Abstract
Cancer is one of the leading causes of death worldwide. In recent years, African countries have been faced with a rapid increase in morbidity and mortality due to this pathology. Management is often complicated by the high treatment costs, side effects and the increasing occurrence of resistance to treatments. The identification of new active ingredients extracted from endemic medicinal plants is definitively an interesting approach for the implementation of new therapeutic strategies: their extraction is often lower cost; their identification is based on an ethnobotanical history and a tradipratic approach; their use by low-income populations is simpler; this can help in the development of new synthetic molecules that are more active, more effective and with fewer side effects. The objective of this review is to document the molecules derived from African medicinal plants whose in vitro anti-cancer activities and the mechanisms of molecular actions have been identified. From the scientific databases Science Direct, PubMed and Google Scholar, we searched for publications on compounds isolated from African medicinal plants and having activity on cancer cells in culture. The data were analyzed in particular with regard to the cytotoxicity of the compounds and their mode of action. A total of 90 compounds of these African medicinal plants were selected. They come from nine chemical groups: alkaloids, flavonoids, polyphenols, quinones, saponins, steroids, terpenoids, xanthones and organic sulfides. These compounds have been associated with several cellular effects: i) Cytotoxicity, including caspase activation, alteration of mitochondrial membrane potential, and/or induction of reactive oxygen species (ROS); ii) Anti-angiogenesis; iii) Anti-metastatic properties. This review points out that the cited African plants are rich in active ingredients with anticancer properties. It also stresses that screening of these anti-tumor active ingredients should be continued at the continental scale. Altogether, this work provides a rational basis for the selection of phytochemical compounds for use in clinical trials.
Keywords: Africa, cancer, phytochemicals, plants, molecular mechanisms
Introduction
Cancer can be defined as an irreversible change in cell homeostasis [1]. This pathology encompasses a range of malignant tumors characterized by rapid and uncontrolled growth of abnormal cells and/or escape to programmed cell death or apoptosis. In advanced stages, cancer cells usually become metastatic, leaving their original primitive site after a phenomenon called epithelial-mesenchymal transition to invade other tissues and form new tumors [2]. Overall cancer survival statistics have improved in recent decades thanks to advances in diagnosis and prognosis, a better understanding of tumor biology, and most importantly, the implementation of new and more efficient treatments. However, the premature mortality rate from cancer has the highest incidence on the African continent due to a significant increase in new cases (more than one million) and especially deaths (more than 700,000) and with a more than doubling increase in new cases and deaths by 2040 [3]. Thus, cancer remains a real public health problem in Africa.
Conventional cancer treatment involve surgery to remove the tumor and followed by radiation therapy when the tumor is located [4]. After metastatic dissemination, cancer chemotherapy, which aims to inhibit the proliferation of tumor cells and induce cancer cell death, is an applicable treatment for many cancers [5]. However, chemotherapy produces various important side effects and can frequently be followed by a multidrug resistance (MDR). The critical point is the access to chemotherapy that remains limited for most African populations due in particular to the availability and accessibility of molecules [6-8]. Faced with these difficulties, many studies have focused on the search for natural bioactive compounds, or even molecules, from plants used in traditional medicine. As an example, the first anticancer agents developed from plants were vinblastine (PubChem: 13342) and vincristine (PubChem: 5978). Both compounds are dimeric alkaloids isolated from the Madagascar periwinkle (Catharanthus roseus), which have significant cytotoxic activity and are used as antineoplastic agents in antitumor therapy [9]. During cell proliferation, they act by inhibiting the metaphase of the cell cycle and binding to microtubules that block mitotic spindle development. In tumor cells, these agents inhibit DNA repair and RNA synthesis machinery [10]. Indeed, plants are reservoirs of new chemical entities, providing a promising avenue for cancer research, in particular based on ethnobotanical data and traditional medical practices. The use of plant-based bioactive compounds to treat cancer is therefore a developing field. Plants in anti-cancer drug discovery would provide access to products that can be obtained more easily, quickly and cheaply, and thus accessible to the greatest number. While the extraction of these compounds is usually environmentally friendly, their overall toxicity needs to be carefully considered. Most are selective, targeting tumor cells while sparing healthy cells [11]. Many phytochemical compounds and their analogues derived from African medicinal plants have been identified and are potential candidates of choice for the treatment of certain cancers, most of them are selective, targeting tumor cells while sparing healthy cells [11]. However, the number of clinical trials with natural bioactive compounds is still limited (Table 1).
Table 1.
Molecules isolated from African medicinal plants in clinical trials as anti-cancer drugs
| Compounds | Phase of clinical trials | Type of cancer/dose/way of administration/mechanism of action | Publications of results |
|---|---|---|---|
| ClinicalTrials.gov ID | |||
| Resveratrol | Phase I | Colon cancer/20 mg daily/oral/modulate patient Wnt signaling pathway | [118] |
| NCT00256334 | |||
| Lycopene | Phase II | Metastatic prostate cancer/30 mg daily/oral/chemotherapy | [156] |
| NCT00068731 | |||
| Quercetin | Phase I | Oral mucus/250 mg quercetin capsules daily for 3 weeks/oral/Chemotherapy | NCT01732393 |
| Isoquercetin | Phase II/III | Pancreatic, lung or colorectal cancers/1000 mg daily for 28 days/oral/prevent venous thromboembolic events in cancer patients | [157] |
| NCT02439385 | |||
| Artesunate (Semi-synthetic derivative of artemisinin) | Phase I | Metastatic breast cancer/200 mg daily/oral/chemotherapy | [158] |
| NCT00764036 | |||
| Curcumin | Phase III | Prostate cancer/1000 mg daily/oral/chemotherapy | NCT03769766 |
| Epigallocatechin gallate | Phase I | Colon or rectum cancer/450 mg twice daily/orally/Evaluate chemopreventive effect in patients | NCT02891538 |
The aim of this review is to identify molecules derived from African plants that can be used in cancer chemotherapy, while describing the associated molecular mechanisms.
Current chemotherapy context (side effects, resistance and costs)
Antitumor therapies depend on the type and stage of the disease. As noted above, current options are surgery, radiation therapy and/or drug treatments [5]. The latter is based on chemotherapy, immunotherapy and targeted therapy [12].
Organs targeted by side effects
Long term treatments with chemotherapy agents entail severe damage or failure of multiple organs: kidneys [13], liver [14,15], heart [16], blood [17], skin [18], nervous system [19], digestive system [20] and reproductive system [21,22].
At the heart level, chemotherapies can lead to hypertrophies, arrhythmias, and/or heart attacks [23]. Doxorubicin, daunorubicin, epirubicin and idarubicin, members of the anthracycline class, have often been reported as cardiotoxic [24]. Indeed, the quinone group of anthracyclines acts on DNA and causes single and double strand breaks, and also generates reactive oxygen species (ROS) toxic to the heart [25]. Cyclophosphamide, ifosfamide, cisplatin, 5-fluorouracil, paclitaxel and docetaxel may also be cardiotoxic [16].
Kidneys, being involved in the elimination of many substances such as urea, uric acid, creatinine and xenobiotics [13], any nephrotoxic compound can alter their plasma concentrations [26]. Several anti-cancer drugs have strong nephrotoxicity, including cisplatin, ifosfamide, mitomycin, methotrexate and gemcitabine [27]. They act directly on nucleic acids causing not only a blockage of DNA and RNA synthesis, but also DNA damages. All these alterations can then lead to decreased creatine clearance, impaired magnesium reabsorption, decreased glomerular filtration rate, acute kidney injury, distal renal tubular acidosis, microangiopathy, chronic renal failure [28,29].
Chemotherapy-induced gastrointestinal toxicity most often causes intense pain, nausea, infections and/or diarrhea [20,30]. Several anti-cancer drugs are responsible for this toxicity, including cisplatin, doxorubicin, cyclophosphamide, methotrexate, bleomycin, 5-fluorouracil, irinotecan, fluorouracil, and capecitabine [27]. The imbalance of the intestinal microbiota (dysbiosis) can also weaken the immune system and reduce the quality of life of patients [20].
The liver is involved in numerous homeostasis including detoxification, drug metabolism and waste excretion [31]. Irinotecan, cisplatin, oxaliplatin, erlotinib, gefitinib, imatinib, azathioprine, 6-mercaptopurine, and 6-thioguanine, cytarabine, and dacarbazine are often associated with high hepatotoxicity [14,15]. Hepatitis, cholestasis, steatosis, veno-occlusive disease, sinusoidal obstruction syndrome and fibrosis are among the most common hepatotoxic side effects of chemotherapy [32,33]. As with other organs, DNA alterations are often the primum movens of these conditions.
The chemotherapeutic molecules, more precisely those of the class of alkylating agents, affect the normal functioning of the gonads, especially in the production of gametes. In men, they not only cause damage to spermatocytes but also act on spermatogonia stem cells, resulting in the inability of cells to repopulate seminiferous tubules after stopping treatment [21,34]. In women, alkylating agents and anthracyclines cause a reduction in the pool of primordial follicles, premature ovarian failure, and oocyte loss [22].
Central or peripheral nervous systems are also secondary targets for some chemotherapy. Peripheral neuropathy can be manifested by paresthesia of the hands and feet, a decrease in deep tendon reflexes, paraparesis or mandibular pain. These clinical manifestations are generally due to the use of platinum-based drugs, particularly cisplatin [35]. Central nerve toxicity is mainly induced by cyclophosphamide, ifosfamide, melphalan, busulfan, aracytin, cytarabine and methotrexate. These drugs, particularly methotrexate and cytarabine, cause leukoencephalopathy and cerebellar dysfunction, leading to cognitive impairment, often transient dementia, and focal deficits [36].
Chemotherapy administration is often associated with skin manifestations that can be grouped into three major groups: alopecia, hand-foot syndrome, and allergic reactions [37]. Alopecia, which refers to hair loss, is caused by anthracyclines, cyclophosphamides, doxorubicin, cisplatin and vinorelbine. These molecules act on the cells of the hair bulb in the renewal phase, causing fragility and hair loss. All chemotherapy products have the potential to cause allergic reactions (rash, erythema, allergic shock) that are manifested by swelling of the face, lips and/or tongue, shortness of breath, fever or severe skin reactions (itching, redness, pimples) [18].
Finally, it is the hematological sphere that is most affected by chemotherapy-induced toxicity since it can affect red blood cells, leukocytes and/or platelets, causing anemia, leukopenia and/or thrombocytopenia [38]. Anemia is prevalent in 30-90% of cancer patients. As red blood cells are involved in the transport of oxygen to various organs of the body, their reduction results primarily in intense fatigue, difficulty breathing, paleness and high blood pressure [17]. The anti-cancer involved are essentially methotrexate and doxorubicin. Leukopenia follows a precipitous decrease in white blood cells caused by alkylating agents, anthracyclines and topoisomerase inhibitors. This decrease in leukocytes reduces the body’s ability to resist infection [39]. Thrombocytopenia, which is responsible for bleeding-related clotting disorders, is more common with mitomycin-C and gemcitabine [40].
Resistance mechanisms
Resistance to cancer treatments is very common. It is responsible for about 90% of cancer patient deaths [41]. This drug resistance of cancer cells is linked to several molecular mechanisms, the main ones being: an abnormal increase in drug efflux; the occurrence of genetic mutations and/or epigenetic alterations; modification/adaptation of molecular targets; increased ability to repair DNA [42] (Figure 1).
Figure 1.
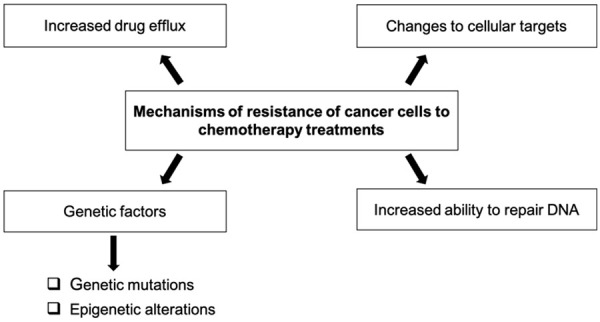
Summary of the main mechanisms of resistance of cancer cells to chemotherapy.
The efflux mechanism of cancer drugs in cancer cells results in a significant reduction in the therapeutic effect of the administered drugs. This resistance is established by a large accumulation of membrane proteins promoting the export of xenobiotics belonging to the superfamily of carrier’s ABCs (ATP-binding cassettes). This multigene family includes ABCA3, ABCB 1 (also known as MDR 1 or P-glycoprotein P-gp), ABCC 1 (MRP 1), ABCC 3 (MRP 3/cMOAT-2), ABCG 2 (ABCP/MXR/BCRP) [2]. The ABCB1-encoded P-glycoprotein is involved in the efflux of doxorubicin, daunorubicin, vincristine, etoposide, colchicine, camptothecin and methotrexate. ABCB1 being overexpressed in cancer cells, cells become chemoresistant. Multidrug-resistant proteins (MRP) are also involved in the efflux of several anti-cancer drugs such as methotrexate (MRP 1, MRP 2 and MRP 4), etoposide (MRP 3, MRP 6), 6-mercaptopurine (MRP 5), 6-thioguanine (MRP 5) and 5-fluorouracil (MRP 8) [43]. Aung et al. (2017) found that resistance genes (ABCA4 and ABCA12) were overexpressed in MCF-7 human breast cancer cells after docetaxel treatment. However, a decrease in their expression is observed when curcumin (phytochemical compound) is associated with docetaxel [44].
Failure of chemotherapy may also be caused by genetic mutations already present or acquired during tumor progression. The most frequently mutated gene encodes the tumor suppressor TP53, qualified as guardian of genome stability. Mutations in the p53 gene most often result in the loss of protein function and changes in the pro-apoptotic balance leading to drug resistance. The correlation between mutation status p53 and cytotoxic drug susceptibility was demonstrated in a study conducted by the National Cancer Institute, which examined 60 cell lines and over 100 cancer drugs [45,46]. Overexpression of p53 mutant with reduced or repealed function is often associated with resistance to the agents tested in the study (cisplatin, temozolomide, doxorubicin, gemcitabine, cetuximab) [47]. In addition, changes in molecular targets and signaling pathways modify the sensitivity of tumor cells to cancer drugs. For example, the mechanism of action of anthracyclines is based on their ability to interact with topoisomerase DNA. Mutations in the TOP1 gene, encoding topoisomerase 1, lead to a decrease in the ability of anthracyclines to interact with their targets [46]. Epigenetic changes, such as DNA methylation (hypermethylation of tumor suppressor genes and hypomethylation of oncogenes), histone modification (methylation, acetylation, phosphorylation, etc.) and alterations in the expression of specific miRNAs are also responsible for resistance to cancer drugs [48]. Another molecular mechanism leading to chemoresistance is the ability of cells to repair damage caused by molecules through DNA repair mechanisms. One of the main pathways is the nucleotide excision (NER) repair pathway involving the endonuclease XPF-ERCC1 [49]. The NER pathway involves several steps: recognition of DNA damage, DNA unfoldment, damage cleavage, polymerization, and ligature [50]. Cancer cells have a higher level of XPF and ERCC1, leading to treatment failure [51].
