Abstract
Importance
Prostate-specific antigen (PSA) screening has potential to reduce prostate cancer mortality but frequently detects prostate cancer that is not clinically important.
Objective
To describe rates of low-grade (grade group 1) and high-grade (grade groups 2-5) prostate cancer identified among men invited to participate in a prostate cancer screening protocol consisting of a PSA test, a 4-kallikrein panel, and a magnetic resonance imaging (MRI) scan.
Design, Setting, and Participants
The ProScreen trial is a clinical trial conducted in Helsinki and Tampere, Finland, that randomized 61 193 men aged 50 through 63 years who were free of prostate cancer in a 1:3 ratio to either be invited or not be invited to undergo screening for prostate cancer between February 2018 and July 2020.
Interventions
Participating men randomized to the intervention underwent PSA testing. Those with a PSA level of 3.0 ng/mL or higher underwent additional testing for high-grade prostate cancer with a 4-kallikrein panel risk score. Those with a kallikrein panel score of 7.5% or higher underwent an MRI of the prostate gland, followed by targeted biopsies for those with abnormal prostate gland MRI findings. Final data collection occurred through June 31, 2023.
Main Outcomes and Measures
In descriptive exploratory analyses, the cumulative incidence of low-grade and high-grade prostate cancer after the first screening round were compared between the group invited to undergo prostate cancer screening and the control group.
Results
Of 60 745 eligible men (mean [SD] age, 57.2 [4.0] years), 15 201 were randomized to be invited and 45 544 were randomized not to be invited to undergo prostate cancer screening. Of 15 201 eligible males invited to undergo screening, 7744 (51%) participated. Among them, 32 low-grade prostate cancers (cumulative incidence, 0.41%) and 128 high-grade prostate cancers (cumulative incidence, 1.65%) were detected, with 1 cancer grade group result missing. Among the 7457 invited men (49%) who refused participation, 7 low-grade prostate cancers (cumulative incidence, 0.1%) and 44 high-grade prostate cancers (cumulative incidence, 0.6%) were detected, with 7 cancer grade groups missing. For the entire invited screening group, 39 low-grade prostate cancers (cumulative incidence, 0.26%) and 172 high-grade prostate cancers (cumulative incidence, 1.13%) were detected. During a median follow-up of 3.2 years, in the group not invited to undergo screening, 65 low-grade prostate cancers (cumulative incidence, 0.14%) and 282 high-grade prostate cancers (cumulative incidence, 0.62%) were detected. The risk difference for the entire group randomized to the screening invitation vs the control group was 0.11% (95% CI, 0.03%-0.20%) for low-grade and 0.51% (95% CI, 0.33%-0.70%) for high-grade cancer.
Conclusions and Relevance
In this preliminary descriptive report from an ongoing randomized clinical trial, 1 additional high-grade cancer per 196 men and 1 low-grade cancer per 909 men were detected among those randomized to be invited to undergo a single prostate cancer screening intervention compared with those not invited to undergo screening. These preliminary findings from a single round of screening should be interpreted cautiously, pending results of the study’s primary mortality outcome.
Trial Registration
ClinicalTrials.gov Identifier: NCT03423303
This preliminary descriptive report compares the detection rates of high-grade and low-grade prostate cancer in men invited for prostate cancer screening vs those of the control group not offered screening.
Key Points
Question
What were the rates of prostate cancer detection among men randomized to be invited to undergo prostate cancer screening compared with a control group not invited to undergo screening?
Findings
In this preliminary report from an ongoing clinical trial, 60 745 men aged 50 through 63 years were randomized either to be invited to undergo prostate cancer screening with a PSA test, a 4-kallikrein panel for those with a PSA of 3.0 ng/mL or higher, and MRI or not to be invited for screening (control group). The risk difference for the group invited to be screened vs the control group was 0.11% for low-grade and 0.51% for high-grade prostate cancer.
Meaning
In this preliminary descriptive report, the screening intervention detected 1 high-grade prostate cancer per 196 men and 1 low-grade prostate cancer per 909 men invited to be screened. These preliminary findings should be interpreted cautiously, pending results of the study’s primary outcome of prostate cancer mortality.
Introduction
Screening for prostate cancer with a prostate-specific antigen (PSA) test has the potential to reduce death from prostate cancer. However, PSA screening frequently detects prostate cancer that is not clinically significant but may result in unnecessary procedures and adverse effects.1,2,3
To increase specificity for high-grade prostate cancer, methods that combine screening tests in an algorithm approach were developed. A kallikrein panel with 4 components (total PSA, free PSA, intact PSA, and human kallikrein-2) reduced the number of men referred to biopsy, while retaining sensitivity for high-grade cancer.4,5 Magnetic resonance imaging (MRI) in men with elevated PSA may reduce the detection of low-grade prostate cancer that is not clinically significant (grade group [GG] 1 or Gleason score <7).6,7
The ProScreen trial tested a screening intervention designed to reduce unnecessary diagnoses of prostate cancer while reducing prostate cancer mortality. The intervention in this clinical trial consisted of an invitation to undergo prostate cancer screening using a 3-phased screening algorithm with PSA, kallikrein panel score, and MRI, with biopsies reserved for men meeting specific thresholds for abnormal results from all 3 tests (except men who tested negative via MRI with a PSA density ≥0.15 ng/mL).8 After initial screening, participants will undergo rescreening every 2 to 6 years, according to their baseline risk.
This preliminary and exploratory report compares rates of high-grade and low-grade prostate cancer diagnoses between the intervention and control group, after the first invitation to participate in prostate cancer screening. Rates of high-grade and low-grade prostate cancer in men invited for prostate cancer screening were compared with those of the control group that was not offered screening.
