Abstract
Recombinant adenovirus vectors have been used to transfer genes to the lungs in animal models, but the extent and duration of primary transgene expression and the ability to achieve expression after repeated vector administration have been limited by the development of antigen-specific immunity to the vector and, in some cases, to vector-transduced foreign proteins. To determine if focused modulation of the immune response could overcome some of these limitations, costimulatory interactions between T cells and B cells/antigen-presenting cells were transiently blocked around the time of vector administration. Systemic treatment at the time of primary-vector administration with a monoclonal antibody (MR1) against murine CD40 ligand, combined with recombinant murine CTLA4Ig and intratracheal coadministration of an adenovirus vector transducing the expression of murine CTLA4Ig, prolonged adenovirus-transduced β-galactosidase expression in the airways for up to 28 days and resulted in persistent alveolar expression for >90 days (the duration of the experiment). Consistent with these results, this treatment regimen reduced local inflammation and markedly reduced the T-cell and T-cell-dependent antibody response to the vector. A secondary adenovirus vector, administered >90 days after the last systemic dose of MR1 and muCTLA4Ig, resulted in alkaline phosphatase expression at levels comparable to those seen with primary-vector administration. Expression of the secondary transgene persisted in the alveoli (but not in the airways) for up to 24 days (the longest period of observation) at levels similar to those observed on days 3 to 4. These results indicate that transient inhibition of costimulatory molecule interactions substantially enhanced gene transfer to the alveoli but was much less effective in the airways. This suggests that there are differences in the efficiency or nature of mechanisms limiting transgene expression in the airways and in the alveoli.
The use of viral vectors for gene transfer in vivo has been complicated by the development of an antigen-specific immune response to the vector and, in the case of proteins foreign to the host, to the gene product. One consequence of this response has been a relatively brief period of transgene expression. This most often results from T-cell-mediated loss of vector DNA from transduced cells or of the transduced cells themselves (1, 30, 43, 44, 48), although expression of some secreted proteins may be lost through the production of antibodies to the protein, which is also T-cell dependent (25, 26, 30). In addition to limiting the duration of transgene expression, T-cell-dependent production of neutralizing antibodies to the viral coat blocks or markedly reduces the efficiency of gene transfer following secondary administration of the vector (1, 3, 17, 40, 43, 44). To improve the efficacy of virus-mediated gene transfer, the immune response must be reduced through manipulation of the vector or modulation of the host immune response. The former approach holds promise for prolonging transgene expression (2, 7, 22, 37) but is less likely to overcome obstacles to secondary vector administration, since antibody is produced in response to proteins present in the administered vector (40, 43).
Some success in modulating the immune response to the vector has been obtained by using cytoablative or broadly immunosuppressive regimens commonly used for organ transplantation, including cyclosporin, FK506, and cyclophosphamide. Cyclosporin prolonged transgene expression minimally when used alone, working effectively only when combined with cyclophosphamide (3, 4); the ability of this regimen to facilitate secondary vector administration was not tested. Similarly, daily administration of FK506 was required to prolong transgene expression in muscle, and antibody production to the vector was only partly reduced (35). In addition to potential toxicity and somewhat limited efficacy, these regimens block both primary and recall responses, thereby impairing preexisting immunity. An alternative strategy to enhance gene transfer is the inhibition of costimulatory interactions between T cells and B cells (17, 18, 38, 41, 45). This approach is based on the observation that the initial activation of T cells is largely dependent both on the engagement of specific antigen receptors on the T cell with peptide antigen-major histocompatibility complexes on the antigen-presenting cell (APC) and on concomitant engagement of CD28 on the T cell with B7 molecules (CD80/CD86) on the APC (8, 21). This is part of a two-way interaction between T cells and APC, since activated T cells express on their surface CD40 ligand, which engages CD40 on the APC, thereby up-regulating B7 molecules (6, 19). In addition, CD40 ligand engages CD40 on B cells, providing a signal necessary for the production of high-affinity immunoglobulin G (IgG) and IgA antibodies and the development of B-cell memory. Blockade of CD28 interactions with B7 molecules through the administration of recombinant murine CTLA4Ig (muCTLA4Ig) prolonged adenovirus (Ad) vector-transduced human α1-antitrypsin gene (hAAT) expression in the liver for more than 5 months (the duration of the experiments), whereas by 6 weeks expression decreased by more than 2 log10 units in control mice (17). However, secondary vector administration was not effective, due to the ultimate development of serum neutralizing antibodies to the vector (17). Treatment with an antibody that blocks CD40/CD40 ligand interaction (MR1) also prolonged transgene expression in the liver (17, 45), but in the one study in which they were compared directly, MR1 was less effective than muCTLA4Ig (17). When mice were treated with muCTLA4Ig and MR1, not only was gene expression prolonged for up to 1 year (the duration of the experiment) in C3H/HeJ and BALB/c mice but also secondary vector administration (at a time when the immunomodulatory effects of the treatment were no longer present) resulted in prolonged and robust expression in more than 50% of the mice (18). Greater efficacy of combined muCTLA4Ig and MR1 treatment than of treatment with either alone has also been observed in studies of solid organ transplantation (20). An important feature favoring treatments that act through inhibition of costimulatory interactions is that they appear preferentially to impair development of the primary response to newly encountered antigens with relative sparing of existing immunity (6, 11, 21).
The present studies sought to determine whether this form of therapy would also enhance the efficacy of gene transfer to the lung. Unlike the liver, the lung is protected by a combination of mucosal and systemic defense mechanisms (23). Two groups found that the blockade of CD40 ligand by treatment with MR1 around the time of vector administration prolonged the duration of first-generation Ad vector-mediated transgene expression in the lung to various degrees and improved the efficacy of transduction after secondary vector administration (28, 45). The present studies confirm and extend these findings. Treatment with muCTLA4Ig and MR1 and coadministration of an Ad vector transducing the expression of muCTLA4Ig at the time of primary vector administration (i) markedly prolonged primary transgene expression in the alveoli but had little effect on the duration of expression in the airways and (ii) allowed secondary vector administration in the absence of additional systemic immunomodulatory therapy at an efficiency comparable to that seen with primary vector administration and prolonged expression of the secondary transgene (but only alveolar expression was prolonged).
MATERIALS AND METHODS
Recombinant Ad vectors.
Construction of the recombinant E1-deficient Ad5 vectors Ad.RSV-βgal (32), Ad.RSV-hAAT (16), and Ad.RSV-muCTLA4Ig (29) has been described previously. Ad.CMV-AlkPhos (H5.010CBALP [43]) was provided by J. Wilson, University of Pennsylvania. Desired recombinants were grown on a large scale and then purified and concentrated by double cesium chloride density centrifugation (10). Wild-type contamination was determined and virus was quantitated by spectrophotometry and plaque assay as described previously (1, 10).