Costs
Beyond the side effects and the development of resistance to treatments, the main difficulty in Africa is the availability and accessibility to essential cancer medicines, new cancer medicines, and supportive treatment. Besides, the participation of African populations in clinical trials remain low-access. Indeed, in some sub-Saharan countries, cancer treatment is not covered by national health-care system, which forces patients to bear the cost of screening and treatment [52]. Relative to chemotherapy treatment, the cost varies depending on the type of cancer, the molecules used, the chemotherapy protocol and the rhythm. On average, the price varies between $170 and $6,700 every three weeks, for 6 sessions, with the possibility of further treatment. This cost is out of reach for most patients, resulting in increased cancer mortality [8]. These difficulties have led to a real decline in the development of new synthetic and seminal drugs. In addition, this has led to the revival of herbal therapies in the form of dietary supplements and preparations of botanical origin. The preventive or therapeutic potential of several phytochemical compounds has been evaluated pharmacologically for several decades [53].
Inventory and anti-cancer molecules of African medicinal plants
Methods
The study protocols
This systematic review was conducted in accordance with the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) guidelines [54].
Selection criteria
Articles were selected by reviewing the relevant titles and abstracts in each electronic database. Full-text articles were reviewed, duplicate and out-of-scope studies were excluded. The inclusion criteria for this review were: I. Full-text articles on molecules isolated from African medicinal plants, and their anti-cancer activities and mechanisms of action; II. Articles published over the past 17 years (2005-2022); III. Written in English.
Systematic research strategy
The systematic search ran from October to November 2022, using three well-known search engines: ScienceDirect, Google Scholar and PubMed. The keywords used in the three search engines were: (“bioactive compounds of African medicinal plants” OR “phytochemicals from African medicinal plants” OR “anti-cancer molecules of African medicinal plants”) AND (“cancer” OR “carcinoma” OR “tumor”), using the appropriate Boolean operators for each database. Research conducted on the ScienceDirect search engine identified 4,752 items containing 1,839 research articles and 1,404 journal articles. Google Scholar gave 3210 journal and research articles. A similar PubMed search yielded a total of 322 results (Figure 2).
Figure 2.
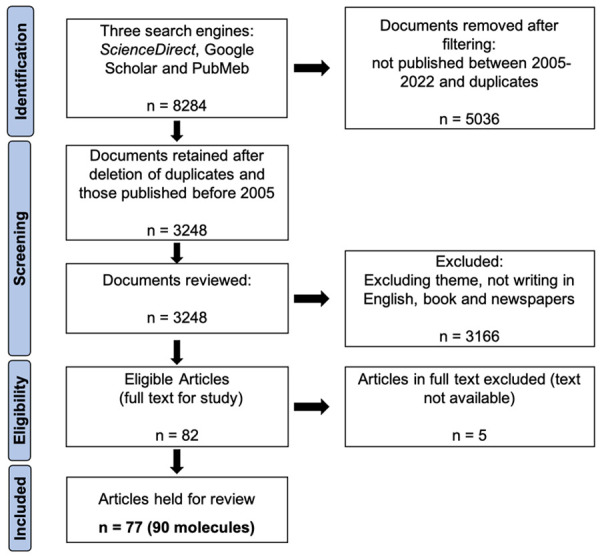
PRISMA diagram for the systematic analysis of research on cancer molecules in African plants 2005-2022.
Results
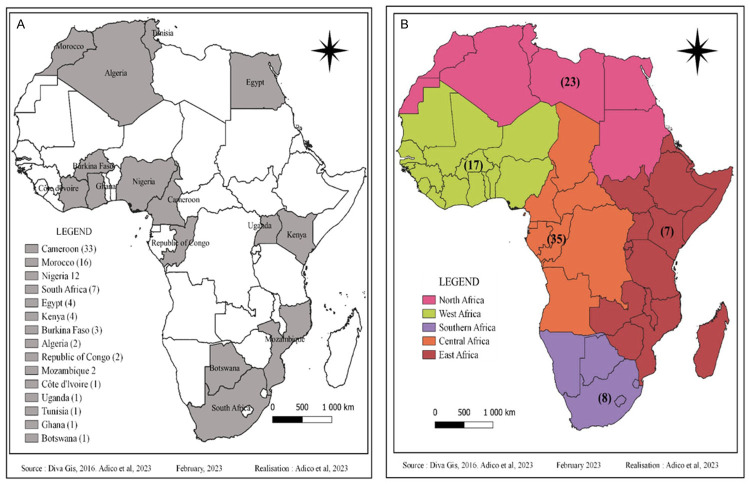
Although Africa has an exceptional plant biodiversity, very few studies have been conducted on the anti-cancer properties and molecular mechanisms of action of bioactive molecules isolated from endemic medicinal plants. In view of the increasing incidence of various cancers and the difficulty of access to synthetic molecules, significant efforts have been made in recent years to develop these studies. Our research identified 77 articles that met the inclusion criteria and were therefore collected for this review (Figure 2). A total of 90 compounds from eight chemical groups were identified for this study: alkaloids, flavonoids, polyphenols, quinones, saponins, steroids, terpenoids, and xanthones. Our data show that 28% of the molecules are terpenoids, followed by flavonoids (22%), phenolic compounds (16%), alkaloids (11%), quinones (8%) and saponins (7%). Steroids, xanthones and organosulfur are the least represented (with 4%, 2% and 2% respectively) (Figure 3). The compounds in our study are derived from medicinal plants from 15 African countries including Algeria, Botswana, Burkina Faso, Cameroon, Congo, Ivory Coast, Egypt, Ghana, Kenya, Morocco, Mozambique, Nigeria, South Africa, Tunisia and Uganda. In this way, Cameroon, Morocco and Nigeria are the countries with the most compounds with 33, 16 and 12 compounds, respectively (Figure 4A). The number of anti-cancer compounds isolated from African plants was grouped according to the geographical area where the plants were collected. Most compounds were isolated from plants harvested in Central Africa (35), followed by North Africa (23), West Africa (17), South Africa (8) and finally East Africa (7) (Figure 4B). Indeed, the predominance of bioactive molecules from Central African plants may be due to the fact that this tropical region of Africa has the greatest diversity of plant species. Also, the Mediterranean climate zone with winter precipitation in North Africa has a great diversity of species, which could explain the proportion obtained in North Africa [55].
Figure 3.
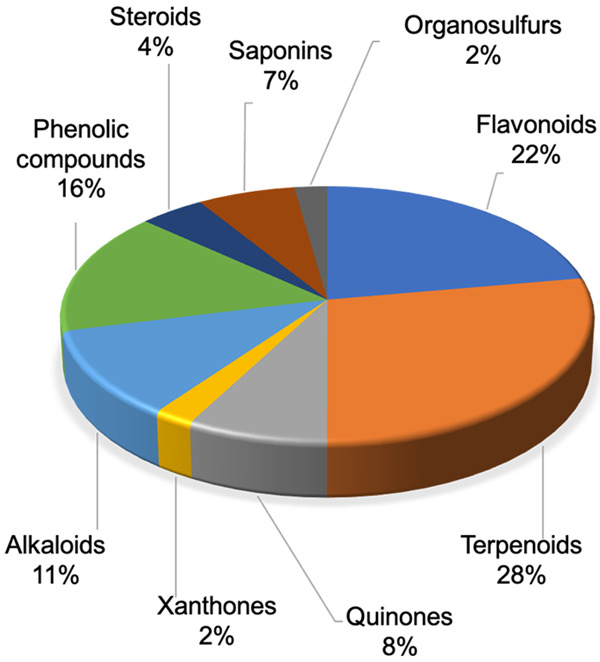
Phytochemical groups of bioactive molecules.
Figure 4.
Number of anti-cancer molecules isolated from African medicinal plants. A. By country; B. By region.
Mechanisms of action of anti-cancer molecules in African medicinal plants
The listed molecules, their PubChem accessibility number available, the cells tested, their activity and the country of collection are shown in Table 2.
Table 2.
Lists of molecules identified by bibliographic research
| Molecules | Plants | Cell lines used | Activities | Country | References |
|
| |||||
| Flavonoids | |||||
|
| |||||
| 2’,4’-dihydroxy-6’-methoxychalcone (1) (PubChem: 67218246) | Erythrina abyssinica | MCF-7 | IC50: 6.30 µM | Kenya | [56] |
| 4’-hydroxy-2’,6’-dimethoxychalcone (2) (PubChem: 71597852) | Polygonum limbatum | CCRF-CEM, CEM/ADR5000, MDA-MB-231-BCRP, HCT116 (p53+/+), HCT116 (p53-/-) | IC50 < 10 µM | Cameroon | [61] |
| Luteolin (3) (PubChem: 5280445) | Cyperus rotundus | HepG2 | IC50: 10.02 µM | Egypt | [57] |
| Isoneorautenol (4) (PubChem: 73649) | Erythrina senegalensis | CCRF-CEM, CEM/ADR5000, MDA-MB-231-pcDNA, MDA-MB-231-BCRP, HCT116 (p53+/+), HCT116 (p53-/-), U87MG, HepG2 | IC50 < 22 M against all cell lines | Cameroon | [159] |
| Quercetin (5) (PubChem: 25130254) | Gaillardia aristata | MCF7 and HCT116 | IC50: 2.2 and 1.7 μg/mL | Egypt | [70] |
| Gancaonin Q (6) (PubChem: 480802) | Genus Dorstenia | CCRF-CEM, CEM/ADR5000, PF-382, HL-60, MCF-7, MiaPaCa-2, SW-680, 786-0, U87MG, A549, Colo-38, HeLa, CaSki, Capan-1 | IC50 < 20 μg/ml | Cameroon | [65] |
| 6,8-diprenyleriodictyol (7) (PubChem: 4063836) | |||||
| 4-hydroxylonchocarpin (8) (PubChem: 5889042) | |||||
| Rutin (9) (PubChem: 5280805) | Buddleja asiatica | HepG2 | IC50: 0.84 µM | Egypt | [69] |
| Dorsmanin F (10) (PubChem: 10527064) | Dorstenia mannii | CCRF-CEM, CEM/ADR5000, MDA-MB-231-pcDNA, MDA-MB-231-BCRP, HCT116 (p53+/+), HCT116 (p53-/-), U87MG, HepG2 | IC50 between 5.34 and 33.30 µM | Cameroon | [63] |
| Poinsettifolin B (11) (PubChem: 11798732) | Dorstenia poinsettifolia | IC50 between 1.94 and 28.92 µM | |||
| Apigenin (12) (PubChem: 5280443) | Gaillardia aristata | HCT116 and MCF7 | IC50: 1.4 and 1.8 μg/Ml | Egypt | [70] |
| Kaempferol-3-methoxy-7-O-α-L-rhamnoside (13) (PubChem: 5280863) | IC50: 2.55 and 5.4 μg/ml | ||||
| Alpinumisoflavone (14) (PubChem: 5490139) | Ficus chlamydocarpa | CCRF-CEM, CEM/ADR5000 | IC50: 9.60 and 5.91 µM | Cameroon | [64] |
| Abyssinone IV (15) (PubChem: 4063835) | Erythrina sigmoidea | CCRF-CEM, CEM/ADR5000, MDA-MB-231-pcDNA, MDA-MB-231-BCRP, HCT116 (p53+/+), HCT116 (p53-/-), U87MG, HepG2, AML12 | IC50 < 30 µM | Cameroon | [62] |
| Sigmoidin I (16) (PubChem: 101667574) | |||||
| 6α-hydroxyphaseollidin (17) (PubChem: 44257482) | |||||
| Sophorapterocarpan A (18) (PubChem: 14017299) | |||||
| Isobavachalcone (19) (PubChem: 5281255) | Dorstenia barteri | CCRF-CEM, CEM/ADR5000, MDA-MB-231-BCRP | IC50: 2.90; 3.73 and 2.93 µM | Cameroon | [160] |
|
| |||||
| Terpenoids | |||||
|
| |||||
| 2’-Hydroxy-4,4’,5’-6’-tetramethoxychalcone (20) (PubChem: 6253276) | Chromolaena odorata | Cal51, MCF7, MDA-MB-231 and HeLa | IC50: 20 mM | Ivory Coast | [72] |
| Betulin (21) (PubChem: 72326) | Parinari curatellifolia | HeLa | IC50: 1.503/ml | Nigeria | [86] |
| Loliolide (22) (PubChem: 100332) | Heliotropium bacciferum | HCT116 and DLD1 | IC50 between 0.236 and 0.395 mM | Algeria | [73] |
| Isololiolide (23) (PubChem: 11019783) | |||||
| Lupeol (24) (PubChem: 259846) | Alstonia boonei | CACO2 | GI50: 50 µg/ml | Nigeria | [77] |
| Tomentosine (25) (PubChem: 6438142) | Inula viscosa | SiHa and HeLa | IC50: 7.10 and 5.87 µM | Morocco | [74] |
| Artemisinin (26) (PubChem: 68827) | Artemisia annua | P815 and BSR | IC50: 12 et 52 µM | Morocco | [161] |
| Galanal A (27) (PubChem: 3050416) | Aframomum arundinanceum | CCRF-CEM, MDA-MB-231-BCRP | IC50: 17.32 and 27.99 μg/mL | Cameroon | [60] |
| Galanal B (28) (PubChem: 3086504) | CCRF-CEM, MDA-MB-231-BCRP | IC50: 19.81 μg/mL | |||
| Carvacrol (29) (PubChem: 10364) | Thymus broussonetii | P-815, K-562, CEM, MCF-7 and MCF-7/gem | IC50 between 0.042 and 0.125 µM | Morocco | [79] |
| Thymol (30) (PubChem: 6989) | IC50 between 0.15 and 0.48 µM | ||||
| Carveol (31) (PubChem: 7438) | IC50 between 0.11 and 0.45 µM | ||||
| Carvone (32) (PubChem: 7439) | IC50 between 0.11 and 0.91 µM | ||||
| Isoeugenol (33) (PubChem: 170833) | IC50 between 0.09 and 0.25 µM | ||||
| Eugenol (34) (PubChem: 3314) | IC50 between 0.09 and 0.87 µM | ||||
| Lycopene (35) (PubChem: 446925) | Carica papaya | HepG2 | IC50: 22.8 µg/ml | Uganda | [81] |
| Curcusone A (36) (PubChem: 175941) | Jatropha curcas | HeLa | IC50 between 0.11 and 0.45 µM | Nigeria | [83] |
| Curcusone B (37) (PubChem: 175944) | |||||
| Curcusone C (38) (PubChem: 175942) | |||||
| Curcusone D (39) (PubChem: 175945) | |||||
| Betulinic acid (40) (PubChem: 64971) | Parinari curatellifolia | HeLa | IC50: 1.503 μg/ml | Nigeria | [86] |
| Ursolic acid (41) (PubChem: 64945) | Rosmarinus officinal | HepG2, Hep3B, Huh7 and HA22T | IC50 = 25 µM | Morocco | [90] |
| Friedelin (42) (PubChem: 91472) | Ficus drupacea | HeLa, MCF-7, Jurkat, HT-29, and T24 | Inhibition of proliferation of all lines. IC50 between 12.81 and 37.21 µM | South Africa | [88] |
| Oleanolic acid (43) (PubChem: 10494) | |||||
| Kaurenoic acid (44) (PubChem: 73062) | Annona senegalensis | 293T, HeLa and PANC-1 | IC50: 0.93; 0.74; 0.52 M | Nigeria | [92] |
| beta-amyrin (45) (PubChem: 225687) | Prunus africana | HEK293, HepG2 and Caco-2 | IC50: 156; 206 and 81 mg/mL | Kenya | [91] |
|
| |||||
| Quinones | |||||
|
| |||||
| Damnacanthal (46) (PubChem: 2948) | Garcinia huillensis | PANC-1 and PSN-1 | PC50 = 4.46 et 3.77 mM | Congo | [96] |
| Nordamacanthal (47) (PubChem: 160712) | PANC-1 | PC50 = 62.5 mM | Congo | ||
