Abstract
The objective of this study was to explore a novel methodology for the synthesis of nanocoated probiotics following their collection and cultivation under optimized conditions, in light of their significant contribution to human health. Probiotics are instrumental in sustaining immune health by modulating the gastrointestinal microbiota and facilitating digestion. However, the equilibrium they maintain can be adversely affected by antibiotic treatments. It is critical to investigate the vulnerability of probiotics to antibiotics, considering the potential implications. This research aimed to assess whether nanoparticle coating could augment the probiotics' resistance to antibiotic influence. A strain of Lactococcus lactis (L. lactis) was isolated, cultured, and comprehensively characterized utilizing state-of-the-art methodologies, including the VITEK® 2 compact system, VITEK® MS, and 16S rRNA gene sequencing. The nanoparticle coating was performed using iron (III) chloride hexahydrate and tannic acid, followed by an evaluation of the probiotics' resistance to a range of antibiotics. The analysis through scanning electron microscopy (SEM) and atomic force microscopy (AFM) demonstrated a partial nanoparticle coating of the probiotics, which was further supported by UV/Vis spectroscopy findings, suggesting enhanced resistance to standard antibiotics. The results revealed that this strain possesses a unique protein profile and is genetically similar to strains identified in various other countries. Moreover, nano-encapsulation notably increased the strain's resistance to a spectrum of standard antibiotics, including Benzylpenicillin, Teicoplanin, Oxacillin, Vancomycin, Tetracycline, Rifampicin, Erythromycin, and Clindamycin. These findings imply that nanoparticle-coated probiotics may effectively counteract the detrimental effects of extended antibiotic therapy, thus preserving their viability and beneficial influence on gastrointestinal health.
Keywords: Antibiotic resistance, Nano-particles, Probiotics, Lactococcus lactis
1. Introduction
The World Health Organization (WHO) defines probiotics as living bacteria that, when taken in the proper dosage, will boost the patient's health, resulting in protection against many diseases (Reid, 2016, Vasiee et al., 2019). Some fermented foods act as sources of many species of probiotic bacteria, as their consumption can produce an adjunct effect on the gut flora (Hotel and Cordoba, 2001).
Probiotics can offer numerous health benefits and applications that serve human health such as decreasing the toxic activity of microbes, preserving the healthy gut microflora, improving the regular operation of the digestive system and fostering overall well-being, and enhancing immunity (Lee et al., 2013).
Several studies suggested that probiotic bacteria have a significant role in treating gut disorders such as antibiotic-associated diarrhea, and travelers' diarrhea. The most frequently used strains are Lactobacillus lactis and Bifidobacterium bacteria, which maintain a healthy digestive tract and support women's vaginal health (Saarela et al., 2002).
Lactococcus is one of the most predominant microbiotas of the gastrointestinal tract. It is the most widely distributed probiotic bacteria in many dietary supplements because of its ability to regulate the intestine's permeability (Vasiee et al., 2019).
In addition, it has fundamental effects on human health, contributing to allergy treatments and enhancing immunity barrier functions. It also plays a vital role in preserving a balance of anti-inflammatory cytokines (Borriello et al., 2003).
A beneficial probiotic should have advantageous effects on the host as it should be free of pathogens, toxins, and non-desirable side effects. Additionally, it should be able to tolerate the gastrointestinal tract acidity, enzymes, and bile salts to survive (Kurokawa et al., 2007).
Nanotechnology deals with the atomic or molecular scale of matter. The factors that define the characteristics of a nanoparticle are its physical and chemical properties, such as surface area, surface charge, hydrophobicity of the surface, thermal stability of the nanoparticle, and antimicrobial activity (Sharma et al., 2019). The microscopic techniques can be used for nano-particle characterization as their shape, size, and location can be observed and investigated by producing nano-layer images. Many observational advanced technologies can be used to examine and characterize the structural properties of nano-structured layers, such as SEM, AFM, transmission electron microscopy/high-resolution transmission electron microscopy (TEM/HRTEM), and scanning tunneling microscopy (STM). The spectroscopic technique (UV/Vis) can be used to study nano-particle interaction with electromagnetic radiations as a function of wavelength (Liong, 2011).
According to (Centurion et al., 2022), many challenges were reported when dealing with probiotics, such as pH, oxygen level effects, and sensitivity to several standard antibiotics. The continuous process of scientific investigation has revealed a wide range of varied strains that are well-suited for the use of probiotics. This is demonstrated by the identification of three strains of Pediococcus spp. obtained from fermented food. These particular strains, which exhibit resilience to acidic conditions and bile salts, have demonstrated exceptional abilities in reducing cholesterol levels and inhibiting the growth of pathogenic strains. This represents a notable advancement in the field of probiotic research. In our case study, we employed a more secure methodology to enhance the characteristics of this advantageous bacterial strain and enable it to circumvent the issue of antibiotic resistance (Vasiee et al., 2020a).
In this study, we focused on evaluating the effects of nano-coating probiotics on antibiotic sensitivity and whether this offers a promising method for protecting probiotics during their delivery challenges across GIT. The differences between nano-coating and non-coating probiotics toward antibiotic resistance were noticed and they were evaluated using SEM, AFM, and UV/vis technologies.
2. Materials and methods
2.1. Collection of probiotic products (probiotic's source)
The fermented dairy products are considered an essential source of Probiotics (Ali et al., 2022). The commercial probiotic dairy products marketed in the Riyadh region, Saudi Arabia (SA) were collected and maintained in adequate conditions for transportation to the microbiology laboratory located at King Saud University (KSU). Collected samples were labeled with specific codes. The collection date, and time were recorded. The chosen product contains probiotic strains with distinct biological properties as the manufacturer's company claims.
2.2. Growth conditions, isolation, purification, and maintenance of probiotics
The collected samples were maintained in suitable physical conditions before isolating probiotic bacteria from it, and they were processed quickly under aerobic and anaerobic conditions (in an anaerobic jar in a CO2 incubator 5 %), and their dilution and cultivation were performed immediately in the selective media Man-Rosgosa-Sharpe Agar (MRS) (Merck, Germany). Incubation was performed at 37 °C for 48–72 hrs., then, the isolates were transferred to new agar plates (Mac Faddin, 1985).
The purified isolated strain was coded as (A). The total viable probiotic cells (colony forming unit per ml) were counted following the usual plate count approach. The purified probiotic strain was also cultivated in MacConkey (Oxoid ®), England) and blood agar (Oxoid ®), England), and its macroscopic characteristics were observed. Isolates were then kept in 30 % sterile glycerol solution and cryopreserved at −80 °C for further investigations (Fontana et al., 2013).
2.3. Identification of probiotics
2.3.1. Macro- and microscopic features
Fresh probiotic isolate and its replicates were obtained after the third sub-culturing on MRS media (the cultured plates were checked every 24 hrs.). The macroscopic features such as the shape, color, and pigmentation were investigated. Moreover, the odor and viscosity of the isolate were assessed during its inoculation under sterile conditions. The microscopic features as cell size, shape, cell aggregation, and Gram stain response were detected by conventional light microscopy (Olympus, Japan) under 1000X of oil immersion objective lens (Shaaban et al., 2012).
2.3.2. Biochemical characterization and antibiotic susceptibility testing (AST) using VITEK® 2 compact system
The identification (ID), and AST of the unknown probiotic isolate (A) were performed using the fully automated VITEK® 2 compact system (BioMérieux, France) (Pan et al., 2022), where the suspension of L. lactis inoculums was prepared by adding a sufficient inoculum of (A) isolate to saline, and the McFarland turbidity of (0.50) was adjusted using DensiChek (BioMerieux, France) (Klare et al., 2007).
The Gram-positive identification card (GP ID) which includes 43 biochemical tests that estimate the bacterial enzymatic activities, carbon source usage, resistance, and inhibition. In addition to, 592 AST card and color-coded indicators which designed to provide accurate AST results and resistance detection were used (Elmaghrabi and Ghozlan, 2019).
