Abstract
Mechanistic research suggests using Cannabis sativa L. (cannabis or marijuana) may increase the risk of cardiometabolic disease, but observational studies investigating associations between cannabis use and myocardial infarction (MI) have reported inconsistent results. Cross-sectional National Health and Nutrition Examination Survey data from five 2-year cycles between 2009 and 2018 and representing 9,769 middle-aged adults (35 to 59 years old) were analyzed. Multivariable logistic regression models accounting for sampling weights and adjusting for cardiovascular risk factors were used to assess associations between a history of monthly cannabis use before MI and a subsequent MI. A quarter of respondents (n = 2,220) reported a history of monthly use >1 year before an MI. A history of MI was reported by 2.1% of all respondents and 3.2.% of those who reported a history of monthly use. In fully adjusted multivariable models, and compared with never use, a history of monthly cannabis use preceding an MI was not associated with an MI (odds ratio [OR] 0.78, 95% confidence interval [CI] 0.35 to 1.71). However, when stratified by recent use, the odds of MI were threefold greater (OR 2.98, 95% CI 1.08 to 8.60) when no use was reported within the past month than when use was reported within the past month. Duration of monthly use was also not significantly associated with MI, including monthly use >10 years (OR 0.78, 95% CI 0.30 to 2.01). In conclusion, in a representative sample of middle-aged US adults, a history of monthly cannabis use >1 year before an MI was not associated with a subsequent physician-diagnosed MI, except for threefold greater odds when cannabis was not used within the past month.
Keywords: myocardial infarction, cardiovascular disease, cannabis, marijuana
Changing societal attitudes toward Cannabis sativa L. (cannabis or marijuana), including consideration of its therapeutic utility,1 have led to changing regulations and increased access in the United States and around the world.2 Therefore, there is greater interest in the long-term effects of cannabis use, including concerns of cardiovascular disease (CVD).
Cardiovascular effects are mediated through cannabinoid receptors, namely, CB1R and CB2R, located in the central nervous and cardiovascular systems. Chronic CB1R activation has been linked with mechanisms of cardiometabolic disease, including profibrotic and proatherogenic effects, whereas CB2R activation has shown anti-inflammatory and antioxidant effects.3 Acute effects include dose-dependent increases in heart rate and cardiac contractility, elevations in myocardial oxygen demand, and alterations in vascular resistance and blood pressure.4
Epidemiologic studies investigating the relation between cannabis use and myocardial infarction (MI) have yielded conflicting results, depending on the study design, population, and measure of cannabis exposure. More specifically, cross-sectional studies have found associations with MI,5,6 coronary artery disease,7 stroke,6,8 and CVD mortality,9 whereas longitudinal studies have shown no association with these outcomes10 or with markers of systemic inflammation,11 CVD risk factors,12 carotid intima-media thickness,13 coronary artery and abdominal artery calcium,14 or abdominal adiposity.15
Previous cross-sectional studies have investigated recency (past month use) and frequency of recent use (days used in the past month), but not duration and timing of use beyond the past month. Thus, we conducted an analysis of National Health and Nutrition Examination Survey (NHANES) data to assess the relation between a history of monthly cannabis use preceding an MI (i.e., long-term use preceding an MI) and self-reported history of physician-diagnosed MI in a nationally representative sample of middle-aged adults in the United States.
Methods
The NHANES is a series of nationally representative, cross-sectional examinations designed to monitor the health of the US population. Participants are selected from the noninstitutionalized, civilian population using a complex, stratified, multistage probability-cluster sampling design for in-home interviews and visits to a mobile examination center.16 Weights in NHANES are formulated to address the survey’s intricate design elements, such as oversampling, nonresponses, and poststratification adjustments to align with the Census Bureau’s total population figures.17
The analytic sample was limited to middle-aged adults (35 to 59 years old) who participated in both the interview and mobile NHANES examinations during 5 2-year cycles between 2009 to 2018. This included 9,769 (unweighted)/91,860,164 (weighted) respondents who answered questions about their cannabis use using the Drug Use Questionnaire (DUQ) and history of physician-diagnosed MI (n = 232) using the Medical Conditions Questionnaire (MCQ). Respondents with relevant missing data from both the DUQ and the MCQ were excluded.
