Abstract
Given the increasing use of bevacizumab in combinatorial drug therapy for a multitude of different cancer types, there is a need for therapeutic drug monitoring to analyze the possible correlation between drug trough concentration, and therapeutic effect and adverse reactions. An ultra-performance liquid chromatography tandem-mass spectrometry method was then developed and validated to determine bevacizumab levels in human plasma samples. Chromatographic separation was achieved on a Shimadzu InertSustainBio C18 HP column, whereas subsequent mass spectrometric analysis was performed using a Shimadzu 8050CL triple quadrupole mass spectrometer equipped with an electro-spray ionization source in the positive ion mode. In total, three multiple reaction monitoring transitions of each of the surrogate peptides were chosen with ‘FTFSLDTSK’ applied as the quantification peptide whereas ‘VLIYFTSSLHSGVPSR’ and ‘STAYLQMNSLR’ were designated as the verification peptides using the Skyline software. This analytical method was then fully validated, with specificity, linearity, lower limit of quantitation, accuracy, precision, stability, matrix effect and recovery calculated. The linearity of this method was developed to be within the concentration range 5–400 µg/ml for bevacizumab in human plasma. Subsequently, eight patients with non-small cell lung cancer (NSCLC) were recruited and injected with bevacizumab over three periods of treatment to analyze their steady-state trough concentration and differences. To conclude, the results of the present study suggest that bevacizumab can be monitored in a therapeutic setting in patients with NSCLC.
Keywords: bevacizumab, monoclonal antibody, nano-surface and molecular-orientation limited proteolysis, ultra-performance liquid chromatography tandem mass spectrometry, therapeutic drug monitoring
Introduction
Bevacizumab is a humanized immunoglobulin G1 monoclonal antibody (mAb) that was the first angiogenesis inhibitor to specifically target vascular endothelial growth factor A. It was approved by the US Food and Drug Administration (FDA) in February 2004. Bevacizumab is typically indicated for the first-line treatment of adult patients with metastatic colorectal cancer (mCRC), unresectable advanced, metastatic or recurrent non-small cell lung cancer (NSCLC) and metastatic breast cancer (1).
Previous pharmacokinetic studies of bevacizumab have reported this drug to have low clearance rates, a limited volume of the central compartment, a long elimination half-life (2–4) and increased area under the curve with inter-patient variations of 4.9-fold (5–7). Although the dosage of bevacizumab is adjusted by body weight, the trough concentration levels frequently vary widely from patient to patient and cannot be easily anticipated. Papachristos et al (8) previously conducted a prospective, real-world study to investigate the relationship between bevacizumab exposure and overall survival (OS) of patients with mCRC. A total of 157 trough concentration samples were analyzed. In total, three distinct groups of patients were identified, with patients who experienced longer OS having markedly higher trough concentration of bevacizumab (8). This observation suggests that it may be of importance to conduct therapeutic drug monitoring (TDM) of bevacizumab during routine clinical treatment.
The selection of a rapid but accurate method for the measurement of bevacizumab in human plasma samples is crucial for the evaluation of exposure-response relationships in efficacy and safety assessments during routine TDM. Classical ligand binding assays have been used to quantify the levels of therapeutic mAbs in biological specimens (9). However, traditional ELISA is not reliable in differentiating between endogenous IgGs and exogenous mAbs. Cross reactions can occur in ELISA because of their similarity in amino acid sequences and protein structures. In such cases, using a mass spectrometry (MS)-based method for quantifying mAbs may confer advantages over immunoassays in terms of accuracy. Although a liquid chromatography (LC)-MS/MS analytical method has been previously developed and validated for detecting bevacizumab in human plasma (10), routine TDM requires a convenient pre-treatment process to simplify flow and shorten the time taken. Iwamoto et al (11) previously proposed a method for bevacizumab analysis by applying nano-surface and molecular-orientation limited (nSMOL) proteolysis.
In the present study, a rapid LC-MS/MS method was designed and fully validated to quantify bevacizumab in human plasma using the nSMOL sample pre-treatment technology. Parameters, including specificity, carry-over, linearity, lower limit of quantitation (LLOQ), accuracy, precision, stability, matrix effect and recovery were calculated. The method was applied to design the trough concentration assay of bevacizumab in eight Chinese patients with NSCLC.
Materials and methods
Chemicals and reagents
Bevacizumab for injection (100 mg; 4 ml/vial; Avastin) was purchased from Roche Diagnostics GmbH. ProteoMass™ Pro14-Arg (P14R) MALDI-MS Standard [internal standard (IS)] was obtained from Sigma-Aldrich; Merck KGaA. The peptides FTFSLDTSK (purity 99.0%), VLIYFTSSLHSGVPSR (purity 99.0%) and STAYLQMNSLR (purity 98.0%) were purchased from GenScript as surrogates after the proteolysis of bevacizumab. A total of 10 individual blank human plasmas (China-Japan Friendship Hospital) were used for validation of specificity/selectivity and the mixture plasmas were used for preparation of calibration standards and quality control samples.
High performance LC (HPLC)-MS grade acetonitrile and formic acid (purity 98.0%) were purchased from Thermo Fisher Scientific, Inc. and Sigma-Aldrich; Merck KGaA, respectively. HPLC-MS grade water was obtained by Milli-Q® Synthesis (MilliporeSigma). nSMOL™ Antibody BA kit, FG beads™ Trypsin DART™ was purchased from Shimadzu Corporation.