The biochemical tests revealed from an unknown (A) strain was compared to the VITEK system databases for its final accurate identification (Pincus, 2006). As well as, the validation of every susceptibility test result with an accurate bacterial phenotypic profile resistance mechanism(s) was provided (Nakasone et al., 2007).
2.3.3. VITEK® mass spectrometry system (VITEK® MS)
The VITEK® MS system (bioMérieux, France) uses the MALDI-TOF (Matrix Assisted Laser Desorption Ionization Time-of-Flight) mass spectrometry technology to analyze particles (mainly proteins) from crystalized sample materials was used. Results were expressed as a mass-to-charge ratio (m/z) and were measured by mass spectrometry after molecular ionization by laser pulses. The mass spectrum was identified and contrasted with a mass spectrum library database. Finally, providing a protein fingerprint was obtained (Lay, 2001, Sandrin et al., 2013). Microbial identification and classification were obtained at the species, genus, and family level, in which the Escherichia coli (E. coli) ATCC 8739 was used as a calibrator microorganism with positive and negative bacterial control (Nanchen et al., 2007, Smart et al., 2010). obtained graph was translated to the identification by bioMérieux algorithm, using the VITEK MS database, which contains 510(k) microbial databases where the diverse list of protein sequences was translated from the mass spectra (Sandrin et al., 2013). Results were interpreted by VITEK MS v3.2, and the identification process was performed three times.
2.3.4. 16S rRNA gene amplification and sequencing
The 16S rRNA gene consists of highly conserved hypervariable sequences, which can be utilized for confirmatory bacterial identification and taxonomic classification (Hugenholtz et al., 1998, O'Sullivan, 2000). A high yield of the total DNA was extracted, a fragment of the gene was amplified, DNA sequencing of the amplified product was performed, and a phylogenetic tree was constructed.
2.3.4.1. Total DNA extraction
The total DNA of the pure isolates was extracted using DNeasy® Blood & Tissue Kit (Qiagen Ltd., Crawley, West Sussex, UK) according to the manufacturer's multi-step instructions. Briefly, bacterial cells were harvested by centrifugation at 7500 rpm/10 min. Bacterial pellets were washed using sterile normal saline, resuspended in 180 µl enzymatic lysis buffer, and incubated at 37 °C/30 min. Next, 25 µl proteinase K and 200 µl buffer AL were added, mixture vortexed, and re-incubated at 56 °C/30 min. A 200 µl ethanol (96 %) volume was then added to the sample with shaking. The mixture was pipetted into the DNeasy Mini spin column and centrifuged. The column was then placed in a new collection tube, followed by a buffer addition step with centrifugation at 6000 rpm/1 min. This step was carried out twice, using 500 µl of AW1 and AW2 buffers, and then centrifuged at 14000 rpm/3 min to dry the DNeasy membrane. Finally, to elute, the Mini spin column was put in a 2 ml microcentrifuge tube, and 100 µl buffer AE was pipetted into DNeasy membrane after incubation at room temperature/1 min and centrifugation. The elution step was repeated to obtain a high DNA yield (Knebelsberger and Stöger, 2012). DNA purity was determined using nanodrop Jenway™ 7200 Visible Scanning Spectrophotometer (Thermo Fisher Scientific Inc., UK).
2.3.4.2. 16S rRNA gene amplification
DNA extraction was then followed by PCR amplification on one or more selected hypervariable areas of the 16S rRNA gene using Genius® Thermal Cycler-FGEN05TD (Techne, Cambridge, UK). The PCR mixture contained Hot StarTaq® Master Mix Kit 250 units (QIAGEN GmbH, Hilden, Germany) and universal primer pair to the 16S rRNA conserved gene sequences (27F: 5′ AGA GTT TGA TCC TGG CTC AG 3′ and 1492R: 5′ GGT TAC CTT GTT ACG ACT T 3′) (El-Desouky et al., 2023). The following thermal cycling conditions were applied: initial denaturation at 95 °C for 15 min, then 35 cycles of denaturation at 95 °C for 30 sec, annealing at 60 °C for 30 sec, extension at 72 °C for 2 min, and a final extension step at 72 °C for 7 min, then the program was held at 4 °C. PCR products were electrophoretically examined using 1.2 % (w/v) agarose gel and visualized by ethidium bromide stain under a UV transilluminator (Major Science, Taiwan.) An image was captured by a digital camera (Nikon, Japan).
2.3.4.3. 16S rRNA gene sequencing
The PCR product (about 1500 bp) was sent to the Qiagen® lab in Korea for sequencing. The PCR process employed the primers 518F (CCAGCAGCCGCGGTAATACG) and 800R (TACCAGGGTATCTAATCC) (Kim et al., 2010). PCR product was subjected to sequencing using the BigDye Terminator Cycle Sequencing Kit. Sequencing reactions were conducted in accordance with the manufacturer's instructions (Applied Biosystems, USA). The sequencing system employed for resolving the sequencing products was the Applied Biosystems model 3730XL, which was automated. Capillary electrophoresis-based sequencing system described herein is widely employed for high-throughput DNA sequencing. The final identity of the probiotic strain was obtained after BLAST analysis by comparing its sequence to those of closely related type strains in the NCBI Gene bank database (https://blast.ncbi.nlm.nih.gov/Blast.cgi). Max Score, total score, query cover, E value, and percentage of identification were used to determine the closest strain to the strain isolated in this work.
2.4. Nano-Coating and characterization of identified probiotic isolate (A)
2.4.1. Synthesis of nano-coated probiotic
According to (Pan et al., 2022), the probiotic isolate was coated by nano-particles by washing 2x105 CFU/mL of probiotics suspension with phosphate buffer solution (PBS) three times, and then it was suspended in a 600 µl PBS solution. Then, 50 µl of (1.25 mg/ml) iron (III) chloride hexahydrate and 50 µl of (5 mg/ml) tannic acid solutions were added to the probiotic's suspension. Finally, 300 µl PBS was added to obtain a 1 ml coated probiotic solution. Vortexing after each step was performed for ten seconds as a crucial step for a successful coating process.
2.4.2. AST of nano-coated probiotic
The suspension of nano-coated L. lactis bacterium was prepared according to Klare et al., 2007, and it was tested for its antibiotic susceptibility by using the VITEK® 2 compact system, and the results were compared to the non-coated probiotics.
2.4.3. Scanning electron microscopy (SEM)
Scanning electron microscopy is a powerful technology that enables the acquisition of high-resolution visual representations of the surface characteristics of isolated probiotic microorganisms (Yassin et al., 2023). Both coated and non-coated probiotic samples were prepared for their investigation by fixation using buffered glutaraldehyde, rinsing with sodium cacodylate, and then post-fixing in osmium tetroxide. After rinsing with distilled water, dehydration of the probiotic samples with graded ethanol, serial concentrations of 25 %, 50 %, 75 %, 90 %, and 100 % was carried out (de Britto et al., 2012). Finally, a drying step using a critical point dryer device was performed to prepare the samples for SEM visualization. Spray-gold was applied to the samples before observation.
The samples' surfaces were photographed using high-energy electron beam scanning. Samples were observed on the high-resolution and low-vacuum SEM model JSM-6380 (JEOL, Japan). Three-dimensional (3D) images were obtained, functional for understanding their surface structure with highly magnified images plus enhanced resolution. The micro- and nano-features of free and treated probiotics were subsequently examined for their discrimination, with information about their purity and spherical morphology provided by this technique (Sharma et al., 2019).
2.4.4. Atomic force microscopy (AFM)
The purpose of using AFM is to measure local characteristics such as height, magnetism, and particle friction where only a small sample's area is scanned (Binnig et al., 1986). In this study, AFM (NanoScope V, 3D taping mode imaging, Bruker SPMs, United States) was used for bacterial ultrastructure investigation, in which the probiotic cells of non-coated and nano-coated probiotics were suspended in 0.89 % serial normal saline and immobilized on a polyether sulfone (PES) surface, which acts as a technical trap for bacterial cells. The PES surface membrane was washed three times with normal saline to remove immobilized cells. Next, an AFM tapping mode (Nasoscope V, Veeco-Bruker SPMs) with a silicon tip (SNL cantilever, Bruker) was used to scan the membrane. The parameters were as follows: a nominal spring constant of 0.3 N/m, a resonance frequency of 65 kHz, and a scanning rate of 0.7 Hz. Nano-Scope Analysis 1.3 software was used to process the photos (Alharbi et al., 2017).
