Abstract
Aim: There is little information on the relationships of serum small dense low-density lipoprotein cholesterol (sdLDL-C) levels and serum triglyceride (TG) levels with cardiovascular events in patients with coronary artery disease (CAD) and type 2 diabetes mellitus (DM) who are receiving statins. The aim of this study was to evaluate the relationships of serum TG levels and sdLDL-C levels as residual risks for cardiovascular events in patients with CAD and type 2 DM who were being treated with statins.
Methods: The subjects were divided into four groups based on TG levels and sdLDL-C levels: sdLDL-C of <40.0 mg/dL and TG of <150 mg/dL, sdLDL-C of ≥ 40.0 mg/dL and TG of <150 mg/dL, sdLDL-C of <40.0 mg/dL and TG of ≥ 150 mg/dL, and sdLDL-C of ≥ 40.0 mg/dL and TG of ≥ 150 mg/dL. During a median follow-up period of 1419 days, cardiovascular events occurred in 34 patients.
Results: The incidences of cardiovascular events were significantly higher in patients with sdLDL-C of ≥ 40.0 mg/dL and TG of <150 mg/dL and in patients with sdLDL-C of ≥ 40.0 mg/dL and TG of ≥ 150 mg/dL, but not in patients with sdLDL-C of <40.0 mg/dL and TG of ≥ 150 mg/dL, than in patients with sdLDL-C of <40.0 mg/dL and TG of <150 mg/dL.
Conclusions: Under the condition of treatment with statins, patients with CAD and type 2 DM who had sdLDL-C levels of ≥ 40.0 mg/dL had a high risk for cardiovascular events even though serum TG levels were controlled at <150 mg/dL.
Keywords: Small dense low-density lipoprotein cholesterol, Triglycerides, Type 2 diabetes mellitus, Statin, Cardiovascular events
Introduction
Diabetes mellitus (DM) is an established risk factor for atherosclerotic cardiovascular disease (ASCVD) 1) . Cardiovascular events caused by ASCVD are the major cause of morbidity and mortality in patients with type 2 DM 2) . It has been shown that patients with a combination of type 2 DM and a history of cardiovascular disease have a very high risk for cardiovascular events 1) . Therefore, the American Diabetes Association (ADA) recommended treatment with high-intensity statins in patients with ASCVD and type 2 DM 2) .
It has been shown that statin treatment reduces the risk of cardiovascular events in patients with coronary artery disease (CAD) by about 20% 3 , 4) . However, residual risks for cardiovascular events remain in patients who were receiving statins. It is well known that high serum triglyceride (TG) levels are residual risks for cardiovascular events 5) . Hypertriglyceridemia is an established cardiovascular residual risk worldwide 6 - 8) . The cut-off value of serum TG for hypertriglyceridemia in guidelines is 150 mg/dL 9 - 11) since previous studies showed that the risk for CVD increased above a cut-off value of 150 mg/dL 12 , 13) . Serum TG levels are increased in most patients with type 2 DM and are predictors of cardiovascular disease 14) .
Several studies have shown that a high level of small dense low-density lipoprotein cholesterol (sdLDL-C) is also a residual risk for cardiovascular disease in patients being treated with statins 15) . sdLDL particles are defined as particles with a diameter of ≤ 25.5 nm and with a density from 1.044 to 1.063 g/mL 16) . In patients with type 2 DM, serum LDL-C levels are usually not increased, but serum sdLDL-C levels are increased as well as serum TG levels 17) . In addition, a previous study showed that LDL particle size has a significant correlation with serum TG levels 18) . sdLDL-C levels are collerated with blood pressure, blood glucose levels, and TG levels 19) .
However, there has been no study on the relationships of serum sdLDL-C levels and TG levels with cardiovascular events in patients with CAD and type 2 DM who are receiving statins.
Aim
Therefore, we evaluated the relationships of serum sdLDL-C levels and TG levels with cardiovascular events in those patients in a multicenter clinical trial.
Methods
Study Patients
Between May 1, 2010 and August 31, 2012, a total of 679 subjects who had been diagnosed with CAD and who had been under regular follow-up at any of the participating institutions for at least 6 months were eligible for enrollment in Flow-mediated Dilation-Japan (FMD-J) Study A. CAD was defined as myocardial infarction, angina pectoris with organic stenosis of at least one coronary artery confirmed by diagnostic imaging (coronary angiography, cardiac nuclear scintigraphy, or coronary computed tomography), or previous percutaneous coronary intervention. The exclusion criteria were as follows: a history of coronary bypass surgery; severe valvular heart disease; arrhythmia that requires treatment (i.e., atrial fibrillation, atrial flutter, permanent pacemaker implantation or frequent ventricular premature beats); severe chronic heart failure (New York Heart Association level of >III); malignancy; undergoing treatment with steroids, nonsteroidal anti-inflammatory drugs, or immunosuppressive drugs; serum creatinine level of >2.5 mg/dL; history of stroke, aortic disease (except peripheral artery disease), or serious liver disease; and judgment of an attending physician that an individual is ineligible for inclusion in the study.
Study Design
This study was a prospective multicenter observational cohort study conducted at 22 university hospitals and affiliated clinics in Japan to examine the usefulness of FMD assessment for management of patients with CAD with a 3-year follow-up period 20 , 21) . In a total number of 679 patients, outcome data were available for 662 patients. Of those patients, 198 patients were included in this study after excluding patients without organic coronary artery stenosis (n=32), without diabetes mellitus (n=388), without statin treatment (n=33) and without sdLDL-C information (n=11) ( Supplementary Fig.1 ) . This study was executed in accordance with the Good Clinical Practice guidelines. All subjects gave written informed consent for participation in the study. The protocol was registered in the University Hospital Medical Information Network Clinical Trials Registry (UMIN000012950).
Supplementary Fig.1.
Flow chart of the study design
Study Procedures
Blood examinations and medical examinations were conducted at the start of this study. Cardiovascular events were monitored annually during the 3-year follow-up period. The participants were managed by their attending physicians who were encouraged to treat cardiovascular risk factors including hypertension, dyslipidemia and DM.
Measurements of Blood Samples and Assessment of Cardiovascular Risk Factors
The subjects fasted the previous night and abstained from drinking alcohol, smoking and consuming caffeine and vitamins for at least 12 hours prior to the study. Each subject was kept in a supine position in a quiet, dark, airconditioned room (23℃ to 26℃) throughout the study. A 23-gauge polyethylene catheter was inserted into the left antecubital vein. Levels of serum total cholesterol, TG, high-density lipoprotein cholesterol (HDL-C), and LDL-C were enzymatically measured (JCA-BM6010, JEOL,Tokyo, Japan). Although the accuracy of the calculation was reduced in patients with diabetes 22) , sdLDL-C was measured by the direct homogenous assay that was previously described 23) . Glucose level was measured by the glucose oxidase immobilized oxygen electrode method (GA09II; A&T, Yokohama, Japan). DM was identified using the ADA criteria 24) . Hypertension was defined as systolic blood pressure of ≥ 140 mm Hg or diastolic blood pressure of ≥ 90 mm Hg, on at least three different occasions in a sitting position, or currently taking antihypertensive medication 25) . Dyslipidemia was identified using the Third Report of the National Cholesterol Education Program 26) . We defined smokers as those who were current smokers. The Framingham risk score was calculated by points of risk factors: age, total cholesterol level, HDL-C level, systolic blood pressure, and smoking status 27) .
Cardiovascular Outcomes
All cardiovascular events were reported to the Efficacy Endpoint Review Committee annually from each institution. The committee adjudicated the end points of death from cardiovascular causes, non-fatal acute coronary syndrome (ACS), non-fatal stroke, angina pectoris, and death from any cause 20 , 28) . Primary cardiovascular events were defined as death from cardiovascular disease, non-fatal ACS, angina pectoris, and non-fatal stroke. Angina pectoris was defined as coronary artery restenosis or de novo coronary artery stenosis, confirmed by diagnostic imaging (coronary angiography, cardiac nuclear scintigraphy, or coronary computed tomography). The committee, consisting of members blinded to any information with regard to vascular function, assessed the appropriateness of clinical judgement of cardiovascular events according to prespecified criteria. The committee could request physicians to provide additional clinical information on cardiovascular events if needed. Any differences in opinion under assessment were resolved by discussion, and the committee finally determined whether the cardiovascular events would be included as outcome events in the analysis. We first assessed the associations of serum sdLDL-C levels and serum TG levels with primary cardiovascular events and then we assessed the associations with first cardiovascular events in four groups according to serum sdLDL-C levels and TG levels.
Statistical Analysis
Results are presented as mean±SD for continuous variables and as percentages for categorical variables. All reported p values were two-sided, and a p value of <0.05 was considered statistically significant. Continuous variables were compared by using Student’s t-test. Univariate linear regression analyses were performed to assess the relationships among the variables. Categorical variables were compared by means of the χ2 test. Receiver-operator characteristic (ROC) curve analysis was performed to assess the sensitivity and specificity of measurements of sdLDL-C and LDL-C for predicting cardiovascular events. Time-to-event end point analysis was performed by using the Kaplan-Meier method. We categorized subjects into two groups according to the cut-off values of serum sdLDL-C levels and TG levels. Cut-off values for serum sdLDL-C were determined according to the highest Youden index from the ROC curves for predicting cardiovascular events. Cut-off values for serum TG were determined according to cut-off values in guidelines: normal (<150 mg/dL) and hypertriglyceridemia (≥ 150 mg/dL) 9 - 11) . The log-rank test was used to compare survival in the groups. We evaluated the associations between serum sdLDL-C level and TG level and primary major cardiovascular events after adjustment for age, sex, and cardiovascular risk factors by using Cox’s proportional hazard regression analysis. The data were processed using JMP pro version 16 (SAS Institute, Cary, NC).
Results
Baseline Clinical Characteristics
The baseline clinical characteristics of the 198 patients are summarized in Table 1 . The mean age of the patients was 64±8 years. Of the 198 patients, 161 patients were men and 37 were women. The mean levels of glucose and hemoglobin A1c (HbA1c) in the patients were 141±45 mg/dL and 7.2±1.0%, respectively. Mean levels of total cholesterol, TG, HDL-C, LDL-C and sdLDL-C were 166±30 mg/dL, 147±111 mg/dL, 50±14 mg/dL, 88±25 mg/dL and 32.4±14.8 mg/dL, respectively. Of the 198 patients, 187 (94.4%) had hypertension, 26 (13.4%) were current smokers, 165 (83.3%) were being treated with anti-diabetic drugs, 149 (75.3%) were being treated with oral anti-diabetic drugs, and 33 (16.7%) were receiving insulin therapy. All of the patients were on statins, 1 (0.5%) was on fibrates, 14 (7.1%) were on eicosapentaenoic acids, and 18 (9.1%) were on ezetimibe.
