Introduction
Testicular seminoma is a rare disease with which management varies among different stages. For patients with bulky metastatic disease, chemotherapy is the treatment of choice 1. Although the response rate is typically outstanding, some patients with metastatic seminoma will harbor residual disease after the chemotherapy and the majority of this residual disease is located in the retroperitoneum. Controversy remains regarding optimal management of residual retroperitoneal disease in post-chemotherapy (PC) seminoma patients where retroperitoneal lymph node dissection (RPLND) is an option for select individuals1,2.
PC-RPLND in seminoma is a notoriously challenging surgery because of the significant desmoplastic reaction that can occur between the regressing tumor and adjacent structures 3. While open surgery is the gold standard for PC-RPLND, there have been studies reporting robot-assisted PC-RPLND. However, the majority of these reports have been in patients with non-seminomatous germ-cell tumors 4, 5. For seminoma patients with a residual solitary retroperitoneal mass, and resection of the mass is intended, robot-assisted surgery may be considered. Nevertheless, despite the operative approach, resection of a seminoma metastasis continues to be a difficult procedure 5. Here we report a case of PC robot-assisted resection of a seminoma metastasis with near-infrared fluorescence (NIRF) using a novel imaging technique utilizing the second window properties of an FDA approved NIR contrast agent, indocyanine green (ICG). The objective was to determine if the seminoma fluoresced after delayed-ICG administration and if NIRF helped the resection.
Case report
Clinical Case
A 29-year-old male with a history of testicular mass and bulky retroperitoneal mass was referred to our institution for the further management of an enlarging pelvic lymph node. The patient had previously undergone a right-sided radical orchiectomy and the final pathology demonstrated an 8 cm seminoma. He received 4 cycles of chemotherapy with EP (etoposide + cisplatin) postoperatively. He had an excellent response to chemotherapy and CT scan demonstrated the bulky retroperitoneal mass had decreased significantly. However, recent follow-up CT scan noted an enlarging right common iliac lymph node (1.6 × 1.9 cm) (Figure 1). Chest CT was normal. Alpha-fetoprotein (AFP) and β-HCG were in normal