Abstract
Voluntary wheel running is a valuable metabolic intervention and well-established measure of physical activity in preclinical rodent models. Herein, we describe detailed assembly instructions and provide necessary resources for researchers to build their own running wheels from commercial-off-the-shelf parts and an open-source program at approximately a tenth of the cost of commercially-available options.
Wheel running is a known voluntary murine behavior1 that is a useful intervention in preclinical models of health and disease. In contrast to other exercise models such as forced swimming3 or treadmill running4, wheel running does not require aversive stimuli to stimulate activity, suggesting that it is distinctly useful in evaluating spontaneous murine behavior. Mice also prefer wheel running to other complex activities, such as mazes when provided with both5, and they express preferences between different saucer shapes6. Indeed, voluntary wheel running is even seen in non-captive mice in the wild when they are provided running wheels7.
The natural predilection of mice to running on a wheel has been applied in translational research, allowing researchers to measure multiple factors contributing to both voluntary and motivated behaviors in preclinical models. Specifically, wheels offer a therapeutic exercise intervention shown to stimulate hippocampal neurogenesis8, reduce tumor incidence and growth9, and alter local EEG patterns during sleep10 in murine models.
Given the clear utility of mouse running wheels in translational research, technology to record wheel revolutions as a measure for mouse activity has been commercialized. These wheels often transmit data wirelessly to a hub and use low-friction bearings. However, their cost can be prohibitive; wheels can be several hundred dollars and a compulsory wireless hub can cost almost a thousand. Furthermore, many experiments often require using several wheels simultaneously - as many as 12 at a time2. As these experiments can last for several weeks, staggering use of limited wheel resources isn’t always plausible for studies on a timeline. The upfront cost for a laboratory attempting such experiments can be prohibitively high, leaving this useful metabolic and behavioral intervention inaccessible to many in the research community.
Lowering the barrier to their use is imperative in making this useful experimental intervention accessible to the broader research community. Thus, we set out to design and construct a homemade running wheel that accurately and reliably counts wheel revolutions for several weeks with the combined benefits of low cost and simple construction. We were able to construct a durable wheel with a precise revolution counter for a fraction of the cost of commercially available wheels, which we used to assess fatigue during the development of cancer cachexia with robust and consistent results, demonstrating the experimental reliability of these constructed wheels11.
Materials
An itemized list of both consumable and non-consumable materials required to assemble the mouse running wheel can be found in Table 1. The pre-fabricated printed circuit board (PCB) can be acquired by going to navigating to https://github.com/m141914/Mouse-Revolution-Counter and following the instructions to either independently manufacture or purchase the circuit boards from an independent manufacturer.
