Abstract
For advanced hepatocellular carcinoma (HCC), the best second-line treatment after first-line treatment with sorafenib is unclear. This study aimed to compared the efficacy of second-line regorafenib (a tyrosine kinase inhibitor) and immune checkpoint inhibitors (ICIs) in patients with advanced HCC after sorafenib therapy. This retrospective study included 89 patients with HCC treated with sorafenib, and then regorafenib (n = 58) or an ICI (n = 31). Treatment response, overall survival (OS) and progression-free survival (PFS) of the 2 groups were compared, and factors associated with post-treatment mortality or disease progression were evaluated. During follow-up period, compared to regorafenib, treatment with an ICI results in a slight increase in a 20% decrease of AFP (35.7% vs. 31.8%), complete response rate (6.5% vs. 0%), objective response rate (16.1% vs. 6.9%), median overall survival (13.3 vs. 5 months), and median PFS (3.0 vs. 2.6 months). Combined locoregional treatment (LRT) (hazard ratio [HR] = 0.40, 95% confidence interval [CI]: 0.15-0.99) during second-line treatment was associated with a decreased risk of post-treatment mortality. After propensity scoring matching, combined LRT during second-line treatment had longer post-treatment OS than patients without combined LRT. A 20% decrease of AFP (HR = 0.54, 95% CI: 0.31-0.94) was associated with a decreased risk of post-treatment disease progression. In conclusions, second-line treatment with regorafenib or ICI prolongs OS in patients with advanced HCC treated with sorafenib. Combined LRT during second-line treatment is associated with decreased post-treatment mortality. A 20% decrease of AFP level may be predictive of a lower rate of disease progression.
Keywords: Combination therapy, hepatocellular carcinoma (HCC), immune checkpoint inhibitor (ICI), locoregional treatment (LRT), regorafenib, second-line treatment, sorafenib
Introduction
Hepatocellular carcinoma (HCC) is the most frequent primary liver cancer, accounting for 75% to 85% of cases [1]. HCC is usually diagnosed at an advanced stage, and thus there are limited treatment options [2]. In patients with advanced disease receiving palliative treatment, the median survival after diagnosis ranges from 6 to 12 months [3]. However, systemic therapy for HCC has undergone revolutionary changes in recent years [4]. Patients with HCC meeting the definition of Barcelona Clinic Liver Cancer classification (BCLC) stage C (advanced stage) are considered optimal candidates for systemic therapy [5]. However, there is a large variation in patients with stage C disease, and the choice between locoregional and systemic therapy can be difficult (such as in a patient with a relatively large tumor who also has tumor-associated main or main branch portal vein thrombosis). Nevertheless, there is little argument that systemic treatment is a reasonable choice for patients with extrahepatic metastatic disease, or those whose tumors have progressed after one or more locoregional treatments (LRTs) [6].
Early studies have indicated that no single chemotherapy agent or combination of agents results in a significant increase in overall survival (OS) [7,8]. As such, research has focused on treatments targeted at pathways thought to be critical to the pathogenesis of HCC. A milestone was the approval of sorafenib for treatment of advanced HCC [9]. Subsequently, regorafenib was found to improve outcomes in patients with HCC who progressed after sorafenib treatment, and other second-line treatment such as ramucirumab and cabozantinib, and immune checkpoint inhibitors (ICIs) were developed [10].
The approval of new drugs for the treatment of advanced HCC offers more treatment options; however, the selection of the best second-line treatment after treatment with sorafenib is unclear although the American Society of Clinical Oncology (ASCO) has developed evidence-based guidelines for the systemic treatment of advanced HCC [11]. Regorafenib as a second-line treatment has shown a survival benefit in patients progressing on sorafenib [12], and nivolumab (an ICI) has showed a median survival of 16 months with an acceptable safety profile in patients who progressed on, or were intolerant to sorafenib [13].
Thus, the purpose of this study was to compare the efficacy of regorafenib and ICIs on survival in patients with advanced HCC after first-line treatment with sorafenib.
Materials and methods
Study design and population
This is a retrospective study using data from a prospectively collected database. The study was approved by the Institutional Review Board of the Chang Gung Memorial Hospital (Approval number: 202002147B0C601). In addition, it waived the need for patient informed consent because the study classified as retrospective study with expedited review. All medical records of the patients diagnosed with HCC treated with sorafenib as the first-line treatment, and subsequently treated with regorafenib or an ICI as second-line treatment between 2015 and 2020 were reviewed (Figure S1).
Exclusion criteria were: 1) Prior systemic therapy other than sorafenib, or sorafenib plus chemotherapy; 2) A second concurrent malignancy; 3) Regorafenib treatment duration < 2 weeks, or ICI treatment < 3 cycles; 4) Participated in another clinical trial; 5) Duplicated records; 6) Treatment no documented clearly in the medical records; 7) Second-line treatment not a tyrosine kinase inhibitor (TKI) or ICI; 8) Treated with sorafenib before 2015. After exclusion, 89 patients were included in the analysis: 58 patients treated with regorafenib and 31 with an ICI (nivolumab n = 22; pembrolizumab n = 6; atezolizumab n = 3).
Baseline demographic and clinical data collected from the medical records were age, sex, history of hepatitis B virus (HBV) or hepatitis C virus (HCV) infection, treatment with transarterial chemoembolization (TACE) and/or radiofrequency ablation (RFA), BCLC classification, cirrhosis, fibrosis-4 (FIB-4) index, metastasis, microvascular invasion (MVI), portal vein invasion (VP) stage, extra-hepatic metastasis (EHM), serum levels of platelets, aspartate aminotransferase (AST), alanine transaminase (ALT), albumin, alpha fetoprotein (AFP), calculated albumin-bilirubin (ALBI) grade, aspartate aminotransferase to platelet ratio (APRI) index, and neutrophil-to-lymphocyte ratio (NLR), combined locoregional treatment (LRT), and tumor burden (up to 7 criteria [14], or up to 11 criteria [15]). Patients were followed up with hepatic ultrasonography and computed tomography (CT) every 3-6 months, and serum AFP and serum protein levels were assessed every month; these data were also extracted from the medical records.
