Abstract
Background:
Prior work has shown that certain modifiable health, Alzheimer’s disease (AD) biomarker, and demographic variables are associated with cognitive performance. However, less is known about the relative importance of these different domains of variables in predicting longitudinal change in cognition.
Objective:
Identify novel relationships between modifiable physical and health variables, AD biomarkers, and slope of cognitive change over two years in a cohort of older adults with mild cognitive impairment (MCI).
Methods:
Metrics of cardiometabolic risk, stress, inflammation, neurotrophic/growth factors, and AD pathology were assessed in 123 older adults with MCI at baseline from the Alzheimer’s Disease Neuroimaging Initiative (mean age = 73.9; SD = 7.6; mean education = 16.0; SD = 3.0). Partial least squares regression (PLSR)—a multivariate method which creates components that best predict an outcome—was used to identify whether these physiological variables were important in predicting slope of change in episodic memory or executive function over two years.
Results:
At two-year follow-up, the two PLSR models predicted, respectively, 20.0% and 19.6% of the variance in change in episodic memory and executive function. Baseline levels of AD biomarkers were important in predicting change in both episodic memory and executive function. Baseline education and neurotrophic/growth factors were important in predicting change in episodic memory, whereas cardiometabolic variables such as blood pressure and cholesterol were important in predicting change in executive function.
Conclusion:
These data-driven analyses highlight the impact of AD biomarkers on cognitive change and further clarify potential domain specific relationships with predictors of cognitive change.
Keywords: Episodic memory, executive function, healthy aging, inflammation, metabolic syndrome, neuronal plasticity, neuroprotection, neuropsychology
INTRODUCTION
By the year 2050, the older adult population in the United States is estimated to reach 87.3 million, a growth doubling the population of adults over 65 years of age in 2012 [1]. The risk of Alzheimer’s disease (AD) increases with age, and the number of AD cases is projected to reach 13.9 million by 2060 [2]. To date, there is no cure for age- or AD-related cognitive decline, a problem highlighting the need to identify variables that are most strongly associated with preserving cognition. Therefore, identifying variables that could serve as targets for intervention to mitigate cognitive decline, particularly among older adults with mild cognitive impairment (MCI), who are at the greatest risk for AD, will be important in maintaining independence and quality of life in our aging population.
There is substantial variability in aging, and multiple health metrics and biomarkers have been shown to positively or negatively influence cognitive decline or conversion to dementia. For instance, plasma and cerebrospinal fluid (CSF) biomarkers are associated with progression of cognitive impairment in older adults with MCI [3–6]. Lower levels of CSF amyloid-β (Aβ42) and higher levels of CSF total tau (t-tau) and phosphorylated tau (p-tau) have been associated with higher rates of conversion to AD [4] and clinical progression in MCI [5]. Other work has shown that these biomarkers are related to cognition [6, 7]. For instance, one study found that lower levels of CSF Aβ42 were associated with poorer episodic memory and that higher levels of CSF t-tau were related to poorer global cognition [7]. Recent work has further suggested that ratios of CSF t-tau/Aβ42 and CSF p-tau/Aβ42 may be more sensitive biomarkers for exploring longitudinal cognition in MCI. In older adults with MCI, higher t-tau/Aβ42 ratios were associated with poorer episodic memory and global cognition [7]. Other work revealed that CSF p-tau/Aβ42 ratios predicted greater declines in episodic memory, executive function, and global cognition in MCI [6]. Thus, there is substantial evidence that AD biomarkers might be relevant predictors of cognitive change in MCI populations.
Other studies have examined the role of neurotrophic/growth factors in the context of cognitive decline. Multiple studies have focused on brain-derived neurotrophic factor (BDNF). For instance, BDNF in older adults has been linked to age-related memory impairment [8] and increased risk of converting to dementia [9]. A recent systematic review concluded that BDNF was consistently associated with neuronal plasticity and memory consolidation [10]. Additionally, authors of this review found that decreased BDNF in aging was related to memory problems across multiple pathologies [10]. Other growth factors, although reported less frequently than BDNF, have also been linked to cognitive and brain decline. For instance, vascular endothelial growth factor (VEGF) has been linked to lower global cognition and severity of cognitive decline in older adults, and lower levels of platelet-derived growth factor (PDGF) might represent more general neuronal loss [11]. In sum, there is accumulating evidence that neurotrophic/growth factors are associated with cognition in aging and MCI.
There is also an established link demonstrating that cardiometabolic (such as blood pressure and cholesterol) and pro-inflammatory variables (such as c-reactive protein and interleukin-6) are negatively associated with cognition [12–15]. Several studies have related metabolic syndrome—which involves high abdominal adiposity or obesity, high blood pressure, high cholesterol, and dyslipidemia, or high amounts of triglycerides, cholesterol or fat phospholipids—to future cognitive impairment [15] and progression from MCI to dementia [14]. A systematic review also found that those with metabolic syndrome consistently had poorer executive function performance on tasks that did not involve language ability [16]. Thus, the current literature indicates that modifiable cardiometabolic variables might be important predictors of changes in executive function in older adults with MCI.
In general, the respective literatures examining AD biomarkers, neurotrophic/growth factors, inflammatory markers, and cardiometabolic factors associated with cognition in MCI populations have remained largely segregated, exploring either a single variable or a small group of variables within a single domain (e.g., examining links between AD biomarkers and cognition without consideration of markers of inflammation and cardiometabolic function). To better understand the complex relationship between health metrics and cognition in MCI, it is important to simultaneously consider an array of physiological variables spanning multiple domains of biomarkers. Few studies have implemented such an approach. A recent study explored CSF levels of neurotrophic/growth factors, inflammatory variables, and AD biomarkers in older adults with and without cognitive impairment [17]. Authors found that variables across all three of these domains were significant predictors of general cognitive function (but did not examine specific cognitive domains). Recent work by our group has further extended the literature by including an additional class of health variables (modifiable cardiometabolic health variables), in addition to consideration of multiple AD biomarkers, neurotrophic/growth factors, inflammatory markers, and domain specific cognitive function. We used a multivariate partial least squares correlation analysis (PLSC) [18] to examine these cross-sectional relationships in a cohort of older adults with MCI [19]. Our results revealed that variables associated with AD pathology and neuroplasticity, as well as education and cortisol (a marker of stress), were largely associated with performance in tasks assessing episodic memory, executive function, processing speed, and language, but not working memory or premorbid IQ. Specifically, better performance on tests within these cognitive domains was associated with increased neurotrophic/growth factor levels (BDNF, PDGF, heparin-binding epidermal growth factor-like growth factor), less AD pathology (plasma tau, CSF t-tau, CSF p-tau181, CSF Aβ42), lower levels of stress (cortisol), and higher education. These findings suggested that variables associated with neuroprotection, neuroplasticity, stress, and AD significantly contribute to declined episodic memory, executive function, and processing speed in older adults with MCI.
One limitation of previous studies, including our own, was the cross-sectional approach used to examine the relationship between multiple health metrics and cognition in MCI. In the current study, we address this limitation and further extend the literature by examining change in cognition over two years. Specifically, we employed partial least squares regression (PLSR) analysis to assess how these different groups of physiological variables might be associated with changes in cognition longitudinally in a cohort of ADNI participants with MCI. In PLSR, a large number of predictor variables is used to predict one or more outcomes [20, 21]. PLSR creates latent variables, or components, that maximize the covariance between the independent (i.e., the predictor) and the dependent (i.e., the predicted outcome) variable(s). This is followed by a regression step, where the latent variables created from linear combinations of the predictor variables are used to predict the outcome(s) [20, 21]. There are several advantages of PLSR, including its ability to analyze collinear data and work well with many predictor variables [22]. For this study, separate PLSRs were run for two cognitive domains: episodic memory and executive function. These cognitive outcomes were selected due to findings from our prior work using cross-sectional PLSC described above [19] as well as substantial work showing that episodic memory and executive function are particularly susceptible to age-related decline [23, 24].
The aims of the present study were to 1) identify which of these modifiable physical, health (i.e., cardiometabolic, inflammation), and AD biomarker metrics predict slope of change over two years in either episodic memory or executive function performance; 2) examine how these physiological variables were related to one another and 3) assess how gender and diagnostic status at two-year follow-up were associated with these physiological variables.
MATERIALS AND METHODS
Participants
Participants with a diagnosis of MCI from the ADNI1 cohort were included in the current study. Full participant inclusion/exclusion criteria are available in [25] and are summarized here: 6th grade or higher education, fluent in English or Spanish, minimum Mini-Mental State Examination (MMSE) of 24, Clinical Dementia Rating of 0.5, subjective memory complaint by subject or study partner, impaired episodic memory, and sufficiently preserved general cognition and functional performance not meeting criteria for AD. Participants with missing data for any variables of interest were excluded, as complete data are necessary for PLSR analysis. One participant classified as MCI with an MMSE score of 23 and one participant with an extremely high and improbable triglycerides value (2,084.0 mg/dL) were excluded. Other ADNI cohorts (ADNIGO, ADNI2, and ADNI3) were excluded from the analysis because these data sets did not include numerous biomarkers of interest (e.g., neurotrophic and growth factors were not available).
The final sample included 123 participants who had composite neuropsychological data available at both baseline and two-year follow-up (age: range = 55.1 – 88.3, M = 73.9, SD = 7.6; education: range = 6 – 20 years, M = 16.0, SD = 3.0; 42 females and 81 males; 119 White, 2 Asian, 2 Black; 121 Non-Hispanic, 2 Hispanic; 54 APOE ε4 negative). Longer follow-up times were not assessed due to participant attrition. That is, cognitive composite scores were available in N = 97, N = 54, and N = 39 participants at 36-, 48-, and 60-month timepoints. Previous studies have demonstrated that significant cognitive decline can occur in MCI at one-year [26] or two-year follow-up [27, 28], and the larger dataset available at 24-month follow-up provided greater generalizability of our study sample to the broader MCI population. Cognitively normal older adults were not included in the present study due to a small sample size with biomarkers and neuropsychological data available at baseline and cognitive data at two-year follow-up (N = 34). These participants were excluded to improve the interpretability of the results, because our goal was to identify the physiological variables important for predicting cognitive change in MCI participants. Study procedures were approved by site-specific Institutional Review Boards, and all participants and/or authorized representatives provided written informed consent consistent with the Declaration of Helsinki.
Neuropsychological assessment
Composite scores of episodic memory and executive function published by ADNI were examined [29, 30]. For the episodic memory composite score, factor analytic methods and item response theory were used to create a score obtained as a weighted set of tests from the ADNI neuropsychological battery (for further details, see [29]). This memory composite score was determined to have good validity [29]. For the executive function composite score, the same statistical techniques were employed, and this score was also determined to have good validity (for further details, see [30]).
