This secondary analysis of a randomized clinical trial assesses whether screening for prostate-specific antigen reduces prostate cancer mortality at 15-year follow-up.
Key Points
Question
In men aged 50 to 69 years, does a single invitation for a prostate-specific antigen (PSA) screening test reduce prostate cancer mortality at 15-year follow-up compared with no invitation for testing?
Findings
In this secondary analysis of a randomized clinical trial of 415 357 men aged 50 to 69 years randomized to a single invitation for PSA screening (n = 195 912) or a control group without PSA screening (n = 219 445) and followed up for a median of 15 years, risk of death from prostate cancer was lower in the group invited to screening (0.69% vs 0.78%; mean difference, 0.09%) compared with the control group.
Meaning
Compared with no invitation for routine PSA testing, a single invitation for a PSA screening test reduced prostate cancer mortality at a median follow-up of 15 years, but the absolute mortality benefit was small.
Abstract
Importance
The Cluster Randomized Trial of PSA Testing for Prostate Cancer (CAP) reported no effect of prostate-specific antigen (PSA) screening on prostate cancer mortality at a median 10-year follow-up (primary outcome), but the long-term effects of PSA screening on prostate cancer mortality remain unclear.
Objective
To evaluate the effect of a single invitation for PSA screening on prostate cancer–specific mortality at a median 15-year follow-up compared with no invitation for screening.
Design, Setting, and Participants
This secondary analysis of the CAP randomized clinical trial included men aged 50 to 69 years identified at 573 primary care practices in England and Wales. Primary care practices were randomized between September 25, 2001, and August 24, 2007, and men were enrolled between January 8, 2002, and January 20, 2009. Follow-up was completed on March 31, 2021.
Intervention
Men received a single invitation for a PSA screening test with subsequent diagnostic tests if the PSA level was 3.0 ng/mL or higher. The control group received standard practice (no invitation).
Main Outcomes and Measures
The primary outcome was reported previously. Of 8 prespecified secondary outcomes, results of 4 were reported previously. The 4 remaining prespecified secondary outcomes at 15-year follow-up were prostate cancer–specific mortality, all-cause mortality, and prostate cancer stage and Gleason grade at diagnosis.
Results
Of 415 357 eligible men (mean [SD] age, 59.0 [5.6] years), 98% were included in these analyses. Overall, 12 013 and 12 958 men with a prostate cancer diagnosis were in the intervention and control groups, respectively (15-year cumulative risk, 7.08% [95% CI, 6.95%-7.21%] and 6.94% [95% CI, 6.82%-7.06%], respectively). At a median 15-year follow-up, 1199 men in the intervention group (0.69% [95% CI, 0.65%-0.73%]) and 1451 men in the control group (0.78% [95% CI, 0.73%-0.82%]) died of prostate cancer (rate ratio [RR], 0.92 [95% CI, 0.85-0.99]; P = .03). Compared with the control, the PSA screening intervention increased detection of low-grade (Gleason score [GS] ≤6: 2.2% vs 1.6%; P < .001) and localized (T1/T2: 3.6% vs 3.1%; P < .001) disease but not intermediate (GS of 7), high-grade (GS ≥8), locally advanced (T3), or distally advanced (T4/N1/M1) tumors. There were 45 084 all-cause deaths in the intervention group (23.2% [95% CI, 23.0%-23.4%]) and 50 336 deaths in the control group (23.3% [95% CI, 23.1%-23.5%]) (RR, 0.97 [95% CI, 0.94-1.01]; P = .11). Eight of the prostate cancer deaths in the intervention group (0.7%) and 7 deaths in the control group (0.5%) were related to a diagnostic biopsy or prostate cancer treatment.
Conclusions and Relevance
In this secondary analysis of a randomized clinical trial, a single invitation for PSA screening compared with standard practice without routine screening reduced prostate cancer deaths at a median follow-up of 15 years. However, the absolute reduction in deaths was small.
Trial Registration
isrctn.org Identifier: ISRCTN92187251
Introduction
In England, the number of men diagnosed with prostate cancer increased by 68% from 28 216 in 2001 to 47 479 in 2019,1 reflecting population aging and increased prostate-specific antigen (PSA) testing.2 In the US, approximately 3.3 million men currently live with a diagnosis of prostate cancer.3 While low-risk prostate cancer progresses slowly and is associated with a low risk of mortality,4,5,6,7 aggressive prostate cancer currently causes approximately 12 000 deaths in the UK and 34 700 deaths in the US annually.3,8 The goal of PSA screening is to reduce prostate cancer mortality by early detection of curable disease. However, uncertainty remains regarding the long-term effect of PSA-based screening on mortality.9,10,11
The Cluster Randomized Trial of PSA Testing for Prostate Cancer (CAP) (N = 415 357) showed that compared with usual care (no screening), an invitation to a single PSA screening test increased the number of prostate cancers diagnosed during the first 18 months of follow-up (the period when PSA testing and subsequent biopsies for men with an elevated level of PSA took place). In this trial, rates of diagnosed prostate cancer in the first 18 months were 2.2 per 1000 person-years in the control group and 10.4 per 1000 person-years in the intervention group (P < .001).10 However, at a median 10-year follow-up, the invitation for a single PSA screening did not reduce prostate cancer mortality compared with the control (0.29% vs 0.30%; rate ratio, 0.96 [95% CI, 0.85-1.08]; P = .50).10 This secondary analysis of the CAP trial describes the effects of this single invitation to a PSA screening test, with subsequent diagnostic tests if the PSA level was 3.0 ng/mL or higher (to convert to micrograms per liter, multiply by 1.0), on the prespecified secondary outcome of prostate-cancer mortality at 15-year follow-up compared with standard practice (no screening).12
Methods
The Derby National Research Ethics Service Committee East Midlands approved this study (ISRCTN92187251). The trial protocol and statistical analysis plan are available in Supplement 1. Participants were enrolled between January 8, 2002, and January 20, 2009. Final follow-up occurred on March 31, 2021. Men who attended PSA testing in the intervention group gave individual written informed consent via the ProtecT study.13 Individual consent was not sought from men in the control group or from nonresponders in the intervention group. Instead, approval for their identification and linkage to routine electronic records was obtained under Section 251 of the National Health Services Act 2006 from the UK Patient Information Advisory Group (now Confidentiality Advisory Group).10 All clinical centers had local research governance approval. This study followed the Consolidated Standards of Reporting Trials (CONSORT) reporting guideline. Study data were collected using REDCap electronic data capture tools hosted at the University of Bristol.
Randomization
The CAP trial was a primary care–based cluster randomized clinical trial that tested the effects of a single invitation for a PSA screening test (eFigure 1 in Supplement 2) compared with usual care (no screening) on the primary outcome of prostate cancer mortality at a median follow-up of 10 years. The primary outcome has been reported previously.10 Between September 25, 2001, and August 24, 2007, 785 eligible general practices in the catchment area of 8 hospitals across England and Wales (located in Birmingham, Bristol, Cambridge, Cardiff, Leeds, Leicester, Newcastle, and Sheffield) were randomized before recruitment (Zelen design) to intervention or control groups, and practices were invited to consent to participate. Randomization was blocked and stratified within groups of 10 to 12 neighboring practices using a computerized random number generator. Because allocation preceded the invitation for practices to participate, it was not possible to conceal allocation. A total of 573 practices (73%), including 271 (68%) randomized to the intervention group and 302 (78%) randomized to the control group, agreed to participate (Figure 1).