Outcome assessment
Primary outcomes included overall survival (OS), and progression-free survival (PFS), treatment response, objective response rate (ORR), disease control rate (DCR) and a decrease of serum AFP of 20% from baseline in patients with regorafenib or immune checkpoint inhibitor. The secondary outcomes were to determine the factors associated with post-treatment OS or post-treatment PFS. Treatment response evaluation was based on the modified Response Evaluation Criteria in Solid Tumors (mRECIST) [16]. ORR was defined as the percentage of patients who achieve a response, which was either be complete response (CR; complete disappearance of lesions) or partial response (PR; reduction in the sum of maximal tumor diameters by at least 30%) [16]. The DCR was defined as the percentage of patients who have achieved a CR, PR, and stable disease [17]. The OS rate was defined as the interval between regorafenib or ICI treatment and the date of death caused by HCC or the date of the last follow-up. PFS was defined as the interval between regorafenib or ICI therapy and the date of diagnosis of the first recurrence or the date of the last follow-up.
Statistical analysis
Continuous variables were expressed as median and interquartile range (IQR) or 95% confidence interval (CI), and compared by the Mann-Whitney U test. Categorical variables were expressed as number and percentage, and were compared with Fisher’s exact test. Univariate and multivariate Cox regression was performed to determine the associations between patient demographic and clinical variables and OS or PFS. The Kaplan-Meier method was used to compare survival times of treatment with regorafenib/ICI or non-LRT/LRT, and survival curves were compared using the log-rank test. Time-to-event was defined as “the follow-up period of event occurrence”. A 2-sided value of P < 0.05 was considered statistically significant. Statistical analyses were performed using SPSS version 25.0 software (SPSS Inc., Chicago, IL, USA).
Results
Patient demographics and clinical characteristics
The baseline demographics and clinical characteristics of 89 patients with HCC are shown in Table 1. There were 72 (80.9%) males and 17 (19.1%) females, with a median age of 63.0 years (IQR: 58.0, 68.0 years). There were no differences in the distributions of age, sex, BCLC stage, MVI, VP stage, beyond the out-to-7 criteria, EHM, prior TACE, prior RFA, history of HBV and HCV, cirrhosis, and baseline NLR, AST, ALT, albumin, AFP, ALBI grade, APRI index, FIB-4 index, and combined LRT and combined other systemic treatment between the regorafenib and ICI groups. Only the proportion of beyond the out-to-11 criteria was significantly different between the 2 groups.
Table 1.
Patient baseline characteristics
| All (n = 89) | Regorafenib (n = 58) | ICI (n = 31) | p-value | |
|---|---|---|---|---|
| Age (years), median (IQR) | 63.0 (58.0, 68.0) | 63.5 (59.0, 71.5) | 61.0 (54.5, 66.5) | 0.10 |
| Sex/male, n (%) | 72 (80.9) | 48 (82.8) | 24 (77.4) | 0.74 |
| BCLC stage, n (%) | 0.24 | |||
| B | 15 (16.9) | 12 (20.7) | 3 (9.7) | |
| C | 74 (83.1) | 46 (79.3) | 28 (90.3) | |
| MVI, n (%) | 46 (51.7) | 25 (43.1) | 21 (67.7) | 0.05 |
| VP stage, n (%) | 0.12 | |||
| VP2 | 5 (10.9) | 3 (12.0) | 2 (9.5) | |
| VP3 | 30 (65.2) | 19 (76.0) | 11 (52.4) | |
| VP4 | 11 (23.9) | 3 (12.0) | 8 (38.1) | |
| Out of up-to-7 criteria, n (%) | 50 (56.2) | 29 (50.0) | 21 (67.7) | 0.17 |
| Out of up-to-11 criteria, n (%) | 39 (43.8) | 20 (34.5) | 19 (61.3) | 0.03* |
| EHM, n (%) | 53 (59.6) | 34 (58.6) | 19 (61.3) | 0.99 |
| Previous TACE, n (%) | 68 (76.4) | 45 (77.6) | 23 (74.2) | 0.92 |
| Previous RFA, n (%) | 25 (28.1) | 18 (31.0) | 7 (22.6) | 0.55 |
| HBV history (%), n (%) | 51 (57.3) | 30 (51.7) | 21 (67.7) | 0.22 |
| HCV history (%), n (%) | 25 (28.1) | 18 (31.0) | 7 (22.6) | 0.55 |
| Cirrhosis, n (%) | 74 (83.1) | 47 (81.0) | 27 (87.1) | 0.67 |
| Baseline NLR, median (IQR) | 3.9 (2.7, 6.3) | 3.77 (2.0, 6.3) | 4.1 (3.1, 6.2) | 0.46 |
| Baseline platelets (103/µL), median (IQR) | 127.0 (92.0, 184.5) | 119.5 (87.5, 163.5) | 143.0 (96.0, 216.0) | 0.33 |
| Baseline AST (U/L), median (IQR) | 54.0 (40.0, 80.0) | 52.0 (39.0, 72.5) | 69.5 (44.5, 87.0) | 0.17 |
| Baseline ALT (U/L), median (IQR) | 41.0 (30.0, 62.0) | 37.0 (30.8, 55.3) | 48.0 (29.5, 73.0) | 0.27 |
| Baseline albumin (g/dL), median (IQR) | 3.8 (3.4, 4.1) | 3.9 (3.5, 4.1) | 3.7 (3.4, 4.1) | 0.70 |
| Baseline AFP (ng/mL), median (IQR) | 280.6 (17.8, 2079.2) | 183.9 (12.3, 1920.8) | 468.0 (146.3, 2162.6) | 0.13 |
| Baseline ALBI II+III, n (%) | 49 (56.3) | 31 (54.4) | 18 (60.0) | 0.78 |
| Baseline APRI, median (IQR) | 1.49 (0.9, 2.0) | 1.5 (1.0, 2.0) | 1.4 (0.9, 1.9) | 0.90 |
| Baseline FIB-4, median (IQR) | 4.47 (2.60, 6.28) | 4.6 (2.7, 6.8) | 4.1 (2.5, 6.0) | 0.31 |
| Combine LRTa, n (%) | 19 (21.3) | 14 (24.1) | 5 (16.1) | 0.54 |
| Combine other systemic treatment, n (%) | 3 (3.4) | 2 (3.4) | 1 (3.2) | 1.00 |
ICI, immune checkpoint inhibitor; BCLC, Barcelona Clinic Liver Cancer classification; MVI, microvascular invasion; VP, portal vein invasion; EHM, extra-hepatic metastasis; TACE, transarterial chemoembolization; RFA, radiofrequency ablation; HBV, hepatitis B virus; HCV, hepatitis C virus; NLR, neutrophil-to-lymphocyte ratio; AST, aspartate aminotransferase; ALT, alanine transaminase; AFP, alpha fetoprotein; ALBI grade, albumin-bilirubin grade; APRI, aspartate aminotransferase to platelet ratio index; FIB-4, fibrosis-4 index; LRT, locoregional treatment; IQR, interquartile range.
