Significance
The innate immune system, especially the Toll pathway, plays a vital role in defending against pathogenic microorganisms, including viruses. Nevertheless, whether the classical Toll immune pathway is involved in maintaining the homeostatic process to ensure the persistent and propagative transmission of arboviruses in insect vectors remains unclear. In our study, we unveiled the molecular mechanism through which Toll-Dorsal-ZN708 (zinc finger protein 708) mediates the maintenance of homeostasis of a plant arbovirus in the insect vector. Specifically, ZN708 is a newly documented zinc finger protein targeted by Dorsal that mediates the downstream antiviral response. In this study, we also present evidence of the antiviral role of the Toll immune system in an insect vector active against plant arboviruses.
Keywords: Rice stripe virus, Laodelphax striatellus, toll immune pathway, dorsal, zinc finger protein
Abstract
Throughout evolution, arboviruses have developed various strategies to counteract the host’s innate immune defenses to maintain persistent transmission. Recent studies have shown that, in addition to bacteria and fungi, the innate Toll-Dorsal immune system also plays an essential role in preventing viral infections in invertebrates. However, whether the classical Toll immune pathway is involved in maintaining the homeostatic process to ensure the persistent and propagative transmission of arboviruses in insect vectors remain unclear. In this study, we revealed that the transcription factor Dorsal is actively involved in the antiviral defense of an insect vector (Laodelphax striatellus) by regulating the target gene, zinc finger protein 708 (LsZN708), which mediates downstream immune-related effectors against infection with the plant virus (Rice stripe virus, RSV). In contrast, an antidefense strategy involving the use of the nonstructural-protein (NS4) to antagonize host antiviral defense through competitive binding to Dorsal from the MSK2 kinase was employed by RSV; this competitive binding inhibited Dorsal phosphorylation and reduced the antiviral response of the host insect. Our study revealed the molecular mechanism through which Toll-Dorsal-ZN708 mediates the maintenance of an arbovirus homeostasis in insect vectors. Specifically, ZN708 is a newly documented zinc finger protein targeted by Dorsal that mediates the downstream antiviral response. This study will contribute to our understanding of the successful transmission and spread of arboviruses in plant or invertebrate hosts.
Innate immunity plays a crucial role in protecting vertebrates and invertebrates against various pathogens, such as bacteria, fungi, and viruses (1). Several evolutionarily conserved immune signaling pathways, including the RNA interference (RNAi), Toll, immune deficiency (IMD), and the Janus kinase-signal transducer and activator of transcription (JAK-STAT) pathways, have been identified in invertebrates (2–5). The Toll and IMD pathways, which are both NF-κB immune effector pathways, are activated because pattern recognition receptors recognize signature molecules of pathogens known as pathogen-associated molecular patterns (6–10). Key components of the canonical Toll immune pathway include the extracellular factor Spatzle (Spz), Toll-like receptors (TLRs), the ligands Tube and MyD88, the kinases Pelle and Cactus, and the transcription factors Dorsal and Dorsal-related immunity factor (Dif). In invertebrates, when pathogens infect hosts, activated Spz binds to the receptor Toll, which is followed by the recruitment of MyD88, Tube, and Pelle to form the receptor proximal oligomeric complex (11–13). In Drosophila, the complex further causes the phosphorylation and degradation of Cactus, which leads to the nuclear translocation of Dorsal and the subsequent regulation of the expression of different antimicrobial peptide genes (14). Previous studies have indicated that the Toll pathway plays a pivotal role in various biological processes, including the development of the embryonic dorsoventral axis in Drosophila, as well as in the response to antibacterial and fungal infections (15–17). Interestingly, recent studies have provided compelling evidence showing that the Toll immune pathway also has antiviral functions in both mammals and model insects (2, 18–23). In vertebrates, TLR7 and TLR13 trigger antiviral responses during viral infection (18–20). In whiteleg shrimp (Litopenaeus vannamei), the Toll-4 receptor recognizes white spot syndrome virus (WSSV) and thus participates in antiviral activities (21). In Drosophila, the Toll-7 receptor interacts with vesicular stomatitis virus (VSV) on the cytoplasmic membrane to induce autophagy (2), while the Toll pathway is activated against dengue virus in various Aedes mosquitoes (22, 23).
During evolution, RNA viruses have also developed various counterdefense strategies to combat the antiviral responses of hosts. RNA silencing suppressors (VSRs) are typical examples of proteins that inhibit host RNAi, thus facilitating the replication of RNA viruses within host cells (24, 25). Moreover, several counterdefense strategies of WSSV fight against host Toll-mediated antiviral defense. In shrimp, multiple NF-κB binding motifs were discovered in the promoters of WSSV genes, and the NF-κB system has been hypothesized to be annexed by WSSV to facilitate its replication in shrimp (26–30). In addition, during WSSV infection, the virus can induce the expression of microRNA in the host (miR-1959). This miRNA targets and inhibits the transcription of the NF-κB inhibitor Cactus, thus leading to the activation of Dorsal to promote viral replication (31).
One of the distinctive characteristics of arbovirus vectors is their ability to tolerate high levels of virus proliferation, and this adaptation plays a critical role in vector competence and disease transmission (32). While the antiviral functions of various immune signaling pathways, such as the RNAi, Toll, IMD, and JAK-STAT pathways, have been demonstrated in arboviral infections, our understanding of the mechanisms by which arboviruses maintain immune homeostasis in insect vectors is still in its early stages (33). For mosquitoes, the most important arbovirus vector, virus-derived nucleic acids effectively enhance the RNAi response mounted by mosquitoes of the genus Aedes against dengue or chikungunya infections, thus promoting the maintenance of immune homeostasis during persistent infection (34). For the plant virus vector whitefly (Bemisia tabaci), two STAT-activated effector genes (BtCD109-2 and BtCD109-3) mediate antiviral activity against tomato yellow leaf curl virus (TYLCV), whereas the coat protein (CP) of TYLCV inhibits the JAK/STAT pathway to maintain persistent viral infection (35). Another example of immune homeostasis in whiteflies is mediated by phosphatidylethanolamine-binding protein, which balances apoptosis and autophagy in B. tabaci to facilitate the transmission of TYLCV (36). Although previous studies have demonstrated that Toll plays essential roles in the antiviral response (3, 22, 23), whether this immune pathway is involved in the homeostatic process required for the persistent transmission of arboviruses in insect vectors has not been determined.
Rice stripe virus (RSV) is a negative-sense RNA virus of the genus Tenuivirus and family Phenuiviridae that causes severe disease in rice crops (37, 38). RSV is transmitted by the insect vector Laodelphax striatellus in a persistent propagative manner (39). When the virus particles are ingested by L. striatellus through feeding on RSV-infected rice plants, RSV disseminates and replicates in the midgut epithelium to establish infection. Subsequently, RSV spreads into the hemolymph and ultimately disseminates to healthy plants after dissemination into the salivary glands (SG). Moreover, RSV virions can also invade the reproductive system of L. striatellus (the ovaries and testes), facilitating vertical transmission to the offspring (39, 40). The developmental time of L. striatellus instars spans approximately 2 wk under typical rearing conditions at 25 °C and exhibiting noteworthy variability with different temperature (41, 42). It has been reported that planthopper instars are more efficient in transmitting tenuiviruses than the adults and the second instar nymph of L. striatellus is commonly used for plant virus acquisition (37, 43, 44). Moreover, persistent propagative transmitted plant virus must undergo a latent period in its insect vector before successful transmission to a healthy plant (39). A previous study indicated that the latent period of RSV ranges from 3 to 10 d (45) while a recent work precisely suggested a latent period of 4 d (96 h) for RSV in L. striatellus (46).
The genome of RSV consists of four RNA segments encoding seven proteins (47, 48). As a persistent infected plant arbovirus in planthopper, RSV and L. striatellus were used as an excellent model for studying the intricate interaction between the plant virus and insect vector. Previous studies have revealed that several antiviral immune pathways are involved in L. striatellus against plant virus infection. Abundant virus-derived small interfering RNAs were detected in L. striatellus during infection with RSV and Rice black-streaked dwarf virus (RBSDV), suggesting activation of the sRNA-mediated RNAi response in host insects (49). Moreover, activation of the c-Jun N-terminal kinase (JNK) promotes the replication and proliferation of RSV (50), whereas silencing of Atg8 inhibits the phosphorylation of the JNK protein in L. striatellus (51). In addition, RSV inhibits the activity of the PPO pathway to maintain persistent proliferation in insect hemolymph (52), and proteins in the importin α family of L. striatellus interact with the nucleocapsid protein (NP) of RSV on the cell membrane, thus promoting RSV transmission (5). A recent report indicated that the JAK-STAT pathway contributes to persistent RSV infection by activating apoptosis in L. striatellus (53). Although our previous study indicated that the Toll pathway can be activated by RSV infection through the interaction between the Toll receptor and viral NP (54), the specific details of the downstream antiviral response responsible for maintaining the persistent viral transmission remain to be explored.
In this study, we revealed that the transcription factor Dorsal was actively involved in the Toll pathway of L. striatellus by regulating the target zinc finger protein 708 (LsZN708) gene, which mediates the downstream antiviral response against RSV infection. In contrast, a counterdefense strategy was employed by RSV, in which the nonstructural protein (NS4) of the virus antagonizes host antiviral defense through competitively binding to Dorsal from the MSK2 kinase and inhibiting Dorsal phosphorylation, thus hindering the antiviral response mediated by the Toll pathway. Our results reveal the molecular mechanism by which the Toll pathway helps to maintain arbovirus homeostasis in the insect vector, which facilitates arbovirus transmission in the field.
