Abstract
OBJECTIVE
While diabetic ketoacidosis (DKA) is common in youth at the onset of the diabetes, the excess costs associated with DKA are unknown. We aimed to quantify the health care services use and medical care costs related to the presence of DKA at diagnosis of diabetes.
RESEARCH DESIGN AND METHODS
We analyzed data from the U.S. MarketScan claims database for 4,988 enrollees aged 3–19 years insured in private fee-for-service plans and newly diagnosed with diabetes during 2010–2016. Youth with and without DKA at diabetes diagnosis were compared for mean health care service use (outpatient, office, emergency room, and inpatient visits) and medical costs (outpatient, inpatient, prescription drugs, and total) for 60 days prior to and 60 days after diabetes diagnosis. A two-part model using generalized linear regression and logistic regression was used to estimate medical costs, controlling for age, sex, rurality, health plan, year, presence of hypoglycemia, and chronic pulmonary condition. All costs were adjusted to 2016 dollars.
RESULTS
At diabetes diagnosis, 42% of youth had DKA. In the 60 days prior to diabetes diagnosis, youth with DKA at diagnosis had less health services usage (e.g., number of outpatient visits: −1.17; P < 0.001) and lower total medical costs (−$635; P < 0.001) compared with youth without DKA at diagnosis. In the 60 days after diagnosis, youth with DKA had significantly greater health care services use and health care costs ($6,522) compared with those without DKA.
CONCLUSIONS
Among youth with newly diagnosed diabetes, DKA at diagnosis is associated with significantly higher use of health care services and medical costs.
Diabetic ketoacidosis (DKA) is an acute and severe complication of diabetes. It is defined by high ketone levels in the blood, which are caused by insulin deficiency. Although it is typically thought to occur in individuals with type 1 diabetes, it can occasionally occur in individuals with type 2 diabetes (1,2). DKA is often present at diagnosis of diabetes in youth, especially in children younger than 5 years of age (3). Presentation of DKA at time of diabetes diagnosis is associated with increased risk of morbidity and mortality (4–8). In addition, DKA at time of diabetes diagnosis is associated with poorer long-term glycemic control (9) and increased risk of subsequent episodes of DKA (10). In the U.S., close to one-third of youth with type 1 diabetes present with DKA at time of diagnosis, and this proportion remained stable from 2002 to 2010 (3). The prevalence of DKA at diagnosis for youth with type 2 diabetes has decreased over this time period from 11.7% to 5.7% (3). It is unknown if these trends have continued.
Besides the health consequences, the financial burden of DKA is substantial. In 2007, among U.S. youth with diabetes being treated with insulin, the average excess annual medical expenditure associated with DKA was estimated to be >$9,000 per year (11). Ideally, hyperglycemia should be identified before metabolic deterioration leading to DKA. Thus, understanding the cost associated with DKA presenting at the time of diabetes diagnosis would provide important information on the financial burden that could potentially be avoided. Therefore, we sought to quantify the medical cost of DKA presenting at the time of diabetes diagnosis among children and adolescents in the 2 months prior to and 2 months after initial diabetes diagnosis.
RESEARCH DESIGN AND METHODS
Data Source
We used the 2010–2016 MarketScan Commercial Claims and Encounters (CCE) Database (Truven Health MarketScan Database, Ann Arbor, MI). This database is comprised of paid medical claims for individuals with private health insurance and their dependents, primarily through employer-sponsored health plans from ~350 payers (12). The Commercial Claims and Encounters Database includes patient-level information on inpatient, outpatient, and drugs claims. The database has been used in previous studies of health care costs, including studies focusing on both clinical and economic outcomes (11,13–15). Health plans include fee-for-service (FFS) and capitated plans. FFS plans include preferred provider organization (PPO) plans, exclusive provider organization plans, point-of service plans, consumer-directed health plans, and indemnity plans. Fully or partially capitated plans include health management organizations and capitated point of service plans.
Study Cohort
The study cohort included youth aged 3–19 years at their first diagnosis with diabetes during the period from 1 January 2001 to 31 December 2016. Diabetes diagnosis was defined by ICD-9-Clinical Modification (CM) (250.xx) or ICD-10-CM (E10 and E11). Use of ICD-9-CM and ICD-10-CM codes to identify diabetes in the pediatric population has high sensitivity and specificity (~90% or greater) (16,17). Figure 1 provides steps we followed to create this cohort. In order to qualify as having newly diagnosed diabetes, enrollees were required to have at least 2 years of continuous enrollment with no diabetes claims codes or antidiabetic prescription medications (therapeutic class codes of 172, 173, and 174), and continuous enrollment at least 2 months after the diabetes diagnosis.
