Significance
While effective in non-pigmented tumors, photodynamic therapy (PDT) has failed in treating melanoma due to the high light attenuation by melanin. Here, an effective approach for ocular melanoma is proposed using the concept of melanin-mediated multi-photon PDT. Experiments were performed comparing cytotoxicity in pigmented versus nonpigmented melanoma cells treated by 1- versus 2-photon PDT using low versus high 2-photon cross-section photosensitizers. The unexpected results show that, under femtosecond pulsed laser irradiation, melanin can absorb 2 and 3 photons and transfer the energy to the photosensitizer. The resulting therapeutic efficacy is demonstrated in vivo in a murine model of conjunctival melanoma, achieving complete tumor eradication and showing the potential of this approach as a minimally invasive therapeutic option.
Keywords: photodynamic therapy, multiphoton, ocular melanoma, melanoma, melanin
Abstract
Photodynamic therapy (PDT) relies on a series of photophysical and photochemical reactions leading to cell death. While effective for various cancers, PDT has been less successful in treating pigmented melanoma due to high light absorption by melanin. Here, this limitation is addressed by 2-photon excitation of the photosensitizer (2p-PDT) using ~100 fs pulses of near-infrared laser light. A critical role of melanin in enabling rather than hindering 2p-PDT is elucidated using pigmented and non-pigmented murine melanoma clonal cell lines in vitro. The photocytotoxicities were compared between a clinical photosensitizer (Visudyne) and a porphyrin dimer (Oxdime) with ~600-fold higher σ2p value. Unexpectedly, while the 1p-PDT responses are similar in both cell lines, 2p activation is much more effective in killing pigmented than non-pigmented cells, suggesting a dominant role of melanin 2p-PDT. The potential for clinical translational is demonstrated in a conjunctival melanoma model in vivo, where complete eradication of small tumors was achieved. This work elucidates the melanin contribution in multi-photon PDT enabling significant advancement of light-based treatments that have previously been considered unsuitable in pigmented tumors.
Photodynamic therapy (PDT) uses light-activated photosensitizers (PS) to induce cell death by necrosis, apoptosis, and/or autophagy and is approved clinically for a range of tumor types, (1, 2) localized infections (3–6) and, in the eye, wet-form age-related macular degeneration (AMD) (7, 8). Melanoma is a pigmented tumor that originates from melanocytes and the cutaneous form is the most lethal skin cancer (9–11), in which the few clinical trials of conventional PDT using molecular PS and low-power continuous wavelength (CW) photoactivation have shown only incomplete responses (12). This is attributed primarily to poor light penetration due to high melanin absorption, although other biophysical and biological effects may also contribute to the limited efficacy (13). In order to overcome the light penetration barrier, we recently reported that topical application of optical clearing agents that reduce the light attenuation through refractive index matching in the tissue, combined with dual-photosensitizer (vascular + cellular) PDT, resulted in tumor eradication in an in vivo intradermal melanoma model in mice (14).
Although melanoma occurs predominantly in the skin, it is also the most prevalent primary intraocular malignancy in adults (15–17), for which current treatments are either globe-preserving treatments or enucleation. The former includes plaque, charged-particle, and stereotactic radiotherapy. Due to associated collateral damage, such as radiation retinopathy, papillopathy, hemorrhage, cataract, macular edema, retinal detachment, and neovascular glaucoma, watchful waiting is recommended for small tumors. Nevertheless, despite high levels of local disease control, 50% of patients develop metastatic disease, resulting in an average survival of only 13.4 mo from time of metastatic diagnosis (18, 19). Other experimental therapies such as thermotherapy, proton beam irradiation, and chemotherapy have been reported but have not led to breakthroughs or clinical translation to date (20–22).
Conventional PDT with low power density (~100 mWcm−2) CW laser irradiation has also been investigated in intraocular tumors as both a primary and adjuvant therapy, but with variable and disappointing responses (12, 23). Yordi and colleagues (23) reviewed 7 trials of Verteporfin (Visudyne™)-mediated PDT as a primary treatment of choroidal melanoma (mean tumor height 3.8 mm) in 162 patients. Partial regression was seen in 92% of non-pigmented tumors but the treatment failed in 81% of pigmented tumors.
Optical clearing would require invasive administration into intraocular tumors, so we investigated here the alternative of two-photon (2p) activation using ultrashort (~100 fs) near-infrared (NIR) laser light. These experiments were performed in cell monolayers in vitro and in an in vivo mouse model of conjunctival melanoma, using both pigmented tumor cells and non-pigmented tumor cells. In addition to the advantage of reduced scattering and melanin absorption using longer wavelength NIR light, multiphoton activation can be highly localized to a near-diffraction-limited volume. We demonstrated this previously by targeting of individual tumor blood vessels (24). This degree of spatial precision is critical in the eye to minimize collateral photodamage, and the full target tumor volume can be treated using x-y-z raster scanning of the focal spot.
