To the Editor:
Idiopathic multicentric Castleman disease (iMCD) is a rare hematologic disorder involving a cytokine storm-driven inflammatory syndrome.1 Interleukin-6 (IL-6) has been implicated in disease pathogenesis and therapies that target IL-6 show efficacy in a portion of iMCD patients.1 Tocilizumab, a monoclonal antibody that targets the IL-6 receptor, was approved to treat iMCD in Japan.1 Siltuximab, a monoclonal antibody that directly targets IL-6, was shown to have a 34% response in the phase II clinical trial and is the only drug approved to treat iMCD in the United States, European Union, and most regions of the world.1 International, consensus treatment guidelines recommend siltuximab first-line1, but it is not effective in all patients, and predictors of response to siltuximab are still under investigation.2
C-reactive protein (CRP), which is elevated in iMCD and one of the minor diagnostic criteria, is also an established biomarker of IL-6 activity3 and normalization of CRP is thought to indicate clinical benefit.1 Further, the open-label, dose-finding phase I study of siltuximab (NCT00412321) found sustained suppression of CRP after administration3 and a post hoc analysis of the phase II siltuximab trial found that CRP normalized a median of 2.0 months post-siltuximab.3
Notably, despite IL-6 being the therapeutic target of siltuximab, IL-6 measurements are not recommended as an indicator of response. Treatment guidelines for iMCD include anecdotal reports that IL-6-directed therapy can cause spurious elevation of serum IL-6.1 However, there is concern that these artificial increases could cause physicians to erroneously infer treatment failure as the potential for siltuximab to cause spuriously elevated IL-6 is not widely known. Indeed, we are aware of physicians who have interpreted increased IL-6 levels after siltuximab administration to indicate disease progression, despite disease control. In fact, a direct quote from a physician in medical records of the ACCELERATE natural history registry of Castleman disease (CD) (NCT02817997) stated that based on a patient’s “rising IL-6 she appears to have progressed on siltuximab.”
We used the ACCELERATE registry to evaluate circulating IL-6 values before and after siltuximab administration, irrespective of response. We also examined the time between final siltuximab dose and normalization of IL-6 values. Additionally, we compared changes in IL-6 values after siltuximab administration to changes in CRP after siltuximab administration to assess whether increased IL-6 values reflected progressive disease activity. Lastly, we analyzed longitudinal serum IL-6 values in patient samples from the siltuximab phase II clinical trial.
To understand the effect of siltuximab administration on IL-6 values, we analyzed IL-6 values before and after siltuximab initiation. Forty-two patients from the ACCELERATE natural history registry were found in February 2023 to have circulating IL-6 values quantified at least once before and after initiating siltuximab and lymph node histopathology consistent with CD. Breakdown by gender was 50% male (n=21) and 50% female (n=21), and mean age was 41.5 years. IL-6 values were extracted directly from medical records. Though they were quantified for clinical purposes using multiple different assays, IL-6 measurements were typically performed using sandwich Enzyme-Linked Immuno-Sorbant Assays (ELISA) or Luminex bead-based assays.4 Since IL-6 values were quantified using different assays, we standardized IL-6 values relative to the upper limits of normal for the assay to adjust for differences between assays, log10 transformed them, and present the results in standardized IL-6 units. We used a paired t-test to detect a difference in log10 transformed IL-6 values pre- and post-siltuximab. When IL-6 was measured multiple times before or after siltuximab administration, we selected the peak value for analysis. For IL-6 values reported to be below the lower limit of detection, half of the lower limit was used. We found that the median circulating IL-6 value rose almost 150-fold after siltuximab treatment (standardized IL-6: 3.79 vs. 557; p < 2.2 × 10−16). Standardized IL-6 values rose in all patients after siltuximab, with all but three patients demonstrating an over five-fold increase from pre-siltuximab IL-6 values.