Results
Dorsal Knockdown Promotes RSV Replication and Transmission in L. striatellus.
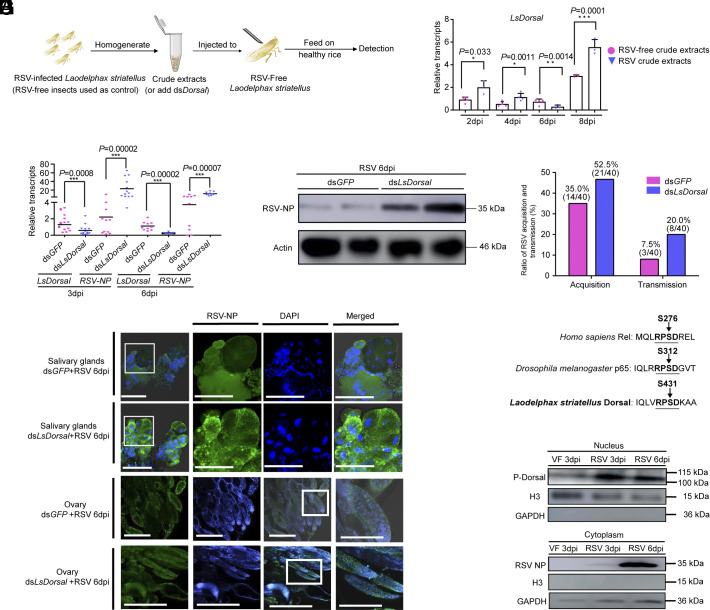
The Dorsal gene in the Toll pathway of insects plays a crucial role in immune response. Three functional domains of Dorsal in L. striatellus were predicted (RHD-n, RHD_dimer, and AidA) with NCBI conserved domain database. To elucidate the function of LsDorsal in RSV infection, RSV-free L. striatellus was injected with RSV crude extracts (derived from infected planthoppers), and the injected L. striatellus were then transferred to healthy rice seedlings to detect LsDorsal transcripts as illustrated in Fig. 1A. The results showed that the transcript level of LsDorsal significantly increased at 2, 4, and 8 days post injection (dpi), whereas a decrease in LsDorsal transcripts was observed at 6 dpi compared to that in the controls (Fig. 1B). Next, a mixture of dsLsDorsal and RSV crude extracts was injected into RSV-free L. striatellus (dsGFP and RSV crude extracts used as control). The relative transcript levels of RSV-NP were significantly increased at 3 and 6 dpi compared to control (Fig. 1C), and the increased viral abundance was further confirmed by measuring the protein levels of RSV-NP at 6 dpi (Fig. 1D). Moreover, RSV acquisition ratio was determined at 3 d after RSV-free L. striatellus was injected with the mixture of dsLsDorsal and RSV crude extracts. The results indicated that RSV acquisition ratio was increased in dsLsDorsal treated planthoppers (52.5%) compared to dsGFP control (35.0%). Subsequently, RSV transmission experiment was performed using the injected (dsLsDorsal and RSV crude extracts) instar nymph planthoppers and viral disease symptom was recorded for each of the single rice seedling 15 d after innoculation. Our results showed that the RSV transmission ratio also increased from 7.5% (dsGFP) to 20.0% (dsLsDorsal) for L. striatellus (Fig. 1E). Consistently, immunofluorescence analysis conducted using dissected SG and ovaries of L. striatellus (6 dpi) exhibited notably increased fluorescence signals of RSV-NP in both tissues of planthoppers treated with dsLsDorsal compared to that in the control (Fig. 1F). These results suggest that LsDorsal might play an essential role in inhibiting RSV replication and transmission in L. striatellus.
Fig. 1.
LsDorsal knockdown promotes RSV replication and transmission in L. striatellus. (A and B) Detection for the transcript levels of LsDorsal injected with crude extracts of RSV-free and RSV-infected L. striatellus at various time points. (C–E) Effects of LsDorsal knockdown on the expression levels of RSV-NP in L. striatellus treated with dsLsDorsal and RSV crude extracts for transcripts at 3 and 6 dpi (C), protein at 6 dpi (D), and ratio of virus acquisition (3 dpi) and transmission (E). (F) Immunofluorescence staining of RSV NP in the SG and ovary of L. striatellus at 6 dpi after treatment with dsGFP or dsLsDorsal and RSV crude extracts. (Scale bar, 50 μm.) (G) Identification of Dorsal phosphorylation site in L. striatellus based on its homologous to Rel (Homo sapiens) and p65 (Drosophila melanogaster). (H) Phosphorylation level of Dorsal protein in the nucleus of L. striatellus at different time points after RSV infection. “VF 3 dpi” indicated that nonviruliferous L. striatellus were injected with RSV-free insect crude extracts for 3 d. “RSV 3 dpi” and “RSV 6 dpi” indicated that nonviruliferous L. striatellus were injected with RSV-infected insect crude extracts for 3 d and 6 d, respectively. H3 and GAPDH antibodies represent specific marker of the cell nucleus and cytoplasm, respectively. Three biological replicates were performed for each of the experiment (10 to 15 L. striatellus for each of the replicate). The t test method was used for significance analysis. * represents significant difference (P < 0.05), ** and *** represent extremely significant difference (P < 0.01 and P < 0.001). The error bars represent the SE of the mean.
The phosphorylation and nuclear translocation of Dorsal are key steps in the activation of the Toll signaling pathway (55). To ascertain the conserved phosphorylated sites of Dorsal in L. striatellus, the predicated amino acid sequence of Dorsal in L. striatellus (LsDorsal), p65 in Drosophila melanogaster (DmDorsal), and Rel in Homo sapiens (HsDorsal) were aligned. The results indicated that the three proteins, LsDorsal, DmDorsal, and HsDorsal, possess “RPSD” phosphorylation sites, located at S431, S312, and S276, respectively (Fig. 1G). Subsequently, the phosphorylation level of LsDorsal protein (p-Dorsal) was evaluated at 3 and 6 dpi following RSV infection in the nucleus of the planthopper cells. The results demonstrated that p-Dorsal levels increased significantly in the nucleus at 3 and 6 dpi, indicating that LsDorsal was phosphorylated and subsequently transported to the nucleus during RSV infection (Fig. 1H).
LsDorsal Binds to the Promoter of the Zinc Finger Protein ZN708 and Regulates Its Expression.
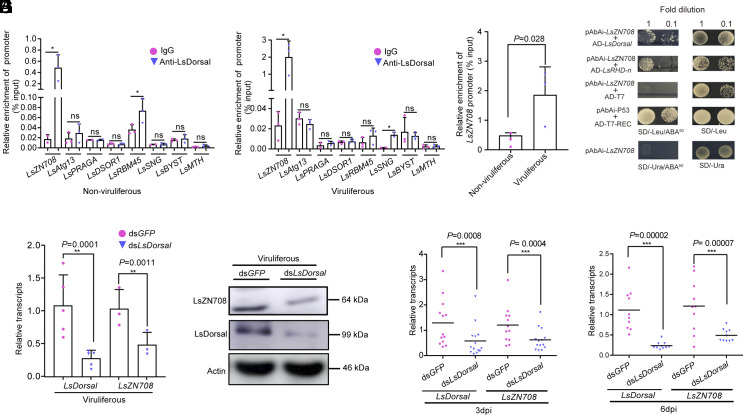
Chromatin immunoprecipitation sequencing was performed to identify the candidate target genes that interacted with LsDorsal in the nucleus. The top eight genes (Zinc Finger Protein ZN708, autophagy-related gene Atg13, RAT Ras associated guanosine triphosphate binding protein RRAGA, Bispecic mitogen-activated protein kinase DSOR1, RNA binding protein 45 RBM45, synaptophysin SNG, Bystin protein BYST, and G-protein-coupled receptors MTH) were selected (based on the q-value cutoff) and their binding ability to LsDorsal was subsequently evaluated by ChIP–qPCR and yeast one-hybrid assays. The results demonstrated that LsDorsal bound specifically to the promoter region of ZN708 in both RSV-free and RSV-infected L. striatellus compared to the control (IgG) (Fig. 2 A and B), which was widely present in diverse arthropods and mammals (SI Appendix, Fig. S1). Moreover, the binding strength of LsDorsal to LsZN708 was significantly higher in viruliferous planthoppers compared to that in the nonviruliferous planthoppers (Fig. 2C). In addition, results of yeast one-hybrid assays showed that LsDorsal and its RHD-n domain (conserved functional domain of LsDorsal) only interacted with the promoter region of LsZN708 (Fig. 2D) and did not interact with the other seven candidate genes (SI Appendix, Fig. S2). Furthermore, knockdown of LsDorsal in viruliferous L. striatellus resulted in the significantly reduced LsZN708 expression at both the transcription (Fig. 2E) and protein (Fig. 2F) level, suggesting that the expression of LsZN708 might be regulated directly by LsDorsal. Consistently, when nonviruliferous planthoppers were injected with a mixture of dsLsDorsal and RSV crude extracts, LsZN708 expression was also significantly downregulated compared to the control (dsGFP+RSV) at 3 (Fig. 2G) and 6 dpi (Fig. 2H), confirming that LsDorsal promotes the expression of LsZN708.
Fig. 2.