2.4.5. Ultraviolet–visible spectrophotometry (UV–Vis)
In this technique, a light beam is directed through the sample, and the UV–vis spectrophotometer measures the quantity of light absorbed concerning the wavelength or frequency function. The concentration of the absorbing component in the sample is directly correlated with the amount of light absorbed (Baldock and Hutchison, 2016).
In this study, analysis was performed by detecting the absorbance within the UV–Vis spectral range of 200–700 nm of coated and non-coated samples using the UV-6300PC spectrophotometer (VWR®, USA) at a resolution of 1 nm. Coated and non-coated probiotics were harvested and washed by centrifugation, and then the bacterial suspensions (O.D 0.65 at 570 nm) were subjected to a wavelength spectrum using UV–Vis spectroscopy. Sterile normal saline (0.89 % sodium chloride) represented the blank control. UV–Vis spectral analysis was expressed as an absorption spectrum graph (Cole and Levine, 2020). Samples were measured in a rectangular quartz cuvette with a one-centimeter length at 25 °C. Every determination was made on three different occasions.
2.5. Design of experiment and statistical analysis
The sample collections were randomly collected according to a completely random design without any discrimination. The phylogenetic tree was obtained by BLAST analysis. The AFM image processing was done with NanoScope Analysis 1.3. Three repetitions of the antibiotic susceptibility tests were conducted, and the standard errors were calculated.
3. Results
3.1. Probiotic identification and characterization
3.1.1. Conventional light microscopy (morphologically)
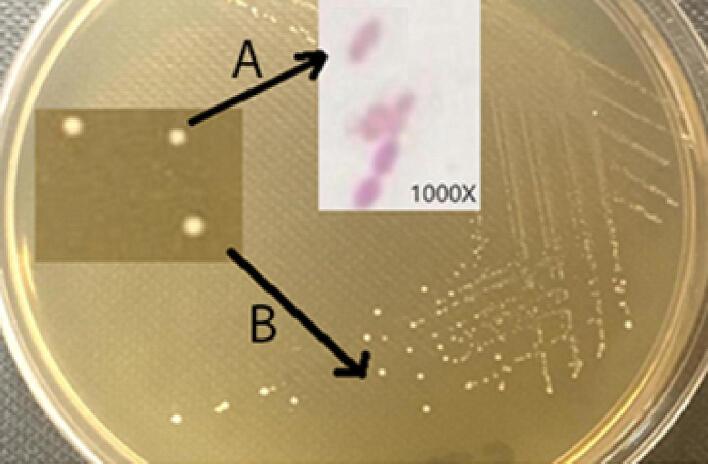
In this study, the identification and characterization of purified probiotic strain (A) were: the suitable selective media for its growth was MRS, no growth was noticed in MacConkey agar, it was grown in both blood and nutrient agar, it was Gram-positive bacterium, its shape was cocci, and some coccobacilli colonies were seen, its aggregation was occurred in pairs and in small chains. It was grown in aerobic and anaerobic conditions (facultative anaerobe), but the optimal and high growth rate was observed under anaerobic conditions. Additionally, it was a non-spore-forming, non-motile bacteria. All of these findings are summarized in Fig. 1 and Table 1.
Fig. 1.
Macroscopic and microscopic features of the probiotic isolate from diary probiotic product as: (a) bacterial colonies in MRS media show their color and morphology; (b) bacterial cells under conventional light microscopy showing their morphology, aggregation, and G-staining response.
Table 2.
The macroscopic and microscopic characteristics of the probiotic bacterium isolated from probiotic products marketed in Riyadh region in Saudi Arabia.
| Feature | Description |
|---|---|
| Colonies morphology | Circular |
| Cells morphology | Cocci and Coccobacilli |
| Aggregation | In pairs and small chains |
| Colonies color | opaque white plastic color |
| Gram Staining | Positive |
| Spore forming | None |
3.1.2. VITEK ® 2 compact system analysis
The fully automated VITEK® 2 compact system was used for identification (ID) and determination of antibiotic susceptibility testing (AST) of isolated probiotic strain, depending on their biochemical characterizations. With VITEK® 2 PC software and GP ID/ 592 AST cards, the final identification was L. lactis. Moreover, its antibiotic sensitivity testing results, shown in Table 3, will be discussed later in comparison to nano-coated probiotics in Table 4.
Table 4.
Antibiotic susceptibility testing data of isolated probiotic (non-coated / free probiotic).
| Antimicrobial | MIC* | Interpretation | Antimicrobial | MIC | Interpretation |
|---|---|---|---|---|---|
| Cefoxitin screen | NEG | – | linezolid | 2 | S |
| Benzylpenicillin | <= 0.03 | S | Teicoplanin | <= 0.5 | S |
| Oxacillin | <= 0.25 | S | Vancomycin | <= 0.5 | S |
| Gentamicin | <= 0.5 | S | Tetracycline | <= 1 | S |
| Tobramycin | <= 1 | S | Tigecycline | <= 0.12 | S |
| Levofloxacin | 0.25 | S | Nitrofurantoin | <= 16 | S |
| Moxifloxacin | <= 0.25 | S | Fusidic acid | 16 | R |
| Inducible clindamycin resistance | NEG | – | Rifampicin | <= 0.5 | S |
| Erythromycin | <= 0.25 | S | Trimethoprim/ sulfamethoxazole | <= 10 | S |
| Clindamycin | <= 0.25 | S |
N = 3, standard error = zero.
Table 5.
Antibiotic susceptibility testing data of nano-coated probiotic (treated probiotic).
| Antimicrobial | MIC* | Interpretation | Antimicrobial | MIC | Interpretation |
|---|---|---|---|---|---|
| Cefoxitin screen | TRM | – | linezolid | >= 8 | |
| Benzylpenicillin | >= 0.5 | R | Teicoplanin | >= 32 | R |
| Oxacillin | >= 4 | R | Vancomycin | <= 32 | R |
| Gentamicin | <= 0.5 | S | Tetracycline | >= 16 | R |
| Tobramycin | <= 1 | S | Tigecycline | >= 2 | |
| Levofloxacin | <=0.12 | S | Nitrofurantoin | 32 | S |
| Moxifloxacin | <= 0.25 | S | Fusidic acid | >= 32 | R |
| Inducible clindamycin resistance | NEG | – | Rifampicin | 4 | R |
| Erythromycin | >= 8 | R | Trimethoprim/ sulfamethoxazole | <= 10 | S |
| Clindamycin | >= 8 | R |
N = 3, standard error = zero.
3.1.3. Protein fingerprint of the probiotic isolate using MALDI-TOF MS
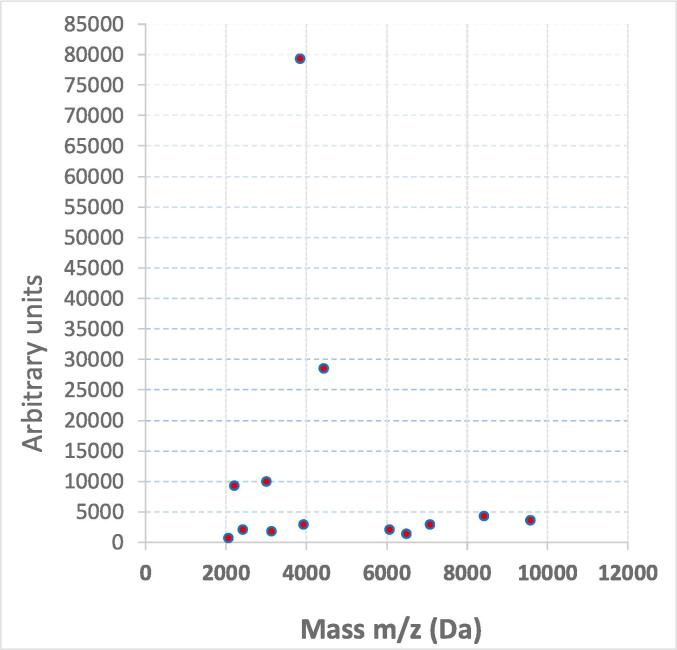
VITEK MS successfully identified the isolated probiotic strain; its protein spectra obtained from the cell lysates were compared to the reference VITEK MS database strains, with good peak content and high reproducibility. Upon the MALDI-TOF MS peak profiles analysis, the peaks obtained in Fig. 2 revealed final identification as L. lactis subsp. lactis, within the acceptable log score value (≥2.0). The 16S rRNA gene detection and sequence analysis subsequently confirmed this result.