Figure 1—
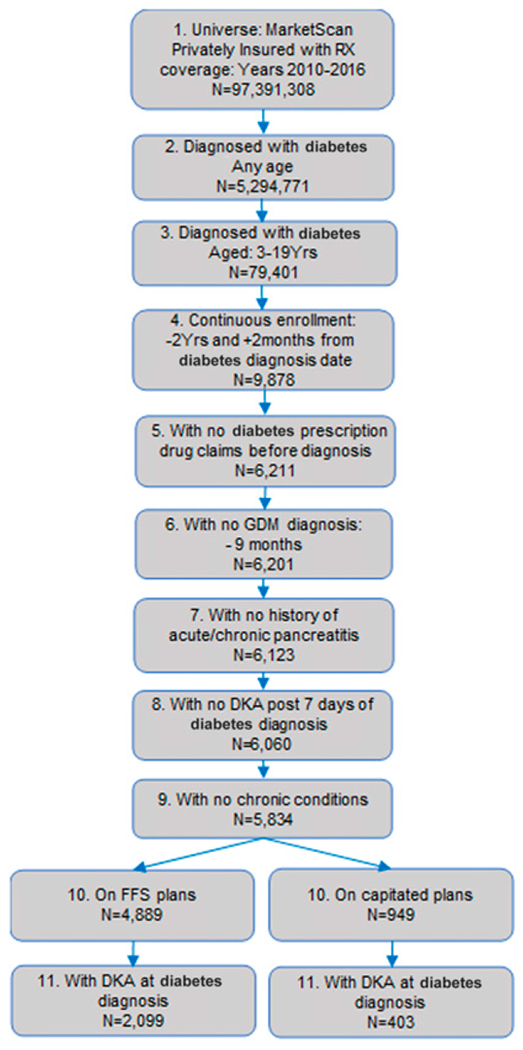
Sample selection for youth <20 years of age with newly diagnosed diabetes in MarketScan, 2010–2016. 1: Number of enrollees in any health plan with length of enrollment >1 day, during 1 January 2010 to 31 December 2016. 2: First diagnosed with diabetes during the period using the algorithm described. 3: Those with age at diagnosis younger than 20 years. 4: Those with continuous enrollment in any health plan 2 years before and 1 year after the diabetes diagnosis. 5: Excluding those who had prescription drug claims for antidiabetes medications before the diagnosis date (Therapeutic class codes 172, 173, and 174). 6: Newly diagnosed with diabetes, excluding those with gestational diabetes mellitus (GDM) before the diagnosis. 7: Excluding those with history of acute and chronic pancreatitis. 8: Excluding those diagnosed with DKA >7 days after diabetes diagnosis. 9: Excluding those with history of chronic conditions, n = 196. Chronic conditions include: congestive heart failure, hemiplegia, HIV/AIDS, any malignancy (including lymphoma and leukemia, except malignant neoplasm of skin), mild liver disease, peripheral vascular disease, renal disease, rheumatic disease, cerebrovascular disease, metastatic solid tumor, and peptic ulcer. 10: Those enrolled in FFS or capitated plans. 11: Those with DKA at diabetes diagnosis (within 7 days). RX, prescription.
We excluded from the sample those with history of acute and chronic pancreatitis, those with gestational diabetes, and those with chronic medical conditions (congestive heart failure [20], hemiplegia [14], HIV/AIDS [2], any malignancy except malignant neoplasm of skin [53], mild liver disease [1], peripheral vascular disease [6], renal disease [33], rheumatic disease [11], cerebrovascular disease [22], metastatic solid tumor [31], peptic ulcer [3], and serious chronic pulmonary disease but not asthma). Individuals with a diagnosis of DKA >7 days after diabetes diagnosis were excluded. We also excluded those enrolled in capitated health plans, because such plans do not include actual payment, making it difficult to account for complete costs during the period. There were 4,889 enrollees on FFS plans available for analysis.