Cardiometabolic, stress, and inflammation variables
BMI (kg/m2), systolic blood pressure (mmHg), diastolic blood pressure (mmHg), pulse rate (per minute), cholesterol (mg/dL), triglycerides (mg/dL), and serum glucose (mg/dL) data were obtained. Insulin (uIU/mL), cortisol (ng/mL), CRP (µg/mL), and interleukin-6 (IL-6) receptor (ng/mL) data were assessed from fasting plasma blood samples. Data were normalized and checked for the defined least detectable dose during the quality control process.
Growth factors and neurotrophic factors
Insulin-like growth factor binding protein (ng/mL), epidermal growth factor (pg/mL), heparin binding epidermal-growth-factor-like growth factor (HB-EGF-like-GF; pg/mL), hepatocyte growth factor (ng/mL), platelet-derived growth factor BB (PDGF; pg/mL), BDNF (ng/mL), and vascular-endothelial growth factor (pg/mL) were analyzed. Data were normalized and checked for the defined least detectable dose during the quality control process.
AD biomarkers
Plasma Apolipoprotein E (apoE; µg/mL), plasma tau (pg/mL), CSF t-tau (pg/mL), CSF p-tau181 (pg/mL), and CSF Aβ42 (pg/mL) were assessed. Only values within the given ranges, which were based on the reported technical limits for each respective assay, were included in the analyses: Aβ42: 200–1700 pg/mL; p-tau181: 8–120 pg/mL; t-tau: 80–1300 pg/mL.
Data processing and analysis
ADNI1 data were scrubbed using the R-language. Raw data files for all blood- and CSF-based biomarkers were checked for imputed values published by ADNI. To ensure data integrity, all analytes with > 10% imputed values were removed. Participants who had missing data or invalid data as indicated by the ADNI manual were excluded.
PLSR R-scripts and functions were developed by co-author H. Abdi and run in R (Version 3.6.1). Several R-packages were used for data analysis (‘PTCA4CATA’, ‘data4PCCAR’, ‘ExPosition’ for PLSR functions, and ‘boot’ for bootstrapping PLSR results) and visualization (‘corrplot’, ‘tidyverse’, ‘ggplot2’). PLSR combines techniques from principal components analysis and multiple linear regression [20]. The goal of a PLSR analysis is to decompose the X-matrix (predictor variables) to create orthogonal components (i.e., optimal linear combinations of the data) that can best predict the outcome (Y-matrix) variable(s). This is done by first creating a correlation matrix between X- and Y-matrices. Next, this covariance matrix is decomposed to generate the X-matrix components. This process generalizes principal components analysis, where the X-matrix is decomposed into optimal linear combinations that are only relevant for the X-matrix, not for both the X- and Y-matrices. Finally, the X-matrix components created from the decomposition of this correlation matrix are used to predict the outcome (Y-matrix) [20]. This made PLSR advantageous over principal components analysis for the present study objectives. Additionally, PLSR was preferred over multiple linear regression because it works well with a large number of multicollinear predictor variables, and uses this multicollinearity to its advantage in its prediction [18, 20]. In multiple linear regression, multicollinear predictor variables are problematic for calculation of regression coefficients and must be removed. However, in PLSR, the researcher can explore how different groups of variables cluster together among different components of the PLSR model. Although PLSR can be run with multiple Y-outcome variables [18, 20], this did not align with the objective of the current study, because our goal was to assess how different physiological variables were weighted and predictive of each cognitive domain (episodic memory and executive function). Therefore, separate PLSR analyses were run per cognitive domain where either slope of change in composite memory or executive function over two years was the Y-matrix, or outcome variable. For both models, physical, health, and AD biomarker variables measured at baseline were the X-matrix (see Table 1 for full list of variables). This approach was implemented to examine how contributions of baseline physiological variables differed in predicting slope of change in episodic memory or executive function over two years.
Table 1.
Descriptive Statistics for Demographic, Neuropsychological, Physical, Health, and AD Biomarker Data at Baseline Entered into the PLSR Analysis
| Demographic Variables | ||
|---|---|---|
|
| ||
| Unit | Mean (SD) | |
|
| ||
| Age | Years | 73.9 (7.6) |
| Education | Years | 16.0 (3.0) |
|
| ||
| Neuropsychological Variables | ||
|
| ||
| Unit | Mean (SD) | |
|
| ||
| ADNI Memory Composite Score | z-score | –0.13 (0.56) |
| ADNI Executive Function Composite Score | z-score | –0.11 (0.81) |
|
| ||
| Cardiometabolic Variables | ||
|
| ||
| Unit | Mean (SD) | |
|
| ||
| Body Mass Index | kg/m2 | 26.1 (3.8) |
| Seated Systolic Blood Pressure | mmHg | 134.5 (18.3) |
| Seated Diastolic Blood Pressure | mmHg | 73.4 (9.4) |
| Seated Pulse Rate | Per minute | 64.6 (9.2) |
| Serum Glucose | mg/dL | 101.8 (33.3) |
| Triglycerides | mg/dL | 157.4 (107.3) |
| Cholesterol | mg/dL | 198.2 (39.0) |
| Insulin* + | uIU/mL | 0.30 (0.31) |
|
| ||
| Inflammatory Variables | ||
|
| ||
| Unit | Mean (SD) | |
|
| ||
| C-reactive Protein*+ | ug/mL | 0.05 (0.46) |
| Cortisol*+ | ng/mL | 2.16 (0.12) |
| Interleukin-6 Receptor* + | ng/mL | 1.45 (0.15) |
|
| ||
| Neurotrophic/Growth Factor Variables | ||
|
| ||
| Unit | Mean (SD) | |
|
| ||
| Insulin-like Growth Factor Binding Protein*+ | ng/mL | 2.00 (0.22) |
| Brain-derived Neurotrophic Factor*+ | ng/mL | 0.28 (0.36) |
| Vascular Endothelial Growth Factor* + | pg/mL | 2.80 (0.13) |
| Epidermal Growth Factor* + | pg/mL | 1.54 (0.58) |
| Heparin-binding Epidermal- Growth-Factor-like Growth Factor *+ | pg/mL | 1.92 (0.36) |
| Hepatocyte Growth Factor*+ | ng/mL | 0.59 (0.10) |
| Platelet-derived Growth Factor BB* + | pg/mL | 3.18 (0.43) |
|
| ||
| AD Biomarker Variables | ||
|
| ||
| Unit | Mean (SD) | |
|
| ||
| Apolipoprotein E* + | ug/mL | 1.68 (0.16) |
| Tau+ | pg/mL | 2.85 (1.82) |
| Aβ42 (CSF) | pg/mL | 735.0 (340.2) |
| Total tau (CSF) | pg/mL | 308.7 (120.7) |
| P-tau181 (CSF) | pg/mL | 30.7 (13.7) |
| P-tau181/Aβ42 | ratio | 0.05 (0.03) |
| Total tau/Aβ42 | ratio | 0.51 (0.28) |
CSF, cerebrospinal fluid
plasma
values are normalized.
To create the PLSR models, the X- and Y-matrix variables (where the X-matrix columns were 29 cardiometabolic, inflammatory, neuroprotective, AD biomarker, and demographic variables and the Y-matrices columns were, respectively, change in episodic memory or executive function) were normalized to have a mean of zero and standard deviation of one. Demographic variables (age and education) were entered as continuous variables. A leave-one-out cross-validation procedure (LOO, also called a jackknife) was completed to prevent overfitting and improve generalizability of the PLSR model [20, 21]. In this procedure, each observation is removed in turn from the X and Y-matrices, and a PLSR model is recreated for each of the remaining observations. The model created by the remaining observations and the variables is used to predict the left-out observation. The predicted observations are stored in a new matrix and a “predicted residual estimated sum of squares” (PRESS) value is created to measure the quality of the prediction, where a lower PRESS value indicates a better prediction. This procedure is repeated and iteratively uses a different number of components (from one to the number of X-matrix predictor variables), and the PRESS value is calculated for each possible number of components [20, 21]. The optimal number of components for the PLSR model is chosen based on a pseudo-scree plot (where the inertia extracted by each of the 29 possible components is plotted using the R-function ‘PlotScree’ from PTCA4CATA package) in which the optimal number of components is indicated by an inertia value greater than 1 divided by the number of components (see the Supplementary Material for pseudo-scree plots). Thus, components with an inertia value greater than 0.034 (1/29 = 0.034) were retained in the PLSR model. Next, the PLSR model is run using this optimal number of components.
PLSR whole-model outcomes
Variable importance on projection scores
Variable importance of projection (VIP) scores were calculated for every X-matrix variable. The VIP score summarizes the contribution of an X-matrix variable to the model, as it represents a combination of the loadings and weights for each X-matrix variable across all components of the PLSR model [31]. Variables with a VIP score > 1 are considered important to the PLS model’s prediction [32]. In other words, if a variable has a VIP score > 1, removing that variable would significantly affect the PLSR model’s prediction. Using outputs from the ‘resPLSR’ R-function (package data4PCCAR), the formula for VIP scores from Akarachantachote and colleagues was employed [33].
BPLS values
BPLS values are conceptually equivalent to regression coefficients in multiple linear regression [20, 21]. BPLS values indicate the strength and directionality for each X-matrix variable in relation to the Y-matrix; a higher absolute value means that variable has a stronger relationship with the outcome [32]. BPLS values are reliable predictors of the outcome if their 95% confidence interval (CI) does not include zero [20, 21]. In other words, a given X-matrix variable is a reliable predictor of the outcome if its BPLS value is significantly different from zero at p < 0.05. To create robust and reliable CIs, bootstrapping with replacement was employed using the ‘boot’ function in R (N = 1,500 iterations). Bootstrapping allowed us to derive standard errors for each BPLS in the model nonparametrically [34].
R2 values
The resPLSR function outputs the percent variance explained in X-matrix as well as the percent variance explained in the Y-matrix per component in the PLSR model. The sum of the percent variance for all components in the model gives the percentage of variance explained for the X and Y-matrices for the whole PLSR model. The percent variance explained in the Y-matrix is analogous to the R2 of the model in multiple linear regression.
