Summary
Background
The aim of this study was to detail incidence rates and relative risks for severe adverse perinatal outcomes by birthweight centile categories in a large Australian cohort of late preterm and term infants.
Methods
This was a retrospective cohort study of singleton infants (≥34+0 weeks gestation) between 2000 and 2018 in Queensland, Australia. Study outcomes were perinatal mortality, severe neurological morbidity, and other severe morbidity. Categorical outcomes were compared using Chi-squared tests. Continuous outcomes were compared using t-tests. Multinomial logistic regression investigated the effect of birthweight centile on study outcomes.
Findings
The final cohort comprised 991,042 infants. Perinatal mortality occurred in 1944 infants (0.19%). The incidence and risk of perinatal mortality increased as birthweight decreased, peaking for infants <1st centile (perinatal mortality rate 13.2/1000 births, adjusted Relative Risk Ratio (aRRR) of 12.96 (95% CI 10.14, 16.57) for stillbirth and aRRR 7.55 (95% CI 3.78, 15.08) for neonatal death). Severe neurological morbidity occurred in 7311 infants (0.74%), with the highest rate (19.6/1000 live births) in <1st centile cohort. There were 75,243 cases of severe morbidity (7.59% livebirths), with the peak incidence occurring in the <1st centile category (12.3% livebirths). The majority of adverse outcomes occurred in infants with birthweights between 10 and 90th centile. Almost 2 in 3 stillbirths, and approximately 3 in 4 cases of neonatal death, severe neurological morbidity or other severe morbidity occurred within this birthweight range.
Interpretation
Although the incidence and risk of perinatal mortality, severe neurological morbidity and severe morbidity increased at the extremes of birthweight centiles, the majority of these outcomes occurred in infants that were apparently “appropriately grown” (i.e., birthweight 10th–90th centile).
Funding
National Health and Medical Research Council, Mater Foundation, Royal Australian College of Obstetricians and Gynaecologists Women’s Health Foundation – Norman Beischer Clinical Research Scholarship, Cerebral Palsy Alliance, University of Queensland Research Scholarship.
Keywords: Birthweight centiles, Small for gestational age, Fetal growth restriction, Late preterm, Term, Perinatal mortality, Stillbirth, Neonatal death, Neonatal mortality, Neonatal morbidity, Asphyxia, Seizures, Acidosis, Placental dysfunction
Research in context.
Evidence before this study
Large cohort studies utilising data obtained from birth registries have demonstrated that infants born at lower birthweight centiles are at greater risk of perinatal mortality, low Apgar score, poorer childhood developmental and educational outcomes. Higher rates of placental dysfunction present at lower birthweight centiles are considered the pathophysiological basis of these findings. Many infants classified as small for gestational age at birth are subject to increased surveillance during the neonatal period and throughout childhood, but it is becoming widely acknowledged that many infants above these thresholds are also vulnerable to adverse outcomes. Furthermore, the birthweight centile threshold associated with increased risk may also vary according to the outcome of interest. We conducted a PubMed search for articles examining the risk of perinatal mortality, adverse neurological outcomes, and other adverse neonatal outcomes up to September 30th 2023. We used search terms including “birthweight”, “centile”, “stillbirth”, “neonatal death”, “hypoxic ischaemic encephalopathy”, “neonatal encephalopathy,” “asphyxia”, “low Apgar score,” “acidosis,” and “neonatal unit admission”, and found very few studies providing granularity on the incidence and risk of severe neurological and other adverse neonatal outcomes across the birthweight centile spectrum.
Added value of this study
In this Australian cohort study of almost 1 million late preterm and term births, we provide increased granularity as to the cumulative incidence and relative risk of perinatal mortality adverse perinatal outcomes across the entire spectrum of birthweight centile categories. We demonstrate a U-shaped distribution in the incidence and relative risk of perinatal mortality, severe neurological morbidity and severe neonatal morbidity across the birthweight centile categories, with no clear thresholds for increased risk. However, we also found that the majority of adverse outcomes occurred in apparently “appropriately grown” infants, with birthweights between 10th–90th centiles.
Implications of all the available evidence
These findings add to the growing body of evidence that birthweight centile thresholds inadequately identify vulnerable infants and that perinatal risk exists on a continuum. It also highlights that imperative of future research to identify biomarkers, ultrasound parameters, and other strategies to identify the “at risk” infant.
Introduction
Placental dysfunction results in poor oxygen and nutrient supply to the fetus and often leads to fetal growth restriction (FGR) and potentially, birth of a small for gestational age (SGA) infant (birthweight <10th centile for gestation).1 SGA infants are at increased risk for stillbirth, neonatal death, and severe neonatal morbidity and in the longer term, for cerebral palsy, social and cognitive problems, anxiety, depression, childhood/adult obesity, cardiovascular and metabolic disease.2, 3, 4, 5 The imperative to prenatally identify these small and vulnerable infants is a key clinical and research priority.3
Although it is convenient to use risk thresholds (such as estimated fetal weight or birthweight <10th centile) to dichotomise infants into low or high-risk populations, this approach disregards the fact that for most biological risk factors the association with adverse outcomes is a continuum.6,7 Risk thresholds tend to be arbitrary constructs, designed to maximise detection of outcomes with acceptable false positive and false negative rates. Whilst this approach is not necessarily unreasonable, it ignores the spread of adverse outcomes associated with values beyond these cut-offs. It is also potentially dangerous because individuals that fall just outside these thresholds may not receive the same level of surveillance or treatment because they are deemed unlikely to be at significant risk of an adverse outcome. This caveat is important—from a perinatal perspective not all FGR infants will be SGA at birth, thus if a weight cutoff of <10th centile is used, some vulnerable infants not meeting this criterion will be missed.8, 9, 10 Given these limitations, information regarding rates and risks of adverse outcomes across the full birthweight centile spectrum of birth is important, and may help healthcare providers individualize care and counsel women prenatally. Therefore, the aim of this study was to detail perinatal mortality (stillbirth and neonatal death), severe neurological and other severe newborn morbidity across all birthweight centile categories in a large Australian birth cohort of late preterm and term infants.
Methods
Study population
This was a retrospective cohort study of late preterm and term singleton infants (i.e., births ≥34+0 weeks gestation) between 2000 and 2018 in Queensland, Australia. We adhered to the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) guidelines.11 We utilized the Queensland Perinatal dataset12 which contains routinely collected, deidentified, maternal and perinatal data of all births in Queensland. The dataset used for analysis was provided by the Statistical Output and Reporting Unit, Statistical Services Branch of Queensland’s Department of Health. Ethical approval was granted by the Metro North Hospital and Health Service Human Research Ethics Committee (number: LNR/219/QRBC/53154). Infants with major structural abnormalities or known genetic or chromosomal abnormalities, twins and higher order multiples, and births with no recorded gestational age, missing or implausible birthweights were excluded from the study population.
Exposure
Birthweight centiles were calculated using Australian population charts13 according to infant sex, and classified into 11 categories: 1) <1st, 2) 1st to <3rd, 3) 3rd to <5th, 4) 5th to <10th, 5) 10th to <25th, 6) 25th to <75th, 7) 75th to <90th, 8) 90th to <95th, 9) 95th to <97th, 10) 97th to ≤99th, and 11) >99th. The 25th to <75th percentile category was selected as the referent group.5
Outcomes
Study outcomes were: 1) Stillbirth, 2) Neonatal Mortality, 3) Severe Neurological Morbidity, 4) Other Severe Morbidity or 5) No Adverse Perinatal Outcome. To avoid interpretative difficulties arising from an infant having multiple outcomes, all outcomes were considered mutually exclusive according to the ranking above, so that an infant could only experience one of the four study outcomes. Stillbirth incorporated both antepartum and intrapartum stillbirths. Neonatal mortality was considered as a binary outcome of death within 28 days of birth. Severe neurological morbidity was defined as neonatal encephalopathy, neonatal seizures, or intraventricular haemorrhage. Other severe morbidity was defined as a composite (corrected for overlap) of Apgar score <4 at 5 min, severe acidosis (cord pH < 7.0), need for severe resuscitation (defined as requiring intermittent positive pressure ventilation via an endotracheal tube, external cardiac massage, administration of ionotropic or chronotropic drugs, or sodium bicarbonate), admission to the neonatal intensive care unit (NICU) for >24 h, sepsis (bacterial, viral and fungal), birth trauma, brachial plexus injury, severe hypoglycemia requiring treatment, or hypothermia. ICD-10 codes relevant to neonatal outcomes are presented in Supplementary Table S1.
Confounders
Confounders of the association between birthweight centile and adverse outcomes were selected based on clinical relevance.14,15 These included maternal health and demographic variables such as age, country of birth, Indigenous status, smoking, illicit drug use, alcohol use, socio-economic status, body mass index (BMI), parity, previous stillbirth, previous caesarean section, antepartum haemorrhage, assisted conception, diabetes mellitus, and hypertension. Maternal health characteristics were determined using ICD-10 codes (Supplementary Table S1). Socio-economic status was ascertained using the socioeconomic index for areas (SEIFA) score. The SEIFA score is generated by the Australian Bureau of Statistics16 and ranks Australian geographical regions according to socioeconomic advantage based on information from the five-yearly census. Relative socioeconomic deprivation is defined as a SEIFA score in the lowest quintile.
