Abstract
Background & Aims:
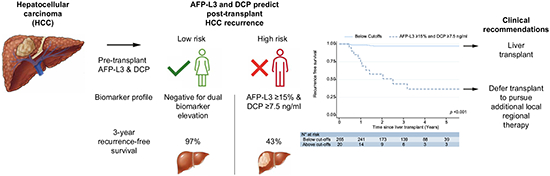
Alpha-fetoprotein (AFP) predicts hepatocellular carcinoma (HCC) recurrence after liver transplant (LT) but remains an imperfect biomarker. The role of DCP (des-gamma-carboxyprothrombin) and AFP-L3 (AFP bound to Lens culinaris agglutinin) in predicting HCC recurrence remains incompletely characterized. AFP-L3 and DCP could identify patients at high risk of post-transplant HCC recurrence and serve as liver transplant exclusion criteria to defer transplant until patients receive additional risk-reducing pre-transplant locoregional therapy.
Methods:
This prospective cohort study included consecutive patients with HCC who underwent LT (within or down-staged to Milan criteria) between 2017 and 2022. Pre-transplant AFP, AFP-L3, and DCP measurements were obtained. The primary endpoint was the ability of biomarkers to predict HCC recurrence-free survival.
Results:
This cohort included 285 patients with a median age of 67 (IQR 63–71). At LT, median biomarker values were AFP 5.0 ng/ml (IQR 3.0–12.1), AFP-L3 6.7% (0.5–13.2), and DCP 1.0 ng/ml (0.3–2.8). Most (94.7%) patients received pre-LT locoregional therapy. After a median post-LT follow-up of 3.1 years, HCC recurrence was observed in 18 (6.3%) patients. AFP-L3 and DCP outperformed AFP with C-statistics of 0.81 and 0.86 respectively, compared with 0.74 for AFP. A dual-biomarker combination of AFP-L3 ≥15% and DCP ≥7.5 predicted 61.1% of HCC recurrences, whereas HCC only recurred in 7 of 265 (2.6%) patients not meeting this threshold. The Kaplan-Meier recurrence-free survival rate at 3 years post-LT was 43.7% for patients with dual-positive biomarkers compared to 97.0% for all others (p <0.001).
Conclusions:
Dual-positivity for AFP-L3 ≥15% and DCP ≥7.5 strongly predicted post-LT HCC recurrence. This model could refine LT selection criteria and identify high-risk patients who require additional locoregional therapy prior to LT.
Keywords: alpha fetoprotein (AFP), AFP-L3, des-gamma-carboxyprothrombin (DCP), liver transplant (LT), hepatocellular carcinoma (HCC)
Graphical abstract
Introduction
Hepatocellular carcinoma (HCC) is a highly fatal tumor that was the sixth most diagnosed cancer and the third leading cause of cancer-related death worldwide in 2020.1 In the Us, ~30% of all liver transplants (LT) are performed for HCC.2 Historically, transplant criteria were solely based on tumor size and number using the Milan criteria which improved survival by reducing HCC recurrence risk.3 More recently, using biomarkers to predict tumor biology has become important in determining LT eligibility for more nuanced allocation of donor livers. Alpha-fetoprotein (AFP) is the most robustly studied tumor marker for liver transplant in HCC and has been shown to predict waitlist dropout, presence of microvascular invasion, and worse tumor differentiation.4–6 In the US, patients with an AFP ≥1,000 ng/ml are not eligible for model for end-stage liver disease (MELD) score exception points until their AFP falls below 500 ng/ml with locoregional therapy (LRT) due to the high-risk of recurrence.7,8 Reducing AFP levels to <100 ng/ml further improves post-LT survival.9
Despite its utility, AFP is an imperfect tumor marker. Most patients with HCC being evaluated for LT have a normal AFP and patients with normal AFP levels still experience post-LT HCC recurrence or waitlist dropout.4,5,10 Despite the exclusion of patients with an AFP ≥1,000 ng/ml, 10–15% of patients still experience post-LT HCC recurrence, highlighting the need for additional prognostic markers to predict unfavorable tumor biology.2,11 Studies have shown improved HCC detection and surveillance using the tumor markers AFP-L3 (fraction of AFP bound to Lens culinaris agglutinin) and DCP/PIVKA-II (des-gamma-carboxyprothrombin, also known as protein induced by vitamin K absence or antagonist II).12 DCP is a form of prothrombin lacking carboxylation due to some HCC cells not expressing the vitamin K dependent carboxylase enzyme.13 Similarly, HCC cells supplied by arterial blood differentially express uridine diphosphate alpha (1,6)-fucosyltransferase that attaches fructose residues to AFP, resulting in differential binding to the reagent Lens culinaris agglutinin, termed AFP-L3.14
While AFP-L3 and DCP have been shown to be useful in HCC diagnosis, the utility of these markers in predicting HCC tumor biology and LT outcomes has not been well established. We previously reported that AFP-L3 levels ≥15% or DCP levels ≥7.5 ng/ml at the time of LT (n = 153) were associated with a 5-and 9-fold greater risk, respectively, of one or more high-risk histopathologic explant features such as microvascular invasion, poorly differentiated tumor grade, and tumor burden beyond Milan LT eligibility criteria.15 We also recently showed AFP-L3 >35% and DCP >7.5 ng/ml were more strongly associated with increased waitlist dropout than AFP.16 The goal of this study is to prospectively evaluate the utility of the biomarkers AFP, AFP-L3, and DCP in prognosticating post-LT outcomes for HCC with a primary outcome of HCC recurrence-free survival (RFS), and a secondary outcome of high-risk explant pathology in a single-center US cohort.
Patients and methods
Study population
Biomarkers were measured within 90 days of LT in consecutive patients with HCC from July 2017 to August 2022. The study received expedited approval by our center’s Institutional Review Board with minimal study risk assignment. Inclusion criteria included diagnosis of HCC based on CT or MRI meeting LI-RADS (Liver Imaging Reporting and Data System) 5 criteria or needle biopsy confirming a histologic diagnosis of HCC if imaging diagnosis was equivocal.17 Patients met either Milan LT criteria or down-staging criteria at the time of listing for LT.3 United Network for Organ Sharing (UNOS) national down-staging criteria include: one lesion >5 cm and ≤8 cm, two or three lesions each ≤5 cm with total tumor diameter ≤8 cm, four or five lesions none >3 cm with total tumor diameter ≤8 cm, and no vascular invasion on imaging or metastatic disease. Patients without vascular invasion or extrahepatic disease but exceeding UNOS down-staging criteria based on tumor number, tumor size, or total tumor diameter (“all-comers”) were considered for LT at our center on a case-by-case basis. AFP ≥1,000 ng/ml was an exclusion criterion for LT unless the level decreased to <500 ng/ml with LRT. Of the 345 patients transplanted at University of California, San Francisco for HCC since July 2017, 21 patients were excluded due to missing biomarkers and 22 patients were excluded due to the presence of mixed HCC-cholangiocarcinoma on pathology. Additionally, warfarin is known to cause artificial elevations of DCP. Therefore, 17 patients taking warfarin at the time of LT biomarker measurements were excluded from the analysis.