DKA at or within 7 days of diabetes diagnosis was identified by ICD-9-CM (250.1x) or ICD-10-CM (E0810 E0910, E1010, and E1310) claim codes. Hypoglycemia was identified using the ICD-9 (251.0, 775.6, 251.2, and 251.1) and ICD-10 (E09649, E08649, E08641, E160, E13649, E08641, E13649, E11641, E10649, E10641, E161, E162, P703, and P704) codes in the 7 days after diabetes diagnosis.
Estimation of Medical Costs
We gathered the total cost incurred during 60 days before the diabetes diagnosis and during 60 days after the diagnosis (including the day of diagnosis). The 60-day window was selected in order to capture all costs associated with diabetes diagnosis, including DKA occurrence. The total cost comprised the sum of that incurred in outpatient care, inpatient care, and prescription medications during the periods. The medical costs are an annual aggregated paid amount for the services incurred for the patient during the study period. To estimate the excess cost attributed to DKA, we used a generalized linear model for outpatient and prescription drug cost with log link and γ distribution. However, because a large proportion of patients did not have an inpatient admission or inpatient expenditures and expenditures were highly skewed, we used a two-part model to estimate the inpatient expenditures attributable to DKA (18). In the first part of the model, we used logistic regression to estimate the probability of incurring expenditures on inpatient care. In the second part, among those who received inpatient care, we used the generalized linear model with log link and γ distribution to estimate the expenditures attributable to DKA. We combined estimates from these two models to predict the cost for youth with or without DKA present at (or near) the time of diabetes diagnosis. We assessed the excess costs attributed to DKA as the difference between the costs for youth with DKA and youth without DKA. The models controlled for age at diabetes diagnosis, sex, metropolitan statistical area status (yes/no), year of diabetes diagnosis, health plan (PPO vs. non-PPO plan), status of hypoglycemia, and status of chronic pulmonary conditions. All costs were adjusted to 2016 dollars using the medical care consumer price index for all urban consumers (https://www.bls.gov/cpi/tables/home.htm). For 95% CIs around the predicted expenditures, we used nonparametric bootstrapping with 1,000 replications. The reported CIs are bias corrected.
We used Stata version 14.0 (StataCorp, College Station, TX) for all analyses.
RESULTS
Of the 4,889 children and adolescents with newly diagnosed diabetes, 2,099 (42.9%) had DKA near the time of diabetes diagnosis. Characteristics of the study population at diabetes diagnosis are reported in Table 1. Youth with DKA were younger (11.3 vs. 12.6 years; P < 0.001) and less likely to be female (45.2% vs. 49.5%; P < 0.01). There were no significant differences between the groups for rural residency status, PPO health plan, and hypoglycemia at diabetes diagnosis.
Table 1—
Characteristics of study population at diabetes diagnosis (n = 4,889) by DKA status, MarketScan 2010–2016
| Variables | With DKA (n = 2,099) |
Without DKA (n = 2,790) |
Excess |
|---|---|---|---|
| Mean age at diabetes diagnosis, years | 11.3 (11.1, 11.4) | 12.6 (12.4, 12.8) | −1.3*** (−1.6, −1.1) |
| Female, % | 45.20 (43.0, 47.3) | 49.50 (47.6, 51.4) | −4.3** (−7.2, −1.5) |
| Rural residency, % | 14.60 (13.1, 16.1) | 13.30 (12.0, 14.5) | 1.30 (−0.6, 3.3) |
| PPO, % | 69.20 (67.3, 71.2) | 69.60 (68.0, 71.5) | −0.40 (−3.1, 2.1) |
| Hypoglycemia, % | 1.60 (01.0, 2.1) | 2.40 (1.8, 2.93) | −0.8 (−1.6, 0.004) |
| Chronic pulmonary condition, % | 3.1 (2.4, 3.8) | 4.6 (3.8, 5.4) | −1.5** (−2.6, −0.4) |
Data in parentheses are 95% CIs. Hypoglycemia was identified within 7 days of diabetes diagnosis. Chronic pulmonary was identified in the last 60 days of diabetes diagnosis.
P < 0.01;
P < 0.001.
Health services usage differed among youth with and without DKA at time of diabetes diagnosis and in the months prior to and after diagnosis. In the 60 days prior to diagnosis, youth with DKA had less use of health care services (Table 2). This corresponded to lower estimated medical costs in the 60 days prior to diagnosis for youth with DKA at time of diagnosis. Overall, in the 60 days prior to diagnosis for youth with DKA, estimated total medical costs were $625 lower and patient copay was $48 lower than corresponding costs for youth without DKA at time of diagnosis (Table 3).