Effect measure modifiers (interactions)
Potential effect measure modifiers of the effect of birthweight centile on the study outcomes included gestational age at birth and mode of birth.
Statistical analysis
Data were analyzed using Stata 18® (Statacorp, College Station, TX, USA). Significance was set at α = 0.05 for all analyses. The cumulative incidence of adverse perinatal outcomes was calculated by dividing the number of events that occurred by the population at risk of the event. Cumulative incidences were determined overall, and then for each birthweight centile category. Preliminary evaluation of the association between potential confounders, effect measure modifiers and components of the outcomes were performed using Fishers exact, chi-squared, t-tests, Wilcoxon rank sum tests, ANOVA or Kruskal–Wallis ANOVAs, depending on the number of outcome categories and the distribution of variables. The distribution of variables was assessed using histograms.
Missing data
Data were missing for country of birth (2/991,042), SEIFA score (8846/991,042) and BMI (370,287/991,042). Data were imputed using Multivariate Imputation by Chained Equations, with one imputation and ten iterations, with year of birth, severe adverse perinatal outcome, maternal age, Indigenous status, smoking, illicit drug use, alcohol use, parity, previous stillbirth, previous caesarean section, antepartum haemorrhage, assisted conception, diabetes mellitus and hypertension as covariates. Cross-tabulations revealed similar proportions of the study outcomes and birthweight centile categories in imputed and original data.
Multinomial logistic regression models
Multivariable multinomial logistic regression models were built to determine Relative Risk Ratios (RRR) and 95% Confidence Intervals (95% CI) for the effect of birthweight centile on adverse perinatal outcomes. Robust standard errors were used to account for clustering at the maternal level because new pregnancies in the same woman are assigned new identification numbers in the Queensland Perinatal Dataset. Models were built using forward selection of all clinically relevant confounders and stepwise backward elimination. Interaction terms were investigated for clinically relevant effect measure modifiers. Final models were selected according to parsimony and Akaike’s and Schwarz’s Bayesian information criteria. Reporting of this study adheres to the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement.11
Ethical approval
Institutional ethical approval was granted by the Metro North Hospital and Health Service Human Research Ethics Committee (Reference number: LNR/219/QRBC/53154).
Role of the funding source
None of the funding parties had any role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Results
Over the study period there were 1,105,612 births in Queensland, of which 991,042 infants were births >34+0 weeks gestation (Fig. 1). Of these, 4.9% (48,172/991,042) were born at late preterm gestations (34+0–36+6 weeks) whilst 27.1% (268,481/991,042) and 54.3% (538,592/991,042) were at early term (37+0–38+6 weeks) and term (39+0–40+6 weeks) gestations respectively. The late term (41+0–41+6 weeks) cohort comprised 13.0% (128,516/991,042) and 0.7% (7281/991,042) were post term (>42+0 weeks).
Fig. 1.
Study flow diagram—inclusion and exclusion criteria.
Table 1 presents the association between birthweight centiles and potential confounders. Rates of women who were <25 years old, nulliparous, BMI <18 kg/m2, had hypertension, antepartum haemorrhage, smokers, used alcohol and illicit drugs, born outside Australia, or reported Aboriginal or Torres Strait Islander (Indigenous) ethnicity were higher in birthweight <25th centile categories. In contrast, rates of diabetes mellitus, BMI >30 kg/m2 and previous caesarean section were higher in birthweight >50th centile categories.
Table 1.
Maternal characteristics.
| Birthweight centile category | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| <1st | 1st–<3rd | 3rd–<5th | 5–<10th | 10–<25th | 25–<50th | 50–<75th | 75–<90th | 90–<95th | 95–<97th | 97-≤99th | >99th | Total | |
| Total birthsa n (%) | 7748 (0.8%) | 15,757 (1.6%) | 16,473 (1.7%) | 43,495 (4.4%) | 136,291 (13.8%) | 240,800 (24.3%) | 258,422 (26.1%) | 158,917 (16.01%) | 55,124 (5.6%) | 22,567 (2.3%) | 21,924 (2.2%) | 13,524 (1.4%) | 991,042 (100.0%) |
| Maternal age (y) | |||||||||||||
| <25 | 2364 (30.5%) | 4639 (29.4%) | 4616 (28.0%) | 11,562 (26.6%) | 33,897 (24.9%) | 55,117 (22.9%) | 54,219 (21.0%) | 31,340 (19.7%) | 10,244 (18.6%) | 4150 (18.4%) | 3822 (17.4%) | 2263 (16.7%) | 218,233 (22.0%) |
| 25–34 | 4016 (51.8%) | 8322 (52.8%) | 9085 (55.2%) | 24,450 (56.2%) | 78,435 (57.5%) | 142,512 (59.2%) | 156,101 (60.4%) | 96,860 (61.0%) | 33,842 (61.4%) | 13,830 (61.3%) | 13,575 (61.9%) | 8301 (61.4%) | 589,329 (59.5%) |
| 35–44 | 1358 (17.5%) | 2780 (17.6%) | 2754 (16.7%) | 7410 (17.0%) | 23,753 (17.4%) | 42,830 (17.8%) | 47,733 (18.5%) | 30,482 (19.2%) | 10,969 (19.9%) | 4555 (20.2%) | 4495 (20.5%) | 2937 (21.7%) | 182,056 (18.4%) |
| >45 | 10 (0.1%) | 16 (0.1%) | 18 (0.1%) | 73 (0.2%) | 206 (0.2%) | 341 (0.1%) | 369 (0.1%) | 235 (0.1%) | 69 (0.1%) | 32 (0.1%) | 32 (0.1%) | 23 (0.2%) | 1424 (0.1%) |
| Born in Australia | 5980 (77.2%) | 11,918 (75.6%) | 12,286 (74.6%) | 32,560 (74.9%) | 103,084 (75.6%) | 186,691 (77.5%) | 205,731 (79.6%) | 129,346 (81.4%) | 45,275 (82.1%) | 18,543 (82.2%) | 18,156 (82.8%) | 11,131 (82.3%) | 780,701 (78.8%) |