Tumor characteristics and recurrence
Explant histopathologic features included maximal tumor diameter, number of tumor nodules, histologic grade of differentiation based on the Edmondson and Steiner criteria and the presence or absence of vascular invasion, based on standardized reporting during the entire study period. High risk pathology was defined as the presence of microvascular invasion, beyond Milan Criteria on explant, or poor tumor differentiation on explant. Only viable tumors in the explant specimen were considered during tumor staging. Information was obtained from explant histology for each patient to calculate the RETREAT (Risk Estimation of Tumor Recurrence After Transplant) score, a validated HCC recurrence prediction model.18 HCC recurrence was determined from query of the Electronic Health Record by radiographic (LI-RADS) or biopsy results.
Statistical analysis and biomarker thresholds
Data were analyzed using Stata version 17 (StataCorp, College Station, TX). The primary outcome was the ability for an individual biomarker or biomarker combination to predict HCC RFS. Secondary outcomes were the ability of biomarker models to predict explant tumor characteristics and the ability of explant characteristics to predict HCC recurrence. Single biomarker cut-offs for AFP (≥100 ng/ml), AFP-L3 (≥15%, ≥25%) and DCP (≥7.5 ng/ml, ≥15 ng/ml) were used. Biomarker combinations investigated included dual-positivity for AFP-L3 ≥15% and DCP ≥7.5 ng/ml and dual-positivity for AFP-L3 ≥25% and DCP ≥15 ng/ml.
Due to the varying post-LT follow-up times in the cohort, a standardized timepoint of 3 years was selected to analyze descriptive statistics, given a median follow-up time of 3.1 years. This method reduced bias by excluding patients with short follow-up times and no recurrence. Two-sample exact Wilcoxon rank-sum (Mann-Whitney) and Chi-squared analyses were employed to evaluate the association biomarker thresholds with explant pathology and the RETREAT score. Univariate Cox-proportional hazards analysis, the Kaplan-Meier method, and the log-rank test were used to evaluate associations between explant characteristics and RFS, along with the ability of individual and combined biomarkers to predict RFS. Area under the receiver-operating characteristic curves and time-dependent mean Harrell’s C-statistic from 100 bootstrap replications quantified individual continuous biomarkers’ ability to predict HCC recurrence. Multivariate Cox-proportional hazards analysis was performed with a dual positive biomarker threshold of AFP-L3 ≥15% and DCP ≥7.5 ng/ml and initial Milan classification at diagnosis.
Results
Patient characteristics
The baseline demographic and clinical characteristics of the 285 patients comprising the cohort are summarized in Table 1. The median age of these patients was 67.0 years (IQR 63.0–71.0), 214 (75.1%) were men, and 60 (21.1%) were Asian/Pacific Islander. Hepatitis B and C were the most common etiologies of liver disease (64.6%), followed by non-alcoholic steatohepatitis (15.4%) and alcohol use (12.3%). In this cohort, 239 (83.9%) patients were within Milan criteria at diagnosis, 32 (11.2%) were within UNOS down-staging criteria, and 14 (4.9%) were within “all-comers” criteria. At time of listing, the median MELD was 11.0 (IQR 8.0–13.0). Most (94.7%) patients underwent LRT with transarterial chemoembolization (TACE) (66.3%) as the most common modality. The median overall biomarker values at LT were AFP 5.0 ng/ml (IQR 3.0–12.1), AFP-L3 6.7% (0.5–13.2), and DCP 1.0 ng/ml (IQR 0.3–2.8; Table 1). Median waitlist time until LT was 1.0 year (IQR 0.6–1.4).
Table 1.
Baseline and waitlist characteristics of study population.
| Patients (N = 285) | |
|---|---|
| Median age at listing (years, IQR) | 67.0 (63.0–71.0) |
| Sex (n, %) | |
| Men | 214 (75.1) |
| Women | 71 (24.9) |
| Race (n, %) | |
| White | 127 (44.6) |
| Hispanic | 67 (23.5) |
| Asian/Pacific Islander | 60 (21.1) |
| African American | 13 (4.6) |
| Other | 18 (6.3) |
| Etiology of liver disease (n, %) | |
| Hepatitis B or C | 184 (64.6) |
| NASH | 44 (15.4) |
| Alcohol Use | 35 (12.3) |
| Other | 22 (7.7) |
| Tumor stage at diagnosis (n, %) | |
| Milan criteria | 239 (83.9) |
| UNOS downstaging | 32 (11.2) |
| All-comers | 14 (4.9) |
| Median number of HCC viable lesions on explant (n, IQR) | 1.0 (0–2.0) |
| Median size of largest tumor on explant (cm, IQR) | 1.0 (0–1.8) |
| Median total viable tumor on explant (cm, IQR) | 1.2 (0–3.0) |
| Locoregional therapy | |
| Ever received (n, %) | 270 (94.7) |
| Median number of treatments (n, IQR) | 2 (1–3) |
| Types of locoregional therapy (n, %) | |
| TACE | 189 (66.3) |
| RFA | 100 (35.1) |
| Y90 | 73 (25.6) |
| Median MELD at listing (n, IQR) | 11.0 (8.0–13.0) |
| Median MELD at transplant (n, IQR) | 17.0 (13.0–21.0) |
| Median waitlist time to LT (years, IQR) | 1.0 (0.6–1.4) |
| Median AFP at LT (ng/ml, IQR) | 5.0 (3.0–12.1) |
| AFP ≥100 ng/ml at LT (n, %) | 12 (4.2) |
| Median AFP-L3 at LT (%, IQR) | 6.7 (0.5–13.2) |
| AFP-L3 ≥15% at LT (n, %) | 57 (20.0) |
| Median DCP at LT (ng/ml, IQR) | 1.0 (0.3–2.8) |
| DCP ≥7.5 ng/ml at LT (n, %) | 40 (14.0) |
AFP, alpha-fetoprotein; AFP-L3, AFP bound to Lens culinaris agglutinin; DCP, des-gamma-carboxyprothrombin; HCC, hepatocellular carcinoma; LT, liver transplant; MELD, model for end-stage liver disease; NASH, non-alcoholic steatohepatitis; RFA, radiofrequency ablation; TACE, transarterial chemoembolization; UNOS, United Network for Organ Sharing.
