Abstract
Currently, the pollution of the Caspian Sea by the oil industry is one of the highest problems in this area. Critically endangered species inhabit this sea, such as sturgeons, whose ecological value is incalculable. Thus, we aimed to evaluate the level of contamination of aliphatic hydrocarbons of petroleum and its relation with several toxic elements directly on sturgeons spines. A total of 40 adult starry sturgeons (Acipenser stellatus) were obtained within a repopulation programme in the northern and southern coastal waters of the Caspian Sea. The marginal pectoral fin was extracted from each fish to determine aliphatic hydrocarbons, arsenic, cadmium, mercury, nickel, lead, and vanadium. Subsequently, the sturgeons were released. Clearly, the presence of hydrocarbons was evidenced in all the sampled areas finding higher concentrations in the northern areas (N1 = 1.35 ± 0.4; N2 = 1.65 ± 0.46; N3 = 1.27 ± 0.40; S1 = 0.61 ± 0.22; S2 = 0.85 ± 0.43 mg/kg). Furthermore, to a greater or lesser extent, some toxic elements, mainly Hg and As, have been linked to aliphatic hydrocarbons.
Keywords: Arsenic, Bioaccumulation, Endangered fish, Heavy metals, Petroleum hydrocarbons
Introduction
The Caspian Sea is the largest enclosed body of water in the world, with a surface of approximately 370,000 km2 and a volume of water of 78,000 km3. It is located between Asia and Europe and is bordered by five countries: Russia, Kazajistan, Turkmenistan, Iran, and Azerbaiyan. Based on its geophysical conditions, this sea can be subdivided into three parts: the northern area with a maximum depth of 30 m and a salinity of below 10 g/L, and the middle and southern areas, with a salinity of nearly 13 g/L and depths of 800 and 1000 m, respectively (Kosarev 2005; Lattuada et al. 2019). It is filled and drained mainly by the rivers Volga (233 km3/year) and Ural (6.6 km3/year), as well as the rainfall (Ramazanova et al. 2022). The fact that it is closed makes its ecosystem less tolerant and more vulnerable to human-generated and spilled environmental pollutants (Ghayebzadeh et al. 2020).
Currently, pollution from crude oil is one of the most important concerns in the Caspian Sea. The first deep wells began to be excavated in the nineteenth century and, from that moment on, both the extensive exploitation of the oil and its transport across the Volga river have originated drastic pollution in this area. During the Soviet period, the oil and gas companies were principally limited to the west and north coasts. After its dissolution, all the countries on its coasts have become involved in this industry (Leroy et al. 2020). Crude oil is classified in four well-defined fractions: aliphatic, aromatic hydrocarbons, resins, and asphaltenes. Aliphatic hydrocarbons represent the greater part of crude oil, and they have no double bonds (Varjani 2017; Oyibo et al. 2018). In addition, the oil may contain a variety of potentially toxic elements like arsenic (As), cadmium (Cd), lead (Pb), nickel (Ni), mercury (Hg), and vanadium (V), which mainly originate in crude oil from the perforation fluid during its extraction, and in the detachment of impurities from the oil products and tanks (Wilhelm and Bloom 2000; Fakhru’l-Razi et al. 2009; Li et al. 2021). To be specific, the most abundant metals in crude oil and its by-products are Ni and V. Both metals are joined in the centre of some aromatic compounds called petroporphyrins, forming V or Ni petroporphyrins (Valencia 2023).
In the Caspian Sea, the total ichthyofauna reaches 76 species and 47 subspecies, in reference to 17 families and 53 genera (Leroy et al. 2020). Among these 76 species, there are five species of sturgeon, including Persian sturgeon (Acipenser persicus), Russian sturgeon (Acipenser gueldenstaedti), starry sturgeon (Acipenser stellatus), boat sturgeon (Acipenser nudiventris), and beluga sturgeon (Huso huso) (Wang et al. 2008). Although sturgeon caviar is currently produced in farms (Bronzi et al. 2019), in the past, approximately 90% of sturgeon caviar comes from this sea (Pourkazemi 2006; Graham and Murphy 2007). However, overfishing and the contamination of the ecosystem have greatly affected sturgeon populations. Actually, in the Caspian Sea, these five sturgeon species have been classified as being “critically endangered species” by the International Union for the Conservation of Nature (UICN 2022). Thus, there is an urgent need for the five surrounding countries to develop a common strategy for the revival of sturgeon populations (FAO 2022).
For all the above, our study aimed to determine the concentrations of total aliphatic hydrocarbons (TAH) and the potentially toxic elements, As, Cd, Hg, Pb, V, and Ni in the pectoral fin of the starry sturgeon (A. stellatus) in different places of the northern and southern areas of the Caspian Sea. We set two main objectives: (i) to evaluate the differences in the concentrations of these elements in the different zones and (ii) to establish the relationships between concentrations of hydrocarbons and toxic elements.