| Aboriginal and Torres Strait Islander | 1114 (14.4%) | 1898 (12.0%) | 1781 (10.8%) | 4182 (9.6%) | 11,014 (8.1%) | 16,038 (6.7%) | 14,700 (5.7%) | 8409 (5.3%) | 2799 (5.1%) | 1142 (5.1%) | 1185 (5.4%) | 962 (7.1%) | 65,224 (6.6%) |
| Smoking | 2200 (28.4%) | 4018 (25.5%) | 3904 (23.7%) | 8844 (20.3%) | 22,466 (16.5%) | 30,728 (12.8%) | 25,806 (10.0%) | 13,041 (8.2%) | 4044 (7.3%) | 1553 (6.9%) | 1448 (6.6%) | 960 (7.1%) | 119,012 (12.0%) |
| Drug use | 199 (2.6%) | 388 (2.5%) | 294 (1.8%) | 662 (1.5%) | 1484 (1.1%) | 1639 (0.7%) | 1240 (0.5%) | 537 (0.3%) | 155 (0.3%) | 46 (0.2%) | 50 (0.2%) | 31 (0.2%) | 6725 (0.7%) |
| Alcohol use | 74 (1.0%) | 95 (0.6%) | 72 (0.4%) | 144 (0.3%) | 367 (0.3%) | 454 (0.2%) | 366 (0.1%) | 158 (0.1%) | 50 (0.1%) | 23 (0.1%) | 19 (0.1%) | 16 (0.1%) | 1838 (0.2%) |
| Lowest SEIFA Quintile | 2116 (27.6%) | 4015 (25.7%) | 4092 (25.1%) | 10,288 (23.9%) | 29,739 (22.0%) | 48,900 (20.5%) | 50,091 (19.6%) | 30,318 (19.2%) | 10,626 (19.4%) | 4436 (19.8%) | 4454 (20.5%) | 2980 (22.2%) | 202,055 (20.6%) |
| Maternal BMI (kg/m2) | |||||||||||||
| ≤18 | 621 (14.9%) | 1383 (15.0%) | 1457 (14.6%) | 3396 (12.7%) | 9265 (11.0%) | 12,984 (8.6%) | 10,370 (6.3%) | 4671 (4.6%) | 1213 (3.5%) | 409 (2.9%) | 333 (2.4%) | 133 (1.6%) | 46,235 (7.4%) |
| 19–24 | 2090 (50.1%) | 4830 (52.4%) | 5154 (51.7%) | 14,254 (53.5%) | 45,019 (53.4%) | 78,176 (52.0%) | 80,525 (49.3%) | 45,173 (44.7%) | 14,263 (40.6%) | 5335 (37.5%) | 4701 (33.9%) | 2351 (27.8%) | 301,871 (48.6%) |
| 25–29 | 833 (20.0%) | 1839 (19.9%) | 1981 (19.9%) | 5416 (20.3%) | 17,651 (21.0%) | 34,262 (22.8%) | 40,422 (24.7%) | 26,908 (26.6%) | 9697 (27.6%) | 3957 (27.8%) | 3959 (28.6%) | 2314 (27.4%) | 149,239 (24.0%) |
| 30–34 | 381 (9.1%) | 733 (7.9%) | 845 (8.5%) | 2248 (8.4%) | 7688 (9.1%) | 15,577 (10.4%) | 19,413 (11.9%) | 14,116 (14.0%) | 5627 (16.0%) | 2484 (17.5%) | 2604 (18.8%) | 1717 (20.3%) | 73,433 (11.8%) |
| 35–39 | 182 (4.4%) | 286 (3.1%) | 328 (3.3%) | 885 (3.3%) | 3076 (3.7%) | 6194 (4.1%) | 8147 (5.0%) | 6292 (6.2%) | 2598 (7.4%) | 1186 (8.3%) | 1326 (9.6%) | 1083 (12.8%) | 31,583 (5.1%) |
| >40 | 67 (1.6%) | 152 (1.6%) | 195 (2.0%) | 456 (1.7%) | 1545 (1.8%) | 3237 (2.2%) | 4554 (2.8%) | 3845 (3.8%) | 1706 (4.9%) | 850 (6.0%) | 940 (6.8%) | 847 (10.0%) | 18,394 (3.0%) |
| Nulliparous | 4080 (52.7%) | 8314 (52.8%) | 8563 (52.0%) | 21,954 (50.5%) | 64,947 (47.7%) | 105,179 (43.7%) | 99,598 (38.5%) | 54,359 (34.2%) | 17,075 (31.0%) | 6455 (28.6%) | 5909 (27.0%) | 3266 (24.1%) | 399,699 (40.3%) |
| Previous stillbirth | 129 (1.7%) | 244 (1.5%) | 228 (1.4%) | 614 (1.4%) | 1875 (1.4%) | 3230 (1.3%) | 3565 (1.4%) | 2276 (1.4%) | 794 (1.4%) | 330 (1.5%) | 319 (1.5%) | 223 (1.6%) | 13,827 (1.4%) |
| Previous caesarean section | 878 (11.3%) | 1777 (11.3%) | 1857 (11.3%) | 5301 (12.2%) | 18,366 (13.5%) | 36,017 (15.0%) | 43,998 (17.0%) | 29,770 (18.7%) | 11,034 (20.0%) | 4782 (21.2%) | 4950 (22.6%) | 3607 (26.7%) | 162,337 (16.4%) |
| Antepartum haemorrhage | 226 (2.9%) | 474 (3.0%) | 506 (3.1%) | 1212 (2.8%) | 3810 (2.8%) | 6467 (2.7%) | 6505 (2.5%) | 3679 (2.3%) | 1091 (2.0%) | 407 (1.8%) | 392 (1.8%) | 221 (1.6%) | 24,990 (2.5%) |
| Assisted conception | 219 (2.8%) | 545 (3.5%) | 547 (3.3%) | 1633 (3.8%) | 5189 (3.8%) | 9278 (3.9%) | 10,089 (3.9%) | 6217 (3.9%) | 2043 (3.7%) | 808 (3.6%) | 772 (3.5%) | 446 (3.3%) | 37,786 (3.8%) |
| Diabetes mellitusb | 411 (5.3%) | 901 (5.7%) | 1073 (6.5%) | 2811 (6.5%) | 9142 (6.7%) | 16,428 (6.8%) | 18,844 (7.3%) | 13,115 (8.3%) | 5182 (9.4%) | 2394 (10.6%) | 2662 (12.1%) | 2709 (20.0%) | 75,672 (7.6%) |
| Hypertensionc | 782 (10.1%) | 1413 (9.0%) | 1384 (8.4%) | 3266 (7.5%) | 8873 (6.5%) | 14,221 (5.9%) | 15,098 (5.8%) | 9754 (6.1%) | 3713 (6.7%) | 1537 (6.8%) | 1657 (7.6%) | 1278 (9.4%) | 62,976 (6.4%) |
Total births = stillbirths + livebirths.
Diabetes includes: gestational diabetes, Type 1 and Type 2 diabetes mellitus.
Hypertension includes: essential hypertension, gestational hypertension and preeclampsia.
Table 2 details the relationship between birthweight centiles and intrapartum outcomes. Spontaneous vaginal birth rates decreased as birthweight centile increased and were lowest for infants with a birthweight >99th centile. Elective caesarean section rates increased as birthweight centile increased and was highest for infants with birthweight >99th centile. This cohort also had the lowest rate of instrumental vaginal birth. Emergency caesarean section rates were highest in the <1st and >99th birthweight centile cohorts. Rates of operative birth (caesarean section or instrumental birth) for non-reassuring fetal status were inversely related to birthweight whilst operative birth for failure to progress was associated with increasing birthweight. Cord prolapse, intrapartum haemorrhage and non-reassuring fetal status rates were highest in the <1st centile category.
Table 2.
Pregnancy outcomes.
| Birthweight centile category | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| <1st | 1st–3rd | 3rd–5th | 5–10th | 10–25th | 25–50th | 50–75th | 75–90th | 90–95th | 95–97th | 97–99th | >99th | Total | |
| Total birthsa n (%) | 7748 (0.8%) | 15,757 (1.6%) | 16,473 (1.7%) | 43,495 (4.4%) | 136,291 (13.8%) | 240,800 (24.3%) | 258,422 (26.1%) | 158,917 (16.01%) | 55,124 (5.6%) | 22,567 (2.3%) | 21,924 (2.2%) | 13,524 (1.4%) | 991,042 (100.0%) |
| Late preterm (34 + 0–36 + 6w) n (%) | 387 (5.0%) | 768 (4.9%) | 779 (4.7%) | 2137 (4.9%) | 6542 (4.8%) | 11,313 (4.7%) | 12,471 (4.8%) | 7862 (4.9%) | 2748 (5.0%) | 1119 (5.0%) | 1051 (4.8%) | 995 (7.4%) | 48,172 (4.9%) |