Methods
Trial Design
The trial protocol can be found in Supplement 1 and the statistical analysis plan in Supplement 2. The protocol was reviewed by the Ethics Committee of the Helsinki University Hospital (HUS 2910/2017). Individuals randomized to an invitation to undergo prostate cancer screening signed informed consent prior to undergoing prostate cancer screening. The ethics committee determined that signed informed consent was not required for other randomized individuals. Permission to collect data on cancer cases from the hospital records and cancer registry was obtained from the National Institute for Health and Welfare (THL 676/2018). Randomization occurred from February 2018 to July 2020. Enrollment (screening invitations) took place between May 2018 and September 2022. First-round prostate cancer screening occurred through December 2022, and prostate cancer diagnoses through March 2023 were included in the analyses. Additional data collection for Gleason grade and treatment occurred through June 30, 2023.
This clinical trial is a randomized, population-based pragmatic prostate cancer screening clinical trial of men aged 50 through 63 years. The age range was chosen to allow at least 2 screening opportunities before age 71 years for men randomized to the intervention. Men were randomized either to be invited to participate in the prostate cancer screening or to a control group that did not receive an invitation for screening. The screening intervention comprised plasma PSA values, a 4-kallikrein panel, and a multiparametric MRI of the prostate. Rescreening interval was risk stratified based on the first-round PSA test results: those with a PSA concentration of 3.0 ng/mL or higher were invited after 2 years, between 1.5 and 2.99 ng/mL after 4 years, and less than 1.5 ng/mL at 6 years. Individuals who declined the screening invitation will be invited to the subsequent screening rounds. The primary end point is prostate cancer mortality. Process outcomes and ancillary end points include incidence of high-grade prostate cancer (GGs 2-5) and low-grade prostate cancer (GG 1), sensitivity of screening for high-grade cancer, incidence of advanced disease (defined as stage T3-T4, or N1, or M1), quality of life, and cost-effectiveness.
This report compares rates of high- and low-grade cancers between men invited for the first round of screening in the intervention group with those in the control group who did not receive an invitation for prostate cancer screening. This report also compares rates of high- and low-grade cancers between men randomized to the screening invitation who participated in prostate cancer screening and men randomized the control group. Study design, statistical analysis plan, and a pilot study evaluating the feasibility and acceptability of the study procedures were published previously.8,9,10
Participants
We identified all men aged 50 through 63 years residing in the cities of Helsinki and Tampere, Finland, at baseline in 2018 through the Population Data Services Agency and randomized them prior to consent (Zelen design)11,12 to the screening invitation or the control group using a 1:3 allocation ratio.
Men with a previous diagnosis of prostate cancer according to the Finnish Cancer Registry were excluded prior to randomization.
Randomization was performed (using computer-generated pseudorandom numbers) by a trial statistician (J. Raitanen) in 4 batches (approximately 15 000 men each time) between February 2018 and July 2020 (Figure 1). Participants and investigators were not masked to randomization assignment.
Figure 1. Flow Diagram of the ProScreen Trial.
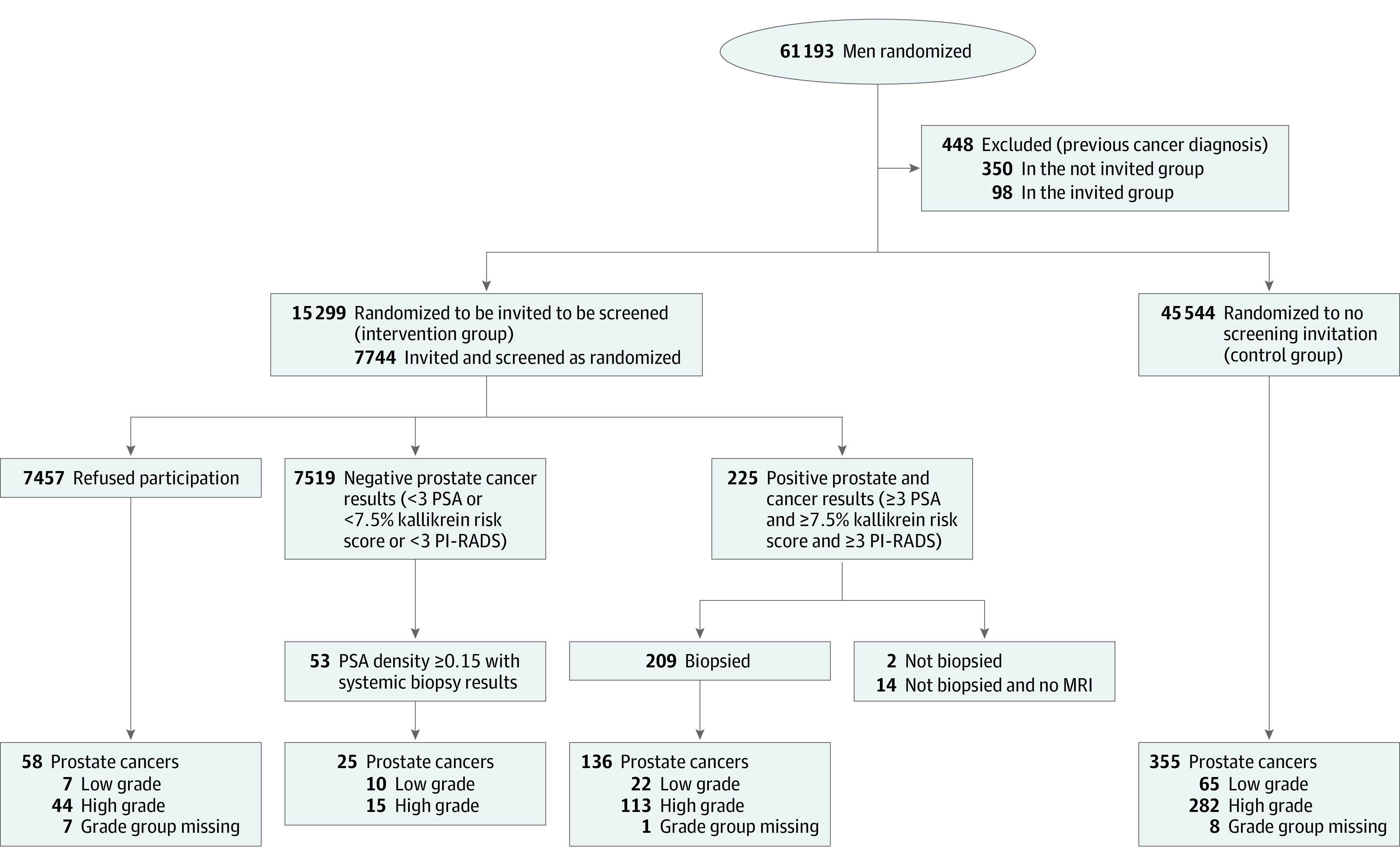
MRI indicates magnetic resonance imaging; PI-RADS, Prostate Imaging and Data Reporting System; and PSA, prostate-specific antigen.