Conventional 1p-PDT uses a range of molecular PS (1, 8, 25, 26) that have very low 2p cross-section, σ2p, typically tens of GM units or lower. As a result, the activation is very inefficient and requires the use of very high doses of light (27). Hence, molecular (24, 28) and nanoparticle (29, 30) agents have been investigated, and have shown tumor responses in various non-pigmented preclinical tumor models. Here, we used a porphyrin dimer, Oxdime (24, 31), developed specifically for 2p-PDT, with σ2p ~ 17,000 GM at a peak wavelength of 920 nm (24). We compared 2p- and 1p-PDT in the clonally identical melanotic and amelanotic melanoma cells, using both Oxdime and the benzoporphyrin photosensitizer Visudyne (32) (σ2p = 31 ± 9 GM at 840 to 890 nm) (27) that is used clinically for AMD (7). This was done in both cell monolayers in vitro (where melanin light attenuation can be ignored) and in an in vivo mouse model of conjunctival melanoma.
The in vitro studies led to the unexpected observation of a dominant role of melanin-mediated photoactivation in the 2p-PDT cytotoxicity, particularly with low σ2p photosensitizer. Additional photophysical studies indicate that the likely mechanism of action is the absorption of the pulsed laser light by melanin, followed by either photosensitizer absorption of the resulting fluorescence emission and/or Förster resonant energy transfer (FRET) between melanin and the photosensitizer. The optical power dependence of the PDT cytotoxicity also suggests a contribution from 3p melanin absorption in addition to 2p activation. Further, we found significant anti-tumor efficacy in vivo, including in some cases complete eradication, in a mouse model of conjunctival melanoma using a standard fs laser scanning microscope for spatially precise light delivery.
This study reports the role of melanin in multi-photon PDT, breaking the paradigm that pigmented tumors are unsuitable to light-activated therapies. Moreover, this was demonstrated in a clinically -relevant model in which complete response with minimal collateral tissue damage is critical and with a photosensitizer that is already approved and widely used in patients for 1p PDT, thereby facilitating clinical translation. The clinical feasibility for intraocular tumors is also made possible by the availability of 2p laser scanning confocal ophthalmoscopy (33). These findings suggest that multiphoton-PDT could be further developed into an effective, minimally invasive treatment for ocular tumors, including pigmented melanoma, either as a stand-alone modality or in combination with surgical excision or radiation treatment. The known immune upregulation of PDT could also be a significant additional benefit in reducing tumor progression and metastatic spread, as we have shown recently in intradermal melanoma treated with 1p-PDT (34).
Results
In Vitro Studies.
Although pigmented B16F10 cells and non-pigmented B78H1 are clonally identical, it was necessary to confirm that any differences in PDT response are related to only the presence or absence of melanin and not to altered photosensitizer uptake and/or intracellular localization. Uptake and subcellular localization measurements were performed only using Visudyne, since the fluorescence of Oxdime is weak and at long NIR wavelengths. SI Appendix, Fig. S1 shows no statistically significant difference (P < 0.05) in total fluorescence signal between the 2 cell lines after 3 h incubation, with the primary localization being in the endoplasmic reticulum, as reported also for other cancer cells (35). These experiments were also carried out with membrane and mitochondrial probes, however, no significant colocalization was observed for either of the cells.
SI Appendix, Fig. S2 shows the cell viability following PDT at varying photosensitizer and light doses. Visudyne 1p-PDT was more effective in both cell lines than Oxdime, which is not surprising given likely differences in uptake and co-localization compared with Visudyne and the known lower singlet oxygen quantum yield. More importantly, for both PS, there was minimal difference between the pigmented and non-pigmented cells, showing that the “intrinsic” photosensitivity of these clonal cell lines is the same. Given that the cells were treated in monolayer culture, there was no significant attenuation of the light dose.
Fig. 1 A and B show examples of changes in cell morphology, including cell membrane blebbing, rounding, and detachment following Visudyne 2p-PDT. Similar responses were seen with Oxdime. The cell viability is plotted as a function of the number of laser scans and corresponding energy density for both PS in Fig. 1C, together with the light-only controls. Light-only 2p irradiation reduced the cell viability by ~10 to 15% in non-pigmented cells and up to ~20% in the pigmented cells. This can be attributed to the well-known photothermal and photomechanical damage mechanisms with fs laser exposure (36).
Fig. 1.
Pulsed-laser PDT responses. (A and B) Micrographs of pigmented and non-pigmented cells treated using Visudyne. (C–E) Normalized cell viability measured by cell morphology (means ± 1 SD, n = 9) for Visudyne (2.5 µM, 3 h incubation), Oxdime (10 µM, 1 h incubation), and corresponding light-only controls. (F) Fluences (Jcm−2) for 50% cell kill.
Overall, Oxdime was more effective in pulsed-laser PDT killing in both cell lines than Visudyne and was 2.4 times greater in pigmented than non-pigmented cells. This is expected, due to its high 2p cross-section. However, as noted in earlier studies in vascular endothelial cells (24, 27), this higher efficacy for Oxdime compared with Visudyne is substantially less than that expected based solely on the ~600-fold greater σ2γ, likely due to different subcellular localization and/or energy transfer efficiency, as discussed below.