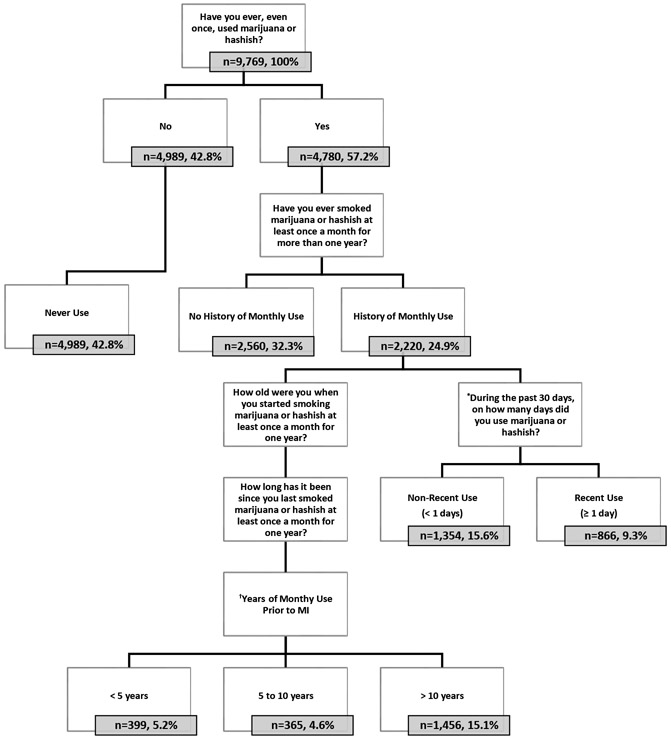
Cannabis use was defined using questions from the DUQ, which surveyed respondents between 18 and 69 years of age (age 59 years for cannabis use).18 Classifications for the primary analysis included “never use,” “no history of monthly use,” or “history of monthly use” (i.e., a history of monthly use >1 year), as outlined in Figure 1. A secondary exposure variable was calculated to measure duration of monthly cannabis use before an MI (Figure 1).
Figure 1. Flowchart of the analytic sample (unweighted).
Notes: subgroups are represented by unweighted frequencies and weighted percentages. *This question was administered to all respondents reporting ever using marijuana or hashish. Only responses from those with a history of monthly use >1 year were analyzed. †Years of monthly cannabis use before MI was determined by responses to the following questions: “How old were you when you started smoking marijuana or hashish at least once a month for one year?” (age in years) and “How long has it been since you last smoked marijuana or hashish at least once a month for one year?” Years of monthly use were calculated by subtracting the number of years since using it monthly for a year from a respondent’s current age and then subtracting the age at which they reported starting using it monthly for a year. If the number of years since a reported MI was greater than the number of years since monthly use, the difference was subtracted from the number of years of monthly use, resulting in years of monthly use before the MI.
For recent use (i.e., within the past month), a categorical variable was created to summarize the frequency of use within the month. Respondents reporting <10 days of use were categorized as “Recent, light” users, 10 to 20 days as “Recent, moderate” users, and >20 days as “Recent, heavy” users.19
Covariates were obtained through self-report and included age, gender, race/ethnicity, education, household income, physical activity, cigarette smoking status, and alcohol use. Age was self-reported in years. Gender was self-reported as male or female. Race/ethnicity was categorized as non-Hispanic White, non-Hispanic Black, Asian and other, or Hispanic (inclusive of Mexican American and Other Hispanic). Height and weight were obtained by trained examiners and were used to compute body mass index (BMI). Comprehensive information on covariates is available in Supplementary Methods and Results.
Biomarkers (total cholesterol [mg/100 ml], high-density lipoprotein [HDL] cholesterol [mg/100 ml], fasting blood glucose [mg/d], HbA1c [%]) were objectively measured by American Society of Clinical Pathologists-certified medical technologists or medical laboratory technicians at the University of Minnesota, Minneapolis, Minnesota. Dyslipidemia was defined as a total cholesterol/HDL ratio >5.0 or use of lipid-lowering medication. Prediabetes was defined as fasting blood glucose ≥100 or HbA1c ≥5.7%. Diabetes was defined as fasting blood glucose ≥126 or HbA1c >6.4% or use of antidiabetic medication.
The 10-year atherosclerotic CVD (ASCVD) risk was calculated using the race- and gender-specific Pooled Cohort Risk Equations developed by the American College of Cardiology (ACC) and American Heart Association (AHA).20 Given that the 10-year ASCVD risk score is only validated in non-Hispanic White and Black subjects, men and women reporting other races/ethnicities were treated as non-Hispanic White (per the equations). Parameters included the natural log of age, age squared, total cholesterol, HDL cholesterol, treated or untreated systolic blood pressure, smoking status, and diabetes status. A unique set of parameters was used for each race- and gender-specific equation, as specified by the ACC/AHA.
Cardiovascular-related medical conditions were defined by questions from the MCQ. Eligible respondents were aged between 20 and 150 years. A self–reported history of MI was determined by response to the following question: “Has a doctor or other health professional ever told you that you…had a heart attack (also called myocardial infarction)?” (“Yes,” “No”). Respondents reporting “Yes” were categorized as having history of MI. The age of the heart attack was determined by response to the following question: “How old were you when you were first told you had a heart attack (also called myocardial infarction)?” The age of the MI was used to determine the years of monthly cannabis use before MI (Figure 1).
NHANES data are weighted using a complex sampling design, so the population provides a representation of the population of the United States for each 2-year survey cycle. Per the guidelines provided by NHANES, weights were used for the smallest subsample used in this study.21 The analytic sample combines data from multiple survey cycles; the weights were adjusted per the instructions provided by NHANES.17
Descriptive statistics were used to characterize the sample (mean and standard error, or median and interquartile range, for continuous variables and unweighted counts and weighted percentages for nominal variables). Multicollinearity between independent variables was assessed in the final multivariable adjusted model using Variance Inflation Factor and Tolerance. Trend analyses were conducted for cannabis use across time using simple linear regression. Data management and statistical analyses were performed using SAS On-Demand for Academics (Copyright 2014 SAS Institute Inc., Cary, North Carolina).