Explant tumor size and number of HCC lesions are summarized in Table 2. On explant pathology, 13 (4.6%) patients had vascular invasion, 47 (16.5%) patients had high-risk pathology, and 40 (14.0%) had tumor size and number beyond stage T2/Milan criteria (under-staged; Table 2). The median RETREAT score was 1.0 (IQR 0–2.0).
Table 2.
Explant characteristics on pathology by dual positivity for AFP-L3 ≥15% and DCP ≥7.5 ng/ml.
| Overall (N = 285) | Negative for dual biomarker elevation (n = 265) | AFP-L3 ≥15% and DCP ≥7.5 ng/ml (n = 20) | p value | |
|---|---|---|---|---|
| Median largest viable tumor on explant (cm, IQR) | 1.0 (0–1.8) | 0.8 (0–1.7) | 2.0 (1.6–2.9) | <0.001¶ |
| Median total viable tumor diameter on explant (cm, IQR) | 1.2 (0–3.0) | 1.0 (0–2.7) | 4.9 (3.1–8.2) | <0.001¶ |
| Median number of viable HCC lesions on explant (n, IQR) | 1.0 (0–2) | 1.0 (0–2) | 4.0 (2.5–5) | <0.001¶ |
| Vascular invasion (n, %) | 13 (4.6) | 5 (1.9) | 8 (40.0) | <0.001* |
| High risk pathology on explant (n, %) | 47 (16.5) | 30 (11.3) | 17 (85.0) | <0.001* |
| Explant outside Milan (n, %) | 40 (14.0) | 28 (10.6) | 12 (60.0) | <0.001* |
| RETREAT score (score, IQR) | 1 (0–2) | 1 (0–2) | 3 (2–4) | <0.001¶ |
| Recurrence-free survival (HR, 95% CI) | – | Reference | 26.6 (10.3–68.9) | <0.001Δ |
| Overall survival (HR, 95% CI) | – | Reference | 8.2 (3.4–19.6) | <0.001Δ |
AFP-L3, AFP bound to Lens culinaris agglutinin; DCP, des-gamma-carboxyprothrombin; HCC, hepatocellular carcinoma; HR, hazard ratio; RETREAT, risk estimation of tumor recurrence after transplant.
Chi squared test.
Two-sample exact Wilcoxon rank-sum (Mann-Whitney) test.
Cox proportional hazards test.
Performance of single biomarkers at predicting HCC recurrence
The median post-LT follow-up was 3.1 years (IQR 1.6–4.3). Early HCC recurrence was observed in 6.3% (n = 18) of patients with a median time to recurrence of 1.0 years (IQR 0.6–1.4) after LT. Of the 21 (7.4%) deaths recorded, 10 (48%) were attributed to HCC recurrence. The overall RFS rates for the entire cohort were 96.7% (95% CI 94.0–98.3) at 1 year, 94.1% (95% CI 90.4–96.4) at 2 years, and 93.0% (95% CI 88.8–95.6) at 3 years. Median biomarker values stratified by recurrence vs. no-recurrence at 3 years post-LT were 29 ng/ml (IQR 9–85) and 5.0 ng/ml (IQR 3.0–14.0) for AFP, 30.7% (IQR 12.5–65.7) and 6.7% (IQR 0.5–12.9) for AFP-L3, and 26.2 ng/ml (IQR 9.5–46.0) and 1.0 ng/ml (IQR 0.4–2.4) for DCP.
Overall area under the receiver-operating characteristic curve analysis of continuous biomarkers predicting HCC recurrence is shown in Fig. 1A. The overall bootstrapped time-dependent C-statistics were AFP at 0.74 (95% CI 0.58–0.89), AFP-L3 at 0.81 (95% CI 0.69–0.93), and DCP at 0.86 (95% CI 0.77–0.96). Sensitivity and specificity of dichotomized biomarker measures for HCC recurrence are shown in Table S1.
Fig. 1. Relationships of AFP-L3 and DCP with HCC recurrence.
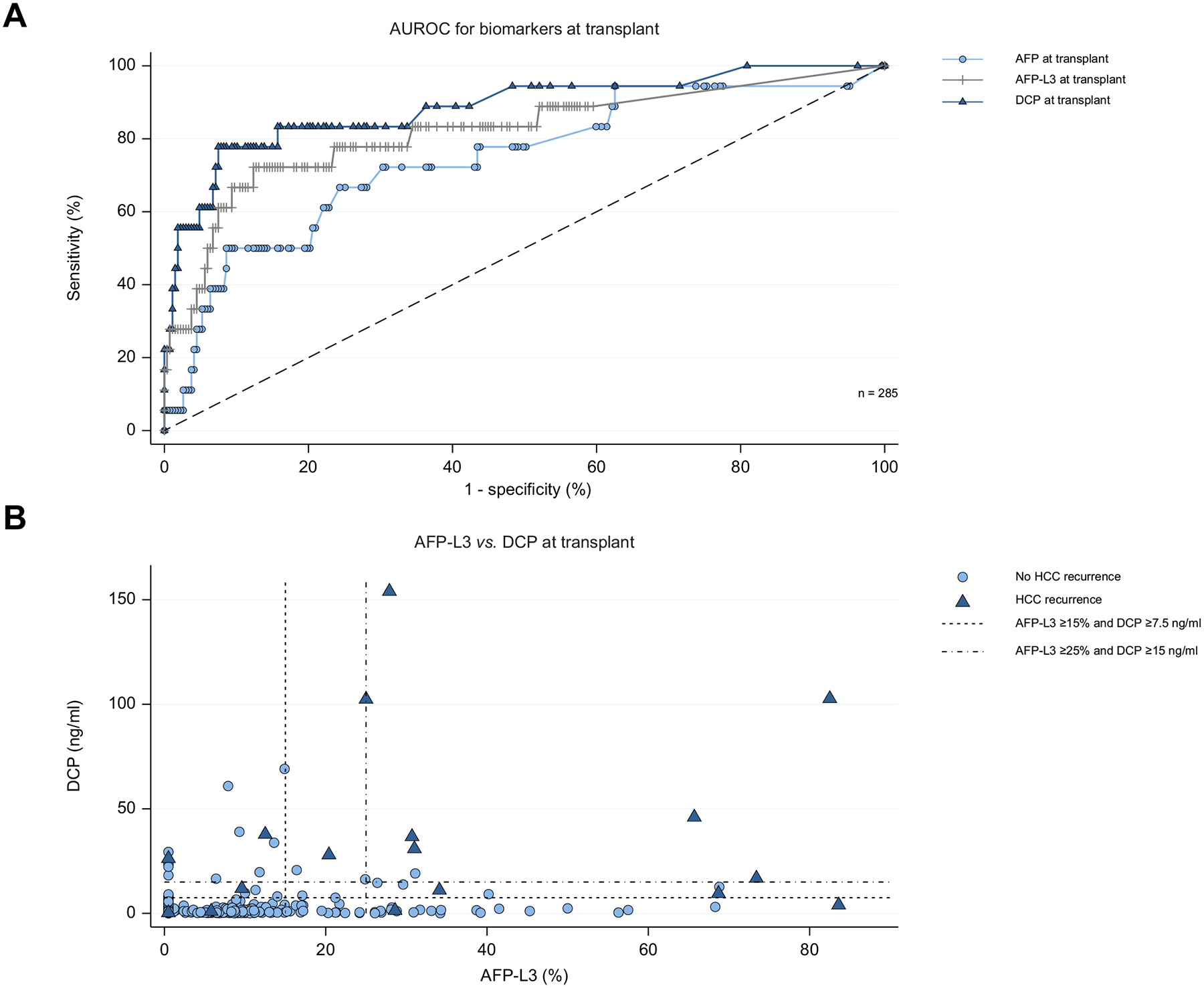
(A) Area under the receiver-operating characteristics curves for continuous AFP, AFP-L3, and DCP relationship with recurrence (n = 285). (B) Relationship between AFP-L3 and DCP values stratified by HCC recurrence. Low (AFP-L3 ≥15% and DCP ≥7.5 ng/ml) cut-off represented by short-dashed line. High (AFP-L3 ≥25% and DCP ≥15 ng/ml) dual biomarker positive model represented by the dash-dot line. AFP, alpha-fetoprotein; AFP-L3, AFP bound to Lens culinaris agglutinin; DCP, des-gamma-carboxyprothrombin.
