Abstract
Despite evidence that live, attenuated simian immunodeficiency virus (SIV) vaccines can elicit potent protection against pathogenic SIV infection, detailed information on the replication kinetics of attenuated SIV in vivo is lacking. In this study, we measured SIV RNA in the plasma of 16 adult rhesus macaques immunized with a live, attenuated strain of SIV (SIVmac239Δnef). To evaluate the relationship between replication of the vaccine virus and the onset of protection, four animals per group were challenged with pathogenic SIVmac251 at either 5, 10, 15, or 25 weeks after immunization. SIVmac239Δnef replicated efficiently in the immunized macaques in the first few weeks after inoculation. SIV RNA was detected in the plasma of all animals by day 7 after inoculation, and peak levels of viremia (105 to 107 RNA copies/ml) occurred by 7 to 12 days. Following challenge, SIVmac251 was detected in all of the four animals challenged at 5 weeks, in two of four challenged at 10 weeks, in none of four challenged at 15 weeks, and one of four challenged at 25 weeks. One animal immunized with SIVmac239Δnef and challenged at 10 weeks had evidence of disease progression in the absence of detectable SIVmac251. Although complete protection was not achieved at 5 weeks, a transient reduction in viremia (approximately 100-fold) occurred in the immunized macaques early after challenge compared to the nonimmunized controls. Two weeks after challenge, SIV RNA was also reduced in the lymph nodes of all immunized macaques compared with control animals. Taken together, these results indicate that host responses capable of reducing the viral load in plasma and lymph nodes were induced as early as 5 weeks after immunization with SIVmac239Δnef, while more potent protection developed between 10 and 15 weeks. In further experiments, we found that resistance to SIVmac251 infection did not correlate with the presence of antibodies to SIV gp130 and p27 antigens and was achieved in the absence of significant neutralizing activity against the primary SIVmac251 challenge stock.
Immunization with live, attenuated strains of simian immunodeficiency virus (SIV) can induce protection against infection with virulent virus (2, 8, 10, 21, 22, 26, 31, 33). Despite these encouraging results, safety concerns persist over the possible use of live, attenuated HIV vaccines in humans (3). As a result, research efforts by several groups have focused on elucidating the underlying mechanisms associated with protective immunity in this model. Although several studies have evaluated the humoral (1, 6, 12, 22, 24, 26, 33) and cellular (13, 16) immune responses in monkeys immunized with live, attenuated SIV, the correlates of protective immunity remain unclear.
Initial studies suggested that maturation of the protective response took a prolonged period of time to develop, raising questions as to the nature of the induced immunity (8, 33). While immune responses to SIV develop within a few weeks following infection with pathogenic strains (28, 34), protection was achieved only after 35 weeks following immunization of macaques with an attenuated macrophagetropic virus (SIV17E-Cl) (8) and 79 weeks after immunization with a triple-deletion mutant (SIVΔ3) (33). In several studies, inoculation of macaques with highly attenuated strains of SIV, which were unable to establish persistent infection in the host, failed to confer significant protection against challenges by pathogenic viruses (11, 21). Taken together, these results suggest that the degree of attenuation of the vaccine strain and its ability to replicate in vivo are critical determinants of the protective effect.
Because sensitive, quantitative methods to measure SIV in plasma have only recently been developed, detailed information on the replication kinetics of live, attenuated SIV in macaques is limited (12). To address this issue, we examined the replication of an attenuated strain of SIV (SIVmac239Δnef) in rhesus macaques by measuring plasma viremia via a quantitative branched DNA (bDNA) assay (9). Plasma viral load was measured frequently following inoculation with SIVmac239Δnef and again after challenge with uncloned SIVmac251. To determine the temporal relationship between replication of the vaccine virus and the onset of protection, animals infected with SIVmac239Δnef were challenged with SIVmac251 at either 5, 10, 15, or 25 weeks after immunization. Data on viral load in the plasma and lymph nodes, as well as on the induction of anti-SIV antibody responses, were then compared with outcome following challenge.
MATERIALS AND METHODS
Macaques.
Twenty adult, female rhesus macaques (Macaca mulatta, 6 to 8 kg) were used in this study. None of the animals had prior exposure to SIV or type D retrovirus, and all were negative for SIV and type D retrovirus antibodies before the start of the experiments. Animal 1490 was also negative for type D retrovirus by PCR. All protocols used in this study were reviewed and approved by an institutional animal care and use committee.
Vaccine and challenge viruses.
Viral stocks of SIVmac239Δnef and SIVmac251 were kindly provided by Ronald Desrosiers (New England Regional Primate Research Center, Harvard Medical School, Southborough, Mass.). Both stocks were grown in rhesus peripheral blood mononuclear cells (PBMC) and titered on CEM×174 cells (19). The SIVmac239Δnef stock had a titer of 4 × 103 50% tissue culture infective doses (TCID50)/ml; the SIVmac251 challenge stock had a titer of 5 × 103 TCID50/ml. The SIVmac251 challenge stock was also titered in macaques and contained 104.5 animal infective doses per ml. Both stocks were negative for foamy virus and type D retrovirus.
Inoculation of vaccine and challenge viruses.
Sixteen rhesus macaques were infected with SIVmac239Δnef by intravenous inoculation of the virus stock (2 × 104 TCID50) on day 0. Four additional macaques served as nonimmunized controls. The immunized and control animals were subsequently challenged by intravenous inoculation of 10 animal infective doses of SIVmac251 in groups of five (four immunized and one control) at either 5, 10, 15, or 25 weeks after immunization with SIVmac239Δnef.
SIV RNA in plasma and lymph nodes.
Blood samples collected in EDTA anticoagulant were used to determine SIV RNA levels in the plasma, using a bDNA signal amplification assay as previously described (9). Briefly, viral particles in 1 ml of plasma were pelleted by centrifugation (23,500 × g for 60 min at 4°C) and detected by using probes that hybridize within the pol region of SIVmac. SIV RNA was quantified by comparison to a standard curve produced by serial dilutions of cell-free SIV-infected cell culture supernatants. The lower quantification limit of this assay is 10,000 SIV RNA copies per ml. To quantify SIV RNA in lymph nodes, quadruplicate samples of peripheral lymph node biopsies were taken. Each sample was frozen at −80°C until processing. Samples were weighed, and the DNA and RNA were extracted in guanidine HCl as previously described (14). SIV RNA extracted from lymphoid tissue specimens was quantified by using the bDNA assay, and the data were expressed as RNA copies per 10 mg of tissue. Individual values for each sample, as well as the geometric mean of the quadruplicate samples, are presented. DNA from lymphoid tissues was further purified (United States Biochemical, Cleveland, Ohio) and used in PCR assays to discriminate between the vaccine and challenge strains as described below.
PBMC isolation and DNA PCR.
