Abstract
Background
The complexity of calculating the Hijdra score has limited its clinical utility in aiding the diagnosis of intracranial ruptured aneurysms.
Objective
This study aimed to investigate the diagnostic and prognostic value of the modified Hijdra score in patients with aneurysmal subarachnoid hemorrhage (aSAH).
Methods
Data from 773 patients with subarachnoid hemorrhage (SAH) at the First People's Hospital of Lianyungang from January 2018 to June 2023 were collected. The modified Hijdra scoring method simplifies the assessment of 10 basal cisterns/cisterns fissures compared to the traditional scoring method, with scores ranging from 0 to 2 for each item, and assigns specific scores to hematomas larger than 1 cm in diameter. The data were divided into an evaluation group (n = 641) and a validation group (n = 132). In the evaluation group, the performance of the modified Hijdra score in diagnosis and prognostic prediction was assessed, while the diagnostic and prognostic prediction efficacy of the modified Hijdra method was evaluated using the validation set.
Results
Among the 641 patients in the evaluation group,550 (85. 8%) were diagnosed with intracranial aneurysms. The modified Hijdra score demonstrated an AUC of 0. 894 for aneurysm diagnosis, with a sensitivity of 98. 0% and a specificity of 64. 8% at a CutOff value of 7. 5. The diagnostic efficacy of the modified Hijdra score was 93. 24%, with a negative predictive value of 84. 29%, while the Hijdra score 's diagnostic efficacy was 85. 34% with a negative predictive value of 48. 89%. The AUC of the modified Hijdra score for predicting prognosis in patients with aneurysms was 0. 824, with a sensitivity of 84. 3% and a specificity of 70. 0% at a CutOff value of 16. 5. In CTA-negative patients, the modified Hijdra score was significantly higher (P < 0. 0001) in patients with aneurysmal SAH (15. 48 ± 3. 93) compared to those with non-aneurysmal SAH (6. 31 ± 4. 52).
Conclusions
The modified Hijdra score is a valuable tool for assisting in the diagnosis and prognosis prediction of aneurysmal subarachnoid hemorrhage.
Keywords: Modified Hijdra score, CT, Aneurysm, Subarachnoid hemorrhage, Prognosis
1. Introduction
Aneurysmal subarachnoid hemorrhage (aSAH) is a critical neurosurgical emergency with high disability and mortality, primarily due to the initial severity and subsequent complications [1,2]. Accurate aneurysms diagnosis is crucial [2,3]. Aneurysms are abnormal arterial formations within the cranial cavity. Conventional diagnostic methods include cranial CTA, MRI, MRA, and DSA, critical for identifying intracranial aneurysms. Yet, these methods have limitations: invasiveness with associated risks, prolonged duration and higher costs, and potential failure in detecting small lesions, inpending timely diagnosis. Notably, about 15%–20% of non-traumatic SAH patients show no vascular abnormalities on cerebral angiography [2,4,5], but repeat angiography in CT-negative cases detects vascular lesions in 13%, and aneurysms or pseudoaneurysms in 4% [[6], [7], [8]]. Nonaneurysmal SAH diagnosis requires excluding other causes through invasive and noninvasive tests, including angiography, CT, and MRI. The necessity of DSA in patients with negative initial CTA is debated, posing significant diagnostic challenges in identifying vascular lesions and distinguishing true non-aneurysmal SAH.
The Hijdra score is a quantifies subarachnoid hemorrhage volume from CT findings, assessing 10 brain pools/splits and 4 ventricles, with scores ranging 0 to 3. Initially used for baseline assessment and prediction of outcomes [9,10], the score has shown improved interobserver agreement [2,6]. In 2020, Kole et al. demonstrated that the Hijdra score's utility in diagnosing SAH patients with cerebral angiography-detected aneurysms and developed a model predicting aneurysm risk in nontraumatic SAH patients [3]. Previous studies identified factors associated with poor prognosis, including age, hypertension, severity according to the World Federation of Neurosurgery Scale (WFNS) classification, and the extent of intracranial hemorrhage [[11], [12], [13]].
This study, enhanced the Hijdra score by simplifying the assessment of brain pools/splits, scoring each from 0 to 2. The study retrospectively analyzed clinical data from 641 patients with spontaneous SAH admitted to the Department of Neurosurgery at the First People's Hospital of Lianyungang from January 2018 to June 2022. The aim was to evaluate the diagnostic and prognostic value of the modified Hijdra score for intracranial aneurysms in SAH patients, involving patients selection meeting inclusion criteria for data collection and analysis.
