Abstract
In this study we investigated the effects of Vpr during human immunodeficiency virus (HIV) infection of proliferating Jurkat T cells by using a vesicular stomatitis virus envelope G glycoprotein pseudotyped HIV superinfection system. We observe that the expression of Vpr results in a severe reduction in the life span of HIV type 1 (HIV-1)-infected dividing T cells in culture. In agreement with a recent report (S. A. Stewart, B. Poon, J. B. M. Jowett, and I. S. Chen, J. Virol. 71:5579–5592, 1997), we show that events characteristic of apoptotic cell death are involved in the Vpr-mediated cytopathic effects. Our results also show that infection with viruses expressing the wild-type vpr gene results in an increase in viral gene expression and production. Interestingly, the effects of Vpr on cell viability and on viral gene expression both correlate with the ability of the protein to induce a cell cycle arrest in the G2/M phase. Mutagenesis analyses show that the C terminus of Vpr is essential for these biological activities. Although the role of Vpr is currently associated with the infection of nondividing cells, our results suggest that Vpr can also directly increase viral replication in vivo in infected dividing T cells. Furthermore, these in vitro observations suggest that Vpr-mediated cytotoxic effects could contribute to the CD4+ depletion associated with AIDS progression.
The human immunodeficiency virus type 1 (HIV-1) accessory gene product Vpr is a 96-amino-acid protein (14 kDa), which is highly conserved in HIV-1, HIV-2, and simian immunodeficiency virus (SIV) (reviewed in references 11 and 35). Several functional roles of Vpr during HIV replication have been suggested (11, 35). Unlike other HIV-1 accessory proteins, Vpr is efficiently incorporated into viral particles through an interaction with the p6 domain of the p55 Gag protein (6, 22, 24, 30). The incorporation of Vpr into progeny virions is highly suggestive of its participation in the early events of viral infection, such as its reported role in the nuclear import of the HIV-1 preintegration complex in nondividing cells (17). De novo-synthesized Vpr plays an additional role in HIV-1 replication since Vpr that is supplied in trans does not fully complement the replication of Vpr-negative viruses in infected macrophages (8). Vpr may upregulate viral gene expression. Several studies have shown that Vpr can modestly transactivate the HIV long terminal repeat (LTR) and heterologous viral promoters in dividing cells (7, 37), and this may be achieved by direct binding to transcription factors TFIIB and Sp1 (1, 37).
In contrast to its well-documented role in nondividing cells, Vpr’s role in HIV replication in dividing cells remains to be fully elucidated. Vpr appears to be dispensable for the in vitro replication of HIV in proliferating T cells and stimulated peripheral blood mononuclear cells (PBMCs) (3, 7, 8, 14, 29, 32). However, Vpr contributes substantially to HIV-1 replication in these cells since viral replication is increased in dividing cells when Vpr is present under certain conditions, such as at very low multiplicities of infection (MOIs) (3, 8, 29).
Recent evidence indicates that Vpr induces a cell cycle arrest in the G2/M phase in human and primate cells and in HIV-1-infected cells (19, 32). Vpr proteins from HIV-2 and SIV also cause a G2 arrest, but this can be species specific (34). Several studies have provided evidence that Vpr-mediated G2 arrest occurs as a consequence of the inhibition of the cyclin-dependent kinase p34cdc2 activity (4, 15, 31). The ability of Vpr to induce cell-cycle G2 arrest was shown to prevent persistent HIV infection in vitro (32). However, it remains to be established whether the Vpr-mediated G2 arrest plays a pivotal role in viral replication in vivo.
HIV-1 infection in vivo is characterized by persistent high-level viral replication with rapid turnover of CD4+ T cells (18, 38). Both direct and indirect mechanisms have been proposed to account for the decline of CD4+ T cells in HIV-1 infection. Cytotoxic effects and apoptotic events in HIV-1-infected cells have been shown to partly account for these phenomena (12, 21). A recent report by Stewart et al. (33) indicated that the induction of G2 arrest by Vpr correlates with the induction of apoptosis, and these authors have proposed that Vpr could contribute to the CD4+ cell depletion in HIV-induced AIDS.
In the present study, we use a single-cycle HIV-1 replication system to further characterize the effect of Vpr on viral replication in dividing T cells. Our results indicate that even though Vpr expression increases HIV-mediated cell killing, it also induces a concomitant increase of viral expression and production among surviving cells. Mutagenic analysis reveals that the carboxyl-terminal domain of Vpr is necessary to induce both the G2 arrest and the cytotoxic effect, suggesting that they are related phenomena. Finally, our results indicate that the Vpr-mediated increase in viral expression also correlates with its ability to mediate the G2 arrest.
MATERIALS AND METHODS
HIV molecular clones.
The HIV-1 envelope-defective proviral plasmids used in this study, including HxBRUR+/Env−, HxBRUR−/Env−, HxBRURV57L/Env−, HxBRURI63F/Env−, and HxBRURI63K/Env, have been described (36). In addition, HxBRURR80A/Env− was constructed by deleting a BglII fragment (nucleotides 6583 to 7163, +1 = start of initiation of transcription of HxBC2) from the Env sequence of an HIV-1 infectious proviral construct HxBRURR80A, in which the amino acid Arg-80 in Vpr was substituted for Ala by a two-step PCR-based method essentially as described previously (41). The nucleotide sequence of the sense mutagenic oligonucleotide is 5′-TCGACATAGCGCAATAGGCGT-3′. HxBRUR77f/Env− was generated by digestion of the SalI site (nucleotide 5331) in HxBRUR+/Env− and the religation following blunt ending with the Klenow fragment of DNA polymerase I. These treatments created a frameshift within the Vpr open reading frame at amino acid position 78, as described previously (41). The HxBH10/Env− provirus clone encodes a truncated Vpr (R72f) (40).
Cell lines, antisera, and chemicals.
The Jurkat T-lymphoid cell line used in this study was maintained in RPMI 1640 medium containing 10% fetal calf serum. Human embryonic kidney 293T cells were propagated in Dulbecco’s modified Eagle medium supplemented with 10% fetal calf serum. The HIV-1-positive human serum 162 and the rabbit anti-Vpr serum used in this study were as previously described (24). Chemicals, including propidium iodide (PI), actinomycin D, and Polybrene were purchased from Sigma Chemical, Inc. (Missisauga, Ontario, Canada). The Annexin V-FITC kit (catalog no. 1828681) was purchased from Boehringer Mannheim, Inc. (Laval, Quebec, Canada).
Infection of Jurkat T cells.
