Abstract
Purpose:
To evaluate recovery of platelet count after transjugular intrahepatic portosystemic shunt (TIPS) creation and patient factors predicting platelet recovery after TIPS creation.
Materials and Methods:
Adults with cirrhosis who underwent TIPS creation at 9 U.S. hospitals from 2010 to 2015 were included in this retrospective analysis. Change in platelets from before TIPS to 4 months after TIPS creation was characterized. Logistic regression was used to assess factors associated with top quartile percentage platelet increase after TIPS. Subgroup analyses were performed among patients with a pre-TIPS platelet count of ≤50 ×109/L.
Results:
A total of 601 patients were included. The median absolute change in platelets was 1 × 109/L (−26 × 109/L to 25 × 109/L). Patients with top quartile percent platelet increase experienced ≥32% platelet increase. In multivariable analysis, pre-TIPS platelet counts (odds ratio [OR], 0.97 per 109/L; 95% CI, 0.97–0.98), age (OR, 1.24 per 5 years; 95% CI, 1.10–1.39), and pre-TIPS model for end-stage liver disease (MELD) scores (OR, 1.06 per point; 95% CI, 1.02–1.09) were associated with top quartile (≥32%) platelet increase. Ninety-four (16%) patients had a platelet count of ≤50 × 109/L before TIPS. The median absolute platelet change was 14 × 109/L (2 × 109/L to 34 × 109/L). Fifty-four percent of patients in this subgroup were in the top quartile for platelet increase. In multivariable logistic regression, age (OR, 1.50 per 5 years; 95% CI, 1.11–2.02) was the only factor associated with top quartile platelet increase in this subgroup.
Conclusions:
TIPS creation did not result in significant platelet increase, except among patients with a platelet count of ≤50 × 109/L before TIPS. Lower pre-TIPS platelet counts, older age, and higher pre-TIPS MELD scores were associated with top quartile (≥32%) platelet increase in the entire cohort, whereas only older age was associated with this outcome in the patient subset with a pre-TIPS platelet count of ≤50 × 109/L.
Transjugular intrahepatic portosystemic shunt (TIPS) creation is an effective intervention for ascites and variceal bleeding, 2 common complications of portal hypertension in patients with cirrhosis (1). Thrombocytopenia is another complication of portal hypertension in patients with cirrhosis that has important implications for periprocedural and peritransplant management of patients with cirrhosis due to perceived risk of bleeding (2–7). However, research regarding the impact of TIPS creation on thrombocytopenia is limited, and the few prior studies investigating platelet recovery after TIPS creation have yielded conflicting results. Some studies (8–13) have reported improvement in thrombocytopenia after TIPS creation. Others reported no significant change in platelets after TIPS creation (14,15) or a trend toward decreased platelet counts after TIPS creation (16).
In addition to these conflicting results, existing studies are conflicting regarding factors, such as patient or procedural variables, that may predict platelet recovery. One study (12) reported that portosystemic pressure gradient was predictive of platelet recovery, whereas another (9) reported no such association. Studies (8,12) are also conflicting on whether pre-TIPS thrombocytopenia severity predicts platelet recovery after TIPS creation. Most of the prior studies have been limited by small sample sizes and to single medical centers. This study is a retrospective analysis of platelet recovery in a multicenter cohort of patients with cirrhosis who underwent TIPS creation (17,18). This study was intended to evaluate platelet count change after TIPS creation and to identify factors associated with platelet count increase after TIPS creation. Thrombocytopenia in portal hypertension is believed to be caused by hypersplenism or splenic sequestration due to congestion in the portal vein (19). The hypothesis of this study is that relief of congestion in the portal vein by TIPS will lead to an improvement in thrombocytopenia.
MATERIALS AND METHODS
Participants
The Advancing Liver Therapeutics Approaches Study and the Advancing Liver Therapeutics Approaches Study Group have been described previously (17,18). In short, adult patients with cirrhosis who underwent TIPS at 9 academic medical centers in the United States from January 1, 2010, through December 31, 2015, were included in this study. This study was approved by the institutional review boards at each of the 9 participating sites (Northwestern University, University of California San Francisco, The Scripps Clinic, University of Wisconsin, University of Florida, University of Arizona, Stanford University, Columbia University, and The University of Chicago).
Clinical Data
Demographic and clinical data were obtained by a direct medical chart review, Common Procedural Terminology codes, International Classification of Diseases −9/10 billing codes, encounter codes, and problem list codes by research personnel at participating study sites and uploaded to a central study database using Research Electronic Data Capture software hosted at the organizing center. Etiologies of cirrhosis were categorized as alcohol-associated liver disease, hepatitis C, nonalcoholic fatty liver disease, or other etiologies. Indications for TIPS were categorized as refractory ascites and/or hepatic hydrothorax, variceal bleeding, portal vein thrombosis/other indications, or multiple indications. Portosystemic pressure gradients were calculated from the difference between portal venous and systemic venous pressures prior to and after TIPS creation. The pre-TIPS main portal vein direction of flow was determined from the pre-TIPS liver ultrasound. Pre-TIPS baseline data were obtained within 2–28 days before TIPS. Post-TIPS data defined as 4 months after TIPS were obtained between 90 and 150 days after TIPS. Platelet count was evaluated at 4 months after TIPS creation to allow sufficient time for platelet counts to recover. Based on the clinical experience of the investigators and prior studies, it can take 3–4 months for refractory ascites to resolve following TIPS creation (20–22). A time frame at least beyond 3 months after TIPS was chosen to allow for post-TIPS recovery and “recalibration.” Although 6 months would have been ideal, follow-up in this retrospective study was variable at 6 months. Therefore, the 4-month time frame was the best time frame to balance these 2 concerns.