Table 1 ∣.
Parts list for mouse wheels per unit and non-consumable tools
| Item | Approximate Cost (USD) |
|---|---|
| Parts | |
| Exercise Saucer | $6.29 |
| 12-14.5cmh clear plexiglass pane | $1.92 |
| JST Power wire | $0.85 |
| ATMEL Attiny85 | $2.09 |
| Pre-Printed MRC PCB | $5.00 |
| 3000mAh Lithium Polymer Battery | $12.50 |
| 10X3mm Neodymium Magnet x11 | $1.43 |
| SSD1306 OLED screen | $4.39 |
| 10kohm resistor | $0.06 |
| Hall effect sensor | $0.40 |
| IR sensor | $1.54 |
| Header Pins x7 | $0.10 |
| Female-Female Dupont Cables x3 | $0.38 |
| Total Parts Cost | $36.95 |
| Tools | |
| TinyAVR USB Programmer | $15.00 |
| Electrical Tape | $6.50 |
| Super Glue | $1.07 |
| IR-emitting remote control | $6.00 |
| Soldering Kit | $17.00 |
| Dremel | $35.00 |
| 6-battery usb charger | $7.00 |
| JST extension cables x6 | $36.36 |
| Arduino IDE | Free Download |
| TinyWireM library | Free Download |
| Tiny4kOLED library | Free Download |
| Total Tools Cost | $123.93 |
Procedure
Wheel counter circuit board programming and soldering. Timing ~20 min:
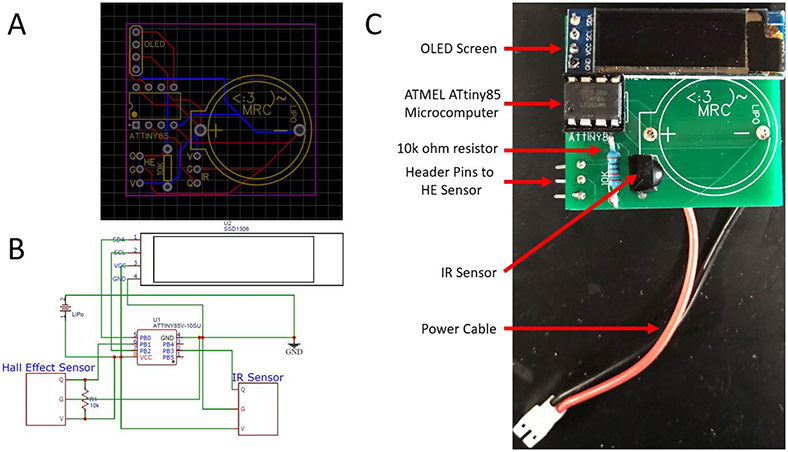
Start by soldering wires, header pins, optical light-emitting diode (OLED) screen, infrared (IR) sensor, and ATtiny85 socket onto the pre-fabricated circuit board as shown in Figure 1. [CAUTION Soldering electronic components involves high temperatures that can cause severe, permanent burn injury as well as property damage. Ensure that all manufacturer safety instructions are followed and that the heated soldering iron is placed in a safe and secure location when not actively used].
Figure 1 ∣. Mouse running wheel PCB schematics and parts layout.
(A) Mouse wheel PCB diagram. (B) Electronic schematic. (C) Fully-constructed PCB with parts labelled.
Once soldering is complete and cooled, insert the ATtiny85 into the TinyUSB programmer with static-free forceps and insert the programmer into a PC USB drive. This can be done with any PC running Windows, Mac OS, or Linux-based operating systems. On the PC, open the Arduino integrated development environment (IDE; https://www.arduino.cc/en/main/software) and under the “tools” header, select “ATtiny85” as the “Processor,” “Internal 1MHz” as the “Clock,” and then “Burn Bootloader.”
Next, download both the TinyWireM (https://github.com/adafruit/TinyWireM) and Tiny4kOLED Arduino libraries (https://github.com/datacute/Tiny4kOLED) and place both library files within the “libraries” folder in the Arduino directory that was made on installation of the Arduino IDE.
In the Arduino IDE, create a new Arduino file by selecting “file” on the toolbar and then “new.” Copy the code online in Supplementary Information, paste it into the blank file, and save. Next, select the “Upload” button, which is the 2nd button from the left just below the toolbar - it appears as a right-facing arrow - in order to send the program to the ATtiny85 microcontroller.
Once “upload complete” is indicated, safely remove the TinyUSB programmer from the PC and remove the ATtiny85. Place the ATtiny85 into the 8-pin socket on the assembled circuit board, ensuring that the depressed dot on the upper left of the microcontroller is on the side farthest from the OLED screen and on the same side as the semi-circular impression in the socket.