Biomarker cut-offs were likewise evaluated at 3 years post-LT, at which time, AFP ≥100 ng/ml was a poor predictor of HCC recurrence in our cohort as only two of nine patients who met this cut-off developed recurrence (Table S1). For this reason, AFP was not included in multi-biomarker models. The prognostic ability of low and high AFP-L3 (≥15% vs. ≥25%) and DCP (7.5 ng/ml vs. 15 ng/ml) thresholds were compared at 3 years post-LT. The higher thresholds increased specificity by excluding 14 false positives for AFP-L3 and six for DCP.
Univariate predictors of early HCC recurrence
Of the biomarker thresholds evaluated, only AFP ≥100 ng/ml at LT was not significantly associated with worse overall RFS (p = 0.17 Fig. 2A, Table S2). At 3 years post-LT, the RFS was 93.3% (95% CI 89.2–96.0) for AFP <100 ng/ml and 83.3% (95% CI 48.2–95.6) for AFP ≥100 ng/ml. AFP-L3 ≥15% was associated with worse overall RFS (p <0.001; Fig. 2B, Table S2). The 3-year RFS was 76.4% (95% CI 61.9–86.0) if positive for AFP-L3 ≥15% and 97.4% (95% CI 93.9–98.9) if negative. DCP had the largest hazard ratio (HR) for risk of RFS among single biomarkers (DCP ≥7.5 vs. <7.5 ng/ml; HR 24.2, 95% CI 8.0–73.7, p <0.001; Fig. 2C; Table S2). DCP ≥7.5 ng/ml had a 3-year RFS of 62.8% (95% CI 44.2–76.7) if positive vs. 98.2% (95% CI 95.3–99.3) if negative.
Fig. 2. Kaplan-Meier survival estimates of single biomarker thresholds.
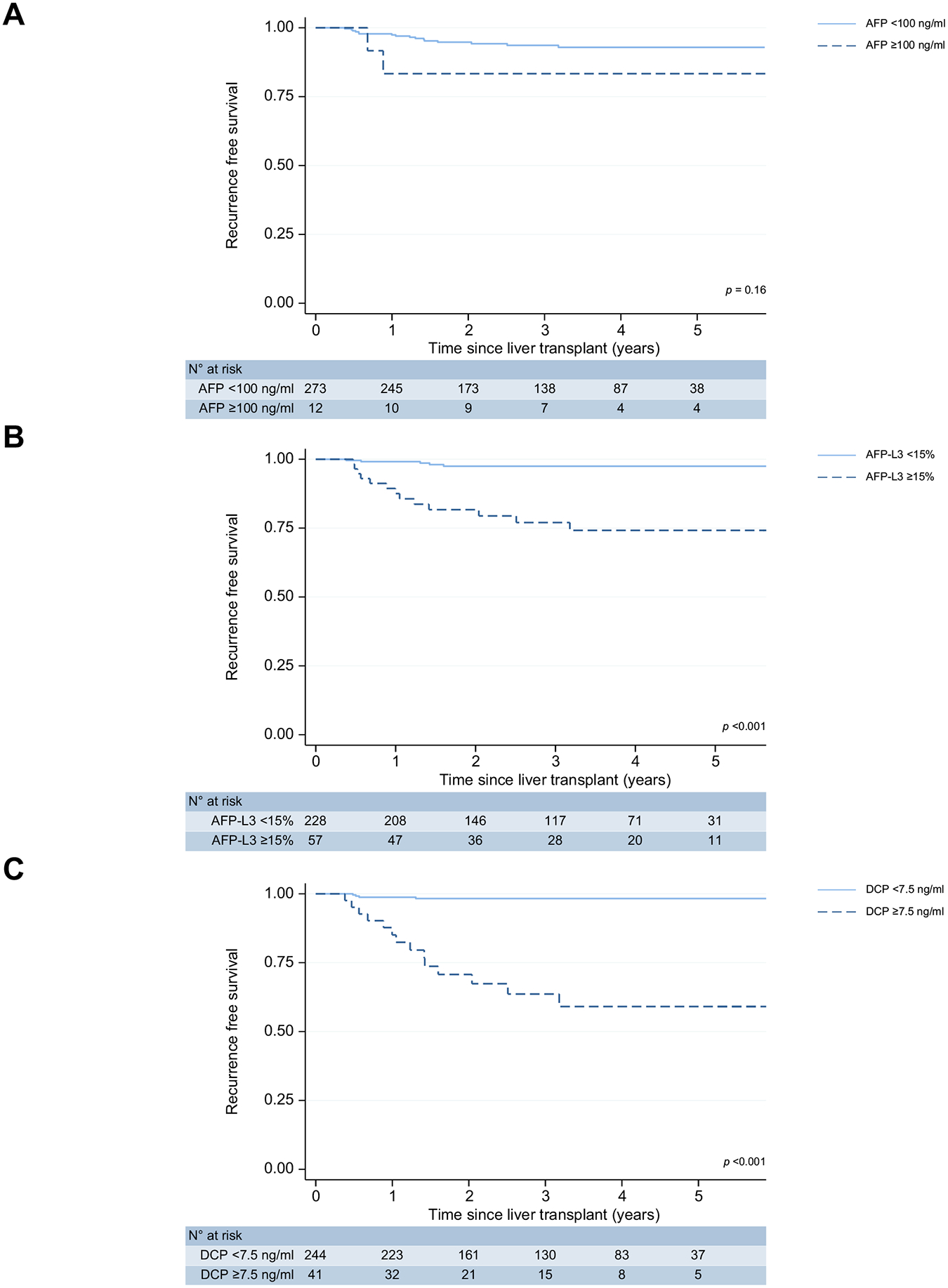
(A) AFP ≥100 ng/ml (B) AFP-L3 ≥15% (C) DCP ≥7.5 ng/ml. AFP, alpha-fetoprotein; AFP-L3, AFP bound to Lens culinaris agglutinin; DCP, des-gamma-carboxyprothrombin.
Baseline characteristics including age, waitlist time, and MELD at listing were not significantly associated with HCC recurrence. Total number of LRT treatments (HR 1.5; 95% CI 1.2–1.8; p <0.001) and total number of TACE treatments (HR 1.6; 95% CI 1.24–1.94; p <0.001) were significantly associated with HCC recurrence. Zero of 71 female patients experienced early HCC recurrence, while 18 of 214 male patients recurred (estimated HR = 1); the effect was statistically significant by the likelihood ratio chi-squared test from the Cox model (p = 0.002). All explant tumor characteristics were significantly associated with worse RFS (Table 2).
Dual-biomarker models improve prediction of early HCC recurrence
At 3 years post-LT the dual positive AFP-L3 ≥15 % and DCP ≥7.5 ng/ml model reduced the percentage of patients who tested positive but did not recur to 3.4% from 16.5% and 10.1% with the AFP-L3 or DCP single biomarker thresholds, respectively. A more stringent dual positive biomarker combination of AFP-L3 ≥25% and DCP ≥15 ng/ml further reduced the false positive rate to 0.4%. AFP-L3 and DCP levels at LT were stratified by HCC recurrence and plotted to visualize the distribution of individual patient biomarker values with respect to the two dual-positive biomarker models (Fig. 1B).
Dual positivity for AFP-L3 and DCP predicts explant pathology