Percent change in platelet counts was chosen rather than absolute change because it takes into account the baseline of the patient because a 10 × 109/L increase is more significant in a patient with a baseline of 30 × 109/L than it is to a patient who starts with a baseline of 80 × 109/L. Change in platelets between before and after TIPS was calculated; then, the percentage change was divided into quartiles. The top quartile for platelet count increase was isolated and assessed for patient factors associated with inclusion in this group: (a) age because thrombocytopenia is associated with age (23), (b) pre-TIPS model for end-stage liver disease (MELD) as an indicator of liver disease severity and the most commonly used metric to assess the risk of hepatic decompensation after TIPS among the investigators, and (c) pre-TIPS platelet count because it is hypothesized that severity of thrombocytopenia is associated with platelet response after TIPS. These variables were selected for multivariable analysis a priori via purposeful selection because the authors believed that these variables could potentially confound the relationship between the primary predictor and the primary outcome.
Procedural Technique
TIPS creation procedures, including target end points, associated variceal/portosystemic shunt embolization, and any additional ancillary interventions, were performed at centers participating in this retrospective study according to individual institutional and operator standards following established techniques. The only procedural variables included in this analysis were pre-TIPS portosystemic gradient and post-TIPS portosystemic gradient (although specific anatomy of portosystemic shunts was not captured).
Statistical Analysis
Categorical variables are presented as percentages, and continuous variables are presented as medians and interquartile ranges (IQRs). Variables were compared by change in platelet count using Wilcoxon rank-sum and chi-square tests, respectively. Patients were categorized as being in the top quartile for platelet increase or not in the top quartile for platelet increase on the basis of percent change in platelet count before TIPS to 4 months after TIPS. Change in platelet count after TIPS creation was assessed using paired t-tests for before TIPS to 4 months after TIPS. Then, the pre-to-post TIPS outcome of interest used in univariable and multivariable logistic regression was being in the top quartile for platelet increase, as defined earlier. The association between being in the top quartile for platelet increase and patient factors, pre-TIPS platelet count, age, and pre-TIPS MELD, was assessed using the multivariable logistic regression at 4 months after TIPS. Given the clinical relevance of severe thrombocytopenia (platelets, ≤50 × 109/L) for perceived risk of bleeding with procedures, subgroup analyses among those with pre-TIPS platelets ≤50 × 109/L were performed (Stata, SE17).
Inclusion of patients in this study is illustrated in Figure 1. From January 1, 2010, through December 31, 2015, 1,260 patients with cirrhosis underwent TIPS creation at the 9 U.S. hospitals participating in this study. Of these 1,260 patients, 184 (15%) died within 4 months and 115 (9%) underwent transplantation within 4 months. Patients who died or underwent transplantation within 4 months after TIPS were excluded from this study. An additional 360 (29%) patients were excluded from this study due to either missing pre-TIPS platelet counts or missing 4 months post-TIPS platelet counts. Therefore, 601 patients with cirrhosis were included in the analysis for this study.
Figure 1.
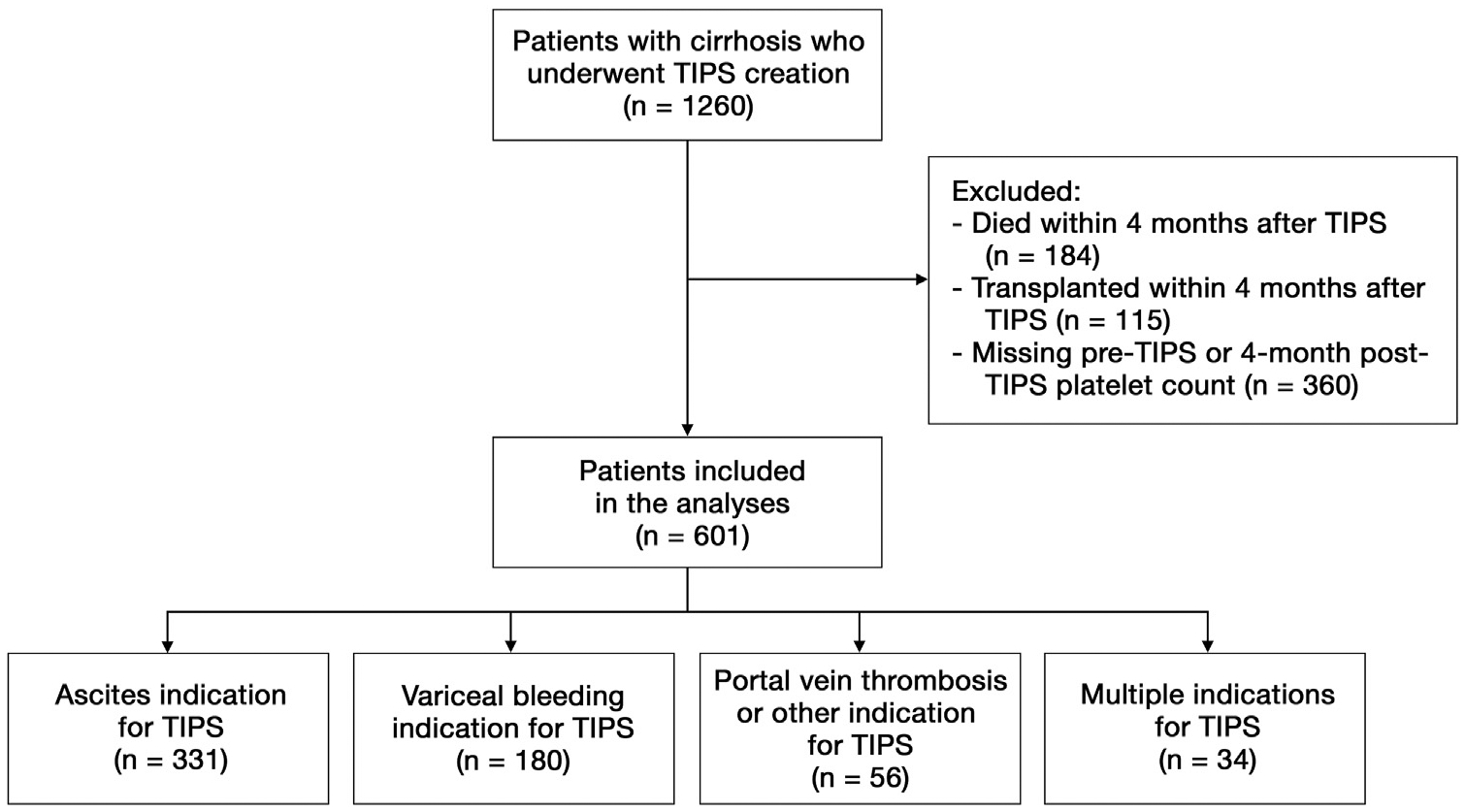
Flow diagram of patients included in the study. TIPS = transjugular intrahepatic portosystemic shunt.