2. Methods
2.1. Study design and subjects
This retrospective cohort study was obtained from institutional review board (KY-20220817001-01). Data was conducted from January 2018 to June 2023, including continuously hospitalized patients for treatment. The hospital's ethics review committee approved the protocol with a waiver for individual consent. Patient data, including clinical, operative, and imaging records, were extracted from the hospital's comprehensive database. Inclusion criteria included adults over 18 with non-traumatic subarachnoid hemorrhage and initial CT scans with cerebral angiography performed at the hospital. Exclusion criteria encompressed a history of cranial surgery, presence of intracranial artifacts, patients with traumatic subarachnoid hemorrhage and those with incomplete data or first CT scans conducted over 24 post-hemorrhage onset.
2.2. Modified Hijdra score calculation method
The modified Hijdra score segments the brain into 10 areas, assessing blood volume in each, with scores from 0 to 2 indicating no, small and medium to large blood amount, respectively. A total score is calculated, with an additional 20 points for hematomas over 1 cm in diameter. Refer to Fig. 1 for details.
Fig. 1.
Example of Modified Hijdra Scale. A 59-year-old patient with subarachnoid hemorrhage caused by a ruptured left posterior communicating A 59-year-old patient with subarachnoid hemorrhage caused by a ruptured left posterior communicating artery aneurysm, as seen on computed tomography scan approximately 3 h after the event.
*The upper right image shows 10 basal cisterns and fissures: A. interhemispheric fissure; B. lateral fissure, outer segment; C. lateral fissure, basal segment; D. suprasellar cistern; E. perimesencephalic cistern; F. quadrigeminal cistern.
**The lower right image shows the score for the amount of bleeding in each basal cistern and fissure region. the Modified Hijdra sum score is 18 points.
2.3. Data collection
Two neurosurgeons independently evaluated initial CT scans, with a neuroradiologist adjudicating significant discrepancies. Assessments used the modified Fisher, Hijdra score, and modified Hijdra score. Patient demographics and clinical characteristics including medical history and scores like GCS and Hunt-Hess, were documented at admission. Surgical history and postoperative complications were also recorded when applicable.
2.4. Statistical analysis
For continuous variables, means and standard deviations were calculated, and t-tests compared aneurysm and non-aneurysm groups. Non-normal variables t were assessed with medians, interquartile range, and the Wilcoxon-Mann-Whitney U test. Categorical variables were described numerically and assessed with chi-square tests. Multiple logistic regression analyzed factors impacting prognosis and Receiver operating characteristic (ROC) curves evaluated the aneurysm score's predictive accuracy. SPSS (IBM SPSS Statistics 24. 0), GraphPad Prism (Version 8. 0. 2), and R software (Version 4. 2. 0) were used for analysis, with a significance threshold of P ≤ 0. 05.
3. Results
During the study period, a total of 773 patients with subarachnoid hemorrhage (SAH) were included in the study. The data were divided into an evaluation group (n = 641) and a validation group (n = 132). Table 1 presents the demographic information of the patients in the evaluation group, with a mean age of 59. 79 ± 11. 97 years. Among the 641 patients who met the inclusion criteria, 550 (85. 8%) were initially diagnosed with aneurysms based on imaging (CT/CTA/DSA). Among these, 521 aneurysms (94. 7% of all aneurysms) were diagnosed using CTA. Among the SAH patients with negative CTA results, 28 (5. 1%) were subsequently diagnosed with aneurysms through repeat DSA examinations. One patient was also diagnosed with an aneurysm after a non-enhanced magnetic resonance angiography (MRA) examination due to concerns about elevated creatinine and poor renal function. Among these patients, 403 (73. 3%) underwent aneurysm clamping. A total of 535 patients had positive CTA findings suggestive of aneurysm. Among these patients, 115 underwent repeat DSA, and 11 (9. 7%) were diagnosed without finding an aneurysm, resulting in false-positive cases. The overall flow of the 192 patients who underwent both CTA and DSA angiography is presented in Fig. 2. Among these patients, 77 had negative CTA findings and underwent DSA review, and 26 patients were subsequently diagnosed with an aneurysm, resulting in false-negative cases.
Table 1.
Characteristics of the aneurysm group and non-aneurysm group (n = 641).