LsDorsal binds to the promoter of the zinc finger protein LsZN708 and regulates its expression. (A and B) Evaluation for the binding ability of LsDorsal to the promoters of eight candidate genes in RSV-free (A) and RSV-infected L. striatellus (B) by Chip-qPCR. (C) Comparative analysis for the LsDorsal binding ability to the LsZN708 promoter in nonviruliferous and viruliferous L. striatellus. (D) Verification for the binding of LsDorsal to the promoter of the Zinc Finger Protein LsZN708 using yeast one-hybrid assay. pAbAi-LsZN708 indicated that the promoter of LsZN708 was constructed to pAbAi vector. AD-LsDorsal and AD-RHD-n suggested that the full length of genes was constructed to pGAD-T7 vector. pAbAi-P53 and AD-T7-REC represented a group of positive control. pAbAi-LsZN708 and AD-T7 represented a group of negative control. ABA screening concentration was set at 50 ng/μL (inhibiting self-activation). The self-activation assay was performed on selective medium SD/-Ura. The different combinations of constructs transformed into yeast cells were grown on selective medium SD/-Leu, and interactions were detected on SD/-Leu/-ABA (50). Pictures were taken after 3 d of incubation at 30 °C. IgG antibody was used as a negative control, and three biological replicates were performed. (E and F) Effects of LsDorsal knockdown on the expression levels of transcript (E) and protein (F) of LsZN708 in the viruliferous L. striatellus injected with dsLsDorsal. (G and H) Effects of LsDorsal knockdown on the transcripts of LsZN708 in nonviruliferous L. striatellus treated with dsGFP or dsLsDorsal and RSV crude extracts at 3 and 6 dpi, respectively. Three biological replicates were performed for each experiment. The t test method was used for significance analysis. * represents significant difference (P < 0.05), ** and *** represent extremely significant difference (P < 0.01 and P < 0.001), n.s. means no significance. The error bars represent the SE of the mean.
ZN708 Knockdown Promotes RSV Replication and Transmission in L. striatellus.
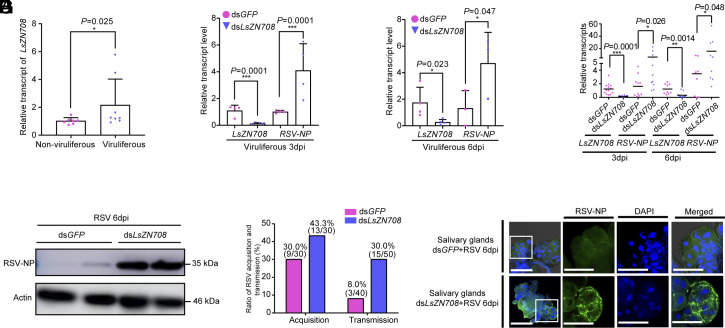
Previous studies have demonstrated that zinc finger protein are actively involved in the host antiviral process in response to various viruses (56–60). In our study, we showed that the relative transcript level of LsZN708 was significantly higher in viruliferous L. striatellus compared to that in nonviruliferous L. striatellus (Fig. 3A). Further analysis indicated that knocking down LsZN708 effectively increased the transcription of RSV-NP in viruliferous planthoppers at 3 and 6 dpi (Fig. 3 B and C). Moreover, when nonviruliferous L. striatellus were treated with a mixture of dsLsZN708 and RSV crude extracts, the relative transcription of RSV-NP was significantly higher at 3 and 6 dpi compared to the control (treated with a mixture of dsGFP and RSV crude extracts) (Fig. 3D). This result was further confirmed by measuring the protein level of RSV-NP, which was obviously increased compared to that in the control at 6 dpi (Fig. 3E).
Fig. 3.
LsZN708 knockdown promotes RSV replication and transmission in L. striatellus. (A) Transcription level of LsZN708 in nonviruliferous and viruliferous L. striatellus. (B and C) Relative transcription levels of RSV-NP in viruliferous L. striatellus treated with dsGFP or dsLsZN708 at 3 dpi and 6 dpi, respectively. (D–F) Effects of dsLsZN708 knockdown on the expression levels of RSV-NP in L. striatellus treated with dsLsZN708 and RSV crude extracts for transcripts at 3 and 6 dpi (D), protein at 6 dpi (E), and ratio of virus acquisition (3 dpi) and transmission (F). (G) Immunofluorescence staining of RSV NP in the SG of L. striatellus treated with dsLsZN708 or dsLsDorsal and RSV crude extracts at 6 dpi. The scale bar represents 50 μm. Three biological replicates were conducted for each experiment. Significance was determined using the t test method. * represents significant difference (P < 0.05), ** and *** represent extremely significant difference (P < 0.01 and P < 0.001). The error bars represent the SE of the mean.
Similarly, the effects of LsZN708 knockdown on RSV acquisition and transmission in L. striatellus were also evaluated. The results showed that both the RSV acquisition ratio (43.3%) and transmission ratio (30.0%) were increased in dsLsZN708 treated planthoppers compared to corresponding controls (dsGFP) (Fig. 3F). Additionally, immunofluorescence analysis of the dissected SG of L. striatellus (6 dpi) demonstrated increased RSV-NP fluorescence in the SG of LsZN708 knockdown planthoppers (Fig. 3G). Similar to the function of LsDorsal, these results suggest that LsZN708 also inhibits RSV replication and transmission in L. striatellus.
Regulation of Downstream Immune-Related Effectors by the Toll-Dorsal Pathway in the Antiviral Response of L. striatellus against RSV Infection.
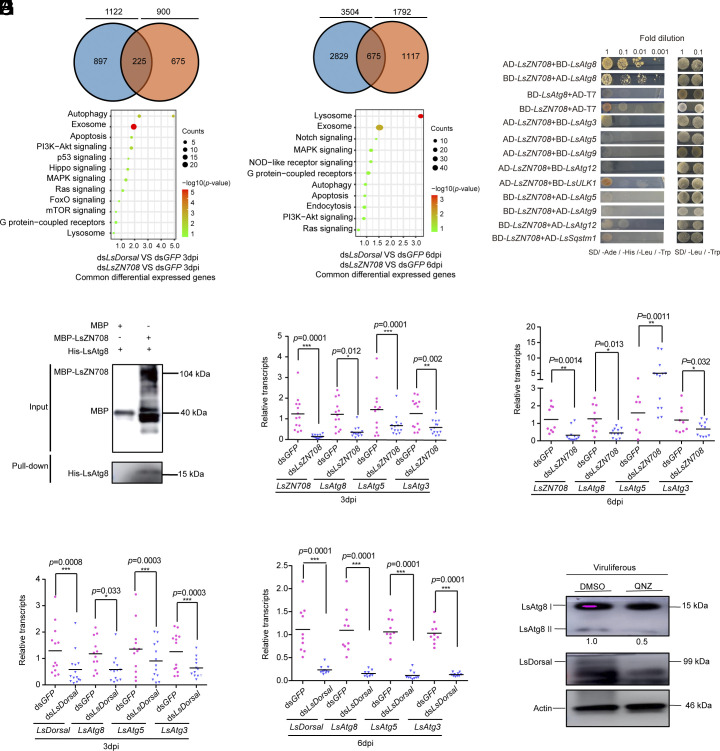
To further elucidate the specific antiviral defense mechanism mediated by Dorsal-ZN708, differentially expressed genes (DEG) analysis was performed for the nonviruliferous planthoppers at 3 and 6 dpi following injection with dsLsDorsal or dsLsZN708 and RSV crude extracts compared to the corresponding controls (a mixture of dsGFP and RSV crude extracts). The results revealed that 1,122 and 900 genes (225 common genes) were differentially expressed for dsLsDorsal and dsLsZN708 treated planthoppers compared to that in the controls at 3 dpi, respectively (Fig. 4A). When these two genes were knockdown at 6 dpi, 3,504 and 1,792 differential expressed genes (675 common genes) (Fig. 4B) were observed for dsLsDorsal and dsLsZN708 knockdown insects, respectively. KEGG pathway enrichment analysis of the common DEGs indicated that two immune-related pathways, autophagy, and exosome, were stably enriched in dsLsDorsal or dsLsZN708 treated planthoppers at both 3 and 6 dpi (Fig. 4 A and B), implying that these two pathways might play essential roles in the antiviral response to RSV infection.
Fig. 4.
Downstream immune-related effectors of toll pathway that potentially involved in the antiviral response of L. striatellus against RSV infection. (A and B) KEGG pathway enrichment analysis of the common DEG in nonviruliferous L. striatellus treated with a dsLsDorsal or dsLsZN708 and RSV crude extracts at 3 dpi (A) and 6 dpi (B). Significant differences were indicated when log2 (fold change) ratio was ≥1 and P ≤ 0.05. (C) The interaction between LsZN708 and autophagy protein LsAtg8 through Y2H assay. The different combinations of constructs transformed into yeast cells were grown on selective medium SD/-Leu/-Trp, and interactions were detected on SD/-Ade/-His/-Leu/-Trp. The images were taken after 3 d of incubation at 30 °C. (D) The interaction between LsZN708 and LsAtg8 protein through an in vitro pull-down assay. MBP-ZN708 fusion protein was used to pull-down with His-Atg8. MBP was used as negative control and His-Atg8 was further detected with anti-His antibody. (E–H) Effects of LsZN708 or LsDorsal knockdown on the transcription levels of autophagy-related genes (LsAtg8, LsAtg5 and LsAtg3) in nonviruliferous L. striatellus treated with dsLsZN708 (E and F) or dsLsDorsal (G and H) and RSV crude extracts at 3 or 6 dpi. (I) Protein level of LsAtg8II in viruliferous L. striatellus treated with QNZ inhibitor for 48 h (DMSO was used as control). Three biological replicates were performed for each experiment. Significance analysis was performed using the t test method. * represents a significant difference (P < 0.05), ** and *** represent extremely significant differences (P < 0.01 and P < 0.001). The error bars represent the SE of the mean.