Baseline characteristics of the 601 patients included in this analysis are presented in Table 1. Two hundred twenty-seven (38%) patients were women, with a median age of 57 years (IQR, 52–62 years) and a median pre-TIPS MELD score of 13 (IQR, 11–17). The most common indications for TIPS creation were as follows: 331 (55%) ascites, 180 (30%) variceal bleeding (encompassing TIPS creation for acute bleeding and primary and secondary prophylaxis), 56 (9%) portal vein thrombosis/other, and 34 (6%) multiple indications.
Table 1.
Characteristics of the 601 Patients with Cirrhosis Included in This Study
| Characteristics | All (N = 601) | By platelet response after TIPS | P value | |
|---|---|---|---|---|
| Not in the top quartile for percent platelet increase, n = 450 (75%) | In the top quartile for percent platelet increase, n = 151 (25%) | |||
| Age (y) | 57 (52–62) | 57 (50–62) | 59 (54–64) | .001 |
| Female | 227 (38) | 169 (38) | 58 (38) | .85 |
| Race/ethnicity | ||||
| Non-Hispanic White | 410 (68) | 308 (68) | 102 (68) | .74 |
| Black | 26 (4) | 22 (5) | 4 (3) | |
| Hispanic | 118 (20) | 85 (19) | 33 (22) | |
| Asian | 10 (2) | 7 (2) | 3 (2) | |
| Other | 37 (6) | 28 (6) | 9 (6) | |
| Etiology of liver disease | ||||
| Alcohol | 184 (31) | 152 (34) | 32 (21) | .02 |
| Hepatitis C | 194 (32) | 140 (31) | 54 (36) | |
| Nonalcoholic fatty liver disease | 114 (19) | 85 (19) | 29 (19) | |
| Other | 109 (18) | 73 (16) | 36 (24) | |
| Indication for TIPS | ||||
| Ascites/hepatic hydrothorax | 331 (55) | 262 (58) | 69 (46) | .002 |
| Variceal bleeding | 180 (30) | 121 (27) | 59 (39) | |
| PVT/other | 56 (9) | 37 (8) | 19 (13) | |
| Multiple | 34 (6) | 30 (7) | 4 (3) | |
| Pre-TIPS platelet count (x109/L) | 89 (63–132) | 103 (74–145) | 63 (46–81) | .0001 |
| MELD | 13 (11–17) | 13 (10–16) | 15 (11–19) | .0002 |
| INR | 1.4 (1.2–1.6) | 1.4 (1.2–1.6) | 1.5 (1.3–1.6) | .002 |
| Total bilirubin (mg/dL) | 1.5 (1.0–2.4) | 1.4 (1.0–2.2) | 1.8 (1.2–3.2) | .0001 |
| Creatinine (mg/dL) | 1.0 (0.8–1.3) | 1.0 (0.8–1.3) | 1.1 (0.8–1.4) | .11 |
| Albumin (g/dL) | 2.9 (2.5–3.3) | 2.8 (2.5–3.2) | 2.9 (2.4–3.4) | .42 |
| Dialysis | 16 (3) | 9 (2) | 7 (5) | .08 |
| Ascites | 441 (74) | 349 (79) | 92 (62) | <.001 |
| Hepatic encephalopathy | 269 (45) | 197 (44) | 72 (48) | .40 |
| Hepatocellular carcinoma | 44 (8) | 26 (6) | 18 (12) | .01 |
| Variceal bleeding within 1 y prior to TIPS | 214 (36) | 153 (34) | 61 (41) | .15 |
| Gastric varices | 105 (17) | 83 (18) | 22 (15) | .28 |
| Pre-TIPS portosystemic gradient | 16 (13–20) | 16 (13–21) | 16 (13–19) | .15 |
| Post-TIPS portosystemic gradient | 6 (4–8) | 6 (4–8) | 6 (4–8) | .19 |
| Change in portosystemic gradient | 10 (7–14) | 11 (8–14) | 10 (7–14) | .31 |
| Pre-TIPS main portal vein flow direction | ||||
| Hepatopetal | 285 (92) | 220 (93) | 65 (88) | .31 |
| Hepatofugal | 15 (5) | 10 (4) | 5 (7) | |
| No flow | 10 (3) | 6 (3) | 4 (5) | |
| Pre-TIPS portal vein diameter | 1.7 (1.4–12) | 1.7 (1.4–13) | 1.7 (1.4–10) | .57 |
| Variceal embolization at TIPS | 149 (26) | 102 (24) | 47 (32) | .04 |
Note–Values are reported as median (interquartile range) or n (%). Patients in the top quartile for percentage platelet increase had ≥32% platelet increase. INR = international normalized ratio; MELD = model for end-stage liver disease; PVT = portal vein thrombosis; TIPS = transjugular intrahepatic portosystemic shunt
RESULTS
For the entire cohort, there was no significant median absolute change in platelet count from before TIPS to after TIPS: 1 × 109/L (IQR, −26 × 109/L to 25 × 109/L). The distribution of percent change in platelet count is shown in Figure 2, and the relationship between pre- and post-TIPS platelet counts is shown in Figure 3. In the subgroup of those with severe thrombocytopenia, there was a significant absolute change in platelet count from before to after TIPS: 14 × 109/L (IQR, 2 × 109/L to 34 × 109/L). The top quartile for platelet change by percentage was calculated to be ≥32% platelet count increase.