Component-specific outcomes (post-hoc analyses)
Individual components of the PLSR model were also analyzed and interpreted. To do this, circles of correlation were produced to explore how the physiological variables were correlated with each component. Each predictor variable is then plotted on a standard coordinate plane created by two components (i.e., a variable positively correlated with both Components 1 and 2 would be in the upper right quadrant of the plot). Circles of correlation also show which predictor variables correlate with one another/cluster together. Additionally, participant observation maps were created to examine the extent to which individual participants were correlated with each component. Participants were either coded by gender or diagnosis at two-year follow-up (where ADNI coded whether participants remained MCI or converted to AD). Bootstrapping (with N = 1,000 iterations) was used to calculate bootstrapped means and bootstrap ratios (BSRs) for each group per component [35]. BSRs indicate whether the group mean, on average, had values significantly different from 0 (|BSR| ≥ 2.0, corresponding to p < 0.05) on a given component. Bootstrapped means and 95% CIs (95% CIs are represented as ellipses plotted around each bootstrapped mean) per group (either male/female or remained MCI/converted to AD at two-year follow-up) were plotted for each graph. Groups are considered to significantly contribute to a given component if their ellipses do not cross 0. If the ellipses of two groups do not overlap along a given axis, then these two groups are significantly different from one another for the given component.
RESULTS
Descriptive statistics for the 123 participants included in the analysis are presented in Table 1. At two-year follow-up, 68 participants retained their MCI diagnostic status, and 55 participants converted from MCI to AD. Descriptive statistics by gender and by diagnostic group at two-year follow-up can be found in the Supplemental Material. PLSR analyses were used to determine the relationship between 29 different physiological and demographic variables and slope of change in episodic memory or executive function at two-year follow-up.
Whole-model outcomes
Episodic memory
The optimal number of components to include in the model was two (see Supplementary Figure 1A). The PLSR model accounted for 20.9% of variance of the X-matrix, which explained 20.0% of the variance in slope of change in episodic memory over two years (Model R2 = 0.200; Fig. 1A). CSF Aβ42 (BPLS = 0.15; 95% CI = [0.01 – 0.29]), CSF t-tau/Aβ42 (BPLS=−0.11; 95% CI = [−0.19 – −0.03]), and education (BPLS = 0.17; 95% CI = [0.01 – 0.34]) had BPLS values that were reliable (BPLS 95% CI did not include 0; see Supplementary Table 1 for BPLS values for all variables). CSF Aβ42 and education were both positively associated with slope of change in episodic memory over two years while CSF t-tau/Aβ42 ratio had a negative association (based on their positive and negative BPLS values). CSF Aβ42, CSF t-tau/Aβ42, and education were also considered important (VIP > 1) to the model, along with several other variables: CSF t-tau, CSF p-tau181, CSF p-tau181/Aβ42, APOE ε4 allele status, BDNF, VEGF, and IL-6 receptor (VIP scores for all variables displayed in Fig. 1A). This model identified a pattern in the data suggesting that higher levels of CSF Aβ42, lower CSF t-tau/Aβ42 ratio, and higher education were reliable and positive predictors of slope of change in episodic memory performance, while baseline levels of multiple AD biomarkers, growth factors, education, and an inflammatory marker were important to the overall PLSR episodic memory model prediction.
Fig. 1.
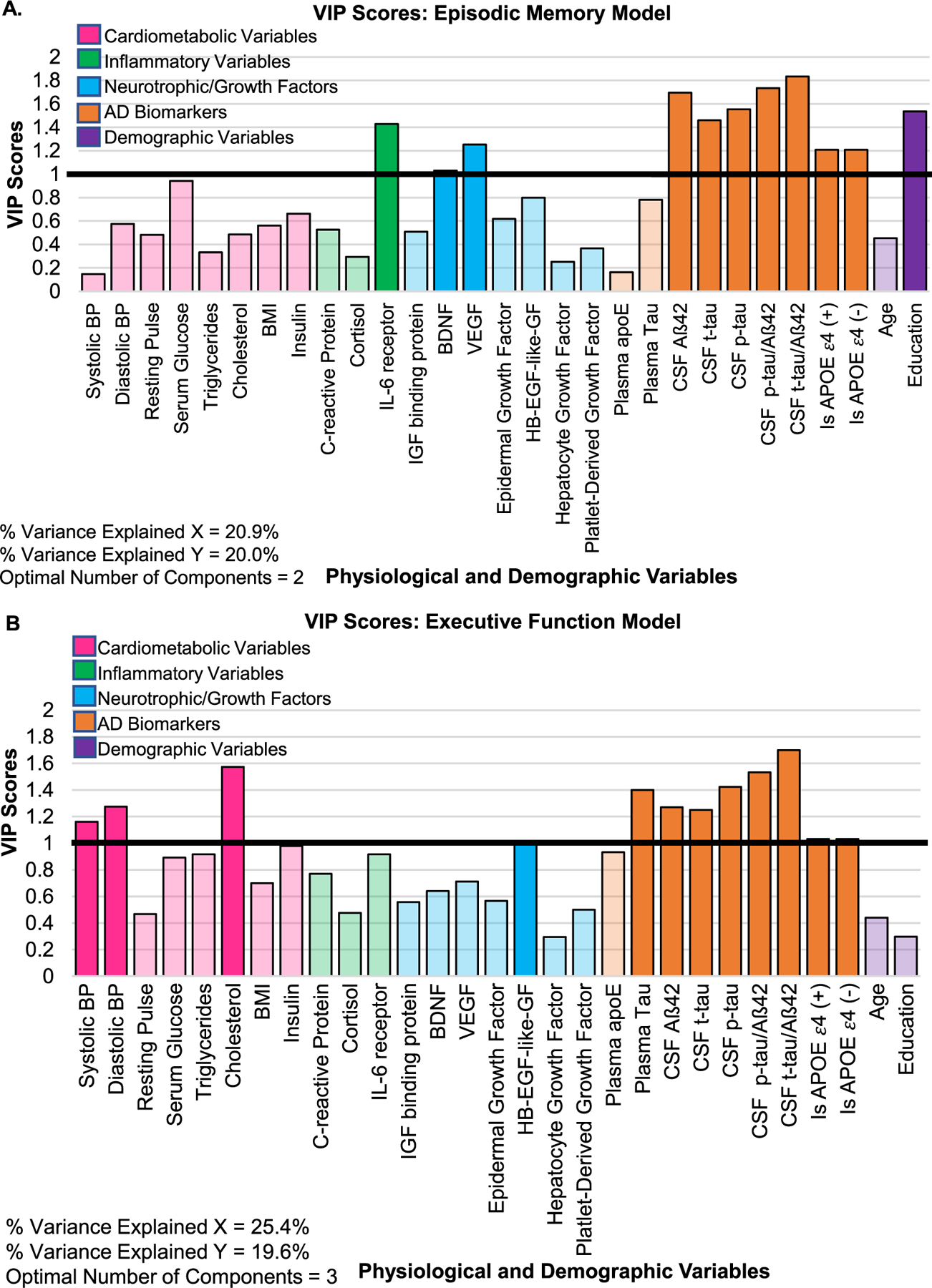
VIP Scores represent whether a given input variable is an important predictor in the PLSR model (across all components). Variables with a VIP score > 1 (highlighted by the black horizontal line at VIP value = 1) are considered important to the PLS model’s prediction. A) VIP scores for all variables entered in the episodic memory model. B) VIP scores for all variables entered in the executive function model. BDNF, brain-derived neurotrophic factor; BMI, body mass index; BP, blood pressure; CSF, cerebrospinal fluid; HB-EGF-like-GF, heparin-binding epidermal growth factor like-growth factor; IL-6, interleukin-6; IGF, insulin-like growth factor; p-tau, phosphorylated tau181; t-tau, total tau; VEGF, vascular endothelial growth factor.
Executive function
The optimal number of components to include in the model was three (see Supplementary Figure 1B). The PLSR model accounted for 25.36% of the variance in the X-matrix, which explained 19.60% of the variance in slope of change in executive function over two years (Model R2 = 0.196; Fig. 1B). CSF t-tau/Aβ42 ratio had a BPLS value that was reliably different from 0 (BPLS=−0.14, 95% CI = [−0.25 – −0.03]) and was negatively associated with slope of change in executive function over two years. CSF t-tau/Aβ42 ratio was also considered important (VIP > 1) to the model, along with several other variables: plasma tau, CSF Aβ42, CSF t-tau, CSF p-tau181, CSF p-tau181/Aβ42, APOE ε4 allele status, systolic blood pressure, diastolic blood pressure, cholesterol, and HB-EGF-like-GF (VIP values displayed in Fig. 1B). Overall, this model identified a pattern suggesting that a lower CSF t-tau/Aβ42 ratio was a reliable and positive predictor of slope of change in executive function, while baseline levels of multiple AD biomarkers, cardiometabolic variables, and a neurotrophic factor were important (VIPs > 1) to the overall prediction of the PLSR executive function model.
Component-specific outcomes and post-hoc analyses
Episodic memory
The variance explained per component was, respectively, 13.1% and 7.9%. In Fig. 2A, Components 1 and 2 are shown, respectively, on the horizontal and vertical axes. Input variables to the right of the vertical axis are positively correlated with Component 1, while variables to the left of the vertical axis are negatively correlated with Component 1. Variables above the horizontal axis are positively correlated with Component 2, while variables below the horizontal axis are negatively correlated with Component 2. Input variables are mapped on the circle (which is a standard coordinate plane) based on their correlation values with Components 1 and 2. As visualized in Fig. 2A, Component 1 was largely associated with AD biomarkers, while Component 2 was largely associated with neurotrophic/growth factors, IL-6-receptor, and education. Specifically, CSF t-tau, CSF p-tau181, CSF t-tau/Aβ42, CSF p-tau181/Aβ42, and positive APOE ε4 allele status were negatively correlated with Component 1, while levels of CSF Aβ42 and negative APOE ε4 allele status were positively correlated with Component 1. BDNF, VEGF, epidermal growth factor, and PDGF were negatively correlated with Component 2, while IL-6 receptor and education were positively correlated with Component 2. Thus, Component 1 represents how AD pathology contributes to the model, while Component 2 represents how education and neuroprotection contribute to the model. Figure 2A illustrates several AD biomarkers and several neurotrophic factors clustering together, showing that these variables are highly correlated with one another, but contribute to the model differentially.
Fig. 2.