Table 1. Clinical Characteristics of Subjects in Groups According to sdLDL-C Levels.
| Variables | Total (n = 198) | sdLDL-C <40.0 mg/dL (n = 144) | sdLDL-C ≥ 40.0 mg/dL (n = 54) | P value |
|---|---|---|---|---|
| Age, yr | 64±8 | 65±8 | 61±8 | 0.01 |
| Gender, men/women | 161/37 | 116/28 | 45/9 | 0.66 |
| Body mass index, kg/m2 | 25.9±4.2 | 25.9±4.3 | 26.0±3.7 | 0.95 |
| Systolic blood pressure, mmHg | 133±18 | 132±18 | 135±15 | 0.28 |
| Diastolic blood pressure, mmHg | 74±10 | 73±10 | 78±10 | 0.01 |
| Heart rate, bpm | 66±12 | 65±11 | 69±12 | 0.053 |
| Total cholesterol, mg/dL | 166±30 | 158±25 | 187±30 | <0.001 |
| TG, mg/dL | 147±111 | 113±55 | 236±163 | <0.001 |
| HDL-C, mg/dL | 50±14 | 51±15 | 46±11 | 0.02 |
| LDL-C, mg/dL | 88±25 | 84±21 | 99±30 | <0.001 |
| sdLDL-C, mg/dL | 32.4±14.8 | 24.9±7.1 | 52.4±10.7 | N/A |
| Glucose, mg/dL | 141±45 | 136±38 | 155±58 | 0.01 |
| Hemoglobin A1c, % | 7.2±1.0 | 7.0±0.9 | 7.5±1.3 | 0.003 |
| Creatinine, mg/dL | 0.86±0.24 | 0.88±0.26 | 0.80±0.15 | 0.03 |
| eGFR, mL/min per 1.73 m2 | 71±18 | 69±18 | 76±17 | 0.02 |
| Current Smoking, n (%) | 26 (13.4) | 21 (14.8) | 5 (9.6) | 0.35 |
| Exercise habit, n (%) | 116 (60.4) | 86 (60.6) | 30 (60.0) | 0.94 |
| Alcohol drink, n (%) | 66 (34.2) | 46 (32.2) | 20 (40.0) | 0.31 |
| Medical history, n (%) | ||||
| Hypertension | 187 (94.4) | 137 (95.1) | 50 (92.6) | 0.49 |
| Dyslipidemia | 198 (100) | 144 (100) | 54 (100) | |
| Previous myocardial infarction | 92 (46.5) | 63 (43.8) | 29 (53.7) | 0.21 |
| Previous angina pectoris | 111 (56.1) | 83 (57.6) | 28 (51.9) | 0.47 |
| Medications, n (%) | ||||
| Antiplatelet drugs | 193 (97.5) | 140 (97.2) | 53 (98.2) | 0.71 |
| Statins | 198 (100) | 144 (100) | 54 (100) | |
| Fibrates | 1 (0.5) | 1 (0.69) | 0 (0) | 0.54 |
| Eicosapentaenoic acids | 14 (7.1) | 11 (7.6) | 3 (5.6) | 0.61 |
| Ezetimibe | 18 (9.1) | 12 (8.3) | 6 (11.1) | 0.54 |
| ACEIs/ARBs | 144 (72.7) | 105 (72.9) | 39 (72.2) | 0.92 |
| β-blockers | 89 (44.9) | 63 (44.8) | 26 (48.2) | 0.58 |
| Calcium-channel blockers | 100 (50.5) | 75 (52.1) | 25 (46.3) | 0.47 |
| Diuretics | 31 (15.7) | 25 (17.4) | 6 (11.1) | 0.28 |
| Any-antidiabetic drugs | 165 (83.3) | 124 (86.1) | 41 (75.9) | 0.09 |
| Oral antidiabetic drugs | 149 (75.3) | 111 (77.1) | 38 (70.4) | 0.33 |
| Insulin | 33 (16.7) | 27 (18.8) | 6 (11.1) | 0.20 |
| Framingham risk score, % | 16±8 | 15±8 | 20±9 | <0.001 |
sdLDL-C indicates small dense low-density lipoprotein cholesterol; TG, triglycerides; HDL-C, high-density lipoprotein cholesterol; LDL-C, low- density lipoprotein cholesterol; eGFR, estimated glomerular filtration rate; ACEIs, angiotensin-converting enzyme inhibitors; ARBs, angiotensin receptor blockers
Relationships of sdLDL-C Levels with Cardiovascular Events
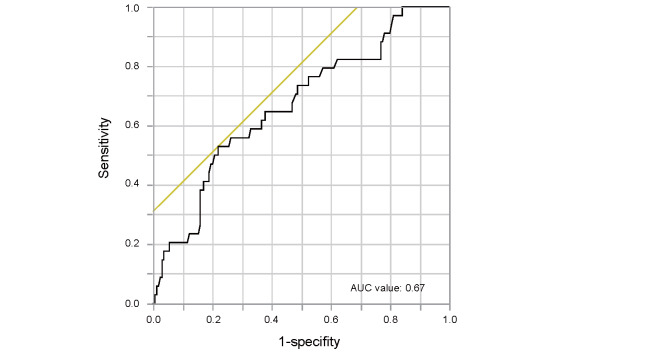
The cut-off value of sdLDL-C levels derived from ROC curve for predicting primary cardiovascular events was 40.0 mg/dL ( Supplementary Fig.2 ) . Therefore, we divided the subjects into two groups according to the cut-off sdLDL-C level of 40.0 mg/dL. Clinical characteristics of the subjects in the two groups according to serum sdLDL-C levels are summarized in Table 1 . Age, diastolic blood pressure, total cholesterol, TG, HDL-C, LDL-C, glucose, HbA1c, creatine, estimated glomerular filtration rate (eGFR) and Framingham risk score were significantly different in the two groups. There were no significant differences in other parameters between the two groups.
Supplementary Fig.2.
Receiver-operator characteristic (ROC) curve of serum small dense low-density lipoprotein cholesterol levels for predicting primary cardiovascular events
Univariate analysis revealed that serum sd-LDL-C levels were positively correlated with diastolic blood pressure, heart rate, total cholesterol, TG, LDL-C, glucose, HbA1c, eGFR, and Framingham risk score and were negatively correlated with age and HDL-C ( Supplementary Table 1 ) .
Supplementary Table 1. Univariate Analysis of Relationships of sdLDL-C with Variables.
| Variables | sdLDL-C | |
|---|---|---|
| r | P value | |
| Age, yr | -0.20 | 0.005 |
| Body mass index, kg/m2 | 0.06 | 0.42 |
| Systolic blood pressure, mmHg | 0.11 | 0.11 |
| Diastolic blood pressure, mmHg | 0.21 | 0.003 |
| Heart rate, bpm | 0.20 | 0.005 |
| Total cholesterol, mg/dL | 0.56 | <0.001 |
| TG, mg/dL | 0.61 | <0.001 |
| HDL-C, mg/dL | -0.19 | 0.01 |
| LDL-C, mg/dL | 0.36 | <0.001 |
| Glucose, mg/dL | 0.24 | <0.001 |
| Hemoglobin A1c, % | 0.32 | <0.001 |
| Creatinine, mg/dL | -0.11 | 0.13 |
| eGFR, mL/min per 1.73 m2 | 0.16 | 0.02 |
| Framingham risk score, % | 0.34 | <0.001 |
sdLDL-C indicates small dense low-density lipoprotein cholesterol; TG, triglycerides; HDL-C, high-density lipoprotein cholesterol; LDL-C, low-density lipoprotein cholesterol; eGFR, estimated glomerular filtration rate; Univariate analysis of the relations between sdLDL-C, TG levels and variables (Pearson’s correlation analysis).
During the follow-up of period, 34 patients had primary cardiovascular events, 3 died from cardiovascular disease, 3 had non-fatal stroke, 22 had angina pectoris and 6 had non-fatal ACS ( Supplementary Table 2 ) . The incidences of primary cardiovascular events, non-fatal stroke, angina pectoris, and non-fatal ACS were significantly higher in the high sdLDL-C group than in the low sdLDL-C group.
Supplementary Table 2. Clinical Outcomes of Subjects in Groups According to sdLDL-C Levels.
| Variables, n (%) | Total (n= 198) | sdLDL-C <40.0 mg/dL (n= 136) | sdLDL-C ≥ 40.0 mg/dL (n= 62) | P value |
|---|---|---|---|---|
| Primary cardiovascular events | 34 (17.2) | 16 (11.1) | 18 (33.3) | <0.001 |
| Death from cardiovascular disease | 3 (1.5) | 2 (1.4) | 1 (1.9) | 0.81 |
| Non-fatal stroke | 3 (1.5) | 0 (0) | 3 (5.6) | 0.004 |
| Angina pectoris | 22 (11.1) | 12 (8.3) | 10 (18.5) | 0.04 |
| Non-fatal acute coronary syndrome | 6 (3.0) | 2 (1.4) | 4 (7.4) | 0.03 |
sdLDL-C indicates small dense low-density lipoprotein cholesterol
Primary cardiovascular events include death from cardiovascular disease, non-fatal acute coronary syndrome, non-fatal stroke, and angina pectoris.
Kaplan-Meier analysis showed that patients with sdLDL-C above the cut-off value of 40.0 mg/dL had significantly higher incidences of primary cardiovascular events (log-rank p<0.001; Fig.1A ), non-fatal ACS (log-rank p=0.03; Fig.1B ), non-fatal stroke (log-rank p=0.005; Fig.1D ), and angina pectoris (log-rank p=0.048; Fig.1E ) than those for patients with sdLDL-C below the cut-off value. Multivariate Cox proportional hazard analysis revealed that sdLDL-C above the cut-off value of 40.0 mg/dL was a significant predictor of higher risk of primary cardiovascular events after adjustment for cardiovascular risk factors including serum TG levels (hazard ratio, 2.89: 95% confidence interval, 1.11-7.48; p=0.03) ( Supplementary Table 3 ) .
Fig.1.
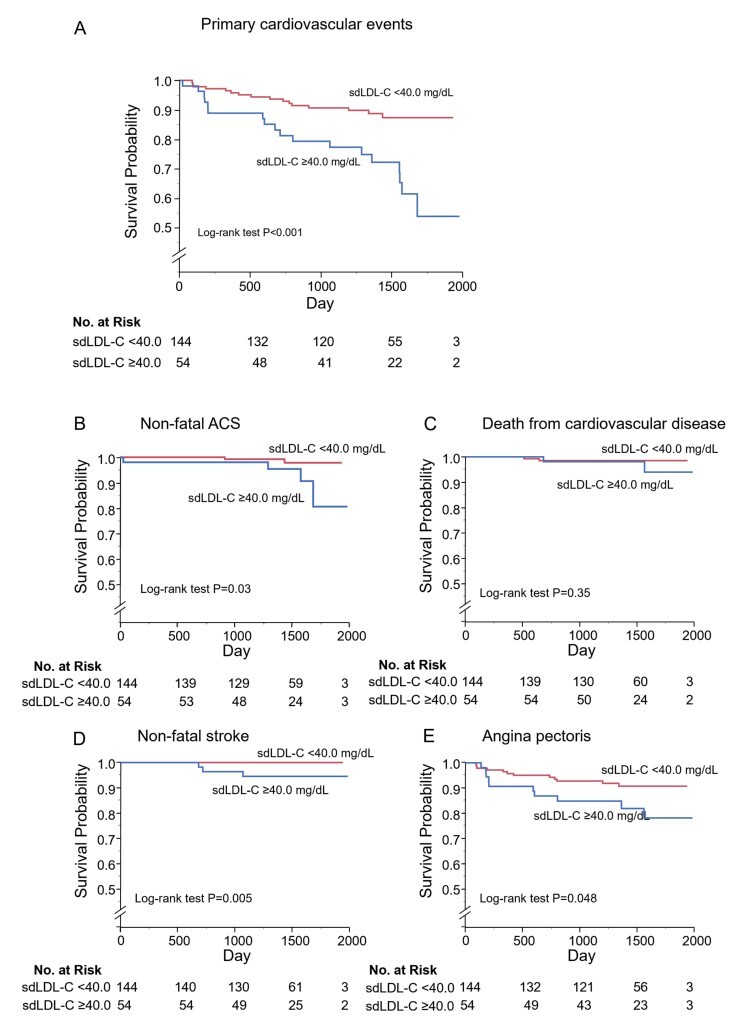
Kaplan-Meier curves of cumulative event-free survival of first major cardiovascular events (A), non-fatal acute coronary syndrome (B), death from cardiovascular disease (C), non-fatal stroke (D), and angina pectoris (E) according to serum small dense low-density lipoprotein cholesterol (sdLDL-C) levels.