Figure 2.
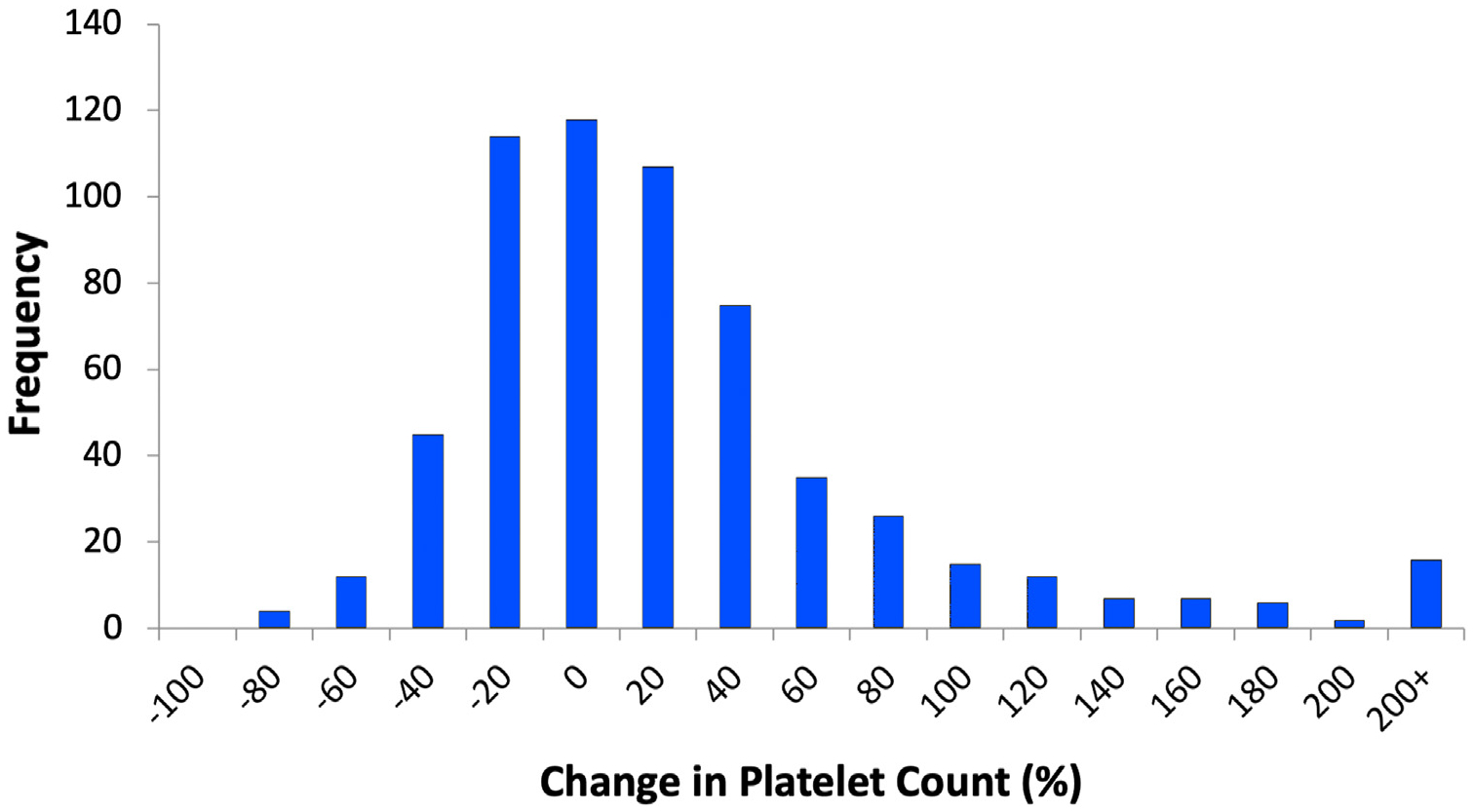
Histogram of change in platelet count at 4 months after transjugular intrahepatic portosystemic shunt (percentage).
Figure 3.