Recombinant reagents to block costimulatory interactions.
muCTLA4Ig, the control monoclonal antibody L6, and the MR1 monoclonal antibody to murine CD40 ligand (CD154) have been described previously (17, 18). These reagents were diluted in pyrogen-free saline and administered to the indicated groups of mice intraperitoneally at doses of 200 μg (muCTLA4Ig or L6) or 250 μg (MR1) on days −1, 2, and 7 relative to the time of primary vector administration (day 0).
Animals and animal procedures.
C3H/HeJ mice were obtained from Jackson Laboratory (Bar Harbor, Maine) and housed under specific-pathogen-free conditions in accordance with institutional guidelines. Mice of ≥8 weeks of age were anesthetized lightly with ketamine-xylazine and, if the level of anesthesia appeared not to be sufficient, also by inhalation of methoxyflurane. The trachea was exposed through a midline incision and cannulated with a 26-gauge needle attached to a tuberculin syringe. The marker gene vector (Ad.RSV-βgal or Ad.CMV-AlkPhos) was administered intratracheally at 5 × 109 PFU in a volume of 100 μl followed by 200 μl of air to ensure delivery into the lungs. Mice also received 2.5 × 109 PFU of Ad.RSV-muCTLA4Ig or, as a control, Ad.RSV-hAAT in the same inoculum as the marker vector. At the indicated intervals, the mice were anesthetized, blood was collected by retro-orbital puncture, and then the mice were sacrificed. The trachea was cannulated, the chest was opened, and bronchoalveolar lavage (BAL) was performed by repeated instillation of phosphate-buffered saline (PBS). The lungs were then inflated and fixed at 23 cm of pressure in 2% paraformaldehyde in PBS. Spleens were collected and processed for analysis as described previously (17).
Histological and histochemical analysis.
β-Galactosidase (βgal) was assessed with 5-bromo-4-chloro-3-indolyl-β-d-galactopyranoside (X-Gal) as the substrate by using previously described methods (24). For assessment of alkaline phosphatase, after fixation of whole lungs in 2% paraformaldehyde, the lungs were cut into smaller pieces, postfixed in 4% paraformaldehyde for 3 h, rinsed, and heated to 70°C for 1 h to inactivate the endogenous enzyme. The tissues were then rinsed in 0.1 M Tris buffer (pH 8.5) and reacted in the same buffer to which nitroblue tetrazolium (1 mg/ml) and 5-bromo-4-chloro-3-indolyl-phosphate (0.1 mg/ml) (Boehringer Mannheim) were added. After development overnight, the tissues were washed in PBS, fixed in 10% buffered formalin, embedded in paraffin, and sectioned. A minimum of three to five random (5-μm-thick) sections totaling >60 mm2 through all portions of the lungs from each animal were analyzed microscopically in a blinded manner. The average values from these sections for each mouse were recorded on a 6-point scale. A score of 0 was assigned to mice in which no cells in any of the airways or alveoli contained βgal or alkaline phosphatase product; for the airways, a score of 1 reflected 20% positive cells, 2 reflected 40% positive cells, increasing to a score of 5 for 100% positive cells; for the alveoli, a score of 2 was assigned to mice for which one or two cells were positive per field, 3 was assigned to mice with three to six positive cells per field, increasing to a score of 5 for 100% positive cells per field. Inflammation was graded by using the criteria developed by Ginsberg et al. (9).
Immunological assays.
Assays to detect neutralizing antibodies and enzyme-linked immunosorbent assays (ELISAs) to detect isotype-specific antibodies to Ad were performed as described previously (17). For the ELISAs, plates were coated with 5 × 108 PFU of UV-inactivated Ad.RSV-βgal and the reactions were developed with isotype-specific, horseradish peroxidase-conjugated anti-murine IgG1, IgG2a, or IgA antibodies (Southern Biotechnology, Birmingham, Ala.) or anti-murine IgM antibodies (Tago/Biosource, Camarillo, Calif.). Serial threefold dilutions of serum or BAL fluid were tested by ELISA with an initial dilution of 1:10 for BAL fluid and 1:25 for serum. Titers are reported as the reciprocal of the highest dilution at which the optical density at 405 nm exceeded that of the preimmune serum by 0.1 or that of negative control BAL fluid by 0.05. Proliferation of and gamma interferon (IFN-γ) and interleukin-4 production by isolated spleen cells were determined as described previously (17).
RESULTS
Combined treatment with muCTLA4Ig and MR1 prolongs transgene expression.
Inhibition of T-cell–APC/B cell costimulatory interactions by systemic administration of muCTLA4Ig around the time of vector administration prolongs the expression of human α1-antitrypsin in hepatocytes of mice given first-generation Ad vectors (17, 18). However, in initial experiments, βgal expression declined to undetectable levels within 28 days both in control and muCTLA4Ig-treated mice that were given Ad.RSV-βgal intratracheally (data not shown). The failure to affect transgene expression might reflect inadequate local concentrations of muCTLA4Ig in the lungs, incomplete inhibition of costimulatory interactions by muCTLA4Ig, or both. Accordingly, the treatment regimen was modified to address these possibilities.
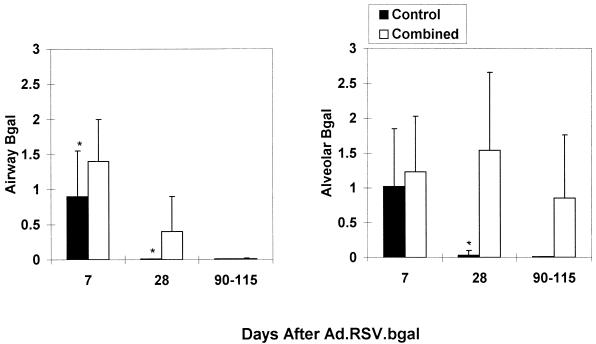
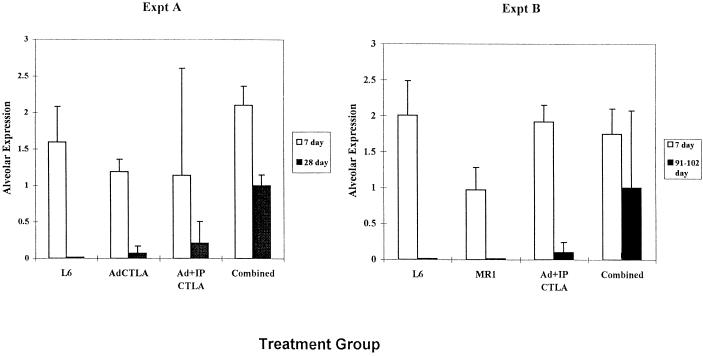
Systemic treatment with muCTLA4Ig and MR1 on days −1, 2, and 7, in combination with intratracheal administration of Ad.RSV-muCTLA4Ig at the time (day 0) of primary vector (Ad.RSV-βgal) administration (combined treatment regimen), enhanced the duration of βgal expression (Fig. 1 and 2). In the combined-treatment group, alveolar expression persisted for the length of the experiment, 90 to 115 days, and was detectable at levels comparable to those observed on day 7 in six of the eight mice examined at this time point. In contrast, expression declined markedly by day 28 in the control group and was not detected in any of the five mice examined at 90 to 115 days. Expression in bronchial epithelial cells was prolonged in the combined-treatment group, but the effect was less marked than in the alveolar epithelium (Fig. 1 and 2): 10 of 11 mice in the combined-treatment group had detectable expression in the bronchial epithelium at 28 days but only 2 of 8 had detectable expression at 90 to 115 days, whereas 11 of 11 mice and 6 of 8 mice had detectable expression in the alveolar epithelium at 28 and 90 to 115 days, respectively. A small number of βgal-positive cells in the alveoli of treated mice appeared to be macrophages, but this was not sufficient to account for the difference in expression compared to the bronchial epithelium. Thus, combined systemic and local blockade of costimulatory interactions enhanced transgene persistence in the alveoli whereas this regimen prolonged airway expression only minimally. Further differing from results in the liver, in which muCTLA4Ig alone markedly prolongs transgene expression (17, 18), the effect of the combined regimen on alveolar expression appeared to be compromised when muCTLA4Ig or MR1 was omitted (Fig. 3).