The primary analysis tested the association between a history of monthly cannabis use (> 1 year) preceding an MI and a history of a subsequent MI. Secondary analyses included duration of monthly use preceding an MI, both as a continuous and a categorical measure (<5 years, 5 to 10 years, and >10 years). Weighted multivariable logistic regression models were used to estimate adjusted odds ratios (ORs) and 95% confidence intervals (CIs), controlling for potential confounders. Variables considered potential confounders in the multivariable analyses were sociodemographic variables (model 1); cigarette and alcohol use and physical activity (model 2); and BMI and the 10-year ASCVD risk (model 3).
Multiplicative, first-order interactions were examined in the multivariable adjusted regression models between history of monthly use and gender, race/ethnicity, cigarette smoking status, recency of use, and age of first use (separately). Interaction terms were considered significant in the final multivariable adjusted model if p <0.10. A p <0.05 was considered significant for all other analyses.
Results
The sample comprised 9,769 respondents, of whom 24.9% (n = 2,220) reported a history of monthly use >1 year (Table 1). A history of MI infarction was reported by 2.1% of all respondents, 1.5% of those who reported never using cannabis, 2.2% of those who denied a history of monthly use, and 3.2.% of those who reported a history of monthly use.
Table 1.
Characteristics of Middle-Aged Adults (35-59 YOA) by Cannabis Use Status, 2009–2018 (n=9,769)
| Overall Sample (n=9,769, 100.0%) |
Never (n=4,989, 42.8%) |
No History of Monthly Use n=2,560 (32.3%) |
History of Monthly Use (n=2,220, 24.9%) |
|
|---|---|---|---|---|
| Socio-demographics, n (%) | ||||
| Sex | ||||
| Male | 4705 (49.3) | 2120 (43.6) | 1240 (47.8) | 1345 (61.0) |
| Age (YOA), mean (SE) | 47.3 (0.1) | 46.9 (0.2) | 47.1 (0.2) | 48.1 (0.2) |
| Race/Ethnicity | ||||
| Non-Hispanic White | 3708 (65.5) | 1234 (52.2) | 1327 (76.3) | 1147 (74.6) |
| Non-Hispanic Black, Asian, Other | 3383 (18.0) | 1805 (20.9) | 806 (14.5) | 772 (17.6) |
| Hispanic | 2678 (16.4) | 1950 (26.9) | 427 (9.3) | 301 (7.8) |
| Education | ||||
| High school or less | 4222 (35.7) | 2328 (39.2) | 826 (26.5) | 1068 (41.7) |
| Some college | 2924 (31.0) | 1238 (27.2) | 904 (32.7) | 782 (35.2) |
| College graduate or above | 2617 (33.3) | 1418 (33.6) | 829 (40.8) | 370 (23.0) |
| Household income, annual $ | ||||
| $0 to 44,999 | 4029 (32.1) | 1997 (34.1) | 929 (25.1) | 1103 (37.7) |
| $45,000 to $99,999 | 2775 (34.4) | 1439 (35.2) | 728 (33.0) | 608 (34.8) |
| ≥ $100,000 | 2093 (33.6) | 997 (30.7) | 746 (41.8) | 350 (27.6) |
| Cigarette smoking status | ||||
| Never | 5514 (55.4) | 3779 (78.2) | 1269 (51.3) | 466 (21.4) |
| Former | 1959 (23.0) | 619 (11.3) | 647 (27.4) | 693 (37.6) |
| Current | 2293 (21.6) | 589 (10.5) | 643 (21.3) | 1061 (40.9) |
| Pack-years, median (IQR) | 6.8 (0.2) | 2.8 (0.2) | 7.0 (0.4) | 13.7 (0.5) |
| Alcohol use, n (%) | ||||
| No days/week | 1536 (15.5) | 786 (17.3) | 380 (12.8) | 370 (16.3) |
| ≤ 2 days/week | 5577 (63.9) | 2725 (70.6) | 1610 (64.2) | 1242 (53.9) |
| > 2 days/week | 1468 (20.7) | 383 (12.1) | 501 (23.0) | 584 (29.8) |
| Phys. Act. Min./wk., median (IQR) | 339.7 (7.5) | 339.8 (10.0) | 311.6 (11.8) | 378.0 (13.8) |
| BMI, kg/m2, mean (SE) | 29.8 (0.1) | 30.0 (0.2) | 29.7 (0.2) | 29.5 (0.2) |
| Cardio-Metabolic Medical history, n (%) | ||||
| Myocardial infarction | 232 (2.1) | 81 (1.5) | 70 (2.2) | 81 (3.2) |
| Coronary heart disease | 160 (1.5) | 53 (0.9) | 56 (1.6) | 51 (2.3) |
| Angina | 152 (1.5) | 58 (1.2) | 37 (1.2) | 57 (2.4) |
| Stroke | 221 (1.7) | 93 (1.8) | 63 (1.6) | 65 (1.8) |
| Heart failure | 163 (1.2) | 72 (1.2) | 40 (0.9) | 51 (1.7) |
| Hypertension | 4822 (47.6) | 2367 (47.0) | 1270 (46.1) | 1185 (50.5) |
| Dyslipidemia | 3140 (32.3) | 1586 (31.0) | 834 (31.8) | 720 (35.1) |
| Diabetes or Pre-diabetes | 4928 (45.6) | 2610 (48.0) | 1199 (40.8) | 1119 (47.8) |
| 10-year ASCVD Risk Score, median (IQR) | 4.6 (0.1) | 4.1 (0.1) | 4.2 (0.2) | 5.8 (0.2) |
