Abstract
Human sera contain high levels of natural antibody (Ab) to Galα1-3Gal, a terminal glycosidic structure expressed on the surface of cells of mammals other than Old World primates. Incorporation of this determinant onto retroviral membranes by passage of viruses in cells encoding α-1-3-galactosyltransferase (GT) renders retroviruses sensitive to lysis by natural Ab and complement in normal human serum (NHS). Plasma membrane-budding viruses representing four additional virus groups were examined for their sensitivities to serum inactivation after passage through human cell lines that lack a functional GT or human cells expressing recombinant porcine GT. The inactivation of lymphocytic choriomeningitis virus (LCMV) by NHS directly correlated with host modification of the virus via expression of Galα1-3Gal and was blocked by incorporation of soluble Galα1-3Gal disaccharide into the inactivation assay. GT-deficient mice immunized to make high levels of Ab to Galα1-3Gal (anti-Gal Ab) were tested for resistance to LCMV passaged in GT-expressing cells. Resistance was not observed, but in vitro analyses of the mouse immune sera revealed that the antiviral activity of the sera was insufficient to eliminate LCMV infectivity on its natural targets of infection, macrophages, which express receptors for Ab and complement. Newcastle disease virus and vesicular stomatitis virus (VSV) were inactivated by NHS regardless of cell passage history, whereas Sindbis virus (SV) passaged in human cells resisted inactivation. Both VSV and SV passaged in Galα1-3Gal-expressing human cells incorporated this sugar moiety onto their major envelope glycoproteins. SV passaged in mouse cells expressing Galα1-3Gal was moderately sensitive to inactivation by NHS. These results indicate that enveloped viruses expressing Galα1-3Gal differ in their sensitivities to NHS and that a potent complement source, such as that in NHS, is required for efficient inactivation of sensitive viruses in vitro and in vivo.
Natural immunity to viruses can be mediated by humoral components of the immune system, including natural antibody (Ab) and complement (11). The complement system is generally activated by Ab that has bound to its target antigen, but it can also be activated by many membrane structures independently of Ab. For example, neutralization of retroviruses and paramyxoviruses has been reported to be mediated by Ab-independent mechanisms via the classical and alternative complement pathways, respectively (42, 44, 45). Retroviruses directly activate human complement through direct interaction of C1q with the murine retrovirus p15e or with the human retrovirus gp41 transmembrane protein (5, 13). Human and nonhuman complement sources act differently; although both human and guinea pig C1q bind the Moloney leukemia virus p15e, the human C1s is required to initiate the complement cascade (4).
Viruses act differently in their sensitivities to human complement. Murine retroviruses passaged through mouse cells are reported to activate complement sufficiently for it to lyse the virions, releasing virion RNA and reverse transcriptase (34, 44, 45). Conversely, activation of complement by human retroviruses passaged through human cells is reported to not be sufficient to lyse the virions. However, the deposition of complement on the human retroviruses results in opsonization of the virions and enhanced infection of complement receptor (CR)-expressing cells (20, 38).
It is clear that Ab is not required for the activation of human complement by retroviruses, because complement activation has been shown with purified viruses or viral proteins and purified complement components (4, 5, 10). It is also unlikely that Ab is essential for the reduction in infectivity and the lysis of nonhuman retroviruses by complement, as there are numerous reports that human serum can inactivate and lyse nonhuman retroviruses passed through human cells (34, 45), through other Old World primate cells (16, 34, 45), or through nonprimate cells lacking reactivity with Ab in human serum (44). In addition, agammaglobulinemic human sera retain the capacity to lyse murine retroviruses (44, 45). Nevertheless, Ab can accelerate the dynamics of complement deposition on membranes, and recent work has indicated that the level of retrovirus inactivation by normal human serum (NHS) can be greatly augmented by natural Ab if the viruses are passed through appropriate cell lines that express the epitope to which the Ab is directed (28, 30, 36).
Human sera contain very high levels of natural Ab specific for a carbohydrate moiety present on the surface of cells from most mammals but not from Old World primates, such as humans (15). This moiety, Galα1-3Galβ1-4GlcNAc-R (Galα1-3Gal), is a product of the α-1-3-galactosyltransferase (GT) enzyme that adds a terminal galactose onto glycoproteins and glycolipids in a specific α1-3 linkage. Humans do not express a functional GT enzyme and instead make high levels of Ab against Galα1-3Gal as a presumed consequence of environmental exposure (14, 15, 32, 39). Recent reports have shown that murine retroviruses and human immunodeficiency virus (HIV) passaged through cells expressing GT assimilate Galα1-3Gal onto the virion gp70 and gp120 envelope proteins, respectively (28, 30, 36). The anti-Galα1-3Gal Ab (anti-Gal) in NHS binds to the virion and greatly augments the ability of complement to lyse the virion. Unquestionably, the incorporation of Galα1-3Gal onto virion surfaces by passaging retroviruses through cells expressing this carbohydrate epitope augments their reactivity with complement in NHS by binding natural Ab, and such a host cell modification may well result in a species-dependent barrier for their transmission.
Earlier work with other enveloped viruses, such as lymphocytic choriomeningitis virus (LCMV), Newcastle Disease virus (NDV), and Sindbis virus (SV), has identified host cell modification of viruses as being important for virion reactivity with NHS (6, 19, 41, 42). Given the new information available on the role of anti-Gal natural Ab in complement-dependent lysis of retroviruses, we have reevaluated the mechanisms of NHS inactivation of a variety of other viruses that acquire their envelopes by budding through the plasma membrane. Our results show remarkably different patterns of inactivation, depending on the virus and the cell in which it is grown.
MATERIALS AND METHODS
Immunization of mice.
GT null (GT-deficient) mice were mice with the 129/Sv (129) background derived from embryonic stem cells deleted by homologous recombination in the GT gene (37). These mice were licensed from the University of Michigan and were developed in a Howard Hughes Medical Institute Laboratory. 129 wild-type (WT) (GT-sufficient) mice used as controls were obtained from Jackson Laboratories. GT-deficient and GT-sufficient mice were immunized at 6 weeks of age with erythrocyte membranes isolated from fresh rabbit blood (Cocalico Biologicals, Inc., Reamstown, Pa.). Whole blood was centrifuged at 3,000 × g for 10 min, and the plasma was discarded. Cells were lysed with 0.1× phosphate-buffered saline (PBS), and membranes were pelleted at 40,000 × g for 30 min. The supernatant was removed, and membranes were resuspended in 0.1× PBS and centrifuged as before. After the procedure was carried out a third time, the membrane pellet was resuspended in 1× PBS and again centrifuged and resuspended in PBS to give 1010 erythrocyte membranes per ml. A total of 0.5 ml was injected intraperitoneally into each mouse. Four total injections were given at 4-week intervals. Anti-Gal Ab levels in the mouse sera were assayed by enzyme-linked immunosorbent assay (ELISA) using Galα1-3Gal-conjugated bovine serum albumin (BSA) in the plate coat.