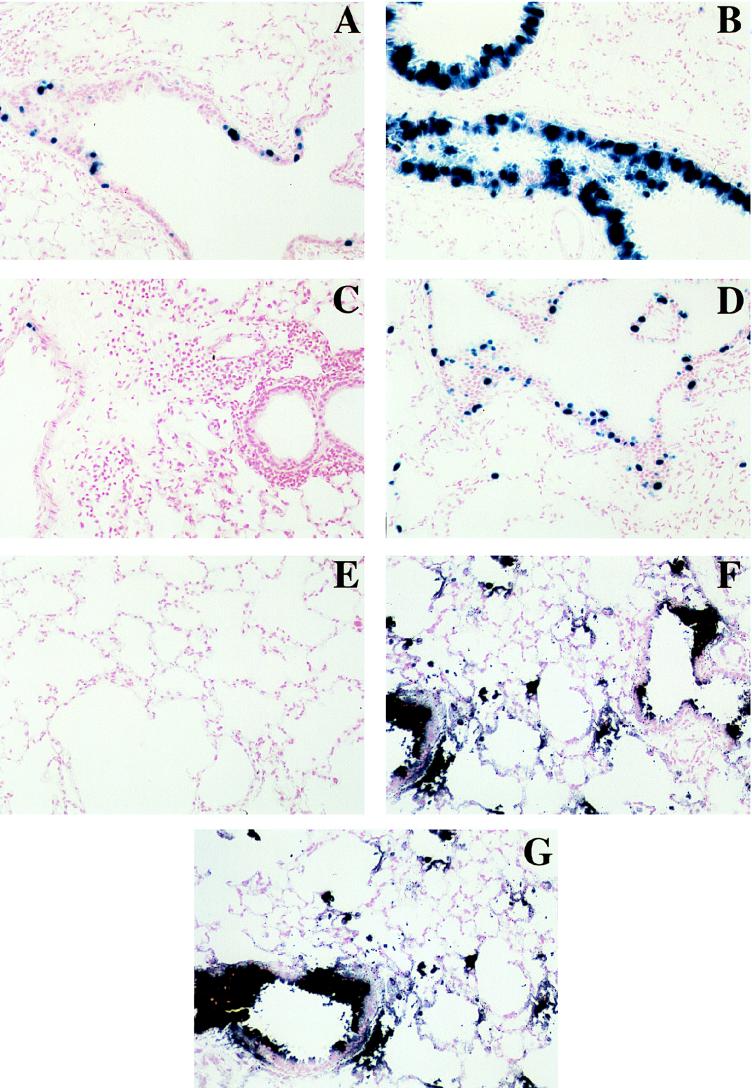
FIG. 1.
Photomicrographs of lungs stained to detect βgal (A to D) or alkaline phosphatase (E to G). Ad.RSV-βgal was administered intratracheally on day 0 to control mice (A and C) or mice treated with the combined regimen (B and D), and βgal expression was assessed 7 (A and B) or 28 (C and D) days after vector administration. For treatment details, see Materials and Methods. Ad.CMV-AlkPhos was administered intratracheally at 90 to 102 days after primary vector administration to control mice (E), mice treated with the combined regimen at the time of primary vector administration (F) or mice receiving Ad.CMV-AlkPhos as primary vector (G), and alkaline phosphatase expression was assessed 3 to 4 days later. For the representative animals shown, the scores (see Materials and Methods and Fig. 2) for airway and alveolar expression of βgal were, respectively, 0.9 and 0.6 (A), 1.8 and 1.9 (B), 0.0 and 0.0 (C), and 1.5 and 2.6 (D) and for alkaline phosphatase, respectively, 1.6 and 1.5 (E), 1.6 and 1.7 (F), and 0.0 and 0.0 (G).
FIG. 2.
The combined-treatment regimen prolongs βgal expression in the lung epithelium. Results are the numerical score for βgal expression based on a scale of 0 to 5 (see Materials and Methods for details of the scoring method). Expression in the bronchial epithelium is shown on the left, and expression in the alveolar epithelium is shown on the right. Data were derived from two to four experiments with 5 to 11 mice for each time point per group and are shown as mean ± standard deviation (SD). ∗, p < 0.05 (control versus combined-treatment group). The combined-treatment group received muCTLA4Ig (200 μg) and MR1 (250 μg) intraperitoneally on days −1, 2, and 7 and Ad.RSV.muCTLA4Ig intratracheally at the time of Ad.RSV.βgal administration. The control group received L6 (200 μg) intraperitoneally on days −1, 2 and 7 and Ad.RSV.hAAT intratracheally at the time of Ad.RSV.βgal administration.
FIG. 3.
Contribution of the components of the combined-treatment regimen to prolonged βgal expression in the alveolar epithelium. Results are the mean ± SD of the numerical score for βgal expression based on a scale of 0 to 5. Data were derived from two experiments with two to four mice per time point per group, except for the MR1 group, which was one experiment with one or two mice per time point. The AdCTLAIg group received Ad.RSV-muCTLA4Ig intratracheally along with Ad.RSV-βgal on day 0; the Ad+IP muCTLA4Ig group received Ad.RSV-muCTLA4Ig intratracheally along with Ad.RSV-βgal on day 0 and recombinant muCTLA4Ig IP on days −1, 2, and 7; the MR1 group received MR1 on days −1, 2, and 7; the control and combined-treatment regimens are described in the legend to Fig. 2.
Successful secondary Ad vector-mediated gene transduction in mice treated with the combined regimen.