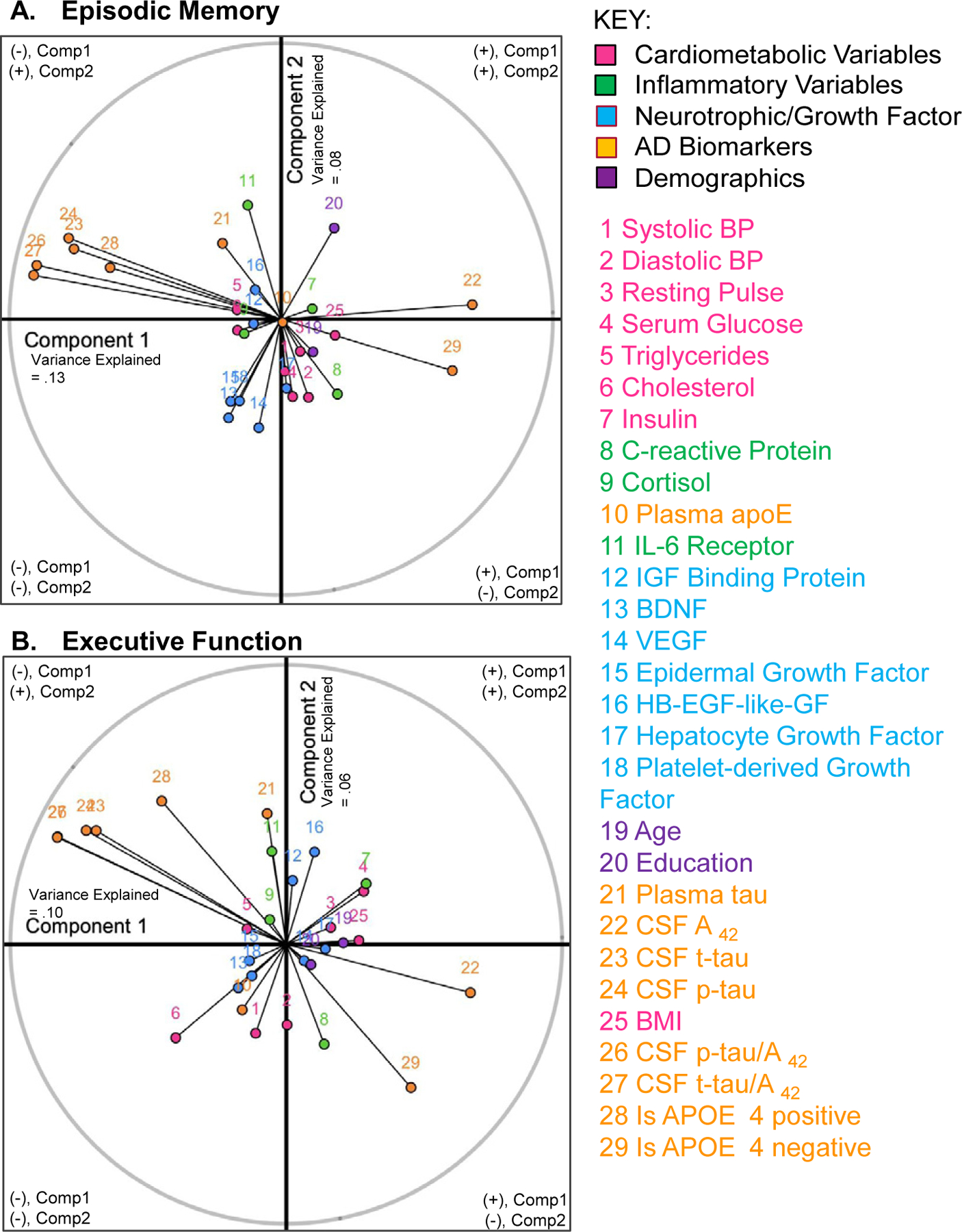
Circle of correlation per model. Figures show how each individual input variable (labelled 1 – 29; see figure legend) is correlated with Components 1 (x-axis) and 2 (y-axis) of each model. Each variable is plotted in a standard coordinate plane. For instance, education (purple #20, A) is positively correlated with both Components 1 and 2, which places it on the right, upper half of the coordinate plane. Education was more highly correlated with Component 2, as shown by nearer distance of #20 from the y-axis, relative to the x-axis. A variable that is perfectly correlated with a given component would touch the edge (gray line) of the circle. A) Episodic Memory: AD biomarkers (orange) are largely associated with Component 1, and neurotrophic factors (blue) and education (purple #20) are largely associated with Component 2. B) Executive Function: AD biomarkers (orange) are associated with both Components 1 and 2, while cardiometabolic variables and HB-EGF-like-GF (#16) are largely associated with Component 2. BDNF, brain-derived neurotrophic factor; BMI, body mass index; BP, blood pressure; CSF, cerebrospinal fluid; HB-EGF-like-GF, heparin-binding epidermal growth factor like-growth factor; IL-6, interleukin-6; IGF, insulin-like growth factor; p-tau, phosphorylated tau181; t-tau, total tau; VEGF, vascular endothelial growth factor.
Next, BSRs and bootstrapped group means were calculated to explore group comparisons between 1) older adults who remained MCI versus converted to AD at two-year follow-up and 2) males and females on Components 1 and 2 of the episodic memory model. Post-hoc analyses revealed that older adults who converted to AD at two-year follow-up were significantly and negatively associated with Component 1 (BSRAD_Comp1 = −2.98; p = 0.01; Fig. 3A) but not Component 2 (BSRAD_Comp2 = −1.78; p = 0.07; Fig. 3A). Older adults who remained MCI at two-year follow-up were not significantly associated with Component 1 (BSRMCI_Comp1 = 1.69; p = 0.09; Fig. 3A) or Component 2 (p > 0.05; Fig. 3A). Together, as shown by the ellipses plotted in Fig. 3A, older adults who remained MCI were significantly different from those who converted to AD. Females were significantly and negatively associated with Component 1 (BSRFComp1 = −2.24; p = 0.02; Fig. 3B) but not Component 2 (p > 0.05; Fig. 3B). Males were not significantly associated with Component 1 or 2 (all ps > 0.05; Fig. 3B). Males and females were not significantly different from one another in this model, as shown by the overlapping ellipses in Fig. 3B. Therefore, females and those who converted to AD at two-year follow-up were significantly and negatively associated with Component 1, which was largely associated with AD pathology.
Fig. 3.
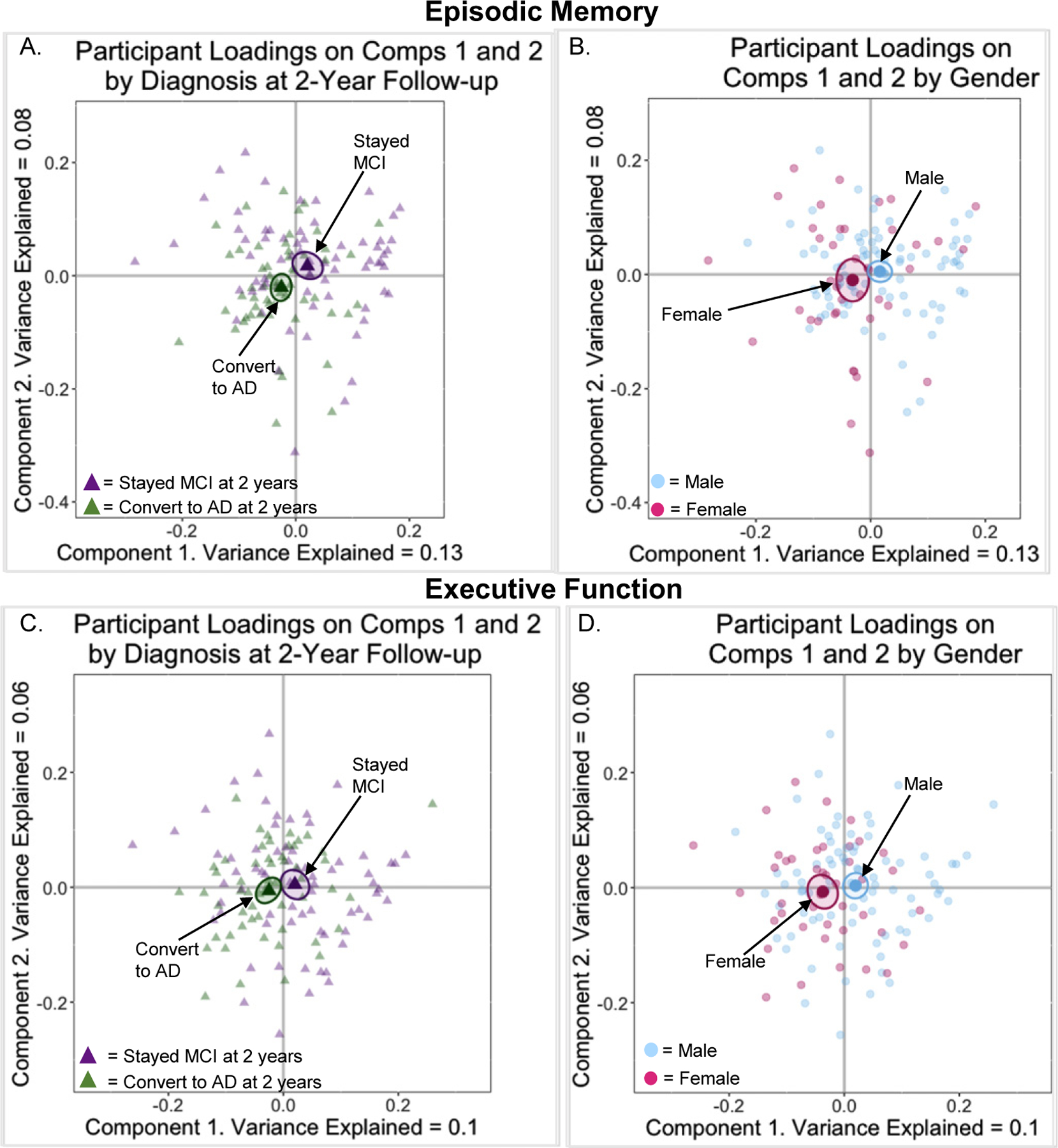
Maps of participant loadings for Components 1 and 2. A) Episodic Memory Model: participants coded by diagnosis at two-year follow-up. B) Episodic Memory Model: participants coded by gender. C) Executive Function Model: participants coded by diagnosis at two-year follow-up. D) Executive Function Model: participants coded by gender.
Executive function
Variance explained in Y per component was, respectively, 9.7%, 6.3%, and 3.6%. For brevity and due to the low percentage of variance explained, component three was not analyzed further. Figure 2B visualizes how each input variable correlated with Components 1 and 2 of the executive function model. Both Components 1 and 2 were associated with AD biomarkers (as evidenced by a 45° angle on the circle of correlation, demonstrating that AD biomarkers were roughly equally correlated with both components). Only Component 2 was associated with cardiometabolic variables and HB-EGF-like-GF (Fig. 2B). Specifically, CSF t-tau, CSF p-tau181, CSF t-tau/Aβ42, CSF p-tau181/Aβ42, and positive APOE ε4 allele status were negatively correlated with Component 1 and positively correlated with Component 2. CSF Aβ42 and negative APOE ε4 allele status were positively correlated with Component 1 and negatively correlated with Component 2. Systolic blood pressure, diastolic blood pressure, and cholesterol were correlated with Component 2 negatively while plasma tau, interleukin-6 receptor, and HB-EGF-like-GF were positively correlated with Component 2. In contrast to the episodic memory model, both Components 1 and 2 revealed how AD pathology contributed to executive function, while Component 2 also demonstrates how cardiometabolic risk and inflammation contribute to the executive function model prediction. Figure 2B shows that several AD biomarkers and several cardiometabolic variables clustered together, suggesting that these variables are highly correlated with one another, but differentially contribute to the model prediction.