Figure 1. Recruitment, Randomization, and Flow of Practices and Patients.
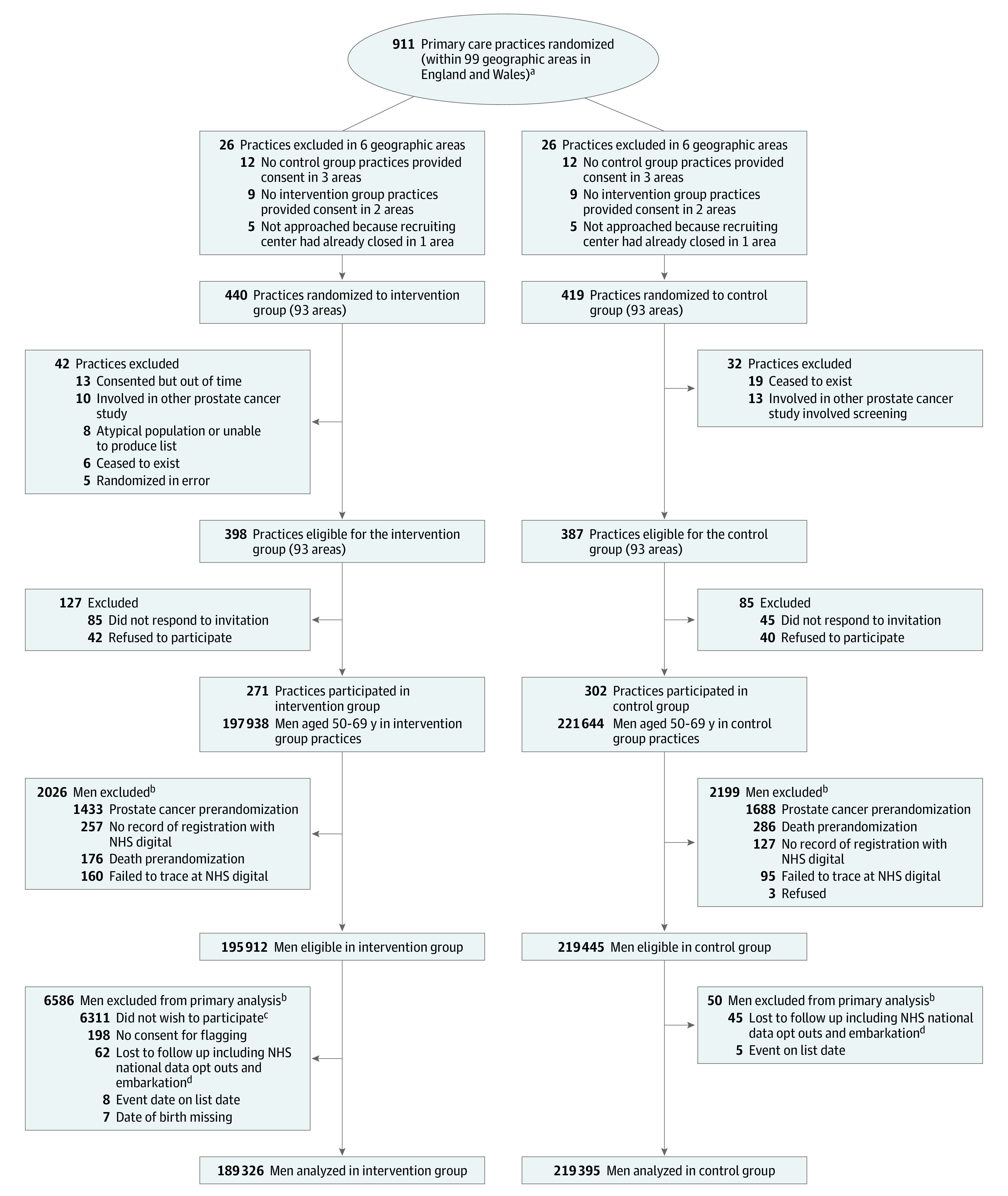
Follow-up was through routine National Health Services (NHS) electronic vital status and cancer registry databases for diagnoses and deaths notified by November 17, 2021, but that occurred up to March 31, 2021.
aPractices were randomized prior to invitation to take part in the trial. Randomization details are given in the Randomization subsection of the Methods section.
bNumbers of men are as of November 17, 2021, and are subject to small changes over time because of continued updates from NHS Digital (eg, changes to the trace status of the men, men newly successfully traced). Note that not all men traced at 15 years were traced at 10 years.
cPseudo-anonymized follow-up.
dNHS digital national data opt-outs (previously type 2 opt-outs) preventing NHS data being used for research.14
Participants
Men aged 50 to 69 years in each participating randomized general practice were included. Men with prostate cancer on or before the randomization date and those registered as a patient with participating practices on a temporary or emergency basis were excluded. Race and ethnicity for men attending the intervention group PSA test clinic were ascertained by a nurse using standardized definitions as one of a range of baseline characteristics to assess generalizability.13 Race and ethnicity were defined using UK Office for National Statistics Census categories and recoded as White and other (all other categories collapsed due to low numbers of participants who were not White). Race and ethnicity data were not available from National Health Services (NHS) routine data that we had access to at the time, so we could not compute these data for the control group.
Intervention
Men in practices randomized to the intervention received a single invitation for a PSA test after counseling. If the resulting PSA level was 3.0 to 19.9 ng/mL, they were offered 10-core transrectal ultrasonography-guided biopsies. All laboratories participated in the UK National External Quality Assessment Service for PSA testing. Test results that did not meet laboratory quality assurance requirements or were lost were considered nonvalid, as were tests for which consent was ambiguous or insufficient blood was obtained. Men in the intervention group diagnosed with localized prostate cancer were invited to participate in a second randomized clinical trial, the ProtecT treatment trial, which randomized participants to active monitoring (consisting of regular PSA testing and clinical review), radical prostatectomy, or radical conformal radiotherapy with neoadjuvant androgen deprivation (eFigure 1 in Supplement 2).15 Men with a PSA level of 20 ng/mL or higher were referred to a urologist and received standard care.