| Early term (37 + 0–38 + 6w) n (%) | 1956 (25.2%) | 4431 (28.1%) | 4510 (27.4%) | 11,982 (27.5%) | 36,574 (26.8%) | 64,309 (26.7%) | 69,758 (27.0%) | 43,545 (27.4%) | 15,115 (27.4%) | 6184 (27.4%) | 6056 (27.6%) | 4061 (30.0%) | 268,481 (27.1%) |
| Term (39 + 0–40 + 6w) n (%) | 4264 (55.0%) | 8317 (52.8%) | 8834 (53.6%) | 23,320 (53.6%) | 74,353 (54.6%) | 131,678 (54.7%) | 141,233 (54.7%) | 860,70 (54.2%) | 29,682 (53.8%) | 12,298 (54.5%) | 11,734 (53.5%) | 6809 (50.3%) | 538,592 (54.3%) |
| Late term (>41 + 0–41 + 6) n (%) | 1085 (14.0%) | 2107 (13.4%) | 2229 (13.5%) | 5734 (13.2%) | 17,845 (13.1%) | 31,726 (13.2%) | 33,087 (12.8%) | 20,259 (12.7%) | 7156 (13.0%) | 2790 (12.4%) | 2924 (13.3%) | 1574 (11.6%) | 128,516 (13.0%) |
| Post term (>42w) n (%) | 56 (0.7%) | 134 (0.9%) | 121 (0.7%) | 322 (0.7%) | 977 (0.7%) | 1774 (0.7%) | 1873 (0.7%) | 1181 (0.7%) | 423 (0.8%) | 176 (0.8%) | 159 (0.7%) | 85 (0.6%) | 7281 (0.7%) |
| SVB n (%) | 4396 (56.7%) | 9622 (61.1%) | 10,197 (61.9%) | 27,057 (62.2%) | 84,267 (61.8%) | 147,003 (61.0%) | 153,860 (59.5%) | 91,270 (57.4%) | 30,582 (55.5%) | 12,038 (53.3%) | 11,308 (51.6%) | 5865 (43.4%) | 587,465 (59.3%) |
| IVB n (%) | 683 (8.8%) | 1585 (10.1%) | 1768 (10.7%) | 4692 (10.8%) | 14,295 (10.5%) | 24,296 (10.1%) | 24,092 (9.3%) | 13,570 (8.5%) | 4409 (8.0%) | 1656 (7.3%) | 1453 (6.6%) | 717 (5.3%) | 93,216 (9.4%) |
| ElCS n (%) | 1296 (17.7%) | 2365 (13.9%) | 2462 (12.4%) | 6694 (11.6%) | 22,680 (11.0%) | 43,111 (11.0%) | 50,816 (11.5%) | 34,159 (12.5%) | 12,618 (13.6%) | 5526 (14.8%) | 5767 (15.5%) | 4559 (17.6%) | 192,053 (11.9%) |
| EmCS n (%) | 1373 (17.7%) | 2184 (13.9%) | 2046 (12.4%) | 5051 (11.6%) | 15,049 (11.0%) | 26,388 (11.0%) | 29,653 (11.5%) | 19,916 (12.5%) | 7515 (13.6%) | 3347 (14.8%) | 3396 (15.5%) | 2383 (17.6%) | 118,301 (11.9%) |
| IVB n (%) | 683 (8.8%) | 1585 (10.1%) | 1768 (10.7%) | 4692 (10.8%) | 14,295 (10.5%) | 24,296 (10.1%) | 24,092 (9.3%) | 13,570 (8.5%) | 4409 (8.0%) | 1656 (7.3%) | 1453 (6.6%) | 717 (5.3%) | 93,216 (9.4%) |
| IVB for NRFS n (% of IVB) | 475 (69.5%) | 1057 (66.7%) | 1074 (60.7%) | 2706 (57.7%) | 7171 (50.2%) | 10,298 (42.4%) | 8765 (36.4%) | 4129 (30.4%) | 1234 (28.0%) | 418 (25.2%) | 354 (24.4%) | 142 (19.8%) | 37,823 (40.6%) |
| IVB for FTP n (% of IVB) | 130 (19.0%) | 329 (20.8%) | 468 (26.5%) | 1325 (28.2%) | 4809 (33.6%) | 9673 (39.8%) | 10,748 (44.6%) | 6733 (49.6%) | 2279 (51.7%) | 883 (53.3%) | 787 (54.2%) | 411 (57.3%) | 38,575 (41.4%) |
| IVB other n (% of IVB) | 78 (11.4%) | 199 (12.6%) | 226 (12.8%) | 661 (14.1%) | 2315 (16.2%) | 4325 (17.8%) | 4579 (19.0%) | 2708 (20.0%) | 896 (20.3%) | 355 (21.4%) | 312 (21.5%) | 164 (22.9%) | 16,818 (18.0%) |
| EmCS n (%) | 1373 (17.7%) | 2184 (13.9%) | 2046 (12.4%) | 5051 (11.6%) | 15,049 (11.0%) | 26,388 (11.0%) | 29,653 (11.5%) | 19,916 (12.5%) | 7515 (13.6%) | 3347 (14.8%) | 3396 (15.5%) | 2383 (17.6%) | 118,301 (11.9%) |
| EmCS for NRFS N (% of EmCS) | 1099 (80.0%) | 1443 (66.1%) | 1188 (58.1%) | 2635 (52.2%) | 6244 (41.5%) | 8492 (32.2%) | 7507 (25.3%) | 4202 (21.1%) | 1400 (18.6%) | 588 (17.6%) | 561 (16.5%) | 405 (17.0%) | 35,764 (30.2%) |
| EmCS for FTP N (% of EmCS) | 168 (12.2%) | 390 (17.9%) | 435 (21.3%) | 1263 (25.0%) | 4686 (31.1%) | 9913 (37.6%) | 12,978 (43.8%) | 9573 (48.1%) | 3771 (50.2%) | 1706 (51.0%) | 1812 (53.4%) | 1185 (49.7%) | 47,880 (40.5%) |
| EmCS for Other N (% of Em CS) | 106 (7.8%) | 351 (16.1%) | 423 (20.7%) | 1153 (22.8%) | 4119 (27.4%) | 7983 (30.3%) | 9168 (30.9%) | 6141 (30.8%) | 2344 (31.2%) | 1053 (31.5%) | 1023 (30.1%) | 793 (33.3%) | 34,657 (29.3%) |
| NRFS n (%) | 3091 (39.9%) | 5095 (32.3%) | 4761 (28.9%) | 11,603 (26.7%) | 31,207 (22.9%) | 48,493 (20.1%) | 46,946 (18.2%) | 26,998 (17.0%) | 9063 (16.4%) | 3868 (17.1%) | 3657 (16.7%) | 2244 (16.6%) | 197,026 (19.9%) |
| Cord Prolapse n (%) | 29 (0.4%) | 45 (0.3%) | 40 (0.2%) | 94 (0.2%) | 240 (0.2%) | 365 (0.2%) | 342 (0.1%) | 237 (0.1%) | 100 (0.2%) | 35 (0.2%) | 41 (0.2%) | 34 (0.3%) | 1602 (0.2%) |
| Intrapartum Haemorrhage n (%) | 21 (0.3%) | 35 (0.2%) | 34 (0.2%) | 137 (0.3%) | 348 (0.3%) | 531 (0.2%) | 515 (0.2%) | 309 (0.2%) | 94 (0.2%) | 41 (0.2%) | 36 (0.2%) | 22 (0.2%) | 2123 (0.2%) |
| Uterine Rupture n (%) | 5 (0.06%) | 3 (0.02%) | 2 (0.01%) | 13 (0.03%) | 46 (0.03%) | 79 (0.03%) | 77 (0.03%) | 49 (0.03%) | 17 (0.03%) | 6 (0.03%) | 7 (0.03%) | 1 (0.01%) | 305 (0.03%) |
| Shoulder Dystocia n (%) | 5 (0.1%) | 7 (0.0%) | 10 (0.1%) | 37 (0.1%) | 217 (0.2%) | 821 (0.3%) | 1869 (0.7%) | 2469 (1.6%) | 1459 (2.6%) | 835 (3.7%) | 1108 (5.1%) | 1057 (7.8%) | 9894 (1.0%) |
SVB: Spontaneous vaginal birth; IVB: Instrumental vaginal birth; FAB: Forceps assisted birth; VAB: Vacuum assisted birth; ElCS: Elective Caesarean Section, EmCS: Emergency Caesarean Section; NRFS: Non-Reassuring Fetal Status; FTP: Failure to progress.
Total births = stillbirths + livebirths.
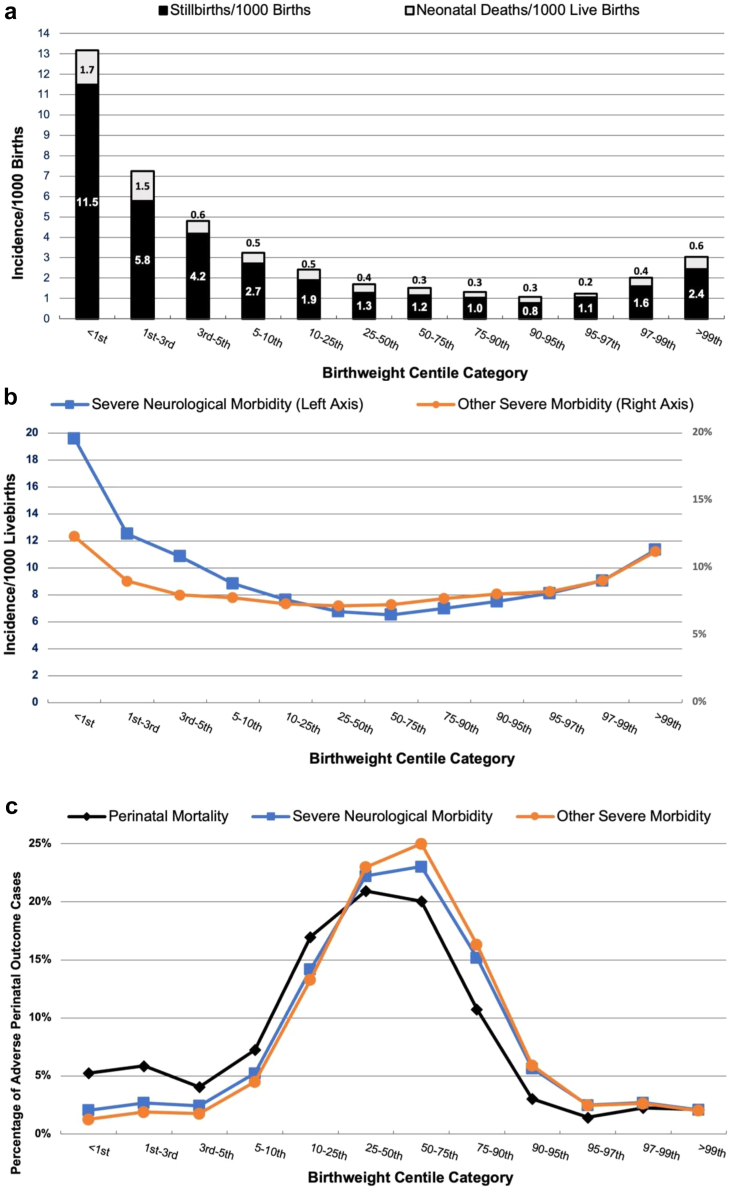
Table 3 and Fig. 2 a-c details the cumulative incidence of perinatal mortality, severe neurological and severe non-neurological morbidity. Perinatal deaths occurred in 1944 infants (0.19%)—1535 (0.15%) were stillbirths and 409 (0.04%) were neonatal deaths. Stillbirth and neonatal death rates increased when birthweight was <50th centile and were highest when birthweight was <3rd centile. Infants with birthweight <1st centile had almost double the stillbirth rate compared to those with birthweights in the 1st–3rd centile category (11.5 vs. 5.8/1000 births). There was a “U” shaped distribution of both stillbirth and neonatal deaths with higher rates at both extremes of birthweight (<1st centile and >99th centile).
Table 3.