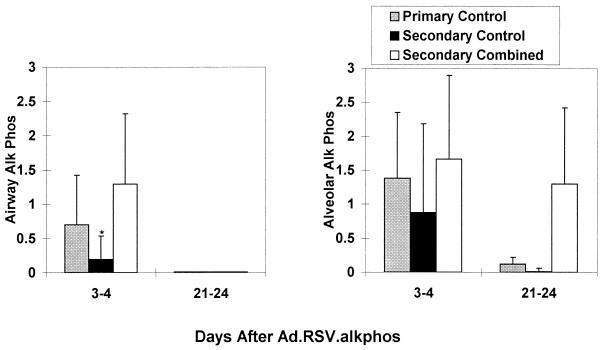
Secondary Ad vector administration does not usually lead to substantive transgene expression, largely due to the induction of neutralizing antibodies to the virus in response to primary vector administration (3, 17, 43). However, mice in the combined-treatment group had robust alkaline phosphatase expression in the bronchial and alveolar epithelium 3 to 4 days after secondary Ad.CMV-AlkPhos administration (Fig. 4, Secondary Combined group), which was comparable to expression in Ad-naive mice (Primary Control group). These mice did not receive Ad.RSV-muCTLA4Ig at the time of secondary vector administration, and the last doses of systemic muCTLA4Ig and MR1 were given more than 90 days earlier, so that the systemic effects of these agents would have waned (18). Although continued local production of muCTLA4Ig from the Ad vector given along with the primary Ad.RSV-βgal vector may have contributed to the efficacy of secondary vector administration, it is not likely to have played a major role, since immunocompetence in the lungs was sufficiently restored that a robust mucosal antibody response occurred after secondary vector administration (see below). To determine if the duration of alkaline phosphatase expression from the secondary vector could be extended without further systemic administration of muCTLA4Ig or MR1, a subset of the secondary combined treatment group were given Ad.RSV-muCTLA4Ig along with Ad.CMV-AlkPhos and expression was analyzed 21 to 24 days later (Fig. 4, Secondary Combined group); alkaline phosphatase expression in the alveoli of these mice was detectable at substantial levels (scores 0.5 and 2.1) but was not detectable in either control group (Fig. 4).
FIG. 4.
Expression of alkaline phosphatase after secondary Ad.CMV-AlkPhos administration to mice treated with the combined regimen at the time of primary vector administration. Results are the mean ± SD of the numerical score for expression based on a scale of 0 to 5. Expression in the bronchial epithelium is shown on the left, and expression in the alveolar epithelium is shown on the right. Data were derived from two experiments with three to five mice per time point per group, with the exception of the 21- to 24-day combined treatment group (two mice). The primary controls had not previously received Ad vector. The other groups received either L6 or no treatment (secondary control) or the combined regimen (secondary combined) on days −1, 2, and 7 relative to the time of primary Ad vector administration 98 to 115 days earlier. ∗, P < 0.05 with respect to the other two groups at that time point.
The combined-treatment regimen modulates but does not ablate the immune response to the vector.
The high efficiency of transduction with secondary vector administration and the persistence of primary transgene expression in mice receiving the combined regimen suggested that the immune response to the vector was impeded. To address first the basis for efficient secondary vector transduction, the anti-Ad antibody levels in serum and BAL fluid were measured. As shown in Table 1, the combined regimen nearly abolished the production of antibodies in serum and BAL fluid at 28 days, since only one of six mice had detectable, low-level antibodies as determined by a sensitive isotype-specific ELISA and neutralizing antibodies were not detected. Although present at low titers compared to controls, by 90 to 102 days the levels of antibody in serum and BAL fluid in most of the mice in the combined-treatment group were detectable. However, consistent with the high efficiency of secondary vector transduction observed in this group (Fig. 4), neutralizing antibodies were not detected in the BAL fluid 90 to 102 days after primary vector administration (Table 1). The antibody titers in BAL fluid rose 21 days after secondary vector administration both in controls and in the combined-treatment group; the combined-treatment group received Ad.RSV-muCTLA4Ig but did not receive systemic MR1 or muCTLA4Ig with the secondary vector. In the absence of continued or repeated exposure to antigen, mucosal antibody wanes more rapidly than does serum antibody (23, 34). Consistent with this, antibody titers in the BAL fluid but not the serum of control mice declined markedly by 90 to 102 days, which correlated with the ability to achieve low-level secondary transduction in this group (Fig. 4).
TABLE 1.
Antibody responsesa
| Mice | Mean titerb of:
|
||||||
|---|---|---|---|---|---|---|---|
| Serum IgG1 | Serum IgG2a | BALF IgA | BALF IgG1 | BALF IgG2a | Serum neut.c | BALF neut.c | |
| Controls | |||||||
| 28 days | >1.8 × 104 (≥1.8 × 104; 4/4) | >1.8 × 104 (≥1.8 × 104; 4/4) | 156 (30–810; 4/4) | 270 (30–810; 4/4) | 1.4 × 103 ([0.3–7.3] × 103; 4/4) | 136 (16,256; 2/2) | 32 (16,64; 2/2) |
| 90–102 days | 4.2 × 103 ([2.0–6.1] × 103; 3/3) | >1.8 × 104 (≥1.8 × 104; 3/3) | 11.4 (5–30; 2/3) | 30.0 (10–90; 2/3) | 562 (0.3–2.4 × 103; 3/3) | 64 (64; n = 1) | <4 (<4; n = 1) |
| 21 days post 2°; 119–136 days post 1° | >1.8 × 104 (≥1.8 × 104; 2/2) | >1.8 × 104 (≥1.8 × 104; 2/2) | 1.4 × 103 ([0.3–7.9] × 103; 2/2) | 1.4 × 103 ([0.3–7.9] × 103; 2/2) | 1.4 × 103 ([0.3–7.9] × 103; 2/2) | NDd | ND |
| Combined treatment | |||||||
| 28 days post 1° | 13.8 (<25–25; 1/6) | 25.9 (<25–2,025; 1/6) | 8.1 (<10–10; 1/6) | 5.6 (<10–270; 1/6) | 9.7 (<10–90; 1/6) | <16 (<16; 0/2) | <4 (<4; 0/2) |
| 90–102 days post 1° | 98.5 (<25–2.0 × 103; 5/7) | 3.8 × 103 (75–1.8 × 104; 7/7) | 28.8 (<10–810; 5/7) | 11.9 (<10–270; 4/7) | 118 (<10–2,430; 5/7) | 5.3 (<16–16; 1/3) | <4 (<4; 0/3) |
| 21 days post 2°; 119– 136 days post 1° | 276 (<25, 6.1 × 103; 1/2) | 272 (<25, 1.8 × 104; 1/2) | 1.4 × 103 ([0.3–7.9] × 103; 2/2) | 270 (90, 810; 2/2) | 4.2 × 103 ([2.4–7.3] × 103; 2/2) | ND | ND |
Isotype-specific anti-adenoviral antibodies were assayed by ELISA on a subset of the mice in the experiments in Fig. 2 and 4; control mice were either treated with the control monoclonal antibody L6 or not treated, with similar results. The combined-treatment group received systemic muCTLAIg and MR1 and intratracheal Ad.RSV-muCTLA4Ig with the primary vector and intratracheal Ad.RSVmuCTLA4Ig with the secondary vector.