PBMC were isolated from whole blood by layering over lymphocyte separation medium (Organon Teknika, Durham, N.C.) followed by standard density gradient centrifugation. Genomic DNA was extracted from 106 cells, and sequences spanning the deleted region of nef were amplified by nested PCR. DNA (1 μg) was added to a PCR mixture containing 50 mM KCl, 10 mM Tris HCl, 0.1% Triton X-100, 2 mM deoxynucleoside triphosphate, 25 mM MgCl2, 2.5 U of Taq DNA polymerase (Promega, Madison, Wis.), and 100 ng each of the outer primers A (5′-CCTACCTACAATATGGGTGGAGC; SIVmac239, nucleotides [nt] 9065 to 9087) (10, 27) and B (5′-CCTCTGACAGGCCTGACTTGCTTCC; nt 9776 to 9800) (10, 27) in a final volume of 50 μl. Amplification was performed for 35 cycles (94°C, 1 min; 60°C, 30 s; 72°C, 45 s), after which 5 μl of the first-round product was transferred to a new reaction mixture containing the inner primers C (5′-CCGTCTGGAGATCTGCGACAGAGACT; nt 9110 to 9135) (27) and D (5′-GGTATCTAACATATGCCTCATAAG; nt 9741 to 9764) (27). Amplification was carried out for an additional 35 cycles under the cycling conditions specified above. PCR products were separated on 1% agarose gels and visualized by ethidium bromide staining. Under these conditions, amplification of SIVmac239Δnef yields an expected product of 472 bp, and amplification of SIVmac251 yields a product of 654 bp. To confirm detection of SIVmac251, PCR was carried with primers A and B, after which 5 μl of the amplified product was transferred to a new reaction mixture containing primer E (5′-GAAACCCAGCTGAAGAGAGAG; nt 9288 to 9310) (27) paired with primer D. Primer E hybridizes within the 182-bp deleted region of SIVmac239Δnef and therefore recognizes only wild-type nef alleles. Protection was defined as the failure to detect the challenge virus by nested PCR under these conditions.
Flow cytometry.
Whole blood collected in EDTA was analyzed for CD4 and CD8 T-cell subsets, using antibodies to CD3 (Biosource International, Camarillo, Calif.) and to CD4 and CD8 (anti-Leu-3a and anti-Leu2a, respectively; Becton Dickinson, San Jose, Calif.). Antibodies (10 μl of each) were added to 50 μl of whole blood and allowed to sit for 15 min at room temperature, after which erythrocytes were lysed by the addition of 450 μl of lysing buffer. Samples were analyzed on a FACS Calibur flow cytometer (Becton Dickinson), and the CD4 and CD8 cell counts per microliter were enumerated relative to a standard count of beads used for calibration.
Detection of antibodies to SIV Env and Gag proteins by enzyme-linked immunosorbent-assay (ELISA).
To detect antibodies to the SIV envelope glycoproteins, a sheep polyclonal antibody to the C terminus of SIV gp130 (D7369; Aalto BioReagents, Dublin, Ireland) was coated onto Immulon II plates (Dynatech, Ltd.) at 5 μg/ml in 0.1 NaHCO3 (pH 8.6). To detect SIV p27, a p27–glutathione S-transferase fusion protein (a gift from Ian Jones, Medical Research Council, London, England) was coated at approximately 5 μg/ml. The plates were then washed with Tris-buffered saline (TBS) and blocked with 2% nonfat milk in TBS for 30 min, after which recombinant gp130 (a gift from James Arthos, National Institutes of Health) was added at 100 ng/ml to the D7369-coated wells. Simian plasma, precleared for platelets, was titrated threefold (starting at 1:100) in TBS–2% milk–20% sheep serum and added to plates precoated with either gp130 or p27 for 1 h at room temperature. After two further TBS washes, goat anti-human immunoglobulin G-alkaline phosphatase conjugate (Accurate Chemicals, Westbury, N.Y.) was added to wells at 1:10,000 in TBS–2% milk–20% sheep serum. After 30 min at room temperature, the plates were washed and developed by using the AMPAK amplification system (Dako, Carpenteria, Calif.), and the A490 was determined. Midpoint titers were calculated and are presented.
Neutralization assays.
Neutralizing antibodies were assessed in a CEMx174 cell-killing assay as described previously (24). Briefly, cell-free virus (50 μl containing 0.5 to 1 ng of p27) was added to multiple dilutions of test plasmas in 100 μl of growth medium in triplicate wells of 96-well microtiter plates and incubated at 37°C for 1 h. Twenty-five microliters was then transferred to a separate plate that contained 100,000 CEMx174 cells in 100 μl of growth medium/well and incubated until syncytium formation and nearly complete cell killing first became apparent by light microscopic examination (usually 6 days). Cell densities were reduced and medium was replaced after 3 days of incubation. Viable cells were quantified by staining with Finter’s neutral red in poly(l-lysine)-coated plates. Percent protection from virus-induced cell killing was calculated by taking the difference in A540 values between test wells (cells, plasma sample, and virus) and virus control wells (cells and virus) and dividing this result by the difference in absorption between cell control wells (cells only) and virus control wells. Neutralization titers are given as the reciprocal of the plasma dilution required to protect 50% of cells from virus-induced killing. T-cell line-adapted (TCLA) SIVmac251 had been passaged repeatedly in H9 cells and was expanded in H9 cells for use in neutralization assays. Assay stocks of primary SIVmac251 and molecularly cloned SIVmac239/nef-open were derived by a single expansion in rhesus PBMC of virus taken directly from vials of animal challenge stocks (24). The animal challenge stocks were of a low passage number in rhesus PBMC exclusively (19). All viruses were provided by Ronald Desrosiers.
RESULTS
Replication of SIVmac239Δnef following inoculation.
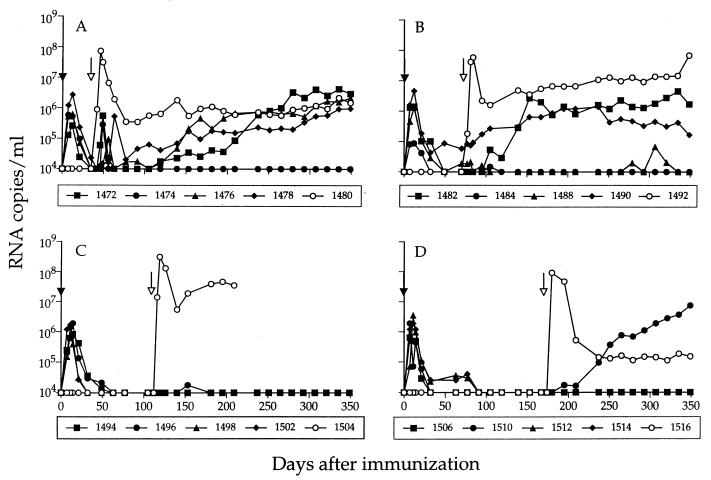
To assess virus replication following inoculation of rhesus macaques with SIVmac239Δnef, plasma samples were obtained at frequent intervals and the levels of SIV RNA were determined by using a quantitative bDNA assay (9). SIV RNA was detected in the plasma of all immunized animals on day 7 after inoculation (Fig. 1), and peak viremia (105 to 107 RNA copies/ml) occurred within 7 to 12 days. SIV RNA was rapidly cleared to undetectable levels by 5 to 7 weeks in all animals, with the exception of one (animal 1490) that maintained detectable plasma viremia throughout the entire study. DNA PCR performed on PBMC samples from the immunized animals confirmed that the replicating virus was SIVmac239Δnef, with no evidence that repair or reversion of the original nef deletion had occurred (Table 1).