Transarterial chemoembolization or radiofrequency ablation.
P < 0.05.
Outcomes after regorafenib and ICI treatment
After treatment, the median follow-up period in regorafenib group was 6.92 months (IQR: 3.73, 11.48 months), and in the ICI group was 8.03 months (IQR: 3.61, 21.95 months) (P > 0.05, Table 2). Compared to the regorafenib group, the ICI group had a slightly higher rate of increase in 20% improvement of AFP (ICI and regorafenib groups: 35.7% vs. 31.8%), CR rate (ICI and regorafenib groups: 6.5% vs. 0%), ORR (ICI and regorafenib groups: 16.1% vs. 6.9%), median OS (ICI and regorafenib groups: 13.3 vs. 5 months), and median PFS (ICI and regorafenib groups: 3.0 vs. 2.6 months); however, the differences were not statistically significant (all, P > 0.05, Table 2 and Figure 1).
Table 2.
Response and outcome assessment between treatment with regorafenib or an immune checkpoint inhibitor
| Review according to mRECIST | Regorafenib (n = 58) | ICI (n = 31) | p-value |
|---|---|---|---|
| AFP decrease 20% from baselinea, median (IQR) | 14 (31.8) | 10 (35.7) | 0.93 |
| Treatment response, n (%) | |||
| Complete response | 0 (0.0) | 2 (6.5) | 0.34 |
| Partial response | 4 (6.9) | 3 (9.7) | |
| Stable disease | 38 (65.5) | 16 (51.6) | |
| Progressive disease | 12 (20.7) | 7 (22.6) | |
| Not evaluable | 4 (6.9) | 3 (9.7) | |
| Objective response rate, n (%) | 4 (6.9) | 5 (16.1) | 0.31 |
| Disease control rate, n (%) | 42 (72.4) | 21 (67.7) | 0.83 |
| Follow up duration (months), median (IQR) | 6.92 (3.73, 11.48) | 8.03 (3.61, 21.95) | 0.39 |
| Overall survival (months), median (95% CI) | 8 (9.2, NA) | 13.3 (8.3, NA) | 0.37 |
| Progression-free survival (months), median (95% CI) | 2.6 (2.4, 9.0) | 3.0 (2.5, 4.7) | 0.72 |
mRECIST, modified Response Evaluation Criteria in Solid Tumors; ICI, immune checkpoint inhibitor; AFP, alpha fetoprotein.
Patients with a baseline AFP level < 10 were excluded from AFP decrease by 20% analysis.
Figure 1.

Kaplan-Meier survival curves for overall survival and progression-free survival. (A) Overall survival and (B) progression-free survival according to combined LRT during second-line treatment. ICI, immune checkpoint inhibitor.
Factors associated with post-treatment mortality
In the univariate Cox regression analysis, combined LRT (hazard ratio [HR] = 0.31, 95% confidence interval [CI]: 0.12-0.81; P = 0.02) and history of HCV (HR = 0.40, 95% CI: 0.16-0.95; P = 0.04) were significantly associated with a decreased risk of post-treatment mortality, while BCLC stage C (stage C vs. stage B: HR = 5.31, 95% CI: 1.28-22.15; P = 0.02), beyond the up-to-7 criteria (HR = 2.29, 95% CI: 1.14-4.59; P = 0.02), and MVI (HR = 1.96, 95% CI: 1.01-3.80; P = 0.05) were significantly associated with an increased risk of post-treatment mortality (Table 3). However, the type of second-line treatment, age, sex, EHM, history of HBV, ALBI grade, FIB-4 index, and 20% improvement of AFP were not significantly associated with post-treatment mortality (all, P > 0.05, Table 3).
Table 3.