Supplementary Table 3. Association of sdLDL-C Levels with Primary Cardiovascular Events During Follow-up.
|
Variable sdLDL-C, mg/dL |
Unadjusted HR (95% CI) P value |
Adjusted* HR (95% CI) P value |
Adjusted† HR (95% CI) P value |
Adjusted‡ HR (95% CI) P value |
Adjusted§ HR (95% CI) P value |
|---|---|---|---|---|---|
| <40.0 | 1 (reference) | 1 (reference) | 1 (reference) | 1 (reference) | 1 (reference) |
| ≥ 40.0 | 3.18 (1.62-6.23) | 3.25 (1.63-6.47) | 3.54 (1.77-7.09) | 3.16 (1.48-6.79) | 2.89 (1.11-7.48) |
| <0.001 | <0.001 | <0.001 | 0.003 | 0.03 |
sdLDL-C indicates small dense low-density lipoprotein cholesterol; CI, confidence interval; HR, hazard ratio.
*adjusted for age, gender.
†adjusted for age, gender, body mass index, hypertension and current smoking.
‡adjusted for age, gender, body mass index, triglyceride.
§adjusted for age, gender, body mass index, systolic blood pressure, triglyceride, low dense lipoprotein cholesterol, glucose and current smoking.
Relationships of TG Levels with Cardiovascular Events
We divided the subjects into two groups according to the cut-off value of serum TG levels of 150 mg/dL. Clinical characteristics of the subjects in the two groups according to serum TG levels are summarized in Supplementary Table 4 . Age, total cholesterol, TG, HDL-C, LDL-C, glucose, HbA1c, use of insulin, and Framingham risk score were significantly different in the two groups. There were no significant differences in other parameters between the two groups.
Supplementary Table 4. Clinical Characteristics of Subjects in Groups According to TG Levels.
| Variables | Total (n= 198) | TG <150 mg/dL (n= 127) | TG ≥ 150 mg/dL (n= 71) | P value |
|---|---|---|---|---|
| Age, yr | 64±8 | 65±8 | 62±9 | 0.01 |
| Gender, men/women | 161/37 | 99/28 | 62/9 | 0.10 |
| Body mass index, kg/m2 | 25.9±4.2 | 25.6±4.1 | 26.6±4.2 | 0.10 |
| Systolic blood pressure, mmHg | 133±18 | 134±18 | 131±16 | 0.43 |
| Diastolic blood pressure, mmHg | 74±10 | 74±11 | 75±10 | 0.75 |
| Heart rate, bpm | 66±12 | 66±11 | 67±11 | 0.88 |
| Total cholesterol, mg/dL | 166±30 | 161±28 | 175±31 | 0.002 |
| TG, mg/dL | 147±111 | 93±28 | 243±137 | N/A |
| HDL-C, mg/dL | 50±14 | 53±14 | 44±12 | <0.001 |
| LDL-C, mg/dL | 88±25 | 89±22 | 85±29 | 0.30 |
| sdLDL-C, mg/dL | 32.4±14.8 | 26.2±11.0 | 43.4±14.2 | <0.001 |
| Glucose, mg/dL | 141±45 | 134±36 | 152±56 | 0.01 |
| Hemoglobin A1c, % | 7.2±1.0 | 7.0±0.9 | 7.4±1.1 | 0.01 |
| Creatinine, mg/dL | 0.86±0.24 | 0.86±0.24 | 0.86±0.25 | 0.92 |
| eGFR, mL/min per 1.73 m2 | 71±18 | 70±19 | 72±17 | 0.41 |
| Current Smoking, n (%) | 26 (13.4) | 13 (10.3) | 13 (19.1) | 0.09 |
| Exercise habit, n (%) | 116 (60.4) | 77 (61.6) | 39 (58.2) | 0.65 |
| Alcohol drink, n (%) | 66 (34.2) | 44 (34.9) | 22 (32.8) | 0.77 |
| Medical history, n (%) | ||||
| Hypertension | 187 (94.4) | 122 (96.1) | 65 (91.6) | 0.18 |
| Previous myocardial infarction | 92 (46.5) | 56 (44.1) | 36 (50.7) | 0.37 |
| Previous angina pectoris | 111 (56.1) | 74 (58.3) | 37 (52.1) | 0.40 |
| Medications, n (%) | ||||
| Antiplatelet drugs | 193 (97.5) | 124 (97.6) | 69 (97.2) | 0.84 |
| Statins | 198 (100) | 127 (100) | 71 (100) | |
| Fibrates | 1 (0.5) | 1 (0.79) | 0 (0) | 0.45 |
| Eicosapentaenoic acids | 14 (7.1) | 10 (7.9) | 4 (5.6) | 0.56 |
| Ezetimibe | 18 (9.1) | 10 (7.9) | 8 (11.3) | 0.43 |
| ACEIs/ARBs | 144 (72.7) | 90 (70.9) | 54 (76.1) | 0.43 |
| β-blockers | 89 (44.9) | 61 (48.0) | 28 (39.4) | 0.24 |
| Calcium-channel blockers | 100 (50.5) | 68 (53.5) | 32 (45.1) | 0.25 |
| Diuretics | 31 (15.7) | 22 (17.3) | 9 (12.7) | 0.39 |
| Any-antidiabetic drugs | 165 (83.3) | 109 (85.8) | 56 (78.9) | 0.21 |
| Oral antidiabetic drugs | 149 (75.3) | 96 (75.6) | 53 (74.7) | 0.88 |
| Insulin | 33 (16.7) | 27 (21.3) | 6 (8.5) | 0.02 |
| Framingham risk score, % | 16±8 | 15±7 | 19±9 | <0.001 |
TG indicates triglycerides; HDL-C, high-density lipoprotein cholesterol; LDL-C, low-density lipoprotein cholesterol; sdLDL-C, small dense low- density lipoprotein cholesterol; eGFR, estimated glomerular filtration rate; ACEIs, angiotensin-converting enzyme inhibitors; ARBs, angiotensin receptor blockers.
Univariate analysis revealed that serum TG levels were positively correlated with body mass index, total cholesterol, sdLDL-C, glucose, HbA1c, and Framingham risk score and were negatively correlated with age and HDL-C ( Supplementary Table 5 ) .
Supplementary Table 5. Univariate Analysis of Relationships of TG Levels with Variables.
| Variables | TG | |
|---|---|---|
| r | P value | |
| Age, yr | -0.25 | <0.001 |
| Body mass index, kg/m2 | 0.15 | 0.04 |
| Systolic blood pressure, mmHg | -0.01 | 0.85 |
| Diastolic blood pressure, mmHg | 0.11 | 0.14 |
| Heart rate, bpm | 0.06 | 0.43 |
| Total cholesterol, mg/dL | 0.30 | <0.001 |
| HDL-C, mg/dL | -0.37 | <0.001 |
| LDL-C, mg/dL | -0.11 | 0.15 |
| sdLDL-C, mg/dL | 0.61 | <0.001 |
| Glucose, mg/dL | 0.26 | <0.001 |
| Hemoglobin A1c, % | 0.22 | 0.003 |
| Creatinine, mg/dL | 0.007 | 0.92 |
| eGFR, mL/min per 1.73 m2 | 0.07 | 0.31 |
| Framingham risk score, % | 0.21 | 0.004 |
TG indicates triglycerides; sdLDL-C, small dense low-density lipoprotein cholesterol; HDL-C, high-density lipoprotein cholesterol; LDL-C, low-density lipoprotein cholesterol; eGFR, estimated glomerular filtration rate; Univariate analysis of the relations between sdLDL-C, TG levels and variables (Pearson’s correlation analysis).
During the follow-up of period, the incidences of primary cardiovascular events and non-fatal ACS were significantly higher in the high TG group than in the low TG group ( Supplementary Table 6 ) .
Supplementary Table 6. Clinical Outcomes of Subjects in Groups According to TG Levels.
| Variables, n (%) | Total (n= 198) | TG <150 mg/dL (n= 127) | TG ≥150 mg/dL (n= 71) | P value |
|---|---|---|---|---|
| Primary cardiovascular events | 34 (17.2) | 15 (11.8) | 19 (26.8) | 0.008 |
| Death from cardiovascular disease | 3 (1.5) | 3 (2.4) | 0 (0) | 0.19 |
| Non-fatal stroke | 3 (1.5) | 1 (0.8) | 2 (2.8) | 0.26 |
| Angina pectoris | 22 (11.11) | 11 (8.7) | 11 (15.5) | 0.14 |
| Non-fatal acute coronary syndrome | 6 (3.0) | 0 (0) | 6 (8.5) | <0.001 |
TG indicates triglycerides.
Primary cardiovascular events include death from cardiovascular disease, non-fatal acute coronary syndrome, non-fatal stroke, and angina pectoris.
Kaplan-Meier analysis showed that patients with serum TG above the cut-off value of 150 mg/dL had significantly higher incidences of primary cardiovascular events (log-rank p=0.01; Supplementary Fig.3A ) and non-fatal ACS (log-rank p=0.001; Supplementary Fig.3B ) than those for patients with serum TG below the cut-off value. Multivariate Cox proportional hazard analysis revealed that hypertriglyceridemia was a significant predictor of a higher risk of primary cardiovascular events after adjustment of cardiovascular risk factors (hazard ratio, 2.56: 95% confidence interval, 1.29-5.13; p=0.008), but after adjustment of sdLDL-C, hypertriglyceridemia was not a significant predictor of primary cardiovascular events (hazard ratio, 1.64: 95% confidence interval, 0.73-3.68; p=0.23) ( Supplementary Table 7 ) .
Supplementary Fig. 3.