Vesicular stomatitis virus envelope G glycoprotein (VSV-G) pseudotyped HIV-1 virus preparations were generated by cotransfection of 293T cells (106) with 12.5 μg of envelope-defective HIV-1 proviral DNA and 25 μg of VSV-G expression plasmid SVCMV-VSV-G (36) by the calcium phosphate coprecipitation method. At 72 h postinfection (p.i.), cell supernatants were collected, preclarified, and ultracentrifuged at 45,000 rpm in a Beckman 60 Ti rotor for 1 h to pellet the pseudotyped virus. Virus was resuspended in RPMI medium and filtered through a 0.45-μm-pore-size filter (Costar, Cambridge, Mass.). Virus stocks were titrated by using the MAGI assay (20). To infect Jurkat cells, 0.5 × 106 cells were incubated with VSV-G pseudotyped virus at different MOIs for 6 h in the presence of Polybrene (10 μg/ml). Infected cells were washed twice and cultured at a density of 0.5 × 106 cells/ml. To monitor viral production, supernatants in different cultures were collected and replaced with fresh medium at each 12-h interval. Virus levels in the supernatants were measured by an HIV reverse transcriptase (RT) assay (40).
Cell cycle analysis and Annexin V-PI double staining.
Cells were harvested at several time points p.i. and tested for DNA content by flow cytometry analysis. Briefly, cells were washed once with phosphate-buffered saline (PBS) and resuspended in 80% ethanol for 30 min on ice. After an additional wash, cells were treated with 180 U of RNase A per ml and subsequently stained with 30 μg of PI per ml in 1 ml of PBS at 37°C for 30 min. The DNA content was then analyzed by FACScan with the Consort 30 software.
The Annexin V-FITC assay was performed as recommended by the manufacturer. Briefly, 0.25 × 106 infected cells were washed once with PBS and then resuspended in Annexin V binding buffer (Annexin V-fluorescein isothiocyanate [FITC] [2.5 μg/ml], 10 mM HEPES-NaOH [pH 7.4], 150 mM NaCl, 5 mM KCl, 1 mM MgCl2, 1.8 mM CaCl2, and PI [1 μg/ml]). After 10 to 15 min of incubation, stained cells were washed twice with binding buffer, resuspended in binding buffer containing 1% paraformaldehyde, and subsequently analyzed by FACScan.
Cell labeling and radioimmunoprecipitation.
At different times p.i., Jurkat cells were metabolically labeled with 100 μCi of [35S]methionine per ml for 2.5 h. After labeling, cells and/or supernatants were lysed in RIPA buffer containing 10 mM Tris-HCl (pH 7.4), 1 mM EDTA, 100 mM NaCl, 1% Triton X-100, 0.1% sodium dodecyl sulfate (SDS), 0.25% sodium deoxycholate, and 0.2% phenylmethylsulfonyl fluoride and immunoprecipitated with the HIV-1-positive human serum 162 as previously described (41). Immunoprecipitates were run on an SDS–12.5% polyacrylamide gel and subsequently analyzed by autoradiography. Densitometric analysis of autoradiograms was performed with a Molecular Dynamics Personal densitometer by using ImageQuant software version 3.22.
RNA extraction and Northern blot hybridization analysis.
Cytosolic RNA was isolated from mock-infected, superinfected Vpr−, or Vpr+-infected Jurkat cells at 6 and 24 h p.i. by a Nonidet P-40 lysis method (28). For virus entry and steady-state mRNA measurements, cells were washed twice with ice-cold PBS and then treated with 0.1% trypsin–0.02% EDTA for 5 min at 37°C. After treatment, cells were washed twice with PBS and then lysed in Nonidet P-40 lysis buffer or they were cultured for an additional 18 h for the assessment of steady-state HIV-1 mRNA levels. RNA from equal numbers of viable cells were fractionated on a 0.8 M formaldehyde agarose gel and then transferred to a Zetaprobe nylon membrane (Bio-Rad, Mississauga, Ontario, Canada). A 285-bp fragment extending from the transcription initiation site (+1) to the major splice donor site was generated by PCR (27) and used to prepare a 32P-labeled HIV-1 probe by the random primer method. A 400-bp probe to GAPDH (glyceraldehyde-3-phosphate dehydrogenase) mRNA was similarly prepared to correct for differences in RNA loading. Hybridization and prehybridization were performed in Church’s buffer (0.5 M Na2HPO4 [pH 7.4], 7% SDS, 1 mM EDTA, 1% bovine serum albumin). Quantitation of the autoradiographic signals was performed with a PhosphorImager (Molecular Dynamics, Sunnyvale, Calif.), using the ImageQuant software.
RESULTS
Expression of Vpr increases cell killing during a single cycle of infection of dividing Jurkat T cells.
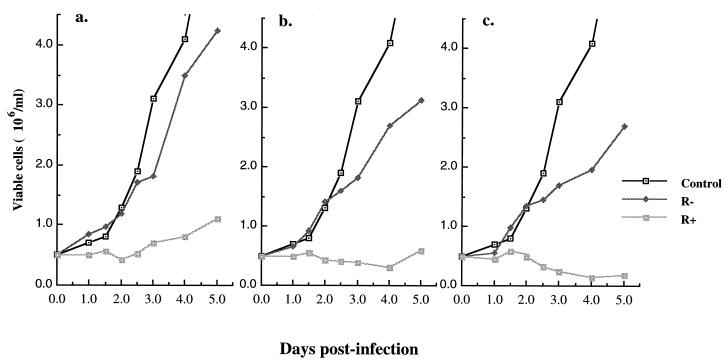
To evaluate the effect of Vpr on cell viability during HIV-1 infection of dividing T cells, we have used a previously described single-cycle HIV-1 superinfection system (4). The VSV-G pseudotyped HIV-1 viral particles were produced by cotransfection of envelope-defective HIV-1 provirus, either HxBRUR+/Env− or HxBRUR−/Env−, with a VSV-G expressor (SVCMV-VSV-G) in 293T cells. Since HIV-1 envelope was not encoded by the HIV-1 proviral constructs, viral spread and envelope-mediated syncytium formation were prevented, leading to a single viral replication cycle. Infection with VSV-G-pseudotyped HIV-1 viruses at high MOI allows for a simultaneous infection of almost all cells in the population (4). To test the effect of Vpr expression on the viability of Jurkat T cells, 0.5 × 106 Jurkat T cells were infected with Vpr+ or Vpr− pseudotyped HIV viruses at MOIs of 5, 10, and 20. Infection of Jurkat T cells by VSV-G pseudotyped HIV-1 viruses was monitored by using specific Gag p24 immunofluorescence assay. At 48 h p.i., the majority of cells (from 80 to more than 95%, depending on the MOI) were positive for p24 (data not shown). At different time intervals after infection, the number of viable cells were counted by trypan blue exclusion assay. As shown in Fig. 1, the number of mock- and Vpr−-infected cells increased throughout the time course of the experiment, although at different rates. In contrast, a severe reduction in the growth rate of cells infected with Vpr+ viruses was observed, a finding that is most likely due to the cytostatic properties of Vpr (Fig. 1). Strikingly, with infection at an MOI of 20, the presence of Vpr resulted not only in cell growth arrest but also in a reduction of the life span of infected cells. Cell depletion was clearly observed at 2.5 days p.i., and at 4 days p.i. only 20% of viable cells remained in the infected cultures (Fig. 1c). The half-life of the infected cells in these cultures was approximately 3 days. Interestingly, the extent of Vpr-mediated cell killing was directly dependent on the infection efficiency, i.e., the number of infected cells expressing Vpr, since an increase in the number of viable cells was observed at day 3 or 5 at MOIs of 5 and 10 (Fig. 1a and b). Overall, these results indicate that the expression of Vpr during HIV-1 infection increases cell killing of infected cells.