FIG. 1.
SIV RNA in plasma following immunization with SIVmac239Δnef and challenge with SIVmac251. Viremia was determined by measuring total SIV RNA in plasma in a bDNA assay (sensitivity of 104 RNA copies/ml). Immunization with SIVmac239Δnef ( ) was performed on day 0. The animals were then challenged with SIVmac251 ( ) at either 5 (A), 10 (B), 15 (C), or 25 (D) weeks. Levels of SIV RNA for the nonimmunized control animal in each challenge group are designated by open symbols.
TABLE 1.
Detection of SIVmac239Δnef and SIVmac251 by DNA PCR
| Challenge group | Detection by DNA
PCRa at indicated days after immunization
|
||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 7 | 49 | 56 | 63 | 77 | 84 | 105 | 112 | 119 | 126 | 139 | 174 | 180 | 195 | 209 | 279 | 293 | |
| 5 week (35b) | |||||||||||||||||
| 1472 | Δnef | Δnef/251 | Δnef/251 | Δnef/251 | 251 | Δnef/251 | Δnef/251 | Δnef/251 | |||||||||
| 1474 | Δnef | Δnef | Δnef/251 | Δnef | Δnef | Δnef | Δnef | Δnef | |||||||||
| 1476 | Δnef | Δnef | Δnef/251 | Δnef | Δnef | Δnef/251 | Δnef/251 | Δnef/251 | |||||||||
| 1478 | Δnef | Δnef | Δnef/251 | Δnef/251 | Δnef | Δnef/251 | Δnef/251 | Δnef/251 | |||||||||
| 1480d | Neg | 251 | 251 | 251 | 251 | 251 | 251 | 251 | |||||||||
| 10 week (70) | |||||||||||||||||
| 1482 | Δnef | Δnef | Δnef | Δnef | Δnef/251 | 251 | 251 | ||||||||||
| 1484 | Δnef | Δnef | Δnef | Δnef | Neg | Δnef/251 | Δnef | ||||||||||
| 1488 | Δnef | Δnef | Δnef | Neg | Neg | Δnef | Neg | ||||||||||
| 1490 | Δnef | Δnef | Δnef | Δnef | Δnef | Neg | Δnef | ||||||||||
| 1492d | Neg | Neg | 251 | 251 | 251 | 251 | 251 | ||||||||||
| 15 week (105) | |||||||||||||||||
| 1494 | Δnef | Δnef | Neg | Δnef | Δnef | Neg | Δnef | ||||||||||
| 1496 | Δnef | Δnef | Δnef | Δnef | Neg | Δnef | Δnef | ||||||||||
| 1498 | Δnef | Neg | Δnef | Δnef | Δnef | Neg | Neg | ||||||||||
| 1502 | Δnef | Δnef | Δnef | Δnef | Δnef | Δnef | Neg | ||||||||||
| 1504d | Neg | 251 | 251 | 251 | 251 | 251 | Deadc | ||||||||||
| 25 week (167) | |||||||||||||||||
| 1506 | Δnef | Neg | Neg | Δnef | Neg | Δnef | |||||||||||
| 1510 | Δnef | Δnef | Neg | Δnef | Neg | 251 | |||||||||||
| 1512 | Δnef | Δnef | Neg | Neg | Δnef | Δnef | |||||||||||
| 1514 | Δnef | Neg | Δnef | Δnef | Δnef | Neg | |||||||||||
| 1516d | Neg | Neg | 251 | 251 | 251 | 251 | |||||||||||
DNA PCR was performed on PBMC samples by using nested primers that span the deleted region in nef as described in Materials and Methods. Neg, no virus detected.
Day of challenge.
Animal 1504 was euthanized 190 days after challenge with SIVmac251 due to poor health. Prominent findings at necropsy included severe glomerulonephritis and hepatic amyloidosis.
Nonimmunized control.
To evaluate the temporal onset of protection, the immunized animals were challenged in groups of five (four immunized and one nonimmunized control) with uncloned SIVmac251 at either 5, 10, 15, or 25 weeks after inoculation of SIVmac239Δnef. DNA PCR was performed on PBMC samples, using two sets of primers to discriminate between the vaccine and challenge viruses. Shortly after challenge, all of the four immunized animals in group A experienced a transient increase in plasma viremia (Fig. 1A), which coincided with detection of SIVmac251 DNA in PBMC (Table 1). Despite evidence that these animals were not protected from challenge, the level of viremia was approximately 100-fold lower than in the nonimmunized control animal (animal 1480), suggesting that virus-suppressive mechanisms may act as early as 5 weeks after immunization. Suppression of virus replication was transient, however, and the levels of plasma SIV RNA subsequently increased in three of four macaques in this group, reaching levels comparable to that of the nonimmunized control by 45 weeks after challenge (Fig. 1A).
SIVmac251 DNA was detected in two of four immunized animals (1482 and 1484) challenged at 10 weeks (Table 1), coincident with a marked increase in plasma viremia in animal 1482 (Fig. 1B). Increasing plasma viremia was also detected in another animal (1490) from this challenge group. However, nested DNA PCR performed on PBMC and lymph node samples from this animal failed to detect the challenge virus, suggesting that plasma viremia was due to replication of the vaccine strain (see below). In the 5- and 10-week challenge groups, SIVmac251 was detected by DNA PCR at only a single time point for 2 animals (1474 and 1484), and the level of plasma viremia has remained below the limit of detection for more than a year after challenge in both animals, suggesting they were able to control replication of the challenge virus. Taken together, these results indicate that protective responses induced by 5 to 10 weeks act to initially control virus replication but provide long-term suppression in only some animals. In contrast, plasma viremia remained undetectable in all of the four immunized animals challenged at 15 weeks (Fig. 1C) and in three of four challenged at 25 weeks (Fig. 1D), suggesting that more potent protection develops between 10 and 15 weeks after immunization. DNA PCR performed on PBMC samples from the immunized animals in these latter groups detected only SIVmac239Δnef (Table 1), with the exception of the one unprotected animal (1510) in the 25-week challenge group, in which SIVmac251 was first detected on day 70 after challenge.
Viral load in lymph nodes.