Selection of surrogate and monitoring peptides
The key to successfully develop a bevacizumab analytical method is the selection of unique signature peptides. Fragments of complementarity-determining region sequences of bevacizumab are typically used as signature surrogate peptides in bottom-up proteomics approaches (12). It is necessary to confirm beforehand that the surrogate peptide candidates are unique sequences from bevacizumab and cannot be generated from human blood samples. Determination of amino acid sequences of bevacizumab was performed in DrugBank (https://go.drugbank.com/drugs/DB00112). The surrogate peptide candidates were determined using Skyline (version 21.2.0.369; skyline.ms/project/home/begin.view) from the variable regions of the light and heavy chains of bevacizumab. A total of three candidate peptides were chosen; FTFSLDTSK was used as the quantification peptide, while VLIYFTSSLHSGVPSR and STAYLQMNSLR were used as qualitative peptides.
Ultra-HPLC (UPLC)-MS/MS conditions
LC was performed on an UPLC unit (Shimadzu Corporation) with a Shimadzu InertSustainBio C18 HP (2.1×100 mm; 3.0-µm particle size). The mobile phase consisted of (A) water containing 0.1% formic acid and (B) acetonitrile containing 0.1% formic acid with a gradient elution. At the beginning, the mobile phase consisted of 5% B and 95% A until 30 sec. Between 0.5 and 3.5 min, the percentage of B changed to 70%. At 3.51 min, the percentage of B increased to 95% and maintained until 4.5 min. At 4.51 min, the percentage of B returned to 5% till the end of 6.0 min. The overall run time was 6.0 min at 0.2 ml/min flow rate. A Shimadzu 8050CL triple quadrupole MS equipped with an electro-spray ionization source (Shimadzu Corporation) was used for mass spectrometric detection. The detection was operated in the positive mode with multiple reaction monitoring (MRM). The dwell time was set to 3.0 msec for each MRM transition. After optimization, the source parameters were set as follows: Atomizing gas, 3 l/min; dry gas, 5 l/min; heat gas, 15 l/min; ion spray voltage, 4,000 V; interface temperature, 300°C; temperature, 150°C and heat block temperature, 400°C. The MRM transitions and specific parameters for all surrogate peptides and IS are listed in Table I. Data acquisition and processing were performed using Labsolutions (version 5.81; Shimadzu Corporation).
Table I.
MRM transitions and specific parameters for the targeted peptides, FTFSLDTSK, VLIYFTSSLHSGVPSR and STAYLQMNSLR, and IS P14R.
| Selected peptide | Region | MRM transition, m/z | Q1 Pre Bias, V | CE, V | Q3 Pre Bias, V |
|---|---|---|---|---|---|
| FTFSLDTSK | H-chain of | 523.30([M+2H]2+)→797.40(y7+)a | −38 | −18 | −34 |
| CDR2 | 523.30([M+2H]2+)→898.50(y8+) | −38 | −20 | −30 | |
| 523.30([M+2H]2+)→650.30(y6+) | −38 | −19 | −34 | ||
| VLIYFTSSLHSGVPSR | L-chain of | 588.50([M+3H]3+)→776.10(y14++) | −30 | −19 | −32 |
| CDR2 | 588.50([M+3H]3+)→939.60(y9+) | −22 | −28 | −26 | |
| 588.50([M+3H]3+)→602.40(y6+) | −22 | −28 | −24 | ||
| STAYLQMNSLR | H-chain of | 642.60([M+2H]2+)→861.30(y7+) | −32 | −23 | −28 |
| CH1 | 642.60([M+2H]2+)→748.30(y7+) | −24 | −22 | −24 | |
| 642.60([M+2H]2+)→620.30(y7+) | −34 | −24 | −24 | ||
| P14R (IS) | - | 512.10→292.30a | −38 | −20 | −20 |
| 512.10→389.30 | −38 | −16 | −28 | ||
| 512.10→757.50 | −38 | −19 | −38 |
Quantitation peptides. IS, internal standard; P14R, Pro14-Arg; MRM, multiple reaction monitoring; Q, quadrupole; CE is short for collision energy; V means voltage.
Stock and working solutions, calibration standards and quality control (QC) sample
Bevacizumab injection (25 mg/ml) was used as a stock solution to make the calibration standards and QC samples. The stock solution of the IS P14R (10 nmol) was diluted to 1 ml using Enhanced Reaction Solution from the nSMOL Antibody BA Kit (cat. no. P/N 225-32250-91; Shimadzu Corporation) to obtain a 10 nmol/ml P14R solution. The surrogate peptides were prepared by dissolving 1.7 mg FTFSLDTSK (net weight, 1.0 mg), 1.5 mg VLIYFTSSLHSGVPSR (net weight, 1.0 mg) and 1.5 mg STAYLQMNSLR (net weight, 1.0 mg) in 1 ml methanol/water (1:1, v/v), respectively. The stock solution of bevacizumab was also further diluted with blank human plasma to obtain the calibration standards and QC samples at the designated concentration levels. The final concentrations of the calibration standards were 5, 10, 20, 50, 100, 200, 300 and 400 µg/ml. The concentrations of the QC samples in plasma were 10, 50, 200 µg/ml and 5 µg/ml for LLOQ and 400 µg/ml for upper LOQ (ULOQ). The IS working solutions were prepared by adding 5 µl P14R (10 nmol/ml) to 995 µl Enhanced Reaction Solution and 1 ml Reaction Solution, which were freshly prepared every time. The bevacizumab and P14R stock solutions were stored at 4 and −80°C, respectively. The calibration standards and QC samples were immediately stored at −80°C.