Univariate and multivariate Cox regression analyses of overall survival
| Variables | Number | Univariate | Multivariate | |||
|---|---|---|---|---|---|---|
|
|
|
|||||
| HR (95% CI) | p-value | HR (95% CI) | p-value | |||
| Sex | Female | 17 | Ref. | |||
| Male | 72 | 1.27 (0.53, 3.05) | 0.59 | |||
| Age | 89 | 0.99 (0.95, 1.02) | 0.42 | |||
| Second-line | Regorafenib | 58 | Ref. | Ref. | ||
| ICI | 31 | 0.75 (0.37, 1.51) | 0.42 | 0.54 (0.26, 1.09) | 0.08 | |
| Combine LRTa | No | 70 | Ref. | Ref. | ||
| Yes | 19 | 0.31 (0.12, 0.81) | 0.02* | 0.40 (0.15, 0.99) | 0.04* | |
| BCLC stage | B | 15 | Ref. | |||
| C | 74 | 5.31 (1.28, 22.15) | 0.02* | 3.64 (0.80, 16.57) | 0.09 | |
| MVI | No | 43 | Ref. | Ref. | ||
| Yes | 46 | 1.96 (1.01, 3.80) | 0.05 | 1.48 (0.73, 2.99) | 0.28 | |
| Out of up-to-7 criteria | No | 39 | Ref. | Ref. | ||
| Yes | 50 | 2.29 (1.14, 4.59) | 0.02* | 1.90 (0.97, 3.81) | 0.06 | |
| EHM | No | 36 | Ref. | |||
| Yes | 53 | 1.59 (0.81, 3.14) | 0.18 | |||
| HBV | No | 38 | Ref. | |||
| Yes | 51 | 1.51 (0.77, 2.98) | 0.23 | |||
| HCV | No | 64 | Ref. | |||
| Yes | 25 | 0.40 (0.16, 0.95) | 0.04* | 0.46 (0.19, 1.12) | 0.09 | |
| ALBI | I | 38 | Ref. | |||
| II+III | 49 | 1.62 (0.83, 3.17) | 0.16 | |||
| FIB-4 | 75 | 0.90 (0.78, 1.03) | 0.14 | |||
| AFP drop 20% from baseline | No | 48 | Ref. | |||
| Yes | 24 | 0.50 (0.23, 1.09) | 0.08 | |||
ICI, immune checkpoint inhibitor; LRT, locoregional treatment; BCLC, Barcelona Clinic Liver Cancer classification; MVI, microvascular invasion; EHM, extra-hepatic metastasis; HBV, hepatitis B virus; HCV, hepatitis C virus; ALBI grade, albumin-bilirubin grade; APRI, aspartate aminotransferase to platelet ratio index; FIB-4, fibrosis-4 index; AFP, alpha fetoprotein; HR, hazard ratio; CI, confidence interval.
Transarterial chemoembolization or radiofrequency ablation.
P < 0.05.
Subsequent multivariate Cox analysis showed that combined LRT during second-line treatment was independently associated with a decreased risk of post-treatment mortality (HR = 0.40, 95% CI: 0.15-0.99; P = 0.04). However, the type of second-line treatment (ICI vs. regorafenib: HR = 0.54, 95% CI: 0.26-1.09; P = 0.08), BCLC stage C (stage C vs. stage B: HR = 3.64, 95% CI: 0.80-16.57; P = 0.09), beyond the up-to-7 criteria (HR = 1.90, 95% CI: 0.97-3.81; P = 0.06), MVI (HR = 1.48, 95% CI: 0.73-2.99; P = 0.28), and history of HCV (HR = 0.46, 95% CI: 0.19-1.12; P = 0.09) were not significantly associated with post-treatment mortality (Table 3).
Kaplan-Meier curve analysis showed that patients with LRT had a longer OS than patients without LRT during second-line treatment (P = 0.02, Figure 2A). Next, we confirmed the effect of combined LRT on post-treatment mortality in patients matched for age, sex, MVI, beyond up-to-7 criteria, EHM, high baseline AFP (> 200 ng/mL), and high baseline ALBI grade (II and III). In propensity scoring matching analysis, 18 patients with LRT and 56 patients had a similar demographic and clinical characteristics during second-line treatment (Table S1). As shown in Figure 2B, after propensity scoring matching patients with combined LRT during second-line treatment had a longer post-treatment OS than non-LRT patients (P = 0.04).
Figure 2.
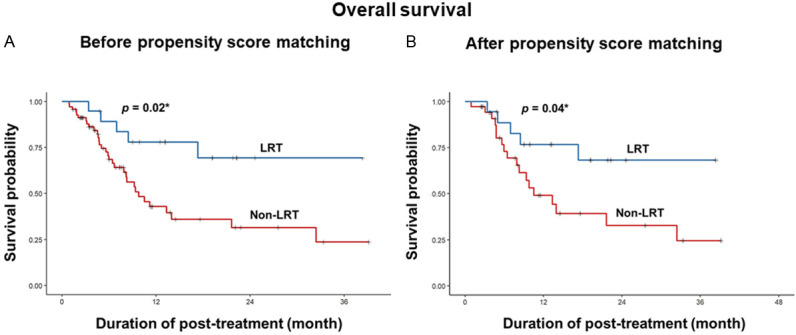
Kaplan-Meier survival curves for overall survival according to the presence combined LRT. (A) Before and (B) after propensity score matching for patient demographic and clinical characteristics. LRT, locoregional treatment. *P < 0.05.
Factors associated with post-treatment disease progression
In the univariate Cox regression analysis, combined LRT (HR = 0.57, 95% CI: 0.32-1.01; P = 0.05), 20% improvement of AFP (HR = 0.54, 95% CI: 0.31-0.94; P = 0.03), and history of HCV (HR = 0.43, 95% CI: 0.22-0.86; P = 0.02) were significantly associated with a decreased risk of post-treatment disease progression, while BCLC stage C (stage C vs. stage B: HR = 3.27, 95% CI: 1.48-7.21; P < 0.01), EHM (HR = 2.18, 95% CI: 1.34-3.56; P < 0.01), MVI (HR = 1.77, 95% CI: 1.12-2.81; P = 0.02), and beyond the up-to-7 criteria (HR = 1.67, 95% CI: 1.04-2.69; P = 0.03) were significantly associated with an increased risk of post-treatment disease progression (Table 4). However, the type of second-line treatment, age, sex, history of HBV, ALBI grade, and FIB-4 index were not significantly associated with post-treatment disease progression (all, P > 0.05, Table 4).