| 2-acetylfuro-1,4-naphtoquinone (48) (PubChem: 10331844) | Newbouldia laevis | MCF-7, HeLa, Caski, PF-382 and colo-38 | IC50: 0.57 µg/ml | Cameroon | [95] |
| Thymoquinone (49) (PubChem: 10281) | Nigella sativa | A-549 and DLD-1 | IC50: 13 and 5.9 μg/mL | Tunisia | [94] |
| Isofuranonaphtoquinone (50) (PubChem: -) | Bulbine frutescens | Jurkat T | At 25 µg/ml, 47% cell viability | Botswana | [98] |
| Plumbagin (51) (PubChem: 10205) | Plumbago zeylanica | A549, SPC212, DLD-1, Caco-2, MCF-7, HepG2, CRL2120 | IC50 between 0.06 and 1.14 µM | Kenya | [97] |
| Rapanone (52) (PubChem: 100659) | Rapanea melanphloes | SPC212 | IC50 = 2.27 µM | ||
|
| |||||
| Xanthones | |||||
|
| |||||
| Cudraxanthone I (53) (PubChem: 86276176) | Milicia excelsa | CCRF-CEM, CEM/ADR5000, MDA-MB-231-pcDNA, MDA-MB-231-BCRP, HCT116 (p53+/+), HCT116 (p53-/-), U87MG, HepG2, | Inhibition of proliferation of all lines. IC50 between 2.78 M and 22.49 M | Cameroon | [100] |
| Xanthone V1 (54) (PubChem: 139068057) | Vismia laurentii | MCF-7, HeLa, Caski, PF-382 et colo-38 | IC50: 0.56 µg/ml | Cameroon | [95] |
|
| |||||
| Alkaloids | |||||
|
| |||||
| Fagaridine chloride (55) (ChemSpider: 8889516) | Fagara tessmannii | CCRF-CEM, CEM/ADR5000, MDA-MB-231-pcDNA, MDA-MB-231-BCRP, HCT116 (p53+/+), HCT116 (p53-/-), U87MG, HepG2, | IC50 between 1.69 µM and 22.06 µM | Cameroon | [102] |
| Nitidine chloride (56) (ChemSpider: 23901) | |||||
| Solamargine (57) (PubChem: 73611) | Solanum aculeastrum | SH-SY5Y | IC50: 15.62 μg/mL | South Africa | [162] |
| Tomatidine (58) (PubChem: 65576) | HeLa | IC50: 141.7 µM | [104] | ||
| Solasodine (59) (PubChem: 442985) | HeLa | IC50: 252.5 µM | |||
| 15-Hydroxyangustilobine A (60) (PubChem: 70698298) | Alstonia boonei | MCF-7 | IC50: 26 µM | Ghana | [105] |
| Berberine (61) (PubChem: 2353) | Berberis vulgaris | MCF-7 | IC50: 8.75 µM | Morocco | [163] |
| Tabernaelegantinine B (62) (ChemSpider: 10222219) | Tabernaemontana elegans | HCT116 | IC50: 0.5 µM | Mozambique | [106] |
| Tabernaelegantinine C (63) (ChemSpider: 10202338) | IC50: 20 µM | ||||
| Arborinine (64) (PubChem: 5281832) | Uapaca togoensis | CCRF-CEM, CEM/ADR5000, MDA-MB-231-pcDNA, HCT116 (p53+/+), HCT116 (p53-/-), U87MG, HepG2 | Inhibition of proliferation of all lines. IC50 < 32 µM | Cameroon | [101] |
|
| |||||
| Phenolic compounds | |||||
|
| |||||
| Epigarcinol (65) (PubChem: -) | Garcinia ovalifolia | HL-60 and PC-3 | IC50: 7 μg/mL | Cameroon | [107] |
| Isogarcinol (66) (PubChem: 11135781) | IC50: 4 and 8 μg/mL | ||||
| Resveratrol (67) (PubChem: 445154) | Commiphora africana | MCF7, A549, PC-3, HepG | IC50: 16.80; 21.74; 17.89 et 17.44 µM | Nigeria | [164] |
| Epigallocatechin gallate (68) (PubChem: 65064) | Camellia sinensis | NIHpATMras | 20 µM inhibited cell growth | Morocco | [90] |
| Isoxanthochymol (69) (PubChem: 10461245) | Hypericum lanceolatum | CCRF-CEM, CEM/ADR5000, MDA-MB-231-pcDNA, MDA-MB-231-BCRP, HCT116 (p53+/+), HCT116 (p53-/-), U87MG, HepG2 | Inhibition of cell line proliferation. IC50 between 0.83 µM and 14.70 µM | Cameroon | [108] |
| Guttiferone E (70) (PubChem: 5352088) | |||||
| Gallic acid (71) (PubChem: 370) | Anogeissus leiocarpus | B16-F10 | GI50: 50 µg/ml | Nigeria | [165] |
| Ellagic acid (72) (PubChem: 5281855) | |||||
| Pycnanthuligenene A (73) (PubChem: 46186375) | Pycnanthus angolensis | CCRF-CEM, CEM/ADR5000, MDA-MB-231-pcDNA, MDA-MB-231-BCRP, HCT116 (p53+/+), HCT116 (p53-/-), U87MG, HepG2 | IC50 5.84 to 65.32 | Cameroon | [64] |
| 6-shogaol (74) (PubChem: 5281794) | Zingiber officinale | A549, SKOV3, SKMEL2, HCT15 | IC50: 17.43; 15.72; 20.94 and 30.05 µg/ml | Morocco | [90] |
| 6-gingerol (75) (PubChem: 442793) | A549, SKOV3, SKMEL2, HCT15 | IC50: 9.58; 18.85; 12.12 and 12.57 µg/ml | |||
| Curcumin (76) (PubChem: 969516) | Curcuma longa | CEM/ADR5000, MDA-MB-231-pcDNA, MDA-MB-231-BCRP, HCT116 (p53+/+), HCT116 (p53-/-), U87MG, HepG2 | IC50 between 6.25 and 19.69 µg/ml | Cameroon | [166] |
| Hypoxoside (77) (PubChem: 13785311) | Hypoxis colchicifolia | INS-1 | IC50: 25 µM | South Africa | [167] |
| Hyperoside (78) (PubChem: 5281643) | MIA PaCa-2 | IC50: 50 µM | |||
|
| |||||
| Steroids | |||||
|
| |||||
| 2”-Oxovoruscharin (79) (PubChem: 11410873) | Calotropis procera | A549, Hs683, U373, HCT-15 and LoVo | IC50: 2.7 nM | Burkina Faso | [122] |
| β-sitosterol (80) (PubChem: 222284) | Parinari curatellifolia | HeLa | IC50: 1.503/ml | Nigeria | [86] |
| UNBS-1450 (81) (PubChem: 11261970) | Calotropis procera | NSCLC | IC50: 10 nM | Egypt | [126] |
| Diosgenin (82) (PubChem: 99474) | Trigonella foenumgraecum | 1547 | IC50: 40 µM (24 h) | Morocco | [86,127] |
|
| |||||
| Saponins | |||||
|
| |||||
| Balanitin 6 (83) (PubChem: 44576182) | Balanite aegyptiaca | NSCLC and U373 | IC50: 0.3 and 0.5 µM | Burkina Faso | [132] |
| Balanitin 7 (84) (PubChem: 10953190) | |||||
| Olean-12-en-3-β-O-D-glucopyranoside (85) (PubChem: -) | Tetrapleura tetraptera | CCRF-CEM, CEM/ADR5000, MDA-MB-231-pcDNA, MDA-MB-231-BCRP, HCT116 (p53+/+), HCT116 (p53-/-), U87MG, HepG2 | Inhibition of proliferation of all lines. IC50 between 10.27 µg/mL and 23.61 µg/mL | Cameroon | [128] |
| Daucosterol (86) (PubChem: 5742590) | Crateva adansonii | PC-3 and DU145 | CC50: 17.45 and 29.25 µg/mL | Cameroon | [131] |
| Ardisiacrispin B (87) (PubChem: 10441164) | Ardisia kivuensis Taton | CCRF-CEM, CEM/ADR5000, MDA-MB-231-pcDNA, MDA-MB-231-BCRP, HCT116 (p53+/+), HCT116 (p53-/-), U87MG, HepG2 | IC50 between 1.20 and 6.76 µM | Cameroon | [129] |
| Progenin III (88) (PubChem: 146157809) | Raphia vinifera | CCF-CEM, CEM/ADR5000, MDA-MB-231-pcDNA, MDA-MB-231-BCRP, HCT116 (p53+/+), HCT116 (p53-/-), U87MG, HepG2 | IC50 between 1.59 and 31.61 µM | Cameroon | [130] |
|
| |||||
| Organosulfur | |||||
|
| |||||
| Allicin (89) (PubChem: 65036) | Allium sativum | HepG2 | IC50: 35 µM | Morocco | [90,133] |
| S-allycysteine (90) (PubChem: 9793905) | Allium sativum | A2780 | IC50 < 6.25 mmol/l (96 h) | [90,134] | |
IC50: median concentration for inhibition of cell survival of 50%; CC50: concentration necessary to obtain 50% of the cytotoxic effect; GI50: concentration necessary for inhibition of cell growth of 50%; PC50: preferential cytotoxicity to obtain 50% of the effect studied. Since cell survival was the parameter measured for each molecule, we can consider these values to be comparable.
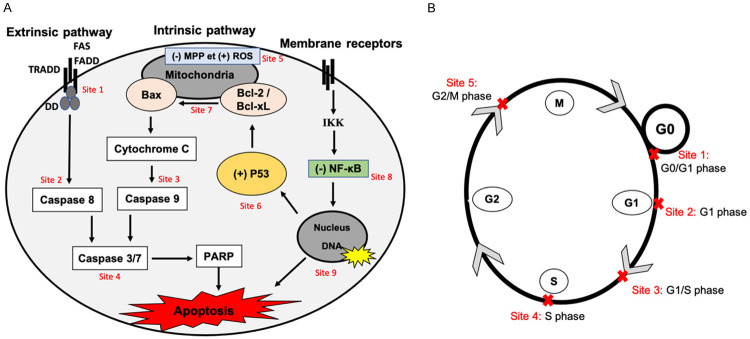
In the following chapters, the numbers refer to the molecules listed in the table. The main sites of action of bioactives molecules from African medicinal plants at the level of apoptotic pathways and different phases of the cell cycle in cancer cells presented in this review are summarized in Figure 5A and 5B.
Figure 5.
Sites of action of the bioactive molecules from African medicinal plants as anticancer agents. A. Therapeutic approaches of bioactive molecules from African medicinal plants targeting apoptosis pathways in cancer cells. Anticancer molecules from African medicinal plants are able to induce cancer cell apoptosis via: The apoptotic extrinsic pathways activation (site 1); activation of caspases 8, -9, -7 and -3 and PARP cleavage (site 2-3-4); apoptotic intrinsic pathways activation by loss of the potential of the mitochondrial membrane (MMP), ROS increase, Bcl2/Bcl-xl anti-apoptotic proteins inhibition, p53 activity and cytochrome c release, (site 5-6-7); inhibition of IKK/NF-κB activity (site 8) and preventing the transcription of anti-apoptotic regulators (site 9). B. Therapeutic approaches of bioactive molecules from African medicinal plants targeting cell cycle in cancer cells. Anticancer molecules from African medicinal plants act mainly on cell cycle to inhibit the proliferation of cancer cells at G0/G1 (site 1), G1 (site 2), G1/S (site 3), S (site 4) and G2/M (site 5).
Flavonoids
Flavonoids are metabolites with polyphenolic structure widely distributed in plants, carrying anti-cancer biological properties. Plants with anti-cancer activity in Africa present flavonoids in the second highest percentage within their entire range of compounds. As examples, 2’,4’-dihydroxy-6’-methoxychalcone (1), isolated by Kuete et al. from Erythrina abyssinica collected in Kenya, induces apoptosis of MCF-7 by activating caspases 3/7 and 9, and decreasing mitochondrial membrane potential which increases the production of reactive oxygen species (ROS) [56]. Luteolin (3), isolated from Cyperus rotundus collected in Egypt, shows significant cytotoxicity against cancer cells HepG2 compared to the reference drug doxorubicin [57]. This compound activates the extrinsic and intrinsic apoptotic pathways (Figure 5A), particularly by increasing the expression of the DR5 receptor in cervical or prostate cancer cells [58]. It activates the intrinsic pathway by inducing DNA damage and activating p53, thus increasing the level of caspases 9 and 3, and the Bcl-2 cleavage in cancer cells. Luteolin can also block the cell cycle in G1 phase [59]. Cameroonian compounds (2), (4), (10), (11), (14), (15), (16), (17), (18) and (19) isolated from Polygonum limbatum (2), Erythrina senegalensis (4), Dorstenia mannii (10), D. poinsettifolia (11), Ficus chlamydocarpa (14), Erythrina sigmoidea (15; 16; 17 and 18) and Dorstenia barteri (19) induce apoptosis of CCRF-CEM leukemia cells mediated by the disruption of mitochondrial membrane potential (MMP) and increased ROS production via the activation of initiator caspases 8 and 9, and effector caspase 3/7 [60-64]. Compound (2) arrests the cell cycle between G0/G1 phase [61]. An anti-angiogenic activity of compounds (6), (7) and (8) isolated from genus Dorstenia (Cameroon), followed by activation of caspase 3/7 induce apoptosis [65]. These molecules could become the basis of new cytotoxic drugs against resistant multifactorial cancers. The anti-cancer effects of quercetin (5) appear to be mediated by stopping the cell cycle in G2/M phases. It also promotes the expression of the Bax, p53, caspase-3 and cytochrome-c genes involved in cell death, while decreasing the expression of AKT and Bcl-2 [66]. Rutin (9) inhibits proliferation by modifying the BAX/Bcl ratio in human glioma CHME cells, to activate the intrinsic apoptotic pathway [67]. It regulates upward the expression of the p53 cancer gene [68]. This compound (9) isolated from Buddleja asiatica leaves (Egypt) has a significant cytotoxic activity against a HepG2 cell line [69]. Compounds (5), (12) and (13), isolated from the Egyptian plant Gaillardia aristata, show cytotoxicity against HCT116 and MCF-7 colon and breast cancer cell lines, respectively [70]. The mechanism of action of apigenin (12) in cancer cells consists mainly in blocking phosphorylation of the JAK2 and STAT3 pathways. Rahmani et al. demonstrated a cytotoxic effect on MDA-MB-453 breast cancer cells by inhibiting the STAT3 signaling pathway through increased caspases 3 and 8 expression levels [71].