| Laboratory measures, mean (SE) | ||||
| Total cholesterol, mg/dL | 201.1 (0.7) | 199.2 (0.8) | 203.3 (1.3) | 201.3 (1.4) |
| HDL, mg/dL | 53.3 (0.3) | 52.6 (0.4) | 54.9 (0.4) | 52.4 (0.5) |
| LDL, mg/dL | 120.8 (0.6) | 121.0 (0.9) | 120.3 (1.2) | 121.3 (1.7) |
| Triglycerides, mg/dL | 131.0 (2.5) | 132.2 (3.5) | 122.6 (3.6) | 139.2 (4.1) |
| Hemoglobin A1c, % | 5.7 (0.0) | 5.7 (0.0) | 5.6 (0.0) | 5.7 (0.0) |
| Medication use, n (%) | ||||
| Antihypertensive (incl. comb.) | 2115 (36.5) | 1025 (39.9) | 567 (33.7) | 523 (35.1) |
| Antilipidemic | 1298 (24.7) | 633 (24.5) | 369 (23.8) | 296 (26.2) |
| Antidiabetic | 895 (13.7) | 504 (17.0) | 218 (11.6) | 173 (11.4) |
Notes: Subgroup sizes represent unweighted frequencies. All other numbers are weighted column percentages, means (with standard errors) or medians (with IQR). Physical activity includes the following domains: Walk/bike for transportation, vigorous recreational activity, moderate recreational activity.
Those with a history of monthly use > 1 year were more likely than were never users to be non-Hispanic White men with less education, lower household income, and more days of alcohol use per week. Notably, subjects with a history of monthly use had a higher prevalence of current cigarette smoking than did those without a history of monthly use, and almost double the median number of pack-years. Despite this, the former reported engaging in more minutes of physical activity per week. Finally, median 10-year ASCVD risk score was significantly higher for those with a history of monthly use than for those without a history of monthly use and those who reported never using cannabis at all. This difference was statistically significant (Tukey adjusted p <0.0001 for both).
Between 2009 and 2018, the percentage of respondents reporting a history of monthly cannabis use fluctuated around 25%, without a statistically significant trend over time (p for trend = 0.69). However, a significant upward trend was seen in recent cannabis use (past month) from 9.0% (95% CI 7.0 to 11.1) in 2009 to 2010 to 13.5% (95% CI 10.9 to 16.2) in 2017 to 2018 (p for trend <0.01), and in recent use by those with a history of monthly use from 7.6% (95% CI 5.7 to 9.6) in 2009 to 2010 to 11.4% (95% CI 8.7 to 14.1) in 2017 to (p for trend = 0.03). Conversely, the percentage reporting a history of MI decreased from 2.5% (SE 0.4) in 2009 to 2010 to 2.2% (SE 0.7) in 2017 to 2018. This trend was not significant (p for trend = 0.931).
Detailed characteristics of respondents reporting ever using cannabis (n = 4,780, 57.2%) are reported in Table 2. Almost 44% of those who reported ever using cannabis also reported a history of monthly use >1 year. Of these, approximately 38% reported recent use (past month).
Table 2.
Cannabis Use Characteristics by Cannabis Use Status, 2009–2018 (4,780, 57.2%)
| Cannabis Use | No History of Monthly Use (n=2,560, 32.3%) |
History of Monthly Use (n=2,220, 24.9%) |
|---|---|---|
| Years of monthly use prior to MI, n (%) | ||
| < 5 yrs. | NA | 399 (21.0) |
| 5 to 10 yrs. | NA | 365 (18.6) |
| > 10 yrs. | NA | 1456 (60.4) |
| Years of monthly use prior to MI, mean (SE) | 0 (0.0) | 16.5 (0.4) |
| Onset of use, mean (SE) | ||
| Age of first use | 18.9 (0.1) | 16.0 (0.1) |
| Age started monthly use | NA | 18.7 (0.2) |
| Recency of use, n (%) | ||
| Past month | 162 (5.1) | 866 (37.5) |
| > Past month | 2398 (94.9) | 1354 (62.5) |
| Frequency of Recent Use | ||
| Days of use, past month, mean (SE) | 3.0 (0.3) | 15.3 (0.6) |
| Days of use, past month, n (%) | ||
| Light (< 10 days/past mo.) | 144 (91.8) | 380 (41.1) |
| Moderate (10-20 days /past mo.) | 15 (7.0) | 175 (19.8) |
| Heavy (> 20 days/past mo.) | 3 (1.2) | 311 (39.1) |
Notes: Subgroup sizes represent unweighted frequencies. All other numbers are weighted column percentages, means (with standard errors) or medians (with IQR). Cannabis use variables are all standard NHANES variables, other than “Years of monthly use prior to MI”, which is a calculation.