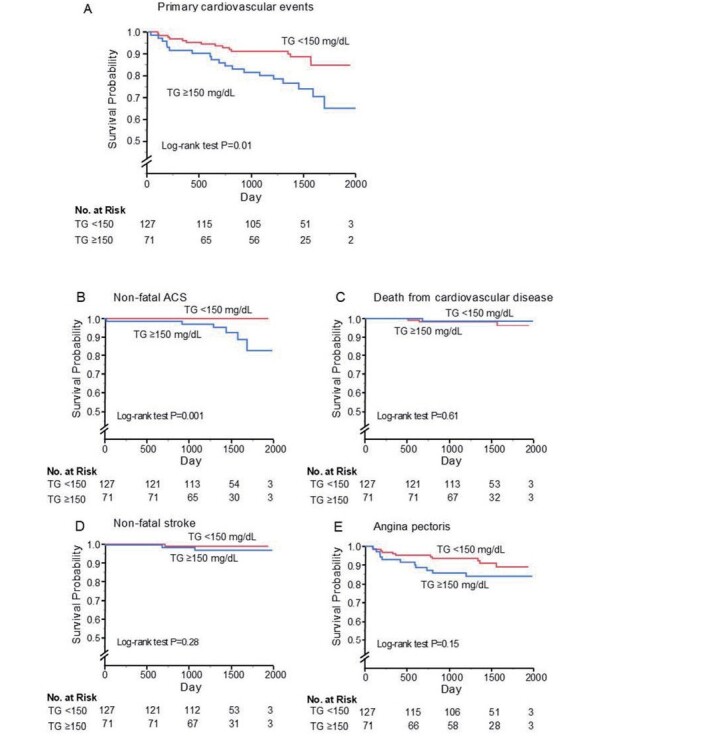
Kaplan-Meier curves of cumulative event-free survival of first major cardiovascular events (A), non-fatal acute coronary artery syndrome (B), death from cardiovascular disease (C), non-fatal stroke (D), and angina pectoris (E) according to serum triglycerides (TG) levels
Supplementary Table 7. Association of TG Levels with Primary Cardiovascular Events During Follow-up.
| Variable TG, mg/dL |
Unadjusted HR (95% CI) P value |
Adjusted* HR (95% CI) P value |
Adjusted† HR (95% CI) P value |
Adjusted‡ HR (95% CI) P value |
Adjusted§ HR (95% CI) P value |
|---|---|---|---|---|---|
| <150 | 1 (reference) | 1 (reference) | 1 (reference) | 1 (reference) | 1 (reference) |
| ≥ 150 | 2.38 (1.21-4.68) | 2.42 (1.21-4.84) | 2.56 (1.29-5.13) | 1.64 (0.73-3.68) | 1.62 (0.68-3.87) |
| 0.01 | 0.01 | 0.008 | 0.23 | 0.28 |
TG indicates triglycerides; CI, confidence interval; HR, hazard ratio.
*adjusted for age, gender.
†adjusted for age, gender, body mass index, hypertension and current smoking.
‡adjusted for age, gender, body mass index, small dense low lipoprotein cholesterol.
§adjusted for age, gender, body mass index, systolic blood pressure, small dense low lipoprotein cholesterol, low dense lipoprotein cholesterol,
glucose and current smoking.
Relationships of the Combination of Serum sdLDL-C Level and TG Level with Cardiovascular Events
Next, we divided the subjects into four groups according to the sdLDL-C cut-off value of 40.0 mg/dL and TG cut-off value of 150 mg/dL: group 1, TG of <150 mg/dL and sdLDL of <40.0 mg/dL; group 2, TG of <150 mg/dL and sdLDL of ≥ 40.0 mg/dL; group 3, TG of ≥ 150 mg/dL and sdLDL-C of <40.0 mg/dL; group 4, TG of ≥ 150 mg/dL and sdLDL-C of ≥ 40.0 mg/dL. Clinical characteristics of the subjects and clinical events are summarized in Table 2 . Age, diastolic blood pressure, total cholesterol, TG, HDL-C, LDL-C, sdLDL-C, glucose, HbA1c, and Framingham risk score were significantly different among the four groups. There were no significant differences in other parameters among the four groups.
Table 2. Clinical Characteristics of Subjects in Groups According to Cut-off Values of sdLDL-C and TG Levels.
| Variables | Total (n = 198) |
Group 1 (n = 112) |
Group 2 (n = 15) |
Group 3 (n = 32) |
Group 4 (n = 39) |
P value |
|---|---|---|---|---|---|---|
| Age, yr | 64±8 | 65±8 | 61±8 | 62±9 | 61±8 | 0.02 |
| Gender, men/women | 161/37 | 89/23 | 10/5 | 27/5 | 35/4 | 0.22 |
| Body mass index, kg/m2 | 25.9±4.2 | 25.7±4.2 | 24.7±3.0 | 26.7±4.7 | 26.5±3.9 | 0.31 |
| Systolic blood pressure, mmHg | 133±18 | 133±19 | 137±13 | 128±16 | 134±16 | 0.32 |
| Diastolic blood pressure, mmHg | 74±10 | 73±11 | 77±8 | 71±7 | 78±10 | 0.03 |
| Heart rate, bpm | 66±12 | 66±11 | 71±11 | 65±11 | 68±12 | 0.19 |
| Total cholesterol, mg/dL | 166±30 | 158±26 | 186±28 | 160±23 | 188±32 | <0.001 |
| TG, mg/dL | 147±111 | 90±27 | 119±22 | 197±44 | 281±172 | N/A |
| HDL-C, mg/dL | 50±14 | 53±14 | 54±8 | 45±14 | 43±11 | <0.001 |
| LDL-C, mg/dL | 88±25 | 86±21 | 109±23 | 75±21 | 95±32 | <0.001 |
| sdLDL-C, mg/dL | 32.4±14.8 | 23.0±6.3 | 50.1±9.4 | 31.4±5.9 | 53.2±11.1 | N/A |
| Glucose, mg/dL | 141±45 | 132±35 | 154±36 | 149±44 | 155±65 | 0.01 |
| Hemoglobin A1c, % | 7.2±1.0 | 7.0±0.8 | 7.6±1.3 | 7.3±0.9 | 7.5±1.3 | 0.007 |
| Creatinine, mg/dL | 0.86±0.24 | 0.87±0.24 | 0.77±0.20 | 0.92±0.33 | 0.81±0.13 | 0.11 |
| eGFR, mL/min per 1.73 m2 | 71±18 | 69±18 | 78±22 | 69±19 | 75±15 | 0.11 |
| Current Smoking, n (%) | 26 (13.4) | 13 (11.7) | 0 (0) | 8 (25.8) | 5 (13.5) | 0.08 |
| Exercise habit, n (%) | 116 (60.4) | 68 (61.3) | 9 (64.3) | 18 (58.1) | 21 (58.3) | 0.97 |
| Alcohol drink, n (%) | 66 (34.2) | 36 (32.1) | 8 (57.1) | 10 (32.2) | 12 (33.3) | 0.31 |
| Medical history, n (%) | ||||||
| Hypertension | 187 (94.4) | 108 (96.4) | 14 (93.3) | 29 (90.6) | 36 (92.3) | 0.55 |
| Dyslipidemia | 198 (100) | 112 (100) | 15 (100) | 32 (100) | 39 (100) | |
| Previous myocardial infarction | 92 (46.5) | 49 (43.8) | 7 (46.7) | 14 (43.8) | 22 (56.4) | 0.58 |
| Previous angina pectoris | 111 (56.1) | 65 (58.0) | 9 (60.0) | 18 (56.3) | 19 (48.7) | 0.77 |
| Medications, n (%) | ||||||
| Antiplatelet drugs | 193 (97.5) | 110 (98.2) | 14 (93.3) | 30 (93.8) | 39 (100) | 0.25 |
| Statins | 198 (100) | 112 (100) | 15 (100) | 32 (100) | 39 (100) | |
| Fibrates | 1 (0.5) | 1 (0.89) | 0 (0) | 0 (0) | 0 (0) | 0.86 |
| Eicosapentaenoic acids | 14 (7.1) | 9 (8.0) | 1 (6.7) | 2 (6.3) | 2 (5.1) | 0.94 |
| Ezetimibe | 18 (9.1) | 10 (8.9) | 0 (0) | 2 (6.3) | 6 (15.4) | 0.30 |
| ACEIs/ARBs | 144 (72.7) | 80 (71.4) | 10 (66.7) | 25 (78.1) | 29 (74.4) | 0.83 |
| β-blockers | 89 (44.9) | 53 (47.3) | 8 (53.3) | 10 (31.3) | 18 (46.2) | 0.37 |
| Calcium-channel blockers | 100 (50.5) | 60 (53.6) | 8 (53.3) | 15 (46.9) | 17 (43.6) | 0.71 |
| Diuretics | 31 (15.7) | 20 (17.9) | 2 (13.3) | 5 (15.6) | 4 (10.3) | 0.72 |
| Any-antidiabetic drugs | 165 (83.3) | 97 (86.6) | 12 (80.0) | 27 (84.4) | 29 (74.4) | 0.35 |
| Oral antidiabetic drugs | 149 (75.3) | 86 (76.8) | 10 (66.7) | 25 (78.1) | 28 (71.8) | 0.77 |
| Insulin | 33 (16.7) | 25 (22.3) | 2 (13.3) | 2 (6.3) | 4 (10.3) | 0.10 |
| Framingham risk score, % | 16±8 | 14±8 | 16±6 | 16±8 | 21±9 | <0.001 |
sdLDL-C indicates small dense low-density lipoprotein cholesterol; TG, triglycerides; HDL-C, high-density lipoprotein cholesterol; LDL-C, low- density lipoprotein cholesterol; eGFR, estimated glomerular filtration rate; ACEIs, angiotensin-converting enzyme inhibitors; ARBs, angiotensin receptor blockers; FMD, flow-mediated vasodilation.
Group 1 indicates TG of <150 mg/dL and sdLDL-C of <40.0 mg/dL; group 2, TG of <150 mg/dL and sdLDL-C of ≥ 40.0 mg/dL; group 3, TG of ≥ 150 mg/dL and sdLDL-C of <40.0 mg/dL; group 4, TG of ≥ 150 mg/dL and sdLDL-C of ≥ 40.0 mg/dL.
During the follow-up period, the incidences of primary cardiovascular events, non-fatal stroke, and non-fatal ACS were significantly higher in the high sdLDL group than in the low sdLDL group ( Supplementary Table 8 ) .
Supplementary Table 8. Associations of sdLDL-C Levels and TG Levels with Primary Cardiovascular Events During Follow-up.
| Variables, n (%) | Group 1 | Group 2 | Group 3 | Group 4 | P value |
|---|---|---|---|---|---|
| TG <150 mg/dL | TG ≥ 150 mg/dL | ||||
|
sdLDL-C <40.0 mg/dL (n= 112) |
sdLDL-C ≥ 40.0 mg/dL (n= 15) |
sdLDL-C <40.0 mg/dL (n= 32) |
sdLDL-C ≥ 40.0 mg/dL (n= 39) |
||
| Primary cardiovascular events | 10 (8.9) | 5 (33.3) | 6 (18.8) | 13 (33.3) | 0.002 |
| Death from cardiovascular disease | 2 (1.8) | 1 (6.7) | 0 (0) | 0 (0) | 0.28 |
| Non-fatal stroke | 0 (0) | 1 (6.7) | 0 (0) | 2 (5.1) | 0.04 |
| Angina pectoris | 8 (7.1) | 3 (20.0) | 4 (12.5) | 7 (18.0) | 0.18 |
| Non-fatal acute coronary syndrome | 0 (0) | 0 (0) | 2 (6.3) | 4 (10.3) | 0.01 |
TG indicates triglycerides; sdLDL-C, small dense low-density lipoprotein cholesterol.