Men in practices randomized to the control group received standard NHS management but did not receive a formal invitation for PSA testing as part of this study.16 We assessed cumulative PSA testing for prostate cancer detection in the control group of the CAP trial by longitudinal analysis of a national primary care database (434 236 men from 558 UK primary care practices).2
Outcomes
The primary outcome of the CAP trial, 10-year prostate cancer mortality, was reported previously.10 Prespecified secondary outcomes were definite or probable prostate cancer mortality at 15-year follow-up, all-cause mortality at 10-year follow-up, all-cause mortality at 15-year follow-up, all-cause mortality at 5-year follow-up, prostate cancer mortality at 5-year follow-up, disease grade and staging, cost-effectiveness, and health-related quality of life. The protocol did not indicate the time point for assessing prostate cancer grade and staging; these were measured at median follow-up time points of 10 years and 15 years. Previously reported outcomes were all-cause mortality at 10-year follow-up,10 disease grade and stage at 10-year follow-up,10 cost-effectiveness,17 and health-related quality of life.18 The current report provides results for the remaining secondary outcomes of definite or probable prostate cancer mortality at 15-year follow-up, all-cause mortality at 15-year follow-up, and disease grade and stage at 15-year follow-up. All-cause and prostate cancer mortality at 5-year follow-up were not published separately, but 5-year follow-up data are shown in Kaplan-Meier curves both in the current report and in the publication of the 10-year primary outcome.10
Outcome Ascertainment
Prostate cancer mortality at 15-year follow-up was ascertained with death certificates from the Office for National Statistics at NHS England and adjudicated by an independent cause of death evaluation committee using clinical information from hospital medical records and following a standardized protocol.19,20 Prostate cancer stage and Gleason grade were obtained from the National Disease Registration Service21 (formerly Public Health England) at NHS England and Public Health Wales22 up to December 31, 2020.
Exploratory Outcomes
Additional outcomes reported here that were described in the published original statistical analysis plan10 were (1) mean age at diagnosis in the allocated groups and (2) a sensitivity analysis redefining the primary outcome. This analysis included (1) definite, probable, possible, and treatment-related prostate cancer mortality and (2) definite and treatment-related prostate cancer mortality.
Post Hoc Outcomes
We estimated differences in the risk of prostate cancer diagnosis between the intervention and control groups at 18 months, 10 years, and 15 years to quantify changes in diagnosis rates over long-term follow-up. We calculated mean sojourn time (the period in which a tumor is asymptomatic but detectable by screening) from microsimulation using estimated transition parameters for single episodes of screening between ages 50 to 69 years and overdiagnosis rates as the difference in the cumulative prostate cancer incidence between screened and unscreened groups over a lifetime (further methodologic details are given in the eMethods in Supplement 2).23,24
Statistical Analysis
The intervention effect at a median 15-year follow-up (March 31, 2021) was analyzed comparing groups as randomized using random-effects Poisson regression to estimate prostate cancer–specific and all-cause mortality rate ratios (RRs) in intervention vs control practices, allowing for clustering within primary care practices and randomization strata. To allow for variation in the incidence of prostate cancer with age, follow-up for each participant was divided into periods within 5-year age groups. We present rates (per 1000 person-years) and Kaplan-Meier estimates of the cumulative risk (per 100 men) of prostate cancer diagnosis and prostate cancer and all-cause mortality.
In prespecified analyses described in the original statistical analysis plan (Supplement 1), we used instrumental methods (generalized method of moments estimator) to estimate the effect of attending the PSA screening clinic at a median 15-year follow-up compared with men in the control group who would have attended the clinic if invited, adjusting for age group and using robust SEs to allow for variation between practices. We also compared mean age, prostate cancer clinical stage (T1/T2, T3, and T4/N1/M1 disease), and Gleason score (6 [low-grade], 7 [intermediate grade], and ≥8 [high grade]) at diagnosis between the intervention and control groups using ordered logistic regression.
Prespecified subgroup analyses investigated variation in the effect of screening on prostate cancer mortality by baseline age group and quintiles of the geographic area–based Index of Multiple Deprivation, a measure of socioeconomic status. An interaction test P value was used to evaluate the evidence against the null hypothesis of equal intervention effect across subgroups.
In accordance with our original analysis plan,10 we did not conduct multiple imputation analyses. The statistical analysis plan did not specify an intention to adjust P values for multiple comparisons; conventional adjustments assume statistical independence between estimates, which was not the case for analyses of the same outcome at 10 and 15 years. All statistical testing was for superiority, and P values were 2-sided. In interpreting the results, we focused on estimated effects and associated 95% CIs. Results were considered statistically significant if the P value was <.05 or not statistically significant if the P value was ≥.05. All trial analyses were conducted using Stata, version 16.1 (StataCorp LLC).
Results
Study Population
A total of 911 primary care practices were randomized in 99 geographic areas. Of these, 126 were subsequently excluded as ineligible (Figure 1).12 Consent rates were 68% (271 of 398) among eligible practices in the intervention group and 78% (302 of 387) among eligible practices in the control group. Overall, 415 357 men (mean [SD] age, 59.0 [5.6] years) registered with these practices were eligible for the intervention (n = 195 912) and control (n = 219 445) groups. Follow-up data for cancer diagnosis and mortality at a median of 15 years after randomization were available for 408 721 of the eligible men (98%), including 189 326 (97%) randomized to the intervention and 219 395 (>99%) randomized to control (Figure 1). In the intervention group, 98% of patients were White and 2% were other race and ethnicity; data were not available for the control group.
Baseline characteristics were similar between intervention and control groups at the practice and individual levels (Table 1). Among people randomized to the intervention who developed prostate cancer (n = 12 013), 9% were missing data for cancer stage and 10% were missing data for Gleason grade. Among people randomized to the control group who developed prostate cancer (n = 12 958), 8% were missing data for cancer stage and 11% were missing data for Gleason grade.
Table 1. Individual- and Practice-Level Characteristics at Baseline Among Consented Primary Care Practices and Men Included in the Analysisa.
| Characteristic | Intervention group | Control group |
|---|---|---|
| Individual characteristics | ||
| Men, No. | 189 326 | 219 395 |
| Age, median (IQR), y | 58.5 (54.3-63.5) | 58.6 (54.3-63.5) |
| Index of Multiple Deprivation score, median (IQR)b | ||
| England | 17.5 (10.1-33.2) | 16.9 (9.8-32.4) |
| Wales | 17.6 (9.2-29.5) | 13.7 (7.1-29) |
| Urban area, %c | 86 | 86 |
| Race and ethnicity, % | ||
| White | 98 | NA |
| Otherd | 2 | NA |
| Practice characteristics | ||
| Practices, No. | 271 | 302 |
| Practice list size, median (IQR)e | 6300 (4150-9107) | 6300 (3793-9000) |
| Urban practices, No. (%) | 244 (90) | 267 (88) |
| Multiple-partner practices, No. (%) | 242 (89) | 267 (88) |
| Partners in practice, No. (%) | ||
| Single partnerf | 21 (8) | 29 (10) |
| Small (2-3 practitioners) | 60 (22) | 61 (20) |
| Medium or large (≥4 practitioners) | 128 (47) | 146 (48) |
| Missing | 62 (23) | 66 (22) |
| QOF points achieved, median (IQR), % [n]g | 98.9 (97.4-99.6) [224] | 99 (97.4-99.7) [266] |
| Index of Multiple Deprivation score, median (IQR) [n] | ||
| England | 21.8 (12.7-44.1) [231] | 23.6 (13.3-46.7) [271] |
| Wales | 18.8 (11.9-22.9) [40] | 20.1 (7.6-34.5) [31] |
| Prevalence, mean (SD), %h | ||
| All cancers | 0.6 (0.3) | 0.5 (0.2) |
| Diabetes | 3.6 (1.0) | 3.7 (1.0) |
| Coronary heart disease | 4.1 (1.4) | 3.9 (1.3) |
| Obesity | 8.0 (2.8) | 7.8 (2.8) |
Abbreviations: NA, not available; QOF, Quality and Outcomes Framework.