Both dual positive models strongly predicted high-risk explant pathology at transplant (Table 2, Table S3). On Cox-proportional analysis both dual positive models were also associated with increased HCC recurrence, and worse all-cause mortality (p <0.001) with higher HRs observed with AFP-L3 ≥25% and DCP ≥15 ng/ml.
Dual positivity for AFP-L3 ≥15% and DCP ≥7.5 ng/ml strongly predicts RFS
The dual positive combination of AFP-L3 ≥15% and DCP ≥7.5 demonstrated superior ability to predict RFS (p <0.001, Fig. 3A) The 3-year RFS was 43.7% (95% CI 20.3–65.2) if dual-positive vs. 97.0% (93.8–98.6) if negative for dual-biomarker elevation. Regarding the AFP-L3 ≥25% and DCP ≥15% model, the 3-year RFS was 11.1% (1.0–38.8) if positive for dual-elevation vs. 96.2% (92.9–98.0) if negative (p <0.001 for overall RFS, Fig. 3B).
Fig. 3. Kaplan-Meier survival estimates of dual-positive biomarker thresholds.
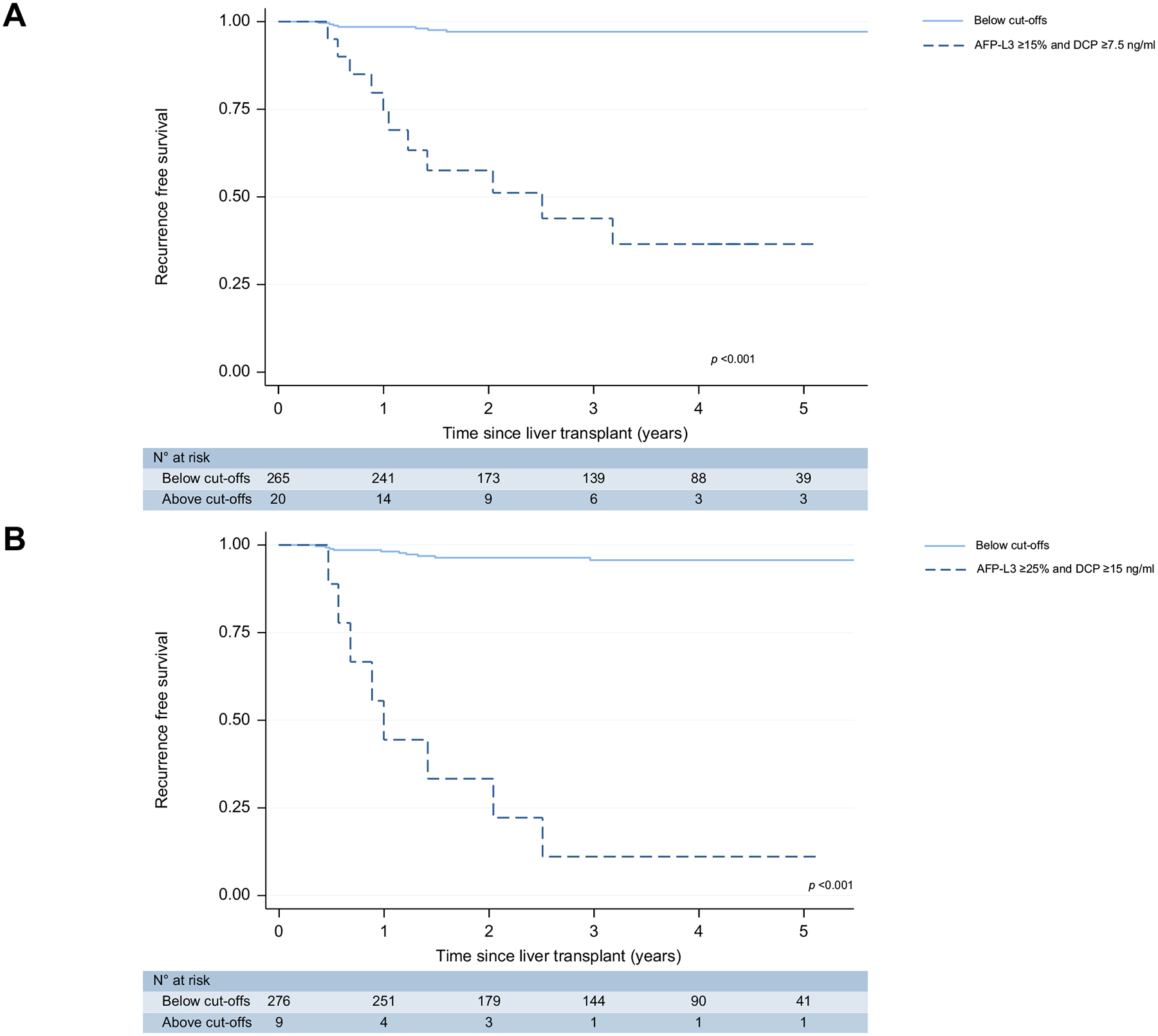
(A) AFP-L3 ≥15% and DCP ≥7.5 ng/ml (B) AFP-L3 ≥25% and DCP ≥15 ng/ml. AFP, alpha-fetoprotein; AFP-L3, AFP bound to Lens culinaris agglutinin; DCP, des-gamma-carboxyprothrombin.
Stratification by initial milan classification
Of the 20 patients who pet the dual-biomarker combination of AFP-L3 ≥15% and DCP ≥7.5, 18 were within Milan criteria of whom 11 recurred and two were UNOS-downstaged, though neither experienced HCC recurrence. None of the 14 “all-comers” patients met the dual-biomarker positive threshold (Table S4). In a subgroup analysis (n = 271) excluding UNOS “all-comers”, we observed a 3-year RFS rate of 98.2% in patients not meeting the dual AFP-L3 and DCP biomarker positive threshold compared to 43.7% in those who did have dual biomarker positivity.
In an exploratory multivariate analysis including the dual biomarker model and initial Milan category at diagnosis, the dual-positive biomarker model was independently associated with worse RFS (HR 44.3; 95% CI 14.0–139.5; p <0.001). UNOS downstaging was not an independent predictor of worse RFS (HR 0.55; 95% CI 0.07–4.2; p = 0.567) though the ‘all-comers’ group was an independent predictor of worse RFS (HR 14.4; 95% CI 3.2–64.4; p <0.001) when compared to a reference group of within Milan (Table S5).
Discussion
Herein, we report the results of the first prospective analysis demonstrating AFP-L3 and DCP strongly predict early HCC recurrence after LT. This study aimed to investigate AFP-L3 and DCP thresholds that could supplement the role of AFP ≥1,000 ng/ml as a LT exclusion criterion and identify patients who would benefit from additional LRT prior to LT. We found that AFP-L3 and DCP were more powerful at predicting early HCC recurrence than AFP with Harrell’s C-statistics of 0.81 and 0.86, respectively, compared to 0.74 for AFP.
An optimal biomarker threshold to serve as an exclusion criterion for LT should identify patients with an unacceptably high risk of early post-transplant HCC recurrence while minimizing the number of patients that test positive but do not experience early HCC recurrence. Presumably, most patients at a high risk of recurrence can receive additional LRT to reduce their tumor burden prior to LT. This strategy judiciously allocates organs by limiting HCC recurrence and allowing patients to receive maximal benefit from a LT. We therefore opted for a dual-positive biomarker combination as single biomarker models resulted in up to 17% of patients without early HCC recurrence testing positive for AFP-L3 or DCP.