Previous studies have demonstrated that the zinc finger protein-mediated autophagy pathway is essential in resistance to different viruses; the key genes of autophagy, including Atg3, Atg5, Atg8, Atg9, Atg12, ULK1, and Sqstm1, are especially important (61–63). In our study, subsequent yeast two-hybrid (Y2H) indicated that planthopper LsZN708 interacts specifically with LsAtg8 and not with the other autophagy-related proteins (Fig. 4C). Furthermore, this interaction was verified by an in vitro pull-down assay. Primarily, recombinant His-Atg8 fusion protein and MBP beads with MBP-ZN708 protein coincubated overnight at 4 °C. Then the MBP beads were washed 5 to 6 times with corresponding buffer, and detected using His-tag antibody by western blot. MBP fusion protein and His-Atg8 fusion protein were used as a negative control (Fig. 4D). Moreover, knockdown of either LsZN708 (Fig. 4 E and F) or LsDorsal (Fig. 4 G and H) resulted in significantly reduced transcription of LsAtg8, LsAtg5, and LsAtg3 in L. striatellus at both 3 and 6 dpi, suggesting regulation of the autophagy pathway by Dorsal-ZN708. Importantly, inhibition of LsDorsal following treatment with a QNZ inhibitor resulted in a remarkable reduction in LsAtg8-II protein levels in RSV-infected L. striatellus (Fig. 4I).
To further investigate the function of autophagy in RSV infection, the level of LsAtg8 transcripts was determined in L. striatellus challenged by RSV infection (injected with RSV crude extract). The results demonstrated that LsAtg8 transcripts of the insects were significantly increased at 2, 4, and 8 dpi compared to the control samples (SI Appendix, Fig. S3A). Subsequently, RSV-infected L. striatellus were treated with 3-MA (an autophagy pathway inhibitor) or Rapamycin (an autophagy pathway activator) through membrane feeding for 24 h and viral accumulation levels were determined by RT-qPCR. The results indicated that the relative transcript levels of RSV-NP was significantly increased when planthoppers were treated with 3-MA (SI Appendix, Fig. S3B) whereas decreased transcripts of RSV-NP were observed with Rapamycin treatment (SI Appendix, Fig. S3C), implying the antiviral role of autophagy pathway in L. striatellus. Furthermore, the relative transcript levels of RSV-NP exhibited a significant increase when RSV-free L. striatellus was injected with a mixture of RSV crude extracts and dsLsAtg8 at 3 and 6 dpi (SI Appendix, Fig. S3D) or dsLsAtg3 at 6 dpi (SI Appendix, Fig. S3E) compared to the control (RSV crude extracts and dsGFP). Moreover, the elevated viral abundance was also confirmed by the protein levels of RSV-NP when treated with dsLsAtg8 (SI Appendix, Fig. S3G) or dsLsAtg3 (SI Appendix, Fig. S3H) at 6 dpi. Conversely, effective silencing of LsSqstm1 (a negative regulator of the autophagy pathway) resulted in a significant decrease in the relative expression levels of RSV-NP transcripts (SI Appendix, Fig. S3F) and protein (SI Appendix, Fig. S3I). Additionally, increased or decreased protein levels of RSV-NP were observed when viruliferous L. striatellus was injected with dsLsAtg3 or dsLsSqstm1 compared to control (dsGFP) (SI Appendix, Fig. S3J), respectively, consolidating the above results. These results collectively suggest that autophagy functions as downstream antiviral effectors within the Toll pathway in planthoppers, which act against RSV infection.
It should be noted that significant enrichment of exosome pathway–related genes were observed in dsLsDorsal and dsLsZN708 knockdown planthoppers (Fig. 4 A and B), and the antiviral roles of these genes against RSV were also evaluated. The results showed that the expression of these genes was significantly downregulated when nonviruliferous L. striatellus was treated with dsLsDorsal or dsLsZN708 and RSV crude extracts (SI Appendix, Fig. S4 A and B). Interestingly, the protein level of RSV-NP was markedly decreased after the knockdown of exosome pathway–related genes (SI Appendix, Fig. S4C); this result was also verified at the transcriptional level (SI Appendix, Fig. S4 D–I). Nevertheless, determining the precise roles of the exosome pathway in the regulation of Toll-Dorsal-ZN708 antiviral immunity still requires further investigation.
Additionally, knockdown of LsToll, an upstream receptor in the Toll pathway, notably decreased the transcript levels of LsDorsal, LsZN708, and LsAtg8 in L. striatellus at both 3 and 6 dpi (SI Appendix, Fig. S5 A and B), concomitant with a significant increase of RSV accumulation at 6 dpi compared to that in the control (SI Appendix, Fig. S5B). These results consolidate our finding that the Dorsal-ZN708 mediated Toll pathway modulates the downstream antiviral response such as autophagy in L. striatellus. Furthermore, to evaluate the effects of RSV persistent infection on the survival of L. striatellus, dsRNA of LsDorsal, LsZN708, or LsAtg8 mixed with the crude extracts of viruliferous planthoppers were injected into virus-free L. striatellus (the mixture of dsGFP and the crude extracts as the control). Survival rates were recorded everyday until 13 d post injection. The results indicated that there was no significant difference in the mortality of L. striatellus treated with the dsLsDorsal mixture (SI Appendix, Fig. S6A) and limited effects on the insect mortality were observed when treated with dsLsZN708 (SI Appendix, Fig. S6B) or dsLsAtg8 (SI Appendix, Fig. S6C) compared to dsGFP. These results suggest that these key genes of Toll pathway have limited effects on the insect susceptibility to RSV infection and the downstream genes (such as LsAtg8) exhibit higher efficacy against RSV.
Non-Structural Protein of RSV (RSV NS4) Participates in the Viral Counterdefense Strategy by Inhibition LsDorsal Phosphorylation in L. striatellus.
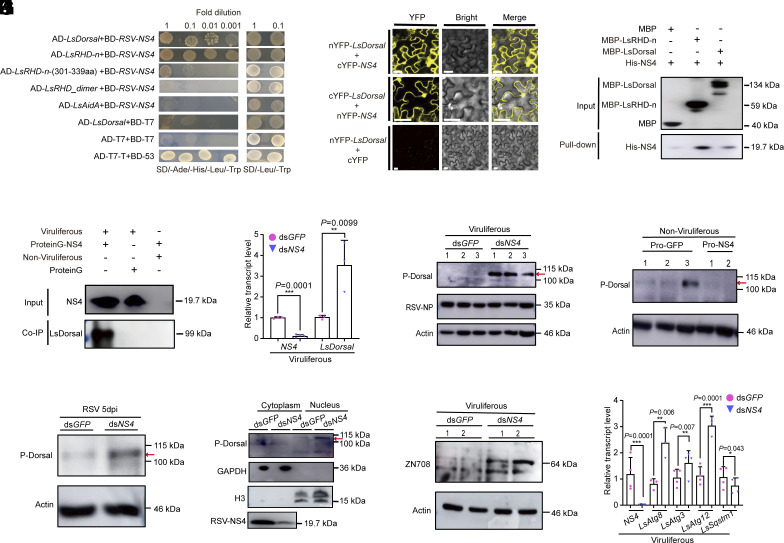
To investigate potential interactions between RSV and LsDorsal, we utilized LsDorsal and its functional domains (RHD-n, RHD_dimer, and Aida) as bait to conduct a Y2H interaction assay with RSV-encoded proteins. The results revealed that, in contrast to the other RSV proteins (SI Appendix, Fig. S7), only NS4 interacted with LsDorsal through its specific RHD-n domain (301 to 339 aa) (Fig. 5A). Bimolecular fluorescence complementation (BiFC) assays were conducted to verify this interaction in tobacco cells. There were strong Yellow fluorescent protein (YFP) fluorescence signals in the nucleus and cytoplasm when nYFP-LsDorsal and cYFP-NS4 or cYFP-LsDorsal and nYFP-NS4 were transiently coexpressed in Nicotiana benthamiana leaves, whereas no detectable signal was observed in the negative control (nYFP-LsDorsal and cYFP) (Fig. 5B). In addition, the interaction was further confirmed by an in vitro pull-down assay. Recombinant His-NS4 fusion protein and MBP beads with MBP-Dorsal or RHD-n protein were coincubated overnight at 4 °C. MBP fusion protein and His-NS4 fusion protein were used as negative control (Fig. 5C). Moreover, a coimmunoprecipitation (Co-IP) assay was performed to verify the interaction between LsDorsal and NS4 in vivo in L. striatellus. Crude extracts of L. striatellus were incubated with ProteinG-NS4 and the coimmunoprecipitated proteins were identified using Dorsal antibody (Fig. 5D). These results conclusively confirm the interaction between LsDorsal and RSV NS4 in L. striatellus.
Fig. 5.