A measure of relative deprivation for small areas; a higher score indicates more deprivation (range, 0-100). English and Welsh scores are not directly comparable. The Index of Multiple Deprivation for the practice refers to the area of the practice, not where patients live.
Based on rural or urban classification 2004, a measure of population density and sparseness; urban was defined as areas with more than 10 000 people.
All other categories were collapsed due to low numbers of participants who were not White.
Total number of individuals registered at primary care practices.
Primary care practices with a single general practitioner registered and practicing there.
Based on 2007-2008 data for England only. Scores are measured from 135 indicators and 1 measure of depth of care (holistic care) and are split across clinical, organizational, patient experience, and additional services domains (maximum score, 1000 points).
Obtained from the clinical domain indicators of QOF. Practices reported counts of patients with each condition and practice list size, enabling calculation of mean prevalence.
Rates of PSA Testing
Overall, 75 694 men (40%) men randomized to the intervention group underwent PSA testing, and 64 425 (34%) had a valid (as defined in the Methods) test result. Of these, 6855 (11%) had a PSA value between 3 and 19.9 ng/mL and were eligible for the ProtecT trial. Of these, 5848 (85%) had a prostate biopsy. Cumulative PSA testing for prostate cancer detection in the control group was indirectly estimated at 10% to 15% over a median 10-year follow-up.2,10
Prostate Cancer Deaths
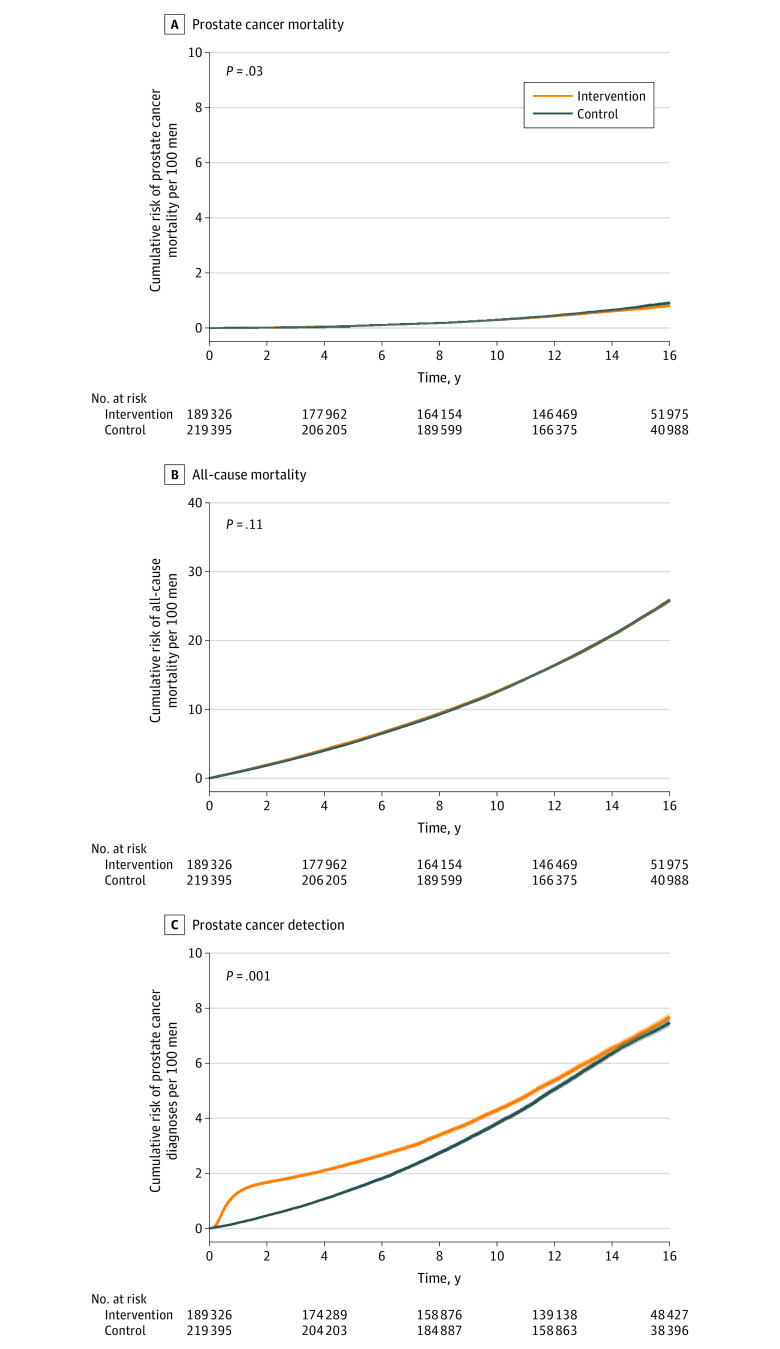
After a median follow-up of 15.4 years (IQR, 14.2-16.4; range, 12.2-19.2), there were 1199 deaths due to prostate cancer (rate, 0.47 [95% CI, 0.45-0.50] per 1000 person-years) in the intervention group and 1451 deaths (rate, 0.50 [95% CI, 0.48-0.53] per 1000 person-years) in the control group (RR, 0.92 [95% CI, 0.85-0.99]; P = .03) (Table 2 and Figure 2A). At a median 15-year follow-up, the cumulative risks of prostate cancer mortality were 0.69% (95% CI, 0.65%-0.73%) in the intervention group and 0.78% (95% CI, 0.73%-0.82%) in the control group (risk difference, −0.09% [95% CI, −0.15% to −0.03%]; P = .02) (Table 2 and eTable 1 in Supplement 2). Using instrumental variable analysis, the prostate cancer mortality RR for the effect of screening among men attending PSA testing clinics was 0.83 (95% CI, 0.68-1.00; P = .053) (Table 2).
Table 2. Effect of the Trial Intervention on Prostate Cancer–Specific and All-Cause Mortality and Prostate Cancer Diagnosis by Random Allocation and Instrumental Variable Analysis After a Median 15-Year Follow-Upa.
| Outcome | Intervention group (n = 189 326; 2 543 298 person-years) | Control group (n = 219 395; 2 885 418 person-years) | Estimated effect of intervention vs control | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Events, No. | Rate, per 1000 person-years (95% CI) | Risk at 15 y, % (95% CI)b | Events, No. | Rate, per 1000 person-years (95% CI) | Risk at 15 y, % (95% CI)b | Risk difference at 15 y, percentage points (95% CI) | Rate ratio (95% CI)c | P valuec,d | ||
| 15-y Prostate cancer mortalitye | ||||||||||
| As randomized | 1199 | 0.47 (0.45 to 0.50) | 0.69 (0.65 to 0.73) | 1451 | 0.50 (0.48 to 0.53) | 0.78 (0.73 to 0.82) | −0.09 (−0.15 to −0.03) | 0.92 (0.85 to 0.99) | .03 | |
| IV analysisf | NA | NA | NA | NA | NA | NA | NA | 0.83 (0.68 to 1.00) | .053 | |
| 15-y All-cause mortality | ||||||||||
| As randomized | 45 084 | 17.7 (17.6 to 17.9) | 23.2 (23.0 to 23.4) | 50 336 | 17.4 (17.3 to 17.6) | 23.3 (23.1 to 23.5) | −0.07 (−0.35 to 0.21) | 0.97 (0.94 to 1.01) | .11 | |
| IV analysisf | NA | NA | NA | NA | NA | NA | NA | 1.01 (0.91 to 1.12) | .85 | |
| 15-y Prostate cancer diagnoses | ||||||||||
| As randomized | 12 013 | 4.88 (4.80 to 4.97) | 7.08 (6.95 to 7.21) | 12 958 | 4.60 (4.52 to 4.68) | 6.94 (6.82 to 7.06) | 0.14 (−0.04 to 0.31) | 1.06 (1.02 to 1.09) | .001 | |
Abbreviations: IV, instrumental variable; NA, not applicable.