Primary cardiovascular events include death from cardiovascular disease, non-fatal acute coronary syndrome, non-fatal stroke, and angina pectoris. Group 1 indicates TG of <150 mg/dL and sdLDL-C of <40.0 mg/dL; group 2, TG of <150 mg/dL and sdLDL-C of ≥ 40.0 mg/dL; group 3, TG of ≥ 150 mg/dL and sdLDL-C of <40.0 mg/dL; group 4, TG of ≥ 150 mg/dL and sdLDL-C of ≥ 40.0 mg/dL.
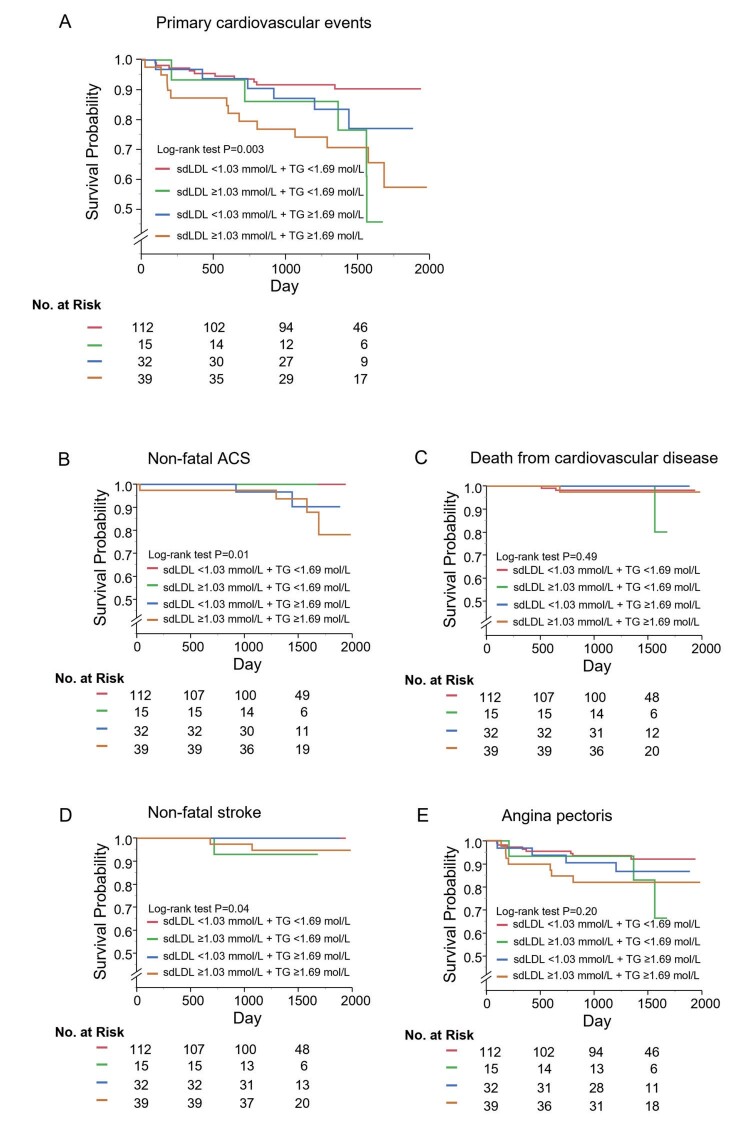
Kaplan-Meier curves for the four groups according to the cut-off values of sdLDL-C and TG showed that primary cardiovascular events (log-rank p=0.003; Fig.2A ), non-fatal ACS (log-rank p=0.01; Fig.2B ) and non-fatal stroke (log-rank p=0.04; Fig.2D ) were significantly different. Multivariate Cox proportional hazard analysis revealed that patients with a combination of sdLDL-C of ≥ 40.0 mg/dL and TG of ≥ 150 mg/dL (hazard ratio, 4.38; 95% CI, 1.78-10.8; p=0.001) and patients with a combination of sdLDL-C of ≥ 40.0 mg/dL and TG of <150 mg/dL (hazard ratio, 5.32; 95% CI, 1.62-17.4; p=0.006) had significantly higher incidences of primary cardiovascular events than did patients with a combination of sdLDL-C of <40.0 mg/dL and TG of <150 mg/dL. There was no significant difference in the incidence of primary cardiovascular events between patients with a combination of sdLDL-C of <40.0 mg/dL and TG of ≥ 150 mg/dL and patients with a combination of sdLDL-C of <40.0 mg/dL and TG of <150 mg/dL (hazard ratio, 2.36; 95% CI, 0.83-6.72; p=0.11) ( Table 3 ) .
Fig.2.
Kaplan-Meier curves of cumulative event-free survival of first major cardiovascular events (A), non-fatal acute coronary syndrome (B), death from cardiovascular disease (C), non-fatal stroke (D), and angina pectoris (E) among the four groups according to serum small dense low-density lipoprotein cholesterol (sdLDL-C) levels and triglycerides (TG) levels.
Table 3. Associations of sdLDL-C Levels and TG Levels with Primary Cardiovascular Events During Follow-up.
|
Variable sdLDL-C mg/dL |
Unadjusted HR (95% CI) P value |
Adjusted* HR (95% CI) P value |
Adjusted† HR (95% CI) P value |
Adjusted‡ HR (95% CI) P value |
|---|---|---|---|---|
| Group 1 | 1 (reference) | 1 (reference) | 1 (reference) | 1 (reference) |
| Group 2 | 4.06 (1.38-11.9) | 4.22 (1.42-12.6) | 4.72 (1.55-14.4) | 5.32 (1.62-17.4) |
| 0.01 | 0.01 | 0.006 | 0.006 | |
| Group 3 | 2.21 (0.80-6.09) | 2.30 (0.83-6.40) | 2.36 (0.84-6.58) | 2.36 (0.83-6.72) |
| 0.12 | 0.11 | 0.10 | 0.11 | |
| Group 4 | 3.97 (1.74-9.06) | 4.17 (1.79-9.70) | 4.47 (1.92-10.4) | 4.38 (1.78-10.8) |
| 0.001 | <0.001 | <0.001 | 0.001 |
sdLDL-C indicates small dense low-density lipoprotein cholesterol; TG, triglycerides; CI, confidence interval; HR, hazard ratio.
*adjusted for age, gender.
†adjusted for age, gender, body mass index, hypertension and current smoking.
‡adjusted for age, gender, body mass index, systolic blood pressure, LDL-C, glucose and current smoking.
Group 1 indicates TG of <150 mg/dL and sdLDL-C of <40.0 mg/dL; group 2, TG of <150 mg/dL and sdLDL-C of ≥ 40.0 mg/dL; group 3, TG of ≥ 150 mg/dL and sdLDL-C of <40.0 mg/dL; group 4, TG of ≥ 150 mg/dL and sdLDL-C of ≥ 40.0 mg/dL.
Relationship of Serum LDL-C Levels with Cardiovascular Events
Finally, we assessed the relationship between LDL-C levels and cardiovascular events. We divided the subjects into two groups according to the cut-off value of serum LDL-C levels of 70 mg/dL. Clinical characteristics of the subjects in the two groups according to LDL-C levels are summarized in Supplementary Table 9 . Total cholesterol, HDL-C, sdLDL-C, use of insulin, and Framingham risk score were significantly different in the two groups. There were no significant differences in other parameters between the two groups.
Supplementary Table 9. Clinical Characteristics of Subjects in Groups According to LDL-C.
| Variables | Total (n= 191) | LDL-C <70 mg/dL (n= 44) | LDL-C ≥ 70 mg/dL (n= 147) | P value |
|---|---|---|---|---|
| Age, yr | 64±8 | 64±8 | 64±9 | 0.92 |
| Gender, men/women | 161/37 | 38/6 | 116/31 | 0.27 |
| Body mass index, kg/m2 | 25.9±4.2 | 25.8±4.2 | 26.0±4.1 | 0.85 |
| Systolic blood pressure, mmHg | 133±18 | 128±18 | 134±17 | 0.06 |
| Diastolic blood pressure, mmHg | 74±10 | 73±9 | 75±10 | 0.25 |
| Heart rate, bpm | 66±12 | 68±12 | 66±11 | 0.27 |
| Total cholesterol, mg/dL | 166±30 | 139±20 | 173±27 | <0.001 |
| TG, mg/dL | 147±111 | 150±90 | 131±63 | 0.12 |
| HDL-C, mg/dL | 50±14 | 50±18 | 50±13 | 0.99 |
| LDL-C, mg/dL | 88±25 | 58±13 | 97±20 | N/A |
| sdLDL-C, mg/dL | 32.4±14.8 | 25.9±12.0 | 33.3±13.6 | 0.002 |
| Glucose, mg/dL | 141±45 | 144±61 | 137±38 | 0.38 |
| Hemoglobin A1c, % | 7.2±1.0 | 7.0±1.0 | 7.2±1.0 | 0.30 |
| Creatinine, mg/dL | 0.86±0.24 | 0.88±0.28 | 0.85±0.23 | 0.44 |
| eGFR, mL/min per 1.73 m2 | 71±18 | 70±17 | 71±18 | 0.79 |
| Current Smoking, n (%) | 26 (13.4) | 9 (20.5) | 17 (11.8) | 0.15 |
| Exercise habit, n (%) | 111 (59.7) | 29 (65.9) | 82 (57.8) | 0.33 |
| Alcohol drink, n (%) | 63 (33.7) | 14 (31.8) | 49 (34.3) | 0.76 |
| Medical history, n (%) | ||||
| Hypertension | 181 (94.8) | 40 (90.9) | 141 (95.9) | 0.19 |
| Dyslipidemia | 191 (100) | 44 (100) | 147 (100) | |
| Previous myocardial infarction | 87 (45.6) | 21 (47.7) | 66 (44.9) | 0.74 |
| Previous angina pectoris | 109 (57.1) | 23 (52.3) | 86 (58.5) | 0.46 |
| Medications, n (%) | ||||
| Antiplatelet drugs | 186 (97.4) | 43 (97.7) | 143 (97.4) | 0.87 |
| Statin | 191 (100) | 44 (100) | 147 (100) | |
| Fibrates | 1 (0.5) | 0 (0) | 1 (0.7) | 0.58 |
| Eicosapentaenoic acids | 14 (7.3) | 3 (6.8) | 11 (7.5) | 0.88 |
| Ezetimibe | 18 (9.4) | 4 (9.1) | 14 (9.5) | 0.93 |
| ACEIs/ARBs | 140 (73.3) | 32 (72.7) | 108 (73.5) | 0.92 |
| β-blockers | 84 (44.0) | 18 (40.9) | 66 (44.9) | 0.64 |
| Calcium-channel blockers | 99 (51.8) | 22 (50.0) | 77 (52.4) | 0.78 |
| Diuretics | 30 (15.7) | 6(13.6) | 24 (16.3) | 0.67 |
| Any-antidiabetic drugs | 159 (83.3) | 39 (88.7) | 120 (81.6) | 0.28 |
| Oral antidiabetic drugs | 143 (74.9) | 33 (75.0) | 110 (74.8) | 0.98 |
| Insulin | 32 (16.8) | 10 (22.7) | 22 (15.0) | 0.23 |
| Framingham risk score, % | 16±8 | 13±7 | 17±8 | 0.01 |
LDL-C indicates low density lipoprotein cholesterol; TG, triglycerides; HDL-C, high-density lipoprotein cholesterol; sdLDL-C, small dense low- density lipoprotein cholesterol; eGFR, estimated glomerular filtration rate; ACEIs, angiotensin-converting enzyme inhibitors; ARBs, angiotensin receptor blockers.