range. After an informed discussion with both urology and medical oncology, the patient elected to move forward with complete excision of the enlarging adenopathy. The patient was enrolled in an IRB-approved trial of intraoperative molecular imaging after informed consent. He received 5 mg/kg of ICG 24 hours intravenously before robot-assisted laparoscopic resection of the adenopathy. No significant adverse events occurred during the infusion.
Figure 1.
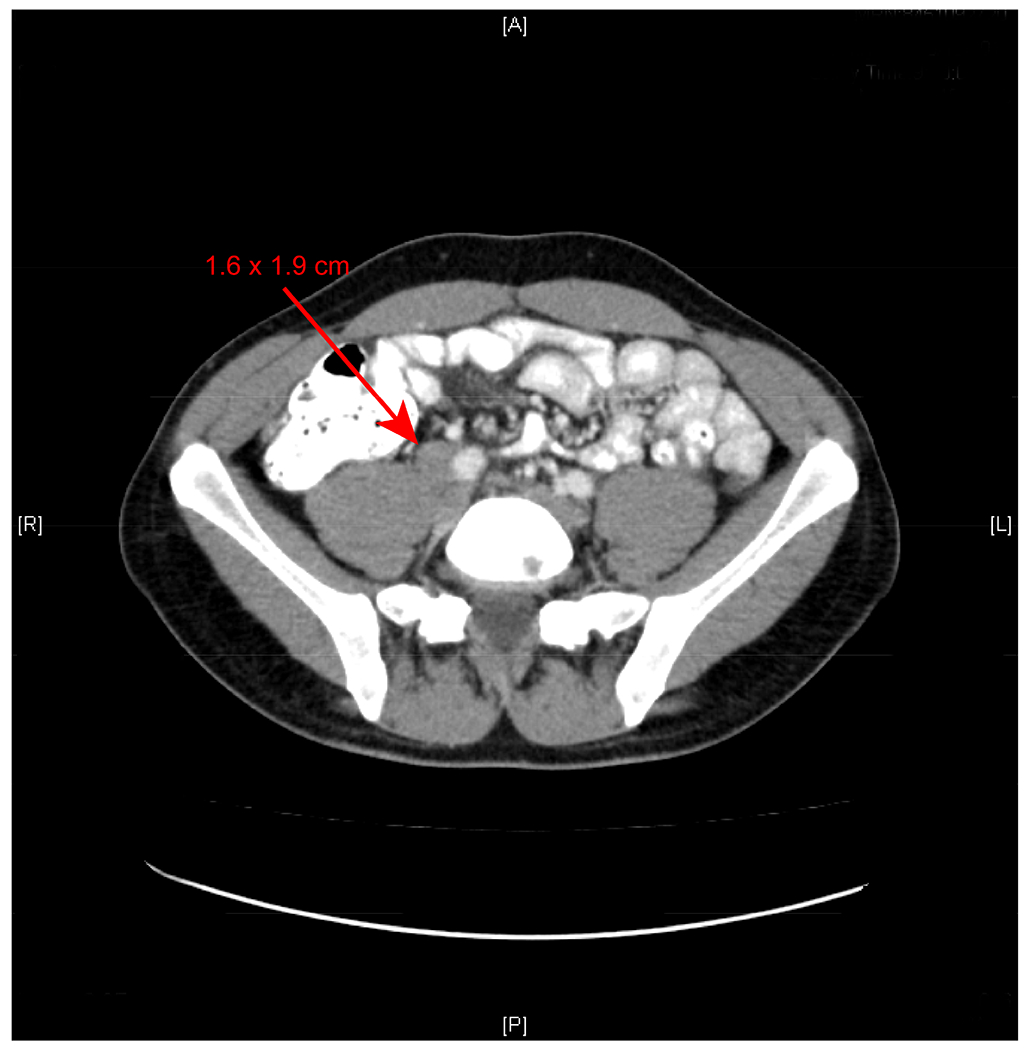
CT scan of the enlarged right common iliac lymph node (1.6 × 1.9 cm).
Surgical Management
The surgery was performed with the DaVinci Xi robot (with the built-in Firefly system). The patient was placed in the Trendelenburg position. A Foley catheter was placed. A Veress needle was used for insufflation. A four-arm transperitoneal approach was utilized and trocar placement was similar to robot-assisted radical prostatectomy. The peritoneum overlying the external iliac and common iliac vessels on the right was carefully incised with monopolar scissors. The ureter and enlarged common iliac node were identified. Next, the Firefly system was turned on and the molecular imaging confirmed positive fluorescence of the node. The ureter was fairly adherent to the medial aspect of the lymph node and sharp dissection was employed to completely mobilize it off the node. The Firefly system was turned on again and confirmed the fluorescence positive node was successfully dissected from the ureter (Figure 2). Then the mass was dissected off the external iliac artery and common iliac artery. Finally, the mass was mobilized off the psoas muscle and remainder of the attachments were divided with a monopolar cautery. The specimen was placed into a retrieval bag and removed intact through the camera port. As a follow-up, the resection bed was inspected with the Firefly system and no residual fluorescence was found. The specimen was placed on a back table and re-imaged with an NIRF visualization system (Visionsense®, Philadelphia, PA). The results showed positive fluorescence of the specimen (Figure 3). In total, the console time was 80 minutes and estimated blood loss was less than 100 ml. No significant intraoperative complications occurred. The postoperative course was uneventful and the patient was discharged postoperative day 1. Final pathologic diagnosis of the mass was metastatic seminoma.
Figure 2.