Wheel enclosure assembly. Timing ~10 min:
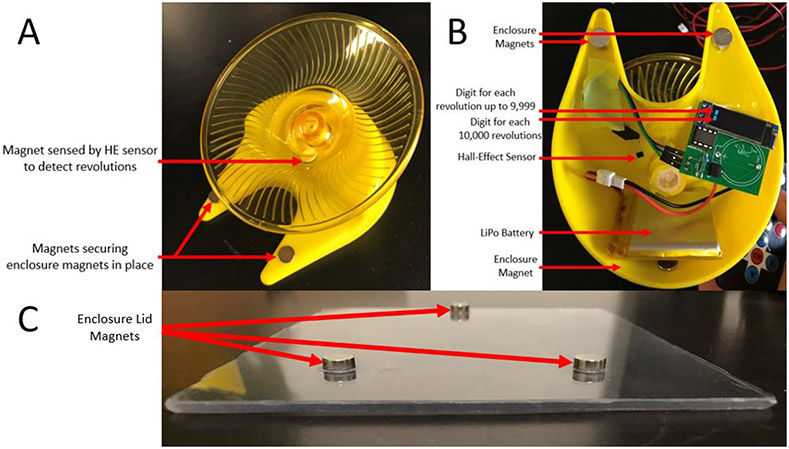
Using a Dremel tool, cut and grind the underside of the mouse wheel base to remove the two midline plastic walls between the main body and white bottom of the wheel axle until it is flush with the yellow plastic bearing. [CAUTION The use of a Dremel tool can cause severe and permanent damage as well as wide dispersion of plastic. Ensure eye protection and an enclosed space such as a hood are used]. Glue neodymium magnets to the following areas on the wheel base:
* 2x under each base strut, in addition to 1 on the upper side of each strut for increased adhesion.
* 1x on the inner aspect of the real wall of the wheel base. Use and extra magnet on the outer side to hold the magnet in place while glue dries.
* 1x magnet at the bottom of the rotating wheel. Ensure that the magnet is as close to the wheel base as possible so that the hall-effect sensor will be within range.
Secure the hall-effect sensor with the sensing (curved) side facing the wheel magnet through the plastic base. Ensure that it is as close to the magnet as possible to ensure reliable sensing, then attach 3 socket-socket DuPont cables to the hall-effect sensor prongs. Attach the other ends of the DuPont cables to the assembled circuit board. Correct wiring is such that the three header pins should line up with the hall-effect prongs if they are both face-up and the hall-effect prongs are pointing toward the header pins. Place a charged lithium-ion battery into the base between the wheel bearing and rear wall of the wheel base.
Using a Dremel tool, cut a 12x14.5cm transparent rectangular pane from a large sheet of plastic and sandwich the plastic pane between the assembled wheel base and 3 neodymium magnets. Use adhesive to glue the 3 neodymium magnets in position on the plastic pane to ensure that they effectively secure the pane to the wheel via the wheel-base magnets. When the adhesive dries, the wheel is ready to be used.
Wheel operation. Timing <5 min:
Turn the wheel on by opening the enclosure to access the inside of the wheel base. Attach the plug Japan Solderless Terminal (JST) coupling to the socket JST coupling soldered to the circuit board and replace the enclosure lid. Place the mouse running wheel within a murine subject habitat that has a transparent bottom.
In order to reset the counter, power must be disconnected from the microcontroller. The device does not have memory capability and should be checked regularly to record data. To display the revolution count, a user can press any button on an IR-emitting remote control while it is facing the IR sensor, which includes most available television remote controls. The wheel displays 2 numbers:
1. The top number counts from 0-9,999. When a revolution occurs after 9,999, this number resets to 0.
2. The bottom number increments by 1 integer value once the top number resets. The bottom number represents 10,000 times its value in revolutions.
Conclusion:
The utility of voluntary wheel-running for preclinical modeling and murine behavior evaluation is well established1. In order to make this valuable research modality available to the broader scientific community, we constructed an accurate and reliable running wheel out of cheap materials. We succeeded at designing a model that can be simply built for less than a tenth of the cost of commercially-available options. This design has been validated against industry standard running wheels in previous studies of pancreatic cancer cachexia11. Additionally, the initial investment into required tools (Dremel, pencil-tip soldering iron) becomes a smaller part of the overall cost with scaling to larger experiments requiring a greater number of wheels.