Geometric mean titer (range; number of mice with detectable antibody/number tested).
The 90- to 102-day neutralizing antibody titers were determined 3 days after secondary vector administration.
ND, not done.
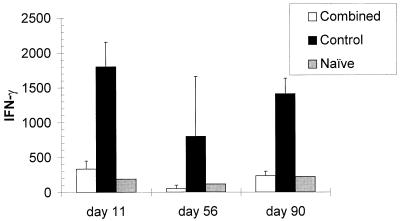
The T-cell response to the Ad vector was markedly reduced in the treated mice for up to 90 days, when measured by the capacity to produce IFN-γ in vitro (Fig. 5). However, in the experiment shown, each of three mice studied on day 11 and two of three mice studied on day 90 from the combined-treatment group produced IFN-γ in amounts (>200 pg/ml) exceeding those observed in Ad-stimulated cell cultures from naive mice (Fig. 5). Similar results were obtained in other experiments and when proliferation in response to Ad vector rather than IFN-γ production was measured (data not shown). The reduced IFN-γ production was not associated with a shift in the nature of the cytokines produced toward a Th2 pattern, since IL-4 was not detected in Ad-stimulated cultures from mice in either group. When evaluated 21 days after secondary vector administration, IFN-γ production by cells from control and treated mice was similar (data not shown). Together with the antibody data (Table 1), these results suggest that even though the immune response of the mice to the vector was not persistently blocked, secondary administration of Ad.CMV-AlkPhos was successful and resulted in prolonged transgene expression in the alveoli.
FIG. 5.
The combined-treatment regimen inhibits but does not ablate T-cell responses to the vector. Shown are results from one experiment, expression data from which are included in those shown in Fig. 2. Spleens were obtained from mice at the indicated times after intratracheal administration of Ad.RSV-βgal. The results of IFN-γ production (in picograms per milliliter) by cells stimulated with Ad.RSV-βgal are shown. Cells from each group produced similar amounts of IFN-γ in response to stimulation with an anti-CD3 monoclonal antibody used as a positive control. Data are the mean ± SD production of IFN-γ by cells from two or three mice per time point.
Similar to these in vitro results, histological analysis of lungs from mice in the combined-treatment group showed a significant reduction (P < 0.05) in peribronchial and alveolar inflammation compared to controls at 7 and 28 days after primary vector administration (data not shown).
DISCUSSION
This study sought to identify an immunomodulatory regimen that would prolong expression after initial Ad vector administration to the lung and overcome the barrier to repeated administration. The combined regimen used was modified from ones used successfully in liver-directed gene therapy by ourselves (17, 18) and by others (45), which are directed at interrupting costimulatory interactions between T cells and APC/B cells. There are important differences in the immunological barriers that must be overcome in the lung, a mucosal compartment, from those in nonmucosal tissues, such as liver and muscle. Perhaps as a consequence, different results were achieved in these tissues. One to three doses of muCTLA4Ig given systemically around the time of administration of an identical Ad vector directing expression of hAAT in the liver prolonged transgene expression for 5 to 6 months or more (the duration of the experiments), while in the absence of such treatment hAAT expression declined by more than 2 log10 units within 6 weeks (1, 17, 18, 30). In contrast, in the present studies, multiple doses of systemic muCTLA4Ig alone had little or no effect on the duration of transgene expression in the airways or alveoli. However, when systemic muCTLA4Ig and MR1 and an Ad vector transducing muCTLA4Ig in the lung were given in combination, transgene expression was prolonged substantially, but only in the alveoli, not in the airways.
One possible difference between this study and the studies of the liver is the nature of the transgene expressed. In the absence of a cytotoxic T-cell (CTL) response to the transgene-encoded protein, the CTL response to vector proteins may be sufficient to eliminate transgene expression in some cases (42, 46) but not in others (2, 7, 36). Conversely, a robust immune response against a protein encoded by a foreign transgene may extinguish expression. This may result from cytotoxic elimination of transgene expression and/or transgene-expressing cells or, in the case of secreted proteins, through antibody-mediated loss of protein expression (2, 25, 26, 30, 33, 34, 36). For example, βgal induces a robust humoral and cellular immune response in mice (15, 36), and loss of transgene expression correlates at least in part with the loss of transgene DNA through a T-cell-mediated process (25, 26, 44). In mice receiving an Ad directing hAAT expression, cytotoxicity (31) and/or a T-cell-dependent antibody response to hAAT is induced in a strain-dependent manner (26, 30). Antibody-mediated elimination of transgene-encoded protein appears to be the major mechanism for loss of hAAT expression in C3H/HeJ mice (26, 30), whereas cytotoxic mechanisms appear to predominate in BALB/c mice (30).
In addition to the potential role of the transgene product, in the present study gene transfer was directed to a different tissue from that in the previous studies. Unlike the liver, the respiratory mucosal epithelium forms an anatomical barrier at which host and microbes interact on a daily basis and is defended both by local mucosal defenses and by recruited systemic defenses (reviewed in reference 23). Thus, one potential mechanism limiting the efficacy of immunomodulatory therapy in the lungs is the need to block local as well as systemic immune mechanisms. This was addressed in part by the use of lung-directed expression of muCTLA4Ig from a recombinant Ad in an attempt to augment local concentrations of the inhibitor. However, this vector alone or in combination with systemic muCTLA4Ig was not sufficient to sustain local transgene expression. Only when local and systemic muCTLA4Ig were combined with systemic MR1 was primary transgene expression substantially prolonged. A regimen of systemic muCTLA4Ig and MR1 without Ad muCTLA4Ig was not tested in the present study, so it is not possible to be certain to what extent the latter component contributed to the efficacy of the regimen. The immune response to a novel antigen encountered in the lung is actually initiated not in the lung parenchyma but in the regional lymph nodes and lung-associated lymphoid tissues, to which antigen or antigen-laden APC from the lung epithelium are carried (23). Thus, it is likely that the principal locus of action of the combined immunomodulatory regimen was in these tissues rather than in the lung parenchyma itself and that systemic muCTLA4Ig and MR1 were the major factors in the efficacy of the regimen. This notion is supported by the robust mucosal antibody response observed at the time of secondary administration, when these mice received Ad.RSV-muCTLA4Ig intratracheally but did not receive systemic muCTLA4Ig or MR1. The greater efficacy of combined systemic treatment with muCTLA4Ig and MR1 has been observed in the context of allogeneic tissue transplants (20), autoimmune diseases (12), and Ad vector readministration to the liver (18), and it appears to reflect a more complete inhibition of T-cell and T-cell-dependent antibody responses by the combination than by either agent alone.