Median follow-up time was 15.43 years (IQR, 14.23-16.43; range, 12.19-19.23). The intervention was a single invitation to prostate-specific antigen screening. Median 10-year estimates can be found in Martin et al.10
The numbers of deaths for the cumulative 15-year risk in the intervention and control groups were 1018 and 1288, respectively.
Adjusted for current age using a lexis diagram approach; variation between randomization cluster and primary care practice were accommodated by random effects in a 3-level model.
Likelihood ratio test of the null hypothesis of no difference between the groups.
Defined as definite or probable prostate cancer death or intervention-related death by an independent cause of death committee.
Instrumental variable analysis to estimate the effect of screening among those attending the prostate-specific antigen testing clinic using a generalized method of moments estimator with random allocation as the instrumental variable.
Figure 2. Effect of the Trial Intervention on the Cumulative Incidence (95% CI) of Prostate Cancer Mortality and Diagnosis and All-Cause Mortality After a Median 15-Year Follow-Up.
P values are from a random-effects Poisson model (see Statistical Analysis subsection of the Methods section).
Overall Survival
There were 45 084 total deaths (23.2% [95% CI, 95% CI, 23.0%-23.4%]) in the intervention group and 50 336 total deaths (23.3% [95% CI, 23.1%-23.5%]) in the control group (RR, 0.97 [95% CI, 0.94-1.01]; P = .11) (Table 2 and Figure 2B). Other causes of death were similar between the 2 groups (eTable 2 in Supplement 2).
Prostate Cancer Grade and Stage
Compared with the control group, men in the intervention group were at higher risk of diagnosis of low-grade prostate cancer (2.2% vs 1.6%; risk difference, 0.58% [95% CI, 0.50%-0.67%]) and at lower risk of high-grade prostate cancers (1.2% vs 1.3%; risk difference, −0.15% [95% CI, −0.22% to −0.08%]) over the 15-year follow-up (P < .001 for trend). There was a higher risk of localized prostate cancers (3.6% vs 3.1%; risk difference, 0.56% [95% CI, 0.44%-0.67%]) and a lower risk of advanced-stage tumors (0.9% vs 1.1%; risk difference, −0.16% [95% CI, −0.22% to −0.10%]) over the 15-year follow-up in the intervention group vs control group (P < .001 for trend) (eTable 3 and eFigures 2 and 3 in Supplement 2).
Exploratory Results
The mortality results were similar when including in the outcome definition prostate cancer–specific deaths judged as possible by the cause of death evaluation committee and when restricting to those judged as definite prostate cancer–specific deaths (eTable 4 in Supplement 2). There was little evidence that the intervention effect differed by age group or socioeconomic status (P ≥ .46 for interaction) (Table 3). Compared with the control group, intervention group men were a mean 1.22 years (95% CI, 1.02-1.42 years; P < .001) younger at prostate cancer diagnosis (eTable 3 in Supplement 2).
Table 3. Exploratory Analysis of Prostate Cancer Mortality Rate Ratios Comparing Intervention vs Control Groups by Age and Area Deprivation Index Score After a Median 15-Year Follow-Up.
| Outcome | Intervention group (n = 189 326; 2 543 298 person-years) | Control group (n = 219 395; 2 885 418 person-years) | Estimated effect of intervention vs control | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Deaths, No. | Rate, per 1000 person-years (95% CI) | Risk at 15 y, % (95% CI) | Deaths, No. | Rate, per 1000 person-years (95% CI) | Risk at 15 y, % (95% CI) | Risk difference at 15 y, percentage points (95% CI) | Rate ratio (95% CI)a | P value for interactiona | |
| Age at baseline, y | |||||||||
| 50-54 | 132 | 0.17 (0.14 to 0.20) | 0.22 (0.18 to 0.27) | 154 | 0.18 (0.15 to 0.21) | 0.25 (0.21 to 0.30) | −0.03 (−0.09 to 0.03) | 0.96 (0.76 to 1.22) | .75 |
| 55-59 | 251 | 0.33 (0.29 to 0.38) | 0.47 (0.41 to 0.54) | 300 | 0.35 (0.31 to 0.39) | 0.54 (0.47 to 0.61) | −0.07 (−0.16 to 0.02) | 0.92 (0.78 to 1.10) | |
| 60-64 | 368 | 0.64 (0.58 to 0.71) | 0.97 (0.87 to 1.09) | 465 | 0.70 (0.64 to 0.77) | 1.10 (1.00 to 1.22) | −0.13 (−0.28 to 0.02) | 0.90 (0.77 to 1.04) | |
| 65 to ≥69b | 448 | 1.05 (0.96 to 1.15) | 1.61 (1.45 to 1.78) | 532 | 1.07 (0.99 to 1.17) | 1.76 (1.60 to 1.93) | −0.15 (−0.38 to 0.08) | 0.98 (0.86 to 1.12) | |
| IMD area deprivation tertile Englandc | |||||||||
| Most affluent | 326 | 0.44 (0.40 to 0.50) | 0.61 (0.54 to 0.69) | 425 | 0.47 (0.43 to 0.52) | 0.71 (0.64 to 0.79) | −0.11 (−0.21 to 0.00) | 0.92 (0.79 to 1.07) | .46 |
| Mid-level | 373 | 0.51 (0.46 to 0.56) | 0.76 (0.68 to 0.85) | 463 | 0.53 (0.49 to 0.58) | 0.84 (0.76 to 0.93) | −0.08 (−0.20 to 0.04) | 0.94 (0.82 to 1.07) | |
| Most deprived | 351 | 0.48 (0.44 to 0.54) | 0.74 (0.66 to 0.83) | 444 | 0.55 (0.50 to 0.61) | 0.86 (0.77 to 0.95) | −0.11 (−0.23 to 0.01) | 0.85 (0.74 to 0.99) | |
| IMD area deprivation tertile Walesd | |||||||||
| Most affluent | 45 | 0.41 (0.31 to 0.55) | 0.52 (0.37 to 0.73) | 43 | 0.34 (0.25 to 0.46) | 0.47 (0.34 to 0.65) | 0.05 (−0.19 to 0.28) | 1.16 (0.76 to 1.77) | .84 |
| Mid-level | 48 | 0.37 (0.28 to 0.49) | 0.62 (0.46 to 0.84) | 36 | 0.40 (0.29 to 0.56) | 0.60 (0.43 to 0.84) | 0.02 (−0.25 to 0.30) | 0.89 (0.55 to 1.43) | |
| Most deprived | 56 | 0.49 (0.37 to 0.63) | 0.66 (0.49 to 0.89) | 39 | 0.41 (0.30 to 0.56) | 0.72 (0.52 to 1.02) | −0.07 (−0.38 to 0.25) | 1.23 (0.82 to 1.85) | |
Abbreviations: IMD, Index of Multiple Deprivation; NA, not applicable.