Next, we identified patients in the cohort who stopped siltuximab and examined their post-siltuximab IL-6 values to determine if IL-6 normalized throughout the remaining available records or before the patient received another IL-6 blocker and investigated the time to normalization of IL-6. Out of 42 patients, 17 had IL-6 values measured after stopping siltuximab. For two patients who received tocilizumab after stopping siltuximab, we used the time to their most recent pre-tocilizumab IL-6 measurement. For the three patients out of 17 whose IL-6 normalized during the study period, the mean time from final siltuximab dose to IL-6 normalization was 588 days (~1.6 years). The mean time from final siltuximab dose to the most recently available IL-6 measurement in the 14 patients with continuously elevated IL-6 was 135.7 day (~4.5 months). The time to normalization we observed is consistent with anecdotal evidence that IL-6 remains elevated for 18–24 months post-final siltuximab dose.1
Next, given that CRP is a well-established biomarker of IL-6 activity, we investigated whether the long-lasting observed increase in IL-6 reflected increased disease activity. We gathered CRP values measured nearest the respective IL-6 values before and after siltuximab, and not more than 30 days from them, when available (n=27). We performed a Wilcoxon signed-rank test to test for a difference in CRP values pre-and post-siltuximab. We found that median pre-siltuximab CRP was 67.8 mg/L, well above the normal range of 10 mg/L and indicative of a state of inflammation. After treatment, median CRP decreased to 5.1 mg/L (p = 8.3 × 10−4), within the normal range, suggesting that IL-6 values rose despite reduced disease and IL-6 activity. 18 patients responded to the regimen they were on when post-siltuximab CRP was measured, 8 did not respond, and 1 had an unknown response.
Separately, to study the effect of siltuximab on IL-6 values over time using an orthogonal platform in an independent cohort, we analyzed longitudinal serum IL-6 values from a serum proteomics study5 performed on patients enrolled in the phase II siltuximab clinical trial (NCT01024036). Serum samples collected on day 1 (Cycle 1, Day 1), 8 (Cycle 1, Day 8) and 64 (Cycle 4, Day 1) of siltuximab (n=53) and placebo (n=26) were analyzed using the SomaLogic SOMAScan DNA aptamer approach, and IL-6 data are presented for 73 patients. IL-6 values were log2 transformed and capped at the 2.5th and 97.5th percentiles.5 We used a linear mixed effects model to determine the interaction between cycle and study arm on IL-6 values. Age and sex were included as covariates, and the patient was included as the random effect. Longitudinal data were available on 49 patients treated with siltuximab, including 17 responders and 32 non-responders, and 24 patients treated with placebo. Interestingly, we found a significant interaction between siltuximab and infusion cycle with IL-6 rising as early as day 8 (p=7.9 × 10−5) and remaining elevated at day 64 (p=1.4 × 10−9). Siltuximab led to significantly higher IL-6 values whereas the placebo did not affect IL-6 values, supporting the hypothesis that siltuximab causes spuriously elevated IL-6.
Anecdotal reports through the CDCN and medical records from the ACCELERATE registry revealed anecdotal evidence that IL-6 values measured after siltuximab administration have been used to assess treatment response in CD patients. This study provides evidence that IL-6 values uniformly increase in patients post-siltuximab despite evidence of disease response with declining CRP values and should not be used to assess response to therapy. In fact, circulating IL-6 levels uniformly rise upon siltuximab administration and can remain elevated for >1 year after stopping siltuximab.
We believe the most likely cause of the elevated IL-6 values is the presence of siltuximab-IL-6 complexes that interfere with measurement of free IL-6.6 These complexes accumulate because they have a longer half-life than free IL-6.6 It is also possible that there is increased production of IL-6 in the presence of siltuximab.6 However, given that CRP is a biomarker of IL-6 activity and it decreases following siltuximab administration,3 we infer that siltuximab causes an artificial increase in IL-6 by binding IL-6 that does not have a functional impact in patients. Of note, there is evidence of a competing effect which results in decreased IL-6 values measured in the presence of siltuximab when using two different commercial Luminex singleplex kits.4 Nonetheless, in this study’s cohort of siltuximab-treated patients, artificial increases in IL-6 values outweighed possible competing downward effects.
These data demonstrate that IL-6 values should not be used as a biomarker of response to siltuximab. In place of IL-6, laboratory tests such as albumin, hemoglobin, platelets, and creatinine should be used.1,3 Additionally, a recent serum proteomics study identified and validated C-X-C Motif Chemokine Ligand-13 (CXCL13) as a predictive biomarker of response to siltuximab as early as day 8 after siltuximab initiation; however, CXCL13 is not yet widely available for clinical use.2 Ultimately, these results indicate that IL-6 values should not be measured after siltuximab administration and should not be used to guide treatment decisions.
Figure 1.