Table 4.
Univariate and multivariate Cox regression analyses for progression-free survival
| Variables | Number | Univariate | Multivariate | |||
|---|---|---|---|---|---|---|
|
|
|
|||||
| HR (95% CI) | p-value | HR (95% CI) | p-value | |||
| Sex | Female | 17 | Ref. | |||
| Male | 72 | 1.00 (0.57, 1.76) | 0.99 | |||
| Age | 89 | 0.97 (0.95, 1.00) | 0.07 | |||
| Second-line | Regorafenib | 58 | Ref. | Ref. | ||
| ICI | 31 | 0.88 (0.53, 1.47) | 0.62 | 0.85 (0.48, 1.53) | 0.60 | |
| Combine LRTa | No | 70 | Ref. | Ref. | ||
| Yes | 19 | 0.57 (0.32, 1.01) | 0.05 | 0.83 (0.40, 1.70) | 0.60 | |
| BCLC stage | B | 15 | Ref. | Ref. | ||
| C | 74 | 3.27 (1.48, 7.21) | < 0.01* | 3.88 (1.38, 10.95) | 0.01* | |
| MVI | No | 43 | Ref. | Ref. | ||
| Yes | 46 | 1.77 (1.12, 2.81) | 0.02* | 0.99 (0.54, 1.82) | 0.98 | |
| Out of up-to-7 criteria | No | 39 | Ref. | Ref. | ||
| Yes | 50 | 1.67 (1.04, 2.69) | 0.03* | 0.81 (0.45, 1.45) | 0.48 | |
| EHM | No | 36 | Ref. | Ref. | ||
| Yes | 53 | 2.18 (1.34, 3.56) | < 0.01* | 0.83 (0.41, 1.68) | 0.60 | |
| HBV | No | 38 | Ref. | |||
| Yes | 51 | 1.52 (0.95, 2.43) | 0.08 | |||
| HCV | No | 64 | Ref. | |||
| Yes | 25 | 0.54 (0.31, 0.94) | 0.03* | 0.59 (0.30, 1.13) | 0.11 | |
| ALBI | I | 38 | Ref. | |||
| II+III | 49 | 1.39 (0.87, 2.23) | 0.17 | |||
| FIB-4 | 75 | 0.93 (0.86, 1.01) | 0.08 | |||
| AFP drop 20% from baseline | No | 48 | Ref. | |||
| Yes | 24 | 0.43 (0.22, 0.86) | 0.02* | 0.39 (0.19, 0.82) | 0.01* | |
ICI, immune checkpoint inhibitor; LRT, locoregional treatment; BCLC, Barcelona Clinic Liver Cancer classification; MVI, microvascular invasion; EHM, extra-hepatic metastasis; HBV, hepatitis B virus; HCV, hepatitis C virus; ALBI grade, albumin-bilirubin grade; APRI, aspartate aminotransferase to platelet ratio index; FIB-4, fibrosis-4; AFP, alpha fetoprotein; HR, hazard ratio; CI, confidence interval.
Transarterial chemoembolization or radiofrequency ablation.
P < 0.05.
Subsequent multivariate Cox analysis showed that a 20% AFP improvement (HR = 0.54, 95% CI: 0.31-0.94; P = 0.03) was independently associated with a decreased risk of post-treatment disease progression, while BCLC stage C (stage C vs. stage B: HR = 0.40, 95% CI: 0.15-0.99; P = 0.04) was independently associated with an increased risk of post-treatment disease progression (Table 4). However, the type of second-line treatment (ICI vs. regorafenib: HR = 0.85, 95% CI: 0.48-1.53; P = 0.60), combined LRT (HR = 0.83, 95% CI: 0.40-1.70; P = 0.60), MVI (HR = 0.99, 95% CI: 0.54-1.82; P = 0.98), beyond the up-to-7 criteria (HR = 0.81, 95% CI: 0.45-1.45; P = 0.48), EHM (HR = 0.83, 95% CI: 0.41-1.68; P = 0.60), and history of HCV (HR = 0.59, 95% CI: 0.30-1.13; P = 0.11) were not significantly associated with post-treatment disease progression (Table 4).
Discussion
The present study compared the efficacy of second-line treatment with regorafenib or an ICI on survival in patients with advanced HCC after first-line treatment with sorafenib. The key findings of the study were as follows. First, second-line treatment with regorafenib or an ICI results in a significant increase in OS. Second, there is a tendency that ICI treatment may result in a lower post-treatment mortality rate than regorafenib; however, the difference was not statistically significant (P = 0.08). Third, combined LRT (TACE or RFA) during second-line treatment may decrease the post-treatment mortality rate, but it did not significantly affect the risk of post-treatment disease progression. Fourth, a 20% decrease of AFP may independently predict a lower risk of post-treatment disease progression.
Sorafenib is a TKI that has long been the standard treatment for advanced HCC based on the BCLC staging system [18]. However, randomized controlled trials (RCTs) demonstrated that sorafenib only slightly extended survival from 7.9 to 10.7 months in patients with BCLC stage C disease [5,19]. In studies of Asia-Pacific patients with advanced HCC, the survival benefit was even lower than the survival benefit observed globally [19,20]. A major cause of mortality in patients with advanced HCC is hepatic dysfunction due to a greater load of intrahepatic tumors and involvement of the portal vein [19,20]. In our study, all patients were treated with sorafenib as first-line treatment, and overall patients had very advanced disease: BCLC stage C 83.1%, EHM 59.6%, MVI 51.7%, later stage of portal vein invasion (Vp3+Vp4: 89.1% in MVIW). Our data showed that second-line treatment with regorafenib or an ICI prolonged the median OS to 8 or 13.3 months, respectively; although OS was not significantly different between the regorafenib and ICI groups. This indicates that second-line treatment with regorafenib or an ICI results in a significant survival benefit in advanced HCC with more aggressive disease and involvement of the portal vein.
