Abstract
Background: Androgen deprivation therapy (ADT) improves local cancer control in unfavorable localized prostate cancer treated with radiotherapy. ADT is known to cause hormonally related symptoms that resolve with testosterone recovery. Hot flashes are particularly burdensome. This study sought to evaluate the timeline of hot flashes following short-course ADT and stereotactic body radiotherapy (SBRT) as well as its relationship with testosterone recovery.
Methods: Institutional IRB approval was obtained for this retrospective review of prospectively collected data (IRB#: 2009-510). ADT was initiated three months prior to the start of SBRT. Hot flashes were self-reported via question 13a of the Expanded Prostate Index Composite (EPIC)-26 prior to ADT initiation, the first day of robotic SBRT, and at each follow-up (one, three, six, nine, 12, 18, 24, and 36 months). The responses were grouped into three relevant categories (no problem, very small-small problem, and moderate-big problem). Scores were transformed to a 0-100 scale with higher scores reflecting less bother. Testosterone levels were measured at each follow-up.
Results: From 2007 to 2010, 122 localized prostate cancer patients (nine low-, 64 intermediate-, and 49 high-risk according to the D’Amico classification) at a median age of 72 years (range 54.5-88.3) were treated with short course ADT (three to six months) and SBRT (35-36.25 Gy) at Georgetown University Hospital. Thirty-two percent were Black and 27% were obese. Seventy-seven percent of patients received three months of ADT. At baseline, 2% of men experienced hot flashes that were a “moderate to big problem” and that proportion peaked at the start of SBRT (45%) before returning to baseline (2%) nine months post-SBRT with a cumulative incidence of 52.4%. The median baseline EPIC-26 hot flash score of 94 declined to 50 at the start of SBRT but this returned to baseline (92) by six months post SBRT. These changes were both statistically and clinically significant (MID = 9.5083, p<0.01). Testosterone recovery (> 230 ng/dL) occurred in approximately 70% of patients by 12 months post SBRT. Resolution of hot flashes correlated with testosterone recovery.
Conclusion: Bothersome hot flashes occur in greater than 50% of men treated with neoadjuvant ADT. Resolution of hot flashes occurs in the majority of patients within one year after treatment. Reassurance of the temporary nature of hot flashes may assist in reducing patient anxiety. Measuring testosterone levels at follow-up visits may allow for anticipatory counseling that may limit the associated bother.
Keywords: prostate cancer, sbrt (stereotactic body radiotherapy), cyberknife, adt (androgen deprivation therapy), hot flashes
Introduction
The current treatment paradigm for unfavorable and high-risk prostate cancer involves a combination of androgen deprivation therapy (ADT) in combination with radiotherapy [1-3]. The addition of ADT to radiotherapy improves prostate cancer-specific mortality and distant metastasis rates in men with unfavorable- and high-risk prostate cancer [4,5]. Despite this, ADT is associated with impaired quality of life and detriments related to adverse side effects. In particular, low testosterone from androgen suppression is associated with hypogonadal symptoms such as fatigue, depression, gynecomastia, weight gain, and hot flashes [1,6].
Hot flashes are one of the most bothersome toxicities to men following ADT, but hot flashes are generally benign findings that self-resolve [7]. Hot flashes can also be treated with a number of pharmacologic agents including gabapentin, Megace, and venlafaxine. About 70% to 80% of men will experience hot flashes while undergoing ADT [8,9]. Due to symptomatic bother, hot flashes may decrease adherence to ADT recommendations [10]. Patients reported quality of life scores often improve to baseline in the months following ADT corresponding to testosterone recovery (TR).
The timeline to TR following androgen deprivation is variable. Previous studies have defined TR as a return to >230 ng/ml [11]. The time to TR is variable and dependent on patient and treatment characteristics including age, race, BMI, and duration of ADT [12,13]. Older and obese men have a shorter time to TR, while black men have a faster time to TR [12,14,15]. The longer duration of ADT correlates with a slower trajectory to TR [12]. We have previously reported that 71% of patients recovered to a eugonadal state around 12 months, with a mean recovery time of four months post-stereotactic body radiotherapy (SBRT) [16]. This study sought to understand the onset and resolution of hot flashes, as well as evaluate the relationship between TR and hot flash onset and resolution.
This article was previously presented as a poster at the 2023 ASTRO Annual Meeting on October 2, 2023.
