Abstract
Nearly twenty-five percent of colorectal cancer (CRC) patients develop metachronous colorectal liver metastasis (CRLM) after curative surgery. Hepatosteatosis is the most prevalent liver condition worldwide, but its impact on the incidence of metachronous CRLM is understudied. In the present study, we aimed to investigate the predictive value of hepatic steatosis on the development of metachronous CRLM. First, a nested case-control study was conducted, enrolling stage I to III CRC patients in the National Colorectal Cancer Cohort (NCRCC) database. Metachronous CRLM patients and recurrence-free patients were matched via propensity-score matching. Fatty liver was identified based on treatment-naïve CT scans and the degree of hepatic fibrosis was scored. Multivariable analysis was conducted to investigate the association between fatty liver and metachronous CRLM. In our database, a total of 414 patients were included. Metachronous CRLM patients had considerably higher rates of hepatic steatosis (30.9% versus 15.9%, P<0.001) and highly fibrotic liver (11.6% versus 2.9%, P=0.001) compared to recurrence-free patients. Multivariable analysis showed that fatty liver (odds ratios [OR]=1.99, 95% confidence interval [CI] 1.19-3.30, P=0.008) and fibrotic liver (OR=4.27, 95% CI 1.54-11.81, P=0.005) were associated with high risk of metachronous CRLM. Further, a systematic literature review was performed to assess available evidence on the association between hepatosteatosis and development of metachronous CRLM. In the systematic review, 1815 patients were pooled from eligible studies, and hepatic steatosis remained a significant risk factor for metachronous CRLM (OR=1.90, 95% CI 1.35-2.66, P<0.001, I2=25.3%). In conclusion, our data suggest that patients with a steatotic liver and a high fibrosis score at CRC diagnosis have elevated risk of developing metachronous CRLM.
Keywords: Hepatic steatosis, colorectal cancer, metachronous liver metastasis, L/S ratio, tumor stage
Introduction
Colorectal cancer (CRC) is the third most prevalent malignancy worldwide, with a predicted annual incidence of 2.5 million by 2035, and a high mortality rate, currently accounting for 900,000 disease-related deaths per year [1,2]. Importantly, 25% of patients will develop metachronous liver metastases (CRLM) even after curative surgeries for the primary tumor [2,3]. Metachronous CRLM is clinically challenging because few patients are eligible for surgery, local recurrence rates are high, and survival rates are extremely low [4,5]. Furthermore, despite advancements in preclinical researches, there remains a lack of reliable clinical predictors for the development of metachronous CRLM.
In contrast to synchronous metastasis, the development of metachronous metastasis relies on the activation of dormant disseminated tumor cells. It is wildly accepted that the dormancy of seeded cells is regulated by the local conditions of the target organ [6,7]. Chronic liver diseases such as steatosis and fibrosis not only produce an inflammatory environment but also hamper immunosurveillance functions against malignancies. On the other hand, chronic liver disease leads to rewiring of hepatic metabolism and a consequent increase in nutrient availability in the liver [8]. Combining these phenomena, chronic liver disease may ultimately result in a nutrient-rich, immunosuppressive microenvironment in favor of activation and growth of metastatic cells [9].
Hepatic steatosis is the most common chronic liver condition worldwide, affecting up to 31.8% of the population [10,11]. It is characterized by excessive lipid storage and, in the advanced stages, necrotic, inflammatory, and fibrotic changes occur [8]. Recent preclinical studies have yielded insights into the supportive role of fatty liver in facilitating cancer cell activation [12,13]. However, there is a lack of consistent clinical evidence concerning the impact of hepatic steatosis on the development of metachronous CRLM after curative resection [14-18]. The literature currently reports mixed results, which are likely attributable to retrospective design, limited sample numbers, heterogeneity in patient baseline parameters, and differences in previously administered anti-tumor treatments. Thus, a nested case-matched study based on a prospectively designed large patient cohort is warranted.
Traditionally, the onset of primary gut tumors and hepatic steatosis were mostly viewed as independent events. In contrast, we recently reported that primary tumor actively “educates” the host liver and induce significant lipid accumulation in animal models prior to their distal colonization [19]. Yet, clinical correlation between primary CRC tumors and incidence of hepatosteatosis is, by far, lacking. In light of this, we conducted a nested case-control study utilizing the large-scale National Colorectal Cancer Cohort (NCRCC) database [20]. We enrolled stage I-III CRC patients who developed metachronous CRLM post-curative surgery and control patients who remained tumor-free after primary tumor resection. Having identified liver steatosis as risk factor for metachronous CRLM in our dataset, a systematic review and meta-analysis were conducted to collate stronger evidence from multiple sources.
Materials and methods
The study consisted of two sections. The first section is a nested case-control study in which colorectal cancer patients who were non-metastatic at disease diagnosis and developed metachronous CRLM were matched with non-metastatic patients with no recurrence. Multidimensional clinical data were analyzed to identify predictive factors for metachronous CRLM. After identifying hepatic steatosis as a risk factor for metachronous CRLM, we conducted a systematic review and meta-analysis to confirm the link.
Design of a nested case-control study and definition of groups
Study cohorts
The NCRCC is an ongoing prospective cohort study in which individuals at high risk of CRC and newly diagnosed CRC patients were recruited. Patients’ baseline characteristics, treatment information, survival endpoints and lifestyle questionnaire were recorded in a prospective fashion, making it an ideal database to conduct a nested case-control study. So far, more than 16,000 CRC patients were recruited. The cohort protocol was elaborated previously [20].
Cases and controls
Patients recruited in NCRCC between June 2015 and December 2019 were consecutively enrolled during identification process. Metastatic disease, patients with unclear stages or non-adenocarcinoma disease were excluded. Those who developed metachronous CRLMs during postoperative survey were identified as cases. The definition of metachronous CRLM is: newly-diagnosed liver metastatic lesion that was radiologically or pathologically confirmed more than six months after curative surgery for primary colorectal cancer. In addition, patients with no evidence of disease recurrence were identified as controls (Figure 1A). Patients then underwent detailed selection before case-matching process. The inclusion criteria were: (1) Age between 18 and 80; (2) Abdominal CT scans at disease diagnosis; (3) No evidence of disease (NED) after curative surgery; (4) Routine follow-up protocols with radiological examination. The exclusion criteria were: (1) Patients with chronic liver disease; (2) History of HBV or HCV infection; (3) History of excessive alcohol consumption (≥30 g per day for men or ≥20 g per day for women) by life style questionnaire at diagnosis of CRC [10,20]; (4) Patients receiving preoperative chemotherapy or treatment at other medical center prior to hospitalization; (5) Sever comorbidities; (6) Patients not adhering to follow-up protocols or lost in follow-up.
Figure 1.