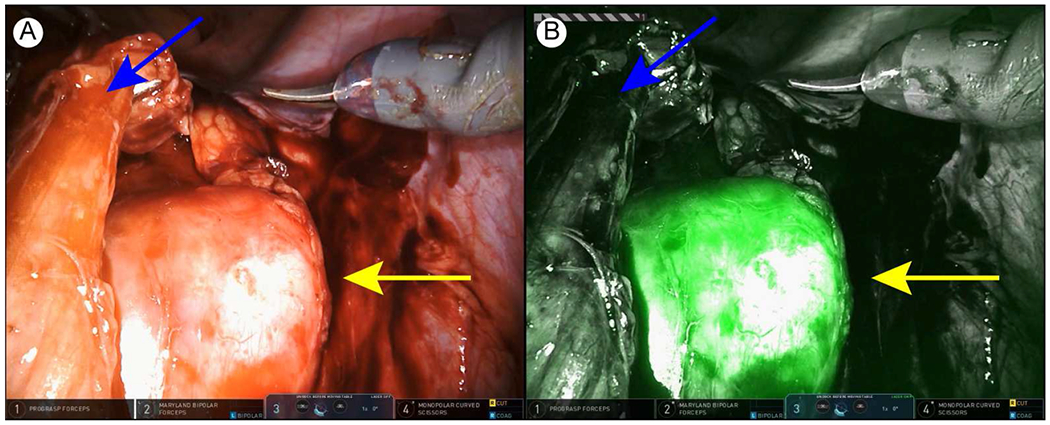
Intraoperative images showing the dissected ureter and enlarged lymph node. (A) White light image. (B) Near-infrared fluorescence image. Yellow arrow, lymph node. Blue arrow, ureter.
Figure 3.
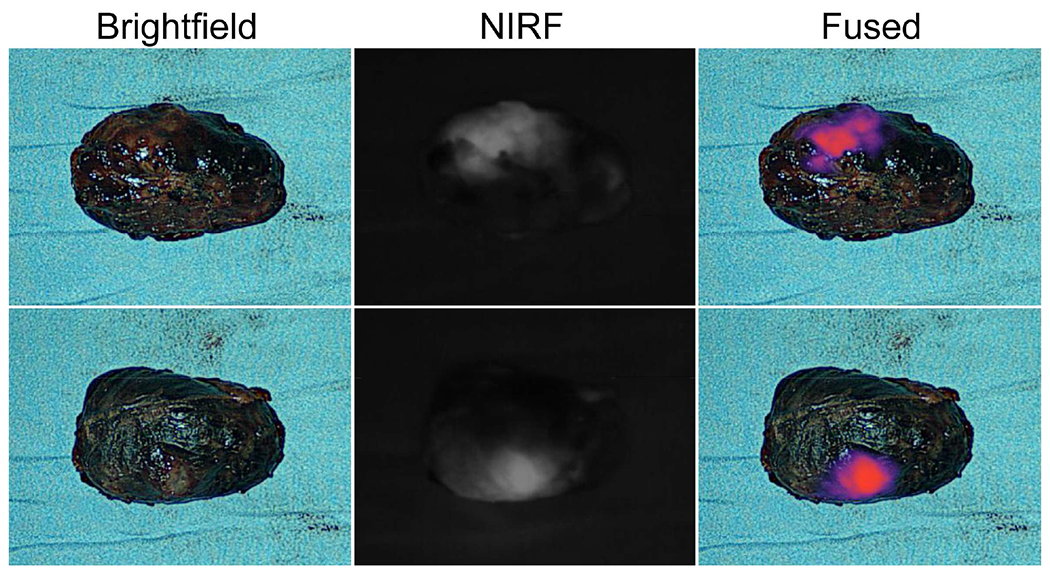
Postoperative specimen.
Discussion
This case demonstrates that robot-assisted resection of seminoma metastasis in the PC setting is a feasible and a safe procedure, which is consistent with the case series (3 cases) from Kamel et al 5. Guidelines recommend FDG-PET for the evaluation of suspicious residual seminoma mass, however, patients with radiographic progressing disease without HCG progression can be considered for surgical resection if possible 1, 6–8. The patient in this case did not receive FDG-PET because the node was actively enlarging. Considering the minimally-invasive features and significant advantages of the Da Vinci platform, including reduced blood loss and shorter recovery time, robot-assisted resection of seminoma metastasis is a viable option for patients provided it can replicate the open technique with regard to complete resection. However, additional strategies to optimize the procedure to enhance complete resection while minimizing risk to surrounding structures are still needed secondary to the desmoplastic reaction that is frequently encountered in the PC seminoma setting. Experience from open PC-RPLND for seminoma has shown that resection of the residual seminoma mass is associated with a higher rate of additional intraoperative procedures and postoperative complications compared with nonseminomatous masses 9.