Unexpectedly, 2p-PDT with Visudyne showed a marked difference in cell kill between the cell lines, the LD50 being >6 times lower in pigmented than non-pigmented cells. By contrast, Oxdime showed only ~2 fold difference. A series of additional experiments, as follows, were carried out in order to elucidate the possible mechanisms behind this striking susceptibility of pigmented cells to Visudyne 2p-PDT.
First, TEM (transmission electron microscopy) images of pigmented melanoma cells and the corresponding 2p light-only treated cells are shown in SI Appendix, Fig. S3. Melanosomes are the most electron-dense structures (dark and round dots) distributed throughout the cytoplasm. In the treated group, changes in melanosome shape were observed in all cells, with blebbing around the melanosome as well as melanosome membrane rupture that may lead to leakage of the melanin and its byproducts that are known to be cytotoxic (37, 38), adding to the PDT effect. Chiarelli-Neto and collaborators investigated capacity of melanin to suppress or generate reactive oxygen species (ROS) under visible light irradiation of hair shafts. Exposure at >400 nm led to melanin degradation but melanin generated singlet oxygen above 532 nm (39). Watanabe et al. (36) studied the effects of laser irradiation of black guinea pig skin. Fs Irradiation at 635 nm disrupted melanosomes and they hypothesized that the observed nuclear damage could be due to release of tyrosinase or melanin precursors (36). Although these studies used different irradiation parameters (pulsed laser, wavelength, laser power), these findings support the hypothesis that photomechanical disruption of melanosomes could result in ROS generated and consequent melanosome membrane oxidation. This could also explain the pronounced shoulder observed in the cell viability assessment for 2p excitation in the light-only controls (Fig. 1C). In order to explore this hypothesis further and to elucidate whether other non-linear phenomena may be involved, the 2p absorption and fluorescence emission were measured.
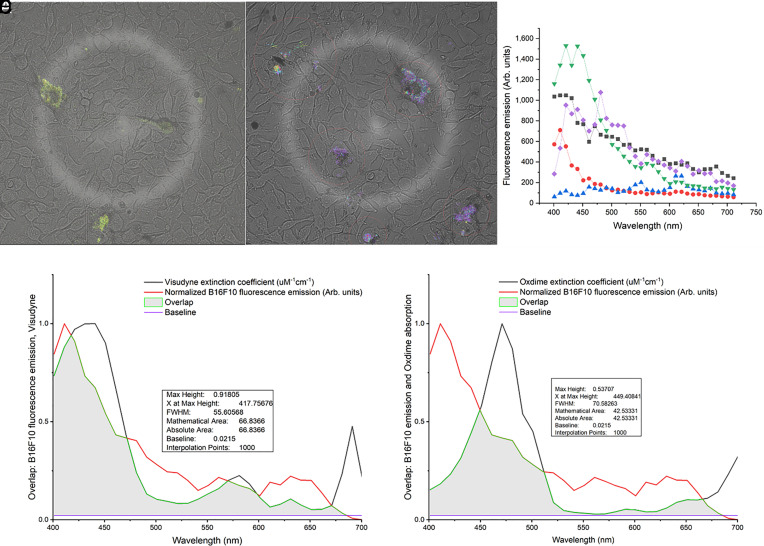
Second, B16F10 pigmented melanoma cells were irradiated with the fs pulsed laser and fluorescence images were taken at multiple wavelengths between 400 and 700 nm (Fig. 2). The melanin spectra peaked in the blue-green region but showed significant inter- and intra-cellular variability (Fig. 2C), likely due to variable melanin granule size and aggregation. The average melanin fluorescence emission overlaps ~67% and ~43% with the absorption spectra of Visudyne (Fig. 2D) and Oxdime (Fig. 2E), respectively, which allows resonant energy transfer.
Fig. 2.
(A and B) Spectral fluorescence imaging of B16F10 cells after fs pulsed irradiation, showing variable inter- and intra-cellular melanin emission. Panel C shows the averaged spectra over five different individual cells. The overlap of the melanin fluorescence emission and the photosensitizer absorption spectra is evident in D and E.
Third, in order to verify that the melanin and PS were being excited through a multiphoton process, the cells were irradiated with and without Visudyne and Oxdime incubation and the fluorescence intensity was plotted as a function of laser power, as shown in Fig. 3. For n-photon absorption, and assuming the same post-absorption photo-physics, -chemistry, and -biology, the fluorescence signal should be proportional to the nth power of the peak incident power density, i.e., ∞ ϕn. Hence, the log–log plot should be linear with a slope of n.
Fig. 3.
Representative log–log plots of fluorescence emission as a function of pulsed laser power for individual cells. (A) B16F10 cells without Visudyne (melanin fluorescence only). (B) B16F10 cells with Visudyne (3 h incubation, 2.5 µM). (C) B78H1 cells with Visudyne (3 h incubation, 2.5 µM). The slopes (±1 SD) for the linear regression fits are listed for each cell.
In the non-pigmented cells with Visudyne (Fig. 3C), the mean n value of 1.95 ± 0.04 indicates purely 2p excitation of the photosensitizer, consistent with our previous observations in vascular endothelial cells (n = 2 ± 0.2) (24). In the pigmented cells with Visudyne, n ranged from 1.95 ± 0.04 to 2.25 ± 0.10, suggesting either a combination of 2- and 3-p melanin excitation together with Visudyne-mediated 2p absorption, and/or melanin-mediated enhancement of the Visudyne excitation. This interpretation is consistent with the fact that variability in this group was also intermediate between the melanin-only (A) and Visudyne-only (C) groups.