Univariate analysis revealed that LDL-C levels were positively correlated with total cholesterol, sdLDL-C and Framingham risk score, and LDL-C levels was negatively correlated with glucose ( Supplementary Table 10 ) . Clinical events on the basis of serum LDL-C levels are summarized in Supplementary Table 11 . During the follow-up period, there were no significant differences in the incidences of primary cardiovascular events between the low LDL-C group and the high LDL-C group. Kaplan-Meier analysis showed that incidences of primary cardiovascular events (log-rank p=0.57; Supplementary Fig.4A ), non-fatal ACS (log-rank p=0.80; Supplementary Fig.4B ), death from cardiovascular disease (log-rank p=0.75; Supplementary Fig. 4C ), non-fatal stroke (log-rank p=0.33; Supplementary Fig.4D ), and angina pectoris (log-rank p=0.99; Supplementary Fig.4E ) were similar in the two groups.
Supplementary Table 10. Univariate Analysis of Relationships of LDL-C Levels with Variables.
| Variables | LDL-C | |
|---|---|---|
| r | P value | |
| Age, yr | -0.07 | 0.35 |
| Body mass index, kg/m2 | 0.06 | 0.44 |
| Systolic blood pressure, mmHg | 0.15 | 0.04 |
| Diastolic blood pressure, mmHg | 0.11 | 0.14 |
| Heart rate, bpm | 0.06 | 0.39 |
| Total cholesterol, mg/dL | 0.86 | <0.001 |
| TG, mg/dL | -0.11 | 0.15 |
| HDL-C, mg/dL | 0.13 | 0.08 |
| sdLDL-C, mg/dL | 0.36 | <0.001 |
| Glucose, mg/dL | -0.19 | 0.01 |
| Hemoglobin A1c, % | 0.07 | 0.32 |
| Creatinine, mg/dL | -0.07 | 0.36 |
| eGFR, mL/min per 1.73 m2 | -0.01 | 0.89 |
| Framingham risk score, % | 0.27 | <0.001 |
LDL-C indicate low-density lipoprotein cholesterol; TG, triglycerides; HDL-C, high-density lipoprotein cholesterol; sdLDL-C, small dense low-density lipoprotein cholesterol; eGFR, estimated glomerular filtration rate; Univariate analysis of the relations between LDL-C, levels and variables (Pearson’s correlation analysis).
Supplementary Table 11. Clinical Outcomes of Subjects in Groups According to LDL-C Levels.
| Variables, n (%) | Total (n= 191) | LDL-C <70 mg/dL (n= 44) | LDL-C ≥70 mg/dL (n= 147) | P value |
|---|---|---|---|---|
| Primary cardiovascular events | 32 (16.8) | 8 (18.2) | 24 (16.3) | 0.77 |
| Death from cardiovascular disease | 4 (2.1) | 1 (2.3) | 3 (2.0) | 0.92 |
| Non-fatal stroke | 3 (1.6) | 0 (1.5) | 3 (2.0) | 0.34 |
| Angina pectoris | 18 (9.4) | 4 (9.1) | 14 (9.5) | 0.93 |
| Non-fatal acute coronary syndrome | 6 (3.1) | 1 (2.2) | 5 (3.4) | 0.71 |
LDL-C indicates low-density lipoprotein cholesterol.
Primary cardiovascular events include death from cardiovascular disease, non-fatal acute coronary syndrome, non-fatal stroke, and angina pectoris.
Supplementary Fig.4.
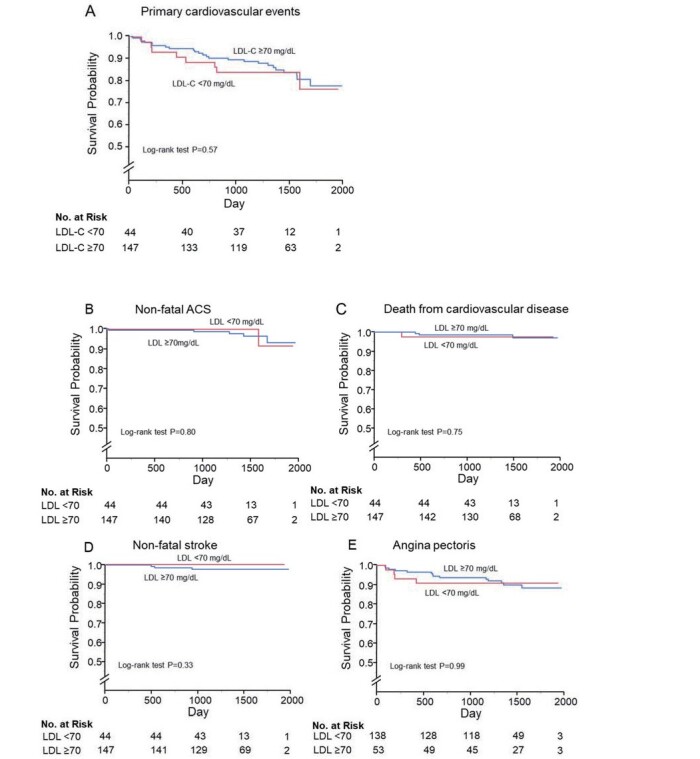
Kaplan-Meier curves of cumulative event-free survival of first major cardiovascular events (A), non-fatal acute coronary syndrome (B), death from cardiovascular disease (C), non-fatal stroke (D), and angina pectoris (E) according to serum lipoprotein cholesterol (LDL-C) levels
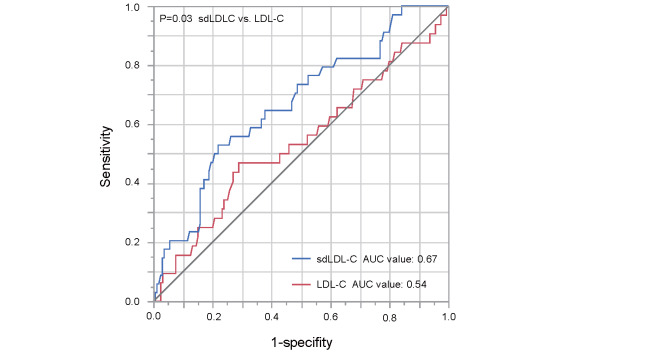
We assessed the ROC curves of serum sdLDL-C and LDL-C levels for predicting primary cardiovascular events. The area under the ROC curve value for sdLDL-C to predict primary cardiovascular events was significantly higher than that for LDL-C (0.67 vs. 0.54, p=0.03) ( Supplementary Fig.5 ) .
Supplementary Fig.5.
Receiver operating characteristic (ROC) curves of serum small dense low-density lipoprotein cholesterol (sdLDL-C) levels and serum lipoprotein cholesterol (LDL-C) levels for predicting primary cardiovascular event
Discussion
In the present study, we demonstrated that high serum sdLDL-C levels were significant predictors of cardiovascular events in patients with established CAD and type 2 DM who were receiving statins. However, serum TG levels were not significant predictors of cardiovascular events in those subjects after adjustment for cardiovascular risk factors including serum sdLDL-C. In addition, the incidence of cardiovascular events was significantly higher in patients with CAD and type 2 DM who had serum sdLDL-C of ≥ 40.0 mg/dL and TG of <150 mg/dL, but not patients with sdLDL-C of <40.0 mg/dL and TG of ≥ 150 mg/dL, than in patients with serum sdLDL-C of <40.0 mg/dL and TG of <150 mg/dL. To our knowledge, this study is the first prospective multicenter study that was carried out to evaluate the roles of sdLDL-C levels and TG levels in the prediction of cardiovascular events in patients with CAD and type 2 DM who were receiving statins.
First, to evaluate the association of serum sdLDL-C levels with cardiovascular events in patients with CAD and type 2 DM who were receiving statins, we divided the subjects into two groups of serum sdLDL-C levels. Sekimoto et al. showed that subjects after ACS with serum sdLDL-C of ≥ 20.9 mg/dL had significantly higher incidences of cardiovascular events than those for patients with sdLDL-C of <20.9 mg/dL 29) . Higashioka et al. showed that subjects without CAD with serum sdLDL-C of ≥ 35.0 mg/dL had significantly higher incidences of cardiovascular events than those for patients with sdLDL-C of <35.0 mg/dL 30) . However, the cut-off value of sdLDL-C levels has not yet been established in patients with CAD and type 2 DM. Therefore, we determined the cut off value of sdLDL-C levels by ROC curves for predicting cardiovascular events. Patients with serum sdLDL-C levels above the cut-off value of 40.0 mg/dL had a significantly higher incidence of primary cardiovascular events than that for patients with sdLDL-C levels below the cut-off value after adjustment for cardiovascular risk factors including serum TG. A previous study showed that serum sdLDL-C levels were correlated with mean intima-media thickness of the carotid artery in patients with type 2 DM 31) . Some studies have shown that increased serum sdLDL-C was significantly associated with a higher incidence of cardiovascular events. The adjusted hazard ratio (95% CI) for cardiovascular events per every 10 mg/dL increase in sdLDL-C was 1.210 (1.003-1.459) in patients with CAD 32) . Jin et al. reported that subjects with elevated serum sdLDL-C and DM had a higher incidence of cardiovascular events than that in subjects without elevated serum sdLDL-C and DM 33) . Interestingly, in the present study, serum LDL-C of ≥ 70 mg/dL was not a significantly higher risk for cardiovascular events. A high LDL-C level is an established cardiovascular risk factor and the benefit of statin treatment is also well established 34) . In the present study, all of the patients were treated with statins, and their mean serum LDL-C levels were controlled under 100 mg/dL and under 70 mg/dL in 44 patients. Serum LDL-C of ≥ 70 mg/dL might not be a significantly higher risk for cardiovascular events in patients with CAD and type 2 DM who are receiving statins. In addition, the area under the ROC curve value for sdLDL-C to predict primary cardiovascular events was significantly higher than that for LDL-C in these patients. A previous study showed that subjects after CAD with serum sdLDL >35 mg/dL had a higher incidence of cardiovascular events than that in subjects with LDL-C >100 mg/dL 35) . This results of this study are consistent with the results of that study. Previous interventional studies showed that dietary interventions 36) , physical exercise 37) , and pharmacological therapy 38) reduced sdLDL-C levels. Ishi et al. showed that high-dose statin therapy (4 mg pitavastatin) resulted in a 20% reduction in sdLDL-C levels compared to low-dose statin therapy (1 mg pitavastatin). Furthermore, in patients with sdLDL-C >34.3 mg/dL with established stable CAD, high-dose statin therapy (4 mg pitavastatin) significantly reduced the risk of major cardiovascular events (cardiovascular death, nonfatal ischemic stroke, nonfatal myocardial infarction, and unstable angina) compared to low-dose statin therapy (1 mg pitavastatin) 38) .