Fig. 2.
MALDI-TOF MS protein fingerprint analysis of isolated probiotics shows the spectra of L. lactis subsp. Lactis, where the ions intensities are on the y-axis, the ions masses (in Daltons) are shown on the x-axis, and the m/z value is the mass-to-charge ratio (this value matches the protein molecular mass for each positive charge).
3.1.4. Probiotic's nucleic acid sequencing (16SrRNA gene sequencing analysis)
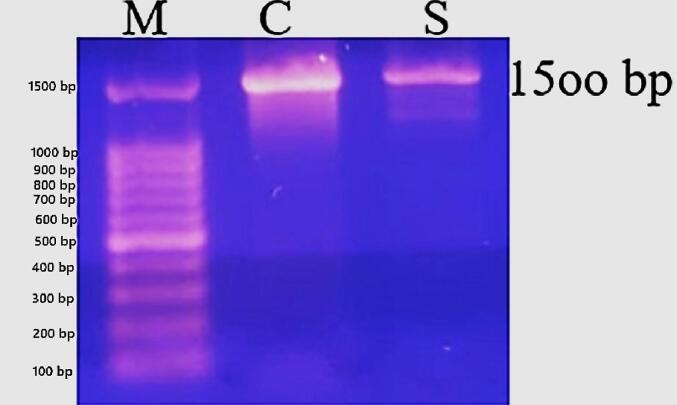
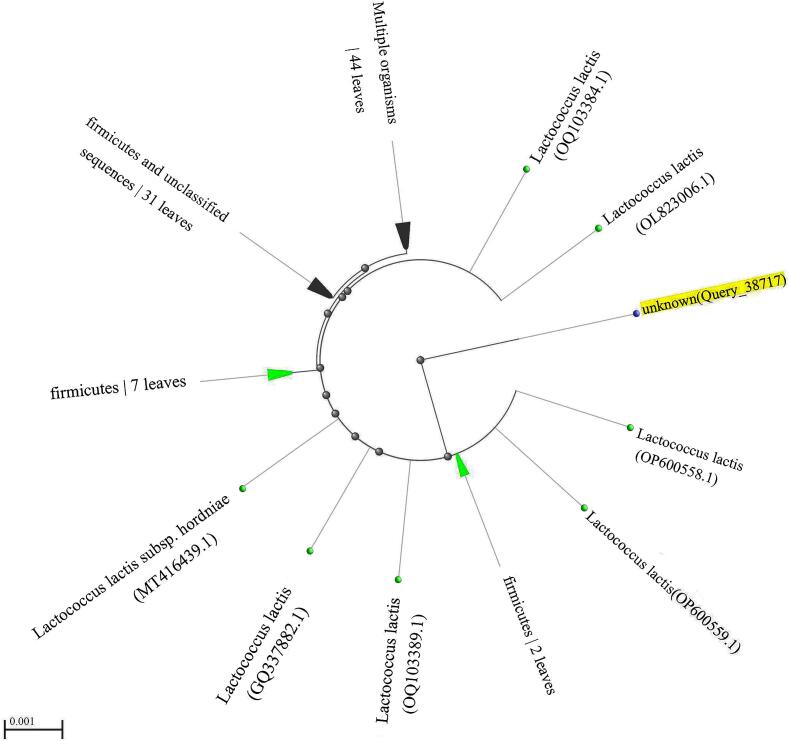
Gel electrophoresis for the 16S rRNA gene amplified using PCR revealed a band of 1500 bp size Fig. 3, distinct and conserved to bacterial identification. The sequencing of part of the 16S rRNA gene and BLAST analysis reported that the isolates were L. lactis strain. The description, scientific name, max score, total score, query cover, E value, percentage identification, accession length, and accession number (MH899252.1, ON746656.1, ON561782.1, OP600559.1, OP600558.1, and MH899238.1) were arranged in Table 2. According to the highest identification rate, the strain isolated in this study is closely related to OP600559.1 and OP600558.1. According to BLAST analysis Fig. 4, the strain isolated in the present work has distinctive characteristics that place it in a single group that distinguishes it from all reference isolates used in the analysis.
Fig. 3.
Ethidium Bromide stained 1.2 % agarose gel electrophoresis showing 16S rRNA gene amplicons (1500 bp). Lane S: Sample under test, Lane C: Positive Control (Staphylococcus aureus ATCC29213), Lane M: 100 bp molecular weight DNA Ladder. Gel was run at 100 Volt/45 min.
Table 3.
ID 16S rRNA strains incorporated in this study. Accession numbers deposited in the GenBank database for reference sequences in this study are shown in the right column.
| Description | Scientific Name | Max Score | Total Score | Query Cover | E value | Per. ident | Acc. Len | Accession |
|---|---|---|---|---|---|---|---|---|
| Lactococcus lactis subsp. lactis strain CE1.29 16S ribosomal RNA gene, partial sequence | Lactococcus lactis subsp. Lactis | 2126 | 2126 | 98 % | 0 | 97.67 | 1236 | MH899252.1 |
| Lactococcus lactis subsp. lactis strain BRM3 16S ribosomal RNA gene, partial sequence | Lactococcus lactis subsp. Lactis | 2108 | 2108 | 96 % | 0 | 97.88 | 1241 | ON746656.1 |
| Lactococcus lactis strain KMCM3 16S ribosomal RNA gene, partial sequence | Lactococcus lactis | 2098 | 2098 | 98 % | 0 | 97.19 | 1502 | ON561782.1 |
| Lactococcus lactis strain AL7 16S ribosomal RNA gene, partial sequence | Lactococcus lactis | 2097 | 2097 | 91 % | 0 | 99.48 | 1442 | OP600559.1 |
| Lactococcus lactis strain AL6 16S ribosomal RNA gene, partial sequence | Lactococcus lactis | 2097 | 2097 | 91 % | 0 | 99.48 | 1375 | OP600558.1 |
| Lactococcus lactis strain CE1.10 16S ribosomal RNA gene, partial sequence | Lactococcus lactis | 2093 | 2093 | 96 % | 0 | 97.71 | 1229 |
MH899238.1 |
Fig. 4.
Phylogenetic Tree ID BLAST Analysis of the tested isolate (yellow highlight). (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
3.2. Assessment of the nano-coated probiotic and investigation of its resistance to several antibiotics
3.2.1. SEM analysis
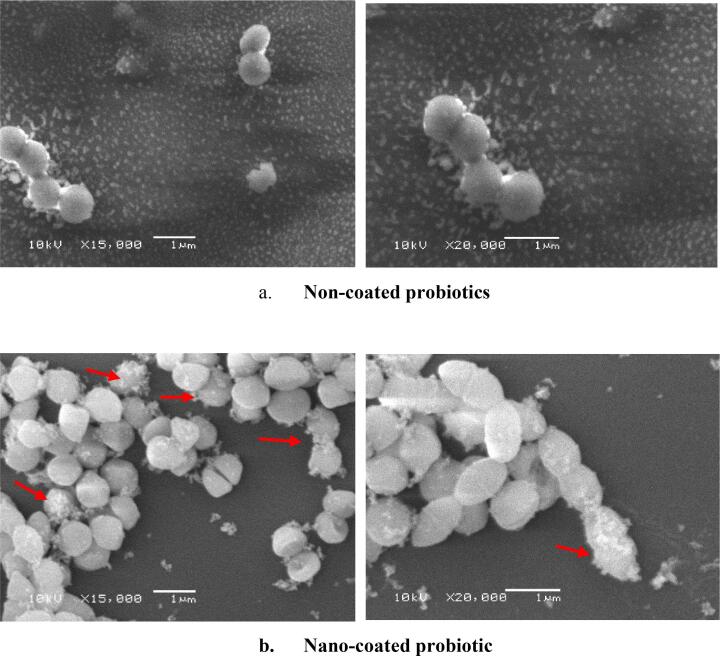
The SEM images of the control (non-coated probiotic) and the sample (nano-coated probiotic) are presented in Fig. 5. According to the observed results, non-coated probiotic particles showed a relatively smooth surface structure with size uniformity Fig. 5(a). Cell aggregations were of low grade, showing bi-layered arrangements per field. On the other hand, the nano-coat layer partially covered the bacterial cells, as shown in Fig. 5(b). Slight morphological changes of the uniform cocci are demonstrated, with some protrusions and indentations among treated cells. Enhanced aggregative distribution with less dispersion is also noted throughout the field.