Men allocated to the screening invitation group were mailed an information package describing the aims and procedures of the trial and a consent form by the trial coordinator (K.N.). Besides written informed consent, the men attending screening provided questionnaire information on previous PSA measurements (for any reason), prior prostate biopsies, and family history of prostate cancer. Men in the control group were not contacted (apart from questionnaires mailed to a random sample of 6026 men [13%] on health care costs and quality of life for later analyses).
Measures of PSA and Kallikrein Panel
Men randomized to the intervention who underwent prostate cancer screening had a 30-mL peripheral venous blood sample drawn and separated into whole blood, plasma, and serum. PSA in blood samples was analyzed by HUSLAB (Helsinki) or FimLab (Tampere) laboratory network. If the PSA value was 3.0 ng/mL or higher, plasma was shipped to Lund University, Wallenberg Research laboratories, at Skåne University Hospital in Malmö, Sweden, for determination of the 4-kallikrein risk score (comprising total PSA, free PSA, intact PSA, and human kallikrein-2). For free and total PSA measures, the dual-label DELFIA Prostatus total/free PSA-Assay (PerkinElmer)13 calibrated against the World Health Organization (WHO) 96/670 (PSA-WHO) and WHO 68/668 (free PSA-WHO) standards was used, and assays for intact PSA and human kallikrein-related peptidase 2 used F(ab’)2 fragments of monoclonal capture antibodies to reduce nonspecific assay interference.14 A prespecified statistical model based on age and the 4-kallikrein markers15 was applied to estimate the probability of high-grade prostate cancer expressed as the kallikrein panel risk score ranging theoretically from 0 to 100% (commercially available as the 4Kscore test in the US). Risk scores of 7.5% or higher were considered abnormally high as recommended in the literature.16 However, prior prostate biopsy or digital rectal examination findings were not included in the score.
Prostate Cancer Outcomes
Men with kallikrein panel risk scores of 7.5% or higher were referred to the urology department at either the Helsinki or Tampere university hospitals. First, a 1.5 tesla (T) or 3 T multiparametric (T2-weighted, diffusion-weighted, and dynamic contrast-enhanced) MRI of the prostate was performed. The MRI images were evaluated by radiology teams including abdominal and general radiologists. The images were graded according to the Prostate Imaging Reporting and Data System (PI-RADS) version 2.1.17 The PI-RADS score for a region of interest indicates the level of suspicion for high-grade prostate cancer on a scale of 1 to 5, where a higher score indicates higher suspicion; scores 3 to 5 were considered to indicate findings suspicious for high-grade prostate cancer. Each region of interest was marked on the MRI images using the DynaCad software (Philips). Men with a PI-RADS score of 3 to 5 were referred to software-targeted transrectal fusion biopsies of the identified lesions, with 2 to 4 cores per region of interest using the UroNav system (Philips). Biopsy specimens were not obtained of prostate tissue not identified as abnormal except in those with a negative MRI result (PI-RADS <3) but elevated PSA density (>0.15 ng/mL2). In these individuals, 10 to 12 systematic prostate biopsy specimens were obtained to avoid missing high-grade cancers.
Each biopsy core was separately evaluated according to the International Society for Urological Pathology guidelines18 by uropathologists at the Helsinki and Tampere university hospitals. GG 1 cancers were regarded as low grade and those in the GG 2 through 5 range were designated as high grade.19
Clinical features including GG, clinical stage, and treatment were abstracted from the hospital records including pathology databases for all cases (including those identified from the cancer registry) by study nurses and the trial coordinator.
Study Outcomes
The primary outcome of the clinical trial is prostate cancer mortality at 10 and 15 years of follow-up. Primary outcome data are currently not available. This report gives preliminary results from the first invitation for prostate cancer screening on detection of high-grade and low-grade cancer, results of the screening tests, and frequency of biopsy referral. Incident prostate cancer cases since randomization were identified from the population-based Finnish Cancer Registry with a high completeness of coverage20 and from the hospital pathology databases. Both sources for diagnoses were used for both trial groups and diagnoses documented in either source were regarded as confirmed cases.
Sample Size Calculation
Pretrial sample-size calculations indicated that with 111 000 men in the trial, a 1:3 randomization provided a statistical power of 0.89 to detect a 25% relative risk reduction in prostate cancer mortality at 15 years (see the statistical analysis plan in Supplement 2).10 No sample size calculations were performed for the outcomes reported in this report.