Terpenoids
Compound (20) isolated from Chromolaena odoratae harvested in Ivory Coast, displays cytotoxic and anticlonogenic effects on several cell lines (Cal51, MCF7 and MDAMB-468). The association of this molecule with the Bcl2 inhibitor ABT737 significantly improves apoptosis in Cal51 breast cancer-derived cells [72]. Loliolide (22) and isololiolide (23) isolated from Heliotropium bacciferum (Algeria) induce cytotoxicity in cancer cell lines HCT116 and DLD1 [73]. Tomentosine (25) isolated from Inula viscosa (Morocco) and galanal A (27) and B (28) isolated from Aframomum arundinanceum (Cameroon) induce apoptosis in cancer cells by generating ROS and promoting cytochrome c release, and increasing Caspase-3. Galanal A (27) and B (28) also reduce Bcl-2 expression, leading to the activation of the intrinsic apoptotic pathway. In addition to inhibiting telomerase expression, the compound (25) stops the cell cycle in G2/M phase [60,74]. The anti-cancer effects of artemisinin (26) isolated from Artemisia annua (Morocco) appear to be mediated by tumor cell cycle arrest by modulating the levels of cyclins and CDK proteins. Thus, the compound triggers G1 phase cell cycle arrest of prostate cancer cells, accompanied by down regulation of CDK2 and CDK4 proteins [75]. Artemisinin (26) and its derivatives also inhibit both tumor cell invasion and metastatic processes of tumor cells by enhancing the matrix metalloproteinases (MMPs) expression and regulating the tumor microenvironment. MMP-2 and MMP-9 are strongly correlated with tumor cell invasion and metastasis [76]. Lupeol (24), isolated from Alstonia boonei, collected in Nigeria, is cytotoxic on CACO2 intestinal cells [77]. The mechanism of action involves a decreased expression of anti-apoptotic proteins Bcl-2 and Bcl-xL, and the induction of the apoptosis of MCF-7 cells [78]. Monoterpenes (29), (30), (31), (32), (33) and (34) isolated from Thymus broussonetii (Morocco) were characterized with anticancer properties [79]. Compounds (29) and (31) induce the cytotoxic effect by stopping the cell cycle in S phase, while compounds (30) and (33) arrest the cell cycle in G0/G1 phase. However, no effects on the cell cycle were observed for carvone (32) and eugenol (34) [80]. Lycopene (35) isolated from Carica papaya (Uganda) inhibits the viability of HepG2 cancer cells [81]. The cytotoxicity of compound (35) is also associated with an inhibition of the cell cycle progression. This occurs through the reduction of cyclin D and p27 levels, leading to cycle arrest in G1 phase of breast and endometrial cancer cells and increases phosphorylation of the anti-oncogenes p53 and Rb [82]. Jatropha curcas, collected in Nigeria, was used to isolate curcusones A (36), B (37), C (38) and D (39) which have cytotoxic activities against cervical HeLa cells [83]. Cytotoxicity of compound (37) is associated with cytochrome c release, activation of caspase-3 and caspase-9, deregulation Bcl-xL/Bax, and decreased survival, resulting in apoptosis [84]. Curcusine C (38) inhibits the metastatic process by reducing the expression of certain MMPs [85]. Betulinic acid (40) isolated from Parinari curatellifolia (Nigeria) [86], triggers cancer cell apoptosis by activating the mitochondrial pathway. In fact, Betulinic acid (40) significantly increases the level of production of reactive oxygen species (ROS) and modifies the mitochondrial membrane potential gradient, followed by the release of cytochrome c, resulting in apoptosis of caspase-dependent cancer cells. It also activates the transcription 3 (STAT3) signaling pathways of cancer cells and nuclear factor NF-κB [87]. Friedelin (42) and oleanolic acid (43) isolated from Ficus drupacea (South Africa) are cytotoxic against several cell lines [88]. Friedelin (42) leads to cancer cell death not only through apoptosis but also by necrosis, increasing the expression of tumor suppressor genes Cdkn1a, pRb2, p53, Nrf2, caspase-3 and decreasing the expression of Bcl-2, mdm2 and PCNA [89]. Ursolic acid (41), isolated from Rosmarinus officinal (Morocco), causes apoptosis through increased expression of Bax and caspases 3 and 8 [67,90]. β-amyrin (45) isolated from Prunus africana (Kenya) induces apoptosis in Caco-2 cell lines [91]. Kaurenoic acid (44) isolated from Annona senegalensis (Nigeria) possesses cytotoxicity and anticancer effect against HeLa and PANC-1 cell lines [92]. The apoptotic effect of β-amyrin (45) was attributed to the increase of Bax and reduction of Bcl-2 in Hep-G2 hepatic cells. It also causes the G2/M cycle arrest and activates p38 and JNK signaling channels [93].
Quinones
The quinones (46), (47), (48), (49), isolated from the plants Garcinia huillensis for (46) and (47) (Congo), Newbouldia laevis for (48) (Cameroon) and Nigella sativa for (49) (Tunisia), respectively, induce apoptosis through irreparable DNA damage and stopping the cell cycle in S phase. Compounds (46) and (47) trigger intrinsic apoptosis through activation of caspases-3/7 and 9, while thymoquinone (49) activates the extrinsic pathway through caspase 8 and 3 [94-96]. Increased ROS production results in decreased MMP in breast cancer cells (MCF-7) treated with plumbagin (51) isolated from Plumbago zeylancia (Kenya) or rapanone (52) from Rapanea melanphloes (Kenya) [97]. Isofuranonaphtoquinone (50) isolated from Bulbine frutescens (Botswana) is considered an excellent anti-cancer agent due to its actions similar to 1,3-bis(2-chloroethyl)-1-nitrosoure (BCNU) [98]. Mechanistic studies suggest that isofuranonaphtoquinone (50) can exert its activity by generating ROS that lead to cell death and inhibition of drug efflux pumps. The efficacy of compound (50) was highest on Jurkat lymphocyte cells when combined with BCNU. Therefore, this compound (50) is a potential candidate for the development of new anti-cancer drugs and a complementary compound in combination therapy regimens [99].
Xanthones
Exposure to cudraxanthone I (53) and xanthone V1 (54) from Milicia excelsa and Vismia laurentii, respectively from Cameroon, increases the expression of caspases 3/7 and blocks the S-phase cell cycle in CCRF-CEM cells. The compound (53) also activates caspases 8 and 9 [95,100].
Alkaloids
Fagaridine chloride (55), nitidine chloride (56) extracted from Fagara tessmannii (Cameroon) and Arborinine (64) isolated from Uapaca togoensis (Cameroon) inhibit the proliferation of CCRF-CEM cells by activating caspases 3/7, 8 and 9 and increasing ROS production. Arborinine (64) also blocks the cell cycle in G0/G1 and S phases [101,102]. These compounds require further studies for the development of new cytotoxic agents against resistant multifactorial cancers. Solamargine (57) isolated from Solanum aculeastrum (South Africa) is an alkaloid with non-selective cytotoxicity against the SH-SY5Y cancer cell lines and P-glycoprotein inhibition [103]. This compound (57) activates the expression of external death receptors, such as tumor necrosis factor receptor I (TNFR-I), FAS receptor, TNFR-I-associated death domain (TRADD) and FAS-associated death domain (FADD) and also increases the intrinsic ratio of Bax to Bcl-2 by increasing and decreasing Bcl-2 and Bcl-xL expressions. These effects cause the release of mitochondrial cytochrome c and the activation of caspases-8, -9 and -3 in breast cancer cells [103]. Tomatidine (58) and solasodine (59) isolated from Solanum aculeastrum (South Africa) inhibit HeLa cell growth by blocking the cell cycle at the G0/G1 phase [104]. Isolated from Alstonia boonei (Ghana), 15-hydroxyangustilobine A (60) induces cell cycle arrest at G2/M phase in MCF-7 breast cancer cells, at least partially triggering apoptosis [105]. The level of expression of caspase-3 was increased when HCT116 cells were treated by tabernaelegantinine B (62) and C (63) isolated from Tabernaemontana elegans (Mozambique). These compounds also cause cell shrinkage and condensation, cell nuclei fragmentation, plasma membrane detachment, and chromatin condensation [106], signs of apoptosis. Berberine (61), isolated from Berberis vulgaris collected in Morocco, inhibits the proliferation of MCF-7 cells by inducing cell apoptosis through the decrease of MMP and increase of ROS production [107].
Phenolic compounds
Compounds épigarcinol (65) and isogarcinol extracted from Garcinia ovalifolia (Cameroon) (66) inhibit HL-60 and PC-3 cell proliferation with an inhibition concentration (IC50) ranging from 4 to 8 µg/mL, depending on the line. These compounds induce G2/S phase cell cycle arrest, MMP disruption, and ROS production in HL-60 cells [107]. Isoxanthochymol (69), guttiferone E (70) and pycnanthulignene A (73), isolated from Hypericum lanceolatum for compounds (69) and (70) Pycnanthus angolensis for compound (73) (Cameroon), degrade the mitochondrial membranes of CCRF-CEM cells. Compounds (69) and (70) cause apoptosis via the activation of caspases 3/7, 8 and 9 as well as the loss of MMP [64,108]. For compound (76) isolated from Curcuma longa (Cameroon), the expression of migration-associated proteins, including matrix metalloproteinase (MMP)-9, NF-κB, and claudin-3, decreases while its concentration increases in colorectal cells (HCT116). Therefore, curcumin (76) promotes cell apoptosis and inhibits tumor cell metastasis by regulating the NF-κB signaling pathway [109]. Zingiber officinale, as common ginger, is a rhizomatous perennial plant used in Africa as a spice in food and beverages. It is also used as a remedy for several digestive disorders [110,111]. As pointed out by Bayala et al. in a study conducted in Burkina Faso [112], the essential oil of this plant is known for its anti-inflammatory properties. Among the compounds isolated, 6-gingerol (75) and 6-shogaol (74) appear to be part of the molecules responsible for many biological effects especially anti-cancer activity [113]. In addition to stopping the cell cycle at the G2/M phase, the autophagy of MCF-7 and T47D lines derived from breast cancers is also inhibited by 6-gingerol (75) [114]. 6-gingerol (75) promotes apoptosis by increasing ROS production, activating the p53 protein and inhibiting the degradation of CDK-inhibiting proteins (p27 and p21) in LoVo colorectal cells [115], and activates caspases 3/7, 8 and 9 in the colorectal cells SW-480 [116]. Two compounds isolated from Anogeissus leiocarpus (Nigeria), gallic acid (71) and ellagic acid (72) decrease the proliferation of human pancreatic cell lines MIA PaCa-2 and PANC-1 by activating caspases 3, 9 and increasing ROS production and reduce MMP. Ellagic acid (72) stimulates apoptosis through inhibition of the nuclear factor NF-κB [117]. Treatment of INS-1 and MIA PaCa-2 cells with hypoxoside (77) and hyperoside (78) isolated from Hypoxis colchicifolia (South Africa) leads to G2/M phase cycle arrest and caspase-3 activation in these cells. Resveratrol (67) isolated from Commiphora africana (Nigeria) has been used in several clinical trials [118]. Its mechanism of action relies not only on S-phase cell cycle arrest and survival inhibition, but also on activation of p53 protein, while inhibiting cyclooxygenase and cytochrome P450 enzymes [119]. The action of epigallocatechin gallate (68) isolated from Camellia sinensis (Morocco) on cancer cells is mainly based on cycle arrest and apoptosis phenomenon, through activation of caspase-3, caspase-9 and PARP-1 in MCF-7 cells. It also has an anti-metastatic activity by down-regulating the MMP-2, MMP-3 and MMP-9 proteins [120,121].
Steroids
2”-Oxovoruscharin (79), isolated from Calotropis procera (Burkina Faso), shows anti-cancer effects against various cell lines (A549, Hs683, U373, HCT-15 and LoVo). It binds and inhibits the Na+/K+ ATPase complex, which results in modulation of different signaling cascades controlling proliferation mechanisms, apoptosis or gene expression [122]. β-sitosterol (80) has antitumor effects against several types of cancer including breast, cervical, prostate, colon and lung cancer [123]. Apoptosis occurs through the activation of caspases 3 and 9, by reducing the levels of Bcl2 and PI3K/Akt proteins, and by decreasing MMP. It also causes cell cycle arrest at S and G2/M phases [124,125]. Two plants harvested in Northern Africa, Calotropis procera (Egypt) and Trigonella foenumgraecum (Morocco), used to isolate UNBS1450 (81) and diosgenin (82) respectively, show cytotoxic activity. UNBS-1450 (81) triggers apoptosis of U937 cells by cleavage of pro-caspases 8, 9, and 3/7, by reducing the expression of the anti-apoptotic protein Mcl-1, and by recruiting pro-apoptotic proteins Bax and Bak [126]. Diosgenin (82) isolated from Trigonella foenumgraecum (Morocco) increases the accumulation of p53 and p21 proteins that stop 1547 cells in the G1 phase [127].
Saponins
Saponins (85), (87) and (88), isolated from Tetrapleura tetraptera, Ardisia kivuensis Taton, Raphia vinifera, respectively (Cameroon), induce apoptosis of CCRF-CEM cells through the decrease of MMP, increase of ROS production and activation of caspases 3/7, 8 and 9. Olean-12-en-3-O-D-glucopyranoside (85) and progenin III (88) block cell cycles at G0/G1 and G1 phases, respectively [128-130]. Daucosterol (86), isolated from Crateva adansonii (Cameroon), inhibits the proliferation of DU145 and PC-3 cells derived from prostate metastases by decreasing the levels of cdk1 and pcdk1, and leading to cycle arrest in G0/G1 phase cycle. In addition, the anti-apoptotic proteins Akt, p-akt and Bcl-2 are decreased, while the pro-apoptotic protein Bax is increased [131]. The combination of balanitin-6 (83) and -7 (84), extracted from Balanite aegyptiaca from Burkina Faso, promotes the depletion of intracellular [ATP], causing the disruption of the actin cytoskeleton and altering cell proliferation and migration of glioblastoma and lung cancer cells lines [132].