| Non-Aneurysm SAH | Aneurysmal SAH | P-value | |
|---|---|---|---|
| Total number of patients | 91 (14. 2%) | 550 (85. 8%) | |
| Age | 60. 7 ± 11. 6 | 59. 6 ± 12. 0 | . 284∗∗ |
| Female | 41 (45. 1%) | 357 (64. 9%) | <. 001‡ |
| Hypertension | 38 (41. 8%) | 328 (59. 6%) | . 001‡ |
| Diabetes | 8 (8. 8%) | 41 (7. 5%) | . 657‡ |
| Hunt-Hess Scale | |||
| 0 | 15 (2. 7%) | 26 (4. 1%) | <. 001‡ |
| 1 | 297 (54. 0%) | 363 (56. 6%) | |
| 2 | 114 (20. 7%) | 117 (18. 3%) | |
| 3 | 97 (17. 6%) | 105 (16. 4%) | |
| 4 | 27 (4. 9%) | 30 (4. 7%) | |
| MFS | |||
| 0 | 5 (5. 5%) | 1 (0. 2%) | <. 001‡ |
| 1 | 45 (49. 5%) | 28 (5. 1%) | |
| 2 | 34 (37. 4%) | 273 (49. 6%) | |
| 3 | 3 (3. 3%) | 123 (22. 4%) | |
| 4 | 4 (4. 4%) | 125 (22. 7%) | |
| Hijdra | 6 (3–15) | 20 (15–25) | <. 001∗∗ |
| M-Hijdra | 4 (2–10) | 14 (11–19) | <. 001∗∗ |
| Hematoma (d ≥ 1 cm) | 1 (1. 1%) | 125 (22. 7%) | <. 001‡ |
Bivariate analysis showed significant differences (P < 0. 05) in the proportion of female patients, hypertension, Hunt-Hess Scale, modified Fisher Bivariate analysis showed significant differences (P < 0. 05) in the proportion of female patients, hypertension, Hunt-Hess Scale, modified Fisher score, Hijdra scores, M-Hijdra scores, and presence of hematoma (diameter≥1 cm) between groups. Statistical comparisons between continuous and categorical variables were performed using Student t-test (∗∗) and chi-squared test (‡).
Fig. 2.
The flow chart shows the situation of SAH patients undergoing CTA and DSA angiography. Percentages represent the percentage of each group class relative to the previous study group.
Table 1 displays the baseline characteristics, disease assessment, and clinical data of patients in both the non-aneurysmal SAH and aneurysmal SAH groups. Among them, the number of patients with aneurysmal SAH was significantly higher than that of non-aneurysmal SAH, and there was no significant age difference between the two groups (P = 0. 284). A higher proportion of women were diagnosed with aneurysmal SAH compared to non-aneurysmal SAH (P < 0. 001), and there was a higher prevalence of hypertension (P = 0. 001). In terms of condition assessment, aneurysmal SAH had a higher Hunt-Hess Scale (HHS) score (P < 0. 001) but a lower modified Fisher score (P < 0. 001). Regarding clinical data, both the HHS and modified Hijdra scores were higher in the aneurysmal SAH group than in the non-aneurysmal SAH group (P < 0. 001), while patients with aneurysmal SAH had a significantly higher proportion of hematomas measuring greater than or equal to 1 cm (P < 0. 001). The difference in diabetes prevalence between the two groups was not significant (P = 0. 657).
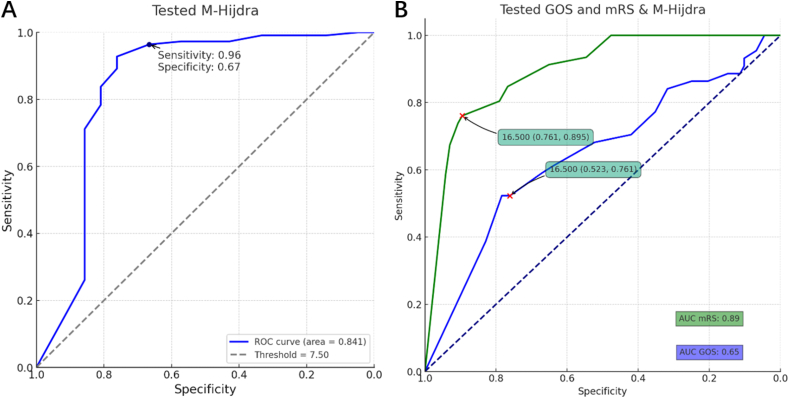
Independent samples t-tests and ROC curve analysis (Fig. 3, Fig. 4) demonstrated that the area under the curve (AUC) for the Hijdra score was 0. 863, and for the modified Hijdra score, it was 0. 894. There was no statistically significant difference between the two scores in diagnosing aneurysms, but a statistically significant difference was observed between the modified Hijdra score for aneurysms and non-aneurysms. The cutoff value of the ROC curve for the modified Hijdra score was determined to be 7. 5, with a diagnostic sensitivity of 98. 0% and a diagnostic specificity of 64. 8%. The t-test (Fig. 4) comparing the diagnostic efficacy, positive predictive value, and negative predictive value of the modified Hijdra score with the Hijdra score revealed a significant difference (P < 0. 0001) in the diagnostic efficacy, with the modified Hijdra score (93. 24%) outperforming the Hijdra score (85. 34%). The positive predictive value of the modified Hijdra score (94. 40%) was not significantly different from that of the Hijdra score (95. 06%) (P = 0. 6835), whereas the negative predictive value of the modified Hijdra score (84. 29%) was significantly higher than that of the Hijdra score (48. 89%) (P < 0. 0001).
Fig. 3.
Independent samples t-tests comparing M-Hijdra scores and Hijdra scores between the Aneurysm group and Non-aneurysm group.
Fig. 4.