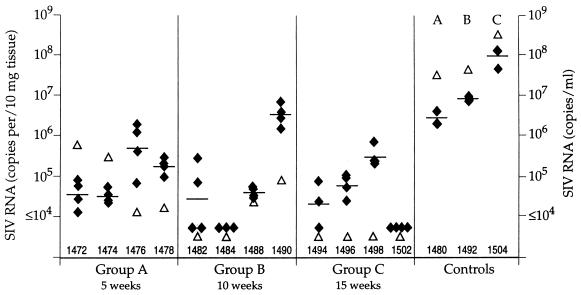
Based on our initial findings, which indicated that immunization with SIVmac239Δnef could reduce the postchallenge viral load in the peripheral blood, we evaluated the viral burden in lymphoid tissues. Lymph node biopsies were performed 2 weeks after challenge, and the samples were used to quantify total SIV RNA by bDNA assay. Because this assay uses probes that hybridize within the SIV pol gene, it detects both SIVmac239Δnef and SIVmac251. Quadruplicate samples were assayed from each lymph node, and the results were expressed as RNA copies/10 mg of tissue. The levels of SIV RNA found in the lymph nodes ranged from 104 to 106 RNA copies/10 mg in the immunized animals (with the exception of animal 1490, in which the viral load approached 107 RNA copies/10 mg) (Fig. 2). Comparison with the nonimmunized controls showed approximately 100-fold-higher levels of SIV RNA in the lymph nodes of the control animals (106 to 108 RNA copies/10 mg) than in those of animals immunized with SIVmac239Δnef. However, it is important to note that this assay detects total SIV RNA in lymph nodes, and a large proportion may represent trapped virions. Overall, these results indicate that immunization with SIVmac239Δnef can reduce the postchallenge viral load in lymph nodes and that this reflects similar changes seen in the blood.
FIG. 2.
Comparison of SIV RNA in the plasma (▵) and lymph nodes (⧫) 2 weeks after challenge with SIVmac251. Lymph node biopsy samples were collected, and the tissues were divided into quadruplicate samples. SIV copy number was determined for each sample by bDNA assay, and the data were normalized per 10 mg of tissue. Individual data points, as well as the geometric mean, are presented for the quadruplicate samples. Paired plasma samples were analyzed for SIV RNA, and the data are expressed as SIV copies per ml. Data for controls depict the results obtained for the nonimmunized animals in each challenge group.
Calculation of total viral load following immunization.
To assess whether the level of SIVmac239Δnef replication is a determinant of protection, we calculated the area under the curve of virus load in plasma during the period prior to challenge (Table 2). For those time points when the virus load was below the limit of detection, the actual value of the detection limit of the bDNA assay was used (104 RNA copies/ml). While this leads to a slight overestimate, the contribution of these data points to the integral is negligible, even for the 25-week challenge group, where the virus load is below the limit of detection for about 100 days prior to challenge (Fig. 1D). Following infection with SIVmac239Δnef, the mean area under the curve was approximately 1.2 × 107 virions for the 16 immunized macaques (Table 2). Comparison of the different challenge groups revealed no significant difference between the groups, with mean values ranging from 9.2 × 106 to 1.5 × 107 virions (Table 2). To estimate the total production of virions in the blood, the area under the curve was multiplied by the clearance rate of free virus and the volume of blood. Assuming an extracellular fluid volume of 0.5 liters (based on a mean body weight of 7.68 kg) and a free virus half-life of 20 min for nef-deleted SIV (35), the mean total production of virus in the blood prior to challenge was approximately 3.0 × 1011 virions, suggesting that SIVmac239Δnef replicates quite extensively in vivo. Despite these findings, the level of virus replication was not significantly different between the different challenge groups (Table 2), implying that total exposure to virions cannot be the sole determinant for protection. Rather, our findings support the idea that the duration of exposure to the vaccine virus is a critical factor for the development of protection.
TABLE 2.
Viral load prior to challenge
| Determination | No. of virions (area under the
curve) on indicated challenge group (n = 4)
|
|||
|---|---|---|---|---|
| 5 week | 10 week | 15 week | 25 week | |
| 1 | 3.0 × 106 | 2.0 × 107 | 1.3 × 107 | 9.5 × 106 |
| 2 | 8.3 × 106 | 1.8 × 106 | 1.8 × 107 | 1.5 × 107 |
| 3 | 8.4 × 106 | 1.3 × 107 | 6.6 × 106 | 2.1 × 107 |
| 4 | 1.7 × 107 | 2.6 × 107 | 8.0 × 106 | 1.1 × 107 |
| Mean | (9.2 ± 5.8) × 106 | (1.5 ± 1.0) × 107 | (1.2 ± 0.7) × 107 | (1.5 ± 0.5) × 107 |
| Mean (n = 16) | (1.2 ± 0.7) × 107 | |||
Detection of binding antibodies to SIV Env (gp130) and Gag (p27) proteins.
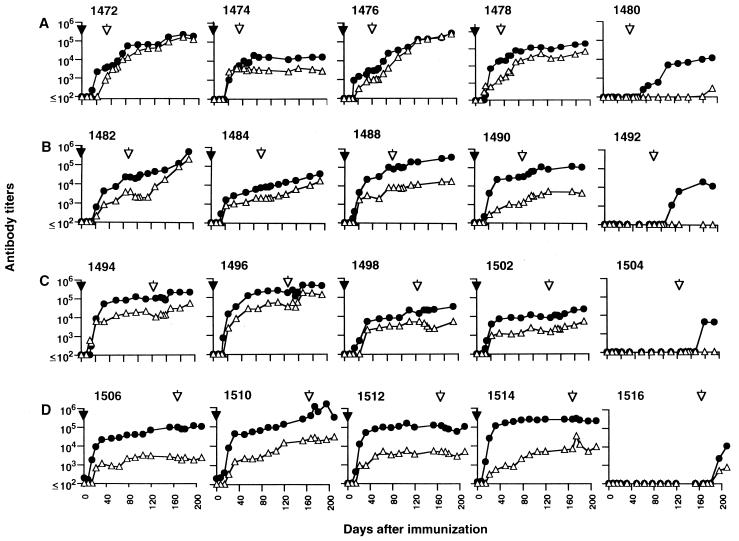
To address possible immune correlates of protection, we evaluated the temporal development of anti-SIV antibodies, both before and after challenge with SIVmac251. Antibodies to SIV gp130 and p27 increased rapidly in the first 2 to 3 weeks in response to immunization with SIVmac239Δnef and in association with a decline in plasma viremia (Fig. 3). All of the animals responded to immunization, although some variation in the magnitude of the response was observed. In some cases this variation was associated with the extent of SIVmac239Δnef replication in vivo, such that those animals with higher initial viremia (e.g., 1490) had more rapid development of antibodies to SIV gp130 compared to those with lower peak viremia (e.g., 1484). A similar association was recently reported for rhesus macaques infected with highly attenuated mutants of SIV (12). However, this phenomenon was not observed for all the animals in our study; several (e.g., 1502 and 1514) had similar levels of plasma viremia following immunization with SIVmac239Δnef but different anti-gp130 antibody responses, suggesting that host factors may also affect the kinetics of anti-SIV antibody development. As expected, specific antibodies were not detected prior to challenge in the nonimmunized controls. Following challenge, there was evidence of an anamnestic response to both Env and Gag in a subset of the animals (notably 1472 and 1476) which was associated with detection of the challenge virus in PBMC and an increase in plasma viremia. However, comparison of the anti-gp130 and anti-p27 antibody titers on the day of challenge did not reveal any obvious correlation with outcome (Fig. 4), and there was no evidence to suggest that unprotected animals were among those with lower antibody responses.