Material and methods
Sampling area and preparation of the samples
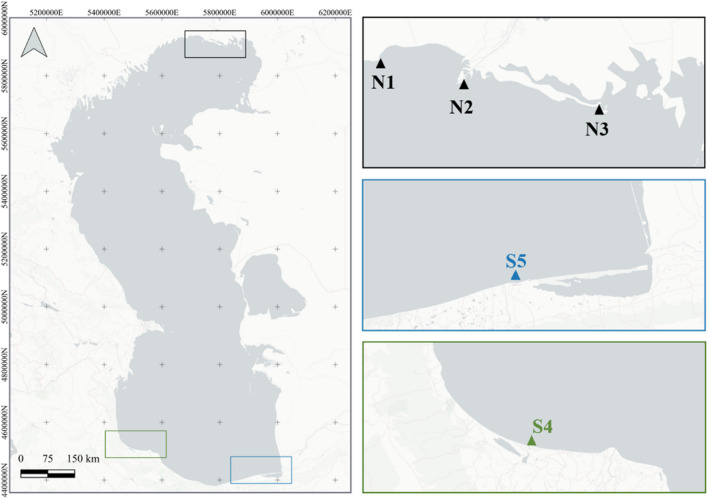
A total of 40 adult starry sturgeons with a mean weight of 6.38 ± 1.55 kg and 134.57 ± 13.57 cm long were obtained within a repopulation programme in the northern and southern coastal waters of the Caspian Sea. Figure 1 shows sampling sites. More precisely, three sampling areas were established in the north (N1, N2, and N3), in which a total of 20 specimens were analyzed (eight, four, and eight, respectively), and two in the south (S4 and S5), where another 20 specimens were evaluated, 10 per sampling area.
Fig. 1.
Different areas in which starry sturgeons are caught in the Caspian Sea
The marginal pectoral fin ray (the most cranial one) was extracted from each fish by cutting it at the articulation point, following Koch et al. (2008). Before taking each sample, the instruments used were rinsed in 1% nitric acid. The samples were frozen at − 80 °C up to analysis. It is important to clarify that this sampling method is not lethal. Fish were not sacrificed for this study but were freed once the samples had been taken. This work was approved by the Ethics Committee of the institution involved, and was carried out in accordance with the European regulations for the protection of animals used for scientific purposes (European Parliament 2010).
Potentially toxic element analyses
The spine samples were digested with a mixture of nitric acid (65% HNO3) and perchloric acid (60% HClO4) (2:1 v/v) in a boiling water bath at 70 °C to decompose all the organic matter. The solution obtained was completed with distilled water until reaching a volume of 50 ml. The toxic element concentrations were quantified by employing an inductively coupled plasma-mass spectrometer (ICP-MS) with Agilent 7500 series equipment. Analytical blanks were processed appropriately, and concentrations were determined using standard solutions that were prepared in the same acidic matrix.
TAH analyses
The concentration of the following aliphatic hydrocarbons was determined: C10, C11, C12, C13, C14, C15, C16, C17, Pristane, C18, Phytane, C19, C20, C21, C22, C23, C24, C25, C26, C27, C28, C29, C30, C31, C32, C33, C34, C35, C36, C37, C38, and C39. After the individual determinations, their concentrations were summed to obtain the TAH value for each sturgeon.
For the extraction, each sample was ground in a clean mortar together with 10 g of anhydrous sodium sulfate until completely dry and homogenized. The sample was extracted using 50 ml of hexane with dichloromethane employed as the extraction solvent in a 1:1 ratio. Ten grams of the homogenized product of the sample was placed in a 50-ml extraction bottle, which was left for 60 min in an ultrasonic bath. The extract was carefully decanted and concentrated to 2 ml using a rotary evaporator kept at 20 °C. The aliphatic hydrocarbons concentrations were determined by means of gas chromatography with Agilent-7890 equipped with a mass spectrometer (Agilent-5975) with a quadrupole type Split-Splitless inlet.
Statistical analysis
Statistical analysis of the data was performed using the SPSS version 25 statistical software. The normality of the data was assessed using the Kolmogorov–Smirnov test. Due to the non-normality of the TAH and toxic element concentrations, the data were analyzed by the Kruskal-Walis non-parametric H test to compare the concentrations between zones. Bonferroni correction was used as a method of multiple comparisons in pairwise analysis. It is important to highlight that Bonferroni correction as a pairwise comparison is a much more conservative method than other statistical analyses (Chen et al. 2017), thus ensuring that the differences found are representative. Besides, to evaluate the differences between north and south in general (∑N1-N3 and ∑S1-S2), the non-parametric Mann–Whitney U test was used.
On the other hand, to establish the relationships between elements, Spearman’s correlation test was applied. In all the cases, a value of p < 0.05 was taken as being significant. Finally, to study the relationships of the elements with hydrocarbons, a principal component analysis (PCA) was carried out in R studio using the FactoMiner and Factoextra libraries. The length and weight data of the animals were included in this analysis.
Results
TAH and toxic element concentrations
The descriptive statistics of the TAH and toxic element concentrations in the different sampling areas of the Caspian Sea are shown in Table 1. Regarding TAH concentrations, significantly higher concentrations were found in zone N1 compared to zone S4. For As, zones N1 and N2 had significantly higher concentrations than zone S5. In the case of Pb and Cd, no differences attributable to location were found. Ni levels were substantially higher in zones N1 and N3 than in zone S5. Finally, V concentrations were higher in N1 than N3 and S5 zones. In general, except for Pb and Cd, some northern location always had a higher concentration of TAH and toxic elements than some southern zone. In fact, on analyzing the samples from the northern and southern areas together, i.e., ∑N1–N3 and ∑S4–S5, it was found that the northern area presented significantly higher concentrations (p < 0.05) of TAH, As, Hg, Ni, and V. Specifically, mean TAH levels in north were 1.380 ± 0.416 mg/kg, while in the south, they were significantly lower, 0.737 ± 0.356 mg/kg. Regarding toxic elements, As levels were 1.943 ± 2.805 µg/kg in north compared to 0.291 ± 0.601 µg/kg in south. North Hg concentrations were 1.076 ± 0.800 µg/kg compered to south Hg levels of 0.520 ± 1.576 µg/kg. Ni concentrations in south were 1.314 ± 1.731 µg/kg compered to higher concentrations in north (3.995 ± 3.837 µg/kg). Finally, V levels were 13.306 ± 5.475 and 1.314 ± 1.731 µg/kg in the north and south of the Caspian Sea, respectively. An interesting fact obtained in this study is that several sturgeons from southern areas had levels of As, Hg, and Cd lower than that of the detection limit of the test.