Nonstructural protein NS4 of RSV (RSV NS4) participates in the viral counterdefense strategy through the inhibition of LsDorsal phosphorylation. (A) Interaction of RSV NS4 with LsDorsal or RHD-n domain (301 to 339 aa) of LsDorsal was confirmed by Y2H assay. (B) BIFC assays verified the interaction between LsDorsal and NS4 in the cell nucleus and cytoplasm of Nicotiana benthamiana leaves. (C) The interaction between LsDorsal and NS4 was confirmed by an in vitro pull-down assay. MBP-Dorsal and MBP-RHD-n proteins were used to pull-down with His-NS4. His-NS4 was further detected with anti-His antibody. (D) The Co-IP assay confirmed the interaction between LsDorsal and NS4 of L. striatellus in vivo. The crude extracts of L. striatellus were prepared and immunoprecipitated by ProteinG-NS4 combinations. The coimmunoprecipitated proteins were detected with LsDorsal antibody. (E and F) Effect of NS4 knockdown on the transcription (E) and phosphorylation (F) levels of LsDorsal in viruliferous L. striatellus (dsGFP as control). (G) Phosphorylation level of LsDorsal in nonviruliferous L. striatellus treated with purified NS4 protein (GFP protein as control). (H) Phosphorylation level of LsDorsal in nonviruliferous L. striatellus treated with dsNS4 and RSV crude extracts at 5 dpi. (I–K) Effect of NS4 knockdown on the protein level of p-Dorsal in the nucleus and cytoplasm (I), the protein level of LsZN708 (J), and relative transcription levels of autophagy genes LsAtg8, LsAtg3, LsAtg12 and LsSqstm1 (K) in viruliferous L. striatellus. Three biological replicates were performed for each experiment. The t test method was used for significance analysis. * represents significant difference (P < 0.05), * and *** represent extremely significant difference (P < 0.01 and P < 0.001), n.s. means no significance. The error bars represent the SE of the mean.
As the phosphorylation of Dorsal is a key regulatory step in the Toll antiviral pathway, it’s interesting to investigate whether RSV NS4 can affect the phosphorylation of LsDorsal and thus interfere with the host subsequent antiviral response activated by LsToll. The results showed that knocking down RSV NS4 in viruliferous L. striatellus resulted in an increase in both the transcript and phosphorylation levels of LsDorsal (Fig. 5 E and F). Conversely, the LsDorsal phosphorylation was clearly decreased in nonviruliferous L. striatellus when injected with purified NS4 protein of RSV compared to that in the GFP control (Fig. 5G). Additionally, the phosphorylation level of LsDorsal was considerably increased at 5 dpi following injection with dsNS4 and RSV crude extracts in nonviruliferous L. striatellus (Fig. 5H). Notably, when NS4 was knocked down in viruliferous L. striatellus, more p-Dorsal protein translocated into the nucleus compared to the cytoplasm, suggesting that NS4 could inhibit the translocation of p-Dorsal (Fig. 5I). Moreover, similar to the effects of LsDorsal, knocking down RSV NS4 in viruliferous L. striatellus led to a significant increase in the level of LsZN708 protein (Fig. 5J) and the transcription of autophagy-related genes (LsAtg8, LsAtg3 and LsAtg12) (Fig. 5K) compared to that in the dsGFP control; however, the expression for the negative regulator gene of autophagy (LsSqstm1) was significantly downregulated (Fig. 5K). In addition, NS4 knockdown also resulted in a significantly decreased viral loads of RSV at both transcription and protein levels in viruliferous L. striatellus (SI Appendix, Fig. S8). These results provided evidence that NS4 is actively involved in the counterdefense mechanism of RSV by inhibiting the phosphorylation of LsDorsal, thereby regulating the expression of LsZN708 and the downstream immune-related effectors.
RSV NS4 Competes with LsMSK2 for Dorsal Binding.
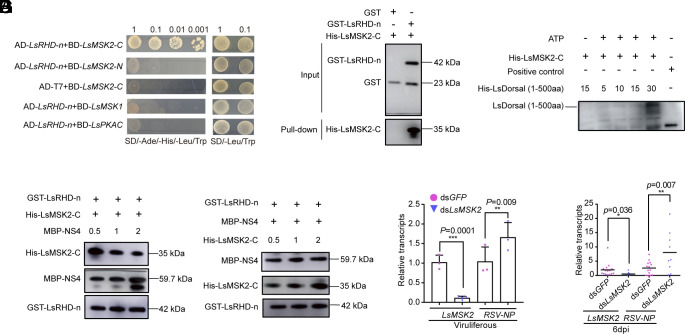
Previous studies have indicated that p65 phosphorylation can be directly targeted to the S276 site by kinases such as Mitogen- and stress-activated protein kinase-1 (MSK1), MSK2, and PKAc in mammals (64–66). In this study, MSK2 of L. striatellus (LsMSK2) was identified and the interaction between the C-terminus of LsMSK2 (C-terminus) and LsDorsal (RHD-n domain) was confirmed by Y2H assay (Fig. 6A). The interaction was further confirmed by an in vitro pull-down assay. Recombinant His-LsMSK2-C fusion protein and GST beads with GST-LsRHD-n protein were coincubated overnight at 4 °C. GST fusion protein and His-LsMSK2-C fusion protein were used as negative control (Fig. 6B). In addition, in vitro phosphorylation experiments showed that, with increasing levels of LsDorsal protein, the level of phosphorylation of LsDorsal protein by LsMSK2 increased steadily (Fig. 6C). To further investigate the binding of NS4 and LsMSK2 to LsDorsal, a competitive assay was performed. The result showed that increased concentrations of MBP-NS4 protein led to a decrease in binding to LsRHD-n and His-MSK2-C (Fig. 6D). However, the concentration of MBP-NS4 bound to LsRHD-n did not significantly change with increasing concentrations of His-MSK2-C protein (Fig. 6E). Furthermore, knockdown of LsMSK2 in viruliferous L. striatellus resulted in a significantly increase in the transcription of RSV-NP (Fig. 6F). Similarly, nonviruliferous L. striatellus injected with dsLsMSK2 and RSV crude extracts also had a significantly increased level of RSV-NP transcripts at 6 dpi (Fig. 6G). These findings suggest that the RSV NS4 protein competes with LsMSK2 for binding to LsDorsal, thereby inhibiting the phosphorylation of LsDorsal, and contributes to the counterdefense and persistent RSV infection in host insects.
Fig. 6.
RSV NS4 competes with LsMSK2 for LsDorsal binding. (A) Interaction between RHD-n domain of LsDorsal and the C-terminus of LsMSK2 kinase confirmed through Y2H assay. (B) An in vitro pull-down assay verified the interaction between LsRHD-n and C-terminus of LsMSK2. GST-RHD-n protein were used to pull-down with His-MSK2-C. His-MSK2-C was further detected with anti-His antibody. (C) The phosphorylation of LsDorsal protein by LsMSK2 kinase protein in vitro. (D) Binding ability of LsMSK2-C to LsRHD-n with the increasing concentration of NS4 protein. (E) Binding ability of NS4 to LsRHD-n with the increasing concentration of LsMSK2-C. (F) Effect of LsMSK2 knockdown on the accumulation level of RSV in viruliferous L. striatellus treated with dsGFP and dsLsMSK2. (G) Effect of LsMSK2 knockdown on the accumulation level of RSV in L. striatellus treated with dsGFP or dsLsMSK2 and RSV crude extracts at 6 dpi. Three biological replicates were performed for each experiment. The t test method was used for significance analysis. * represents significant difference (P < 0.05), ** and *** represent extremely significant difference (P < 0.01 and P < 0.001). The error bars represent the SE of the mean.
The Broad-Spectrum Antiviral Roles of the Toll Pathway against other Rice Viruses in Planthoppers.
To further investigate the potential antiviral role of the canonical Toll pathway in other arboviruses and insect vectors, the combination of L. striatellus and RBSDV, as well as another rice planthopper species (Nilaparvata lugens) and its transmitted virus (Rice ragged stunt virus, RRSV), were evaluated in this study. The results indicated that the LsRHD-n domain of LsDorsal can also interact with the P10 protein of RBSDV in L. striatellus, as evidenced by the Y2H assay (SI Appendix, Fig. S9A). In addition, the interaction was verified by an in vitro pull-down assay which included recombinant GST-RHD-n and His-RBSDV-P10 fusion protein. The combination of GST and His-RBSDV-P10 fusion protein was used as the negative control (SI Appendix, Fig. S9B). Additionally, the relative expression of LsDorsal was significantly upregulated when nonviruliferous L. striatellus was injected with RBSDV crude extracts at 4 dpi (SI Appendix, Fig. S9C). Similar to the results of RSV, after nonviruliferous insects were treated with dsLsDorsal and RBSDV crude extracts at 6 dpi, the relative transcription of RBSDV-P10 increased significantly (SI Appendix, Fig. S9D), whereas a significant decrease in the level of LsZN708 transcripts was observed in L. striatellus (SI Appendix, Fig. S9E). Notably, various expression levels of autophagy-related genes were observed after treatment, with decreased level of LsAtg5, increased level of LsAtg8, and no significant change for LsAtg3 (SI Appendix, Fig. S9E); this result implies that the downstream immune-related effectors of the Toll pathway might be diverse for L. striatellus following challenges by different RNA viruses.
Additionally, for the combination of N. lugens and RRSV, an interaction assay was conducted between RRSV proteins and Toll or Dorsal of N. lugens. Our results showed that only a specific region of NlToll, 96-891 aa, interacted with the P8 protein of RRSV in N. lugens (SI Appendix, Fig. S10A), but not for NlDorsal (SI Appendix, Fig. S10 E–G) or the other region of NlToll (SI Appendix, Fig. S10D). Furthermore, the relative transcript level of RRSV P8 significantly increased in N. lugens injected with a mixture of dsNlDorsal or dsNlToll and RRSV crude extracts at 6 dpi compared to the dsGFP control (SI Appendix, Fig. S10 B and C), suggesting that RRSV P8 might also activate the antiviral Toll pathway in N. lugens by interacting with the NlToll protein.