Fig. 3A shows the results for the pigmented cells in the absence of photosensitizer, where the fluorescence is attributed to melanin itself. The slopes ranged from 2.05 ± 0.03 to 2.86 ± 0.08, the variation likely due to melanin heterogeneity [e.g., stage of maturity and granule size (40–42)]. These values indicate a significant contribution from both 3 as well as 2p absorption, which to our knowledge has never been demonstrated directly in cells.
For the final set of mechanistic experiments, cells were incubated with both photosensitizer and a fluorescent reporter dichlorodihydrofluorescein diacetate (DCFDA, Sigma-Aldrich, USA) to measure ROS generation (Fig. 4). At the same time, photobleaching of the photosensitizer presumed to be ROS-mediated was measured by the decrease in its fluorescence, the two fluorophores being distinguished spectrally.
Fig. 4.
Photosensitizer photobleaching (A–C and G–I) and ROS generation (D–F and G–I) during multi-photon PDT using 3 different average laser powers of 29, 42, and 55 mW at 865 nm for Visudyne (A–F) and 25, 36, and 48 mW for Oxdime (G–I). Each plot is normalized to the value following the first laser scan at a given power. The photobleaching rates (β, cm2 J−1) were calculated by fitting to a single exponential form, I = I0 e−βE. Error bars correspond to SD, n = 4 each over 50 cells.
ROS generation was seen clearly in the pigmented cells with both Visudyne and Oxdime, while only Oxdime generated significant ROS in the non-pigmented cells. Photobleaching was seen with both PS, with somewhat higher rates in the presence of melanin, especially for lower laser power. These results suggest that melanin itself contributes significantly to the ROS generation, either by direct photophysical activation, as seen in the light-only controls, and/or by energy transfer to the photosensitizer. Visudyne is a poor 2p photosensitizer so that, as previously reported in (non-pigmented) vascular endothelial cells, (24, 27) high energy density is required to achieve significant excitation. This was confirmed by the photobleaching being observed only at the highest energy density in the non-pigmented cells (Fig. 4C), while in pigmented cells similar photobleaching rates were measured for all laser powers used (Fig. 4 A–C). The approximately twofold higher Visudyne photobleaching in the melanotic than amelanotic cells suggests that the reabsorption of melanin fluorescence and/or FRET from melanin to Visudyne makes a significant contribution to the photosensitizer excitation. Oxdime, having ~600x higher 2p cross-section than Visudyne, results in a higher photobleaching rate in both cell lines (Fig. 4 G–I). Indeed, there is little difference in ROS generation (Fig. 4 J–L) between pigmented and non-pigmented cells, which is consistent with the cell viability results. Hence, the high 2p cross-section of Oxdime appears to overwhelm the contribution from melanin-mediated photochemistry that makes a significant contribution with Visudyne.
In Vivo Studies.
After conjunctival tumor growth, the photosensitizer was administered by tail-vein injection, followed by raster scanning of the focal spot of the 2p confocal microscope through a target tumor volume of approximately 400 µm × 400 µm laterally ×1,000 µm in depth (20× objective, 100 fs, 90 MHz, 865 or 910 nm for Visudyne and Oxdime, respectively, SI Appendix, Fig. S4). Examples of whole-eye immunohistochemistry sections at 24 h post-treatment are shown in SI Appendix, Fig. S5.
The tumors presented as a contiguous mass in the conjunctiva, with no evidence of TUNEL staining in the untreated or light-only controls. There was also no evidence of apoptosis or necrotic hematoxylin and eosin (H&E) cell death in the non-pigmented tumors after treatment with either photosensitizer. However, intense TUNEL staining was seen in all sections in the treated pigmented tumors with both PS. Similar results were seen in all 5 mice treated with each tumor type. These findings confirm the in solu and in vitro results and suggest melanin-mediated 2p-PDT as a unique strategy to treat pigmented lesions.
In some cohorts (N = 3), well-localized pigmented tumor enabled full-lesion treatment, and those sections are shown 48 h later in Fig. 5.
Fig. 5.
H&E-stained sections of smaller pigmented tumors at 48 h post-treatment. Each row shows different sections for the same eye and black arrows indicate the tumor. (A) light-only, (B) 2p-PDT with 0.5 mg/kg Visudyne, (C) with 0.8 mg/kg Visudyne. Although some residual tumor is still present in A and B, complete ablation is seen at the highest photosensitizer concentration (C). (Scale bar indicates 2 mm.)
The animals (N = 3) treated with 2p-PDT and 0.8 mg/kg Visudyne did not present any visible residual tumor (Fig. 5C), while residual tumors was seen at the lower Visudyne dose (Fig. 5B) and light-only (Fig. 5A). However, we note that full-volume light irradiation treatment is technically challenging since the objective focal length limits the treatment depth to ~ 2 mm with the current optical setup. Hence, only a subset of mice had tumors accessible for full treatment.