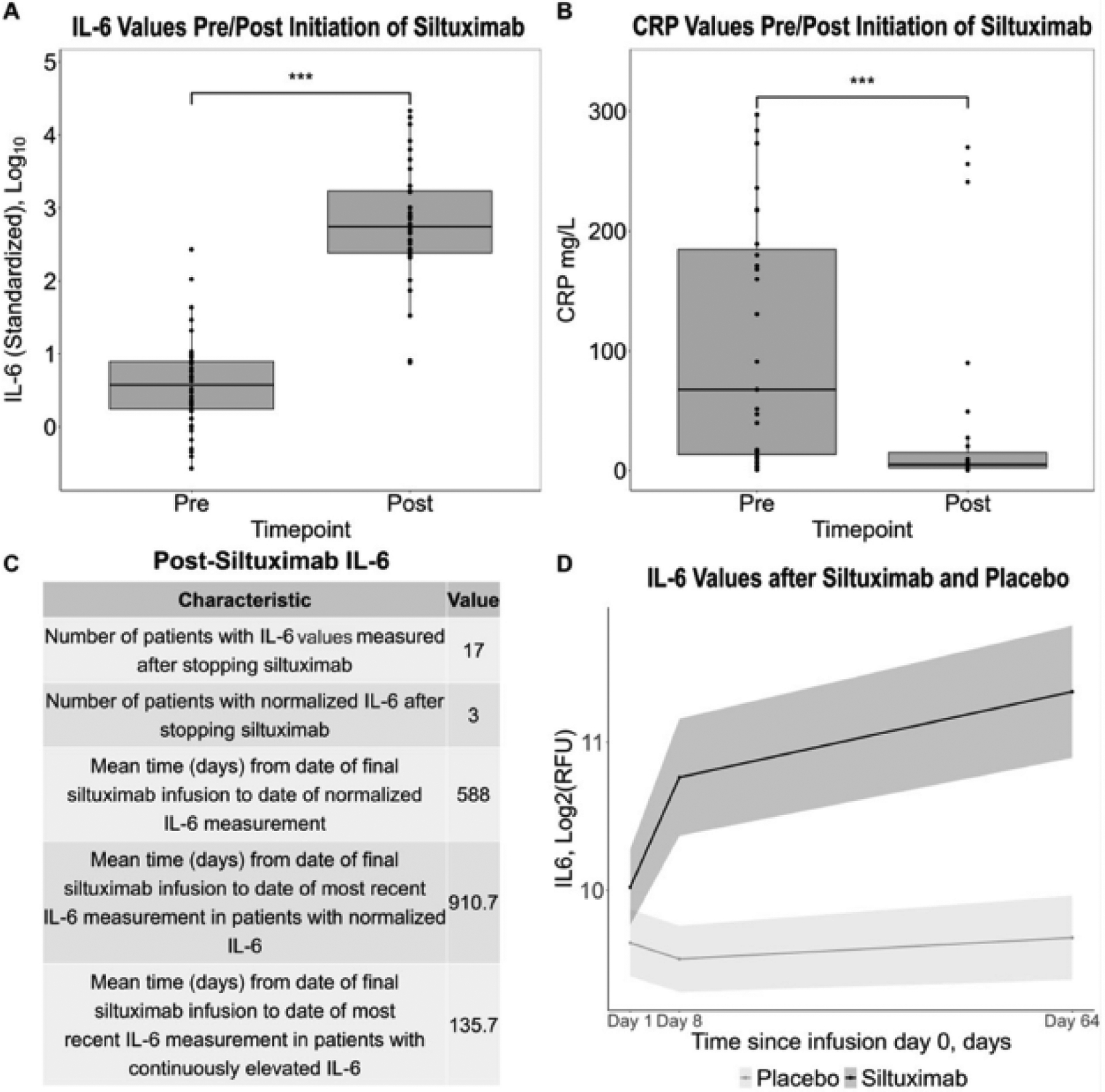
A. Box plots show an increase in median (IQR) standardized interleukin-6 (IL-6) values after initiation of siltuximab 557 (243–1707) compared to before siltuximab 3.79 (1.75–7.92) (p<2.2 × 10−16). B. Box plots show a decrease in median C-reactive protein (CRP) after initiation of siltuximab 5.1 (2.5–15.1) mg/L compared to before siltuximab 67.8 (13.6–185) mg/L (p=8.3 × 10−4). C. A table demonstrating the number of patients whose IL-6 values ever normalized after stopping siltuximab and the duration of time until normalization. D. A linear mixed effects model used to investigate the effect of the interaction between cycle and study arm on IL-6 values (n=73), adjusted for age and sex with the patient as the random effect. A significant interaction effect was observed between cycle and study arm at day 8 (p=7.9 × 10−5) and day 64 (p=1.4 × 10−9) with continued siltuximab infusions leading to significantly greater IL-6.