Discussion
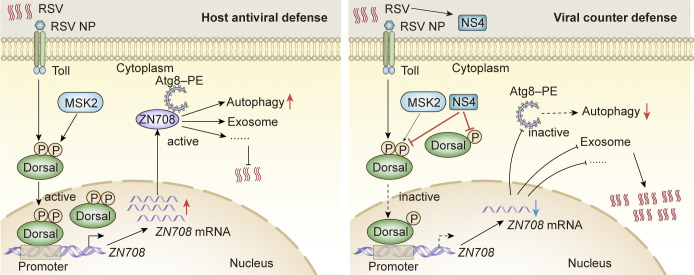
Accumulating evidence has indicated that the innate Toll-Dorsal immune system plays a crucial role in resisting viral infections in invertebrates such as Drosophila and mosquitoes (3, 21–23, 67). Throughout evolution, arboviruses have evolved different antidefense strategies that target the innate immune system of the hosts to maintain persistent transmission (32, 33, 36). Nevertheless, the precise roles of the classical Toll immune pathway in sustaining the persistent and propagative transmission of arboviruses in insect vectors remains unclear. Our previous results showed that the Toll immune pathway was successfully activated and participated in the antiviral process by directly binding the RSV-NP to the LsToll receptor of L. striatellus (54). In the present study, as illustrated in the schematic diagram (Fig. 7), we revealed the molecular mechanism of the Toll-Dorsal pathway mediated homeostasis in the persistent transmission of a plant arbovirus in its insect vector. LsDorsal played a role in the antiviral defense of L. striatellus by regulating a new target gene, LsZN708, thus inducing the production of downstream immune response pathways against RSV infection, such as the autophagy and exosome pathways. In contrast, RSV also developed an antidefense strategy by using its NS4 protein to antagonize the host Toll antiviral defense by competitively binding LsDorsal with LsMSK2 kinase to inhibit LsDorsal phosphorylation (Fig. 7).
Fig. 7.
The schematic diagram indicating toll-dorsal pathway mediated homeostasis for the persistent transmission of RSV in L. striatellus. The Toll pathway is activated by the interaction between the Toll receptor of L. striatellus and RSV NP. Then, Dorsal participated in the antiviral defense of L. striatellus by regulating the target gene LsZN708 and induced the downstream immune response pathways (such as autophagy) against RSV infection (Left). Conversely, RSV also developed antidefense strategy by using its NS4 protein to antagonize the host Toll antiviral defense through competitively binding to LsDorsal with LsMSK2 kinase, resulting in the inhibition of LsDorsal phosphorylation and translocation to the nucleus (Right).
The transcription factor Dorsal is a key component in the Toll immune pathway, and Dorsal plays a critical role in resisting virus infections (21, 29, 67, 68). A previous study conducted in L. Vannamei suggested that knockdown of Dorsal resulted in a significant increase in the accumulation of WSSV (21). In Drosophila, mutation of Dorsal and Dif increased the accumulation of Drosophila C Virus in viral oral infection and led to a higher mortality rate in the host insect (67). In A. albopictus, knockdown of the transcription factor Rel1 increased the accumulation of DEV-2 (69). Our results showed that the transcript level of LsDorsal increased significantly in nonviruliferous L. striatellus injected with RSV crude extracts at 2, 4, and 8 dpi (Fig. 1 A and B). In addition, knockdown of LsDorsal resulted in an increase in the accumulation of RSV and RSV acquisition and transmission in L. striatellus (Fig. 1 C–H). These findings confirmed that Dorsal in L. striatellus plays an essential role against RSV infection, which is consistent with the antiviral functions of other members in the NF-κB family (e.g., Dif, or Rel1) (67, 70). Interestingly, after 6 dpi, the transcript level of LsDorsal decreased significantly upon injection with RSV crude extracts (Fig. 1B); this response may be related to RSV counterdefense, which helps to maintain its proliferation in L. striatellus.
The Dorsal/REL regulate the transcription of AMPs by binding to the κB sites of their promoter regions in the nucleus, thereby inducing the production of various immune effectors. In Drosophila, the promoter activity of AMP genes can be activated by the Rel homology domain (RHD) of Dorsal, Dif, and Relish (71), while for L. Vannamei, Dorsal binds to the promoters of the AMP genes (ALF1 and LYZ1) to regulate their transcription (21). Additionally, the relish of mosquito has been demonstrated to bind to the κB sites of promoters of AMP genes (72, 73). Whole genome analysis has been utilized to identify over 40 target genes of Dorsal that encode a wide range of cell signaling proteins and transcription factors (68). Interestingly, rather than the commonly reported AMP genes, our results revealed that Dorsal of L. striatellus bound to the promoter of a zinc finger protein, LsZN708, and increased its transcription and protein levels (Fig. 2). Moreover, the binding of LsDorsal to the promoter of LsZN708 in viruliferous L. striatellus was significantly higher than that in nonviruliferous L. striatellus (Fig. 2C), and subsequent experiments indicated that the expression of LsZN708 might be regulated directly by Dorsal in L. striatellus (Fig. 2 D–H). These findings suggest that infection by different viruses might result in evolutionary variations in target effector genes of the Toll immune pathway, consequently leading to the binding of distinct promoter regions by Dorsal in the nucleus of host insects. The zinc finger antiviral proteins (ZAPs) are mammalian host restriction factors that inhibit the replication of various viruses through recruiting the exosome to degrade the substrate of viruses including alphaviruses (57, 59), filoviruses (58), retroviruses (56), orthohepadnaviruses (60). Consistently, knockdown of LsZN708 led to a significant increase in the accumulation of RSV, as well as an increase in RSV acquisition and transmission in L. striatellus (Fig. 3), suggesting that LsZN708 plays an important role against RSV infection.
In addition to the reported exosome that was recruited by ZAPs to degrade viral RNA (74), autophagy has also been demonstrated to be a downstream signaling pathway of the Toll immune system against viral infection. A previous study showed that MyD88-mediated autophagy can be induced by TLR7 to eliminate intracellular microbes (75). Furthermore, the accumulation of VSV was effectively inhibited through the autophagy pathway mediated by Toll7 in Drosophila (76). Interestingly, our KEGG analysis also showed that autophagy and exosome are stably enriched in LsDorsal and LsZN708 knockdown L. striatellus (Fig. 4 A and B), and subsequent experiments suggested that autophagy act as an important downstream antiviral effector to limit the accumulation of RSV (Fig. 4 C–F and SI Appendix, Fig. S3); however, determining the roles of exosome in combating RSV infection will require further investigation (SI Appendix, Fig. S4). Moreover, a recent study has demonstrated that ZAPs regulate autophagy in cells and induce the degradation of viral RNA (61). Therefore, our results provided reliable evidence that Dorsal-ZN708 regulates immune effectors (i.e., autophagy) against RSV infection. The Dorsal-ZN708-autophagy mediated Toll antiviral pathway was also identified in L. striatellus infected with another plant virus, RBSDV (SI Appendix, Fig. S9), suggesting that this pathway might be a general antiviral mechanism in L. striatellus.
The coevolution of arboviruses and their hosts has led to the development of various viral counterdefense strategies and some viral proteins, such as VSRs, are used to inhibit the antiviral mechanisms of the hosts, thereby allowing the maintenance of persistent infection. For example, a viral protein of Barley stripe mosaic virus disrupts vacuolar acidification and inhibits host autophagy degradation to facilitate infection in plants (77). Moreover, the JAK/STAT immune pathway regulates the balance between whiteflies and geminiviruses. The CP of TYLCV interacts with STAT to inhibit its nuclear translocation and promote accumulation and transmission of TYLCV (35). In our study, a RSV counterdefense strategy in L. striatellus involves competitive binding of the viral protein NS4 to host LsDorsal with LsMSK2 kinase to inhibit Dorsal phosphorylation (Figs. 5 and 6), thus the downstream antiviral response is inhibited. The phosphorylation of Dorsal plays a critical role in regulating the transcription of target genes in the nucleus (55). In mammals, the key phosphorylation sites for p65 are S276 (RPSD), S468, and S536 (66, 78, 79). In contrast, the highly conserved serine site S312 is essential for Dorsal phosphorylation in Drosophila (55). Furthermore, the serine sites of Dorsal also play a role in the antiviral mechanism of L. Vannamei (21). In this study, comparative analysis indicated that Dorsal of L. striatellus also has a conserved serine site of “RPSD” at S431 (Fig. 1G) and protein level of phosphorylated Dorsal increased following RSV challenge (Fig. 1H), implying the conserved antiviral function of LsDorsal at this site of “RPSD”. Additionally, RSV NS4 interacted with LsDorsal and NS4 knockdown resulted in a significant increase in the phosphorylation of LsDorsal in viruliferous L. striatellus (Fig. 5 A–I), thereby increasing the expression of LsZN708 and activating autophagy (Fig. 5 J and K); this result suggests that the Dorsal-ZN708 antiviral pathway in L. striatellus was hijacked by RSV through the viral protein NS4. Phosphokinases, such as MSK1 and MSK2, are involved in the phosphorylation of serine site S276 in the p65 of mammalian cells (80). In this study, LsMSK2 was successfully identified in L. striatellus and the interaction between LsMSK2 and LsDorsal was confirmed; however, LsDorsal was then competitively bound by RSV NS4 (Fig. 6 A–E), indicating that the phosphorylation of Dorsal may vary in response to different viruses within the hosts.