Sample pretreatment
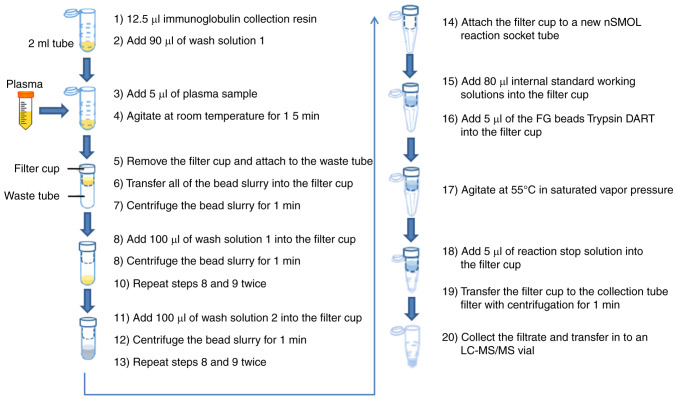
Plasma samples were prepared using an nSMOL antibody BA kit to obtain the surrogate peptides (11). The study design is summarized in Fig. 1. The specific experimental procedures were listed as follows: After sufficiently evenly diffusing the immunoglobulin collection resin with vortex, 12.5 µl bead slurry was aliquoted into 2-ml sample tubes before adding 90 µl wash solution 1 (binding solution). A total of 5 µl plasma sample was added into the 2-ml sample tubes, which included calibration curve, QC or clinical samples; the mixture was gently agitated at room temperature for 15 min by a micro tube mixer; the filter cup was removed from the filter tube (Ultrafree-MC; GV 0.22-µm; MilliporeSigma) and which was attached to the waste tube (Micro tube 2 ml; Sarstedt, Inc.); all of the bead slurry were transferred into the filter cup and centrifuged at room temperature under 10,000 × g for 1 min; 100 µl wash solution 1 (binding solution) was added into the filter cup and the bead slurry was centrifuged at room temperature under 10,000 × g for 1 min, which procedure was repeated twice; 100 µl wash solution 2 was added into the filter cup and the bead slurry was centrifuged at room temperature under 10,000 × g for 1 min, which was repeated twice as well; the filter cup was attached to a new nSMOL reaction socket tube (Shimadzu Corporation); after sufficiently evenly diffusing 5 µl of FG beads Trypsin DART with vortex, the Trypsin DART and 80 µl IS working solutions were then added into the filter cup; the solution was gently (700 rpm) agitated at 55°C in saturated vapor pressure for 5 h for enzymatic digestion; 5 µl of reaction stop solution was added into the filter cup; the filtrate was collected and transferred to an LC-MS/MS vial, after the filter cup was transferred to the collection tube with centrifugation at 12,000 × g for 1 min.
Figure 1.
Graphic description and methodology.
Method validation
The method was fully validated for specificity/selectivity, linearity, precision, accuracy, recovery, matrix effect and stability according to The International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use guideline-Bioanalytical method validation M10 (13), the European Medicines Agency-Guideline on bioanalytical method validation (14) and the US FDA (15).
The specificity/selectivity of this method was investigated by analyzing 10 individual human blank plasma samples. Each blank sample was tested for interferences by using the proposed pre-treatment procedures and LC-MS/MS conditions.
The carry-over of surrogate peptides and IS were evaluated by injecting blank samples after the ULOQ samples to compare the peak area of blank samples and LLOQ samples at the retention time.
The linearity and LLOQ for the three surrogate peptides were assessed by plotting their peak area ratios with the IS vs. their respective concentrations. The criterion was that the deviation of each back-calculated concentration had to be within ±20% of the nominal value except for the LLOQ, which had to be within ±25%.
Accuracy and precision were determined by analyzing five concentration levels (5, 10, 50, 200 and 400 µg/ml) for bevacizumab at LLOQ, low QC (LQC), middle QC (MQC), high (HQC) and ULOQ in the plasma. A total of five replicates of every QC sample were analyzed on six separate validation days. The intra- and inter-day accuracy should be within ±20% of the theoretical concentrations (LLOQ within ±25%). The intra- and inter-day precision should be <20% for QC samples (LLOQ should be <25%).
The stability of bevacizumab in the human plasma samples was evaluated under different temperature and timing conditions. The short-term stability was evaluated by determining QC samples at three concentration levels, which were kept at room temperature for a period that exceeded the routine pre-treatment time of the samples (~6 h). The auto-sampler stability was measured by re-analyzing the QC samples kept in the auto-sampler (4°C) for 72 h. The freeze and thaw stability of the QC samples was tested after three freeze (−80°C) and thaw (room temperature) cycles. Their long-term stability was assessed by analyzing storage at a low temperature (−80°C) for 26 days.
The validation processes of the matrix effect and recovery were performed according to the method of Jiang et al (16). The matrix effects for surrogate peptides with their IS P14R were assessed by using 10 different individual human plasma samples and comparing the ratio of the peak area in the presence of matrix (measured by analyzing blank matrix after extraction and then spiked with FTFSLDTSK, VLIYFTSSLHSGVPSR and STAYLQMNSLR) to the peak area in the absence of matrix (pure analyte and IS solution). The matrix effect was evaluated by comparing the average peak areas of spiked samples after extraction to those of corresponding working solutions at the same concentration. The inter-individual variability of the IS-normalized matrix factor expressed by relative standard deviation (RSD) should be <15%.
Recoveries of the pre-treatment method were analyzed at three QC concentrations by comparing the mean peak areas of pre-treated QC samples (n=10) with those of post-extracted blank plasma samples (n=10) spiked with working solutions. The recoveries of the IS were determined using the same method.
Data processing
Data acquisition and processing were conducted by using Labsolutions software (version 5.81; Shimadzu Corporation). Descriptive statistics [mean, SD, RSD and relative error (RE)] were calculated by using Microsoft Office (version 2016; Microsoft Corporation).
Patients
The analytical method was used to monitor human plasma levels of bevacizumab in patients with NSCLC. All the procedures were conducted in accordance with the Declaration of Helsinki, and the present study was approved by the Medical Ethics Committee of China-Japan Friendship Hospital (Beijing, China). A total of 8 patients with NSCLC were enrolled in this research from June 2020 to December 2020 in China-Japan Friendship Hospital. Plasma samples were collected by venipuncture into evacuated EDTA-anticoagulated blood collection tubes after injecting bevacizumab over 3 periods of treatment and centrifuged at 1,811 × g for 5 min. All samples were stored at −80°C until analysis.