FIG. 1.
Vpr increases cell killing during single-cycle infection of dividing Jurkat T cells. Jurkat T cells were mock infected or infected with VSV-G pseudotyped Vpr+ and Vpr− HIV-1 viruses at MOIs of 5, 10, and 20 (respectively presented in panels a, b, and c). At different time intervals after infection, the number of viable cells was counted by trypan blue exclusion assay. The values represented means from duplicate samples. Similar data were obtained in two independent experiments.
Investigation of the mechanism(s) involved in the killing of infected T cells mediated by Vpr.
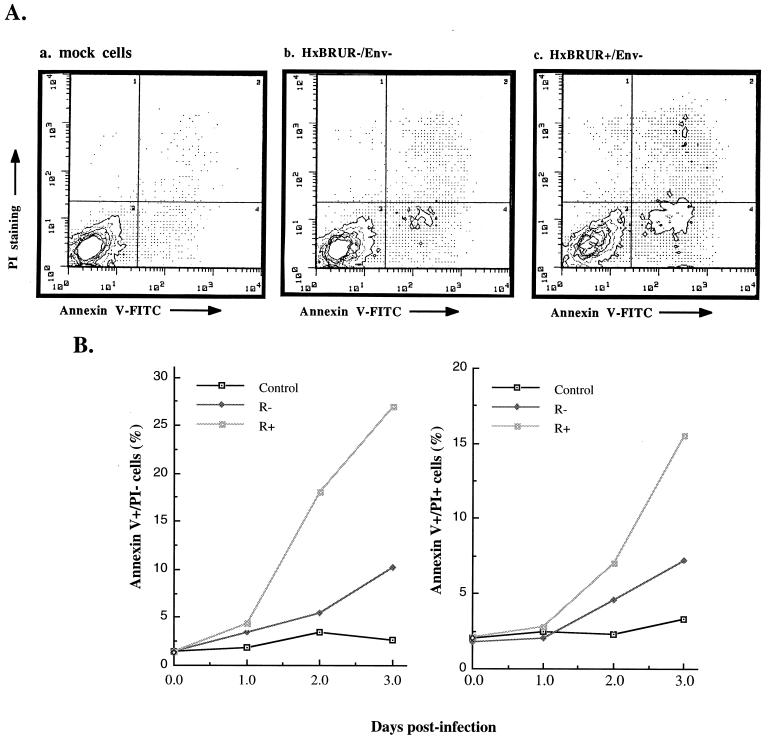
To investigate the mechanism(s) underlying the Vpr-mediated cell killing, we evaluated the level of apoptotic cells and necrotic cells after infection of Jurkat T cells with VSV-G pseudotyped Vpr+ and Vpr− HIV viruses. As previously shown, the translocation of the phospholipid phosphatidylserine on the outer layer of the plasma membrane is one of the earliest features of cells undergoing apoptosis and can easily be detected through binding with Annexin V (26). In order to distinguish between apoptotic and necrotic cells, we costained cells with Annexin V-FITC and PI, a vital dye, and measured the cell-associated fluorescence with a fluorescence-activated cell sorter. At different times after infection, the percentage of apoptotic (Annexin-V+ PI−), necrotic (Annexin-V+ PI+), or viable (Annexin-V− PI−) cells within the population was determined (Fig. 2). At 24 h p.i., the percentages of apoptotic and necrotic cells in infected cultures were similar to that of the control culture (Fig. 2B). However, induction of apoptotic cells and necrotic cells by viral infection was clearly detected at 48 h p.i. and increased thereafter in all infected cultures compared to noninfected Jurkat cell cultures (Fig. 2). Strikingly, the presence of Vpr is associated with a dramatic increase in the percentage of apoptotic cells (26 versus 10% in Vpr+ or Vpr− HIV-1-infected cultures) and necrotic cells (16 versus 6% in Vpr+ or Vpr− HIV-1-infected cultures) after 3 days of infection (Fig. 2B). These results indicate that the expression of Vpr induces a concomitant increase in levels of both apoptotic and necrotic cells during VSV-G pseudotyped HIV-1 infection in dividing Jurkat T cells. To provide additional evidence of Vpr-mediated apoptosis, we attempted to detect the presence of DNA fragmentation in Jurkat T cells infected with pseudotyped HIV-1 viruses. Our results revealed that a DNA fragmentation pattern showing a characteristic nucleosomal-length ladder was detected in Jurkat T cells infected with Vpr+ pseudotyped virus at 3 days p.i. but was not observed in cells infected with Vpr− viruses or in mock-infected cells (data not shown). These observations are consistent with the recent findings from Stewart et al. (33) demonstrating that expression of Vpr, either alone or in the context of a viral infection, induces apoptosis.
FIG. 2.
Vpr induces apoptosis during single-cycle infection of dividing Jurkat T cells. (A) Jurkat T cells were infected with VSV-G pseudotyped Vpr+ or Vpr− HIV-1 viruses. At 48 h p.i., the infected or mock-infected cells were costained with Annexin V and PI and analyzed by flow cytometry. The axes represent the cell-associated fluorescence intensity of Annexin V (x axis) and PI (y axis). (B) The percentage of Annexin-V+ PI+ or Annexin-V+ PI− cells within the different infected cell populations were evaluated by Annexin V-PI costaining and flow cytometry analysis at 1, 2, and 3 days p.i.
Vpr-mediated cell killing correlates with Vpr’s ability to induce G2 arrest and requires the C-terminal domain of the protein.