Discussion
These results demonstrate the efficacy of multi-photon PDT treatment of melanoma cells in vitro and of tumors grown in immunodeficient mice. Both the in vitro and in vivo data show that it is possible with melanotic melanoma to use a clinically- approved photosensitizer with small 2p cross-section (~<30 GM units), where the melanin serves as an intermediate. For non-pigmented tumors, such as amelanotic melanoma as well as potentially other ocular tumors such as retinoblastoma, it is likely that “designer” PS of much higher σ2p will be required, as demonstrated here with Oxdime σ2p = 17,000).
These observations suggest that there are several factors involved 2p-PDT of melanoma. In order to elucidate this systematically, we propose that the effective multiphoton photosensitizer-mediated PDT dose can be expressed as
| [1] |
where I is the incident laser fluence (Jcm−2), is the 2p cross section of the photosensitizer, [PS] is the photosensitizer concentration, and the function f relates the probability of cell death to the photodynamically effective dose. Additional factors likely contribute to cell death in the pigmented cells. The first is direct energy transfer to the photosensitizer from melanin that is activated by a combination of 2- and 3p absorption. An alternative pathway is indirect energy transfer via photosensitizer absorption of the fluorescence emitted by the melanin. Thus, cell death also depends on melanin concentration, [M], and a constant factor, κ, that is a measure of the energy transfer efficiency. Other potential contributors include ROS photogeneration in melanin leading to photosensitizer-independent multiphoton cytotoxicity, as well as cytotoxicity due to light-induced release of melanin byproducts. Since we currently have not attempted to distinguish between these two pathways, we combine them into a single constant, ξ. Thus,
| [2] |
Note that f and f’ are likely different functions corresponding to the different cell-death pathways between ROS and melanin byproducts (39, 41, 42).
Wachter et al. (43) reported that most of the light energy absorbed by melanin is converted to heat by phonon-phonon coupling but that, under specific irradiation conditions, melanin could generate phototoxic products (43). Baptista et al. (39) described the effect of UV (355 nm) and visible light (532 nm) on epithelial cell lines with different melanin content (without exogenous photosensitizer) and found that cells with lower melanin were more susceptible to UVB compared to highly pigmented cells measured by mitochondria activity, which is consistent with the UV photo-protection role of melanin. Conversely, the most pigmented cells were more sensitive to visible light. Moreover, singlet oxygen generated under visible light irradiation, as measured by phosphorescence at 1,032 nm, increased in the highly -pigmented cells, indicating a role for melanin in ROS photogeneration (39). Free radical generation, including singlet oxygen, could also be related to melanosome photodamage and account for some of the higher susceptibility of pigmented than non-pigmented melanoma to multi-photon PDT.
A third potential contributing factor arises from how melanin is synthesized. This is mediated by tyrosinase hydroxylase activity that involves tyrosine (TYR) and tyrosinase-related proteins 1 and 2 (TYR1, TYR2) being converted to 3,4-dihydroxyphenylalanine (DOPA), followed by the conversion into DOPAquinone by DOPA oxidase (38). During these processes, two main toxic byproducts are produced, namely 5,6-dihydroxyindole and 5,6-dihydroxyindole-2-carbolic acid (37). To circumvent this potential toxicity, melanin is synthesized in melanosomes, which are membrane protein-bound organelles that prevent leakage of the toxic products into the cytoplasm (38). Davids and Kleeman suggested that melanosome-targeted photosensitizer would increase the efficacy of PDT since, if the photosensitizer could penetrate the melanosomes, PDT membrane damage would cause leakage of these cytotoxic intermediates, increasing the treatment response (38). However, since these intermediates are generated in late-maturing melanin synthesis (stages 3 and 4), this effect would not be expected to increase equally in all cells in a non-synchronized population such as used here. Investigation of hypericin-mediated 1p PDT in non-pigmented and pigmented melanoma cells has suggested that, even targeting the melanosomes, PDT was more efficient in killing non-pigmented than pigmented melanoma cells, emphasizing that the leakage of the cytotoxic intermediates may be only an additive effect, but not be the main contributor the cell death (38). By contrast, in the present studies, 2p-PDT was more efficient in pigmented than non-pigmented melanoma cells in which, rather than using a melanosome-targeted photosensitizer, the light itself caused melanosome membrane breakage, as seen in the TEM images (SI Appendix, Fig. S3). Hence, direct multiphoton absorption and multiphoton-PDT had an additive effect, increasing cell death in pigmented relative to non-pigmented cells. In addition, we hypothesize that, following melanosomal membrane rupture, melanin itself may be released into the cytoplasm. This was also considered by Watanabe et al. (36). The melanin can then be excited by multiphoton absorption, emitting green fluorescence which is absorbed by the Visudyne or Oxdime, thereby also favoring multiphoton-PDT cell death in pigmented cells. This can be seen in the ROS measurements with Visudyne-mediated treatment.