BSRs and bootstrapped group means were calculated to explore group comparisons between 1) older adults who remained MCI versus converted to AD at two-year follow-up and 2) males and females on Components 1 and 2 of the executive function model. This post-hoc analysis revealed that older adults who converted to AD at two-year follow-up were significantly and negatively associated with Component 1 (BSRAD_Comp1 = −2.46; p = 0.02; Fig. 3C) but not Component 2 (p > 0.05; Fig. 3C). Older adults who remained MCI at two-year follow-up were not significantly associated with Components 1 (BSRMCI_Comp1 = 1.71, p = 0.09; Fig. 3C) or 2 (p > 0.05; Fig. 3C). Those who remained MCI were not significantly different from those who converted to AD in this model, as shown by the overlapping ellipses in Fig. 3C. Females were significantly and negatively associated with Component 1 (BSRFComp1 = −3.01; p = 0.004; Fig. 3D) but not Component 2 (p > 0.05; Fig. 3D). Males were not significantly associated with either Component 1 (BSRM_Comp1 = 1.94; p = 0.06; Fig. 3D) or 2 (p > 0.05; Fig. 3D). As shown by the bootstrapped CIs plotted in Fig. 3D, males and females were significantly different from one another in this model. Similar to the episodic memory model, females and those who converted to AD at two-year follow-up were significantly and negatively associated with Component 1.
DISCUSSION
To summarize the results, whole-model PLSR analyses explained roughly 20% of the variance in slope of change in episodic memory and slope of change in executive function performance over two years in older adults with MCI. A critical aspect of the current study includes the multivariate, data driven approach used to consider the multiple classes of variables and their impact on domain specific changes in cognition over two years. The results indicated that AD biomarkers were important for predicting cognitive change over two years in both episodic memory and executive function. Other variables exhibited domain specific relationships. Variables associated with neural plasticity (BDNF, VEGF), inflammation (IL-6-receptor), and education were important predictors for change in episodic memory but not executive function. In contrast, variables associated with cardiometabolic function (blood pressure, cholesterol) and HB-EGF-like-GF were important predictors of change in executive function but not episodic memory. Post-hoc analyses revealed that females and those who converted to AD at two-year follow-up were significantly and negatively associated with Component 1 in both models, which was predominately associated with AD biomarkers in the episodic memory model. Thus, participants who negatively load on Component 1 in the episodic memory model were more likely to be female, convert to AD at two-year follow-up, and have higher levels of AD biomarkers associated with AD pathology (i.e., higher levels of CSF t-tau, CSF p-tau181, CSF t-tau/Aβ42, CSF p-tau181/Aβ42, lower levels of CSF Aβ42, and a positive APOE ε4 allele status).
Episodic memory
Results demonstrated that AD biomarkers were important predictors of slope of change in episodic memory at two-year follow-up. Specifically, our finding that higher CSF Aβ42 was a positive predictor of change in episodic memory is consistent with prior work showing that levels of CSF Aβ42 predicted change in memory over two years in those with early and late MCI [36], as well as research showing that older adults with lower CSF Aβ42 had steeper declines in memory over a three-year follow-up [37]. This result could be due to underlying structural changes in the brain, evidenced by prior work showing that lower CSF Aβ42 at baseline was associated with increased rates of brain atrophy and cortical thinning [38], as well as significant changes in brain volume over one-year follow-up in MCI subjects [39]. Additional work has shown that brain atrophy was a significant mediator between baseline levels of CSF Aβ42 and memory declines in older adults with early and late MCI, an effect suggesting that brain atrophy, specifically in the medial temporal regions of the brain, may be the mechanism underlying changes in cognition associated with baseline levels of CSF Aβ42 [36]. This finding also supports our prior work showing that CSF Aβ42 was a positive predictor of multiple domains of cognition, including episodic memory, cross-sectionally [19].
Additionally, the CSF t-tau/Aβ42 ratio was a negative predictor of change in episodic memory. To our knowledge, this may be one of the first reports linking CSF t-tau/Aβ42 ratio to longitudinal change in objective assessment of episodic memory performance in MCI. These data strengthen the findings of a recent cross-sectional study, which demonstrated that older adults with a higher CSF t-tau/Aβ42 ratio had worse verbal episodic memory performance [7], as well as earlier work showing that a higher CSF t-tau/Aβ42 ratio predicted change in cognition as assessed by the clinical dementia rating scale [40]. Other research exploring plasma rather than levels of CSF t-tau and CSF Aβ42 in those with amnestic MCI found that using both of these biomarkers led to better predictions of current and future episodic memory performance than exploring either alone, although they did not explore the ratio [41]. Multiple studies have shown that CSF t-tau/Aβ42 ratio is a reliable predictor of conversion from MCI to AD [42], including one study which found that older adults who converted from MCI to AD at two-year follow-up had higher levels of CSF t-tau/Aβ42 at baseline [43]. CSF t-tau/Aβ42 ratio may represent a potentially promising biomarker for identifying older adults with MCI who are most at risk for a decline in episodic memory.
CSF t-tau, CSF p-tau181, CSF p-tau181/Aβ42, and APOE ε4 allele status were additional variables related to AD pathology that were considered important (VIP > 1) but not reliable (BPLS values not significantly different from zero at p < 0.05) predictors in the episodic memory model. This result suggests when explored together, these variables were important but not independent predictors of change in episodic memory. Research has established that these variables are important in exploring trajectories of normal aging to AD [44, 45], and APOE ε4 allele status is a well-known predictor of cognitive decline. Additionally, prior cross-sectional work in our laboratory revealed that CSF t-tau and CSF p-tau181 were negative predictors of multiple cognitive domains including episodic memory [19]. However, our results revealed that when these AD-related variables were explored simultaneously, CSF t-tau, CSF p-tau181, CSF p-tau181/Aβ42, and APOE ε4 allele status were relatively inferior predictors of change in episodic memory when compared to CSF Aβ42 and CSF t-tau/Aβ42.
Post-hoc analyses of episodic memory revealed that participants who were female or converted to AD at two-year follow-up significantly and negatively loaded on Component 1, which was largely correlated with AD biomarkers indicative of worse pathology (Fig. 2A). This pattern is consistent with current literature exploring AD biomarkers across the cognitively normal to AD spectrum, which has found that CSF Aβ42 declines first, then CSF t-tau and p-tau181, followed by changes in memory [36, 45]. Thus, one could speculate that participants who negatively loaded on Component 1 may have been at a higher risk of converting to AD at two-year follow-up due to their baseline AD biomarker values, which was potentially followed by brain atrophy leading to steeper declines in memory and thus a diagnosis of AD at follow-up [36, 38, 39]. These post-hoc results are also consistent with work showing that in older adults with MCI, females with levels of CSF Aβ42 and CSF t-tau indicative of worse pathology had steeper declines in memory as well as hippocampal volume when compared to men [46]. This suggests that women may have a higher likelihood of converting to AD than men, even with similar levels of pathology.
In addition to AD biomarkers, education was considered an important (VIP > 1) and reliable positive (BPLS significantly different from zero at p < 0.05) predictor of slope of change in episodic memory. This finding supports recent work showing that higher education positively impacted future memory and language declines in White older adults without dementia at baseline, as our sample was predominately White [47]. Prior work in our laboratory exploring cross-sectional associations between education and cognition using PLSC also showed that education was positively related to multiple cognitive domains including episodic memory [19]. This result also aligns with multiple studies, including a meta-analysis, that show that lower levels of education put participants at greater risk for developing dementia [48, 49]. Several studies have shown that education may be used as a proxy of cognitive reserve [48, 50]—defined as an individual’s characteristics that preserve cognitive function despite age-related or pathology-related brain changes in aging [51]. However, education is one of many factors that can contribute to an individual’s cognitive reserve [49]. Thus, our finding that education was positively associated with trajectories of episodic memory performance suggests that older adults with higher levels of education have attenuated decline in episodic memory over two years, even at the MCI stage—an effect which may be due to higher education increasing cognitive reserve.
Neurotrophic/growth factors BDNF and VEGF were important (VIP > 1) but not reliable (BPLS values not significantly different from zero at p < 0.05) predictors of episodic memory. Thus, BDNF and VEGF were not reliable predictors of change in episodic memory but are important enough to the model that removing these two markers would affect the model prediction. Recent work has suggested that plasma levels of BDNF might not be useful to explore in MCI, because plasma BDNF levels could not distinguish between healthy older adult and MCI groups, and did not correlate with changes in multiple cognitive domains including memory and executive function [52]. The current results were inconsistent with these findings, as plasma BDNF was important in predicting episodic memory change in MCI. But this finding does align with our prior analysis using PLSC, which revealed that plasma BDNF was significantly and positively associated with cognition cross-sectionally. Other cross-sectional work has shown that lower serum levels of BDNF were associated with memory impairment but not executive dysfunction in community dwelling older adults [8]. Our results are consistent with those findings and further strengthen and extend this pattern to longitudinal changes, as we found that BDNF was important in predicting change in episodic memory but not executive function. Prior work has also shown that VEGF was one of six biomarkers in a profile that distinguished AD participants from cognitively normal participants and correlated with current but not future severity of cognitive impairment [11]. Other work has shown that increased VEGF levels in AD were associated with poorer memory and executive function, but only in APOE ε4 carriers [53]. Thus, our finding that VEGF was an important but not a reliable predictor of episodic memory in MCI could be due to differences between APOE ε4 genotype or its relevance to cognition in AD, which may create differences in findings for those with MCI due to AD rather than MCI due to another type of dementia [53]. Given the conflicting results regarding plasma BDNF and VEGF for predicting cognition in MCI, it is clear that additional studies are needed to clarify the relationships between these growth factors and decline in episodic memory.