To establish the relationship between Vpr-mediated G2-arrest and Vpr-induced cell killing activities, we evaluated the ability of six VSV-G pseudotyped HIV-1 viruses expressing different Vpr alleles to mediate these cellular effects. The mutations were introduced in the previously identified leucine-isoleucine-rich region (LR domain) and in the arginine-rich C-terminal domain of Vpr. Substitution mutations were designed to affect highly conserved amino acids in Vpr. In the LR domain, Val-57 was substituted for Leu (RV57L) and Ile-63 was substituted for Phe (RI63F) or Lys (RI63K) as described previously (36, 41). In the C-terminal domain, the amino acid Arg-80 was changed to Ala (RR80A), as this mutation was previously shown to abolish both the Vpr-induced cell cycle G2 arrest (9) and apoptosis (33). Also, two previously described C-terminal frameshift mutants, R77f and R72f, which remove the last 19 and 24 amino acids, respectively, were used in this study (41). All of these Vpr mutants were showed to be abundantly expressed in the VSV-G pseudotyped HIV infection system (data not shown).
We tested each of these Vpr mutants for their ability to induce G2 arrest. Jurkat T cells were infected with VSV-G pseudotyped HIV viruses harboring the different Vpr mutants, and cell cycle analysis was performed at 48 and 72 h p.i. Among the three Vpr mutants harboring substitutions in the LR domain, RV57L and RI63F mediated a G2 arrest as efficiently as wild-type Vpr, whereas RI63K showed an impaired ability to induce G2 arrest (Table 1). In contrast, all the C-terminal Vpr mutants tested (R77f, R72f, and RR80A) lost their ability to induce G2 arrest in infected Jurkat T cells (Table 1).
TABLE 1.
Cell cycle G2 arrest ability of wild-type Vpr and mutants in Jurkat T cellsa
| Time p.i. (h) | Cell cycle G2 arrest ability (G2/M:G1 ratio) of:
|
||||||||
|---|---|---|---|---|---|---|---|---|---|
| Mock | R− | R+ | V57L | I63F | I63K | R80A | R77f | R72f | |
| 48 | 0.45 | 0.43 | 2.60 | 2.40 | 2.23 | 0.98 | 0.47 | 0.55 | 0.47 |
| 72 | 0.28 | 0.32 | 1.79 | 1.89 | 1.73 | 1.30 | 0.33 | 0.39 | 0.37 |
Cell-associated DNA content profiles of infected Jurkat T cells were determined by measurement of PI DNA staining as described in Materials and Methods. The ability of each Vpr mutant to induce G2 arrest was determined by calculating the G2/M:G1 ratio, as described previously (9). Mock, mock infected; R− and R+, Vpr− and Vpr+, respectively.
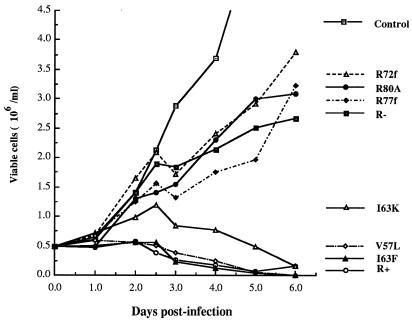
The viability of cells infected with VSV-G pseudotyped HIV viruses harboring wild-type or mutated Vpr was also analyzed. As shown in Fig. 3, RV57L and RI63F induced killing of infected Jurkat T cells as efficiently as did wild-type Vpr, whereas mutant RI63K showed a slightly impaired cytopathic effect. In contrast, viruses harboring the three C-terminal Vpr mutations R77f, R72f and RR80A and the Vpr− virus induced cell killing to a similar extent (Fig. 3). From these results we conclude that the ability of Vpr to induce cell killing in HIV-infected T cells closely correlates with its ability to disrupt cell cycle progression. Moreover, both of these Vpr-associated phenotypes require the C-terminal domain of the protein.
FIG. 3.
Effects of different Vpr mutants on cell killing in Jurkat T cells. Induction of the cytopathic effect associated with different vpr alleles is shown. After infection with the indicated pseudotyped HIV-1 viruses, the number of viable cells in the cultures was determined at the indicated time intervals.
Effect of Vpr on HIV viral production in dividing T cells.
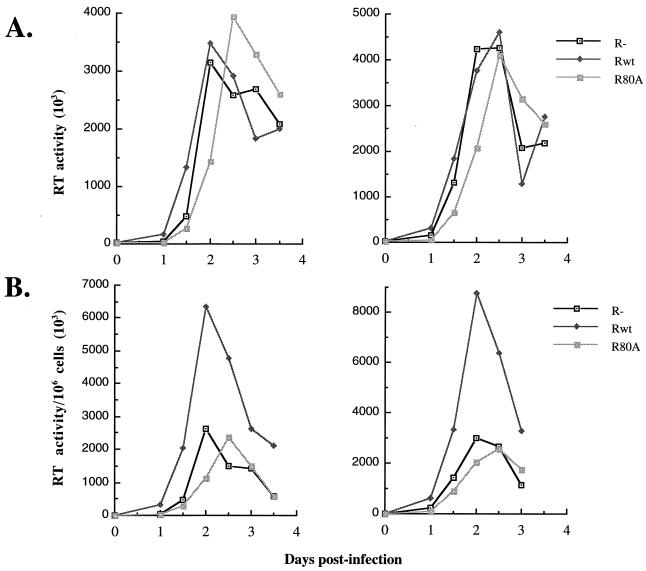
We (this study) and others have shown that Vpr induces G2 arrest, apoptosis, and cell killing in dividing cells (15, 19, 31–33). However, how these Vpr functions affect viral production remains to be evaluated. In the next series of experiments, we investigated the effect of Vpr on viral replication in dividing Jurkat T cells infected with VSV-G pseudotyped Vpr+ or Vpr− HIV-1 viruses at MOIs of 5 or 10. At 12 h intervals p.i., supernatants were collected and viral production was evaluated by measuring virion-associated RT activity. As depicted in Fig. 4A, only minor differences in the level of viral production were observed in the cultures after infection of cells with Vpr+ or Vpr− viruses when the RT activities were expressed per unit volume of supernatant (RT activity/50 μl). However, since our results showed that the expression of Vpr mediated cell killing, the value of RT activity was also expressed per number of viable cells in the cultures (RT activity/50 μl/106 cells). When the RT activities were calculated relative to the number of viable cells, the results revealed that the contribution of each viable cell to viral production increased by approximately threefold in the presence of Vpr (Fig. 4B). Similar differences were observed at each time point in the kinetic studies.
FIG. 4.