Perinatal mortality, severe neurological and other severe morbidity by birthweight centiles.
| Birthweight centile category | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| <1st | 1st–<3rd | 3rd–<5th | 5–<10th | 10–<25th | 25–<50th | 50–<75th | 75–<90th | 90–<95th | 95–<97th | 97–99th | >99th | Total | |
| Perinatal mortality n | 102 | 114 | 79 | 141 | 330 | 407 | 390 | 209 | 59 | 28 | 44 | 41 | 1944 |
| % BWC categorya | 5.3% | 5.9% | 4.1% | 7.3% | 17.0% | 21.0% | 20.1% | 10.8% | 3.0% | 1.4% | 2.3% | 2.1% | 100% |
| n/1000 total births | 13.2 | 7.2 | 4.8 | 3.2 | 2.4 | 1.7 | 1.5 | 1.3 | 1.1 | 1.2 | 2.0 | 3.0 | 2.0 |
| Stillbirths n | 89 | 91 | 69 | 118 | 258 | 308 | 301 | 166 | 43 | 24 | 35 | 33 | 1535 |
| % BWC categorya | 5.8% | 5.9% | 4.5% | 7.7% | 16.8% | 20.1% | 19.6% | 10.8% | 2.8% | 1.6% | 2.3% | 2.1% | 100.0% |
| (n/1000 births) | 11.5 | 5.8 | 4.2 | 2.7 | 1.9 | 1.3 | 1.2 | 1 | 0.8 | 1.1 | 1.6 | 2.4 | 1.5 |
| Neonatal deaths n | 13 | 23 | 10 | 23 | 72 | 99 | 89 | 43 | 16 | 4 | 9 | 8 | 409 |
| % BWC categorya | 3.2% | 5.6% | 2.4% | 5.6% | 17.6% | 24.2% | 21.8% | 10.5% | 3.9% | 1.0% | 2.2% | 2.0% | 100.0% |
| (n/1000 live births) | 1.7 | 1.5 | 0.6 | 0.5 | 0.5 | 0.4 | 0.3 | 0.3 | 0.3 | 0.2 | 0.4 | 0.6 | 0.4 |
| Severe neurologicalmorbidityn | 150 | 196 | 178 | 383 | 1037 | 1625 | 1684 | 1111 | 413 | 183 | 198 | 153 | 7311 |
| % BWC categorya | 2.1% | 2.7% | 2.4% | 5.2% | 14.2% | 22.2% | 23.0% | 15.2% | 5.6% | 2.5% | 2.7% | 2.1% | 100.0% |
| (n/1000 live births) | 19.6 | 12.5 | 10.9 | 8.8 | 7.6 | 6.8 | 6.5 | 7.0 | 7.5 | 8.1 | 9.0 | 11.3 | 7.4 |
| Other severe morbidityn | 954 | 1420 | 1315 | 3381 | 10,006 | 17,287 | 18,806 | 12,273 | 4438 | 1861 | 1985 | 1517 | 75,243 |
| % BWC categorya | 1.3% | 1.9% | 1.7% | 4.5% | 13.3% | 23.0% | 25.0% | 16.3% | 5.9% | 2.5% | 2.6% | 2.0% | 100.0% |
| % Live births | 12.3% | 9.0% | 8.0% | 7.8% | 7.3% | 7.2% | 7.3% | 7.7% | 8.1% | 8.2% | 9.1% | 11.2% | 7.6% |
BWC: Birthweight centile.
Stillbirth includes both antepartum and intrapartum stillbirths. Severe Neurological Morbidity was defined as neonatal encephalopathy, neonatal seizures, or intraventricular haemorrhage. Other Severe Morbidity is a composite (corrected for overlap) of Apgar score <4 at 5 min, severe acidosis (cord pH < 7.0), need for severe resuscitation, admission to the neonatal intensive care unit, sepsis, birth trauma, brachial plexus injury, severe hypoglycemia requiring treatment, or hypothermia.
% BWC category—refers to the percentage of cases for each outcome in the respective BWC category.
Fig. 2.
Perinatal outcomes: a: Perinatal mortality, b: Severe neurological and other severe morbidity. c: Proportion of adverse outcomes by birth weight centile categories.
Severe neurological morbidity occurred in 7311 infants (0.74%), with the highest rate (19.6/1000 live births) in <1st centile cohort and the lowest rate observed in the 50th–75th centile group (6.5/1000 live births). Rates of other severe morbidity were highest in the <1st centile category (12.3% of live births) and lowest in the 25–<50th category. For both severe neurological and other severe morbidity, there was also a U-shaped pattern of distribution peaking at the extremes of birthweight centile. Fig. 2c and Supplementary Fig. S3b shows that most cases of perinatal mortality, severe neurological and other severe morbidity occurred in the 10th–90th centile categories.
Multivariable multinomial logistic regression was undertaken to investigate the relationship between birthweight centile and adverse perinatal outcomes after considering potential confounders and effect measure modifiers (Table 4 and Fig. 3). There were no significant interactions between birthweight centile and gestational age and non-reassuring fetal status, cord prolapse, intrapartum haemorrhage, uterine rupture and shoulder dystocia. The only interaction that was significant was between birthweight centile and mode of birth and thus incorporated in the model (Supplementary Table S4).
Table 4.
Multinomial logistic regression analyses of the effect of birthweight centile on perinatal outcomes.
| Univariable analysis | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Perinatal outcomes |
Stillbirth |
Neonatal death |
Severe neurological morbidity |
Other severe morbidity |
No severe adverse outcome |
||||
| Birthweight centile | RRR (95% CI) | p-value | RRR (95% CI) | p-value | RRR (95% CI) | p-value | RRR (95% CI) | p-value | RRR (95% CI) |
| <1st | 10.25 (8.2, 12.83) | <0.001 | 4.85 (2.76, 8.52) | <0.001 | 3.18 (2.7, 3.75) | <0.001 | 1.85 (1.73, 1.99) | <0.001 | Ref |
| 1st–<3rd | 4.89 (3.92, 6.1) | <0.001 | 4 (2.6, 6.17) | <0.001 | 1.94 (1.68, 2.24) | <0.001 | 1.29 (1.22, 1.36) | <0.001 | Ref |
| 3rd–<5th | 3.49 (2.72, 4.48) | <0.001 | 1.64 (0.87, 3.1) | 0.128 | 1.66 (1.42, 1.93) | <0.001 | 1.12 (1.06, 1.19) | <0.001 | Ref |
| 5th–<10th | 2.25 (1.84, 2.74) | <0.001 | 1.42 (0.92, 2.19) | 0.114 | 1.34 (1.21, 1.49) | <0.001 | 1.09 (1.05, 1.13) | <0.001 | Ref |
| 10th–<25th | 1.56 (1.35, 1.8) | <0.001 | 1.41 (1.07, 1.85) | 0.014 | 1.15 (1.07, 1.24) | <0.001 | 1.02 (1, 1.04) | 0.115 | Ref |
| 25th–75th | Ref | Ref | Ref | Ref | Ref | Ref | Ref | Ref | Ref |
| 75th–<90th | 0.86 (0.73, 1.02) | 0.088 | 0.72 (0.52, 1.01) | 0.054 | 1.06 (0.99, 1.14) | 0.091 | 1.07 (1.05, 1.1) | <0.001 | Ref |
| 90th–<95th | 0.65 (0.47, 0.88) | 0.006 | 0.78 (0.47, 1.3) | 0.335 | 1.14 (1.03, 1.26) | 0.012 | 1.12 (1.09, 1.16) | <0.001 | Ref |
| 95th–<97th | 0.88 (0.59, 1.33) | 0.549 | 0.48 (0.18, 1.28) | 0.142 | 1.24 (1.07, 1.44) | 0.005 | 1.15 (1.1, 1.21) | <0.001 | Ref |
| 97th–≤99th | 1.34 (0.95, 1.88) | 0.093 | 1.12 (0.57, 2.18) | 0.749 | 1.39 (1.21, 1.61) | <0.001 | 1.28 (1.22, 1.34) | <0.001 | Ref |
| >99th | 2.11 (1.48, 2.99) | <0.001 | 1.65 (0.81, 3.36) | 0.164 | 1.8 (1.53, 2.11) | <0.001 | 1.63 (1.55, 1.72) | <0.001 | Ref |
| Multivariable analysis | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Perinatal outcomes |
Stillbirth |
Neonatal death |
Severe neurological morbidity |
Other severe morbidity |
No severe adverse outcome |
||||
| Birthweight Centile | aRRR (95% CI) | p-value | aRRR (95% CI) | p-value | aRRR (95% CI) | p-value | aRRR (95% CI) | p-value | aRRR (95% CI) |
| <1st | 12.96 (10.14, 16.57) | <0.001 | 7.55 (3.78, 15.08) | <0.001 | 2.01 (1.5, 2.71) | <0.001 | 1.65 (1.49, 1.82) | <0.001 | Ref |
| 1st–<3rd | 5.2 (4.05, 6.69) | <0.001 | 3.65 (1.88, 7.08) | <0.001 | 1.64 (1.32, 2.04) | <0.001 | 1.1 (1.02, 1.19) | 0.019 | Ref |
| 3rd–<5th | 3.48 (2.6, 4.65) | <0.001 | 1.72 (0.7, 4.26) | 0.238 | 1.48 (1.19, 1.85) | 0.001 | 1.01 (0.93, 1.09) | 0.858 | Ref |
| 5th–<10th | 2.23 (1.77, 2.8) | <0.001 | 1.43 (0.76, 2.7) | 0.263 | 1.12 (0.95, 1.32) | 0.173 | 0.99 (0.94, 1.04) | 0.593 | Ref |
| 10th–<25th | 1.59 (1.34, 1.88) | <0.001 | 1.58 (1.08, 2.33) | 0.019 | 0.94 (0.85, 1.05) | 0.287 | 0.94 (0.91, 0.97) | <0.001 | Ref |
| 25th–75th | Ref | Ref | Ref | Ref | Ref | Ref | Ref | Ref | Ref |
| 75th–<90th | 0.69 (0.55, 0.86) | 0.001 | 0.61 (0.36, 1.03) | 0.066 | 1.15 (1.04, 1.26) | 0.007 | 1.13 (1.09, 1.16) | <0.001 | Ref |
| 90th–<95th | 0.58 (0.39, 0.87) | 0.008 | 0.56 (0.23, 1.38) | 0.206 | 1.32 (1.14, 1.53) | <0.001 | 1.22 (1.17, 1.28) | <0.001 | Ref |
| 95th–<97th | 0.74 (0.42, 1.28) | 0.276 | 0.85 (0.27, 2.68) | 0.783 | 1.32 (1.05, 1.65) | 0.017 | 1.3 (1.21, 1.39) | <0.001 | Ref |
| 97th–≤99th | 0.89 (0.53, 1.5) | 0.672 | 1.19 (0.44, 3.27) | 0.73 | 1.86 (1.53, 2.27) | <0.001 | 1.48 (1.38, 1.58) | <0.001 | Ref |
| >99th | 1.37 (0.77, 2.44) | 0.278 | 1.72 (0.54, 5.46) | 0.36 | 2.77 (2.22, 3.47) | <0.001 | 1.86 (1.7, 2.02) | <0.001 | Ref |
RRR: Relative Risk Ratio. aRRR: Adjusted Relative risk ratio; adjusted for lowest quintile of SEIFA, BMI, nulliparity, drug use, maternal history of stillbirth, antepartum haemorrhage, diabetes mellitus and hypertension in pregnancy, and incorporating an interaction term for method of birth and birthweight centile.