In the present study, the univariate Cox regression analysis showed that the type of second-line treatment (regorafenib or ICI) was not significantly associated with risk of post-treatment mortality (P = 0.42). However, multivariate Cox regression analysis showed there was a tendency that ICIs treatment may be associated with lower post-treatment mortality than treatment with regorafenib; however, the difference did not reach statistical significance (P = 0.08, Table 3). These results suggest the possibility that second-line treatment with an ICI could be more effective in patients with first-line sorafenib treatment, especially in patients with combined LRT, BCLC stage B, no MVI, no beyond the up-to-7 criteria, and history of HCV.
Targeted agents in conjunction with LRT have been extensively investigated in clinical trials, and have shown promise [10]. Additionally, treatment with ICIs combined with LRT is expected to improve the median OS in patients with advanced HCC [21]. The release of neoantigens induced by LRT-associated tumor necrosis may augment the response to ICIs [21]. Duffy et al. [22] treated 32 patients (BCLC B/C with progressive disease at enrollment, 75% sorafenib experienced) with TACE or RFA, followed by the ICI tremelimumab (an anti-CTLA-4 antibody) and reported a PR in 26% of patients, a time to tumor progression (TTP) of 7.4 months, and OS of 12.3 months. The present study expanded these findings and further compared the risk of post-treatment mortality and disease progression between HCC patients (BCLC B/C with progressive disease at enrollment, 100% sorafenib experienced) with combined LRT (TACE or RFA) and without combined LRT during second-line treatment (regorafenib or an ICI). Our data showed that patients with combined LRT had a decreased risk of post-treatment mortality when compared to patients without combined LRT, while combined LRT did not significantly affect post-treatment disease progression. Moreover, after propensity scoring matching for demographics and clinical characteristics, patients with combined LRT during second-line treatment had a longer post-treatment OS than patients without combined LRT. Based on these findings, we suggest that combined LRT (TACE or RFA) during second-line treatment (regorafenib or an ICI) may be effective in patients treated with first-line sorafenib. To the best of our knowledge, no study has focused on the impact of second-line treatment with regorafenib or an ICI combined LRT (TACE or RFA) in advanced HCC on mortality after first-line treatment with sorafenib.
Multiple biomarkers may play a role in diagnosis and post-treatment outcomes of HCC, including AFP [23], fucosyltransferase 1 (FUT1) [23], death-associated protein kinase 1 (DAPK1) mRNA [24], and beta-1,3-galactosyltransferase 5 (B3GALT5) [23]. Notably, a retrospective study reported that preoperative serum AFP level > 100 ng/mL is associated with a higher rate of HCC recurrence after hepatectomy [25]. Additionally, higher post-treatment serum AFP level is associated with a high risk of HCC recurrence after treatment [26,27]. We demonstrated for the first time that a 20% decrease of AFP level was independently predictive of decreased post-treatment disease progression in patients with advanced HCC treated with sorafenib and then regorafenib or an ICI. Based on these results, we suggest that a post-treatment decrease of AFP by 20% should be considered an appropriate response to second-line treatment with regorafenib or an ICI.
Currently, few studies have examined if the addition of LRT (TACE or RFA) to systemic therapy is associated with decreased PFS. Nevertheless, it is worth noting that there was no statistical difference in PFS of patients receiving TACE combined with a TKI and ICI compared with that of patients receiving treatment without TACE [28]. The results of the present study demonstrated that combined LRT (TACE or RFA) during second-line treatment with regorafenib or an ICI did not significantly decrease post-treatment disease progression when compared without combined LRT. A potential reason for this result may be that more patients in the LRT group were defined as having disease progression due to new hepatic foci, rather than enlargement of the primary tumor, and this may not necessarily have been due to failure of LRT [28,29].
There are several limitations to this study that should be considered. First, this was a retrospective study at a single center with a relatively small sample size. Second, the survival estimates in the study may be somewhat limited by the duration of treatment. Third, there was a high proportion of patients with BCLC stage C and HBV-related HCC; thus, the results may not be generalizable to patient populations.
Conclusion
Second-line treatment with regorafenib or an ICI prolongs OS in patients with advanced HCC after first-line treatment with sorafenib, and there is a tendency that ICI therapy may be associated with decreased post-treatment mortality compared to treatment with regorafenib. Patients receiving combined LRT during second-line treatment have lower post-treatment mortality than patients without combined LRT. Moreover, a 20% decrease of AFP from baseline may predict a lower risk of disease progression. We recommend that an early combination of these treatments should be considered for improving the survival of advanced HCC due to increasing their efficacy through a synergistic mechanism.
Acknowledgements
The authors thank all the members of the Cancer Center, Chang Gung Memorial Hospital, for their invaluable help including. The authors also appreciate for their assistance in data acquisition and cleaning. Grants from Chang Gung Medical Research Fund (CMRPG3M0391), National Science Council, Taiwan (NMRPG3N6011). NSTC 112-2314-B-182A-016-MY3, and National Science Council, Taiwan (NMRPG3N6011).
Disclosure of conflict of interest
None.