Flow chart of patient inclusion, case-matching and data gathering process. A: The National Colorectal Cancer Cohort (NCRCC) database was utilized. Stage I to III colorectal cancer patient with metachronous colorectal liver metastasis (CRLM) and patients with no disease recurrence during more than 3 years of survey were identified; B: The identified patients underwent screening process and eligible patients in both groups were matched according to baseline information, disease stage and tumor location via propensity-score-matching PSM.
Then, a one-to-one propensity-score matching (PSM) strategy was used. In detail, baseline information (i.e., sex, age, and overweight), systematic metabolic disease (i.e., diabetes) and disease information such as primary tumor location and disease stage were used for matching. The schematics of PSM was in Figure 1B.
Clinical data gathering
Clinical data were extracted from electrical medical record system (EMRS). Platelet count was extracted from blood routine at admission. Liver function indicators including blood bilirubin, alanine transaminase (ALT), aspartate transaminase (AST), albumin and blood bile acid were extracted from serum biochemicals. Additionally, blood lipid metabolism-related indicators including total cholesterol, triglyceride, free fatty acid and lipoproteins were recorded. Genetic features including Ras mutation, Braf mutation were verified via PCR or sequencing and were extracted from patient records. MMR status was extracted from patients’ immunohistochemistry (IHC) exams.
CT attenuation measurement of the liver and spleen
All CT scans were performed with second-generation dual-source scanner (Statel: SOMATOM Definition AS and Sensation 16, Siemens Medical Solutions, Forchheim, Germany). Non-enhanced series of abdominal CT scan at disease diagnosis was used for measurement, and the measuring strategy was in accordance to our previous research [21]. Briefly, regions of interest (ROIs) sizing 1 cm2 were manually drawn at eight anatomical segmentations of liver, avoiding blood vessels, bile duct and surface areas and the mean CT attenuation in Hounsfield unit (Hu) was calculated. ROIs were drawn at upper, middle and lower one third of spleen to get the mean spleen attenuation. Then, L/S ratio was defined as mean liver attenuation divided by mean spleen attenuation. Patients with L/S ratio lower than 1.1 was identified as fatty liver. The repetitive images of steatosis and normal liver were in Figure S1.
Calculation of liver fibrotic score
Hepatic fibrosis is typical in advanced-stage steatosis. Thus, the liver fibrotic score was also calculated for each patient. The NAFLD fibrotic scoring (NFS) system was as follow:
NFS = -1.675 + 0.037 * Age + 0.094 * BMI + 1.13 * Diabetes + 0.99 * (AST/ALT) - 0.013 * Platelet - 0.66 * Albumin
The cut-off value of -1.455 and 0.672 was used for differentiation of fibrotic levels [22,23].
Statistical analysis
For binary variables, Pearson’s Chi-test was used. Student’s t-test was used to compare consecutive variables. Rank-sum-test was used for comparison between hierarchical variables. Univariate logistic regression was used to screen risk factors for metachronous CRLM and baseline information and variables presenting p-value of less than 0.05 was included in multivariate logistic regression. To exclude the effect of collinearity on logistic regression, variables with strong correlation, i.e. L/S ratio and NAFLD, NFS level and highly fibrotic liver, were not included in a single analysis. SPSS version 19.0 (SPSS Inc, Chicago, IL), R version 4.1.2 (R Foundation for Statistical Computing, Vienna, Austria) and Graphpad prism 9 were used in this study. Binary or hierarchical variables were presented as number (percentage) whereas consecutive variables were presented as mean±SD or median with interquartile range (IQR) if not normally distributed. A p value of less than 0.05 was regarded as statistically significant.
Methods for systematic review and meta-analysis
Data sources, searches, extraction, and quality assessment
We searched PubMed, Medline, Web of Science, Embase, Cochrane and Google Scholar for records concerning the association between liver steatosis and colorectal cancer metachronous liver metastasis. The last search update was on June 2023. The following terms were used: (“Fatty liver” or “hepatic steatosis” or “steatohepatitis” or “non-alcoholic fatty liver disease” or “NAFLD” or “non-alcoholic steatohepatitis” or “NASH” or “fibrosis”) and (“colorectal cancer” or “colorectal carcinoma” or “colorectal neoplasm” or “colorectal tumor” or “colon tumor” or “colon cancer” or “rectal cancer” or “rectal tumor”) and (“liver metastasis”). After removing duplicates, records were sorted by type and were manually screened by title and abstract. The inclusion criteria were: (1) case-control or case-cohort study concerning hepatic steatosis and metachronous CRLM; (2) contained data for calculation of odds ratio (OR) and 95% confidence interval (CI); The exclusion criteria were: (1) missing relevant outcomes; (2) studies concerning synchronous CRLM; (3) Duplicated data from a single center; (4) fibrotic liver unrelated to liver steatosis; (5) data not available. All eligible records were retrieved for full text for further evaluation. Our workflow was demonstrated in Figure S2. Then, all included studies were evaluated for research quality using a modified Newcastle-Ottawa Scale (NOS) available in Table S1.
Data synthesis and analysis
Lastly, all eligible studies with available data were analyzed for publication bias via Egger’s test and Begg’s test. And researches with significant publication bias would be excluded from further analysis. The I2 value was used to evaluate data heterogeneities among studies included. For I2>50% and p-value <0.05, a significantly heterogeneous data pool was considered and subject to random effect model. Else, a fixed model was used. STATA version 12.0 (Stata Corporation, College Station, Texas, USA) were used for data processing.
Results
Characteristics of included patients with or without metachronous CRLM
During the inclusion period, 6309 patients were enrolled in the cohort, among whom 4264 patients had stage I to III disease. After more than three years of follow-up, 273 CRC patients with metachronous CRLM were identified. In addition, 3868 cases of recurrence-free CRC patients were identified as the control group (Figure 1A). After PSM, 207 metachronous patients and 207 control patients with matched baseline information and disease stages were included for investigation (Figure 1B).
Consistent with the global epidemiology of CRC, in our dataset, around 74% of patients were men and 36% of patients were aged 70 or older (Table 1). In agreement with Asian population characteristics, the rate of overweight was low. In terms of disease characteristics, 70.3% of tumors were left-sided and 65.7% of patients had stage III disease. Noticeably, the tumor burden in the metachronous CRLM group was significantly higher compared to the control group (35.7% patients had T4 tumors, in contrast to 16.9% in the control group). In patients with available genetic information, the metachronous group had a higher rate of Ras-mutation, while the rate of dMMR was not statistically different (Table 1). Strikingly, the metachronous CRLM group exhibited a significantly higher incidence of steatosis (30.9% versus 15.9%, respectively, P<0.001) at CRC presentation. Further, we used NFS to determine the extent of liver fibrosis and the cutoff for stratification was consistent with published studies [22]. Interestingly, the rate of highly fibrotic liver was four-fold higher in the metachronous group (Table 1, 11.6 versus 2.9%, P=0.001).
Table 1.