Finally, IL-6 receptor was considered an important (VIP > 1) but not reliable (BPLS value did not significantly differ from zero at p < 0.05) predictor of slope of change in episodic memory at two-year follow-up. This is consistent with research exploring CSF levels of IL-6-receptor in ADNI, which found that three of 83 assessed CSF proteins were significant predictors of global cognition in MCI patients at four-year follow-up [54]. However, IL-6-receptor was no longer significant after controlling for multiple comparisons. Similarly, our results show that plasma IL-6 receptor is one of the 12 important variables out of 29 predictors of change in episodic memory, but did not have a reliable BPLS value, a pattern indicating that plasma IL-6 receptor was not a reliable predictor on its own. Other work has shown decreases in serum levels of IL-6 receptor in AD patients compared to healthy controls, suggesting that higher levels IL-6-receptor may be beneficial [55]. However, few studies have explored plasma levels of IL-6-receptor in the context of predicting episodic memory or global cognition, highlighting the need to explore this variable further.
Executive function
Our findings demonstrated that AD biomarkers were important and negative predictors of slope of change in executive function at two-year follow-up. Specifically, lower CSF t-tau/Aβ42 ratio was the only reliable and positive predictor of change in executive function (BPLS significantly different from 0 at p < 0.05). Similar to our finding in the episodic memory model, we believe this is one of the first reports linking CSF t-tau/Aβ42 ratio to longitudinal change in objective assessment of executive function performance in MCI. These results support recent work which revealed that in those with late MCI, lower levels of CSF Aβ42 and higher CSF t-tau (which would produce a higher ratio) were significant predictors of change in executive function, with lateral temporal lobe atrophy mediating this relationship [36]. Additionally, recent work in those with subjective cognitive decline demonstrated that lower baseline levels of CSF Aβ42 and higher CSF t-tau were associated with steeper declines in tests of attention and executive functions [56]. Cross-sectional work exploring both CSF t-tau and Aβ42 found that older adults with MCI that had lower CSF t-tau and higher CSF Aβ42 had better performance in attention tasks, which is a component of executive function [57]. Prior research has shown that CSF t-tau/Aβ42 ratio in MCI subjects was significantly different between normal and control participants, was significantly and negatively related to longitudinal changes in brain volume in age-vulnerable regions [39], and predicted conversion from amnestic MCI to dementia [42]. Thus, one could infer that participants with a higher baseline CSF t-tau/Aβ42 ratio (indicative of worse AD pathology) might have negative slopes of change in executive function at two-year follow-up due to being in the later stage of MCI [36], decreases in brain volume [39], or being at greater risk of converting to AD [42].
CSF Aβ42, CSF t-tau, CSF p-tau181, CSF p-tau181/Aβ42, and APOE ε4 allele status were additional variables related to AD pathology that were considered important (VIP > 1) but not reliable (BPLS values not significantly different from zero at p < 0.05) predictors for executive function. As noted in the episodic memory model discussion, prior research has shown that these variables were important predictors of change from cognitively normal to AD status [44, 45]. Additionally, as discussed in the episodic memory model discussion, prior work in our lab using PLSC indicates that CSF Aβ42, CSF t-tau, and CSF p-tau181 are significantly associated with several cognitive domains including executive function cross-sectionally [19]. However, only one executive function task (Trail Making Test Trail B) was significant in this prior analysis, an effect which is not as robust as using the ADNI executive function composite score employed for the present study. The present findings show that, when explored simultaneously, CSF t-tau/Aβ42 is a better predictor of executive function than the other explored AD-related variables (CSF Aβ42, CSF t-tau, CSF p-tau181, CSF p-tau181/Aβ42, and APOE ε4 allele status).
The executive function model revealed that HB-EGF-like-GF was important (VIP > 1) but not reliable (BPLS value not significantly different from zero at p < 0.05) predictor of executive function at two-year follow-up. Interestingly, our previous cross-sectional study showed that HB-EGF-like-GF was positively associated with multiple cognitive domains, including executive function, in a cohort of participants with MCI, a pattern suggesting potential neuroprotective benefits [19]. Very few studies have explored HB-EGF-like-GF in relation to human aging and cognition [17, 58]. In one study exploring cognitively normal and MCI participants, higher levels of CSF HB-EGF-like-GF were associated with greater AD pathology, demonstrated by lower levels of CSF Aβ42 and higher levels of CSF t-tau [58], which conflicted with our prior finding. Other studies exploring mice supported this relationship, demonstrating that HB-EGF-like-GF caused a cascade of cellular events leading to neuroinflammation and subsequent increases in Aβ in the brain [59, 60]. However, other work exploring mice has shown that lack of HB-EGF-like-GF was associated with decreased neurogenesis in the hippocampus, which aligns with our prior cross-sectional finding that plasma HB-EGF-like-GF may have a neuroprotective effect [61]. The current study showed that HB-EGF-like-GF was not a reliable predictor of executive function on its own, although it was important to predicting change in executive function in this MCI cohort. Unfortunately, the directionality of this relationship cannot be probed further, as the BPLS value was not significantly different from zero, and thus cannot be interpreted. The available literature has demonstrated that it is possible that plasma HB-EGF-like-GF contributes to cognitive changes in humans, but future human subjects research should further explore this biomarker to assess its potential role in predicting cognitive change.
Modifiable cardiometabolic variables including diastolic blood pressure, systolic blood pressure, and cholesterol were important (VIP > 1) but not reliable (BPLS values not significantly different from zero at p < 0.05) predictors of change in executive function at two-year follow-up. This is consistent with research showing that metabolic syndrome symptoms as a whole, rather than individual symptoms, predicted small but negative effects on executive function and language but not episodic memory in community dwelling older adults [62]. In terms of individual cardiometabolic variables, a recent longitudinal population-based study of those ages 5 to 95 years found that mean systolic blood pressure over time was associated with significant declines in executive function at follow-up (average of 12.4 years; [63]).
Post-hoc analyses of executive function revealed that participants who were female or converted to AD at two-year follow-up significantly and negatively loaded on Component 1 (Fig. 2B). AD biomarkers indicative of worse pathology were approximately equally correlated with both Components 1 and 2 of this model. However, post-hoc analyses for gender and diagnosis at two-year follow-up were not significant for Component 2. Thus, there was not a clear interpretation for a significant and negative loading on Component 1 (females and those converting to AD at two-year follow-up), and as such we will not interpret these results further in order to avoid an over-interpretation of the data.
Limitations and strengths
The present study had several limitations. First, the ADNI sample lacks racial diversity and is highly educated—a configuration which may limit generalizability. This work also did not explore a few biomarkers of interest such as IL-6 (rather than IL-6 receptor) and tumor necrosis factor-alpha due to data either not being available in the ADNI sample or due to high amounts (> 10%) of imputed biomarker values in the MCI sample who had neuropsychological data available. The current report did not explore other modifiable cardiometabolic/metabolic syndrome variables of interest in aging such as high-density lipoprotein cholesterol, low-density lipoprotein cholesterol, waist circumference, body fat percentage, etc., as these data were not available in this cohort. Bidirectional associations (e.g., does cognitive decline precede change in a given modifiable variable?) were not assessed because several of the biomarkers examined at baseline were either only assessed at baseline (cardiometabolic variables such as triglycerides, cholesterol and serum glucose and AD biomarker plasma tau) or were only assessed at baseline and 12-month follow-up but not 24-month follow up (inflammatory markers such as insulin, interleukin-6 receptor, c-reactive protein, and neurotrophic/growth factors such as BDNF and VEGF). However, these variables are likely modifiable and not simply a consequence of disease progression as several large population-based studies have shown that higher levels of cardiovascular, metabolic, and inflammatory variables were predictors of memory decline [64], executive function decline [65], and incident dementia at follow-up [64]. In the few studies that have assessed bi-directional relationships between modifiable variables and cognition in older adults, higher BMI predicted longitudinal declines in cognition, but cognition did not predict change in BMI [66]. Lastly, the present study had a relatively small sample size (N = 123) and a large number of predictors (I = 29), which may limit the generalizability of the current findings to the broader MCI population. In particular, important but less reliable predictors such as cardiometabolic variables and neurotrophic/growth factors that exhibited domain-specific findings for episodic memory and executive function respectively, may not generalize to the broader MCI population.
Overall, the current study had multiple strengths. A unique multivariate approach, PLSR, was implemented, which allowed for the simultaneous exploration of several physiological and demographic variables of interest without violating traditional statistical assumptions needed for other multivariate analyses such as multiple linear regression. This approach is new relative to much of the extant literature, which examines a single variable or class of variables and examines global cognition or a single cognitive domain. This work also explored longitudinal cognitive change in MCI, where older adults are at greatest risk of converting to dementia.
Conclusions
In summary, the simultaneous assessment of AD biomarkers, neurotrophic/growth factors, cardiometabolic variables, and demographics revealed that AD biomarkers were important and reliable negative predictors of change in both episodic memory and executive function over two years in older adults with MCI. Specifically, CSF Aβ42, CSF t-tau/Aβ42, and education were reliable predictors of change in episodic memory whereas CSF t-tau/Aβ42 was a reliable predictor of change in executive function. There was also evidence of domain specificity. Variables associated with neuroplasticity and cognitive reserve were important for change in episodic memory, whereas variables associated with cardiometabolic health were important in predicting executive function. These findings provide preliminary evidence for the suggestion that interventions targeting neurotrophic/growth factors and cognitive reserve may be relatively more effective in slowing decline in episodic memory, whereas interventions targeting cardiometabolic health variables may be relatively more effective in slowing decline in executive function. Together, variables associated with neuroplasticity and modifiable cardiometabolic health variables were important for predicting change in episodic memory and executive function respectively but were not reliable predictors on their own. AD biomarkers, however, were reliable predictors on their own for both cognitive domains, and thus may carry relatively more importance in predicting these two cognitive outcomes in older adults with MCI, which should be considered when designing future research.
Supplementary Material
ACKNOWLEDGMENTS
We would like to thank Jena Moody for her contributions including sharing her knowledge of the ADNI database.
FUNDING
This research was supported by the National Institute on Aging (NIA) of the National Institutes of Health (NIH) R01AG068882 (awarded to SMH) and R21AG056921 (awarded to SMH); and The Ohio State University Discovery Themes Chronic Brain Injury Initiative (awarded to SMH).