Although the combined treatment regimen resulted in sustained expression of βgal in the alveolar epithelium, expression was not sustained in the airway epithelium. The basis for this difference is not certain. Ad vectors are known to exhibit different degrees of tropism for lung epithelial cells, e.g., being relatively high for basal and low for differentiated columnar epithelium (13, 47). However, since airway expression persists in T-cell and combined-immunodeficient mouse strains, the loss of expression in the airway but not the alveoli is not likely simply to reflect a difference in tropism or in the rate of non-immune-system-mediated cell turnover (43, 44, 48). The immune response to the vector was markedly attenuated but not completely blocked by the combined regimen, and so it remains possible that airway epithelial cells were either more readily recognized or more readily eliminated by the attenuated immune response of the treated mice. Supporting these possibilities, there is a more extensive network of dendritic APC and lymphoid aggregates in the airway parenchyma than in the alveoli, there is a greater abundance of macrophages in the alveolar area (which primarily inhibit rather than augment T-cell responses), and there are differences in the circulatory supply, which may be associated with differences in endothelial adhesins for leukocytes (23, 27).
The persistence of transgene expression in mice treated with the combined regimen is similar to that reported previously by J. Wilson and colleagues (39, 43) for mice with different forms of T-cell immunodeficiency. However, in their studies, expression persisted for 28 to 90 days in the airway epithelium as well. The basis for the greater persistence in the airways in their studies is not obvious, although it might reflect strain-related differences between the C3H/HeJ mice used in the present study and the C57BL/6 and 129/Sv mice used in their studies. They also observed prolonged expression of βgal or alkaline phosphatase transgenes from first-generation Ad vectors at levels >80% of peak for 28 days and ∼50% of peak for 31 to 60 days, following either depletion of CD4+ T cells with a monoclonal antibody or the use of MR1 treatment alone, respectively. Scaria et al. (28) also found that MR1 administered around the time of primary vector administration prolonged first-generation Ad-mediated βgal expression in the lung, but the results were less striking than in the studies by Yang et al. (40): βgal expression declined ∼10-fold in the MR1-treated mice over the 30-day period of analysis; the cell type(s) expressing βgal was not determined. In the present study, expression in mice treated with the combined regimen was >80% of peak for 90 to 115 days in the alveoli and ∼50% and <10% of peak in the airways at 28 and 90 to 115 days, respectively. The transgenes and dosing regimens used for MR1 were similar in these three studies, but differences in the mouse strains and minor differences in the vector sequences preclude direct comparison of the results.
A second goal of these studies was to define a regimen that allowed efficient secondary Ad-mediated gene transfer. This was achieved. Gene transfer was at least as efficient in mice that received combined treatment around the time of primary vector administration as in naive mice, even when no further immunomodulatory therapy was given. Further, in the subset of mice that was monitored for 21 days, alveolar expression of the secondary transgene was sustained. This latter group did receive Ad.RSV-muCTLA4Ig at the time of secondary vector administration, although the role of this vector in prolonging expression of the secondary transgene is not clear. Two other groups achieved transduction after secondary Ad vector administration to mice that received MR1 alone around the time of primary vector administration, 30 to 90 (40) or 50 (28) days earlier. The efficiency of transduction was ∼50% (40) to 90% (28) of that achieved in naive mice, but the duration of secondary transgene expression was not studied. The ability to achieve secondary gene transfer in these studies and in the present study correlated best with reduced amounts of anti-Ad antibodies in BAL fluid of treated mice and is consistent with other data indicating that BAL, but not serum, antibody blocks transduction. A limited degree of secondary vector transduction occurred in the control group in the present study and in certain other studies (28, 36). This appears to reflect the fact that mucosal antibody wanes over time, falling to concentrations insufficient to neutralize the virus fully. Accordingly, if the need for repeated gene transfer is infrequent, the extent to which the production of neutralizing antibody must be blocked at the time of primary vector administration might be lower.
These studies support the notion that focused immunomodulatory therapy at the time of initial vector administration to the lung can substantially extend transgene expression and allow efficient secondary transduction. These studies were done with first-generation Ad vectors directing the expression of foreign, immunogenic marker proteins in the lungs. Despite the immunogenicity of the vectors and transgenes, the combined regimen of systemic costimulatory molecule blockade with muCTLA4Ig and MR1 and local Ad vector-directed muCTLAIg achieved both of the primary goals: transgene expression was substantially prolonged, particularly in the alveoli, and secondary vector administration was fully efficient. Further refinements that enhance vector tropism for the relevant cell populations in the lung and reduce vector immunogenicity are important goals if gene defects in the lungs are to be corrected (5, 14, 47). It is likely that less intense immunomodulatory regimens will be needed in the context of less immunogenic vectors.
ACKNOWLEDGMENTS
This work was supported in part by grants from the National Institutes of Health (DK51807, DK47754, and AI37107). D.S. was the recipient of the National Hemophilia Association’s Judith Graham Pool Fellowship.