Cells.
L-929, a mouse fibroblast cell line derived from C3H mice, BHK-21, a Syrian hamster cell fibroblast line, N115, a neuroblastoma line from strain A mice, HeLa, a human epithelial cell line, and Vero, an African green monkey kidney cell line, are well-characterized cell lines cultivated on monolayers as previously described (8, 42). SK-N-MC (HTB 10), a human melanoma cell line (from the American Type Culture Collection, Rockville, Md.), was maintained as monolayers in RPMI medium (Mediatech, Inc., Herndon, Va.) supplemented with 10% fetal bovine serum (FBS), 100 penicillin (IU/ml), streptomycin (100 mg/ml), 2 mM glutamine, and 1 mM sodium pyruvate (R10 medium). GT-WT and GT-KO are mouse embryonic fibroblast lines cultured from GT-sufficient 129 WT mice and GT-deficient transgenic mice, respectively. They were cultivated as monolayers in Dulbecco modified Eagle medium (Gibco BRL, Grand Island, N.Y.) supplemented with penicillin, streptomycin, glutamine, pyruvate, and 10% FBS (D10 medium) as described above.
Generation of GT-modified SK-N-MC cells.
The full-length porcine GT cDNA (39) was cloned into the pLXSN retroviral vector, and amphotropic retroviral particles were produced through the intermediate ecotropic packaging cell line GPE+86 (23). Briefly, GPE+86 cells were transfected with pLXSN containing the GT gene (pLGTSN) or pLXSN without insert, using the calcium phosphate method (2). Transfected cells were selected in D10 medium containing G418 (500 μg/ml, active weight). Transfectants were pooled, and a 24-h supernatant was harvested from cells at about 90% confluence. The ecotropic virus stock was used to transduce the amphotropic packaging cell line PA317 (26), which was also selected as a pool in G418. A 24-h supernatant was harvested from these cells at 90% confluence, and the supernatant was filtered through a 0.2-μm-pore-size filter and stored at −70°C. SK-N-MC cells were transduced with pLGTSN or pLXSN by adding 1 ml of amphotropic virus to cells in 9 ml of R10 medium containing Polybrene (8 μg/ml). Following an overnight incubation, virus was removed, and cells were selected as a pool in R10 medium containing G418 (500 μg/ml). The GT-modified cells were subcloned by limiting dilution and screened for surface expression of Galα1-3Gal by fluorescein isothiocyanate (FITC) staining (see below). One clone expressing high levels of the Galα1-3Gal epitope on its surface, designated SK-GT, was used in this study. The SK-N-MC cells transduced with pLXSN were selected as a pool and served as control cells (designated SK). Transduced cells were maintained in R10 medium containing G418 (500 μg/ml).
Immunofluorescence and cell killing assays.
Cells (5 × 105) were reacted with polyclonal anti-Gal Ab (20 μg/ml) previously purified from human serum (30). FITC-conjugated goat anti-human immunoglobulin G (IgG) and FITC-conjugated goat anti-human IgM (Zymed Laboratories, South San Francisco, Calif.) were combined for use as secondary Ab (7.5 μg/ml [final concentration] for each). Fluorescence was measured in a FACSort (Becton Dickinson Immunocytometry Systems, San Jose, Calif.).
Viruses, propagation, and purification.
The following viruses were used in these studies: herpes simplex virus type 1 (HSV-1), KOS1.1 strain (3); LCMV, Armstrong strain (8); NDV, AV strain (25); SV, originally from E. Pfefferkorn, Dartmouth Medical School (40); and vesicular stomatitis virus (VSV), Indiana strain (6). For purification, 75- or 150-mm2 tissue culture flasks containing about 75% confluent monolayers of SK or SK-GT cells were infected with virus as described above and harvested at the peak of infection. On harvest, the culture fluid was twice cleared of cell debris by centrifugation at 2,000 rpm (900 × g) for 10 min. The supernatants were then layered onto discontinuous gradients of 25 and 75% Renografin-76 (Squibb Diagnostics, New Brunswick, N.J.) diluted in TES buffer (0.01 M Tris-HCl, 0.1 M NaCl, 0.001 M EDTA [pH 7.4]) as described previously (43). The samples were spun to equilibrium, and the 25%/75% interphase band was recovered, diluted in TES buffer plus 0.1% BSA, and pelleted in an SW41 Beckman centrifuge rotor at 35,000 rpm for 60 min in a Beckman ultracentrifuge. The pellet was resuspended in a standard sodium dodecyl sulfate-urea acrylamide gel preparation buffer and run on gels for Western analyses.
Western blots.
Gradient-purified VSV and SV were disrupted, and their proteins were size fractionated via sodium dodecyl sulfate-polyacrylamide gel electrophoresis on a 12% gel. Viral samples were normalized for protein content by Coomassie blue staining of the gel. Proteins were transferred to nitrocellulose and exposed to either IB4 lectin or antiviral Ab in Western blots as described previously (28, 30). IB4 lectin selectively binds to Galα1-3Gal and is used like an Ab to detect expression of this sugar (30). Monoclonal Abs (MAbs) SV2 and SV127 to SV envelope proteins (used in combination) were a generous gift from Diane Griffin (Johns Hopkins University), and the anti-VSV MAb, which recognizes the VSV G envelope protein, was kindly provided by Kathy Hardgrave (Oklahoma Medical Research Foundation).
Serum inactivation studies.
Virus was diluted in complete minimal essential medium (MEM) with 10% FBS to a concentration of 105 PFU/ml, and 50 μl was mixed with 50 μl of MEM, freshly thawed NHS, or serum whose complement was inactivated by heating at 56°C for 30 min. Some experiments were miniaturized, with half of each volume used. The NHS was a human serum pool (Diamedix, Cambridge, Mass.) frozen at −70°C until use. The samples were incubated at 37°C for 45 min, 200 μl of MEM was added, and the reaction was stopped by putting the samples on ice. The virus was then titrated directly for plaque formation on Vero cell monolayers. In the disaccharide blocking studies, 10 μl of virus at a titer of 5 × 105 PFU/ml was added to 40 μl of sera and 50 μl of disaccharide diluted in Hanks balanced salt solution (HBSS). The disaccharides were either Galα1-3Gal (Dextra Laboratories, Reading, England) or sucrose (Sigma Chemical Co., St. Louis, Mo.) at a concentration of 10 mg/ml before mixture (30). In some other experiments, mouse serum was substituted for NHS.
PEC.
In some experiments serum-treated virus was added onto freshly isolated 129 mouse peritoneal exudate cells (PEC) previously adhered to 16-well plastic plates 2 to 4 h before infection. The PEC had been isolated by peritoneal lavage with RPMI 1640 and seeded at 106 cells per well. Before infection, the medium was removed, and replaced with 1 ml of complete RPMI 1640 (R10 medium without the pyruvate), to which virus was added in 100 μl. After a 2- to 4-h adsorption period, the culture fluid containing unadsorbed virus was removed and replaced with 1 ml of RPMI medium. At 22 to 26 h postinfection, the culture fluid was harvested and used for viral titrations on Vero cell monolayers.