Data collection and sharing for this project was funded by the Alzheimer’s Disease Neuroimaging Initiative (ADNI) (National Institutes of Health Grant U01 AG024904) and DOD ADNI (Department of Defense award number W81XWH-12–2-0012). ADNI is funded by the National Institute on Aging, the National Institute of Biomedical Imaging and Bioengineering, and through generous contributions from the following: AbbVie, Alzheimer’s Association; Alzheimer’s Drug Discovery Foundation; Araclon Biotech; BioClinica, Inc.; Biogen; Bristol-Myers Squibb Company; CereSpir, Inc.; Cogstate; Eisai Inc.; Elan Pharmaceuticals, Inc.; Eli Lilly and Company; EuroImmun; F. Hoffmann-La Roche Ltd and its affiliated company Genentech, Inc.; Fujirebio; GE Healthcare; IXICO Ltd.; Janssen Alzheimer Immunotherapy Research & Development, LLC.; Johnson & Johnson Pharmaceutical Research & Development LLC.; Lumosity; Lundbeck; Merck & Co., Inc.; Meso Scale Diagnostics, LLC.; NeuroRx Research; Neurotrack Technologies; Novartis Pharmaceuticals Corporation; Pfizer Inc.; Piramal Imaging; Servier; Takeda Pharmaceutical Company; and Transition Therapeutics. The Canadian Institutes of Health Research is providing funds to support ADNI clinical sites in Canada. Private sector contributions are facilitated by the Foundation for the National Institutes of Health (http://www.fnih.org). The grantee organization is the Northern California Institute for Research and Education, and the study is coordinated by the Alzheimer’s Therapeutic Research Institute at the University of Southern California. ADNI data are disseminated by the Laboratory for Neuro Imaging at the University of Southern California.
Footnotes
CONFLICT OF INTEREST
The authors do not have any conflicts of interest.
SUPPLEMENTARY MATERIAL
The supplementary material is available in the electronic version of this article: https://dx.doi.org/10.3233/JAD-221084.
DATA AVAILABILITY
The data supporting the findings of this study are openly available in the Alzheimer’s Disease Neuroimaging Initiative database, https://adni.loni.usc.edu/.
REFERENCES
- [1].Rios M (2014) For immediate release: Tuesday, May 06, 2014. Census.gov 2012–2014. [Google Scholar]
- [2].Matthews KA, Xu W, Gaglioti AH, Holt JB, Croft JB, Mack D, McGuire LC (2019) Racial and ethnic estimates of Alzheimer’s disease and related dementias in the United States (2015–2060) in adults aged≥65 years. Alzheimers Dement 15, 17–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Brookmeyer R, Abdalla N (2018) Estimation of lifetime risks of Alzheimer’s disease dementia using biomarkers for preclinical disease. Alzheimers Dement 14, 981–988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Diniz BSO, Pinto JA, Forlenza OV (2008) Do CSF total tau, phosphorylated tau, and b-amyloid 42 help to predict progression of mild cognitive impairment to Alzheimer’s disease? A systematic review and meta-analysis of the literature. World J Biol Psychiatry 9, 172–182. [DOI] [PubMed] [Google Scholar]
- [5].Hansson O, Seibyl J, Stomrud E, Zetterberg H, Trojanowski JQ, Bittner T, Lifke V, Corradini V, Eichenlaub U, Batrla R, Buck K, Zink K, Rabe C, Blennow K, Shaw LM (2018) CSF biomarkers of Alzheimer’s disease concord with amyloid-β PET and predict clinical progression: A study of fully automated immunoassays in BioFINDER and ADNI cohorts. Alzheimers Dement 14, 1470–1481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Prakash RS, McKenna MR, Gbadeyan O, Andridge R, Scharre DW (2020) p-tau/Aβ42 ratio associates with cognitive decline in Alzheimer’s disease, mild cognitive impairment, and cognitively unimpaired older adults. medRxiv 2020.10.13.20211375. [Google Scholar]
- [7].Matura S, Köhler J, Reif A, Fusser F, Karakaya T, Scheibe M, Ehret F, Hartmann D, Kang JS, Mayer C, Prvulovic D, Pantel J (2020) Intrinsic functional connectivity, CSF biomarker profiles and their relation to cognitive function in mild cognitive impairment. Acta Neuropsychiatr 32, 206–213. [DOI] [PubMed] [Google Scholar]
- [8].Mizoguchi Y, Yao H, Imamura Y, Hashimoto M, Monji A (2020) Lower brain-derived neurotrophic factor levels are associated with age-related memory impairment in community-dwelling older adults: The Sefuri study. Sci Rep 10, 16442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Weinstein G, Beiser AS, Choi SH, Preis SR, Chen TC, Vorgas D, Au R, Pikula A, Wolf PA, DeStefano AL, Vasan RS, Seshadri S (2014) Serum brain-derived neurotrophic factor and the risk for dementia: The Framingham Heart Study. JAMA Neurol 71, 55–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Miranda M, Morici JF, Zanoni MB, Bekinschtein P (2019) Brain-derived neurotrophic factor: A key molecule for memory in the healthy and the pathological brain. Front Cell Neurosci 13, 363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Hu WT, Chen-Plotkin A, Arnold SE, Grossman M, Clark CM, Shaw LM, Pickering E, Kuhn M, Chen Y, McCluskey L, Elman L, Karlawish J, Hurtig HI, Siderowf A, Lee VM- Y, Soares H, Trojanowski JQ (2010) Novel CSF biomarkers for Alzheimer’s disease and mild cognitive impairment. Acta Neuropathol (Berl) 119, 669–678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Assuncao N, Sudo FK, Drummond C, De Felice FG, Mattos P (2018) Metabolic syndrome and cognitive decline in the elderly: A systematic review. PLoS One 13, e0194990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Femminella GD, Taylor-Davies G, Scott J, Edison P (2018) Do cardiometabolic risk factors influence amyloid, tau, and neuronal function in APOE4 carriers and non-carriers in Alzheimer’s disease trajectory? J Alzheimers Dis 64, 981–993. [DOI] [PubMed] [Google Scholar]
- [14].Gao Q, Gwee X, Feng L, Nyunt MSZ, Feng L, Collinson SL, Chong MS, Lim WS, Lee TS, Yap P, Yap KB, Ng TP (2018) Mild cognitive impairment reversion and progression: Rates and predictors in community-living older persons in the Singapore Longitudinal Ageing Studies Cohort. Dement Geriatr Cogn Disord Extra 8, 226–237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Yaffe K, Kanaya A, Lindquist K, Simonsick EM, Harris T, Shorr RI, Tylavsky FA, Newman AB (2004) The metabolic syndrome, inflammation, and risk of cognitive decline. J Am Med Assoc 292, 2237–2242. [DOI] [PubMed] [Google Scholar]
- [16].Alcorn T, Hart E, Smith AE, Feuerriegel D, Stephan BCM, Siervo M, Keage HAD (2019) Cross-sectional associations between metabolic syndrome and performance across cognitive domains: A systematic review. Appl Neuropsychol Adult 26, 186–199. [DOI] [PubMed] [Google Scholar]
- [17].Meyer P-F, Savard M, Poirier J, Morgan D, Breitner J (2019) Hypothesis: Cerebrospinal fluid protein markers suggest a pathway toward symptomatic resilience to AD pathology. Alzheimers Dement 15, 1160–1171. [DOI] [PubMed] [Google Scholar]
- [18].Krishnan A, Williams LJ, McIntosh AR, Abdi H (2011) Partial least squares (PLS) methods for neuroimaging: A tutorial and review. Neuroimage 56, 455–475. [DOI] [PubMed] [Google Scholar]
- [19].Stark J, Palombo DJ, Hayes JP, Hiersche KJ, Hasselbach AN, Hayes SM, Alzheimer’s Disease Neuroimaging Initiative (2022) Partial least squares analysis of Alzheimer’s disease biomarkers, modifiable health variables, and cognition in older adults with mild cognitive impairment. J Int Neuropsychol Soc 28, 781–789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Abdi H (2010) Partial least squares regression and projection on latent structure regression (PLS Regression). Wiley Interdiscip Rev Comput Stat 2, 97–106. [Google Scholar]
- [21].Abdi H, Williams LJ (2013) Partial least squares methods: Partial least squares correlation and partial least square regression. In Methods in Molecular Biology, Reisfeld B, Mayeno AN, eds. Humana Press, Clifton, N.J., pp. 549–570. [DOI] [PubMed] [Google Scholar]
- [22].Van Roon P, Zakizadeh J, Chartier S (2014) Partial least squares tutorial for analyzing neuroimaging data. Quant Methods Psychol 10, 200–215. [Google Scholar]
- [23].Buckner RL (2004) Memory and executive function in aging and ad: Multiple factors that cause decline and reserve factors that compensate. Neuron 44, 195–208. [DOI] [PubMed] [Google Scholar]
- [24].Tromp D, Dufour A, Lithfous S, Pebayle T, Després O (2015) Episodic memory in normal aging and Alzheimer disease: Insights from imaging and behavioral studies. Ageing Res Rev 24, 232–262. [DOI] [PubMed] [Google Scholar]
- [25].(2010) ADNI Procedures Manual. 1–120. [Google Scholar]
- [26].Ottoy J, Niemantsverdriet E, Verhaeghe J, De Roeck E, Struyfs H, Somers C, wyffels L, Ceyssens S, Van Mossevelde S, Van den Bossche T, Van Broeckhoven C, Ribbens A, Bjerke M, Stroobants S, Engelborghs S, Staelens S (2019) Association of short-term cognitive decline and MCI-to-AD dementia conversion with CSF, MRI, amyloid- and 18F-FDG-PET imaging. Neuroimage Clin 22, 101771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Nordlund A, Rolstad S, Klang O, Edman Ǻ, Hansen S, Wallin A (2010) Two-year outcome of MCI subtypes and aetiologies in the Göteborg MCI study. J Neurol Neurosurg Psychiatry 81, 541–546. [DOI] [PubMed] [Google Scholar]
- [28].Grimmer T, Wutz C, Drzezga A, Forster S, Forstl H, Ortner M, Perneczky R, Kurz A (2013) The usefulness of amyloid imaging in predicting the clinical outcome after two years in subjects with mild cognitive impairment. Curr Alzheimer Res 10, 82–85. [PubMed] [Google Scholar]
- [29].Crane PK, Carle A, Gibbons LE, Insel P, Mackin RS, Gross A, Jones RN, Mukherjee S, Curtis SMK, Harvey D, Weiner M, Mungas D (2012) Development and assessment of a composite score for memory in the Alzheimer’s Disease Neuroimaging Initiative (ADNI). Brain Imaging Behav 6, 502–516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Gibbons LE, Carle AC, Mackin RS, Harvey D, Mukherjee S, Insel P, Curtis SMK, Mungas D, Crane PK (2012) A composite score for executive functioning, validated in Alzheimer’s Disease Neuroimaging Initiative (ADNI) participants with baseline mild cognitive impairment. Brain Imaging Behav 6, 517–527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Eriksson S, Lundquist A, Gustafson Y, Lundin-Olsson L (2009) Comparison of three statistical methods for analysis of fall predictors in people with dementia: Negative binomial regression (NBR), regression tree (RT), and partial least squares regression (PLSR). Arch Gerontol Geriatr 49, 383–389. [DOI] [PubMed] [Google Scholar]