Fig. 3.
The adjusted relative risk ratio and 95% of confidence interval of stillbirth, neonatal death, severe neurological morbidity and other severe morbidity across birthweight centile categories. aRRR—adjusted Relative Risk Ratio. Adjusted for lowest quintile of SEIFA, body mass index, nulliparity, drug use, maternal history of stillbirth, antepartum haemorrhage, diabetes mellitus and hypertension in pregnancy, and incorporating an interaction term for birthweight centile and method of birth.
Infants at lower birthweight centiles, particularly those <5th centile, were at greater relative risk of perinatal mortality (Fig. 3). The relative risk for stillbirth was significantly increased when birthweight fell below the 25th centile and peaked for infants with birthweight <1st centile (aRRR 12.96, 95% CI 10.14–16.57, p < 0.001). Similarly, the relative risk of neonatal death was significantly increased when birthweight fell below the 5th centile, and greatest for birthweight <1st centile (aRRR 7.55, 95% CI 3.78–15.08). The relative risk of severe neurological morbidity and other severe morbidity was highest for infants with birthweight >99th centile (aRRR 2.77, 95% CI 2.22–3.47, p < 0.001 and aRRR 1.86, 95% CI 1.70–2.02, p < 0.001, respectively) (Table 4, Fig. 3).
Discussion
In this descriptive study of more 1 million births in Australia, we demonstrate the following key findings. Firstly, perinatal death rates are greatest in the birthweight <1st centile cohort. Secondly, rates of severe neurological and other severe newborn morbidity follow a U-shaped distribution with peaks at the extremes of birthweight centiles. Thirdly, the incidence of all study outcomes (perinatal mortality, neurological and other morbidity) increased when birthweight was <25th centile or >97th centile. Finally, although the cumulative incidence of adverse outcomes was greatest when birthweight was <10th centile, most adverse outcomes actually occurred in infants that were apparently “appropriately grown” (i.e., birthweight 10th–90th centile). Almost two in three stillbirths and approximately three in four cases of neonatal death, severe neurological or other severe morbidity occurred within this birthweight range.
Our finding that rates of SGA infants are higher in women who are younger (<25 years), of low BMI, nulliparous, Aboriginal or Torres Strait Islander ethnicity, smokers, use alcohol or illicit drugs, or experience an antepartum haemorrhage, are consistent with other publications.17,18 Our results also confirm that Indigenous women are more likely to give birth to an SGA infant. A similar demographic association was also reported in a smaller study by McEwen et al.19 from the Northern Territory in Australia—a geographical region with a large Aboriginal or Torres Strait Islander population. The relationship between increasing birthweight, maternal BMI and diabetes mellitus is also consistent, as obesity and hyperglycemia are known risk factors for increased fetal growth.20 The correlation between birthweight centile and previous caesarean section is likely to reflect the effect of parity on increasing infant birthweight.21,22 Interestingly however, despite our large cohort we did not see an association between maternal age and low birthweight as reported in other studies.22,23
We included late preterm infants in our study cohort because although they are greater risk of perinatal mortality and severe morbidity compared to term infants, the absolute incidence of these complications is low and generally similar to term infants.24 The exception to this relates to other complications, such as NICU admission, hypoglycaemia, hypothermia, respiratory morbidity and sepsis, which are acknowledged complications of prematurity.25 However, as late preterm infants comprised only 4.9% of our study cohort, and the incidence of late preterm births was consistent across the birthweight centiles (Table 2), the influence of prematurity on our study outcomes is likely to be small.
Rates of intrapartum sentinel events (cord prolapse, intrapartum haemorrhage or uterine rupture), which are strongly associated with severe hypoxic injury and adverse outcomes26,27 were also relatively low in our cohort and therefore their contribution to the overall incidence of adverse perinatal outcomes was small. The higher rates of cord prolapse and intrapartum haemorrhage in the lower birthweight centile cohorts is likely to reflect the higher rates of malpresentation and hypertension in this group.28,29
Our findings highlight the vulnerability of smaller infants, particularly to asphyxial complications (either antenatal or intrapartum). Our epidemiological data are consistent with other studies showing higher rates of histological10,30 and ultrasound31, 32, 33 abnormalities in keeping with placental dysfunction that are present at lower birthweight centiles.10,34 Prenatal identification of these infants may improve outcomes.3 Although third trimester ultrasound can improve prenatal detection rates of SGA infants,35, 36, 37 caution is required because this may result in increased rates of obstetric intervention particularly iatrogenic preterm/early term birth38 and may also be, from a population perspective, not cost effective.39
Intrapartum uterine contractions (against a background of pre-existing poor placental function), results in impaired oxygen and nutrient transfer to the fetus increasing the likelihood of compromise.40 This is reflected by higher rates of operative birth for non-reassuring fetal status as seen at lower birthweight centiles in our study. We also observed that rates of operative birth for failure to progress increased as birthweight centile increased, and that after adjusting for confounders and considering the interaction of mode of birth, the relative risk of severe neurological and other severe morbidity was greatest for infants with a birthweight >99th centile. Adverse outcomes in larger infants may reflect obstructed labour and complications related to operative birth. Vasak41 and others42,43 have suggested that a Darwinian process may exist in later gestations to limit fetal growth, to improve the likelihood of successful vaginal birth, reduce maternal morbidity, and optimise maternal survival. It may also explain why we and others21,44 found that nulliparous and younger women are over-represented at the lower birthweight centiles. From a biological perspective, these women are in the earlier stages of their reproductive life and have future reproductive potential if birth can proceed free of complications related to obstructed labour.
Strengths of our study include the large cohort which allowed the analysis of rarer adverse outcomes to be determined across birthweight centiles. Our findings are also generalisable to other high-income countries and provides granular information that obstetric practitioners can use to guide care and inform women. However, it is important that healthcare professionals communicate pregnancy risks in a sensible and balanced manner to women and their families without causing undue alarm. Furthermore, women should not be offered unnecessary obstetric intervention such as preterm or early term birth without robust evidence that such interventions result in a reduction in adverse outcomes. We were unable to account for the effects of interventions or treatment on perinatal outcomes or changes in clinical practice over the study period. Treatment effect may have had a more pronounced effect at the extremes of birthweight centiles where interventions such as planned birth, mode of birth, and intensity of fetal surveillance may have altered outcomes. Although perinatal mortality rates were stable during the study period, we observed an increase in rates of severe neurological and other severe morbidity (Supplementary Fig. S6) in the latter half. We postulate that this may be due to changes reporting practices over the study period or possibly the consequences of obstetric intervention at earlier gestations for a variety of perceived maternal and perinatal risks. A further potential limitation is our use of birthweight charts13 that may have included preterm small infants. We were also not able to ascertain the interval between fetal demise in utero and gestation at birth.