RESULTS
Expression of Galα1-3Gal on cell lines.
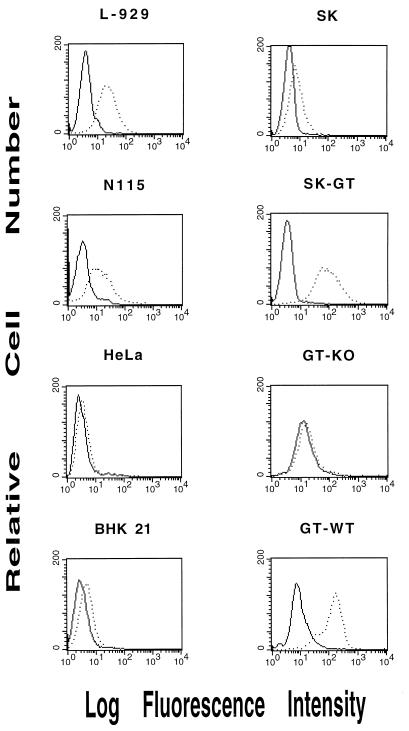
We reported previously that LCMV passaged in L-929 cells, but not in N115, HeLa, or BHK-21 cells, was effectively inactivated by NHS (42). To determine if this pattern of serum sensitivity correlated directly with Galα1-3Gal expression on the host cell surface, these cell lines, as well as the human melanoma cell line SK-N-MC modified to express GT, were reacted with purified anti-Gal and analyzed by flow cytometry. As expected, the unmodified human SK cells and human HeLa cells did not react with anti-Gal (Fig. 1). Similarly, the hamster cell line BHK-21, shown previously to be deficient in Galα1-3Gal expression (17), did not react with anti-Gal. In contrast, the mouse L-929 and modified human SK-GT cells reacted strongly with the anti-Gal. The mouse N115 cell line was weakly positive for Galα1-3Gal expression. These cell lines were also incubated at 37°C for 30 min with NHS, and cell viability was measured. There was a direct correlation between cell surface expression of Galα1-3Gal and cell killing in NHS (data not shown).
FIG. 1.
Expression of Galα1-3Gal on cultured cells. Cells used in these experiments were stained with purified anti-Gal Ab and analyzed by flow cytometry as described in Materials and Methods. Peaks depict Galα1-3Gal expression on the surfaces of L-929, N115, HeLa, BHK-21, SK-N-MC (SK) cells or SK-N-MC cells transduced with recombinant GT (SK-GT), and cells from GT-deficient (GT-KO) or GT-sufficient (GT-WT) mice.
Inactivation of LCMV passaged through different cell lines.
Passage of LCMV through L-929 cells resulted in a virus sensitive to inactivation by fresh but not heat-inactivated NHS (Table 1, experiment 1). Our previous studies had shown that this inactivation was mediated by an undefined natural Ab and by the classical complement pathway, which is heat sensitive (42). Passage of LCMV through HeLa and BHK-21 cells resulted in virus that resisted inactivation by NHS. Our earlier work also showed that passage of LCMV in N115 cells led to virus resistant to NHS, and it is noteworthy that N115 cells only dimly express Galα1-3Gal and are themselves resistant to lysis by NHS (Fig. 1). Passage of LCMV through SK-GT cells resulted in virus highly sensitive to inactivation, but passage of LCMV through unmodified SK cells led to resistant virus. These data suggest that incorporation of Galα1-3Gal onto the virion surface renders LCMV susceptible to the anti-Gal natural Ab in NHS.
TABLE 1.
Inactivation of LCMV by NHSa
| Expt | Virus | Inhibitor | Log10 PFU after treatment
|
||
|---|---|---|---|---|---|
| NHS | Heat-inactivated NHS | MEM | |||
| 1 | LCMV-BHK | 4.0 | 3.9 | 4.2 | |
| LCMV-HeLa | 5.0 | 4.9 | 4.8 | ||
| LCMV-L-929 | 1.5 | 3.9 | 4.2 | ||
| LCMV-SK | 4.3 | 4.5 | 4.5 | ||
| LCMV-SK-GT | 2.4 | 4.0 | 4.2 | ||
| 2 | LCMV-L-929 | HBSS | <1 | 3.7 | |
| Galα1-3Gal | 2.5 | 3.9 | |||
| Sucrose | <1 | 3.7 | |||
| LCMV-SK | HBSS | 3.9 | 4.3 | ||
| Galα1-3Gal | 4.1 | 4.2 | |||
| Sucrose | 3.9 | 4.0 | |||
| LCMV-SK-GT | HBSS | 2.0 | 3.8 | ||
| Galα1-3Gal | 3.3 | 3.7 | |||
| Sucrose | 2.0 | 3.7 | |||
| 3 | LCMV-GT-WT | 1.6 | 4.3 | 4.0 | |
| LCMV-GT-KO | 3.4 | 3.6 | 3.4 | ||
| LCMV-SK | 4.2 | 4.2 | 4.1 | ||
| LCMV-SK-GT | 2.1 | 4.0 | 3.7 | ||
LCMV passaged in different cell lines was treated with fresh or heat-inactivated NHS or with MEM for 1 h at 37°C and titrated for PFU. In expt 2, either HBSS, soluble Galα1-3Gal, or sucrose was included in the inactivation assay.
To confirm that the sensitivity of LCMV passed through L-929 or SK-GT cells was indeed associated with the incorporation and targeting of Galα1-3Gal moieties, soluble Galα1-3Gal was added into the inactivation assays in order to consume the anti-Gal natural Ab. As controls, viruses were incubated either with HBSS or with a control disaccharide, sucrose. Incorporation of either disaccharide into the assays had no effect on the serum sensitivity of LCMV passed through SK cells (Table 1, experiment 2). Conversely, the inclusion of soluble Galα1-3Gal markedly inhibited the serum inactivation of LCMV passaged in SK-GT cells. The inclusion of soluble Galα1-3Gal into assays with LCMV passaged through L-929 cells also inhibited the inactivation by NHS, though it should be noted that in three experiments this inhibition was never as complete as it was with virus passaged through the SK-GT cells (Table 1, experiment 2). We interpret these experiments to mean that the incorporation of the Galα1-3Gal epitope into LCMV during its growth in cells renders it sensitive to anti-Gal natural Ab-dependent complement inactivation, but the LCMV passaged through mouse L-929 cells, unlike LCMV passaged through human SK-GT cells, may express additional epitopes that could also be targeted by NHS. Alternatively, differences in the level of Galα1-3Gal incorporation into the virion or other differences in membrane structure associated with sensitivity to complement might have influenced the results with LCMV passaged through L-929 cells. LCMV was therefore also passaged in cell lines derived from either a GT-deficient mouse (GT-KO) or a GT-sufficient mouse (GT-WT). The GT-KO cell line is a 3T3-like cell that is totally devoid of the Galα1-3Gal epitope and completely resistant to lysis by NHS (Fig. 1). LCMV passaged through GT-WT cells was inactivated about 500-fold by NHS, whereas virus passaged through GT-KO cells was very resistant (Table 1, experiment 3). This result indicates that Galα1-3Gal is the major target of serum inactivation of mouse cell-passaged LCMV and that any other targets are of minimal importance.