FIG. 3.
Antibody responses to SIV gp130 (•) and p27 (▵) measured by ELISA following immunization with SIVmac239Δnef ( ) and challenge with SIVmac251 ( ) of animals in 5-week (A), 10-week (B), 15-week (C), and 25-week (D) challenge groups. Data for nonimmunized control animals (1480, 1492, 1504, and 1516) are shown on the far right.
FIG. 4.
Antibody titers to SIV gp130 and p27 measured by ELISA on the day of challenge for each of the 16 macaques immunized with SIVmac239Δnef. The results are presented based on the outcome following challenge, irrespective of the challenge group (unprotected [n = 7] or protected [n = 9]).
Neutralizing antibody responses to primary and TCLA SIVmac251.
It is not yet clear whether protection conferred by SIVmac239Δnef correlates with the presence of SIV-neutralizing antibodies. Such a correlation was found in one study (33) but has not been supported by others (1, 22, 24, 26, 30). In part, these discrepancies may result from the use of either TCLA-adapted or primary SIV stocks, which have different sensitivities to antibody neutralization (23). To address this question, we tested plasma from animals immunized with SIVmac239Δnef for neutralization activity against both a TCLA stock of SIVmac251 and the primary SIVmac251 stock used for challenge. Our results show that antibodies capable of neutralizing the TCLA virus arise within the first few weeks following immunization (Table 3). In general, the increase in neutralization titer paralleled the replication kinetics of the vaccine virus, such that plasma from animals with higher viremia tended to have higher titers of neutralizing activity. However, neutralizing titers to TCLA SIVmac251 on the day of challenge were not associated with the ability to resist infection by the challenge virus. We next evaluated neutralizing activity against the primary SIVmac251 challenge stock. Little or no neutralizing activity was detected against the primary virus in any of the animals over the entire course of observation (over 25 weeks). Moreover, at 15 weeks, when neutralizing activity to the challenge virus was low or absent, all of the four animals resisted SIVmac251 infection, showing that neutralizing antibodies are not associated with protection in this model.
TABLE 3.
Neutralizing antibody responses on the day of challenge
| Challenge group | Neutralizing antibody
responsea
|
Protection? | ||
|---|---|---|---|---|
| TCLA SIVmac251 | Primary SIVmac251 | Cloned SIVmac239 | ||
| 5 week | ||||
| 1472 | 231 | 15 | <4 | No |
| 1474 | 304 | 9 | <4 | No |
| 1476 | 1,093 | <4 | <4 | No |
| 1478 | 1,014 | 9 | <4 | No |
| 1480b | <20 | <4 | <4 | No |
| 10 week | ||||
| 1482 | 1,499 | 22 | 9 | No |
| 1484 | 409 | 29 | 10 | No |
| 1488 | 2,613 | 8 | 7 | Yes |
| 1490 | 1,549 | <4 | 10 | Yes |
| 1492b | <20 | <4 | <4 | No |
| 15 week | ||||
| 1494 | 3,288 | 22 | 26 | Yes |
| 1496 | 3,904 | 25 | 10 | Yes |
| 1498 | 898 | <4 | <4 | Yes |
| 1502 | 350 | <4 | <4 | Yes |
| 1504b | <20 | <4 | <4 | No |
| 25 week | ||||
| 1506 | 774 | 9 | 16 | Yes |
| 1510 | 3,625 | <4 | 29 | No |
| 1512 | 2,747 | <4 | 20 | Yes |
| 1514 | 9,039 | 74 | 23 | Yes |
| 1516b | <20 | <4 | <4 | No |
Reciprocal serum dilution to yield a 50% reduction in virus-induced cell killing in CEMx174 cells.
Nonimmunized control animal.
Disease progression associated with increased replication of SIVmac239Δnef.
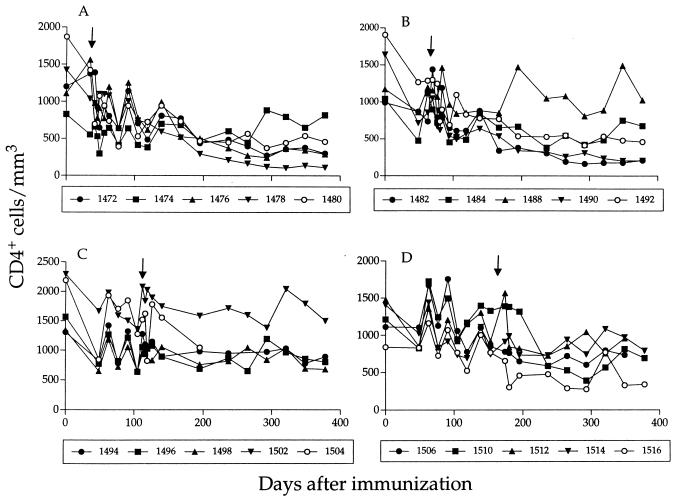
For the majority of unprotected animals, a dramatic increase in the levels of SIV in plasma and a decline in the number of circulating CD4+ T cells (Fig. 5) was associated with consistent detection of SIVmac251 by DNA PCR (Table 1). The one notable exception was animal 1490 (10-week challenge group), in which a high viral load and declining CD4+ T cells occurred in the absence of detectable SIVmac251. Following immunization with SIVmac239Δnef, the level of plasma viremia in this animal reached 5 × 106 RNA copies/ml, the highest for 16 immunized macaques (Fig. 1). Viremia subsequently declined by almost 2 logs; however, SIV RNA was readily detectable in plasma at all time points, in contrast to all other animals, in which viremia consistently dropped below the limit of detection. Following challenge with SIVmac251, the level of SIV RNA in plasma continued to gradually increase, reaching a peak of approximately 106 RNA copies/ml, after which it declined slightly (Fig. 1). DNA PCR performed on multiple, sequential PBMC samples failed to detect wild-type nef but rather identified several shorter PCR products (Fig. 6). One of these products corresponded in size to the expected band for SIVmac239Δnef and was the predominant band seen in samples obtained early after immunization (Fig. 6A). However, multiple shorter products were identified in later PBMC samples obtained several weeks after immunization, suggesting that additional deletions in nef had occurred. A similar, shorter PCR product was also identified in later samples derived from sequential plasma cocultures from animal 1490 (Fig. 6B). Amplification and sequencing of the nef gene from later PBMC samples confirmed the presence of the original 182-bp nef deletion and also identified a second 112-bp deletion in the upstream U3 region of the 3′ long terminal repeat (data not shown), similar to that described earlier by Kirchoff et al. (17). In contrast to the results of Kirchoff et al., which suggested that additional deletions in U3 develop only after a prolonged period of infection, we found evidence of additional nef deletions early after infection with SIVmac239Δnef, and by 12 weeks the multiply deleted form had become the major variant detected by PCR.