Materials and methods
Patient selection
Patients eligible for inclusion in this study had histologically confirmed localized prostate cancer treated with a combination of short-course ADT and SBRT and had a minimum of three years of follow-up. Approval from the MedStar Georgetown University Institutional Review Board was obtained for a retrospective review of prospectively collected data in our institutional database (IRB#: 2009-510).
Treatment planning and delivery
All patients received between three to six months of ADT consisting of the luteinizing hormone-releasing hormone agonist, leuprolide (22.5 mg). In general, SBRT was delivered three months after the first injection to maximize prostate size reduction and minimize radiation dose to surrounding healthy tissues [17]. SBRT was delivered utilizing the CyberKnife robotic radiosurgical system (Accuray, Inc., Sunnyvale, CA, USA). Required fiducial placement, treatment planning magnetic resonance imaging (MRI), and computed tomography (CT) simulation procedures have been previously described [18,19]. The clinical target volume (CTV) was defined as the prostate and proximal seminal vesicles. The CTV was expanded 5 mm in all directions except 3 mm posteriorly to generate the planning target volume (PTV). Patients were treated to a prescription dose was 35-36.25 Gy to the PTV delivered in five fractions over two weeks based on treatment planning performed using Multiplan (Accuray Inc., Sunnyvale, CA, USA). Treatment beams that directly traversed the testis were blocked and scatter dose was kept to a minimum [17]. In general, patients began treatment between two and and weeks after the treatment planning scans.
Follow-up and statistical analysis
Serum testosterone levels were obtained before the first SBRT treatment and during routine follow-up visits every three months for the first year and every six months thereafter. In general, serum samples were collected in the morning to limit the impact of circadian variance [17]. TR was defined as reaching a serum testosterone level of at least 230 ng/mL. Hot flash bother was assessed using the Expanded Prostate Index Composite (EPIC)-26: Question 13a prior to ADT initiation, the first day of SBRT, and at each follow-up. The EPIC questionnaire asks patients to assess both sexual and hormonal function, and question 13a specifically assesses hot flashes at these time points.
To statistically compare changes in EPIC question scores at each time point, the level of responses was assigned a score and transformed onto a 0-100 scale with lower scores reflecting worsening sexual or hormonal symptoms [19]. The minimally important difference (MID=9.5) utilized for the question 13a response was determined by half plus or minus the standard deviation from baseline values [20,21].
Results
One hundred twenty-two patients with localized prostate cancer treated with short-course ADT and prostate SBRT at Georgetown University Hospital from 2013 to 2019 were included in this analysis. Their baseline characteristics are summarized in Table 1. Our patients were ethnically diverse with a median age of 72 years (range 54.5-88.3). Thirty-two percent were black and 27% were obese. The patients had high levels of comorbidities with 52% of patients having a Charlson-Comorbidity Index of one or more. Using the D’Amico risk classification, nine patients were low-, 64 intermediate-, and 49 high-risk. Approximately 77% of patients received three months of ADT.
Table 1. Patient characteristics and treatment.
SBRT - stereotactic body radiotherapy; AUA - American Urologic Association
| Percent of Patients | |
| (n=122) | |