Statistical analysis
Descriptive statistics were performed for the data of patients. Microsoft Excel (Microsoft® Office® 2016, Microsoft Corp., USA) was utilized for data analysis (mean values, SDs and RSDs). A weighted least square fit was used for the standard calibrations.
Results
Method validation
Specificity/selectivity
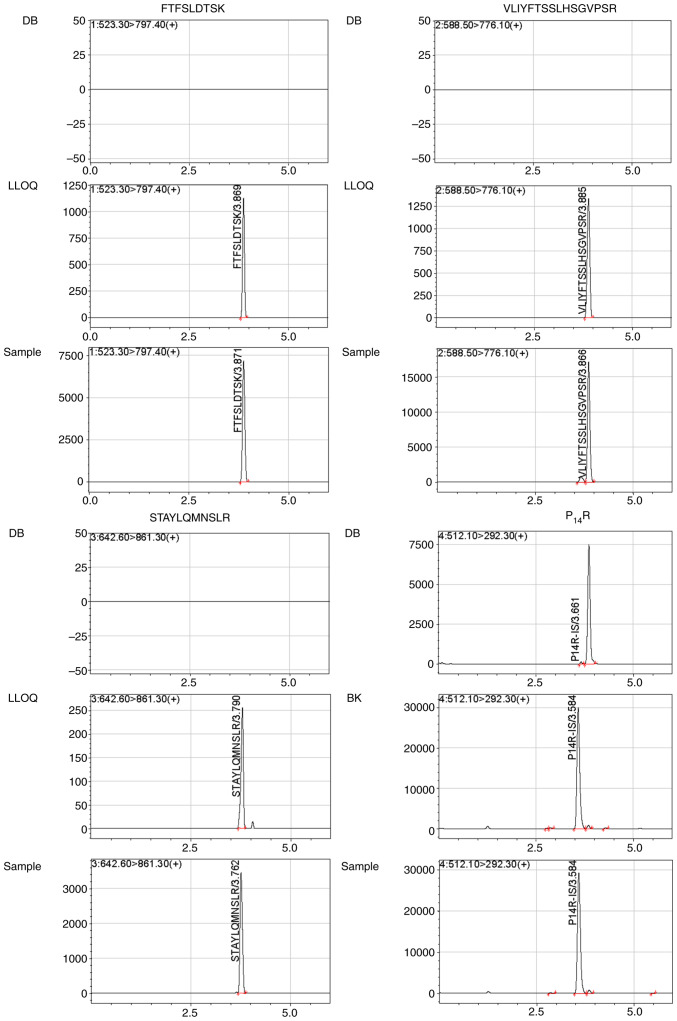
The analysis of 10 healthy independent blank human plasma samples showed that there were no endogenous interference peaks observed during the retention time of the surrogate peptides. Representative chromatograms of double blank with no surrogate peptide of interest or IS (Fig. 2) showed the absence of any interference during the retention time for surrogate peptides and IS. Blank human plasma from 10 individuals were individually extracted to determine if any interferences detected at the retention times were <20% for FTFSLDTSK, VLIYFTSSLHSGVPSR and STAYLQMNSLR, and if they were 5% for the IS in LLOQ samples.
Figure 2.
Representative chromatograms for FTFSLDTSK, VLIYFTSSLHSGVPSR, STAYLQMNSLR and there IS P14R: (DB) a blank plasma sample; (BK) a blank plasma sample with IS; (LLOQ) a plasma LLOQ sample with IS; (Sample) a plasma sample from a NSCLC patient after injecting bevacizumab 3 periods.
Carry-over
There was no interference observed at the retention time of the surrogate peptides and IS after ULOQ sample injection.
Linearity and LLOQ
Calibration curves were created by plotting the peak area ratios of the various surrogate peptides to IS vs. nominal concentration of the analyte standards with a weighting factor of 1/x2 applied to all calibration curves. The correlation coefficient (r2) of all the calibration curves was >0.99. The typical regression equations of these curves were calculated as follows: i) FTFSLDTSK in human plasma (y=0.00116185×-0.00128682;r2=0.9907); ii) VLIYFTSSLHSGVPSR in human plasma(y=0.00684308×+0.00390712; r2=0.9953); and iii) STAYLQMNSLR in human plasma (y=0.00132500×-0.000746389; r2=0.9971).
The LLOQ of 5 µg/ml bevacizumab in human plasma was eligible with 11.80–18.84% of RSD and −2.95–2.47% of RE. Typical chromatograms of LLOQs for FTFSLDTSK, VLIYFTSSLHSGVPSR and STAYLQMNSLR in plasma are shown in Fig. 2.
Accuracy and precision
The accuracy and precision for FTFSLDTSK, VLIYFTSSLHSGVPSR and STAYLQMNSLR after bevacizumab proteolysis are summarized in Table II. The intra- and inter-day accuracy range was −7.48–9.04%, and the intra- and inter-day precision range was 1.63–18.84% in five QC samples (LLOQ, LQC, MQC, HQC and ULOQ). The validated method was reliable and reproducible for the quantification of bevacizumab.
Table II.