FIG. 5.
CD4+ T-cell counts for immunized and control animals in the 5-week (A), 10-week (B), 15-week (C), and 25-week (D) challenge groups. The results for nonimmunized control animals are depicted by open symbols. , day of challenge with SIVmac251.
FIG. 6.
DNA PCR analysis of sequential PBMC and plasma samples from animal 1490. Sequences in nef were amplified by nested DNA PCR using primers that span the deleted region in SIVmac239Δnef as described in Materials and Methods. (A) Sequential PBMC samples obtained before and after challenge with SIVmac251 (70*, day of challenge); (B) plasma samples from sequential time points following coculture with CEMx174 cells. M, molecular size markers; (+), amplification of nef sequences from rhesus PBMC used to propagate the SIVmac239Δnef immunization stock; (−), no DNA control.
DISCUSSION
In this study, we examined in detail the replication of a live, attenuated strain of SIV (SIVmac239Δnef) in rhesus macaques and determined the temporal relationship between replication of the attenuated virus and the onset of protection against challenge with pathogenic SIVmac251. Our results indicate that protective responses capable of reducing the postchallenge viral load in both plasma and lymph nodes are induced as early as 5 weeks after immunization with SIVmac239Δnef, while more potent protection develops between 10 and 15 weeks. Antibodies directed against SIV Env and Gag proteins could be detected within the first few weeks after immunization; however, there was no apparent relationship between antibody titer and subsequent protection from challenge. Moreover, protection was achieved in the absence of detectable antibodies capable of neutralizing the primary SIVmac251 challenge virus in vitro, raising questions as to the significance of neutralizing antibody responses in this model. Overall, our results suggest that protective responses can develop much more rapidly than was previously observed with more highly attenuated SIV strains (8, 33) and support the idea that the ability of live, attenuated vaccines to elicit protection is closely linked to their replicative capacity in vivo.
Based on earlier reports, increased attenuation of SIV as a result of successive deletion of virus auxiliary genes (12), or through recombination of SIV env genes (8, 21), results in a decrease in replicative capacity in vivo and extends the period required for protective antiviral immunity to develop. We found that SIVmac239Δnef, which is attenuated as a result of a large 182-bp deletion in the nef gene (10), replicates efficiently in rhesus macaques in the first few weeks following inoculation. However, we observed considerable variation in the levels of SIVmac239Δnef replication among the immunized animals (notably 1484 and 1490), with almost a 2-log range in peak values between different animals. Similar differences in viral replication patterns have been reported for macaques infected with pathogenic SIV (20), and these differences have been correlated with in vitro permissiveness of host cells to infection (20). Since all of the animals in our study were inoculated with the same virus under similar conditions, our results imply that inherent host factors can also have a considerable impact on the ability of live, attenuated SIV vaccines to replicate in vivo. Given that the replicative ability of the vaccine virus can influence the development of antiviral immunity (8, 12, 21, 33), it is possible that host factors which restrict replication of a live, attenuated vaccine in some individuals may prolong the period required for protective immunity to develop, while in others, replication of the same attenuated strain may be enhanced, leading to pathogenicity. Anecdotally, the one animal in our study with the highest level of viremia during acute infection (1490) failed to control replication of the vaccine virus, resulting in an increase in viral load and a sustained drop in CD4+ T cells.
In all but one animal (1490), SIVmac239Δnef was rapidly reduced to undetectable levels within 5 to 7 weeks. In assessing possible correlates of protection, we found that the decline in viremia was associated with an increase in the titer of antibodies to SIV Env and Gag proteins. Binding antibody titers increased rapidly in the first few weeks following immunization and were sustained at high levels for many months. It was notable that animals immunized with SIVmac239Δnef all developed anti-p27 antibody responses with kinetics similar to those against gp130. Previous studies have shown that sustained antibody responses to HIV-1 Gag proteins correlate with a favorable clinical outcome, while the failure to generate anti-Gag antibodies, or the loss of this response, is an early indicator of disease progression (4, 7, 15, 32, 36). Our results indicate that anti-p27 antibody titers per se do not correlate with protection in animals immunized with SIVmac239Δnef. However, the lack of strong anti-Gag antibody responses in the nonimmunized control animals following SIVmac251 challenge suggests early immune dysfunction that was not evident in animals infected with SIVmac239Δnef.
The magnitude and duration of anti-SIV antibody responses in the immunized animals is consistent with ongoing replication of SIVmac239Δnef or, alternatively, continuous antigenic stimulation as a result of virions trapped on follicular dendritic cells in lymphoid tissues (6). While our results cannot discriminate between these two possibilities, we were able to detect SIVmac239Δnef in PBMC many months after immunization, and abundant SIV RNA was found in the lymph nodes of animals that were protected from SIVmac251 challenge. DNA PCR performed on lymph node samples from the immunized animals revealed the presence of SIVmac239Δnef (data not shown), indicating persistence of the vaccine virus in both blood and tissues.
Despite high titers of antibodies against SIV envelope glycoproteins, plasma from the immunized animals failed to neutralize the primary SIVmac251 stock used for challenge, although some neutralizing activity was measured against a TCLA stock. These results are consistent with earlier studies of human immunodeficiency virus type 1 (HIV-1) (25) and confirm observed differences in the neutralization sensitivity of primary and TCLA SIV strains (23). This may also explain discrepancies in the literature with regard to the role of neutralizing antibodies in mediating protection by live, attenuated SIV vaccines (2, 22, 26, 30, 33). Because very few epitopes on primary HIV-1 envelopes are accessible to antibodies, and the immunogenicity of the mature oligomer on virions is generally low (reviewed in reference 5), it is perhaps not surprising that antisera from the immunized macaques failed to neutralize the primary SIVmac251 challenge virus in vitro. In vivo, passive transfer of immune sera from protected macaques to recipient animals has yielded mixed results; protection was conferred in some studies (8) but not others (1). Again variation in the virus strain used for challenge, the relative neutralization sensitivity of the challenge strain, and the source of cells used for virus propagation may all contribute to the observed differences in outcome.
Recent reports have shown that vigorous cytotoxic T-lymphocyte (CTL) activity is present as early as 14 days after infection with SIVmac239Δnef, coincident with a drop in the viral load (16). Similar observations have been made in macaques infected with pathogenic SIVmac251 (34) and in humans infected with HIV-1 (18), providing strong evidence that CTL play a key role in reducing the viral load during acute infection. It is not yet clear whether CTL also contribute to the protective responses observed with live, attenuated SIV vaccines. Assessment of the contribution of CD8+ cells, either through in vitro measurement of CTL activity (13, 16) or through severe depletion by CD8-specific monoclonal antibodies in vivo (29), has not yielded conclusive results. Given that CTL arise rapidly during primary infection, one would expect to see protection developing coincident with a decline in viremia. Although we observed a reduction in postchallenge viremia as early as 5 weeks following immunization with SIVmac239Δnef, more potent protection did not develop until 10 to 15 weeks later, well after viremia was reduced to undetectable levels. It is possible that SIV-specific CTL affect the initial reduction in viremia through the clearance of infected cells, while resistance to infection with the challenge virus depends on a broadening or maturation of the cellular response over time.