Clinical translation of this modality to treat ocular tumors should be feasible. First, using an already approved photosensitizer such as Visudyne facilitates regulatory approval. For novel PS with high higher σ2p, whether molecular-based like Oxdime and others (31) or nanoparticle-based as proposed, for example, by Gao et al. (44), extensive preclinical validation (in vivo pharmacokinetics, biodistribution, toxicology) and materials scale-up and good manufacturing practice production would be required. Hence, at least for pigmented lesions that form the majority (~85%) of ocular melanomas, the melanin-mediated mechanisms shown here are advantageous. In terms of pulsed excitation to target intraocular tumors, 2p confocal laser scanning ophthalmoscopy has been demonstrated (45) and other devices are under development that would allow computer-controlled 3D scanning of the focal spot through the target volume. Although capital costs will be high and there is a relatively small clinical market, there is a significant unmet clinical need for this technology.
Conclusions
Using both pigmented and non-pigmented melanomas, as well as PS with high 2p cross-section and a conventional and well-established 1p photosensitizer with much lower higher σ2p, has led to the unexpected observation of significantly higher photocytotoxicity in pigmented than non-pigmented cells, particularly using the low σ2p photosensitizer. Subsequent investigation of this difference has revealed the key role played by melanin when using fs-pulsed laser excitation and suggests several mechanisms or pathways to account for the observed effects, including contribution from 3p absorption.
This work opens up the possibility of a unique effective, minimally-invasive, and highly targeted treatment of devastating melanomas in the eye. The use of 2p excitation allows highly conformal treatment that should minimize off-target damage to other eye structures compared to conventional therapies (including 1p PDT). This would apply also in non-pigmented ocular tumors such as retinoblastoma, a childhood disease with high rates of blindness and vision impairment post-treatment (46, 47). For non-pigmented tumors, it is likely that new high σ2p PS will be required to avoid the need for very high light doses and hence long treatment times. Finally, there is increasing evidence that PDT can up-regulate the immune system, even when treatment is localized to the tumor, leading to reduced metastases and increased survival (48). This could be important also for cancers in the eye since, for example, ~50% of ocular melanoma patients die from metastatic disease even after local treatment, including enucleation (18, 19). We are currently investigating this for cutaneous melanoma using 1p PDT and plan to evaluate its applicability in the intraocular treatment setting and for 2p activation in pigmented and non-pigmented tumors.
Materials and Methods
The studies were done using clonal B16F10 (pigmented) and B78H1 (non-pigmented) melanoma cells, both in vitro and injected into the conjunctival of nude mice. Light treatment was delivered via a 200-fs laser scanning confocal microscope. In vitro responses were assessed by PrestoBlue live/dead assay, cell morphology, photosensitizer photobleaching, and ROS fluorescence reporter quantification. In vivo responses were assessed by histopathology. Details are provided in SI Appendix, Supplementary Material.
Supplementary Material
Appendix 01 (PDF)
Acknowledgments
We thank Prof. Pier Luigi Lollini, Universitá Di Bologna, Italy, for donating the amelanotic B78H1 cells, and the EM Facility, University of Sao Paulo at Ribeirao Preto for performing the TEM preparation and imaging. This research was funded by the Princess Margaret Cancer Center Foundation Invest-in-Research Fund (B.C.W.), the Princess Margaret Cancer Center Excellence Fellowship Award (L.P.), a joint research program between Universidade de São Paulo, Brazil, and the University of Toronto, ON, Canada (CEPOF/FAPESP 2013-07276-1 and CNPq Program 305795/2016-3), Cancer Prevention and Research Institute of Texas (CPRIT, M20301556), Governor’s University Research Initiative (GURI, M230930) and CRI (02-292034). L.P. was also supported by a scholarship from the National Council of Scientific and Technological Development CNPq, Science Without Borders Program and Coordination for the Improvement of Higher Level Personnel (Capes, Brazil), and by a Banting Fellowship from the Canadian Institutes of Health Research. Additional funding was provided by the Henry Farrugia Research Fund (Y.Y.) and the Vision Science Research Program Award (S.K.).
Author contributions
L.P., Y.Y., V.S.B., C.K., and B.C.W. designed research; L.P., S.K., S.P., C.C., R.R., C.K., and B.C.W. performed research; L.P., S.P., S.K., Y.Y., V.S.B., C.K., and B.C.W. analyzed data; and L.P., S.P., S.K., Y.Y., V.S.B., C.K., and B.C.W. wrote the paper.
Competing interests
The authors declare no competing interest.
Footnotes
Reviewers: K.C., University of Pennsylvania; and A.R., Universität Ulm.
Contributor Information
Vanderlei S. Bagnato, Email: vander@ifsc.usp.ca.
Cristina Kurachi, Email: cristina@ifsc.usp.br.
Brian C. Wilson, Email: brian.wilson@uhn.ca.
Data, Materials, and Software Availability
All study data are included in the article and/or SI Appendix.