Clinical and pathological characteristics of included metachronous CRLM patients and matched non-metastatic patients
| Characteristics | Total patiens | Metachronous CRLM | Non-metastatic | p-value | |||
|---|---|---|---|---|---|---|---|
|
|
|
|
|||||
| N/median value | %/IQR | N/median value | %/IQR | N/median value | %/IQR | ||
| Total | 414 | 100 | 207 | 100 | 207 | 100 | |
| Sex ratio (M:F) | 74.6 | 75.4 | 73.9 | 0.821b | |||
| Age (years) | >0.999a | ||||||
| ≤70 | 265 | 64.0 | 133 | 64.3 | 132 | 63.8 | |
| >70 | 149 | 36.0 | 74 | 35.7 | 75 | 36.2 | |
| Overweight | 0.426a | ||||||
| No | 387 | 93.5 | 196 | 94.7 | 191 | 92.3 | |
| Yes | 27 | 6.5 | 11 | 5.3 | 16 | 7.7 | |
| Diabetes | >0.999a | ||||||
| No | 351 | 84.8 | 175 | 84.5 | 176 | 85 | |
| Yes | 63 | 15.2 | 32 | 15.5 | 31 | 15 | |
| Location | >0.999a | ||||||
| Right | 123 | 29.7 | 60 | 29 | 63 | 30.4 | |
| Left | 291 | 70.3 | 147 | 71 | 144 | 69.6 | |
| Disease stage | 0.745c | ||||||
| Stage I | 32 | 7.7 | 18 | 8.7 | 14 | 6.8 | |
| Stage II | 110 | 26.6 | 54 | 26.1 | 56 | 27.1 | |
| Stage III | 272 | 65.7 | 135 | 65.2 | 137 | 66.2 | |
| T stage | |||||||
| T1 | 11 | 2.7 | 3 | 1.4 | 8 | 3.9 | <0.001* |
| T2 | 39 | 9.4 | 15 | 7.2 | 24 | 11.6 | |
| T3 | 255 | 61.6 | 115 | 55.6 | 140 | 67.6 | |
| T4 | 109 | 26.3 | 74 | 35.7 | 35 | 16.9 | |
| N stage | 0.577c | ||||||
| N0 | 142 | 34.3 | 73 | 35.3 | 69 | 33.3 | |
| N1 | 178 | 43.0 | 80 | 38.6 | 98 | 47.3 | |
| N2 | 94 | 22.7 | 54 | 26.1 | 40 | 19.3 | |
| Ras status | / | ||||||
| Wild type | 40 | 9.7 | 24 | 11.6 | 16 | 7.7 | |
| Mutation | 41 | 9.9 | 30 | 14.5 | 11 | 5.3 | |
| Not available | 333 | 80.4 | 153 | 73.9 | 18 | 87.0 | |
| Braf status | / | ||||||
| Wild type | 76 | 18.4 | 50 | 24.2 | 25 | 12.6 | |
| Mutation | 4 | 1.0 | 3 | 1.4 | 1 | 0.5 | |
| Not available | 334 | 80.7 | 154 | 74.4 | 180 | 87.0 | |
| MMR status | 0.058c | ||||||
| pMMR | 257 | 62.1 | 131 | 63.3 | 126 | 60.9 | |
| dMMR | 31 | 7.5 | 10 | 4.8 | 21 | 10.1 | |
| Not available | 126 | 30.4 | 66 | 31.9 | 60 | 29.0 | |
| Platelet | 227 | 182-278 | 223 | 185-276 | 227 | 179-279 | 0.407b |
| Total Bilirubin | 11.4 | 8.5-14.9 | 11.2 | 8.4-14.1 | 11.5 | 8.5-15.2 | 0.945b |
| Direct Bilirubin | 2.3 | 1.7-3.3 | 2.2 | 1.7-3.4 | 2.3 | 1.7-3.2 | 0.114b |
| Indirect Bilirubin | 8.9 | 6.5-11.5 | 8.8 | 6.4-11.2 | 9 | 6.6-11.9 | 0.542b |
| ALT (U/L) | 15 | 45251.0 | 14 | 11-21 | 14 | 11-21 | 0.858b |
| AST (U/L) | 21 | 17-25 | 20 | 16-25 | 20 | 17-24 | 0.656b |
| Albumin (g/L) | 40.3 | 36.4-43.1 | 39.9 | 35.3-43.2 | 40.4 | 36.8-42.8 | 0.029*,b |
| Total Bile Acid (umol/L) | 3.2 | 1.7-5.3 | 3.3 | 1.9-5.4 | 3 | 1.5-5.1 | 0.067b |
| Total Cholesterol (mmol/L) | 4.39 | 3.70-5.17 | 4.47 | 3.68-5.21 | 4.33 | 3.72-5.14 | 0.821b |
| Triglyceride (mmol/L) | 1.14 | 0.91-1.63 | 1.17 | 0.96-1.66 | 1.10 | 0.90-1.62 | 0.481b |
| HDL (mmol/L) | 1.10 | 0.94-1.32 | 1.10 | 0.93-1.27 | 1.09 | 0.94-1.36 | 0.626b |
| LDL (mmol/L) | 2.43 | 1.99-3.00 | 2.4 | 1.97-3.01 | 2.41 | 1.99-2.97 | 0.230b |
| L/S Ratio | 1.20 | 1.11-1.30 | 1.17 | 1.08-1.27 | 1.23 | 1.15-1.32 | <0.001*,b |
| NAFLD | <0.001*,a | ||||||
| No | 317 | 76.6 | 143 | 69.1 | 174 | 84.1 | |
| Yes | 97 | 23.4 | 64 | 30.9 | 33 | 15.9 | |
| NFS level | 0.391c | ||||||
| Low | 185 | 44.7 | 93 | 44.9 | 92 | 44.4 | |
| Intermediate | 199 | 48.1 | 90 | 43.5 | 109 | 52.7 | |
| High | 30 | 7.2 | 24 | 11.6 | 6 | 2.9 | |
| Highly fibrotic liver | |||||||
| No | 384 | 92.8 | 183 | 88.4 | 201 | 97.1 | 0.001*,a |
| Yes | 30 | 7.2 | 24 | 11.6 | 6 | 2.9 | |
Pearson x2-test;
Student’s t-test;
Rank-sum test;
Statistically significant.
Categorical value was presented as N and (%), continuous value was presented as median value and (interquartile range [IQR]). CRLM: Colorectal cancer liver metastasis; ALT: Alanine transaminase; AST: Aspartate aminotransferase; HDL: High-density lipoprotein; LDL: Low-density lipoprotein; NAFLD: Non-alcoholic fatty liver disease; NFS: NAFLD fibrosis score.