Although, fortunately rare in high income countries, adverse perinatal outcomes still have catastrophic, life-altering impacts for infants and their families. Furthermore, despite the higher risk of perinatal morbidity and mortality in small infants, from a healthcare perspective, the overall quantum of complications is greatest in the apparently normal size cohort (infants with birthweight 10–90th centile, Fig. 2c). Almost 70% of aa perinatal mortality and 75% of all severe neurological morbidity cases occurred in “normal size” infants (Table 4, Fig. 2c). Our results concur with the view that the continuum of perinatal risks extends beyond a specific birthweight threshold10,34 and thus simply dichotomising thresholds may not be the best approach to improving outcomes. Identifying the “at risk” infant is however difficult. Although fetal size, reduced intrauterine growth velocity8,45 and evidence of cerebral redistribution32,46,47 are associated with greater odds of perinatal morbidity and mortality8,45,48, 49, 50 other factors, such as placental derived biomarkers51 may need to be considered. The use of iterative and automated artificial intelligence techniques currently evaluated in other areas of science and medicine52, 53, 54 could be future strategies to identify pregnancies and infants at risk of adverse outcomes.
Contributors
SK conceived the study, provided overall supervision, reviewed and revised all versions of the manuscript. KC compiled the database and analyzed the data. TT, JH, VC and SK analyzed the findings. TT prepared the first draft and KC contributed to the statistical analysis section. All authors had access to all data in the study, contributed to writing of the manuscript, and had final responsibility to submit for publication.
Data sharing statement
All code, scripts and data used to produce the results in this article will be available to any researcher provided appropriate ethics approval, inter-institutional data sharing agreements and other regulatory requirements are in place. Additional specific approval from the Data Custodian of the Statistical Services Branch of Queensland’s Department of Health will also be required.
Declaration of interests
The authors report no conflict of interest.
Acknowledgements
SK is supported by the Mater Foundation and receives research funding from the Australian Medical Research Future Fund and the National Health and Medical Research Council. TT is supported by a University of Queensland Research Scholarship, The Royal Australian and New Zealand College of Obstetricians and Gynaecologists' Women’s Health Foundation - Norman Beischer Clinical Research Scholarship, and the Research Foundation of Cerebral Palsy Alliance.
Footnotes
Supplementary data related to this article can be found at https://doi.org/10.1016/j.lanwpc.2024.101054.
Appendix ASupplementary data
References
- 1.Aplin J.D., Myers J.E., Timms K., Westwood M. Tracking placental development in health and disease. Nat Rev Endocrinol. 2020;16(9):479–494. doi: 10.1038/s41574-020-0372-6. [DOI] [PubMed] [Google Scholar]
- 2.Fung C., Zinkhan E. Short- and long-term implications of small for gestational age. Obstet Gynecol Clin North Am. 2021;48(2):311–323. doi: 10.1016/j.ogc.2021.02.004. [DOI] [PubMed] [Google Scholar]
- 3.Ashorn P., Ashorn U., Muthiani Y., et al. Small vulnerable newborns-big potential for impact. Lancet. 2023;401(10389):1692–1706. doi: 10.1016/S0140-6736(23)00354-9. [DOI] [PubMed] [Google Scholar]
- 4.Lawn J.E., Ohuma E.O., Bradley E., et al. Small babies, big risks: global estimates of prevalence and mortality for vulnerable newborns to accelerate change and improve counting. Lancet. 2023;401(10389):1707–1719. doi: 10.1016/S0140-6736(23)00522-6. [DOI] [PubMed] [Google Scholar]
- 5.Adanikin A., Lawlor D.A., Pell J.P., Nelson S.M., Smith G.C.S., Iliodromiti S. Association of birthweight centiles and early childhood development of singleton infants born from 37 weeks of gestation in Scotland: a population-based cohort study. PLoS Med. 2022;19(10) doi: 10.1371/journal.pmed.1004108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hyperglycemia and adverse pregnancy outcomes. N Engl J Med. 2008;358(19):1991–2002. doi: 10.1056/NEJMoa0707943. [DOI] [PubMed] [Google Scholar]
- 7.Giannoni A., Baruah R., Leong T., et al. Do optimal prognostic thresholds in continuous physiological variables really exist? Analysis of origin of apparent thresholds, with systematic review for peak oxygen consumption, ejection fraction and BNP. PLoS One. 2014;9(1) doi: 10.1371/journal.pone.0081699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Larsen M.L., Schreiber V., Krebs L., Hoei-Hansen C.E., Kumar S. The magnitude rather than the rate of decline in fetal growth is a stronger risk factor for perinatal mortality in term infants. Am J Obstetr Gynecol MFM. 2023;5(2) doi: 10.1016/j.ajogmf.2022.100780. [DOI] [PubMed] [Google Scholar]
- 9.Iliodromiti S., Mackay D.F., Smith G.C., et al. Customised and noncustomised birth weight centiles and prediction of stillbirth and infant mortality and morbidity: a cohort study of 979,912 term singleton pregnancies in scotland. PLoS Med. 2017;14(1) doi: 10.1371/journal.pmed.1002228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Damhuis S.E., Kamphof H.D., Ravelli A.C.J., Gordijn S.J., Ganzevoort W.J. Perinatal mortality rate and adverse perinatal outcomes presumably attributable to placental dysfunction in (near) term gestation: a nationwide 5-year cohort study. PLoS One. 2023;18(5) doi: 10.1371/journal.pone.0285096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.von Elm E., Altman D.G., Egger M., Pocock S.J., Gøtzsche P.C., Vandenbroucke J.P. Strengthening the reporting of observational studies in epidemiology (STROBE) statement: guidelines for reporting observational studies. BMJ. 2007;335(7624):806–808. doi: 10.1136/bmj.39335.541782.AD. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Perinatal data collection (PDC) manual 2016-2017. Queensland Health. 2016. https://www.health.qld.gov.au/hsu/collections/pdc [Google Scholar]
- 13.Dobbins T.A., Sullivan E.A., Roberts C.L., Simpson J.M. Australian national birthweight percentiles by sex and gestational age, 1998–2007. Med J Aust. 2012;197(5):291–294. doi: 10.5694/mja11.11331. [DOI] [PubMed] [Google Scholar]
- 14.Heinze G., Wallisch C., Dunkler D. Variable selection–a review and recommendations for the practicing statistician. Biom J. 2018;60(3):431–449. doi: 10.1002/bimj.201700067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Moons K.G.M., Wolff R.F., Riley R.D., et al. PROBAST: a tool to assess risk of bias and applicability of prediction model studies: explanation and elaboration. Ann Intern Med. 2019;170(1):W1–w33. doi: 10.7326/M18-1377. [DOI] [PubMed] [Google Scholar]
- 16.Socio-economic indexes for areas (SEIFA) 2011. https://www.abs.gov.au/ausstats/abs@.nsf/DetailsPage/2033.0.55.0012011
- 17.Moraitis A.A., Wood A.M., Fleming M., Smith G.C.S. Birth weight percentile and the risk of term perinatal death. Obstet Gynecol. 2014;124(2 Pt 1):274–283. doi: 10.1097/AOG.0000000000000388. [DOI] [PubMed] [Google Scholar]
- 18.Melamed N., Baschat A., Yinon Y., et al. FIGO (International Federation of Gynecology and Obstetrics) initiative on fetal growth: best practice advice for screening, diagnosis, and management of fetal growth restriction. Int J Gynecol Obstet. 2021;152(S1):3–57. doi: 10.1002/ijgo.13522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.McEwen E.C., Guthridge S.L., He V.Y., McKenzie J.W., Boulton T.J., Smith R. What birthweight percentile is associated with optimal perinatal mortality and childhood education outcomes? Am J Obstet Gynecol. 2018;218(2s):S712–S724. doi: 10.1016/j.ajog.2017.11.574. [DOI] [PubMed] [Google Scholar]
- 20.McCloskey K., Ponsonby A.-L., Collier F., et al. The association between higher maternal pre-pregnancy body mass index and increased birth weight, adiposity and inflammation in the newborn. Pediatric Obesity. 2018;13(1):46–53. doi: 10.1111/ijpo.12187. [DOI] [PubMed] [Google Scholar]
- 21.Hinkle S.N., Albert P.S., Mendola P., et al. The association between parity and birthweight in a longitudinal consecutive pregnancy cohort. Paediatr Perinat Epidemiol. 2014;28(2):106–115. doi: 10.1111/ppe.12099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Swamy G.K., Edwards S., Gelfand A., James S.A., Miranda M.L. Maternal age, birth order, and race: differential effects on birthweight. J Epidemiol Commun Health. 2012;66(2):136–142. doi: 10.1136/jech.2009.088567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Odibo A.O., Nelson D., Stamilio D.M., Sehdev H.M., Macones G.A. Advanced maternal age is an independent risk factor for intrauterine growth restriction. Am J Perinatol. 2006;23(5):325–328. doi: 10.1055/s-2006-947164. [DOI] [PubMed] [Google Scholar]