| Age (years): Median 77 (55–88) | |
| 60–69 | 6.40% |
| 70–79 | 40% |
| >80 | 53% |
| Race | |
| White | 52% |
| Black | 32% |
| Other | 16% |
| Prostate Volume (cc) | Median 41.1 (7 - 160) |
| Body Mass Index (kg/m²) | |
| <18.5 | 1% |
| 18.5–24.9 | 23% |
| 25.0–29.9 | 48% |
| 30.0–34.9 | 20% |
| 35.0–39.9 | 4% |
| 40.0–44.9 | 3% |
| Charlson Comorbidity Index | |
| 0 | 48% |
| 1 | 25% |
| 2 | 16% |
| 3 | 11% |
| Risk Group (D’Amico) | |
| Low | 7% |
| Intermediate | 52% |
| High | 40% |
| Hormone Therapy | |
| 3 months | 77% |
| 4 months | 8% |
| 6 months | 15% |
| SBRT Dose | |
| 35 | 34% |
| 36.25 | 66% |
| AUA Baseline | |
| 0–7 (Mild) | 39% |
| 8–19 (Moderate) | 45% |
| ≥20 (Severe) | 16% |
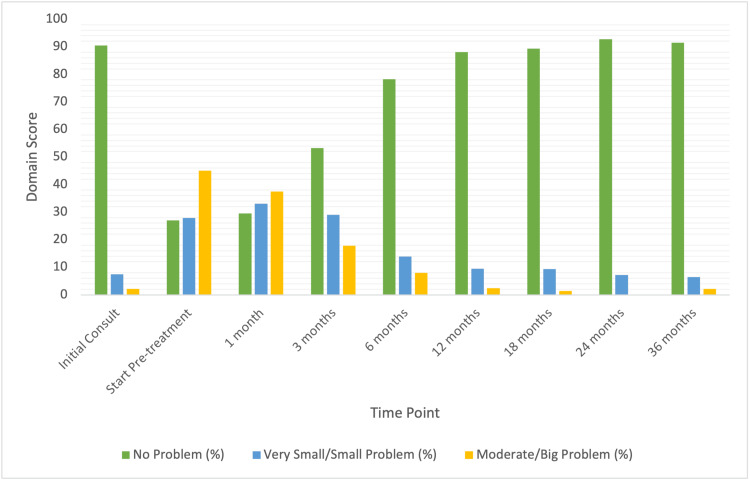
The prevalence of hot flashes prior to and after SBRT treatment is shown in Table 2. At the time of initial consult, 90% of patients reported no problem with their hot flashes. Only 7% endorsed a very small/small problem, and 2% endorsed a moderate/big problem with hot flashes. At time of ADT, 73% of patients reported hot flashes; 28% described hot flashes as very small or a small problem and 45% as a moderate to big problem. Scores of patient-reported hot flashes decreased significantly following treatment. The bother of hot flashes (20%) remained clinically and statistically significant until the six-month time point (MID = (9.5, p<0.01), but after this time point, hot flash bother returned to baseline levels.
Table 2. Hot flashes bother following short course ADT and SBRT by time point.
ADT - androgen deprivation therapy; SBRT - stereotactic body radiotherapy
| Initial Consult | Start Pre-Treatment | 1 month | 3 months | 6 months | 9 months | 12 months | 18 months | 24 months | 36 months | |
| No problem (%) | 90 | 27 | 29 | 53 | 78 | 88 | 89 | 93 | 91 | 90 |
| Very Small/Small Problem (%) | 7 | 28 | 33 | 29 | 14 | 10 | 9 | 7 | 6 | 7 |
| Moderate/Big Problem (%) | 2 | 45 | 38 | 18 | 8 | 2 | 1 | 0 | 2 | 2 |
| Patient Response (N) | 94 | 122 | 112 | 107 | 101 | 84 | 75 | 69 | 84 | 94 |
| P-value | >.01 | >.01 | >.01 | >.01 | .52 | .58 | .34 | .93 | 1 |
The hot flash mean reported EPIC scores can be seen in Table 3. Hot flashes were not bothersome at baseline, with the mean score being 94.3. Hot flash bother increased to 50.9 at time of radiation start. Following SBRT, scores increased gradually to 55 and 73 at one and three months post-SBRT, respectively, and returned to baseline levels by 12 months (p=0.58). The hot flash mean scores remained stable at this level for the three-year follow-up period, eventually reaching 97 at 36 months (Table 3).
Table 3. Hot flash mean reported score following short course ADT and SBRT for prostate cancer.
ADT - androgen deprivation therapy; SBRT - stereotactic body radiotherapy
| Time | Score | Confidence Interval (95%) |
| Baseline Score | 94.3 | 90.5-98.1 |
| SBRT start | 50.9 | 47.1-54.7 |
| 1 month | 55.4 | 51.6-59.2 |
| 3 months | 73.0 | 69.3-76.8 |
| 6 months | 87.3 | 83.5-91.1 |
| 12 months | 92.5 | 88.7-96.3 |
| 18 months | 93.4 | 89.6-97.2 |
| 24 months | 95.9 | 92.1-99.7 |
| 36 months | 97.0 | 93.2-100.8 |
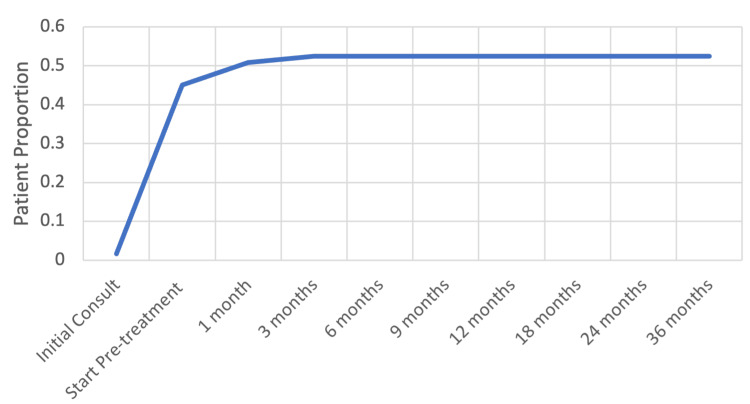
Patients reporting a very small/small amounts of bother and moderate/severe bother peaked at one month and pre-treatment (corresponding to receipt of ADT), respectively, and decreased steadily thereafter (Figure 1). High levels of bother occurred closer to ADT receipt before falling to lower levels of bother, then back to baseline levels (Figure 1). The cumulative incidence of hot flashes seen in our patient population was 52.4% for “moderate to big problem” (Figure 2).