Finally, we divided the subjects into four groups according to the serum sdLDL-C cut-off value of 40.0 mg/dL and TG cut-off value of 150 mg/dL and assessed cardiovascular events. Interestingly, patients with serum sdLDL-C of ≥ 40.0 mg/dL and TG of <150 mg/dL had a higher incidence of cardiovascular events than did patients with serum sdLDL-C of <40.0 mg/dL and TG of <150 mg/dL. However, patients with serum sdLDL-C of <40.0 mg/dL and TG of ≥ 150 mg/dL did not have a higher incidence of cardiovascular events than that in patients with serum sdLDL-C of <40.0 mg/dL and TG of <150 mg/dL. These findings suggest that even though serum TG levels were controlled at <150 mg/dL, some subjects had serum sdLDL-C of ≥ 40.0 mg/dL and had a high risk for cardiovascular events.
This study has some limitations. First, we measured serum sdLDL-C levels only once when the subjects were enrolled. Repeated measurements of serum sdLDL-C levels and assessment of the change in serum sdLDL-C levels would enable more specific conclusions concerning the role of sdLDL-C levels in cardiovascular events to be drawn. In addition, the cut-off value of serum sdLDL-C for cardiovascular events in patients with CAD and type 2 DM has not been established. The results of the present study may be useful for determining the cut-off of value serum sdLDL-C for cardiovascular events in patients with CAD and type 2 DM. Second, it has been shown that statins decrease serum sdLDL-C levels 38 , 39) . In the present study, we included patients with statin treatment and excluded patients without statin treatment to assess the residual risk for cardiovascular events under the condition of treatment with statins. Future studies are needed to evaluate the association of sdLDL-C levels with cardiovascular evets in a general population. Third, we cannot completely exclude subjects with familial hypercholesterolemia and combined hyperlipidemia. In the present study, we did not have information on untreated LDL-C levels, presence of tendon and skin xanthomas, family history of premature CAD, FH causative genes, and dose of the statin. Such information would enable more specific conclusions concerning the roles of sdLDL-C and TG in cardiovascular events to be drawn. Finally, all of the patients were Japanese. The doses of statins and effects of statins vary in different countries. Asians are often treated with lower statin doses than those for Caucasian. Indeed, the Japanese Lipid Intervention Trial showed that statin doses were about one-fourth of those in Western countries to obtain similar levels of LDL-C in Japanese with hypercholesterolemia 40) .
Conclusions
In conclusion, patients with CAD and type 2 DM who had sdLDL-C levels of ≥ 40.0 mg/dL and were taking statins had a high risk for cardiovascular events even though serum TG levels were controlled at <150 mg/dL. We should pay attention to not only serum TG levels but also sdLDL-C levels as residual risks for cardiovascular events under the condition of treatment with statins in patients with CAD and type 2 DM.
Acknowledgements
The authors would like to thank all of the subjects who participated in this study. In addition, we thank Megumi Wakisaka, Ki-ichiro Kawano and Satoko Michiyama for their excellent secretarial assistance.
Role of Authors
Yukihito Higashi and Takayuki Yamaji drafting the article and conception of this study; Takahiro Harada, Masato Kajikwa, Tatsuya Maruhashi, Shinji Kishimoto, Farina Mohamad Yusoff, Chikara Goto, Ayumu Nakashima, Hirofumi Tomiyama, Bonpei Takase, Takahide Kohro, Toru Suzuki, Tomoko Ishizu, Shinichiro Ueda, Tsutomu Yamazaki, Tomoo Furumoto, Kazuomi Kario, Teruo Inoue, Shinji Koba, Kentaro Watanabe, Yasuhiko Takemoto, Takuzo Hano, Masataka Sata,, Yutaka Ishibashi, Koichi Node, Koji Maemura, Yusuke Ohya, Taiji Furukawa, Hiroshi Ito, and Akira Yamashina, measuring the brachial artery diameter and FMD; Yukiko Nakano, Kenichi Yoshimura, and Kazuaki Chayama, revising the article critically for important intellectual content.
Sources of Founding
Grant-in-Aid of Japanese Arteriosclerosis Prevention Fund (to Y.Higashi).
Conflict of Interest
All authors have no conflicts of interests to report.
Trial Registration Number
The protocol was registered in the University Hospital Medical Information Network Clinical Trials Registry (UMIN000012950).
References
- 1).Haffner SM, Lehto S, Rönnemaa T, Pyörälä K and Laakso M: Mortality from coronary heart disease in subjects with type 2 diabetes and in nondiabetic subjects with and without prior myocardial infarction. The New England journal of medicine, 1998; 339: 229-234 [DOI] [PubMed] [Google Scholar]
- 2).10. Cardiovascular Disease and Risk Management: Standards of Medical Care in Diabetes-2019. Diabetes Care, 2019; 42: S103-s123 [DOI] [PubMed] [Google Scholar]
- 3).Cannon CP, Braunwald E, McCabe CH, Rader DJ, Rouleau JL, Belder R, Joyal SV, Hill KA, Pfeffer MA and Skene AM: Intensive versus moderate lipid lowering with statins after acute coronary syndromes. The New England journal of medicine, 2004; 350: 1495-1504 [DOI] [PubMed] [Google Scholar]
- 4).Pitt B, Waters D, Brown WV, van Boven AJ, Schwartz L, Title LM, Eisenberg D, Shurzinske L and McCormick LS: Aggressive lipid-lowering therapy compared with angioplasty in stable coronary artery disease. Atorvastatin versus Revascularization Treatment Investigators. The New England journal of medicine, 1999; 341: 70-76 [DOI] [PubMed] [Google Scholar]
- 5).Kajikawa M, Maruhashi T, Kishimoto S, Matsui S, Hashimoto H, Takaeko Y, Yusoff FM, Kihara Y, Chayama K, Goto C, Noma K, Nakashima A, Tomiyama H, Takase B, Kohro T, Suzuki T, Ishizu T, Ueda S, Yamazaki T, Furumoto T, Kario K, Inoue T, Koba S, Watanabe K, Takemoto Y, Hano T, Sata M, Ishibashi Y, Node K, Maemura K, Ohya Y, Furukawa T, Ito H, Ikeda H, Yamashina A and Higashi Y: Target of Triglycerides as Residual Risk for Cardiovascular Events in Patients With Coronary Artery Disease - Post Hoc Analysis of the FMD-J Study A. Circulation journal : official journal of the Japanese Circulation Society, 2019; 83: 1064-1071 [DOI] [PubMed] [Google Scholar]
- 6).Miller M, Cannon CP, Murphy SA, Qin J, Ray KK and Braunwald E: Impact of triglyceride levels beyond low-density lipoprotein cholesterol after acute coronary syndrome in the PROVE IT-TIMI 22 trial. Journal of the American College of Cardiology, 2008; 51: 724-730 [DOI] [PubMed] [Google Scholar]
- 7).Hokanson JE and Austin MA: Plasma triglyceride level is a risk factor for cardiovascular disease independent of high-density lipoprotein cholesterol level: a meta-analysis of population-based prospective studies. J Cardiovasc Risk, 1996; 3: 213-219 [PubMed] [Google Scholar]
- 8).Wang J, Shen X, He S, An Y, Gong Q, Li H, Zhang B, Shuai Y, Chen Y, Hu Y and Li G: Hypertriglyceridaemia predicts subsequent long-term risk of cardiovascular events in Chinese adults: 23-year follow-up of the Daqing Diabetes Study. Diabetes Metab Res Rev, 2019; 35: e3163 [DOI] [PubMed] [Google Scholar]
- 9).Executive Summary of The Third Report of The National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, And Treatment of High Blood Cholesterol In Adults (Adult Treatment Panel III). Jama, 2001; 285: 2486-2497 [DOI] [PubMed] [Google Scholar]
- 10).Mach F, Baigent C, Catapano AL, Koskinas KC, Casula M, Badimon L, Chapman MJ, De Backer GG, Delgado V, Ference BA, Graham IM, Halliday A, Landmesser U, Mihaylova B, Pedersen TR, Riccardi G, Richter DJ, Sabatine MS, Taskinen MR, Tokgozoglu L and Wiklund O: 2019 ESC/EAS Guidelines for the management of dyslipidaemias: lipid modification to reduce cardiovascular risk. Eur Heart J, 2020; 41: 111-188 [DOI] [PubMed] [Google Scholar]
- 11).Kinoshita M, Yokote K, Arai H, Iida M, Ishigaki Y, Ishibashi S, Umemoto S, Egusa G, Ohmura H, Okamura T, Kihara S, Koba S, Saito I, Shoji T, Daida H, Tsukamoto K, Deguchi J, Dohi S, Dobashi K, Hamaguchi H, Hara M, Hiro T, Biro S, Fujioka Y, Maruyama C, Miyamoto Y, Murakami Y, Yokode M, Yoshida H, Rakugi H, Wakatsuki A and Yamashita S: Japan Atherosclerosis Society (JAS) Guidelines for Prevention of Atherosclerotic Cardiovascular Diseases 2017. J Atheroscler Thromb, 2018; 25: 846-984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12).Madsen CM, Varbo A and Nordestgaard BG: Unmet need for primary prevention in individuals with hypertriglyceridaemia not eligible for statin therapy according to European Society of Cardiology/European Atherosclerosis Society guidelines: a contemporary population-based study. Eur Heart J, 2018; 39: 610-619 [DOI] [PubMed] [Google Scholar]
- 13).Sarwar N, Sandhu MS, Ricketts SL, Butterworth AS, Di Angelantonio E, Boekholdt SM, Ouwehand W, Watkins H, Samani NJ, Saleheen D, Lawlor D, Reilly MP, Hingorani AD, Talmud PJ and Danesh J: Triglyceride-mediated pathways and coronary disease: collaborative analysis of 101 studies. Lancet (London, England), 2010; 375: 1634-1639 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14).Nordestgaard BG and Varbo A: Triglycerides and cardiovascular disease. Lancet (London, England), 2014; 384: 626-635 [DOI] [PubMed] [Google Scholar]