REFERENCES
- 1.Barr D, Tubb J, Ferguson D, Scaria A, Lieber A, Wilson C, Perkins J, Kay M A. Strain related variations in adenovirally mediated transgene expression from mouse hepatocytes in vivo: comparisons between immunocompetent and immunodeficient inbred strains. Gene Ther. 1995;2:151–155. [PubMed] [Google Scholar]
- 2.Chen H H, Mack L M, Kelly R, Ontell M, Kochanek S, Clemens P R. Persistence in muscle of an adenoviral vector that lacks all viral genes. Proc Natl Acad Sci USA. 1997;94:1645–1650. doi: 10.1073/pnas.94.5.1645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dai Y, Schwarz E M, Gu D, Zhang W W, Sarvetnick N, Verma I M. Cellular and humoral immune responses to adenoviral vectors containing factor IX gene: tolerization of factor IX and vector antigens allows for long-term expression. Proc Natl Acad Sci USA. 1995;92:1401–1405. doi: 10.1073/pnas.92.5.1401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Engelhardt J F, Ye X, Doranz B, Wilson J M. Ablation of E2A in recombinant adenoviruses improves transgene persistence and decreases inflammatory response in mouse liver. Proc Natl Acad Sci USA. 1994;91:6196–6200. doi: 10.1073/pnas.91.13.6196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fasbender A, Zabner J, Chill’on M, Moninger T O, Puga A P, Davidson B L, Welsh M J. Complexes of adenovirus with polycationic polymers and cationic lipids increase the efficiency of gene transfer in vitro and in vivo. J Biol Chem. 1997;272:6479–6489. doi: 10.1074/jbc.272.10.6479. [DOI] [PubMed] [Google Scholar]
- 6.Foy T M, Aruffo A, Bajorath J, Buhlmann J E, Noelle R J. Immune regulation by CD40 and its ligand GP39. Annu Rev Immunol. 1996;14:591–617. doi: 10.1146/annurev.immunol.14.1.591. [DOI] [PubMed] [Google Scholar]
- 7.Gao G P, Yang Y, Wilson J M. Biology of adenovirus vectors with E1 and E4 deletions for liver-directed gene therapy. J Virol. 1996;70:8934–8943. doi: 10.1128/jvi.70.12.8934-8943.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gause W C, Mitro V, Via C, Linsley P, Urban J F, Jr, Greenwald R J. Do effector and memory T helper cells also need B7 ligand costimulatory signals? J Immunol. 1997;159:1055–1058. [PubMed] [Google Scholar]
- 9.Ginsberg H S, Moldawer L L, Sehgal P B, Redington M, Kilian P L, Chanock R M, Prince G A. A mouse model for investigating the molecular pathogenesis of adenovirus pneumonia. Proc Natl Acad Sci USA. 1991;88:1651–1655. doi: 10.1073/pnas.88.5.1651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Graham F, Prevec L. Adenovirus-based expression vectors and recombinant vectors. Bio/Technology. 1992;20:363–390. doi: 10.1016/b978-0-7506-9265-6.50022-1. [DOI] [PubMed] [Google Scholar]
- 11.Grewal I S, Flavell R A. The CD40 ligand. At the center of the immune universe? Immunol Res. 1997;16:59–70. doi: 10.1007/BF02786323. [DOI] [PubMed] [Google Scholar]
- 12.Griggs N D, Agersborg S S, Noelle R J, Ledbetter J A, Linsley P S, Tung K S. The relative contribution of the CD28 and gp39 costimulatory pathways in the clonal expansion and pathogenic acquisition of self-reactive T cells. J Exp Med. 1996;183:801–810. doi: 10.1084/jem.183.3.801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Grubb B R, Pickles R J, Ye H, Yankaskas J R, Vick R N, Engelhardt J F, Wilson J M, Johnson L G, Boucher R C. Inefficient gene transfer by adenovirus vector to cystic fibrosis airway epithelia of mice and humans. Nature. 1994;371:802–806. doi: 10.1038/371802a0. [DOI] [PubMed] [Google Scholar]
- 14.Ilan Y, Droguett G, Chowdhury N R, Li Y, Sengupta K, Thummala N R, Davidson A, Chowdhury J R, Horwitz M S. Insertion of the adenoviral E3 region into a recombinant viral vector prevents antiviral humoral and cellular immune responses and permits long-term gene expression. Proc Natl Acad Sci USA. 1997;94:2587–2592. doi: 10.1073/pnas.94.6.2587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Juillard V, Villefroy P, Godfrin D, Pavirani A, Venet A, Guillet J G. Long-term humoral and cellular immunity induced by a single immunization with replication-defective adenovirus recombinant vector. Eur J Immunol. 1995;25:3467–3473. doi: 10.1002/eji.1830251239. [DOI] [PubMed] [Google Scholar]
- 16.Kay M A, Graham F, Leland F, Woo S L. Therapeutic serum concentrations of human alpha-1-antitrypsin after adenoviral-mediated gene transfer into mouse hepatocytes. Hepatology. 1995;21:815–819. [PubMed] [Google Scholar]
- 17.Kay M A, Holterman A X, Meuse L, Gown A, Ochs H D, Linsley P S, Wilson C B. Long-term hepatic adenovirus-mediated gene expression in mice following CTLA4Ig administration. Nat Genet. 1995;11:191–197. doi: 10.1038/ng1095-191. [DOI] [PubMed] [Google Scholar]
- 18.Kay M A, Meuse L, Gown A M, Linsley P, Hollenbaugh D, Aruffo A, Ochs H D, Wilson C B. Transient immunomodulation with anti-CD40 ligand antibody and CTLA4Ig enhances persistence and secondary adenovirus-mediated gene transfer into mouse liver. Proc Natl Acad Sci USA. 1997;94:4686–4691. doi: 10.1073/pnas.94.9.4686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Laman J D, Claassen E, Noelle R J. Functions of CD40 and its ligand, gp39 (CD40L) Crit Rev Immunol. 1996;16:59–108. doi: 10.1615/critrevimmunol.v16.i1.40. [DOI] [PubMed] [Google Scholar]
- 20.Larsen C P, Elwood E T, Alexander D Z, Ritchie S C, Hendrix R, Tucker B-C, Cho H R, Aruffo A, Hollenbaugh D, Linsley P S, Winn K J, Pearson T C. Long-term acceptance of skin and cardiac allografts after blocking CD40 and CD28 pathways. Nature. 1996;381:434–438. doi: 10.1038/381434a0. [DOI] [PubMed] [Google Scholar]
- 21.Lenschow D J, Walunas T L, Bluestone J A. CD28/B7 system of T cell costimulation. Annu Rev Immunol. 1996;14:233–258. doi: 10.1146/annurev.immunol.14.1.233. [DOI] [PubMed] [Google Scholar]
- 22.Lieber A, He C, Kay M A. Adenoviral preterminal protein stabilizes mini-adenoviral genomes in vitro and in vivo. Nat Biotechnol. 1997;15:1383–1387. doi: 10.1038/nbt1297-1383. [DOI] [PubMed] [Google Scholar]
- 23.Lipscomb M F, Bice D E, Lyons C R, Schuyler M R, Wilkes D. The regulation of pulmonary immunity. Adv Immunol. 1995;59:369–455. doi: 10.1016/S0065-2776(08)60634-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.MacGregor G R, Nolan G P, Fiering S, Roederer M, Herzenberg L A. Use of E. coli lac Z as a reporter gene. Methods Mol Biol. 1989;7:1–19. doi: 10.1385/0-89603-178-0:217. [DOI] [PubMed] [Google Scholar]
- 25.Michou A I, Santoro L, Christ M, Julliard V, Pavirani A, Mehtali M. Adenovirus-mediated gene transfer: influence of transgene, mouse strain and type of immune response on persistence of transgene expression. Gene Ther. 1997;4:473–482. doi: 10.1038/sj.gt.3300412. [DOI] [PubMed] [Google Scholar]
- 26.Morral N, O’Neal W, Zhou H, Langston C, Beaudet A. Immune responses to reporter proteins and high viral dose limit duration of expression with adenoviral vectors: comparison of E2a wild type and E2a deleted vectors. Hum Gene Ther. 1997;8:1275–1286. doi: 10.1089/hum.1997.8.10-1275. [DOI] [PubMed] [Google Scholar]
- 27.Quiding J-M, Nordstrom I, Granstrom G, Kilander A, Jertborn M, Butcher E C, Lazarovits A I, Holmgren J, Czerkinsky C. Differential expression of tissue-specific adhesion molecules on human circulating antibody-forming cells after systemic, enteric, and nasal immunizations. A molecular basis for the compartmentalization of effector B cell responses. J Clin Investig. 1997;99:1281–1286. doi: 10.1172/JCI119286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Scaria A, St. George J A, Gregory R J, Noelle R J, Wadsworth S C, Smith A E, Kaplan J M. Antibody to CD40 ligand inhibits both humoral and cellular immune responses to adenoviral vectors and facilitates repeated administration to mouse airway. Gene Ther. 1997;4:611–617. doi: 10.1038/sj.gt.3300431. [DOI] [PubMed] [Google Scholar]
- 29.Showalter D B, Meuse L, Wilson C B, Linsley P S, Kay M A. Constitutive expression of murine CTLA4Ig from a recombinant adenovirus vector results in prolonged transgene expression. Gene Ther. 1997;4:853–860. doi: 10.1038/sj.gt.3300466. [DOI] [PubMed] [Google Scholar]
- 30.Showalter, D. B., C. L. Himeda, B. L. Winther, C. B. Wilson, and M. A. Kay. Strain variation in duration of adenovirus mediated transgene expression in mice: Identification of different immune clearance mechanisms. Submitted for publication.