Our results indicate that suppression of pathogenic SIV infection can occur rapidly after immunization with SIVmac239Δnef and that this is associated with significant replication of the vaccine virus in vivo. Estimates of the total virus load of SIVmac239Δnef in plasma prior to challenge indicate that approximately 3.0 × 1011 virions are produced during the immunization period. However, comparison of the different challenge groups revealed no significant difference in total viral load between the groups, indicating that total exposure to virions (or viral antigens) cannot be the sole determinant for protection. Rather, our results support the idea that the duration of time between immunization and challenge is critical for protective immunity to develop and that this may be dependent on continuous replication of the attenuated strain. It is not yet known whether replication of the vaccine virus must be sustained in order to maintain protective immunity, or whether the initial antigenic stimulation achieved in the first 10 to 15 weeks following immunization is sufficient to impart long-lasting immunity. This will be an important question to address in future studies and will provide information this is directly relevant to the design of alternative vaccine strategies.
ACKNOWLEDGMENTS
We thank J. Booth, B. Clas, M. Bilska, C. Wingfield, and J. Keeperman for technical assistance, and we thank W. Chen for preparation of the figures.
This work was supported by NIH grants (AI42454, AI41373, and AI35166) and the Aaron Diamond Foundation.
REFERENCES
- 1.Almond N, Cocoran T, Hull R, Walker B, Rose J, Sangster R, Silvera K, Silvera P, Cranage M, Rud E, Stott E J. Mechanisms of protection induced by attenuated simian immunodeficiency virus. I. Protection cannot be transferred with immune serum. J Gen Virol. 1997;78:1919–1922. doi: 10.1099/0022-1317-78-8-1919. [DOI] [PubMed] [Google Scholar]
- 2.Almond N, Kent K, Cranage M, Rud E, Clarke B, Stott E J. Protection by attenuated simian immunodeficiency virus in macaques against challenge with virus-infected cells. Lancet. 1995;345:1342–1344. doi: 10.1016/s0140-6736(95)92540-6. [DOI] [PubMed] [Google Scholar]
- 3.Baba T W, Jeong Y S, Penninck D, Bronson R, Greene M F, Ruprecht R M. Pathogenicity of live, attenuated SIV after mucosal infection of neonatal macaques. Science. 1995;267:1820–1825. doi: 10.1126/science.7892606. [DOI] [PubMed] [Google Scholar]
- 4.Binley J M, Klasse P J, Cao Y, Jones I M, Markowitz M, Ho D D, Moore J P. Differential regulation of the antibody response to Gag and Env proteins of human immunodeficiency virus type 1. J Virol. 1997;71:2799–2809. doi: 10.1128/jvi.71.4.2799-2809.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Burton, D. R., and D. Montefiori. 1997. The antibody response in HIV-1 infection. AIDS 11(Suppl. A):587–598. [PubMed]
- 6.Chakrabarti L, Baptiste V, Khatissan E, Cumont M-C, Aubertin A-M, Montagnier L, Hurtrel B. Limited viral spread and rapid immune responses in lymph nodes of macaques inoculated with attenuated simian immunodeficiency virus. Virology. 1995;213:535–548. doi: 10.1006/viro.1995.0026. [DOI] [PubMed] [Google Scholar]
- 7.Cheingsong-Popov R, Panagiotidi C, Bowcock S, Aronstam A, Wadsworth J, Weber J. Relation between humoral responses to HIV gag and env proteins at seroconversion and clinical outcome of HIV infections. Br Med J. 1991;302:23–26. doi: 10.1136/bmj.302.6767.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Clements J E, Montelare R C, Zink M C, Amedee A M, Miller S, Trichel A M, Hagerski B, Hauer D, Martin L N, Bohm R P, Murphey-Corb M. Cross-protective immune responses induced in rhesus macaques by immunization with attenuated macrophage-tropic simian immunodeficiency virus. J Virol. 1995;69:2737–2744. doi: 10.1128/jvi.69.5.2737-2744.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dailey P J, Zamround M, Kelso R, Kolberg J, Urdea M. Quantitation of simian immunodeficiency virus (SIV) RNA in plasma of acute and chronically infected macaques using a branched DNA (bDNA) signal amplification assay. J Med Primatol. 1995;24:209. [Google Scholar]
- 10.Daniel M D, Kirchoff F, Czajak S C, Sehgal P K, Desrosiers R C. Protective effects of a live, attenuated SIV vaccine with a deletion in the nefgene. Science. 1992;258:1938–1941. doi: 10.1126/science.1470917. [DOI] [PubMed] [Google Scholar]
- 11.Denesvre C, Le Grand R, Boisson-Cans F, Chakrabarti L, Hurtrel B, Vaslin B, Dormont D, Sonigo P. Highly attenuated SIVmac142 is immunogenic but does not protect against SIVmac251 challenge. AIDS Res Hum Retroviruses. 1995;11:1397–1406. doi: 10.1089/aid.1995.11.1397. [DOI] [PubMed] [Google Scholar]
- 12.Desrosiers R C, Lifson J D, Gibbs J S, Czajak S C, Howe A Y M, Arthur L O, Johnson R P. Identification of highly attenuated mutants of simian immunodeficiency virus. J Virol. 1998;72:1431–1437. doi: 10.1128/jvi.72.2.1431-1437.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dittmer U, Niblein T, Bodemer W, Petry H, Sauermann U, Stahl-Hennig C, Hunsmann G. Cellular immune response of rhesus monkeys infected with a partially attenuated nefdeletion mutant of the simian immunodeficiency virus. Virology. 1995;212:392–397. doi: 10.1006/viro.1995.1496. [DOI] [PubMed] [Google Scholar]
- 14.Harris M, Patenaude P, Cooperberg P, Filipenko D, Thorne A, Raboud J, Rae S, Dailey P, Chernoff D, Todd J, Conway B, Montaner J S G the INCAS Study Group. Correlation of viral load in plasma and lymph node tissue in HIV infection. J Infect Dis. 1997;176:1388–1392. doi: 10.1086/517328. [DOI] [PubMed] [Google Scholar]
- 15.Hogervorst E, Jurrians S, de Wolf F, van Wijk A, Wiersma A, Valk M, Roos M, van Gemen B, Coutinho R, Miedema F, Goudsmit J. Predictors for non- and slow-progression in human immunodeficiency virus (HIV) type 1 infection: low viral RNA copy numbers in serum and maintenance of high HIV-1 p24-specific but not V3-specific antibody levels. J Infect Dis. 1995;171:811–821. doi: 10.1093/infdis/171.4.811. [DOI] [PubMed] [Google Scholar]