Organosulfur
Two organosulfur compounds, allicin (89) and S-allycystein (90) isolated from the Moroccan plant Allium sativum, showed anti-cancer activity on HepG2 and A2780 cells, respectively [90]. Chu et al., showed that allicin (89) decreases cytoplasmic p53, PI3K/mTOR and Bcl-2 levels [133]. S-allycystein (90) causes the G1/S phase arrest and induces apoptosis, accompanied by a decrease in pro-caspase-3, Parp-1 and Bcl-2, and an increase in active caspase-3 and Bax. S-allycystin significantly decreases the accumulation of Wnt5a, p-AKT and c-Jun proteins, which are key proteins involved in proliferation and metastatic processes [134].
Opportunities for traditional or modern use of anti-cancer molecules
The use of anti-cancer molecules from African medicinal plants to treat cancer is accepted as part of a medical intervention. The main reasons for this acceptance are not only the small side effects induce by these molecules, but above all their relatively low cost and effectiveness against cancer cells. Many of African medicinal plants have the ability to fight a wide range of cancers, inhibiting enzymes involved in tumor development and with anti-proliferative effects. But, at the same time inducing antioxidant effects and repairing DNA damage of healthy cells [135]. Indeed, between 2002 and 2005, the World Health Organization adopted its first ever strategy to promote traditional medicine with the aim of reducing global cancer mortality. This framework also aims to deepen the potential risks related to the active principles of plant extracts: the identification of secondary metabolites, the identification of mechanisms of action and the evaluation of safety [136]. Thus, in view of the growing interest of populations, especially African, for traditional medicines, the WHO has adopted its new strategy (2014-2023) for the treatment of diseases, especially chronic ones such as cancer. This strategy has two major objectives: (1) to support states seeking to leverage the contribution of traditional medicine to health, well-being and care; (2) promote the safe and effective use of traditional medicine through regulation of products, practices and practitioners [137]. Over the years, several studies have shown that some bioactive substances from the African flora are promising candidates for the development of new drugs. There are also many new cancer molecules currently in clinical development with good selectivity against cancer cell lines and molecular targets associated with cancer. In particular beta-sitosterol, betulinic acid, curcumin, resveratrol, lycopene, 2’-oxovoruscharine and UNBS1450 [99].
Our work thus found that the identification of isolated active biological extracts from endemic African plants is a rational approach to the development of new anti-cancer drugs. It is therefore necessary for researchers on the African continent to continue to work on identifying biomolecules that can treat cancer so that the most economically vulnerable population can easily access them. The strengthening of collaboration between research teams in all African regions must be accelerated in order to promote the identification of new molecules, their therapeutic and dosage targets and the anti-cancer mechanisms of action. This collaboration will ultimately lead technology transfer that is essential to sustain this biomedical research.
Key technologies and methods of synthetic biology research on medicinal natural products
Here, we have reviewed the complexity of essential oils from native African plant in terms of their metabolites, with potential applications as anticancer molecules. Given that, the high demand for plant medicinal products and at the same time the difficulties of cultivation, abundance of metabolites, purification and chemical synthesis need alternative methods of production of active compounds are necessary. Great progress with the use of host microorganisms has been made recently focusing on the production of complex plant-derivate molecules such as terpenoids using Escherichia coli [138] or Saccharomyces cerevisiae [139]. However, the use of engineering in host microbes still represents a challenge due to the introduction of plant-derived genetics and metabolic machinery, as well as the potential that the product be toxic to the host.
In this direction, plants synthetic biology allows the sustainable production of phytochemicals by a direct isolation of molecules from plant resources. The use of plant platforms, whether at the cellular level (cell cultures), tissues (hairy root culture) or from whole plant, provides greater cost-efficiency for the production of phytochemicals, thanks to i) quick and easy cultivation not affected by environmental factors; ii) efficient energy dependence in terms of sources of carbon, light, water, and essential nutrients; iii) the similarity of metabolic pathways and genetic machinery between plants; iv) the use of different root, stem or leaf tissues would facilitate the production of natural products; v) the use of combinatorial engineering strategies (overexpression of enzymes, tissue-specific production of metabolites) that can be used to enhance the production and isolation of specific metabolites.
Efficiency in plant cell culture and synthetic biology has already been demonstrated for the production of natural products in the cosmetic, food, and pharmaceutical industries [140-142]. Besides, general strategies to enhance plant metabolite production have recently been reviewed in [143].
A good example of this is the use of the tobacco plant or Nicotiana benthamiana which, through the expression of different genes of interest, has allowed the production of alkaloids, terpenoids, flavonoids and phenolic derivatives [144-151], which are metabolites enriched in essential oils.
It is still a big challenge to use plant synthetic biology for the production of high value essential oil. Mainly because the knowledge on the production processes of these compounds by plants is very scarce. In the same way, the introduction of complex enzymatic systems would activate regulatory mechanisms, at the genetic and metabolic levels that could interfere or alter the structure of the molecules that are required to be produced by plant engineering [152,153].
Recently new tools of genome editing by CRISPR method have been applied for gene edition in plants showing great efficiency [154,155]. Which will allow a rapid advance in the generation of molecules and natural products by plants biology synthetic.
Conclusion
Compounds isolated from African medicinal plants clearly constitute a promising source of powerful anticancer agents. This review summarizes the data obtained on molecules identified in the literature as potential pharmacological agents, focusing on their mechanisms of molecular action. Although some of anti-cancer compounds described in this review are already in clinical trials, most of them should be further investigated to prove their effectiveness and to explore their potential in combination therapies, especially to find ways to overcome resistance to conventional drugs. Considering the diversity of African medicinal plants and the efficacy of their molecules, it is imperative that researchers around the world, and particularly those on the African continent, continue to work on identifying bioactive molecules usable in cancer treatment.
Acknowledgements
This work is supported by Institute Génétique, Reproduction, Développement, UMR CNRS 6293, INSERM U1103 of Université Clermont Auvergne, France; Laboratoire de Biologie Moléculaire et Génétique (LABIOGENE) of Université Joseph KI-ZERBO, Burkina Faso; Ecole Normale Supérieure, Burkina Faso and Centre de Recherche Biomoléculaire Pietro Annigoni (CERBA), Burkina Faso.
Disclosure of conflict of interest
None.
References
- 1.Abotaleb M, Liskova A, Kubatka P, Büsselberg D. Therapeutic potential of plant phenolic acids in the treatment of cancer. Biomolecules. 2020;10:221. doi: 10.3390/biom10020221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mbaveng AT, Kuete V, Efferth T. Potential of Central, Eastern and Western Africa medicinal plants for cancer therapy: spotlight on resistant cells and molecular targets. Front Pharmacol. 2017;8:343. doi: 10.3389/fphar.2017.00343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71:209–249. doi: 10.3322/caac.21660. [DOI] [PubMed] [Google Scholar]
- 4.Nwodo JN, Ibezim A, Simoben CV, Ntie-Kang F. Exploring cancer therapeutics with natural products from African medicinal plants, part II: alkaloids, terpenoids and flavonoids. Anticancer Agents Med Chem. 2016;16:108–127. doi: 10.2174/1871520615666150520143827. [DOI] [PubMed] [Google Scholar]
- 5.Wang JJ, Lei KF, Han F. Tumor microenvironment: recent advances in various cancer treatments. Eur Rev Med Pharmacol Sci. 2018;22:3855–3864. doi: 10.26355/eurrev_201806_15270. [DOI] [PubMed] [Google Scholar]
- 6.Kirtane AR, Kalscheuer SM, Panyam J. Exploiting nanotechnology to overcome tumor drug resistance: challenges and opportunities. Adv Drug Deliv Rev. 2013;65:1731–1747. doi: 10.1016/j.addr.2013.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yi X, Yang K, Liang C, Zhong X, Ning P, Song G, Wang D, Ge C, Chen C, Chai Z, Liu Z. Imaging-guided combined photothermal and radiotherapy to treat subcutaneous and metastatic tumors using Iodine-131-doped copper sulfide nanoparticles. Adv Funct Mater. 2015;25:4689–4699. [Google Scholar]
- 8.Coulidiati TH. Burden of cancer and role of traditional medicine in Burkina Faso. IJCAM. 2019;12:194–200. [Google Scholar]
- 9.Noble RL. The discovery of the vinca alkaloids-chemotherapeutic agents against cancer. Biochem Cell Biol. 1990;68:1344–1351. [PubMed] [Google Scholar]
- 10.Keglevich P, Hazai L, Kalaus G, Szántay C. Modifications on the basic skeletons of vinblastine and vincristine. Molecules. 2012;17:5893–5914. doi: 10.3390/molecules17055893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Iqbal J, Abbasi BA, Mahmood T, Kanwal S, Ali B, Shah SA, Khalil AT. Plant-derived anticancer agents: a green anticancer approach. Asian Pac J Trop Biomed. 2017;7:1129–1150. [Google Scholar]
- 12.Nikolaou M, Pavlopoulou A, Georgakilas AG, Kyrodimos E. The challenge of drug resistance in cancer treatment: a current overview. Clin Exp Metastasis. 2018;35:309–318. doi: 10.1007/s10585-018-9903-0. [DOI] [PubMed] [Google Scholar]
- 13.Chiruvella V, Annamaraju P, Guddati AK. Management of nephrotoxicity of chemotherapy and targeted agents: 2020. Am J Cancer Res. 2020;10:4151–4164. [PMC free article] [PubMed] [Google Scholar]
- 14.Floyd J, Mirza I, Sachs B, Perry MC. Hepatotoxicity of chemotherapy. Semin Oncol. 2006;33:50–67. doi: 10.1053/j.seminoncol.2005.11.002. [DOI] [PubMed] [Google Scholar]
- 15.Oun R, Moussa YE, Wheate NJ. The side effects of platinum-based chemotherapy drugs: a review for chemists. Dalton Trans. 2018;47:6645–6653. doi: 10.1039/c8dt00838h. [DOI] [PubMed] [Google Scholar]
- 16.Dumas G, Canet E. Effets cardiovasculaires graves des chimiothérapies, thérapies ciblées et des traitements immunosuppresseurs. Réanimation. 2016;25:123–136. [Google Scholar]
- 17.Knight K, Wade S, Balducci L. Prevalence and outcomes of anemia in cancer: a systematic review of the literature. Am J Med. 2004;116(Suppl 7A):11S–26S. doi: 10.1016/j.amjmed.2003.12.008. [DOI] [PubMed] [Google Scholar]
- 18.Auffret N. Complications cutanées des chimiothérapies - chemotherapy cutaneous toxicity. 2007 [Google Scholar]
- 19.James SE, Burden H, Burgess R, Xie Y, Yang T, Massa SM, Longo FM, Lu Q. Anti-cancer drug induced neurotoxicity and identification of Rho pathway signaling modulators as potential neuroprotectants. Neurotoxicology. 2008;29:605–612. doi: 10.1016/j.neuro.2008.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Secombe KR, Coller JK, Gibson RJ, Wardill HR, Bowen JM. The bidirectional interaction of the gut microbiome and the innate immune system: implications for chemotherapy-induced gastrointestinal toxicity. Int J Cancer. 2019;144:2365–2376. doi: 10.1002/ijc.31836. [DOI] [PubMed] [Google Scholar]
- 21.Boekelheide K. Mechanisms of toxic damage to spermatogenesis. J Natl Cancer Inst Monogr. 2005:6–8. doi: 10.1093/jncimonographs/lgi006. [DOI] [PubMed] [Google Scholar]
- 22.Ben-Aharon I, Shalgi R. What lies behind chemotherapy-induced ovarian toxicity? Reproduction. 2012;144:153–163. doi: 10.1530/REP-12-0121. [DOI] [PubMed] [Google Scholar]
- 23.Minami M, Matsumoto S, Horiuchi H. Cardiovascular side-effects of modern cancer therapy. Circ J. 2010;74:1779–1786. doi: 10.1253/circj.cj-10-0632. [DOI] [PubMed] [Google Scholar]
- 24.Qiao X, van der Zanden SY, Wander DPA, Borràs DM, Song JY, Li X, van Duikeren S, van Gils N, Rutten A, van Herwaarden T, van Tellingen O, Giacomelli E, Bellin M, Orlova V, Tertoolen LGJ, Gerhardt S, Akkermans JJ, Bakker JM, Zuur CL, Pang B, Smits AM, Mummery CL, Smit L, Arens R, Li J, Overkleeft HS, Neefjes J. Uncoupling DNA damage from chromatin damage to detoxify doxorubicin. Proc Natl Acad Sci U S A. 2020;117:15182–15192. doi: 10.1073/pnas.1922072117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sawyer DB. Anthracyclines and heart failure. N Engl J Med. 2013;368:1154–1156. doi: 10.1056/NEJMcibr1214975. [DOI] [PubMed] [Google Scholar]