Figure 1. Hot flashes bother following SBRT for prostate cancer by time period.
Expanded Prostate Cancer Index Composite (EPIC) 13a scores before and after stereotactic body radiotherapy (SBRT) treatment. Patients were stratified into three groups: no problem, very small-small problem, and moderate-big problem.
Figure 2. Cumulative incidence of hot flashes before and after SBRT and ADT for prostate cancer.
ADT - androgen deprivation therapy; SBRT - stereotactic body radiotherapy
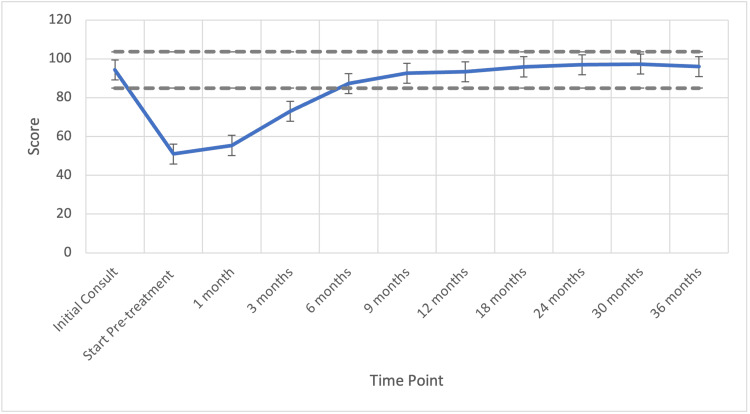
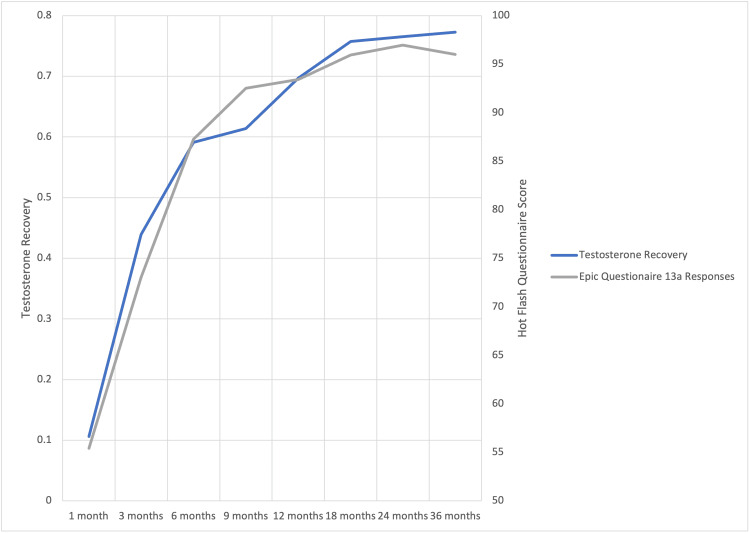
The mean time to resolution of clinically and statistically significant hot flashes was six months post-SBRT (MID = 9.51, p<0.01, Figure 3). By six months, testosterone recovered in 60% of patients (Figure 4). There was a strong correlation of 98.6% (R^2=99.7%) between TR and hot flash score, with both values rising and eventually plateauing as time progressed.
Figure 3. Hot flash reported score over time .
Gray lines represent MID (Minimally important difference).
Figure 4. Testosterone and hot flashes over time.