Intra- and inter-day accuracy and precision of QCs, LLOQ and ULOQ samples in plasma.
| Nominal concentration, µg/ml | Intra-day a (n=5) | RSD, % | RE, % | Inter-daya (n=5, 6 daysb) | RSD, % | RE, % |
|---|---|---|---|---|---|---|
| FTFSLDTSK | ||||||
| 5 | 4.85±0.59 | 12.20 | −2.95 | 5.02±0.81 | 16.18 | 0.41 |
| 10 | 9.36±1.02 | 10.84 | −6.43 | 9.69±1.28 | 13.18 | −3.09 |
| 50 | 47.74±3.08 | 6.45 | −4.52 | 51.43±5.92 | 11.51 | 2.86 |
| 200 | 196.09±7.08 | 3.61 | −1.95 | 192.79±16.41 | 8.51 | −3.60 |
| 400 | 402.44±18.24 | 4.53 | 0.61 | 370.10±32.57 | 8.80 | −7.48 |
| VLIYFTSSLHSGVPSR | ||||||
| 5 | 5.10±0.70 | 13.70 | 1.92 | 5.12±0.74 | 14.39 | 2.47 |
| 10 | 9.70±0.34 | 3.48 | −3.02 | 9.42±1.212 | 12.93 | −5.76 |
| 50 | 51.10±4.48 | 8.76 | 2.20 | 48.86±4.47 | 9.15 | −2.28 |
| 200 | 218.08±8.62 | 3.95 | 9.04 | 192.04±21.09 | 10.98 | −3.98 |
| 400 | 394.60±6.44 | 1.63 | −1.35 | 375.16±27.13 | 7.23 | −6.21 |
| STAYLQMNSLR | ||||||
| 5 | 4.90±0.58 | 11.80 | −2.04 | 5.09±0.96 | 18.84 | 1.71 |
| 10 | 9.66±1.05 | 10.85 | −3.37 | 10.04±1.46 | 14.53 | 0.44 |
| 50 | 52.76±4.77 | 9.04 | 5.52 | 49.81±5.35 | 10.73 | −0.38 |
| 200 | 204.29±13.40 | 6.56 | 2.15 | 192.66±16.84 | 8.74 | −3.67 |
| 400 | 415.61±24.71 | 5.95 | 3.90 | 399.65±31.18 | 7.80 | −0.09 |
Data are shown as mean ± SD.
Independent validation days for accuracy and precision. QC, quality control; LLOQ, lower limit of quantitation; ULOQ, upper limit of quantitation; RE, relative error; RSD, relative standard deviation.
Stability
Next, the stability of bevacizumab in human plasma was investigated under different storage and processing conditions (Table III). Bevacizumab was found to be stable in human plasma following three freeze-thaw cycles (from −80°C to 25°C) and storage at −80°C for 26 days. In addition, bevacizumab remained stable during short-term storage at 25°C for 6 h and auto-sampler stability was maintained at 4°C for 72 h.
Table III.
Stability results of selected peptides (FTFSLDTSK, VLIYFTSSLHSGVPSR, STAYLQMNSLR) in plasma in different conditions.
| Nominal concentration, µg/ml | FTFSLDTSKa | RSD, % | RE, % | VLIYFTSSLHSGVPSRa | RSD, % | RE, % | STAYLQMNSLRa | RSD, % | RE, % |
|---|---|---|---|---|---|---|---|---|---|
| Short-term (25°C for 6 h) | |||||||||
| 10 | 9.74±1.11 | 11.35 | −2.57 | 9.84±1.25 | 12.69 | −1.62 | 9.70±1.18 | 12.21 | −3.00 |
| 50 | 49.75±2.82 | 5.67 | −0.50 | 47.86±1.27 | 2.65 | −4.29 | 51.08±5.24 | 10.27 | 2.15 |
| 200 | 186.33±3.38 | 1.81 | −6.84 | 189.08±7.56 | 4.00 | −5.46 | 219.35±14.93 | 6.81 | 9.67 |
| Auto-sampler (4°C for 72 h) | |||||||||
| 10 | 9.20±1.14 | 12.36 | −8.04 | 11.21±0.81 | 7.18 | 12.10 | 9.81±1.40 | 14.30 | −1.87 |
| 50 | 49.80±4.26 | 8.56 | −0.40 | 48.44±3.79 | 7.83 | −3.12 | 55.81±4.79 | 8.58 | 11.63 |
| 200 | 192.51±7.40 | 3.84 | −3.75 | 185.69±5.06 | 2.72 | −7.16 | 213.34±4.50 | 2.11 | 6.67 |
| Freeze thaw (−80-25°C for 3 cycles) | |||||||||
| 10 | 9.17±1.18 | 12.86 | −8.26 | 9.18±0.59 | 6.43 | −8.17 | 9.35±0.98 | 10.49 | −6.51 |
| 50 | 47.21±5.71 | 12.09 | −5.59 | 46.44±1.67 | 3.61 | −7.12 | 45.63±3.46 | 7.57 | −8.74 |
| 200 | 190.14±11.93 | 6.27 | −4.93 | 191.43±4.24 | 2.21 | −4.28 | 189.56±9.47 | 4.99 | −5.22 |
| Long term (−80°C for 26 days) | |||||||||
| 10 | 10.03±0.98 | 9.78 | 0.27 | 8.79±0.76 | 8.62 | −12.08 | 9.31±1.11 | 11.94 | −6.90 |
| 50 | 48.81±3.68 | 7.55 | −2.37 | 44.88±4.72 | 10.52 | −10.24 | 47.86±4.78 | 9.99 | −4.29 |
| 200 | 191.94±7.10 | 3.70 | −4.03 | 191.56±6.63 | 3.46 | −4.22 | 189.65±14.77 | 7.79 | −5.18 |
Data are shown as mean ± SD. RSD, relative standard deviation; RE, relative error.
Matrix effect and recovery
The range of IS-normalized matrix effects in human plasma was 41.87–44.24% for FTFSLDTSK, 53.48–59.52% for VLIYFTSSLHSGVPSR and 47.67–49.65% for STAYLQMNSLR. The matrix effect of every surrogate peptide was in the same level for the three different QC concentrations. The recovery range in plasma was 22.11–23.59% for FTFSLDTSK, 21.78–24.65% for VLIYFTSSLHSGVPSR and 22.74–23.29% for STAYLQMNSLR. The RSD of IS-normalized matrix effects and recovery was <20% (Table IV).