Statistical Analyses
The analyses of this preliminary and descriptive report were not prespecified in the statistical analysis plan. The risk of high-grade and low-grade prostate cancer was reported as cumulative incidence, with the number of cases relative to number of men, with the follow-up period starting at the date of randomization. The number of cases relative to the duration of total follow-up time in person-years (incidence density) was not used because screening always induces lead time due to earlier detection and therefore results in poor comparability of rates between the trial arms.
The main analysis was based on the study population as allocated by randomization (all men randomized to the screening invitation included in the intervention group), with the effect of screening invitation quantified as risk difference between the entire intervention and control groups as an indicator of the population-level impact of the screening program. An ancillary analysis comparing cumulative incidence among screened men in the intervention group with all men in the control group (per-protocol analysis) is presented as an indicator of the performance of the screening process. Missing values were imputed with expected frequencies based on the observed GG distribution (see eAppendix 1 in Supplement 3).
Exact 95% CIs for the cumulative incidences and for their differences and ratios in group comparisons were calculated using Poisson regression with Stata statistical software version 18 (StataCorp).
Results
Participants
A total of 295 randomized participants had a prior diagnosis of prostate cancer and were excluded. In addition, some previously diagnosed prostate cancer among randomized participants were identified after randomization due to delayed cancer registration (98 in the screening and 350 in the control group). After exclusions, 60 745 men were eligible.
Of the eligible men, 45 544 were assigned to the control group and 15 201 to the screening invitation group. The mean (SD) age at entry was 57.3 (4.0) years in both groups. Information on GG was missing for 16 cancer cases (2.8%) and the main analysis is based on a complete case analysis with an ancillary analysis including imputation of the missing GGs is reported in the eTable 4 in Supplement 3.
Men randomized to the screening invitation group (n = 353, 2.3%) who had emigrated or died after randomization were therefore not invited but were included in the analysis. Of the 15 201 men invited to screening, 7744 (51%) had participated by December 31, 2022. The proportion of individuals who participated increased with age from 47.3% at ages 50 through 54 years to 54.5% at 60 through 64 years. The proportion who participated was 49.3% in Helsinki and 55.5% in Tampere. Of the participants randomized to the intervention who completed study questionnaires, 2894 (44%) reported previous PSA measurements and 195 (3%) prior prostate biopsies (Table 1). Similar or slightly higher proportions were also reported by men in the control group (eTable 1 in Supplement 3).
Table 1. Characteristics of the ProScreen Trial Population.
| Group, No. (%) | |||
|---|---|---|---|
| Screening | Control (n = 45 544) | ||
| Invited men who underwent screening (n = 7744) |
All invited men (n = 15 201) |
||
| Age, y | |||
| 50-54 | 2413 (31) | 5104 (34) | 15 755 (35) |
| 55-59 | 2896 (37) | 5668 (37) | 16 537(36) |
| 60-64 | 2435 (31) | 4429 (29) | 13 252(29) |
| Center | |||
| Helsinki | 5506 (71) | 11 171 (73) | 33 114 (73) |
| Tampere | 2238 (29) | 4030 (27) | 12 430 (27) |
| Previous PSA (n = 6513)a | |||
| <12 mo | 855 (13) | ||
| 12-24 mo | 832 (13) | ||
| 3-5 y | 791 (12) | ||
| >5 y | 406 (6) | ||
| Never | 3629 (56) | ||
| Previous prostate biopsy (n = 6513)a | 195 (3) | ||
| Prostate cancer in a first-degree relative (n = 6513)a | 927 (14) | ||
Abbreviation: PSA, prostate-specific antigen.
Men in the group invited to screening were mailed a survey. The numbers represent the number who answered the question.
Among participants randomized to the intervention who participated in screening, the median PSA value was 0.94 ng/mL (IQR, 0.58-1.67 ng/mL) with PSA values of 3.0 ng/mL or higher in 752 men (9.7%). The 4-kallikrein panel was analyzed for the men with PSA values of 3.0 ng/mL or higher and the median kallikrein panel risk score was 11.7% (IQR, 6.6%-21.3%). Of those measurements, 526 (70%) were positive findings (kallikrein panel risk score ≥7.5%).
Of 526 men with PSA values of 3 ng/mL or higher and kallikrein panel scores of 7.5% or higher, 509 (97%) underwent MRI. Of these, 211 (41%) had a PI-RADS score of 3 to 5, indicating lesions suggesting presence of high-grade cancer, and were referred for prostate biopsy. Targeted transrectal prostate biopsies (only) were performed in 209 men (99%) with a positive MRI result, 2.7% of the participants. All 53 men with a negative MRI result (PI-RADS <3) but PSA density of 0.15 ng/L2 or higher (median, 0.20 ng/mL2; IQR, 0.17-0.27 ng/mL2) had a systematic biopsy (10-12 cores). A total of 3.4% of the participants (262 of 7744) underwent biopsy.
Cancer Detection
Of the targeted biopsies, 136 (65%) showed prostate cancer (approximately 1.5 biopsies to detect a cancer). Of the prostate cancers identified, 22 were low grade (GG 1), 113 were high grade (52 GG 2 and 61 GG 3-5), and 1 was missing a GG (Figure 1). In addition, 25 cancers (47%, 10 GG 1, 11 GG 2, and 4 GG 3-5) were detected in systematic biopsies of the 53 men with PSA density of 0.15 ng/mL or higher (eTable 2 in Supplement 3). The overall cancer detection was 2.07% (161 of 7744, 95% CI, 1.76%-2.40%). Detection of high-grade cancer increased with age, PSA value, the 4-kallikrein panel risk score, and the PI-RADS score, but was comparable in men who reported previous PSA measurements and those who did not (1.9% vs 1.5%; eTable 2 in Supplement 3).