Testosterone recovery and Epic 13a scores after stereotactic body radiotherapy (SBRT) treatment. Patients were scored from 0 to 100 and testosterone recovery was measured as cumulative incidence across time
Discussion
Hot flashes are a known complication of ADT, which adversely affects quality of life in men with prostate cancer. While on ADT, 52% of patients reported hot flashes as being a moderate to big problem. Hot flash bother may have significant implications on adherence to treatment recommendations [22]. It is important to communicate with patients about the expected effects of androgen deprivation and discuss the expected recovery with each treatment.
Hot flashes are secondary to the release of hypothalamic catecholamines (i.e., norepinephrine) in response to the decreased LH and FSH [1]. Here, we demonstrate a significant correlation between TR and symptomatic hot flashes. We have previously reported that the median time to recovery of testosterone levels corresponds to approximately four months following ADT cessation [16]. Previous studies have demonstrated TR after short ADT to range from 13 weeks to two years [11,23]. In the present study, we demonstrated that the mean time to resolution of clinically and statistically significant hot flashes was six months post-SBRT (MID = 9.5, p<0.01, Figure 3) corresponding with 60% TR. There is some evidence that shorter-acting GnRH antagonists result in a faster recovery to baseline testosterone levels, which may be an area for further research [24].
Our results add to previous results demonstrating the transient nature of these side effects [6]. The prevalence of hot flashes peaked at the pre-treatment time point corresponding with receipt of ADT. We demonstrate that short-term ADT may contribute to hot flash symptoms for up to six months prior to recovery to baseline. Hot flashes were rare at time points greater than two years post-SBRT. Hormone replacement therapy is contraindicated in this patient population. However, for significant bother, megestrol acetate, venlafaxine, or gabapentin can be trialed [25-27]. Utilizing testosterone levels can further assist in determining a return to an eugonadal state which may coincide with the resolution of hot flashes and improvement in EPIC-26 scores.
Limitations of this study include its retrospective design. Men likely only reported hot flashes to their physician when bothersome to them and/or their partner. Additionally, a portion of our patients were obese and older, which could confound data as they are likely at a lower baseline for testosterone, and less recovered back to a eugonadal state (66%). Likewise, a portion of our patients were Black, which also could contribute as those patients had a higher rate of return to eugonadal state (85%). Further, we did not collect baseline testosterone levels prior to ADT. Hence, the proportion of baseline hypogonadal patients is unknown. The course of ADT given to patients was also variable, and while most received short courses, there may be some further confounding for those with longer courses of ADT (i.e., six months). Men who had hypogonadism at baseline may have had slower TR and more prolonged hot flash systems [15,16].
Conclusions
Hot flashes are a bothersome self-limiting symptom experienced by a small percentage of men following prostate SBRT. SBRT and short-term ADT are proven to be safe and effective for treating localized prostate cancer. Hot flashes and testosterone are closely associated with one another, with TR coinciding with a resolution of hot flashes. Reassurance of the short duration of hot flashes may assist in reducing patient anxiety. Hot flashes followed the TR trend closely, and follow-up monitoring of testosterone levels may allow for guidance to limit bother of these temporary changes.
The authors have declared financial relationships, which are detailed in the next section.
Sean Collins declare(s) personal fees from Accuray. SC serves as clinical consultant to Accuray Inc. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Funding Statement
This work was supported by The James and Theodore Pedas Family Foundation. The Department of Radiation Medicine at Georgetown University Hospital receives a grant from Accuray to support a research coordinator. We gratefully acknowledge the grant R01MD012767 from the National Institute on Minority Health and Health Disparities (NIMHD), NIH to SC.
Author Contributions
Concept and design: Sarthak Shah, Sean P. Collins, Nima Aghdam
Acquisition, analysis, or interpretation of data: Sarthak Shah, Abigail Pepin, Simran Jatar, Jessica Hsueh, Lindsey Gallagher, Malika T. Danner, Alan Zwart, Marilyn Ayoob, Thomas M. Yung, Deepak Kumar, Paul D. Leger, Nancy A. Dawson, Suy Simeng, Sean P. Collins
Drafting of the manuscript: Sarthak Shah, Abigail Pepin, Sean P. Collins
Critical review of the manuscript for important intellectual content: Sarthak Shah, Abigail Pepin, Simran Jatar, Jessica Hsueh, Lindsey Gallagher, Malika T. Danner, Alan Zwart, Marilyn Ayoob, Thomas M. Yung, Deepak Kumar, Paul D. Leger, Nancy A. Dawson, Suy Simeng, Sean P. Collins, Nima Aghdam
Supervision: Abigail Pepin, Sean P. Collins
Human Ethics
Consent was obtained or waived by all participants in this study. MedStar Georgetown University Institutional Review Board issued approval 2009-510
Animal Ethics
Animal subjects: All authors have confirmed that this study did not involve animal subjects or tissue.