Replication of LCMV in mice expressing Ab to Galα1-3Gal.
To test the hypothesis that natural Ab-mediated complement inactivation of viruses passaged through cells expressing Galα1-3Gal may play an important role in natural resistance to virus infections in vivo, we examined the replication of LCMV in mice expressing Ab to this epitope. This experiment was complicated by the fact that mice endogenously express Galα1-3Gal and cannot be immunized against it because they are immunologically tolerant. Passive transfer of Ab into normal mice also is not feasible, as it would react with the recipient’s tissues. To overcome these obstacles GT-sufficient and GT-deficient (GT-KO) 129 mice (37) were immunized with rabbit erythrocytes, which are a rich source of Galα1-3Gal (15). As assessed by binding to Galα1-3Gal-conjugated BSA by ELISA (data not shown), the GT-deficient mice developed high Ab titers to Galα1-3Gal, equaling or surpassing the anti-Gal titers in NHS. Both IgG and IgM anti-Gal Ab were present in the sera of these mice. The immunized GT-sufficient mice, because of immunological tolerance, did not make a detectable Ab response to the epitope. These mice were inoculated with LCMV passaged either in SK cells or in SK-GT cells and examined for viral titers in the spleens 3 days later. The mice received either a standard dose of 5 × 104 PFU, which we use for assays on NK cell and cytotoxic T-cell function (8), or a lower dose of 5 × 102 PFU, in case any effects could more readily be seen at limiting amounts of virus. To ensure exposure of virus to high concentrations of serum components, virus was inoculated intravenously.
It was anticipated that in this experiment, LCMV passaged through SK-GT cells would replicate poorly in the GT-deficient mice expressing Ab to Galα1-3Gal, but the results failed to support this hypothesis, as there were no particular patterns in viral replication that could not be explained by minor differences in input inocula of the two viral stocks (Table 2). Of further note is that the individual Ab-positive GT-deficient mice had over fivefold variations in their Ab titers (data not shown), but the reproducibility in viral titers within that group of mice was extremely high, as shown by the very low standard deviation, and did not correlate at all with the Ab titers.
TABLE 2.
Replication of LCMV in mice containing Anti-Gal Aba
| Virus | Dose | Log10 PFU/spleen (mean ± SD)
|
|
|---|---|---|---|
| GT-sufficient (Ab−) mice | GT-deficient (Ab+) mice | ||
| LCMV-SK | 5 × 104 | 6.5 ± 0.15 | 6.1 ± 0.19 |
| LCMV-SK-GT | 5 × 104 | 5.7 ± 0.03 | 5.5 ± 0.06 |
| LCMV-SK | 5 × 102 | 5.2 ± 0.13 | 4.6 ± 0.03 |
| LCMV-SK-GT | 5 × 102 | 4.0 ± 0.13 | 3.4 ± 0.01 |
Mice were immunized with sheep erythrocyte membranes to induce Ab to Galα1-3Gal. No Abs were detected in immunized GT-sufficient 129 WT mice, but high titers were elicited in GT-deficient (GT-KO) mice, which recognize Galα1-3Gal as a foreign antigen. Mice were inoculated intravenously with 5 × 104 (n = 4 per group) or 5 × 102 (n = 2 per group) PFU of LCMV passaged in SK or SK-GT cells and titrated for PFU/spleen at 3 days postinfection.
This result led us to question whether our fundamental hypothesis was wrong or whether mouse serum lacked the capacity to inactivate LCMV even in the presence of Ab to the Galα1-3Gal epitope. Therefore, in vitro assays were performed to assess the ability of anti-Gal in the serum from GT-deficient mice to neutralize LCMV passaged in cells expressing Galα1-3Gal. Fresh serum from Ab-positive mice reduced the infectivity of LCMV passaged in SK-GT cells as measured by PFU in Vero cells by 0.7 to 0.8 log10 compared to the normal mouse serum controls (Table 3, experiments 1 and 2). Under the same conditions, NHS more dramatically reduced LCMV infectivity, by 1.3 to 1.6 log10 PFU. Although the NHS was frozen and thawed before use, the mouse immune serum needed to be freshly isolated to have any significant antiviral activity. After one freeze-thaw, the immune mouse serum lost its ability to inactivate LCMV, even though it could still mediate the inactivation of LCMV by a guinea pig serum complement (gp C) source (e.g., inactivation of LCMV passaged in SK-GT cells [log10 PFU]: MEM, 3.7; control mouse serum, 3.6; immune mouse serum, 3.5; gp C, 3.7; immune mouse serum plus gp C, 2.9). This result demonstrates the well-known phenomenon that complement levels from laboratory mouse strains are quite low and lose activity after freeze-thawing (46). The ability of the immune mouse serum to sensitize virus to inactivation by gp C-containing serum is evidence that the mouse serum contains anti-Gal Ab that survived the freeze-thaw cycle.
TABLE 3.
Inactivation of LCMV with immune mouse seruma
| Expt | Virus | Treatment | Log10 PFU
|
|
|---|---|---|---|---|
| Direct assay | PEC yield | |||
| 1 | LCMV-SK | NHS | 3.7 | 3.5 |
| MEM | 3.8 | 3.3 | ||
| GT-def 1 (Ab+) | 3.8 | 3.5 | ||
| GT-def 2 (Ab+) | 3.8 | 3.6 | ||
| GT-suf (Ab−) | 3.7 | 3.5 | ||
| LCMV-SK-GT | NHS | 1.7 | <1 | |
| MEM | 3.0 | 2.1 | ||
| GT-def 1 (Ab+) | 2.5 | 2.5 | ||
| GT-def 2 (Ab+) | 2.5 | 2.7 | ||
| GT-suf (Ab−) | 3.3 | 2.8 | ||
| 2 | LCMV-SK | NHS | 3.9 | 2.0 |
| ΔNHS | 4.0 | 2.3 | ||
| MEM | 4.2 | 2.4 | ||
| GT-def (Ab+) | 4.1 | 2.7 | ||
| GT-suf (Ab−) | 4.0 | 3.2 | ||
| LCMV-SK-GT | NHS | 2.3 | <1 | |
| ΔNHS | 3.8 | 2.3 | ||
| MEM | 3.9 | 2.6 | ||
| GT-def (Ab+) | 3.1 | 2.1 | ||
| GT-suf (Ab−) | 3.8 | 2.4 | ||
LCMV passaged in different cell lines was treated with NHS, heat-inactivated NHS (ΔNHS), MEM, or serum from GT-sufficient 129 WT (GT-suf) or GT-deficient (GT-def) mice. All mice had been previously immunized against sheep erythrocytes, but only the GT-deficient mice developed anti-Gal Ab responses. Samples were either titrated directly on Vero cells or used to infect 129 mouse PEC. Virus released into the PEC culture fluid 1 day postinfection was then titrated for PFU on Vero cells.