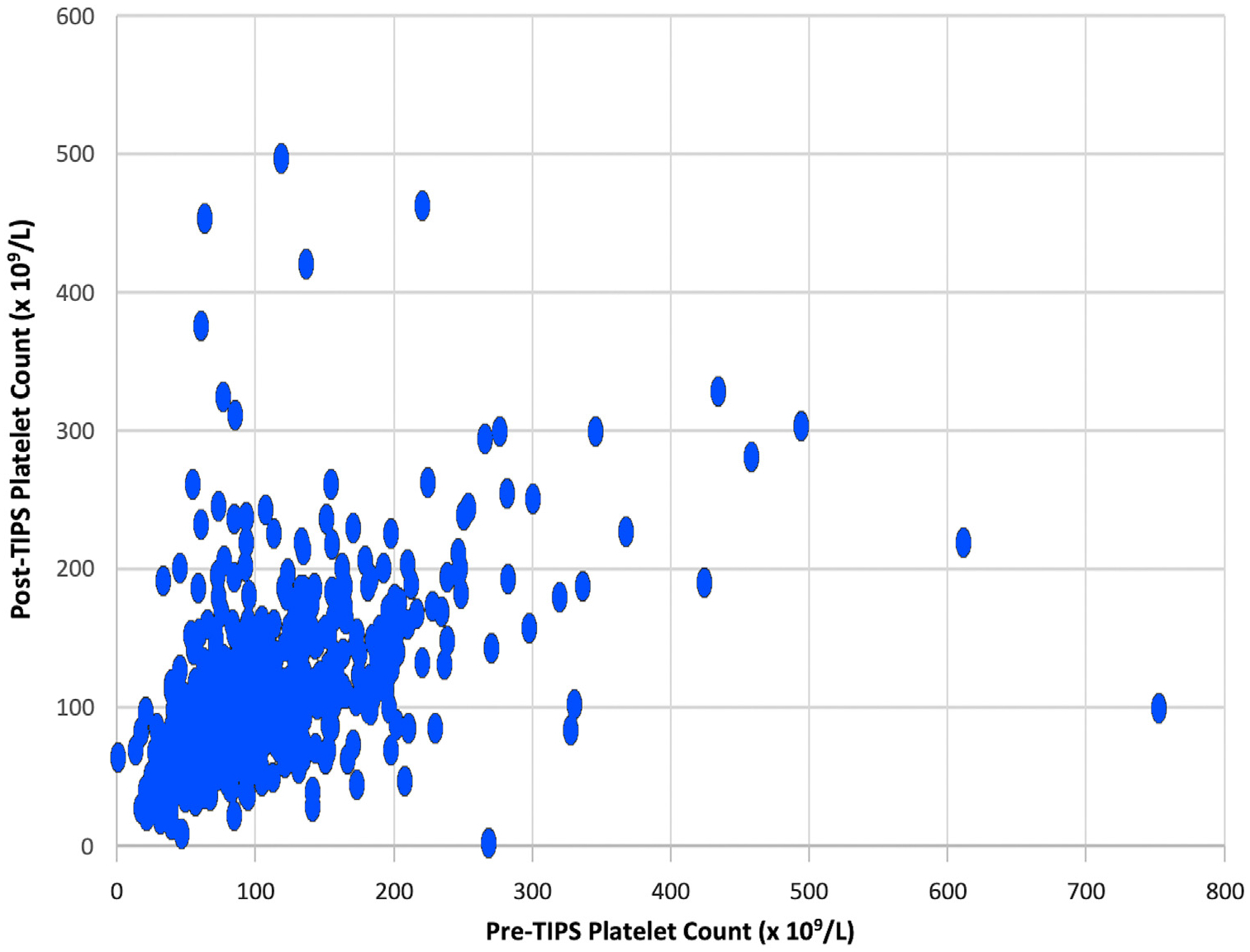
Four months post-TIPS platelet count versus pre-TIPS platelet counts. TIPS = transjugular intrahepatic portosystemic shunt.
The 601 patients were categorized as being in the top quartile for platelet increase (≥32% platelet count increase) or not in the top quartile for platelet increase at 4 months after TIPS (Table 1). The 2 groups were similar by platelet response categories in sex, race, pre-TIPS portosystemic gradient, pre-TIPS main portal vein direction of flow, pre-TIPS portal vein diameter, presence of hepatic encephalopathy, and presence of a variceal bleed within 1 year prior to TIPS (P > .05).
Compared with those who did not experience a pre- to post-TIPS change in platelet count of ≥32%, those who did experience a ≥32% increase in platelet count were more likely to be older (58 vs 57 years; P = .001), have an “other” etiology of cirrhosis (24% vs 16%; P = .02), have an indication for TIPS of variceal bleeding (39% vs 27%; P = .002) or portal vein thrombosis/other (13% vs 8%; P = .002), have a lower pre-TIPS platelet count (63 vs 103; P < .001), have a higher pre-TIPS MELD score (15 vs 13; P < .001), undergo variceal embolization at the time of TIPS (32% vs 24%; P = .04), and have hepatocellular carcinoma (12% vs 6%; P = .01).
For the entire cohort, the results of univariable and multivariable logistic regression analyses of factors predictive of being in the top quartile for platelet increase 4 months after TIPS creation are presented in Table 2. In univariable logistic regression, older age, lower pre-TIPS platelet count, and higher pre-TIPS MELD were significantly associated with being in the top quartile for platelet increase. In multivariable analysis, these patient factors remained significantly associated with being in the top quartile for platelet increase: age (odds ratio [OR], 1.24 per 5 years; 95% CI, 1.10–1.39), pre-TIPS platelet count (OR, 0.97 per 109/L; 95% CI, 0.97–0.98), and pre-TIPS MELD (OR, 1.06 per point; 95% CI, 1.02–1.09).
Table 2.
Univariable and Multivariable Models to Assess Factors Associated with a Top Quartile Increase in Platelets 4 Months after Transjugular Intrahepatic Portosystemic Shunt Creation in the Entire Cohort
| Odds ratios for a top quartile increase in platelets (95% CI); P value | ||
|---|---|---|
| Univariable model | Multivariable model | |
| Pre-TIPS platelets (×109/L) | 0.98 (0.97–0.98); <.001 | 0.97 (0.97–0.98); <.001 |
| Age, per 5 y | 1.18 (1.06–1.31); .003 | 1.24 (1.10–1.39); <.001 |
| Pre-TIPS MELD | 1.06 (1.03–1.09); <.001 | 1.06 (1.02–1.09); .003 |
MELD = model for end-stage liver disease; TIPS = transjugular intrahepatic portosystemic shunt.