- 31.Song W, Kong H L, Traktman P, Crystal R G. Cytotoxic T lymphocyte responses to proteins encoded by heterologous transgenes transferred in vivo by adenoviral vectors. Hum Gene Ther. 1997;8:1207–1217. doi: 10.1089/hum.1997.8.10-1207. [DOI] [PubMed] [Google Scholar]
- 32.Stratford-Perricaudet L D, Makeh I, Perricaudet M, Briand P. Widespread long-term gene transfer to mouse skeletal muscles and heart. J Clin Investig. 1992;90:626–630. doi: 10.1172/JCI115902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tripathy S K, Black H B, Goldwasser E, Leiden J M. Immune responses to transgene-encoded proteins limit the stability of gene expression after injection of replication-defective adenovirus vectors. Nat Med. 1996;2:545–550. doi: 10.1038/nm0596-545. [DOI] [PubMed] [Google Scholar]
- 34.van Ginkel F W, McGhee J R, Liu C, Simecka J W, Yamamoto M, Frizzell R A, Sorscher E J, Kiyono H, Pascual D W. Adenoviral gene delivery elicits distinct pulmonary-associated T helper cell responses to the vector and to its transgene. J Immunol. 1997;159:685–693. [PubMed] [Google Scholar]
- 35.Vilquin J T, Gu’erette B, Kinoshita I, Roy B, Goulet M, Gravel C, Roy R, Tremblay J P. FK506 immunosuppression to control the immune reactions triggered by first-generation adenovirus-mediated gene transfer. Hum Gene Ther. 1995;6:1391–1401. doi: 10.1089/hum.1995.6.11-1391. [DOI] [PubMed] [Google Scholar]
- 36.Wadsworth S C, Zhou H, Smith A E, Kaplan J M. Adenovirus vector-infected cells can escape adenovirus antigen-specific cytotoxic T-lymphocyte killing in vivo. J Virol. 1997;71:5189–5196. doi: 10.1128/jvi.71.7.5189-5196.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wang Q, Greenburg G, Bunch D, Farson D, Finer M H. Persistent transgene expression in mouse liver following in vivo gene transfer with a delta E1/delta E4 adenovirus vector. Gene Ther. 1997;4:393–400. doi: 10.1038/sj.gt.3300404. [DOI] [PubMed] [Google Scholar]
- 38.Wilson C, Kay M A. Immunomodulation to enhance gene therapy. Nat Med. 1995;1:887–889. doi: 10.1038/nm0995-887. [DOI] [PubMed] [Google Scholar]
- 39.Yang Y, Ertl H C, Wilson J M. MHC class I-restricted cytotoxic T lymphocytes to viral antigens destroy hepatocytes in mice infected with E1-deleted recombinant adenoviruses. Immunity. 1994;1:433–442. doi: 10.1016/1074-7613(94)90074-4. [DOI] [PubMed] [Google Scholar]
- 40.Yang Y, Greenough K, Wilson J M. Transient immune blockade prevents formation of neutralizing antibody to recombinant adenovirus and allows repeated gene transfer to mouse liver. Gene Ther. 1996;3:412–420. [PubMed] [Google Scholar]
- 41.Yang Y, Haecker S E, Su Q, Wilson J M. Immunology of gene therapy with adenoviral vectors in mouse skeletal muscle. Hum Mol Genet. 1996;5:1703–1712. doi: 10.1093/hmg/5.11.1703. [DOI] [PubMed] [Google Scholar]
- 42.Yang Y, Jooss K U, Su Q, Ertl H C, Wilson J M. Immune responses to viral antigens versus transgene product in the elimination of recombinant adenovirus-infected hepatocytes in vivo. Gene Ther. 1996;3:137–144. [PubMed] [Google Scholar]
- 43.Yang Y, Li Q, Ertl H C, Wilson J M. Cellular and humoral immune responses to viral antigens create barriers to lung-directed gene therapy with recombinant adenoviruses. J Virol. 1995;69:2004–2015. doi: 10.1128/jvi.69.4.2004-2015.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yang Y, Nunes F A, Berencsi K, Gonczol E, Engelhardt J F, Wilson J M. Inactivation of E2a in recombinant adenoviruses improves the prospect for gene therapy in cystic fibrosis. Nat Genet. 1994;7:362–369. doi: 10.1038/ng0794-362. [DOI] [PubMed] [Google Scholar]
- 45.Yang Y, Su Q, Grewal I S, Schilz R, Flavell R A, Wilson J M. Transient subversion of CD40 ligand function diminishes immune responses to adenovirus vectors in mouse liver and lung tissues. J Virol. 1996;70:6370–6377. doi: 10.1128/jvi.70.9.6370-6377.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yang Y, Su Q, Wilson J M. Role of viral antigens in destructive cellular immune responses to adenovirus vector-transduced cells in mouse lungs. J Virol. 1996;70:7209–7212. doi: 10.1128/jvi.70.10.7209-7212.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zabner J, Freimuth P, Puga A, Fabrega A, Welsh M J. Lack of high affinity fiber receptor activity explains the resistance of ciliated airway epithelia to adenovirus infection. J Clin Investig. 1997;100:1144–1149. doi: 10.1172/JCI119625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zsengeller Z K, Wert S E, Hull W M, Hu X, Yei S, Trapnell B C, Whitsett J A. Persistence of replication-deficient adenovirus-mediated gene transfer in lungs of immune-deficient (nu/nu) mice. Hum Gene Ther. 1995;6:457–467. doi: 10.1089/hum.1995.6.4-457. [DOI] [PubMed] [Google Scholar]