Of the screen-detected cancers, 32 (20%) were low-grade and 128 (80%) high-grade (63 GG 2 and 65 GG 3-5, respectively, and 1 case was missing a GG). The yield of high-grade cancers was 1.65% (128 of 7744; 95% CI, 1.37%-1.94%) and that for low-grade cancer was 0.41% (32 of 7744; 95% CI, 0.27%-0.56%; Figure 2). A detailed description of the cases is provided in eTables 2 through 4 in the Supplement 3.
Figure 2. Summary of the Results.
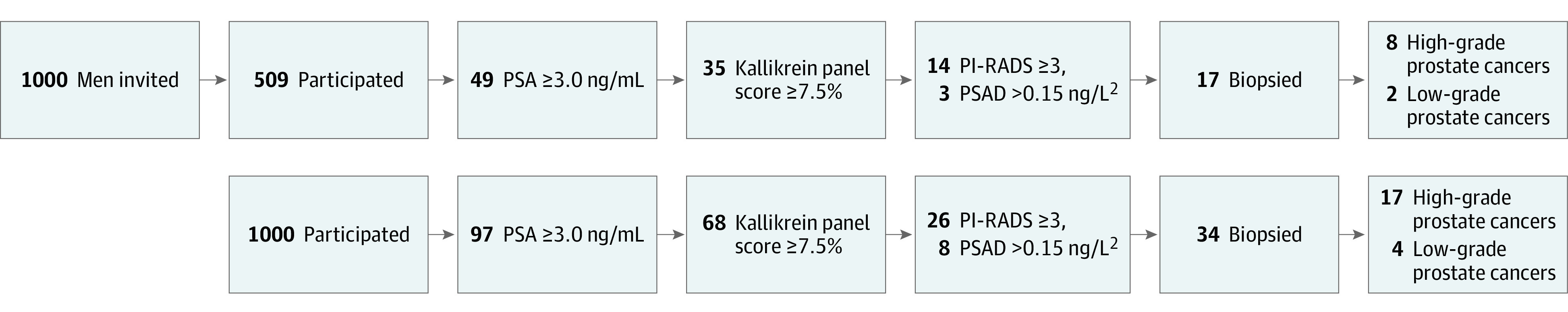
Screening results expressed per 1000 invited men (upper panel) and per 1000 screened men (lower panel). PSA indicates prostate-specific antigen.
Among the 7457 men allocated to the intervention group, who did not participate, 58 prostate cancers (7 GG 1 [cumulative incidence, 0.1%], 44 GG 2-5 [cumulative incidence, 0.6%], and 8 with missing GGs) were diagnosed between randomization and the end of 2022.
In the entire intervention group, 39 low-grade cancers (18%; cumulative incidence, 0.26%) and 172 high-grade cancers (79%; cumulative incidence, 1.13%) were diagnosed, with 8 cases (3.7%) lacking GG.
Among the 45 544 men in the control group, 355 new prostate cancers were detected during a median follow-up of 3.2 years from randomization until end of 2022. Of those, 65 were low grade (18%) and 282 high grade (79%) with 8 cases (2.3%) lacking GGs. This corresponds to a cumulative incidence of 0.14% and 0.62%, respectively (Table 2).
Table 2. Prostate Cancers Diagnosed by Trial Group, Screening Participation, and Grade Group.
| Grade group | Screening group, No. (%) | Control group, No. (%) (n = 45 544) | Risk difference, % (95% CI) | ||
|---|---|---|---|---|---|
| Invited men who underwent screening (n = 7744) | All invited men (n = 15 201) | Invited men who underwent screening vs control group | All invited men vs control group | ||
| All cancers | 161 (2.08) | 219 (1.44) | 355 (0.78) | 1.30 (0.97 to 1.63) | 0.66 (0.45 to 0.87) |
| With known grade group | 160 (2.07) | 211 (1.39) | 347 (0.76) | 1.30 (0.97 to 1.63) | 0.62 (0.42 to 0.83) |
| Low grade (grade group 1) | 32 (0.41) | 39 (0.26) | 65 (0.14) | 0.27 (0.12 to 0.42) | 0.11 (0.03 to 0.20) |
| High grade (grade group 2-5) | 128 (1.65) | 172 (1.13) | 282 (0.62) | 1.03 (0.74 to 1.33) | 0.51 (0.33 to 0.70) |
| Detailed classification by grade group | |||||
| 1 | 32 (0.41) | 39 (0.26) | 65 (0.14) | 0.27 (0.12 to 0.42) | 0.11 (0.03 to 0.20) |
| 2 | 63 (0.81) | 76 (0.50) | 121 (0.27) | 0.55 (0.34 to 0.75) | 0.23 (0.11 to 0.35) |
| 3 | 45 (0.58) | 57 (0.37) | 62 (0.14) | 0.44 (0.27 to 0.62) | 0.24 (0.14 to 0.34) |
| 4-5 | 20 (0.26) | 39 (0.26) | 99 (0.22) | 0.04 (−0.08 to 0.16) | 0.04 (−0.05 to 0.13) |
| Missing | 1 (0.01) | 8 (0.05) | 8 (0.02) | ||
Comparison of All Participants Randomized to Intervention vs Control Group
The main analysis contrasting the entire screening group (including participants randomized to screening who did not take part in screenings) with the control group, ie, randomly assigned populations yielded a risk difference of 0.11% (95% CI, 0.03%-0.20%) for low-grade cancer and 0.51% (95% CI, 0.33%-0.70%) for high-grade cancer. For any prostate cancer, the risk difference was 0.66% (95% CI, 0.45%-0.87%), corresponding to a risk ratio of 1.85 (95% CI, 1.56-2.19).