Hepatic steatosis was identified as a strong predictor for metachronous CRLM
Univariate logistic regression was used to explore correlations between patient characteristics and the development of metachronous CRLM. Due to the nested case-matched study design, patients’ baseline information, tumor location and disease stage were not associated with metachronous CRLM, while T4 tumor was identified as a risk factor (Table S2). Higher albumin levels showed a minor protective effect. Liver steatosis and highly fibrotic liver were strong risk factors for metachronous CRLM. In multivariate logistic regression analysis, liver steatosis (Figure 2 and Table S2, OR 1.990, 95% CI 1.199-3.302, P<0.001), highly fibrotic liver (OR 4.269, 95% CI 1.543-11.810, P=0.005) and T4 tumor (OR 4.438, 95% CI 1.056-18.647, P=0.042) were identified as independent risk factors for metachronous CRLM.
Figure 2.

Logistic regression was used to determine the risk factors for metachronous colorectal liver metastasis. Multivariate logistic regression analysis of factors presenting p-value <0.1 during univariate logistic regression, and was adjusted with baseline characteristics and tumor location. OR: odds ratio; CI: confidence interval; *: statistically significant.
Hepatic steatosis is correlated with advanced tumor stages
Our previous preclinical studies and other tumor models have highlighted the modulatory effect of the primary tumor on distal organs, including the liver. Therefore, we sought to explore the clinical evidence linking CRC advancement and liver steatosis [19,24]. Indeed, stage III patients displayed a significantly higher incidence of hepatic steatosis compared to early-stage CRC patients (Figure 3A). Interestingly, when patients were differentiated according to T stage, we found a significant negative correlation between tumor penetration depth and L/S ratio (Figure 3B, Pearson correlation -0.247, P<0.001). These results suggested a potential correlation between gross tumor stage, especially tumor invasion depth, and the development of hepatic steatosis.
Figure 3.
A Positive correlation between hepatic steatosis and disease aggressiveness was observed. A: Patients were stratified by disease stages at colorectal cancer diagnosis. Fraction of patients presenting hepatic steatosis in each stage was calculated. Additionally, comparison was made between early stage (stage I and II) and advanced stage (stage III) patients; B: The violin plot of liver-spleen attenuation ratio in patients stratified by T stages. Dashed line represents the trend of median Liver-spleen ratio (L/S ratio) alteration with advancing T stages. Pearson correlation test suggested a significant negative correlation between T stage and L/S ratio. *: p value <0.05; **: p value <0.01.
Hepatic steatosis is identified as a risk factor for metachronous CRLM via systematic review
To further confirm the connection between liver steatosis and the development of metachronous CRLM, we conducted a meta-analysis (schematics in Figure S2). Four studies were identified and the pooled data included both Eastern and Western populations (Table 2) [14-17]. Two studies used L/S ratio on CT scans to identify liver steatosis, one study (Kondo et al. [16]) used NFS, and one study (Xi et al. [25]) used ultrasound. The quality scores ranged from five to eight (Table 2). In publication bias analysis, the study by Murino et al. [15] presented significant publication bias and was therefore excluded from further analysis (P=0.025, Figure 4A and 4B). Combining the remaining studies, the final data pool consisted of 1815 patients from four medical centers, among whom 285 patients were diagnosed with liver steatosis. No significant heterogeneity was identified in the final included studies, and a fixed effects model was adopted (I2=25.3%, P=0.260, Figure 4C). In the pooled data, hepatic steatosis remained a significant risk factor for metachronous CRLM compared to non-steatosis liver (Figure 4C, OR 1.90, 95% CI 1.35-2.66, P<0.001).
Table 2.
Characteristics of studies included in the meta-analysis
| ID | Study | Country | Database | Period | Quality score | Sample size (Fatty liver/Normal liver) | Diagnostic method for fatty liver | Age (years) | Male (%) | Overweight (%) | Tumor location (%) | Disease stage (n) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Besutti et al. [14] | Italy | local population-based Cancer Registry and from the electronic medical records of all the Local Health Authority hospitals of the province | 2010 to 2016 | 5 | 202 (60/142) | L/S ratio | 68±14 | 59 | NA | NA | Stage II (77) |
| Stage III (125) | ||||||||||||
| 2 | Murono et al. [15]a | Japan | University of Tokyo Hospital | 2004 to 2011 | 7 | 603 (63/540) | L/S ratio | 67±11 | 60.4 | 22.1 | Colon (63.5) | Stage I (160) |
| Rectum (36.5) | Stage II (197) | |||||||||||
| Stage III (172) | ||||||||||||
| 3 | Kondo et al. [16] | Japan | Keio University Hospital | 2000 to 2011 | 7 | 956 (73/876) | NFS | Fibrotic liver 75.3±9.3 | 59.4 | 22.0 | Right colon (35.3) | Stage I (293) |
| Normal liver 64.9±11.7 | Left colon (42.7) | Stage II (327) | ||||||||||
| Rectum (22.0) | Stage III (333) | |||||||||||
| 4 | Xi et al. [17] | China | Renji Hospital, Shanghai Jiaotong University | 1993 to 2002 | 5 | 243 (55/188) | Ultrasound | Fatty liver 64±10.8 | 56.4 | NA | NA | NA |
| Normal liver 63±13.2 | ||||||||||||
| 5 | NCRCC database | China | Sencond Affiliated Hospital of Zhejiang University School of Medicine | 2012 to 2022 | 8 | 414 (97/317) | L/S ratio | 63.5±12.6 | 75.6 | 6.5 | Right colon (29.7) | Stage I (32) |
| Left colon b(70.3) | Stage II (110) | |||||||||||
| Stage III (272) |
Study with publication bias form Egger’s test;
Rectum was included in the left colon in this study.
L/S ratio: Liver-spleen ratio on CT scans; NFS: NAFLD fibrosis score; NCRCC: National Colorectal Cancer Cohort.
Figure 4.
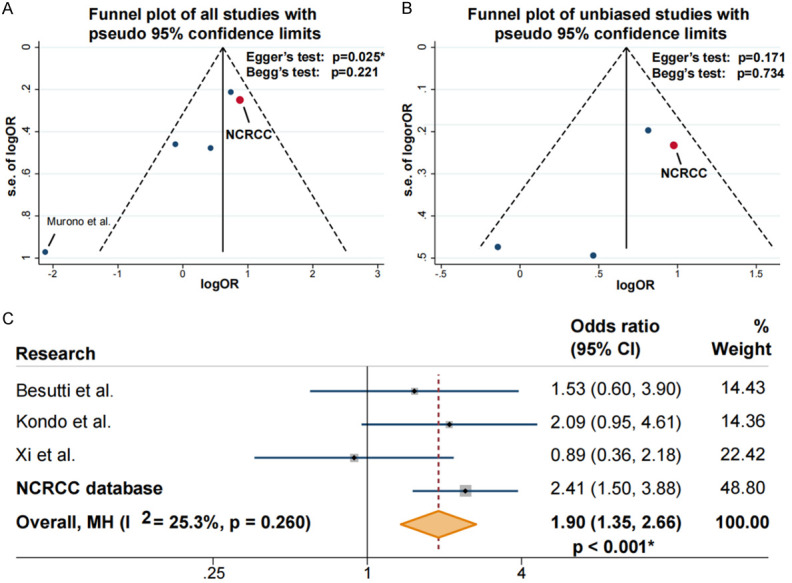
Funnel plot of studies identified in systematic research and forest plot of odds ratio (OR) for liver steatosis on metachronous colorectal liver metastasis (CRLM) by meta-analysis. A: Funnel plot of studies identified in systematic research. In all identified studies, one study presented significant publication bias via Egger’s test. B: Studies with no publication bias was selected for following analysis. C: No significant heterogeneity was identified in our database (National Colorectal Cancer Cohort, NCRCC) and other studies (I2<50%, P=0.260), and a fixed effect model was adopted for analysis. In pooled data, hepatic steatosis at cancer diagnosis was risk factor for developing metachronous CRLM (OR=1.90, 95% confidence intervals [CI] 1.35-2.66, P<0.001).