Frequencies and percentages of MI history are reported according to cannabis use characteristics in Table 3. Those with a history of monthly cannabis use had the greatest percentage of MIs (n = 81, 3.2%), which was largely driven by those with a history of 5 to 10 years of monthly use before their MI (n = 14, 3.7%).
Table 3.
Cannabis Use Characteristics by Myocardial Infarction Status, 2009–2018 (n=9,769)
| Cannabis Use | MI (n=232, 2.1%) |
No MI (n=9,537, 97.9%) |
|---|---|---|
| Never | 81 (1.5) | 4908 (98.5) |
| No History of Monthly Use | 70 (2.2) | 2490 (97.8) |
| History of Monthly Use | 81 (3.2) | 2139 (96.8) |
| Duration of monthly use | ||
| < 5 yrs. | 13 (2.5) | 386 (97.5) |
| 5 to 10 yrs. | 14 (3.7) | 351 (96.3) |
| > 10 yrs. | 54 (3.3) | 1402 (96.7) |
| Years of monthly use prior to MI, mean (SE) | 18.9 (2.2) | 16.4 (0.4) |
| Onset of use, mean (SE) | ||
| Age of first use | 17.3 (0.5) | 17.7 (0.1) |
| Age started monthly use | 17.4 (0.7) | 18.7 (0.2) |
| Recency of use, n (%) | ||
| Past month | 35 (2.7) | 993 (97.3) |
| > Past month | 116 (2.6) | 3636 (97.4) |
| Frequency of Recent Use | ||
| Days of use, past month, mean (SE) | 14.0 (2.2) | 13.5 (0.6) |
| Days of use, past month, n (%) | ||
| Light (< 10 days/past mo.) | 15 (2.4) | 509 (97.6) |
| Moderate (10-20 days /past mo.) | 7 (4.7) | 183 (95.3) |
| Heavy (> 20 days/past mo.) | 13 (2.2) | 301 (97.8) |
| Years since monthly use, n (%) | ||
| < 5 yrs. | 35 (2.9) | 992 (97.1) |
| 5 to 10 yrs. | 10 (3.3) | 242 (96.7) |
| > 10 yrs. | 36 (3.5) | 905 (96.5) |
| Frequency of Monthly Use | ||
| Time per Month | ||
| Once/month | 7 (3.4) | 152 (96.6) |
| 2-3 times/month | 8 (1.2) | 313 (98.8) |
| 4-8 times/month | 19 (2.7) | 543 (97.3) |
| 9-24 times/month | 22 (4.3) | 514 (95.7) |
| 25-30 times/month | 25 (3.7) | 613 (96.3) |
| Joints or Pipes per Day | ||
| 1 joint or pipe/day | 29 (2.3) | 921 (97.7) |
| 2 joints or pipes/day | 24 (3.6) | 657 (96.4) |
| 3-5 joints or pipes/day | 21 (3.3) | 432 (96.7) |
| ≥ 6 joints or pipes/day | 6 (7.2) | 116 (92.8) |
Notes: Subgroup sizes represent unweighted frequencies. All other numbers in the table are weighted column percentages.
The odds of MI by cannabis use status are reported in Table 4. In multivariable logistic regression models adjusted for sociodemographic variables, the odds of reporting a history of a MI were significantly greater among those with and without a history of monthly cannabis use (OR 1.69, 95% CI 1.17 to 2.45 and 1.58, 95% CI 1.08 to 2.30), respectively, than among those who reported never using cannabis. After adjusting for cigarette smoking, alcohol use, and physical activity, the odds became nonsignificant for both groups (no history of monthly use: OR 0.81, 95% CI 0.41 to 1.60 and history of monthly use: OR 0.78, 95% CI 0.35 to 1.71). After adjusting for cardiovascular risk factors, the odds remained nonsignificant for both groups.
Table 4.