Fig. 5.
Scanning electron microscopy examination of a. non-coated probiotic and b. nano-coated probiotic cells. Secondary electron imaging SEM revealed a very high-resolution detailed image with a size of 1 nm. The non-coated probiotic cells (a) appear ideally shaped and have a nano-layer-free surface. The morphological changes are clearly seen in nano-coated probiotic cells (b), with a nano-layer surrounding some cells (The red arrows denote some nano-coated probiotic cells). (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
3.2.2. AFM analysis
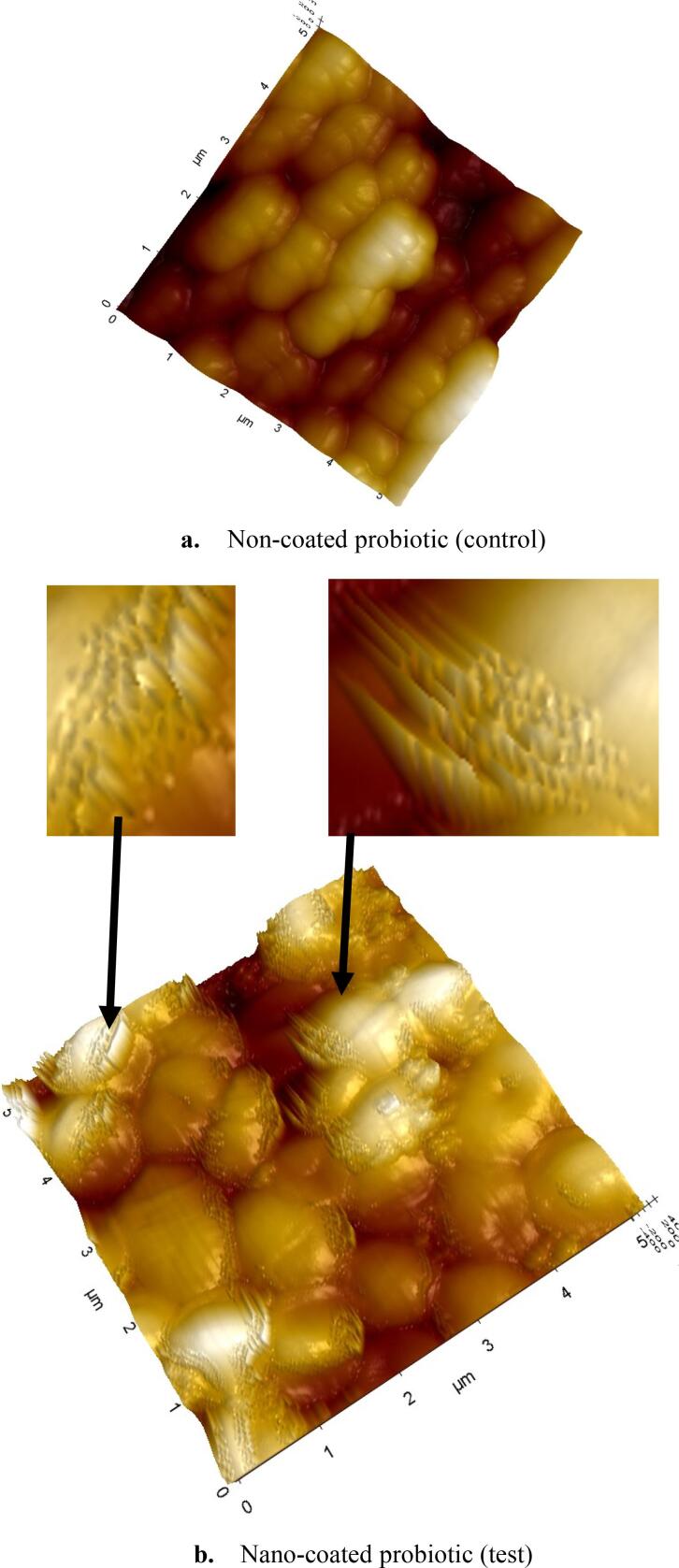
We were able to image bacterial cells with excellent resolution by using AFM and modified PES membranes as mechanical traps. Primary physical characteristics of non-coated and nano-coated L. lactis were observable, providing insight into the three-dimensional structure of the bacterial cells at the micro- and nanoscale (see Fig. 6).
Fig. 6.
AFM microscopic and ultrastructural features show the divergence between free and treated probiotics as: (a) non-coated probiotic; (b) nano-coated probiotic cells, as black arrows indicate that the nano-layer partially covers some cells. The figure does not show a fully coated cell.
3.2.3. AST analysis by VITEK® 2 compact system
The susceptibility testing information of free and treated probiotics revealed from VITEK® 2 compact system analysis is listed in Table 4, Table 5. After interpretation of the results, it was found that the nano-coated probiotics became resistant to many different standard antibiotics mentioned in Table 5, where it had become resistant to Benzylpenicillin, Oxacillin, Erythromycin, Clindamycin, Teicoplanin, Vancomycin, Tetracycline, Fusidic acid, and Rifampicin (written in bold in Table 4) after had been sensitive to them except for Fusidic acid before its coating by the nano-layers. Table 3 illustrates these findings.
4. Discussion
Previous research verified that, probiotics have effective and beneficial roles in human health as the prophylaxis and treatment of many diseases (Ehrmann et al., 2002). In our study, the findings revealed L. lactis subsp. lactis. is significant bacterium in the dairy industry, and frequently employed to produce fermented dairy products. Various factors influence fermented dairy products, including flavor, texture, and shelf life.
Certain strains of L. lactis have been investigated for their potential as probiotics, which can positively impact gastrointestinal well-being. It is suggested that this may positively impact the equilibrium of the gut microbiota, hence aiding in the preservation of a well-functioning digestive system (Vrdoljak et al., 2022).
Several clinical studies have examined the potential application of L. lactis strains, namely L. lactis subsp. lactis, in the management of several health disorders, including irritable bowel syndrome (IBS) and inflammatory bowel diseases (IBD) (Mileriene et al., 2023).
There are many promising technologies that could improve the properties of this microbe, for example the genetically modified strain of L. lactis, which has the Ama r 2 gene, demonstrated strong probiotic characteristics such as resilience to acid and bile, strong attachment to both E. coli and intestinal cells, and the ability to counteract E. coli infections. The results indicate that it has the potential to be a dependable substitute for probiotics in therapeutic applications, regardless of the presence of the allergen Ama r 2 gene (Vasiee et al., 2019). It has been assessed the potential of recombinant probiotic bacteria as an oral vaccine against Amaranthus retroflexus pollen allergy. After correcting vector structure, Lactococcus lactis NZ1330 successfully expressed the allergen Ama r 2, confirmed by western blot analysis. Oral immunotherapy using probiotic ice cream significantly reduced serum IgE levels and enhanced Th1 and Treg responses, suggesting its efficacy in allergy treatment (Vasiee et al., 2020b).