References
- 1.Evaluating the prevalence and predictive factors of vasomotor and psychological symptoms in prostate cancer patients receiving hormonal therapy: results from a single institution experience. Challapalli A, Edwards SM, Abel P, Mangar SA. Clin Transl Radiat Oncol. 2018;10:29–35. doi: 10.1016/j.ctro.2018.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ten-year follow-up of radiation therapy oncology group protocol 92-02: a phase III trial of the duration of elective androgen deprivation in locally advanced prostate cancer. Horwitz EM, Bae K, Hanks GE, et al. J Clin Oncol. 2008;26:2497–2504. doi: 10.1200/JCO.2007.14.9021. [DOI] [PubMed] [Google Scholar]
- 3.Short-term androgen-deprivation therapy improves prostate cancer-specific mortality in intermediate-risk prostate cancer patients undergoing dose-escalated external beam radiation therapy. Zumsteg ZS, Spratt DE, Pei X, et al. Int J Radiat Oncol Biol Phys. 2013;85:1012–1017. doi: 10.1016/j.ijrobp.2012.07.2374. [DOI] [PubMed] [Google Scholar]
- 4.Effect of androgen deprivation on long-term outcomes of intermediate-risk prostate cancer stratified as favorable or unfavorable: a secondary analysis of the RTOG 9408 randomized clinical trial. Zumsteg ZS, Spratt DE, Daskivich TJ, Tighiouart M, Luu M, Rodgers JP, Sandler HM. JAMA Netw Open. 2020;3:0. doi: 10.1001/jamanetworkopen.2020.15083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Combined androgen deprivation therapy and radiation therapy for locally advanced prostate cancer: a randomised, phase 3 trial. Warde P, Mason M, Ding K, et al. Lancet. 2011;378:2104–2111. doi: 10.1016/S0140-6736(11)61095-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.The relationship between hot flashes and testosterone recovery after 12 months of androgen suppression for men with localised prostate cancer in the ascende-RT trial. Dosani M, Morris WJ, Tyldesley S, Pickles T. Clin Oncol (R Coll Radiol) 2017;29:696–701. doi: 10.1016/j.clon.2017.06.009. [DOI] [PubMed] [Google Scholar]
- 7.Antiandrogen monotherapy in patients with localized or locally advanced prostate cancer: final results from the bicalutamide Early Prostate Cancer programme at a median follow-up of 9.7 years. Iversen P, McLeod DG, See WA, Morris T, Armstrong J, Wirth MP. BJU Int. 2010;105:1074–1081. doi: 10.1111/j.1464-410X.2010.09319.x. [DOI] [PubMed] [Google Scholar]
- 8.Estrogenic side effects of androgen deprivation therapy. Guise TA, Oefelein MG, Eastham JA, Cookson MS, Higano CS, Smith MR. https://pubmed.ncbi.nlm.nih.gov/18231613/ Rev Urol. 2007;9:163–180. [PMC free article] [PubMed] [Google Scholar]
- 9.Androgen deprivation therapy-associated vasomotor symptoms. Jones JM, Kohli M, Loprinzi CL. Asian J Androl. 2012;14:193–197. doi: 10.1038/aja.2011.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Changes in physical functioning and muscle strength in men receiving androgen deprivation therapy for prostate cancer: a controlled comparison. Gonzalez BD, Jim HS, Small BJ, et al. Support Care Cancer. 2016;24:2201–2207. doi: 10.1007/s00520-015-3016-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Androgen suppression and radiation vs radiation alone for prostate cancer: a randomized trial. D'Amico AV, Chen MH, Renshaw AA, Loffredo M, Kantoff PW. JAMA. 2008;299:289–295. doi: 10.1001/jama.299.3.289. [DOI] [PubMed] [Google Scholar]
- 12.A nomogram for testosterone recovery after combined androgen deprivation and radiation therapy for prostate cancer. Spiegel DY, Hong JC, Oyekunle T, Waters L, Lee WR, Salama JK, Koontz BF. Int J Radiat Oncol Biol Phys. 2019;103:834–842. doi: 10.1016/j.ijrobp.2018.11.007. [DOI] [PubMed] [Google Scholar]
- 13.Identification and functional analysis of consensus androgen response elements in human prostate cancer cells. Horie-Inoue K, Bono H, Okazaki Y, Inoue S. Biochem Biophys Res Commun. 2004;325:1312–1317. doi: 10.1016/j.bbrc.2004.10.174. [DOI] [PubMed] [Google Scholar]