Adjusted Odds Ratios for MI by Cannabis Use Status, 2009-2018
| OR (95% CI) | |
|---|---|
| Unadjusted (n=9,769) | |
| Never | 1.00 Reference |
| No History of Monthly Use | 1.50 (1.06-2.13)* |
| History of Monthly Use | 2.23 (1.56-3.20)* |
| Duration of monthly | |
| < 5 yrs. | 1.71 (0.84-3.45) |
| 5 to 10 yrs. | 2.60 (1.24-5.42)* |
| > 10 yrs. | 2.31 (1.48-3.59)* |
| Model 1 (M1)*: Adjusted for socio-demographic variables (n=8,893) | |
| Never | 1.00 Reference |
| No History of Monthly Use | 1.58 (1.08-2.30)* |
| History of Monthly Use | 1.69 (1.17-2.45)* |
| Duration of monthly | |
| < 5 yrs. | 1.83 (0.89-3.76) |
| 5 to 10 yrs. | 2.48 (1.15-5.35)* |
| > 10 yrs. | 1.49 (0.95-2.34) |
| Model 2 (M2)**: Adjusted for cigarette, alcohol use & physical activity (n=4,584) | |
| Never | 1.00 Reference |
| No History of Monthly Use | 0.81 (0.41-1.60) |
| History of Monthly Use | 0.78 (0.35-1.71) |
| Duration of monthly | |
| < 5 yrs. | 0.81 (0.26-2.55) |
| 5 to 10 yrs. | 0.91 (0.26-3.14) |
| > 10 yrs. | 0.74 (0.29-1.91) |
| Model 3 (M3)***: Adjusted for CVD risk factors (n=2,238) | |
| Never | 1.00 Reference |
| No History of Monthly Use | 0.52 (0.21-1.29) |
| History of Monthly Use | 0.75 (0.34-1.64) |
| Duration of monthly | |
| < 5 yrs. | 0.67 (0.22-2.06) |
| 5 to 10 yrs. | 0.72 (0.17-3.01) |
| > 10 yrs. | 0.78 (0.30-2.01) |
Notes: Odds ratios and 95% CI for reporting a prior MI by cannabis use status. Unweighted frequencies and weighted percentages reported.* Socio-demographic variables: age, sex, race/ethnicity, education, and household income.** All variables in M1 plus cigarette and alcohol use and physical activity.*** ASCVD risk and BMI.
A secondary analysis was performed to investigate the duration of monthly use preceding the MI as both a categorical and continuous measure (years of monthly use before MI). No association was found between years of monthly use and history of MI in the fully adjusted multivariable logistic regression model at any of the 3 levels of the categorical measure (<5 years, 5 to 10 years, >10 years) or for the continuous measure expressed in decades of use (10 years).
Models exploring potential effect modification by gender, race/ethnicity, cigarette smoking status, and age of first cannabis use were all nonsignificant (Supplementary Table 1). However, a significant interaction was found between a history of monthly cannabis use and recency of use for self-reported MI. Specifically, a history of monthly use among those denying use in the past month was associated with threefold greater odds of MI than among those with no history of monthly use (OR 2.98, 95% CI 1.08 to 8.60, p <0.03).
Discussion
In a nationally representative sample of middle-aged US adults, a history of monthly cannabis use (>1 year) before experiencing an MI was not associated with a history of self-reported physician-diagnosed MI after adjusting for sociodemographic characteristics, alcohol and cigarette use, physical activity, BMI, and cardiovascular risk factors, except when stratified by use within in the past month. In the latter case, the odds were threefold greater when use was not reported within the past month. Duration of monthly use preceding the MI was also not associated, including monthly use >10 years.
The lack of association in our study may be unexpected given the observation that those with a history of cannabis use were more likely to be male, smoke more cigarettes, drink more alcohol, and have a greater 10-year ASCVD risk. In contrast, they were also found to engage in more physical activity. Other observational studies, including other studies using NHANES data, have reported that cannabis users engage in more physical activity22 and have more favorable BMI profiles,12,23 lower concentrations of selected lipid biomarkers,8,12 and better glycemic parameters.23 It remains unclear whether these favorable factors counterbalance potential risks.
Our results diverge from previous research in the Behavioral Risk Factor Surveillance System survey in US adults, which reported elevated odds of MI among young adults (18 to 44 years old),5 and those of Shah et al,6 who reported increased odds of MI or coronary artery disease (CAD) among adults (18 to 74 years old) naive to cigarette smoking. Importantly, both studies investigated recent use (30 days before survey administration), including frequency of recent use, but did not assess long-term use or discriminate between cannabis use before or after the MI.
The greater odds of MI or CAD reported by Shah et al6 were driven by a single method of cannabis use (smoking), which, if causative, may be attributable to increases in oxidative stress, carbon monoxide, and other byproducts of cannabis combustion.24 This finding holds implications for future investigations, given the range of cannabis products accessible to consumers where cannabis has been legalized and regulated. Unfortunately for our study, the method of cannabis use was not available because it was only recently added to the NHANES DUQ in the 2019-to-2020 survey cycle.25
The finding that respondents with a history of monthly cannabis use, but no recent use, had significantly greater odds of MI is somewhat unexpected given the known acute cardiovascular effects of cannabis,26,27 other studies reporting associations with recent use, and the shorter duration of exposure in this group (average years of use before MI: 11.9 years vs 23.9 years for those who reported past month use). One might hypothesize smaller odds of MI owing to less cumulative exposure.
It is conceivable that subjects with a history of monthly cannabis use may have ceased usage earlier owing to health-related concerns that may have otherwise contributed to an increased risk of CVD. If this phenomenon exists, it may be comparable to the “smoker’s paradox” evident in tobacco research, in which cigarette smokers may receive more intensive medical interventions at a younger age and consequently exhibit better outcomes than do current smokers or never smokers.28 This finding warrants further investigation.
Study strengths include using a nationally representative sample and a relatively large sample size, which was balanced across sociodemographic and other characteristics. Another strength is the focus on a history of monthly use (long-term) as a measure of duration of exposure (preceding the reported MI) as opposed to recency of cannabis use (past month use) and frequency of recent use (days used in the past month), which fail to account for both the duration and timing of the exposure beyond the past month.