We identified a dual-positive AFP-L3 ≥15% and DCP ≥7.5 ng/ml model that represented 7% of the cohort with a 3-year HCC RFS rate of 44% (compared to a 3-year RFS rate of 97% in all others). This model showed excellent discriminative capacity at identifying patients at high risk of early HCC recurrence, with a lower false positive rate of 3%. We also considered a more stringent model of AFP-L3 ≥25% and DCP ≥15 ng/ml, which was associated with a 3-year RFS rate of 11% and further reduced the number of patients testing positive but not experiencing recurrence to <1%. These dual-biomarker models show a discriminative power similar to the MoRAL (model to predict tumor recurrence after living donor LT) score (3-year RFS of ~60% if MoRAL positive and ~85% if MoRAL negative).24 Similarly, the Kyoto criteria that utilizes DCP was associated with a 5-year post-transplant recurrence rate of 51% if outside Kyoto vs. 4% if within Kyoto criteria.19
The AFP-L3 ≥25% and DCP ≥15 ng/ml model mirrors the stringent approach of using AFP ≥1,000 ng/ml to select the highest risk patients for LT exclusion, though only nine patients met these criteria. Importantly, the less stringent dual-biomarker model, AFP-L3 ≥15% and DCP ≥7.5 ng/ml, was still strongly predictive of HCC recurrence. We favor this model as clinically a 56% estimated risk of recurrence at 3 years is sufficient to exclude a patient from transplant and uses commonly accepted clinical cut-offs for straightforward interpretation. Lastly, while our study’s median follow-up time of 3 years with a median time to recurrence of 1 year captures early recurrences with poor prognosis, we are cognizant that some patients will develop late HCC recurrence in the future. Therefore, adopting the dual-positivity for AFP-L3 ≥15% and DCP ≥7.5 ng/ml model will conservatively allow more patients to receive additional LRT prior to LT to decrease the chance of late recurrences. Studies have demonstrated that LRT reduces AFP-L3 levels and improves survival.20 Rising AFP-L3 levels post-transplant also predict HCC recurrence, hinting that decreasing AFP-L3 levels with LRT prior to LT may reduce the risk of HCC recurrence.21 A small retrospective study likewise showed DCP reduction after TACE improved survival in a subgroup of patients with DCP levels >3.8 ng/ml.22
Prior research from our group showed that AFP-L3, and DCP correlated with high-risk explant pathology.15 This report confirms that high-risk explant pathology correlates with increased risk of HCC recurrence and furthermore shows that dual-positivity for AFP-L3 ≥15% and DCP ≥7.5 ng/ml predicted all high-risk explant characteristics evaluated in our study. This supports our theory that high AFP-L3 and DCP levels predict unfavorable tumor biology. The RETREAT score which currently uses AFP and explant characteristics to predict the risk of post-LT HCC recurrence may also be improved with the addition of AFP-L3 and DCP.