Discussion
In this study, we utilized the NCRCC database to conduct a nested case-control study and incorporated comprehensive genetic information, pathological status, imaging features, serum markers and baseline information to investigate the risk factors for metachronous CRLM at primary tumor diagnosis. To preclude chemotherapy-induced liver pathology, the CT scans of treatment-naive patients were investigated. The metachronous CRLM group had a significantly higher rate of hepatic steatosis at cancer diagnosis. In the subsequent analysis, liver steatosis and a high degree of liver fibrosis were identified as strong risk factors for metachronous CRLM. The harmful effect of hepatic steatosis on the development of metachronous CRLM was further supported by our systematic review.
Past observational studies have suggested that patients with liver steatosis at CRC diagnosis have a greater tendency to develop metachronous CRLM; however, none of these were case-matched studies and the conclusions were ambiguous [15-17]. The inconsistency in previous results could be due to unbalanced patient baseline characteristics, differences in tumor location, and suboptimal data quality (Table S2). Our present study is based on the NCRCC database, which is by far the largest CRC patient database in China. Therefore, our analysis included the full spectrum of disease information, imaging data, pathological reports, and baseline information which were gathered in a prospective manner. On top of that, the current study adopted a nested case-matched design, minimizing confounding factors such as overweight, age-related liver steatosis, and tumor sidedness. Our study, in combination with others, provides substantial evidence or the role of hepatic steatosis on the onset and multiple outcomes of CRLM. For example, in the dataset of Lv et al. [26], fatty liver was reported to be the biggest risk factor for synchronous CRLM, outcompeting elevated tumor markers and lymph node status. The altered liver milieu also impacts surgical outcomes of CRLM: our recent study showed that hepatic steatosis was associated with significantly worse recurrence-free survival compared to the non-steatotic control group [21]. Fatty liver also affects the results of systemic CRLM treatment, as fatty liver can impair the detoxification of chemotherapy drugs, and thus potentially increase morbidity during chemotherapy or immunotherapy [27-29]. Moreover, the compromised liver function resulting from hepatic steatosis can result in dose delay, therapy readjustment, and hospitalization in other cancer types [30,31].
There is increasing preclinical evidence that CRC actively steers the liver towards steatosis to facilitate its colonization [19,32]. Interestingly, our results also highlighted that patients with advanced-stage CRC (stage III) were prone to fatty liver, and the extent of steatosis showed significant correlation with T stages (Figure 3). In the majority of literatures investigating the potential link between CRLM and liver steatosis, the onset of gut tumors and histological changes in the liver were viewed as independent factors. However, given that the carcinogenesis of conventional CRC takes years if not decades, and that early-stage colorectal cancers are rarely symptomatic, the tumor would have a long-term influence on the liver to create pathological changes [33,34]. This notion is reflected by our data and the study by Aktas et al., in which 105 non-metastatic CRC patients were matched with 94 patients with no history of cancer, and the liver densities of both groups on abdominal CT were measured. Interestingly, CRC-bearing patients showed significantly lower liver densities compared to controls, suggesting the presence of histological change towards fatty liver disease [35]. Consistently, Manzano et al. and Caldwell et al. also reported the accumulation of liver lipid and the presence of fibrosis in orthotopic cancer-bearing mouse models [36,37].
Though cancer has long been advocated as a systemic disease, the mechanisms of the crosstalk between the primary tumor and target organs remain unclear, and little is known about how this interorgan communication facilitates tumor colonization. One potential mechanism involves the diversity of factors that are actively shed by tumor cells into the circulation, including circular RNA (circRNA) and extracellular vesicles (EVs) encasing heat-shock proteins, miRNA, or fatty acids; these molecules may serve as messengers for inter-organ communication [19,38-40]. While smaller molecules are less liver-targeted, we and others have reported that tumor-shed EVs showed high organotropism towards the liver and may be the key factor in liver metabolic remodeling [19,41]. For instance, Silva et al. reported that tumor-derived EVs enriched in macrophage migration inhibitory factor (MIF) were engulfed by liver macrophages, which then triggered fibrotic pathways in hepatic stellate cells and resulted in a pro-inflammatory milieu that supports tumor cell colonization [42]. Consistent with this notion, studies have reported increased liver metastasis in a mouse model of fatty liver injected with a colon cancer cell line. Furthermore, tumor cells presented a more aggressive growth pattern in steatotic liver [43,44]. One intriguing mechanism for this enhanced aggressiveness of CRLM in steatotic liver was recently elucidated by Wang et al., involving increased EV secretion from hepatocytes from fatty liver. These EVs fostered an immunosuppressive microenvironment and promoted oncogenic pathways in metastatic cells that ultimately resulted in tumor outgrowth [45]. Additionally, it is now known that metastatic tumor cells also undergo metabolic reprogramming and upregulate scavenger transporters to facilitate lipid uptake as well as increase fatty acid oxidation to support their survival and migration [46-48]. Thus, the steatotic liver becomes the “fertile crescent” for the migrating tumor cells. In short, our data support the theory that the tumor itself may actively remodel target organ to create a more favorable premetastatic niche, while hijacking liver’s metabolic capability to facilitate its own colonization process. However, clinical evidence on the close relationship between fatty liver and CRLM remains limited, and there is a clear and urgent need for more clinical studies.
The current study has its limitations. Firstly, in relation to our meta-analysis, studies regarding liver steatosis and metachronous CRLM were few and inter-study publication bias was found. Secondly, due to missing data concerning the Ras and Braf status of the patients, a direct comparison to study the effect of primary tumor genetics on liver steatosis was not feasible. Nevertheless, the metachronous CRLM group seemed to present a relatively higher incidence of Ras-mutation (Table 1).
Conclusion
In this nested case-matched study, we reported that liver steatosis and high levels of hepatic fibrosis at disease diagnosis were risk factors for the development of metachronous CRLM. The adverse effect of fatty liver on CRLM was verified via systematic review.