In summary, the mechanism underlying Toll mediated homeostasis to maintain a persistent and propagative transmitted plant arbovirus in L. striatellus was revealed in this study (Fig. 7). These results will contribute to a better understanding of the arms race between arboviruses and insect vectors during coevolution. However, further investigation is necessary to elucidate the specific roles of autophagy-related genes and to understand the collaboration between autophagy and other downstream immune effectors (such as exosomes) in sustaining persistent infections of arboviruses in insect vectors.
Materials and Methods
A full description of materials and methods, including RSV-Infected and RSV-Free Insect Materials, dsRNA Microinjection, qRT-PCR, ChIP-qPCR, Immunohistochemistry, Y2H, Antibody Preparation, Pull-Down, Western Blotting, Co-IP, BiFC, and RSV Acquisition/Transmission, is provided in SI Appendix, Supplemental Materials and Methods. Primers used in this study are listed in SI Appendix, Table S1.
Supplementary Material
Appendix 01 (PDF)
Acknowledgments
We thank Professor Zong-Tao Sun (Ningbo University, China) for his valuable and constructive suggestions for improving the manuscript. This work was supported by the National Natural Science Foundation of China (U20A2036, 32270146), the National Key Research and Development Plan in the 14th five-year plan (2021YFD1401100: H.J.-H. and C.-X.Z.), and K. C. Wong Magna Fund in Ningbo University.
Author contributions
G.L., B.-J.X., C.-X.Z., J.-P.C., and J.-M.L. designed research; Y.-J.H., Q.-Z.M., Y.-H.Q., G.-Y.J., H.-T.W., and Y.-Z.T. performed research; H.-J.H. and J.-M.L. analyzed data; and Y.-J.H., B.-J.X., J.-P.C., and J.-M.L. wrote the paper.
Competing interests
The authors declare no competing interest.
Footnotes
This article is a PNAS Direct Submission. N.S. is a guest editor invited by the Editorial Board.
Contributor Information
Chuan-Xi Zhang, Email: chxzhang@zju.edu.cn.
Jian-Ping Chen, Email: jianpingchen@nbu.edu.cn.
Jun-Min Li, Email: lijunmin@nbu.edu.cn.
Data, Materials, and Software Availability
All study data are included in the article and/or SI Appendix.
Supporting Information
References
- 1.Janeway C. A. Jr., Medzhitov R., Innate immune recognition. Annu. Rev. Immunol. 20, 197–216 (2002). [DOI] [PubMed] [Google Scholar]
- 2.Nakamoto M., et al. , Virus recognition by Toll-7 activates antiviral autophagy in Drosophila. Immunity 36, 658–667 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zambon R. A., Nandakumar M., Vakharia V. N., Wu L. P., The Toll pathway is important for an antiviral response in Drosophila. Proc. Natl. Acad. Sci. U.S.A. 102, 7257–7262 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bang I. S., JAK/STAT signaling in insect innate immunity. Entomol. Res. 49, 339–353 (2019). [Google Scholar]
- 5.Zhao W., et al. , The nucleocapsid protein of rice stripe virus in cell nuclei of vector insect regulates viral replication. Protein Cell. 13, 360–378 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Debnath L. R., Prem R., Achintya K., The mechanisms of innate immunity in insects. BN Seal J. Sci. 9, 146–159 (2017). [Google Scholar]
- 7.Gay N. J., Gangloff M., Structure and function of Toll receptors and their ligands. Annu. Rev. Biochem. 76, 141–165 (2007). [DOI] [PubMed] [Google Scholar]
- 8.Hultmark D., Drosophila immunity: Paths and patterns. Curr. Opin. Immunol. 15, 12–19 (2003). [DOI] [PubMed] [Google Scholar]
- 9.Palmer W. H., Hadfield J. D., Obbard D. J., RNA-interference pathways display high rates of adaptive protein evolution in multiple invertebrates. Genetics 208, 1585–1599 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kumar H., Kawai T., Akira S., Pathogen recognition in the innate immune response. Biochem. J. 420, 1–16 (2009). [DOI] [PubMed] [Google Scholar]
- 11.Horng T., Medzhitov R., Drosophila MyD88 is an adapter in the Toll signaling pathway. Proc. Natl. Acad. Sci. U.S.A. 98, 12654–12658 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Towb P., Bergmann A., Wasserman S. A., The protein kinase Pelle mediates feedback regulation in the Drosophila Toll signaling pathway. Development 23, 4729–4736 (2001). [DOI] [PubMed] [Google Scholar]
- 13.Sun H., Bristow B. N., Qu G., Wasserman S. A., A heterotrimeric death domain complex in Toll signaling. Proc. Natl. Acad. Sci. U.S.A. 99, 12871–12876 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lemaitre B., Nicolas E., Michaut L., Reichhart J.-M., Hoffmann J. A., The dorsoventral regulatory gene cassette spätzle/Toll/cactus controls the potent antifungal response in Drosophila adults. Cell 86, 973–983 (1996). [DOI] [PubMed] [Google Scholar]
- 15.Moussian B., Roth S., Dorsoventral axis formation in the Drosophila embryo—shaping and transducing a morphogen gradient. Curr. Biol. 15, R887–R899 (2005). [DOI] [PubMed] [Google Scholar]
- 16.Lemaitre B., The road to Toll. Nat. Rev. Immunol. 4, 521–527 (2004). [DOI] [PubMed] [Google Scholar]
- 17.Michel T., Reichhart J.-M., Hoffmann J. A., Royet J., Drosophila Toll is activated by Gram-positive bacteria through a circulating peptidoglycan recognition protein. Nature 414, 756–759 (2001). [DOI] [PubMed] [Google Scholar]
- 18.Crozat K., Beutler B., TLR7: A new sensor of viral infection. Proc. Natl. Acad. Sci. U.S.A. 101, 6835–6836 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Diebold S. S., Kaisho T., Hemmi H., Akira S., C. Reis e Sousa, Innate antiviral responses by means of TLR7-mediated recognition of single-stranded RNA. Science 303, 1529–1531 (2004). [DOI] [PubMed] [Google Scholar]
- 20.Shi Z., et al. , A novel Toll-like receptor that recognizes vesicular stomatitis virus. J. Biol. Chem. 286, 4517–4524 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Li H., et al. , RNAi screening identifies a new Toll from shrimp Litopenaeus vannamei that restricts WSSV infection through activating Dorsal to induce antimicrobial peptides. PLoS Pathog. 14, e1007109 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ramirez J. L., Dimopoulos G., The Toll immune signaling pathway control conserved anti-dengue defenses across diverse Ae. aegypti strains and against multiple dengue virus serotypes. Dev. Comp. Immunol. 34, 625–629 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Xi Z., Ramirez J. L., Dimopoulos G., The Aedes aegypti toll pathway controls dengue virus infection. PLOS Pathog. 4, e1000098 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.O’Neal S. T., Samuel G. H., Adelman Z. N., Myles K. M., Mosquito-borne viruses and suppressors of invertebrate antiviral RNA silencing. Viruses 6, 4314–4331 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Li F., Wang A., RNA-targeted antiviral immunity: More than just RNA silencing. Trends Microbiol. 27, 792–805 (2019). [DOI] [PubMed] [Google Scholar]
- 26.Huang X.-D., et al. , Shrimp NF-κB binds to the immediate-early gene ie1 promoter of white spot syndrome virus and upregulates its activity. Virology 406, 176–180 (2010). [DOI] [PubMed] [Google Scholar]
- 27.Qiu W., et al. , Identification, characterization, and function analysis of the NF-κB repressing factor (NKRF) gene from Litopenaeus vannamei. Dev. Comp. Immunol. 76, 83–92 (2017). [DOI] [PubMed] [Google Scholar]
- 28.Qiu W., et al. , Litopenaeus vannamei NF-κB is required for WSSV replication. Dev. Comp. Immunol. 45, 156–162 (2014). [DOI] [PubMed] [Google Scholar]
- 29.Ren Q., et al. , Two white spot syndrome virus microRNAs target the dorsal gene to promote virus infection in Marsupenaeus japonicus shrimp. J. Virol. 91, e02261-16 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chen Y. H., et al. , Two Litopenaeus vannamei HMGB proteins interact with transcription factors LvSTAT and LvDorsal to activate the promoter of white spot syndrome virus immediate-early gene ie1. Mol. Immunol. 48, 793–799 (2011). [DOI] [PubMed] [Google Scholar]
- 31.Zuo H., et al. , A microRNA-mediated positive feedback regulatory loop of the NF-κB pathway in Litopenaeus vannamei. J. Immunol. 196, 3842–3853 (2016). [DOI] [PubMed] [Google Scholar]
- 32.Lambrechts L., Saleh M.-C., Manipulating mosquito tolerance for arbovirus control. Cell Host Microbe 26, 309–313 (2019). [DOI] [PubMed] [Google Scholar]
- 33.Oliveira J. H., Bahia A. C., Vale P. F., How are arbovirus vectors able to tolerate infection? Dev. Comp. Immunol. 103, 103514 (2020). [DOI] [PubMed] [Google Scholar]
- 34.Goic B., et al. , Virus-derived DNA drives mosquito vector tolerance to arboviral infection. Nat. Commun. 7, 12410 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wang Y. M., et al. , A balance between vector survival and virus transmission is achieved through JAK/STAT signaling inhibition by a plant virus. Proc. Natl. Acad. Sci. U.S.A. 119, e2122099119 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wang S., Guo H., Zhu-Salzman K., Ge F., Sun Y., PEBP balances apoptosis and autophagy in whitefly upon arbovirus infection. Nat. Commun. 13, 846 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Falk B. W., Tsai J. H., Biology and molecular biology of viruses in the genus Tenuivirus. Annu. Rev. Phytopathol. 36, 139–163 (1998). [DOI] [PubMed] [Google Scholar]