The strengths of this study include a prospective study design, homogenous study population, few patients missing biomarker measurements, and well-designed LT selection criteria for HCC at a high-volume transplant center. Our study with a median post-LT follow-up time of 3 years captures early HCC recurrences that occur in the first 2 years and accounts for the majority of all post-LT recurrences.23 Early recurrences are also known to carry an inferior prognosis compared with late recurrences.24
However, our study was limited by a relatively small number of recurrences and thus we were unable to perform substantive multivariate analyses. This cohort (n = 285 with 18 recurrences) is larger than the study that resulted in AFP ≥1,000 ng/ml being adopted as an exclusion criterion for LT (n = 211 with 19 recurrences).5 It will be important to confirm these findings in larger, multi-center prospective studies with multivariate analyses. Since AFP-L3 and DCP are not routinely measured at most centers it is difficult investigate their role in LT. We advocate for broader testing of AFP-L3 and DCP at diagnosis, listing, and transplant. If these biomarkers were measured every 3 months like AFP, more granular analyses of the effect of these biomarkers would be possible. Due to the known artificial elevations in DCP from taking warfarin, the dual-biomarker model is not applicable to patients taking warfarin.
In conclusion, this first prospective study analyzing the discriminative power of AFP, AFP-L3 and DCP to predict early post-LT recurrence demonstrates patients meeting a dual-positive combination of AFP-L3 ≥15% and DCP ≥7.5 ng/ml have a poor 3-year RFS rate of 44%. This high-risk of early recurrence indicates that these patients are likely not appropriate candidates for LT without additional pre-transplant LRT. These biomarkers are promising candidates to further refine LT exclusion criteria to complement the use of AFP to optimize organ allocation.
Supplementary Material
Highlights.
Even when excluding patients outside Milan criteria with AFP ≥1,000 ng/ml, a subset still experiences post-transplant HCC recurrence.
A dual-positive biomarker model of AFP-L3 ≥15% and DCP ≥7.5 ng/ml strongly predicts the risk of early HCC recurrence.
AFP-L3 and DCP may help further refine liver transplant criteria for patients with HCC.
High-risk patients should receive additional tumor treatment prior to transplant to optimize the use of scarce organs.
Impact and implications.
Alpha-fetoprotein (AFP) is used to predict hepatocellular carcinoma (HCC) recurrence after liver transplant, but it remains an imperfect biomarker. In this prospective study, the biomarkers DCP (des-gamma-carboxyprothrombin) and AFP-L3 (AFP bound to Lens culinaris agglutinin) strongly predicted early HCC recurrence and outperformed AFP. A dual-biomarker combination of AFP-L3 ≥15% and DCP ≥7.5 predicted the majority of recurrences and could be used to further refine liver transplant eligibility criteria.
Financial support
This work is supported in part by a grant from the to the University of California, San Francisco Liver Center (P30 DK026743).
Conflicts of interest
Neil Mehta has served on advisory boards for WAKO Diagnostics and has received institutional research funding from WAKO Diagnostics, Glycotest, and Target Pharmasolutions. Francis Yao has received institutional research funding from WAKO Diagnostics. None of the other authors have any relevant potential conflicts of interest to disclose.
Please refer to the accompanying ICMJE disclosure forms for further details.
Abbreviations
- AFP
alpha-fetoprotein
- AFP-L3
AFP bound to Lens culinaris agglutinin
- DCP
des-gamma-carboxyprothrombin
- HR
hazard ratio
- HCC
hepatocellular carcinoma
- LRT
locoregional therapy
- LT
liver transplant
- MELD
model for end-stage liver disease
- RETREAT
risk estimation of tumor recurrence after transplant
- RFS
recurrence-free survival
- TACE
transarterial chemoembolization
- UNOS
United Network for Organ Sharing
Footnotes
Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jhep.2023.08.020
Data availability statement
The miRNA deep sequencing raw data has been deposited in Gene Expression Omnibus with the accession number GSE232919. All data included in this study are available upon reasonable request by contact with the corresponding author.