Table 2—
Mean health service usage and prevalence of chronic pulmonary condition 60 days before and after diabetes diagnosis among those with and without DKA at diagnosis
| 60 days before diabetes diagnosis |
60 days after diabetes diagnosis |
|||||
|---|---|---|---|---|---|---|
| With DKA | Without DKA | Excess | With DKA | Without DKA | Excess | |
| Services use | ||||||
| Number of outpatient visits | 1.15 (1.6, 1.25) | 2.34 (2.18, 2.50) | −1.19*** (−1.39, −0.98) | 4.49 (4.38, 4.61) | 4.42 (4.25, 4.59) | 0.07**** (−0.15, 0.29) |
| Number of office visits | 0.59 (0.55, 0.63) | 0.89 (0.85, 0.94) | −0.31*** (−0.37, −0.24) | 2.34 (2.27, 2.40) | 2.00 (1.94, 2.06) | 0.33*** (0.24, 0.42) |
| Number of ER visits | 0.12 (0.10, 0.13) | 0.16 (0.14, 0.18) | −0.04** (−0.06, −0.01) | 0.26 (0.24, 0.29) | 0.22 (0.20, 0.24) | 0.04** (0.02, 0.07) |
| Number of inpatient admissions | 0.002 (0.000, 0.005) | 0.02 (0.01, 0.03) | −0.02*** (−0.02, −0.01) | 1.01 (1.00, 1.01) | 0.66 (0.64, 0.68) | 0.35*** (0.32, 0.38) |
| Length of stay, days | 0.004 (0.000, 0.008) | 0.10 (0.06, 0.14) | −0.09*** (−0.15, −0.05) | 2.48 (2.41, 2.55) | 1.99 (1.71, 2.66) | 0.49** (0.17, 0.81) |
|
| ||||||
| Medical conditions | ||||||
| Chronic pulmonary, % | 3.1 (2.4, 3.8) | 4.6 (3.8, 5.4) | −1.5** (−2.6, −0.04) | 10.04 (8.5, 11.1) | 9.8 (8.9, 11.2) | −0.2**** (−1.9, 1.5) |
Data in parentheses are 95% CIs. Outpatient visits includes office visits, ER visits, and additional laboratory or clinical outpatient visits. ER, emergency room.
P < 0.01;
P < 0.001;
P > 0.05 (NS).
Table 3—
Estimated mean medical costs (in 2016 dollars) 60 days before and after diabetes diagnosis among those with and without DKA at diagnosis
| 60 days before diabetes diagnosis |
60 days after diabetes diagnosis |
|||||
|---|---|---|---|---|---|---|
| Costs | With DKA | Without DKA | Excess | With DKA | Without DKA | Excess |
| Outpatient# | 567.7 (442.5, 683.8) | 849.8 (741.5, 987.7) | −282.2* (−430.8, −132.7) | 2,692.1 (2,439.2, 2,954.3) | 1,638.6 (1,506.3, 1,785.7) | 1,053.5* (792.5, 1,345.5) |
|
| ||||||
| Inpatient## | 22.0 (2.2, 62.3) | 311.1 (144.2, 616.4) | −289.0* (−616.9, −123.2) | 12,951.1 (12,403.8, 13,462.3) | 8,305.3 (7,256.3, 9,764.1) | 4,645.8* (3,100.3, 5,641.9) |
|
| ||||||
| Prescription drugs# | 71.5 (54.9, 94.9) | 125.2 (104.2, 149.4) | −53.7* (−86.1, −27.4) | 2,353.9 (2,282.8, 2,425.4) | 1,401.4 (1,344.0, 1,464.1) | 952.6* (858.6, 1,050.1) |
|
| ||||||
| Total# | 661.2 (519.2, 806.5) | 1,286.1 (1,043.8, 1,605.2) | −624.9* (−958.5, −362.2) | 17,997.2 (17,363.4, 18,630.4) | 11,345.3 (10,249.6, 12,768.5) | 6,651.9* (5,117.0, 7,687.0) |
|
| ||||||
| Total copay# | 110.8 (97.6, 125.4) | 158.6 (143.2, 176.4) | −47.8* (−69.1, −26.0) | 2,499.9 (2,420.8, 2,576.9) | 1,398.4 (1,330.0, 1,451.2) | 1,101.4* (1,010.6, 1,220.3) |
Data in parentheses are bias-corrected bootstrap 95% CIs with 1,000 replications.