Vpr increases HIV viral production in dividing Jurkat T cells. Jurkat T cells were infected with VSV-G pseudotyped Vpr+, Vpr−, or RR80A HIV-1 viruses at an MOI of 5 (left panels) or 10 (right panels). At each 12-h interval, the supernatants were collected and the virion-associated RT activities per volume of supernatants (RT activity/50 μl) (A) or the virion-associated RT activities relative to 106 viable cells (RT activity/50 μl/106 cells) (B) were determined. Values were representative of the data obtained from three independent experiments.
Interestingly, when the same experiments were carried out with the RR80A mutant, which is unable to mediate cell cycle arrest, the RT activities were comparable to those obtained with Vpr− viruses (Fig. 4). These results suggest that the induction of a G2 arrest by Vpr may indeed stimulate viral production.
Vpr stimulates viral protein and RNA expression in dividing T cells.
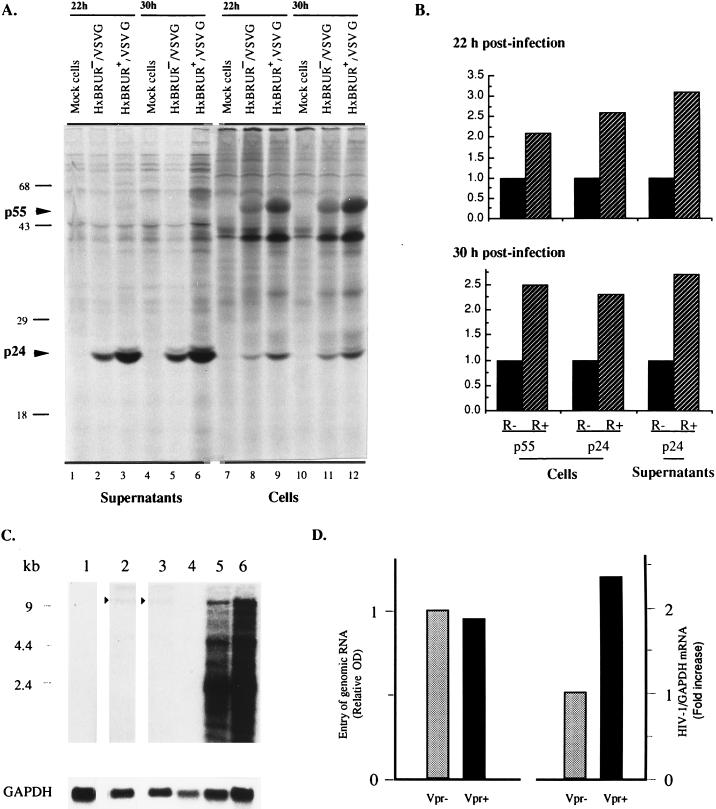
We next analyzed whether the Vpr-mediated increase in viral production was the result of an increase in viral protein synthesis. At 22 and 30 h p.i., equal numbers of viable cells from VSV-G pseudotyped Vpr+ or Vpr− HIV-infected cultures were labeled with [35S]methionine and the HIV viral proteins in cells, and supernatants were immunoprecipitated with an HIV-positive human serum and analyzed on a SDS–12% polyacrylamide gel. The data shown in Fig. 5A clearly show that at these two time points preceding the detection of the apparent cytopathic effects, the presence of Vpr results in an increase of viral protein synthesis. Quantitative analysis of immunoprecipitated protein by densitometry reveals that a two- to threefold increase in the level of viral proteins, including Gag precursor p55 and the cleaved product p24, was observed in both cells and supernatants from cultures infected with Vpr+ pseudotyped viruses, compared to cultures infected with Vpr− pseudotyped viruses (Fig. 5B). These results strongly suggest that Vpr increases viral production as a consequence of an increased level of viral protein synthesis in infected T cells.
FIG. 5.
Vpr increases HIV viral gene expression in dividing Jurkat T cells. (A) Expression of viral protein in Jurkat T cells infected with VSV-G pseudotyped Vpr+ and Vpr− HIV-1 viruses at MOIs of 10. At 22 and 30 h p.i., equivalent numbers of infected trypan blue-negative cells from different cultures were metabolically labeled with [35S]methionine. The viral proteins in both cell lysates and lysed supernatants were immunoprecipitated with an HIV-positive human serum and analyzed by SDS-polyacrylamide gel electrophoresis and autoradiography. (B) Densitometric analysis of the autoradiograms presented in panel A. The densitometric values of Gag p55 and p24 proteins from Vpr− HIV-1-infected cells were arbitrarily set to 1. The densitometric values were highly reproducible. The standard deviation was less than 5%. (C) Expression of HIV-1 RNA in dividing Jurkat T cells. Jurkat cells were mock infected (lanes 1 and 4) or infected with Vpr− (lanes 2 and 5) or Vpr+ (lanes 3 and 6) VSV-G pseudotyped HIV-1 particles at an MOI of 10 for 6 h. Cytoplasmic RNA was isolated from cells (lanes 1 to 3), or cells were cultured for an additional 18 h for steady-state mRNA measurements (lanes 4 to 6). RNA was fractionated, transferred to a nylon membrane, and probed sequentially with a 32P-labeled 285-bp probe for the HIV-1 5′ leader sequence and a 400-bp probe for GAPDH. The signals for internalized genomic RNA following the adsorption period are indicated by the arrowheads. (D) Results of densitometric scanning of internalized genomic RNA signals of panel C, lanes 2 and 3, and of the total of the spliced and unspliced RNA signals in lanes 5 and 6 of panel C. All signals were related to GAPDH. Relative optical density and fold increase in HIV-1/GAPDH mRNA were both related to the Vpr− lane (set to 1). The densitometric values were highly reproducible. The standard deviation was less than 5%.
To further understand the mechanism(s) involved in Vpr-mediated stimulation of viral production, we then analyzed the level of viral RNA expression. For that purpose, we first determined the levels of entry of viral genomic RNA during the adsorption period. After an adsorption period of 6 h with VSV-G pseudotyped Vpr+ and Vpr− HIV-1 viruses, cells were extensively washed and treated with trypsin and EDTA in order to remove bound, noninternalized virus particles, and then cytosolic cellular RNA was extracted. For steady-state mRNA analyses, cells were cultured for an additional 18 h. As shown in Fig. 5C and D, the entry of viral genomic RNA in cells infected with Vpr+ and Vpr− viruses was comparable, as determined by Northern blot analysis as described in Materials and Methods (compare lanes 2 to 3). In addition, cytosolic RNA was extracted from equal numbers of viable cells at 24 h p.i., and steady-state HIV-1-specific RNA levels in infected cells were analyzed by Northern blot analysis. Densitometric analyses from three independent experiments demonstrate that the presence of Vpr results in an average of 2.4-fold (range, 2.1- to 2.7-fold) increase in the number of full-length and spliced viral RNA species in HIV-1-infected cells (Fig. 5C, compare lanes 5 to 6; Fig. 5D, right graph). These results strongly suggest that Vpr increases viral production by increasing the level of viral RNA in infected dividing T cells.