Adjustment for age stratum and practice cluster effects apart from age, which was not adjusted for age stratum.
By the time some men underwent PSA testing, they were 70 years of age.
Scores range from 0 to 100, with higher scores indicating higher levels of deprivation. Tertile 1 has scores from 1.08 to 12.17, tertile 2 has scores from 12.18 to 25.95, and tertile 3 has scores from 25.97 to 79.98.
Scores range from 0 to 100, with higher scores indicating higher levels of deprivation. Tertile 1 has scores from 1.40 to 10.30, tertile 2 has scores from 10.40 to 23.30, and tertile 3 has scores from 23.40 to 78.90.
Post Hoc Results
After a median 15-year follow-up, there were 12 013 prostate cancer diagnoses in the intervention group (4.88 [95% CI, 4.80-4.97] per 1000 person-years; cumulative risk, 7.08% [95% CI, 6.95%-7.21%]) and 12 958 in the control group (4.60 [95% CI, 4.52-4.68] per 1000 person-years; cumulative risk, 6.94% [95% CI, 6.82%-7.06%]) (Table 2 and Figure 2C). Differences in the risks of prostate cancer diagnosis between the intervention and control groups varied markedly during follow-up. Cumulative risk differences per 1000 men for the intervention vs control groups were 12.23 (95% CI, 11.63-12.84) at 18 months, 4.80 (95% CI, 3.53-6.07) at 10 years, 1.38 (95% CI, −0.38 to 3.14) at 15 years, and 0.86 (95% CI, −1.80 to 3.53) at 18 years (eTable 1 in Supplement 2).
For the group aged 50 to 54 years compared with the group aged 65 to 69 years, the mean sojourn time was 12.1 years (95% CI, 12.1-12.2 years) vs 15.3 years (95% CI, 15.2-15.3 years). The mean probability of overdiagnosis in these groups was 9.2% (95% CI, 8.9%-9.4%) vs 20.8% (95% CI, 20.6%-21.0%) (eTable 5 and eFigures 4-6 in Supplement 2).
Adverse Events
Among the deaths due to prostate cancer, 8 in the intervention group (0.7%) and 7 in the control group (0.5%) were related to a diagnostic biopsy or prostate cancer treatment.10 Other adverse events were reported previously.9,11
Discussion
In this secondary analysis of a cluster randomized clinical trial of 415 357 men aged 50 to 69 years, compared with usual care, a single invitation to undergo a PSA test led to an absolute reduction in prostate cancer mortality of 0.09% after a median follow-up of 15 years. However, the magnitude of the effect was small. There was no effect on overall survival. Policymakers considering screening for prostate cancer should consider this small reduction in deaths against the potential adverse effects associated with overdiagnosis and overtreatment of prostate cancer.6,25
The CAP trial previously reported no benefit of a single invitation to PSA screening on the primary outcome of prostate cancer mortality at a median follow-up of 10 years.10 Prostate-specific antigen testing is increasingly common,2 particularly among men older than 60 years,2,26 and definitive evidence on the benefits and harms of PSA screening remain unclear.25 Analyses reported here are important because of the need for a longer follow-up period to evaluate the effect of PSA detection of prostate cancers,5 particularly as findings from the ProtecT trial showed no difference in mortality irrespective of treatment over 15 years.6
The magnitude of reduction in prostate cancer mortality was smaller than the a priori defined effect size considered important for clinical and public health benefit.12 The harms of PSA testing include overdiagnosis; biopsy complications9; adverse treatment effects on urinary, sexual, and bowel function11; and the potential to miss an aggressive prostate cancer.10 The clinical trial’s single invitation to a PSA screening test aimed to minimize overdiagnosis and overtreatment compared with other screening trials, but overdiagnosis was still observed after a median 15-year follow-up. The European Randomized Study of Prostate Cancer Screening (ERSPC) randomized clinical trial (N = 162 243), which combined data from 7 centers with different protocols and screening strategies, reported that PSA screening conducted every 2 to 4 years (mean of 1.4 tests per participant) reduced prostate cancer mortality after 16 years (RR, 0.80 [95% CI, 0.72-0.89]).27 The Prostate, Lung, Colorectal and Ovarian (PLCO) randomized clinical trial (N = 76 683) reported little evidence of prostate cancer mortality benefit after 17 years with annual PSA testing compared with usual care (RR, 0.93 [95% CI, 0.81-1.08])28 but was limited by high rates of PSA testing in the control group (mean of 2.7 routine PSA tests over the trial’s 6-year intervention period29) and only 35% adherence to recommendations for diagnostic biopsy.30 The Stockholm clinical trial compared 1-time PSA screening and diagnostic investigations if the PSA level was higher than 10 ng/mL with an unscreened control group.31 It demonstrated overdiagnosis of prostate cancer (persistent excess in cumulative prostate cancer incidence in the screening intervention group throughout follow-up), without reduced prostate cancer mortality after 20-year follow-up. Multiple screening tests implemented in the ERSPC and PLCO trials increased overdiagnosis,32 with evidence of a strong positive correlation between the extent of the absolute prostate cancer mortality reduction achieved by the screening intervention and the extent of overdiagnosis (quantified as the risk difference in cumulative incidence of prostate cancer between the trial arms).33
Strengths and Limitations
This study has several strengths. First, compared with randomizing individual patients, recruitment in general practice clusters is expected to minimize volunteer bias and reduce contamination in the control group, in which the intervention effects also cause greater screening in the control group. Cumulative PSA testing in the control arm of this clinical trial was indirectly estimated at 10% to 15% over the median 10-year follow-up, consistent with current UK policy not to recommend screening. A priori estimates suggested that the effect on statistical power of ever undergoing PSA testing during follow-up in the control group (contamination) would be minimal unless the PSA testing rate reached 20%.12 Second, all practices followed the same screening and diagnosis protocol, providing consistent results. Third, among those with an elevated PSA level, adherence with recommendations for biopsy was high at 85%, similar to the rate in the ERSPC trial (81%) and higher than the rate in the PLCO trial (35%). This feature of the clinical trial would likely improve the potential effectiveness of screening, which depends on patients’ willingness to undergo subsequent diagnostic tests. Fourth, the large sample size of this trial contributed to excellent statistical power to detect a clinically meaningful effect size (a prostate cancer mortality RR of 0.87), assuming that PSA testing in the intervention arm was between 35% and 50% and that less than 20% of the control group had PSA testing.12 Fifth, the comprehensive national electronic health record linkage of all the men in this clinical trial helped attain a follow-up rate of 98% over the median 15-year follow-up period.