In the present investigation, a more reliable methodology was employed to enhance the characteristics of the beneficial bacterial strain, enabling it to circumvent the challenge of antibiotic susceptibility. it was found that to possess strong susceptibility spectra against most standard antibiotics. The enhancement of probiotic’s biological properties was performed after its coating by nanoparticles, and it was investigated by VITEK® 2 Compact System and morphologically confirmed by SEM and AFM technologies. Additionally, the UV–Vis spectra revealed findings about the UV–Vis spectra of both coated and non-coated samples that matched all previous tests results.
Traditional identification techniques are not only time-consuming and tedious, but they are also highly vulnerable to human error, unstable reagents, and variable laboratory circumstances. Being fast, with an accuracy of up to 99 % (Bowen et al., 2020). The VITEK MS represented a valuable tool for probiotic identification in our study.
An increasing number of fingerprints are continuously added to databases to improve identification accuracy as VITEK-MS is being developed and used (Mesureur et al., 2018, Quéro et al., 2019).
Sequencing data identified the isolate to the subspecies level at 98 % confidence. We were able to locate its phylogenetic genetic relatedness to close relatives. So far, L. lactis has been divided into four subspecies: L. lactis ssp. Lactis, L. lactis ssp. cremoris, L. lactis ssp. hordniae, and L. lactis ssp. tructae (Stiles and Holzapfel, 1997). A thorough analysis of the genotypic and phenotypic diversity of a considerable number of strains originating from dairy and nondairy origins verified the presence of the two principal genomic lineages, lactis and cremoris (Rademaker et al., 2007).
Numerous studies using various DNA techniques have documented the broad diversification of L. lactis. Multi-strain microarrays were used in a comparative genome hybridization investigation to identify if genes and gene clusters were present in these strains. Their findings confirmed that L. lactis is a highly versatile species in terms of its genome and that niche adaptation is a factor in its diversification (Quiberoni et al., 2001).
After coating of L. lactis subsp. lactis by nanoparticles using iron (III) chloride hexahydrate and tannic acid, the evaluation of AST of both non-coated and nano-coated probiotics, and differentiation of their susceptibilities to antibiotics were conducted by the VITEK® 2 compact system.
Sometimes, antibiotics cannot be effective in infectious diseases because of the resistance of pathogens to antibiotic/s (Scott, 2009). In this study, we found that L. lactis subsp. lactis has a strong sensitivity to all tested antibiotics except fusidic acid. On the other hand, after its coating by nanoparticles, it became resistant to all tested antibiotics except gentamicin, tobramycin, levofloxacin, moxifloxacin, nitrofurantoin, and trimethoprim/sulfamethoxazole.
As antibiotic resistance is crucial for the growth and survival of probiotics in the intestinal tract, previous research (Tu et al., 2022) concluded that the isolated probiotic gained antibiotic resistance after its nano-particle coating, and our findings support these data. Hence, comparing the demonstrated AST findings in Table 4, Table 5 confirmed the successful coating process and its efficacy, enhancing its resistance effects to almost most antibiotics that were sensitive to them before its nano-coating.
Several techniques have been developed to observe and characterize the surface and structural properties of nano-structured material and cells, including SEM, TEM/HRTEM, AFM, and STM. Meanwhile, the interaction of nano-particles with electromagnetic radiations as a function of wavelength could be investigated using spectroscopic techniques (Sharma et al., 2019).
When analyzing SEM results, we noticed that nano-coated probiotics have strengthened cell aggregation. Enhanced aggregative distribution is an expected observation characteristic for nano-coated cells using self-assembly techniques (Zhang et al., 2023), such as Tannic acid/Ferric iron used in this study. This indicates more robust adhesive properties and biofilm-producing capabilities encountered by the probiotic, a condition that may be beneficially adopted for different therapeutic purposes in vivo. This correlates with a recent study, which revealed 1.4X enhanced adhesive capabilities of nano-treated probiotics on an intestinal epithelial cell model. Conversely, another study revealed that nano-treatment did not affect the adhesion of probiotics to intestinal mucosal surfaces compared to uncoated cells (Pan et al., 2022). On a different scope, (Spirescu et al., 2021) reported decreased biofilm/aggregating capabilities of pathogenic bacteria on nano-treated surfaces.
In our study, Partial nano-coating was also noticed using AFM. This distinguishable differentiation character between nano-coated and non-coated probiotics samples is an observation that matches the SEM and UV/Vis spectrophotometry results. Moreover, the slight decrease in cell length noticed among nano-coated cells may suggest cell shrinkage that may have occurred due to the dehydration steps undertaken during the nano-coating process.
Similar to our study, (Rosa Teixeira et al., 2013) reported morphological changes among nano-treated cells, suggesting cell membrane disruptions that may have developed either during the coating multi-step process or due to chemical interactions of reagents with the cell membrane itself.
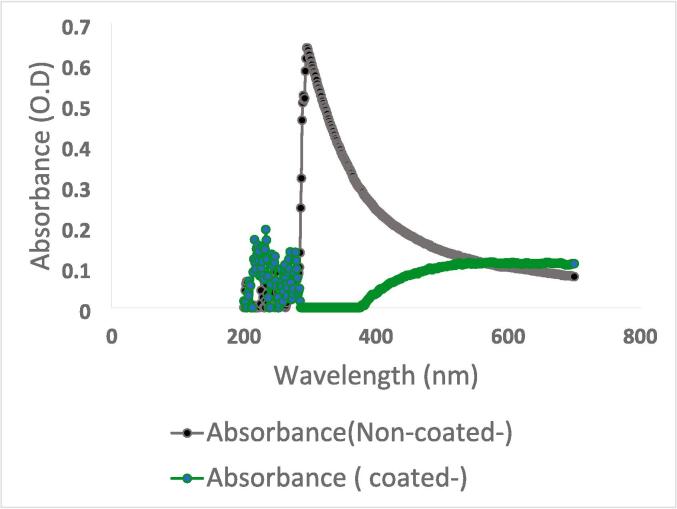
Fig. 7 presents the UV–Vis spectra of non-coated and nano-coated probiotic cells. The nano-coated cells exhibited a broad peak beginning at ∼550 nm. The overall transmittance of nano-coated layers was enhanced at most wavelengths, probably due to the probiotic size shrinkage and the cell surface smoothness acquired by the probiotic upon nano-treatment. The findings here, which have excellent linkage with SEM and AFM, reflected the partial coating of probiotic particles.
Fig. 7.
UV–Vis Spectral analysis of non-coated (gray plot) versus nano-coated (green plot) probiotics. The wavelength (nm) is expressed on the horizontal x-axis, whereas the absorbance is represented on the vertical y-axis. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
5. Conclusions
The design of probiotics, by their coating by nano-layers, can modulate their properties by enhancing their biological activities to resist numerous standard antibiotics. These findings were examined by the VITEK® 2 compact, which compared nano-coated and non-coated probiotics' AST information. Consequently, assuming that the treated probiotics can survive and persist in their passage across the gastrointestinal tract (GIT) may have advantageous effects on the human health.
6. Future perspective
Upon our findings, the future directions of this research may focus on the investigation of the effects of nano-coated probiotics on resistance to simulated gastric and intestinal fluids. In addition to, the toxicity testing of nano-coated probiotics against cancer and normal cell lines.
Funding
This work was supported by Researchers Supporting Project number (RSP2024R70), King Saud University, Riyadh, Saudi Arabia.
Author Contributions
NA, JK and ME contributed to the study Conception and design; Supervision was conducted by NA and JK; Material preparation was done by AA, SK and ME; data collection, and the manuscript’s first draft Writing, reviewing, and editing were carried out by ME; Formal analysis was done by JK and ME; Software adjustment was performed by AA, and AR. All authors read and approved the final manuscript.
CRediT authorship contribution statement
Marwa M. Elmaghrabi: Investigation, Methodology, Writing – original draft, Writing – review & editing, Data curation. Naiyf S. Alharbi: Conceptualization, Data curation, Funding acquisition, Project administration, Supervision, Validation, Visualization. Ahmed S. Alobaidi: Resources. Adel A. Abdulmanea: Validation. Shine Kadaikunnan: Resources. Asmaa A. Ramadan: Formal analysis. Jamal M. Khaled: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Supervision, Validation.