Estimated using a generalized linear model with log link and γ distribution.
Estimated using a two-part model: first part is logistic regression, and second part is a generalized linear model with log link and γ distribution.
P < 0.001.
In the 60 days after diabetes diagnosis, youth with DKA at time of diabetes diagnosis had significantly greater use of health services and costs compared with youth without DKA at time of diagnosis. Youth with DKA at diabetes diagnosis, on average, had 0.34 more office visits, 0.35 more inpatient admissions, and 0.49 more days in the hospital compared with youth without DKA at time of diagnosis (Table 2). This corresponded to higher medical costs for outpatient, inpatient, and prescription drugs. Overall, youth with DKA at time of diabetes diagnosis had $6,652 more in medical costs (P < 0.001) during the 60 days after diagnosis, mainly attributed to excess costs on inpatient care followed by outpatient and prescription drugs. Youth with DKA at diabetes diagnosis also had $1,101 more in copays (P < 0.001) compared with youth who did not have DKA at diagnosis (Table 3).
CONCLUSIONS
While youth with DKA at time of diabetes diagnosis used fewer health services and had lower medical costs prior to diagnosis, they experienced greater use of health services and greater medical costs in the 60 days after diagnosis. The excess costs after diabetes diagnosis in youth with DKA was 10 times greater than their lower costs prior to diabetes diagnosis.
In 2007, medical costs of youth with diabetes with DKA were reported to be more than $2,800 greater for one episode of DKA and almost $6,000 greater for two or more episodes of DKA compared with youth who did not have DKA (11). We report in this study that the excess medical costs within the first 60 days after diagnosis were $6,652 for DKA at diabetes diagnosis compared with youth with no DKA at diabetes diagnosis. Previous studies have found that individuals who have DKA at diabetes diagnosis are more likely to have additional DKA episodes (10,19,20) than those who develop it later. In addition, youth with DKA at time of diagnosis are more likely to have additional DKA episodes in subsequent years, often have greater insulin requirements for the first year after diagnosis (10), and tend to have higher levels of A1C (9,10,21). Thus, one would expect additional excess medical costs for youth who have DKA at diabetes diagnosis past the 60 days after diagnosis that we examined in this study.
In this privately insured population, the incidence of DKA at diabetes diagnosis was 42%, ~13 percentage points higher than that observed in the SEARCH for Diabetes in Youth study (3). This difference may be due to differences in methods of ascertainment, differences in the population studied, or changes over time. The SEARCH study is conducted in five clinical sites in the U.S. using children and adolescents enrolled in a population-based registry of diabetes; enrollees are privately insured, publicly insured, or have no health insurance coverage. However, the incidence of DKA at diabetes diagnosis observed in SEARCH was last reported for 2010. Evidence from Colorado in 2012 suggests that among youths with type 1 DKA at diagnosis, trends differ by insurance status, with an increase among those with private insurance (increase of 2.5% per year) and a decrease among those with public insurance (decrease of 1.3% per year) (22). It is unknown if incidence of DKA at diagnosis has changed in the larger U.S. youth population during the past decade. Worldwide, the reported incidence of DKA at diabetes diagnosis among youth has ranged from 15% to 67% (23–30). The incidence of DKA at diagnosis seems to have either declined (25,29) or remained unchanged (3,27,30).
Our finding that youth with DKA at diabetes diagnosis were less likely to use health care services in the 2 months prior to diagnosis has been observed in other populations. Among youth with type 1 diabetes in Ontario, Canada, those who had DKA at time of diagnosis were less likely to have visited a medical provider in the weeks prior to diabetes diagnosis compared with those without DKA at diabetes diagnosis (23). It is important to note that in this study, youth who were diagnosed with diabetes, regardless of DKA at onset, had more health care encounters prior to diabetes diagnosis than a control group of youth with asthma, another common chronic disease of childhood (23). It is likely that youth seeking care are more likely to be diagnosed with diabetes earlier, possibly resulting in the prevention of DKA. It is also possible that youth who present with DKA at diabetes diagnosis have a more rapid onset of diabetes resulting in DKA at or near the time of diabetes diagnosis.