For the subset of patients with severe pre-TIPS thrombocytopenia (n = 94, 16% of entire cohort), the results of univariable and multivariable logistic regression analyses of factors predictive of being in the top quartile for platelet increase 4 months after TIPS creation are presented in Table 3. In univariable logistic regression, age was significantly associated with being in the top quartile for platelet increase (P < .05), whereas pre-TIPS platelet count and pre-TIPS MELD score were not (P > .05). This remained true for multivariable logistic regression as well: age (OR, 1.50 per 5 years; 95% CI, 1.11–2.02), pre-TIPS platelet count (OR, 0.97 per 109/L; 95% CI, 0.92–1.01), and pre-TIPS MELD score (OR, 1.00 per point; 95% CI, 0.93–1.09). Additionally, the median platelet count changed significantly from 40 to 53 (P < .0001) in this subset, with 54% being in the top quartile for platelet increase. Moreover, 50 (53%) patients in this severe thrombocytopenia cohort experienced an increase in platelet count that brought them over the threshold for severe thrombocytopenia (50 × 109/L).
Table 3.
Univariable and Multivariable Models to Assess Factors Associated with a Top Quartile Increase in Platelets 4 Months after Transjugular Intrahepatic Portosystemic Shunt Creation in Those with Severe Pre–Transjugular Intrahepatic Portosystemic Shunt Thrombocytopenia (Platelets ≤ 50 × 109/L)
| Odds ratios for a top quartile increase in platelets (95% CI); P value | ||
|---|---|---|
| Univariable model | Multivariable model | |
| Pre-TIPS platelets (×109/L) | 0.96 (0.92–1.01); .11 | 0.97 (0.92–1.01); .13 |
| Age, per 5 y | 1.49 (1.12–1.99); .006 | 1.50 (1.11–2.02); .008 |
| Pre-TIPS MELD | 1.00 (0.92–1.08); .97 | 1.00 (0.93–1.09); .93 |
MELD = model for end-stage liver disease; TIPS = transjugular intrahepatic portosystemic shunt.
DISCUSSION
In this large, multicenter study of 601 patients with cirrhosis who underwent TIPS creation, there was not a statistically significant change in platelet count 4 months after TIPS creation. However, in the subset of patients with a pre-TIPS platelet count of <50 × 109/L, there was a significant increase in platelets 4 months after TIPS, with 54% achieving a ≥32% increase in their platelet count and 53% crossing the threshold to no longer have severe thrombocytopenia. In the analysis of the entire cohort, older age, lower pre-TIPS platelet count, and higher pre-TIPS MELD score were associated with a significant increase in platelet count after TIPS independent of each other in the multivariable analysis. In the subgroup analysis of patients with a pre-TIPS platelet count of ≤50 × 109/L, older age was the only factor associated with a significant increase in platelet count after TIPS.
Prior studies investigating the effect of TIPS on thrombocytopenia have reported conflicting results. Some studies (8–13) have reported improvement in thrombocytopenia after TIPS creation. In a study (8) of 74 patients with cirrhosis who underwent TIPS creation, there was an average increase in platelet counts of 22%. Another study (9) of 55 patients with cirrhosis found a median platelet count increase of 19.7%. In addition, there was a study (10) in which 34 (75%) of 45 patients with cirrhosis and thrombocytopenia (defined as a platelet count of <100 × 109/L) showed an increase in platelet count after TIPS creation, with a mean platelet count change from 83 × 109/L ± 4 to 100.8 × 109/L ± 5.4. Additionally, in a study (11) of 11 patients with portal hypertension, all patients experienced a statistically significant improvement in platelet counts. Another study (12) of 21 patients with cirrhosis found an increase in mean platelet counts from 95 × 109/L ± 44 before TIPS to 123 × 109/L ± 91 after TIPS, although the difference was only significant when a portosystemic pressure gradient of <12 mm Hg was achieved. Furthermore, a study (13) of 23 patients found a significant increase in platelet counts from 85.9 × 109/L ± 8.4 before TIPS to 135.3 × 109/L ± 16.8 after TIPS. Although these studies found a significant increase in platelet counts after TIPS creation, others reported no significant change in platelets after TIPS creation (14,15) or a trend toward decreased platelet counts after TIPS creation (16). No significant changes in platelet counts after TIPS were found in 2 studies: one of 62 patients with cirrhosis (14) and another of 60 patients with cirrhosis (15). Finally, one study found that platelet count tended to decrease after TIPS from 120.1 × 109/L ± 72.1 to 99.8 × 109/L ± 51.4, although the findings were not statistically significant (16). Although prior studies have conflicted in whether cohorts as a whole experienced a significant change in platelet count, all studies have reported that subsets of patients with cirrhosis, such as those with severe pre-TIPS thrombocytopenia or those with lower post-TIPS portosystemic gradient, do experience a significant increase in platelets after TIPS creation (8).
In this study, lower pre-TIPS platelet count predicted a greater increase in platelet count after TIPS. This finding is consistent with 2 prior studies investigating the effect of TIPS on thrombocytopenia that showed variation in platelet response (8,9). Prior studies investigating change in platelets after TIPS creation have been limited in sample size. The main strength of this study in comparison with prior literature is the large sample size, allowing analysis of factors predictive of platelet recovery after TIPS creation and subgroup analysis of a sizable cohort with severe thrombocytopenia (platelet count, ≤ 50 × 109/L) prior to TIPS creation.