- 15).Hoogeveen RC, Gaubatz JW, Sun W, Dodge RC, Crosby JR, Jiang J, Couper D, Virani SS, Kathiresan S, Boerwinkle E and Ballantyne CM: Small dense low-density lipoprotein-cholesterol concentrations predict risk for coronary heart disease: the Atherosclerosis Risk In Communities (ARIC) study. Arterioscler Thromb Vasc Biol, 2014; 34: 1069-1077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16).Austin MA, King MC, Vranizan KM and Krauss RM: Atherogenic lipoprotein phenotype. A proposed genetic marker for coronary heart disease risk. Circulation, 1990; 82: 495-506 [DOI] [PubMed] [Google Scholar]
- 17).Feingold KR, Grunfeld C, Pang M, Doerrler W and Krauss RM: LDL subclass phenotypes and triglyceride metabolism in non-insulin-dependent diabetes. Arterioscler Thromb, 1992; 12: 1496-1502 [DOI] [PubMed] [Google Scholar]
- 18).Hirano T, Oi K, Sakai S, Kashiwazaki K, Adachi M and Yoshino G: High prevalence of small dense LDL in diabetic nephropathy is not directly associated with kidney damage: a possible role of postprandial lipemia. Atherosclerosis, 1998; 141: 77-85 [DOI] [PubMed] [Google Scholar]
- 19).Hirano T, Kodera R, Hirashima T, Suzuki N, Aoki E, Hosoya M, Oshima T, Hayashi T, Koba S, Ohta M, Satoh N and Ito Y: Metabolic Properties of Lowdensity Lipoprotein (LDL) Triglycerides in Patients with Type 2 Diabetes, Comparison with Small Dense LDL-Cholesterol. J Atheroscler Thromb, 2022; 29: 762-774 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20).Tomiyama H, Kohro T, Higashi Y, Takase B, Suzuki T, Ishizu T, Ueda S, Yamazaki T, Furumoto T, Kario K, Inoue T, Koba S, Watanabe K, Takemoto Y, Hano T, Sata M, Ishibashi Y, Node K, Maemura K, Ohya Y, Furukawa T, Ito H and Yamashina A: A multicenter study design to assess the clinical usefulness of semi-automatic measurement of flow-mediated vasodilatation of the brachial artery. Int Heart J, 2012; 53: 170-175 [DOI] [PubMed] [Google Scholar]
- 21).Maruhashi T, Soga J, Fujimura N, Idei N, Mikami S, Iwamoto Y, Iwamoto A, Kajikawa M, Matsumoto T, Oda N, Kishimoto S, Matsui S, Hashimoto H, Takaeko Y, Yamaji T, Harada T, Han Y, Aibara Y, Mohamad Yusoff F, Hidaka T, Kihara Y, Chayama K, Noma K, Nakashima A, Goto C, Tomiyama H, Takase B, Kohro T, Suzuki T, Ishizu T, Ueda S, Yamazaki T, Furumoto T, Kario K, Inoue T, Koba S, Watanabe K, Takemoto Y, Hano T, Sata M, Ishibashi Y, Node K, Maemura K, Ohya Y, Furukawa T, Ito H, Ikeda H, Yamashina A and Higashi Y: Increased arterial stiffness and cardiovascular risk prediction in controlled hypertensive patients with coronary artery disease: post hoc analysis of FMD-J (Flow-mediated Dilation Japan) Study A. Hypertens Res, 2020; [DOI] [PubMed] [Google Scholar]
- 22).Hirano T and Ito Y: Accuracy of Small Dense Low-density Lipoprotein-cholesterol Concentration Estimated via Sampson’s Equation in Healthy Subjects and Patients with Diabetes. J Atheroscler Thromb, 2023; 30: 979-989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23).Ito Y, Fujimura M, Ohta M and Hirano T: Development of a homogeneous assay for measurement of small dense LDL cholesterol. Clin Chem, 2011; 57: 57-65 [DOI] [PubMed] [Google Scholar]
- 24).2. Classification and Diagnosis of Diabetes. Diabetes Care, 2017; 40: S11-s24 [DOI] [PubMed] [Google Scholar]
- 25).Shimamoto K, Ando K, Fujita T, Hasebe N, Higaki J, Horiuchi M, Imai Y, Imaizumi T, Ishimitsu T, Ito M, Ito S, Itoh H, Iwao H, Kai H, Kario K, Kashihara N, Kawano Y, Kim-Mitsuyama S, Kimura G, Kohara K, Komuro I, Kumagai H, Matsuura H, Miura K, Morishita R, Naruse M, Node K, Ohya Y, Rakugi H, Saito I, Saitoh S, Shimada K, Shimosawa T, Suzuki H, Tamura K, Tanahashi N, Tsuchihashi T, Uchiyama M, Ueda S and Umemura S: The Japanese Society of Hypertension Guidelines for the Management of Hypertension (JSH 2014). Hypertens Res, 2014; 37: 253-390 [Google Scholar]
- 26).Klose G, Beil FU, Dieplinger H, von Eckardstein A, Foger B, Gouni-Berthold I, Heigl F, Koenig W, Kostner GM, Landmesser U, Laufs U, Leistikow F, Marz W, Noll G, Parhofer KG, Paulweber B, Riesen WF, Schaefer JR, Steinhagen-Thiessen E, Steinmetz A, Toplak H, Wanner C and Windler E: New AHA and ACC guidelines on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk. Wien Klin Wochenschr, 2014; 126: 169-175 [DOI] [PubMed] [Google Scholar]
- 27).Wilson PW, Castelli WP and Kannel WB: Coronary risk prediction in adults (the Framingham Heart Study). Am J Cardiol, 1987; 59: 91g-94g [DOI] [PubMed] [Google Scholar]
- 28).Maruhashi T, Soga J, Fujimura N, Idei N, Mikami S, Iwamoto Y, Iwamoto A, Kajikawa M, Matsumoto T, Oda N, Kishimoto S, Matsui S, Hashimoto H, Aibara Y, Mohamad Yusoff F, Hidaka T, Kihara Y, Chayama K, Noma K, Nakashima A, Goto C, Tomiyama H, Takase B, Kohro T, Suzuki T, Ishizu T, Ueda S, Yamazaki T, Furumoto T, Kario K, Inoue T, Koba S, Watanabe K, Takemoto Y, Hano T, Sata M, Ishibashi Y, Node K, Maemura K, Ohya Y, Furukawa T, Ito H, Ikeda H, Yamashina A and Higashi Y: Endothelial Dysfunction, Increased Arterial Stiffness, and Cardiovascular Risk Prediction in Patients With Coronary Artery Disease: FMD-J (Flow-Mediated Dilation Japan) Study A. J Am Heart Assoc, 2018; 7: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29).Sekimoto T, Koba S, Mori H, Sakai R, Arai T, Yokota Y, Sato S, Tanaka H, Masaki R, Oishi Y, Ogura K, Arai K, Nomura K, Kosaki R, Sakai K, Tsujita H, Kondo S, Tsukamoto S, Tsunoda F, Shoji M, Matsumoto H, Hamazaki Y and Shinke T: Small Dense Low-Density Lipoprotein Cholesterol: A Residual Risk for Rapid Progression of Non-Culprit Coronary Lesion in Patients with Acute Coronary Syndrome. J Atheroscler Thromb, 2021; 28: 1161-1174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30).Higashioka M, Sakata S, Honda T, Hata J, Shibata M, Yoshida D, Goto K, Kitazono T, Osawa H and Ninomiya T: The Association of Small Dense Low-Density Lipoprotein Cholesterol and Coronary Heart Disease in Subjects at High Cardiovascular Risk. J Atheroscler Thromb, 2021; 28: 79-89 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31).Inukai T, Yamamoto R, Suetsugu M, Matsumoto S, Wakabayashi S, Inukai Y, Matsutomo R, Takebayashi K and Aso Y: Small low-density lipoprotein and small low-density lipoprotein/total low-density lipoprotein are closely associated with intima-media thickness of the carotid artery in Type 2 diabetic patients. J Diabetes Complications, 2005; 19: 269-275 [DOI] [PubMed] [Google Scholar]
- 32).Sakai K, Koba S, Nakamura Y, Yokota Y, Tsunoda F, Shoji M, Itoh Y, Hamazaki Y and Kobayashi Y: Small dense low-density lipoprotein cholesterol is a promising biomarker for secondary prevention in older men with stable coronary artery disease. Geriatr Gerontol Int, 2018; 18: 965-972 [DOI] [PubMed] [Google Scholar]
- 33).Jin JL, Zhang HW, Cao YX, Liu HH, Hua Q, Li YF, Zhang Y, Wu NQ, Zhu CG, Xu RX, Gao Y, Li XL, Cui CJ, Liu G, Sun J, Dong Q, Guo YL and Li JJ: Association of small dense low-density lipoprotein with cardiovascular outcome in patients with coronary artery disease and diabetes: a prospective, observational cohort study. Cardiovasc Diabetol, 2020; 19: 45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34).Baigent C, Keech A, Kearney PM, Blackwell L, Buck G, Pollicino C, Kirby A, Sourjina T, Peto R, Collins R and Simes R: Efficacy and safety of cholesterol-lowering treatment: prospective meta-analysis of data from 90,056 participants in 14 randomised trials of statins. Lancet (London, England), 2005; 366: 1267-1278 [DOI] [PubMed] [Google Scholar]
- 35).Nishikura T, Koba S, Yokota Y, Hirano T, Tsunoda F, Shoji M, Hamazaki Y, Suzuki H, Itoh Y, Katagiri T and Kobayashi Y: Elevated small dense low-density lipoprotein cholesterol as a predictor for future cardiovascular events in patients with stable coronary artery disease. J Atheroscler Thromb, 2014; 21: 755-767 [DOI] [PubMed] [Google Scholar]
- 36).Wang L, Bordi PL, Fleming JA, Hill AM and Kris-Etherton PM: Effect of a moderate fat diet with and without avocados on lipoprotein particle number, size and subclasses in overweight and obese adults: a randomized, controlled trial. J Am Heart Assoc, 2015; 4: e001355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37).Kotani K, Tsuzaki K, Sakane N and Taniguchi N: The Correlation Between Small Dense LDL and Reactive Oxygen Metabolites in a Physical Activity Intervention in Hyperlipidemic Subjects. J Clin Med Res, 2012; 4: 161-166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38).Ishii J, Kashiwabara K, Ozaki Y, Takahashi H, Kitagawa F, Nishimura H, Ishii H, Iimuro S, Kawai H, Muramatsu T, Naruse H, Iwata H, Tanizawa-Motoyama S, Ito H, Watanabe E, Matsuyama Y, Fukumoto Y, Sakuma I, Nakagawa Y, Hibi K, Hiro T, Hokimoto S, Miyauchi K, Ohtsu H, Izawa H, Ogawa H, Daida H, Shimokawa H, Saito Y, Kimura T, Matsuzaki M and Nagai R: Small Dense Low-Density Lipoprotein Cholesterol and Cardiovascular Risk in Statin-Treated Patients with Coronary Artery Disease. J Atheroscler Thromb, 2022; 29: 1458-1474 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39).Nishikido T, Oyama J, Keida T, Ohira H and Node K: High-dose statin therapy with rosuvastatin reduces small dense LDL and MDA-LDL: The Standard versus high-dose therApy with Rosuvastatin for lipiD lowering (SARD) trial. J Cardiol, 2016; 67: 340-346 [DOI] [PubMed] [Google Scholar]
- 40).Matsuzaki M, Kita T, Mabuchi H, Matsuzawa Y, Nakaya N, Oikawa S, Saito Y, Sasaki J, Shimamoto K and Itakura H: Large scale cohort study of the relationship between serum cholesterol concentration and coronary events with low-dose simvastatin therapy in Japanese patients with hypercholesterolemia. Circulation journal: official journal of the Japanese Circulation Society, 2002; 66: 1087-1095 [DOI] [PubMed] [Google Scholar]