In recent years, there has been interest in population-based screening for type 1 diabetes in order to identify and control it and to prevent DKA and other complications. Prospective longitudinal studies of children at high risk of developing type 1 diabetes have reported that screening and repeated testing for type 1 diabetes auto-antibodies resulted in reduction in DKA rates at diagnosis (31–33). One study estimated the costs and benefits of screening children at birth for HLA genotype and follow-up of those found to have high-risk HLA with islet cell auto-antibody testing every 6 months until they reach 5 years of age. This study concluded that screening for preventing DKA at the onset of diabetes was not economically advantageous (34). The ongoing Fr1Da Study in Bavaria, Germany, is aiming to screen children 2–5 years of age for type 1 diabetes markers, with one of the goals being to prevent acute complications at diagnosis (35). This study may provide additional insights on the feasibility and cost-effectiveness of screening in the general population.
Given 23,200 annual incident cases of diabetes among youth in the U.S. (28), and assuming 30–40% of these cases present with DKA at diabetes diagnosis, annual excess medical costs could range from $46 million to $63 million. Interventions to reduce DKA at diagnosis may include public health information campaigns to increase public awareness of DKA; however, these campaigns had mixed results in Austria (36), and their potential to reduce DKA in the U.S. is unknown.
There are a number of limitations of this study. First, DKA is based on ICD codes and not confirmed by medical record review or laboratory results that may result in misclassification (37). Next, the study population was limited to youth with private insurance coverage in FFS plans. One study found that youth with DKA at diabetes diagnosis are less likely to have health insurance (3). Therefore, our study population likely has better access to health care compared with youth who are uninsured or covered under Medicaid, although how this may influence cost is unknown. Further, our analysis did not distinguish between type 1 and type 2 diabetes; influence of diabetes type on the use of health care services and associated costs around the time of diagnosis is unknown. We also were unable to explore additional factors that may be associated with health care services, costs, and DKA, such as race/ethnicity, parental education, and household income. Finally, costs were restricted to medical expenditures from administrative claims. We were unable to account for other costs, such as cost of care provided by family members and human capital losses such as loss of income and decreased productivity.
To our knowledge, this is the first study to examine the use of health care services and cost attributable to DKA among youth with newly diagnosed diabetes. The excess use of health care services and associated medical care costs in the 2 months after diabetes diagnosis for those who present with DKA at diabetes diagnosis were substantial. These costs could potentially be avoided if DKA is averted through timely diabetes diagnosis and management.
Acknowledgments
The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
Footnotes
Duality of Interest. No potential conflicts of interest relevant to this article were reported.
Prior Presentation. Preliminary findings of this study were presented at the 78th Scientific Sessions of the American Diabetes Association, Orlando, FL, 22–26 June 2018.
References
- 1.Newton CA, Raskin P. Diabetic ketoacidosis in type 1 and type 2 diabetes mellitus: clinical and biochemical differences. Arch Intern Med 2004;164:1925–1931 [DOI] [PubMed] [Google Scholar]