ROC curve of M-Hijdra scores and Hijdra scores predicting aneurysm presence in patients.
When comparing CTA with the modified Hijdra score for the diagnosis of aneurysms in SAH patients (Fig. 5), the diagnostic sensitivity of CTA (94. 32%) was significantly different from that of the modified Hijdra score (98. 00%) (P = 0. 0015), and the diagnostic specificity of CTA (84. 27%) significantly differed from that of the modified Hijdra score. The prognosis of SAH patients predicted by the Hijdra score and modified Hijdra score is illustrated in Fig. 8. The AUC value for the GOS prognostic assessment was 0. 833, with a sensitivity of 70. 6% and specificity of 85. 7% at a cutoff value of 16. 5 for the modified Hijdra score. The AUC value for the modified Rankin Scale prognostic assessment was 0. 824, with a sensitivity of 84. 2% and specificity of 70. 0%. For the Hijdra score, the AUC value for GOS prognostic assessment was 0. 798, with a sensitivity of 73. 9% and specificity of 76. 8% at a cutoff value of 21. 5, and the AUC value for modified Rankin Scale prognostic assessment was 0. 794, with a sensitivity of 75. 8% and specificity of 74. 1%. A significant difference (P < 0. 0001) was observed in the modified Hijdra score (Fig. 9) between SAH patients with no aneurysm diagnosed on the initial CTA contrast imaging examination (6. 31 ± 4. 52) and those diagnosed with aneurysms on repeat DSA (15. 48 ± 3. 93) When comparing CTA with the modified Hijdra score for diagnosing aneurysms in SAH patients (Fig. 6), there was a significant difference in diagnostic sensitivity between CTA (94.32%) and the modified Hijdra score (98.00%) (P = 0.0015). Additionally, there was a notable disparity in diagnostic specificity between CTA (84.27%) and the modified Hijdra score (64.84%) (P = 0.0035).
Fig. 5.
The comparison results of diagnostic efficiency, positive predictive value, and negative predictive value between the modified and original Hijdra scoring systems.
* "DE" means "diagnostic efficiency", "PPV" means " positive predictive value" and "NPV" means "negative predictive value".
Fig. 8.
ROC curves of Hijdra scores and M-Hijdra scores in predicting outcomes in SAH patients.
Fig. 9.
The comparison of the Modified Hijdra score between patients with and without aneurysms among CTA-negative participants.
Fig. 6.
Comparison of diagnostic sensitivity and specificity between CTA and M-Hijdra scores.
* "TPR" means "True Positive Rate","TNR" means "True Negative Rate".
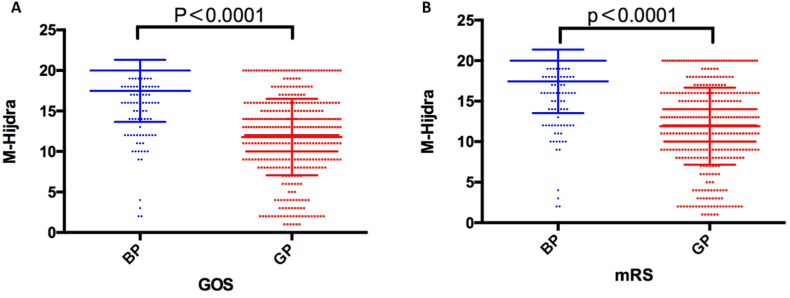
The prognosis of patients with subarachnoid hemorrhage (SAH) was assessed using the GOS scale and the modified Rankin scale (Fig. 7). A significant difference was observed in the score distribution of the modified Hijdra score between patients with a better prognosis (GOS score>3) and those with a poorer prognosis (GOS score ≤3)(P < 0. 0001). Similarly, the modified Hijdra score showed a significant difference between patients with a better prognosis (mRS≤3) and those with a poorer prognosis (mRS>3) according to the modified Rankin scale (P < 0. 0001).
Fig. 7.
Comparison of better and worse prognoses based on GOS and mRS scores.
*"BP" means "bad prognoses", and "GP" means "good prognoses".
The results of the univariate analysis (Table 2) indicated that age, hypertension, HHS classification, comorbidities, clamping procedure, unplanned secondary procedure, modified Fisher classification, and modified Hijdra score were factors influencing the prognosis of aneurysm. Furthermore, the multifactorial logistic regression analysis (Table 3) demonstrated that HHS classification, complications, clamping surgery, unplanned secondary surgery, and modified Hijdra score were influential factors affecting the prognosis of aneurysm. In Fig. 10, the ROC curve evaluation of the modified Hijdra score demonstrates good diagnostic and prognostic performance in the validation dataset.
Table 2.