This pilot case demonstrates that intraoperative NIFR imaging might be a useful adjunct to facilitate identification of surgical borders of a PC mass. Intraoperative molecular imaging has been increasingly used in the field of surgical oncology and has been demonstrated to be a useful supplement to the standard surgical approach 10-12. Intraoperative molecular imaging may be useful to help locate the primary tumor or metastasis and provide guidance through the procedure, such as identification of positive margins as well as protecting normal structures 10, 13–16. In robot-assisted laparoscopic surgery, surgeons are largely dependent on visual cues and feedback. Enhanced visualization and additional visual information from NIRF imaging may provide the surgeon with a significant advantage in the safe and efficient removal of tumors adjacent to vital structures.
In this study, we explored the use of a known NIR contrast agent, ICG, using a novel method of injection. Our group has been studying novel methods of intraoperative imaging using ICG by investigating the enhanced permeability and retention effect (EPR). The EPR effect explains small molecular contrast agents such as ICG can accumulate in solid tumors due to leaky capillaries, differences in oncotic tissue pressures and release of local cytokines such as bradykinin 17, 18. The EPR effects allow for tumor specificity without receptor targeting. The ICG induced NIRF usage in robot-assisted surgery for urologic cancers has been reported and has shown promising results, even though the ICG administration method that we utilized has not been previously applied to this disease 11. To our knowledge, this is the first case of PC robot-assisted laparoscopic resection of seminoma metastasis with intraoperative molecular imaging.
The Da Vinci surgical robot (Si and Xi) has a built-in visualization system (Firefly) that can detect ICG NIRF as an overlaid, green pseudocolor in real time. The major advantage of the system is that console surgeon can easily toggle between the white light and NIRF, which requires no extra setup or additional operative time. In addition, ICG is a very safe compound without ionizing radiation and has been in medical use for almost 60 years. Therefore, the barriers to implement ICG NIRF in robotic surgery is minimal and it can potentially augment the information available during a surgical resection. In our case, the seminoma was confirmed florescent 24 hours after the ICG injection. In the meantime, major vessels, ureter, and other surrounding structures were not fluorescent. Intraoperative molecular imaging provided valuable real-time information through the resection. Due to the serious fibrosis between the mass and surround structures, sharp dissection was heavily utilized and NIRF imaging was useful in identifying the tumor margin relative to important adjacent anatomic structures. The main limitations of this technique are the potentially false-positive and negative fluorescence. Future studies are needed to confirm the predictive value of this technique as well as the possibility of expanding its usage to non-seminoma.
Conclusions
This case report demonstrates that robot-assist resection of the seminoma metastasis in the PC setting is feasible, and the intraoperative molecular imaging might be helpful for the procedure. However, larger case series are needed to confirm our findings.
Clinical Practice Points.
Post-chemotherapy retroperitoneal lymph node dissection (PC-RPLND) for seminoma metastasis is a challenging surgical scenario.
Most of the PC-RPLND cases for seminoma metastasis in the literature were open surgery.
In this case, we used a known near-infrared contrast agent indocyanine green with a novel method of injection to facilitate the robot-assisted resection of a seminoma metastasis in the PC setting.
To the best of our knowledge, this is the first case of PC robot-assisted laparoscopic resection of seminoma metastasis with intraoperative molecular imaging.