The reason for the association found between both lower pre-TIPS platelet count and higher MELD score and greater post-TIPS platelet recovery in the overall cohort is unclear. Although one hypothesis could be that higher MELD scores and lower pre-TIPS platelet count are indicative of increased severity of portal hypertension, no associations between pre-TIPS portosystemic pressure gradient or change in the portosystemic gradient and platelet recovery were found in the multivariable analysis. Additionally, information on extrahepatic native portosystemic shunts was not captured in this study, so the true severity of portal hypertension in this cohort could not be assessed. It is also possible that there is a reversible component to liver disease or acute-on-chronic liver failure in the patients with higher MELD scores. Further investigation of this hypothesis is necessary with a cohort that includes a greater number of patients with higher MELD scores.
This study has a number of limitations. First, this study was a retrospective analysis and was limited by the availability of clinical data. In addition, some of the data were extracted using International Classification of Diseases-9/10 codes, which could have led to a degree of inaccuracy compared with manual chart review. Analyses of the available data also could have been subject to selection bias because complete data sets were not available for all participants. In addition, the small numbers of patients in each of the “other” categories for etiology of cirrhosis preclude the ability to evaluate specific etiologies within this category. Finally, the data set did not include the collection of certain variables, such as white blood cell count, detailed information about stent type, type of variceal bleed, timing of platelet count collection with respect to acute bleeding events, information on splenic embolization, or use of medications that may induce thrombocytopenia, that may be considered in future work to explore the mechanism or more technical aspects related to the association that was identified in this study. Future work should also include assessment of craniocaudal diameter to explore the underlying mechanism of the association between spleen size and thrombocytopenia because splenic sequestration is a proposed mechanism for thrombocytopenia in patients with cirrhosis.
Overall, patients with cirrhosis did not experience a significant change in platelet count after TIPS creation. However, substantial improvement in platelet counts was observed in some subgroups of patients, namely, those with lower pre-TIPS platelet counts, who were older, and who had higher MELD scores. Of particular clinical relevance, more than half of the patients with severe pre-TIPS thrombocytopenia experienced an increase in platelet count to >50 × 109/L, the threshold that is considered to be associated with a clinically meaningful risk of bleeding. This study offers the community additional information about the indirect benefits of TIPS creation and lays the foundation for future work investigating mechanisms for platelet recovery after TIPS.
RESEARCH HIGHLIGHTS.
This multicenter retrospective study of 601 patients who underwent transjugular intrahepatic portosystemic shunt (TIPS) creation delineated no significant increase in platelet count after TIPS creation (median absolute change, 1 × 109/L).
The subset of patients with a pre-TIPS creation platelet count of <50 × 109/L did experience a significant platelet increase (median absolute platelet change, 14 × 109/L).
Patients with lower pre-TIPS creation platelet counts, of older age, and with higher pre-TIPS model for end-stage liver disease scores were most likely to experience the greatest platelet count increase after TIPS creation.
ACKNOWLEDGMENTS
The authors acknowledge the American Society for Transplantation Liver and Intestinal Community of Practice Education Subcommittee for providing a forum for investigators to collaborate on the enclosed study. The authors also thank Ms. Cynthia Padilla and Ms. Dyanna Gregory for their invaluable contributions to the conduct of the Advancing Liver Therapeutics Approaches (ALTA) study. This manuscript has been reviewed by the ALTA Study Group for scientific content and consistency of data interpretation with previous ALTA publications. This study was funded by National Institutes of Health KL2TR001870 (to J.G.) and P30DK026743 (to J.G. and J.C.L.). The ALTA Study Group, however, is funded by an investigator-initiated grant from W.L. Gore and Associates. The Northwestern Research Electronic Data Capture is funded, in part, by the National Center for Advancing Translational Sciences of the National Institutes of Health research grant UL1TR001422 to the Northwestern University Clinical and Translational Sciences Institute. The sponsor (W.L. Gore & Associates) had no input into the overall design and conduct of the ALTA study.
J.G. receives research grant support from Merck. J.B. receives investigator-initiated grant support and consulting fees from W.L. Gore & Associates. E.S. holds stocks or stock options in Abbott. S.P. receives grant support from Genfit and Target PharmaSolutions. L.V. receives receives investigator-initiated and educational grant support from W.L. Gore & Associates (Newark, DE), the manufacturer of the transjugular intrahepatic portosystemic shunt Viatorr stent; reports grants from Intercept Pharmaceuticals, Enanta Pharmaceuticals, and NGM Pharmaceuticals; receives consulting fees from Noble Insights and Gerson Lehrman Group; receives payment for expert testimony from Wapner Newman and Mercaldo Law Firm; and holds leadership roles in the American Association for the Study of Liver Diseases, American Society for Transplantation, and Liver Transplantation (editorial). J.C.L. receives investigator-initiated research grant support from Gore Medical. K.P.K. receives consulting fees from and reports participation on the Data Safety Monitoring Board or Advisory Board of Becton, Dickinson, &Company. None of the other authors have identified a conflict of interest.