Univariate analysis of factors influencing prognosis in aneurysmal subarachnoid hemorrhage (n = 641).
| β | OR | OR_2.5% | OR_97.5% | SE | Z value | P value | |
|---|---|---|---|---|---|---|---|
| Age | −0.03732 | 0.963371 | 0.948376 | 0.978123 | 0.007868 | −4.74308 | <0.0001 |
| Female | 0.007785 | 1.007816 | 0.707828 | 1.441495 | 0.181232 | 0.042958 | 0.9657 |
| Hypertension | −0.72981 | 0.482 | 0.332466 | 0.692034 | 0.186753 | −3.9079 | 0.0001 |
| Diabetes | −0.13018 | 0.877934 | 0.474152 | 1.698732 | 0.323244 | −0.40274 | 0.6871 |
| Hunt-Hess Scale | −1.60698 | 0.200492 | 0.154835 | 0.255247 | 0.127331 | −12.6205 | <0.0001 |
| Comorbidities | −1.79757 | 0.165702 | 0.078655 | 0.331084 | 0.363335 | −4.94741 | <0.0001 |
| Aneurysm clipping surgery | 1.051069 | 2.860709 | 2.009036 | 4.08832 | 0.181077 | 5.80453 | <0.0001 |
| Unplanned secondary surgery | −2.49146 | 0.082789 | 0.012539 | 0.325197 | 0.786853 | −3.16637 | 0.0015 |
| Modifeid Fisher score | −1.49397 | 0.22448 | 0.17475 | 0.284285 | 0.123965 | −12.0515 | <0.0001 |
| Modified Hijdra score | −0.32385 | 0.723357 | 0.682278 | 0.763872 | 0.028785 | −11.2507 | <0.0001 |
Table 3.
Multivariate logistic regression analysis of factors influencing prognosis in aneurysmal subarachnoid hemorrhage (n = 641).
| β | OR | OR_2.5% | OR_97.5% | SE | Z value | P value | |
|---|---|---|---|---|---|---|---|
| Age | −0.00849 | 0.991546 | 0.968671 | 1.014647 | 0.011796 | −0.71971 | 0.4717 |
| Hypertension | −0.41949 | 0.657382 | 0.380129 | 1.126004 | 0.276244 | −1.51855 | 0.1289 |
| Hunt-Hess Scale | −1.17486 | 0.308861 | 0.225724 | 0.415414 | 0.15528 | −7.56609 | <0.0001 |
| Comorbidities | −2.15426 | 0.115989 | 0.035237 | 0.369494 | 0.59682 | −3.60956 | 0.0003 |
| Aneurysm clipping surgery | 1.659187 | 5.255037 | 2.949215 | 9.577592 | 0.299612 | 5.537777 | <0.0001 |
| Unplanned secondary surgery | −2.22961 | 0.107571 | 0.01047 | 0.833285 | 1.086477 | −2.05214 | 0.0402 |
| Modifeid Fisher score | −0.18682 | 0.82959 | 0.495679 | 1.39163 | 0.262149 | −0.71266 | 0.4761 |
| Modifeid Hijdra score | −0.23472 | 0.790795 | 0.707327 | 0.879356 | 0.055231 | −4.24971 | <0.0001 |
Fig. 10.
Roc curve of the modified Hijdra score in Aneurysm diagnosis and prognostic prediction.
4. Discussion
In this study, we aimed to compare the efficacy of the modified Hijdra score with the Hijdra score in diagnosing aneurysms and assessing the prognosis of patients with aneurysms. To our knowledge, this is the first study to evaluate the prognostic value of the modified Hijdra score in patients with aneurysms. Our findings suggest that the modified Hijdra score outperforms the Hijdra score in diagnosing aneurysms and serves as an independent risk factor for the prognosis of patients with aneurysms.
The modified Fisher score showed a significant difference between aneurysmal and non-aneurysmal patients [14]; however, there was poor interobserver agreement [15]. In contrast, the modified Hijdra score exhibits better interobserver agreement [2,16] and provides an independent assessment of 10 brain pools or fissures. This makes the modified Hijdra score more user-friendly in clinical applications while maintaining a higher diagnostic efficacy compared to the Hijdra score.
Previous studies have indicated that intracerebroventricular hematoma does not significantly contribute to the prognosis of patients with aneurysms. Instead, intracerebral hematoma has been identified as an independent risk factor for poor prognosis [2,16]. The Hijdra score has been shown to predict the risk of aneurysms in patients with SAH, and our study confirmed that the modified Hijdra score can also predict the prognostic outcome in patients with aneurysms.
Non-aneurysmal subarachnoid hemorrhage is a diagnosis reached through exclusion, necessitating a series of invasive and non-invasive tests to rule out aneurysms and other potential causes. Patients with undiagnosed aneurysms in the context of SAH have a higher rate of disability and mortality [5]. Conversely, patients with non-aneurysmal SAH misdiagnosed as aneurysms often receive more aggressive treatment and attention, resulting in a significantly reduced risk of rebleeding [4,17]. Among the 28 patients with SAH who initially had negative CTA results but were subsequently diagnosed with aneurysms on repeat DSA examination, the mean modified Hijdra score was 15. 32 ± 3. 97, indicating a high risk of aneurysm, which aligns with the examination findings.