Table 1.
Toxic element and TAH concentrations in starry sturgeons in different areas of the Caspian Sea
| Zone | Parameter | n | Mean | SD | Median | Min | Max | |
|---|---|---|---|---|---|---|---|---|
| North | N1 | TAH (mg/kg)a | 8 | 1.351 | 0.402 | 1.437 | 0.784 | 2.015 |
| As (µg/kg)a | 3.248 | 3.753 | 1.487 | 0.000 | 8.804 | |||
| Cd (µg/kg) | 0.268 | 0.152 | 0.214 | 0.077 | 0.506 | |||
| Hg (µg/kg)a | 1.502 | 1.117 | 1.031 | 0.447 | 3.676 | |||
| Ni (µg/kg)a | 5.732 | 4.650 | 3.956 | 0.000 | 13.501 | |||
| Pb (µg/kg) | 10.485 | 4.912 | 8.065 | 7.084 | 21.441 | |||
| V (µg/kg)a | 17.782 | 6.214 | 15.590 | 14.269 | 32.952 | |||
| N2 | TAH (mg/kg)a | 4 | 1.651 | 0.458 | 1.719 | 1.030 | 2.134 | |
| As (µg/kg)a | 1.994 | 2.597 | 0.710 | 0.667 | 5.890 | |||
| Cd (µg/kg) | 0.191 | 0.082 | 0.194 | 0.090 | 0.285 | |||
| Hg (µg/kg)a,b | 0.856 | 0.146 | 0.812 | 0.741 | 1.059 | |||
| Ni (µg/kg)a,b | 2.243 | 1.608 | 1.911 | 0.695 | 4.456 | |||
| Pb (µg/kg) | 11.474 | 4.911 | 10.253 | 7.165 | 18.226 | |||
| V (µg/kg)a,b | 12.134 | 0.642 | 12.239 | 11.263 | 12.795 | |||
| N3 | TAH (mg/kg)a | 8 | 1.273 | 0.403 | 1.315 | 0.411 | 1.796 | |
| As (µg/kg)a,b | 0.611 | 0.635 | 0.473 | 0.000 | 1.615 | |||
| Cd (µg/kg) | 0.177 | 0.031 | 0.181 | 0.116 | 0.207 | |||
| Hg (µg/kg)a,b | 0.761 | 0.364 | 0.640 | 0.373 | 1.472 | |||
| Ni (µg/kg)a | 3.134 | 3.342 | 2.377 | 0.000 | 9.470 | |||
| Pb (µg/kg) | 8.078 | 2.864 | 6.963 | 5.957 | 14.740 | |||
| V (µg/kg)b | 9.415 | 1.259 | 9.494 | 7.195 | 10.763 | |||
| South | S4 | TAH (mg/kg)b | 10 | 0.609 | 0.215 | 0.578 | 0.427 | 1.122 |
| As (µg/kg)a,b | 0.404 | 0.716 | 0.115 | 0.000 | 2.319 | |||
| Cd (µg/kg) | 0.270 | 0.269 | 0.188 | 0.064 | 1.001 | |||
| Hg (µg/kg)b | 0.817 | 2.180 | 0.112 | 0.000 | 7.013 | |||
| Ni (µg/kg)ab | 2.245 | 1.713 | 1.871 | 0.000 | 4.474 | |||
| Pb (µg/kg) | 8.767 | 3.446 | 7.669 | 5.284 | 16.551 | |||
| V (µg/kg)a,b | 14.497 | 11.237 | 10.170 | 7.186 | 44.076 | |||
| S5 | TAH (mg/kg)a,b | 10 | 0.851 | 0.427 | 0.790 | 0.143 | 1.759 | |
| As (µg/kg)b | 0.178 | 0.494 | 0.000 | 0.000 | 1.578 | |||
| Cd (µg/kg) | 0.126 | 0.059 | 0.103 | 0.064 | 0.246 | |||
| Hg (µg/kg)b | 0.224 | 0.545 | 0.000 | 0.000 | 1.758 | |||
| Ni (µg/kg)b | 0.382 | 1.210 | 0.000 | 0.000 | 3.827 | |||
| Pb (µg/kg) | 7.919 | 3.110 | 7.529 | 4.096 | 15.838 | |||
| V (µg/kg)b | 6.390 | 3.568 | 5.203 | 2.391 | 13.049 | |||
a,b When at least one letter is not shared in the same compound, there are statistically significant differences (p < 0.05)
Correlation analysis between TAH and toxic elements
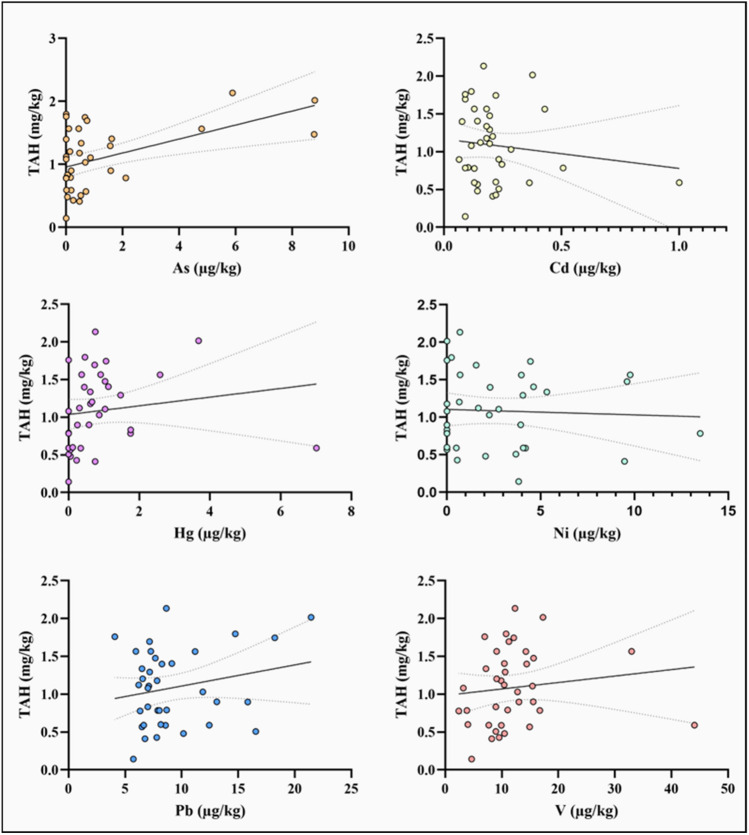