Declaration of competing interest
The authors declare that they have no relevant financial or non-financial interests that could influence the findings presented in this paper.
Acknowledgments
The authors extend their appreciation to the Researchers Supporting Project number (RSP2024R70), King Saud University, Riyadh, Saudi Arabia.
Contributor Information
Marwa M. Elmaghrabi, Email: marvenmomo@yahoo.com.
Jamal M. Khaled, Email: gkhaled@ksu.edu.sa.
References
- Alharbi N.S., Khaled J.M., Alzaharni K.E., Mothana R.A., Alsaid M.S., Alhoshan M., Dass L.A., Kadaikunnan S., Alobaidi A.S. Effects of Piper cubeba L. essential oil on methicillin-resistant Staphylococcus aureus: an AFM and TEM study. J. Mol. Recognit. 2017;30(1):e2564. doi: 10.1002/jmr.2564. [DOI] [PubMed] [Google Scholar]
- Ali M.A., Kamal M.M., Rahman M.H., Siddiqui M.N., Haque M.A., Saha K.K., Rahman M.A. Functional dairy products as a source of bioactive peptides and probiotics: current trends and future prospectives. J. Food Sci. Technol. 2022;59(4):1263–1279. doi: 10.1007/s13197-021-05091-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baldock B.L., Hutchison J.E. UV–visible spectroscopy-based quantification of unlabeled DNA bound to gold nanoparticles. Anal. Chem. 2016;88(24):12072–12080. doi: 10.1021/acs.analchem.6b02640. [DOI] [PubMed] [Google Scholar]
- Binnig G., Quate C.F., Gerber C. Atomic force microscope. Phys. Rev. Lett. 1986;56(9):930. doi: 10.1103/PhysRevLett.56.930. [DOI] [PubMed] [Google Scholar]
- Borriello S.P., Hammes W.P., Holzapfel W., Marteau P., Schrezenmeir J., Vaara M., Valtonen V. Safety of probiotics that contain lactobacilli or bifidobacteria. Clin. Infect. Dis. 2003;36(6):775–780. doi: 10.1086/368080. [DOI] [PubMed] [Google Scholar]
- Bowen T., Yingang X., Xue J., Qiang D., Junhong L., Hongbing T., Xiaodong H. Rapid detection of microbial mass spectra VITEK-MS for Campylobacter jejuni and Listeria monocytogenes. Food Anal. Methods. 2020;13(2):412–419. [Google Scholar]
- Centurion F., Merhebi S., Baharfar M., Abbasi R., Zhang C., Mousavi M., Xie W., Yang J., Cao Z., Allioux F.M., Harm G.F. Cell-mediated biointerfacial phenolic assembly for probiotic nano encapsulation. Adv. Funct. Mater. 2022;32(26):2200775. [Google Scholar]
- Cole K., Levine B.S. Ultraviolet-visible spectrophotometry. Princip. Forens. Toxicol. 2020:127–134. [Google Scholar]
- de Britto D., de Moura M.R., Aouada F.A., Mattoso L.H., Assis O.B. N, N, N-trimethyl chitosan nanoparticles as a vitamin carrier system. Food Hydrocoll. 2012;27(2):487–493. [Google Scholar]
- Ehrmann M.A., Kurzak P., Bauer J., Vogel R.F. Characterization of lactobacilli towards their use as probiotic adjuncts in poultry. J. Appl. Microbiol. 2002;92(5):966–975. doi: 10.1046/j.1365-2672.2002.01608.x. [DOI] [PubMed] [Google Scholar]
- El-Desouky R.A., Abdallah N.A., Hussien H.A., Abdelaal S.S., Saeed A.M. Preparation of chitosan/ginger and chitosan/garlic microcapsules by using emulsion cross-linking method. Egypt. J. Chem. 2023;66(13):973–982. [Google Scholar]
- Elmaghrabi, M., Ghozlan, H., 2019. Antibacterial activity of essential oils and antibiotics on bacterial strains isolated from infected urinary tract, J. Ecosyst. Ecogr. 9 | doi: 10.4172/2157-7625-C1-044.
- Fontana L., Bermudez-Brito M., Plaza-Diaz J., Munoz-Quezada S., Gil A. Sources, isolation, characterisation and evaluation of probiotics. Br. J. Nutr. 2013;109(S2):S35–S50. doi: 10.1017/S0007114512004011. [DOI] [PubMed] [Google Scholar]
- Hotel A.C.P., Cordoba A. Health and nutritional properties of probiotics in food including powder milk with live lactic acid bacteria. Prevention. 2001;5(1):1–10. [Google Scholar]
- Hugenholtz P., Goebel B.M., Pace N.R. Impact of culture-independent studies on the emerging phylogenetic view of bacterial diversity. J. Bacteriol. 1998;180(18):4765–4774. doi: 10.1128/jb.180.18.4765-4774.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim H.M., Hwang C.Y., Cho B.C. Arcobacter marinus sp. nov. Int. J. Syst. Evol. Microbiol. 2010;60(3):531–536. doi: 10.1099/ijs.0.007740-0. [DOI] [PubMed] [Google Scholar]
- Klare I., Konstabel C., Werner G., Huys G., Vankerckhoven V., Kahlmeter G., Hildebrandt B., Müller-Bertling S., Witte W., Goossens H. Antimicrobial susceptibilities of Lactobacillus, Pediococcus and Lactococcus human isolates and cultures intended for probiotic or nutritional use. J. Antimicrob. Chemother. 2007;59(5):900–912. doi: 10.1093/jac/dkm035. [DOI] [PubMed] [Google Scholar]
- Knebelsberger T., Stöger I. DNA extraction, preservation, and amplification. DNA Barcodes: Methods Protocols. 2012:311–338. doi: 10.1007/978-1-61779-591-6_14. [DOI] [PubMed] [Google Scholar]
- Kurokawa K., Itoh T., Kuwahara T., Oshima K., Toh H., Toyoda A., Takami H., Morita H., Sharma V.K., Srivastava T.P., Taylor T.D. Comparative metagenomics revealed commonly enriched gene sets in human gut microbiomes. DNA Res. 2007;14(4):169–181. doi: 10.1093/dnares/dsm018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lay J.O., Jr MALDI-TOF mass spectrometry of bacteria. Mass Spectrom. Rev. 2001;20(4):172–194. doi: 10.1002/mas.10003. [DOI] [PubMed] [Google Scholar]
- Lee I.C., Tomita S., Kleerebezem M., Bron P.A. The quest for probiotic effector molecules—unraveling strain specificity at the molecular level. Pharmacol. Res. 2013;69(1):61–74. doi: 10.1016/j.phrs.2012.09.010. [DOI] [PubMed] [Google Scholar]
- Liong M.T., editor. vol. 21. Springer Science & Business Media; 2011. (Probiotics: Biology, Genetics and Health Aspects). [Google Scholar]
- Mac Faddin, J.F., 1985. Media for isolation-cultivation-identification-maintenance of medical bacteria. (No Title).