Table IV.
Matrix effect and recovery of FTFSLDTSK, VLIYFTSSLHSGVPSR, STAYLQMNSLR and P14R.
| Nominal concentration of bevacizumab, µg/ml | Selected peptides FTFSLDTSK | VLIYFTSSLHSGVPSR | STAYLQMNSLR | IS P14R | ||||
|---|---|---|---|---|---|---|---|---|
|
|
|
|
|
|||||
| Mean ± SD | RSD,% | Mean ± SD | RSD,% | Mean ± SD | RSD, % | Mean ± SD | RSD,% | |
| Matrix effect, % (n=10) | ||||||||
| 10 | 32.98±3.27 | 4.82 | 46.06±6.53 | 6.15 | 37.21±3.86 | 5.04 | 82.30±3.28 | 10.14 |
| 50 | 31.83±1.67 | 6.28 | 43.84±3.44 | 5.27 | 38.46±2.57 | 4.95 | ||
| 200 | 34.80±1.31 | 4.1 | 42.12±0.99 | 3.24 | 36.93±1.26 | 3.47 | ||
| IS-normalized matrix factor, % (n=10) | ||||||||
| 10 | 41.87±3.47 | 5.49 | 59.52±7.16 | 6.55 | 48.03±4.96 | 4.93 | ||
| 50 | 41.89±2.54 | 8.65 | 57.60±3.21 | 7.66 | 49.65±4.28 | 7.25 | ||
| 200 | 44.24±4.18 | 6.41 | 53.48±3.90 | 6.24 | 47.67±3.95 | 5.6 | ||
| Recovery, % (n=10) | ||||||||
| 10 | 22.86±1.10 | 15.61 | 23.15±1.42 | 14.17 | 22.74±1.28 | 12.79 | 77.47±4.49 | 6.05 |
| 50 | 22.11±1.39 | 5.24 | 21.78±1.15 | 7.84 | 23.29±1.47 | 8.79 | ||
| 200 | 23.59±0.97 | 3.78 | 24.65±0.80 | 2.34 | 22.91±0.94 | 2.97 | ||
IS, internal standard; P14R, Pro14-Arg.
Application of the method in the TDM of patients with NSCLC
The validated LC-MS/MS method was applied successfully in a trough concentration assay after injecting eight patients with NSCLC bevacizumab over three periods of treatment. Representative chromatograms of a plasma sample from a patient with NSCLC at 2 h after injecting bevacizumab for three periods of treatment are shown in Fig. 2. Patient characteristics are shown in Table V. The bevacizumab trough concentration in the eight patients with NSCLC and dose are shown in Table VI.
Table V.
Patient characteristics.
| Patient | Age, years | Sex | Height, cm | Weight, kg | BMI, kg/m2 | BSA, m2 | Disease stage | Combined pharmacotherapy |
|---|---|---|---|---|---|---|---|---|
| 1 | 36 | F | 172 | 52 | 17.6 | 1.562 | IV-B | PEM, CBDCA, ICO |
| 2 | 61 | F | 162 | 70 | 26.5 | 1.734 | IV-B | ICO |
| 3 | 50 | F | 170 | 82 | 28.4 | 1.934 | IV-B | OSI |
| 4 | 66 | M | 175 | 72.5 | 23.7 | 1.843 | IV-A | PTX |
| 5 | 64 | F | 168 | 53.6 | 19.0 | 1.558 | IV-B | AFA |
| 6 | 68 | F | 160 | 56.5 | 22.1 | 1.546 | IV-B | OSI |
| 7 | 82 | F | 155 | 49 | 20.4 | 1.48 | IV | STM |
| 8 | 56 | M | 168 | 63 | 22.3 | 1.678 | IV-A | PEM, CBDCA |
BMI, body mass index; BSA, body surface area; ICO, icotinib; PEM, pemetrexed; CBDCA, carboplatin; OSI, osimertinib; PTX, paclitaxel; AFA, afatinib; STM, sintilimab; M, male; F, female.
Table VI.
Quantification of bevacizumab in the plasma of patients, adjusted doses, tumor response and adverse events.
| Patient | Dose of bevacizumab, mg | Trough concentration, µg/ml | Tumor response after three courses of treatment | Bevacizumab-induced adverse events |
|---|---|---|---|---|
| 1 | 400 | 35.36 | PD | N/A |
| 2 | 500 | 32.05 | PD | Gum bleeding |
| 3 | 500 | 62.27 | SD | Gum bleeding |
| 4 | 500 | 33.48 | SD | N/A |
| 5 | 400 | 23.22 | SD | Gum bleeding, hemorrhinia |
| 6 | 500 | 40.65 | PD | N/A |
| 7 | 300 | 29.23 | PD | N/A |
| 8 | 500 | 37.60 | SD | Hemorrhinia |
Short-term efficacy evaluation index. SD, stable disease; PD, progressive disease; N/A, not applicable.
Patient demographic characteristics
Table V shows the characteristics of the eight patients. Patients had been diagnosed with either postoperative recurrence or metastatic NSCLC, all of which were classified as adenocarcinoma. In total, 20 genes associated with lung cancer were identified by next-generation sequencing (NovaSeq 6000; Illumina, Inc.) for personality usage of molecular targeted drugs, including ALK, ATM, BRAF, BRCA1, BRCA2, DDR2, EGFR, ERBB2, HRAS, KIT, KRAS, MET, NARS, NTRK1, NTRK2, NTRK3, PDGFRA, PIK3CA, RET and ROS1, in the pathology laboratory of the China-Japan Friendship Hospital. The genetic results of the patients were retrieved from their electronic medical records. Patient 1, 2, 3 and 5 had EGFR 19 exon deletion. Patient 4 and 6 had EGFR 20 exon and 21 exon mutation, respectively. Patient 7 had an ERBB2 20 exon insertion mutation and patient 8 had no gene mutations.