Results of Spearman’s correlation analysis are shown in Table 2. Regarding the correlation between toxic elements and hydrocarbons, only Hg and V had a positive correlation with TAH. In this regard, the r values were 0.436 and 0.327 for Hg and V, respectively. In the case of As, its p-value was 0.056, a value that slightly exceeded the confidence level (p < 0.05), so we decided not to take it as a conclusive result. Figure 2 shows the scatter graphs between TAH and determined toxic elements, where different correlations can be seen. On the other hand, a high correlation between different toxic elements is proven, as shown in Table 2.
Table 2.
Spearman’s correlation analysis between TAH and toxic elements in starry sturgeon
| Compound | TAH | As | Cd | Hg | Ni | Pb | V | ||
|---|---|---|---|---|---|---|---|---|---|
| Rho de Spearman | TAH | r | 1 | 0.316 | − 0.120 | 0.436** | 0.035 | 0.119 | 0.327* |
| Sig | - | 0.056 | 0.478 | 0.007 | 0.838 | 0.481 | 0.048 | ||
| As | r | 1 | 0.468** | 0.623** | 0.426** | 0.351* | 0.613** | ||
| Sig | - | 0.002 | 0.000 | 0.006 | 0.026 | 0.000 | |||
| Cd | r | 1 | 0.593** | 0.396* | 0.343* | 0.236 | |||
| Sig | - | 0.000 | 0.011 | 0.030 | 0.143 | ||||
| Hg | r | 1 | 0.479** | 0.339* | 0.426** | ||||
| Sig | - | 0.002 | 0.032 | 0.006 | |||||
| Ni | r | 1 | 0.088 | 0.341* | |||||
| Sig | - | 0.590 | 0.031 | ||||||
| Pb | r | 1 | 0.325* | ||||||
| Sig | - | 0.041 | |||||||
| V | r | 1 | |||||||
| Sig | - | ||||||||
*The correlation is significant with a confidence level of 0.05 (bilateral)
**The correlation is significant with a confidence level of 0.01 (bilateral)
Fig. 2.
Scatter graphs between TAH and toxic elements in starry sturgeon samples from the Caspian Sea
Principal component analysis
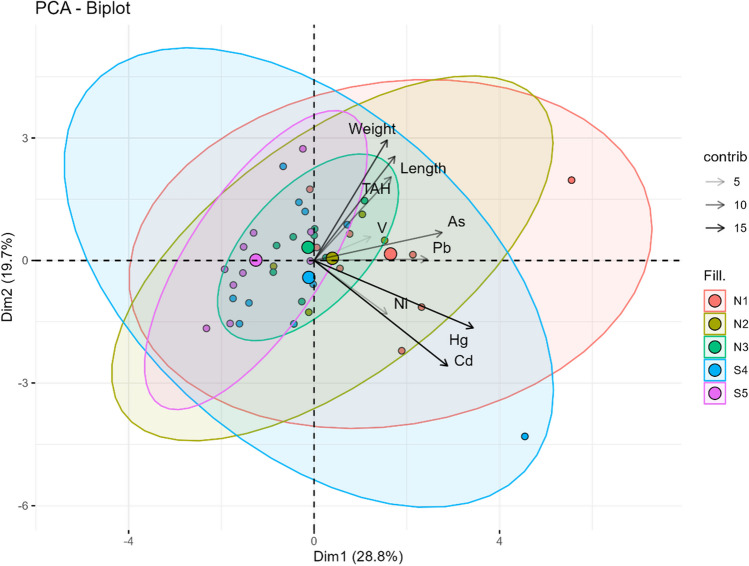
In the PCA, four principal components (PCs) were extracted, whose eigenvalues exceeded the unity. The explained variance percentages for PC1, PC2, PC3, and PC4 were 28.8, 19.7, 14.3, and 13.7, respectively. With these four components were explained the 76.5% of the variance. Table 3 shows the loadings of height, weight, TAH, and toxic elements with each components. All loadings were positive for PC1 exceeding at all times 0.3, implying a strong involvement of all variables in this component. In the case of PC2, length, weight, TAH, As, and V had a positive loading, while the other variables were negatives or neutral such as Pb. Both components, PC1 and PC2, explained the 48.5% of the variance. Figure 3 shows the spatial distribution of each location with respect to these two components indicating, in addition, the contribution of each variable.