Limitations include a cross-sectional design, the age limit of the NHANES Drug Use Questionnaire (59 years old for cannabis use), and cannabis use and MI being based on self-report. Finally, and despite attempts to account for the magnitude of use (days of use per month, years of monthly use, joints or pipes per day), the actual amount of cannabis (grams of dried flower or extracted oil), and/or cannabinoids (milligrams of phytocannabinoids such as tetrahydrocannabinol, cannabidiol, and so on), and the method of use (e.g., inhalation, ingestion, and so on) were not available.
In conclusions, in a representative sample of middle-aged US adults, a history of monthly cannabis use for more than a year before an MI was not linked to a subsequent physician-diagnosed MI, after accounting for cardiovascular risk factors. However, when considering recent use, the odds were 3 times greater if no use was reported in the past month. The length of monthly use before the MI, including use >10 years, also showed no association. The evidence base for cardiovascular harms is conflicting and limited by the ability to accurately quantify use, especially the method of use, dose, and potency. Given the expanding access to cannabis products in the United States and around the world, more research, particularly longitudinal and experimental studies, is needed.
Supplementary Material
Acknowledgment
The authors thank Julie Denenberg for help with data analysis.
This study was funded by a T32 Grant, number T32HL079891 from National Institutes of Health, Bethesda, Maryland.
Footnotes
Declaration of Competing Interest
Dr. Corroon is a member of the Board of Directors of CV Sciences Inc., a manufacturer of hemp-derived cannabidiol products. The remaining authors have no competing interests to declare.
Supplementary materials
Supplementary material associated with this article can be found in the online version at https://doi.org/10.1016/j.amjcard.2023.07.065.
References
- 1.The Health Effects of Cannabis and Cannabinoids: The Current State of Evidence and Recommendations for Research. Washington: National Academies of sciences Engineering, and Medicine. The National Academies Press; 2017. [PubMed] [Google Scholar]
- 2.Bahji A, Stephenson C. International perspectives on the implications of cannabis legalization: a systematic review & thematic analysis. Int J Environ Res Public Health 2019;16:3095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Page RL 2nd, Allen LA, Kloner RA, Carriker CR, Martel C, Morris AA, Piano MR, Rana JS, Saucedo JF, American Heart Association Clinical Pharmacology Committee and Heart Failure and Transplantation Committee of the Council on Clinical Cardiology; Council on Basic Cardiovascular Sciences; Council on Cardiovascular and Stroke Nursing; Council on Epidemiology and Prevention; Council on Lifestyle and Cardiometabolic Health; and Council on Quality of Care and Outcomes Research. Medical marijuana, recreational cannabis, and cardiovascular health: a scientific statement from the American Heart Association. Circulation 2020;142:e131–e152. [DOI] [PubMed] [Google Scholar]
- 4.Pacher P, Steffens S, Haskó G, Schindler TH, Kunos G. Cardiovascular effects of marijuana and synthetic cannabinoids: the good, the bad, and the ugly. Nat Rev Cardiol 2018;15:151–166. [DOI] [PubMed] [Google Scholar]
- 5.Ladha KS, Mistry N, Wijeysundera DN, Clarke H, Verma S, Hare GMT, Mazer CD. Recent cannabis use and myocardial infarction in young adults: a cross-sectional study 2021. CMAJ 2021;193:E1377–E1384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shah S, Patel S, Paulraj S, Chaudhuri D. Association of marijuana use and cardiovascular disease: a behavioral risk factor surveillance system data analysis of 133,706 US adults. Am J Med 2021;134:614–620.e1. [DOI] [PubMed] [Google Scholar]
- 7.Skipina TM, Patel N, Upadhya B, Soliman EZ. Cannabis use is associated with prevalent coronary artery disease. Am J Med Sci 2022;364:304–308. [DOI] [PubMed] [Google Scholar]
- 8.Parekh T, Pemmasani S, Desai R. Marijuana use among young adults (18–44 years of age) and risk of stroke: a behavioral risk factor surveillance system survey analysis. Stroke 2020;51:308–310. [DOI] [PubMed] [Google Scholar]
- 9.Sun Y, Liu B, Wallace RB, Bao W. Association of cannabis use with All-Cause and cause-specific mortality among younger- and middle-aged U.S. adults. Am J Prev Med 2020;59:873–879. [DOI] [PubMed] [Google Scholar]