The design is limited in comparison to commercially available options due to required assembly and familiarization with soldering. Additionally, the lack of memory capacity combined with lack of wireless data transmission requires regular counter monitoring to prevent loss of data capture in the event of battery depletion.
Despite its limitations, however, this design significantly lowers the barrier to entry for utilizing wheel-running in basic science research, making it accessible to a larger proportion of the scientific community. This will enable a rapid expansion of similar research, increasing volume of results and scientific knowledge.
Supplementary Material
Figure 2 ∣. Running wheel construction and design.
(A) Superior view of mouse running wheel with suggested magnet locations. (B) Bottom-side view of completed mouse running wheel enclosure). (C) Transparent enclosure lid with 3-point magnet placement.
Acknowledgements:
This work was supported by NCI CA184324 (Marks), the Brenden‐Colson Center for Pancreatic Care (Marks), and NCI CA254033 (Olson).
Footnotes
DM is a consultant for Pfizer, Inc. and Alkermes, Inc. DM is a consultant, has received grant funding, and has equity in Tensive Controls, Inc. JE and BO declare that they have no conflict of interest.
References:
- 1.Goh J & Ladiges W Voluntary Wheel Running in Mice. Curr Protoc Mouse Biol 5, 283–290, doi: 10.1002/9780470942390.mo140295 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sugihara M. et al. Usefulness of running wheel for detection of congestive heart failure in dilated cardiomyopathy mouse model. PLoS One 8, e55514, doi: 10.1371/journal.pone.0055514 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Can A. et al. The mouse forced swim test. J Vis Exp, e3638, doi: 10.3791/3638 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bouganim S & Bergdahl A Constructing an inexpensive and versatile homemade rodent treadmill. Lab Anim (NY) 46, 67–69, doi: 10.1038/laban.1196 (2017). [DOI] [PubMed] [Google Scholar]
- 5.Brant DH & Kavanau JL 'Unrewarded' Exploration and Learning of Complex Mazes by Wild and Domestic Mice. Nature 204, 267–269, doi: 10.1038/204267a0 (1964). [DOI] [PubMed] [Google Scholar]
- 6.Yu K. et al. A nonmyeloablative chimeric mouse model accurately defines microglia and macrophage contribution in glioma. Neuropathol Appl Neurobiol, doi: 10.1111/nan.12489 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Meijer JH & Robbers Y Wheel running in the wild. Proceedings. Biological sciences 281, doi: 10.1098/rspb.2014.0210 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Garrett L, Lie DC, Hrabe de Angelis M, Wurst W & Holter SM Voluntary wheel running in mice increases the rate of neurogenesis without affecting anxiety-related behaviour in single tests. BMC Neurosci 13, 61, doi: 10.1186/1471-2202-13-61 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pedersen L. et al. Voluntary Running Suppresses Tumor Growth through Epinephrine- and IL-6-Dependent NK Cell Mobilization and Redistribution. Cell Metab 23, 554–562, doi: 10.1016/j.cmet.2016.01.011 (2016). [DOI] [PubMed] [Google Scholar]
- 10.Vyazovskiy VV, Ruijgrok G, Deboer T & Tobler I Running wheel accessibility affects the regional electroencephalogram during sleep in mice. Cereb Cortex 16, 328–336, doi: 10.1093/cercor/bhi110 (2006). [DOI] [PubMed] [Google Scholar]
- 11.Olson B, Z. X, Norgard MA, Levasseur PR, Butler JT, Buenafe A, Burfeind KG, Michaelis KA, Pelz KR, Mendez H, Edwards J, Krasnow SM, Grossberg AJ, & Marks DL. Lipocalin 2 mediates appetite suppression during pancreatic cancer cachexia. Nat Commun 12, 2057, doi: 10.1038/s41467-021-22361-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Michaelis KA et al. Establishment and characterization of a novel murine model of pancreatic cancer cachexia. J Cachexia Sarcopenia Muscle 8, 824–838, doi: 10.1002/jcsm.12225 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.