Acknowledgments:
Part of this work was funded by the National Institutes of Health (R01 CA193556)
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosure: No conflicts of interest
References
- 1.Albers P, Albrecht W, Algaba F, et al. Guidelines on testicular cancer: 2015 update. Eur Urol 2015; 68(6):1054–68. [DOI] [PubMed] [Google Scholar]
- 2.Daneshmand S, Albers P, Fossa SD, et al. Contemporary management of postchemotherapy testis cancer. Eur Urol 2012; 62(5):867–76. [DOI] [PubMed] [Google Scholar]
- 3.Sim A, Aufderklamm S, Halalsheh O, et al. Surgical removal of retroperitoneal tumors after chemotherapy treated testicular tumors. Curr Urol Rep 2014; 15(11):456. [DOI] [PubMed] [Google Scholar]
- 4.Cheney SM, Andrews PE, Leibovich BC, et al. Robot-assisted retroperitoneal lymph node dissection: technique and initial case series of 18 patients. BJU Int 2015; 115(1):114–20. [DOI] [PubMed] [Google Scholar]
- 5.Kamel MH, Littlejohn N, Cox M, et al. Post-Chemotherapy Robotic Retroperitoneal Lymph Node Dissection: Institutional Experience. J Endourol 2016; 30(5):510–9. [DOI] [PubMed] [Google Scholar]
- 6.Bilen MA, Hariri H, Leon C, et al. Positive FDG-PET/CT scans of a residual seminoma after chemotherapy and radiotherapy: case report and review of the literature. Clin Genitourin Cancer 2014; 12(4):e147–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Muller J, Schrader AJ, Jentzmik F, et al. [Assessment of residual tumours after systemic treatment of metastatic seminoma: (1)(8)F-2-fluoro-2-deoxy-D-glucose positron emission tomography - meta-analysis of diagnostic value]. Urologe A 2011; 50(3):322–7. [DOI] [PubMed] [Google Scholar]
- 8.Motzer RJ, Jonasch E, Agarwal N, et al. Testicular Cancer, Version 2.2015. J Natl Compr Canc Netw 2015; 13(6):772–99. [DOI] [PubMed] [Google Scholar]
- 9.Mosharafa AA, Foster RS, Leibovich BC, et al. Is post-chemotherapy resection of seminomatous elements associated with higher acute morbidity? J Urol 2003; 169(6):2126–8. [DOI] [PubMed] [Google Scholar]
- 10.Singhal S The Future of Surgical Oncology: Image-Guided Cancer Surgery. JAMA Surg 2016; 151(2):184–5. [DOI] [PubMed] [Google Scholar]
- 11.Autorino R, Zargar H, White WM, et al. Current applications of near-infrared fluorescence imaging in robotic urologic surgery: a systematic review and critical analysis of the literature. Urology 2014; 84(4):751–9. [DOI] [PubMed] [Google Scholar]
- 12.Keating JJ, Okusanya OT, De Jesus E, et al. Intraoperative Molecular Imaging of Lung Adenocarcinoma Can Identify Residual Tumor Cells at the Surgical Margins. Mol Imaging Biol 2016; 18(2):209–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Keating J, Tchou J, Okusanya O, et al. Identification of breast cancer margins using intraoperative near-infrared imaging. J Surg Oncol 2016; 113(5):508–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Keating JJ, Kennedy GT, Singhal S. Identification of a subcentimeter pulmonary adenocarcinoma using intraoperative near-infrared imaging during video-assisted thoracoscopic surgery. J Thorac Cardiovasc Surg 2015; 149(3):e51–3. [DOI] [PubMed] [Google Scholar]
- 15.Okusanya OT, DeJesus EM, Jiang JX, et al. Intraoperative molecular imaging can identify lung adenocarcinomas during pulmonary resection. J Thorac Cardiovasc Surg 2015; 150(1):28–35 e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Guzzo TJ, Jiang J, Keating J, et al. Intraoperative Molecular Diagnostic Imaging Can Identify Renal Cell Carcinoma. J Urol 2016; 195(3):748–55. [DOI] [PubMed] [Google Scholar]
- 17.Jiang JX, Keating JJ, Jesus EM, et al. Optimization of the enhanced permeability and retention effect for near-infrared imaging of solid tumors with indocyanine green. Am J Nucl Med Mol Imaging 2015; 5(4):390–400. [PMC free article] [PubMed] [Google Scholar]
- 18.Singhal S, Nie S, Wang MD. Nanotechnology applications in surgical oncology. Annu Rev Med 2010; 61:359–73. [DOI] [PMC free article] [PubMed] [Google Scholar]