- [32].Kosse NM, De Groot MH, Vuillerme N, Hortobágyi T, Lamoth CJC (2015) Factors related to the high fall rate in long-term care residents with dementia. Int Psychogeriatr 27, 803–814. [DOI] [PubMed] [Google Scholar]
- [33].Akarachantachote N, Chadcham S, Saithanu K (2014) Cutoff threshold of variable importance in projection for variable selection. Int J Pure Appl Math 94, 307–322. [Google Scholar]
- [34].Efron B, Tibshirani RJ (1994) An Introduction to the Bootstrap, Chapman and Hall/CRC, New York. [Google Scholar]
- [35].Hesterberg T (2011) Bootstrap. WIREs Comp Stat 3, 497–526. [Google Scholar]
- [36].Fletcher E, Filshtein TJ, Harvey D, Renaud A, Mungas D, DeCarli C (2018) Staging of amyloid β, t-tau, regional atrophy rates, and cognitive change in a nondemented cohort: Results of serial mediation analyses. Alzheimers Dement (Amst) 10, 382–393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Ho JK, Nation DA (2018) Neuropsychological profiles and trajectories in preclinical Alzheimer’s disease. J Int Neuropsychol Soc 24, 693–702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Tosun D, Schuff N, Truran-Sacrey D, Shaw LM, Trojanowski JQ, Aisen P, Peterson R, Weiner MW, Alzheimer’s Disease Neuroimaging Initiative (2010) Relations between brain tissue loss, CSF biomarkers, and the ApoE genetic profile: A longitudinal MRI study. Neurobiol Aging 31, 1340–1354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Fjell AM, Walhovd KB, Fennema-Notestine C, McEvoy LK, Hagler DJ, Holland D, Brewer JB, Dale AM, Alzheimer’s Disease Neuroimaging Initiative (2010) CSF biomarkers in prediction of cerebral and clinical change in mild cognitive impairment and Alzheimer’s disease. J Neurosci 30, 2088–2101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Fagan AM, Roe CM, Xiong C, Mintun MA, Morris JC, Holtzman DM (2007) Cerebrospinal fluid tau/beta-amyloid(42) ratio as a prediction of cognitive decline in nondemented older adults. Arch Neurol 64, 343–349. [DOI] [PubMed] [Google Scholar]
- [41].Park J-C, Han S-H, Yi D, Byun MS, Lee JH, Jang S, Ko K, Jeon SY, Lee Y-S, Kim YK, Lee DY, Mook-Jung I (2019) Plasma tau/amyloid-β1–42 ratio predicts brain tau deposition and neurodegeneration in Alzheimer’s disease. Brain 142, 771–786. [DOI] [PubMed] [Google Scholar]
- [42].Vemuri P, Wiste HJ, Weigand SD, Shaw LM, Trojanowski JQ, Weiner MW, Knopman DS, Petersen RC, Jack CR (2009) MRI and CSF biomarkers in normal, MCI, and AD subjects: Predicting future clinical change. Neurology 73, 294–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Brys M, Pirraglia E, Rich K, Rolstad S, Mosconi L, Switalski R, Glodzik-Sobanska L, De Santi S, Zinkowski R, Mehta P, Pratico D, Saint Louis LA, Wallin A, Blennow K, de Leon MJ (2009) Prediction and longitudinal study of CSF biomarkers in mild cognitive impairment. Neurobiol Aging 30, 682–690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Jack CR, Knopman DS, Jagust WJ, Petersen RC, Weiner MW, Aisen PS, Shaw LM, Vemuri P, Wiste HJ, Weigand SD, Lesnick TG, Pankratz VS, Donohue MC, Trojanowski JQ (2013) Update on hypothetical model of Alzheimer’s disease biomarkers. Lancet Neurol 12, 207–216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Wang H-F, Shen X-N, Li J-Q, Suckling J, Tan C-C, Wang Y-J, Feng L, Zhang C, Tan L, Dong Q, Touchon J, Gauthier S, Yu J-T, Alzheimer’s Disease Neuroimaging Initiative (2020) Clinical and biomarker trajectories in sporadic Alzheimer’s disease: A longitudinal study. Alzheimers Dement (Amst) 12, e12095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Koran MEI, Wagener M, Hohman TJ (2017) Sex differences in the association between AD biomarkers and cognitive decline. Brain Imaging Behav 11, 205–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Avila JF, Rentería MA, Jones RN, Vonk JMJ, Turney I, Sol K, Seblova D, Arias F, Hill-Jarrett T, Levy S, Meyer O, Racine AM, Tom SE, Melrose RJ, Deters K, Medina LD, Carrión CI, Díaz-Santos M, Byrd DR, Chesebro A, Colon J, Igwe KC, Maas B, Brickman AM, Schupf N, Mayeux R, Manly JJ (2021) Education differentially contributes to cognitive reserve across racial/ethnic groups. Alzheimers Dement 17, 70–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Meng X, D’Arcy C (2012) Education and dementia in the context of the cognitive reserve hypothesis: A systematic review with meta-analyses and qualitative analyses. PLoS One 7, e38268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Stern Y (2013) Cognitive reserve in ageing. Lancet Neurol 11, 1006–1012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Jefferson AL, Gibbons LE, Rentz DM, Carvalho JO, Manly J, Bennett DA, Jones RN (2011) A life course model of cognitive activities, socioeconomic status, education, reading ability, and cognition. J Am Geriatr Soc 59, 1403–1411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Cabeza R, Albert M, Belleville S, Craik FIM, Duarte A, Grady CL, Lindenberger U, Nyberg L, Park DC, Reuter-Lorenz PA, Rugg MD, Steffener J, Rajah MN (2018) Maintenance, reserve and compensation: The cognitive neuroscience of healthy ageing. Nat Rev Neurosci 19, 701–710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Balietti M, Giuli C, Casoli T, Fabbietti P, Conti F (2020) Is blood brain-derived neurotrophic factor a useful biomarker to monitor mild cognitive impairment patients? Rejuvenation Res 23, 411–419. [DOI] [PubMed] [Google Scholar]
- [53].Alvarez XA, Alvarez I, Aleixandre M, Linares C, Muresanu D, Winter S, Moessler H (2018) Severity-related increase and cognitive correlates of serum VEGF levels in Alzheimer’s disease ApoE4 carriers. J Alzheimers Dis 63, 1003–1013. [DOI] [PubMed] [Google Scholar]
- [54].Khan W, Aguilar C, Kiddle SJ, Doyle O, Thambisetty M, Muehlboeck S, Sattlecker M, Newhouse S, Lovestone S, Dobson R, Giampietro V, Westman E, Simmons A (2015) A subset of cerebrospinal fluid proteins from a multi-analyte panel associated with brain atrophy, disease classification and prediction in Alzheimer’s disease. PLoS One 10, e0134368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Angelis P, Scharf S, Mander A, Vajda F, Christophidis N (1998) Serum interleukin-6 and interleukin-6 soluble receptor in Alzheimer’s disease. Neurosci Lett 244, 106–108. [DOI] [PubMed] [Google Scholar]
- [56].Verberk IMW, Hendriksen HMA, van Harten AC, Wesselman LMP, Verfaillie SCJ, van den Bosch KA, Slot RER, Prins ND, Scheltens P, Teunissen CE, Van der Flier WM (2020) Plasma amyloid is associated with the rate of cognitive decline in cognitively normal elderly: The SCIENCe project. Neurobiol Aging 89, 99–107. [DOI] [PubMed] [Google Scholar]
- [57].Nordlund A, Rolstad S, Klang O, Lind K, Pedersen M, Blennow K, Edman A, Hansen S, Wallin A (2008) Episodic memory and speed/attention deficits are associated with Alzheimer-typical CSF abnormalities in MCI. J Int Neuropsychol Soc 14, 582–590. [DOI] [PubMed] [Google Scholar]
- [58].Meyer PF, Savard M, Poirier J, Labonté A, Rosa-Neto P, Weitz TM, Town T, Breitner J (2018) Bi-directional association of cerebrospinal fluid immune markers with stage of Alzheimer’s disease pathogenesis. J Alzheimers Dis 63, 577–590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Ashok A, Rai NK, Raza W, Pandey R, Bandyopadhyay S (2016) Chronic cerebral hypoperfusion-induced impairment of Aβ clearance requires HB-EGF-dependent sequential activation of HIF1α and MMP9. Neurobiol Dis 95, 179–193. [DOI] [PubMed] [Google Scholar]
- [60].Maurya SK, Mishra J, Abbas S, Bandyopadhyay S (2016) Cypermethrin stimulates GSK3β-dependent Aβ and p-tau proteins and cognitive loss in young rats: Reduced HB-EGF signaling and downstream neuroinflammation as critical regulators. Mol Neurobiol 53, 968–982. [DOI] [PubMed] [Google Scholar]
- [61].Sasaki K, Omotuyi OI, Ueda M, Shinohara K, Ueda H (2015) NMDA receptor agonists reverse impaired psychomotor and cognitive functions associated with hippocampal Hbegf-deficiency in mice. Mol Brain 8, 83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Philippou E, Michaelides MP, Constantinidou F (2018) The role of metabolic syndrome factors on cognition using latent variable modeling: The neurocognitive study on aging. J Clin Exp Neuropsychol 40, 1030–1043. [DOI] [PubMed] [Google Scholar]
- [63].Levine DA, Gross AL, Briceño EM, Tilton N, Kabeto MU, Hingtgen SM, Giordani BJ, Sussman JB, Hayward RA, Burke JF, Elkind MSV, Manly JJ, Moran AE, Kulick ER, Gottesman RF, Walker KA, Yano Y, Gaskin DJ, Sidney S, Yaffe K, Sacco RL, Wright CB, Roger VL, Allen NB, Galecki AT (2020) Association between blood pressure and later-life cognition among black and white individuals. JAMA Neurol 77, 810–819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Cederwald BF von, Josefsson M, Wåhlin A, Nyberg L, Karalija N (2022) Association of cardiovascular risk trajectory with cognitive decline and incident dementia. Neurology 98, e2013–e2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Power MC, Rawlings A, Sharrett AR, Bandeen-Roche K, Coresh J, Ballantyne CM, Pokharel Y, Michos ED, Penman A, Alonso A, Knopman D, Mosley TH, Gottesman RF (2018) Association of midlife lipids with 20-year cognitive change: A cohort study. Alzheimers Dement 14, 167–177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Karlsson IK, Zhan Y, Gatz M, Reynolds CA, Dahl Aslan AK (2021) Change in cognition and body mass index in relation to preclinical dementia. Alzheimers Dement (N Y) 7, e12176. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data supporting the findings of this study are openly available in the Alzheimer’s Disease Neuroimaging Initiative database, https://adni.loni.usc.edu/.