This study has several limitations. First, the screening intervention involved a single invitation for a PSA screening test, which is not typical of organized screening programs. Some advanced prostate cancers that might have been identified in subsequent screening rounds were likely missed. Second, NHS electronic records were used to identify prostate cancer, resulting in missing data for clinical characteristics and possible delays in recording diagnoses. Third, prostate cancer mortality at 15 years was a secondary outcome. Fourth, since this clinical trial began, newer diagnostic methods34 and more effective treatments for advanced and metastatic prostate cancer35 have been identified. Fifth, few Black men, who are at higher risk of prostate cancer, were included.36
Conclusions
In this secondary analysis of a randomized clinical trial, a single invitation for PSA screening compared with standard practice without routine screening reduced prostate cancer deaths at a median follow-up of 15 years. However, the absolute reduction in deaths was small.
Educational Objective: To identify the key insights or developments described in this article.
-
This study describes the effects of prostate cancer screening on prostate cancer mortality at 15-year follow-up. What screening was done?
An invitation to a single prostate-specific antigen (PSA) test with biopsy if PSA level was elevated.
Annual physical examination combined with biannual ultrasonography evaluation.
Biennial PSA testing for 10 years.
-
What were the outcomes at 15 years?
A markedly lower risk of prostate cancer mortality in the screened group that was also reflected in overall mortality.
A small reduction in risk of prostate cancer mortality in the screened group with a nonsignificant difference in overall mortality.
No difference in either prostate cancer mortality or overall mortality in the screened group.
-
Which of the following was a limitation of this work?
Despite clear screening protocols, adherence with recommendations for biopsy was less than 50%.
Newer diagnostic methods and more effective treatments for advanced and metastatic prostate cancer have since been identified.
Recruitment by clusters of general practices may have led to large-scale contamination of the control group.
Trial Protocol and Statistical Analysis Plan
eMethods.
eTable 1. Prostate cancer-specific diagnoses and mortality and all-cause mortality at 10-years, 15-years and 18-years post-randomization (and at 18 months for prostate cancer diagnoses) by random allocation and an as randomized estimate of the difference between groups
eTable 2. Underlying causes of death in intervention vs control groups at 15-year median follow-up (not including prostate cancer)
eTable 3. Effect of the CAP trial intervention on characteristics of prostate cancer cases at diagnosis
eTable 4. Sensitivity analyses employing alternative definitions of prostate cancer deaths
eTable 5. Estimated mean and median sojourn time and probability of overdiagnosis
eFigure 1. CAP trial design
eFigure 2. Cumulative incidence of prostate cancer by TNM stage at diagnosis
eFigure 3. Cumulative incidence of prostate cancer by Gleason score at diagnosis
eFigure 4. Comparing simulated data to empirical data for the cumulative prostate cancer incidence and cancer-specific and all-other cause mortality risk among the screened men and the unscreened group
eFigure 5. Comparison number of subjects per 100, 000 cohorts at death from all causes by ages between simulated data and CAP data
eFigure 6. Transition diagram for multi-state survival models
CAP Trial Group
Data Sharing Statement
References
- 1.NHS Digital . Cancer registration statistics, England 2019. Accessed May 30, 2022. https://www.cancerdata.nhs.uk/incidence_and_mortality
- 2.Young GJ, Harrison S, Turner EL, et al. Prostate-specific antigen (PSA) testing of men in UK general practice: a 10-year longitudinal cohort study. BMJ Open. 2017;7(10):e017729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.National Cancer Institute . Surveillance Epidemiology, and End Results Programme. Cancer stat facts 2023. Accessed May 15, 2023. https://https://seer.cancer.gov/statfacts/
- 4.Johansson JE, Andrén O, Andersson SO, et al. Natural history of early, localized prostate cancer. JAMA. 2004;291(22):2713-2719. doi: 10.1001/jama.291.22.2713 [DOI] [PubMed] [Google Scholar]
- 5.Bill-Axelson A, Holmberg L, Garmo H, et al. Radical prostatectomy or watchful waiting in prostate cancer—29-year follow-up. N Engl J Med. 2018;379(24):2319-2329. doi: 10.1056/NEJMoa1807801 [DOI] [PubMed] [Google Scholar]
- 6.Hamdy FC, Donovan JL, Lane JA, et al. ; ProtecT Study Group . Fifteen-year outcomes after monitoring, surgery, or radiotherapy for prostate cancer. N Engl J Med. 2023;388(17):1547-1558. doi: 10.1056/NEJMoa2214122 [DOI] [PubMed] [Google Scholar]
- 7.Wilt TJ, Brawer MK, Jones KM, et al. ; Prostate Cancer Intervention versus Observation Trial (PIVOT) Study Group . Radical prostatectomy versus observation for localized prostate cancer. N Engl J Med. 2012;367(3):203-213. doi: 10.1056/NEJMoa1113162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cancer Research UK . Prostate cancer statistics. 2021. Accessed May 24, 2021. https://www.cancerresearchuk.org/health-professional/cancer-statistics/statistics-by-cancer-type/prostate-cancer/
- 9.Rosario DJ, Lane JA, Metcalfe C, et al. Short term outcomes of prostate biopsy in men tested for cancer by prostate specific antigen: prospective evaluation within ProtecT study. BMJ. 2012;344:d7894. doi: 10.1136/bmj.d7894 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Martin RM, Donovan JL, Turner EL, et al. ; CAP Trial Group . Effect of a low-intensity PSA-based screening intervention on prostate cancer mortality: the CAP randomized clinical trial. JAMA. 2018;319(9):883-895. doi: 10.1001/jama.2018.0154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Donovan JL, Hamdy FC, Lane JA, et al. Patient-reported outcomes 12 years after localized prostate cancer treatment. NEJM Evid. 2023;2(4):a2300018. doi: 10.1056/EVIDoa2300018 [DOI] [PubMed] [Google Scholar]
- 12.Turner EL, Metcalfe C, Donovan JL, et al. ; CAP trial group . Design and preliminary recruitment results of the Cluster randomised trial of PSA testing for prostate cancer (CAP). Br J Cancer. 2014;110(12):2829-2836. doi: 10.1038/bjc.2014.242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Donovan JL, Opmeer B, Young GJ, et al. ; ProtecT Study Group . Factors associated with trial recruitment, preferences, and treatments received were elucidated in a comprehensive cohort study. J Clin Epidemiol. 2019;113:200-213. doi: 10.1016/j.jclinepi.2019.05.036 [DOI] [PubMed] [Google Scholar]
- 14.NHS . Opt out of sharing your health records. Accessed November 20, 2023. https://www.nhs.uk/using-the-nhs/about-the-nhs/opt-out-of-sharing-your-health-records/
- 15.Hamdy FC, Donovan JL, Lane JA, et al. ; ProtecT Study Group . 10-Year outcomes after monitoring, surgery, or radiotherapy for localized prostate cancer. N Engl J Med. 2016;375(15):1415-1424. doi: 10.1056/NEJMoa1606220 [DOI] [PubMed] [Google Scholar]