Ancillary Analyses With Imputation of Missing Values and Per-Protocol Analysis
A modified analysis of the randomly assigned populations with imputation of the missing GG (n = 16 [2.6%]) is shown in eTable 5 in Supplement 3.
A supplementary analysis comparing the 7744 men randomized to the intervention who underwent screening with the control group (per-protocol analysis) gave a risk difference of 0.27% (95% CI, 0.12%-0.41%) for low-grade cancers and 1.03% (95% CI, 0.74%-1.33%) for high-grade cancers (Table 2). For any prostate cancer, the risk difference was 1.30% (95% CI, 0.97%-1.63%). Compared with the control group, localized prostate cancer was more common in the screen-detected participants (88% vs 78% localized), but less common among participants randomized to screening who did not take part in screenings (53%; eTable 6 in Supplement 3). Treatment for identified prostate cancer was more commonly active surveillance in the group randomized to screening, compared with the control group (32% vs 24%). Treatment for identified prostate cancer was less often androgen deprivation therapy in the group randomized to screening, compared with the control group (3% vs 7%), and especially in screen-detected cases (eTable 7 in Supplement 3).
Discussion
In this preliminary and exploratory report from a population-based randomized clinical trial, an invitation for a single round of a screening protocol that combined a PSA test, a kallikrein panel risk score, and MRI before targeted biopsy resulted in a biopsy rate of 1.4% among men invited for screening (2.7% in the screened men) and detected 1 additional high-grade prostate cancer per 196 men invited to screening, compared with the control group that was not invited to screening. The intervention detected 1 additional low-grade prostate cancer per 909 men invited to screening, compared with the control group. However, these results were descriptive and should be interpreted provisionally, pending results from the trial on the primary outcome of prostate cancer mortality.
The detection rate of high-grade cancer (1.7%) among men randomized to intervention who underwent screening was comparable with that in the initial screening round of the European Randomized Study of Screening for Prostate Cancer (ERSPC) trial (1.8%),21 despite the younger population in the current trial, while detection of low-grade cancer was lower (0.4% vs 3.2%, respectively). In the UK Cluster Randomized Trial of PSA Testing for Prostate Cancer (CaP)22 trial with a single screening round, detection of high-grade cancer in screened men was higher than rates reported herein (2.7% for men aged 50-69 years), whereas the US Prostate, Lung, Colorectal and Ovarian (PLCO)23 reported a lower detection of high-grade cancer at the first screen (0.5%), although both the populations in the CaP and PLCO trials were older, with higher expected prevalence, than the population in the current study.
In the current trial, 9.7% of the men who underwent screening had PSA values of 3 ng/mL or higher. The kallikrein panel used as a reflex test reduced the proportion of men referred to MRI to 6.8%, and after MRI, 2.7% were referred to biopsy. In addition, 0.7% of participants randomized to the intervention who took part in screening were referred to systematic biopsies due to elevated PSA density despite a negative MRI result to minimize potential for missing high-grade cancers, with 40% of the cases detected in these men representing low-grade cancer compared with 16% of the cases in targeted biopsies.
Recently, 2 randomized clinical trials were published evaluating an MRI-based pathway in a screening setting. The Göteborg2 screening trial24 randomized 11 986 men aged 50 to 60 years attending screening to an experimental group with PSA followed by MRI and targeted biopsies only if PSA values were 3.0 ng/mL or higher or to the control group with systematic and targeted biopsies. They reported detection rates of 0.9% vs 1.1% for high-grade cancer, which is lower than in the current study (1.7%). The detection of low-grade cancer, on the other hand, was somewhat higher in Göteborg2 (0.6%) compared with the current trial (0.4%). The proportions of men biopsied were comparable (2.8% in Göteborg2 vs 2.7% in the current trial).
In the STHLM3-MRI study,25 1532 screened men aged 50 to 74 years with a PSA value of 3 ng/mL or higher were randomized to either a standard diagnostic pathway including a systematic biopsy without MRI or to an experimental arm including MRI with both targeted and systematic biopsy in men with a PI-RADS score of 3 or more. The proportion of low-grade cancer (18%) in the MRI group was comparable with the screened men in the current trial (20%), although a direct comparison is challenging due to different study designs.
The control group in this trial was not invited to screening and hence the comparator represents the current screening practice in the population. Consequently, the results inform about the absolute population-level effect of introducing an invitation for screening to a population undergoing prostate cancer screening as part of their usual health care. The results are directly applicable to populations with a similar prevalence of PSA testing. The reports from 2 Swedish trials (Göteborg2 and Sthlm3-MRI) compared different diagnostic approaches within a screened cohort and did not include a nonscreened control group.24,25 Their results provide information about the effects of adding MRI to a PSA-based screening regimen among men attending screening.
The strengths of this trial include comprehensive inclusion of the target population (covering all men in the source population), a larger number of randomized men than in the 2 Swedish trials, a high participation rate (51% of the men invited to screening attended compared with 26% in the Sthlm3-MRI study25 and 47% in the Göteborg2 trial24), and 97% to 99% adherence with diagnostic examinations. A Zelen design with randomization before consent, which is possible because the Finnish regulation did not require consent from the control population, allowed inclusion of the entire target population.11,12
Limitations
This study has several limitations. First, the absolute differences between the 2 randomized groups were small and their clinical importance remains unclear. Second, prior screening reported by a substantial proportion of the participants may have reduced cancer detection compared with a population that had not undergone prior screening for prostate cancer, although the findings did not show material differences between participants with and without previous PSA tests. Third, the results were based on a single invitation for screening, so some high-grade prostate cancers were likely missed, although they may be identified after subsequent screening invitations. Fourth, no data on cancers missed at screening is available and interval cancer incidence is needed to assess sensitivity of the screening protocol (ability to detect cases or completeness of cancer detection).