- 14.Testosterone suppression with luteinizing hormone-releasing hormone (LHRH) agonists in patients receiving radiotherapy for prostate cancer. Wilke D, Patil N, Hollenhorst H, Bowes D, Rutledge R, Ago C. Pharmacotherapy. 2018;38:327–333. doi: 10.1002/phar.2084. [DOI] [PubMed] [Google Scholar]
- 15.Kinetics of serum androgen normalization and factors associated with testosterone reserve after limited androgen deprivation therapy for nonmetastatic prostate cancer. Gulley JL, Aragon-Ching JB, Steinberg SM, et al. J Urol. 2008;180:1432–1437. doi: 10.1016/j.juro.2008.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Testosterone as a biomarker for quality of life (QOL) following androgen deprivation therapy (ADT) and stereotactic body radiotherapy (SBRT) Shah S, Pepin A, Forsthoefel M, et al. Cureus. 2023;15:0. doi: 10.7759/cureus.44440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Low incidence of new biochemical and clinical hypogonadism following hypofractionated stereotactic body radiation therapy (SBRT) monotherapy for low- to intermediate-risk prostate cancer. Oermann EK, Suy S, Hanscom HN, et al. J Hematol Oncol. 2011;4:12. doi: 10.1186/1756-8722-4-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Management of complications of androgen deprivation therapy in the older man. Mohile SG, Mustian K, Bylow K, Hall W, Dale W. Crit Rev Oncol Hematol. 2009;70:235–255. doi: 10.1016/j.critrevonc.2008.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sanda MG, Wei JT, Litwin MS. Scoring instructions for the expanded prostate cancer index composite short form (EPIC-26) Ann Arbor, MI: University of Michigan; 2002. [Google Scholar]
- 20.Stereotactic body radiation therapy (SBRT) for clinically localized prostate cancer: the Georgetown University experience. Chen LN, Suy S, Uhm S, et al. Radiat Oncol. 2013;8:58. doi: 10.1186/1748-717X-8-58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Minimally important difference for the expanded prostate cancer index composite short form. Skolarus TA, Dunn RL, Sanda MG, Chang P, Greenfield TK, Litwin MS, Wei JT. Urology. 2015;85:101–105. doi: 10.1016/j.urology.2014.08.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Androgen-deprivation therapy alone versus combined with radiation therapy or chemotherapy for nonlocalized prostate cancer: a systematic review and meta-analysis. Lei JH, Liu LR, Wei Q, Song TR, Yang L, Meng Y, Han P. Asian J Androl. 2016;18:102–107. doi: 10.4103/1008-682X.150840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Recovery of serum testosterone after neoadjuvant androgen deprivation therapy and radical radiotherapy in localized prostate cancer. Murthy V, Norman AR, Shahidi M, et al. BJU Int. 2006;97:476–479. doi: 10.1111/j.1464-410X.2006.06013.x. [DOI] [PubMed] [Google Scholar]
- 24.Treatment of nonmetastatic castration-resistant prostate cancer: focus on second-generation androgen receptor inhibitors. Saad F, Bögemann M, Suzuki K, Shore N. Prostate Cancer Prostatic Dis. 2021;24:323–334. doi: 10.1038/s41391-020-00310-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Megestrol acetate for the prevention of hot flashes. Loprinzi CL, Michalak JC, Quella SK, et al. N Engl J Med. 1994;331:347–352. doi: 10.1056/NEJM199408113310602. [DOI] [PubMed] [Google Scholar]
- 26.Venlafaxine for the treatment of hormonal therapy-induced hot flashes in a male patient. Singh H, Sharma A. Prim Care Companion CNS Disord. 2011;13:0. doi: 10.4088/PCC.10l01039blu. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gabapentin for the management of hot flashes in prostate cancer survivors: a longitudinal continuation study - NCCTG Trial N00CB. Moraska AR, Atherton PJ, Szydlo DW, et al. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3075822/ J Support Oncol. 2010;8:128–132. [PMC free article] [PubMed] [Google Scholar]