- 2.Vellanki P, Umpierrez GE. Diabetic ketoacidosis: a common debut of diabetes among African Americans with type 2 diabetes. Endocr Pract 2017;23:971–978 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dabelea D, Rewers A, Stafford JM, et al. ; SEARCH for Diabetes in Youth Study Group. Trends in the prevalence of ketoacidosis at diabetes diagnosis: the SEARCH for diabetes in youth study. Pediatrics 2014;133:e938–e945 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cengiz E, Xing D, Wong JC, et al. ; T1D Exchange Clinic Network. Severe hypoglycemia and diabetic ketoacidosis among youth with type 1 diabetes in the T1D Exchange clinic registry. Pediatr Diabetes 2013;14:447–454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Klingensmith GJ, Tamborlane WV, Wood J, et al. Diabetic ketoacidosis at diabetes onset: still an all too common threat in youth. J Pediatr 2013;162:330–334.e1 [DOI] [PubMed] [Google Scholar]
- 6.Umpierrez G, Korytkowski M. Diabetic emergencies - ketoacidosis, hyperglycaemic hyperosmolar state and hypoglycaemia. Nat Rev Endocrinol 2016;12:222–232 [DOI] [PubMed] [Google Scholar]
- 7.White PC, Dickson BA. Low morbidity and mortality in children with diabetic ketoacidosis treated with isotonic fluids. J Pediatr 2013;163:761–766 [DOI] [PubMed] [Google Scholar]
- 8.Wolfsdorf JI, Glaser N, Agus M, et al. ISPAD Clinical Practice Consensus Guidelines 2018: diabetic ketoacidosis and the hyperglycemic hyperosmolar state. Pediatr Diabetes 2018;19(Suppl. 27):155–177 [DOI] [PubMed] [Google Scholar]
- 9.Duca LM, Wang B, Rewers M, Rewers A. Diabetic ketoacidosis at diagnosis of type 1 diabetes predicts poor long-term glycemic control. Diabetes Care 2017;40:1249–1255 [DOI] [PubMed] [Google Scholar]
- 10.Shalitin S, Fisher S, Yackbovitch-Gavan M, et al. Ketoacidosis at onset of type 1 diabetes is a predictor of long-term glycemic control. Pediatr Diabetes 2018;19:320–328 [DOI] [PubMed] [Google Scholar]
- 11.Shrestha SS, Zhang P, Barker L, Imperatore G. Medical expenditures associated with diabetes acute complications in privately insured U.S. youth. Diabetes Care 2010;33:2617–2622 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.IBM Watson Health. IBM MarketScan Research Databases for Health Services Researchers (white paper) [Internet], 2019. Available from https://www.ibm.com/downloads/cas/6KNYVVQ2. Accessed 25 September 2019
- 13.Liu J, Wang R, Ganz ML, Paprocki Y, Schneider D, Weatherall J. The burden of severe hypoglycemia in type 1 diabetes. Curr Med Res Opin 2018;34:171–177 [DOI] [PubMed] [Google Scholar]
- 14.Liu J, Wang R, Ganz ML, Paprocki Y, Schneider D, Weatherall J. The burden of severe hypoglycemia in type 2 diabetes. Curr Med Res Opin 2018;34:179–186 [DOI] [PubMed] [Google Scholar]
- 15.Rhoads GG, Orsini LS, Crown W, Wang S, Getahun D, Zhang Q. Contribution of hypoglycemia to medical care expenditures and short-term disability in employees with diabetes. J Occup Environ Med 2005;47:447–452 [DOI] [PubMed] [Google Scholar]
- 16.Chi GC, Li X, Tartof SY, Slezak JM, Koebnick C, Lawrence JM. Validity of ICD-10-CM codes for determination of diabetes type for persons with youth-onset type 1 and type 2 diabetes. BMJ Open Diabetes Res Care 2019;7:e000547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lawrence JM, Black MH, Zhang JL, et al. Validation of pediatric diabetes case identification approaches for diagnosed cases by using information in the electronic health records of a large integrated managed health care organization. Am J Epidemiol 2014;179:27–38 [DOI] [PubMed] [Google Scholar]
- 18.Manning WG, Mullahy J. Estimating log models: to transform or not to transform? J Health Econ 2001;20:461–494 [DOI] [PubMed] [Google Scholar]
- 19.Malik FS, Hall M, Mangione-Smith R, et al. Patient characteristics associated with differences in admission frequency for diabetic ketoacidosis in United States children’s hospitals. J Pediatr 2016;171:104–110 [DOI] [PubMed] [Google Scholar]
- 20.Mays JA, Jackson KL, Derby TA, et al. An evaluation of recurrent diabetic ketoacidosis, fragmentation of care, and mortality across Chicago, Illinois. Diabetes Care 2016;39:1671–1676 [DOI] [PubMed] [Google Scholar]
- 21.Fredheim S, Johannesen J, Johansen A, et al. ; Danish Society for Diabetes in Childhood and Adolescence. Diabetic ketoacidosis at the onset of type 1 diabetes is associated with future HbA1c levels. Diabetologia 2013;56:995–1003 [DOI] [PubMed] [Google Scholar]