DISCUSSION
The functional role of Vpr during HIV-1 infection of dividing cells is not fully elucidated. Many studies have documented Vpr-mediated G2 arrest in different types of dividing cells, including dividing T and HeLa cells (9, 15, 31, 32), and equally numerous studies have indicated that viral replication is generally not affected by Vpr in T cells (8, 14, 32). However, experimental approaches used in several studies have not been able to clearly distinguish between both the positive and what appear to be the deleterious effects of Vpr on viral production in dividing cells.
In this study, we have selected a single-cycle superinfection system using VSV-G pseudotyped HIV-1 viruses to achieve simultaneous infection of nearly 100% of cells in culture. This has allowed us to monitor various cellular parameters occurring during infection of dividing Jurkat T cells in the presence of Vpr but in the absence of the HIV envelope gene products which are major determinants of cytopathicity. We determined the time course of Vpr-mediated G2/M arrest, apoptosis, and cell killing during HIV-1 infection of dividing T cells. The sequence of these three cellular responses likely reflects their dependence on a common, as-yet-unknown Vpr signaling pathway. Indeed, the first manifestation of a G2 arrest in cells was observed at an early time p.i. (16 h p.i.) (data not shown), whereas cell killing and apoptosis were detected only at 48 h p.i. Whether another mechanism(s), in addition to apoptosis, is involved in the Vpr-mediated cytopathic effect observed during HIV replication in dividing T cells is still unknown. Indeed, the expression of Vpr induces an increase of necrotic (Annexin-V+ PI+) cells in the infected cultures. However, this accumulation of necrotic cells could represent a late stage of apoptosis as observed in other systems (10).
The functional relationship that exists between these cellular responses is also pointed out by the loss of the three biological activities upon mutations of the C-terminal portion of Vpr. All of the Vpr mutants tested in our study (RR80A, R77f, and R72f) which are unable to induce a G2 arrest also lost their ability to induce cell killing. Recently, the RR80A Vpr mutant was also found to be defective in G2 arrest and apoptosis-promoting activities (33). These studies indicate that the C-terminal domain of Vpr constitutes an important functional determinant for these Vpr phenotypes.
As observed in our experiments, induction of apoptosis was also detected in the absence of Vpr, suggesting that another viral gene product(s) is involved in this phenomenon. In particular, gp120, Tat, and recently Nef were reported to induce apoptosis and cell toxicity in a variety of cell systems (16, 21, 39). However, gp120 and Nef are not encoded in our provirus DNA, suggesting that Tat or another uncharacterized viral protein(s) could be involved. In fact, a proper combination of factors could likely affect the final cellular response to Vpr. Interestingly, a recent study has demonstrated that Vpr is able to induce apoptosis in PBMCs but could otherwise protect the cells from T-cell-receptor-triggered apoptosis (2).
The single-cycle superinfection VSV-G pseudotype HIV-1 system was also used to monitor, in parallel, parameters of viral replication in dividing Jurkat T cells, including viral gene expression and viral production. In these evaluations, we have taken into account the effect of Vpr on cell viability. Our results show that there is a quantitative correlation between the Vpr-induced levels of HIV-1 mRNA, protein synthesis, and viral production after infection.
It is well documented that the presence of Vpr during HIV infection of nondividing cells results in increased viral production and this is likely to be achieved through two steps in the viral cycle, namely, at the level of nuclear transport of the viral DNA and secondly after de novo Vpr synthesis (8). Vpr was shown to increase expression of a reporter gene under the control of HIV LTR in dividing cells (7, 37). Thus, Vpr expressed in the context of a viral infection could possibly act in a similar fashion. Interestingly, the Vpr-mediated enhancement of viral expression in our studies correlates with previous observations in a transient Vpr expression system (7). Thus, we favor the scenario in which Vpr participates to increase viral replication both in dividing and nondividing cells by increasing the level of viral RNA. Even though the exact mechanism by which Vpr increases viral expression is still unclear, it is possible that this effect could be due to an increase of viral transcription. Indeed, Vpr was shown to directly bind to the general transcription factors TF-IIB and Sp1 in in vitro assays (1, 37).
Interestingly, the Vpr-mediated increase in viral expression appears to be related to Vpr’s ability to induce G2 arrest. Using pseudotyped virus containing the RR80A mutant, we observed a reduction in the ability of the protein to induce the G2 arrest, which correlates with a concomitant reduction in the capacity to stimulate viral production. In this respect, a previous study has demonstrated that treatment of cells with 2-aminopurine, a product which induces a cell cycle arrest in the G2 phase, also results in an increase in transcription from different promoters, including the HIV LTR promoter (25). The observed fold increase of expression from HIV-1 promoter following the treatment with 2-aminopurine was comparable to that found in our study. This suggests that the G2 phase could provide a transcriptional environment which is favorable for HIV LTR expression. Indeed, Vpr could both create a favorable transcriptional environment and directly participate in the assembly of the transcriptional machinery on the HIV promoter, leading subsequently to enhanced HIV-1 replication in dividing cells.
The in vitro data presented here suggests that Vpr might act similarly in vivo at the level of gene expression to increase viral replication in dividing T cells. In addition, our data suggest that Vpr could also affect the viability of HIV-infected CD4+ T cells in vivo. The question arises as to whether the ability of Vpr to induce apoptosis and cell killing provides an advantageous environment for viral production in vivo. Several studies have demonstrated the occurrence of single cell killing and apoptosis in HIV-infected T cells and particularly in infected CD4+ T memory cells (5, 12). In infected individuals, the specific killing of immune cells, including memory T cells, could contribute to limit the HIV-directed immune response, providing an advantage for viral replication. In addition, apoptosis of HIV-1-infected cells could also theoretically increase the transmission of viral infection to macrophages through apoptotic body phagocytosis (23). Moreover, in addition to its effect on viral replication, Vpr may also play a role in AIDS progression by contributing to HIV-mediated depletion of CD4+ T cells.
We note that after the original submission of this report, Goh et al. also established a relationship between the abilities of Vpr to arrest cells in G2 and to increase viral gene expression (13).
ACKNOWLEDGMENTS
We thank Checroune Florent and Serge Senechal for technical support and Luchino Cohen for helpful discussions.