These experiments showed that freshly isolated immune mouse sera could inactivate LCMV passed through SK-GT cells, even though the levels were not as profound as with NHS. However, if aliquots from these same serum-treated viral preparations were used to initiate infection of adherent PEC, only the treatment with NHS caused an inhibition in the 24-h yield (Table 3, experiments 1 and 2). In the two experiments shown, NHS completely eliminated the 24-h yield of LCMV passaged through SK-GT cells but had no effect on LCMV passed through SK cells. In contrast, the 24-h yield of LCMV passaged through SK-GT cells and exposed to anti-Gal-Ab-containing fresh mouse serum was indistinguishable from the titers in control samples. Macrophages, which have receptors for mouse Ab and complement, are a primary target for the LCMV infection in vivo (24), and it appears that exposure of LCMV to the immune mouse serum could not block its infection of macrophages, which are the predominant cell in the adherent PEC population. These results indicate that natural Ab to Galα1-3Gal by itself is insufficient to control infection by a virus expressing the epitope and that a potent complement source may be needed for the efficacy of the Ab in vivo. The results also indicate that the inactivation of LCMV by mouse serum containing anti-Gal Ab is unlikely to be due to a virolytic mechanism, as a lysed virus would not maintain its infectivity for macrophages.
Patterns of inactivation of other enveloped viruses.
An evaluation of NHS inactivation of other enveloped viruses putatively modified by passage through cells either expressing or not expressing the Galα1-3Gal epitope was performed, and different patterns of inactivation were noted (Table 4). HSV passaged in either SK-GT or unmodified SK cells was totally inactivated by NHS, and this inactivation did not require complement, as heat-inactivated NHS also completely inactivated the virus (Table 4, experiment 1). This result could be explained by the fact that HSV is a ubiquitous human virus, and NHS contain high levels of anti-HSV neutralizing antibodies. The presence of anti-HSV Ab in a separately tested NHS preparation that gave the same pattern of inactivation was confirmed by exposing HSV-infected SK cells to NHS followed by an FITC-labeled anti-human Ig. We present these data with HSV to contrast them to studies with viruses that are not ubiquitous human pathogens. VSV, which has previously been shown to be inactivated by the classical complement pathway mediated by autologous natural Ab (6), was inactivated by NHS, regardless of the passage history of the virus (Table 4, experiments 1, 2, and 4). In contrast to HSV, heat inactivation of the serum considerably reduced the inactivation, suggesting a complement-mediated event. Similarly, NDV, which previously has been shown to be inactivated by the alternative complement pathway in the absence of Ab (41, 42), was inactivated over 100-fold by fresh NHS but not by heat-inactivated serum, again regardless of its passage history. Previous work with NDV has shown that host modification of the virus plays a role in its sensitivity to lysis by NHS (41), but in this case the virus was grown in similar cell lines either expressing Galα1-3Gal or not, and that defined variable did not alter the sensitivity of NDV to inactivation. NDV and VSV both had patterns of inactivation distinct from those of LCMV and the retroviruses, but SV demonstrated yet another pattern. SV passed through either SK-GT or SK cells resisted inactivation by NHS. Host modification of sialic acid determinants on SV has previously been implicated in its ability to activate human or gp C (19), but here it is clear that modulating only the cell expression of Galα1-3Gal did not affect the resistance of SV to neutralization by NHS.
TABLE 4.
Inactivation of heterologous viruses by NHSa
| Expt | Virus | Log10 PFU after treatment
|
||
|---|---|---|---|---|
| NHS | Heat-inactivated NHS | MEM | ||
| 1 | HSV-SK | <1 | <1 | 3.5 |
| HSV-SK-GT | <1 | <1 | >3.7 | |
| SV-SK | 3.2 | 3.5 | 3.6 | |
| SV-SK-GT | 3.4 | 3.3 | 3.3 | |
| VSV-SK | 2.2 | 3.6 | 4.1 | |
| VSV-SK-GT | 2.0 | 4.0 | 3.9 | |
| 2 | NDV-SK | 1.8 | 3.8 | 3.7 |
| NDV-SK-GT | <1 | 3.9 | 4.2 | |
| SV-SK | 3.4 | 3.6 | 3.5 | |
| SV-SK-GT | 3.5 | 3.4 | 3.7 | |
| VSV-SK | 2.2 | 3.6 | 4.3 | |
| VSV-SK-GT | 1.8 | 3.9 | 4.0 | |
| 3 | NDV-SK-STOCK 1 | <1 | 3.0 | 3.1 |
| NDV-SK-STOCK 2 | 1.7 | 4.0 | 4.1 | |
| NDV-SK-GT-STOCK 1 | <1 | 3.4 | 3.4 | |
| NDV-SK-GT-STOCK 2 | 1.5 | 4.1 | 4.2 | |
| 4 | SV-GT-WT | 2.9 | 4.1 | 4.2 |
| SV-GT-KO | 4.2 | 4.4 | 4.4 | |
| VSV-GT-WT | <1 | 4.0 | 4.1 | |
| VSV-GT-KO | 1.0 | 4.0 | 4.1 | |
| 5 | SV-GT-WT | 3.5 | 4.0 | 4.3 |
| SV-GT-WT + nanase | 3.4 | 4.3 | 4.4 | |
| SV-GT-KO | 4.2 | 4.3 | 4.4 | |
| SV-GT-KO + nanase | 3.8 | 4.1 | 4.2 | |
| SV-SK | 3.9 | 3.8 | 3.8 | |
| SV-SK + nanase | 4.2 | 3.9 | 4.0 | |
| SV-SK-GT | 3.6 | 3.7 | 3.7 | |
| SV-SK-GT + nanase | 3.8 | 3.8 | 3.7 | |
Viruses passaged through human SK and SK-GT or mouse GT-KO and GT-WT cells were treated with sera as described in Materials and Methods and assayed for PFU on Vero cell monolayers. In experiment 5, viruses, where designated, were incubated 1:1 with 0.3 U of Clostridium perfringens neuraminidase (nanase) (Sigma) per ml for 1 h at 37°C before treatment with sera.