- 26.Dasari S, Tchounwou PB. Cisplatin in cancer therapy: molecular mechanisms of action. Eur J Pharmacol. 2014;740:364–378. doi: 10.1016/j.ejphar.2014.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Van den Boogaard WMC, Komninos DSJ, Vermeij WP. Chemotherapy side-effects: not all DNA damage is equal. Cancers (Basel) 2022;14:627. doi: 10.3390/cancers14030627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kintzel PE. Anticancer drug-induced kidney disorders. Drug Saf. 2001;24:19–38. doi: 10.2165/00002018-200124010-00003. [DOI] [PubMed] [Google Scholar]
- 29.Kooijmans EC, Bökenkamp A, Tjahjadi NS, Tettero JM, van Dulmen-den Broeder E, van der Pal HJ, Veening MA. Early and late adverse renal effects after potentially nephrotoxic treatment for childhood cancer. Cochrane Database Syst Rev. 2019;3:CD008944. doi: 10.1002/14651858.CD008944.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mitchell EP. Gastrointestinal toxicity of chemotherapeutic agents. Semin Oncol. 2006;33:106–120. doi: 10.1053/j.seminoncol.2005.12.001. [DOI] [PubMed] [Google Scholar]
- 31.Grigorian A, O’Brien CB. Hepatotoxicity secondary to chemotherapy. J Clin Transl Hepatol. 2014;2:95–102. doi: 10.14218/JCTH.2014.00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Torrisi JM, Schwartz LH, Gollub MJ, Ginsberg MS, Bosl GJ, Hricak H. CT findings of chemotherapy-induced toxicity: what radiologists need to know about the clinical and radiologic manifestations of chemotherapy toxicity. Radiology. 2011;258:41–56. doi: 10.1148/radiol.10092129. [DOI] [PubMed] [Google Scholar]
- 33.Thatishetty AV, Agresti N, O’Brien CB. Chemotherapy-induced hepatotoxicity. Clin Liver Dis. 2013;17:671–686. doi: 10.1016/j.cld.2013.07.010. [DOI] [PubMed] [Google Scholar]
- 34.Levi M, Hasky N, Stemmer SM, Shalgi R, Ben-Aharon I. Anti-Müllerian hormone is a marker for chemotherapy-induced testicular toxicity. Endocrinology. 2015;156:3818–3827. doi: 10.1210/en.2015-1310. [DOI] [PubMed] [Google Scholar]
- 35.Wolf S, Barton D, Kottschade L, Grothey A, Loprinzi C. Chemotherapy-induced peripheral neuropathy: prevention and treatment strategies. Eur J Cancer. 2008;44:1507–1515. doi: 10.1016/j.ejca.2008.04.018. [DOI] [PubMed] [Google Scholar]
- 36.Orbach D, Brisse H, Doz F. Toxicité neurologique centrale des chimiothérapies: état des connaissances actuelles. Arch Pediatr. 2003;10:533–539. doi: 10.1016/s0929-693x(03)00105-2. [DOI] [PubMed] [Google Scholar]
- 37.Fondrinier É, Pezet D, Gamelin É. Prise en charge et surveillance du patient cancéreux. Issy-les-Moulineaux: Masson; 2004. [Google Scholar]
- 38.Testart-Paillet D, Girard P, You B, Freyer G, Pobel C, Tranchand B. Contribution of modelling chemotherapy-induced hematological toxicity for clinical practice. Crit Rev Oncol Hematol. 2007;63:1–11. doi: 10.1016/j.critrevonc.2007.01.005. [DOI] [PubMed] [Google Scholar]
- 39.Lyman GH, Abella E, Pettengell R. Risk factors for febrile neutropenia among patients with cancer receiving chemotherapy: a systematic review. Crit Rev Oncol Hematol. 2014;90:190–199. doi: 10.1016/j.critrevonc.2013.12.006. [DOI] [PubMed] [Google Scholar]
- 40.Kuter DJ. Treatment of chemotherapy-induced thrombocytopenia in patients with non-hematologic malignancies. Haematologica. 2022;107:1243–1263. doi: 10.3324/haematol.2021.279512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bukowski K, Kciuk M, Kontek R. Mechanisms of multidrug resistance in cancer chemotherapy. Int J Mol Sci. 2020;21:3233. doi: 10.3390/ijms21093233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Luqmani YA. Mechanisms of drug resistance in cancer chemotherapy. Med Princ Pract. 2005;14(Suppl 1):35–48. doi: 10.1159/000086183. [DOI] [PubMed] [Google Scholar]
- 43.Dean M. ABC transporters, drug resistance, and cancer stem cells. J Mammary Gland Biol Neoplasia. 2009;14:3–9. doi: 10.1007/s10911-009-9109-9. [DOI] [PubMed] [Google Scholar]
- 44.Aung TN, Qu Z, Kortschak RD, Adelson DL. Understanding the effectiveness of natural compound mixtures in cancer through their molecular mode of action. Int J Mol Sci. 2017;18:656. doi: 10.3390/ijms18030656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Huang Y, Sadée W. Membrane transporters and channels in chemoresistance and -sensitivity of tumor cells. Cancer Lett. 2006;239:168–182. doi: 10.1016/j.canlet.2005.07.032. [DOI] [PubMed] [Google Scholar]
- 46.Marin JJ, Romero MR, Martinez-Becerra P, Herraez E, Briz O. Overview of the molecular bases of resistance to chemotherapy in liver and gastrointestinal tumours. Curr Mol Med. 2009;9:1108–1129. doi: 10.2174/156652409789839125. [DOI] [PubMed] [Google Scholar]
- 47.Hientz K, Mohr A, Bhakta-Guha D, Efferth T. The role of p53 in cancer drug resistance and targeted chemotherapy. Oncotarget. 2017;8:8921–8946. doi: 10.18632/oncotarget.13475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wilting RH, Dannenberg JH. Epigenetic mechanisms in tumorigenesis, tumor cell heterogeneity and drug resistance. Drug Resist Updat. 2012;15:21–38. doi: 10.1016/j.drup.2012.01.008. [DOI] [PubMed] [Google Scholar]
- 49.Gentile F, Elmenoufy AH, Ciniero G, Jay D, Karimi-Busheri F, Barakat KH, Weinfeld M, West FG, Tuszynski JA. Computer-aided drug design of small molecule inhibitors of the ERCC1-XPF protein-protein interaction. Chem Biol Drug Des. 2020;95:460–471. doi: 10.1111/cbdd.13660. [DOI] [PubMed] [Google Scholar]
- 50.Rastogi RP, Richa, Kumar A, Tyagi MB, Sinha RP. Molecular mechanisms of ultraviolet radiation-induced DNA damage and repair. J Nucleic Acids. 2010;2010:592980. doi: 10.4061/2010/592980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Rosell R, Taron M, Ariza A, Barnadas A, Mate JL, Reguart N, Margel M, Felip E, Méndez P, García-Campelo R. Molecular predictors of response to chemotherapy in lung cancer. Semin Oncol. 2004;31(Suppl 1):20–27. doi: 10.1053/j.seminoncol.2003.12.011. [DOI] [PubMed] [Google Scholar]
- 52.Diallo M, Fall D, Mballo I, Niang CI, Charfi ME. Coûts médicaux directs de traitement du cancer du sein à l’Institut Joliot Curie de l’Hôpital Aristide Le Dantec de Dakar, Sénégal. Pan Afr Med J. 2022:18. doi: 10.11604/pamj.2022.42.266.32967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Akhtar MF, Saleem A, Rasul A, Faran Ashraf Baig MM, Bin-Jumah M, Abdel Daim MM. Anticancer natural medicines: an overview of cell signaling and other targets of anticancer phytochemicals. Eur J Pharmacol. 2020;888:173488. doi: 10.1016/j.ejphar.2020.173488. [DOI] [PubMed] [Google Scholar]
- 54.Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, Shamseer L, Tetzlaff JM, Akl EA, Brennan SE, Chou R, Glanville J, Grimshaw JM, Hróbjartsson A, Lalu MM, Li T, Loder EW, Mayo-Wilson E, McDonald S, McGuinness LA, Stewart LA, Thomas J, Tricco AC, Welch VA, Whiting P, Moher D. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372:n71. doi: 10.1136/bmj.n71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kuete V, Efferth T. African flora has the potential to fight multidrug resistance of cancer. Biomed Res Int. 2015;2015:914813. doi: 10.1155/2015/914813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kuete V, Omosa LK, Midiwo JO, Karaosmanoglu O, Sivas H. Cytotoxicity of naturally occurring phenolics and terpenoids from Kenyan flora towards human carcinoma cells. J Ayurveda Integr Med. 2019;10:178–184. doi: 10.1016/j.jaim.2018.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Samra RM, Soliman AF, Zaki AA, Ashour A, Al-Karmalawy AA, Hassan MA, Zaghloul AM. Bioassay-guided isolation of a new cytotoxic ceramide from Cyperus rotundus L. S Afr J Bot. 2021;139:210–216. [Google Scholar]
- 58.Horinaka M, Yoshida T, Shiraishi T, Nakata S, Wakada M, Nakanishi R, Nishino H, Matsui H, Sakai T. Luteolin induces apoptosis via death receptor 5 upregulation in human malignant tumor cells. Oncogene. 2005;24:7180–7189. doi: 10.1038/sj.onc.1208874. [DOI] [PubMed] [Google Scholar]
- 59.Lin Y, Shi R, Wang X, Shen HM. Luteolin, a flavonoid with potential for cancer prevention and therapy. Curr Cancer Drug Targets. 2008;8:634–646. doi: 10.2174/156800908786241050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kuete V, Ango PY, Yeboah SO, Mbaveng AT, Mapitse R, Kapche GD, Ngadjui BT, Efferth T. Cytotoxicity of four aframomum species (A. arundinaceum, A. alboviolaceum, A. kayserianum and A. polyanthum) towards multi-factorial drug resistant cancer cell lines. BMC Complement Altern Med. 2014;14:340. doi: 10.1186/1472-6882-14-340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kuete V, Nkuete AH, Mbaveng AT, Wiench B, Wabo HK, Tane P, Efferth T. Cytotoxicity and modes of action of 4’-hydroxy-2’,6’-dimethoxychalcone and other flavonoids toward drug-sensitive and multidrug-resistant cancer cell lines. Phytomedicine. 2014;21:1651–1657. doi: 10.1016/j.phymed.2014.08.001. [DOI] [PubMed] [Google Scholar]
- 62.Kuete V, Sandjo LP, Djeussi DE, Zeino M, Kwamou GM, Ngadjui B, Efferth T. Cytotoxic flavonoids and isoflavonoids from Erythrina sigmoidea towards multi-factorial drug resistant cancer cells. Invest New Drugs. 2014;32:1053–1062. doi: 10.1007/s10637-014-0137-y. [DOI] [PubMed] [Google Scholar]
- 63.Kuete V, Mbaveng AT, Zeino M, Ngameni B, Kapche GD, Kouam SF, Ngadjui BT, Efferth T. Cytotoxicity of two naturally occurring flavonoids (dorsmanin F and poinsettifolin B) towards multi-factorial drug-resistant cancer cells. Phytomedicine. 2015;22:737–743. doi: 10.1016/j.phymed.2015.04.007. [DOI] [PubMed] [Google Scholar]
- 64.Kuete V, Mbaveng AT, Nono EC, Simo CC, Zeino M, Nkengfack AE, Efferth T. Cytotoxicity of seven naturally occurring phenolic compounds towards multi-factorial drug-resistant cancer cells. Phytomedicine. 2016;23:856–863. doi: 10.1016/j.phymed.2016.04.007. [DOI] [PubMed] [Google Scholar]
- 65.Kuete V, Ngameni B, Wiench B, Krusche B, Horwedel C, Ngadjui BT, Efferth T. Cytotoxicity and mode of action of four naturally occuring flavonoids from the genus Dorstenia: gancaonin Q, 4-hydroxylonchocarpin, 6-prenylapigenin, and 6,8-diprenyleriodictyol. Planta Med. 2011;77:1984–1989. doi: 10.1055/s-0031-1280023. [DOI] [PubMed] [Google Scholar]
- 66.Bishayee K, Ghosh S, Mukherjee A, Sadhukhan R, Mondal J, Khuda-Bukhsh AR. Quercetin induces cytochrome-c release and ROS accumulation to promote apoptosis and arrest the cell cycle in G2/M, in cervical carcinoma: signal cascade and drug-DNA interaction. Cell Prolif. 2013;46:153–163. doi: 10.1111/cpr.12017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Yan X, Hao Y, Chen S, Jia G, Guo Y, Zhang G, Wang C, Cheng R, Hu T, Zhang X, Ji H. Rutin induces apoptosis via P53 up-regulation in human glioma CHME cells. Transl Cancer Res. 2019;8:2005–2013. doi: 10.21037/tcr.2019.09.07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Efeyan A, Serrano M. p53: guardian of the genome and policeman of the oncogenes. Cell Cycle. 2007;6:1006–1010. doi: 10.4161/cc.6.9.4211. [DOI] [PubMed] [Google Scholar]
- 69.Mohamed M, Abdou A, Saad A, Ibrahim M. Cytotoxic activity of Buddleja asiatica. Life Science Journal. 2013;10:2773–2777. [Google Scholar]
- 70.Salama MM, Kandil ZA, Islam WT. Cytotoxic compounds from the leaves of Gaillardia aristata Pursh. growing in Egypt. Nat Prod Res. 2012;26:2057–62. doi: 10.1080/14786419.2011.606219. [DOI] [PubMed] [Google Scholar]
- 71.Rahmani AH, Alsahli MA, Almatroudi A, Almogbel MA, Khan AA, Anwar S, Almatroodi SA. The potential role of apigenin in cancer prevention and treatment. Molecules. 2022;27:6051. doi: 10.3390/molecules27186051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Kouamé PB, Jacques C, Bedi G, Silvestre V, Loquet D, Barillé-Nion S, Robins RJ, Tea I. Phytochemicals isolated from leaves of Chromolaena odorata: impact on viability and clonogenicity of cancer cell lines. Phytother Res. 2013;27:835–840. doi: 10.1002/ptr.4787. [DOI] [PubMed] [Google Scholar]
- 73.Aïssaoui H, Mencherini T, Esposito T, De Tommasi N, Gazzerro P, Benayache S, Benayache F, Mekkiou R. Heliotropium bacciferum forssk. (Boraginaceae) extracts: chemical constituents, antioxidant activity and cytotoxic effect in human cancer cell lines. Nat Prod Res. 2019;33:1813–1818. doi: 10.1080/14786419.2018.1437433. [DOI] [PubMed] [Google Scholar]
- 74.Merghoub N, El Btaouri H, Benbacer L, Gmouh S, Trentesaux C, Brassart B, Attaleb M, Madoulet C, Wenner T, Amzazi S, Morjani H, El Mzibri M. Tomentosin induces telomere shortening and caspase-dependant apoptosis in cervical cancer cells. J Cell Biochem. 2017;118:1689–1698. doi: 10.1002/jcb.25826. [DOI] [PubMed] [Google Scholar]
- 75.Jia J, Qin Y, Zhang L, Guo C, Wang Y, Yue X, Qian J. Artemisinin inhibits gallbladder cancer cell lines through triggering cell cycle arrest and apoptosis. Mol Med Rep. 2016;13:4461–4468. doi: 10.3892/mmr.2016.5073. [DOI] [PubMed] [Google Scholar]
- 76.Li D, Zhang J, Zhao X. Mechanisms and molecular targets of artemisinin in cancer treatment. Cancer Invest. 2021;39:675–684. doi: 10.1080/07357907.2021.1954190. [DOI] [PubMed] [Google Scholar]