Conclusions
In this preliminary descriptive report from a randomized clinical trial, compared with a control group not offered screening, a single prostate cancer screening intervention that included a PSA test, a kallikrein panel for patients with PSA values of 3.0 ng/mL or higher, and an MRI, the screening intervention detected 1 additional high-grade cancer per 196 men invited to screening and 1 low-grade cancer per 909 men invited to the screening group. These preliminary findings should be interpreted provisionally, pending results of the primary mortality outcome.
Trial protocol
Statistical Analysis Plan
eMethod. Imputation method for replacing missing Grade Group values
eTable 1. Previous PSA test and prostate biopsies among men in the control group. Results from a mail survey to a random sample of 2009 men with 574 participants
eTable 2. Numbers of prostate cancer detected at screening by Grade Group, age group, PSA, kallikrein panel risk score, and MRI PI-RADS score
eTable 3. Detailed Grade Group classification of cases detected at screening by biopsy type (targeted or systematic)
eTable 4. Age, PSA and kallikrein panel risk score (median with interquartile range) for men with screen-detected Grade Group 1 cancer, Grade Group 2–5 cancer and benign biopsy result (excluding one case with a missing Gleason score)
eTable 5. Analysis of cancer detection by trial group with imputation of missing Grade Group based on the stratum-specific observed relative frequencies (by trial group and screening attendance)
eTable 6. Clinical TNM stage in cancers detected at screening, among men invited to screening who did not participate, and in the control group. Incident cases in the non-screened groups include those detected in surgery for benign prostatic hyperplasia (one in men invited to screening who did not participate and fourteen in the control group)
eTable 7. Primary treatment of prostate cancers by trial group, number of cases (%)
Nonauthor Collaborators. ProScreen Trial Investigators
Data Sharing Statement
References
- 1.Ilic D, Djulbegovic M, Jung JH, et al. Prostate cancer screening with prostate-specific antigen (PSA) test: a systematic review and meta-analysis. BMJ. 2018;362:k3519. doi: 10.1136/bmj.k3519 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fenton JJ, Weyrich MS, Durbin S, Liu Y, Bang H, Melnikow J. Prostate-specific antigen-based screening for prostate cancer. JAMA. 2018;319(18):1914-1931. doi: 10.1001/jama.2018.3712 [DOI] [PubMed] [Google Scholar]
- 3.Paschen U, Sturtz S, Fleer D, Lampert U, Skoetz N, Dahm P. Assessment of prostate-specific antigen screening: an evidence-based report by the German Institute for Quality and Efficiency in Health Care. BJU Int. 2022;129(3):280-289. doi: 10.1111/bju.15444 [DOI] [PubMed] [Google Scholar]
- 4.Braun K, Sjöberg DD, Vickers AJ, Lilja H, Bjartell AS. A four-kallikrein panel predicts high-grade cancer on biopsy: independent validation in a community cohort. Eur Urol. 2016;69(3):505-511. doi: 10.1016/j.eururo.2015.04.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vickers A, Vertosick EA, Sjöberg DD, et al. Value of intact prostate specific antigen and human kallikrein 2 in the 4 kallikrein predictive model: an individual patient data meta-analysis. J Urol. 2018;199(6):1470-1474. doi: 10.1016/j.juro.2018.01.070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kasivisvanathan V, Rannikko AS, Borghi M, et al. ; PRECISION Study Group Collaborators . MRI-targeted or standard biopsy for prostate cancer diagnosis. N Engl J Med. 2018;378(19):1767-1777. doi: 10.1056/NEJMoa1801993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Drost FH, Osses D, Nieboer D, et al. Prostate magnetic resonance imaging, with or without magnetic resonance imaging–targeted biopsy, and systematic biopsy for detecting prostate cancer: a Cochrane systematic review and meta-analysis. Eur Urol. 2020;77(1):78-94. doi: 10.1016/j.eururo.2019.06.023 [DOI] [PubMed] [Google Scholar]
- 8.Auvinen A, Rannikko A, Taari K, et al. A randomized trial of early detection of clinically significant prostate cancer (ProScreen): study design and rationale. Eur J Epidemiol. 2017;32(6):521-527. doi: 10.1007/s10654-017-0292-5 [DOI] [PubMed] [Google Scholar]
- 9.Rannikko A, Leht M, Mirtti T, et al. Population-based randomized trial of screening for clinically significant prostate cancer ProScreen: a pilot study. BJU Int. 2022;130(2):193-199. doi: 10.1111/bju.15683 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nevalainen J, Raitanen J, Natunen K, et al. Early detection of clinically significant prostate cancer: protocol summary and statistical analysis plan for the ProScreen randomised trial. BMJ Open. 2024;14(1):e075595. doi: 10.1136/bmjopen-2023-075595 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zelen M. A new design for randomized clinical trials. N Engl J Med. 1979;300(22):1242-1245. doi: 10.1056/NEJM197905313002203 [DOI] [PubMed] [Google Scholar]
- 12.Simon GE, Shortreed SM, DeBar LL. Zelen design clinical trials: why, when, and how. Trials. 2021;22(1):541. doi: 10.1186/s13063-021-05517-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mitrunen K, Pettersson K, Piironen T, Björk T, Lilja H, Lövgren T. Dual-label one-step immunoassay for simultaneous measurement of free and total prostate-specific antigen concentrations and ratios in serum. Clin Chem. 1995;41(8 pt 1):1115-1120. doi: 10.1093/clinchem/41.8.1115 [DOI] [PubMed] [Google Scholar]