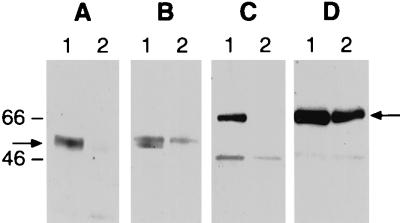
The apparent indifference of SV and VSV to passage in SK cells lacking or expressing Galα1-3Gal led us to question whether Galα1-3Gal was excluded from their virion surfaces. Western blot analyses were performed on purified virus, using either a lectin specific for the Galα1-3Gal epitope (IB4 lectin) or Ab to the viral envelope proteins. VSV passaged through SK-GT cells but not through SK cells contained a Galα1-3Gal-expressing protein of about 70 kDa (Fig. 2). This represents the major VSV envelope glycoprotein G, as demonstrated by its reactivity with Ab to G protein (12). VSV passed through SK cells expressed the 70-kDa G protein but did not react with the lectin. Examination of SV passed through SK-GT cells revealed a 53-kDa protein expressing Galα1-3Gal. The reactivity of a MAb pool to the SV envelope proteins indicated that this band represents the major glycosylated envelope protein E2 of SV (21, 33). Of interest is that the incorporation of Galα1-3Gal into the SV envelope protein may have altered its processing, as the protein appeared as a double band with SK-GT-passaged SV but a single band with SK-passaged SV. We have not further investigated this observation. Thus, both VSV and SV were shown to incorporate the Galα1-3Gal determinant onto virion glycoproteins, yet the presence of this epitope did not influence their inactivation by or resistance to NHS. Preliminary observations with the more difficult to purify (from these cells) LCMV revealed an IB4 lectin-binding band in the position of the LCMV GP1 protein. Because the reactivity of LCMV with Ab to Galα1-3Gal appeared quite clear in our inactivation studies, we have not further analyzed the LCMV virion for its incorporation of the moiety.
FIG. 2.
Expression of Galα1-3Gal on virion proteins. Purified SV (A and B) and VSV (C and D) passaged in SK-GT (lanes 1) or SK (lanes 2) cells were analyzed by Western blotting for expression of viral envelope proteins by binding of anti-viral Ab (B and D) or for expression of Galα1-3Gal by binding of IB4 lectin (A and C) as described in Materials and Methods. Sizes are indicated in kilodaltons.
Given that host cell differences have previously been correlated with the ability of SV to activate complement in the absence of Ab (19) and some viruses have been shown to incorporate anti-complement regulatory proteins into their virions when passaged through certain human cell lines (27), we examined the inactivation of SV passaged through mouse GT-WT and GT-KO cell lines. After this passage, the inactivation of SV resembled that of LCMV in that SV passaged through the GT-WT cells was inactivated, whereas SV passaged through GT-KO cells was resistant (Table 4, experiment 4). The inactivation of SV passaged through GT-WT cells by NHS was not as pronounced as the inactivation of LCMV passaged through the same cells, but it was nevertheless significantly different from the inactivation of SV passed through SK-GT cells. To determine if this sensitivity to inactivation may have been due to differences in sialic acid content between the human and mouse cell lines, SV passaged through these lines was treated 1:1 with 0.3 U of neuraminidase per ml before exposure to NHS. Although 0.5 ml of this same neuraminidase solution removed a sialic acid-dependent CD45 epitope from splenocytes (detectable by MAb CZ-1 [7]) (data not shown), the treatment of SV in the present experiment had no significant effect on the pattern of viral inactivation (Table 4, experiment 5).
DISCUSSION
It is well established that enveloped viruses assimilate virtually unmodified cellular lipids into their virions and that their glycoproteins have glycosylation patterns similar to those of the glycoproteins of the cells in which they are passaged (29). It would therefore be expected that viruses passaged through cells containing a functional GT and expressing Galα1-3Gal should themselves express this carbohydrate epitope, and data shown here for VSV and SV (Fig. 2) and published elsewhere for HIV (28) and murine retroviruses (30, 36) support this conclusion. What is surprising is that the various enveloped viruses studied displayed different patterns of inactivation by NHS. LCMV was similar to the retroviruses in that Ab to Galα1-3Gal greatly augmented its inactivation by complement in NHS. In contrast, SV passed through GT-expressing human cells was resistant to inactivation by NHS, while NDV and VSV were inactivated by NHS, regardless of whether the Galα1-3Gal epitope was present.
Factors involved in Ab and complement inactivation of viruses include the amount of Ab bound to the virion, the proximity of Ab binding to the lipid bilayer (1), whether complement regulatory factors are incorporated into the virion (27), and the nature of virion lipid and carbohydrate structures (which could be influenced by the type of cellular membrane through which the virion buds and by such factors as the degree of sialation) (19, 21, 29). The fact that NDV and VSV were inactivated by NHS regardless of their modification with Galα(1-3)Gal can be explained by previous work showing that NDV can directly activate complement in the absence of Ab (41, 42) and that VSV binds to a distinct human natural IgM Ab that remains undefined but, based on the present study, is probably not specific for Galα1-3Gal (6). An explanation for the resistance of SV to NHS, even after it has incorporated Galα1-3Gal into its virion, is more problematic. Previous work has shown that SV could be lysed by complement in the presence of antiviral Ab (35). Other work showed that purified SV virions could activate the complement system in the absence of Ab, that the levels of activation depended on the cells through which SV was passaged, and that the activation was significantly enhanced by treatment of the virions with neuraminidase to cleave off sialic acid determinants (19). In this present study, neuraminidase treatment of SV passed through SK-GT cells did not render the virions significantly more sensitive to NHS (Table 4, experiment 5), suggesting that high levels of sialation were not responsible for its resistance to lysis. Although SV virions acquire host cell plasma membrane lipids and glycosylation patterns, the ratio of cholesterol to phospholipids is much higher than in cell membranes, and the SV membranes are more densely packed, less fluid, and have greater curvature than cellular membranes (21, 22, 29). It is possible that these properties contribute to the resistance of the virus to inactivation by NHS. Additionally, some viruses, such as HIV, acquire species-restricted complement regulatory factors from the cells through which they are passed (27). We detected significant expression of the complement regulatory factors CD55 and CD59 on SK-N-MC cells by immunofluorescence (data not shown). This seemed like an unlikely mechanism to explain the resistance of SV to inactivation by NHS, because the SK-GT cells themselves were quite sensitive to lysis (Fig. 2). Nevertheless, we examined the sensitivity of SV passaged through mouse GT-WT cells to NHS and found that it was sensitive to inactivation. Whether this means that SV acquired species-restricted anti-complement factors from human SK-GT cells but not from mouse GT-KO cells remains unresolved, but it demonstrates further the complexity of host modification factors determining whether or not a given virus is sensitive to inactivation by NHS.