Overall, our study highlights the superiority of the modified Hijdra score in diagnosing aneurysms and predicting the prognosis of patients with aneurysms. The use of this modified score can aid in better risk stratification and treatment decision-making for patients with SAH.
Patients with subarachnoid hemorrhage (SAH) who initially test negative for aneurysms on CTA but have a modified Hijdra score greater than 7. 5 are at high risk of having an aneurysm and should undergo a second angiogram or DSA [8,18]. A study by Kalra et al., in 2015 suggested that the risk of aneurysm could be ruled out after an initial negative CTA in patients with SAH [19]. However, aneurysms may still be present in some patients despite a negative initial CTA. Our findings indicate that SAH patients with undiagnosed aneurysms who undergo DSA typically have a modified Hijdra score of no more than 7. 5. When the modified Hijdra score exceeds 7. 5, aneurysms are diagnosed in 61. 4% of patients during repeat DSA, even with a negative initial CTA. Therefore, a modified Hijdra score of less than 7. 5 effectively excludes the possibility of an aneurysm and reduces the need for further medical evaluation. Conversely, if the modified Hijdra score is greater than 7. 5, even with a negative CTA, angiography is strongly recommended to confirm the presence or absence of an aneurysm, ensuring prompt medical intervention for patients with aneurysms.
The modified Hijdra score (>16. 5) is an independent prognostic risk factor for patients with aneurysms and exhibits similar prognostic predictive value as the Hijdra score. In 2019, a meta-analysis by van der Steen et al. confirmed the significant association between the Hijdra scale and poor prognosis in SAH, particularly in cases of delayed cerebral ischemia, hydrocephalus, and cerebral vasospasm, which were independent predictors of prognosis at 6 months [16,[20], [21], [22], [23]]. The prognostic value of the modified Hijdra score in predicting outcomes in patients with aneurysmal SAH was supported by studies demonstrating its improved diagnostic efficacy. In a study by Van der Jagt et al. [22,24] involving 168 patients with intracerebral hemorrhage, the presence of a confined hematoma around the ruptured aneurysm indicated the responsible vessel for the rupture in only 15% of patients. However, our study found that 19. 50% of patients with aneurysms larger than 1 cm exhibited a hematoma within the brain parenchyma, all of whom were diagnosed with aneurysms during DSA review. This subset of patients had an average Hijdra score of 24. 4, indicating the presence of aneurysm risk. This finding contrasts with the optimized assessment of cerebral parenchymal hematomas provided by the modified Hijdra score. Models quantifying total blood volume have demonstrated better predictive value for patients with aSAH [24], while the modified Hijdra score was significantly predictive for patients with non-traumatic subarachnoid hemorrhage in the presence of parenchymal hematoma.
In this study, we investigated the factors influencing the prognosis of patients with ruptured aneurysms. Our findings revealed that HHS classification, presence of complications, aneurysm clamping, unplanned secondary surgery, and modified Hijdra score were independent risk factors affecting the prognosis of patients with ruptured aneurysms. HHS classification can guide the timing of surgery in patients with aneurysms [23]. According to Konczalla et al., in 2018, early aggressive medical intervention in patients with Hunt Hess grade V SAH coma significantly improves survival [25], highlighting its association with the prognosis of SAH patients. Previous studies have emphasized the importance of surgical intervention following aneurysm rupture, and our study further underscores the crucial role of early surgical treatment in improving prognosis, although the optimal surgical approach remains controversial [26]. Surgically treated aneurysms generally have a more favorable prognosis. While previous studies have identified female gender and Fisher score as predictors of prognosis in patients with aneurysmal subarachnoid hemorrhage [14,27], these factors did not show predictive significance in our study, possibly due to inter-ethnic differences and selection bias in our subject population.
4.1. Limitations
Despite the excellent predictive performance of the modified Hijdra score in assessing the prognosis of patients with aSAH, the score has certain limitations and shortcomings. For instance, it relies solely on the clinical characteristics of patients and does not account for the influence of other factors such as genetic and environmental factors. Furthermore, our study was based on a regression analysis with a relatively small sample size, warranting further validation with a larger sample in future studies. Prospective studies should also be conducted to further establish the reliability of the score.
5. Conclusion
In conclusion, the modified Hijdra score is a valid and user-friendly clinical tool that can aid in the diagnosis of intracranial aneurysms and serve as an independent risk factor for predicting the prognosis of aneurysms.
Availability of data and material
The datasets used or analyzed during the current study are available from the corresponding author on reasonable request.
CRediT authorship contribution statement
Rongjie Wu: Writing – original draft, Methodology, Data curation. Fangbo Hu: Software, Formal analysis, Data curation. Changtao Liu: Supervision, Project administration. Jingshan Liang: Writing – review & editing, Supervision, Resources, Funding acquisition, Conceptualization.