Table 3.
Loadings between principal components of PCA and length, weight, TAH, and toxic elements determined in sturgeon
| Variable | PC 1 | PC 2 | PC 3 | PC 4 |
|---|---|---|---|---|
| Length | 0.4143844 | 0.606657537 | − 0.4987555 | 0.309265161 |
| Weight | 0.3746787 | 0.701472591 | − 0.4382613 | − 0.001685339 |
| TAH | 0.3952502 | 0.487070566 | 0.5261645 | − 0.308614944 |
| As | 0.6565125 | 0.162913141 | 0.5109190 | − 0.085034259 |
| Cd | 0.6843315 | − 0.614540093 | − 0.3078367 | − 0.024759511 |
| Hg | 0.8159013 | − 0.393086210 | − 0.2170338 | − 0.152485259 |
| Ni | 0.3741725 | − 0.312059554 | 0.1725340 | 0.607038169 |
| Pb | 0.5800106 | 0.007527265 | 0.0808575 | − 0.406745431 |
| V | 0.2903158 | 0.137716950 | 0.3574629 | − 0.481581355 |
Fig. 3.
Spatial distribution in PC1 and PC2 of length, weight, TAH, and toxic elements determined in sturgeon in different locations in the Caspian Sea
Discussion
The release of oil and oil-related products into the environment can destroy an ecosystem because these compounds are highly persistent, have a low biodegradability, are bioaccumulative, and possess a high biotoxicity (Chen et al. 2015; Lee et al. 2019; Ossai et al. 2020). The genetic reserves of sturgeon in the Caspian Sea form one of the largest natural resources in the world, and play a fundamental role in this sea’s ecosystem because, mainly, they are predators (Fazli et al. 2022). These fish live at the bottom of the sea and their feeding pattern, which is based on the ingestion of the benthic organisms found in its sediments, increases the risk of exposure to many pollutants, such as metals or crude oil hydrocarbons (Poorbagher et al. 2017). Moreover, these species are very long-lived, between 50 and 75 years (Doroshov 2019), so they are exposed to pollutants for a long time and can be good bioindicators of pollution. Particularly, we have observed in the PCA that variables such as weight and length (attributes related to the age of the sturgeon) are strongly associated with the presence of TAH in them. Therefore, detecting the concentrations of these contaminants in these species can provide highly valuable information for assessing the level of pollution to which these animals are exposed. That information could be used to establish area management actions, that will help to conserve this highly valued species, that is currently, under great threat.
Another key aspect is the matrix employed for TAH and metal determination. These sturgeons are “protected species” so sacrificing animals to determine concentrations in muscle and other organs is not justified. The use of bone samples from the marginal pectoral fin is a validated and minimally harmful method for sturgeons, and is mainly used to determine their age and growth (Baremore 2014; Bakhshalizadeh et al. 2021). In addition, several studies have reported that bone tissue is a good matrix to address the concentration of accumulative substances like toxic elements, since the concentrations obtained in this tissue are higher than those found in muscle tissue, and could be compared to those obtained in liver tissue (Edem et al. 2009; Adeosun et al. 2015). Future research could also consider the collection of blood samples through a non-invasive method to add information about the health of the sturgeons.
Regarding the presence of hydrocarbons in marine sediments, Readman et al. (2002) found that there is no contamination when their concentration is below 10 mg/kg, but that the sediments are polluted when their concentration is over 100 mg/kg. Shirneshan et al. (2017) reported concentrations of TAH in sediments in several areas in the south-western Caspian Sea, which varied from 19.75 to 996.23 mg/kg, which are considered relatively high. There are very few studies on the concentrations of aliphatic hydrocarbon in fish. De Mora et al. (2010) obtained concentrations of TAH in the muscles of hamour and tuna, which ranged between 0.56 and 9.9 mg/kg in the ROPME Sea Area, in which the oil industry is also strongly developed. The concentrations obtained in our study are in the range found by these researchers. On the other hand, the proximity to the Volga river, which carries a lot of waste, suggests that it could be responsible for the higher levels of TAH and some elements in northern areas. However, despite the high concentrations of TAH in Caspian Sea sediments, based on those obtained in our work, it is suggested that, surprisingly, they are not accumulated in excess in the sturgeons spine and that their concentration in other organs could be higher. According to Mohsenpour (2020) hydrocarbons accumulate in the liver and gall bladder after they enter the body orally. These authors also pointed out that one of the main adverse effects of oil hydrocarbons is that they alter and reduce fish fertility. In general, fish early life stages and juveniles across taxa may be more susceptible to the effects of oil due to their immaturity and developing physiologies (Takeshita et al. 2021). These adverse effects clearly can negatively affect repopulation programs of various sturgeon species.