- 24.Mitha A., Chen R., Altman M., Johansson S., Stephansson O., Bolk J. Neonatal morbidities in infants born late preterm at 35-36 Weeks of gestation: a Swedish nationwide population-based study. J Pediatr. 2021;233:43–50.e5. doi: 10.1016/j.jpeds.2021.02.066. [DOI] [PubMed] [Google Scholar]
- 25.Elaine M.B., Samantha J., Bradley M., et al. Neonatal outcomes and delivery of care for infants born late preterm or moderately preterm: a prospective population-based study. Arch Dis Child Fetal Neonatal Ed. 2015;100(6):F479. doi: 10.1136/archdischild-2014-307347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.McIntyre S., Blair E., Badawi N., Keogh J., Nelson K.B. Antecedents of cerebral palsy and perinatal death in term and late preterm singletons. Obstet Gynecol. 2013;122(4):869–877. doi: 10.1097/AOG.0b013e3182a265ab. [DOI] [PubMed] [Google Scholar]
- 27.Badawi N., Keogh J.M. Causal pathways in cerebral palsy. J Paediatr Child Health. 2013;49(1):5–8. doi: 10.1111/jpc.12068. [DOI] [PubMed] [Google Scholar]
- 28.Allen V.M., Joseph K., Murphy K.E., Magee L.A., Ohlsson A. The effect of hypertensive disorders in pregnancy on small for gestational age and stillbirth: a population based study. BMC Pregnancy Childbirth. 2004;4(1):17. doi: 10.1186/1471-2393-4-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pilliod R.A., Caughey A.B. Fetal malpresentation and malposition: diagnosis and management. Obstet Gynecol Clin North Am. 2017;44(4):631–643. doi: 10.1016/j.ogc.2017.08.003. [DOI] [PubMed] [Google Scholar]
- 30.Melamed N., Hiersch L., Aviram A., Keating S., Kingdom J.C. Customized birth-weight centiles and placenta-related fetal growth restriction. Ultrasound Obstet Gynecol. 2021;57(3):409–416. doi: 10.1002/uog.23516. [DOI] [PubMed] [Google Scholar]
- 31.Prior T., Mullins E., Bennett P., Kumar S. Prediction of intrapartum fetal compromise using the cerebroumbilical ratio: a prospective observational study. Am J Obstet Gynecol. 2013;208(2):124.e1–124.e6. doi: 10.1016/j.ajog.2012.11.016. [DOI] [PubMed] [Google Scholar]
- 32.Bendall A., Schreiber V., Crawford K., Kumar S. Predictive utility of the fetal cerebroplacental ratio for hypoxic ischaemic encephalopathy, severe neonatal morbidity and perinatal mortality in late-preterm and term infants. Aust N Z J Obstet Gynaecol. 2023;63(4):491–498. doi: 10.1111/ajo.13668. [DOI] [PubMed] [Google Scholar]
- 33.Morales-Roselló J., Khalil A., Morlando M., Bhide A., Papageorghiou A., Thilaganathan B. Poor neonatal acid-base status in term fetuses with low cerebroplacental ratio. Ultrasound Obstet Gynecol. 2015;45(2):156–161. doi: 10.1002/uog.14647. [DOI] [PubMed] [Google Scholar]
- 34.Coutinho C.M., Melchiorre K., Thilaganathan B. Stillbirth at term: does size really matter? Int J Gynecol Obstet. 2020;150(3):299–305. doi: 10.1002/ijgo.13229. [DOI] [PubMed] [Google Scholar]
- 35.Sovio U., White I.R., Dacey A., Pasupathy D., Smith G.C.S. Screening for fetal growth restriction with universal third trimester ultrasonography in nulliparous women in the pregnancy outcome prediction (POP) study: a prospective cohort study. Lancet. 2015;386(10008):2089–2097. doi: 10.1016/S0140-6736(15)00131-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Henrichs J., Verfaille V., Jellema P., et al. Effectiveness of routine third trimester ultrasonography to reduce adverse perinatal outcomes in low risk pregnancy (the IRIS study): nationwide, pragmatic, multicentre, stepped wedge cluster randomised trial. BMJ. 2019;367:l5517. doi: 10.1136/bmj.l5517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Caradeux J., Martinez-Portilla R.J., Peguero A., Sotiriadis A., Figueras F. Diagnostic performance of third-trimester ultrasound for the prediction of late-onset fetal growth restriction: a systematic review and meta-analysis. Am J Obstet Gynecol. 2019;220(5):449–459.e19. doi: 10.1016/j.ajog.2018.09.043. [DOI] [PubMed] [Google Scholar]
- 38.Monier I., Blondel B., Ego A., Kaminiski M., Goffinet F., Zeitlin J. Poor effectiveness of antenatal detection of fetal growth restriction and consequences for obstetric management and neonatal outcomes: a French national study. BJOG. 2015;122(4):518–527. doi: 10.1111/1471-0528.13148. [DOI] [PubMed] [Google Scholar]
- 39.Wilson E., Wastlund D., Moraitis A.A., Smith G. Late pregnancy ultrasound to screen for and manage potential birth complications in nulliparous women: a cost-effectiveness and value of information analysis. Value Health. 2021;24:513–521. doi: 10.1016/j.jval.2020.11.005. [DOI] [PubMed] [Google Scholar]
- 40.Turner J.M., Mitchell M.D., Kumar S.S. The physiology of intrapartum fetal compromise at term. Am J Obstet Gynecol. 2020;222(1):17–26. doi: 10.1016/j.ajog.2019.07.032. [DOI] [PubMed] [Google Scholar]
- 41.Vasak B., Koenen S.V., Koster M.P.H., et al. Human fetal growth is constrained below optimal for perinatal survival. Ultrasound Obstet Gynecol. 2015;45(2):162–167. doi: 10.1002/uog.14644. [DOI] [PubMed] [Google Scholar]
- 42.Gluckman P.D., Hanson M.A. Maternal constraint of fetal growth and its consequences. Semin Fetal Neonatal Med. 2004;9(5):419–425. doi: 10.1016/j.siny.2004.03.001. [DOI] [PubMed] [Google Scholar]
- 43.Gluckman P.D., Hanson M.A., Low F.M. Evolutionary and developmental mismatches are consequences of adaptive developmental plasticity in humans and have implications for later disease risk. Philos Trans R Soc Lond B Biol Sci. 2019;374(1770) doi: 10.1098/rstb.2018.0109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kozuki N., Lee A.C.C., Silveira M.F., et al. The associations of parity and maternal age with small-for-gestational-age, preterm, and neonatal and infant mortality: a meta-analysis. BMC Publ Health. 2013;13(3):S2. doi: 10.1186/1471-2458-13-S3-S2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Pacora P., Romero R., Jung E., et al. Reduced fetal growth velocity precedes antepartum fetal death. Ultrasound Obstet Gynecol. 2021;57(6):942–952. doi: 10.1002/uog.23111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Conde-Agudelo A., Villar J., Kennedy S.H., Papageorghiou A.T. Predictive accuracy of cerebroplacental ratio for adverse perinatal and neurodevelopmental outcomes in suspected fetal growth restriction: systematic review and meta-analysis. Ultrasound Obstet Gynecol. 2018;52(4):430–441. doi: 10.1002/uog.19117. [DOI] [PubMed] [Google Scholar]
- 47.Akolekar R., Ciobanu A., Zingler E., Syngelaki A., Nicolaides K.H. Routine assessment of cerebroplacental ratio at 35–37 weeks' gestation in the prediction of adverse perinatal outcome. Am J Obstet Gynecol. 2019;221(1):65.e1-.e18. doi: 10.1016/j.ajog.2019.03.002. [DOI] [PubMed] [Google Scholar]
- 48.Chatzakis C., Papaioannou G.-K., Eleftheriades M., Makrydimas G., Dinas K., Sotiriadis A. Perinatal outcome of appropriate-weight fetuses with decelerating growth. J Matern Fetal Neonatal Med. 2021;34(20):3362–3369. doi: 10.1080/14767058.2019.1684470. [DOI] [PubMed] [Google Scholar]
- 49.Gordijn S.J., Beune I.M., Thilaganathan B., et al. Consensus definition of fetal growth restriction: a Delphi procedure. Ultrasound Obstet Gynecol. 2016;48(3):333–339. doi: 10.1002/uog.15884. [DOI] [PubMed] [Google Scholar]
- 50.Hong J., Crawford K., Jarrett K., Triggs T., Kumar S. Five-minute Apgar score and risk of neonatal mortality, severe neurological morbidity and severe non-neurological morbidity in term infants – an Australian population-based cohort study. Lancet Reg Health Western Pac. 2024;44 doi: 10.1016/j.lanwpc.2024.101011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hong J., Kumar S. Circulating biomarkers associated with placental dysfunction and their utility for predicting fetal growth restriction. Clin Sci (Lond) 2023;137(8):579–595. doi: 10.1042/CS20220300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Leibig C., Brehmer M., Bunk S., Byng D., Pinker K., Umutlu L. Combining the strengths of radiologists and AI for breast cancer screening: a retrospective analysis. Lancet Digit Health. 2022;4(7):e507–e519. doi: 10.1016/S2589-7500(22)00070-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Soltan A.A.S., Yang J., Pattanshetty R., et al. Real-world evaluation of rapid and laboratory-free COVID-19 triage for emergency care: external validation and pilot deployment of artificial intelligence driven screening. Lancet Digit Health. 2022;4(4):e266–e278. doi: 10.1016/S2589-7500(21)00272-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Callender T., van der Schaar M. Automated machine learning as a partner in predictive modelling. Lancet Digit Health. 2023;5(5):e254–e256. doi: 10.1016/S2589-7500(23)00054-7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.