Declaration of competing interest
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests: Jingshan Liang reports financial support was provided by Project of Health and Science of Lianyungang City (202002). Jingshan Liang reports financial support was provided by Postdoctoral Research Funding of Jiangsu Province (SBSH01). If there are other authors, they declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Contributor Information
Rongjie Wu, Email: chrio185@163.com.
Fangbo Hu, Email: 962134860@qq.com.
Changtao Liu, Email: lctgz@163.com.
Jingshan Liang, Email: neuroliang@163.com.
References
- 1.Jabbarli R., Reinhard M., Shah M., et al. Early vasospasm after aneurysmal subarachnoid hemorrhage predicts the Occurrence and severity of Symptomatic vasospasm and delayed cerebral ischemia. Cerebrovasc. Dis. 2016;41(5–6):265–272. doi: 10.1159/000443744. [DOI] [PubMed] [Google Scholar]
- 2.Said M., Odensass S., Gümüs M., et al. Comparing radiographic scores for prediction of complications and outcome of aneurysmal subarachnoid hemorrhage: which performs best? Eur. J. Neurol. 2023;30(3):659–670. doi: 10.1111/ene.15634. [DOI] [PubMed] [Google Scholar]
- 3.Kole M.J., Shea P., Albrecht J.S., et al. Utility of the Hijdra sum score in predicting risk of aneurysm in patients with subarachnoid hemorrhage: a Single-Center Experience with 550 patients. Neurosurgery. 2020;86(6):783–791. doi: 10.1093/neuros/nyz346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sadigh G., Menon R.K., Bhojak M., et al. Radiological management of angiographically negative, spontaneous intracranial subarachnoid hemorrhage: a multicenter study of utilization and diagnostic yield. Neurosurg. 2019;85(1):126–133. doi: 10.1093/neuros/nyy225. [DOI] [PubMed] [Google Scholar]
- 5.Mohan M., Islim A.I., Rasul F.T., et al. Subarachnoid haemorrhage with negative initial neurovascular imaging: a systematic review and meta-analysis. Acta Neurochir. 2019;161(10):2013–2026. doi: 10.1007/s00701-019-04025-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Woo P.Y.M., Tse T.P.K., Chan R.S.K., et al. Computed tomography interobserver agreement in the assessment of aneurysmal subarachnoid hemorrhage and predictors for clinical outcome. J. Neurointerventional Surg. 2017;9(11):1118–1124. doi: 10.1136/neurintsurg-2016-012576. [DOI] [PubMed] [Google Scholar]
- 7.Heit J.J., Pastena G.T., Nogueira R.G., et al. Cerebral angiography for evaluation of patients with CT angiogram-negative subarachnoid hemorrhage: an 11-year experience. AJNR Am J Neuroradiol. 2016;37(2):297–304. doi: 10.3174/ajnr.A4503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Agid R., Andersson T., Almqvist H., et al. Negative CT angiography findings in patients with spontaneous subarachnoid hemorrhage: when is digital subtraction angiography still needed? Am. J. Neuroradiol. 2010;31(4):696–705. doi: 10.3174/ajnr.A1884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hijdra A., Brouwers P., Vermeulen M., Vangijn J. Grading the amount of blood on computed tomograms after subarachnoid hemorrhage. Stroke. 1990;21(8):1156–1161. doi: 10.1161/01.STR.21.8.1156. [DOI] [PubMed] [Google Scholar]
- 10.Bretz J.S., Von Dincklage F., Woitzik J., et al. The Hijdra scale has significant prognostic value for the functional outcome of Fisher grade 3 patients with subarachnoid hemorrhage. Clin. Neuroradiol. 2017;27(3):361–369. doi: 10.1007/s00062-016-0509-0. [DOI] [PubMed] [Google Scholar]
- 11.Jaja B.N.R., Saposnik G., Lingsma H.F., et al. Development and validation of outcome prediction models for aneurysmal subarachnoid haemorrhage: the SAHIT multinational cohort study. BMJ. 2018;18:j5745. doi: 10.1136/bmj.j5745. Published online January. [DOI] [PubMed] [Google Scholar]
- 12.Inagawa T. Risk factors for cerebral vasospasm following aneurysmal subarachnoid hemorrhage: a review of the literature. World Neurosurg. 2016;85:56–76. doi: 10.1016/j.wneu.2015.08.052. [DOI] [PubMed] [Google Scholar]