- 16.Burford D, Kirby M, Austoker J. NHS Cancer Screening Programmes. 2010. Accessed May 24, 2021. http://www.cancerscreening.nhs.uk/prostate/pcrmp02.pdf
- 17.Keeney E, Sanghera S, Martin RM, et al. Cost-effectiveness analysis of prostate cancer screening in the UK: a decision model analysis based on the CAP Trial. Pharmacoeconomics. 2022;40(12):1207-1220. doi: 10.1007/s40273-022-01191-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Donovan JL, Hamdy FC, Lane JA, et al. ; ProtecT Study Group . Patient-reported outcomes after monitoring, surgery, or radiotherapy for prostate cancer. N Engl J Med. 2016;375(15):1425-1437. doi: 10.1056/NEJMoa1606221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Turner EL, Metcalfe C, Donovan JL, et al. Contemporary accuracy of death certificates for coding prostate cancer as a cause of death: is reliance on death certification good enough? a comparison with blinded review by an independent cause of death evaluation committee. Br J Cancer. 2016;115(1):90-94. doi: 10.1038/bjc.2016.162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Williams NJ, Hill EM, Ng SY, et al. ; CAP Cause of Death Committee . Standardisation of information submitted to an endpoint committee for cause of death assignment in a cancer screening trial—lessons learnt from CAP (Cluster randomised triAl of PSA testing for Prostate cancer). BMC Med Res Methodol. 2015;15(1):6. doi: 10.1186/1471-2288-15-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.NHS England. National Disease Registration Service. 2023. Accessed November 21, 2023. https://digital.nhs.uk/services/national-disease-registration-service
- 22.Public Health Wales . Data and analysis. 2023. Accessed November 21, 2023. https://phw.nhs.wales/services-and-teams/observatory/data-and-analysis/
- 23.Pashayan N, Duffy SW, Pharoah P, et al. Mean sojourn time, overdiagnosis, and reduction in advanced stage prostate cancer due to screening with PSA: implications of sojourn time on screening. Br J Cancer. 2009;100(7):1198-1204. doi: 10.1038/sj.bjc.6604973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bhatt R, van den Hout A, Pashayan N. A multistate survival model of the natural history of cancer using data from screened and unscreened population. Stat Med. 2021;40(16):3791-3807. doi: 10.1002/sim.8998 [DOI] [PubMed] [Google Scholar]
- 25.Ilic D, Djulbegovic M, Jung JH, et al. Prostate cancer screening with prostate-specific antigen (PSA) test: a systematic review and meta-analysis. BMJ. 2018;362:k3519. doi: 10.1136/bmj.k3519 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Vickers A, O’Brien F, Montorsi F, et al. Current policies on early detection of prostate cancer create overdiagnosis and inequity with minimal benefit. BMJ. 2023;381:e071082. doi: 10.1136/bmj-2022-071082 [DOI] [PubMed] [Google Scholar]
- 27.Hugosson J, Roobol MJ, Månsson M, et al. ; ERSPC investigators . A 16-yr follow-up of the European Randomized Study of Screening for Prostate Cancer. Eur Urol. 2019;76(1):43-51. doi: 10.1016/j.eururo.2019.02.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pinsky PF, Miller E, Prorok P, Grubb R, Crawford ED, Andriole G. Extended follow-up for prostate cancer incidence and mortality among participants in the Prostate, Lung, Colorectal and Ovarian randomized cancer screening trial. BJU Int. 2019;123(5):854-860. doi: 10.1111/bju.14580 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gulati R, Tsodikov A, Wever EM, et al. The impact of PLCO control arm contamination on perceived PSA screening efficacy. Cancer Causes Control. 2012;23(6):827-835. doi: 10.1007/s10552-012-9951-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Grubb RL III, Pinsky PF, Greenlee RT, et al. Prostate cancer screening in the Prostate, Lung, Colorectal and Ovarian cancer screening trial: update on findings from the initial four rounds of screening in a randomized trial. BJU Int. 2008;102(11):1524-1530. doi: 10.1111/j.1464-410X.2008.08214.x [DOI] [PubMed] [Google Scholar]
- 31.Lundgren PO, Kjellman A, Norming U, Gustafsson O. Long-term outcome of a single intervention population based prostate cancer screening study. J Urol. 2018;200(1):82-88. doi: 10.1016/j.juro.2018.01.080 [DOI] [PubMed] [Google Scholar]
- 32.Heijnsdijk EAM, de Carvalho TM, Auvinen A, et al. Cost-effectiveness of prostate cancer screening: a simulation study based on ERSPC data. J Natl Cancer Inst. 2014;107(1):366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Auvinen A, Moss SM, Tammela TLJ, et al. Absolute effect of prostate cancer screening: balance of benefits and harms by center within the European Randomized Study of Prostate Cancer Screening. Clin Cancer Res. 2016;22(1):243-249. doi: 10.1158/1078-0432.CCR-15-0941 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ahmed HU, El-Shater Bosaily A, Brown LC, et al. ; PROMIS study group . Diagnostic accuracy of multi-parametric MRI and TRUS biopsy in prostate cancer (PROMIS): a paired validating confirmatory study. Lancet. 2017;389(10071):815-822. doi: 10.1016/S0140-6736(16)32401-1 [DOI] [PubMed] [Google Scholar]
- 35.de Bono J, Mateo J, Fizazi K, et al. Olaparib for metastatic castration-resistant prostate cancer. N Engl J Med. 2020;382(22):2091-2102. doi: 10.1056/NEJMoa1911440 [DOI] [PubMed] [Google Scholar]
- 36.Ben-Shlomo Y, Evans S, Ibrahim F, et al. ; PROCESS study group . The risk of prostate cancer amongst black men in the United Kingdom: the PROCESS cohort study. Eur Urol. 2008;53(1):99-105. doi: 10.1016/j.eururo.2007.02.047 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Trial Protocol and Statistical Analysis Plan
eMethods.
eTable 1. Prostate cancer-specific diagnoses and mortality and all-cause mortality at 10-years, 15-years and 18-years post-randomization (and at 18 months for prostate cancer diagnoses) by random allocation and an as randomized estimate of the difference between groups
eTable 2. Underlying causes of death in intervention vs control groups at 15-year median follow-up (not including prostate cancer)
eTable 3. Effect of the CAP trial intervention on characteristics of prostate cancer cases at diagnosis
eTable 4. Sensitivity analyses employing alternative definitions of prostate cancer deaths
eTable 5. Estimated mean and median sojourn time and probability of overdiagnosis
eFigure 1. CAP trial design
eFigure 2. Cumulative incidence of prostate cancer by TNM stage at diagnosis
eFigure 3. Cumulative incidence of prostate cancer by Gleason score at diagnosis
eFigure 4. Comparing simulated data to empirical data for the cumulative prostate cancer incidence and cancer-specific and all-other cause mortality risk among the screened men and the unscreened group
eFigure 5. Comparison number of subjects per 100, 000 cohorts at death from all causes by ages between simulated data and CAP data
eFigure 6. Transition diagram for multi-state survival models
CAP Trial Group
Data Sharing Statement