ABBREVIATIONS
- IQR
interquartile range
- MELD
model for end-stage liver disease
- OR
odds ratio
- TIPS
transjugular intrahepatic portosystemic shunt
Footnotes
Level of evidence: 4 (SIR-D)
REFERENCES
- 1.LaBerge JM. Transjugular intrahepatic portosystemic shunt—role in treating intractable variceal bleeding, ascites, and hepatic hydrothorax. Clin Liver Dis 2006; 10:583–598, ix. [DOI] [PubMed] [Google Scholar]
- 2.Caldwell SH, Hoffman M, Lisman T, et al. Coagulation disorders and hemostasis in liver disease: pathophysiology and critical assessment of current management. Hepatology 2006; 44:1039–1046. [DOI] [PubMed] [Google Scholar]
- 3.Northup PG, Garcia-Pagan JC, Garcia-Tsao G, et al. Vascular liver disorders, portal vein thrombosis, and procedural bleeding in patients with liver disease: 2020 practice guidance by the American Association for the Study of Liver Diseases. Hepatology 2021; 73:366–413. [DOI] [PubMed] [Google Scholar]
- 4.Hayashi H, Beppu T, Shirabe K, Maehara Y, Baba H. Management of thrombocytopenia due to liver cirrhosis: a review. World J Gastroenterol 2014; 20:2595–2605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Moore AH. Thrombocytopenia in cirrhosis: a review of pathophysiology and management options. Clin Liver Dis (Hoboken) 2019; 14:183–186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gangireddy VG, Kanneganti PC, Sridhar S, Talla S, Coleman T. Management of thrombocytopenia in advanced liver disease. Can J Gastroenterol Hepatol 2014; 28:558–564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gallo P, Terracciani F, Di Pasquale G, Esposito M, Picardi A, Vespasiani-Gentilucci U. Thrombocytopenia in chronic liver disease: physiopathology and new therapeutic strategies before invasive procedures. World J Gastroenterol 2022; 28:4061–4074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Massoud OI, Zein NN. The effect of transjugular intrahepatic portosystemic shunt on platelet counts in patients with liver cirrhosis. Gastroenterol Hepatol (N Y) 2017; 13:286–291. [PMC free article] [PubMed] [Google Scholar]
- 9.Gschwantler M, Vavrik J, Gebauer A, et al. Course of platelet counts in cirrhotic patients after implantation of a transjugular intrahepatic portosystemic shunt—a prospective, controlled study. J Hepatol 1999; 30:254–259. [DOI] [PubMed] [Google Scholar]
- 10.Pursnani KG, Sillin LF, Kaplan DS. Effect of transjugular intrahepatic portosystemic shunt on secondary hypersplenism. Am J Surg 1997; 173: 169–173. [DOI] [PubMed] [Google Scholar]
- 11.Alvarez OA, Lopera GA, Patel V, Encarnacion CE, Palmaz JC, Lee M. Improvement of thrombocytopenia due to hypersplenism after transjugular intrahepatic portosystemic shunt placement in cirrhotic patients. Am J Gastroenterol 1996; 91:134–137. [PubMed] [Google Scholar]
- 12.Lawrence SP, Lezotte DC, Durham JD, Kumpe DA, Everson GT, Bilir BM. Course of thrombocytopenia of chronic liver disease after transjugular intrahepatic portosystemic shunts (TIPS). A retrospective analysis. Dig Dis Sci 1995; 40:1575–1580. [DOI] [PubMed] [Google Scholar]
- 13.Jalan R, Redhead DN, Allan PL, Hayes PC. Prospective evaluation of haematological alterations following the transjugular intrahepatic portosystemic stent-shunt (TIPSS). Eur J Gastroenterol Hepatol 1996; 8: 381–385. [DOI] [PubMed] [Google Scholar]
- 14.Jabbour N, Zajko A, Orons P, Irish W, Fung JJ, Selby RR. Does transjugular intrahepatic portosystemic shunt (TIPS) resolve thrombocytopenia associated with cirrhosis? Dig Dis Sci 1998; 43: 2459–2462. [DOI] [PubMed] [Google Scholar]
- 15.Sanyal AJ, Freedman AM, Purdum PP, Shiffman ML, Luketic VA. The hematologic consequences of transjugular intrahepatic portosystemic shunts. Hepatology 1996; 23:32–39. [DOI] [PubMed] [Google Scholar]
- 16.Karasu Z, Gurakar A, Kerwin B, et al. Effect of transjugular intrahepatic portosystemic shunt on thrombocytopenia associated with cirrhosis. Dig Dis Sci 2000; 45:1971–1976. [DOI] [PubMed] [Google Scholar]
- 17.Ge J, Lai JC, Boike JR, et al. Nonalcoholic fatty liver disease and diabetes mellitus are associated with post-transjugular intrahepatic portosystemic shunt renal dysfunction: an Advancing Liver Therapeutic Approaches Group Study. Liver Transpl 2021; 27:329–340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Boike JR, Mazumder NR, Kolli KP, et al. Outcomes after TIPS for ascites and variceal bleeding in a contemporary era—an ALTA Group study. Am J Gastroenterol 2021; 116:2079–2088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Peck-Radosavljevic M Thrombocytopenia in chronic liver disease. Liver Int 2017; 37:778–793. [DOI] [PubMed] [Google Scholar]
- 20.Parvinian A, Bui JT, Knuttinen MG, Minocha J, Gaba RC. Transjugular intrahepatic portosystemic shunt for the treatment of medically refractory ascites. Diagn Interv Radiol 2014; 20:58–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gaba RC, Parvinian A. How quickly does ascites respond to TIPS? Clinical follow-up of a cohort of eighty patients. Diagn Interv Radiol 2014; 20:364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wong F, Sniderman K, Liu P, Blendis L. The mechanism of the initial natriuresis after transjugular intrahepatic portosystemic shunt. Gastroenterology 1997; 112:899–907. [DOI] [PubMed] [Google Scholar]
- 23.Jones CI. Platelet function and ageing. Mamm Genome 2016; 27: 358–366. [DOI] [PMC free article] [PubMed] [Google Scholar]