Acknowledgements
We acknowledge all patients enrolled in this research and authors of included literatures. This work was supported by the grant from National Natural Science Foundation of China (No. 82072624); The Fundamental Research Funds for the Central Universities (No. 226-2022-00009); Key R&D Program of Zhejiang (No. 2023C03049); Zhejiang Provincial Natural Science Foundation of China under Grant (No. Q23H260015) and the National Natural Science Foundation of China (Grant No. 82103498).
Written consents were obtained from all participants enrolled in this study.
Disclosure of conflict of interest
None.
Abbreviations
- CRC
colorectal cancer
- CRLM
colorectal liver metastasis
- NCRCC
National Colorectal Cancer Cohort
- NED
no evidence of disease
- PSM
propensity-score matching
- EMRS
electrical medical record system
- ALT
alanine transaminase
- AST
aspartate transaminase
- IHC
immunohistochemistry
- ROIs
regions of interest
- Hu
Hounsfield unit
- NFS
NAFLD fibrotic scoring
- IQR
interquartile range
- NOS
Newcastle-Ottawa Scale
- OR
odds ratio
- CI
confidence interval
Supporting Information
References
- 1.Dekker E, Tanis PJ, Vleugels JLA, Kasi PM, Wallace MB. Colorectal cancer. Lancet. 2019;394:1467–1480. doi: 10.1016/S0140-6736(19)32319-0. [DOI] [PubMed] [Google Scholar]
- 2.Biller LH, Schrag D. Diagnosis and treatment of metastatic colorectal cancer: a review. JAMA. 2021;325:669–685. doi: 10.1001/jama.2021.0106. [DOI] [PubMed] [Google Scholar]
- 3.Shin AE, Giancotti FG, Rustgi AK. Metastatic colorectal cancer: mechanisms and emerging therapeutics. Trends Pharmacol Sci. 2023;44:222–236. doi: 10.1016/j.tips.2023.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Riesco-Martinez MC, Modrego A, Espinosa-Olarte P, La Salvia A, Garcia-Carbonero R. Perioperative chemotherapy for liver metastasis of colorectal cancer: lessons learned and future perspectives. Curr Treat Options Oncol. 2022;23:1320–1337. doi: 10.1007/s11864-022-01008-5. [DOI] [PubMed] [Google Scholar]
- 5.Hugen N, van de Velde CJH, de Wilt JHW, Nagtegaal ID. Metastatic pattern in colorectal cancer is strongly influenced by histological subtype. Ann Oncol. 2014;25:651–657. doi: 10.1093/annonc/mdt591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Manjili MH, Khazaie K. Pattern recognition of tumor dormancy and relapse beyond cell-intrinsic and cell-extrinsic pathways. Semin Cancer Biol. 2022;78:1–4. doi: 10.1016/j.semcancer.2021.12.008. [DOI] [PubMed] [Google Scholar]
- 7.Attaran S, Bissell MJ. The role of tumor microenvironment and exosomes in dormancy and relapse. Semin Cancer Biol. 2022;78:35–44. doi: 10.1016/j.semcancer.2021.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tilg H, Adolph TE, Dudek M, Knolle P. Non-alcoholic fatty liver disease: the interplay between metabolism, microbes and immunity. Nat Metab. 2021;3:1596–1607. doi: 10.1038/s42255-021-00501-9. [DOI] [PubMed] [Google Scholar]
- 9.Li Z, Ji BW, Dixit PD, Tchourine K, Lien EC, Hosios AM, Abbott KL, Rutter JC, Westermark AM, Gorodetsky EF, Sullivan LB, Vander Heiden MG, Vitkup D. Cancer cells depend on environmental lipids for proliferation when electron acceptors are limited. Nat Metab. 2022;4:711–723. doi: 10.1038/s42255-022-00588-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Powell EE, Wong VW, Rinella M. Non-alcoholic fatty liver disease. Lancet. 2021;397:2212–2224. doi: 10.1016/S0140-6736(20)32511-3. [DOI] [PubMed] [Google Scholar]
- 11.Younossi ZM, Koenig AB, Abdelatif D, Fazel Y, Henry L, Wymer M. Global epidemiology of nonalcoholic fatty liver disease-Meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology. 2016;64:73–84. doi: 10.1002/hep.28431. [DOI] [PubMed] [Google Scholar]
- 12.Peinado H, Zhang H, Matei IR, Costa-Silva B, Hoshino A, Rodrigues G, Psaila B, Kaplan RN, Bromberg JF, Kang Y, Bissell MJ, Cox TR, Giaccia AJ, Erler JT, Hiratsuka S, Ghajar CM, Lyden D. Pre-metastatic niches: organ-specific homes for metastases. Nat Rev Cancer. 2017;17:302–317. doi: 10.1038/nrc.2017.6. [DOI] [PubMed] [Google Scholar]
- 13.Barcellos-Hoff MH, Lyden D, Wang TC. The evolution of the cancer niche during multistage carcinogenesis. Nat Rev Cancer. 2013;13:511–518. doi: 10.1038/nrc3536. [DOI] [PubMed] [Google Scholar]
- 14.Besutti G, Damato A, Venturelli F, Bonelli C, Vicentini M, Monelli F, Mancuso P, Ligabue G, Pattacini P, Pinto C, Rossi PG. Baseline liver steatosis has no impact on liver metastases and overall survival in rectal cancer patients. BMC Cancer. 2021;21:253. doi: 10.1186/s12885-021-07980-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Murono K, Kitayama J, Tsuno NH, Nozawa H, Kawai K, Sunami E, Akahane M, Watanabe T. Hepatic steatosis is associated with lower incidence of liver metastasis from colorectal cancer. Int J Colorectal Dis. 2013;28:1065–1072. doi: 10.1007/s00384-013-1656-2. [DOI] [PubMed] [Google Scholar]
- 16.Kondo T, Okabayashi K, Hasegawa H, Tsuruta M, Shigeta K, Kitagawa Y. The impact of hepatic fibrosis on the incidence of liver metastasis from colorectal cancer. Br J Cancer. 2016;115:34–39. doi: 10.1038/bjc.2016.155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hu X, Marietta A, Dai WX, Li YQ, Ma XJ, Zhang L, Cai SJ, Peng JJ. Prediction of hepatic metastasis and relapse in colorectal cancers based on concordance analyses with liver fibrosis scores. Clin Transl Med. 2020;9:13. doi: 10.1186/s40169-020-0264-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kondo T, Okabayashi K, Hasegawa H, Ishii Y, Tsuruta M, Ishida T, Matsui S, Kitagawa Y. The impact of hepatic steatosis on the incidence of liver metastasis from colorectal cancer. Dis Colon Rectum. 2014;57:e293. [Google Scholar]