- 13.Couret D., Boussen S., Cardoso D., et al. Computed Tomography in the Assessment of Aneurysmal Subarachnoid Haemorrhage for Clinical Outcome: an Observational Cohort Study. 2020. Published online February 17. [DOI] [Google Scholar]
- 14.Paez-Granda D., Parrilla G., Diaz-Perez J., Espinosa de Rueda M., Garcia-Villalba B., Zamarro J. Are modified Fisher Scale and bleeding pattern helpful predictors of neurological complications in non-aneurysmal subarachnoid hemorrhage? Neuroradiology. 2021;63(2):253–257. doi: 10.1007/s00234-020-02524-7. [DOI] [PubMed] [Google Scholar]
- 15.Melinosky C., Kincaid H., Claassen J., Parikh G., Badjatia N., Morris N.A. The modified Fisher scale lacks interrater reliability. Neurocrit Care. 2021;35(1):72–78. doi: 10.1007/s12028-020-01142-8. [DOI] [PubMed] [Google Scholar]
- 16.van der Steen W.E., Leemans E.L., van den Berg R., et al. Radiological scales predicting delayed cerebral ischemia in subarachnoid hemorrhage: systematic review and meta-analysis. Neuroradiology. 2019;61(3):247–256. doi: 10.1007/s00234-019-02161-9. [DOI] [PubMed] [Google Scholar]
- 17.Delgado Almandoz JE., Jagadeesan B.D., Refai D., et al. Diagnostic yield of computed tomography angiography and magnetic resonance angiography in patients with catheter angiography-negative subarachnoid hemorrhage. J. Neurosurg. 2012;117(2):309–315. doi: 10.3171/2012.4.JNS112306. [DOI] [PubMed] [Google Scholar]
- 18.Hui F.K., Tumialan L.M., Tanaka T., Cawley C.M., Zhang Y.J. Clinical differences between angiographically negative, diffuse subarachnoid hemorrhage and perimesencephalic subarachnoid hemorrhage. Neurocrit Care. 2009;11(1):64–70. doi: 10.1007/s12028-009-9203-2. [DOI] [PubMed] [Google Scholar]
- 19.Kalra V.B., Wu X., Matouk C.C., Malhotra A. Use of follow-up imaging in isolated perimesencephalic subarachnoid hemorrhage A meta-analysis. Stroke. 2015;46(2):401–406. doi: 10.1161/STROKEAHA.114.007370. [DOI] [PubMed] [Google Scholar]
- 20.Dupont S.A., Wijdicks E.F.M., Manno E.M., Lanzino G., Rabinstein A.A. Prediction of angiographic vasospasm after aneurysmal subarachnoid hemorrhage: value of the Hijdra sum scoring system. Neurocrit Care. 2009;11(2):172–176. doi: 10.1007/s12028-009-9247-3. [DOI] [PubMed] [Google Scholar]
- 21.Masson A., Boulouis G., Janot K., et al. Acute hydrocephalus and delayed cerebral infarction after aneurysmal subarachnoid hemorrhage. Acta Neurochir. 2022;164(9):2401–2408. doi: 10.1007/s00701-022-05321-8. [DOI] [PubMed] [Google Scholar]
- 22.van der Jagt M., Hasan D., Bijvoet H.W.C., et al. Validity of prediction of the site of ruptured intracranial aneurysms with CT. Neurology. 1999;52(1):34–39. doi: 10.1212/WNL.52.1.34. [DOI] [PubMed] [Google Scholar]
- 23.Hunt W.E., Kosnik E.J. Timing and perioperative care in intracranial aneurysm surgery. Clin. Neurosurg. 1974;21:79–89. doi: 10.1093/neurosurgery/21.cn_suppl_1.79. [DOI] [PubMed] [Google Scholar]
- 24.Aldrich E.F., Higashida R., Hmissi A., et al. Thick and diffuse cisternal clot independently predicts vasospasm-related morbidity and poor outcome after aneurysmal subarachnoid hemorrhage. J. Neurosurg. 2021;134(5):1553–1561. doi: 10.3171/2020.3.JNS193400. [DOI] [PubMed] [Google Scholar]
- 25.Konczalla J., Seifert V., Beck J., et al. Outcome after Hunt and Hess Grade V subarachnoid hemorrhage: a comparison of pre-coiling era (1980-1995) versus post-ISAT era (2005-2014) J. Neurosurg. 2018;128(1):100–110. doi: 10.3171/2016.8.JNS161075. [DOI] [PubMed] [Google Scholar]
- 26.Stienen M.N., Germans M., Burkhardt J.K., et al. Predictors of in-hospital death after aneurysmal subarachnoid hemorrhage: analysis of a nationwide database (Swiss SOS [Swiss study on aneurysmal subarachnoid hemorrhage]) Stroke. 2018;49(2):333–340. doi: 10.1161/STROKEAHA.117.019328. [DOI] [PubMed] [Google Scholar]
- 27.Witsch J., Kuohn L., Hebert R., et al. Early prognostication of 1-year outcome after subarachnoid hemorrhage: the FRESH score validation. J Stroke Cerebrovasc. 2019;28(10) doi: 10.1016/j.jstrokecerebrovasdis.2019.06.038. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used or analyzed during the current study are available from the corresponding author on reasonable request.