- 14.Väisänen V, Peltola MT, Lilja H, Nurmi M, Pettersson K. Intact free prostate-specific antigen and free and total human glandular kallikrein 2. elimination of assay interference by enzymatic digestion of antibodies to F(ab’)2 fragments. Anal Chem. 2006;78(22):7809-7815. doi: 10.1021/ac061201+ [DOI] [PubMed] [Google Scholar]
- 15.Bryant RJ, Sjoberg DD, Vickers AJ, et al. Predicting high-grade cancer at ten-core prostate biopsy using four kallikrein markers measured in blood in the ProtecT study. J Natl Cancer Inst. 2015;107(7):djv095. doi: 10.1093/jnci/djv095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Vertosick EA, Häggström C, Sjöberg DD, et al. Prespecified 4-kallikrein marker model at age 50 or 60 for early detection of lethal prostate cancer in a large population based cohort of asymptomatic men followed for 20 years. J Urol. 2020;204(2):281-288. doi: 10.1097/JU.0000000000001007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Turkbey B, Rosenkrantz AB, Haider MA, et al. Prostate Imaging and Data Reporting System 2 (PIRADS 2.1). Eur Urol. 2019;76:340-351. doi: 10.1016/j.eururo.2019.02.033 [DOI] [PubMed] [Google Scholar]
- 18.van Leenders GJLH, van der Kwast TH, Grignon DJ, et al. ; ISUP Grading Workshop Panel Members . The 2019 International Society for Urological Pathology (ISUP) consensus conference on grading of prostatic carcinoma. Am J Surg Pathol. 2020;44(8):e87-e99. doi: 10.1097/PAS.0000000000001497 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Moore CM, Kasivisvanathan V, Eggener S, et al. ; START Consortium . Standards of reporting for MRI-targeted biopsy studies (START) of the prostate: recommendations from an International Working Group. Eur Urol. 2013;64(4):544-552. doi: 10.1016/j.eururo.2013.03.030 [DOI] [PubMed] [Google Scholar]
- 20.Leinonen MK, Miettinen J, Heikkinen S, Pitkäniemi J, Malila N. Quality measures of the population-based Finnish Cancer Registry indicate sound data quality for solid malignant tumours. Eur J Cancer. 2017;77:31-39. doi: 10.1016/j.ejca.2017.02.017 [DOI] [PubMed] [Google Scholar]
- 21.van der Cruijsen-Koeter IW, Roobol MJ, Wildhagen MF, van der Kwast TH, Kirkels WJ, Schröder FH. Tumor characteristics and prognostic factors in two subsequent screening rounds with four-year interval within prostate cancer screening trial, ERSPC Rotterdam. Urology. 2006;68(3):615-620. doi: 10.1016/j.urology.2006.03.015 [DOI] [PubMed] [Google Scholar]
- 22.Martin RM, Donovan JL, Turner EL, et al. ; CAP Trial Group . Effect of a low-intensity PSA-based screening intervention on prostate cancer mortality: The CAP randomized clinical trial. JAMA. 2018;319(9):883-895. doi: 10.1001/jama.2018.0154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Grubb RL III, Pinsky PF, Greenlee RT, et al. Prostate cancer screening in the PROSTATE, LUNG, COLORECTAL AND OVARIAN cancer screening trial: update on findings from the initial four rounds of screening in a randomized trial. BJU Int. 2008;102(11):1524-1530. doi: 10.1111/j.1464-410X.2008.08214.x [DOI] [PubMed] [Google Scholar]
- 24.Hugosson J, Månsson M, Wallström J, et al. ; GÖTEBORG-2 Trial Investigators . Prostate cancer screening with PSA and MRI followed by targeted biopsy only. N Engl J Med. 2022;387(23):2126-2137. doi: 10.1056/NEJMoa2209454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Eklund M, Jäderling F, Discacciati A, et al. ; STHLM3 consortium . MRI-targeted or standard biopsy in prostate cancer screening. N Engl J Med. 2021;385(10):908-920. doi: 10.1056/NEJMoa2100852 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Trial protocol
Statistical Analysis Plan
eMethod. Imputation method for replacing missing Grade Group values
eTable 1. Previous PSA test and prostate biopsies among men in the control group. Results from a mail survey to a random sample of 2009 men with 574 participants
eTable 2. Numbers of prostate cancer detected at screening by Grade Group, age group, PSA, kallikrein panel risk score, and MRI PI-RADS score
eTable 3. Detailed Grade Group classification of cases detected at screening by biopsy type (targeted or systematic)
eTable 4. Age, PSA and kallikrein panel risk score (median with interquartile range) for men with screen-detected Grade Group 1 cancer, Grade Group 2–5 cancer and benign biopsy result (excluding one case with a missing Gleason score)
eTable 5. Analysis of cancer detection by trial group with imputation of missing Grade Group based on the stratum-specific observed relative frequencies (by trial group and screening attendance)
eTable 6. Clinical TNM stage in cancers detected at screening, among men invited to screening who did not participate, and in the control group. Incident cases in the non-screened groups include those detected in surgery for benign prostatic hyperplasia (one in men invited to screening who did not participate and fourteen in the control group)
eTable 7. Primary treatment of prostate cancers by trial group, number of cases (%)
Nonauthor Collaborators. ProScreen Trial Investigators
Data Sharing Statement