A.J.M. and J.F. are recipients of an AIDS postdoctoral fellowship and a studentship, respectively, from the National Health Research and Development Program (NHRDP) of Canada. E.A.C. is a recipient of a National Health Scientist award from NHRDP. This work was supported by grants from MRC and FCAR to E.A.C.
REFERENCES
- 1.Agostini I, Navarro J M, Rey F, Bouhamdan M, Spire B, Vigne R, Sire J. The human immunodeficiency virus type 1 Vpr transactivator: cooperation with promoter-bound activator domains and binding to TFIIB. J Mol Biol. 1996;261:599–606. doi: 10.1006/jmbi.1996.0485. [DOI] [PubMed] [Google Scholar]
- 2.Ayyavoo V, Mahboubi A, Mahalingam S, Ramalingam R, Kudchodkar S, Williams W V, Green D R, Weiner D B. HIV-1 Vpr suppresses immune activation and apoptosis through regulation of nuclear factor κB. Nat Med. 1997;3:1117–1123. doi: 10.1038/nm1097-1117. [DOI] [PubMed] [Google Scholar]
- 3.Balliet J W, Kolson D L, Eiger G, Kim F M, McGann K A, Srinivasan A, Collman R. Distinct effects in primary macrophages and lymphocytes of the human immunodeficiency virus type 1 accessory genes vpr, vpu, and nef: mutational analysis of a primary HIV-1 isolate. Virology. 1994;200:623–631. doi: 10.1006/viro.1994.1225. [DOI] [PubMed] [Google Scholar]
- 4.Bartz S R, Rogel M E, Emerman M. Human immunodeficiency virus type 1 cell cycle control: Vpr is cytostatic and mediates G2 accumulation by a mechanism which differs from DNA damage checkpoint control. J Virol. 1996;70:2324–2331. doi: 10.1128/jvi.70.4.2324-2331.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chun T-W, Chadwick K, Margolick J, Siliciano R F. Differential susceptibility of naive and memory CD4+ T cells to the cytopathic effects of infection with human immunodeficiency virus type 1 strain LAI. J Virol. 1997;71:4436–4444. doi: 10.1128/jvi.71.6.4436-4444.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cohen E A, Dehni G, Sodroski J G, Haseltine W A. Human immunodeficiency virus vpr product is a virion-associated regulatory protein. J Virol. 1990;64:3097–3099. doi: 10.1128/jvi.64.6.3097-3099.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cohen E A, Terwilliger E F, Jalinoos Y, Proulx J, Sodroski J G, Haseltine W A. Identification of HIV-1 Vpr product and function. J Acquired Immune Defic Syndr. 1990;1:11–18. [PubMed] [Google Scholar]
- 8.Connor R I, Chen B K, Choe S, Landau N R. Vpr is required for efficient replication of human immunodeficiency virus type-1 in mononuclear phagocytes. Virology. 1995;206:935–944. doi: 10.1006/viro.1995.1016. [DOI] [PubMed] [Google Scholar]
- 9.Di Marzio P, Choe S, Ebright M, Knoblauch R, Landau N R. Mutational analysis of cell cycle arrest, nuclear localization and virion packaging of human immunodeficiency virus type 1 Vpr. J Virol. 1995;69:7909–7916. doi: 10.1128/jvi.69.12.7909-7916.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Echaniz P, de Juan M D, Cuadrado E. DNA staining changes associated with apoptosis and necrosis in blood lymphocytes of individuals with HIV infection. Cytometry. 1995;19:164–170. doi: 10.1002/cyto.990190211. [DOI] [PubMed] [Google Scholar]
- 11.Emerman M. HIV-1, Vpr and the cell cycle. Curr Biol. 1996;6:1096–1103. doi: 10.1016/s0960-9822(02)00676-0. . (Review.) [DOI] [PubMed] [Google Scholar]
- 12.Glynn J M, McElligott D L, Mosier D E. Apoptosis induced by HIV infection in H9 T cells is blocked by ICE-family protease inhibition but not by a Fas (CD95) antagonist. J Immunol. 1996;157:2754–2758. [PubMed] [Google Scholar]
- 13.Goh W C, Rogel M E, Kinsey C M, Michael S F, Fultz P N, Nowak M A, Hahn B H, Emerman M. HIV-1 Vpr increases viral expression by manipulation of the cell cycle: a mechanism for selection of Vpr in vivo. Nat Med. 1998;4:65–71. doi: 10.1038/nm0198-065. [DOI] [PubMed] [Google Scholar]
- 14.Hattori N, Michaels F, Fargnoli K, Marcon L, Gallo R C, Franchini G. The human immunodeficiency virus type 2 vpr gene is essential for productive infection of human macrophages. Proc Natl Acad Sci USA. 1990;87:8080–8084. doi: 10.1073/pnas.87.20.8080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.He J, Choe S, Walker R, Di Marzio P, Morgan D O, Landau N R. Human immunodeficiency virus type 1 viral protein R (Vpr) arrests cells in the G2 phase of the cell cycle by inhibiting p34cdc2 activity. J Virol. 1995;69:6705–6711. doi: 10.1128/jvi.69.11.6705-6711.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.He J-L, deCastro C M, Vandenbark G R, Busciglio J, Gabuzda D. Astrocyte apoptosis induced by HIV-1 transactivation of the c-kit protooncogene. Proc Natl Acad Sci USA. 1997;94:3954–3959. doi: 10.1073/pnas.94.8.3954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Heinzinger N K, Bukrinsky M I, Haggerty S A, Ragland A M, Kewalramani V, Lee M-A, Gendelman H E, Ratner L, Stevenson M, Emerman M. The Vpr protein of human immunodeficiency virus type 1 influences nuclear localization of viral nucleic acids in nondividing host cells. Proc Natl Acad Sci USA. 1994;91:7311–7315. doi: 10.1073/pnas.91.15.7311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ho D D, Neuman A U, Perelson A S, Chen W, Leonard J M, Markowitz M. Rapid turnover of plasma virions and CD4 lymphocytes in HIV-1 infection. Science. 1995;373:123–126. doi: 10.1038/373123a0. [DOI] [PubMed] [Google Scholar]