- 19.Wang G, Li J, Bojmar L, Chen H, Li Z, Tobias GC, Hu M, Homan EA, Lucotti S, Zhao F, Posada V, Oxley PR, Cioffi M, Kim HS, Wang H, Lauritzen P, Boudreau N, Shi Z, Burd CE, Zippin JH, Lo JC, Pitt GS, Hernandez J, Zambirinis CP, Hollingsworth MA, Grandgenett PM, Jain M, Batra SK, DiMaio DJ, Grem JL, Klute KA, Trippett TM, Egeblad M, Paul D, Bromberg J, Kelsen D, Rajasekhar VK, Healey JH, Matei IR, Jarnagin WR, Schwartz RE, Zhang H, Lyden D. Tumour extracellular vesicles and particles induce liver metabolic dysfunction. Nature. 2023;618:374–382. doi: 10.1038/s41586-023-06114-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhu Y, Hu Y, Kong X, Xiao Q, Pan Z, Zheng Z, Wei Y, Ziqiang W, Wang D, Chen J, Chen K, Zheng S, Wang M, Wu X, Ding K NCRCC Research Group. Cohort profile: The National Colorectal Cancer Cohort (NCRCC) study in China. BMJ Open. 2021;11:e051397. doi: 10.1136/bmjopen-2021-051397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chen H, Dai S, Fang Y, Chen L, Jiang K, Wei Q, Ding K. Hepatic steatosis predicts higher incidence of recurrence in colorectal cancer liver metastasis patients. Front Oncol. 2021;11:631943. doi: 10.3389/fonc.2021.631943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Srivastava A, Gailer R, Tanwar S, Trembling P, Parkes J, Rodger A, Suri D, Thorburn D, Sennett K, Morgan S, Tsochatzis EA, Rosenberg W. Prospective evaluation of a primary care referral pathway for patients with non-alcoholic fatty liver disease. J Hepatol. 2019;71:371–378. doi: 10.1016/j.jhep.2019.03.033. [DOI] [PubMed] [Google Scholar]
- 23.Pereyra D, Rumpf B, Ammann M, Perrodin SF, Tamandl D, Haselmann C, Stift J, Brostjan C, Laengle F, Beldi G, Gruenberger T, Starlinger P. The combination of APRI and ALBI facilitates preoperative risk stratification for patients undergoing liver surgery after neoadjuvant chemotherapy. Ann Surg Oncol. 2019;26:791–799. doi: 10.1245/s10434-018-07125-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Baracos VE, Martin L, Korc M, Guttridge DC, Fearon KCH. Cancer-associated cachexia. Nat Rev Dis Primers. 2018;4:17105. doi: 10.1038/nrdp.2017.105. [DOI] [PubMed] [Google Scholar]
- 25.Xi ZF, Fan ZP, Qiu DK, Zeng MD. The relationship between fatty liver disease and liver metastases from colorectal cancer. Chinese Journal of Digestion. 2009;29:157–160. [Google Scholar]
- 26.Lv Y, Zhang HJ. Effect of non-alcoholic fatty liver disease on the risk of synchronous liver metastasis: analysis of 451 consecutive patients of newly diagnosed colorectal cancer. Front Oncol. 2020;10:251. doi: 10.3389/fonc.2020.00251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Makri E, Goulas A, Polyzos SA. Epidemiology, pathogenesis, diagnosis and emerging treatment of nonalcoholic fatty liver disease. Arch Med Res. 2021;52:25–37. doi: 10.1016/j.arcmed.2020.11.010. [DOI] [PubMed] [Google Scholar]
- 28.Ramanathan R, Ali AH, Ibdah JA. Mitochondrial dysfunction plays central role in nonalcoholic fatty liver disease. Int J Mol Sci. 2022;23:7280. doi: 10.3390/ijms23137280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Goldman O, Adler LN, Hajaj E, Croese T, Darzi N, Galai S, Tishler H, Ariav Y, Lavie D, Fellus-Alyagor L, Oren R, Kuznetsov Y, David E, Jaschek R, Stossel C, Singer O, Malitsky S, Barak R, Seger R, Erez N, Amit I, Tanay A, Saada A, Golan T, Rubinek T, Sang Lee J, Ben-Shachar S, Wolf I, Erez A. Early infiltration of innate immune cells to the liver depletes HNF4α and promotes extrahepatic carcinogenesis. Cancer Discov. 2023;13:1616–1635. doi: 10.1158/2159-8290.CD-22-1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ghosh S, Chen ML, Weinberg J, Fikre T, Ko NY. Hepatitis C virus infection and chemotherapy in breast cancer: a retrospective chart analysis. Oncologist. 2020;25:845–852. doi: 10.1634/theoncologist.2020-0172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pfister D, Núñez NG, Pinyol R, Govaere O, Pinter M, Szydlowska M, Gupta R, Qiu M, Deczkowska A, Weiner A, Müller F, Sinha A, Friebel E, Engleitner T, Lenggenhager D, Moncsek A, Heide D, Stirm K, Kosla J, Kotsiliti E, Leone V, Dudek M, Yousuf S, Inverso D, Singh I, Teijeiro A, Castet F, Montironi C, Haber PK, Tiniakos D, Bedossa P, Cockell S, Younes R, Vacca M, Marra F, Schattenberg JM, Allison M, Bugianesi E, Ratziu V, Pressiani T, D’Alessio A, Personeni N, Rimassa L, Daly AK, Scheiner B, Pomej K, Kirstein MM, Vogel A, Peck-Radosavljevic M, Hucke F, Finkelmeier F, Waidmann O, Trojan J, Schulze K, Wege H, Koch S, Weinmann A, Bueter M, Rössler F, Siebenhüner A, De Dosso S, Mallm JP, Umansky V, Jugold M, Luedde T, Schietinger A, Schirmacher P, Emu B, Augustin HG, Billeter A, Müller-Stich B, Kikuchi H, Duda DG, Kütting F, Waldschmidt DT, Ebert MP, Rahbari N, Mei HE, Schulz AR, Ringelhan M, Malek N, Spahn S, Bitzer M, Ruiz de Galarreta M, Lujambio A, Dufour JF, Marron TU, Kaseb A, Kudo M, Huang YH, Djouder N, Wolter K, Zender L, Marche PN, Decaens T, Pinato DJ, Rad R, Mertens JC, Weber A, Unger K, Meissner F, Roth S, Jilkova ZM, Claassen M, Anstee QM, Amit I, Knolle P, Becher B, Llovet JM, Heikenwalder M. NASH limits anti-tumour surveillance in immunotherapy-treated HCC. Nature. 2021;592:450–456. doi: 10.1038/s41586-021-03362-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhang C, Wang XY, Zhang P, He TC, Han JH, Zhang R, Lin J, Fan J, Lu L, Zhu WW, Jia HL, Zhang JB, Chen JH. Cancer-derived exosomal HSPC111 promotes colorectal cancer liver metastasis by reprogramming lipid metabolism in cancer-associated fibroblasts. Cell Death Dis. 2022;13:57. doi: 10.1038/s41419-022-04506-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nguyen LH, Goel A, Chung DC. Pathways of colorectal carcinogenesis. Gastroenterology. 2020;158:291–302. doi: 10.1053/j.gastro.2019.08.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Alonso-Abreu I, Alarcón-Fernández O, Gimeno-García AZ, Romero-García R, Carrillo-Palau M, Nicolás-Pérez D, Jiménez A, Quintero E. Early colonoscopy improves the outcome of patients with symptomatic colorectal cancer. Dis Colon Rectum. 2017;60:837–844. doi: 10.1097/DCR.0000000000000863. [DOI] [PubMed] [Google Scholar]