- Mesureur J., Arend S., Cellière B., Courault P., Cotte-Pattat P.J., Totty H., Deol P., Mick V., Girard V., Touchberry J., Burrowes V. A MALDI-TOF MS database with broad genus coverage for species-level identification of Brucella. PLoS Neglected Trop. Diseases. 2018;12(10):e0006874. doi: 10.1371/journal.pntd.0006874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mileriene J., Aksomaitiene J., Kondrotiene K., Asledottir T., Vegarud G.E., Serniene L., Malakauskas M. Whole-genome sequence of Lactococcus lactis Subsp. lactis LL16 confirms safety, probiotic potential, and reveals functional traits. Microorganisms. 2023;11(4):1034. doi: 10.3390/microorganisms11041034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakasone I., Kinjo T., Yamane N., Kisanuki K., Shiohira C.M. Laboratory-based evaluation of the colorimetric VITEK-2 Compact system for species identification and of the Advanced Expert System for detection of antimicrobial resistances: VITEK-2 Compact system identification and antimicrobial susceptibility testing. Diagn. Microbiol. Infect. Dis. 2007;58(2):191–198. doi: 10.1016/j.diagmicrobio.2006.12.008. [DOI] [PubMed] [Google Scholar]
- Nanchen, A., Fuhrer, T. and Sauer, U., 2007. Determination of metabolic flux ratios from 13 C-experiments and gas chromatography-mass spectrometry data: protocol and principles. Metabolomics: Methods and protocols, pp.177–197. [DOI] [PubMed]
- O'Sullivan D.J. Methods for analysis of the intestinal microflora. Curr. Issues Intest. Microbiol. 2000;1(2):39–50. [PubMed] [Google Scholar]
- Pan J., Gong G., Wang Q., Shang J., He Y., Catania C., Birnbaum D., Li Y., Jia Z., Zhang Y., Joshi N.S. A single-cell nanocoating of probiotics for enhanced amelioration of antibiotic-associated diarrhea. Nat. Commun. 2022;13(1):2117. doi: 10.1038/s41467-022-29672-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pincus, D.H., 2006. Microbial identification using the bioMérieux Vitek® 2 system. Encyclopedia of Rapid Microbiological Methods. Bethesda, MD: Parenteral Drug Association, 2006, pp. 1–32.
- Quéro L., Girard V., Pawtowski A., Tréguer S., Weill A., Arend S., Cellière B., Polsinelli S., Monnin V., van Belkum A., Vasseur V. Development and application of MALDI-TOF MS for identification of food spoilage fungi. Food Microbiol. 2019;81:76–88. doi: 10.1016/j.fm.2018.05.001. [DOI] [PubMed] [Google Scholar]
- Quiberoni A., Rezaïki L., El Karoui M., Biswas I., Tailliez P., Gruss A. Distinctive features of homologous recombination in an ‘old’microorganism, Lactococcus Lactis. Res. Microbiol. 2001;152(2):131–139. doi: 10.1016/s0923-2508(01)01183-4. [DOI] [PubMed] [Google Scholar]
- Rademaker J.L., Herbet H., Starrenburg M.J., Naser S.M., Gevers D., Kelly W.J., Hugenholtz J., Swings J., van Hylckama Vlieg J.E. Diversity analysis of dairy and nondairy Lactococcus lactis isolates, using a novel multilocus sequence analysis scheme and (GTG) 5-PCR fingerprinting. Appl. Environ. Microbiol. 2007;73(22):7128–7137. doi: 10.1128/AEM.01017-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reid G. Probiotics: definition, scope and mechanisms of action. Best Pract. Res. Clin. Gastroenterol. 2016;30(1):17–25. doi: 10.1016/j.bpg.2015.12.001. [DOI] [PubMed] [Google Scholar]
- Rosa Teixeira K.I., Araújo P.V., Almeida Neves B.R., Bohorquez Mahecha G.A., Sinisterra R.D., Cortés M.E. Ultrastructural changes in bacterial membranes induced by nano-assemblies β-cyclodextrin chlorhexidine: SEM, AFM, and TEM evaluation. Pharm. Dev. Technol. 2013;18(3):600–608. doi: 10.3109/10837450.2011.649853. [DOI] [PubMed] [Google Scholar]
- Saarela M., Lähteenmäki L., Crittenden R., Salminen S., Mattila-Sandholm T. Gut bacteria and health foods—the European perspective. Int. J. Food Microbiol. 2002;78(1–2):99–117. doi: 10.1016/s0168-1605(02)00235-0. [DOI] [PubMed] [Google Scholar]
- Sandrin T.R., Goldstein J.E., Schumaker S. MALDI TOF MS profiling of bacteria at the strain level: a review. Mass Spectrom. Rev. 2013;32(3):188–217. doi: 10.1002/mas.21359. [DOI] [PubMed] [Google Scholar]
- Scott G. Antibiotic resistance. Medicine. 2009;37(10):551–556. [Google Scholar]
- Shaaban M.T., Ghozlan H.A., El Maghraby M.M. Susceptibility of bacteria infecting urinary tract to some antibiotics and essential oils. J. Appl. Pharmaceut. Sci. 2012;Issue:90–98. [Google Scholar]
- Sharma S., Jaiswal S., Duffy B., Jaiswal A.K. Nanostructured materials for food applications: spectroscopy, microscopy and physical properties. Bioengineering. 2019;6(1):26. doi: 10.3390/bioengineering6010026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smart K.F., Aggio R.B., Van Houtte J.R., Villas-Bôas S.G. Analytical platform for metabolome analysis of microbial cells using methyl chloroformate derivatization followed by gas chromatography–mass spectrometry. Nat. Protoc. 2010;5(10):1709–1729. doi: 10.1038/nprot.2010.108. [DOI] [PubMed] [Google Scholar]
- Spirescu V.A., Șuhan R., Niculescu A.G., Grumezescu V., Negut I., Holban A.M., Oprea O.C., Bîrcă A.C., Vasile B.Ș., Grumezescu A.M., Bejenaru L.E. Biofilm-resistant nanocoatings based on ZnO nanoparticles and linalool. Nanomaterials. 2021;11(10):2564. doi: 10.3390/nano11102564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stiles M.E., Holzapfel W.H. Lactic acid bacteria of foods and their current taxonomy. Int. J. Food Microbiol. 1997;36(1):1–29. doi: 10.1016/s0168-1605(96)01233-0. [DOI] [PubMed] [Google Scholar]
- Tu B., Cao N., Zhang B., Zheng W., Li J., Tang X., Su K., Li J., Zhang Z., Yan Z., Li D. Synthesis and biological evaluation of novel fusidic acid derivatives as two-in-one agent with potent antibacterial and anti-inflammatory activity. Antibiotics. 2022;11(8):1026. doi: 10.3390/antibiotics11081026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vasiee A., Mortazavi S.A., Sankian M., Yazdi F.T., Mahmoudi M., Shahidi F. Antagonistic activity of recombinant Lactococcus lactis NZ1330 on the adhesion properties of Escherichia coli causing urinary tract infection. Microb. Pathog. 2019;133 doi: 10.1016/j.micpath.2019.103547. [DOI] [PubMed] [Google Scholar]
- Vasiee A., Falah F., Behbahani B.A., Tabatabaee-Yazdi F. Probiotic characterization of Pediococcus strains isolated from Iranian cereal-dairy fermented product: Interaction with pathogenic bacteria and the enteric cell line Caco-2. J. Biosci. Bioeng. 2020;130(5):471–479. doi: 10.1016/j.jbiosc.2020.07.002. [DOI] [PubMed] [Google Scholar]
- Vasiee A., Falah F., Sankian M., Tabatabaei-Yazdi F., Mortazavi S.A. Oral immunotherapy using probiotic ice cream containing recombinant food-grade Lactococcus lactis which inhibited allergic responses in a BALB/c mouse model. J. Immunol. Res. 2020 doi: 10.1155/2020/2635230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vrdoljak M., Tudor Kalit M., Dolenčić Špehar I., Radeljević B., Jelić M., Mandinić S., Frece J., Kalit S. Effects of the autochthonous probiotic bacteria lactobacillus plantarum B and Lactococcus lactis Subsp. lactis S1 on the proteolysis of Croatian cheese ripened in a lambskin sack (sir iz Mišine) Fermentation. 2022;8(8):382. [Google Scholar]
- Yassin M.T., Al-Otibi F.O., Al-Askar A.A., Elmaghrabi M.M. Synergistic anticandidal effectiveness of greenly synthesized zinc oxide nanoparticles with antifungal agents against nosocomial candidal pathogens. Microorganisms. 2023;11(8):1957. doi: 10.3390/microorganisms11081957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang T., Shang C., Du T., Zhuo J., Wang C., Li B., Xu J., Fan M., Wang J., Zhang W. Cytoprotection of probiotics by nanoencapsulation for advanced functions. Trends Food Sci. Technol. 2023;142 [Google Scholar]