References
- [1].Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2021;71(3):209–249. 10.3322/caac.21660. [DOI] [PubMed] [Google Scholar]
- [2].Sapisochin G, Bruix J. Liver transplantation for hepatocellular carcinoma: outcomes and novel surgical approaches. Nat Rev Gastroenterol Hepatol 2017;14(4):203–217. 10.1038/nrgastro.2016.193. [DOI] [PubMed] [Google Scholar]
- [3].Mazzaferro V, Regalia E, Doci R, Andreola S, Pulvirenti A, Bozzetti F, et al. Liver transplantation for the treatment of small hepatocellular carcinomas in patients with cirrhosis. N Engl J Med 1996;334(11):693–699. 10.1056/NEJM199603143341104. [DOI] [PubMed] [Google Scholar]
- [4].Mehta N, Dodge JL, Goel A, Roberts JP, Hirose R, Yao FY. Identification of liver transplant candidates with hepatocellular carcinoma and a very low dropout risk: implications for the current organ allocation policy. Liver Transpl 2013;19(12):1343–1353. 10.1002/lt.23753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Hameed B, Mehta N, Sapisochin G, Roberts JP, Yao FY. Alpha-fetoprotein level > 1000 ng/ml as an exclusion criterion for liver transplantation in patients with hepatocellular carcinoma meeting the Milan criteria. Liver Transpl 2014;20(8):945–951. 10.1002/lt.23904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Duvoux C, Roudot-Thoraval F, Decaens T, Pessione F, Badran H, Piardi T, et al. Liver transplantation for hepatocellular carcinoma: a model including α-fetoprotein improves the performance of Milan criteria. Gastroenterology 2012;143(4):986–994.e3. 10.1053/j.gastro.2012.05.052. quiz e14–15. [DOI] [PubMed] [Google Scholar]
- [7].Liver and Intestinal Organ Transplantation Committee. OPTN/UNOS policy notice modification to hepatocellular carcinoma (HCC) extension criteria. OPTN/UNOS; 2018. [Google Scholar]
- [8].Halazun KJ, Rosenblatt RE, Mehta N, Quirino L, Hajifathalian K, Goren A, et al. Dynamic α-fetoprotein response and outcomes after liver transplant for hepatocellular carcinoma. JAMA Surg 2021;156(6):559–567. 10.1001/jamasurg.2021.0954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Mehta N, Dodge JL, Roberts JP, Hirose R, Yao FY. Alpha-fetoprotein decrease from > 1,000 to < 500 ng/ml in patients with hepatocellular carcinoma leads to improved posttransplant outcomes. Hepatology 2019;69(3):1193–1205. 10.1002/hep.30413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Agopian VG, Harlander-Locke MP, Markovic D, Zarrinpar A, Kaldas FM, Cheng EY, et al. Evaluation of patients with hepatocellular carcinomas that do not produce α-fetoprotein. JAMA Surg 2017;152(1):55–64. 10.1001/jamasurg.2016.3310. [DOI] [PubMed] [Google Scholar]
- [11].Goldman ML, Zhou K, Dodge JL, Yao F, Mehta N. Lower Alpha-Fetoprotein Threshold of 500 ng/ml for Liver Transplantation May Improve Posttransplant Outcomes in Patients With Hepatocellular Carcinoma. Liver Transplantation. n/a(n/a). doi: 10.1002/lt.26392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Johnson PJ, Pirrie SJ, Cox TF, Berhane S, Teng M, Palmer D, et al. The detection of hepatocellular carcinoma using a prospectively developed and validated model based on serological biomarkers. Cancer Epidemiol Biomarkers Prev 2014;23(1):144–153. 10.1158/1055-9965.EPI-13-0870. [DOI] [PubMed] [Google Scholar]
- [13].De J, Shen Y, Qin J, Feng L, Wang Y, Yang L. A systematic Review of des-γ-carboxy prothrombin for the diagnosis of primary hepatocellular carcinoma. Medicine (Baltimore) 2016;95(17):e3448. 10.1097/MD.0000000000003448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Noda K, Miyoshi E, Kitada T, Nakahara S, Gao C-X, Honke K, et al. The enzymatic basis for the conversion of nonfucosylated to fucosylated α-fetoprotein by acyclic retinoid treatment in human hepatoma cells: activation of α1–6 fucosyltransferase. TBI 2002;23(4):202–211. 10.1159/000067253. [DOI] [PubMed] [Google Scholar]
- [15].Kotwani P, Chan W, Yao F, Mehta N. DCP and AFP-L3 are complementary to AFP in predicting high-risk explant features: results of a prospective study. Clin Gastroenterol Hepatol Published Online January 29, 2021. 10.1016/j.cgh.2021.01.043.S1542-S3565(21)93-98. [DOI] [PubMed] [Google Scholar]
- [16].Mehta N, Kotwani P, Norman J, Shui A, Li JL, Saxena V, et al. AFP-L3 and DCP are superior to AFP in predicting waitlist dropout in hepatocellular carcinoma patients: results of a prospective study. Liver Transplant. 10.1097/LVT.0000000000000149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Mitchell DG, Bruix J, Sherman M, Sirlin CB. LI-RADS (liver imaging reporting and Data System): summary, discussion, and consensus of the LI-RADS management working group and future directions. Hepatology 2015;61(3):1056–1065. 10.1002/hep.27304. [DOI] [PubMed] [Google Scholar]
- [18].Mehta N, Heimbach J, Harnois DM, Sapisochin G, Dodge J, Lee D, et al. Validation of a risk estimation of tumor recurrence after transplant (RETREAT) score for hepatocellular carcinoma recurrence after liver transplant. JAMA Oncol 2017;3(4):493–500. 10.1001/jamaoncol.2016.5116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Kaido T, Ogawa K, Mori A, Fujimoto Y, Ito T, Tomiyama K, et al. Usefulness of the Kyoto criteria as expanded selection criteria for liver transplantation for hepatocellular carcinoma. Surgery 2013;154(5):1053–1060. 10.1016/j.surg.2013.04.056. [DOI] [PubMed] [Google Scholar]
- [20].Huang C, Sheng S, Sun X, Liu J, Huang G. Lens culinaris agglutinin-reactive α-fetoprotein decline after transcatheter arterial chemoembolization in patients with hepatocellular carcinoma predicts survival. Clin Chim Acta 2014;431:232–238. 10.1016/j.cca.2014.02.009. [DOI] [PubMed] [Google Scholar]
- [21].Shawky Elsawabi A, Abdel K, Ibrahim W, Saleh S, Massoud Y, Abdelbary M, et al. α-Fetoprotein (AFP)-L3% and transforming growth factor B1 (TGFB1) in prognosis of hepatocellular carcinoma after radiofrequency. Egypt Liver. J 2019;9(1):8. 10.1186/s43066-019-0008-5. [DOI] [Google Scholar]
- [22].Lee YK, Kim SU, Kim DY, Ahn SH, Lee LH, Lee DY, et al. Prognostic value of α-fetoprotein and des-γ-carboxy prothrombin responses in patients with hepatocellular carcinoma treated with transarterial chemoembolization. BMC Cancer 2013;13:5. 10.1186/1471-2407-13-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Toso C, Cader S, Mentha-Dugerdil A, Meeberg G, Majno P, Morad I, et al. Factors predicting survival after post-transplant hepatocellular carcinoma recurrence. J Hepatobiliary Pancreat Sci 2013;20(3):342–347. 10.1007/s00534-012-0528-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Portolani N, Coniglio A, Ghidoni S, Giovanelli M, Benetti A, Tiberio GAM, et al. Early and late recurrence after liver resection for hepatocellular carcinoma. Ann Surg 2006;243(2):229–235. 10.1097/01.sla.0000197706.21803.a1. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The miRNA deep sequencing raw data has been deposited in Gene Expression Omnibus with the accession number GSE232919. All data included in this study are available upon reasonable request by contact with the corresponding author.