Crude oils mainly consist of hydrocarbons but a small amount (generally under 1%) is composed of inorganic compounds, including metals or metalloids (Shahat et al. 2018). V is usually the most abundant trace metal in oil samples, and its concentration can reach up to 1500 mg/kg, although in some crude oils there is less than 0.1 mg/kg (Amorim et al. 2007). On the other hand, Ni concentrations may reach 340 mg/kg in crude oil. Ni/V ratios have been used to classify those in crude oils (Barwise 1990). In the Caspian Sea, V can be trapped in the oil production process, while fuel is burned, although generally its concentration in the water is insignificant and it does not affect its quality (Tasmagambetova et al. 2019). Concentrations of Ni found in sturgeons generally agree with the values reported previously in different tissues of other species (0.1–8.00 mg/kg) in the Caspian Sea (Sheikhzadeh and Hamidian 2021). This suggest that the concentrations of TAH, the most abundant compounds in crude oil, are probably closely related to the concentrations of Ni and V since these two metals should be the most abundant. In the present work, the Spearman correlation, which focuses on evaluating the relationship between pairs of variables, for example, TAH and V, and PCA, which identifies general patterns in a multidimensional dataset, has been used. Using both approaches provides a more comprehensive understanding of the relationships between TAH and toxic elements in the study. In the Spearman correlation, positive correlations have been found between Hg and V with respect to TAH. Meanwhile, in the PCA, all elements have shown a strong correlation with TAH in PC1, particularly Hg, and only As and V in PC2. This could imply that all the elements share some more or less close environmental relationship, with As, Hg, and V being the ones most related to petroleum. Apart from the harmful effects of oil itself, the adverse effects of As on fish health include various mechanisms of acute and chronic toxicity, ranging from enzymatic, genetic, and immune system failures, which alter normal biological functions, resulting in the direct initiation of diseases or, at least, in the body’s predisposition to them (Kumari et al. 2016). Regarding this concern, Acolas et al. (2020) determined As concentrations of less than 0.25 µg/mL in European sturgeon (Acipenser sturio) in the Gironde estuary in France. Although it is true that they used a different biological matrix, such as blood, these are much lower concentrations than those determined in any area of this study. On the other hand, Hg extreme toxicity, long-distance migration, and bioaccumulation are a serious problem for ecosystems. At the moment, several methods are being applied to Hg capture since it is a major challenge in petroleum and natural gas processing (Warrag et al. 2018; Huo et al. 2019). Overall, oil pollution, already highly concerning on its own, carries with it the pollution of toxic elements that further worsens this environmental issue in the Caspian Sea.
Conclusions
Monitoring contaminants directly on protected species, in addition to the environment where they live, can add information on the bioaccumulation of pollutants, such as hydrocarbons. It should be noted that monitoring these species requires non-lethal methods, which can pose challenges to the study. In this case, the presence of aliphatic hydrocarbons from oil in all samples of sturgeon spines has been evidenced in the southern and northern Caspian Sea, particularly with higher concentrations in the northern areas. Furthermore, to a greater or lesser extent, some toxic elements, mainly Hg and As, have been linked to TAH, which aggravates this environmental problem due to their toxicity. So, proposing new studies on sturgeons themselves using other non-lethal methods or evaluating their environment is mandatory to recover valuable and unique species such as sturgeons. Finally, it should be emphasized that restrictions must be taken against pollution in the Caspian Sea.
Author contribution
S. B. and N. A.-S. contributed to the conception and design of the study. Data collection and sampling were carried out by B. N., T. K., and A. A. Laboratory and statistical analysis were performed by S. B., A. A., N. A.-S., and R. M.-M. The first draft of the document was written and supervised by S. B. and N. A.-S. All authors commented on previous versions of the article and read and approved the final article.
Funding
Funding for open access publishing: Universidad de Córdoba/CBUA.
Data availability
The data that support the findings of this study are available from the corresponding author.
Declarations
Ethics approval
All the applicable international, national, and/or institutional guidelines for the care and use of animals were followed. The use and care of fish in this study were approved by the University of Guilan from the point of Ethical issues (1400.8.3–89122/15p).