Supporting Information
References
- 1.Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394–424. doi: 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
- 2.Sim HW, Knox J. Hepatocellular carcinoma in the era of immunotherapy. Curr Probl Cancer. 2018;42:40–48. doi: 10.1016/j.currproblcancer.2017.10.007. [DOI] [PubMed] [Google Scholar]
- 3.Alqahtani A, Khan Z, Alloghbi A, Said Ahmed TS, Ashraf M, Hammouda DM. Hepatocellular carcinoma: molecular mechanisms and targeted therapies. Medicina (Kaunas) 2019;55:526. doi: 10.3390/medicina55090526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wang J, Pillai A. Systemic therapy for hepatocellular carcinoma. Clin Liver Dis (Hoboken) 2021;17:337–340. doi: 10.1002/cld.1058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Llovet JM, Zucman-Rossi J, Pikarsky E, Sangro B, Schwartz M, Sherman M, Gores G. Hepatocellular carcinoma. Nat Rev Dis Primers. 2016;2:16018. doi: 10.1038/nrdp.2016.18. [DOI] [PubMed] [Google Scholar]
- 6.Gbolahan OB, Schacht MA, Beckley EW, LaRoche TP, O’Neil BH, Pyko M. Locoregional and systemic therapy for hepatocellular carcinoma. J Gastrointest Oncol. 2017;8:215–228. doi: 10.21037/jgo.2017.03.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gish RG, Porta C, Lazar L, Ruff P, Feld R, Croitoru A, Feun L, Jeziorski K, Leighton J, Gallo J, Kennealey GT. Phase III randomized controlled trial comparing the survival of patients with unresectable hepatocellular carcinoma treated with nolatrexed or doxorubicin. J. Clin. Oncol. 2007;25:3069–3075. doi: 10.1200/JCO.2006.08.4046. [DOI] [PubMed] [Google Scholar]
- 8.Yeo W, Mok TS, Zee B, Leung TW, Lai PB, Lau WY, Koh J, Mo FK, Yu SC, Chan AT, Hui P, Ma B, Lam KC, Ho WM, Wong HT, Tang A, Johnson PJ. A randomized phase III study of doxorubicin versus cisplatin/interferon alpha-2b/doxorubicin/fluorouracil (PIAF) combination chemotherapy for unresectable hepatocellular carcinoma. J Natl Cancer Inst. 2005;97:1532–1538. doi: 10.1093/jnci/dji315. [DOI] [PubMed] [Google Scholar]
- 9.Llovet JM, Ricci S, Mazzaferro V, Hilgard P, Gane E, Blanc JF, de Oliveira AC, Santoro A, Raoul JL, Forner A, Schwartz M, Porta C, Zeuzem S, Bolondi L, Greten TF, Galle PR, Seitz JF, Borbath I, Haussinger D, Giannaris T, Shan M, Moscovici M, Voliotis D, Bruix J SHARP Investigators Study Group. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med. 2008;359:378–390. doi: 10.1056/NEJMoa0708857. [DOI] [PubMed] [Google Scholar]
- 10.Huang A, Yang XR, Chung WY, Dennison AR, Zhou J. Targeted therapy for hepatocellular carcinoma. Signal Transduct Target Ther. 2020;5:146. doi: 10.1038/s41392-020-00264-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gordan JD, Kennedy EB, Abou-Alfa GK, Beg MS, Brower ST, Gade TP, Goff L, Gupta S, Guy J, Harris WP, Iyer R, Jaiyesimi I, Jhawer M, Karippot A, Kaseb AO, Kelley RK, Knox JJ, Kortmansky J, Leaf A, Remak WM, Shroff RT, Sohal DPS, Taddei TH, Venepalli NK, Wilson A, Zhu AX, Rose MG. Systemic therapy for advanced hepatocellular carcinoma: ASCO Guideline. J. Clin. Oncol. 2020;38:4317–4345. doi: 10.1200/JCO.20.02672. [DOI] [PubMed] [Google Scholar]
- 12.Bruix J, Qin S, Merle P, Granito A, Huang YH, Bodoky G, Pracht M, Yokosuka O, Rosmorduc O, Breder V, Gerolami R, Masi G, Ross PJ, Song T, Bronowicki JP, Ollivier-Hourmand I, Kudo M, Cheng AL, Llovet JM, Finn RS, LeBerre MA, Baumhauer A, Meinhardt G, Han G RESORCE Investigators. Regorafenib for patients with hepatocellular carcinoma who progressed on sorafenib treatment (RESORCE): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. 2017;389:56–66. doi: 10.1016/S0140-6736(16)32453-9. [DOI] [PubMed] [Google Scholar]
- 13.El-Khoueiry AB, Sangro B, Yau T, Crocenzi TS, Kudo M, Hsu C, Kim TY, Choo SP, Trojan J, Welling TH Rd, Meyer T, Kang YK, Yeo W, Chopra A, Anderson J, Dela Cruz C, Lang L, Neely J, Tang H, Dastani HB, Melero I. Nivolumab in patients with advanced hepatocellular carcinoma (CheckMate 040): an open-label, non-comparative, phase 1/2 dose escalation and expansion trial. Lancet. 2017;389:2492–2502. doi: 10.1016/S0140-6736(17)31046-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bolondi L, Burroughs A, Dufour JF, Galle PR, Mazzaferro V, Piscaglia F, Raoul JL, Sangro B. Heterogeneity of patients with intermediate (BCLC B) hepatocellular carcinoma: proposal for a subclassification to facilitate treatment decisions. Semin Liver Dis. 2012;32:348–359. doi: 10.1055/s-0032-1329906. [DOI] [PubMed] [Google Scholar]