- 77.Nana F, Sandjo LP, Keumedjio F, Ambassa P, Malik R, Kuete V, Rincheval V, Choudhary MI, Ngadjui BT. Ceramides and cytotoxic constituents from Ficus glumosa Del. (Moraceae) J Braz Chem Soc. 2012;23:482–487. [Google Scholar]
- 78.Pitchai D, Roy A, Ignatius C. In vitro evaluation of anticancer potentials of lupeol isolated from Elephantopus scaber L. on MCF-7 cell line. J Adv Pharm Technol Res. 2014;5:179–84. doi: 10.4103/2231-4040.143037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Bouyahya A, Belmehdi O, Benjouad A, Ameziane El Hassani R, Amzazi S, Dakka N, Bakri Y. Pharmacological properties and mechanism insights of Moroccan anticancer medicinal plants: what are the next steps? Ind Crops Prod. 2020;147:19. [Google Scholar]
- 80.Jaafari A, Tilaoui M, Mouse HA, M’bark LA, Aboufatima R, Chait A, Lepoivre M, Zyad A. Comparative study of the antitumor effect of natural monoterpenes: relationship to cell cycle analysis. Rev Bras Farmacogn. 2012;22:534–540. [Google Scholar]
- 81.Omara T, Kiprop AK, Ramkat RC, Cherutoi J, Kagoya S, Moraa Nyangena D, Azeze Tebo T, Nteziyaremye P, Nyambura Karanja L, Jepchirchir A, Maiyo A, Jematia Kiptui B, Mbabazi I, Kiwanuka Nakiguli C, Nakabuye BV, Chepkemoi Koske M. Medicinal plants used in traditional management of cancer in Uganda: a review of ethnobotanical surveys, phytochemistry, and anticancer studies. Evid Based Complement Alternat Med. 2020;2020:3529081. doi: 10.1155/2020/3529081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Nahum A, Hirsch K, Danilenko M, Watts CK, Prall OW, Levy J, Sharoni Y. Lycopene inhibition of cell cycle progression in breast and endometrial cancer cells is associated with reduction in cyclin D levels and retention of p27Kip1 in the cyclin E-cdk2 complexes. Oncogene. 2001;20:3428–3436. doi: 10.1038/sj.onc.1204452. [DOI] [PubMed] [Google Scholar]
- 83.Aiyelaagbe OO, Hamid AA, Fattorusso E, Taglialatela-Scafati O, Schröder HC, Müller WE. Cytotoxic activity of crude extracts as well as of pure components from Jatropha species, plants used extensively in African traditional medicine. Evid Based Complement Alternat Med. 2011;2011:134954. doi: 10.1155/2011/134954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Muangman S, Thippornwong M, Tohtong R. Anti-metastatic effects of curcusone B, a diterpene from Jatropha curcas. In Vivo. 2005;19:265–268. [PubMed] [Google Scholar]
- 85.An J, Li L, Zhang X. Curcusone C induces apoptosis in endometrial cancer cells via mitochondria-dependent apoptotic and ERK pathway. Biotechnol Lett. 2021;43:329–338. doi: 10.1007/s10529-020-03027-4. [DOI] [PubMed] [Google Scholar]
- 86.Halilu ME, October N, Balogun M, Namrita L, Abubakar MS. Studies of in vitro antioxidant and cytotoxic activities of extracts and isolated compounds from Parinari curatellifolia (Chrysobalanaceae) Journal of Natural Sciences Research. 2013;3:16. [Google Scholar]
- 87.Zhang X, Hu J, Chen Y. Betulinic acid and the pharmacological effects of tumor suppression (Review) Mol Med Rep. 2016;14:4489–4495. doi: 10.3892/mmr.2016.5792. [DOI] [PubMed] [Google Scholar]
- 88.Yessoufou K, Elansary HO, Mahmoud EA, Skalicka-Woźniak K. Antifungal, antibacterial and anticancer activities of Ficus drupacea L. stem bark extract and biologically active isolated compounds. Ind Crops Prod. 2015;74:752–758. [Google Scholar]
- 89.Subash-Babu P, Li DK, Alshatwi AA. In vitro cytotoxic potential of friedelin in human MCF-7 breast cancer cell: regulate early expression of Cdkn2a and pRb1, neutralize mdm2-p53 amalgamation and functional stabilization of p53. Exp Toxicol Pathol. 2017;69:630–636. doi: 10.1016/j.etp.2017.05.011. [DOI] [PubMed] [Google Scholar]
- 90.Alami Merrouni I, Elachouri M. Anticancer medicinal plants used by Moroccan people: ethnobotanical, preclinical, phytochemical and clinical evidence. J Ethnopharmacol. 2021;266:113435. doi: 10.1016/j.jep.2020.113435. [DOI] [PubMed] [Google Scholar]
- 91.Maiyo F, Moodley R, Singh M. Phytochemistry, cytotoxicity and apoptosis studies of β-sitosterol-3-oglucoside and β-amyrin from Prunus africana. Afr J Tradit Complement Altern Med. 2016;13:105–112. doi: 10.21010/ajtcam.v13i4.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Okoye T. Kaurenoic acid isolated from the root bark of annona senegalensis induces cytotoxic and antiproliferative effects against PANC-1 and HeLa cells. AJEA. 2014;4:579–589. [Google Scholar]
- 93.Wen S, Gu D, Zeng H. Antitumor effects of beta-amyrin in Hep-G2 liver carcinoma cells are mediated via apoptosis induction, cell cycle disruption and activation of JNK and P38 signalling pathways. J BUON. 2018;23:965–970. [PubMed] [Google Scholar]
- 94.Bourgou S, Pichette A, Marzouk B, Legault J. Bioactivities of black cumin essential oil and its main terpenes from Tunisia. S Afr J Bot. 2010;76:210–216. [Google Scholar]
- 95.Kuete V, Wabo HK, Eyong KO, Feussi MT, Wiench B, Krusche B, Tane P, Folefoc GN, Efferth T. Anticancer activities of six selected natural compounds of some Cameroonian medicinal plants. PLoS One. 2011;6:e21762. doi: 10.1371/journal.pone.0021762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Dibwe DF, Awale S, Kadota S, Tezuka Y. Damnacanthal from the congolese medicinal plant Garcinia huillensis has a potent preferential cytotoxicity against human pancreatic cancer PANC-1 cells. Phytother Res. 2012;26:1920–1926. doi: 10.1002/ptr.4672. [DOI] [PubMed] [Google Scholar]
- 97.Kuete V, Omosa LK, Tala VR, Midiwo JO, Mbaveng AT, Swaleh S, Karaosmanoğlu O, Sivas H. Cytotoxicity of plumbagin, rapanone and 12 other naturally occurring quinones from Kenyan flora towards human carcinoma cells. BMC Pharmacol Toxicol. 2016;17:60. doi: 10.1186/s40360-016-0104-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Tambama P, Abegaz B, Mukanganyama S. Antiproliferative activity of the Isofuranonaphthoquinone Isolated from Bulbine frutescens against Jurkat T cells. Biomed Res Int. 2014;2014:752941. doi: 10.1155/2014/752941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Osafo N, Boakye YD, Agyare C, Obeng S, Foli JE, Minkah PAB. African plants with antiproliferative properties. In: Badria FA, editor. Natural Products and Cancer Drug Discovery. 2017. pp. 1–23. [Google Scholar]
- 100.Kuete V, Sandjo LP, Ouete JL, Fouotsa H, Wiench B, Efferth T. Cytotoxicity and modes of action of three naturally occurring xanthones (8-hydroxycudraxanthone G, morusignin I and cudraxanthone I) against sensitive and multidrug-resistant cancer cell lines. Phytomedicine. 2014;21:315–322. doi: 10.1016/j.phymed.2013.08.018. [DOI] [PubMed] [Google Scholar]
- 101.Kuete V, Sandjo LP, Seukep JA, Zeino M, Mbaveng AT, Ngadjui B, Efferth T. Cytotoxic compounds from the fruits of Uapaca togoensis towards multifactorial drug-resistant cancer cells. Planta Med. 2015;81:32–38. doi: 10.1055/s-0034-1383362. [DOI] [PubMed] [Google Scholar]
- 102.Mbaveng AT, Damen F, Çelik İ, Tane P, Kuete V, Efferth T. Cytotoxicity of the crude extract and constituents of the bark of Fagara tessmannii towards multi-factorial drug resistant cancer cells. J Ethnopharmacol. 2019;235:28–37. doi: 10.1016/j.jep.2019.01.031. [DOI] [PubMed] [Google Scholar]
- 103.Shiu LY, Chang LC, Liang CH, Huang YS, Sheu HM, Kuo KW. Solamargine induces apoptosis and sensitizes breast cancer cells to cisplatin. Food Chem Toxicol. 2007;45:2155–2164. doi: 10.1016/j.fct.2007.05.009. [DOI] [PubMed] [Google Scholar]
- 104.Koduru S, Grierson DS, van de Venter M, Afolayan AJ. Anticancer activity of steroid alkaloids isolated from solanum aculeastrum. Pharm Biol. 2007;45:613–618. [Google Scholar]
- 105.Spiegler V, Greiffer L, Jacobtorweihen J, Asase A, Lanvers-Kaminsky C, Hempel G, Agyare C, Hensel A. In vitro screening of plant extracts traditionally used as cancer remedies in Ghana-15-hydroxyangustilobine A as the active principle in Alstonia boonei leaves. J Ethnopharmacol. 2021;265:113359. doi: 10.1016/j.jep.2020.113359. [DOI] [PubMed] [Google Scholar]
- 106.Mansoor TA, Borralho PM, Dewanjee S, Mulhovo S, Rodrigues CM, Ferreira MJ. Monoterpene bisindole alkaloids, from the African medicinal plant Tabernaemontana elegans, induce apoptosis in HCT116 human colon carcinoma cells. J Ethnopharmacol. 2013;149:463–470. doi: 10.1016/j.jep.2013.06.051. [DOI] [PubMed] [Google Scholar]
- 107.Pieme CA, Ambassa P, Yankep E, Saxena AK. Epigarcinol and isogarcinol isolated from the root of Garcinia ovalifolia induce apoptosis of human promyelocytic leukemia (HL-60 cells) BMC Res Notes. 2015;8:700. doi: 10.1186/s13104-015-1596-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Kuete V, Tchakam PD, Wiench B, Ngameni B, Wabo HK, Tala MF, Moungang ML, Ngadjui BT, Murayama T, Efferth T. Cytotoxicity and modes of action of four naturally occuring benzophenones: 2,2’,5,6’-tetrahydroxybenzophenone, guttiferone E, isogarcinol and isoxanthochymol. Phytomedicine. 2013;20:528–536. doi: 10.1016/j.phymed.2013.02.003. [DOI] [PubMed] [Google Scholar]
- 109.Xiang L, He B, Liu Q, Hu D, Liao W, Li R, Peng X, Wang Q, Zhao G. Antitumor effects of curcumin on the proliferation, migration and apoptosis of human colorectal carcinoma HCT-116 cells. Oncol Rep. 2020;44:1997–2008. doi: 10.3892/or.2020.7765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Srivastava KC, Mustafa T. Ginger (Zingiber officinale) in rheumatism and musculoskeletal disorders. Med Hypotheses. 1992;39:342–348. doi: 10.1016/0306-9877(92)90059-l. [DOI] [PubMed] [Google Scholar]
- 111.Park EJ, Pezzuto JM. Botanicals in cancer chemoprevention. Cancer Metastasis Rev. 2002;21:231–255. doi: 10.1023/a:1021254725842. [DOI] [PubMed] [Google Scholar]
- 112.Bayala B, Bassole IH, Gnoula C, Nebie R, Yonli A, Morel L, Figueredo G, Nikiema JB, Lobaccaro JM, Simpore J. Chemical composition, antioxidant, anti-inflammatory and anti-proliferative activities of essential oils of plants from Burkina Faso. PLoS One. 2014;9:e92122. doi: 10.1371/journal.pone.0092122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Akimoto M, Iizuka M, Kanematsu R, Yoshida M, Takenaga K. Anticancer effect of ginger extract against pancreatic cancer cells mainly through reactive oxygen species-mediated autotic cell death. PLoS One. 2015;10:e0126605. doi: 10.1371/journal.pone.0126605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Bawadood AS, Al-Abbasi FA, Anwar F, El-Halawany AM, Al-Abd AM. 6-shogaol suppresses the growth of breast cancer cells by inducing apoptosis and suppressing autophagy via targeting notch signaling pathway. Biomed Pharmacother. 2020;128:110302. doi: 10.1016/j.biopha.2020.110302. [DOI] [PubMed] [Google Scholar]
- 115.Lin CB, Lin CC, Tsay GJ. 6-gingerol inhibits growth of colon cancer cell LoVo via induction of G2/M arrest. Evid Based Complement Alternat Med. 2012;2012:326096. doi: 10.1155/2012/326096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Bansal M, Singh N, Pal S, Dev I, Ansari KM. Chemopreventive role of dietary phytochemicals in colorectal cancer. In: Advances in Molecular Toxicology. Elsevier; 2018. pp. 69–121. [Google Scholar]
- 117.Edderkaoui M, Odinokova I, Ohno I, Gukovsky I, Go VL, Pandol SJ, Gukovskaya AS. Ellagic acid induces apoptosis through inhibition of nuclear factor kappa B in pancreatic cancer cells. World J Gastroenterol. 2008;14:3672–80. doi: 10.3748/wjg.14.3672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Nguyen AV, Martinez M, Stamos MJ, Moyer MP, Planutis K, Hope C, Holcombe RF. Results of a phase I pilot clinical trial examining the effect of plant-derived resveratrol and grape powder on Wnt pathway target gene expression in colonic mucosa and colon cancer. Cancer Manag Res. 2009;1:25–37. [PMC free article] [PubMed] [Google Scholar]
- 119.Wu H, Chen L, Zhu F, Han X, Sun L, Chen K. The cytotoxicity effect of resveratrol: cell cycle arrest and induced apoptosis of breast cancer 4T1 cells. Toxins (Basel) 2019;11:731. doi: 10.3390/toxins11120731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Zan L, Chen Q, Zhang L, Li X. Epigallocatechin gallate (EGCG) suppresses growth and tumorigenicity in breast cancer cells by downregulation of miR-25. Bioengineered. 2019;10:374–382. doi: 10.1080/21655979.2019.1657327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Aggarwal V, Tuli HS, Tania M, Srivastava S, Ritzer EE, Pandey A, Aggarwal D, Barwal TS, Jain A, Kaur G, Sak K, Varol M, Bishayee A. Molecular mechanisms of action of epigallocatechin gallate in cancer: recent trends and advancement. Semin Cancer Biol. 2022;80:256–275. doi: 10.1016/j.semcancer.2020.05.011. [DOI] [PubMed] [Google Scholar]
- 122.Van Quaquebeke E, Simon G, André A, Dewelle J, El Yazidi M, Bruyneel F, Tuti J, Nacoulma O, Guissou P, Decaestecker C, Braekman JC, Kiss R, Darro F. Identification of a novel cardenolide (2”-Oxovoruscharin) from Calotropis procera and the hemisynthesis of novel derivatives displaying potent in vitro antitumor activities and high in vivo tolerance: structure-activity relationship analyses. J Med Chem. 2005;48:849–856. doi: 10.1021/jm049405a. [DOI] [PubMed] [Google Scholar]