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Acolas ML, Davail B, Gonzalez P, et al. Health indicators and contaminant levels of a critically endangered species in the Gironde estuary, the European sturgeon. Environ Sci Pollut Res. 2020;27:3726–3745. doi: 10.1007/s11356-019-05139-5. [DOI] [PubMed] [Google Scholar]
- Adeosun FI, Akinyemi AA, Idowu AA, et al. The effects of heavy metals concentration on some commercial fish in Ogun River, Opeji, Ogun State, Nigeria. Afr J Environ Sci Technol. 2015;9:365–370. doi: 10.5897/ajest2014.1659. [DOI] [Google Scholar]
- Amorim F, Welz B, Costa A, et al. Determination of vanadium in petroleum and petroleum products using atomic spectrometric techniques. Talanta. 2007;72:349–359. doi: 10.1016/j.talanta.2006.12.015. [DOI] [PubMed] [Google Scholar]
- Bakhshalizadeh S, Bani A, Abdolmalaki S, Ponce-Palafox JT. Marking fin spine of juvenile sturgeon (Acipenseriformes: Acipenseridae) using oxytetracycline and the comparing of growth performance. Arquivo Brasileiro De Medicina Veterinária e Zootecnia. 2021;73:902–908. doi: 10.1590/1678-4162-12270. [DOI] [Google Scholar]
- Baremore IE. Rosati JDA (2014) Validated, minimally deleterious method for aging sturgeon. Fish Bull. 2014;112:274–282. doi: 10.7755/fb.112.4.4. [DOI] [Google Scholar]
- Barwise AJG. Role of nickel and vanadium in petroleum classification. Energy Fuels. 1990;4:647–652. doi: 10.1021/ef00024a005. [DOI] [Google Scholar]
- Bronzi P, Chebanov M, Michaels JT, et al. Sturgeon meat and caviar production: global update 2017. J Appl Ichthyol. 2019;35:257–266. doi: 10.1111/jai.13870. [DOI] [Google Scholar]
- Chen M, Xu P, Zeng G, Yang C, Huang D, Zhang J. Bioremediation of soils contaminated with polycyclic aromatic hydrocarbons, petroleum, pesticides, chlorophenols and heavy metals by composting: applications, microbes and future research needs. Biotechnol Adv. 2015;33:745–755. doi: 10.1016/j.biotechadv.2015.05.003. [DOI] [PubMed] [Google Scholar]
- Chen SY, Feng Z, Yi X. A general introduction to adjustment for multiple comparisons. J Thorac Dis. 2017;9:1725–1729. doi: 10.21037/jtd.2017.05.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Mora S, Tolosa I, Fowler SW, Villeneuve JP, Cassi R, Cattini C. Distribution of petroleum hydrocarbons and organochlorinated contaminants in marine biota and coastal sediments from the ROPME sea area during 2005. Mar Pollut Bull. 2010;60:2323–2349. doi: 10.1016/j.marpolbul.2010.09.021. [DOI] [PubMed] [Google Scholar]
- Doroshov SI (2019) Biology and culture of sturgeon Acipenseriformes. In: Muir JF, Roberts RJ (eds) Recent Advances in Aquaculture. Springer, Boston, pp 251–274. 10.4324/9780429303937-7
- Edem CA, Osabor V, Iniama G, Etiuma R, Eke J. Distribution of heavy metals in bones, gills, livers and muscles of (tilapia) Oreochromis niloticus from Henshaw town beach market in Calabar Nigeria. Pak J Nutr. 2009;8:1209–1211. doi: 10.3923/pjn.2009.1209.1211. [DOI] [Google Scholar]
- Fakhru’l-Razi A, Pendashteh A, Abdullah LC, Biak DRA, Madaeni SS, Abidin ZZ. Review of technologies for oil and gas produced water treatment. J Hazard Mater. 2009;170:530–551. doi: 10.1016/j.jhazmat.2009.05.044. [DOI] [PubMed] [Google Scholar]
- FAO (2022) Fishery and aquaculture country profiles Islamic Republic of Iran. Fisheries and Aquaculture. Available at https://www.fao.org/fishery/en/facp/IRN. Accessed 19 Jan 2023
- Fazli H, Behrouz Khoshghalb MR, Abdolmaleki SA. Multi-species modeling approach to consider the effects of environmental parameters on Caspian sturgeon fishes stock status. Reg Stud Marine Sci. 2022;56:102666. doi: 10.1016/j.rsma.2022.102666. [DOI] [Google Scholar]
- Ghayebzadeh M, Aslani H, Taghipour H, Mousavi S. Contamination of the Caspian Sea southern coast sediments with microplastics: a marine environmental problem. Mar Pollut Bull. 2020;160:111620. doi: 10.1016/j.marpolbul.2020.111620. [DOI] [PubMed] [Google Scholar]
- Graham LJ, Murphy BR. The decline of the Beluga sturgeon: a case study about fisheries management. J Nat Resour Life Sci Educ. 2007;36:66–75. doi: 10.2134/jnrlse2007.36166x. [DOI] [Google Scholar]
- Huo Q, Wang Y, Chen H, Han L, Wang J, Bao W, Chang L, Xie K. ZnS/AC sorbent derived from the high sulfur petroleum coke for mercury removal. Fuel Process Technol. 2019;191:36–43. doi: 10.1016/j.fuproc.2019.03.025. [DOI] [Google Scholar]
- IUCN (2022) The IUCN red list of threatened species. Available at https://www.iucnredlist.org/search?query=Sturgeons&searchType=species. Accessed 19 Jan 2023
- Koch JD, Schreck WJ, Quist MC. Standardised removal and sectioning locations for Shovelnose sturgeon fin rays. Fish Manage Ecol. 2008;15:139–145. doi: 10.1111/j.1365-2400.2008.00594.x. [DOI] [Google Scholar]
- Kosarev AN (2005) Physico-geographical conditions of the Caspian Sea. In: Kostianoy AG, Kosarev AN (eds) The Caspian Sea Environment. The Handbook of Environmental Chemistry, vol 5P. Springer, Berlin, Heidelberg, pp 5–31. 10.1007/698_5_002
- Kumari B, Kumar V, Sinha AK, Ahsan J, Ghosh AK, Wang H, DeBoeck G. Toxicology of arsenic in fish and aquatic systems. Environ Chem Lett. 2016;15:43–64. doi: 10.1007/s10311-016-0588-9. [DOI] [Google Scholar]