- 15.Kim JH, Shim JH, Lee HC, Sung KB, Ko HK, Ko GY, Gwon DI, Kim JW, Lim YS, Park SH. New intermediate-stage subclassification for patients with hepatocellular carcinoma treated with transarterial chemoembolization. Liver Int. 2017;37:1861–1868. doi: 10.1111/liv.13487. [DOI] [PubMed] [Google Scholar]
- 16.Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, Dancey J, Arbuck S, Gwyther S, Mooney M, Rubinstein L, Shankar L, Dodd L, Kaplan R, Lacombe D, Verweij J. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1) Eur J Cancer. 2009;45:228–247. doi: 10.1016/j.ejca.2008.10.026. [DOI] [PubMed] [Google Scholar]
- 17.Sznol M. Reporting disease control rates or clinical benefit rates in early clinical trials of anticancer agents: useful endpoint or hype? Curr Opin Investig Drugs. 2010;11:1340–1341. [PubMed] [Google Scholar]
- 18.Llovet JM, Villanueva A, Lachenmayer A, Finn RS. Advances in targeted therapies for hepatocellular carcinoma in the genomic era. Nat Rev Clin Oncol. 2015;12:408–424. doi: 10.1038/nrclinonc.2015.103. [DOI] [PubMed] [Google Scholar]
- 19.Sung PS, Park HL, Yang K, Hwang S, Song MJ, Jang JW, Choi JY, Yoon SK, Yoo IR, Bae SH. (18)F-fluorodeoxyglucose uptake of hepatocellular carcinoma as a prognostic predictor in patients with sorafenib treatment. Eur J Nucl Med Mol Imaging. 2018;45:384–391. doi: 10.1007/s00259-017-3871-5. [DOI] [PubMed] [Google Scholar]
- 20.Kim DY, Kim HJ, Han KH, Han SY, Heo J, Woo HY, Um SH, Kim YH, Kweon YO, Lim HY, Yoon JH, Lee WS, Lee BS, Lee HC, Ryoo BY, Yoon SK. Real-life experience of sorafenib treatment for hepatocellular carcinoma in Korea: from GIDEON data. Cancer Res Treat. 2016;48:1243–1252. doi: 10.4143/crt.2015.278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kudo M. Immuno-oncology in hepatocellular carcinoma: 2017 update. Oncology. 2017;93(Suppl 1):147–159. doi: 10.1159/000481245. [DOI] [PubMed] [Google Scholar]
- 22.Duffy AG, Ulahannan SV, Makorova-Rusher O, Rahma O, Wedemeyer H, Pratt D, Davis JL, Hughes MS, Heller T, ElGindi M, Uppala A, Korangy F, Kleiner DE, Figg WD, Venzon D, Steinberg SM, Venkatesan AM, Krishnasamy V, Abi-Jaoudeh N, Levy E, Wood BJ, Greten TF. Tremelimumab in combination with ablation in patients with advanced hepatocellular carcinoma. J Hepatol. 2017;66:545–551. doi: 10.1016/j.jhep.2016.10.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kuo HH, Lin RJ, Hung JT, Hsieh CB, Hung TH, Lo FY, Ho MY, Yeh CT, Huang YL, Yu J, Yu AL. High expression FUT1 and B3GALT5 is an independent predictor of postoperative recurrence and survival in hepatocellular carcinoma. Sci Rep. 2017;7:10750. doi: 10.1038/s41598-017-11136-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Li L, Guo L, Wang Q, Liu X, Zeng Y, Wen Q, Zhang S, Kwok HF, Lin Y, Liu J. DAPK1 as an independent prognostic marker in liver cancer. PeerJ. 2017;5:e3568. doi: 10.7717/peerj.3568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chong CC, Lee KF, Ip PC, Wong JS, Cheung SY, Wong J, Ho SC, Lai PB. Pre-operative predictors of post-hepatectomy recurrence of hepatocellular carcinoma: can we predict earlier? Surgeon. 2012;10:260–266. doi: 10.1016/j.surge.2011.07.004. [DOI] [PubMed] [Google Scholar]
- 26.Tanaka Y, Ogawa E, Huang CF, Toyoda H, Jun DW, Tseng CH, Hsu YC, Enomoto M, Takahashi H, Furusyo N, Yeh ML, Iio E, Yasuda S, Lam CP, Lee DH, Haga H, Yoon EL, Ahn SB, Wong G, Nakamuta M, Nomura H, Tsai PC, Jung JH, Song DS, Dang H, Maeda M, Henry L, Cheung R, Yuen MF, Ueno Y, Eguchi Y, Tamori A, Yu ML, Hayashi J, Nguyen MH REAL-C Investigators. HCC risk post-SVR with DAAs in East Asians: findings from the REAL-C cohort. Hepatol Int. 2020;14:1023–1033. doi: 10.1007/s12072-020-10105-2. [DOI] [PubMed] [Google Scholar]
- 27.Watanabe T, Tokumoto Y, Joko K, Michitaka K, Horiike N, Tanaka Y, Tada F, Kisaka Y, Nakanishi S, Yamauchi K, Ochi H, Hiraoka A, Yagi S, Yukimoto A, Hirooka M, Abe M, Hiasa Y. AFP and eGFR are related to early and late recurrence of HCC following antiviral therapy. BMC Cancer. 2021;21:699. doi: 10.1186/s12885-021-08401-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hu Y, Pan T, Cai X, He QS, Zheng YB, Huang MS, Jiang ZB, Chen JW, Wu C. Addition of transarterial chemoembolization improves outcome of tyrosine kinase and immune checkpoint inhibitors regime in patients with unresectable hepatocellular carcinoma. J Gastrointest Oncol. 2023;14:1837–1848. doi: 10.21037/jgo-23-486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bruix J, Reig M, Rimola J, Forner A, Burrel M, Vilana R, Ayuso C. Clinical decision making and research in hepatocellular carcinoma: pivotal role of imaging techniques. Hepatology. 2011;54:2238–2244. doi: 10.1002/hep.24670. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