- 123.Bao X, Zhang Y, Zhang H, Xia L. Molecular mechanism of β-sitosterol and its derivatives in tumor progression. Front Oncol. 2022;12:926975. doi: 10.3389/fonc.2022.926975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Vundru SS, Kale RK, Singh RP. β-sitosterol induces G1 arrest and causes depolarization of mitochondrial membrane potential in breast carcinoma MDA-MB-231 cells. BMC Complement Altern Med. 2013;13:280. doi: 10.1186/1472-6882-13-280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Zhang S, Mercado-Uribe I, Xing Z, Sun B, Kuang J, Liu J. Erratum: generation of cancer stem-like cells through the formation of polyploid giant cancer cells. Oncogene. 2014;33:116–28. doi: 10.1038/onc.2013.96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Juncker T, Schumacher M, Dicato M, Diederich M. UNBS1450 from Calotropis procera as a regulator of signaling pathways involved in proliferation and cell death. Biochem Pharmacol. 2009;78:1–10. doi: 10.1016/j.bcp.2009.01.018. [DOI] [PubMed] [Google Scholar]
- 127.Moalic S, Liagre B, Corbière C, Bianchi A, Dauça M, Bordji K, Beneytout JL. A plant steroid, diosgenin, induces apoptosis, cell cycle arrest and COX activity in osteosarcoma cells. FEBS Lett. 2001;506:225–230. doi: 10.1016/s0014-5793(01)02924-6. [DOI] [PubMed] [Google Scholar]
- 128.Mbaveng AT, Chi GF, Bonsou IN, Ombito JO, Yeboah SO, Kuete V, Efferth T. Cytotoxic phytochemicals from the crude extract of Tetrapleura tetraptera fruits towards multi-factorial drug resistant cancer cells. J Ethnopharmacol. 2021;267:113632. doi: 10.1016/j.jep.2020.113632. [DOI] [PubMed] [Google Scholar]
- 129.Mbaveng AT, Ndontsa BL, Kuete V, Nguekeu YMM, Çelik İ, Mbouangouere R, Tane P, Efferth T. A naturally occuring triterpene saponin ardisiacrispin B displayed cytotoxic effects in multi-factorial drug resistant cancer cells via ferroptotic and apoptotic cell death. Phytomedicine. 2018;43:78–85. doi: 10.1016/j.phymed.2018.03.035. [DOI] [PubMed] [Google Scholar]
- 130.Mbaveng AT, Chi GF, Nguenang GS, Abdelfatah S, Tchangna Sop RV, Ngadjui BT, Kuete V, Efferth T. Cytotoxicity of a naturally occuring spirostanol saponin, progenin III, towards a broad range of cancer cell lines by induction of apoptosis, autophagy and necroptosis. Chem Biol Interact. 2020;326:109141. doi: 10.1016/j.cbi.2020.109141. [DOI] [PubMed] [Google Scholar]
- 131.Zingue S, Gbaweng Yaya AJ, Michel T, Ndinteh DT, Rutz J, Auberon F, Maxeiner S, Chun FK, Tchinda AT, Njamen D, Blaheta RA. Bioguided identification of daucosterol, a compound that contributes to the cytotoxicity effects of Crateva adansonii DC (capparaceae) to prostate cancer cells. J Ethnopharmacol. 2020;247:112251. doi: 10.1016/j.jep.2019.112251. [DOI] [PubMed] [Google Scholar]
- 132.Gnoula C, Mégalizzi V, De Nève N, Sauvage S, Ribaucour F, Guissou P, Duez P, Dubois J, Ingrassia L, Lefranc F, Kiss R, Mijatovic T. Balanitin-6 and -7: diosgenyl saponins isolated from Balanites aegyptiaca Del. display significant anti-tumor activity in vitro and in vivo. Int J Oncol. 2008;32:5–15. [PubMed] [Google Scholar]
- 133.Chu YL, Ho CT, Chung JG, Rajasekaran R, Sheen LY. Allicin induces p53-mediated autophagy in Hep G2 human liver cancer cells. J Agric Food Chem. 2012;60:8363–8371. doi: 10.1021/jf301298y. [DOI] [PubMed] [Google Scholar]
- 134.Xu YS, Feng JG, Zhang D, Zhang B, Luo M, Su D, Lin NM. S-allylcysteine, a garlic derivative, suppresses proliferation and induces apoptosis in human ovarian cancer cells in vitro. Acta Pharmacol Sin. 2014;35:267–274. doi: 10.1038/aps.2013.176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Okaiyeto K, Oguntibeju OO. African herbal medicines: adverse effects and cytotoxic potentials with different therapeutic applications. Int J Environ Res Public Health. 2021;18:5988. doi: 10.3390/ijerph18115988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Organisation Mondiale de la Santé. Stratégie de l’OMS pour la médecine traditionnelle pour 2002-2005. Genève: Organisation mondiale de la Santé; 2002. [Google Scholar]
- 137.Organisation Mondiale de la Santé. Stratégie de l’OMS pour la médecine traditionnelle pour 2014-2023. Genève: Organisation mondiale de la Santé; 2013. [Google Scholar]
- 138.Meadows AL, Hawkins KM, Tsegaye Y, Antipov E, Kim Y, Raetz L, Dahl RH, Tai A, Mahatdejkul-Meadows T, Xu L, Zhao L, Dasika MS, Murarka A, Lenihan J, Eng D, Leng JS, Liu CL, Wenger JW, Jiang H, Chao L, Westfall P, Lai J, Ganesan S, Jackson P, Mans R, Platt D, Reeves CD, Saija PR, Wichmann G, Holmes VF, Benjamin K, Hill PW, Gardner TS, Tsong AE. Rewriting yeast central carbon metabolism for industrial isoprenoid production. Nature. 2016;537:694–697. doi: 10.1038/nature19769. [DOI] [PubMed] [Google Scholar]
- 139.Shukal S, Chen X, Zhang C. Systematic engineering for high-yield production of viridiflorol and amorphadiene in auxotrophic Escherichia coli. Metab Eng. 2019;55:170–178. doi: 10.1016/j.ymben.2019.07.007. [DOI] [PubMed] [Google Scholar]
- 140.Roberts SC. Production and engineering of terpenoids in plant cell culture. Nat Chem Biol. 2007;3:387–395. doi: 10.1038/nchembio.2007.8. [DOI] [PubMed] [Google Scholar]
- 141.Ochoa-Villarreal M, Howat S, Hong S, Jang MO, Jin YW, Lee EK, Loake GJ. Plant cell culture strategies for the production of natural products. BMB Rep. 2016;49:149–158. doi: 10.5483/BMBRep.2016.49.3.264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Halder M, Sarkar S, Jha S. Elicitation: a biotechnological tool for enhanced production of secondary metabolites in hairy root cultures. Eng Life Sci. 2019;19:880–895. doi: 10.1002/elsc.201900058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Liu X, Zhang P, Zhao Q, Huang AC. Making small molecules in plants: a chassis for synthetic biology-based production of plant natural products. J Integr Plant Biol. 2023;65:417–443. doi: 10.1111/jipb.13330. [DOI] [PubMed] [Google Scholar]
- 144.Li J, Mutanda I, Wang K, Yang L, Wang J, Wang Y. Chloroplastic metabolic engineering coupled with isoprenoid pool enhancement for committed taxanes biosynthesis in Nicotiana benthamiana. Nat Commun. 2019;10:4850. doi: 10.1038/s41467-019-12879-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Reed J, Osbourn A. Engineering terpenoid production through transient expression in Nicotiana benthamiana. Plant Cell Rep. 2018;37:1431–1441. doi: 10.1007/s00299-018-2296-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.De La Peña R, Sattely ES. Rerouting plant terpene biosynthesis enables momilactone pathway elucidation. Nat Chem Biol. 2021;17:205–212. doi: 10.1038/s41589-020-00669-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Forestier ECF, Czechowski T, Cording AC, Gilday AD, King AJ, Brown GD, Graham IA. Developing a Nicotiana benthamiana transgenic platform for high-value diterpene production and candidate gene evaluation. Plant Biotechnol J. 2021;19:1614–1623. doi: 10.1111/pbi.13574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Delatte TL, Scaiola G, Molenaar J, de Sousa Farias K, Alves Gomes Albertti L, Busscher J, Verstappen F, Carollo C, Bouwmeester H, Beekwilder J. Engineering storage capacity for volatile sesquiterpenes in Nicotiana benthamiana leaves. Plant Biotechnol J. 2018;16:1997–2006. doi: 10.1111/pbi.12933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Lee AR, Kwon M, Kang MK, Kim J, Kim SU, Ro DK. Increased sesqui- and triterpene production by co-expression of HMG-CoA reductase and biotin carboxyl carrier protein in tobacco (Nicotiana benthamiana) Metab Eng. 2019;52:20–28. doi: 10.1016/j.ymben.2018.10.008. [DOI] [PubMed] [Google Scholar]
- 150.Polturak G, Grossman N, Vela-Corcia D, Dong Y, Nudel A, Pliner M, Levy M, Rogachev I, Aharoni A. Engineered gray mold resistance, antioxidant capacity, and pigmentation in betalain-producing crops and ornamentals. Proc Natl Acad Sci U S A. 2017;114:9062–9067. doi: 10.1073/pnas.1707176114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Nakatsuka T, Abe Y, Kakizaki Y, Yamamura S, Nishihara M. Production of red-flowered plants by genetic engineering of multiple flavonoid biosynthetic genes. Plant Cell Rep. 2007;26:1951–1959. doi: 10.1007/s00299-007-0401-0. [DOI] [PubMed] [Google Scholar]
- 152.Kunakom S, Eustáquio AS. Natural products and synthetic biology: where we are and where we need to go. mSystems. 2019;4:e00113–19. doi: 10.1128/mSystems.00113-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Zhu X, Liu X, Liu T, Wang Y, Ahmed N, Li Z, Jiang H. Synthetic biology of plant natural products: from pathway elucidation to engineered biosynthesis in plant cells. Plant Commun. 2021;2:100229. doi: 10.1016/j.xplc.2021.100229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Wang X, Ye L, Lyu M, Ursache R, Löytynoja A, Mähönen AP. An inducible genome editing system for plants. Nat Plants. 2020;6:766–772. doi: 10.1038/s41477-020-0695-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Mohanta TK, Bashir T, Hashem A, Abd Allah EF, Bae H. Genome editing tools in plants. Genes (Basel) 2017;8:399. doi: 10.3390/genes8120399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Jatoi A, Burch P, Hillman D, Vanyo JM, Dakhil S, Nikcevich D, Rowland K, Morton R, Flynn PJ, Young C, Tan W North Central Cancer Treatment Group. A tomato-based, lycopene-containing intervention for androgen-independent prostate cancer: results of a phase II study from the North Central Cancer Treatment Group. Urology. 2007;69:289–294. doi: 10.1016/j.urology.2006.10.019. [DOI] [PubMed] [Google Scholar]
- 157.Zwicker JI, Schlechter BL, Stopa JD, Liebman HA, Aggarwal A, Puligandla M, Caughey T, Bauer KA, Kuemmerle N, Wong E, Wun T, McLaughlin M, Hidalgo M, Neuberg D, Furie B, Flaumenhaft R CATIQ Investigators11. Targeting protein disulfide isomerase with the flavonoid isoquercetin to improve hypercoagulability in advanced cancer. JCI Insight. 2019;4:e125851. doi: 10.1172/jci.insight.125851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Von Hagens C, Walter-Sack I, Goeckenjan M, Osburg J, Storch-Hagenlocher B, Sertel S, Elsässer M, Remppis BA, Edler L, Munzinger J, Efferth T, Schneeweiss A, Strowitzki T. Prospective open uncontrolled phase I study to define a well-tolerated dose of oral artesunate as add-on therapy in patients with metastatic breast cancer (ARTIC M33/2) Breast Cancer Res Treat. 2017;164:359–369. doi: 10.1007/s10549-017-4261-1. [DOI] [PubMed] [Google Scholar]
- 159.Kuete V, Sandjo LP, Kwamou GM, Wiench B, Nkengfack AE, Efferth T. Activity of three cytotoxic isoflavonoids from erythrina excelsa and erythrina senegalensis (neobavaisoflavone, sigmoidin H and isoneorautenol) toward multi-factorial drug resistant cancer cells. Phytomedicine. 2014;21:682–688. doi: 10.1016/j.phymed.2013.10.017. [DOI] [PubMed] [Google Scholar]
- 160.Kuete V, Mbaveng AT, Zeino M, Fozing CD, Ngameni B, Kapche GD, Ngadjui BT, Efferth T. Cytotoxicity of three naturally occurring flavonoid derived compounds (artocarpesin, cycloartocarpesin and isobavachalcone) towards multi-factorial drug-resistant cancer cells. Phytomedicine. 2015;22:1096–1102. doi: 10.1016/j.phymed.2015.07.006. [DOI] [PubMed] [Google Scholar]
- 161.Tilaoui M, Mouse HA, Jaafari A, Zyad A. Differential effect of artemisinin against cancer cell lines. Nat Prod Bioprospect. 2014;4:189–196. doi: 10.1007/s13659-014-0024-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Burger T, Mokoka T, Fouché G, Steenkamp P, Steenkamp V, Cordier W. Solamargine, a bioactive steroidal alkaloid isolated from Solanum aculeastrum induces non-selective cytotoxicity and P-glycoprotein inhibition. BMC Complement Altern Med. 2018;18:137. doi: 10.1186/s12906-018-2208-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.El khalki L, Tilaoui M, Jaafari A, Ait Mouse H, Zyad A. Studies on the dual cytotoxicity and antioxidant properties of berberis vulgaris extracts and its main constituent berberine. Adv Pharmacol Sci. 2018;2018:3018498. doi: 10.1155/2018/3018498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Segun PA, Ogbole OO, Ismail FMD, Nahar L, Evans AR, Ajaiyeoba EO, Sarker SD. Resveratrol derivatives from Commiphora africana (A. Rich.) Endl. display cytotoxicity and selectivity against several human cancer cell lines. Phytother Res. 2019;33:159–166. doi: 10.1002/ptr.6209. [DOI] [PubMed] [Google Scholar]
- 165.AlQathama A, Ezuruike UF, Mazzari ALDA, Yonbawi A, Chieli E, Prieto JM. Effects of selected Nigerian medicinal plants on the viability, mobility, and multidrug-resistant mechanisms in liver, colon, and skin cancer cell lines. Front Pharmacol. 2020;11:546439. doi: 10.3389/fphar.2020.546439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Mbaveng AT, Manekeng HT, Nguenang GS, Dzotam JK, Kuete V, Efferth T. Cytotoxicity of 18 Cameroonian medicinal plants against drug sensitive and multi-factorial drug resistant cancer cells. J Ethnopharmacol. 2018;222:21–33. doi: 10.1016/j.jep.2018.04.036. [DOI] [PubMed] [Google Scholar]
- 167.Boukes GJ, van de Venter M. The apoptotic and autophagic properties of two natural occurring prodrugs, hyperoside and hypoxoside, against pancreatic cancer cell lines. Biomed Pharmacother. 2016;83:617–626. doi: 10.1016/j.biopha.2016.07.029. [DOI] [PubMed] [Google Scholar]