- 38.Wei T.-Y., et al. , Genetic diversity and population structure of rice stripe virus in China. J. Gen. Virol. 90, 1025–1034 (2009). [DOI] [PubMed] [Google Scholar]
- 39.Hogenhout S. A., E.-D. Ammar, A. E. Whitfield, M. G. Redinbaugh, Insect vector interactions with persistently transmitted viruses. Annu. Rev. Phytopathol. 46, 327–359 (2008). [DOI] [PubMed] [Google Scholar]
- 40.Xu Y., Fu S., Tao X., Zhou X., Rice stripe virus: Exploring molecular weapons in the arsenal of a negative-sense RNA virus. Annu. Rev. Phytopathol. 59, 351–371 (2021). [DOI] [PubMed] [Google Scholar]
- 41.Xiao L., Huang L.-L., He H.-M., Xue F.-S., Tang J.-J., Life history responses of the small brown planthopper Laodelphax striatellus to temperature change. J. Therm Biol. 115, 103626 (2023). [DOI] [PubMed] [Google Scholar]
- 42.Huang H.-J., et al. , Planthopper salivary sheath protein LsSP1 contributes to manipulation of rice plant defenses. Nat. Commun. 14, 737 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Li L., Li H., Dong H., Wang X., Zhou G., Transmission by Laodelphax striatellus Fallen of Rice black-streaked dwarf virus from frozen infected rice leaves to healthy plants of rice and maize. J. Phytopathol. 159, 1–5 (2011). [Google Scholar]
- 44.Moya Fernández M. B., Liu W., Zhang L., Hajano J.-U.-D., Wang X., Interplay of rice stripe virus and rice black streaked dwarf virus during their acquisition and accumulation in insect vector. Viruses 13, 1121 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hsieh C., Transmission of rice stripe virus by Laodelphax striatellus Fallen in Taiwan. Plant Protect. Bull. 15, 153–162 (1973). [Google Scholar]
- 46.Zhao W., Yang P., Kang L., Cui F., Different pathogenicities of Rice stripe virus from the insect vector and from viruliferous plants. New Phytol. 210, 196–207 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kakutani T., Hayano Y., Hayashi T., Minobe Y., Ambisense segment 4 of rice stripe virus: Possible evolutionary relationship with phleboviruses and uukuviruses (Bunyaviridae). J. Gen. Virol. 71, 1427–1432 (1990). [DOI] [PubMed] [Google Scholar]
- 48.Hayano Y., Kakutani T., Hayashi T., Minobe Y., Coding strategy of rice stripe virus: Major nonstructural protein is encoded in viral RNA segment 4 and coat protein in RNA complementary to segment 3. Virology 177, 372–374 (1990). [DOI] [PubMed] [Google Scholar]
- 49.Li J., et al. , Characterization of rice black-streaked dwarf virus- and rice stripe virus-derived siRNAs in singly and doubly infected insect vector Laodelphax striatellus. PLoS One 8, e66007 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wang W., et al. , The c-Jun N-terminal kinase pathway of a vector insect is activated by virus capsid protein and promotes viral replication. eLife 6, e26591 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Yu Y. L., et al. , Laodelphax striatellus Atg8 facilitates Rice stripe virus infection in an autophagy-independent manner. Insect Sci. 28, 315–329 (2021). [DOI] [PubMed] [Google Scholar]
- 52.Chen X., et al. , A plant virus ensures viral stability in the hemolymph of vector insects through suppressing prophenoloxidase activation. Mbio 11, e01453-20 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zhang Y., et al. , The JAK-STAT pathway promotes persistent viral infection by activating apoptosis in insect vectors. PLoS Pathog. 19, e1011266 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.He Y. J., et al. , Activation of toll immune pathway in an insect vector induced by a plant virus. Front. Immunol. 11, 613957 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Drier E. A., Huang L. H., Steward R., Nuclear import of the Drosophila Rel protein Dorsal is regulated by phosphorylation. Genes Dev. 13, 556–568 (1999). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lee H., et al. , Zinc-finger antiviral protein mediates retinoic acid inducible gene I–like receptor-independent antiviral response to murine leukemia virus. Proc. Natl. Acad. Sci. U.S.A. 110, 12379–12384 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kozaki T., et al. , Role of zinc-finger anti-viral protein in host defense against Sindbis virus. Int. Immunol. 27, 357–364 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Müller S., et al. , Inhibition of filovirus replication by the zinc finger antiviral protein. J. Virol. 81, 2391–2400 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Bick M. J., et al. , Expression of the zinc-finger antiviral protein inhibits alphavirus replication. J. Virol. 77, 11555–11562 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Mao R., et al. , Inhibition of hepatitis B virus replication by the host zinc finger antiviral protein. PLoS Pathog. 9, e1003494 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Viret C., Duclaux-Loras R., Nancey S., Rozières A., Faure M., Selective autophagy receptors in antiviral defense. Trends Microbiol. 29, 798–810 (2021). [DOI] [PubMed] [Google Scholar]
- 62.Chauhan S., et al. , ZKSCAN3 Is a Master Transcriptional Repressor of Autophagy (Cell Press, 2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Wang L.-L., et al. , The autophagy pathway participates in resistance to tomato yellow leaf curl virus infection in whiteflies. Autophagy 12, 1560–1574 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Vermeulen L., De Wilde G., Van Damme P., Berghe W. V., Haegeman G., Transcriptional activation of the NF-κB p65 subunit by mitogen-and stress-activated protein kinase-1 (MSK1). EMBO J. 22, 1313–1324 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Jacks K. A., Koch C. A., Differential regulation of mitogen-and stress-activated protein kinase-1 and-2 (MSK1 and MSK2) by CK2 following UV radiation. J. Biol. Chem. 285, 1661–1670 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Okazaki T., et al. , Phosphorylation of serine 276 is essential for p65 NF-κB subunit-dependent cellular responses. Biochem. Biophys. Res. Commun. 300, 807–812 (2003). [DOI] [PubMed] [Google Scholar]
- 67.Ferreira A. G., et al. , The Toll-dorsal pathway is required for resistance to viral oral infection in Drosophila. PLoS Pathog. 10, e1004507 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Zehavi Y., Kuznetsov O., Ovadia-Shochat A., Juven-Gershon T., Core promoter functions in the regulation of gene expression of Drosophila dorsal target genes. J. Biol. Chem. 289, 11993–12004 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Wei Y., et al. , Vector competence for DENV-2 among Aedes albopictus (Diptera: Culicidae) populations in China. Front. Cell Infect. Microbiol. 11, 649975 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kingsolver M. B., Huang Z., Hardy R. W., Insect antiviral innate immunity: Pathways, effectors, and connections. J. Mol. Biol. 425, 4921–4936 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Chowdhury M., et al. , An in vitro study of NF-κB factors cooperatively in regulation of Drosophila melanogaster antimicrobial peptide genes. Dev. Comp. Immunol. 95, 50–58 (2019). [DOI] [PubMed] [Google Scholar]
- 72.Shin S. W., et al. , REL1, a homologue of Drosophila dorsal, regulates toll antifungal immune pathway in the female mosquito Aedes aegypti. J. Biol. Chem. 280, 16499–16507 (2005). [DOI] [PubMed] [Google Scholar]
- 73.Shin S. W., Kokoza V., Ahmed A., Raikhel A. S., Characterization of three alternatively spliced isoforms of the Rel/NF-κB transcription factor Relish from the mosquito Aedes aegypti. Proc. Natl. Acad. Sci. U.S.A. 99, 9978–9983 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Guo X., Ma J., Sun J., Gao G., The zinc-finger antiviral protein recruits the RNA processing exosome to degrade the target mRNA. Proc. Natl. Acad. Sci. U.S.A. 104, 151–156 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Delgado M. A., Elmaoued R. A., Davis A. S., Kyei G., Deretic V., Toll-like receptors control autophagy. EMBO J. 27, 1110–1121 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Shelly S., Lukinova N., Bambina S., Berman A., Cherry S., Autophagy is an essential component of Drosophila immunity against vesicular stomatitis virus. Immunity 30, 588–598 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Yang M., et al. , A viral protein disrupts vacuolar acidification to facilitate virus infection in plants. EMBO J. 41, e108713 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Hu J., Nakano H., Sakurai H., Colburn N. H., Insufficient p65 phosphorylation at S536 specifically contributes to the lack of NF-κB activation and transformation in resistant JB6 cells. Carcinogenesis 25, 1991–2003 (2004). [DOI] [PubMed] [Google Scholar]
- 79.Schwabe R. F., Sakurai H., IKKβ phosphorylates p65 at S468 in transactivaton domain 2. FASEB J. 19, 1758–1760 (2005). [DOI] [PubMed] [Google Scholar]
- 80.Vermeulen L., De Wilde G., Notebaert S., Berghe W. V., Haegeman G., Regulation of the transcriptional activity of the nuclear factor-κB p65 subunit. Biochem. Pharmacol. 64, 963–970 (2002). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix 01 (PDF)
Data Availability Statement
All study data are included in the article and/or SI Appendix.