Supporting Information
References
- 1.Allison R. R., et al. , Photosensitizers in clinical PDT. Photodiagn. Photodyn. Ther. 1, 27–42 (2004). [DOI] [PubMed] [Google Scholar]
- 2.NCI, Photodynamic therapy to treat cancer (NCI, 2011) (27 January 2023). [Google Scholar]
- 3.Andersen R., Loebel N., Hammond D., Wilson M., Treatment of periodontal disease by photodisinfection compared to scaling and root planing. J. Clin. Dent. 18, 34–38 (2007). [PubMed] [Google Scholar]
- 4.O’Riordan K., et al. , Photoinactivation of Mycobacteria in vitro and in a new murine model of localized Mycobacterium bovis BCG-induced granulomatous infection. Antimicrob. Agents Chemother. 50, 1828–1834 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pires L., et al. , Translational feasibility and efficacy of nasal photodynamic disinfection of SARS-CoV-2. Sci. Rep. 12, 14438 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lei X., Liu B., Huang Z., Wu J., A clinical study of photodynamic therapy for chronic skin ulcers in lower limbs infected with Pseudomonas aeruginosa. Arch. Dermatol. Res. 307, 49–55 (2015). [DOI] [PubMed] [Google Scholar]
- 7.Verteporfin In Photodynamic Therapy Study Group, Verteporfin therapy of subfoveal choroidal neovascularization in age-related macular degeneration: Two-year results of a randomized clinical trial including lesions with occult with no classic choroidal neovascularization—verteporfin in photodynamic therapy report 2. Am. J. Ophthalmol. 131, 541–560 (2001). [DOI] [PubMed] [Google Scholar]
- 8.Bressler N. M., Bressler S. B., Photodynamic therapy with verteporfin (Visudyne): Impact on ophthalmology and visual sciences. Invest. Ophthalmol. Vis. Sci. 41, 624–628 (2000). [PubMed] [Google Scholar]
- 9.Cancer. Net, Melanoma—Statistics (13 April 2023) (2012).
- 10.Bedogni B., Powell M. B., Hypoxia, melanocytes and melanoma—Survival and tumor development in the permissive microenvironment of the skin. Pigment Cell Melanoma Res. 22, 166–174 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Corrie P., Hategan M., Fife K., Parkinson C., Management of melanoma. Br. Med. Bull. 111, 149–162 (2014). [DOI] [PubMed] [Google Scholar]
- 12.Ms T., A review of photodynamic therapy for intraocular tumors. J. Anal. Bioanal. Techn. 4, 1 (2014). [Google Scholar]
- 13.Huang Y.-Y., et al. , Melanoma resistance to photodynamic therapy: New insights. Biol. Chem. 394, 239–250 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pires L., et al. , Dual-agent photodynamic therapy with optical clearing eradicates pigmented melanoma in preclinical tumor models. Cancers 12, 1956 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Papastefanou V. P., Cohen V. M. L., Papastefanou V. P., Cohen V. M. L., Uveal melanoma. J. Skin Cancer 2011, e573974 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tarlan B., Kıratlı H., Uveal melanoma: Current trends in diagnosis and management. Turk. J. Ophthalmol. 46, 123–137 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bock F., et al. , Novel anti(lymph)angiogenic treatment strategies for corneal and ocular surface diseases. Prog. Retin. Eye Res. 34, 89–124 (2013). [DOI] [PubMed] [Google Scholar]
- 18.Singh A. D., Turell M. E., Topham A. K., Uveal melanoma: Trends in incidence, treatment, and survival. Ophthalmology 118, 1881–1885 (2011). [DOI] [PubMed] [Google Scholar]
- 19.Collaborative Ocular Melanoma Study Group, Development of metastatic disease after enrollment in the COMS trials for treatment of choroidal melanoma: Collaborative ocular melanoma study group report no. 26. Arch. Ophthalmol. 123, 1639–1643 (2005). [DOI] [PubMed] [Google Scholar]
- 20.Damato B., Kacperek A., Errington D., Heimann H., Proton beam radiotherapy of uveal melanoma. Saudi J. Ophthalmol. 27, 151–157 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mashayekhi A., et al. , Primary transpupillary thermotherapy for choroidal melanoma in 391 cases: Importance of risk factors in tumor control. Ophthalmology 122, 600–609 (2015). [DOI] [PubMed] [Google Scholar]
- 22.Yang J., Manson D. K., Marr B. P., Carvajal R. D., Treatment of uveal melanoma: Where are we now? Ther. Adv. Med. Oncol. 10, 1758834018757175 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yordi S., Soto H., Bowen R. C., Singh A. D., Photodynamic therapy for choroidal melanoma: What is the response rate? Surv. Ophthalmol. 66, 552–559 (2021). [DOI] [PubMed] [Google Scholar]
- 24.Collins H. A., et al. , Blood-vessel closure using photosensitizers engineered for two-photon excitation. Nat. Photonics 2, 420–424 (2008). [Google Scholar]
- 25.Goyan R. L., Cramb D. T., Near-infrared two-photon excitation of protoporphyrin IX: Photodynamics and photoproduct generation. Photochem. Photobiol. 72, 821–827 (2000). [DOI] [PubMed] [Google Scholar]
- 26.Songca S. P., Mbatha B., Solubilization of meso-tetraphenylporphyrin photosensitizers by substitution with fluorine and with 2,3-dihydroxy-1-propyloxy groups. J. Pharm. Pharmacol. 52, 1361–1367 (2000). [DOI] [PubMed] [Google Scholar]