Bevacizumab trough concentration and adverse events
In total, eight analytes collected after ≥3 periods of treatment were treated as the bevacizumab trough concentration. The median bevacizumab trough concentration was found to be 34.42 µg/ml, with a range of 23.22–62.27 µg/ml. Tumor responses in all of the patients were evaluated. Patient 1, 2, 6 and 7 were considered as progressive disease, whereas patient 3, 4, 5 and 8 were shown to exhibit stable disease. The most common adverse effects observed were gum bleeding and hemorrhinia with 50% incidence rate.
Discussion
In the present study, a determination method of bevacizumab in human plasma by UPLC-MS/MS method was fully validated. The method meets the requirements for the quantitative measurements of bevacizumab human plasma sample, which was also successfully applied in a trough concentration analysis of eight patients with NSCLC.
A short and stable isotope-labeled peptide, P14R, was chosen as the IS in the present study due to availability. In addition, it has been previously used for the quantification of antibody-based drugs for biological analysis (17). The method reported in the present study allows for a throughput of ≥100 samples per day on a single LC-MS/MS platform due to a relatively short injection to injection time of 6 min during the chromatography step. P14R was a chemically-synthesized stable isotope labeled (SIL) peptide which was an alternative selection. SIL peptides with flanking amino acids on their C- and N-terminals were used as IS to minimize the variability during sample processing and detection.
Evaluation of processing recovery contains four different parts, namely pre-digestion recovery, digestion recovery, post-digestion recovery and overall digestion. Each procedure can result in recovery loss. The recovery of the present method was at 20–25%, which to the best of our knowledge, has not been previously reported. Although it remains unclear at present which of the procedures resulted in recovery loss, sensitivity of the present method was sufficient for the detection of bevacizumab in human plasma samples. Optimizing this aspect of the protocol is required in future studies.
Subsequently, in the present study, eight patients were injected with 7.5 mg/kg body weight bevacizumab. In particular, five patients were injected with 500 mg bevacizumab, with a trough concentrations range of 32.054–62.266 µg/ml, which was variable among individuals. The trough concentrations in the two patients injected with 400 mg bevacizumab were 33.726 and 23.217 µg/ml, respectively, whereas the trough concentration of one patient injected with 300 mg bevacizumab was 29.225 µg/ml. The individual differences of bevacizumab trough concentrations suggested that TDM is promoted for bevacizumab in adjusting the therapeutic strategy. Additional plasma samples from patients with NSCLC are required for detecting the bevacizumab trough concentration and explore the TDM range for individualized drug administration.
TDM-guided approach has been proved to improve efficacy and reduce toxicity for several classes of small-molecule and therapeutic antibody drugs. In the case of bevacizumab in NSCLC, the TDM approach shows the potential to provide possibilities for patients with NSCLC to achieve the goal of personalized treatment. Additional samples are required to investigate the rational bevacizumab TDM range for different subtypes of NSCLC and to identify the influence factors of bevacizumab pharmacokinetics.
Acknowledgements
Not applicable.
Glossary
Abbreviations
- UPLC-MS/MS
ultra-performance liquid chromatography tandem mass spectrometry
- LLOQ
lower limit of quantitation
- OS
overall survival
- mCRC
metastatic colorectal cancer
- NSCLC
non-small cell lung cancer
- TDM
therapeutic drug monitoring
- mAb
monoclonal antibodies
- nSMOL
nano-surface and molecular-orientation limited
- MRM
multiple reaction monitoring
- P14R
Pro14-Arg
- QC
quality control
- LQC
low quality control
- MQC
middle quality control
- HQC
high quality control
- IS
internal standard
- RE
relative error
- RSD
relative standard deviation
Funding Statement
The present study was supported by National High Level Hospital Clinical Research Funding (grant no. 2022-NHLHCRF-LX-01-0303), CAMS Innovation Fund for Medical Sciences (grant no. 2021-I2M-1-012), Research Fund of China-Japan Friendship Hospital (grant no. 2019-2-QN-66), Bethune Charitable Foundation (grant no. B-19-H-20200622) and National Key Research and Development Program of China (grant no. 2020YFC2005504).
Availability of data and materials
The data generated in the present study may be requested from the corresponding author.
Authors' contributions
BL and XLZ contributed to the study conception and design and prepared the materials. BL optimized the methodology and wrote the original draft. MY conducted clinical evaluation. XXW, WQC and LS performed the method validation. XBZ, PML and LHL were responsible for recording clinical data and interpretation of the data. HKL, XYL and GW performed the data analysis and statistics. XLZ reviewed and edited the manuscript and gave final approval of the version to be published. BL, MY and PML acquired funding. BL, MY and XLZ confirm the authenticity of all the raw data. All authors have read and approved the final version of the manuscript.
Ethics approval and consent to participate
Research involving human subjects complied with all relevant national regulations, institutional policies and is in accordance with the tenets of the Helsinki Declaration, and has been approved by the Institutional Review Board of China-Japan Friendship Hospital (approval no. 2021-111-K69; Beijing, China).