Acknowledgements and Funding Statement
The ACCELERATE natural history registry has received funding from Janssen Pharmaceuticals (2016-2018), EUSA Pharma LLC (US), which has merged with Recordati Rare Diseases Inc. (2018-2022), and the U.S. Food & Drug Administration (RF01FD007632) (2022-Present). D.C.F has also received research funding relevant to this project from the NIH National Heart, Lung, & Blood Institute (R01HL141408). The authors wish to thank the patients and their families for participating in the ACCELERATE natural history registry. We wish to thank the Castleman Disease Collaborative Network (CDCN) and the ACCELERATE registry team for their support. We wish to thank the volunteers for the CDCN who have supported this research, including Mary Zuccato and Mileva Repasky. We wish to thank Shawnee Bernstein, Nathan Hersh, Gerard Hoeltzel, and Jeremy Zuckerberg for their contributions to this study. We wish to thank former ACCELERATE registry team members, including Faizaan Ahkter, Erin Napier, Eric Haljasmaa, Katharine Floess, Mark-Avery Tamakloe, Victoria Powers, Alexander Gorzewski, Johnson Khor, Reece Williams, Jasira Ziglar, Amy Liu, Saishravan Shyamsundar, Criswell Lavery, and Bridget Austin.
Conflict of Interest Disclosure
D.C.F. has received funding for the ACCELERATE registry and consulting fees from EUSA Pharma, study drug with no associated research funding from Pfizer for the clinical trial of sirolimus (NCT03933904), and has two provisional patents pending related to the diagnosis and treatment of iMCD, including one related to CXCL13 as a biomarker in iMCD. J.D.B. has received consulting fees from EUSA Pharma. F.v.R has received consulting fees from EUSA Pharma, GlaxoSmithKline, Karyopharm and Takeda and has received research funding from Janssen Pharmaceuticals and Bristol Myers Squibb. F.v.R. also sits on the advisory boards of Adicent Bio, Bristol Myers Squibb, Castleman Disease Collaborative Network, EUSA Pharma, GlaxoSmithKline, Janssen Pharmaceutical, Kite Pharma and Secura. All other authors report no conflicts of interest.
Footnotes
Ethical Approval
The ACCELERATE natural history registry has received ethical approval from the University of Pennsylvania IRB.
Data Availability Statement
All source data reported in this study are available by contacting the ACCELERATE team at accelerate@uphs.upenn.edu.
References
- 1.van Rhee F, Voorhees P, Dispenzieri A, et al. International, evidence-based consensus treatment guidelines for idiopathic multicentric Castleman disease. Blood. 2018;132(20):2115–2124. doi: 10.1182/blood-2018-07-862334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pierson SK, Katz L, Williams R, et al. CXCL13 is a predictive biomarker in idiopathic multicentric Castleman disease. Nat Commun. 2022;13(1):7236. doi: 10.1038/s41467-022-34873-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Van Rhee F, Rosenthal A, Kanhai K, et al. Siltuximab is associated with improved progression-free survival in idiopathic multicentric Castleman disease. Blood Adv. 2022;6(16):4773–4781. doi: 10.1182/bloodadvances.2022007112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chen F, Teachey DT, Pequignot E, et al. Measuring IL-6 and sIL-6R in serum from patients treated with tocilizumab and/or siltuximab following CAR T cell therapy. J Immunol Methods. 2016;434:1–8. doi: 10.1016/j.jim.2016.03.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pierson SK, Shenoy S, Oromendia AB, et al. Discovery and validation of a novel subgroup and therapeutic target in idiopathic multicentric Castleman disease. Blood Adv. 2021;5(17):3445–3456. doi: 10.1182/bloodadvances.2020004016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wang W, Wang X, Doddareddy R, et al. Mechanistic Pharmacokinetic/Target Engagement/Pharmacodynamic (PK/TE/PD) Modeling in Deciphering Interplay Between a Monoclonal Antibody and Its Soluble Target in Cynomolgus Monkeys. AAPS J. 2014;16(1):129–139. doi: 10.1208/s12248-013-9545-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All source data reported in this study are available by contacting the ACCELERATE team at accelerate@uphs.upenn.edu.