- 16.Johnson R P, Glickman R L, Yang J Q, Kaur A, Dion J T, Mulligan M J, Desrosiers R C. Induction of vigorous cytotoxic T-lymphocyte responses by live attenuated simian immunodeficiency virus. J Virol. 1997;71:7711–7718. doi: 10.1128/jvi.71.10.7711-7718.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kirchoff F, Kestler H W, Desrosiers R C. Upstream U3 sequences in simian immunodeficiency virus are selectively deleted in vivo in the absence of an intact nefgene. J Virol. 1994;68:2031–2037. doi: 10.1128/jvi.68.3.2031-2037.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Koup R A, Safrit J T, Cao Y, Andrews C A, Wu Y, McLeod G, Borkowsky W, Farthing C, Ho D D. Temporal association of cellular immune response with the initial control of viremia in primary human immunodeficiency virus type 1 syndrome. J Virol. 1994;68:4650–4655. doi: 10.1128/jvi.68.7.4650-4655.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lewis M G, Bellah S, McKinnin K, Yalley-Ogunro J, Zack P M, Elkins W R, Desrosiers R C, Eddy G A. Titration and characterization of two rhesus-derived SIVmac challenge stocks. AIDS Res Hum Retroviruses. 1994;10:213–220. doi: 10.1089/aid.1994.10.213. [DOI] [PubMed] [Google Scholar]
- 20.Lifson J D, Nowak M A, Goldstein S, Rossio J L, Kinter A, Vasquez G, Wiltrout T A, Brown C, Schneider D, Wahl L, Lloyd A L, Williams J, Elkins W R, Fauci A S, Hirsch V M. The extent of early viral replication is a critical determinant of the natural history of simian immunodeficiency virus infection. J Virol. 1997;71:9508–9514. doi: 10.1128/jvi.71.12.9508-9514.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lohman B L, McChesney M B, Miller C J, McGowan E, Joye S M, Van Rompay K K A, Reay E, Antipa L, Pedersen N C, Marthas M L. A partially attenuated simian immunodeficiency virus induces host immunity that correlates with resistance to pathogenic virus challenge. J Virol. 1994;68:7021–7029. doi: 10.1128/jvi.68.11.7021-7029.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Marthas M L, Sutjipto S, Miller C J, Higgins J, Torten J, Unger R E, Marx P A, Pedersen N C. Efficacy of live-attenuated and whole-inactivated SIV vaccines against intravenous and vaginal challenge. In: Brown F, Chanock R M, Ginsberg H S, Lerner R A, editors. Vaccines 92. Cold Spring Harbor, N.Y: Cold Spring Harbor Press; 1992. pp. 117–122. [Google Scholar]
- 23.Means R E, Greenough T, Desrosiers R C. Neutralization sensitivity of cell culture-passaged simian immunodeficiency virus. J Virol. 1997;71:7895–7902. doi: 10.1128/jvi.71.10.7895-7902.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Montefiori D C, Baba T W, Li A, Bilska M, Ruprecht R M. Neutralizing and infection-enhancing antibody responses do not correlate with the differential pathogenicity of SIVmac239Δnef in adult and infant rhesus monkeys. J Immunol. 1996;157:5528–5535. [PubMed] [Google Scholar]
- 25.Moore J P, Cao Y, Qing L, Sattentau Q J, Pyati J, Koduri R, Robinson J, Barbas C F I, Burton D R, Ho D D. Primary isolates of human immunodeficiency virus type 1 are relatively resistant to neutralization by monoclonal antibodies to gp120. J Virol. 1995;69:101–109. doi: 10.1128/jvi.69.1.101-109.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Norley S, Beer B, Binninger-Schinzel D, Cosma C, Kurth R. Protection from pathogenic SIVmac challenge following short-term infection with a nef-deficient attenuated virus. Virology. 1996;219:195–205. doi: 10.1006/viro.1996.0237. [DOI] [PubMed] [Google Scholar]
- 27.Regier D A, Desrosiers R C. The complete nucleotide sequence of a pathogenic molecular clone of simian immunodeficiency virus. AIDS Res Hum Retroviruses. 1990;6:1221–1231. doi: 10.1089/aid.1990.6.1221. [DOI] [PubMed] [Google Scholar]
- 28.Reimann K A, Tenner-Racz K, Racz P, Montefiori D C, Yasutomi Y, Ransil B J, Letvin N L. Immunopathogenic events in acute infection of rhesus monkeys with simian immunodeficiency virus of macaques. J Virol. 1994;68:2362–2370. doi: 10.1128/jvi.68.4.2362-2370.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shibata R, Matano T, Siemon C, Connors M, Lane H C, Martin M. Abstracts of the 9th Annual Meeting of the National Cooperative Vaccine Development Groups for AIDS. Conference on Advances in AIDS Vaccine Development, Bethesda, Md. 1997. Effect of CD8 cells on replication of a superchallenged SHIV in macaque monkeys; p. 151. [Google Scholar]
- 30.Shibata R, Siemon C, Czajak S C, Desrosiers R C, Martin M A. Live, attenuated simian immunodeficiency virus vaccines elicit potent resistance against a challenge with a human immunodeficiency virus type 1 chimeric virus. J Virol. 1997;71:8141–8148. doi: 10.1128/jvi.71.11.8141-8148.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Stahl-Hennig C, Dittmer U, NiBlein T, Petry H, Jurkiewicz E, Fuchs D, Wachter H, Mätz-Rensing K, Kuhn E M, Kaup F J, Rud E W, Hunsmann G. Rapid development of vaccine protection in macaques by live-attenuated simian immunodeficiency virus. J Gen Virol. 1996;77:2969–2981. doi: 10.1099/0022-1317-77-12-2969. [DOI] [PubMed] [Google Scholar]
- 32.Strathdee S A, Frank J W, McLaughlin J, Leblanc M, Major C, O’Shaughnessy M V, Rudd E S. Quantitative measures of human immunodeficiency virus-specific antibodies predict progression to AIDS. J Infect Dis. 1995;172:1375–1379. doi: 10.1093/infdis/172.5.1375. [DOI] [PubMed] [Google Scholar]
- 33.Wyand M S, Manson K H, M. G-M, Montefiori D, Desrosiers R C. Vaccine protection by a triple-deletion mutant of simian immunodeficiency virus. J Virol. 1996;70:3724–3733. doi: 10.1128/jvi.70.6.3724-3733.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yasutomi Y, Reimann K A, Lord C I, Miller M D, Letvin N L. Simian immunodeficiency virus-specific CD8+lymphocyte response in acutely infected rhesus monkeys. J Virol. 1993;67:1707–1711. doi: 10.1128/jvi.67.3.1707-1711.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhang, L. Q. and D. D. Ho. Personal communication.
- 36.Zwart G, van der Hoek L, Valk M, Cornelissen M T E, Baan E, Dekker J, Koot M, Kuiken C L, Goudsmit J. Antibody responses to HIV-1 envelope and gag epitopes in HIV-1 seroconvertors with rapid versus slow disease progression. Virology. 1994;201:285–293. doi: 10.1006/viro.1994.1293. [DOI] [PubMed] [Google Scholar]