Patient consent for publication
Written informed consent was obtained from all individuals included in the present study for publication of their data and associated images.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Shih T, Lindley C. Bevacizumab: An angiogenesis inhibitor for the treatment of solid malignancies. Clin Ther. 2006;28:1779–1802. doi: 10.1016/j.clinthera.2006.11.015. [DOI] [PubMed] [Google Scholar]
- 2.Lu JF, Bruno R, Eppler S, Novotny W, Lum B, Gaudreault J. Clinical pharmacokinetics of bevacizumab in patients with solid tumors. Cancer Chemother Pharmacol. 2008;62:779–786. doi: 10.1007/s00280-007-0664-8. [DOI] [PubMed] [Google Scholar]
- 3.Li J, Gupta M, Jin D, Xin Y, Visich J, Allison DE. Characterization of the long-term pharmacokinetics of bevacizumab following last dose in patients with resected stage II and III carcinoma of the colon. Cancer Chemother Pharmacol. 2013;71:575–580. doi: 10.1007/s00280-012-2031-7. [DOI] [PubMed] [Google Scholar]
- 4.Tabrizi MA, Tseng CM, Roskos LK. Elimination mechanisms of therapeutic monoclonal antibodies. Drug Discov Today. 2006;11:81–88. doi: 10.1016/S1359-6446(05)03638-X. [DOI] [PubMed] [Google Scholar]
- 5.Gao B, Yeap S, Clements A, Balakrishnar B, Wong M, Gurney H. Evidence for therapeutic drug monitoring of targeted anticancer therapies. J Clin Oncol. 2012;30:4017–4025. doi: 10.1200/JCO.2012.43.5362. [DOI] [PubMed] [Google Scholar]
- 6.Glade Bender JL, Adamson PC, Reid JM, Xu L, Baruchel S, Shaked Y, Kerbel RS, Cooney-Qualter EM, Stempak D, Chen HX, et al. Phase I trial and pharmacokinetic study of bevacizumab in pediatric patients with refractory solid tumors: A children's oncology group study. J Clin Oncol. 2008;26:399–405. doi: 10.1200/JCO.2007.11.9230. [DOI] [PubMed] [Google Scholar]
- 7.Wu JY, Wu XN, Ding L, Zhao YB, Ai B, Li Y, Hu X, Cheng G. Phase I safety and pharmacokinetic study of bevacizumab in Chinese patients with advanced cancer. Chin Med J (Engl) 2010;123:901–906. [PubMed] [Google Scholar]
- 8.Papachristos A, Kemos P, Kalofonos H, Sivolapenko G. Correlation between bevacizumab exposure and survival in patients with metastatic colorectal cancer. Oncologist. 2020;25:853–858. doi: 10.1634/theoncologist.2019-0835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ternant D, Cézé N, Lecomte T, Degenne D, Duveau AC, Watier H, Dorval E, Paintaud G. An enzyme-linked immunosorbent assay to study bevacizumab pharmacokinetics. Ther Drug Monit. 2010;32:647–652. doi: 10.1097/FTD.0b013e3181ef582a. [DOI] [PubMed] [Google Scholar]
- 10.Legeron R, Xuereb F, Chaignepain S, Gadeau AP, Claverol S, Dupuy JW, Djabarouti S, Couffinhal T, Schmitter JM, Breilh D. A new reliable, transposable and cost-effective assay for absolute quantification of total plasmatic bevacizumab by LC-MS/MS in human plasma comparing two internal standard calibration approaches. J Chromatogr B Analyt Technol Biomed Life Sci. 2017;1070:43–53. doi: 10.1016/j.jchromb.2017.10.042. [DOI] [PubMed] [Google Scholar]
- 11.Iwamoto N, Umino Y, Aoki C, Yamane N, Hamada A, Shimada T. Fully validated LCMS bioanalysis of bevacizumab in human plasma using nano-surface and molecular-orientation limited (nSMOL) proteolysis. Drug Metab Pharmacokinet. 2016;31:46–50. doi: 10.1016/j.dmpk.2015.11.004. [DOI] [PubMed] [Google Scholar]
- 12.Todoroki K, Mizuno H, Sugiyama E, Toyo'oka T. Bioanalytical methods for therapeutic monoclonal antibodies and antibody-drug conjugates: A review of recent advances and future perspectives. J Pharm Biomed Anal. 2020;179:112991. doi: 10.1016/j.jpba.2019.112991. [DOI] [PubMed] [Google Scholar]
- 13.Chen J, Zheng X, Liu DY, Zhao Q, Wu YW, Tan FL, Wang YX, Jiang J, Hu P. Therapeutic effects and adverse drug reactions are affected by icotinib exposure and CYP2C19 and EGFR genotypes in Chinese non-small cell lung cancer patients. Asian Pac J Cancer Prev. 2014;15:7195–7200. doi: 10.7314/APJCP.2014.15.17.7195. [DOI] [PubMed] [Google Scholar]
- 14.Liu D, Jiang J, Zhang L, Tan F, Wang Y, Zhang D, Hu P. Clinical pharmacokinetics of icotinib, an anti-cancer drug: Evaluation of dose proportionality, food effect, and tolerability in healthy subjects. Cancer Chemother Pharmacol. 2014;73:721–727. doi: 10.1007/s00280-014-2398-8. [DOI] [PubMed] [Google Scholar]
- 15.Liang W, Wu X, Fang W, Zhao Y, Yang Y, Hu Z, Xue C, Zhang J, Zhang J, Ma Y, et al. Network meta-analysis of erlotinib, gefitinib, afatinib and icotinib in patients with advanced non-small-cell lung cancer harboring EGFR mutations. PLoS One. 2014;9:e85245. doi: 10.1371/journal.pone.0085245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jiang H, Zeng J, Titsch C, Voronin K, Akinsanya B, Luo L, Shen H, Desai DD, Allentoff A, Aubry AF, et al. Fully validated LC-MS/MS assay for the simultaneous quantitation of coadministered therapeutic antibodies in cynomolgus monkey serum. Anal Chem. 2013;85:9859–9867. doi: 10.1021/ac402420v. [DOI] [PubMed] [Google Scholar]
- 17.Brun V, Masselon C, Garin J, Dupuis A. Isotope dilution strategies for absolute quantitative proteomics. J Proteomics. 2009;72:740–749. doi: 10.1016/j.jprot.2009.03.007. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data generated in the present study may be requested from the corresponding author.