- 19.Jowett J B, Planelles V, Poon B, Shah N P, Chen M L, Chen I S. The human immunodeficiency virus type 1 vpr gene arrests infected T cells in the G2 + M phase of the cell cycle. J Virol. 1995;69:6304–6313. doi: 10.1128/jvi.69.10.6304-6313.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kimpton J, Emerman M. Detection of replication-competent and pseudotyped human immunodeficiency virus with a sensitive cell line on the basis of activation of an integrated β-galactosidase gene. J Virol. 1992;66:2232–2239. doi: 10.1128/jvi.66.4.2232-2239.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kolesnitchenko V, Wahl L M, Tian H, Sunila I, Tani Y, Hartmann D-P, Cossman J, Raffeld M, Orenstein J, Samelson L E, Cohen D I. Human immunodeficiency virus 1 envelope-initiated G2-phase programmed cell death. Proc Natl Acad Sci USA. 1995;92:11889–11893. doi: 10.1073/pnas.92.25.11889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kondo E, Mammano F, Cohen E A, Göttlinger H G. The p6gag domain of human immunodeficiency type 1 is sufficient for the incorporation of Vpr into heterologous viral particles. J Virol. 1995;69:2759–2764. doi: 10.1128/jvi.69.5.2759-2764.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kornbluth R S. Significance of T cell apoptosis for macrophages in HIV infection. J Leukocyte Biol. 1994;56:247–256. doi: 10.1002/jlb.56.3.247. [DOI] [PubMed] [Google Scholar]
- 24.Lavallée C, Yao X-J, Ladha A, Göttlinger H, Haseltine W A, Cohen E A. Requirement of the Pr55gag precursor for incorporation of the Vpr product into human immunodeficiency type 1 viral particles. J Virol. 1994;68:1926–1934. doi: 10.1128/jvi.68.3.1926-1934.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Maio J J, Graham G J, Brown F L. Induction of G2 arrest and gene expression by 2-aminopurine in human U937 promonocyte-macrophage cells. Exp Cell Res. 1995;219:442–448. doi: 10.1006/excr.1995.1250. [DOI] [PubMed] [Google Scholar]
- 26.Martin J S, Reutelingsperger C P M, McGahon A J, Rader J A, van Schie R C A A, LaFace D M, Green D R. Early redistribution of plasma membrane phosphatidylserine is a general feature of apoptosis regardless of the initiating stimulus: inhibition by overexpression of BcL-2 and Ab1. J Exp Med. 1995;182:1545–1556. doi: 10.1084/jem.182.5.1545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Miele G, Mouland A J, Harrison G P, Cohen E A, Lever A M L. The human immunodeficiency virus type 1 5′ packaging signal structure affects translation but does not function as an internal ribosome entry site. J Virol. 1996;70:944–951. doi: 10.1128/jvi.70.2.944-951.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mouland A J, Hendy G N. Regulation of synthesis and secretion of chromogranin A by calcium and 1,25-dihydroxycholecalciferol in cultured bovine parathyroid cells. Endocrinology. 1991;128:441–449. doi: 10.1210/endo-128-1-441. [DOI] [PubMed] [Google Scholar]
- 29.Ogawa K, Shibata R, Kiyomasu T, Higuchi I, Kishida Y, Ishimoto A, Adachi A. Mutational analysis of the human immunodeficiency virus vpr open reading frame. J Virol. 1989;63:4110–4114. doi: 10.1128/jvi.63.9.4110-4114.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Paxton W, Connor R I, Landau N R. Incorporation of Vpr into human immunodeficiency virus type 1 virions: requirement for the p6 region of Gag and mutational analysis. J Virol. 1993;67:7229–7237. doi: 10.1128/jvi.67.12.7229-7237.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Re F, Braaten D, Franke E K, Luban J. Human immunodeficiency virus type 1 Vpr arrests the cell cycle in G2 by inhibiting the activation of p34cdc2-cyclin B. J Virol. 1995;69:6859–6864. doi: 10.1128/jvi.69.11.6859-6864.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rogel M E, Wu L I, Emerman M. The human immunodeficiency virus type 1 Vpr gene prevents cell proliferation during chronic infection. J Virol. 1995;69:882–888. doi: 10.1128/jvi.69.2.882-888.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Stewart S A, Poon B, Jowett J B M, Chen I S. Human immunodeficiency virus type 1 Vpr induces apoptosis following cell cycle arrest. J Virol. 1997;71:5579–5592. doi: 10.1128/jvi.71.7.5579-5592.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Stivahtis G L, Soares M A, Vodicka M A, Hahn B H, Emerman M. Conservation and host specificity of Vpr-mediated cell cycle arrest suggest a fundamental role in primate lentivirus evolution and biology. J Virol. 1997;71:4331–4338. doi: 10.1128/jvi.71.6.4331-4338.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Subbramanian R A, Cohen E A. Molecular biology of the human immunodeficiency virus accessory proteins. J Virol. 1994;68:6831–6835. doi: 10.1128/jvi.68.11.6831-6835.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Subbramanian, R. A., X.-J. Yao, H. Dilhuydy, N. Rougeau, D. Bergeron, Y. Robitaille, and E. A. Cohen. Human immunodeficiency virus type 1 Vpr localization: nuclear transport of a viral protein modulated by a putative amphipathic helical structure and its relevance to biological activity. J. Mol. Biol., in press. [DOI] [PubMed]
- 37.Wang L, Mukherjee S, Jia F, Narayan O, Zhao L J. Interaction of virion protein Vpr of human immunodeficiency virus type 1 with cellular transcription factor Sp1 and trans-activation of viral long terminal repeat. J Biol Chem. 1995;270:25564–25569. doi: 10.1074/jbc.270.43.25564. [DOI] [PubMed] [Google Scholar]
- 38.Wei X, Ghosh S K, Taylor M E, Johnson V A, Emini E A, Deutsch P, Lifson J D, Bohoeffer S, Norwak M A, Hahn B H, Saag M S, Shaw G M. Viral dynamics in human immunodeficiency virus type 1 infection. Nature. 1995;373:117–122. doi: 10.1038/373117a0. [DOI] [PubMed] [Google Scholar]
- 39.Westendorp M O, Shatrov V A, Schulze-Osthoff K, Frank R, Kraft M, Los M, Krammer P H, Dröge W, Lehmann V. HIV-1 Tat potentiates TNF-induced NF-κB activation and cytotoxicity by altering the cellular redox state. EMBO J. 1995;14:546–554. doi: 10.1002/j.1460-2075.1995.tb07030.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yao X-J, Göttlinger H, Haseltine W A, Cohen E A. Envelope glycoprotein and CD4 independence of Vpu-facilitated human immunodeficiency virus type 1 capsid export. J Virol. 1992;66:5119–5126. doi: 10.1128/jvi.66.8.5119-5126.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yao X-J, Subbramanian R A, Rougeau N, Boisvert F, Bergeron D, Cohen E A. Mutagenic analysis of human immunodeficiency virus type 1 Vpr: role of a predicted N-terminal alpha-helical structure in Vpr nuclear localization and virion incorporation. J Virol. 1995;69:7032–7044. doi: 10.1128/jvi.69.11.7032-7044.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]