- 35.Aktas E, Uzman M, Yildirim O, Sahin B, Buyukcam F, Aktas B, Yilmaz B, Yildirim AM, Basyigit S, Yeniova O, Kefeli A, Aribas BK. Assessment of hepatic steatosis on contrast enhanced computed tomography in patients with colorectal cancer. Int J Clin Exp Med. 2014;7:4342–4346. [PMC free article] [PubMed] [Google Scholar]
- 36.Altea-Manzano P, Doglioni G, Liu Y, Cuadros AM, Nolan E, Fernández-García J, Wu Q, Planque M, Laue KJ, Cidre-Aranaz F, Liu XZ, Marin-Bejar O, Van Elsen J, Vermeire I, Broekaert D, Demeyer S, Spotbeen X, Idkowiak J, Montagne A, Demicco M, Alkan HF, Rabas N, Riera-Domingo C, Richard F, Geukens T, De Schepper M, Leduc S, Hatse S, Lambrechts Y, Kay EJ, Lilla S, Alekseenko A, Geldhof V, Boeckx B, de la Calle Arregui C, Floris G, Swinnen JV, Marine JC, Lambrechts D, Pelechano V, Mazzone M, Zanivan S, Cools J, Wildiers H, Baud V, Grünewald TGP, Ben-David U, Desmedt C, Malanchi I, Fendt SM. A palmitate-rich metastatic niche enables metastasis growth via p65 acetylation resulting in pro-metastatic NF-κB signaling. Nat Cancer. 2023;4:344–364. doi: 10.1038/s43018-023-00513-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rosa-Caldwell ME, Brown JL, Lee DE, Wiggs MP, Perry RA Jr, Haynie WS, Caldwell AR, Washington TA, Lo WJ, Greene NP. Hepatic alterations during the development and progression of cancer cachexia. Appl Physiol Nutr Metab. 2020;45:500–512. doi: 10.1139/apnm-2019-0407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhang G, Liu Z, Ding H, Zhou Y, Doan HA, Sin KWT, Zhu ZJ, Flores R, Wen Y, Gong X, Liu Q, Li YP. Tumor induces muscle wasting in mice through releasing extracellular Hsp70 and Hsp90. Nat Commun. 2017;8:589. doi: 10.1038/s41467-017-00726-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lu L, Liu Y, Zhang G, Xu Y, Hu D, Ji G, Xu H. The circRNA expression profile of colorectal inflammatory cancer transformation revealed potential predictive biomarkers. Aging (Albany NY) 2022;14:9280–9299. doi: 10.18632/aging.204406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Becker A, Thakur BK, Weiss JM, Kim HS, Peinado H, Lyden D. Extracellular vesicles in cancer: cell-to-cell mediators of metastasis. Cancer Cell. 2016;30:836–848. doi: 10.1016/j.ccell.2016.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kostallari E, Valainathan S, Biquard L, Shah VH, Rautou PE. Role of extracellular vesicles in liver diseases and their therapeutic potential. Adv Drug Deliv Rev. 2021;175:113816. doi: 10.1016/j.addr.2021.05.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Costa-Silva B, Aiello NM, Ocean AJ, Singh S, Zhang H, Thakur BK, Becker A, Hoshino A, Mark MT, Molina H, Xiang J, Zhang T, Theilen TM, García-Santos G, Williams C, Ararso Y, Huang Y, Rodrigues G, Shen TL, Labori KJ, Lothe IM, Kure EH, Hernandez J, Doussot A, Ebbesen SH, Grandgenett PM, Hollingsworth MA, Jain M, Mallya K, Batra SK, Jarnagin WR, Schwartz RE, Matei I, Peinado H, Stanger BZ, Bromberg J, Lyden D. Pancreatic cancer exosomes initiate pre-metastatic niche formation in the liver. Nat Cell Biol. 2015;17:816–826. doi: 10.1038/ncb3169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Masaki S, Hashimoto Y, Kunisho S, Kimoto A, Kitadai Y. Fatty change of the liver microenvironment influences the metastatic potential of colorectal cancer. Int J Exp Pathol. 2020;101:162–170. doi: 10.1111/iep.12371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Heinrich B, Brown ZJ, Diggs LP, Vormehr M, Ma C, Subramanyam V, Rosato U, Ruf B, Walz JS, McVey JC, Wabitsch S, Fu Q, Yu SJ, Zhang Q, Lai CW, Sahin U, Greten TF. Steatohepatitis impairs T-cell-directed immunotherapies against liver tumors in mice. Gastroenterology. 2021;160:331–345. e336. doi: 10.1053/j.gastro.2020.09.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wang Z, Kim SY, Tu W, Kim J, Xu A, Yang YM, Matsuda M, Reolizo L, Tsuchiya T, Billet S, Gangi A, Noureddin M, Falk BA, Kim S, Fan W, Tighiouart M, You S, Lewis MS, Pandol SJ, Di Vizio D, Merchant A, Posadas EM, Bhowmick NA, Lu SC, Seki E. Extracellular vesicles in fatty liver promote a metastatic tumor microenvironment. Cell Metab. 2023;35:1209–1226. e13. doi: 10.1016/j.cmet.2023.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Nath A, Li I, Roberts LR, Chan C. Elevated free fatty acid uptake via CD36 promotes epithelial-mesenchymal transition in hepatocellular carcinoma. Sci Rep. 2015;5:14752. doi: 10.1038/srep14752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Pascual G, Avgustinova A, Mejetta S, Martín M, Castellanos A, Attolini CS, Berenguer A, Prats N, Toll A, Hueto JA, Bescós C, Di Croce L, Benitah SA. Targeting metastasis-initiating cells through the fatty acid receptor CD36. Nature. 2017;541:41–45. doi: 10.1038/nature20791. [DOI] [PubMed] [Google Scholar]
- 48.Antalis CJ, Uchida A, Buhman KK, Siddiqui RA. Migration of MDA-MB-231 breast cancer cells depends on the availability of exogenous lipids and cholesterol esterification. Clin Exp Metastasis. 2011;28:733–741. doi: 10.1007/s10585-011-9405-9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.