The availability of GT-deficient mice, which can be immunized to mount Ab responses to Galα1-3Gal, led us to test our hypothesis that species-dependent differences in natural Abs to cellular membranes may play important roles in natural immunity against enveloped viruses (42). However, even though sera from the immunized mice had some moderate antiviral activity against LCMV passaged through GT-expressing cells, immune mice did not restrict the replication of that virus in vivo. Work on Ab-independent complement activation by human retroviruses has shown that the activation of complement through this mechanism is insufficient to trigger virolysis and consequently serves as an opsonin for viral infection of cells expressing CR (38). We therefore questioned whether the inactivation of LCMV vis à vis its growth in vero cells would be equivalent to its inactivation vis à vis its growth in macrophages, which express Fc receptors and CR and are the natural targets for LCMV in vivo (24). Table 3 shows that treatment with the same mouse sera that inhibited virus growth on Vero cells did not inhibit the viral infectivity on PEC. Complement levels in laboratory mice are much lower than in wild mice, guinea pigs, rabbits, or humans, and infectious virus-Ab-complement complexes can be isolated from the sera of LCMV persistently infected mice (46). Thus, the GT-deficient mouse model, due to its low complement activity, may not be an appropriate model to test the role of anti-Gal in immunity to viruses crossing species barriers, despite mounting anti-Gal Ab titers similar to that in humans. This model does, however, illustrate the probable importance of a highly efficient complement source, as the anti-Gal Ab that was present could apparently bind to the LCMV virions but was ineffective in the weak complement milieu of the mouse. The importance of complement in the control of viral infections has further been illustrated in studies in animals depleted of complement components (18) and is predicted by the observations that many viruses encode CR or complement regulatory proteins and that some viruses incorporate cellular complement regulatory proteins into their membranes (9, 27, 31).
ACKNOWLEDGMENT
This work was supported by PHS research grants AI17672 and CA34461 to R.M.W.
REFERENCES
- 1.Almeida J D, Waterson A P. The morphology of virus-antibody interaction. Adv Virus Res. 1969;15:307–338. doi: 10.1016/S0065-3527(08)60878-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ausubel F M, Brent R, Kingston R E, Moore D D, Seidman J G, Smith J A, Struhl K, editors. Current protocols in molecular biology. I. New York, N.Y: John Wiley & Sons; 1991. [Google Scholar]
- 3.Babu J S, Thomas J, Kanagat S, Morrison L A, Knipe D M, Rouse B T. Viral replication is required for induction of ocular immunopathology by herpes simplex virus. J Virol. 1996;70:101–107. doi: 10.1128/jvi.70.1.101-107.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bartholomew R M, Esser A F. Differences in activation of human and guinea pig complement by retroviruses. J Immunol. 1998;121:1748–1751. [PubMed] [Google Scholar]
- 5.Bartholomew R M, Esser A F, Muller-Eberhard H J. Lysis of oncornaviruses by human serum: isolation of the viral component (C1) receptor and identification as p15E. J Exp Med. 1978;147:844–853. doi: 10.1084/jem.147.3.844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Beebe D P, Cooper N R. Neutralization of vesicular stomatitis virus (VSV) by human complement requires a natural IgM antibody present in human serum. J Immunol. 1981;126:1562–1568. [PubMed] [Google Scholar]
- 7.Brutkiewicz R R, O’Donnell C L, Maciaszek J W, Welsh R M, Vargas-Cortes M. The mAb CZ-1 identifies a mouse CD45-associated epitope expressed on IL-2-responsive cells. Eur J Immunol. 1993;23:2427–2433. doi: 10.1002/eji.1830231008. [DOI] [PubMed] [Google Scholar]
- 8.Bukowski J F, Woda B A, Habu S, Okumura K, Welsh R M. Natural killer cell depletion enhances virus synthesis and virus induced hepatitis in vivo. J Immunol. 1983;131:1531–1538. [PubMed] [Google Scholar]
- 9.Cooper N R. Complement evasion strategies of microorganisms. Immunol Today. 1991;12:327–331. doi: 10.1016/0167-5699(91)90010-Q. [DOI] [PubMed] [Google Scholar]
- 10.Cooper N R, Jensen F C, Welsh R M, Oldstone M B A. Lysis of RNA tumor viruses by human serum: direct antibody independent triggering of the classical complement pathway. J Exp Med. 1976;144:970–984. doi: 10.1084/jem.144.4.970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cooper N R, Welsh R M. Antibody and complement dependent viral neutralization. Semin Immunopathol. 1979;2:285–310. doi: 10.1007/BF00198721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Crise B, Ruusala A, Zagouras P, Shaw A, Rose J K. Oligomerization of glycolipid-anchored and soluble forms of the vesicular stomatitis virus glycoprotein. J Virol. 1989;63:5328–5333. doi: 10.1128/jvi.63.12.5328-5333.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ebenbichler C F, Thielens N M, Vornhagen R, Marschang P, Arlaud G J, Dierich M P. Human immunodeficiency virus type 1 activates the classical pathway of complement by direct C1 binding through specific sites in the transmembrane glycoprotein gp41. J Exp Med. 1991;174:1417–1424. doi: 10.1084/jem.174.6.1417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Galili U, Mandrell R E, Hamadeh R M, Shohet S B, Griffis J M. Interaction between human natural anti-α-galactosyl immunoglobulin G and bacteria of the human flora. Infect Immun. 1988;56:1730–1737. doi: 10.1128/iai.56.7.1730-1737.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Galili U, Shohet S B, Kobrin E, Stults C L M, Macher B A. Man, apes, and old world monkeys differ from other mammals in the expression of α-galactosyl epitopes on nucleated cells. J Biol Chem. 1988;263:17755–17762. [PubMed] [Google Scholar]
- 16.Gallagher R E, Schrecker A W, Walter C A, Gallo R C. Oncornavirus lytic activity in the serum of gibbon apes. J Natl Cancer Inst. 1978;60:677–682. doi: 10.1093/jnci/60.3.677. [DOI] [PubMed] [Google Scholar]
- 17.Goochee C F, Gramer M J, Andersen D C, Baher J B, Rasmussen J R. The oligosaccharides of glycoproteins: bioprocess factors affecting oligosaccharide structure and their effect on glycoprotein properties. Bio/Technology. 1991;9:1347–1355. doi: 10.1038/nbt1291-1347. [DOI] [PubMed] [Google Scholar]
- 18.Hicks J T, Ennis F A, Kim E, Verbonitz M. The importance of an intact complement pathway in recovery from a primary viral infection. Influenza in decomplemented and in C5-deficient mice. J Immunol. 1978;121:1437–1445. [PubMed] [Google Scholar]