- Lattuada M, Albrecht C, Wilke T. Differential impact of anthropogenic pressures on Caspian Sea ecoregions. Mar Pollut Bull. 2019;142:274–281. doi: 10.1016/j.marpolbul.2019.03.046. [DOI] [PubMed] [Google Scholar]
- Lee Y, Lee Y, Jeon CO (2019) Biodegradation of naphthalene, BTEX, and aliphatic hydrocarbons by Paraburkholderia aromaticivorans BN5 isolated from petroleum-contaminated soil. Sci Rep 9:860. 10.1038/s41598-018-36165-x [DOI] [PMC free article] [PubMed]
- Leroy SAG, Lahijani HAK, Crétaux JF, Aladin NV, Plotnikov IS (2020) Past and current changes in the largest lake of the world: the Caspian Sea. In: Mischke S (eds) Large Asian Lakes in a Changing World. Springer Water. Springer, Cham, pp 65–107. 10.1007/978-3-030-42254-7_3
- Li J, Lin F, Li K, Zheng F, Yan B, Che L, Tian W, Chen G, Yoshikawa K. A critical review on energy recovery and non-hazardous disposal of oily sludge from petroleum industry by pyrolysis. J Hazard Mater. 2021;406:124706. doi: 10.1016/j.jhazmat.2020.124706. [DOI] [PubMed] [Google Scholar]
- Mohsenpour R. Investigation of oil pollution on aquatic animals and methods of its prevention. J Aquac Marine Biol. 2020;9:160–165. doi: 10.15406/jamb.2020.09.00291. [DOI] [Google Scholar]
- Ossai IC, Ahmed A, Hassan A, Hamid FS. Remediation of soil and water contaminated with petroleum hydrocarbon: a review. Environ Technol Innov. 2020;17:100526. doi: 10.1016/j.eti.2019.100526. [DOI] [Google Scholar]
- Oyibo JN, Wegwu MO, Uwakwe AA, Osuoha JO. Analysis of total petroleum hydrocarbons, polycyclic aromatic hydrocarbons and risk assessment of heavy metals in some selected finfishes at Forcados Terminal, Delta State, Nigeria. Environ Nanotechnol, Monit Manag. 2018;9:128–135. doi: 10.1016/j.enmm.2017.11.002. [DOI] [Google Scholar]
- Parliament E. Directive 2010/63/EU of 22 September 2010 on the protection of animals used for scientific purposes. OJEU. 2010;L276:33–79. [Google Scholar]
- Poorbagher H, Hosseini SV, Hosseini SM, Aflaki F. Regenstein, J. M. Metal accumulation in Caspian sturgeons with different feeding niches, condition factor, body size and age. Microchem J. 2017;132:43–48. doi: 10.1016/j.microc.2017.01.003. [DOI] [Google Scholar]
- Pourkazemi M. Caspian Sea sturgeon conservation and fisheries: past present and future. J Appl Ichthyol. 2006;22:12–16. doi: 10.1111/j.1439-0426.2007.00923.x. [DOI] [Google Scholar]
- Ramazanova E, Bahetnur Y, Yessenbayeva K, Lee SH, Lee W. Spatiotemporal evaluation of water quality and risk assessment of heavy metals in the Northern Caspian Sea bounded by Kazakhstan. Mar Pollut Bull. 2022;181:113879. doi: 10.1016/j.marpolbul.2022.113879. [DOI] [PubMed] [Google Scholar]
- Readman JW, Fillmann G, Tolosa I, Bartocci J, Villeneuve JP, Catinni C, Mee LD. Petroleum and PAH contamination of the Black Sea. Mar Pollut Bull. 2002;44:48–62. doi: 10.1016/s0025-326x(01)00189-8. [DOI] [PubMed] [Google Scholar]
- Shahat A, Hassan HMA, El-Shahat MF, El Shahawy O, Awual MdR. Visual nickel(II) ions treatment in petroleum samples using a mesoporous composite adsorbent. Chem Eng J. 2018;334:957–967. doi: 10.1016/j.cej.2017.10.105. [DOI] [Google Scholar]
- Sheikhzadeh H, Hamidian AH. Bioaccumulation of heavy metals in fish species of Iran: a review. Environ Geochem Health. 2021;43:3749–3869. doi: 10.1007/s10653-021-00883-5. [DOI] [PubMed] [Google Scholar]
- Shirneshan G, Bakhtiari AR, Memariani M. Identifying the source of petroleum pollution in sediment cores of Southwest of the Caspian Sea using chemical fingerprinting of aliphatic and alicyclic hydrocarbons. Mar Pollut Bull. 2017;115:383–390. doi: 10.1016/j.marpolbul.2016.12.022. [DOI] [PubMed] [Google Scholar]
- Takeshita R, Bursian SJ, Colegrove KM, et al. A review of the toxicology of oil in vertebrates: what we have learned following the Deepwater Horizon oil spill. J Toxicol Environ Health, Part B. 2021;24:355–394. doi: 10.1080/10937404.2021.1975182. [DOI] [PubMed] [Google Scholar]
- Tasmagambetova AI, Tovassarov AD, Bihan-Poudec AC, Bissariyeva SS, Akberliyev AB. Assessment of the current state of the Caspian Sea and the Caspian Seal habitat analysis. Eurasian Chemico-Technol J. 2019;21:165. doi: 10.18321/ectj827. [DOI] [Google Scholar]
- Valencia D. Chemical bonding and aromaticity analyses of petroporphyrins with vanadium or nickel. Fuel. 2023;333:126344. doi: 10.1016/j.fuel.2022.126344. [DOI] [Google Scholar]
- Varjani SJ. Microbial degradation of petroleum hydrocarbons. Biores Technol. 2017;223:277–286. doi: 10.1016/j.biortech.2016.10.037. [DOI] [PubMed] [Google Scholar]
- Wang W, Batterman S, Chernyak S, Nriagu J. Concentrations and risks of organic and metal contaminants in Eurasian Caviar. Ecotoxicol Environ Saf. 2008;71:138–148. doi: 10.1016/j.ecoenv.2007.06.007. [DOI] [PubMed] [Google Scholar]
- Warrag SEE, Fetisov EO, van Osch DJGP, Harwood DB, Kroon MC, Siepmann JI, Peters CJ. Mercury capture from petroleum using deep eutectic solvents. Ind Eng Chem Res. 2018;57:9222–9230. doi: 10.1021/acs.iecr.8b00967. [DOI] [Google Scholar]
- Wilhelm SM, Bloom N. Mercury in petroleum. Fuel Process Technol. 2000;63:1–27. doi: 10.1016/s0378-3820(99)00068-5. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author.