:1747–1752. doi: 10.2527/jas.2013-7131 [DOI] [PubMed] [Google Scholar]
- Thomas, J. M., Locke J. W. C., Bonacker R. C., Knickmeyer E. R., Wilson D. J., Vishwanath R., Arnett A. M., Smith M. F., and Patterson D. J... 2019. Evaluation of SexedULTRA 4M™ sex-sorted semen in timed artificial insemination programs for mature beef cows. Theriogenology. 123:100–107. doi: 10.1016/j.theriogenology.2018.09.039 [DOI] [PubMed] [Google Scholar]
- Umehara, T., Tsujita N., Zhu Z., Ikedo M., and Shimada M... 2020. A simple sperm-sexing method that activates TLR7/8 on X sperm for the efficient production of sexed mouse or cattle embryos. Nat. Protocols. 15:2645–2667. doi: 10.1038/s41596-020-0348-y [DOI] [PubMed] [Google Scholar]
- Vas, J., Chojnacki R. M., and Andersen I. L... 2019. Search behavior in goat (Capra hircus) kids from mothers kept at different animal densities throughout pregnancy. Front. Vet. Sci. 6:21. doi: 10.3389/fvets.2019.00021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vicens, A., Borziak K., Karr T. L., Roldan E. R. S., and Dorus S... 2017. Comparative sperm proteomics in mouse species with divergent mating systems. Mol. Biol. Evol. 34:1403–1416. doi: 10.1093/molbev/msx084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, X., Lou Z., Dong X., Yang W., Peng Y., Yin B., Gong Y., Yuan J., Zhou W., Bartlam M.,. et al. 2009. Crystal structure of the C-terminal domain of human DPY-30-like protein: a component of the histone methyltransferase complex. J. Mol. Biol. 390:530–537. doi: 10.1016/j.jmb.2009.05.061 [DOI] [PubMed] [Google Scholar]
- Wolff, J. H., and Mikkelsen J. G... 2022. Delivering genes with human immunodeficiency virus-derived vehicles: still state-of-the-art after 25 years. J. Biomed. Sci. 29:79. doi: 10.1186/s12929-022-00865-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xi, J. F., Wang X. -Z., Zhang Y. -S., Jia B., Li C. -C., Wang X. -H., and Ying R. -W... 2019. Sex control by Zfy siRNA in the dairy cattle. Anim. Reprod. Sci. 200:1–6. doi: 10.1016/j.anireprosci.2018.05.015 [DOI] [PubMed] [Google Scholar]
- Xie, Y., Xu Z., Wu Z., and Hong L... 2020. Sex manipulation technologies progress in livestock: a review. Front. Vet. Sci. 7:481. doi: 10.3389/fvets.2020.00481 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu, X., Ahmed T., Wang L., Cao X., Zhang Z., Wang M., Lv Y., Kanwal S., Tariq M., Lin R.,. et al. 2022. The mTORC1-eIF4F axis controls paused pluripotency. EMBO Rep. 23:e53081. doi: 10.15252/embr.202153081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang, Z., Shah K., Khodadadi-Jamayran A., and Jiang H... 2019. Control of hematopoietic stem and progenitor cell function through epigenetic regulation of energy metabolism and genome integrity. Stem Cell Rep. 13:61–75. doi: 10.1016/j.stemcr.2019.05.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, J., An X., Han Y., Ma R., Yang K., Zhang L., Chi J., Li W., Llobet-Navas D., Xu Y.,. et al. 2016. Overexpression of JARID1B promotes differentiation via SHIP1/AKT signaling in human hypopharyngeal squamous cell carcinoma. Cell Death Dis. 7:e2358. doi: 10.1038/cddis.2016.262 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.