- 22.Rewers A, Dong F, Slover RH, Klingensmith GJ, Rewers M. Incidence of diabetic ketoacidosis at diagnosis of type 1 diabetes in Colorado youth, 1998-2012. JAMA 2015;313:1570–1572 [DOI] [PubMed] [Google Scholar]
- 23.Bui H, To T, Stein R, Fung K, Daneman D. Is diabetic ketoacidosis at disease onset a result of missed diagnosis? J Pediatr 2010;156:472–477 [DOI] [PubMed] [Google Scholar]
- 24.Choleau C, Maitre J, Filipovic Pierucci A, et al. ; AJD Study Group. Ketoacidosis at diagnosis of type 1 diabetes in French children and adolescents. Diabetes Metab 2014;40:137–142 [DOI] [PubMed] [Google Scholar]
- 25.Hekkala A, Knip M, Veijola R. Ketoacidosis at diagnosis of type 1 diabetes in children in northern Finland: temporal changes over 20 years. Diabetes Care 2007;30:861–866 [DOI] [PubMed] [Google Scholar]
- 26.Komulainen J, Lounamaa R, Knip M, Kaprio EA, Akerblom HK; Childhood Diabetes in Finland Study Group. Ketoacidosis at the diagnosis of type 1 (insulin dependent) diabetes mellitus is related to poor residual beta cell function. Arch Dis Child 1996;75:410–415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Neu A, Hofer SE, Karges B, Oeverink R, Rosenbauer J, Holl RW; DPV Initiative and the German BMBF Competency Network for Diabetes Mellitus. Ketoacidosis at diabetes onset is still frequent in children and adolescents: a multicenter analysis of 14,664 patients from 106 institutions. Diabetes Care 2009;32:1647–1648 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rewers A, Klingensmith G, Davis C, et al. Presence of diabetic ketoacidosis at diagnosis of diabetes mellitus in youth: the Search for Diabetes in Youth Study. Pediatrics 2008;121:e1258–e1266 [DOI] [PubMed] [Google Scholar]
- 29.Samuelsson U, Stenhammar L. Clinical characteristics at onset of type 1 diabetes in children diagnosed between 1977 and 2001 in the south-east region of Sweden. Diabetes Res Clin Pract 2005;68:49–55 [DOI] [PubMed] [Google Scholar]
- 30.Schober E, Rami B, Waldhoer T; Austrian Diabetes Incidence Study Group. Diabetic ketoacidosis at diagnosis in Austrian children in 1989-2008: a population-based analysis. Diabetologia 2010;53:1057–1061 [DOI] [PubMed] [Google Scholar]
- 31.Barker JM, Barriga KJ, Yu L, et al. ; Diabetes Autoimmunity Study in the Young. Prediction of autoantibody positivity and progression to type 1 diabetes: Diabetes Autoimmunity Study in the Young (DAISY). J Clin Endocrinol Metab 2004;89:3896–3902 [DOI] [PubMed] [Google Scholar]
- 32.Barker JM, Goehrig SH, Barriga K, et al. ; DAISY Study. Clinical characteristics of children diagnosed with type 1 diabetes through intensive screening and follow-up. Diabetes Care 2004;27:1399–1404 [DOI] [PubMed] [Google Scholar]
- 33.Elding Larsson H, Vehik K, Bell R, et al. ; TEDDY Study Group; SEARCH Study Group; Swediabkids Study Group; DPV Study Group; Finnish Diabetes Registry Study Group. Reduced prevalence of diabetic ketoacidosis at diagnosis of type 1 diabetes in young children participating in longitudinal follow-up. Diabetes Care 2011;34:2347–2352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Meehan C, Fout B, Ashcraft J, Schatz DA, Haller MJ. Screening for T1D risk to reduce DKA is not economically viable. Pediatr Diabetes 2015;16:565–572 [DOI] [PubMed] [Google Scholar]
- 35.Raab J, Haupt F, Scholz M, et al. ; Fr1da Study Group. Capillary blood islet autoantibody screening for identifying pre-type 1 diabetes in the general population: design and initial results of the Fr1da study. BMJ Open 2016;6:e011144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fritsch M, Schober E, Rami-Merhar B, Hofer S, Frohlich-Reiterer E, Waldhoer T; Austrian Diabetes Incidence Study Group. Diabetic ketoacidosis at diagnosis in Austrian children: a population-based analysis, 1989-2011. J Pediatr 2013;163:1484–1488.e1 [DOI] [PubMed] [Google Scholar]
- 37.VanderWeele J, Pollack T, Oakes DJ, et al. Validation of data from electronic data warehouse in diabetic ketoacidosis: caution is needed. J Diabetes Complications 2018;32:650–654 [DOI] [PubMed] [Google Scholar]