- 27.Khurana M., et al. , Quantitative in vitro demonstration of two-photon photodynamic therapy using photofrin® and visudyne®. Photochem. Photobiol. 83, 1441–1448 (2007). [DOI] [PubMed] [Google Scholar]
- 28.Starkey J. R., et al. , New two-photon activated photodynamic therapy sensitizers induce xenograft tumor regressions after near-IR laser treatment through the body of the host mouse. Clin. Cancer Res. 14, 6564–6573 (2008). [DOI] [PubMed] [Google Scholar]
- 29.Shen Y., Shuhendler A. J., Ye D., Xu J.-J., Chen H.-Y., Two-photon excitation nanoparticles for photodynamic therapy. Chem. Soc. Rev. 45, 6725–6741 (2016). [DOI] [PubMed] [Google Scholar]
- 30.Zhang P., et al. , Noncovalent ruthenium(II) complexes–single-walled carbon nanotube composites for bimodal photothermal and photodynamic therapy with near-infrared irradiation. ACS Appl. Mater. Interfaces 7, 23278–23290 (2015). [DOI] [PubMed] [Google Scholar]
- 31.Khurana M., et al. , Biodistribution and pharmacokinetic studies of a porphyrin dimer photosensitizer (oxdime) by fluorescence imaging and spectroscopy in mice bearing xenograft tumors. Photochem. Photobiol. 88, 1531–1538 (2012). [DOI] [PubMed] [Google Scholar]
- 32.Verteporfin Roundtable 2000 and 2001 Participants, Treatment of age-related macular degeneration with photodynamic therapy (TAP) study group principal investigators, Verteporfin in photodynamic therapy (VIP) study group principal investigators, Guidelines for using verteporfin (visudyne) in photodynamic therapy to treat choroidal neovascularization due to age-related macular degeneration and other causes. Retina (Philadelphia, Pa.) 22, 6–18, (2002). [DOI] [PubMed] [Google Scholar]
- 33.Boguslawski J., et al. , In vivo imaging of the human eye using a 2-photon-excited fluorescence scanning laser ophthalmoscope. J. Clin. Invest. 132, e154218 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wilson B. C., Calcada C., Pires L., “Photodynamic therapy approaches for melanoma” in International Photodynamic Association Conference (International Association Conference Annals, Tampere, Finland, 2023). [Google Scholar]
- 35.Wang M., et al. , Verteporfin is a promising anti-tumor agent for cervical carcinoma by targeting endoplasmic reticulum stress pathway. Front. Oncol. 10, 1781 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Watanabe S., et al. , Comparative studies of femtosecond to microsecond laser pulses on selective pigmented cell injury in skin. Photochem. Photobiol. 53, 757–762 (1991). [DOI] [PubMed] [Google Scholar]
- 37.Simon J. D., Peles D., Wakamatsu K., Ito S., Current challenges in understanding melanogenesis: Bridging chemistry, biological control, morphology, and function. Pigment Cell Melanoma Res. 22, 563–579 (2009). [DOI] [PubMed] [Google Scholar]
- 38.Davids L. M., Kleemann B., Combating melanoma: The use of photodynamic therapy as a novel, adjuvant therapeutic tool. Cancer Treat. Rev. 2010, 466–475 (2017), 10.1016/j.ctrv.2010.11.007. [DOI] [PubMed] [Google Scholar]
- 39.Chiarelli-Neto O., et al. , Melanin photosensitization and the effect of visible light on epithelial cells. PLoS One 9, e113266 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Maranduca M. A., et al. , Synthesis and physiological implications of melanic pigments. Oncol. Lett. 17, 4183–4187 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Plonka P. M., Slominski A. T., Pajak S., Urbanska K., Transplantable melanomas in gerbils (meriones unguiculatus). II: Melanogenesis. Exp. Dermatol. 12, 356–364 (2003). [DOI] [PubMed] [Google Scholar]
- 42.Sarna M., Zadlo A., Czuba-Pelech B., Urbanska K., Nanomechanical phenotype of melanoma cells depends solely on the amount of endogenous pigment in the cells. Int. J. Mol. Sci. 19, 607 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wachter E. A., Petersen M. G., Dees C., “Photodynamic therapy with ultrafast lasers”. Proc. SPIE 3616, Commercial and Biomedical Applications of Ultrafast Lasers, 66-74 (1999).
- 44.Gao D., Agayan R. R., Xu H., Philbert M. A., Kopelman R., Nanoparticles for two-photon photodynamic therapy in living cells. Nano. Lett. 6, 2383–2386 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hunter J. J., Merigan W. H., Schallek J. B., Imaging retinal activity in the living eye. Annu. Rev. Vis. Sci. 5, 15–45 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Nahum M. P., et al. , Long-term follow-up of children with retinoblastoma. Pediatric Hematol. Oncol. 18, 173–179 (2001). [DOI] [PubMed] [Google Scholar]
- 47.Stacey A. W., et al. , The incidence of binocular visual impairment and blindness in children with bilateral retinoblastoma. Ocular Oncol. Pathol. 5, 1–7 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Falk-Mahapatra R., Gollnick S. O., Photodynamic therapy and immunity: An update. Photochem. Photobiol. 96, 550–559 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix 01 (PDF)
Data Availability Statement
All study data are included in the article and/or SI Appendix.