- 19.Hirsch R L, Griffin D E, Winkelstein J A. Host modification of Sindbis virus sialic acid content influences alternative complement pathway activation and virus clearance. J Immunol. 1981;127:1740–1743. [PubMed] [Google Scholar]
- 20.Hoshino H, Tanaka H, Miwa M, Okada H. Human T-cell leukemia virus is not lysed by human serum. Nature. 1984;310:324–325. doi: 10.1038/310324a0. [DOI] [PubMed] [Google Scholar]
- 21.Keegstra K, Sefton B, Burke D. Sindbis virus glycoproteins: effect of the host cell on oligosaccharides. J Virol. 1975;16:613–620. doi: 10.1128/jvi.16.3.613-620.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lenard J. Lipids of alphaviruses. In: Schlesinger R W, editor. The togaviruses. New York, N.Y: Academic Press; 1980. pp. 335–341. [Google Scholar]
- 23.Markowitz D, Goff S, Bank A. A safe packaging line for gene transfer: separating viral genes on two different plasmids. J Virol. 1988;62:1120–1124. doi: 10.1128/jvi.62.4.1120-1124.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Matloubian M, Kolhekar S R, Somasundaram T, Ahmed R. Molecular determinants of macrophage tropism and viral persistence: importance of single amino acid changes in the polymerase and glycoprotein of lymphocytic choriomeningitis virus. J Virol. 1993;67:7340–7349. doi: 10.1128/jvi.67.12.7340-7349.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.McGinnes L W, Morrison T G. Disulfide bond formation is a determinant of glycosylation site usage in the hemagglutinin-neuraminidase glycoprotein of Newcastle disease virus. J Virol. 1997;71:3083–3089. doi: 10.1128/jvi.71.4.3083-3089.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Miller A D, Buttimore C. Redesign of retrovirus packagining cell lines to avoid recombination leading to helper virus production. Mol Cell Biol. 1997;6:2895–2902. doi: 10.1128/mcb.6.8.2895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Montefiori D C, Cornell R J, Zhou J Y, Hirsch V M, Johnson P R. Complement control proteins, CD46, CD55, and CD59, as common surface constituents of human and simian immunodeficiency viruses and possible targets for vaccine protection. Virology. 1994;205:85–92. doi: 10.1006/viro.1994.1622. [DOI] [PubMed] [Google Scholar]
- 28.Reed D J, Lin X, Thomas T D, Birks C W, Tang J, Rother R P. Alteration of glycosylation renders HIV sensitive to inactivation by normal human serum. J Immunol. 1997;159:4356–4361. [PubMed] [Google Scholar]
- 29.Renkonen O, Kaariainen L, Simons K, Gahmberg C G. The lipid composition of Semliki Forest virus and of plasma membranes of the host cells. Virology. 1971;46:318–426. doi: 10.1016/0042-6822(71)90033-x. [DOI] [PubMed] [Google Scholar]
- 30.Rother R P, Fodor W L, Springhorn J P, Birks C W, Setter E, Sandrin M S, Squinto S P, Rollins S A. A novel mechanism of retrovirus inactivation in human serum mediated by anti-α-galactosyl natural antibody. J Exp Med. 1995;182:1345–1355. doi: 10.1084/jem.182.5.1345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rother R P, Rollins S A, Fodor W L, Albrecht J-C, Setter E, Fleckenstein B, Squinto S P. Inhibition of complement-mediated cytolysis by the terminal complement inhibitor of herpesvirus saimiri. J Virol. 1994;68:730–737. doi: 10.1128/jvi.68.2.730-737.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rother R P, Squinto S P. The α-galactosyl epitope: a sugar coating that makes viruses and cells unpalatable. Cell. 1996;86:185–188. doi: 10.1016/s0092-8674(00)80090-2. [DOI] [PubMed] [Google Scholar]
- 33.Sefton B, Gaffney B J. Effect of viral proteins on the fluidity of membrane lipids in Sindbis virus. J Mol Biol. 1974;90:343–358. doi: 10.1016/0022-2836(74)90378-7. [DOI] [PubMed] [Google Scholar]
- 34.Sherwin S A, Benveniste R E, Todaro G J. Complement-mediated lysis of C-type virus: effect of primate and human sera on various retroviruses. Int J Cancer. 1978;21:6–11. doi: 10.1002/ijc.2910210103. [DOI] [PubMed] [Google Scholar]
- 35.Stollar V. Immune lysis of Sindbis virus. Virology. 1975;66:620–624. doi: 10.1016/0042-6822(75)90235-4. [DOI] [PubMed] [Google Scholar]
- 36.Takeuchi Y, Porter C D, Strahan K M, Preece A F, Gustafsson K, Cosset F-L, Weiss R A, Collins M K L. Sensitization of cells and retroviruses to human serum by (α1-3) galactosyltransferase. Nature. 1996;379:85–88. doi: 10.1038/379085a0. [DOI] [PubMed] [Google Scholar]
- 37.Thall A D, Maly P, Lowe J B. Oocyte Gal alpha 1,3Gal epitopes implicated in sperm adhesion to the zona pellucida glycoprotein ZP3 are not required for fertilization in the mouse. J Biol Chem. 1995;270:21437–21440. doi: 10.1074/jbc.270.37.21437. [DOI] [PubMed] [Google Scholar]
- 38.Thieblemont N, Haeffner-Cavaillon N, Ledur A, L’Age-Stehr J, Ziegler-Heitbrock H W, Kazatchkine M D. CR1 (CD35) and CR3 (CD11b/CD18) mediate infection of human monocytes and monocytic cell lines with complement-opsonized HIV independently of CD4. Clin Exp Immunol. 1993;92:106–113. doi: 10.1111/j.1365-2249.1993.tb05955.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Vaughan H A, Loveland B E, Sandrin M S. Galα(1,3)gal is the major xenoepitope expressed on pig endothelial cells recognized by naturally occurring cytotoxic human antibodies. Transplantation. 1994;58:879–882. doi: 10.1097/00007890-199410270-00003. [DOI] [PubMed] [Google Scholar]
- 40.Waite M R F, Pfefferkorn E R. Effect of altered osmotic pressure on the growth of Sindbis virus. J Virol. 1968;2:759–760. doi: 10.1128/jvi.2.7.759-760.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wedgwood R J, Ginsberg H S, Pillemer L. The properdin system and immunity. IV. The inactivation of Newcastle disease virus by the properdin system. J Exp Med. 1956;104:707–725. doi: 10.1084/jem.104.5.707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Welsh R M. Host cell modification of lymphocytic choriomeningitis virus and Newcastle disease virus altering viral inactivation by human complement. J Immunol. 1977;118:348–354. [PubMed] [Google Scholar]
- 43.Welsh R M, Buchmeier M J. Protein analysis of defective interfering lymphocytic choriomeningitis virus and persistently infected cells. Virology. 1979;96:503–515. doi: 10.1016/0042-6822(79)90107-7. [DOI] [PubMed] [Google Scholar]
- 44.Welsh R M, Cooper N R, Jensen F C, Oldstone M B A. Human serum lyses RNA tumor viruses. Nature. 1975;257:612–614. doi: 10.1038/257612a0. [DOI] [PubMed] [Google Scholar]
- 45.Welsh R M, Jensen F C, Cooper N R, Oldstone M B A. Inactivation and lysis of oncornaviruses by human serum. Virology. 1976;74:432–440. doi: 10.1016/0042-6822(76)90349-4. [DOI] [PubMed] [Google Scholar]
- 46.Welsh R M, Lampert P W, Burner P A, Oldstone M B A. Antibody-complement interactions with purified lymphocytic choriomeningitis virus. Virology. 1976;73:59–71. doi: 10.1016/0042-6822(76)90060-x. [DOI] [PubMed] [Google Scholar]