- 10.Reis JP, Auer R, Bancks MP, Goff DC Jr, Lewis CE, Pletcher MJ, Rana JS, Shikany JM, Sidney S. Cumulative lifetime marijuana use and incident cardiovascular disease in middle age: the coronary artery risk development in young adults (CARDIA) study. Am J Public Health 2017;107:601–606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Alshaarawy O, Sidney S, Auer R, Green D, Soliman EZ, Goff DC Jr, Anthony JC. Cannabis use and markers of systemic inflammation: the coronary artery risk development in young adults study. Am J Med 2019;132:1327–1334.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rodondi N, Pletcher MJ, Liu K, Hulley SB, Sidney S. Coronary Artery Risk Development in Young Adults (CARDIA) Study. Marijuana use, diet, body mass index, and cardiovascular risk factors (from the CARDIA study). Am J Cardiol 2006;98:478–484. [DOI] [PubMed] [Google Scholar]
- 13.Jakob J, von Wyl R, Stalder O, Pletcher MJ, Vittinghoff E, Tal K, Rana JS, Sidney S, Reis JP, Auer R. Cumulative marijuana use and carotid intima-media thickness at middle age: the CARDIA study. Am J Med 2021;134:777–787. e9. [DOI] [PubMed] [Google Scholar]
- 14.Auer R, Sidney S, Goff D, Vittinghoff E, Pletcher MJ, Allen NB, Reis JP, Lewis CE, Carr J, Rana JS. Lifetime marijuana use and subclinical atherosclerosis: the Coronary artery Risk Development in Young Adults (CARDIA) study. Addiction 2018;113:845–856. [DOI] [PubMed] [Google Scholar]
- 15.Bancks MP, Auer R, Carr JJ, Goff DC Jr, Kiefe C, Rana JS, Reis J, Sidney S, Terry JG, Schreiner PJ. Self-reported marijuana use over 25 years and abdominal adiposity: the Coronary artery Risk Development in Young Adults (CARDIA) Study. Addiction 2018;113:689–698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.CDC. About the National Health and Nutrition Examination Survey. Available at: https://www.cdc.gov/nchs/nhanes/about_nhanes.htm. Accessed on October, 13, 2022
- 17.CDC. NHANES tutorials - weighting module. Available at: https://wwwn.cdc.gov/nchs/nhanes/tutorials/weighting.aspx. Accessed on June 23, 2022.
- 18.CDC. National Health and Nutrition Examination Survey 2017-2018 Drug Use Data Documentation, Codebook, and Frequencies. Available at: https://wwwn.cdc.gov/Nchs/Nhanes/2017-2018/DUQ_J.htm. Accessed on June 13, 2022.
- 19.Vidot DC, Bispo JB, Hlaing WM, Prado G, Messiah SE. Moderate and vigorous physical activity patterns among marijuana users: results from the 2007–2014 National Health and Nutrition Examination Surveys. Drug Alcohol Depend 2017;178:43–48. [DOI] [PubMed] [Google Scholar]
- 20.Goff DC Jr, Lloyd-Jones DM, Bennett G, Coady S, D’Agostino RB, Gibbons R, Greenland P, Lackland DT, Levy D, O’Donnell CJ, Robinson JG, Schwartz JS, Shero ST, Smith SC Jr, Sorlie P, Stone NJ, Wilson PW, Jordan HS, Nevo L, Wnek J, Anderson JL, Halperin JL, Albert NM, Bozkurt B, Brindis RG, Curtis LH, DeMets D, Hochman JS, Kovacs RJ, Ohman EM, Pressler SJ, Sellke FW, Shen WK, Smith SC Jr, Tomaselli GF, American College of Cardiology/American Heart Association Task Force on Practice Guidelines. 2013 ACC/AHA guideline on the assessment of cardiovascular risk: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation 2014;129(suppl 2):S49–S73. [DOI] [PubMed] [Google Scholar]
- 21.Akinbami LJ, Chen TC, Davy O, Ogden CL, Fink S, Clark J, Riddles MK, Mohadjer LK. National Health and Nutrition Examination Survey, 2017-March 2020 Prepandemic File: Sample Design, Estimation, and Analytic Guidelines. Vital Health Stat 1 2022:(190):1–36. [PubMed] [Google Scholar]
- 22.Ong LQ, Bellettiere J, Alvarado C, Chavez P, Berardi V. Cannabis use, sedentary behavior, and physical activity in a nationally representative sample of US adults. Harm Reduc J 2021;18:48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rajavashisth TB, Shaheen M, Norris KC, Pan D, Sinha SK, Ortega J, Friedman TC. Decreased prevalence of diabetes in marijuana users: cross-sectional data from the National Health and Nutrition Examination Survey (NHANES) III. BMJ Open 2012;2:e000494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wu TC, Tashkin DP, Djahed B, Rose JE. Pulmonary hazards of smoking marijuana as compared with tobacco. N Engl J Med 1988;318:347–351. [DOI] [PubMed] [Google Scholar]
- 25.2019-2020 NHANES Questionnaire Instruments - Drug Use. Available at: https://wwwn.cdc.gov/nchs/data/nhanes/2019-2020/questionnaires/DUQ-ACASI-K-508.pdf. Accessed on May 23, 2022.
- 26.Mittleman MA, Lewis RA, Maclure M, Sherwood JB, Muller JE. Triggering myocardial infarction by marijuana. Circulation 2001;103:2805–2809. [DOI] [PubMed] [Google Scholar]
- 27.Chetty K, Lavoie A, Deghani P. A literature review of cannabis and myocardial infarction-what clinicians may not be aware of. CJC Open 2020;3:12–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Aune E, Røislien J, Mathisen M, Thelle DS, Otterstad JE. The "smoker’s paradox" in patients with acute coronary syndrome: a systematic review. BMC Med 2011;9:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.